ABSTRACT
Background
Maternal nutrition influences fetal development and may permanently alter (“program”) offspring body composition and metabolism, thereby influencing later risk of diabetes and cardiovascular (cardiometabolic) disease. The prevalence of cardiometabolic disease is rising rapidly in India.
Objectives
To test the hypothesis that supplementing low-income Indian women with micronutrient-rich foods preconceptionally and during pregnancy has a beneficial impact on the children's body composition and cardiometabolic risk marker profiles.
Methods
Follow-up of 1255 children aged 5–10 y whose mothers took part in the Mumbai Maternal Nutrition Project [Project “SARAS”; International Standard Randomised Controlled Trial Number (ISRCTN)62811278]. Mothers were randomly assigned to receive a daily micronutrient-rich snack or a control snack of lower micronutrient content, both made from local foods, in addition to normal diet, from before pregnancy until delivery. Children's body composition was assessed using anthropometry and DXA. Their blood pressure, plasma glucose, insulin, and lipid concentrations were measured. Outcomes were compared between allocation groups with and without adjustment for confounding factors.
Results
Overall, 15% of children were stunted, 34% were wasted, and 3% were overweight. In the intention-to-treat analysis, there were no differences in body composition or risk markers between children in the intervention and control groups. Among children whose mothers started supplementation ≥3 mo before conception (the “per protocol” sample) the intervention increased adiposity among girls, but not boys. BMI in girls was increased relative to controls by 2% (95% CI: 1, 4; P = 0.01); fat mass index by 10% (95% CI: 3, 18; P = 0.004); and percent fat by 7% (95% CI: 1, 13; P = 0.01) unadjusted, with similar results in adjusted models.
Conclusions
Overall, supplementing women with micronutrient-rich foods from before pregnancy until delivery did not alter body composition or cardiometabolic risk markers in the children. Subgroup analyses showed that, if started ≥3 mo before conception, supplementation may increase adiposity among female children.
Keywords: maternal micronutrient supplementation, randomized controlled trial, India, children's body composition, children's glucose, children's insulin, children's lipids, DOHaD
Introduction
Ischemic heart disease (IHD) and type 2 diabetes (T2DM) are leading causes of disability and death worldwide (1). Although mortality from IHD is falling in the UK and other high-income countries, a trend attributed to both a falling incidence and improving medical treatment, it is increasing in low- and middle-income countries (LMICs) (1). The prevalence of T2DM is rising in all countries, along with obesity, but the most rapid increases are in LMICs, despite relatively low obesity rates (2, 3).
Around 30 y ago Barker, Hales, and others showed in a series of birth cohort studies that lower birth weight is associated with a higher risk of IHD and T2DM in adult life (4, 5). They proposed that fetal undernutrition is an important risk factor for cardiometabolic disease in later life, due to impaired development of metabolic tissues such as the pancreas, liver, kidneys, and skeletal muscle (6, 7); this became known as the “fetal programming” hypothesis. The same cohort studies showed that the highest risk of disease occurs in people who were small at birth but later became overweight (5, 8). This led to the concept that suboptimal fetal development results in reduced “metabolic capacity” throughout life, which leads to disease at a lower threshold of “metabolic load,” for example from later life obesity (9). This could explain high rates of cardiometabolic disease, out of proportion to current obesity levels, in LMICs where maternal undernutrition and low birth weight remain common problems (9).
Animal experiments, showing that undernourishing mothers leads to both fetal growth restriction and adult hypertension and diabetes in the offspring, support the fetal programming concept (10, 11). However, evidence for developmental programming in humans is still largely based on observational studies. Randomized controlled trials (RCTs) of nutritional interventions in undernourished women during pregnancy have shown that protein-energy and/or micronutrient supplements increase birth weight (12, 13). Follow-up of the children has shown reductions in blood pressure (14), fasting glucose (15), insulin resistance (16), LDL cholesterol (15), triglyceride concentrations (17), arterial stiffness (16), metabolic syndrome (17), and adiposity (18). However, these changes have been small, inconsistent across studies, and sometimes transient (19, 20) and some studies showed no beneficial effect on cardiometabolic outcomes (21, 22). Most of these trials started the nutritional intervention between 12 and 20 weeks of gestation, which would have missed events in early pregnancy that are potentially important for programming, such as placental development, the period of rapid fetal organogenesis, and periconceptional epigenetic changes (23).
The Mumbai Maternal Nutrition Project [Project “SARAS”, International Standard Randomised Controlled Trial Number [ISRCTN]62811278] was an RCT of a food-based micronutrient supplement, starting preconceptionally, for women from low-income families living in slum communities in Mumbai, India (24). The intervention was a daily snack made from micronutrient-rich local foods as a supplement to the women's normal diet. It reduced the incidence of gestational diabetes (25) and among women who started the supplement ≥3 mo prior to conception, increased birth weight, with larger effects among women who had a higher preconception BMI (24). We have now followed up the children to measure body composition and cardiometabolic risk markers at the age of 5–10 y. We hypothesized that the children of women in the intervention group would have lower cardiometabolic risk markers (blood pressure, serum lipids, plasma glucose, insulin resistance), and a healthier body composition (greater height and lean mass and lower body fat%) than children of mothers in the control group.
Methods
The trial
Project SARAS was a nonblinded individually randomized nutritional supplementation trial among women who were recruited before pregnancy in 2006–2011 (ISRCTN62811278) (24). The intervention was a daily snack made fresh each day in a trial kitchen from local micronutrient-rich vegetarian foods (green leafy vegetables, fruit, and milk) (26). Control snacks contained foods of lower micronutrient content (e.g. potato and onion). The aim was for women to take one snack every alternate day or more, for ≥3 mo before conception, and throughout pregnancy. On average, intervention snacks contained 10–23% of the WHO Reference Nutrient Intake (RNI) for β-carotene, riboflavin and vitamin B-12, folate, calcium, and iron, and 0.7 MJ of energy and 6 g of protein, compared with 0–7% RNI for the micronutrients, 0.4 MJ of energy, and 2 g of protein in control snacks (26, 27). Women were offered one snack daily; intake was supervised and the amount eaten was recorded (none, at least half, all). Women who became pregnant continued supplementation until delivery. All women were prescribed daily iron (100 mg) and folic acid (500 μg) supplements from the diagnosis of pregnancy, as per Indian government guidelines (28). Data were analyzed according to “intention-to-treat” (all women randomly assigned) and in the “per-protocol” subset of women who started supplementation >3 mo before conception. Of 6513 women recruited, 2291 became pregnant, leading to 1962 live singleton deliveries between 2007 and 2012.
Children's follow-up
The “SARAS KIDS” follow-up study took place in 2013–2018, when the children were aged 5–10 y. Ethics approval was obtained from the Intersystem Biomedica Ethics Committee, Mumbai (ISBEC/NR-54/KM/JVJ/2013). Community health workers recontacted families by telephone or home visit, explained the study, and invited parents and children to attend a local clinic for investigations. Informed parental consent and the children's assent were obtained. All investigations were carried out on 1 d except for blood samples, which were done on a separate day after an overnight fast.
Anthropometry
Weight was measured once to the nearest 10 g using digital scales (ATCO Ltd). Height was measured once to the nearest millimeter using a wall-mounted stadiometer (Microtoise, CMS Instruments). Head and midupper-arm circumferences were each measured 3 times to the nearest millimeter using anthropometric tapes, and the mean value used in the analysis. Biceps, triceps, subscapular, and supra-iliac skinfolds were each measured to the nearest millimeter 3 times using Holtain skinfold callipers (CMS Instruments), and the mean value used in the analysis.
Blood pressure and pulse rate
Systolic and diastolic blood pressures and pulse rate were measured using an Omron 705IT digital monitor. Three measurements were made after the child had been seated for 5 min, removing and reapplying the cuff between measurements. The 3 values were averaged for analysis.
