Abstract
Despite stringent testing protocols, there always remains a chance of a delayed haemolytic transfusion reaction (DHTR) occurring as a result of an undetected or unknown antibody. In this systemic review and meta-analysis, we aimed to investigate improvements to patient outcomes that could be achieved through the implementation of a national antibody registry. A series of searches through PubMed and SCOPUS identified a collection of articles with relevant information, restricted to full text, English language articles available through the RMIT Library service. 25 articles were considered for the review, four of these found to have relevant, extractable data for use in the meta-analysis. Alloantibody evanescence rates were analysed for the potential for reducing DHTRs associated with transfusion services, returning significant results indicating antibody evanescence rates of up to 68.4% in one study, with p-values less than 0.001. Due to the small number of included studies however, the interference values were quite high for these analyses at greater than 90% for each. Additional, beneficial side-effects of such a system were also considered, along with reductions in DHTRs. In conclusion it was determined that a National antibody registry would contribute to improving patient outcomes, however further studies could be performed to determine a stronger correlation, and exact levels of improvement that could be achieved.
Keywords: antibody evanescence, DHTR, national antibody register, blood bank
Introduction
According to the Australian National Blood Authority Haemovigilance report for 2017–18, 84 delayed haemolytic transfusion reactions (DHTRs) were reported between 2013 and 20181, which represents 2.7% of all adverse events recorded in that time period. According to the US FDA, DHTRs are reported as the number one cause of transfusion related deaths, and up to 10% resulted in serious morbidity, or worse, in the UK between 2006 and 20142. Not all incompatible blood transfusions will result in DHTRs, just as not all antibodies are clinically significant, and not all patients will become alloimmunised to antigen positive blood, however avoiding these situations is the best strategy in avoiding adverse patient outcomes.
The difficulty often faced when unit matching is that not all antibodies remain detectable. Previous studies have concluded that only 25–41% of clinically significant antibodies become undetectable, or evanesce, over time3, however more recent studies looking at “hospital acquired” alloantibodies have suggested that depending on the time from the immunisation event, the rate of evanescence can range from 36% within a year, to almost 70% within ten years1.
Antibodies “disappearing” is quite an obstacle to effective blood matching, but with good record keeping and Laboratory Information Systems (LIS), hospitals can refer to historical data of their patients, as far back as records keeping allows. However, due to the nature of modern society, people do not often stay in the same house, city, or even state for the entirety of their lives. In the US, records indicate that upwards of 10% of transfusion recipients were screened for, or received transfusions at more than one hospital4, and more disturbingly, several studies have shown that records from different hospitals for the same patient have inconsistent blood screening results5.
These two factors are major contributors to DHTR and adverse patient outcomes. Many other countries, including the Netherlands6,7, France8, Germany9 and the United States2,5,10,11 have implemented registries and databases, some Government directed at a national level, some at a regional level, because of private blood banking companies supplying to a number of hospitals in the US healthcare system. These databases have been useful in identifying fragmented and inconsistent medical records, historical evidence of clinically significant alloantibodies, and even in the detection of bedside errors such as “Wrong Blood in Tube” (WBIT) errors12. In this review we will take a closer look at some of the causes of DHTRs, and steps that have been, and can be taken to effectively reduce the risks associated with blood transfusions.
Antibody evanescence
Blood group antibodies, like many other antibodies, vary in their detectability, but thanks to the efficient work of memory T-cells, antibody levels can be raised in response to a challenge from viral, bacterial, or any other form of antigen. The problem blood bankers are becoming more aware of now, is the variability and frequency in non-ABO blood group antibodies. One study published in 2000 reported that approximately 25–41% of blood group antibodies evanesce3, however several other studies have looked more specifically at hospital induced antibodies, for example in response to alloimmunisation after an incompatible RBC transfusion.
Stack and Tormey published a study in which they designed an algorithm that accurately predicted the Fractional Antibody Detection Rate (FADR), based on recorded screening of 100 patients that developed antibodies that evanesced after receiving transfusions through the Veteran Affairs hospital network in the US2. An earlier study by Tormey and Stack investigated antibody persistence and evanescence in men10. This study was directed at men to isolate alloimmunisation that was a result of transfusion alone, and not pregnancy related. The study from Harm et al. looked at alloimmunisation within the Sickle Cell Disease (SCD) patient population13. DHTR’s can be a huge problem within the SCD population, as they are often required to have regular and sometimes urgent transfusions and are particularly prone to negative side effects of a DHTR, and greater likelihood of developing further alloantibodies.
Medical record accuracy and availability
Another obstacle in the effort to improve patient outcomes is the accuracy and availability of patient historical data. A 2014 investigation5 screened 100 patients receiving transfusions, and found that a significant number had had prior transfusions performed at one of 24 other sites around the country (USA). Of 200 patients within the same hospital who were confirmed to have formed alloantibodies due to a prior transfusion, more than half had inconsistencies in their records across the multiple transfusions.
