Abstract
Background
Vitamin K is a term that comprises a family of structurally related quinones, phylloquinone (PK) and the menaquinones (MKn), that share a common naphthoquinone ring but vary in sidechain length (n) and saturation. Dietary PK is a biosynthetic precursor to tissue menaquinone-4 (MK4), but little is known about the absorption and metabolism of dietary MKn.
Objective
To characterize the absorption and metabolism of dietary MKn relative to PK.
Methods
In the 4-week diet study, 10-week-old male and female C57BL/6 mice were pair-fed a vitamin K deficient diet (control) or a diet supplemented with 5.0 μmol/kg total PK, MK4, and/or MK9 (separately and in combination). In the 1-week stable isotope study, 12-week-old mice were pair-fed diets containing 2.2 μmol/kg PK (unlabeled control), 2H7PK, 13C11MK4, 2H7MK7, or 2H7MK9. Vitamin K tissue content was quantified by HPLC and/or LC-MS, and concentrations were compared by sex and diet group using 2-factor ANOVA.
Results
Regardless of the form(s) of vitamin K provided in the diet, tissue MK4 concentrations did not differ across equimolar supplemented groups in the kidney, adipose, reproductive organ, bone, or pancreas in either males or females in the diet study (all P values > 0.05). Isotopic labeling confirmed the naphthoquinone ring of MK4 in tissues originated from the administered dietary PK or MKn. Despite equimolar supplementation, accumulation of the administered dietary form differed across diet groups in small intestinal segments (all P values < 0.002) and the liver (P < 0.001). Female mice had greater total vitamin K than males in every tissue examined (P < 0.05).
Conclusions
Dietary PK, MK4, MK7, and MK9 all served as precursors to tissue MK4 in mice. This study expands our understanding of vitamin K metabolism and supports a common conversion mechanism of all dietary vitamin K forms to MK4. Further investigation of the metabolism and physiological roles of MK4 that may be independent of classical vitamin K function is warranted.
Key words: vitamin K, menaquinone, menaquinone-4, phylloquinone, UbiA prenyltransferase domain-containing protein 1, diet, metabolism
See corresponding editorial on page 917.
Introduction
Vitamin K is a term that comprises a family of the structurally related vitamers, phylloquinone (PK), and the menaquinones (MKn). All forms of vitamin K possess a 2-methyl-1,4-naphthoquinone ring (commonly referred to as menadione) with a sidechain at the 3-position, but are distinguished from each another by the length and saturation of the sidechain. Whereas PK possesses a mostly saturated phytyl sidechain, MKn possess unsaturated isoprenoid sidechains that range in length, where “n” refers to the number of repeating 5-carbon isoprenyl units (1).
PK is produced by plants and is commonly called vitamin K1 (2). MKn are often collectively referred to as vitamin K2. This misnomer has caused confusion in the literature, as evidenced by MKn being referred to or misunderstood as a single entity and by the erroneous assumption that all MKn are similar in origin (1,3). The majority of MKn (MK5–MK13) are bacterially produced (4,5). In contrast, MK4 is not commonly synthesized by bacteria (4) but rather is synthesized in tissues of animals following ingestion of PK (6, 7), and can thus be more correctly understood as a tissue vitamin K subtype. Briefly, the phytyl sidechain of PK is removed by an unknown enzyme, liberating menadione as an intermediate. The prenyltransferase UbiA prenyltransferase domain-containing protein 1 (UBIAD1) then transfers the geranylgeranyl group from geranylgeranyl pyrophosphate to menadione to form MK4 (6,8, 9). Menadione is sometimes referred to as vitamin K3, though in the absence of a sidechain it is technically a provitamin K form. Although menadione is not approved for human consumption, it is often used as the vitamin K source in animal chow, as it is transformed to MK4 in tissues (9,10).
The only firmly established physiological function for vitamin K is as a cofactor for the enzyme gamma-glutamyl carboxylase (GGCX) (3,11). GGCX is responsible for the activation of a group of proteins collectively termed vitamin K–dependent proteins, which have diverse functions (12). All vitamin K forms can function as a cofactor for GGCX (3), though efficacy may decrease with an increasing sidechain length (13). However, given that both PK and MK4 have similar efficacy as cofactors for GGCX (13), the conversion of PK to MK4 in vivo suggests that MK4 may have additional physiological roles. There is also some evidence that, like PK, oral MK4 undergoes sidechain cleavage and reprenylation to reform MK4 (6). Moreover, menadione is excreted in urine following ingestion of PK, MK4, and MK7 (14), suggesting a common conversion mechanism may exist for all dietary vitamin K forms to MK4 (15). However, this notion has not been conclusively established.
Current requirements for vitamin K are based on adequate dietary intakes of PK, which is found in leafy green vegetables and vegetable oils and has historically been considered the predominant dietary source of vitamin K (16). However, MKn are more prevalent in the food system than previously recognized—for example, present in fermented foods, dairy, and meat—and thus may be relevant contributors to vitamin K status (1,17, 18). To date, robust bioavailability studies have only been conducted for PK, and comparable data are lacking for MKn (1,3). Proposals for separate dietary requirements for “vitamin K2” (19) are currently premature because we lack the rigorous studies needed to fill gaps in understanding the biologic basis for putative claims that there are unique health benefits of MKn relative to PK (20).
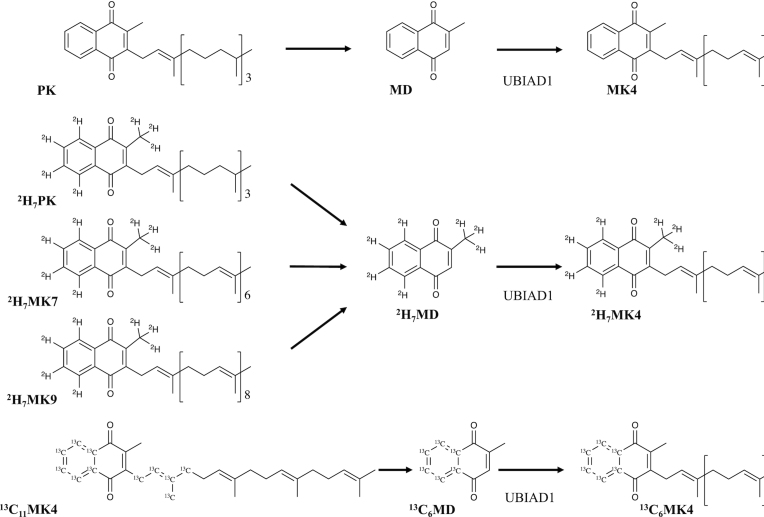
One such fundamental gap is a comparison of the relative bioavailability of vitamin K forms from the diet. The original purpose of the current study was to compare the absorption and tissue-specific accumulation of dietary vitamin K forms of varying length and saturation (PK, MK4, and/or MK9) in male and female C57BL/6 mice, which we hypothesized would vary by vitamin K form. However, regardless of the dietary vitamin K form administered in the diet, the predominant form in most tissues was instead MK4, which was furthermore equivalent in concentration in most tissues across diet groups. To further examine these unexpected findings, we then conducted a second study leveraging stable isotope–labeled vitamin K forms (2H7PK, 13C11MK4, 2H7MK7, or 2H7MK9) to test the hypothesis that PK and MKn alike serve as dietary precursors to MK4 (Figure 1), which was confirmed by the work herein.
FIGURE 1.
Proposed conversion mechanisms for the MK4 formation for the administered vitamin K forms supplemented in the stable isotope study. It is postulated that all vitamin K forms undergo complete side chain cleavage to MD, followed by UBIAD1-catalyzed prenylation with a geranylgeranyl group (via geranylgeranyl pyrophosphate) to form MK4. The cleavage enzyme is currently unidentified. Abbreviations: MD, menadione; MK4, menaquinone-4; MK7, menaquinone-7; MK9, menaquinone-9; PK, phylloquinone.
Methods
Animal models and pair-feeding protocol
Eight-week-old male and female C57BL/6 mice were purchased from Charles River Laboratories and were fed an AIN-93G diet (21) (TD.94,045; Envigo) ad libitum for 2–4 weeks prior to the study initiation to allow mice to acclimate to the new facilities and ensure animal health prior to the study initiation. Once started on study diets (as further described below), mice were group pair-fed to decrease variability both within and between diet groups (22). Briefly, all mice in each group were given a weighed portion of food. On the next day, any remaining food was weighed back, and the weight of food provided to all mice was decreased to the mean daily consumption of the group with the least food consumption. If no food remained, the quantity was increased by 0.5 g per day until individual mice did not eat the entire portion of food. This procedure was repeated daily until the experiment was concluded. Mice were given water ad libitum, and were individually housed in conventional cages. We have previously shown that housing mice in suspended wire cages, meant to prevent coprophagy, results in poor health of the animals and does not influence tissue vitamin K concentrations compared to conventional housing (23). At the conclusion of the studies, the mice were euthanized with isoflurane (administered with a precision vaporizer; 3%–5%), and blood and tissues were collected. All animal experiments and protocols were approved by the Institutional Animal Care and Use Committee at the Human Nutrition Research Center on Aging at Tufts University.
Preparation of study diets
Study diets were mixed in-house to customize and ensure diet vitamin K concentrations. Commercial rodent diets often contain menadione, which is converted to MK4 in vivo (10). A vitamin K–deficient basal mix (TD.120,060; Envigo) containing no menadione was used as the base for all study diets, the composition of which has been described previously (23). Purified vitamin K forms were obtained from Sigma-Aldrich (PK, 2H7PK, MK4, MK9) and IsoSciences (13C11MK4, 2H7MK7, 2H7MK9). The purified PK and 13C11MK4 contained both cis and trans isomers (12% and 20% cis, respectively), whereas all other purified vitamin K forms contained >98% of the trans isomer. As the trans isomer is the biologically active isomer (24), diet vitamin K concentrations were formulated on molar concentrations of the trans isomer of vitamin K forms.
Vitamin K forms were first solubilized in tocopherol-stripped corn oil (5% diet; CA.160,160; Envigo) and then mixed into a vitamin K–deficient basal diet mix (95% diet). Vitamin K concentrations in diets were measured by LC-MS, as described previously (25). For the diet study (as described below), supplemented diets were formulated to an equimolar target of 5.0 μmol trans vitamin K/kg diet, and total (trans + cis) diet concentrations were as follows (mean ± SD): vitamin K–deficient diet (control), 0.0206 ± 0.00198 mg PK/kg; PK diet, 3.20 ± 0.932 mg PK/kg; MK4 diet, 2.15 ± 0.0184 MK4 mg/kg; MK9 diet, 3.82 ± 0.598 MK9 mg/kg; and PK/MK4/MK9 diet, 0.924 ± 0.173 PK, 0.821 ± 0.129 MK4, and 1.31 ± 0.214 MK9 mg/kg. For the stable isotope study (as described below), diets were formulated to an equimolar target of 2.2 μmol trans vitamin K/kg diet, and total (trans + cis) diet concentrations were as follows (mean ± SD): unlabeled PK (control) diet, 1.16 ± 0.199 mg PK/kg; 2H7PK diet, 1.33 ± 0.143 mg 2H7PK/kg; 13C11MK4 diet, 1.69 ± 0.485 mg 13C11MK4/kg; 2H7MK7 diet, 1.90 ± 0.171 mg 2H7MK7/kg; and 2H7MK9 diet, 1.94 ± 0.840 mg 2H7MK9/kg. All diet concentration data expressed in molar units and as the trans isomer only are included in Supplemental Table 1.