Plasma glucose, insulin, and lipids
Parents were asked to ensure that the child had nothing to eat or drink except water for ≥8 h before blood sampling. They were supplied with lidocaine anesthetic cream and shown how to apply this to the venepuncture site before leaving home. Fasting venous blood samples were taken for plasma glucose and insulin and serum lipid concentrations and additional samples were taken at 30 min after an oral glucose load of 1.75 g/kg anhydrous glucose dissolved in 300 mL of water for glucose and insulin, and after 120 min for glucose. Samples were placed on ice and centrifuged at 559 × g at room temerature for 25 minutes within 1 h. Plasma glucose concentrations were measured in a commercial laboratory (Dr Dharap's Laboratory, Dadar, Mumbai) using the glucose oxidase and peroxidase method, on the day of collection, using Accurex kits (Accurex Biomedical Ltd) and an EM200 autoanalyzer (Transasia Biomedicals Ltd). Plasma insulin and lipid samples were stored at –80°C and assayed at the end of the study in the laboratory of the Diabetes Unit, King Edward Memorial Hospital, Pune. Insulin was measured using ELISA kits (Mercodia AB, SE-754 50) and a Victor X4 multilabel plate reader (Perkin Elmer Life Sciences); their detection limit is 1.0 mU/L (ISO11843-Part-4). The kit is calibrated against the 1st International Reference Preparation 66/304. Inter- and intra-assay CVs were <7%. All the lipids were measured on an automated analyzer (Dialab) using Dialab ready-to-use kits. LDL cholesterol was measured by a 2-step enzymatic selective protection method. HDL cholesterol was measured using a homogeneous method without centrifugation steps; antibodies against human lipoproteins form antigen-antibody complexes with LDL, VLDL, and chylomicrons in a way that only HDL cholesterol is selectively determined by an enzymatic measurement. Triglycerides were measured using standard enzymatic kits. Inter- and intrabatch CVs for all 3 lipid measurements were <5%. Insulin sensitivity (HOMA-S) was estimated using the iHOMA2 online calculator (29). The insulogenic index {ln[Insulin(30-min/fasting)/Glucose (30-min/fasting)]} and the product of insulogenic index and insulin sensitivity, calculated as (insulogenic index + ln HOMA-S) were calculated as measures of pancreatic β-cell function.
Body composition
Whole-body and regional fat and lean mass were measured using DXA. Scans were carried out at the Department of Radiology, Nanavati Hospital, Vile Parle, Mumbai on a Lunar Prodigy fan beam DXA scanner, using pediatric software. The machine was calibrated daily according to the manufacturer's instructions. Hand grip strength was measured as a marker of muscle function using a Jamar dynamometer. Three measurements were made with each hand, and the maximum value used in the analysis.
Socioeconomic status
The family's socioeconomic status (SES) was assessed using the Standard of Living Index (SLI) questionnaire, developed for India's National Family Health Survey, which creates a score based on the size and quality of housing and amenities and ownership of land and household assets; a higher score reflects higher SES (30).
Definitions
Height and BMI were converted into z-scores based on the WHO 2007 standard (31, 32). Stunting was defined as a height-for-age <2 SDs below the WHO median. BMI-for-age was categorized as wasting if <2 SD below the WHO median; “normal” if between –2 and +1 SD of the WHO median; and overweight/obese if >1 SD above the WHO median.
Statistical methods
Descriptive data are presented as mean ± SD for continuous normally distributed variables, median (IQR) for skewed variables, and n (%) for categorical variables. We tested the representativeness of the study sample by comparing maternal and newborn characteristics of: 1) the children studied with the remainder of births in the original trial and 2) within the study sample, between intervention and control groups. For comparisons of child outcomes between allocation groups, skewed variables were log-transformed, and all variables (except WHO z-scores and their derivatives) were adjusted for the child's age and sex. They were compared using 2-sided 2 sample t-tests for continuous variables and chi-square tests for categorical variables in: 1) the full intention-to-treat sample and in 2) the per protocol subgroup. We tested for interactions between allocation group and maternal prepregnant BMI and height as continuous variables, and the child's sex. Differences between groups are presented as mean difference and 95% CIs for normal continuous variables, as a multiplicative difference for log-transformed variables, and as ORs for binary outcomes. Statistical significance was set at P < 0.005 for comparisons of outcomes between intervention and control groups, using the Bonferroni correction for multiple testing and based on 10 “families” of outcomes (height, adiposity, lean/muscle, blood pressure, 3 lipid variables, glucose, and indices of insulin sensitivity and secretion). We used a significance level of P < 0.05 for interaction tests. Significant differences in outcomes between allocation groups were further examined using multiple linear regression, adjusting for potential confounders including maternal age, BMI, height, and parity, SES, and the child's birth weight and gestation. Kernel density plots were used to examine and compare the distribution of selected variables between allocation groups. Analyses were carried out using Stata SE v16.1 (33) and R v3.6.0 (34).
Results
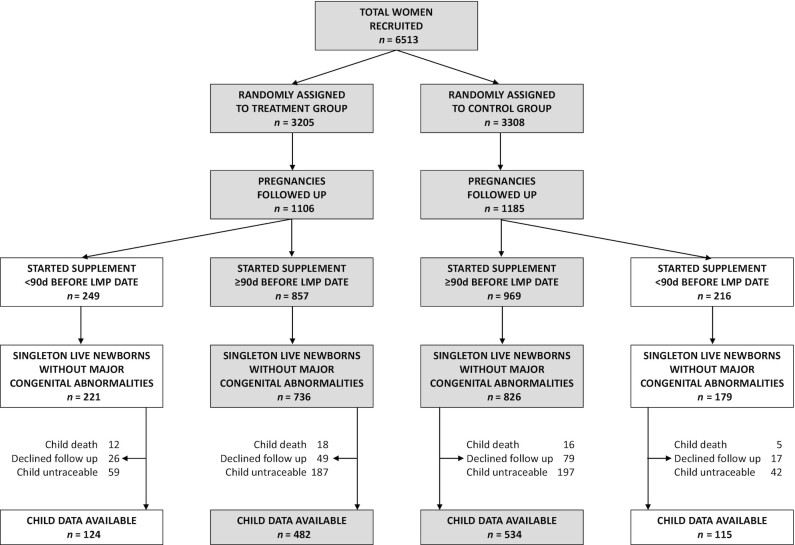
Of the 1962 live singleton births in the trial, 51 children died, 485 could not be retraced, and 171 declined to take part in the follow-up, leaving 1255 children (66% of survivors) who were studied (Figure 1). Their height- and BMI-for-age were low (mean WHO z-scores –1.0 and –1.5, respectively); 18% of boys and 13% of girls were stunted and 34% of boys and girls were wasted. Only 4% of boys and 2% of girls were overweight or obese (Table 1). Girls were more adipose than boys, whereas boys had a higher lean mass and grip strength. Boys had higher systolic blood pressure, fasting glucose concentration, and insulin sensitivity, whereas girls had a higher pulse rate, LDL cholesterol, and triglycerides (Table 1). Children studied were similar to those who were lost to follow-up in the proportions in each allocation group, maternal prepregnancy height, BMI, and gestational diabetes status, birth weight and sex ratio, but their mothers were older and of higher SES (Table 2). Among the children studied, maternal age, height, and Standard of Living Index (SLI) score were similar between the control and intervention groups, whereas maternal BMI was slightly lower in the intervention group (Table 2).
FIGURE 1.
Participant flowchart. LMP, last menstrual period.
TABLE 1.