From another point of view, accurate historical data has been shown to be extremely useful in the fight against DHTRs. Over a 17 year period, 94 “Wrong Blood in Tube errors” (WBIT) were detected, 57% of these were detected through ABO type comparisons with historical data available on the patient14. Central Transfusion Services (CTS) - Pittsburgh has recorded a 38% improvement in the detection and correction of WBIT errors and blood bank typing errors through the comparison of historical data from other hospitals that operate within the CTS - Pittsburgh network.
Would a regional or national antibody register contribute to improving patient outcomes? The data available from the many existing networks and databases around the world all suggest that great improvements are available. In the US, CTS has several networks in cities and states such as Pittsburgh, Seattle, and Connecticut, as well as their Veteran Administration (VA) network for the military. The South East of France has the French Service Alps Mediterranean Blood Establishment (EFSAM), which connects 14 blood banks to 149 hospitals8. In the Netherlands the national Transfusion Register of Irregular Antibodies and Cross-Match Problems (TRIX) has been operating since 2007, and has been invaluable in the detection of incorrect blood typing, detection of evanesced antibodies, and the data has even be utilised to investigate antibody distributions within the population to optimise screening and panel cells7. Since 2011, DHTRs reported in the Netherlands have halved, and other networks have reported improved blood banks efficiencies, with some systems requiring as little as one fifth the amount of time to source two or three antigen negative units15.
With all these improvements in error detection and avoidance, as well as improved patient safety and blood bank efficiency, there is little room for doubt that a national or regional antibody registry would greatly contribute to improved patient outcomes.
Materials and Methods
Study design
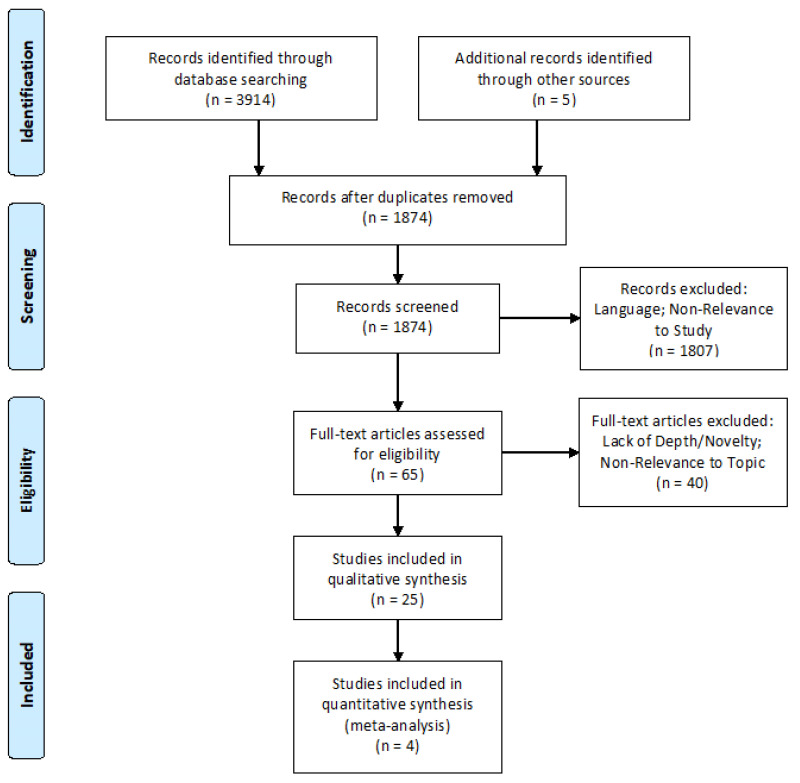
To investigate and justify the need for a national antibody database, and a strategy to achieve this, this study investigated multiple factors that have illustrated the potential for improvement in similar systems in other countries. This investigation specifically focuses on antibody evanescence, the occurrence of DHTR’s as a result of incompatible blood, and the potential economic benefits to individual hospitals, blood bank services, and the wider healthcare system through the minimisation of the often severe negative impact of incompatible blood transfusion. The “Preferred Reporting Items for Systematic Reviews and Meta-Analyses” (PRISMA) guidelines16 were used to ensure that a suitably wide net was cast in the search for relevant information, and to ensure that included information was also suitably relevant to the investigation being undertaken. A PRISMA flow chart, outlining the search strategies and inclusion/exclusion criteria was constructed (Figure 1).
Figure 1.
Prisma flow chart
Strategy for literature search
An initial search was performed through PubMed and SCOPUS to identify potential themes for the review. Once these themes were identified, the following keyword and phrase searches were used with liberal use of Boolean operators, predominantly “AND” and “NOT” in order to ensure a wide and thorough search of the databases, whilst minimising overlap with non-relevant studies: “Transfusion associated fatality”, “Antibody associated transfusion fatality”, “Transfusion Medicine Record Fragmentation”, “Antibody evanescence”, “Antibody record discrepancies”, “Alloimmunised delayed haemolytic transfusion reaction”, “Centralised transfusion database”, “Regional transfusion antibody register”, “National transfusion antibody database”, and “National transfusion antibody register”. To ensure relevant and peer reviewed results the Searches were performed in PubMed and Scopus, results were not actively screened by age, however when information was provided in multiple papers, the most recent was prioritised. Relevant citations within these articles were also investigated, and included where they were deemed to add valuable insight into the investigation.
