Abstract
Background
During pregnancy iron can be obtained from the diet, body iron stores, or iron released from RBC catabolism. Little is known about the relative use of these sources to support fetal iron acquisition.
Objectives
To describe longitudinal change in iron absorption and enrichment across gestation and partitioning of RBC iron to the fetus.
Methods
Fifteen pregnant women ingested an oral stable iron isotope (57Fe) in the second trimester (T2) of pregnancy (weeks 14–16) to label the RBC pool, and a second oral stable isotope (58Fe) in the third trimester (T3) (weeks 32–35). Absorption was measured at T2 and T3. Change in RBC 57Fe enrichment was monitored (18.8–26.6 wk) to quantify net iron loss from this pool. Iron transfer to the fetus was determined based on RBC 57Fe and 58Fe enrichment in umbilical cord blood at delivery.
Results
Iron absorption averaged 9% at T2 and increased significantly to 20% (P = 0.01) by T3. The net increase in iron absorption from T2 to T3 was strongly associated with net loss in maternal total body iron (TBI) from T2 to T3 (P = 0.01). Mean time for the labeled RBC 57Fe turnover based on change in RBC enrichment was 94.9 d (95% CI: 43.5, 207.1 d), and a greater decrease in RBC 57Fe enrichment was associated with higher iron absorption in T2 (P = 0.001). Women with a greater decrease in RBC 57Fe enrichment transferred more RBC-derived iron to their fetus (P < 0.05).
Conclusions
Iron absorption doubled from T2 to T3 as maternal TBI declined. Women with low TBI had a greater decrease in RBC iron enrichment and transferred more RBC-derived iron to their neonate. These findings suggest maternal RBC iron serves as a significant source of iron for the fetus, particularly in women with depleted body iron stores. Am J Clin Nutr 2022;115:1069–1079.
Keywords: pregnancy, neonate, stable isotope, iron absorption, hemoglobin, anemia, iron deficiency
Introduction
The developing fetus relies on placental transfer of iron from maternal sources such as recently absorbed dietary iron, mobilization of body iron reserves, or iron released from catabolized RBCs. Whereas human and animal studies have assessed transfer of dietary iron to the fetus (1, 2, 3, 4, 5, 6), little is known about the relative proportion of fetal iron that can be obtained from the maternal RBC pool. In addition, little is known about factors that influence maternal RBC catabolism or RBC iron turnover across pregnancy. More work on understanding the dynamics of iron partitioning between the mother and her placental/fetal unit is needed to ensure that the mother has enough iron during pregnancy along with adequate neonatal iron endowment at birth.
Iron requirements increase by ∼7-fold during pregnancy to >7 mg/d in the third trimester (7). In recognition of these increased iron demands, the RDA for iron increases from 18 mg/d in nonpregnant women to 27 mg/d during pregnancy (8). This requirement was in part informed by early iron absorption estimations that were based on the acute recovery of an orally administered isotope in serum, an approach that does not reflect longer term utilization of iron for RBC production (9). Early studies were dependent on less precise mass spectrometric approaches. Furthermore, data on maternally absorbed iron did not account for absorbed iron that was rapidly transferred to the fetus (10). Further understanding of maternal iron dynamics across gestation using current methodology is needed.
The majority of iron in the body is contained within the RBC pool. This pool recycles ∼20 mg of iron daily as RBCs are catabolized. In nonpregnant women, iron that is released is either reutilized to support erythropoietic demands or sequestered into storage reserves. During pregnancy, some of this iron can be shunted to the fetus to support fetal iron demands. The lifespan of a RBC has been monitored in older studies using intravenous radioactive chromium (51Cr) or biotinylated labeled RBCs (11, 12, 13, 14, 15, 16, 17). These studies estimated RBC lifespan to be ∼120 ±∼30 d in adult men and women. Analogous data in pregnant women are lacking, as are data on fetal use of iron released from the maternal RBC pool.
The developing fetus relies on maternal iron sources for adequate iron accretion in utero. It is likely that some of this iron is obtained from the maternal RBC pool (18), but to date our understanding of iron recycling from RBC catabolism comes primarily from radioisotope studies in adult men (11, 12, 13). To fill these gaps in knowledge, the goals of this study were to: 1) obtain longitudinal measures of maternal iron absorption from early to late gestation; 2) evaluate change in RBC stable iron isotope enrichment and determinants of this change across pregnancy; and 3) evaluate possible correlations between change in RBC stable iron isotope enrichment and the amount of stable iron isotope recovered in the newborn at birth.
Methods
Participants
Twenty-three women were recruited from University of Rochester Medical Center Midwifery Group and from the Rochester Adolescent Maternity Program (RAMP) in Rochester, NY early in gestation (13–15 wk of gestation). Women were eligible to participate if they had an uncomplicated pregnancy, were carrying a singleton, were not previously treated for lead exposure, and did not have any malabsorption diseases, hemoglobinopathies, or were taking a medication that might alter iron homeostasis. One participant withdrew before receiving the first iron tracer dose, 3 participants were ineligible due to subsequent health complications across gestation [gallbladder removal (n = 2), Gestational Diabetes Mellitus (n = 1)], and dosing errors occurred in 4 women resulting in a final study population of 15 women. The study was approved by the Institutional Review Board of Cornell University and the University of Rochester Research Subjects Review Board, and written consent was obtained from all participants.
Study procedure and isotope dosing
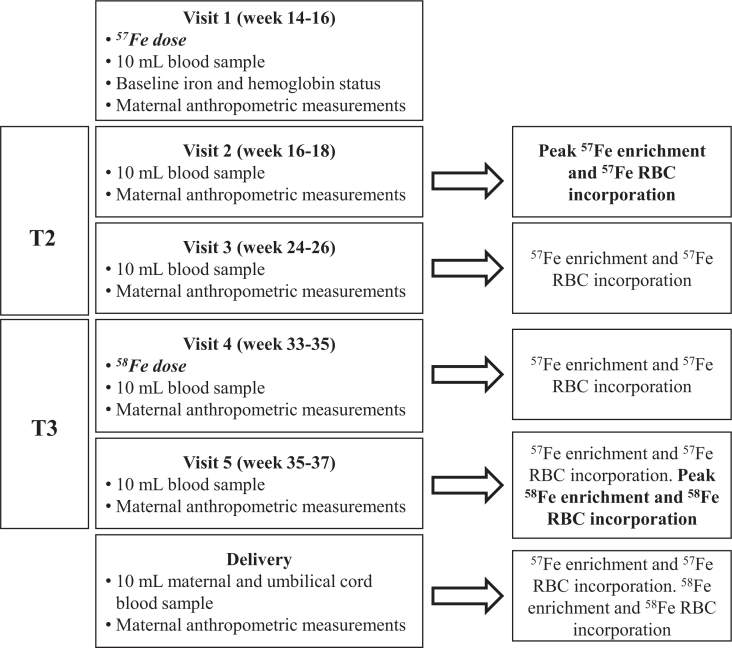
This study was carried out from 2012 to 2015. All participants were asked to discontinue iron or prenatal supplement use 2 d prior to each stable isotope dosing study. Figure 1 depicts the timeline for the study and the study measures collected at each visit (V1–V5). During the second trimester (T2) of pregnancy (V1: 14–16 wk of gestation), women came to the RAMP clinic or Midwifery Group office after an overnight fast. Their height and weight were measured with a stadiometer and calibrated scale. A venous blood sample (5 mL) was collected to determine baseline iron status. To label the maternal RBC pool, each woman ingested a 20-mg dose of 57Fe as ferrous sulfate in a small amount of flavored syrup (Humco). Women were then given a packaged lunch (canned vegetable soup and pretzels) and were instructed to wait ≥1.5 h before consuming this meal. Women returned for 5 more visits over gestation to have blood samples taken: V2 (weeks 16–18), V3 (weeks 24–26), V4 (weeks 33–35), V5 (weeks 35–37), and at delivery. Subsequent measures of 57Fe RBC enrichment were obtained in V3, V4, V5, and at delivery to monitor the change in RBC enrichment from weeks 16–18 of pregnancy until delivery.