Characteristics of the study sample1
| Intention-to-treat sample (all children studied, maximum n = 1255) | Per protocol sample (children of mothers who started supplementation ≥3 mo before conception, maximum n = 1016) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Boys | Girls | Boys | Girls | |||||||
| Outcome | n | n | P 2 | n | n | P2 | ||||
| Anthropometry | ||||||||||
| Height z-score | 670 | –1.0 ± 1.1 | 584 | –1.0 ± 0.9 | 0.44 | 552 | –1.0 ± 1.1 | 463 | –1.0 ± 0.9 | 0.45 |
| Stunted,3n (%) | 670 | 118 (17.6) | 584 | 76 (13.0) | 0.03 | 552 | 98 (17.8) | 463 | 61 (13.2) | 0.05 |
| BMI z-score | 670 | –1.5 ± 1.2 | 584 | –1.6 ± 1.1 | 0.49 | 552 | –1.5 ± 1.2 | 463 | –1.6 ± 1.1 | 0.29 |
| BMI categories,3n (%) | ||||||||||
| Wasting | 670 | 231 (34.5) | 584 | 196 (33.6) | 0.73 | 552 | 187 (33.9) | 463 | 163 (35.2) | 0.66 |
| Normal BMI | 670 | 414 (61.8) | 584 | 377 (64.6) | 0.31 | 552 | 344 (62.3) | 463 | 292 (63.1) | 0.81 |
| Overweight/obese | 670 | 25 (3.7) | 584 | 11 (1.9) | 0.05 | 552 | 21 (3.8) | 463 | 8 (1.7) | 0.05 |
| Sum of skinfolds,4 mm | 670 | 20.9 (18.2, 23.8) | 584 | 23.3 (20.2, 26.8) | <0.001 | 552 | 20.8 (18.0, 23.8) | 463 | 23.0 (20.0, 26.7) | <0.001 |
| Grip strength, kg | 669 | 7.0 ± 1.6 | 584 | 6.2 ± 1.4 | <0.001 | 551 | 7.0 ± 1.6 | 463 | 6.2 ± 1.4 | <0.001 |
| Body composition (DXA) | ||||||||||
| Fat mass,4 kg | 660 | 2.1 (1.7, 2.8) | 567 | 2.8 (2.1, 3.5) | <0.001 | 545 | 2.1 (1.7, 2.7) | 450 | 2.8 (2.1, 3.4) | <0.001 |
| Lean mass, kg | 660 | 13.5 ± 1.6 | 567 | 12.3 ± 1.4 | <0.001 | 545 | 13.5 ± 1.6 | 450 | 12.3 ± 1.4 | <0.001 |
| Percent fat,4 % | 660 | 12.9 (10.6, 15.9) | 567 | 17.2 (14.0, 20.5) | <0.001 | 545 | 13.0 (10.6, 15.9) | 450 | 17.0 (13.9, 20.2) | <0.001 |
| Cardiometabolic risk markers | ||||||||||
| Systolic BP, mmHg | 660 | 93.7 ± 8.6 | 583 | 91.9 ± 8.7 | <0.001 | 543 | 93.8 ± 8.6 | 462 | 92.0 ± 8.6 | <0.001 |
| Diastolic BP, mmHg | 660 | 56.4 ± 7.8 | 583 | 56.4 ± 7.0 | 0.95 | 543 | 56.4 ± 7.7 | 462 | 56.4 ± 7.1 | 0.94 |
| Pulse rate, beats/min | 667 | 95.7 ± 11.3 | 583 | 98.5 ± 11.5 | <0.001 | 549 | 95.9 ± 11.1 | 462 | 98.7 ± 11.8 | <0.001 |
| LDL cholesterol, mmol/L | 651 | 2.29 ± 0.61 | 559 | 2.42 ± 0.67 | <0.001 | 535 | 2.29 ± 0.59 | 444 | 2.43 ± 0.64 | <0.001 |
| HDL cholesterol, mmol/L | 651 | 1.08 ± 0.23 | 560 | 1.06 ± 0.22 | 0.17 | 535 | 1.07 ± 0.23 | 445 | 1.07 ± 0.22 | 0.62 |
| Triglycerides,4 mmol/L | 651 | 0.82 (0.68, 1.02) | 560 | 0.88 (0.72, 1.11) | 0.01 | 535 | 0.82 (0.68, 1.01) | 445 | 0.88 (0.72, 1.11) | 0.01 |
| Fasting glucose, mmol/L | 660 | 4.72 ± 0.56 | 572 | 4.62 ± 0.54 | 0.001 | 544 | 4.74 ± 0.54 | 454 | 4.62 ± 0.54 | <0.001 |
| 120-min glucose, mmol/L | 634 | 4.59 ± 0.87 | 549 | 4.73 ± 1.05 | 0.01 | 521 | 4.60 ± 0.84 | 434 | 4.73 ± 0.95 | 0.03 |
| HOMA-S4 | 644 | 238 (151, 422) | 552 | 207 (126, 323) | <0.001 | 528 | 244 (153, 414) | 437 | 207 (129, 347) | 0.004 |
| Insulogenic index | 639 | 1.7 ± 1.1 | 545 | 1.5 ± 1.2 | 0.003 | 525 | 1.7 ± 1.1 | 434 | 1.5 ± 1.2 | 0.004 |
| Disposition index | 632 | 7.1 ± 1.7 | 538 | 6.7 ± 1.8 | <0.001 | 518 | 7.1 ± 1.6 | 427 | 6.7 ± 1.8 | 0.001 |
Values are mean ± SD unless otherwise specified. All body composition and cardiometabolic outcomes were adjusted for the child's age except for z-scores and BMI categories. HOMA-S: insulin sensitivity by Homeostasis Model Assessment.
P value denotes the significance of differences between boys and girls
Categorical variables are expressed as number nand (%).
Skewed variables are expressed as median and (IQR).
BP: Blood pressure.
TABLE 2.
Representativeness of the study sample: maternal and newborn characteristics for the children included in the study sample compared with those lost to follow-up and, within the study sample, compared between maternal allocation groups1
| Included in this study maximum n = 1255 | Lost to follow-up maximum n = 707 | ||||
|---|---|---|---|---|---|
| Variable | n | n | P 2 | ||
| Among all live singleton births in the original trial: | |||||
| Allocation group,3n (%) | |||||
| Control | 649 | (51.7) | 356 | (50.4) | 0.56 |
| Intervention | 606 | (48.3) | 351 | (49.6) | |
| Maternal age, y | 1255 | 24.5 ± 3.9 | 707 | 23.5 ± 3.4 | <0.001 |
| Maternal height, cm | 1255 | 151.3 ± 5.5 | 706 | 151.6 ± 5.4 | 0.19 |
| Maternal prepregnancy BMI,4 kg/m2 | 1254 | 19.8 (17.8, 22.6) | 706 | 19.8 (17.9, 22.1) | 0.64 |
| Maternal SLI score | 1221 | 25.7 ± 5.7 | 683 | 23.7 ± 6.4 | <0.001 |
| Maternal GDM status,3n (%) | |||||
| No GDM | 660 | (89.8) | 230 | (91.3) | 0.50 |
| GDM | 75 | (10.2) | 22 | (8.7) | |
| Child's birthweight, g | 960 | 2611 ± 381 | 407 | 2611 ± 419 | 0.92 |
| Child's sex,3n (%) | |||||
| Male | 671 | (53.5) | 233 | (53.2) | 0.53 |
| Female | 584 | (46.5) | 205 | (46.8) | |
| Among children studied in this follow-up: | Control group maximum n = 649 | Intervention group maximum n = 606 | |||
| Maternal age, y | 649 | 24.7 ± 3.9 | 606 | 24.4 ± 3.8 | 0.17 |
| Maternal height, cm | 649 | 151.2 ± 5.4 | 606 | 151.3 ± 5.6 | 0.91 |
| Maternal prepregnancy BMI,4 kg/m2 | 649 | 19.9 (17.9, 22.6) | 606 | 19.6 (17.7, 22.5) | 0.04 |
| Maternal SLI score | 629 | 25.7 ± 5.6 | 592 | 25.7 ± 5.8 | 0.90 |
| Maternal GDM status,3n (%) | |||||
| No GDM | 336 | (87.7) | 324 | (92.1) | 0.05 |
| GDM | 47 | (12.3) | 28 | (8.0) | |
| Child's birth weight, g | 499 | 2594 ± 393 | 461 | 2629 (368) | 0.11 |
| Child's sex,3n (%) | |||||
| Male | 282 | (56.3) | 248 | (53.6) | 0.40 |
| Female | 219 | (43.7) | 215 | (46.4) | |
Values are mean ± SD unless otherwise specified.
P values denote the significance of differences between the groups shown
Categorical variables are expressed as number n and (%).
Skewed variables are expressed as median and (IQR).
GDM, maternal gestational diabetes mellitus; SLI: Standard of Living Index.
Effect of the intervention
There were no significant differences in any of the outcomes between children whose mothers were in the control and intervention groups, in either the intention-to-treat or per protocol samples (Table 3). The results were similar when the sample was limited to women who were fully adherent with supplementation (Supplemental Table 1).
TABLE 3.
