ABSTRACT
Background
Inflammation during pregnancy may aggravate iron deficiency (ID) by increasing serum hepcidin and reducing iron absorption. This could restrict iron transfer to the fetus, increasing risk of infant ID and its adverse effects.
Objectives
We aimed to assess whether iron bioavailability and/or iron transfer to the fetus is impaired in overweight/obese (OW) pregnant women with adiposity-related inflammation, compared with normal-weight (NW) pregnant women.
Methods
In this prospective study, we followed NW (n = 43) and OW (n = 40) pregnant women who were receiving iron supplements from the 14th week of gestation to term and followed their infants to age 6 mo. We administered 57Fe and 58Fe in test meals mid-second and mid-third trimester, and measured tracer kinetics throughout pregnancy and infancy.
Results
In total, 38 NW and 36 OW women completed the study to pregnancy week 36, whereas 30 NW and 27 OW mother–infant pairs completed the study to 6 mo postpartum. Both groups had comparable iron status, hemoglobin, and serum hepcidin throughout pregnancy. Compared with the NW, the OW pregnant women had 1) 43% lower fractional iron absorption (FIA) in the third trimester (P = 0.033) with median [IQR] FIA of 23.9% [11.4%–35.7%] and 13.5% [10.8%–19.5%], respectively; and 2) 17% lower maternal–fetal iron transfer from the first tracer (P = 0.051) with median [IQR] maternal–fetal iron transfer of 4.8% [4.2%–5.4%] and 4.0% [3.6%–4.6%], respectively. Compared with the infants born to NW women, infants born to OW women had lower body iron stores (BIS) with median [IQR] 7.7 [6.3–8.8] and 6.6 [4.6–9.2] mg/kg body weight at age 6 mo, respectively (P = 0.024). Prepregnancy BMI was a negative predictor of maternal–fetal iron transfer (β = −0.339, SE = 0.144, P = 0.025) and infant BIS (β = −0.237, SE = 0.026, P = 0.001).
Conclusions
Compared with NW, OW pregnant women failed to upregulate iron absorption in late pregnancy, transferred less iron to their fetus, and their infants had lower BIS. These impairments were associated with inflammation independently of serum hepcidin.
This trial was registered at clinicaltrials.gov as NCT02747316.
Keywords: inflammation, overweight, women, pregnancy, infancy, iron, deficiency, absorption, hepcidin, maternal–fetal transfer
See corresponding editorial on page 985 and article on page 1069.
Introduction
During pregnancy, additional iron is needed to support placental and fetal growth, the increase in maternal RBC mass, and to compensate for blood losses at delivery (1). As a result, the requirement for absorbed iron increases ∼10-fold from 0.8 mg/d in the first trimester to 7.5 mg/d in the third trimester (1). This increased requirement is often not covered by iron stores and dietary iron intake, and ∼38% of pregnant women worldwide are anemic (2), most due to iron deficiency (ID) (3). Maternal iron deficiency anemia (IDA) is linked to higher maternal morbidity, preterm birth, low birth weight, and reduced maternal–fetal iron transfer, which may impair cognitive development in the offspring (4).
In low- and middle-income countries, it is estimated that 31.5% of women aged 20–49 y are overweight/obese (OW) (5). In the United States, nearly two-thirds of 20- to 39-y-old women are OW (6) and 36% are obese (7). In nonpregnant women, OW increases the risk of ID (8) because excess body fat, particularly abdominal fat (9), produces inflammatory cytokines which increase hepatic hepcidin production (8–10). High circulating hepcidin blocks the release of iron from enterocytes and macrophages (10). Hepcidin synthesis is increased by inflammation, but reduced by ID and hypoxia (10). In normal-weight (NW) pregnancy, circulating hepcidin falls by pregnancy week 20 and remains suppressed until term (11, 12). The cause of hepcidin suppression during pregnancy is unclear, but decreasing body iron stores (BIS) may play a role (12). In pregnant women with OW and ID, inflammation could induce hepcidin synthesis despite low iron stores; this could reduce iron absorption and would be particularly detrimental in late pregnancy. However, the relative strength of these opposing signals on hepcidin in pregnant women is uncertain (13, 14).
In studies comparing hepcidin and iron status in NW and OW pregnant women, some found links between OW, higher hepcidin, and lower iron status (15–19), whereas others found no difference in serum hepcidin or iron status (17, 20, 21). Studies examining the effects of being OW during pregnancy on maternal–fetal iron transfer are also equivocal: whereas some studies have reported reduced iron status in newborn cord blood (15, 17), others have not (18). Thus, whether maternal obesity impairs iron absorption during pregnancy and/or newborn iron endowment remains unclear.
In this prospective study, we administered iron tracers in NW and OW pregnant women to quantify iron absorption during the second and third trimesters. We then used the circulating isotopic signature to calculate maternal–fetal iron transfer and dietary iron absorption by the infant, and assessed infant iron stores to age 6 mo. Our hypotheses were 1) in NW pregnant women, serum hepcidin would decrease over the course of pregnancy and iron absorption would increase in the third trimester compared with the second trimester; 2) in contrast, in OW pregnant women with inflammation, serum hepcidin would not decrease during pregnancy and, therefore, iron absorption would not increase in the third trimester; and 3) as a result, maternal–fetal iron transfer would be lower in OW pregnant women, and iron stores in infants born to OW mothers would be lower than in infants born to NW mothers. Primary objectives were to assess 1) iron absorption in the second and third trimesters; 2) iron transfer to the newborn; and 3) infants’ iron status over the first 6 mo of life. Secondary objectives were to assess 1) changes in iron and inflammation parameters throughout pregnancy and 3 and 6 mo postpartum; and 2) dietary iron absorption in infants over the first 6 mo of life.
Methods
Subjects
Healthy pregnant women were recruited from the prenatal clinics of the University Hospital Zurich, Switzerland; the Hospital Regional Materno Infantil de Alta Especialidad in Monterrey, Mexico; and Siriraj Hospital Bangkok, Thailand. Based on their prepregnancy BMI, we enrolled 43 NW and 40 OW pregnant women into the study (Figure 1). Inclusion criteria for the study (NCT02747316) were 1) week of pregnancy 14 ± 3; 2) age 18–45 y; 3) prepregnancy BMI (in kg/m 2) between 18.5 and 24.9 in the NW group, and >27.5 in the OW group; 4) singleton pregnancy; 5) no chronic illness and no significant medical conditions other than obesity; 6) nonsmoking; and 7) no regular use of medication. Written informed consent was obtained from all women. The protocol was approved by the ethics committees of the Canton of Zurich and ETH Zurich, Switzerland; the University of Monterrey, Mexico; and the Siriraj Institutional Review Board, Mahidol University in Bangkok.
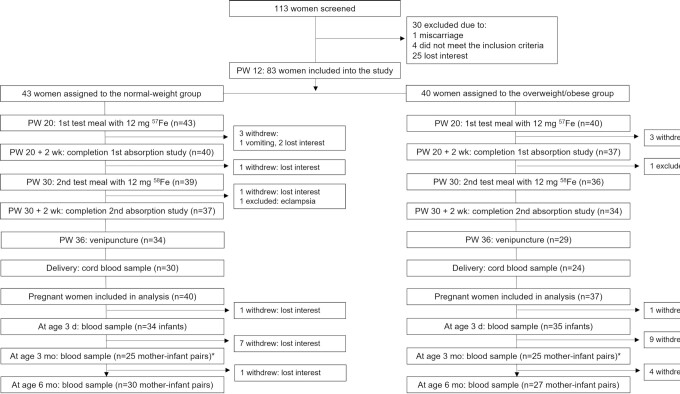
FIGURE 1.
Study design. *the ethics committee in Thailand did not approve an assessment at age 3 mo. PW, pregnancy week.
Study design
At screening, we recorded self-reported prepregnancy body weight (kg), measured height (m), calculated prepregnancy BMI, and assigned subjects to the NW or the OW group. On the first visit (pregnancy week 14 ± 3; appointments throughout the day), we measured weight to the nearest 0.1 kg on a digital scale and height to the nearest 1.0 cm with the use of a stadiometer (22) and collected venous blood for analysis of hemoglobin (Hb), iron status, inflammation, and hepcidin. We then provided all women with a local multivitamin/mineral supplement containing iron and folic acid. New supplements were given out at each prenatal visit, compliance was recorded, and the supplements were taken until term. Any additional iron recommended by the woman's doctor during pregnancy was recorded. During the second visit (pregnancy week 20 ± 2; 08:00 ± 1 h; fasting), we repeated the measures in visit 1 and the women consumed a stable iron-isotope labeled test meal under standardized conditions and close supervision (Figure 1). Women were asked not to take their iron-containing supplements in the 48 h before the test meal. Afterwards, subjects were instructed not to eat or drink for 2 h. We labeled the test meals with 12 mg 57Fe as ferrous sulfate added directly into the test meals just before consumption. The test meal consisted of a white-flour bread roll (∼90 g), topped with butter (15 g) and honey (∼30 g), given with 300 mL of distilled water. On the third visit, 14 d after the first test meal was consumed, we measured body weight and collected venous blood (throughout the day) for analysis of Hb and erythrocyte iron isotopic composition. On the fourth visit (pregnancy week 30 ± 2; 08:00 ± 1 h; fasting), we repeated the measures in visit 2 and gave a second test meal labeled with 58Fe. On the fifth visit, 14 d after the second test meal was consumed, we repeated the measures in visit 3. On the sixth visit (pregnancy week 36 ± 2; throughout the day), we repeated the measures in visit 1. Study assessments and measurements were standardized across the 3 study sites.