FIGURE 1.
Study timeline for dosing and iron analysis showing the study design and when each study visit occurred over the course of gestation. Arrows indicate the study measures obtained at each of the scheduled visits. T, trimester.
At V4, after the fasting blood sample was obtained, women ingested a second oral stable iron isotope (1.1 mg 58Fe and 18.9 mg iron as ferrous sulfate for a final total iron dose of 20 mg). They returned home with the same standardized lunch meal and were again instructed to wait ≥1.5 h before consuming this meal. Iron absorption in the third trimester (T3) was measured 2 wk postdosing using the V5 sample based on 58Fe RBC enrichment. At delivery, a cord blood sample (30 mL) was collected for analysis of neonatal iron status biomarkers and to determine the net transfer of 57Fe and 58Fe to the fetus based on neonatal RBC enrichment at birth.
Laboratory analysis
All newborn iron status indicators were measured using cord blood samples. Hemoglobin (Hb) concentrations were measured using a HemoCue Analyzer at the University of Rochester. Maternal anemia was defined as Hb concentration <11.0 g/dL in T1 and T3, and <10.5 g/dL in T2. Data were also evaluated using CDC race-adjusted cutoffs for black women [<10.2 g/dL in T1 and T3 and <9.7 g/dL in T2 (19)]. Using either the race-adjusted or the nonadjusted cutoffs did not change any of the key findings so all data presented are non–race-adjusted. Neonatal anemia at birth was defined when cord blood Hb was <13 g/dL (19, 20). Cord blood Hb measures are known to be significantly correlated with neonatal venous Hb measures (21). Serum was separated from whole blood and stored at − 80°C until used. Serum ferritin (SF) and soluble transferrin receptor (sTfR) were measured using commercially available kits (Ramco Inc). Hepcidin and erythropoietin were measured by commercially available ELISA kits (Bachem; R&D systems, respectively). The limit of detection (LOD) for the hepcidin assay was 0.39 ng/mL, and for statistical purposes a value of 0.195 ng/mL was assigned and values <0.39 ng/mL were classified as undetectable. All analytes were measured once the study was completed except for erythroferrone (ERFE), which was measured in 2019 when a commercially available ELISA for this hormone became available (Intrinsic Lifesciences). Prior research using this assay has found that ERFE measures were appropriately elevated in serum from thalassemia patients that had been stored at −80 C for 8–9 y (22). The ERFE kit provides quantitative measures below the stated LOD (1.5 ng/mL), and absolute values were used for statistical analysis. Total body iron (TBI) was calculated from SF and sTfR as previously described (23, 24). All samples were run in duplicate.
Iron isotope preparation
Iron isotopes (57Fe at 94.69% enrichment and 58Fe at 93.34% enrichment) were purchased in metal form (Trace Sciences International) and were converted into a ferrous sulfate solution containing ascorbic acid at a 2:1 ratio as previously detailed (25). The isotopic composition of each tracer solution was validated using a Triton TI Magnetic Sector Thermal Ionization Mass Spectrometer (Thermo Fisher Scientific), and the total iron content of each tracer solution was measured using atomic absorption spectrophotometry (Perkin-Elmer Analyst 800).
Isolation and measurement of iron isotope
Maternal and umbilical cord blood (1 mL) was digested with 2 mL ultrapure nitric acid (Ultrex; JT Baker). The digested residue was reconstituted in 2 mL ultrapure hydrochloric acid (Ultrex; JT Baker) and iron was extracted using anion exchange chromatography. The eluate was dried on a hot plate and reconstituted in 30 μL 3% ultrapure nitric acid (Ultrex; JT Baker). The extracted iron (8 μL) was loaded onto a degassed rhenium (H Cross) filament with 6 μL silica gel (Sigma-Aldrich) and 2 μL phosphoric acid (Sigma-Aldrich). Iron isotopic ratios (57/56Fe, 58/56Fe, and 54/56Fe) were measured using a Triton TI Magnetic Sector Thermal Ionization Mass Spectrometer (Thermo Fisher Scientific).
Calculation of RBC iron enrichment
Maternal blood samples taken 2 wk postdosing (V2) were used to measure maternal RBC iron enrichment. The 2-wk postdosing sample collection time is based on early radiotracer data obtained in men that found the majority of ingested tracer was incorporated into RBC by 2 wk postdosing (26). The delta percentage (Δ%) excess of each iron isotope was calculated as the degree to which the iron isotopic ratios (57/56Fe or 58/56Fe) differed from the baseline natural abundance ratios (0.02317 and 0.00307, respectively). Maternal circulating iron during pregnancy was calculated using measured maternal Hb concentration (grams per deciliter) at each study visit, the estimated iron content of Hb (3.47 g/kg) (27), maternal weight (kilograms) at each study visit, and an assumed blood volume of 70 mL/kg (28). Using the % excess and circulating iron concentration, the net quantity (milligrams) of tracer incorporated into the maternal RBC pool was calculated after assuming that 80% of absorbed iron was incorporated into erythrocytes, following similar methodology that has been utilized in pregnant women (29, 30, 31, 32).
Neonatal RBC iron incorporation was calculated using the same approach as detailed above except that the assumed blood volume was estimated as 80 mL/kg (1). We assumed that 80% of iron was incorporated into neonatal RBCs based on data obtained from neonatal autopsy studies at birth (20, 33, 34, 35, 36, 37). This amount is lower than the value of 90% that is often used in similar studies carried out in older infants (38). Should actual incorporation be closer to the 90% estimate used in older infants this would serve to increase the net amount of iron transferred to the fetus.
We previously reported that absorption estimations are underestimated when only utilizing iron isotope incorporated into maternal RBCs (10), because some tracer is rapidly transferred to the fetus (2). Therefore, absorption was calculated as the net quantity (milligrams) of tracer incorporated into the maternal RBC pool at 2 wk and the tracer recovered in the baby at delivery divided by the tracer dose consumed by the mother.
Approaches to evaluate change in RBC enrichment across pregnancy
Change in RBC 57Fe enrichment across gestation was determined by 3 methods: 1) the net change in RBC enrichment was calculated as observed difference between the V2 and delivery measure; 2) the slope of change was estimated for each woman individually using all timepoints; and 3) random slope and intercept models were also used to assess longitudinal changes in RBC enrichment over time. To further quantify the change in RBC enrichment, the time to clear half of the RBC iron tracer was calculated from using the equation: T1/2 = ln(2)/λ, where λ is the observed slope of change in RBC enrichment from V2 to V4 when observed change over time was linear for all women. Additionally, length of time for the labeled RBC iron to turnover (τ) was calculated using the equation ln(2)/λ = τ ln(2). The RBC enrichment AUC for each participant was also calculated using the trapezoidal method (39). The total net iron released from the maternal RBC pool was calculated using maternal circulating iron, as discussed above, and the percentage of iron tracer (milligrams) released from RBCs. This method assumes the 57Fe tracer is incorporated into RBCs in an identical fashion to native dietary iron. Decreases in RBC enrichment across gestation can occur as a consequence of dilution of tracer from an increase in the size of the iron pool as well as from a loss of iron tracer as RBCs are catabolized and the tracer released is transferred to the neonate.
Approaches to evaluate net transfer of iron to the neonate and iron partitioning
Net transfer of 57Fe and 58Fe to the fetus was examined using 3 approaches. Net transfer was calculated as the net milligrams of tracer recovered in neonatal RBCs presented as a fraction of 1) the mass of maternal iron tracer ingested (milligrams), 2)the mass of absorbed iron tracer recovered in maternal RBCs 2 wk postdosing (milligrams), and 3) the mass of 57Fe tracer lost from the maternal RBC pool from V2 to delivery (milligrams).
Iron partitioning of 57Fe between the maternal and neonatal compartment was assessed. Iron partitioning to the neonatal compartment was calculated as the net 57Fe recovered (milligrams) in neonatal RBCs as a fraction of the total 57Fe recovered (milligrams) in the maternal and neonatal compartments (maternal RBC pool 2 wk postdosing + neonatal RBC pool at birth). Similarly, iron partitioning to the neonatal compartment was calculated as net 58Fe (milligrams) recovered in the neonatal RBC pool as a fraction of the total 58Fe recovered in both the maternal and neonatal compartments (maternal RBC pool + neonatal RBC pool).