Diet study (utilizing unlabeled vitamin K forms)
Fifty male and 50 female 10-week-old C57BL/6 mice were acclimated on a vitamin K–deficient diet for 4 weeks. Mice were then randomized to 5 pair-fed groups with maintenance on a vitamin K–deficient diet (control) or a supplemented diet containing 5 μmol/kg of PK, MK4, or MK9 or an equimolar combination of PK/MK4/MK9 for 4 weeks. Mice were pair-fed, and the supplemented dose represents 2.3-fold the recommended vitamin K content of standard rodent diets (10). In humans, twice the minimum recommended PK intake would mimic the PK intake on a healthy diet (26). Mice were 18 weeks of age at the time of fasted sacrifice, and blood and tissues were harvested. The gastrointestinal tract was flushed with PBS buffer to remove intestinal contents, and was segmented: the small intestine was collected from below the stomach to above the cecum and cut into 3 parts (approximating the duodenum, jejunum, and ileum). Immediately after collection, all tissues were frozen in liquid nitrogen and stored at −80°C until the time of analysis. Three male mice in the vitamin K–deficient (control) group died prior to the completion of the study, so data from 7 mice are reported for that group (all other groups retained 10 mice).
Another parallel, proof-of-concept diet arm was included to demonstrate absorption from a rich, food-based source of MKn (freeze-dried pork; 24% of diet). These data are included in Supplemental Table 2, but were not considered statistically because they were not matched to the other diet arms on vitamin K or macronutrient contents.
Stable isotope study (utilizing labeled vitamin K forms)
For 6 weeks, 35 male and 35 female 12-week-old C57BL/6 mice were acclimated on a vitamin K–sufficient (2.2 μmol/kg unlabeled PK) diet. Mice were then randomized to 5 pair-fed groups, with maintenance on the unlabeled PK diet (control) or a diet containing 2.2 μmol/kg of the stable isotopically labeled vitamin K forms—2H7PK, 13C11MK4, 2H7MK7, or 2H7MK9—for 1 week (placement of the isotopic labeling is shown in Figure 1). A lower supplemented dose was chosen for this study to match the standard recommended vitamin K content for rodents [molar equivalent to 1.0 mg PK/kg diet (10)]. The mice were 19 weeks of age at the time of nonfasted sacrifice, and blood and tissues were collected. The collection and storage of tissues (with additional collection of the colon, segmented from below the cecum) were as described above. One female mouse in the control group was not included in the analysis due to a protocol error (n = 6 for control; all other groups, n = 7).
Extraction and detection of vitamin K forms
Tissues were homogenized prior to extraction. Intestine, bone, and muscle samples were treated with liquid nitrogen and homogenized with a mortar and pestle over dry ice. All other tissues (liver, kidney, brain, adipose, pancreas, lung, reproductive organs) were homogenized in PBS buffer using a Powergen homogenizer (Fisher Scientific) as previously described (23). Serum was prepared as reported previously (25,27). As samples from the stable isotope study were collected nonfasted, some tissues (adipose, ileum, and colon) required an additional lipase step [as previously described (28)] for adequate purification. All HPLC-grade solvents (Fisher Scientific Inc.) were used for extraction and chromatography procedures.
For the diet study, unlabeled tissue vitamin K forms (PK and MK4–MK13) were measured by LC-MS (Agilent series 1200 HPLC; Agilent 6130 Quadrupole MSD) using the previously described method (25). If tissues contained only MK4 and/or PK (or these forms at concentrations <30 pmol/g), PK and MK4 were measured by reversed-phase HPLC (27) with lower limits of detection (LLODs) of 0.1 pmol/g for both PK and MK4 (liver, kidney, adipose, reproductive organ, bone, brain, lung, and muscle) or the modified C30 LC-MS method (duodenum, jejunum, and ileum), as described in detail below, to obtain better sensitivity.
For the stable isotope study, the MS system was required for differentiation of stable isotope–labeled compounds, and separation of stereoisomers required the use of a C30 column. Therefore, unlabeled and labeled tissue vitamin K forms (PK and MK4–MK9) in tissues were measured on the same LC-MS above using a ProntoSil C30 column (5 μm; 250 mm × mm) (29), with the following modifications. The mobile phase was solvent A (100% methanol) and solvent B (100% methylene chloride). A linear gradient was run as follows: 0% solvent B at 0.0 minutes to 2% solvent B at 15.0 minutes, to 3% solvent B at 22.50 minutes, to 10% solvent B at 25.0 minutes, to 30% solvent B at 55.0 minutes at 1.0 mL/min. From 55.0–60.0 minutes, the flow rate was increased to 1.2 mL/min to remove lipophilic compounds, remaining at 30% of solvent B. At 60.1 minutes, solvent B was decreased to 0% and the flow rate was returned to 1.0 mL/min. The cycle was complete at 65.0 minutes. Labeled compounds of interest were selected at the indicated mass-to-charge ratios (Supplemental Figure 1). K1,25 (m/z 521.6) was used as an internal standard. Tissues that were identified to contain only MK4 and/or PK were run using an abbreviated method to minimize the unnecessary use of solvents. The total run time was 32.0 minutes, and MK5 (m/z 513.4) was used as an internal standard. In tissues except adipose, the LLODs for the C30 methods were 5 pmol/g for unlabeled PK isomers, MK4, and all MK5–MK9, and were 2.5 pmol/g for 2H7MK4, 13C11MK4, 13C6MK4, and 2H7PK. In adipose, the determined C30 LLODs were 10 pmol/g for unlabeled PK isomers, MK4, and all MK5–MK9, and were 5 pmol/g for 2H7MK4, 13C11MK4, 13C6MK4, and 2H7PK.
Calibration standards for the stable isotope study contained labeled and unlabeled vitamin K forms, including the expected stable isotope products: 13C6MK4 (kindly donated by Isosciences) and 2H7MK4 (Sigma-Aldrich). Responses of all stable isotope forms were standardized to the area of the corresponding unlabeled form in the calibration standard. Tissue concentrations reported refer to the trans isomer only, unless otherwise specified.
Statistical analyses
Sample size calculations for the diet study were based on published data from our laboratory of kidney PK concentrations of vitamin K–deficient and vitamin K–sufficient male C57BL/6 mice (23). With an effect size of 3.30 pmol/g tissue and an SD of 1.56, after accounting for the comparison of multiple means, it was determined that 10 mice per group would provide 80% power to detect a difference by diet group at an α of 0.05. To align with the NIH initiative to account for sex as a biological variable (30), both male and female mice were included (n = 10 each sex). As the purpose of the stable isotope study was to examine the conversion of dietary MKn to MK4, sample size calculations were based on duodenal MK4 concentrations of MK9-supplemented males as compared to vitamin K–deficient males in the diet study [no stable isotope–labeled MK4 was anticipated in the unlabeled PK (control) group]. With an effect size of 8.54 pmol/g tissue and an SD of 3.87, after accounting for the comparison of multiple means, it was determined that 7 mice per group would provide 80% power to detect a difference by diet group at an α of 0.05. Again, both male and female mice were included.
Concentration data were assessed for normality, and ln-transformed to improve normality prior to statistical testing. To avoid the transformation of 0, nondetectable (ND) concentration values were replaced with the LLOD/2. For each tissue, data are displayed by sex and diet group. All tabular data are presented as geometric means ± SEMs. Notation of ND within the tables signifies that for the specified tissue, no animal within the group contained the vitamin K form at a detectable level. If detectable levels of a compound were detected in some, but not all, of the animals within a group, the geometric mean ± SEM, with the ND substitution as above, were presented.
The effects of sex and diet group on concentrations of vitamin K forms were analyzed by 2-factor ANOVA with an interaction term. Significance testing was evaluated using 2-tailed tests at an alpha level of 0.05. If the interaction term was significant, the main effects of sex and diet group were not interpreted in the full model and analyses by diet group were stratified on sex. If the interaction was not significant, the interaction term was dropped from the model and the effect of diet group was averaged over sex. Pairwise comparisons by diet group were conducted using Tukey's Honestly Significant Difference to account for multiple comparisons. Pairwise comparisons by diet group are presented in the tables with superscript letters (where a > b > c > d) for males and females separately (if analyses were not stratified on sex, the superscripts represent the effect of diet group averaged over sex, and are thus parallel for males and females). All analyses were done in R, version 3.6.3 (2020–02-29).
Results
Vitamin K forms supplemented in the diet accumulate in small intestine and liver tissues
In the diet study, all forms of vitamin K (PK, MK4, and/or MK9) provided in the diet accumulated in duodenal and liver tissues (Table 1). The accumulation of vitamin K forms was in part reflective of the form(s) supplemented in the diet, as indicated by footnote 2 in Table 1. In the duodenum, PK concentrations were higher in the PK-supplemented group compared to all other groups in both male and female mice (all pairwise P values < 0.001). Similarly, MK9 concentrations were higher in the MK9-supplemented group in both male and female mice (all P values < 0.05). In fasted jejunal and ileal tissue, only PK and MK4 were present. The accumulation of vitamin K forms in liver was also reflective of forms supplemented in the diets. In both male and female mice, liver PK concentrations were greater in the PK-supplemented group (all pairwise P values < 0.001), MK4 concentrations were greater in the MK4-supplemented group (all P values < 0.05), and MK9 concentrations were greater in the MK9-supplemented group (all P values < 0.03) compared to all other groups (Table 1). Despite equimolar supplementation of PK, MK4, and MK9, the dietary vitamin K forms appeared to accumulate to unequal concentrations in small intestine and liver tissues across supplemented groups. However, concentrations of the dietary forms were not formally compared to each other, as dietary MK4 could not be distinguished from metabolized MK4 (as discussed below), and MK9 was also present in livers of mice not supplemented with MK9.
TABLE 1.