At delivery we collected a blood sample from the umbilical vein, then at infant age 3 ± 2 d we collected a capillary blood sample from the heel of the newborns for analysis of Hb, erythrocyte iron isotopic composition, iron status, and inflammation. At ages 3 and 6 mo, we measured infants’ and mothers’ body weight and we collected a capillary blood sample from the heel of the infant and a venous blood sample from the mother for analysis of Hb, erythrocyte iron isotopic composition, iron status, and inflammation. At ages 3 and 6 mo, mothers answered a standardized health questionnaire on mother and infant status and filled out a food frequency questionnaire (FFQ) on their infant's diet. If mothers came late for these postnatal study visits, we collected samples as long as their infant's age was ≤7 d, between 3 and 5 mo, and between 6 and 8 mo at each successive visit. We only assessed infants from the Thai subgroup at ages 3 d and 6 mo, because the ethics committee in Thailand did not approve an assessment at age 3 mo, and no data on feeding practices in Thailand were collected at 6 mo. We encouraged all women to at least partially breastfeed their infants for the first 6 postnatal months (23).
Laboratory analysis
At the 3 study sites, on the day of blood collection, we measured Hb by using a Coulter Counter or a HemoCue® Hb 201+, and separated and froze whole blood and serum aliquots. Samples from Mexico and Thailand were transferred on dry ice to ETH Zurich. At ETH Zurich, we measured soluble transferrin receptor (sTfR), serum ferritin (SF), and high-sensitivity C-reactive protein (CRP) and α-1 glycoprotein (AGP) by using a multiplex immunoassay (24), hepcidin by using immunoassay (DRG Instruments GmbH), and IL-6 by using immunoassay (R&D Systems). SF and sTfR were adjusted for inflammation using the Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia (BRINDA) regression (25). BIS were calculated using the sTfR:SF ratio (26). Fractional iron absorption (FIA) was adjusted to an SF concentration of 20 µg/L for between-group comparisons (27). Low birth weight was defined as <2500 g, whereas very low birth weight was defined as <1500 g (28). Prevalence of anemia was defined as Hb < 11.0 g/dL, ID as SF < 15 µg/L and/or sTfR > 8.3 mg/L, and IDA as ID and anemia (29, 30).
The labeled 57Fe- and 58Fe-ferrous sulfate solutions were prepared at ETH Zurich as previously described (31). The shifts of iron isotopic ratios in erythrocytes were measured by multicollector inductively coupled plasma MS as previously described (31). The amount of iron circulating in the blood was calculated on the basis of the blood volume and Hb. Blood volume was calculated taking into account varying blood volume at different values of BMI (32) and plasma expansion during pregnancy (33) (see Supplemental Material). FIA was calculated using the principles of isotope dilution (34).
Statistical analysis
We assumed an SD of 0.24 on log-transformed erythrocyte iron incorporation data based on a previous study in NW and OW nonpregnant women done at ETH Zurich (35), a power of 95%, and an α of 0.05. We calculated that a sample size of 27 subjects/group would allow us to detect a difference in FIA of 30% between the groups. Considering the long study duration and the variable course of pregnancy, we anticipated a dropout rate of 50%, resulting in a final sample size of 41 women/group.
Statistical analyses were conducted with the use of SPSS (IBM SPSS statistics, version 22) as described in the Supplemental Material. We used linear mixed-effect model (LMM) analysis with post hoc Bonferroni correction to assess the effects of group and time on maternal and infant variables. The slope of the first label concentration calculated from week of pregnancy 22 to week 36 was used to estimate total iron absorption during this period (36). We used linear regression analyses to assess the effect of prepregnancy BMI and inflammation on several variables. Maternal fetal transfer was determined using the circulating isotopic labels and total circulating iron. We assumed the first tracer, administered in approximately pregnancy week 20 to the mothers, had uniformly equilibrated during gestation in the newborn. Therefore, during infancy, we determined the fraction of total body iron absorbed per day (kabs), i.e., the rate of dilution of the first administered tracer, as described previously (36) (see Supplemental Material). P < 0.05 was considered statistically significant.
Results
Subject characteristics and attrition
We began recruiting in February 2016 and completed the study in April 2019. We screened 113 women for the study; 30 were not included because they declined participation (n = 25), did not meet the inclusion criteria (n = 4), or had an early miscarriage (n = 1). Thus, 83 women were enrolled, and we assigned 43 women to the NW group and 40 women to the OW group (Figure 1). From the 3 study sites, Switzerland, Mexico, and Thailand, we included 24, 13, and 6 NW women and 4, 30, and 6 OW women, respectively. Five women lost interest in the study before iron absorption from the first test meal was assessed; 1 woman vomited the first test meal. Thus, we analyzed data from the first iron absorption study from 77 women: 40 in the NW group, 37 in the OW group. At entry, in the NW and OW groups, mean ± SD age was 29 ± 6 and 30 ± 6 y (P = 0.526). At entry, in the NW and OW groups, median [IQR] prepregnancy weight and prepregnancy BMI were 57.3 kg [52.0–61.1 kg] and 77.5 kg [72.0–91.6 kg] and 21.6 [20.5–23.5 kg/m2] and 31.6 [28.8–34.9 kg/m2], respectively (for both, P < 0.001). Three women subsequently dropped out of the study: 1 NW woman lost interest in the study, 1 NW woman withdrew because of eclampsia, and 1 OW woman withdrew owing to miscarriage. Seventy-four women completed the study to pregnancy week 36 (NW, n = 38; OW, n = 36). The iron content from the daily supplements ranged from 30 to 80 mg and estimated mean ± SD daily iron intake was 53 ± 19 mg in the NW women and 59 ± 8 mg in the OW women (P = 0.139). We collected a capillary blood sample from 62 infants at age 3 d (NW, n = 31; OW; n = 31), from 46 at age 3 mo (NW, n = 23; OW, n = 23), and from 54 at age 6 mo (NW, n = 29; OW, n = 25). The overall dropout rate during pregnancy was 11% and during infancy it was 35%.
Anthropometric, iron, and inflammation parameters during pregnancy
Table 1 shows anthropometric, iron, and inflammation parameters during pregnancy by group. There were significant group effects on weight, blood volume, plasma volume, RBC volume, and inflammation parameters, with higher IL-6 and CRP in the OW group (for all, P < 0.01). There were significant time effects on weight, blood volume, plasma volume, RBC volume, Hb, SF, sTfR, BIS, hepcidin, IL-6, CRP, and AGP (for all, P < 0.001). There were significant time-by-group interactions on weight, plasma volume, and RBC volume (for all, P ≤ 0.05); the percentage increase in plasma volume (P = 0.014) was greater in the NW than in the OW women.
TABLE 1.