Statistical analysis
Participant characteristics and iron status indicators are presented as the mean ± SD, geometric mean (95% CI), or frequency and proportion. Possible changes in iron status indicators and participant characteristics between baseline and delivery were evaluated using t test, Wilcoxon rank sum test, or χ2 test as appropriate. Change in absorption was calculated as the difference in RBC iron enrichment between T2 and T3. Similarly, change in iron status indicators was calculated as the difference between each indicator from dosing week in T2 and T3. Random slope and intercept models were used to assess change in RBC enrichment across gestation. Data from women who were not dosed with a second stable iron isotope at V4 (n = 2) were not included in the longitudinal data analysis. For individual timepoint analyses, analyses were run with, and without, these 2 women to determine if significant findings differed. Because none of the significant findings differed, data from these 2 women were included in all nonlongitudinal analyses. Linear regression models were used to assess correlation between variables and determinants, and standardized β coefficients (sβ) and SEs were reported. For multiple regression models, dependent variables with bivariate correlation P values <0.2 were tested simultaneously and eliminated by backward selection until only statistically significant variables remained. Statistical analyses were conducted with JMP Pro 14.
Results
Subject characteristics and iron status
A total of 15 women completed the study (Supplemental Figure 1). Maternal and neonatal characteristics are presented in Table 1. One participant delivered at week 35, before the second stable iron isotope could be administered, and an additional woman missed the second dosing visit. One of the participants in the study was an adolescent (aged 17 y), and the rest were >20 y of age. Based on self-report, none of the study volunteers were current cigarette smokers. Two neonates were born prematurely (13%) and 1 of the preterm neonates was classified as low birthweight (7%). At birth, 33% of newborns were anemic (cord Hb <13 g/dL). Data on maternal and neonatal iron status are presented in Table 2. Maternal iron status decreased significantly from baseline (∼week 15) to delivery (SF, P = 0.03; TBI, P = 0.01), but prevalence of anemia did not significantly differ between baseline and delivery (P = 0.23).
TABLE 1.
Maternal and neonatal characteristics1
| Maternal characteristics | Value (n = 15) |
|---|---|
| Age at entry, y | 27.31 ± 4.14 |
| Race: white, % | 67 |
| Ethnicity: non-Hispanic, % | 86 |
| Parity ≥ 1, % | 56 |
| Prepregnancy BMI, kg/m2 | 25.12 ± 7.99 |
| Gestational weight gain, kg | 11.58 ± 11.02 |
| Newborn characteristics | (n = 15) |
| Gestational age, wk | 39.23 ± 1.86 |
| Birthweight, g | 3282.44 ± 451.37 |
| LBW, % [n] | 6.7 [1/15] |
| Sex: female, % | 44 |
Data presented as mean ± SD or percentage. LBW, low birthweight: defined as birthweight <2500 g.
TABLE 2.
Longitudinal changes in maternal and neonatal iron status1
| Trimester 2 (visit 1: 57Fe dose) | Trimester 3 (visit 4: 58Fe dose) | Delivery | Neonate | |
|---|---|---|---|---|
| Gestational age, wk | 15.0 ± 0.7 | 34.0 ± 0.7 | 39.4 ± 1.8 | 39.4 ± 1.8 |
| n | 15 | 13 | 15 | 15 |
| Weight, kg | 68.9 ± 21.2a | 77.7 ± 18.8b | 79.9 ± 20.7c | 3.4 ± 0.3 |
| Hemoglobin, g/dL | 12.2 ± 1.9 | 11.0 ± 1.3 | 11.6 ± 1.2 | 13.7 ± 2.3 |
| Anemia, % | 7a | 54b | 23a | 33 |
| Serum ferritin, ug/L | 50.1 [30.6, 82.2]a | 22.7 [12.1, 42.8]b | 33.8 [17.6, 64.6]c | 205.8 [141.5, 299.3] |
| Serum ferritin <12 ug/L, % | 7 | 25 | 8 | — |
| Transferrin receptor, mg/L | 3.6 [2.6, 5.0]a | 4.9 [3.6, 6.6]b | 5.3 [3.83, 7.4]c | 6.2 ± 1.8 |
| Total body iron, mg/kg | 8.0 ± 3.4a | 4.0 ± 3.5ab | 5.2 ± 4.1b | 11.3 ± 3.0 |
| Erythroferrone, ng/mL | 0.7 [0.4, 1.5] | 0.8 [0.4, 1.7] | 0.7 [0.4, 1.3] | 1.6 [0.8, 3.3] |
| Erythropoietin, mIU/mL | 18.5 [12.4, 27.7]a | 30.4 [17.6, 52.3]b | 29.5 [16.3, 53.2]a | 27.4 [16.6, 45.0] |
| Hepcidin, ng/mL | 10.8 ± 7.5 | 5.4 ± 5.1 | — | 36.0 ± 18.1 |
| Undetectable hepcidin, % | 7 | 0 | — | 0 |
Data are presented as mean ± SD for normally distributed data or geometric mean [95% CI] for nonnormally distributed data. Values with different superscripts within a row significantly differ from one another, P < 0.05. Neonatal iron status was determined using umbilical cord blood. Undetectable hepcidin was classified as values <0.39 ng/mL.
Iron absorption across gestation
Maternal iron absorption at T2 (15.0 ± 0.7 wk) averaged 8.7% (95% CI: 5.0, 15.4%) and more than doubled across the ∼19-wk period that elapsed from T2 to T3 (at 34.0 ± 0.8 wk) to 20.3% (95% CI: 13.82, 26.86%) (P = 0.01) (Tables 3 and 4). Our data on maternal iron absorption were compared with previous published iron isotope data in pregnant women using orally administered iron tracers (Tables 4 and 5). Results reported in the study by Hahn et al. (40) were adjusted to assume 80% of absorbed iron was incorporated into RBCs, because their original publication assumed 100% of orally absorbed isotope was incorporated into RBCs.
TABLE 3.
Iron enrichment, weight, and hemoglobin measures across gestation1
| Visit 1 (weeks 14–16) | Visit 2 (weeks 16–18) | Visit 3 (weeks 24–26) | Visit 4 (weeks 33–35) | Visit 5 (weeks 35–37) | Delivery (weeks 35–41) | Neonate | |
|---|---|---|---|---|---|---|---|
| Trimester | 2 | 3 | |||||
| n | 15 | 15 | 13 | 13 | 13 | 15 | 15 |
| Weight, kg | 68.9 ± 21.2 | 70.1 ± 20.6 | 70.2 ± 17.1 | 77.7 ± 18.8 | 77.9 ± 19.1 | 79.8 ± 20.7 | 3.31 ± 0.5 |
| Hb, g/dL | 12.2 ± 1.9 | 11.9 ± 1.5 | 11.2 ± 1.0 | 11.0 ± 1.3 | 11.5 ± 1.3 | 11.6 ± 1.2 | 13.7 ± 2.3 |
| 57Fe Δ% excess | — | 3.2 ± 2.1 | 2.9 ± 2.1 | 2.1 ± 1.3 | 2.1 ± 1.3 | 2.1 ± 1.2 | 2.2 ± 1.4 |
| 57Fe, mg | — | 1.0 [0.6, 1.8] | 0.9 [0.5, 1.6] | 0.7 [0.4, 1.3] | 0.7 [0.4, 1.4] | 0.8 [0.5, 1.3] | 0.1 ± 0.04 |
| 57Fe RBC enrichment, % | — | 8.7* [5.0, 15.4] | 7.7 [4.5, 13.3] | 5.5 [2.9, 10.4] | 5.9 [3.0, 11.7] | 7.2 [4.4, 11.7] | 0.5 ± 0.3 |
| 58Fe Δ% excess | — | — | — | — | 2.4 [1.6, 3.7] | 2.8 [2.1, 3.9] | 3.6 ± 1.9 |
| 58Fe, mg | — | — | — | — | 0.2 [0.1, 0.2] | 0.2 [0.1, 0.2] | 0.01 ± 0.01 |
| 58Fe RBC enrichment, % | — | — | — | — | 20.3 ± 8.7* | 21.8 ± 10.5 | — |
Data presented as mean ± SD for normally distributed data or geometric mean [95% CI] for nonnormally distributed data. Hb, hemoglobin; Δ%, delta percentage.