Vitamin K concentrations of intestinal segments and livers of mice fed a vitamin K–deficient control diet or a diet supplemented with 5 μmol/kg PK, MK4, MK9, or a combination (PK/MK4/MK9) for 4 weeks (diet study)1
| Males | Females | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diet group | Diet group | 2-way ANOVA P values | ||||||||||||
| Tissue | VK form | VKD, control, n = 7 | PK, n = 10 | MK4, n = 10 | MK9, n = 10 | PK/MK4/MK9, n = 10 | VKD, control n = 10 | PK, n = 10 | MK4, n = 10 | MK9, n = 10 | PK/MK4/MK9, n = 10 | Sex | Group | Sex × Group |
| Duodenum | PK | NDb | 14.0a ± 1.782 | NDb | NDb | 2.68b ± 0.2602 | ND c | 32.7a ± 4.662 | NDc | NDc | 10.8b ± 1.772 | <0.001 | ||
| MK4 | NDc | 12.3 b ± 1.16 | 24.8a ± 3.722 | 9.85 b ± 1.68 | 12.9b ± 1.372 | ND b | 45.0 a ± 5.75 | 48.0a ± 11.92 | 32.5a ± 4.07 | 43.7a ± 4.632 | <0.001 | |||
| MK9 | NDb | NDb | NDb | 4.38a ± 2.642 | NDb,2 | NDc | NDc | NDc | 11.6a ± 2.772 | 5.01b ± 0.7812 | <0.001 | |||
| Jejunum | PK | 4.34b ± 1.42 | 10.5a ± 1.342 | 5.04b ± 1.09 | 4.97b ± 1.38 | 5.69b ± 1.552 | 5.29b ± 0.964 | 19.7a ± 3.032 | 6.29b ± 1.46 | 4.59b ± 0.852 | 10.1b ± 1.202 | 0.010 | <0.001 | 0.301 |
| MK4 | 6.55b ± 3.20 | 13.6b ± 2.85 | 25.4a ± 2.662 | 11.6b ± 2.36 | 15.7b ± 1.732 | 4.89b ± 1.74 | 33.8a ± 5.03 | 46.2a ± 4.612 | 28.7 a ± 3.40 | 39.3a ± 4.292 | 0.008 | |||
| MK9 | ND | ND | ND | ND2 | ND2 | ND | ND | ND | ND2 | ND2 | ||||
| Ileum | PK | 3.16c ± 0.594 | 11.3a ± 0.7522 | 2.87c ± 0.750 | 2.77c ± 0.440 | 4.95b ± 0.6772 | 2.79c ± 0.500 | 12.6a ± 1.342 | 2.84c ± 0.640 | 3.22c ± 0.506 | 7.42b ± 1.122 | 0.163 | <0.001 | 0.326 |
| MK4 | NDb | 25.7a ± 3.34 | 17.6a ± 7.512 | 12.8a ± 2.53 | 20.3a ± 3.412 | 3.03b ± 0.593 | 46.3a ± 9.55 | 50.7a ± 13.42 | 43.6 a ± 8.18 | 49.1a ± 5.432 | <0.001 | <0.001 | 0.144 | |
| MK9 | ND | ND | ND | ND2 | ND2 | ND | ND | ND | ND2 | ND2 | ||||
| Liver | PK | 4.38c ± 0.228 | 30.0a ± 3.422 | 4.74c ± 0.173 | 4.78c ± 0.322 | 9.72b ± 0.6262 | 5.38c ± 0.367 | 87.1a ± 7.612 | 5.60c ± 0.299 | 5.49c ± 0.238 | 27.5b ± 2.802 | <0.001 | ||
| MK4 | 1.99c ± 0.326 | 9.92b ± 0.915 | 15.1a ± 1.582 | 7.51b ± 0.546 | 9.53b ± 0.6742 | 3.52c ± 0.375 | 28.4b ± 2.18 | 48.7a ± 4.892 | 28.0b ± 3.05 | 34.4b ± 2.282 | <0.001 | |||
| MK9 | 4.67b ± 1.20 | 4.67b ± 2.06 | 4.66b ± 1.41 | 16.4a ± 3.142 | 6.43b ± 1.422 | 4.07c ± 2.84 | 3.85c ± 1.75 | 2.91c ± 0.382 | 192a ± 25.92 | 46.9b ± 4.162 | <0.001 | |||
Values are geometric means ± SEMs; data are shown as pmol/g tissue. Superscript letters indicate pairwise comparisons by diet group across a row, where a > b > c > d for males and females separately, and geometric means sharing a common superscript are not significantly different from each other (Tukey's Honestly Significant Difference; P < 0.05). If analyses were not stratified on sex (i.e., the interaction term was nonsignificant), the effect of diet group was averaged over sex, and thus superscripts for males and females are parallel. PK and MK4 in liver were measured by HPLC with an LLOD of 0.01 pmol/g for both. The HPLC method does not differentiate stereoisomers. All other concentrations reported were measured by the C30 LC-MS assay with an LLOD of 5 pmol/g each for PK, MK4, and MK9. Abbreviations: LLOD, lower limit of detection; MK4, menaquinone-4; MK9, menaquinone-9; ND, nondetectable; PK, phylloquinone; VK, vitamin K; VKD, vitamin K deficient.
Indicates the form was administered in the group diet.
Tissue MK4 accumulated to generally equal concentrations among supplemented groups
Regardless of the form of vitamin K supplemented in the diet study, MK4 accumulated to equivalent concentrations within sex across groups in the kidney, adipose, reproductive organ, bone, and pancreas of all supplemented mice, and all exceeded tissue MK4 concentrations of the vitamin K–deficient control groups, as shown in Table 2. In the brain, lung, and muscle, MK4 concentrations were comparable among supplemented groups within sex, but several statistically significant pairwise differences were observed (Table 2). Besides MK4, PK was the only other vitamin K form detected in extraintestinal and extrahepatic tissues (Table 2).
TABLE 2.
Vitamin K concentrations of extraintestinal and extrahepatic tissues of mice fed a vitamin K–deficient control diet or a diet supplemented with 5 μmol/kg PK, MK4, MK9, or a combination (PK/MK4/MK9) for 4 weeks (diet study)1
| Males | Females | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diet group | Diet group | 2-way ANOVA P values | ||||||||||||
| Tissue | VK form | VKD, control, n = 7 | PK, n = 10 | MK4, n = 10 | MK9, n = 10 | PK/MK4/MK9, n = 10 | VKD, control, n = 10 | PK, n = 10 | MK4, n = 10 | MK9, n = 10 | PK/MK4/MK9, n = 10 | Sex | Group | Sex × Group |
| Kidney | PK | 0.512c ± 0.134 | 3.89a ± 0.416 | 0.381c ± 0.134 | 0.282c ± 0.151 | 0.815b ± 0.230 | 0.596c ± 0.175 | 9.95a ± 1.22 | 0.294c ± 0.153 | 0.313c ± 0.177 | 3.51b ± 0.450 | 0.0530 | <0.001 | 0.168 |
| MK4 | 1.88b ± 6.33 | 48.1a ± 2.59 | 50.4a ± 2.27 | 24.1a ± 5.42 | 44.5a ± 2.72 | 25.6b ± 6.42 | 240a ± 17.1 | 233a ± 11.8 | 227a ± 9.74 | 231a ± 9.45 | <0.001 | <0.001 | 0.688 | |
| Adipose | PK | 0.0756b ± 0.121 | 0.663a ± 0.760 | NDb | NDb | 0.0789b ± 0.475 | 0.0660c ± 0.0750 | 16.9a ± 9.06 | 0.0858c ± 1.11 | NDc | 9.24b ± 2.54 | <0.001 | ||
| MK4 | 2.32b ± 2.44 | 10.7a ± 5.90 | 19.4a ± 7.34 | 7.38a ± 1.46 | 22.1a ± 11.2 | 6.44b ± 2.79 | 77.4a ± 40.6 | 164a ± 59.9 | 87.9a ± 36.9 | 169a ± 32.8 | <0.001 | <0.001 | 0.509 | |
| Reproductive organ | PK | NDa | 0.0987a ± 0.193 | NDa | NDa | NDa | 0.233b,c ± 0.840 | 11.9a ± 1.22 | NDc | 0.0915c ± 1.28 | 1.19b ± 0.825 | <0.001 | ||
| MK4 | 13.0b ± 1.61 | 118a ± 4.86 | 105a ± 3.75 | 99.6a ± 5.29 | 119a ± 5.09 | 12.0b ± 1.36 | 185a ± 9.33 | 192a ± 15.3 | 148a ± 13.6 | 153a ± 12.4 | <0.001 | |||
| Bone | PK | NDb | 0.208a ± 0.302 | NDb | 0.0665b ± 0.0820 | 0.0670b ± 0.0890 | 0.128b ± 0.131 | 0.474a ± 0.350 | 0.121b ± 0.140 | 0.0719b ± 0.141 | 0.123b ± 0.145 | 0.0135 | 0.002 | 0.847 |
| MK4 | 0.406b ± 0.502 | 5.33a ± 3.57 | 12.8a ± 1.52 | 9.55a ± 1.40 | 14.4a ± 7.98 | 4.05b ± 0.895 | 46.8a ± 3.85 | 41.9a ± 3.39 | 46.9a ± 11.5 | 42.8a ± 4.07 | <0.001 | <0.001 | 0.389 | |
| Pancreas2 | PK | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | |||
| MK4 | NDb | 143a ± 14.7 | 141a ± 10.1 | 135a ± 13.2 | 153a ± 13.9 | NDb | 559a ± 12.2 | 483a ± 34.3 | 511a ± 20.1 | 502a ± 12.3 | <0.001 | |||
| Brain | PK | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | |||
| MK4 | 3.95c ± 0.349 | 75.2a ± 5.86 | 66.2b ± 4.78 | 64.7a,b ± 3.78 | 67.0a,b ± 5.00 | 15.6c ± 0.905 | 342a ± 15.3 | 277b ± 12.3 | 295a,b ± 10.4 | 323a,b ± 17.8 | <0.001 | <0.001 | 0.539 | |
| Lung | PK | 2.06b,c ± 0.407 | 3.85a ± 0.296 | 1.61c ± 0.162 | 1.69c ± 0.174 | 2.58a,b ± 0.284 | 1.80c ± 0.190 | 7.56a ± 0.768 | 1.70c ± 0.172 | 1.96c ± 0.137 | 3.15b ± 0.409 | 0.0033 | ||
| MK4 | 1.97c ± 0.367 | 14.5a,b ± 1.66 | 19.8a ± 1.94 | 11.2b ± 1.15 | 13.7a,b ± 2.11 | 3.74b ± 0.160 | 58.7a ± 3.84 | 55.0a ± 8.05 | 44.4a ± 2.08 | 54.0a ± 6.84 | <0.001 | |||
| Muscle | PK | 0.193a,b ± 0.246 | 0.168a,b ± 0.200 | 0.0628b ± 0.0440 | NDb | 0.383a ± 0.164 | NDb | 1.07a ± 0.744 | 0.089b ± 0.0735 | 0.0945b ± 0.154 | 0.186a,b ± 0.280 | 0.0052 | ||
| MK4 | 1.00c ± 0.318 | 6.84b ± 0.251 | 12.0a ± 1.86 | 5.83b ± 0.487 | 7.22b ± 0.857 | 1.46c ± 0.157 | 38.1a ± 3.10 | 34.4a,b ± 3.09 | 27.9b ± 1.10 | 32.9a,b ± 1.99 | <0.001 | |||
Values are geometric means ± SEMs; data are shown as pmol/g tissue. Superscript letters indicate pairwise comparisons by diet group across a row, where a > b > c > d for males and females separately, and geometric means sharing a common superscript are not significantly different from each other (Tukey's Honestly Significant Difference; P < 0.05). If analyses were not stratified on sex (ie, the interaction term was nonsignificant), the effect of diet group was averaged over sex and thus superscripts for males and females are parallel. Abbreviations: LLOD, lower limit of detection; MK4, menaquinone-4; MK9, menaquinone-9; ND, nondetectable; PK, phylloquinone; VK, vitamin K; VKD, vitamin K deficient.