Outcomes at 5–10 y according to allocation group1
| Intention-to-treat sample (all children studied, maximum n = 1255) | Per protocol sample (children of mothers who started supplementation ≥3 mo before conception, maximum n = 1016) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control group | Intervention group | Control group | Intervention group | |||||||||
| Outcome | n | n | P 1 | P 2 | n | n | P 1 | P 2 | ||||
| Anthropometry | ||||||||||||
| Height z-score | 649 | –1.0 ± 1.0 | 605 | –1.0 ± 1.0 | 0.78 | 0.72 | 534 | –1.0 ± 1.0 | 481 | –1.0 ± 1.0 | 0.91 | 0.88 |
| Stunted, n (%) | 649 | 97 (15.0) | 605 | 97 (16.0) | 0.60 | — | 534 | 85 (15.9) | 481 | 74 (15.4) | 0.82 | — |
| BMI z-score | 649 | –1.6 ± 1.1 | 605 | –1.5 ± 1.2 | 0.45 | 0.16 | 534 | –1.6 ± 1.1 | 481 | –1.5 ± 1.2 | 0.23 | 0.04 |
| BMI categories,3n (%) | ||||||||||||
| Wasting | 649 | 224 (34.5) | 605 | 203 (33.6) | 0.72 | — | 534 | 190 (35.6) | 481 | 160 (33.3) | 0.44 | — |
| Normal BMI | 649 | 411 (63.3) | 605 | 380 (62.8) | 0.85 | — | 534 | 332 (62.2) | 481 | 304 (63.2) | 0.74 | — |
| Overweight/obese | 649 | 14 (2.2) | 605 | 22 (3.6) | 0.12 | — | 534 | 12 (2.3) | 481 | 17 (3.5) | 0.22 | — |
| Sum of skinfolds,4 mm | 649 | 21.9 (19.1, 24.9) | 605 | 22.0 (19.1, 25.5) | 0.36 | 0.18 | 534 | 21.7 (19.0, 24.8) | 481 | 21.8 (19.0, 25.4) | 0.36 | 0.02 |
| Grip strength, kg | 648 | 6.7 ± 1.6 | 605 | 6.6 ± 1.5 | 0.63 | 0.88 | 533 | 6.7 ± 1.6 | 481 | 6.6 ± 1.5 | 0.87 | 0.88 |
| Body composition (DXA) | ||||||||||||
| Fat mass,4 kg | 637 | 2.4 (1.9, 3.1) | 590 | 2.5 (1.9, 3.1) | 0.40 | 0.06 | 525 | 2.3 (1.9, 3.0) | 470 | 2.5 (1.9, 3.1) | 0.31 | 0.01 |
| Lean mass, kg | 637 | 13.0 ± 1.5 | 590 | 13.0 ± 1.5 | 0.97 | 0.72 | 525 | 13.0 ± 1.5 | 470 | 13.0 ± 1.5 | 0.62 | 0.53 |
| Percent fat,4 % | 637 | 14.8 (12.0, 18.0) | 590 | 15.0 (12.3, 18.2) | 0.40 | 0.08 | 525 | 14.7 (11.9, 17.9) | 470 | 14.9 (12.3, 18.0) | 0.39 | 0.02 |
| Cardiometabolic risk markers | ||||||||||||
| Systolic BP, mmHg | 641 | 92.9 ± 8.4 | 602 | 92.8 ± 8.9 | 0.75 | 0.34 | 526 | 92.9 ± 8.5 | 479 | 93.1 ± 8.8 | 0.75 | 0.14 |
| Diastolic BP, mmHg | 641 | 56.2 ± 7.3 | 602 | 56.6 ± 7.4 | 0.28 | 0.60 | 526 | 56.1 ± 7.3 | 479 | 56.8 ± 7.5 | 0.14 | 0.28 |
| Pulse rate, beats/min | 646 | 97.7 ± 11.5 | 604 | 96.3 ± 11.3 | 0.03 | 0.46 | 531 | 98.1 ± 11.2 | 480 | 96.2 ± 11.5 | 0.01 | 0.44 |
| LDL cholesterol, mmol/L | 627 | 2.38 ± 0.65 | 583 | 2.31 ± 0.61 | 0.05 | 0.18 | 515 | 2.39 ± 0.63 | 464 | 2.32 ± 0.59 | 0.07 | 0.29 |
| HDL cholesterol, mmol/L | 627 | 1.07 ± 0.23 | 584 | 1.07 ± 0.22 | 0.69 | 0.14 | 515 | 1.07 ± 0.23 | 465 | 1.07 ± 0.23 | 0.71 | 0.14 |
| Triglycerides,4 mmol/L | 627 | 0.85 (0.68, 1.05) | 584 | 0.84 (0.70, 1.07) | 0.98 | 0.89 | 515 | 0.85 (0.69, 1.05) | 465 | 0.84 (0.70, 1.07) | 0.69 | 0.49 |
| Fasting glucose, mmol/L | 640 | 4.68 ± 0.52 | 592 | 4.67 ± 0.58 | 0.54 | 0.85 | 526 | 4.69 ± 0.52 | 472 | 4.69 ± 0.55 | 0.91 | 0.74 |
| 120-min glucose, mmol/L | 610 | 4.66 ± 0.88 | 573 | 4.65 ± 1.04 | 0.89 | 0.74 | 500 | 4.65 ± 0.87 | 455 | 4.67 ± 0.92 | 0.82 | 0.65 |
| HOMA-S4 | 619 | 223 (142, 387) | 577 | 223 (137, 376) | 0.56 | 0.71 | 507 | 225 (148, 403) | 458 | 228 (137, 367) | 0.45 | 0.74 |
| Insulogenic index | 614 | 1.63 ± 1.11 | 570 | 1.50 ± 1.16 | 0.06 | 0.66 | 507 | 1.66 ± 1.13 | 452 | 1.53 ±1.17 | 0.08 | 0.99 |
| Disposition index | 606 | 7.0 ± 1.7 | 564 | 6.9 ± 1.7 | 0.17 | 0.82 | 499 | 7.0 ± 1.7 | 446 | 6.9 ± 1.7 | 0.19 | 0.96 |
,2Values are mean ± SD unless otherwise specified. All body composition and cardiometabolic outcomes were adjusted for the child's age and sex except for Z-scores. P1: significance of difference between control and intervention groups; P2: significance of interaction between allocation group and sex. BP: blood pressure; HOMA-S: insulin sensitivity by Homeostasis Model Assessment.
Categorical variables are expressed as number (n) and (%).
Skewed variables are expressed as median and (IQR).
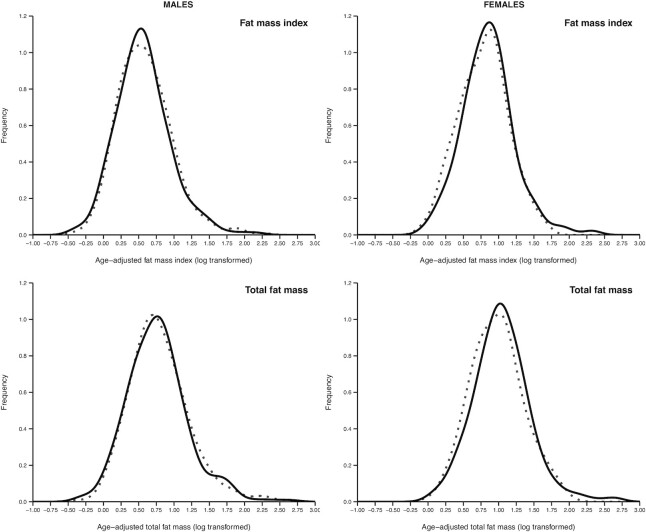
In the per protocol sample, there were significant sex interactions (allocation group × child's sex) for the adiposity outcomes: BMI, skinfolds, and fat mass and percent fat measured by DXA (Table 3). Among girls only, these adiposity measures were higher in the intervention group (Table 4); BMI was increased by 2% (95% CI: 1, 4; P = 0.01); fat mass index by 10% (95% CI: 3, 18; P = 0.004); and percent fat by 7% (95% CI: 1, 13; P = 0.01). The prevalence of wasting was decreased, and that of normal BMI and overweight/obesity increased, though none of these effects were statistically significant (Table 4). Kernel density plots suggested an approximately symmetrical right shift in fat mass and fat mass index (Figure 2). Regression analysis, adjusting for confounding factors, showed that the increased adiposity among girls in the intervention group remained significant after adjusting for maternal characteristics, and may be partly influenced by the higher birth weight in the intervention group (shown for fat mass index in Table 5 and for other adiposity measures in Supplemental Table 2). There were no interactions between allocation group and maternal BMI or height.
TABLE 4.