Indicates the values that represent iron absorption.
TABLE 4.
Absorption estimations from stable iron isotope studies across gestation1
| Stable isotope (57Fe or 54Fe as FeSO4) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Whittaker et al.2,3, (62) (n = 9) | Barrett et al2,3,6 (9) (n = 12) | Whittaker et al2,4 (32) (n = 5) | O’Brien et al4 (42) (n = 43) | Delaney et al4 (10) (n = 5) | Young et al.4,6 (28) (n = 20) | Current study2,4 (n = 15) | Koenig et al.4 (30) (n = 50) | |
| Total iron load, mg | 5.23 | 6 | 10 | 60 | 9 | 9 | 20 | 8.4 |
| Weeks of gestation | Geometric mean [range] | Geometric mean [range] | Geometric mean [range] | Median [range] | Median [range] | Median [range] | Median [range] | Median [IQR] |
| <10 | — | — | — | — | — | — | — | — |
| 10–14 | 7.6 [1.2–21.8] | 7.2 [4.9–10.9] | 11.8 [4.4–24.8] | — | — | — | 8.5 [5.0–27.6] | — |
| 15–19 | — | — | — | — | — | — | — | — |
| 20–24 | 21.1 [8.6–58.4] | 36.3 [27.6–47.3] | — | — | — | — | — | — |
| 25–29 | — | — | — | — | — | — | — | — |
| 30–34 | — | — | — | 13.98 [0.8–33.2] | 37.91 [19.0–62.1] | 40.49 [18.3–62.9] | — | 9.8 [0–20] |
| >35 | 36.3 [18.4–55.6] | 66.1 [57.1–76.2] | 49.0 [38.2–77.2] | 9.32 [1.3–15.6] | — | — | 18.8 [6.2–34.2] | — |
| Fold change weeks 10–14 to >35 | 4.8 | 9.2 | 4.2 | — | — | — | 2.2 | — |
Compared with nonpregnant women (DRI 18% absorption).
Longitudinal study measures across gestation.
Serum iron measured shortly after dosing to calculate absorption.
RBC iron incorporation used to calculate absorption.
5 Absorption measures using whole body counter.
Iron isotope dose given with food.
TABLE 5.
Absorption estimations from radioactive iron isotope studies across gestation1
| Radioactive isotope (59Fe as FeSO4, FeCl2, or ferric ammonium citrate) | |||||
|---|---|---|---|---|---|
| Balfour et al.4 (63) (n = 13) | Svanberg et al2,5 (64) (n = 30) | Svanberg et al2,5 (64) (n = 30) Iron treated | Hahn et al.4 (65) (n = 12–56) | Svanberg et al2,5,6 (66) (n = 22) | |
| Total iron load, mg | 1–122 | 100 | 100 | 2–9 | 4 |
| Week of gestation | Percentage uptake [range] | Mean [range] | Mean [range] | Median percentage uptake | Mean [range] |
| <10 | — | — | — | 13.8 | — |
| 10–14 | 2.7 | 6.5 [1.2–11] | 6.7 [3.4–14.5] | 13.8 | 1.5 [0–6.7] |
| 15–19 | 4.2 | — | — | 21.3 | — |
| 20–24 | 3.2 | 9.2 [3.2–17.9] | 6.0 [1.1–13.1] | 40 | 5.8 [0–15.4] |
| 25–29 | 2.5 | — | — | 41.3 | — |
| 30–34 | 16.4 | — | — | 50.6 | — |
| >35 | 9.1 [2.2–27.7] | 14.3 [5.9–24.7] | 8.6 [2.7–15.0] | 51.3 | 14.6 [5.7–28.3] |
| Fold change weeks 10–14 to >35 | 3.3 | 2.2 | 1.3 | 3.7 | 9.7 |
Compared with nonpregnant women (DRI 18% absorption). Hahn et al. (65) estimations were adjusted to assume 80% incorporation into RBCs because 100% incorporation was used in their publication.
Longitudinal study/measures across gestation.
3 Serum iron measured shortly after dosing to calculate absorption.
RBC iron incorporation used to calculate absorption.
Absorption measures using whole body counter.
Iron isotope dose given with food.
The mean increase in absorption from T2 to T3 was 8.5 ± 11.6%, and the mean change in TBI from T2 to T3 was − 4 ± 4 mg/kg. For every 1 mg/kg decrease in total body iron from T2 to T3, iron absorption increased on average by 1.4%, and change in absorption was significantly associated with change in maternal TBI status from T2 to T3. Change in TBI from T2 to T3 explained 58% of variance in the change of iron absorption from T2 to T3, whereas change in maternal weight from T2 to T3 explained 43% of variance (P = 0.02). There was a trend for the change in iron absorption to be associated with the change in maternal hepcidin from T2 to T3 (P = 0.06). The observed increase in iron absorption was not significantly influenced by maternal age (P = 0.5) or observed change in Hb from T2 to T3 (P = 0.3). TBI at time of dosing explained 57% of variance in iron absorption in T2 (P = 0.003) and 34% of variance in iron absorption in T3 (P = 0.05), and TBI explained more variance in iron absorption than either SF (26%) or sTfR (18%) alone. Iron absorption at either timepoint was not significantly associated with Hb concentration (P = 0.3, P = 0.9).
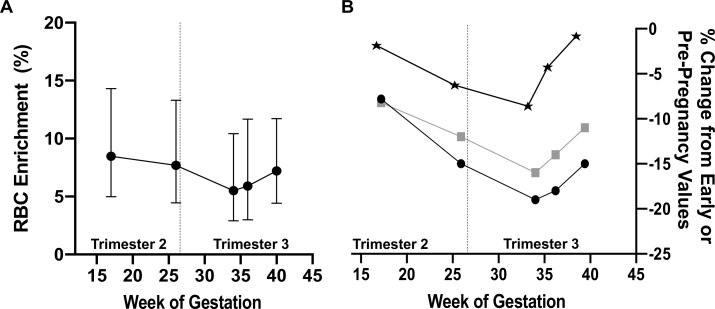
Change in maternal red blood cell enrichment across gestation
To obtain an estimate of iron released from the maternal RBC pool across gestation, changes in RBC 57Fe enrichment were monitored over a 150-d period postdosing (Figure 2, Table 3). Change in RBC 57Fe enrichment was nonlinear (P = 0.02), because there was a linear decrease in RBC enrichment from V2 to V4 in all women followed by a slight increase in RBC enrichment at V5 and delivery. Because tracer recovery is based on estimates of maternal RBC mass, this nonlinear change in RBC enrichment can be driven by the observed change in plasma volume that leads to an increase in Hb concentrations in late gestation (Figure 2, Table 3). Our observed changes in RBC 57Fe enrichment and Hb across gestation mirror reported data on changes in hematocrit across gestation (Figure 2)(41). When assessing the decrease in RBC 57Fe enrichment from V2 to V4, RBC enrichment decreased on average by 20%, which translates to a 0.03% decrease in RBC 57Fe enrichment per day over118d(P = 0.08). Because RBC 57Fe enrichment was strongly inversely associated with TBI, controlling for TBI across gestation strengthened the relation between RBC enrichment decrease per day (0.04%/d, P = 0.04). Using the linear portion of the RBC 57Fe enrichment disappearance (V2 to V4), the time to clear half of the RBC 57Fe tracer was 66 d (95% CI: 30, 144 d). Using this calculation, the length of labeled RBC 57Fe turnover was 94.9 d (95% CI: 43.5, 207.1 d). Women with greater change in RBC 57Fe enrichment from V2 to V4 absorbed significantly more iron in T2 (β = –0.84, SE = 0.2, P = 0.001). RBC 57Fe enrichment increased on average by 5% from V4 to delivery, and greater increases were observed in women who exhibited greater increases in SF (sβ = 0.57, SE = 0.01, P = 0.05) or hepcidin (sβ = 0.66, SE = 1.1, P = 0.04).