Study selection
The inclusion/exclusion criteria for this study was connected to the themes identified in the study design. At the first stage of screening, and articles in which the summary or abstract portrayed a strong association with an area of transfusion medicine not related to the previously outlined themes were removed from the potentials list. This screened list was then subjected to an in-depth review via the full text. Any studies that were not available in full text via the RMIT Library service was excluded, as well as any studies where no English Language version was available. Articles that provided a superficial review or brief mention of the themes were also excluded, as this information was available in other included articles. To be included, articles were required to show evidence of a broad study including appropriate population sizes, and present data in a logical and meaningful manner. Studies surrounding antibody evanescence were required to acknowledge the occurrence and relevance of hospital acquired antibodies as a separate metric to those acquired through pregnancy or other avenues. Data surrounding DHTRs needed to be specific to transfusion related causes, as compared to transplant or pregnancy associations.
Data extraction and management
Data was grouped into relevant studies, and results tabulated for comparison and investigation with Microsoft Excel. Each study was investigated for suitability via a “Strengthening the Reporting of Observational studies in Epidemiology” (STROBE) checklist17 (Table I).
Table I.
The STROBE checklist used to investigate and evaluate all articles used in this review for suitability and fitness of purpose
| Author, yearref | Provides location | Provides timeframe | Provides eligibility criteria | Provides number of participants | Provides detailed study methods | Provides outcome data | Provides valid results and discussion | Conflict of interest statement | Presents limitations/ further research |
|---|---|---|---|---|---|---|---|---|---|
| Stack, 2016 2 | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Tormey, 2009 10 | Y | Y | Y | Y | Y | Y | Y | Y | N |
| Delaney, 2013 4 | Y | Y | Y | Y | Y | Y | Y | Y | N |
| Unni, 2014 5 | Y | Y | Y | Y | Y | Y | Y | Y | N |
| MacIvor, 2009 12 | Y | Y | Y | Y | Y | Y | Y | N | N |
| Harm, 2014 13 | Y | Y | Y | Y | Y | Y | Y | N | N |
| Thonier, 2019 18 | Y | N | Y | Y | Y | Y | Y | Y | N |
| Ferrera-Tourenc, 2015 8 | Y | Y | Y | Y | Y | Y | Y | Y | N |
| van Gammeren, 2019 7 | Y | Y | Y | Y | Y | Y | Y | Y | N |
| Denomme, 2020 15 | Y | Y | Y | N/A | Y | Y | Y | Y | N |
| Ditomasso, 2012 19 | Y | Y | Y | N/A | N | Y | Y | N | Y |
| van Sambeeck, 2018 20 | Y | Y | N/A | N/A | Y | Y | Y | Y | Y |
| Portegys, 2018 9 | Y | Y | Y | Y | Y | Y | Y | Y | N |
| Makroo, 2017 21 | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Franchini, 2019 22 | N/A | Y | Y | Y | N | Y | Y | Y | N |
| Schwickerath, 2010 23 | Y | Y | Y | Y | Y | Y | Y | Y | N |
| Ghezelbash, 2017 24 | Y | Y | N/A | N/A | Y | Y | Y | Y | Y |
| Yazer, 2007 11 | Y | Y | N/A | N/A | N/A | Y | Y | N | N |
| Hatano, 2012 25 | Y | Y | N/A | Y | Y | Y | Y | N | N |
| Flegel, 2015 26 | Y | Y | Y | Y | Y | Y | Y | Y | N |
| Montpetit, 2006 27 | Y | Y | N | Y | Y | Y | Y | N | Y |
| Woo Shin, 2018 28 | Y | Y | Y | Y | Y | Y | Y | N | N |
| Ali, 2017 29 | N/A | N/A | N/A | N/A | Y | Y | Y | N | Y |
| Ji Hong, 2015 30 | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Hauser, 2019 6 | Y | Y | Y | Y | Y | Y | Y | Y | N |
Y: yes; N: no; N/A: not applicable.
Statistical analysis
Data extracted from the studies was tabulated to aid in interpretation and visualisation of the perceived benefits a national or regional database could provide. A Comparison of Proportions was applied to data extracted surrounding the evanescence of select antibodies, using OpenMeta[Analyst] (Tufts® EPCTM, Medford, MA, USA). These results were calculated and presented based on 95% confidence intervals and assessed based on the returned p values.
Results
Search results and study eligibility
After carefully considering the outlined inclusion and exclusion criteria, a total of 25 studies were determined to be eligible for this review from the search terms utilised, as outlined in the provided PRISMA flowchart16 (Figure 1). These studies covered a range of topics deemed to be relevant to this study, including antibody evanescence rates, medical record fragmentation, and the prevalence of DHTRs related to errors caused by, or related to, the previous two topics. Due to the nature of the research all studies were performed retrospectively, but covered a range of clinical conditions, geographical regions, and even a variety of public health policies. The range of results was, however, somewhat limited as there are a limited number of regions in the world that currently employ national or regional antibody registries, and studies were of course limited to what the available data could elucidate.