Adiposity measurements in the children according to the mother's allocation group, stratified by sex (per protocol sample)1
| Boys | Girls | |||||||
|---|---|---|---|---|---|---|---|---|
| Outcome | Control n = 299 | Intervention n = 253 | Difference (intervention-control)2 (95% CI) | P 3 | Control n = 235 | Intervention n = 228 | Difference (intervention-control)2 (95% CI) | P 3 |
| Anthropometry | ||||||||
| BMI,4 kg/m2 | 13.5 (12.8, 14.1) | 13.4 (12.8, 14.0) | 1.00 (0.98, 1.01) | 0.62 | 13.0 (12.3, 13.9) | 13.2 (12.6, 14.1) | 1.02 (1.01, 1.04) | 0.01 |
| BMI z-score (WHO) | –1.5 ± 1.2 | –1.5 ± 1.2 | –0.0 (–0.2, 0.1) | 0.62 | –1.7 ± 1.1 | –1.4 ± 1.2 | 0.3 (0.0, 0.5) | 0.02 |
| BMI categories,5n (%) | ||||||||
| Wasting | 100 (33.4) | 87 (34.4) | 1.04 (0.73, 1.49) | 0.82 | 90 (38.3) | 73 (32.0) | 0.76 (0.52, 1.11) | 0.16 |
| Normal BMI | 189 (63.2) | 155 (61.3) | 0.92 (0.65, 1.30) | 0.64 | 143 (60.9) | 149 (65.4) | 1.21 (0.83, 1.77) | 0.32 |
| Overweight/obese | 10 (3.3) | 11 (4.4) | 1.31 (0.55, 3.15) | 0.54 | 2 (0.9) | 6 (2.6) | 3.15 (0.63, 15.77) | 0.14 |
| Biceps skinfold,4 mm | 4.4 (3.9, 5.3) | 4.3 (3.9, 5.1) | 0.98 (0.94, 1.02) | 0.28 | 4.8 (4.1, 5.5) | 5.0 (4.3, 6.0) | 1.05 (1.01, 1.10) | 0.02 |
| Triceps skinfold,4 mm | 6.9 (5.8, 7.9) | 6.7 (5.8, 7.9) | 0.99 (0.94, 1.03) | 0.61 | 7.3 (6.1, 8.4) | 7.6 (6.4, 9.1) | 1.06 (1.01, 1.11) | 0.01 |
| Subscapular skinfold,4 mm | 5.4 (4.8, 6.3) | 5.3 (4.7, 6.3) | 0.98 (0.94, 1.02) | 0.25 | 5.9 (5.0, 6.7) | 6.2 (5.4, 7.4) | 1.07 (1.03, 1.12) | 0.002 |
| Suprailiac skinfold,4 mm | 4.0 (3.3, 4.8) | 3.9 (3.3, 4.6) | 0.98 (0.94, 1.03) | 0.53 | 4.6 (3.8, 5.5) | 4.6 (4.0, 5.7) | 1.02 (0.97, 1.07) | 0.55 |
| Sum of skinfolds,4 mm | 20.8 (18.3, 24.2) | 20.6 (17.8, 23.6) | 0.98 (0.94, 1.02) | 0.37 | 22.5 (19.7, 25.7) | 23.3 (20.3, 28.0) | 1.05 (1.01, 1.10) | 0.01 |
| Body composition (DXA) | ||||||||
| Fat mass,4 kg | 2.13 (1.71, 2.83) | 2.15 (1.63, 2.66) | 0.97 (0.90, 1.04) | 0.37 | 2.55 (2.01, 3.21) | 2.86 (2.23, 3.62) | 1.11 (1.03, 1.19) | 0.01 |
| Fat mass index,4 kg/m2 | 1.8 (1.4, 2.3) | 1.7 (1.4, 2.2) | 0.97 (0.91, 1.03) | 0.32 | 2.2 (1.7, 2.7) | 2.4 (1.8, 2.9) | 1.10 (1.03, 1.18) | 0.004 |
| Lean mass, kg | 13.5 ± 1.7 | 13.5 ± 1.7 | –0.0 (–0.3, 0.3) | 0.95 | 12.3 ± 1.4 | 12.4 ± 1.4 | 0.1 (–0.1, 0.4) | 0.36 |
| Lean mass index, kg/m2 | 11.0 ± 0.7 | 11.0 ± 0.7 | 0.0 (–0.1, 0.1) | 0.99 | 10.1 ± 0.7 | 10.2 ± 0.7 | 0.1 (–0.1, 0.2) | 0.26 |
| Percent fat,4 % | 13.0 (10.8, 16.3) | 12.9 (10.4, 15.6) | 0.98 (0.92, 1.03) | 0.36 | 16.8 (13.6, 19.8) | 17.2 (14.7, 20.6) | 1.07 (1.01, 1.13) | 0.01 |
| Android fat,4 kg | 0.15 (0.11, 0.20) | 0.14 (0.11, 0.18) | 0.98 (0.90, 1.06) | 0.61 | 0.18 (0.13, 0.23) | 0.19 (0.15, 0.25) | 1.10 (1.01, 1.20) | 0.03 |
| Gynoid fat,4 kg | 0.57 (0.48, 0.73) | 0.56 (0.44, 0.67) | 0.97 (0.91, 1.03) | 0.26 | 0.69 (0.57, 0.81) | 0.72 (0.60, 0.88) | 1.06 (1.00, 1.12) | 0.04 |
Values are mean ± SD unless otherwise specified. All outcomes are adjusted for the child's age.
Differences between allocation groups: for continuous normally distributed variables, these are expressed as raw values in the intervention group minus those in the control group, with 95% CIs. For skewed variables3, which were log-transformed for the analysis, the differences are exponentiated, and indicate the multiplicative difference between control and intervention groups; for example: a value of 1.07 means that the outcome was 7% higher in the intervention group than in the control group, whereas a value of 0.97 means that the outcome was 3% lower in the intervention group. For categorical variables2, the differences between groups are expressed as ORs, with the control group as the reference category.
P values denote the significance of differences between control and intervention groups.
Skewed variables are expressed as median and (IQR).
Categorical variables are expressed as number (n) and (%).
FIGURE 2.
Kernel density plot of fat mass and fat mass index by intervention group in girls and boys.
TABLE 5.
Multiple linear regression analysis of allocation group as a predictor of log fat mass index in girls only, adjusted for maternal and newborn characteristics (per protocol sample)
| Model 1, N = 438 | Model 2, N = 317 | |||||
|---|---|---|---|---|---|---|
| Exposure | Linear regression coefficient | 95% CIs | P value | Linear regression coefficient | 95% CIs | P value |
| Maternal | ||||||
| Allocation group (control = 0, intervention = 1) | 0.1041 | 0.037, 0.170 | 0.003 | 0.0851 | 0.004, 0.167 | 0.04 |
| Age, y | –0.009 | –0.018, –0.000 | 0.05 | –0.016 | –0.028, –0.005 | 0.005 |
| BMI, kg/m2 | 0.025 | 0.016, 0.034 | <0.001 | 0.024 | 0.013, 0.036 | <0.001 |
| Height, cm | –0.004 | –0.010, 0.002 | 0.20 | –0.005 | –0.012, 0.002 | 0.18 |
| Parity – primiparous | –0.059 | –0.139, 0.020 | 0.15 | –0.054 | –0.158, 0.050 | 0.31 |
| Parity – multiparous | –0.112 | –0.218, –0.005 | 0.04 | –0.115 | –0.243, 0.014 | 0.08 |
| Socioeconomic status score | –0.000 | –0.007, 0.006 | 0.91 | –0.003 | –0.010, 0.005 | 0.49 |
| Child | ||||||
| Age, y | 0.053 | 0.006, 0.0101 | 0.03 | 0.032 | –0.029, 0.093 | 0.31 |
| Birth weight, kg | — | — | — | 0.128 | 0.111, 0.245 | 0.03 |
| Gestational age, weeks | — | — | — | –0.012 | –0.035, 0.010 | 0.29 |
For allocation group, the regression coefficient represents the difference in log fat mass between the intervention and control groups. To translate this into a more meaningful value the coefficient is antilogged (exponentiated: values become 1.11 for Model 1 and 1.09 for Model 2) and this value indicates the multiplicative difference between control and intervention groups; for example: an exponentiated value of 1.11 means that fat mass index was 11% higher in the intervention group than in the control group.
Discussion
Summary of findings
This study examined the impact of a preconceptional maternal nutritional intervention in an RCT on cardiometabolic risk markers and body composition in the children. The intervention, a micronutrient-rich food supplement from before conception and throughout pregnancy, had no effect overall on the children's cardiometabolic risk markers or body composition. In the subgroup of children whose mothers started supplementation ≥3 mo before conception, girls had a higher BMI and were more adipose in the intervention group compared with controls.
Cardiometabolic risk markers
Possible reasons for a lack of effect on risk markers are that: 1) the intervention did not sufficiently improve maternal nutritional status; 2) a nutritional intervention alone is not sufficient to improve fetal development among women living with multiple environmental stresses likely to influence outcomes (poverty, overcrowding, pollution, inadequate sanitation); 3) a lack of obesity among these children meant that risk markers remained low in both groups; 4) the children were too young to see an effect; or 5) maternal diet and nutrition are not important influences on children's cardiometabolic risk markers. We chose a food-based supplement based on findings from the Pune Maternal Nutrition Study, and for greater acceptability and potential greater scalability in future, but the “dose” of micronutrients that it supplied was low (maximum 23% RNI) compared with other nutritional interventions used in trials, such as the United Nations International Multiple Micronutrient Preparation (UNIMMAP) tablet (100% RNI). In a separate study among nonpregnant women in Mumbai we showed that the SARAS supplement increased circulating β-carotene (35) and n–3 fatty acid concentrations (36), but did not significantly alter ferritin, retinol, ascorbate, folate, or vitamin B-12 status (35). Additionally, despite our efforts to make the snacks tasty and varied, it was challenging to sustain full adherence to supplementation over the long period of time required in a preconceptional trial. For these reasons, the effect of preconceptional nutritional supplementation requires testing in further trials, and it will be interesting to see the results of several completed or ongoing preconceptional trials which set out to deliver higher doses of multiple micronutrients in tablet or other ready-made form and achieved higher compliance rates (37–41). Ultimately, sustainable ways of improving diet quality, using food, will be necessary. Improved maternal diet on its own may not be sufficient to achieve optimal fetal development; in preventing childhood stunting, another widespread problem in LMICs caused by complex multiple exposures, combinations of interventions, targeting both health and nutrition outcomes, have proved most successful (42). That improved maternal nutrition alone may not be sufficient for optimal fetal development in the face of multiple environmental challenges is recognized in the ongoing HeLTI (Healthy Life Trajectory Initiative) and WINGS (Women and Infants Integrated Growth Study) randomized trials, which aim to improve maternal mental health as well as nutrition, and reduce infection and environmental pollution (43, 44).