FIGURE 2.
Change in RBC enrichment across gestation. (A) The observed changes in RBC 57Fe enrichment (%) over gestation in 15 pregnant women who received oral 57Fe at weeks 14–16 of gestation. Data are presented as geometric mean and 95% CI. (B) The observed percentage change in hemoglobin from visit 1 onwards (black stars), at each of the study timepoints. The percentage change from prepregnancy values of hematocrit in non–iron-supplemented pregnant women (black circles, n= 39) and in iron-supplemented pregnant women (gray squares, n= 39) are presented as adapted from Vricella (41).
Net mass of 57Fe present in the RBC pool was calculated at each sampling point. An average of 11% (95% CI: 2.6, 20.9%) of the total 57Fe mass incorporated into maternal RBCs was lost over the ∼150-d period from V2 to delivery (0.12%/d, P = 0.03). Assuming the 57Fe tracer behaves in the same manner as does the native Fe in RBCs, this magnitude of loss would reflect a net Fe loss of 295 mg (95% CI: 145, 602 mg) from the RBC pool over the ∼150 d. The AUC for RBC 57Fe enrichment across the 150-d period was also calculated. The AUC was greater in women who absorbed more 57Fe in T2 (sβ = 0.87, SE = 0.01, P < 0.001), and these women also experienced a significantly greater decrease in RBC enrichment over time (P < 0.001).
Net transfer of iron released from the maternal RBC iron pool to the neonatal compartment
The net quantity of 57Fe recovered in neonatal RBCs was 60 ± 40 μg, which translates to 0.5% (95% CI: 0.28, 0.66%) of the 20-mg 57Fe dose consumed by the mother at week 15 of gestation. In these women that absorbed ∼8% of their oral iron dose, the 60 μg recovered in neonatal RBCs translates to 5.9% (95% CI: 4.5, 7.2%) of the amount of 57Fe absorbed by the mother. Additionally, the 60 μg 57Fe recovered in neonatal RBCs reflects 26% (95% CI: 10.2, 67.0%) of the amount of 57Fe released from maternal RBCs based on change in RBC 57Fe enrichment over time. When put into perspective in relation to the total mass of iron lost from the maternal RBC pool, this translates to an uptake of 87.1 ± 32.4 mg, or 33% of the total 295 mg iron released from the maternal RBC pool. One-third of the iron released from the maternal RBC pool was diverted to the fetus and not available to be reutilized in support of maternal RBC formation.
Net 57Fe recovered in neonatal RBC (milligrams) was strongly positively associated with the amount of iron absorbed by the mother at T2 (R2 = 0.78, P < 0.001) and with maternal 57Fe AUC (R2 = 0.73, P = 0.0002). Women who exhibited a greater decrease in RBC 57Fe enrichment over the 150 d between dosing and delivery [calculated either as a slope from V2 to delivery or as change in 57Fe (milligrams) over this interval], transferred more 57Fe to their neonate (slope sβ = −0.69, SE = 0.6, P = 0.02; change sβ = −0.76, SE = 0.02, P = 0.003). This was a stronger association than seen between the decrease in maternal 57Fe enrichment and maternal TBI at delivery (sβ = − 0.48, SE = 0.002, P = 0.12) or change in TBI across pregnancy (sβ =−0.38, SE = 0.003, P = 0.25).
Net transfer of dietary iron to the neonatal compartment in T3
The net 58Fe recovered in neonatal RBCs was 10 ± 10 μg, which reflects 1.3% (95% CI: 0.90, 1.71%) of the 58Fe dose consumed by the mother at approximately week 34 of gestation. With the average absorption at this time being nearly 20%, the 10 μg recovered in neonatal RBCs translates to 9.0% (95% CI: 5.8, 13.9%) of the amount of 58Fe absorbed by the mother. Net 58Fe recovered in the neonatal RBCs was not as strongly associated with absorbed iron in T3 (P = 0.06) as seen with 57Fe. However, net 58Fe recovered in neonatal RBCs was inversely associated with maternal delivery SF (sβ =−0.70, SE = 0.01, P = 0.03), and was more strongly associated with SF than TBI (sβ =−0.61, SE < 0.01, P = 0.06).
Iron partitioning between maternal and neonatal compartments
The total amounts of each of the 57Fe and 58Fe tracers recovered in the maternal and neonatal compartments were summed, and the relative fraction of the total iron partitioned to the maternal or neonatal compartments was calculated. Of the total 57Fe recovered in the mother 2 wk postdosing and in the newborn, 4.7% (95% CI: 3.6, 5.8%) was partitioned to the neonatal compartment. The fraction of total 58Fe recovered that was partitioned to the neonatal compartment was higher, although this did not reach statistical significance (6.5%; 95% CI: 4.4, 9.8%; P = 0.12). Neonates with a larger fraction of total 57Fe recovered in their RBCs also had a higher fraction of total 58Fe recovered in their RBCs (R2 = 0.75, P = 0.006). Partitioning of each isotope to the neonatal compartment was positively associated with neonatal ERFE concentrations at birth (57Fe: sβ = 0.61, SE = 0.7, P = 0.03; 58Fe: sβ = 0.56, SE = 1.1, P = 0.06), although only partitioning of 57Fe was associated with maternal ERFE (57Fe: sβ = 0.64, SE = 0.5, P = 0.02; 58Fe: sβ = 0.32, SE = 1.4, P = 0.33). Women with greater decreases in TBI across gestation partitioned a greater fraction of orally ingested iron to their neonatal compartment (57Fe, P = 0.03; 58Fe, P = 0.08).
Discussion
To our knowledge this is the first longitudinal isotope study that sought to quantify the kinetics of RBC iron enrichment across pregnancy. Furthermore, these longitudinal data, and use of 2 stable iron isotopes, allowed changes in maternal iron absorption to be evaluated from T2 to T3 as a function of decreasing maternal iron stores. We report that mean length of time for labeled RBC iron turnover was 95 d during pregnancy, and women with quicker RBC iron turnover absorbed more iron in early gestation and transferred a larger net fraction of absorbed iron to their neonate.
In T2 of pregnancy, iron absorption averaged ∼ 9% and doubled to >20% in T3. The increase in absorption is consistent with early data, and the observed 2.2-fold increase in absorption falls within the range of increases noted previously (range 1.3–10-fold increase) (Tables 3 and 4). In the current study, the percentage iron absorption at T3 (20%) was lower than previous stable iron isotope studies (range: 36–66%) (10, 28, 32, 35). This could be due to the total load of iron given (20 mg), which was higher than that used in previous studies (5–10 mg), and iron absorption is known to decrease as the load of iron ingested increases (40). Additionally, the women in this study appeared to be more iron replete (higher SF or TBI) than those in previous studies. Maternal iron absorption is inversely associated with maternal iron status (9, 10, 32, 42). This finding is also evident in the current study, where women had greater increases in iron absorption from T2 to T3, when they also exhibited a greater loss in total body iron. Although we did observe inverse associations between SF and iron absorption, maternal TBI was more strongly predictive of maternal iron absorption than was hepcidin, a finding that is consistent with other data in nonpregnant adults (43). The 2.2-fold increase in iron absorption we observed from T2 to T3 falls within the range that has been reported in other studies, although the range reported in the published literature is wide.
RBCs are anucleate and have a finite lifespan. Catabolism occurs when RBCs become senescent, and iron is released from catabolized RBCs to be recycled or stored. During pregnancy length of time for labeled RBC 57Fe turnover averaged 95 d, although there was a wide range of variability (95% CI: 43.5, 207.1 d). Although we did not see a linear decrease in RBC iron enrichment over pregnancy, our change in RBC iron enrichment mirrored observed changes in maternal Hb and was also consistent with expected shifts in plasma volume reported from normative data (41)(Figure 1). Interestingly, our data on length of time for RBC iron turnover based on change in 57Fe enrichment were comparable to the few data published on RBC lifespan during pregnancy. Only 1 study to our knowledge has estimated RBC lifespan at 120 d in a small group of pregnant women (n = 6); this study removed RBCs, labeled them ex vivo with 51Cr, and then reinfused them into the participants and monitored change in enrichment over time (44). Studies in pregnant rats that also labeled RBCs ex vivo with 51Cr found that RBC lifespan was shortened by 9% during pregnancy compared with values obtained in nonpregnant rats (36.9 d to 33.6 d, P = 0.001) (45). Further studies are needed to better quantify RBC lifespan and change in RBC iron enrichment in larger sample sizes that include both pregnant and nonpregnant women.