Quality assessment and study fitness
All studies considered for this review were evaluated for the suitability, utilising a STROBE checklist17 which hampers the assessment of its strengths and weaknesses and of a study’s generalizability. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE [Table I]). The studies, where appropriate, gave clear participant eligibility and selection criteria, as well as sample sizes. Where the studies were retrospective and geographically removed from one another, dates and locations were provided, as well as medical institutions that the records were obtained from.
Antibody evanescence
A major hurdle in accurate transfusion unit matching is the detectability of antibodies in the patient’s system. Several studies investigated the frequency at which a range of clinically significant antibodies evanesced, as well as the time periods that this evanescence occurred over. A study from Tormey and Stack, 200910 separated pre-existing antibodies from hospital acquired alloantibodies in order to determine the evanescence of these hospital acquired antibodies. By singling out male patients, they were able to remove induction events through previous pregnancies, and from the data they had access to were able to identify how long after an immunising event an antibody could be expected to remain detectable. They determined that of the 108 evanescent alloantibodies, 50% of them had become undetectable by conventional laboratory methods within 6 months.
A follow up study from Stack and Tormey, 20162 succeeded in developing an equation to determine the likelihood that an antibody will become undetectable. Using data from their previous 2009 study, they produced a series of very accurate lines of best fit for the evanescence of antibodies at the available time points, and from these lines of best fit developed an equation that allowed them to estimate an average likelihood of all alloantibodies becoming undetectable over all time periods, as well as at each of the individual time periods. The equation was constructed thusly: 0 times the number of transfused units that either had follow up testing within the 30 day induction period, or no follow up testing at all (271 units), plus 0.518 times the number of transfused units with follow up screening between 30 to 112 days (58 units), plus 0.536 times the number of units with follow up after the 112 day period (161 units), plus 0.858 times the number of units that had follow up testing in both the 30–112 day period, and after the 112 day period (71 units), divided by the total number of transfused units in the study (561 units). With this ability to take into account that reduced likelihood of evanescence in the first month, and greater likelihood after 6–12 months, they were able to determine that over all observed time periods, 31.6% of antibodies in all of the observed blood group systems will remain detectable by standard methods, all others are highly likely to become undetectable.
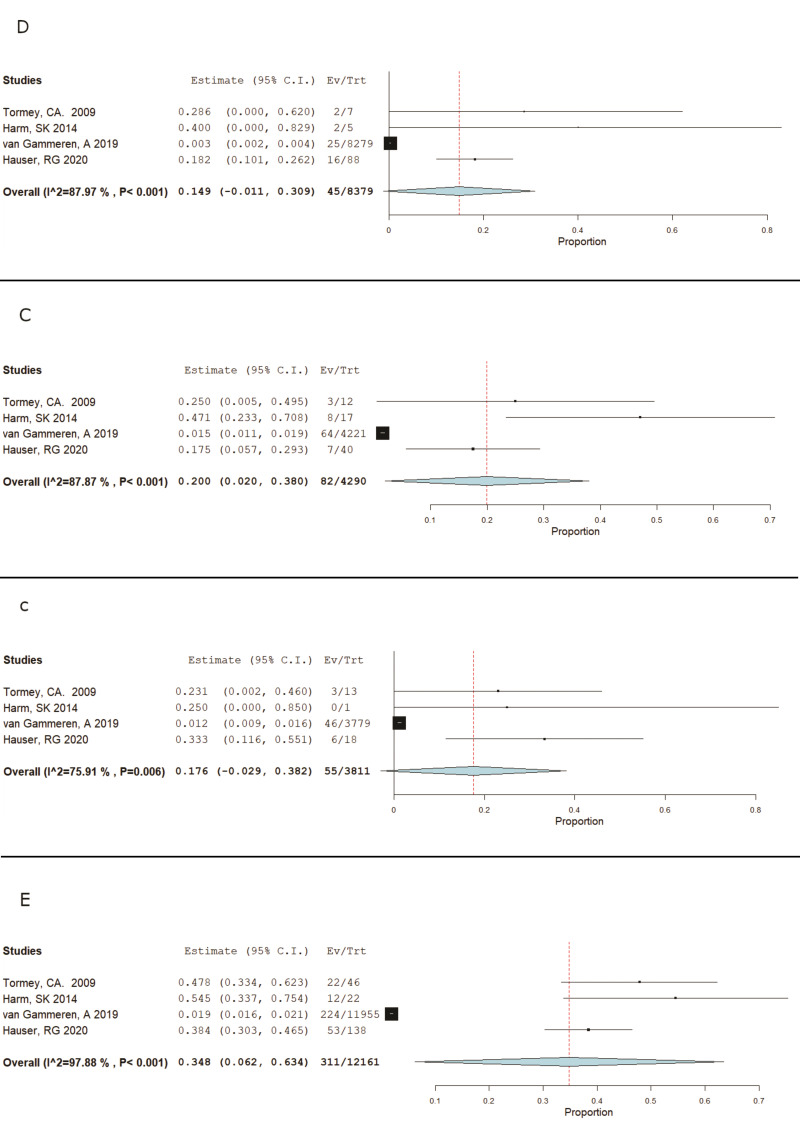
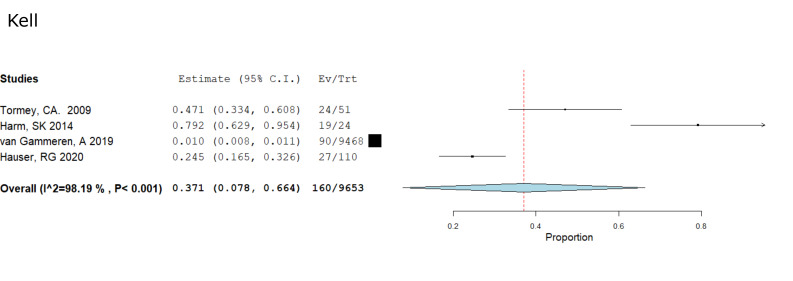
Four studies were identified that provided a more in-depth investigation into the evanescence of “hospital-acquired” alloantibodies. Pooling the data from these four studies, Tormey CA, 200910; Harm SK, 201413; van Gammeren AJ, 20197; and Hauser RG, 20206; we were able to compile Forest Plots to determine and visualise the occurrence and significance of D, C, c, E, and Kell antibody evanescence (Figures 2, 3), all of which showed significance with p values less than 0.05(p<0.001 for K, D C, and E, and p=0.006 for c). These calculations give us the confidence to say that there is a significant likelihood that any one of these antibodies, when considered individually or in conjunction with each other, have a very real and significant likelihood of evanescence and becoming undetectable by current laboratory methods.
Figure 2.
RH meta analysis