Anthropometric, hematological, iron, and inflammation parameters in pregnancy in NW and OW women1
| P value | |||||||
|---|---|---|---|---|---|---|---|
| End-first trimester2 | Mid-second trimester3 | Mid-third trimester3 | End-third trimester2 | Group | Time | Group*time | |
| Gestational age, wk | NA | NA | NA | ||||
| NW | 12.5 [12.0–13.5] (n = 40) | 19.8 [19.0–20.6] (n = 40) | 30.0 [29.4–30.2] (n = 39) | 36.1 [35.2–37.1] (n = 34) | |||
| OW | 13.0 [12.0–13.8] (n = 37) | 20.0 [18.8–21.3] (n = 37) | 30.0 [29.0–31.0] (n = 36) | 36.1 [35.1–36.5] (n = 29) | |||
| Weight, kg | <0.001 | <0.001 | <0.001 | ||||
| NW | 58.9 [52.7–64.1] (n = 37) | 61.0 [54.6–68.8] (n = 38) | 67.2 [60.1–75.1] (n = 38) | 71.2 [61.8–77.5] (n = 34) | |||
| OW | 78.0 [72.5–93.8]*** (n = 37) | 80.3 [74.7–95.3] (n = 37) | 82.7 [77.9–96.7] (n = 36) | 85.9 [80.5–100.3] (n = 29) | |||
| Blood volume,4 mL | <0.001 | <0.001 | 0.5910 | ||||
| NW | 4140 [3933–4512] (n = 40) | 4915 [4567–5242] (n = 38) | 6226 [5813–6751] (n = 38) | 6544 [6043–7002] (n = 34) | |||
| OW | 4783 [4597–5429]*** (n = 37) | 5525 [5287–6263] (n = 37) | 6878 [6550–7635] (n = 36) | 7173 [6720–7749] (n = 29) | |||
| Plasma volume,4 mL | 0.0036 | <0.001 | 0.0535 | ||||
| NW | 2591 [2466–2783] (n = 27) | 3129 [2829–3362] (n = 28) | 3952 [3722–4373] (n = 34) | 3921 [3524–4426] (n = 30) | |||
| OW | 2895 [2714–3134]*** (n = 33) | 3393 [3216–3770] (n = 34) | 4296 [3977–4577] (n = 34) | 4278 [3793–4611] (n = 28) | |||
| RBC volume,4 mL | <0.001 | <0.001 | 0.0274 | ||||
| NW | 1493 [1361–1586] (n = 27) | 1707 [1542–1857] (n = 28) | 2250 [2025–2399] (n = 34) | 2356 [2193–2525] (n = 30) | |||
| OW | 1913 [1721–2118]*** (n = 33) | 2106 [1945–2431] (n = 34) | 2657 [2468–2899] (n = 34) | 2969 [2634–3311] (n = 28) | |||
| Hemoglobin, g/dl | 0.0789 | <0.001 | 0.1996 | ||||
| NW | 12.2 [11.7–12.9] (n = 40) | 11.5 [10.8–12.1] (n = 40) | 11.7 [11.3–12.4] (n = 39) | 12.3 [11.8–12.8] (n = 34) | |||
| OW | 12.3 [11.7–13.0] (n = 37) | 11.9 [11.4–12.5] (n = 37) | 12.1 [11.6–12.5] (n = 35) | 12.6 [12.1–13.2] (n = 29) | |||
| SF adjusted, µg/L | 0.5842 | <0.001 | 0.4222 | ||||
| NW | 27.7 [17.3–48.2] (n = 40) | 22.8 [13.2–31.6] (n = 40) | 15.0 [7.9–27.2] (n = 39) | 18.2 [10.8–25.4] (n = 34) | |||
| OW | 30.6 [16.6–64.4] (n = 37) | 20.9 [12.5–45.5] (n = 37) | 10.3 [7.7–23.0] (n = 35) | 13.0 [8.8–22.5] (n = 29) | |||
| SF, µg/L | 0.7693 | <0.001 | 0.4939 | ||||
| NW | 41.2 [25.3–78.3] (n = 40) | 28.1 [17.4–48.6] (n = 40) | 20.4 [9.4–31.3] (n = 39) | 20.1 [13.1–30.4] (n = 34) | |||
| OW | 49.6 [25.6–107.7] (n = 37) | 32.3 [17.7–72.0] (n = 37) | 15.7 [10.8–28.4] (n = 35) | 18.0 [11.9–30.6] (n = 29) | |||
| sTfR adjusted, g/L | 0.1932 | <0.001 | 0.3838 | ||||
| NW | 3.8 [3.2–4.2] (n = 40) | 3.7 [3.2–4.5] (n = 40) | 4.6 [3.8–5.6] (n = 39) | 4.9 [4.1–5.9] (n = 34) | |||
| OW | 3.8 [3.3–4.5] (n = 37) | 4.2 [3.7–5.0] (n = 37) | 4.9 [4.1–5.7] (n = 35) | 5.0 [4.3–5.7] (n = 29) | |||
| sTfR, g/L | 0.2186 | <0.001 | 0.5322 | ||||
| NW | 3.9 [3.2–4.4] (n = 40) | 4.0 [3.3–4.6] (n = 40) | 4.6 [3.8–5.9] (n = 39) | 4.9 [4.1–5.9] (n = 34) | |||
| OW | 4.0 [3.4–4.8] (n = 37) | 4.3 [3.8–5.0] (n = 37) | 5.0 [4.1–5.7] (n = 35) | 5.0 [4.4–5.7] (n = 29) | |||
| BIS adjusted, mg/kg BW | 0.3792 | <0.001 | 0.5443 | ||||
| NW | 5.7 [3.6–8.5] (n = 40) | 4.8 [3.4–6.6] (n = 40) | 2.2 [0.8–5.1] (n = 39) | 3.2 [1.3–5.0] (n = 34) | |||
| OW | 5.8 [3.1–9.1] (n = 37) | 4.3 [1.0–7.5] (n = 37) | 1.6 [0.2–4.3] (n = 35) | 1.7 [0.3–4.2] (n = 29) | |||
| BIS, mg/kg BW | 0.9017 | <0.001 | 0.5909 | ||||
| NW | 7.0 [5.2–9.4] (n = 40) | 5.5 [4.3–7.2] (n = 40) | 3.7 [1.2–5.9] (n = 39) | 3.8 [2.3–5.3] (n = 34) | |||
| OW | 8.0 [4.3–10.8] (n = 37) | 5.5 [2.7–9.1] (n = 37) | 2.5 [1.2–5.3] (n = 35) | 2.9 [1.6–5.3] (n = 29) | |||
| Anemia, % (n) | NA | NA | NA | ||||
| NW | 7.5 (3) | 27.5 (11) | 12.5 (5) | 2.5 (1) | |||
| OW | 2.7 (1) | 8.1 (3) | 8.1 (3) | 0 (0) | |||
| ID, % (n) | NA | NA | NA | ||||
| NW | 15.0 (6) | 27.5 (11) | 47.5 (19) | 35.0 (14) | |||
| OW | 18.9 (7) | 37.8 (14) | 62.2 (23) | 48.6 (18) | |||
| IDA, % (n) | NA | NA | NA | ||||
| NW | 0 (0) | 2.5 (1) | 2.5 (1) | 2.5 (1) | |||
| OW | 2.7 (1) | 0 (0) | 8.1 (3) | 0 (0) | |||
| Hepcidin, nM | 0.9462 | <0.001 | 0.4400 | ||||
| NW | 3.09 [1.06–5.33] (n = 40) | 1.25 [0.66–2.16] (n = 40) | 0.64 [0.35–0.89] (n = 39) | 0.91 [0.41–1.67] (n = 34) | |||
| OW | 2.16 [0.61–5.10] (n = 37) | 1.54 [0.69–3.65] (n = 37) | 0.55 [0.32–1.22] (n = 35) | 1.16 [0.60–1.97] (n = 29) | |||
| IL-6, pg/mL | 0.0003 | <0.001 | 0.4354 | ||||
| NW | 1.41 [1.03–1.95] (n = 40) | 1.74 [1.10–2.43] (n = 40) | 1.90 [1.19–3.15] (n = 39) | 2.30 [1.39–3.17] (n = 34) | |||
| OW | 2.37 [1.91–3.85]*** (n = 36) | 3.12 [1.96–4.42] (n = 37) | 2.54 [1.80–3.54] (n = 36) | 3.30 [2.60–6.06] (n = 29) | |||
| CRP, mg/L | 0.0003 | 0.0004 | 0.3883 | ||||
| NW | 4.41 [2.52–11.50] (n = 40) | 4.42 [2.58–7.91] (n = 40) | 4.04 [2.24–7.10] (n = 39) | 3.31 [1.82–7.55] (n = 34) | |||
| OW | 8.82 [4.54–19.34]* (n = 37) | 12.31 [6.76–19.84] (n = 37) | 9.55 [3.37–14.38] (n = 36) | 10.36 [3.64–15.23] (n = 29) | |||
| AGP, g/L | 0.0872 | <0.001 | 0.9257 | ||||
| NW | 0.50 [0.40–0.70] (n = 40) | 0.42 [0.33–0.59] (n = 40) | 0.40 [0.29–0.48] (n = 39) | 0.37 [0.31–0.44] (n = 34) | |||
| OW | 0.53 [0.45–0.68] (n = 37) | 0.47 [0.38–0.60] (n = 37) | 0.42 [0.35–0.53] (n = 36) | 0.41 [0.32–0.50] (n = 29) | |||
| RBP, µmol/L | 0.7679 | 0.6504 | 0.2890 | ||||
| NW | 1.5 [1.3–1.7] (n = 40) | 1.5 [1.3–1.7] (n = 40) | 1.5 [1.3–1.8] (n = 39) | 1.5 [1.4–1.9] (n = 34) | |||
| OW | 1.5 [1.2–2.0] (n = 37) | 1.7 [1.2–2.2] (n = 37) | 1.5 [1.1–2.2] (n = 36) | 1.6 [1.3–2.0] (n = 29) | |||
Values are median [IQR] unless otherwise indicated. SF, sTfR, and BIS are adjusted for inflammation (Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia). Definitions for anemia, ID, and IDA are given in the text. Baseline characteristics at end-first trimester between the 2 groups were compared by using independent-sample t tests for normally distributed data and independent-sample Mann–Whitney U test for nonnormally distributed data. Linear mixed-effect model analysis was performed over all 4 time points with group and time as fixed effects using post hoc Bonferroni correction. *,***Different between groups: *P < 0.05; ***P < 0.001. AGP, α-1-glycoprotein; BIS, body iron stores; BW, body weight; CRP, C-reactive protein; ID, iron deficiency; IDA, iron deficiency anemia; NA, not applicable; NW, normal-weight; OW, overweight/obese; RBP, retinol-binding protein; SF, serum ferritin; sTfR, soluble transferrin receptor.
Variable daytime.
08:00 ± 1 h, fasting.
Parameters estimated using algorithms.
Iron absorption during pregnancy
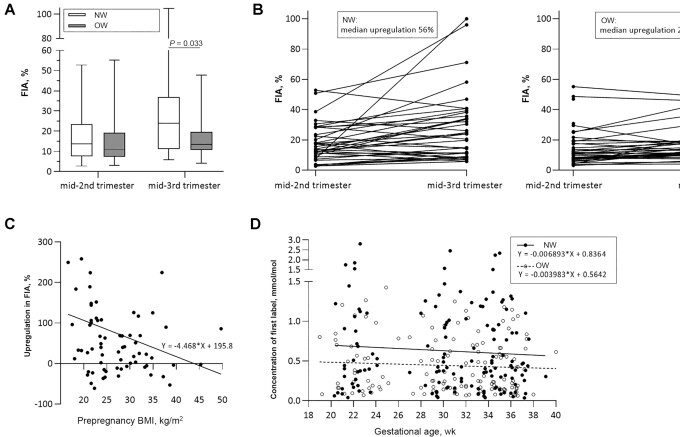
Figure 2 shows the group differences in iron absorption. The median [IQR] FIA (%) in the NW and OW groups were 13.6 [8.2–23.0] and 11.1 [7.6–19.0] in the second trimester and 23.9 [11.4–35.7] and 13.5 [10.8–19.5] in the third trimester, respectively. In LMM analysis, correcting for iron status and the mother's age, there were significant group (P = 0.046) and time (P < 0.001) effects on FIA, but no time-by-group interaction (P = 0.362). In the third trimester, post hoc test showed a significantly higher FIA in the NW group than in the OW group (P = 0.033) (Figure 2A). The increase in median [IQR] FIA from the second to the third trimester was 56% [−2% to 120%] in the NW group, and 24% [−5% to 69%] in the OW group (P = 0.204) (Figure 2B). The percentage increase in FIA from the second to the third trimester was negatively correlated with prepregnancy BMI (rs = −0.235, P = 0.050) (Figure 2C). The slope of circulating isotopically labeled 57Fe concentration from pregnancy week 22 to 36 (reflecting overall iron absorption) was more negative in the NW group than in the OW group with a median [IQR] of −0.0044 [−0.0112 to −0.0023] and −0.0029 [−0.0089 to −0.0011], respectively, but this was not statistically significant (P = 0.197) (Figure 2D).