The developing fetus relies on maternal iron stores and efficient placental transport of iron across gestation. The current study found that iron recycled from the maternal RBC pool provides a significant source of iron for the fetus, and reliance on this pool of iron is greatest in women with low body iron. The majority of RBC catabolism occurs extravascularly, releasing ∼ 20 mg iron back into circulation as diferric transferrin (46), and the placenta is known to upregulate transferrin receptor in relation to maternal iron status so it can better capture diferric transferrin (47). However, 10–20% of RBC catabolism occurs intravascularly, releasing free Hb and heme, which bind to their respective carriers (13, 46). The placenta also expresses a number of heme transport proteins (48, 49, 50, 51, 52, 53, 54, 55). Whether the iron released from the maternal RBC pool is transferred to the neonatal compartment as diferric transferrin or within a heme-containing protein is unknown, but the maternal RBC pool appears to be a significant source of iron for the fetus, and this pool is utilized to a far greater extent in women with depleted iron reserves.
The fetus relies on maternal iron sources for its accretion of this mineral in utero, such as from maternal dietary sources. The current study demonstrated that greater maternal decreases in TBI were associated with a greater fetal uptake of maternally absorbed tracer. This finding is consistent with previous studies demonstrating that maternal iron status was inversely associated with iron transfer to the fetus (3, 10, 29, 47). The current study is the first in which maternal or neonatal ERFE has been assessed in relation to fetal iron transfer and partitioning. Neonatal ERFE concentrations were positively associated with partitioning of iron from both the maternal RBC pool and dietary iron. ERFE is known to decrease circulating hepcidin concentrations when erythropoietic iron demand is high (56). Previous studies have measured neonatal hepcidin, and found that this indicator explained the greatest variation in neonatal iron status (10, 57, 58, 59), but these studies were published before an ERFE assay was commercially available (22). Recent animal data have demonstrated that fetal hepatic hepcidin acts in an autocrine fashion to secure in utero liver iron stores (60). Due to the mediatory role of ERFE in hepcidin suppression, umbilical cord ERFE could be indicative of increased neonatal iron demand in utero.
Although this study assessed RBC iron dynamics in pregnancy and iron transfer to the fetus that was derived from the maternal RBC pool, there are limitations. The current sample size was limited, and we do not have data on iron absorption and change in RBC enrichment across a similar interval of time in nonpregnant women to serve as a control. We dosed women with a large load of iron (20 mg), which could lead to an underestimation of true iron absorption. We were only able to assess change in RBC iron enrichment. Alterations in the rate of RBC production, plasma volume expansion and Hb concentrations across gestation add variability to our estimates. In addition, the labeled RBC pool consists of recently synthesized RBCs, whereas the nonlabeled RBC pool consists of RBCs at variable stages of their lifespan. Similar to previous stable iron isotope studies during pregnancy (1, 30, 42, 47), we assumed a constant blood volume as a function of maternal weight across gestation, although we know that plasma and blood volume increase from 12 to 28 wk of gestation and then plasma volume expansion plateaus whereas Hb production continues to increase (41), with plasma volume nearly doubling in T3 compared with nonpregnant values (61). We were also unable to distinguish the relative amount of neonatal 57Fe that was derived from rapid transfer of maternally absorbed tracer compared with that obtained after it was released from the maternal RBC pool. Further studies are needed with larger sample sizes to confirm these results.
In conclusion, the current longitudinal study has found that iron absorption increases across gestation as a function of decreasing maternal TBI. We also report for the first time that labeled RBC iron turnover during pregnancy averages 95 d and is quicker in women with diminished iron stores. The women with greater decreases in RBC iron enrichment transfer a greater net amount of iron to their neonates, suggesting in times of increased iron demand maternal RBC iron becomes a significant source for fetal iron.
Acknowledgments
The authors’ responsibilities were as follows—KMD, CC: analyzed and interpreted the data and wrote the manuscript; KOO’B: designed the research, performed experiments, analyzed and interpreted the data, and wrote the manuscript; RG, EKP: were responsible for the clinical implementation of the studies, and assisted with the design of the research, analysis and interpretation of the data, and the preparation of the manuscript; and all authors: read and approved the final manuscript. The authors report no conflicts of interest.
Data Availability
Data described in the manuscript, code book, and analytic code will not be made available because of the confidential nature of the data collected.
Footnotes
This study was funded by the US National Institutes of Health (NIH) National Institute of Digestive and Kidney Diseases (NIDDK) grant T32-DK007158.
Supplemental Figure 1 is available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
References
- 1.Young ME, Griffin I, Pressman E, McIntyre AW, Cooper E, McNanley T, Harris ZL, Westerman M, O’Brien KO. Maternal hepcidin is associated with placental transfer of iron derived from dietary heme and nonheme sources. J Nutr. 2012;142(1):33–39. doi: 10.3945/jn.111.145961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pommerenke WT, Hahn PF, Bale WF, Balfour WM. Transmission of radio-active iron to the human fetus. Am J Physiol (1898–1976) 1942;137(1):164–170. [Google Scholar]
- 3.O’Brien KO, Zavaleta N, Abrams SA, Caulfield LE. Maternal iron status influences iron transfer to the fetus during the third trimester of pregnancy. Am J Clin Nutr. 2003;77(4):924–930. doi: 10.1093/ajcn/77.4.924. [DOI] [PubMed] [Google Scholar]
- 4.Murray MJ, Stein N. Contribution of maternal rat iron stores to fetal iron in maternal iron deficiency and overload. J Nutr. 1971;101(11):1583–1587. doi: 10.1093/jn/101.11.1583. [DOI] [PubMed] [Google Scholar]
- 5.Murray MJ, Stein N. The contribution of maternal iron stores to fetal iron in rats. J Nutr. 1970;100(9):1023–1025. doi: 10.1093/jn/100.9.1023. [DOI] [PubMed] [Google Scholar]
- 6.Sangkhae V, Fisher AL, Wong S, Koenig MD, Tussing-Humphreys L, Chu A, Lelic M, Ganz T, Nemeth E. Effects of maternal iron status on placental and fetal iron homeostasis. J Clin Invest. 2019;130(2):625–640. doi: 10.1172/JCI127341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bothwell TH. Iron requirements in pregnancy and strategies to meet them. Am J Clin Nutr. 2000;72(1):257S–264S. doi: 10.1093/ajcn/72.1.257S. [DOI] [PubMed] [Google Scholar]
- 8.Institute of Medicine Panel on Micronutrients . National Academies Press; 2001. Dietary reference intakes for vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. [PubMed] [Google Scholar]