Figure 3.
Kell meta analysis
Medical record accuracy and availability
Of similar impact to the disappearance of alloantibodies is the apparent disappearance of patient hospital records. Multiple studies in the United States have investigated and compared medical records for patients across distinct hospital networks. This is of significant concern as populations are experiencing more pressure and ability to move within and between communities, often receiving medical care from a variety of providers, especially those with chronic conditions. A study by Unni et al.5 isolated a small group of 100 patients within the US’s VA hospital network. Of these 100 patients, 59 had history of receiving one or more transfusions, and 23 of these patients had received a transfusion from at least one of 24 alternate locations. A further investigation within this same network identified 42 patients that had received antibody screens at two of the networked hospitals, 64% of these records were found to be discrepant, the primary cause being detection of antibodies at only one of the two hospitals. It has been suggested that it is becoming more common for patients to attend multiple facilities, and from a selection of 150 Sickle Cell Disease patients it was found that the median number of hospitals patients received transfusions at was 313.
Error and processing time reductions
Often the largest opportunity for error lies within the category of human error. In 2006, a report from CTS Pittsburgh identified 94 individual cases of WBIT errors12. 57% of these errors were automatically identified when ABO typing was able to be compared to historical data. In this same report, the CTS network claimed a 38% improvement in the detection of errors involving WBIT, or major ABO typing errors through comparisons with historical data that was collected at other blood banks within the system.
Another benefit observed through the introduction of a central database was observed by a network of 16 hospital that introduced a cloud-based blood banking system developed by the Versiti Blood Research Institute in Wisconsin. Their records were able to show that the time required for their blood bank staff to search for a likely compatible blood unit, and perform the manual typing and cross match was reduced by up to 5 times in cases were a three antigen combination was required, in some cases reducing a 2+ hour search with multiple failed cross matches down to a 30 second online search and 25 minutes to confirm the phenotypes and cross match15 (Table II).
Table II.
Reduction of type and screen using online database search tool
| Number of Ag’s | Ag’s | Queries | Database query and typing (30 min each) | Manual type/screen (min avg per Batch) | Time for manual (X) |
|---|---|---|---|---|---|
| One | K− | 248 | 30 | 30 | 1.0 |
| S− | 91 | 30 | 40 | 1.3 | |
| Fya− | 304 | 30 | 50 | 1.7 | |
| Jka− | 477 | 30 | 60 | 2.0 | |
| Fyb− | 29 | 30 | 75 | 2.5 | |
| s− | 3 | 30 | 105 | 3.5 | |
| Two | E− K− | 120 | 30 | 30 | 1.0 |
| K− S− | 4 | 30 | 40 | 1.3 | |
| Fya− K− | 26 | 30 | 50 | 1.7 | |
| Jkb− K− | 22 | 30 | 60 | 2.0 | |
| Fya− Jka− | 3 | 30 | 75 | 2.5 | |
| Jkb− S− | 1 | 30 | 90 | 3.0 | |
| C− Fya− | 10 | 30 | 105 | 3.5 | |
| C− Jka− | 18 | 30 | 120 | 4.0 | |
| c− Jkb− | 1 | 30 | 135 | 4.5 | |
| Three | E− K− S− | 26 | 30 | 50 | 1.7 |
| C− E− K− | 177 | 30 | 75 | 2.5 | |
| C− K− S− | 9 | 30 | 75 | 2.5 | |
| E− Fyb− K− | 4 | 30 | 105 | 3.5 | |
| E− Jka− S | 3 | 30 | 120 | 4.0 | |
| c− E− Fya− | 2 | 30 | 120 | 4.0 | |
| C− Fyb− K− | 1 | 30 | 135 | 4.5 |
Time savings were observed when utilising the online database search function for typing and crossmatching units, compared to visually selecting a potentially compatible unit and performing a type and crossmatch. The database query was calculated to require less than 1 minute, with the remainder of the of the required time spent confirming crossmatch compatibility.