Body composition
Daughters of women in the intervention group who started supplementation well before conception (>3 mo) were more adipose than daughters of women in the control group. The trial was designed to test this group separately (24), the rationale being that we would expect around 3 mo of supplementation to be required to achieve its full impact on maternal nutritional status. The effect of the intervention on adiposity in girls was physiologically significant, at ∼10% increase in fat mass index. Overall, the prevalence of wasting was 34% among the study children, whereas that of overweight/obesity was only 3%, and increased adiposity may therefore indicate more optimal nutrition. Greater adiposity provides opportunity for better future childhood and pubertal statural growth and (in girls) later reproductive outcomes. However, a gain in body fat without concomitant gains in height and lean mass could also have adverse cardiometabolic effects in adult life. Greater adiposity could reflect advanced maturation; it was not possible to determine this, although the differences in adiposity between allocation groups were not greater at older ages. Some of these possibilities will become clear with further follow-up. Most previous trials (all starting in midpregnancy) using multiple micronutrients (14, 45, 46), protein energy (16, 22), or n–3 fatty acids (47–49) reported no increase in adiposity in the children. However, in 3 multiple micronutrient supplementation trials in Burkina Faso, Nepal, and Bangladesh, children of mothers who received multiple micronutrients had higher BMI or weight-for-height z-scores at age 30 mo, 8.5 y, and 9 y, respectively (50, 19, 51). In the Nepal trial, as in Mumbai, this effect was present only in girls (19). In the Burkina Faso trial the positive effect on weight-for-height was accompanied by an increase in height (50).
Programming of adiposity
There is observational evidence in humans and interventional evidence in experimental animals that maternal undernutrition during pregnancy increases later adiposity in the children/offspring. Exposure of previously well-nourished women to the Dutch Famine in early gestation was associated with greater adult adiposity in their children, of both sexes (52, 53). In rats, both dietary restriction (either global restriction or a low-protein diet) and overfeeding of mothers during pregnancy increases adiposity in the adult offspring (11, 54–56). None of these dietary experiences or experimental manipulations remotely corresponds to our intervention in Mumbai (supplementation of mothers, many of whom were chronically undernourished, with physiological doses of micronutrient-rich foods) but they show that adipose tissue is “programmable” by maternal diet in pregnancy, including under- and overfeeding. Animal studies have shown that various mechanisms play a role in such experimental programming, including altered appetite (e.g. hyperphagia), food choices (e.g. junk food preference), reduced physical activity or resting energy expenditure, altered concentrations of or sensitivity to hormones (e.g. cortisol and leptin) or inflammatory markers, impaired mitochondrial function, altered mesenchymal stem cell commitment (to adipocyte as opposed to muscle/bone/cartilage lineages), and epigenetic changes (11, 54–56). Perhaps the closest animal experiment to our study was the “thrifty jerry” rat model, in which rats were globally undernourished for many generations, followed by recuperation onto normal feeding (57). During the undernourished phase, newborn pups were smaller than controls but became excessively adipose as adults. After a return to normal feeding (ad libitum standard chow) birth weight was restored to control levels, but adult adiposity remained, and exceeded that in the multigenerationally undernourished offspring. This was associated with epigenetic changes in the insulin-2 promoter region, which persisted after recuperation (57). Unlike our study, the increased adiposity among recuperated offspring was associated with elevated glucose, insulin, and lipid concentrations.
Sex differences
There is extensive literature from experimental animals reporting sex differences in phenotypic outcomes in offspring following maternal nutritional deprivation or overfeeding (58, 59). For example in rats, maternal protein deprivation during pregnancy consistently leads to raised adult blood pressure in male but not female offspring. There are isolated examples of sex differences in the human developmental programming literature, but no consistent pattern has emerged linking particular exposures or outcomes to one or other sex (58, 59). Apart from the Nepal trial described above (19), none of the child follow-ups from maternal supplementation trials in pregnancy have reported sex differences in cardiometabolic or body composition outcomes, but only a minority formally tested for sex differences. Mechanisms underlying sex differences in developmental programming in animals are still poorly understood (58–60). The fact that we observed a sex difference in the effect on adiposity only in the per protocol sample of children suggests that the critical period of exposure was periconceptional or in very early pregnancy, possibly related to sex differences in periconceptional gene expression or epigenetic characteristics in the embryo or placenta (59). In rodents, both maternal nutrient restriction and overfeeding lead to sex-specific changes in DNA methylation in the placenta (60).
Strengths and limitations
We studied a large sample of children, and cardiometabolic risk markers and body composition were measured using standard methods. A limitation was that we studied only 64% of the children born in the original trial. The greatest loss to follow-up was from families moving out of the study area, either through migration or relocation after local authority slum clearance. These losses were minimized by community health workers continually updating mobile phone numbers and attempting to retain contact with parents. We reimbursed families’ expenses to come to the clinic from the main relocation areas ∼20–30 km away. The children studied were similar to those lost to follow-up in key characteristics, but their mothers were older and of higher SES (Table 2). This could be because, in our experience, better-off families were more likely to own rather than rent their dwelling and therefore less likely to get moved out, and more likely to have a permanent mobile phone number. However, SES did not differ between allocation groups and our results were unchanged after adjusting for maternal age and SES and other potential confounding factors.
Conclusions and implications
The intervention, a preconception and pregnancy daily snack made from micronutrient-rich local foods, which increased birth weight and reduced the incidence of gestational diabetes did not alter the children's cardiometabolic risk markers. Girls of mothers who started the intervention >3 mo before conception had a higher BMI, were less likely to be wasted, and were more adipose. We do not know the significance of this for future health outcomes and will continue to follow-up these children.
Supplementary Material
ACKNOWLEDGEMENTS
We thank the SARAS parents and children, and the community health workers and research/laboratory staff who contributed to this study.
The authors’ responsibilities were as follows—the research was designed and overseen by: CHDF, RDP, NB, BMM, AAJ, and KK; it was conducted by: SAS, MG, HC, SHK, DSB, DP, AHK, HS, and PJC; statistical analysis was performed by: MJJ and CdiG; the manuscript was written by: SAS and CHDF who have primary responsibility for its final content; and all authors: read and approved the final manuscript.
Notes
The original trial was supported by grants from United States Agency for International Development (USAID), the Wellcome Trust, the Parthenon Trust, and ICICI Bank Ltd., Mumbai. The current study was funded by Medical Research Council Research Grant number: MR/M005186/1. None of the funders played a role in study design or implementation, or analysis and interpretation of data.
Author disclosures: The authors report no conflicts of interest.
Supplemental Tables 1 and 2 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/jn/.
Abbreviations used: HOMA, homeostasis model assessment; IHD, ischemic heart disease; ISRCT, International Standard Randomised Controlled Trial Number; LMIC, low- or middle-income country; T2DM, type 2 diabetes mellitus; RCT, randomized controlled trial; RNI, reference nutrient intake; SARAS, Mumbai Maternal Nutrition Project; SES, socioeconomic status; SLI, Standard of Living Index.
Contributor Information
Sirazul Ameen Sahariah, Centre for the Study of Social Change, Mumbai, India.
Meera Gandhi, Centre for the Study of Social Change, Mumbai, India.
Harsha Chopra, Centre for the Study of Social Change, Mumbai, India.
Sarah H Kehoe, Medical Research Council Lifecourse Epidemiology Centre, University of Southampton, Southampton, United Kingdom.
Matthew J Johnson, Medical Research Council Lifecourse Epidemiology Centre, University of Southampton, Southampton, United Kingdom.
Chiara di Gravio, Medical Research Council Lifecourse Epidemiology Centre, University of Southampton, Southampton, United Kingdom.
Deepak Patkar, Nanavati Hospital, Mumbai, India.
Harshad Sane, Centre for the Study of Social Change, Mumbai, India.
Patsy J Coakley, Medical Research Council Lifecourse Epidemiology Centre, University of Southampton, Southampton, United Kingdom.
Aarti H Karkera, Nanavati Hospital, Mumbai, India.
Dattatray S Bhat, Diabetes Unit, KEM Hospital, Pune, India.
Nick Brown, International Center for Maternal and Child Health, Uppsala University, Uppsala, Sweden.
Barrie M Margetts, Public Health Nutrition, University of Southampton, Southampton, United Kingdom.
Alan A Jackson, National Institute of Health Research, Biomedical Research Centre, Southampton, United Kingdom.
Kalyanaraman Kumaran, Medical Research Council Lifecourse Epidemiology Centre, University of Southampton, Southampton, United Kingdom; Epidemiology Research Unit, CSI Holdsworth Memorial Hospital, Mysore, South India.
Ramesh D Potdar, Centre for the Study of Social Change, Mumbai, India.
Caroline H D Fall, Medical Research Council Lifecourse Epidemiology Centre, University of Southampton, Southampton, United Kingdom.
Data Availability
Data described in the manuscript, code book, and analytic code will be made available upon request pending application and approval.