- 9.Barrett JF, Whittaker PG, Williams JG, Lind T. Absorption of non-haem iron from food during normal pregnancy. BMJ. 1994;309(6947):79–82. doi: 10.1136/bmj.309.6947.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Delaney KM, Guillet R, Pressman EK, Caulfield LE, Zavaleta N, Abrams SA, O’Brien KO. Iron absorption during pregnancy is underestimated when iron utilization by the placenta and fetus is ignored. Am J Clin Nutr. 2020;112(3):576–585. doi: 10.1093/ajcn/nqaa155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Noyes WD, Bothwell TH, Finch CA. The role of the reticuloendothelial cell in iron metabolism. Br J Haematol. 1960;6(1):43–55. doi: 10.1111/j.1365-2141.1960.tb06216.x. [DOI] [PubMed] [Google Scholar]
- 12.Garby L, Noyes WD. Studies on hemoglobin metabolism. II. Pathways of hemoglobin iron metabolism in normal man. J Clin Invest. 1959;38(9):1484–1486. doi: 10.1172/JCI103926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garby L, Noyes WD. Studies on hemoglobin metabolism. I. The kinetic properties of the plasma hemoglobin pool in normal man. J Clin Invest. 1959;38(9):1479–1483. doi: 10.1172/JCI103925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Recommended method for radioisotope red-cell survival studies. International Committee for Standardization in Haematology. Br J Haematol. 1980;45(4):659–666. doi: 10.1111/j.1365-2141.1980.tb07189.x. [DOI] [PubMed] [Google Scholar]
- 15.Eadie GS, Brown IW. The potential life span and ultimate survival of fresh red blood cells in normal healthy recipients as studied by simultaneous Cr51 tagging and differential hemolysis. J Clin Invest. 1955;34(4):629–636. doi: 10.1172/JCI103112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mock DM, Matthews NI, Zhu S, Strauss RG, Schmidt RL, Nalbant D, Cress GA, Widness JA. Red blood cell (RBC) survival determined in humans using RBCs labeled at multiple biotin densities. Transfusion (Paris) 2011;51(5):1047–1057. doi: 10.1111/j.1537-2995.2010.02926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mock DM, Lankford GL, Widness JA, Burmeister LF, Kahn D, Strauss RG. Measurement of red cell survival using biotin-labeled red cells: validation against 51Cr-labeled red cells. Transfusion (Paris) 1999;39(2):156–162. doi: 10.1046/j.1537-2995.1999.39299154729.x. [DOI] [PubMed] [Google Scholar]
- 18.Muckenthaler MU, Rivella S, Hentze MW, Galy B. A red carpet for iron metabolism. Cell. 2017;168(3):344–361. doi: 10.1016/j.cell.2016.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.CDC Recommendations to prevent and control iron deficiency in the united states. MMWR Recomm Rep. 1998;47(Rr-3):1–29. [PubMed] [Google Scholar]
- 20.Orkin SHN, David N, Ginsburg DG, Look AT, Fisher D, Lux S. 8th ed. Saunders; Philadelphia, PA: 2014. Nathan and Oski’s hematology and oncology of infancy and childhood. [Google Scholar]
- 21.Jopling J, Henry E, Wiedmeier SE, Christensen RD. Reference ranges for hematocrit and blood hemoglobin concentration during the neonatal period: data from a multihospital health care system. Pediatrics. 2009;123(2):e333–e337. doi: 10.1542/peds.2008-2654. [DOI] [PubMed] [Google Scholar]
- 22.Ganz T, Jung G, Naeim A, Ginzburg Y, Pakbaz Z, Walter PB, Kautz L, Nemeth E. Immunoassay for human serum erythroferrone. Blood. 2017;130(10):1243–1246. doi: 10.1182/blood-2017-04-777987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cook JD, Flowers CH, Skikne BS. The quantitative assessment of body iron. Blood. 2003;101(9):3359–3363. doi: 10.1182/blood-2002-10-3071. [DOI] [PubMed] [Google Scholar]
- 24.Mei Z, Cogswell ME, Looker AC, Pfeiffer CM, Cusick SE, Lacher DA, Grummer-Strawn LM. Assessment of iron status in US pregnant women from the National Health and Nutrition Examination Survey (NHANES), 1999-2006. Am J Clin Nutr. 2011;93(6):1312–1320. doi: 10.3945/ajcn.110.007195. [DOI] [PubMed] [Google Scholar]
- 25.Kastenmayer P, Davidsson L, Galan P, Cherouvrier F, Hercberg S, Hurrell RF. A double stable isotope technique for measuring iron absorption in infants. Br J Nutr. 1994;71(3):411–424. doi: 10.1079/bjn19940148. [DOI] [PubMed] [Google Scholar]
- 26.Bothwell TH, Charlton RW, Cook JD, Finch CA. Blackwell Scientific Publications; Oxford, UK: 1979. Iron metabolism in man. [Google Scholar]
- 27.Fomon SJ, Ziegler EE, Serfass RE, Nelson SE, Frantz JA. Erythrocyte incorporation of iron is similar in infants fed formulas fortified with 12 mg/L or 8 mg/L of iron. J Nutr. 1997;127(1):83–88. doi: 10.1093/jn/127.1.83. [DOI] [PubMed] [Google Scholar]
- 28.Young MF, Griffin I, Pressman E, McIntyre AW, Cooper E, McNanley T, Harris ZL, Westerman M, O’Brien KO. Utilization of iron from an animal-based iron source is greater than that of ferrous sulfate in pregnant and nonpregnant women. J Nutr. 2010;140(12):2162–2166. doi: 10.3945/jn.110.127209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tussing-Humphreys L, LaBomascus B, O’Brien K, Nemeth E, Sangkhae V, Steffen AD, Castellanos K, DeMartelly V, Ruchob R, Welke L, et al. Prepregnancy obesity does not impact placental iron trafficking. J Nutr. 2021;151(9):2646–2654. doi: 10.1093/jn/nxab191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koenig MD, Klikuszowian E, O’Brien KO, Pauls H, Steffen A, DeMartelly V, Ruchob R, Welke L, Hemphill N, LaBomascus B, et al. Prepregnancy obesity is not associated with iron utilization during the third trimester. J Nutr. 2020;150(6):1397–1404. doi: 10.1093/jn/nxaa065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Young MF, Griffin I, Pressman E, McIntyre AW, Cooper E, McNanley T, Harris ZL, Westerman M, O’Brien KO. Maternal hepcidin is associated with placental transfer of iron derived from dietary heme and nonheme sources. J Nutr. 2012;142(1):33–39. doi: 10.3945/jn.111.145961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Whittaker PG, Barrett JF, Lind T. The erythrocyte incorporation of absorbed non-haem iron in pregnant women. Br J Nutr. 2001;86(3):323–329. doi: 10.1079/bjn2001390. [DOI] [PubMed] [Google Scholar]
- 33.Georgieff MK, Landon MB, Mills MM, Hedlund BE, Faassen AE, Schmidt RL, Ophoven JJ, Widness JA. Abnormal iron distribution in infants of diabetic mothers: spectrum and maternal antecedents. J Pediatr. 1990;117(3):455–461. doi: 10.1016/s0022-3476(05)81097-2. [DOI] [PubMed] [Google Scholar]
- 34.Rao R, Georgieff MK. Iron in fetal and neonatal nutrition. Semin Fetal Neonatal Med. 2007;12(1):54–63. doi: 10.1016/j.siny.2006.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barrett JF, Whittaker PG, Williams JG, Lind T. Absorption of non-haem iron in normal women measured by the incorporation of two stable isotopes into erythrocytes. Clin Sci (Lond) 1992;83(2):213–219. doi: 10.1042/cs0830213. [DOI] [PubMed] [Google Scholar]
- 36.Widdowson EM, Spray CM. Chemical development in utero. Arch Dis Child. 1951;26(127):205–214. doi: 10.1136/adc.26.127.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Siddappa AM, Rao R, Long JD, Widness JA, Georgieff MK. The assessment of newborn iron stores at birth: a review of the literature and standards for ferritin concentrations. Neonatology. 2007;92(2):73–82. doi: 10.1159/000100805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rios E, Hunter RE, Cook JD, Smith NJ, Finch CA. The absorption of iron as supplements in infant cereal and infant formulas. Pediatrics. 1975;55(5):686–693. [PubMed] [Google Scholar]
- 39.Gabrielsson J, Weiner D. Non-compartmental analysis. Methods Mol Biol. 2012;929:377–389. doi: 10.1007/978-1-62703-050-2_16. [DOI] [PubMed] [Google Scholar]