Discussion
When taken together, the included studies have highlighted and investigated flaws in transfusion services provided around the world, many of which have the potential to be significantly reduced through the introduction of a National registry. Antibody evanescence was found to occur with greater speed and regularity than previously suggested, and DHTR’s as a result of the antibodies was seen to have reduced in many of the countries that have introduced their own versions of a national or regional registry. When combined with ABO typing being included in the registry, pre-laboratory errors were seen to be reduced through the early detection of WBIT errors.
Antibody evanescence is not a new concept. Around the world entire vaccination schedules have been developed around the knowledge that some antibodies will evanesce faster than others, and all at different rates in different populations and individuals, so it should come as no surprise that the same effect was observed with antibodies to almost every non-ABO blood group system. Routine pre-transfusion screenings are unable to detect evanesced antibodies, and unfortunately in this case, out of sight does not mean out of mind. Any patient with a history of previous transfusions, transplants or pregnancies are at risk of having developed antibodies that may not show up in an antibody screen and/or crossmatch. This makes for a very high-risk gamble, as per the calculations by Stack and Tormey2 there is only a 31.6% likelihood that non-ABO antibodies will be detectable. One issue noticed in the development of this algorithm involves the timescales the team had to work with. As with all the studies included with this review, this article utilised a retrospective study, and as such they had to work with the testing schedules set by the hospital at the time of testing. Many patients were seen to have no follow up testing post transfusion, and some with a gap in follow up as large as 10 years. The authors of this study observed that in most cases, follow-up antibody screening only occurred when and if the patient required another transfusion. So while approximately 50% of the antibodies had evanesced at some point in the first 6–12 months, the other half evanesced at some point in the 9 years following that10. This does not take away from the fact that the antibodies evanesced, but it does make it difficult to pinpoint a time at which evanescence becomes a greater risk to patients receiving ongoing transfusions. While the data presented here does suggest clinical significance with p values less than 0.05 for each of the antibodies examined in the meta-analysis, the overall results returned a very high level of heterogeneity, with inconsistency (I2) values ranging from 75 to 98%. This is likely to be a result of the small number of studies included in the analysis, and the small sample sizes in most of the studies will have contributed greatly to this effect. As the data for this meta-analysis was extracted from retrospective studies, the sample sizes were limited by what had often been observed and recorded several years in the past, and a lack of meaningful studies in these areas reduced the ability to include more studies in the analysis. Future studies involving transfusion patients could include regular antibody screenings to get a more accurate and complete picture of what antibodies are being induced, evanescing, and the timelines involved in these complex processes.
Many of the complications surrounding the medical records of patients often relates to the patient’s ability and need to attend multiple medical facilities during their lifetime. With an increasingly mobile population, both nationally and globally, the medical industry needs to adapt to this situation. Several of the included studies recorded patients with records at multiple hospitals, and often these records were discrepant. With the alarmingly regularity at which antibodies have been seen to become undetectable, it is difficult to say with any confidence whether these records do not match due to laboratory errors, or are simply a by-product of evanescence. Regardless, historical information relating to clinically significant antibodies is priceless to blood bank staff when considering crossmatch unit compatibility, and avoiding DHTR’s. One system containing all the patient’s history may not eliminate the dangers of DHTR’s, but will go a long way to improving the safety and outcomes. Most LIS’s allow staff to search for patient records based on a range of identifiers, including name, DOB, and Medicare numbers. While there are several reasons behind a patient changing their name, including spelling errors on admission, a combination of DOB and Medicare numbers would enable staff to be confident that they are looking at the correct patient, where evidence of a name change exists. An example system is “My Health Record”, which holds personal and medical information for any Australian patient who has not opted out of having their results recorded. While this system may have potential uses in consolidating results, it is not directly linked into LIS’s, and does not have the ability to automatically notify staff of an existing record, or discrepant information in another hospital’s LIS.
Of great concern to the Australian population, as well as most other nations, is that of privacy and consent. The previous example of the “My Health Record” system created an entry for everyone, unless they had “opted out” by a certain date. Even with this date having passed, people are within their legal right to request that their record, and all results be deleted from the system. Similarly, with the Dutch “TRIX” database, patients are actively informed that their information and results will be stored in the database during medical counselling pre-transfusion, and they have the right to have their records deleted at any time. In both countries, strict laws exist surrounding what can be stored in the system, who can add information to the system, and who can retrieve information from the system. While the My Health Record does not currently store pre transfusion screening results in a lab-friendly format, its introduction required the development and introduction of protective laws that would be highly relevant to an Australian antibody registry, should one be developed separate to the My Health Record. Similar to the Dutch model, patients exercising their right to “opt-out” of the database should be counselled on the risks of not being included, especially if they have a high potential for multiple transfusions, but also the benefits that would come with inclusion in the system, with “opting out” being an informed decision, requiring more than simply ticking a box on a website, but a formal request through an authorised medical provider.