FIGURE 2.
FIA in NW and OW pregnant women. (A) FIA in NW and OW pregnant women during the second (NW: n = 39; OW: n = 37) and third trimesters (NW: n = 37; OW: n = 34), analyzed using independent-sample t test. Lines and boxes show the median and IQR, whiskers show the range. (B) Upregulation in FIA from the second to the third trimester in NW and OW pregnant women. The increase in median [IQR] FIA from the second to the third trimester was 56% [−2% to 120%] in the NW group (n = 36) and 24% [−5% to 69%] in the OW group (n = 34) (P = 0.204). Analyzed using independent-sample t test. (C) Negative Spearman correlation between prepregnancy BMI and upregulation in FIA from the second to the third trimester, rs = −0.235, P = 0.050 (NW: n = 36; OW: n = 34). (D) The slope of circulating label concentration from pregnancy week 22 to 36 (reflecting overall iron absorption) was more negative in the NW group (n = 38) than in the OW group (n = 36), but this was not statistically significant (P = 0.197). Analyzed using independent-sample t test. FIA, fractional iron absorption; NW, normal-weight; OW, overweight/obese.
Predictors of iron status and iron absorption in pregnancy
In multiple regression analyses (Table 2), prepregnancy BMI was not a significant predictor of serum hepcidin, but it was a significant positive predictor of both sTfR (P = 0.002) and CRP (P < 0.001). Serum hepcidin (P < 0.001) and sTfR (P < 0.001) were significant negative and positive predictors of FIA, respectively, whereas CRP was a significant negative predictor of overall FIA (P = 0.005) and of FIA in the third trimester (P = 0.029), independently of serum hepcidin.
TABLE 2.
Predictors of maternal serum hepcidin and iron status during pregnancy from week 12 to 361
| B | SE of B | Standardized β | |
|---|---|---|---|
| sTfR: R2 = 0.176 | |||
| Gestational age | 0.266 | 0.037 | 0.387*** |
| Prepregnancy BMI | 0.187 | 0.060 | 0.167** |
| CRP: R2 = 0.200 | |||
| Gestational age | −0.307 | 0.135 | −0.120* |
| Prepregnancy BMI | 1.788 | 0.219 | 0.430*** |
| Serum hepcidin: R2 = 0.212 | |||
| Gestational age | −0.782 | 0.166 | −0.268*** |
| Prepregnancy BMI | 0.068 | 0.253 | 0.014 |
| sTfR | −1.221 | 0.245 | −0.287*** |
| Overall FIA: R2 = 0.472 | |||
| sTfR | 0.919 | 0.220 | 0.274*** |
| Hepcidin | −0.382 | 0.049 | −0.508*** |
| CRP | −0.156 | 0.055 | −0.177** |
| FIA second trimester: R2 = 0.514 | |||
| sTfR | 0.995 | 0.355 | 0.247** |
| Hepcidin | −0.542 | 0.079 | −0.599*** |
| CRP | −0.094 | 0.088 | −0.091 |
| FIA third trimester: R2 = 0.243 | |||
| sTfR | 0.535 | 0.217 | 0.276* |
| Hepcidin | −0.139 | 0.050 | −0.303** |
| CRP | −0.122 | 0.054 | −0.242* |
Dependent variables are not indented, whereas explanatory variables are. Analyzed using linear regression analyses. ***P < 0.001; **P < 0.01; *P < 0.05. CRP, C-reactive protein; FIA, fractional iron absorption; sTfR, soluble transferrin receptor.
Maternal–fetal iron transfer
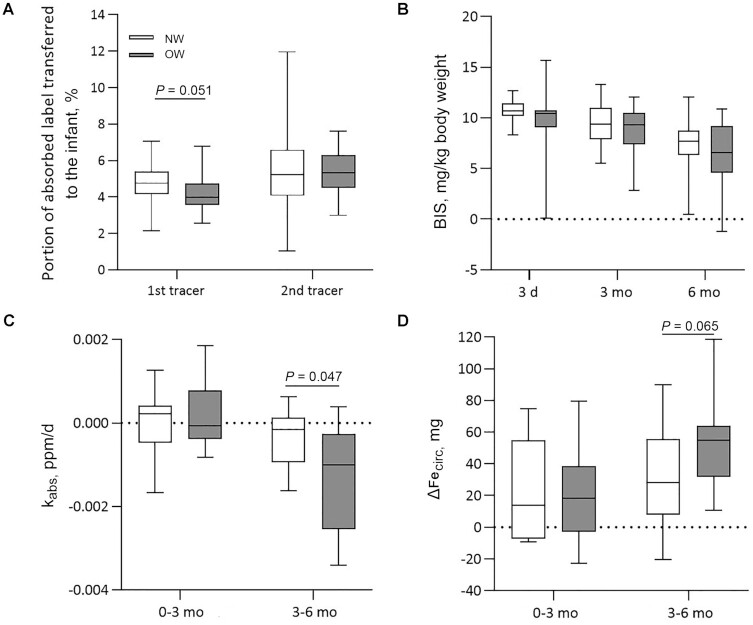
Figure 3A shows maternal–fetal iron transfer by group. There was a trend for NW women transferring a higher percentage of first tracer to their infants than OW women, with median [IQR] 4.8% [4.2%–5.4%] and 4.0% [3.6%–4.6%], respectively (P = 0.051), but this was not true for the second tracer, with median [IQR] 5.2% [4.2%–6.3%] and 5.3% [4.6%–6.2%], respectively (P = 0.965). NW women transferred a significantly higher percentage of total circulating iron to their infants than did OW women (P = 0.014) with a median [IQR] total circulating iron transferred of 5.9% [5.4%–6.4%] and 5.2% [4.2%–5.9%], respectively.
FIGURE 3.
Maternal–fetal iron transfer, iron status, and dietary iron absorption in infants born to NW and OW women ≤6 mo of age. Lines and boxes show the median and IQR, whiskers show the range. (A) Percentage of tracer transferred from the mother to the fetus, assessed in infant blood samples at 3 d of age. First administered tracer given in approximately PW 20 (NW: n = 21; OW: n = 22) and second administered tracer given in approximately PW 32 (NW: n = 22; OW: n = 22). NW women transferred a higher percentage of first tracer (P = 0.051) to their infants than did OW women. Analyzed using independent-sample t test. (B) Linear mixed-effect model analysis showed significant group (P = 0.024) and time (P < 0.001) effects on infants’ BIS over the first 6 mo of life with higher BIS in infants born to NW mothers (NW: n = 31; OW: n = 31). (C) kabs, calculated as the rate of dilution of the first administered tracer during the first and second 3 mo of life (NW: n = 12; OW: n = 11), was significantly more negative in infants of the OW group than in those of the NW group (P = 0.047). Analyzed using independent-sample t test. (D) ΔFecirc during the first and second 3 mo of infants’ life (NW: n = 12; OW: n = 11) showed a trend for being higher in the infants of the OW group (P = 0.065). Analyzed using independent-sample t test. BIS, body iron stores; kabs, fraction of total body iron absorbed per day; NW, normal-weight; OW, overweight/obese; PW, pregnancy week; ΔFecirc, changes in circulating iron.
Anthropometric, iron, and inflammation parameters in cord blood and in infancy
In the NW and OW groups, 76% (n = 29) and 60% (n = 21) of women had a vaginal delivery, 2.5% (n = 1) and 11% (n = 4) of infants were born preterm, and 5% (n = 2) and 14% (n = 5) of infants were born with low birth weight, respectively. Table 3 shows anthropometrics, breastfeeding practices, and iron and inflammation parameters in cord blood, at ages 3 d, 3 mo, and 6 mo, by group. Serum hepcidin was higher in cord blood in the NW group (P = 0.030). The numbers of exclusively and partially breastfed infants were comparable between groups. There was no significant group effect (P = 0.375) or group-by-time interaction (P = 0.651) on Hb. However, there were significant group effects on sTfR (P = 0.046) and BIS (P = 0.024) and a borderline significant group effect on SF (P = 0.095). Figure 3B indicates the trend for lower BIS over the first 6 mo of life in infants born to OW and NW women. BIS from birth to 6 mo were inversely correlated with prepregnancy BMI (rs = −0.172, P = 0.029).
TABLE 3.