- 40.Hahn PF, Caruthers EL, Darby WJ, Sheppard CW. The effect of gestation and dosage level on absorption of radioactive iron in 380 human pregnancies. Proc Am Fed Clin Res. 1947;3:33. [PubMed] [Google Scholar]
- 41.Vricella LK. Emerging understanding and measurement of plasma volume expansion in pregnancy. Am J Clin Nutr. 2017;106(Suppl 6):1620S–1625S. doi: 10.3945/ajcn.117.155903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.O’Brien KO, Zavaleta N, Caulfield LE, Yang DX, Abrams SA. Influence of prenatal iron and zinc supplements on supplemental iron absorption, red blood cell iron incorporation, and iron status in pregnant Peruvian women. Am J Clin Nutr. 1999;69(3):509–515. doi: 10.1093/ajcn/69.3.509. [DOI] [PubMed] [Google Scholar]
- 43.Zimmermann MB, Troesch B, Biebinger R, Egli I, Zeder C, Hurrell RF. Plasma hepcidin is a modest predictor of dietary iron bioavailability in humans, whereas oral iron loading, measured by stable-isotope appearance curves, increases plasma hepcidin. Am J Clin Nutr. 2009;90(5):1280–1287. doi: 10.3945/ajcn.2009.28129. [DOI] [PubMed] [Google Scholar]
- 44.Pritchard JA, Adams RH. Erythrocyte production and destruction during pregnancy. Am J Obstet Gynecol. 1960;79(4):750–757. doi: 10.1016/0002-9378(60)90633-5. [DOI] [PubMed] [Google Scholar]
- 45.Lurie S, Danon D. Life span of erythrocytes in late pregnancy. Obstet Gynecol. 1992;80(1):123–126. [PubMed] [Google Scholar]
- 46.Knutson M, Wessling-Resnick M. Iron metabolism in the reticuloendothelial system. Crit Rev Biochem Mol Biol. 2003;38(1):61–88. doi: 10.1080/713609210. [DOI] [PubMed] [Google Scholar]
- 47.Young MF, Pressman E, Foehr ML, McNanley T, Cooper E, Guillet R, Orlando M, McIntyre AW, Lafond J, O’Brien KO. Impact of maternal and neonatal iron status on placental transferrin receptor expression in pregnant adolescents. Placenta. 2010;31(11):1010–1014. doi: 10.1016/j.placenta.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 48.Qiu A, Jansen M, Sakaris A, Min SH, Chattopadhyay S, Tsai E, Sandoval C, Zhao R, Akabas MH, Goldman ID. Identification of an intestinal folate transporter and the molecular basis for hereditary folate malabsorption. Cell. 2006;127(5):917–928. doi: 10.1016/j.cell.2006.09.041. [DOI] [PubMed] [Google Scholar]
- 49.Solanky N, Requena Jimenez A, D’Souza SW, Sibley CP, Glazier JD. Expression of folate transporters in human placenta and implications for homocysteine metabolism. Placenta. 2010;31(2):134–143. doi: 10.1016/j.placenta.2009.11.017. [DOI] [PubMed] [Google Scholar]
- 50.Cao C, Pressman EK, Cooper EM, Guillet R, Westerman M, O’Brien KO. Placental heme receptor LRP1 correlates with the heme exporter FLVCR1 and neonatal iron status. Reproduction. 2014;148(3):295–302. doi: 10.1530/REP-14-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Best CM, Pressman EK, Cao C, Cooper E, Guillet R, Yost OL, Galati J, Kent TR, O’Brien KO. Maternal iron status during pregnancy compared with neonatal iron status better predicts placental iron transporter expression in humans. FASEB J. 2016;30(10):3541–3550. doi: 10.1096/fj.201600069R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jensen PH, Moestrup SK, Gliemann J. Purification of the human placental alpha 2-macroglobulin receptor. FEBS Lett. 1989;255(2):275–280. doi: 10.1016/0014-5793(89)81105-6. [DOI] [PubMed] [Google Scholar]
- 53.Hvidberg V, Maniecki MB, Jacobsen C, Hojrup P, Moller HJ, Moestrup SK. Identification of the receptor scavenging hemopexin-heme complexes. Blood. 2005;106(7):2572–2579. doi: 10.1182/blood-2005-03-1185. [DOI] [PubMed] [Google Scholar]
- 54.Jaacks LM, Young MF, Essley BV, McNanley TJ, Cooper EM, Pressman EK, McIntyre AW, Orlando MS, Abkowitz JL, Guillet R, et al. Placental expression of the heme transporter, feline leukemia virus subgroup C receptor, is related to maternal iron status in pregnant adolescents. J Nutr. 2011;141(7):1267–1272. doi: 10.3945/jn.110.135798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Keel SB, Doty RT, Yang Z, Quigley JG, Chen J, Knoblaugh S, Kingsley PD, Domenico ID, Vaughn MB, et al. A heme export protein is required for red blood cell differentiation and iron homeostasis. Science. 2008;319(5864):825–828. doi: 10.1126/science.1151133. [DOI] [PubMed] [Google Scholar]
- 56.Kautz L, Jung G, Valore EV, Rivella S, Nemeth E, Ganz T. Identification of erythroferrone as an erythroid regulator of iron metabolism. Nat Genet. 2014;46(7):678–684. doi: 10.1038/ng.2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ru Y, Pressman EK, Guillet R, Katzman PJ, Vermeylen F, O’Brien KO. Umbilical cord hepcidin concentrations are positively associated with the variance in iron status among multiple birth neonates. J Nutr. 2018;148(11):1716–1722. doi: 10.1093/jn/nxy151. [DOI] [PubMed] [Google Scholar]
- 58.Delaney KM, Guillet R, Pressman EK, Ganz T, Nemeth E, O’Brien KO. Umbilical cord erythroferrone is inversely associated with hepcidin, but does not capture the most variability in iron status of neonates born to teens carrying singletons and women carrying multiples. J Nutr. 2021;151(9):2590–2600. doi: 10.1093/jn/nxab156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jones AD, Shi Z, Lambrecht NJ, Jiang Y, Wang J, Burmeister M, Li M, Lozoff B. Maternal overweight and obesity during pregnancy are associated with neonatal, but not maternal, hepcidin concentrations. J Nutr. 2021;151(8):2296–2304. doi: 10.1093/jn/nxab133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kämmerer L, Mohammad G, Wolna M, Robbins PA, Lakhal-Littleton S. Fetal liver hepcidin secures iron stores in utero. Blood. 2020;136(13):1549–1557. doi: 10.1182/blood.2019003907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Aguree S, Gernand AD. Plasma volume expansion across healthy pregnancy: a systematic review and meta-analysis of longitudinal studies. BMC Pregnancy Childbirth. 2019;19(1):508. doi: 10.1186/s12884-019-2619-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Whittaker PG, Lind T, Williams JG. Iron absorption during normal human pregnancy: a study using stable isotopes. Br J Nutr. 1991;65(3):457–463. doi: 10.1079/bjn19910104. [DOI] [PubMed] [Google Scholar]
- 63.Balfour WM, Hahn PF, Bale WF, Pommerenke WT, Whipple GH. Radioactive iron absorption in clinical conditions: normal, pregnancy, anemia and hemochromatosis. J Exp Med. 1942;76(1):15–30. doi: 10.1084/jem.76.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Svanberg B, Arvidsson B, Norrby A, Rybo G, Sölvell L. Absorption of supplemental iron during pregnancy—a longitudinal study with repeated bone-marrow studies and absorption measurements. Acta Obstet Gynecol Scand. 1975;54(s48):87–108. doi: 10.3109/00016347509156332. [DOI] [PubMed] [Google Scholar]
- 65.Hahn PF, Carothers EL, Darby WJ, Martin M, Sheppard CW, Cannon RO, Beam S, Densen PM, Peterson JC, McClellan GS. Iron metabolism in human pregnancy as studied with radioactive isotope, Fe59. Am J Obstet Gynecol. 1951;61(3):477–486. doi: 10.1016/0002-9378(51)91394-4. [DOI] [PubMed] [Google Scholar]
- 66.Svanberg B, Arvidsson B, Bjorn-Rasmussen E, Hallberg L, Rossander L, Swolin B. Dietary iron absorption in pregnancy – a longitudinal study with repeated measurements of non-haeme iron absorption from whole diet. Acta Obstet Gynecol Scand. 1975;54(s48):43–68. doi: 10.3109/00016347509156330. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data described in the manuscript, code book, and analytic code will not be made available because of the confidential nature of the data collected.