Conclusions
The findings from this study show just a few of the many benefits that could be gained through the implementation of a system that can track clinically significant antibodies. The tracking of antibodies from induction to evanescence will not only provide clinicians with important information when performing blood matching services, they could also prove to be a source of vital information in future research, as was the case with this review. With nearly 3% of transfused units resulting in a DHTR, and 10% of those resulting in catastrophic outcomes, there is clear evidence that a national antibody register would contribute to improving patient outcomes. Reductions in processing times will also lead to improved outcomes, which flows on to improvements in staff efficiency, allowing for more effective laboratory performance, which will also contribute to improving patient outcomes. A reduction in DHTR’s will reduce the stress put on the healthcare system, as even when not fatal, these reactions can require significant attention from clinicians, as well as significant follow up investigation, reducing staff efficiency.
Studies from countries such as the United States of America, the Netherlands, and several other European countries have provided clear insights into how their systems have worked, and currently work, and a variety of comparable frameworks are in development in other countries around the world, including but not limited to South Korea, Iran, and India.
While there is clear evidence of the perceived benefits of such a system, further research could be performed in key areas to better quantify the health and financial benefits that could be realised through the introduction of such a database: further research surrounding the timelines of non-ABO blood group antibodies from induction to evanescence would provide valuable information and allow for the development of clinical guidelines surrounding post-transfusion follow-ups; research into the longevity of the associated T-memory cells, to identify whether evanescence is the end of the road, or does the sensitisation wear-off over a long enough period of time?; and at what cost would this system come at? With suggestions of improved efficiency after implementation, maintenance of the system would potentially cover itself, but the development and implementation of the initial system may prove to be prohibitively expensive if not approached correctly.
However, when armed with all the information currently available, there is enough data to confirm that a National antibody register would contribute to improving patient outcomes.
Footnotes
The Authors declare no conflicts of interest.
References
- 1.National Blood Authority [Internet] Australian haemovigilance report - Data for 2017–18. [Accessed on 31/08/2020]. p. 13. Available at: https://www.blood.gov.au/system/files/Australian-Haemovigilance-Report-2017-18-FINAL.pdf.
- 2.Stack G, Tormey CA. Detection rate of blood group alloimmunization based on real-world testing practices and kinetics of antibody induction and evanescence. Transfusion. 2016;56:2662–7. doi: 10.1111/trf.13704. [DOI] [PubMed] [Google Scholar]
- 3.Schonewille H, Haak HL, van Zijl AM. RBC antibody persistence. Transfusion. 2000;40:1127–31. doi: 10.1046/j.1537-2995.2000.40091127.x. [DOI] [PubMed] [Google Scholar]
- 4.Delaney M, Dinwiddie S, Nester TN, AuBuchon JA. The immunohematologic and patient safety benefits of a centralized transfusion database. Transfusion. 2013;53:771–6. doi: 10.1111/j.1537-2995.2012.03789.x. [DOI] [PubMed] [Google Scholar]
- 5.Unni N, Peddinghaus M, Tormey CA, Stack G. Record fragmentation due to transfusion at multiple health care facilities: a risk factor for delayed hemolytic transfusion reactions. Transfusion. 2014;54:98–103. doi: 10.1111/trf.12251. [DOI] [PubMed] [Google Scholar]
- 6.Hauser RG, Hendrickson JE, Tormey CA. TRIX with treats: the considerable safety benefits of a transfusion medicine registry. Transfusion. 2019;59:2489–92. doi: 10.1111/trf.15449. [DOI] [PubMed] [Google Scholar]
- 7.van Gammeren AJ, Bos AG, Som N, et al. A National Transfusion Register of irregular antibodies and cross (x)-match problems: TRIX, a 10-year analysis. Transfusion. 2019;59:2559–66. doi: 10.1111/trf.15351. [DOI] [PubMed] [Google Scholar]
- 8.Ferrera-Tourenc V, Lassale B, Chiaroni J, Dettori I. Unreliable patient identification warrants ABO typing at admission to check existing records before transfusion. Transfus Clin Biol. 2015;22:66–70. doi: 10.1016/j.tracli.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 9.Portegys J, Rink G, Bloos P, et al. Towards a Regional Registry of extended typed blood donors: molecular typing for blood group, platelet and granulocyte antigens. Transfus Med Hemotherapy. 2018;45:331–40. doi: 10.1159/000493555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tormey CA, Stack G. The persistence and evanescence of blood group alloantibodies in men. Transfusion. 2009;49:505–12. doi: 10.1111/j.1537-2995.2008.02014.x. [DOI] [PubMed] [Google Scholar]