Anthropometric, hematological, iron, and inflammation parameters in umbilical vein blood and in infants over the first 6 mo postpartum born to NW and OW mothers1
| Infants’ age | P values | ||||||
|---|---|---|---|---|---|---|---|
| Cord blood | 3 d | 3 mo | 6 mo | Group | Time | Group*time | |
| (Gestational) age | NA | NA | NA | ||||
| NW | 39.4 [38.1–40.2] (n = 40) | 3 [2–3] (n = 29) | 3.8 [3.5–4.3] (n = 23) | 6.5 [6.1–7.0] (n = 29) | |||
| OW | 38.6 [37.3–39.4] (n = 36) | 2 [1–3] (n = 31) | 3.7 [3.4–4.2] (n = 23) | 6.7 [6.5–7.5] (n = 27) | |||
| Sex, M/F | NA | NA | NA | ||||
| NW | 17/202 | 13/17 | 10/9 | 11/15 | |||
| OW | 16/163 | 14/15 | 9/10 | 12/12 | |||
| (Birth) weight, kg | 0.8427 | <0.001 | 0.1592 | ||||
| NW | 3.3 [2.9–3.6] (n = 38) | 3.0 [2.7–3.4]4 (n = 31) | 6.3 [5.8–6.9] (n = 21) | 7.7 [7.0–8.3] (n = 28) | |||
| OW | 3.2 [3.0–3.5] (n = 35) | 3.0 [2.7–3.3]4 (n = 32) | 6.5 [5.7–7.3] (n = 23) | 8.4 [7.6–9.1] (n = 28) | |||
| Exclusively breastfed, % (n) | NA | NA | NA | ||||
| NW | NA | NA | 40 (10) | 35 (10) | |||
| OW | NA | NA | 30 (7) | 25 (6) | |||
| Partially breastfed, % (n) | NA | NA | NA | ||||
| NW | NA | NA | 35 (8) | 35 (8) | |||
| OW | NA | NA | 30 (8) | 30 (7) | |||
| Hemoglobin, g/dl | 0.3753 | <0.001 | 0.6508 | ||||
| NW | 15.1 [14.6–16.0] (n = 27) | 17.1 [15.8–19.7] (n = 21) | 11.5 [10.6–12.2] (n = 22) | 11.1 [10.7–12.3] (n = 29) | |||
| OW | 15.1 [13.3–16.8] (n = 27) | 18.0 [16.6–19.1] (n = 25) | 11.8 [11.0–12.4] (n = 23) | 11.9 [11.4–12.6] (n = 27) | |||
| SF, µg/L | 0.0952 | <0.001 | 0.4327 | ||||
| NW | 144.9 [122.2–168.9] (n = 30) | 193.3 [177.5–207.6] (n = 31) | 123.9 [78.6–160.9] (n = 23) | 73.2 [50.3–105.8] (n = 29) | |||
| OW | 136.5 [110.3–164.2] (n = 24) | 194.6 [177.9–203.3] (n = 31) | 108.9 [75.6–161.9] (n = 23) | 50.6 [33.1–107.1] (n = 25) | |||
| sTfR, g/L | 0.0459 | <0.001 | 0.4680 | ||||
| NW | 6.5 [5.4–8.2] (n = 30) | 6.7 [5.3–8.1] (n = 31) | 5.4 [4.9–5.9] (n = 23) | 5.7 [4.9–6.6] (n = 29) | |||
| OW | 6.5 [5.1–8.4] (n = 24) | 7.8 [5.6–10.1] (n = 31) | 5.7 [5.0–7.0] (n = 23) | 5.8 [5.3–6.5] (n = 25) | |||
| BIS, mg/kg BW | 0.0238 | <0.001 | 0.9399 | ||||
| NW | 9.7 [8.3–10.9] (n = 30) | 10.7 [10.2–11.4] (n = 31) | 9.4 [7.9–11.0] (n = 23) | 7.7 [6.3–8.8] (n = 29) | |||
| OW | 9.6 [8.3–10.2] (n = 24) | 10.4 [9.1–10.8] (n = 31) | 9.3 [7.4–10.5] (n = 23) | 6.6 [4.6–9.2] (n = 25) | |||
| RBP, µmol/L | 0.7975 | <0.001 | 0.3350 | ||||
| NW | 0.85 [0.62–1.01] (n = 30) | 0.71 [0.60–0.89] (n = 31) | 0.87 [0.64–1.08] (n = 23) | 0.94 [0.77–1.10] (n = 29) | |||
| OW | 0.67 [0.60–0.83] (n = 24) | 0.69 [0.60–0.83] (n = 31) | 0.88 [0.72–1.05] (n = 23) | 1.02 [0.83–1.20] (n = 25) | |||
| Hepcidin, nM | NA | NA | NA | ||||
| NW | 11.64 [8.97–15.59]* (n = 30) | NA | NA | NA | |||
| OW | 6.65 [4.55–11.44] (n = 27) | NA | NA | NA | |||
| IL-6, pg/mL | NA | NA | NA | ||||
| NW | 2.56 [1.64–5.36] (n = 30) | NA | NA | NA | |||
| OW | 2.53 [1.86–6.21] (n = 26) | NA | NA | NA | |||
| CRP, mg/L | 0.8764 | <0.001 | 0.4177 | ||||
| NW | 0.15 [0.05–0.31] (n = 30) | 1.27 [0.59–5.16] (n = 31) | 0.26 [0.12–0.43] (n = 23) | 0.24 [0.10–1.38] (n = 29) | |||
| OW | 0.12 [0.07–0.42] (n = 24) | 0.90 [0.42–2.89] (n = 31) | 0.26 [0.15–0.57] (n = 23) | 0.28 [0.16–0.67] (n = 25) | |||
| AGP, g/L | 0.8038 | <0.001 | 0.6768 | ||||
| NW | 0.09 [0.06–0.19] (n = 30) | 0.22 [0.15–0.28] (n = 31) | 0.29 [0.25–0.48] (n = 23) | 0.42 [0.30–0.70] (n = 29) | |||
| OW | 0.09 [0.07–0.20] (n = 24) | 0.19 [0.15–0.30] (n = 31) | 0.35 [0.26–0.49] (n = 23) | 0.47 [0.41–0.76] (n = 25) | |||
Values are median [IQR] unless otherwise indicated. LMM includes infant samples from 3 d to 6 mo. LMM analyses on SF, sTfR, and BIS include AGP as a covariate. Iron status parameters are not adjusted for inflammation. The numbers of exclusively and partially breastfed infants were comparable between groups. *Different between groups (P = 0.030). AGP, α-1-glycoprotein; BIS, body iron stores; BIS, body iron stores; BW, body weight; CRP, C-reactive protein; LMM, linear mixed-effect model; NA, not applicable; NW, normal-weight; OW, overweight/obese; RBP, retinol-binding protein; SF, serum ferritin; sTfR, soluble transferrin receptor.
Sex of 1 infant unknown.
Sex of 4 infants unknown.
If infant's weight at age 3 d was not recorded, we calculated infant's weight at 3 d = birth weight minus 6%.
Iron absorption during infancy
From birth to 3 mo, kabs in infants from both groups was not significantly different from 0 (infants of the NW group, P = 0.394; infants of the OW group, P = 0.861) (Figure 3C). In contrast, from 3 to 6 mo, kabs differed from 0 in both groups (infants of the NW group, P = 0.048; infants of the OW group, P = 0.006) and kabs was significantly more negative in infants of the OW group than in those of the NW group (P = 0.047) (Figure 3C). LMM analysis showed no group effect (P = 0.154), but a significant time effect (P < 0.001) and a significant time-by-group interaction (P = 0.026) on kabs. Changes in circulating iron (ΔFecirc) over the first and second 3 mo of life are shown in Figure 3D; ΔFecirc showed a trend for being higher in the infants of the OW group (P = 0.065). LMM analysis showed no group effect (P = 0.139) and no group-by-time interaction (P = 0.173) but a significant time effect (P = 0.023) on ΔFecirc.
Predictors of iron status and iron absorption in infancy
In multiple regression analyses (Table 4), prepregnancy BMI was the strongest negative predictor of transfer of the first tracer (P = 0.025). CRP was the only significant predictor of percentage of circulating iron transferred from the mother to the infant (P = 0.005). Prepregnancy BMI (P = 0.001) and infant's age (P < 0.001) were significant negative predictors of infant BIS from birth to 6 mo.
TABLE 4.
Predictors of maternal–fetal iron transfer and infant BIS over the first 6 mo1
| Variables | B | SE of B | Standardized β |
|---|---|---|---|
| Percent first tracer transferred from mother to infant: R2 = 0.289 | |||
| Prepregnancy BMI | −0.336 | 0.144 | −0.339* |
| Hepcidin mother PW 20 | 0.087 | 0.032 | 0.390** |
| sTfR mother PW 20 | −0.042 | 0.155 | −0.041 |
| Percent second tracer transferred from mother to infant: R2 = 0.094 | |||
| Prepregnancy BMI | 0.339 | 0.289 | 0.184 |
| Hepcidin mother PW 30 | −0.046 | 0.065 | −0.111 |
| STfR mother PW 30 | 0.277 | 0.285 | 0.158 |
| Percent circulating Fe transferred from mother to infant: R2 = 0.208 | |||
| sTfR mother PW 36 | 0.117 | 0.128 | 0.154 |
| Hepcidin mother PW 36 | 0.018 | 0.036 | 0.084 |
| C-reactive protein mother PW 36 | −0.086 | 0.029 | −0.457** |
| BIS: R2 = 0.303 | |||
| Prepregnancy BMI | −0.091 | 0.026 | −0.237** |
| Infant's age | −0.016 | 0.002 | −0.497*** |
Dependent variables are not indented, whereas explanatory variables are. Analyzed using linear regression analyses. ***P < 0.001; **P < 0.01; *P < 0.05. BIS, body iron stores; PW, pregnancy week; sTfR, soluble transferrin receptor.