PK and MK4 in the pancreas were measured by C18 LC-MS method with an LLOD of 30 pmol/g for both. All other tissues were measured by HPLC with an LLOD of 0.1 pmol/g for both PK and MK4.
The naphthoquinone ring of MK4 originates from the dietary administered vitamin K form
In the stable isotope study, isotopic labeling of the vitamin K forms allowed for confirmation that MK4 in tissues originated from the vitamin K provided in the diet (henceforth referred to as the administered vitamin K form), differentiation between untransformed dietary MK4 and metabolized MK4, and administered MKn from those that may originate from the gut microbiota. Thus, concentrations of supplemented dietary vitamin K forms could be compared across equimolar-supplemented diet groups.
In all groups supplemented with stable isotope–labeled forms of dietary vitamin K, stable isotope-labeled MK4 was detected in tissues (Table 3). In male and female mice supplemented with deuterium-labeled forms (2H7PK, 2H7MK7, and 2H7MK9), the labeled MK4 in tissue retained the deuterium labeling on the naphthoquinone ring (2H7MK4; Figure 1). In male and female mice supplemented with 13C11MK4, 13C6MK4 was detected in tissue, again demonstrating conservation of the naphthoquinone ring of the administered vitamin K form (Figure 1).
TABLE 3.
Vitamin K concentrations of tissues from mice fed a diet containing 2.2 μmol/kg unlabeled PK (control), 2H7PK, 13C11MK4, 2H7MK7, or 2H7MK9 for 1 week (stable isotope study)1
| Males | Females | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group | Group | 2-way ANOVA P values | ||||||||||||
| Tissue | VK form2 | Control, unlabeled PK, n = 7 | 2H7PK, n = 7 | 13C11MK4, n = 7 | 2H7MK7,n = 7 | 2H7MK9, n = 7 | Control, unlabeled PK, n = 6 | 2H7PK, n = 7 | 13C11MK4, n = 7 | 2H7MK7, n = 7 | 2H7MK9, n = 7 | Sex | Group | Sex × Group |
| Duodenum | Unlabeled MK4 | 12.9a ± 2.13 | 5.96b ± 0.731 | NDc | 4.93b ± 0.789 | 4.99b ± 0.880 | 30.3a ± 4.44 | 9.47b ± 1.93 | 18.2a,b ± 2.39 | 12.9b ± 2.58 | 10.6b ± 1.64 | <0.001 | ||
| Administered VK form | 13.9a ± 2.02 | 10.4a ± 1.69 | 10.7a ± 2.58 | 5.01b ± 0.810 | 8.64a,b ± 2.58 | 16.0a ± 2.38 | 22.6a ± 3.85 | 13.6a ± 2.76 | 9.60b ± 1.22 | 14.1a,b ± 1.45 | <0.001 | <0.001 | 0.35 | |
| Labeled MK4 | NDc | 10.3a ± 1.02 | 4.80b ± 1.04 | 8.24a ± 0.773 | 8.94a ± 1.28 | NDb | 30.0a ± 4.39 | 26.5a ± 2.22 | 29.4a ± 3.72 | 23.1a ± 2.83 | 0.03 | |||
| Total VK | 28.3a ± 1.86 | 27.7a ± 1.88 | 18.6a ± 3.06 | 18.8a ± 1.68 | 23.6a ± 3.73 | 46.9a ± 6.02 | 64.5a ± 7.38 | 60.5a ± 3.30 | 53.0a ± 6.26 | 49.0a ± 4.02 | 0.037 | |||
| % Labeled MK4 (of total VK) | 0%c | 37.1%a,b ± 3.05% | 25.8%b ± 3.44% | 43.9%a ± 2.86% | 37.9%a,b ± 4.06% | 0%c | 46.5%a,b ± 3.03% | 43.8%b ± 2.77% | 55.6%a ± 1.06% | 47.3a,b ± 2.40% | 0.026 | |||
| Jejunum | Unlabeled MK4 | 8.29a ± 0.467 | 4.57b ± 1.09 | 4.98b ± 0.887 | 3.90b ± 0.940 | 5.09b ± 1.46 | 23.2a ± 6.95 | 9.12b ± 3.70 | 8.11b ± 0.959 | 4.65b ± 2.63 | 4.36b ± 1.66 | 0.006 | <0.001 | 0.135 |
| Administered VK form | 8.05a ± 1.32 | 3.33b ± 0.913 | 7.61a ± 1.53 | NDb | NDb | 16.3a ± 2.64 | 13.1a ± 5.12 | 15.1a ± 2.64 | 9.71a ± 1.08 | 2.93b ± 0.729 | 0.002 | |||
| Labeled MK4 | NDb | 5.10a ± 0.511 | 5.05a ± 0.883 | 6.22a ± 0.536 | 7.57a ± 0.927 | NDc | 21.8a ± 3.14 | 13.4b ± 1.16 | 17.9a,b ± 2.52 | 15.5a,b ± 1.45 | <0.001 | |||
| Total VK | 16.9a ± 1.43 | 13.8a,b ± 1.65 | 18.6a ± 2.31 | 10.4a,b ± 1.33 | 12.9b ± 2.26 | 40.1a ± 9.21 | 45.9a,b ± 11.4 | 37.3a ± 4.18 | 33.9a,b ± 4.91 | 23.4b ± 3.91 | <0.001 | 0.006 | 0.099 | |
| % Labeled MK4 (of total VK) | 0%c | 36.9%b ± 4.84% | 27.2%b ± 2.72% | 60.0%a ± 4.29% | 58.7%a ± 3.82% | 0%d | 47.5%b ± 2.90% | 36.0%c ± 1.43% | 52.8%b ± 3.02% | 66.3%a ± 3.42% | 0.033 | |||
| Ileum | Unlabeled MK4 | 10.1a ± 1.40 | 4.87b ± 0.902 | 2.80b ± 0.429 | 2.81b ± 0.442 | 3.27b ± 0.752 | 41.7a ± 4.98 | 16.5b ± 3.56 | 16.9b ± 2.59 | 16.5b ± 4.33 | 13.2b ± 2.62 | <0.001 | <0.001 | 0.437 |
| Administered VK form | 7.67a ± 0.647 | 2.89c ± 0.629 | 4.54b ± 0.382 | 3.08c ± 0.500 | 2.89b,c ± 0.643 | 11.6a ± 1.68 | 3.62c ± 0.758 | 7.96b ± 0.871 | 3.97c ± 1.34 | 6.60b,c ± 1.63 | <0.001 | <0.001 | 0.343 | |
| Labeled MK4 | NDc | 10.4a ± 0.653 | 6.69b ± 0.708 | 10.9a ± 0.635 | 11.0a ± 1.03 | NDc | 33.8a ± 1.64 | 17.1b ± 3.18 | 36.1a ± 2.19 | 32.2a ± 2.46 | <0.001 | |||
| Total VK | 18.1a,b ± 1.67 | 18.8a ± 1.18 | 14.2b ± 1.22 | 17.0a,b ± 1.23 | 17.8a,b ± 1.09 | 53.7a,b ± 6.22 | 55.5a ± 4.78 | 44.8b ± 5.19 | 59.0a,b ± 4.77 | 54.8a,b ± 4.74 | <0.001 | 0.028 | 0.886 | |
| % Labeled MK4 (of total VK) | 0%c | 55.4%a ± 3.77% | 47.2%b ± 2.45% | 64.3%a ± 2.57% | 61.6%a ± 4.06% | 0%c | 60.9%a ± 3.34% | 38.2%b ± 5.87% | 61.3%a ± 3.79% | 58.8%a ± 5.04% | 0.454 | <0.001 | 0.553 | |
| Colon | Unlabeled MK4 | 11.9a ± 1.42 | 4.20b ± 0.796 | 4.41b ± 0.886 | 3.45b ± 0.965 | 3.11b ± 0.539 | 38.8a ± 6.67 | 18.3b ± 1.99 | 21.9b ± 2.68 | 19.2b ± 5.03 | 17.4b ± 3.41 | <0.001 | <0.001 | 0.463 |
| Administered VK form | NDa | NDa | 4.91a ± 0.417 | 3.70a ± 5.24 | 5.96a ± 9.22 | 13.2a ± 1.11 | 5.97b,c ± 0.881 | 11.0a,b ± 1.96 | 3.13c ± 1.37 | 3.17c ± 1.54 | <0.001 | |||
| Labeled MK4 | NDb | 8.58a ± 0.989 | 7.04a ± 0.609 | 8.91a ± 0.622 | 8.03a ± 0.702 | NDb | 37.8a ± 3.04 | 25.0a ± 1.32 | 36.6a ± 5.83 | 27.9a ± 4.96 | <0.001 | |||
| Total VK | 11.9 ± 1.42 | 13.5 ± 0.850 | 16.9 ± 1.18 | 17.8 ± 5.84 | 22.5 ± 8.47 | 53.2 ± 6.40 | 63.0 ± 4.02 | 59.2 ± 3.37 | 59.9 ± 10.9 | 50.2 ± 7.22 | <0.001 | 0.279 | 0.080 | |
| % Labeled MK4 (of total VK) | 0%c | 63.7%a ± 6.00% | 41.7%b ± 3.02% | 50.0%a,b ± 7.03% | 35.7%a,b ± 9.45% | 0%c | 60.0%a ± 1.48% | 42.3%b ± 1.66% | 61.2%a,b ± 1.14% | 55.6%a,b ± 3.52% | 0.163 | <0.001 | 0.336 | |
| Liver | Unlabeled MK4 | ND | ND | ND | ND | ND | 8.51a ± 2.20 | 2.80b ± 0.429 | NDb | NDb | 2.76b ± 0.357 | <0.001 | ||
| Administered VK form | 20.4a ± 4.68 | 20.9a ± 4.13 | 10.5a,b ± 0.628 | 6.27b ± 2.02 | 17.5a ± 2.84 | 48.6b ± 10.2 | 63.2a,b ± 10.5 | 18.1c ± 1.92 | 81.3a,b ± 7.57 | 104a ± 23.2 | <0.001 | |||
| Labeled MK4 | NDb | 3.88a ± 0.519 | 4.55a ± 0.672 | 4.08a ± 1.00 | 4.90a ± 1.58 | NDb | 16.9a ± 1.55 | 13.7a ± 0.656 | 16.4a ± 1.44 | 15.8a ± 0.773 | <0.001 | |||