- 11.Yazer M. The Pittsburgh centralized transfusion model: less is more. Transfusion. 2007;47(Suppl 2):164S–168S. doi: 10.1111/j.1537-2995.2007.01378.x. [DOI] [PubMed] [Google Scholar]
- 12.MacIvor D, Triulzi DJ, Yazer MH. Enhanced detection of blood bank sample collection errors with a centralized patient database. Transfusion. 2009;49:40–3. doi: 10.1111/j.1537-2995.2008.01923.x. [DOI] [PubMed] [Google Scholar]
- 13.Harm SK, Yazer MH, Monis GF, et al. A centralized recipient database enhances the serologic safety of RBC Transfusions for patients with sickle cell disease. Am J Clin Pathol. 2014;141:256–61. doi: 10.1309/AJCP47QAAXTOZEKJ. [DOI] [PubMed] [Google Scholar]
- 14.Figueroa PI, Ziman A, Wheeler C, et al. Nearly two decades using the check-type to prevent ABO-incompatible transfusions. Am J Clin Pathol. 2006;126:422–6. doi: 10.1309/C6U7VP87GC030WMG. [DOI] [PubMed] [Google Scholar]
- 15.Denomme GA, Reinders S, Bensing KM, et al. Use of a cloud-based search engine of a centralized donor database to identify historical antigen-negative units in hospital inventories. Transfusion. 2020;60:417–23. doi: 10.1111/trf.15638. [DOI] [PubMed] [Google Scholar]
- 16.Moher D, Liberati A, Tetzlaff J, Altman DG Prisma Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.von Elm E, Altman DG, Egger M, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. 2007;147:573–7. doi: 10.7326/0003-4819-147-8-200710160-00010. [DOI] [PubMed] [Google Scholar]
- 18.Thonier V. Immuno-hematological findings in Delayed Hemolytic Transfusion Reaction (DHTR) Transfus Clin Biol. 2019;26:102–8. doi: 10.1016/j.tracli.2019.02.006. [DOI] [PubMed] [Google Scholar]
- 19.Ditomasso J, Liu Y, Heddle NM. The Canadian Transfusion Surveillance System: what is it and how can the data be used? Transfus Apher Sci. 2012;46:329–35. doi: 10.1016/j.transci.2012.03.024. [DOI] [PubMed] [Google Scholar]
- 20.van Sambeeck JHJ, de Wit PD, Luken J, et al. A conceptual framework for optimizing blood matching strategies: balancing patient complications against total costs incurred. Front Med. 2018;5:199. doi: 10.3389/fmed.2018.00199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Makroo RN, Kakkar B, Agrawal S, et al. Retrospective analysis of forward and reverse ABO typing discrepancies among patients and blood donors in a tertiary care hospital. Transfus Med. 2019;29:103–9. doi: 10.1111/tme.12506. [DOI] [PubMed] [Google Scholar]
- 22.Franchini M, Forni GL, Marano G, et al. Red blood cell alloimmunisation in transfusion-dependent thalassaemia: a systematic review. Blood Transfus. 2019;17:4–15. doi: 10.2450/2019.0229-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schwickerath V, Kowalski M, Menitove JE. Regional registry of patient alloantibodies: first-year experience. Transfusion. 2010;50:1465–70. doi: 10.1111/j.1537-2995.2010.02629.x. [DOI] [PubMed] [Google Scholar]
- 24.Ghezelbash B, Moghaddam M, Aghazadeh S. Challenges of Establishing a National Rare Donor Program in Iran. Int J Hematol stem cell Res. 2018;12:213–9. [PMC free article] [PubMed] [Google Scholar]
- 25.Hatano Y, Otsuka S, Chousa M, et al. Fatal delayed hemolytic transfusion reaction associated with anti-Dib and anti-E. Transfus Apher Sci. 2012;47:263–8. doi: 10.1016/j.transci.2012.07.020. [DOI] [PubMed] [Google Scholar]
- 26.Flegel WA, Gottschall JL, Denomme GA. Implementing mass-scale red cell genotyping at a blood center. Transfusion. 2015;55:2610–5. doi: 10.1111/trf.13168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Montpetit A, Phillips MS, Mongrain I, et al. High-throughput molecular profiling of blood donors for minor red blood cell and platelet antigens. Transfusion. 2006;46:841–8. doi: 10.1111/j.1537-2995.2006.00805.x. [DOI] [PubMed] [Google Scholar]
- 28.Shin DW, Kim H, Chung Y, et al. Establishment and utilization of a transfusion recipient Registry in Korea. Am J Clin Pathol. 2018;150:154–61. doi: 10.1093/ajcp/aqy044. [DOI] [PubMed] [Google Scholar]
- 29.Ali RS, Hafez TF, Ali AB, Abd-Alsabour N. Blood bag: a web application to manage all blood donation and transfusion processes. 2017 International Conference on Wireless Communications, Signal Processing and Networking (WiSPNET); 2017; IEEE; pp. 2125–30. [Google Scholar]
- 30.Hong YJ, Chung Y, Hwang SM, et al. Genotyping of 22 blood group antigen polymorphisms and establishing a national recipient registry in the Korean population. Ann Hematol. 2016;95:985–91. doi: 10.1007/s00277-016-2645-7. [DOI] [PubMed] [Google Scholar]