Maternal variables postpartum
Supplemental Table 1 shows iron and inflammation status in NW women and OW women at 3 and 6 mo postpartum. There were significant group effects on weight, sTfR, CRP, and AGP (for all, P < 0.05), but no group effects on Hb or SF.
Discussion
Main findings
Our main findings are that, compared with the NW group, 1) the OW group had higher IL-6 and CRP concentrations, but did not have higher serum hepcidin; 2) despite not having higher serum hepcidin, the OW group had lower iron absorption in late pregnancy; 3) despite having lower iron absorption late in pregnancy, the OW group did not have lower Hb or iron status; 4) despite not having lower iron status, the OW group had lower maternal–fetal iron transfer. During infancy, infants of the OW group had higher dietary iron absorption, comparable Hb, but lower BIS over 0–6 mo compared with infants of the NW group. These findings should be considered in the context that our participants received daily oral iron supplements from pregnancy week 14 to term.
Changes in iron status, inflammation, and hepcidin in pregnancy
In the first trimester, iron biomarkers in both the NW and OW groups indicated iron sufficiency (Table 1). Over pregnancy, despite iron supplementation, there were comparable declines in iron status in the OW and NW groups although nearly all women remained nonanemic at term, consistent with previous studies (1). As an acute-phase reactant, SF may have been confounded by inflammation and may not necessarily reflect a change in iron status; CRP and IL-6 were sharply higher in the OW group, consistent with previous studies (17, 21). In contrast, sTfR does not change during gestation unless maternal erythropoiesis is iron-deficient and it is less affected by inflammation (12). Thus, the increase in sTfR in both groups (Table 1) indicates onset of iron-deficient erythropoiesis as iron stores empty (37) and BIS in the OW group were ∼50% lower at term than in the NW group (Table 1). Previous studies in OW pregnant women reported varying results: several found OW was associated with lower iron status (17, 18, 16), another reported no difference compared with NW women (21), and yet another reported that OW predicted higher maternal iron status (20).
In our study, serum hepcidin decreased over pregnancy in both groups to a nadir in the mid-third trimester (Table 1), consistent with previous studies in NW and OW pregnancy (12, 38). Notably, despite higher inflammation in the OW group, including higher IL-6 [the main inducer of hepcidin during inflammation (39)], there were no group differences in maternal hepcidin over gestation, and inflammation was not a significant predictor of serum hepcidin (Table 2). This is in agreement with previous studies in healthy pregnancies, where maternal hepcidin concentrations were correlated with iron status parameters (19, 40–43) but not with inflammation markers (40, 41). Previous studies comparing serum hepcidin in OW and NW pregnant women differ: some studies found hepcidin was mildly elevated in OW compared with NW pregnant women (15, 21), whereas others did not (20). These conflicting results may be explained by the fact that net hepcidin concentrations are determined by the relative strength of the opposing stimuli of maternal inflammation and iron depletion (13, 14), and these varied between studies. In OW women, hypoxia may further suppress hepcidin through hypoxia-inducible factor-2 [HIF-2α] (44, 45) and erythroferrone (46).
Iron absorption
Despite comparable serum hepcidin in the NW and OW groups in late pregnancy, the OW group had lower iron absorption in the third trimester (Figure 2A). Supporting this, the slope of the circulating tracer abundance from pregnancy week 22 to 36 was slightly more negative in the NW group (Figure 2D); this greater dilution of the tracer suggests greater overall iron absorption and/or mobilization of iron stores in the NW group (Figure 2D). Although the cause of impaired iron absorption in the OW group is uncertain, our data suggest inflammation, independent of hepcidin, may have played a role: CRP was a negative predictor of FIA, independently of serum hepcidin, and its effect on FIA was strongest in the third trimester (Table 2). Animal data support an independent role of inflammation; in mice, stimulation of Toll-like receptors 2 and 6 triggered profound decreases in ferroportin in macrophages, liver, and spleen without changing hepcidin expression (47). Also, ferroportin mutant mice with a disrupted hepcidin/ferroportin regulation respond to injection of the Toll-like receptor 2 and 6 ligands by ferroportin downregulation and a reduction of serum iron (47). Whether these pathways are important during human pregnancy is uncertain.
Our maternal absorption values (in the range of 12%–23%) are comparable with previous studies using stable isotopes (48) and radioisotopes of iron (49, 50) in pregnant women. In a study of US pregnant women (n= 50; 21 OW, 29 NW; 38% anemia), at pregnancy weeks 31–33, median [IQR] iron absorption was 11.2% [8.3%] in the NW group and 7.7% [8.9%] in the OW group; the 45% higher absorption in the NW group was not significant (P = 0.23) (20). In contrast, in another study of US pregnant women (n = 18, 50% NW, 50% OW) (51), at pregnancy weeks 32–35 mean iron absorption was 40.4%; absorption was not correlated with prepregnancy BMI or serum hepcidin (51). In a study of UK NW pregnant women (n = 9) iron absorption was 21.1% and 37.4% at pregnancy week 24 and 36, respectively (52). Compared with these latter 2 studies, our FIA values are lower and may be more physiological, in that we administered the label in a test meal matrix, whereas most previous studies administered a labeled ferrous sulfate solution containing ascorbic acid (11, 20, 48). A recent study suggested that during pregnancy, quantifying iron absorption based on the amount of orally administered iron tracer that is incorporated into maternal RBCs underestimates maternally absorbed iron, because it fails to account for absorbed iron that is transferred to the fetus or retained within the placenta, which was estimated to be ∼10% (53). We did not try to account for absorbed tracer which may have been retained in the fetus and placenta because we were uncertain about the accuracy of this correction. Therefore, we may have determined maternal iron utilization in our study rather than true maternal iron absorption, but we feel this likely was a small difference that did not affect our overall calculations or conclusions.
Maternal–fetal iron transfer
Despite no significant differences in iron stores or serum hepcidin compared with the NW group, the OW group transferred a lower percentage of tracer given in the second trimester, but not tracer given in the third trimester (Figure 3). Prepregnancy BMI was a significant predictor of transfer of the first tracer and of estimated BIS in the newborn (Table 4). Notably, in both NW and OW women, iron transfer to the fetus was a fairly constant fraction of the amount of iron absorbed from the test meals. Several studies have assessed whether OW is a determinant of maternal–fetal iron transfer by estimating newborn iron status from cord blood iron parameters (11, 15, 17–19, 21, 54). In a study of Spanish pregnant women (18), 43% of whom were OW, maternal BMI and maternal hepcidin were not correlated with newborn (cord blood) iron status. In a study of pregnant US adolescents (21), 40% of whom were OW, maternal BMI had no clear negative impact on newborn (cord blood) iron status. In contrast, other studies in US pregnant women (n = 30) (15, 54) reported lower newborn iron status (cord blood) was predicted by obesity during pregnancy. Our estimates of maternal–fetal transfer, assessed using newborn tracer abundance, are consistent with a study of US pregnant women (n = 19) (51) that estimated mean ± SD transfer to be 4.1% ± 1.6%. In our study, maternal and newborn hepcidin were not significant predictors of circulating iron transferred to the infant, but CRP was; this suggests inflammation independent of its effect on serum hepcidin may be associated with impaired maternal–fetal transfer. Notably, we measured variables both in cord blood and in the newborn at age 3 d, and, despite correlations, the absolute values varied widely (Table 3). These findings suggest interpreting hepcidin and iron biomarkers from only cord blood samples as true “newborn” values may be problematic (see Supplemental Material).
Iron absorption and iron status during infancy
Because the newborns had been isotopically labeled in utero by the tracer given to their mothers, we were able to calculate kabs, i.e., the rate of dilution of the first administered tracer (36). kabs reflects iron absorbed and used for erythropoiesis from all exogenous (non-enriched) sources. kabs from 0 to 3 mo in infants from both groups was not significantly different from 0 (Figure 3C), suggesting that during this time infants were using mainly endogenous iron from equilibrated stores for erythropoiesis rather than exogenous dietary iron. In contrast, from 3 to 6 mo kabs significantly differed from 0 in both groups, and kabs was significantly less negative in infants of the NW group than in those of the OW group (Figure 3C). This pattern likely reflects utilization of mainly equilibrated (enriched) iron stores early in infancy during exclusive breastfeeding and then, between 3 and 6 mo, increasing use of exogenous sources of iron as complementary foods/formula are introduced (55). Our findings suggest infants of the OW mothers absorbed more exogenous iron from 3 to 6 mo.
Although evaluation of iron status during early infancy is challenging (55), our data suggest that infants of OW women had more iron-deficient erythropoiesis and lower BIS over 0–6 mo than infants born to NW women (Table 3). Taken together, our findings of lower BIS and greater iron-deficient erythropoiesis in the infants of the OW group despite greater absorption of dietary iron suggest they may have had lower birth iron stores. This would be consistent with our finding of reduced maternal–fetal iron transfer in the OW pregnant women from the tracer given in the second trimester. Despite this, the infants of the OW group had normal Hb concentrations and were not anemic, suggesting that, although BIS were reduced compared with infants of the NW group, they were still sufficient to support erythropoiesis and normal Hb concentrations during rapid growth.