| Total VK | 20.4a,b ± 4.68 | 25.3a ± 4.10 | 15.1a,b ± 1.18 | 11.1b ± 2.62 | 23.5a ± 2.88 | 57.3b ± 12.3 | 82.7a,b ± 9.89 | 32.0c ± 2.30 | 98.5a,b ± 7.66 | 122a ± 23.0 | <0.001 | |||
| % Labeled MK4 (of total VK) | 0%c | 15.3%b ± 2.92% | 30.1%a ± 2.29% | 36.9%a ± 6.94% | 20.9%a,b ± 5.34% | 0%c | 20.5%b ± 2.83% | 42.7%a ± 2.30% | 16.7%b ± 2.02% | 12.9%b ± 2.53% | <0.001 | |||
| Brain | Unlabeled MK4 | 21.1a ± 1.50 | 10.9b ± 1.08 | 8.01b ± 0.632 | 7.46b ± 0.755 | 8.97b ± 0.499 | 112a ± 19.9 | 61.0b ± 4.84 | 60.2b ± 6.24 | 57.6b ± 5.89 | 51.7b ± 4.76 | <0.001 | <0.001 | 0.20 |
| Administered VK form | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ||||
| Labeled MK4 | NDc | 18.2a ± 1.70 | 11.7b ± 0.84 | 13.3b ± 0.935 | 15.9b ± 0.506 | NDd | 93.0a ± 7.42 | 62.2c ± 5.37 | 85.8a,b ± 8.35 | 72.6a,b ± 6.64 | <0.001 | |||
| Total VK | 21.1 ± 1.50 | 29.1 ± 2.75 | 19.7 ± 1.41 | 20.9 ± 1.56 | 24.9 ± 0.944 | 112 ± 19.9 | 154 ± 12.0 | 122 ± 11.5 | 143 ± 14.2 | 124 ± 11.3 | <0.001 | 0.144 | 0.447 | |
| % Labeled MK4 (of total VK) | 0%c | 62.5%a,b ± 0.844% | 59.1%b ± 1.34% | 63.9%a ± 1.67% | 63.9%a ± 0.900% | 0%c | 60.4%a ± 0.737% | 50.8%b ± 0.838% | 59.8%a ± 0.509% | 58.4%a ± 0.510% | <0.001 | |||
| Pancreas | Unlabeled MK4 | 92.1a ± 6.07 | 50.5b ± 2.75 | 42.2b,c ± 3.11 | 43.7b,c ± 3.15 | 39.7c ± 2.28 | 359a ± 23.0 | 159b ± 4.96 | 154b,c ± 2.05 | 139b,c ± 5.63 | 143c ± 8.84 | <0.001 | <0.001 | 0.268 |
| Administered VK form | NDa | NDa | 2.18a ± 0.332 | NDa | NDa | NDb | NDb | 5.49a ± 0.553 | NDb | NDb | <0.001 | |||
| Labeled MK4 | NDc | 76.6a ± 2.41 | 55.4b ± 3.88 | 71.0a ± 4.19 | 63.3a,b ± 3.58 | NDc | 276a ± 8.27 | 188b ± 6.85 | 242a ± 11.4 | 231a ± 18.4 | <0.001 | |||
| Total VK | 92.1b ± 6.07 | 127a ± 4.94 | 99.9b ± 6.94 | 115a,b ± 6.57 | 103b ± 5.29 | 359b ± 23.0 | 435a ± 11.8 | 348b ± 6.67 | 382a,b ± 14.4 | 374b ± 26.3 | <0.001 | <0.001 | 0.637 | |
| % Labeled MK4 (of total VK) | 0%c | 60.2%a ± 0.777% | 55.5%b ± 0.772% | 61.7%a ± 1.53% | 61.3%a ± 1.15% | 0%c | 63.5%a ± 0.673% | 54.0%b ± 1.07% | 63.4%a ± 1.17% | 61.7%a ± 1.02% | 0.249 | <0.001 | 0.154 | |
Values are geometric mean ± SEM; data are shown as pmol/g tissue (except % labeled MK4 rows). Superscript letters indicate pairwise comparisons by diet group across a row, where a > b > c > d for males and females separately, and geometric means sharing a common superscript are not significantly different from each other (Tukey's Honestly Significant Difference; P < 0.05). If analyses were not stratified on sex (i.e., the interaction term was nonsignificant), the effect of diet group was averaged over sex, and thus superscripts for males and females are parallel. All vitamin K concentrations were measured using C30 LC-MS assay with the following LLODs: 5 pmol/g for unlabeled PK, unlabeled MK4, 2H7MK7, and 2H7MK9 and 2.5 pmol/g for 2H7PK, 2H7MK4, 13C11MK4, and 13C6MK4. Abbreviations: LLOD, lower limit of detection; MK4, menaquinone-4; MK7, menaquinone-7; MK9, menaquinone-9; ND, nondetectable; PK, phylloquinone; VK, vitamin K.
“Administered VK form” refers to the VK form supplemented within that group (PK in the control group, 2H7PK in the 2H7PK group, and so on). “Labeled MK4” for the 13C11MK4 group refers to the generated 13C6MK4 only, not a sum of administered 13C11MK4 and generated 13C6MK4.
Administered vitamin K forms obtained from the diet accumulate unequally in tissues
In the duodenum, the accumulation of the administered vitamin K form was greater in the unlabeled PK (control) and the 2H7PK- and 13C11MK4-supplemented groups as compared to the 2H7MK7-supplemented group (all P values < 0.05), but no group had concentrations significantly different from that in the 2H7MK9 group, in either male or female mice (Table 3). Pairwise comparisons revealed significant differences in concentrations of the administered vitamin K form by diet group in the jejunum and ileum. However, these differences were not consistent across segments of the small intestine and differed by sex (Table 3). In the colon, there were significant differences in concentrations of the administered vitamin K form by diet group in female mice, but not in male mice (Table 3).
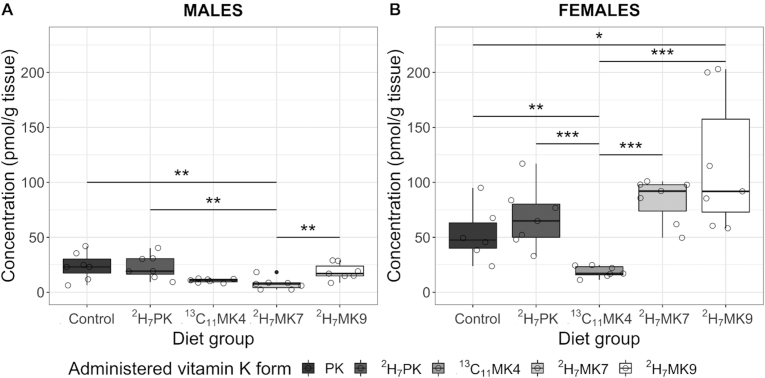
Accumulation of the administered vitamin K form in the liver also differed by diet group, but the response differed by sex (Figure 2). In males, liver accumulation of the administered vitamin K form was significantly less in the 2H7MK7 group as compared to the unlabeled PK (control) and 2H7PK- and 2H7M9-supplemented groups (all P values < 0.01), but did not significantly differ from the 13C11MK4 group (P = 0.38). However, in females, the 3C11MK4-supplemented group had significantly lower administered vitamin K form accumulation as compared to all other groups (all P values < 0.01), and the 2H7MK9-supplemented group had significantly more accumulated administered vitamin K form as compared to the control group (P < 0.05; Figure 2).
FIGURE 2.
Box-and-whisker plots of administered vitamin K forms in the livers of (A) male and (B) female C57BL/6 mice fed a diet containing 2.2 μmol/kg unlabeled PK (control), 2H7PK, 13C11MK4, 2H7MK7, or 2H7MK9 for 1 week (stable isotope study). n = 7 for all groups except female control group (n = 6). Bars represent the medians and the whiskers represent the IQRs; filled circles represent outliers and hollow circles represent individual data points. Pairwise comparisons are significant at P values of *<0.05, **<0.01, ***<0.001; pairwise comparisons are nonsignificant (P > 0.05) if not marked. Abbreviations: MK4, menaquinone-4; MK7, menaquinone-7; MK9, menaquinone-9; PK, phylloquinone.
The administered vitamin K forms PK, 2H7PK, and 13C11MK4, but not 2H7MK7 or 2H7MK9, were present in the adipose tissue of the respective supplemented groups (Table 4). Small amounts of administered 13C11MK4 were detected in the pancreas of 13C11MK4-supplemented mice, but no other administered vitamin K forms were detected in the pancreas (Table 3).
TABLE 4.