References
- 1. Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, Barengo NC, Beaton AZ, Benjamin EJ, Benziger CPet al. Global burden of cardiovascular diseases and risk factors, 1990–2019; update from the GBD 2019 study. J Am Coll Cardiol. 2020;76(25):2982–3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. NCD Risk Factor Collaboration (NCD-RisC) . Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4·4 million participants. Lancet North Am Ed. 2016;387(10027):1513–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. International Diabetes Federation Diabetes Atlas, 9th Edition; 2019. [Internet]. [Accessed 2021 Mar 3]. Available from: www.diabetetesatlas.org.
- 4. Barker DJ, Winter PD, Osmond C, Margetts B, Simmonds SJ. Weight in infancy and death from ischaemic heart disease. Lancet North Am Ed. 1989;334(8663):577–80. [DOI] [PubMed] [Google Scholar]
- 5. Hales CN, Barker DJP, Clark PMS, Cox LJ, Fall CHD. Fetal and infant growth and impaired glucose tolerance at age 64 years. BMJ. 1991;303(6809):1019–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Barker DJ, Gluckman PD, Godfrey KM, Harding JE, Owens JA, Robinson JS. Fetal nutrition and cardiovascular disease in adult life. Lancet North Am Ed. 1993;341(8850):938–41. [DOI] [PubMed] [Google Scholar]
- 7. Hales CN, Barker DJ. Type 2 (non-insulin-dependent) diabetes mellitus: the thrifty phenotype hypothesis. Diabetologia. 1992;35(7):595–601. [DOI] [PubMed] [Google Scholar]
- 8. Eriksson JG, Forsén T, Tuomilehto J, Osmond C, Barker DJ. Early growth and coronary heart disease in later life: longitudinal study. BMJ. 2001;322(7292):949–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wells JC, Sawaya AL, Wibaek R, Mwangome M, Poullas MS, Yajnik CS, Demaio A. The double burden of malnutrition: aetiological pathways and consequences for health. Lancet North Am Ed. 2020;395(10217):75–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Edwards LJ, Coulter CL, Symonds ME, McMillen IC. Prenatal undernutrition, glucocorticoids and the programming of adult hypertension. Clin Exp Pharmacol Physiol. 2001;28(11):938–41. [DOI] [PubMed] [Google Scholar]
- 11. Fernandez-Twinn DS, Hjort L, Novakovic B, Ozanne SE, Saffery R. Intrauterine programming of obesity and type 2 diabetes. Diabetologia. 2019;62(10):1789–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ceesay SM, Prentice AM, Cole TJ, Foord F, Weaver LT, Poskitt EM, Whitehead RG. Effects on birth weight and perinatal mortality of maternal dietary supplements in rural Gambia: 5 year randomised controlled trial. BMJ. 1997;315(7111):786–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fall CHD, Fisher DJ, Osmond C, Margetts BM, the Maternal Micronutrient Supplementation Study Group (MMSSG) . Multiple micronutrient supplementation during pregnancy in low-income countries: a meta-analysis of effects on birth size and length of gestation. Food Nutr Bull. 2009;30(4_suppl4):S533–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vaidya A, Saville N, Shrestha BP, Costello AM, Manandhar DS, Osrin D. Effects of antenatal multiple micronutrient supplementation on children's weight and size at 2 years of age in Nepal: follow-up of a double-blind randomised controlled trial. Lancet North Am Ed. 2008;371(9611):492–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ekström E-C, Lindström E, Raqib R, El Arifeen S, Basu S, Brismar K, Selling K, Persson L-A. Effects of prenatal micronutrient and early food supplementation on metabolic status of the offspring at 4.5 years of age. The MINIMat randomized trial in rural Bangladesh. Int J Epidemiol. 2016;45(5):1656–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kinra S, Rameshwar Sarma KV, Ghafoorunissa, Mendu VVR, Ravikumar R, Mohan V, Wilkinson IB, Cockcroft JR, Davey Smith G, Ben-Shlomo Y. Effect of integration of supplemental nutrition with public health programmes in pregnancy and early childhood on cardiovascular risk in rural Indian adolescents: long term follow-up of Hyderabad nutrition trial. BMJ. 2008;337(jul25 1):a605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Stewart CP, Christian P, Schulze KJ, Leclerq SC, West KP Jr, Khatry SK. Antenatal micronutrient supplementation reduces metabolic syndrome in 6- to 8-year-old children in rural Nepal. J Nutr. 2009;139(8):1575–81. [DOI] [PubMed] [Google Scholar]
- 18. Stewart CP, Christian P, LeClerq SC, West KP Jr, Khatry SK. Antenatal supplementation with folic acid + iron + zinc improves linear growth and reduces peripheral adiposity in school-age children in rural Nepal. Am J Clin Nutr. 2009;90(1):132–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Devakumar D, Chaube SS, Wells JCK, Saville NM, Ayres JG, Manandhar DS, Costello A, Osrin D. Effect of antenatal multiple micronutrient supplementation on anthropometry and blood pressure in mid-childhood in Nepal: follow-up of a double-blind randomised controlled trial. The Lancet Global Health. 2014;2(11):e654–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kinra S, Gregson J, Prabhakaran P, Gupta V, Walia GK, Bhogadi S, Gupta R, Aggarwal A, Mallinson PAC, Kulkarni Bet al. Effect of supplemental nutrition in pregnancy on offspring's risk of cardiovascular disease in young adulthood: long-term follow-up of a cluster trial from India. PLoS Med. 2020;17(7):e1003183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hawkesworth S, Walker CG, Sawo Y, Fulford AJC, Jarjou LMA, Goldberg GR, Prentice A, Prentice AM, Moore SE. Nutritional supplementation during pregnancy and offspring cardiovascular disease risk in the Gambia. Am J Clin Nutr. 2011;94(suppl_6):1853S–60S. [DOI] [PubMed] [Google Scholar]
- 22. Hawkesworth S, Wagatsuma Y, Kahn AI, Hawlader MDH, Fulford AJC, El Arifeen S, Persson L-A, Moore SE. Combined food and micronutrient supplements during pregnancy have limited impact on child blood pressure and kidney function in rural Bangladesh. J Nutr. 2013;143(5):728–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lillycrop KA, Burdge GC. Epigenetic mechanisms linking early nutrition to long term health. Best Pract Res Clin Endocrinol Metab. 2012;26(5):667–76. [DOI] [PubMed] [Google Scholar]
- 24. Potdar RD, Sahariah SA, Gandhi M, Kehoe SH, Brown N, Sane H, Dayama M, Jha S, Lawande A, Coakley PJet al. Improving women's diet quality pre-conceptionally and during gestation: effects on birth weight and prevalence of LBW; a randomized controlled efficacy trial in India (Mumbai maternal nutrition project). Am J Clin Nutr. 2014;100(5):1257–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sahariah SA, Potdar RD, Gandhi M, Kehoe SH, Brown N, Sane H, Coakley PJ, Marley-Zagar E, Chopra H, Shivshankaran Det al. A daily snack containing green leafy vegetables, fruit and milk before and during pregnancy prevented gestational diabetes in a randomized controlled trial in Mumbai, India. J Nutr. 2016;146(7):1453S–60S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shivshankaran D, Gurumurthy S, Kehoe S, Chheda PS, Margetts BM, Muley-Lotankar P, Agarwal A, Brown N, Sahariah SA, Taskar Vet al. Developing micronutrient-rich snacks for pre-conception and antenatal health: the Mumbai maternal nutrition project (MMNP). In: Thompson B, Amoroso L (eds) Combating Micronutrient Deficiencies: Food-based Approaches. CAB International, Wallingford, UK/Food and Agriculture Organization of the United Nations, Rome, 2011, pp. 214–23.. Chapter 12. [Internet]. Available from: http://www.fao.org/docrep/013/am027e/am027e00.pdf. [Google Scholar]
- 27. World Health Organization . Vitamin and mineral requirements in human nutrition. Joint FAO/WHO Expert Consultation on Human Vitamin and Mineral Requirements. 2nd ed.Geneva (Switzerland): World Health Organization; 2004. [Google Scholar]
- 28. Government of India . Guidelines for control of iron deficiency anaemia; national iron+ initiative: towards infinite potential in an anaemia free India. National Rural Health Mission, Adolescent Division, Ministry of Health and Family Welfare, Government of India; 2013.
- 29. University of Oxford . iHOMA2 (interactive HOmeostatsis Model Assessment) online calculator. [Internet]. [Accessed 2021 Dec 17]. Available from: https://www.phc.ox.ac.uk/research/technology-outputs/ihoma2. [Google Scholar]
- 30. International Institute for Population sciences (IIPS) and Operations Research Centre (ORC) Macro 2001 (1998-99) . National Family Health survey (NFHS-2), India. Maharashtra Mumbai: IIPS; 2001. [Google Scholar]
- 31. World Health Organization . Child growth standards. [Internet]. [Accessed 2021 Dec 17]. Available from: https://www.who.int/tools/child-growth-standards.