Strengths and limitations
The strengths of this study are as follows: 1) we prospectively assessed iron metabolism at 4 time points during the second and third trimesters; 2) we administered different iron tracers in the second and third trimesters allowing us to distinguish their individual kinetics; 3) the tracers were given at low concentrations in meals, allowing us to describe physiological iron absorption patterns; 4) we assessed maternal–fetal iron transfer not only in cord blood but also in the newborn at age 3 d; and 5) we measured infant iron status and tracer kinetics in infants to age 6 mo. Limitations of our study include the following: 1) attrition was high after delivery; 2) iron tracer incorporation into erythrocytes and changes in blood volume during pregnancy are uncertain, and our assumptions could have biased our calculations; 3) analysis of the primary and secondary outcomes was not adjusted for multiplicity; 4) enrollment of NW and OB women was not balanced within the 3 study sites; and 5) our subjects received daily iron supplementation throughout pregnancy and nearly all were nonanemic; our findings may have been different in nonsupplemented women, and this limits the generalizability of our findings.
Conclusions
In summary, our findings indicate that, compared with NW women, OW pregnant women fail to upregulate iron absorption in late pregnancy, transfer less iron to their fetus, and their infants have lower BIS. These impairments are associated with inflammation independently of serum hepcidin. However, possibly because they were receiving iron supplements, these impairments in iron metabolism had no negative impact on Hb or anemia risk in OW mothers and their infants. Future research could investigate 1) the potential role and pathways of the effects of inflammatory adipokines during pregnancy; and 2) whether OW pregnant women and their infants with poorer iron status than in our study and/or without iron supplementation are at greater risk of IDA with its associated adverse outcomes.
Supplementary Material
Acknowledgments
We thank K Wang (ETH Zurich) for assistance in the laboratory and C Speich (ETH Zurich) for assistance with the data analysis. We also thank the participating maternity nurses at the hospitals.
The authors’ responsibilities were as follows—IH-A and MBZ: conceived the study; NUS, KC-G, DL-C, KQ-L, ACC-L, and SG: conducted the study; NUS, IH-A, CZ, and MBZ: analyzed the data; NUS, IH-A, and MBZ: wrote the first draft of the manuscript; and all authors: contributed to the editing and the finalization of the manuscript and read and approved the manuscript as submitted. The authors report no conflicts of interest.
Notes
Supported by Swiss National Science Foundation (SNF) grant 320030_156449 (to IH-A) and the ETH Zurich Laboratory of Human Nutrition.
Supplemental Material and Supplemental Table 1 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Abbreviations used: AGP, α-1 glycoprotein; BIS, body iron stores; CRP, C-reactive protein; FIA, fractional iron absorption; Hb, hemoglobin; ID, iron deficiency; IDA, iron deficiency anemia; kabs, fraction of total body iron absorbed per day; LMM, linear mixed-effect model; NW, normal-weight; OW, overweight/obese; SF, serum ferritin; sTfR, soluble transferrin receptor; ΔFecirc, changes in circulating iron.
Contributor Information
Nicole U Stoffel, Laboratory of Human Nutrition, Department of Health Science and Technology, ETH Zurich, Zürich, Switzerland.
Michael B Zimmermann, Laboratory of Human Nutrition, Department of Health Science and Technology, ETH Zurich, Zürich, Switzerland; Medical Research Council Human Immunology Unit, Medical Research Council Weatherall Institute of Molecular Medicine, University of Oxford and John Radcliffe Hospital, Oxford, United Kingdom.
Ana C Cepeda-Lopez, Department of Basic Sciences, School of Medicine, Universidad de Monterrey, Monterrey, Mexico; Sam and Ann Barshop Institute for Longevity and Aging Studies, University of Texas Health Science Center at San Antonio, TX, USA.
Karla Cervantes-Gracia, Department of Basic Sciences, School of Medicine, Universidad de Monterrey, Monterrey, Mexico.
Daniel Llanas-Cornejo, Department of Basic Sciences, School of Medicine, Universidad de Monterrey, Monterrey, Mexico.
Christophe Zeder, Laboratory of Human Nutrition, Department of Health Science and Technology, ETH Zurich, Zürich, Switzerland.
Siriporn Tuntipopipat, Institute of Nutrition, Mahidol University, Bangkok, Thailand.
Sakita Moungmaithong, Siriraj Hospital, Mahidol University, Bangkok, Thailand.
Narumon Densupsoontorn, Siriraj Hospital, Mahidol University, Bangkok, Thailand.
Katharina Quack Loetscher, Obstetrics & Gynecology, University Hospital Zurich, Zürich, Switzerland.
Sueppong Gowachirapant, Institute of Nutrition, Mahidol University, Bangkok, Thailand.
Isabelle Herter-Aeberli, Laboratory of Human Nutrition, Department of Health Science and Technology, ETH Zurich, Zürich, Switzerland.
Data Availability
Data described in the article, code book, and analytic code will not be made available for ethical reasons and data protection.
References
- 1. Bothwell TH. Iron requirements in pregnancy and strategies to meet them. Am J Clin Nutr. 2000;72(1):257S–64S. [DOI] [PubMed] [Google Scholar]
- 2. WHO . The global prevalence of anaemia in 2011. Geneva, Switzerland: World Health Organization; 2015. [Google Scholar]
- 3. Kassebaum NJ, Jasrasaria R, Naghavi M, Wulf SK, Johns N, Lozano R, Regan M, Weatherall D, Chou DP, Eisele TPet al. A systematic analysis of global anemia burden from 1990 to 2010. Blood. 2014;123(5):615–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lynch S, Pfeiffer CM, Georgieff MK, Brittenham G, Fairweather-Tait S, Hurrell RF, McArdle HJ, Raiten DJ. Biomarkers of Nutrition for Development (BOND)—iron review. J Nutr. 2018;148(suppl_1):1001S–67S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Finucane MM, Stevens GA, Cowan MJ, Danaei G, Lin JK, Paciorek CJ, Singh GM, Gutierrez HR, Lu Y, Bahalim ANet al. National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9.1 million participants. Lancet. 2011;377(9765):557–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999-2008. JAMA. 2010;303(3):235–41. [DOI] [PubMed] [Google Scholar]
- 7. Hales CM, Fryar CD, Carroll MD, Freedman DS, Ogden CL. Trends in obesity and severe obesity prevalence in US youth and adults by sex and age, 2007-2008 to 2015-2016. JAMA. 2018;319(16):1723–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cepeda-Lopez AC, Aeberli I, Zimmermann MB. Does obesity increase risk for iron deficiency? A review of the literature and the potential mechanisms. Int J Vitam Nutr Res. 2010;80(45):263–70. [DOI] [PubMed] [Google Scholar]
- 9. Stoffel NU, El-Mallah C, Herter-Aeberli I, Bissani N, Wehbe N, Obeid O, Zimmermann MB. The effect of central obesity on inflammation, hepcidin, and iron metabolism in young women. Int J Obes. 2020;44(6):1291–300. [DOI] [PubMed] [Google Scholar]
- 10. Sangkhae V, Nemeth E. Regulation of the iron homeostatic hormone hepcidin. Adv Nutr. 2017;8(1):126–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. O'Brien KO, Zavaleta N, Abrams SA, Caulfield LE. Maternal iron status influences iron transfer to the fetus during the third trimester of pregnancy. Am J Clin Nutr. 2003;77(4):924–30. [DOI] [PubMed] [Google Scholar]
- 12. Fisher AL, Nemeth E. Iron homeostasis during pregnancy. Am J Clin Nutr. 2017;106(Supplement 6):1567S–74S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Stoffel NU, Lazrak M, Bellitir S, El Mir N, El Hamdouchi A, Barkat A, Zeder C, Moretti D, Aguenaou H, Zimmermann MB. The opposing effects of acute inflammation and iron deficiency anemia on serum hepcidin and iron absorption in young women. Haematologica. 2019;104(6):1143–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sangkhae V, Nemeth E. To induce or not to induce: the fight over hepcidin regulation. Haematologica. 2019;104(6):1093–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dao MC, Sen S, Iyer C, Klebenov D, Meydani SN. Obesity during pregnancy and fetal iron status: is hepcidin the link?. J Perinatol. 2013;33(3):177–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Flynn AC, Begum S, White SL, Dalrymple K, Gill C, Alwan NA, Kiely M, Latunde-Dada G, Bell R, Briley ALet al. Relationships between maternal obesity and maternal and neonatal iron status. Nutrients. 2018;10(8):1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jones AD, Zhao G, Jiang Y-p, Zhou M, Xu G, Kaciroti N, Zhang Z, Lozoff B. Maternal obesity during pregnancy is negatively associated with maternal and neonatal iron status. Eur J Clin Nutr. 2016;70(8):918–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Garcia-Valdes L, Campoy C, Hayes H, Florido J, Rusanova I, Miranda MT, McArdle HJ. The impact of maternal obesity on iron status, placental transferrin receptor expression and hepcidin expression in human pregnancy. Int J Obes. 2015;39(4):571–8. [DOI] [PubMed] [Google Scholar]