Vitamin K concentrations of adipose tissues from mice fed a diet containing 2.2 μmol/kg unlabeled PK (control), 2H7PK, 13C11MK4, 2H7MK7, or 2H7MK9 for 1 week (stable isotope study)1
| Males | Females | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group | Group | 2-way ANOVA P values | ||||||||||||
| Tissue | VK form2 | Control (unlabeled PK) n = 7 | 2H7PK n = 7 | 13C11MK4 n = 7 | 2H7MK7 n = 7 | 2H7MK9 n = 7 | Control (unlabeled PK) n = 6 | 2H7PK n = 7 | 13C11MK4 n = 7 | 2H7MK7 n = 7 | 2H7MK9 n = 7 | Sex | Group | Sex × Group |
| Mesenteric | Unlabeled MK4 | 16.2a ± 10.2 | 5.81b ± 1.33 | 10.2a,b ± 2.72 | 10.2a,b ± 2.58 | 7.05b ± 1.28 | 103a ± 13.2 | 72.3b ± 10.7 | 79.3a,b ± 11.7 | 71.1a,b ± 4.52 | 37.3b ± 7.75 | <0.001 | <0.001 | 0.313 |
| Administered VK | 9.84a ± 3.71 | 4.11b ± 0.732 | 10.9a ± 1.76 | NDb | NDb | 34.2a,b ± 7.51 | 18.2b ± 2.76 | 40.9a ± 4.88 | NDc | NDc | <0.001 | |||
| Labeled MK4 | NDb | 7.51a ± 2.35 | 4.44a,b ± 1.59 | 4.12a,b ± 1.19 | 4.03a,b ± 0.721 | NDc | 88.2a ± 11.8 | 42.1b ± 7.30 | 75.0a,b ± 9.13 | 49.3a,b ± 11.6 | <0.001 | |||
| Total VK | 29.3a ± 9.94 | 18.6a ± 2.99 | 26.6a ± 5.56 | 14.6a,b ± 3.49 | 11.2b ± 1.88 | 140a ± 19.9 | 184a ± 20.7 | 167a ± 16.9 | 147a,b ± 12.3 | 88.4b ± 17.4 | <0.001 | <0.001 | 0.221 | |
| % Labeled MK4 (of total VK) | 0%c | 40.4%a ± 6.05% | 16.7%b ± 2.11% | 28.3%a ± 3.17% | 36.0%a ± 2.75% | 0%c | 47.9%a ± 3.30% | 25.1%b ± 2.64% | 50.9%a ± 2.54% | 44.7%a ± 4.13% | 0.029 | |||
| Perigonadal | Unlabeled MK4 | 10.2a ± 4.75 | 5.83a,b ± 0.679 | 5.56a,b ± 0.538 | 5.31b ± 0.296 | 5.51b ± 0.361 | 61.4a ± 11.1 | 49.0a,b ± 4.64 | 48.1a,b ± 6.14 | 48.6b ± 10.3 | 42.9b ± 6.67 | <0.001 | 0.015 | 0.715 |
| Administered VK | 10.3a ± 1.42 | NDb | 7.26a ± 1.14 | NDb | NDb | 41.7a ± 5.34 | 12.8b ± 1.56 | 31.9a ± 3.92 | NDc | NDc | <0.001 | |||
| Labeled MK4 | ND | ND | ND | ND | ND | NDc | 34.2a ± 2.38 | 17.4b ± 2.13 | 41.1a ± 3.89 | 33.1a ± 4.91 | <0.001 | |||
| Total VK | 22.4a ± 4.96 | 5.83c ± 0.679 | 13.1b ± 1.39 | 5.31c ± 0.296 | 5.51c ± 0.361 | 106a ± 12.4 | 97.1a ± 6.20 | 99.5a ± 8.41 | 91.4a ± 13.2 | 77.5a ± 10.0 | <0.001 | |||
| % Labeled MK4 (of total VK) | 0% | 0% | 0% | 0% | 0% | 0%c | 35.2%a ± 1.80% | 17.4%b ± 1.58% | 45.0%a ± 3.76% | 42.7%a ± 3.98% | <0.001 | |||
| Subcutaneous | Unlabeled MK4 | 12.5a ± 1.94 | 6.49b ± 1.27 | 8.87b ± 2.15 | 9.79a,b ± 1.58 | 6.29b ± 1.17 | 147a ± 13.9 | 81.5b ± 6.89 | 64.0b ± 6.81 | 95.8a,b ± 9.92 | 92.3b ± 11.6 | <0.001 | <0.001 | 0.159 |
| Administered VK | 9.96a ± 1.10 | 3.24b ± 0.632 | 7.96a ± 1.32 | NDb | NDb | 48.9a ± 5.98 | 17.4c ± 1.66 | 34.0b ± 3.66 | NDd | NDd | <0.001 | |||
| Labeled MK4 | NDa | 3.60a ± 1.31 | NDa | 2.93a ± 0.729 | NDa | NDc | 75.0a ± 5.23 | 33.5b ± 5.31 | 87.0a ± 10.0 | 73.6a ± 12.0 | <0.001 | |||
| Total VK | 23.2a ± 2.23 | 13.8a ± 2.66 | 17.2a ± 3.15 | 13.1a ± 1.79 | 6.29b ± 1.17 | 196a ± 18.7 | 176a,b ± 8.17 | 135b ± 11.2 | 185a,b ± 15.9 | 171a,b ± 15.7 | <0.001 | |||
| % Labeled MK4 (of total VK) | 0%b | 26.2%a ± 3.49% | 0%b | 22.4%a ± 4.11% | 0%b | 0%c | 42.5%a ± 2.34% | 24.9%b ± 2.67% | 47.0%a ± 3.23% | 43.0%a ± 4.95% | <0.001 | |||
Values are geometric means ± SEMs; data are shown as pmol/g tissue. Superscript letters indicate pairwise comparisons by diet group across a row, where a > b > c > d for males and females separately, and geometric means sharing a common superscript are not significantly different from each other (Tukey's Honestly Significant Difference; P < 0.05). If analyses were not stratified on sex (i.e., the interaction term was nonsignificant), the effect of diet group was averaged over sex, and thus superscripts for males and females are parallel. All vitamin K concentrations were measured using C30 LC-MS assay for adipose with the following LLODs: 10 pmol/g for unlabeled PK, unlabeled MK4, 2H7MK7, and 2H7MK9 and 5 pmol/g for 2H7PK, 2H7MK4, 13C11MK4, and 13C6MK4. Abbreviations: LLOD, lower limit of detection; MK4, menaquinone-4; MK7, menaquinone-7; MK9, menaquinone-9; ND, nondetectable; PK, phylloquinone; VK, vitamin K.
“Administered VK form” refers to the VK form supplemented within that group (PK in the control group, 2H7PK in the 2H7PK group, and so on). “Labeled MK4” for the 13C11MK4 group refers to the generated 13C6MK4 only, not a sum of administered 13C11MK4 and generated 13C6MK4.
Approximately half of tissue MK4 is turned over in 1 week in the pancreas and brain
Labeled MK4 as a percentage of total vitamin K in tissues was examined to investigate an organ preference for MK4 and to estimate MK4 turnover in the brain and pancreas, where MK4 was the only vitamin K form present. In the pancreas and brain, stable isotope-supplemented groups had approximately half the unlabeled MK4 concentrations as compared to the unlabeled PK (control) group, and around 50%–60% of the total MK4 was the resultant labeled MK4 (Table 3), suggesting approximately half of the MK4 in these tissues is turned over and replaced by MK4 originating from dietary vitamin K within 1 week of feeding in both male and female mice. In tissues where administered vitamin K forms were also present (intestinal, hepatic, and adipose tissues), the percentages of labeled MK4 exhibited a wider variance across diet groups (Tables 3 and 4).
Vitamin K forms were below the limits of detection in serum
No vitamin K forms were detected in fasted serum in the diet study. In nonfasted serum samples collected from the stable isotope study, concentrations of all vitamin K forms were below the LLODs of the assay, and therefore could not be reported here with accuracy.
Vitamin K forms not supplemented in the diet were present in the colon and liver
In addition to the MK9 present in the livers of mice not supplemented with MK9 in the diet study (Table 1), menaquinone-10 (MK10) was detected in variable amounts in liver tissue and menaquinone-6 (MK6) was detected in variable amounts in colon tissue (data not shown). Both forms are known to be produced by common bacterial genera within the intestinal microbiota.
Discussion
At physiologically relevant dietary concentrations in mice, PK and multiple MKn of varying lengths were generally equivalent precursors for tissue MK4. Furthermore, MK4 was the dominant vitamin K form found in most tissues. These findings advance our understanding of the metabolism of dietary vitamin K forms (Figure 1), and suggest that the common metabolite MK4 may either have additional functions beyond the established roles for vitamin K, including but not limited to roles in the prevention of cancer and in vascular maintenance (3, 31), or could simply be a means of regulation of vitamin K in the body.
What is known to date about vitamin K absorption and metabolism is largely based on studies of PK, and has been reviewed in detail (3,15). Vitamin K is absorbed via an active process involving the cholesterol receptor Niemann-Pick C1-Like 1(NPC1L1) (32), and possibly scavenger receptors (33,34). Current evidence regarding the generation of MK4 suggests that the side chain of dietary vitamin K is removed during intestinal absorption, and that menadione is a circulating precursor to MK4 formation in target tissues. In mice, MK4 accumulated in brain tissue when PK was administered orally, but not when administered intravenously or intercerebroventricularly (8). In contrast, menadione administered via any of the above routes resulted in MK4 accumulation (8). These results implicated the intestine as a critical locale in the conversion, and suggested that some tissues are able to synthesize MK4 locally at the tissue if supplied a liberated naphthoquinone precursor. Furthermore, menadione has been measured indirectly in serum following oral administration of PK (9). Our data are consistent with this developing paradigm. Here, we detected converted MK4 in intestinal tissue, supporting that sidechain cleavage—and also some reprenylation—does occur in the intestine. Our observation that even dietary MK4 is transformed to MK4 is consistent with previous reporting by Nakagawa et al. (6), and suggests the cleavage enzyme does not discern different vitamin K forms. Serum concentrations of vitamin K were below the limits of detection here, which could be interpreted as support that menadione (and not circulating administered vitamin K or MK4 generated in the intestine) is the circulating precursor to tissue MK4. Alternatively, this may also be related to species differences in lipoprotein metabolism, as mice are considered HDL dominant (35, 36) and vitamin K associates with triglyceride-rich lipoproteins in humans (37). Lastly, the absent or low concentrations of most administered vitamin K forms in tissues besides the liver and intestine, in both fasted and nonfasted conditions, suggest that most extraintestinal/extrahepatic tissues do not carry out the entire transformation of administered vitamin K forms to MK4 locally, or else this conversion occurs very rapidly.
Beyond the intestine and liver, PK was the only vitamin K form besides MK4 detected in tissues. Although we did not detect any PK in the brain or pancreas here, it is acknowledged that PK has previously been reported in these tissues in a similarly designed study in C57BL/6 mice (8). These differing results may be due to differences in diet, particularly concentrations and/or forms of vitamin K, and possibly also vitamin E, as high concentrations of tocopherol may interfere with vitamin K absorption and metabolism (38). Whereas PK and MK4 may have distinct roles in tissue, it is also possible that uptake mechanisms into some tissues do not differentiate between them due to their structural similarity, as PK can be considered a partially saturated species of MK4 (3). The vast majority (95%–100%) of the labeled tissue MK4 in the pancreas and brain of the 13C11MK4-supplemented group was the transformed 13C6MK4, not the administered 13C11MK4. This seemingly wasteful transformation may instead be indicative of some form of regulatory control of vitamin K. In contrast, our data suggest that some tissues may not be able to differentiate administered MK4 from reformed MK4. Adipose tissues of animals supplemented with 13C11MK4 contained both administered 13C11K4 and 13C6MK4.