- 32. WHO fact sheet on overweight and obesity. [Internet]. [Accessed 2021 Dec 17]. Available from: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight.
- 33. StataCorp . Stata statistical software: release 16. College Station (TX): StataCorp LLC; 2019. [Internet]. [Accessed 2021 Dec 15]. Available from: https://www.stata.com/last.
- 34. R Core Team . R: a language and environment for statistical computing. R Foundation for Statistical Computing; 2020. Vienna (Austria). [Internet]. [Accessed 2021 Dec 15]. Available from: https://www.R-project.org/last. [Google Scholar]
- 35. Kehoe S, Chopra H, Sahariah SA, Bhat DS, Munshi R, Young S, Brown N, Tarwande D, Gandhi M, Margetts BMet al. Effects of a food-based intervention on markers of micronutrient status in low-income Indian women. Br J Nutr. 2015;113(5):813–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chopra HV, Kehoe SH, Sahariah SA, Sane HN, Cox VA, Tarwade DV, Margetts BM, Potdar RD, Fall CHd, Joshi SR. Effect of a daily snack containing green leafy vegetables on women's fatty acid status – a randomized controlled trial in Mumbai, India. Asia Pacific J Clin Nutr. 2018;27(4):804–17. [DOI] [PubMed] [Google Scholar]
- 37. Hambidge KM, Westcott JE, Garcés A, Figueroa L, Goudar SS, Dhaded SM, Pasha O, Ali SA, Tshefu A, Lokangaka Aet al. and the Women First Preconception Trial Study Group. A multicountry randomized controlled trial of comprehensive maternal nutrition supplementation initiated before conception: the women first trial. Am J Clin Nutr. 2019;109(2):457–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nguyen PH, Lowe AE, Martorell R, Nguyen H, Pham H, Nguyen S, Harding KB, Neufeld LM, Reinhart GA, Ramakrishnan U. Rationale, design, methodology and sample characteristics for the Vietnam pre-conceptual micronutrient supplementation trial (PRECONCEPT): a randomized controlled study. BMC Public Health. 2012;12(1):898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kumaran K, Yajnik P, Lubree H, Joglekar C, Bhat D, Katre P, Joshi S, Ladkat R, Fall CHD, Yajnik CS. The Pune rural intervention in young adolescents (PRIYA) study: design and methods. BMC Nutrition. 2017;3(1):41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Owens S, Gulati R, Fulford AJ, Sosseh F, Denison FC, Brabin BJ, Prentice AM. Periconceptional multiple-micronutrient supplementation and placental function in rural Gambian women: a double-blind, randomized, placebo-controlled trial. Am J Clin Nutr. 2015;102(6):1450–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Godfrey KM, Cutfield W, Chan S-Y, Baker PN, Chong Y-S, the NiPPeR Study Group . Nutritional intervention preconception and during pregnancy to maintain healthy glucose metabolism and offspring health (“NiPPeR”): study protocol for a randomised controlled trial. Trials. 2017;18(1):131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hossain M, Choudhury N, Abdullah KAB, Mondal P, Jackson AA, Walson J, Ahmed T. Evidence-based approaches to childhood stunting in low and middle income countries: a systematic review. Arch Dis Child. 2017;102(10):903–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kumaran K, Krishnaveni GV, Kumar GS, Manohar PP, Antonisamy B, Atkinson A, Balasubramaniam R, Bandsma RHJ, Bhutta ZA, Chandak GRet al. Protocol for a cluster randomised trial evaluating a multifaceted intervention starting preconceptionally. Early interventions to support trajectories for healthy life in India (EINSTEIN): a healthy life trajectories initiative (HeLTI) study. BMJ Open. 2021;11(2):e045862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Taneja S, Chowdhury R, Dhabhai N, Mazumder S, Upadhyay RP, Sharma S, Dewan R, Mittal P, Chellani H, Bahl Ret al. Impact of an integrated nutrition, health, water sanitation and hygiene, psychosocial care and support intervention package delivered during the pre- and peri-conception period and/or during pregnancy and early childhood on linear growth of infants in the first two years of life, birth outcomes and nutritional status of mothers: study protocol of a factorial, individually randomized controlled trial in India. Trials. 2020;21(1):127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wang W, Yan H, Zeng L, Cheng Y, Wang D, Qi L. No effect of maternal micronutrient supplementation on early childhood growth in rural western China: 30 month follow-up evaluation of a double blind, cluster randomized controlled trial. Eur J Clin Nutr. 2012;66(2):261–8. [DOI] [PubMed] [Google Scholar]
- 46. Kumordzie SM, Adu-Afarwuah S, Arimond M, Young RR, Adom T, Boatin R, Ocansey ME, Okronipa H, Prado EL, Oaks BMet al. Maternal and infant lipid-based nutritional supplementation increases height of Ghanaian children at 4–6 years only if the mother was not overweight before conception. J Nutr. 2019;149(5):847–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Brei C, Stecher L, Much D, Karla M-T, Amann-Gassner U, Shen J, Ganter C, Karampinos DC, Brunner S, Hauner H. Reduction of the n–6:n–3 long-chain PUFA ratio during pregnancy and lactation on offspring body composition: follow-up results from a randomized controlled trial up to 5 y of age. Am J Clin Nutr. 2016;103(6):1472–81. [DOI] [PubMed] [Google Scholar]
- 48. Gutierrez-Gomez Y, Stein AD, Ramakrishnan U, Barraza-Villarreal A, Moreno-Macias H, Aguilar-Salinas C, Romieu I, Rivera JA. Prenatal docosahexaenoic acid supplementation does not affect non-fasting serum lipid and glucose concentrations of offspring at 4 years of age in a follow-up of a randomized controlled clinical trial in Mexico. J Nutr. 2017;147(2):242–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Muhlhausler BS, Yelland LN, McDermott R, Tapsell L, McPhee A, Gibson RA, Makrides M. DHA supplementation during pregnancy does not reduce BMI or body fat mass in children: follow-up of the DHA to optimize mother infant outcome randomized controlled trial. Am J Clin Nutr. 2016;103(6):1489–96. [DOI] [PubMed] [Google Scholar]
- 50. Roberfroid D, Huybregts L, Lanou H, Ouedraogo L, Henry M-C, Meda N, Kolsteren P, the MISAME study group . Impact of prenatal multiple micronutrients on survival and growth during infancy: a randomized controlled trial. Am J Clin Nutr. 2012;95(4):916–24. [DOI] [PubMed] [Google Scholar]
- 51. Mannan T, Ahmed S, Akhtar E, Roy AK, Haq MA, Roy A, Kippler M, Ekström E-C, Wagatsuma Y, Raqib R. Maternal micronutrient supplementation and long term health impact in children in rural Bangladesh. PLoS One. 2016;11(8):e0161294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ravelli AC, van der Meulen J, Osmond C, Barker DJ, Bleker OP. Obesity at the age of 50 y in men and women exposed to famine prenatally. Am J Clin Nutr. 1999;70(5):811–6. [DOI] [PubMed] [Google Scholar]
- 53. Ravelli GP, Stein ZA, Susser MW. Obesity in young men after famine exposure in utero and early infancy. N Engl J Med. 1976;295(7):349–53. [DOI] [PubMed] [Google Scholar]
- 54. Isganaitis E. Developmental programming of body composition: update on evidence and mechanisms. Curr Diab Rep. 2019;19(8):60. [DOI] [PubMed] [Google Scholar]
- 55. Parlee SD, MacDougald OA. Maternal nutrition and risk of obesity in offspring: the Trojan horse of developmental plasticity. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease. 2014;1842(3):495–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Rajender Rao K, Padmavathi IJN, Raghunath M. Maternal micronutrient restriction programs the body adiposity, adipocyte function and lipid metabolism in offspring: a review. Rev Endocr Metab Disord. 2012;13(2):103–8. [DOI] [PubMed] [Google Scholar]
- 57. Hardikar AA, Satoor SN, Karandikar MS, Keech AC, Jenkins AJ, Yajnik CS. Multigenerational undernutrition increases susceptibility to obesity and diabetes that is not reversed after dietary recuperation. Cell Metab. 2015;22(2):312–9. [DOI] [PubMed] [Google Scholar]
- 58. Aiken CE, Ozanne SE. Sex differences in developmental programming models. Reproduction. 2013;145(1):R1–R13. [DOI] [PubMed] [Google Scholar]
- 59. Dearden L, Bouret SG, Ozanne SE. Sex and gender differences in developmental programming of metabolism. Molecular Metabolism. 2018;15:8–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Tarrade A, Panchenko P, Junien C, Gabory A. Placental contribution to nutritional programming of health and diseases: epigenetics and sexual dimorphism. J Exp Biol. 2015;218(1):50–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data described in the manuscript, code book, and analytic code will be made available upon request pending application and approval.