- 19. Young MF, Griffin I, Pressman E, McIntyre AW, Cooper E, McNanley T, Harris ZL, Westerman M, O'Brien KO. Maternal hepcidin is associated with placental transfer of iron derived from dietary heme and nonheme sources. J Nutr. 2012;142(1):33–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Koenig MD, Klikuszowian E, O'Brien KO, Pauls H, Steffen A, DeMartelly V, Ruchob R, Welke L, Hemphill N, LaBomascus Bet al. Prepregnancy obesity is not associated with iron utilization during the third trimester. J Nutr. 2020;150(6):1397–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cao C, Pressman EK, Cooper EM, Guillet R, Westerman M, O'Brien KO. Prepregnancy body mass index and gestational weight gain have no negative impact on maternal or neonatal iron status. Reprod Sci. 2016;23(5):613–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gibson RS. Principles of nutritional assessment. 2nd ed. New York: Oxford University Press; 2005. [Google Scholar]
- 23. WHO . Exclusive breastfeeding for six months best for babies everywhere. [Internet]. Geneva, Switzerland: World Health Organization; 2011 [cited June 2020]; Available from: https://www.who.int/mediacentre/news/statements/2011/breastfeeding_20110115/en/. [Google Scholar]
- 24. Erhardt JG, Estes JE, Pfeiffer CM, Biesalski HK, Craft NE. Combined measurement of ferritin, soluble transferrin receptor, retinol binding protein, and C-reactive protein by an inexpensive, sensitive, and simple sandwich enzyme-linked immunosorbent assay technique. J Nutr. 2004;134(11):3127–32. [DOI] [PubMed] [Google Scholar]
- 25. Namaste SML, Rohner F, Huang J, Bhushan NL, Flores-Ayala R, Kupka R, Mei Z, Rawat R, Williams AM, Raiten DJet al. Adjusting ferritin concentrations for inflammation: Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia (BRINDA) project. Am J Clin Nutr. 2017;106(Suppl 1):359S–71S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cook JD, Flowers CH, Skikne BS. The quantitative assessment of body iron. Blood. 2003;101(9):3359–63. [DOI] [PubMed] [Google Scholar]
- 27. Cook JD, Dassenko SA, Lynch SR. Assessment of the role of nonheme-iron availability in iron balance. Am J Clin Nutr. 1991;54(4):717–22. [DOI] [PubMed] [Google Scholar]
- 28. WHO . International statistical classification of diseases and related health problems, 10th revision, 2010 edition. Geneva, Switzerland: World Health Organization; 2010. [Google Scholar]
- 29. WHO . Serum ferritin concentrations for the assessment of iron status and iron deficiency in populations. [Internet]. Geneva, Switzerland: World Health Organization; 2011; [cited 3 July, 2018]. Available from: http://www.who.int/vmnis/indicators/serum_ferritin.pdf. [Google Scholar]
- 30. WHO . Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. [Internet]. Geneva, Switzerland: World Health Organization; 2011; [cited 3 July, 2018]. Available from: http://www.who.int/vmnis/indicators/haemoglobin.pdf. [Google Scholar]
- 31. Hotz K, Walczyk T. Natural iron isotopic composition of blood is an indicator of dietary iron absorption efficiency in humans. J Biol Inorg Chem. 2013;18(1):1–7. [DOI] [PubMed] [Google Scholar]
- 32. Lemmens HJ, Bernstein DP, Brodsky JB. Estimating blood volume in obese and morbidly obese patients. Obes Surg. 2006;16(6):773–6. [DOI] [PubMed] [Google Scholar]
- 33. Aguree S, Gernand AD. Plasma volume expansion across healthy pregnancy: a systematic review and meta-analysis of longitudinal studies. BMC Pregnancy Childbirth. 2019;19(1):508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Walczyk T, Davidsson L, Zavaleta N, Hurrell RF. Stable isotope labels as a tool to determine the iron absorption by Peruvian school children from a breakfast meal. Fresenius J Anal Chem. 1997;359(4–5):445–9. [Google Scholar]
- 35. Cepeda-Lopez AC, Melse-Boonstra A, Zimmermann MB, Herter-Aeberli I. In overweight and obese women, dietary iron absorption is reduced and the enhancement of iron absorption by ascorbic acid is one-half that in normal-weight women. Am J Clin Nutr. 2015;102(6):1389–97. [DOI] [PubMed] [Google Scholar]
- 36. Speich C, Wegmüller R, Brittenham GM, Zeder C, Cercamondi CI, Buhl D, Prentice AM, Zimmermann MB, Moretti D. Measurement of long-term iron absorption and loss during iron supplementation using a stable isotope of iron (57Fe). Br J Haematol. 2021;192(1):179–89. [DOI] [PubMed] [Google Scholar]
- 37. Carriaga MT, Skikne BS, Finley B, Cutler B, Cook JD. Serum transferrin receptor for the detection of iron deficiency in pregnancy. Am J Clin Nutr. 1991;54(6):1077–81. [DOI] [PubMed] [Google Scholar]
- 38. Koenig MD, Tussing-Humphreys L, Day J, Cadwell B, Nemeth E. Hepcidin and iron homeostasis during pregnancy. Nutrients. 2014;6(8):3062–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nemeth E, Rivera S, Gabayan V, Keller C, Taudorf S, Pedersen BK, Ganz T. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J Clin Invest. 2004;113(9):1271–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. van Santen S, Kroot JJ, Zijderveld G, Wiegerinck ET, Spaanderman ME, Swinkels DW. The iron regulatory hormone hepcidin is decreased in pregnancy: a prospective longitudinal study. Clin Chem Lab Med. 2013;51(7):1395–401. [DOI] [PubMed] [Google Scholar]
- 41. Schulze KJ, Christian P, Ruczinski I, Ray AL, Nath A, Wu LS-F, Semba RD. Hepcidin and iron status among pregnant women in Bangladesh. Asia Pac J Clin Nutr. 2008;17(3):451–6. [PMC free article] [PubMed] [Google Scholar]
- 42. Bah A, Pasricha S-R, Jallow MW, Sise EA, Wegmuller R, Armitage AE, Drakesmith H, Moore SE, Prentice AM. Serum hepcidin concentrations decline during pregnancy and may identify iron deficiency: analysis of a longitudinal pregnancy cohort in The Gambia. J Nutr. 2017;147(6):1131–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rehu M, Punnonen K, Ostland V, Heinonen S, Westerman M, Pulkki K, Sankilampi U. Maternal serum hepcidin is low at term and independent of cord blood iron status. Eur J Haematol. 2010;85(4):345–52. [DOI] [PubMed] [Google Scholar]
- 44. Foti DP, Brunetti A. Editorial: “Linking hypoxia to obesity.”. Front Endocrinol. 2017;8:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mastrogiannaki M, Matak P, Mathieu JR, Delga S, Mayeux P, Vaulont S, Peyssonnaux C. Hepatic hypoxia-inducible factor-2 down-regulates hepcidin expression in mice through an erythropoietin-mediated increase in erythropoiesis. Haematologica. 2012;97(6):827–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Arezes J, Foy N, McHugh K, Sawant A, Quinkert D, Terraube V, Brinth A, Tam M, Lavallie E, Taylor Set al. Erythroferrone inhibits the induction of hepcidin by BMP6. Blood. 2018;132(14):1473–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Guida C, Altamura S, Klein FA, Galy B, Boutros M, Ulmer AJ, Hentze MW, Muckenthaler MU. A novel inflammatory pathway mediating rapid hepcidin-independent hypoferremia. Blood. 2015;125(14):2265–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. O'Brien KO, Zavaleta N, Caulfield LE, Yang D-X, Abrams SA. Influence of prenatal iron and zinc supplements on supplemental iron absorption, red blood cell iron incorporation, and iron status in pregnant Peruvian women. Am J Clin Nutr. 1999;69(3):509–15. [DOI] [PubMed] [Google Scholar]
- 49. Svanberg B, Arvidsson B, Bjorn-Rasmussen E, Hallberg L, Rossander L, Swolin B. Dietary iron absorption in pregnancy - a longitudinal study with repeated measurements of non-haeme iron absorption from whole diet. Acta Obstet Gynecol Scand. 1975;54(s48):43–68. [DOI] [PubMed] [Google Scholar]
- 50. Hahn PF, Carothers EL, Darby WJ, Martin M, Sheppard CW, Cannon RO, Beam AS, Densen PM, Peterson JC, McClellan GS. Iron metabolism in human pregnancy as studied with the radioactive isotope Fe59. Am J Obstet Gynecol. 1951;61(3):477–86. [DOI] [PubMed] [Google Scholar]
- 51. Young MF, Griffin I, Pressman E, McIntyre AW, Cooper E, McNanley T, Harris ZL, Westerman M, O'Brien KO. Utilization of iron from an animal-based iron source is greater than that of ferrous sulfate in pregnant and nonpregnant women. J Nutr. 2010;140(12):2162–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Whittaker PG, Lind T, Williams JG. Iron absorption during normal human pregnancy: a study using stable isotopes. Br J Nutr. 1991;65(3):457–63. [DOI] [PubMed] [Google Scholar]
- 53. Delaney KM, Guillet R, Pressman EK, Caulfield LE, Zavaleta N, Abrams SA, O'Brien KO. Iron absorption during pregnancy is underestimated when iron utilization by the placenta and fetus is ignored. Am J Clin Nutr. 2020;112(3):576–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Phillips AK, Roy SC, Lundberg R, Guilbert TW, Auger AP, Blohowiak SE, Coe CL, Kling PJ. Neonatal iron status is impaired by maternal obesity and excessive weight gain during pregnancy. J Perinatol. 2014;34(7):513–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Institute of Medicine . Dietary Reference Intakes for vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. Washington (DC): National Academy Press; 2001. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data described in the article, code book, and analytic code will not be made available for ethical reasons and data protection.