Despite the general equivalency of the supplemented vitamin K forms as precursors for MK4 and the apparent preference of most tissues for MK4 as the predominant vitamin K form, differential tissue distribution and concentrations of the administered vitamin K forms suggest that beyond this conversion, there do exist some differences in metabolism of individual vitamin K forms. Here, unequal concentrations of the administered vitamin K forms across groups were observed in both the intestine and liver. Differences in hepatic turnover of vitamin K forms are known in the literature, with slower turnover of longer sidechain MKn thought to be related to their increased lipophilicity, and thus greater affinity for membranes (39, 40, 41). Although we did not measure vitamin K at detectable levels in the serum of mice, vitamin K is present at detectable levels in human circulation (42, 43, 44) and there appear to be differences in plasma kinetics by vitamin K form (45, 46, 47). For example, a study conducted in human subjects reported differences in peak circulating concentrations and the AUC following equimolar oral doses of PK, MK4, and MK9, with concentrations of MK4 and MK9 reported to be less than 20% of the response of PK (45). Others have reported that MK7 and MK9 have longer half lives in plasma as compared to other vitamin K forms (45, 46, 47). However, circulating concentrations or persistence in plasma are not necessarily indicative of bioavailability to tissues, and functional measurements may be more informative of activity. A study by Niemeier et al. (48) demonstrated increased carboxylation of the vitamin K–dependent protein osteocalcin in mice following administration of chylomicron remnants rich in PK, suggesting that PK carried on lipoproteins is bioavailable to and utilized by osteoclasts. Similarly, the fraction of carboxylated osteocalcin in circulation was demonstrated to be increased in humans following oral supplementation of both PK and MK7 (49). In light of the current study, future research should examine whether conversion to MK4 may be an intermediate step in the activation of vitamin K–dependent proteins in bone, or whether species differences may exist.
Sex differences were consistently observed in vitamin K concentrations of tissues, in accordance with previous reports (8,23,50). This was largely an effect of magnitude, wherein female mice had higher vitamin K concentrations as compared to male mice. It is consistently observed in rodents that females are more robust against vitamin K deficiency than males, likely related to the effects of estrogen (51,52), as estrogen has been demonstrated in rats to increase intestinal absorption of vitamin K (53). Despite differences in absolute abundance by sex, in the pancreas and brain approximately half the total MK4 was turned over within 1 week in both males and females. However, in the liver, accumulations of the administered vitamin K form across groups were not parallel in male and female mice. In female mice, hepatic vitamin K forms accumulated generally in accordance with the theory of lipophilicity, as discussed above (with the mostly saturated sidechain of PK also rendering it more lipophilic than MK4). This pattern was not consistent in males; therefore, there may be other hormonal or sex chromosomal influences on the vitamin K metabolism.
In addition to their presence in the diet, MKn are also produced by some gut bacterial taxa (54), but their contribution to the vitamin K status has long been speculative (44,55,56). The data presented here suggest that absorption of MKn produced by bacteria residing within the intestinal tract is minimal. Although some MK6 was detected in colon tissue, which may be evidence of some direct absorption, mice in the vitamin K–deficient group had very low tissue concentrations of any form of vitamin K. Further, in mice supplemented with stable isotopes, approximately half of the MK4 in tissue was replaced with labeled MK4 within a week, confirming the MK4 originated from dietary vitamin K. The MK9 and MK10 detected in livers of mice not supplemented with those forms may have become available via coprophagy. However, even in the presence of coprophagy, without adequate dietary vitamin K, 3 male mice in the vitamin K–deficient group of the diet study died prior to completion of the study, which indicates that the majority of vitamin K to meet requirements comes from the diet.
This study has several strengths. The use of stable isotopes allowed us to definitively determine the origin of tissue MK4. The equimolar dosing designs allowed us to compare conversion efficiencies between vitamin K forms, and the sequential studies demonstrate the reproducibility of our findings. This study also has several limitations. MK7 was added as a supplemented vitamin K form only in the second study; thus, results for MK7 did not benefit from internal replication, nor was the MK7 metabolism assessed in the fasted state. Tissue expression of UBIAD1 was not evaluated, which might have lent insight into differences in MK4 accumulation between tissues, and although it is the proposed intermediate, menadione was not measured in this study. Menadione is extremely reactive and challenging to measure due to its reactivity, and to our knowledge no assay exists to measure it directly in serum or tissues.
In conclusion, this study definitively demonstrates both dietary PK and multiple forms of MKn serve as precursors to tissue MK4. Further research should focus on identification and characterization of the cleavage enzyme, and the physiologic roles and regulation of MK4.
Acknowledgments
he authors’ responsibilities were as follows—JLE, XF, JPK, CJH, JBM, and SLB: conceptualized and designed the study; JLE and XF: conducted the research; JLE: performed the statistical data analysis and wrote the manuscript; JPK, CJH, and JBM: provided critical guidance on the data analysis and review; RAD-B: provided critical review and interpretation; SLB: had primary responsibility for the final content; and all authors: read and approved the final manuscript.
Footnotes
Supported by the USDA Agricultural Research Service under Cooperative Agreement No. 58-8050-9-004, NIH/National Institute of Diabetes and Digestive and Kidney Diseases (grant number T32 DK062032 to JLE), and the Iowa Pork Producers Association National Pork Board(grant 17-003 to XF).
Author disclosures: The authors report no conflicts of interest.
Any opinions, findings, conclusions, or recommendations expressed in this publication are those of the authors and do not necessarily reflect the views of the USDA, the US Army, or Department of Defense. Any citations of commercial organizations and trade names in this report do not constitute an official Department of the Army endorsement or approval of the products or services of these organizations.
Supplemental Tables 1 and 2 and Supplemental Figure 1 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/jn/.
Supplementary Material
References
- 1.Walther B, Karl JP, Booth SL, Boyaval P. Menaquinones, bacteria, and the food supply: the relevance of dairy and fermented food products to vitamin K requirements. Adv Nutr. 2013;4:463–473. doi: 10.3945/an.113.003855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Booth SL. Vitamin K: food composition and dietary intakes. Food Nutr Res. 2012;56:5505. doi: 10.3402/fnr.v56i0.5505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shearer MJ, Newman P. Recent trends in the metabolism and cell biology of vitamin K with special reference to vitamin K cycling and MK-4 biosynthesis. J Lipid Res. 2014;55:345–362. doi: 10.1194/jlr.R045559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Collins MD, Jones D. Distribution of isoprenoid quinone structural types in bacteria and their taxonomic implications. Microbiol Rev. 1981;45:316–354. doi: 10.1128/mr.45.2.316-354.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bentley R, Meganathant R. Biosynthesis of vitamin K (menaquinone) in bacteria. Microbiol Rev. 1982;46:241–280. doi: 10.1128/mr.46.3.241-280.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nakagawa K, Hirota Y, Sawada N, Yuge N, Watanabe M, Uchino Y, Okuda N, Shimomura Y, Suhara Y, Okano T. Identification of UBIAD1 as a novel human menaquinone-4 biosynthetic enzyme. Nature. 2010;468:117–121. doi: 10.1038/nature09464. [DOI] [PubMed] [Google Scholar]
- 7.Al Rajabi A, Booth SL, Peterson JW, Choi SW, Suttie JW, Shea MK, Miao B, Grusak MA, Fu X. Deuterium-labeled phylloquinone has tissue-specific conversion to menaquinone-4 among Fischer 344 male rats. J Nutr. 2012;142:841–845. doi: 10.3945/jn.111.155804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Okano T, Shimomura Y, Yamane M, Suhara Y, Kamao M, Sugiura M, Nakagawa K. Conversion of phylloquinone (vitamin K1) into menaquinone-4 (vitamin K2) in mice: two possible routes for menaquinone-4 accumulation in cerebra of mice. J Biol Chem. 2008;283:11270–11279. doi: 10.1074/jbc.M702971200. [DOI] [PubMed] [Google Scholar]
- 9.Hirota Y, Tsugawa N, Nakagawa K, Suhara Y, Tanaka K, Uchino Y, Takeuchi A, Sawada N, Kamao M, Wada A, et al. Menadione (vitamin K3) is a catabolic product of oral phylloquinone (vitamin K1) in the intestine and a circulating precursor of tissue menaquinone-4 (vitamin K2) in rats. J Biol Chem. 2013;288:33071–33080. doi: 10.1074/jbc.M113.477356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fu X, Booth SL, Smith DE. Vitamin K contents of rodent diets: a review. J Am Assoc Lab Anim Sci. 2007;46:8–12. [PubMed] [Google Scholar]
- 11.Furie BC, Furie B. Structure and mechanism of action of the vitamin K-dependent gamma-glutamyl carboxylase: recent advances from mutagenesis studies. Thromb Haemost. 1997;78:595–598. [PubMed] [Google Scholar]
- 12.Berkner KL, Runge KW. The physiology of vitamin K nutriture and vitamin K-dependent protein function in atherosclerosis. J Thromb Haemost. 2004;2:2118–2132. doi: 10.1111/j.1538-7836.2004.00968.x. [DOI] [PubMed] [Google Scholar]
- 13.Buitenhuis HC, Soute BAM, Vermeer C. Comparison of the vitamins K1, K2, and K3 as cofactors for the hepatic vitamin K-dependent carboxylase. Biochim Biophys Acta. 1990;1034:170–175. doi: 10.1016/0304-4165(90)90072-5. [DOI] [PubMed] [Google Scholar]
- 14.Thijssen HHW, Vervoort LMT, Schurgers LJ, Shearer MJ. Menadione is a metabolite of oral vitamin K. Br J Nutr. 2006;95:260–266. doi: 10.1079/bjn20051630. [DOI] [PubMed] [Google Scholar]
- 15.Shearer MJ, Okano T. Key pathways and regulators of vitamin K function and intermediary metabolism. Annu Rev Nutr. 2018;38:127–151. doi: 10.1146/annurev-nutr-082117-051741. [DOI] [PubMed] [Google Scholar]
- 16.Institute of Medicine Panel on Micronutrients. Dietary references intakes for vitamin K, arsenic, chromium, copper, iodine, iron, manganese, molybeum, silicon, vanadium, and zinc. Washington (DC): National Academies Press; 2001. [PubMed]
- 17.Fu X, Shen X, Finnan EG, Haytowitz DB, Booth SL. Measurement of multiple vitamin K forms in processed and fresh-cut pork products in the U.S. food supply. J Agric Food Chem. 2016;64:4531–4535. doi: 10.1021/acs.jafc.6b00938. [DOI] [PubMed] [Google Scholar]
- 18.Fu X, Harshman SG, Shen X, Haytowitz DB, Philip Karl J, Wolfe BE, Booth SL. Multiple vitamin K forms exist in dairy foods. Curr Dev Nutr. 2017;1:e000638. doi: 10.3945/cdn.117.000638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Akbulut AC, Pavlic A, Petsophonsakul P, Halder M, Maresz K, Kramann R, Schurgers L. Vitamin K2 needs an RDI separate from vitamin K1. Nutrients. 2020;12:1852. doi: 10.3390/nu12061852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shea MK, Berkner KL, Ferland G, Fu X, Holden RM, Booth SL. Perspective: evidence before enthusiasm–a critical review of the potential cardiovascular benefits of vitamin K. Adv Nutr. 2021;12:632. doi: 10.1093/advances/nmab004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reeves PG. Components of the AIN-93 diets as improvements in the AIN-76A diet. J Nutr. 1997;127:838S–841S. doi: 10.1093/jn/127.5.838S. [DOI] [PubMed] [Google Scholar]
- 22.Kim K-C, Jang H, Sauer J, Zimmerly EM, Liu Z, Chanson A, Smith DE, Friso SI, Choi S-W. Folate supplementation differently affects uracil content in DNA in the mouse colon and liver. Br J Nutr. 2011;105:688–693. doi: 10.1017/S0007114510004332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harshman SG, Fu X, Karl JP, Barger K, Lamon-Fava S, Kuliopulos A, Greenberg AS, Smith D, Shen X, Booth SL. Tissue concentrations of vitamin K and expression of key enzymes of vitamin K metabolism are influenced by sex and diet but not housing in C57Bl6 mice. J Nutr. 2016;146:1521–1527. doi: 10.3945/jn.116.233130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Knauer TE, Siegfried C, Willingham AK, Matschiner JT. Metabolism and biological activity of cis- and trans-phylloquinone in the rat. J Nutr. 1975;105:1519–1524. doi: 10.1093/jn/105.12.1519. [DOI] [PubMed] [Google Scholar]
- 25.Karl JP, Fu X, Dolnikowski GG, Saltzman E, Booth SL. Quantification of phylloquinone and menaquinones in feces, serum, and food by high-performance liquid chromatography-mass spectrometry. J Chromatogr B. 2014;963:128–133. doi: 10.1016/j.jchromb.2014.05.056. [DOI] [PubMed] [Google Scholar]
- 26.Harshman SG, Finnan EG, Barger KJ, Bailey RL, Haytowitz DB, Gilhooly CH, Booth SL. Vegetables and mixed dishes are top contributors to phylloquinone intake in US adults: data from the 2011–2012 NHANES. J Nutr. 2017;147:1308–1313. doi: 10.3945/jn.117.248179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Davidson KW, Sadowski JA. Determination of vitamin K compounds in plasma or serum by high-performance liquid chromatography using postcolumn chemical reduction and fluorimetric detection. Methods Enzymol. 1997;282:408–421. doi: 10.1016/s0076-6879(97)82124-6. [DOI] [PubMed] [Google Scholar]
- 28.Shea MK, Booth SL, Gundberg CM, Peterson JW, Waddell C, Dawson-Hughes B, Saltzman E. Adulthood obesity is positively associated with adipose tissue concentrations of vitamin K and inversely associated with circulating indicators of vitamin K status in men and women. J Nutr. 2010;140:1029–1034. doi: 10.3945/jn.109.118380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fu X, Peterson JW, Hdeib M, Booth SL, Grusak MA, Lichtenstein AH, Dolnikowski GG. Measurement of deuterium-labeled phylloquinone in plasma by high-performance liquid chromatography/mass spectrometry. Anal Chem. 2009;81:5421–5425. doi: 10.1021/ac900732w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.National Institutes of Health. Consideration of sex as a biological variable in NIH-funded research. Notice Number: NOT-OD-15-102 [Internet]. 2015. Available from: https://grants.nih.gov/grants/guide/notice-files/not-od-15-102.html.
- 31.Shearer MJ, Newman P. Metabolism and cell biology of vitamin K. Thromb Haemost. 2008;100:530–547. [PubMed] [Google Scholar]
- 32.Takada T, Yamanashi Y, Konishi K, Yamamoto T, Toyoda Y, Masuo Y, Yamamoto H, Suzuki H. NPC1L1 is a key regulator of intestinal vitamin K absorption and a modulator of warfarin therapy. Sci Transl Med. 2015;7:275ra23. doi: 10.1126/scitranslmed.3010329. [DOI] [PubMed] [Google Scholar]
- 33.Yamanashi Y, Takada T, Kurauchi R, Tanaka Y, Komine T, Suzuki H. Transporters for the intestinal absorption of cholesterol, vitamin E, and vitamin K. J Atheroscler Thromb. 2017;24:347–359. doi: 10.5551/jat.RV16007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goncalves A, Margier M, Roi S, Collet X, Niot I, Goupy P, Caris-Veyrat C, Reboul E. Intestinal scavenger receptors are involved in vitamin K1 absorption. J Biol Chem. 2014;289:30743–30752. doi: 10.1074/jbc.M114.587659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Camus MC, Chapman JM, Forgez P, Laplaud PM. Distribution and characterization of the serum lipoproteins and apoproteins in the mouse, Mus musculus. J Lipid Res. 1983;24:1210–1228. [PubMed] [Google Scholar]
- 36.Gordon SM, Li H, Zhu X, Shah AS, Lu LJ, Davidson WS. A comparison of the mouse and human lipoproteome: suitability of the mouse model for studies of human lipoproteins. J Proteome Res. 2015;14:2686–2695. doi: 10.1021/acs.jproteome.5b00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lamon-Fava S, Sadowski JA, Davidson KW, O'Brien ME, McNamara JR, Schaefer EJ. Plasma lipoproteins as carriers of phylloquinone (vitamin K1) in humans. Am J Clin Nutr. 1998;67:1226–1231. doi: 10.1093/ajcn/67.6.1226. [DOI] [PubMed] [Google Scholar]
- 38.Tovar A, Ameho CK, Blumberg JB, Peterson JW, Smith D, Booth SL. Extrahepatic tissue concentrations of vitamin K are lower in rats fed a high vitamin E diet. Nutr Metab. 2006;3:2–7. doi: 10.1186/1743-7075-3-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Duello TJ, Matschiner JT. Characterization of vitamin K from human liver. J Nutr. 1972;102:331–335. doi: 10.1093/jn/102.3.331. [DOI] [PubMed] [Google Scholar]
- 40.Usui Y, Tanimura H, Nishimura N, Kobayashi N, Okanoue T, Ozawa K. Vitamin K concentrations in the plasma and liver of surgical patients. Am J Clin Nutr. 1990;51:846–852. doi: 10.1093/ajcn/51.5.846. [DOI] [PubMed] [Google Scholar]
- 41.Will BH, Suttie JW. Comparative metabolism of phylloquinone and menaquinone-9 in rat liver. J Nutr. 1992;122:953–958. doi: 10.1093/jn/122.4.953. [DOI] [PubMed] [Google Scholar]
- 42.Shea M, Booth S. Concepts and controversies in evaluating vitamin K status in population-based studies. Nutrients. 2016;8:8. doi: 10.3390/nu8010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ellis JL, Fu X, Al Rajabi A, Grusak MA, Shearer MJ, Naumova EN, Saltzman E, Barger K, Booth SL. Plasma response to deuterium-labeled vitamin K intake varies by TG response, but not age or vitamin K status, in older and younger adults. J Nutr. 2019;149:18–25. doi: 10.1093/jn/nxy216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shearer MJ, Fu X, Booth SL. Vitamin K nutrition, metabolism, and requirements: current concepts and future research. Am Soc Nutr. 2012;3:182–195. doi: 10.3945/an.111.001800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schurgers LJ, Vermeer C. Differential lipoprotein transport pathways of K-vitamins in healthy subjects. Biochim Biophys Acta. 2002;1570:27–32. doi: 10.1016/s0304-4165(02)00147-2. [DOI] [PubMed] [Google Scholar]
- 46.Sato T, Schurgers LJ, Uenishi K. Comparison of menaquinone-4 and menaquinone-7 bioavailability in healthy women. Nutr J. 2012;11:93. doi: 10.1186/1475-2891-11-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schurgers LJ, Teunissen KJF, Hamulyák K, Knapen MHJ, Vik H, Vermeer C. Vitamin K–containing dietary supplements: comparison of synthetic vitamin K1 and natto-derived menaquinone-7. Blood. 2007;109:3279–3283. doi: 10.1182/blood-2006-08-040709. [DOI] [PubMed] [Google Scholar]
- 48.Niemeier A, Niedzielska D, Secer R, Schilling A, Merkel M, Enrich C, Rensen PCN, Heeren J. Uptake of postprandial lipoproteins into bone in vivo: Impact on osteoblast function. Bone. 2008;43:230–237. doi: 10.1016/j.bone.2008.03.022. [DOI] [PubMed] [Google Scholar]
- 49.Schurgers LJ, Teunissen KJF, Hamulyák K, Knapen MHJ, Vik H, Vermeer C. Vitamin K-containing dietary supplements: comparison of synthetic vitamin K1 and natto-derived menaquinone-7. Blood. 2007;109:3279–3283. doi: 10.1182/blood-2006-08-040709. [DOI] [PubMed] [Google Scholar]
- 50.Huber AM, Davidson KW, O'Brien-Morse ME, Sadowski JA. Gender differences in hepatic phylloquinone and menaquinones in the vitamin K-deficient and -supplemented rat. Biochim Biophys Acta. 1999;1426:43–52. doi: 10.1016/s0304-4165(98)00121-4. [DOI] [PubMed] [Google Scholar]
- 51.Mellette SJ. Interrelationships between vitamin K and estrogenic hormones. Am J Clin Nutr. 1961;9:109–116. doi: 10.1093/ajcn/9.4.109. [DOI] [PubMed] [Google Scholar]
- 52.Matschiner JT, Willingham AK. Influence of sex hormones on vitamin K deficiency and epoxidation of vitamin K in the rat. J Nutr. 1974;104:660–665. doi: 10.1093/jn/104.6.660. [DOI] [PubMed] [Google Scholar]
- 53.Jolly DW, Craig C, Nelson TE. Estrogen and prothrombin synthesis: effect of estrogen on absorption of vitamin K1. Am J Physiol. 1977;232:H12–7. doi: 10.1152/ajpheart.1977.232.1.H12. [DOI] [PubMed] [Google Scholar]
- 54.Ramotar K, Conly JM, Chubb H, Louie TJ. Production of menaquinones by intestinal anaerobes. J Infect Dis. 1984;150:213–218. doi: 10.1093/infdis/150.2.213. [DOI] [PubMed] [Google Scholar]
- 55.Hill MJ. Intestinal flora and endogenous vitamin synthesis. Eur J Cancer Prev. 1997;6:S43–5. doi: 10.1097/00008469-199703001-00009. [DOI] [PubMed] [Google Scholar]
- 56.Suttie JW. The importance of menaquinones in human nutrition. Annu Rev Nutr. 1995;15:399–417. doi: 10.1146/annurev.nu.15.070195.002151. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.