Abstract
Background
Polycythaemia vera is a myeloproliferative neoplasm characterised by a high incidence of thrombosis. The contribution of platelets, key players in haemostasis, in this setting is still unclear. So far, the majority of studies have been focussed on specific platelet abnormalities but not on their actual capacity to form thrombi. The aim of this study was to characterise, ex vivo under flow conditions, the capacity of platelets from patients with polycythaemia vera to adhere to collagen and induce thrombus formation.
Materials and methods
Thirty-nine patients and 30 healthy controls were studied. Thrombus formation was induced by perfusing whole blood over a collagen-coated surface, in a parallel-plate flow chamber coupled to a fluorescent microscope. This dynamic system enables platelet adhesion and thrombus formation to be followed in real time and also allows measurements of the extent of the thrombus and platelet surface antigen expression. Laboratory data were analysed in the light of the patients’ main haematological parameters and therapies.
Results
Platelet adhesion was significantly greater in patients than in control subjects. Patient thrombi were usually larger and more complex than those formed by control platelets. A significant positive correlation was found between platelet adhesion and both the haematocrit and red blood cell count. These parameters remained significantly correlated with platelet adhesion also after multivariable analysis adjusted for gender, age, therapy and JAK2V617F allele burden. Furthermore, subjects with a haematocrit >45% had significantly greater platelet adhesion than subjects with a haematocrit <45%.
Discussion
Our data indicate that increased platelet adhesion participates in the thrombotic diathesis of patients with polycythaemia vera, and that the haematocrit level can affect the adhesive and thrombus forming capacities of platelets.
Keywords: polycythaemia vera, thrombosis, platelet adhesiveness, haematocrit, erythrocytes
Introduction
Polycythaemia vera (PV), one of the most common Philadelphia-negative myeloproliferative neoplasms, is characterised by haematopoietic stem cell-derived clonal proliferation of the erythroid, megakaryocyte, and granulocytic lineages and by its distinct molecular signature (i.e. the JAK2V617F mutation).
The clinical course of PV is typically complicated by a high rate of arterial and venous thromboses, and a tendency to evolve into myelofibrosis or acute myeloid leukemia1. Arterial thrombosis occurs more frequently than venous thrombosis and accounts for 60% of all vascular events. In addition, transient platelet aggregates may clog small vessels and lead to microvascular disturbances. Overall, cardiovascular complications are the major cause of mortality and morbidity in this disease. Therefore, the major goal of PV treatment is to prevent thrombosis, through the modification of cardiovascular risk factors, and use of aspirin, phlebotomy, and cytoreduction2. However, these strategies reduce but do not abolish the risk of thrombosis.
The pathogenesis of thrombosis in PV is complex and multifactorial. Several mechanisms contributing to PV-associated coagulopathy have been described, including quantitative and qualitative alterations of circulating blood cells arising from the clonal proliferation of haematopoietic stem cells1,3–5. The contribution of platelets to the pathogenesis of thrombosis in PV is still unclear. The formation of a platelet thrombus at sites of vascular damage is fundamental in normal haemostasis, but might lead to vessel occlusion and ischaemia in uncontrolled conditions. In PV, a high platelet count is not a major determinant of the risk of thrombosis, while qualitative abnormalities of platelets have been increasingly recognised as important contributors to the onset of hypercoagulability in these patients.
Previous work by our group and others demonstrated that platelets circulate in an activated state in PV patients6,7, as revealed by the findings of increased surface expression of tissue factor, the main activator of blood coagulation, and of P-selectin, the counter-receptor of P-selectin glycoligand-1 (PSGL-1) on leucocytes and platelets themselves8. The binding of platelet P-selectin to PSGL-1 activates neutrophils and induces the expression of surface CD11b and release of cathepsin-G from neutrophil granules9,10. In addition, the P-selectin/PSGL-1 interaction mediates the release of procoagulant microparticles from platelets and leucocytes, which accumulate in the developing thrombi10. Accordingly, the heterophilic platelet-leucocyte aggregates are increased in the plasma of patients with PV6, as are the levels of circulating procoagulant microparticles11. Furthermore, platelets from PV patients have an elevated thrombin generation potential and retain an increased capacity to aggregate despite antiplatelet therapy with aspirin12. However, so far, no studies have investigated whether, among these alterations, the adhesive capacity of platelets from patients with PV is increased.
In the present study, in a group of PV patients, we aimed to provide a functional characterisation of platelet adhesive properties and thrombus formation capacity, as evaluated under flow conditions at an arterial shear rate. To study this, we utilised a system recently designed by researchers at Maastricht University. This system combines a proprietary parallel-plate flow chamber, designed and produced in Maastricht, with a brightfield and fluorescent microscope: it allows thrombus formation to be observed in real time, and at the end of perfusion, the extent of the thrombi can be quantified by image analysis, and the expression of any platelet surface antigen of interest can be measured by means of fluorescent markers13.
To our knowledge this is the first time that this approach has been utilised in PV patients to obtain quantitative information on the extent and phenotypic characteristics of platelet thrombi.
Materials and methods
Study population
Thirty-nine consecutive patients with PV (22 males and 17 females; median age: 65 years, range: 38–83) were enrolled at our institution after giving informed consent. PV was diagnosed according to the 2008 World Health Organization (WHO) classification system14. At enrolment, PV pharmacological therapy and history of thrombotic/haemorrhagic events were recorded. Thirty healthy subjects (15 males and 15 females; median age: 44 years, range: 27–61), without a history of thrombo-haemorrhagic events, acted as the control group: none of these had symptoms of active infection or inflammatory diseases, or had been taking any antiplatelet or anti-inflammatory agent in the 15 days before blood withdrawal. All investigations were approved by the local Ethics Committee (Comitato di Bioetica, ASST Papa Giovanni XXIII Hospital, Bergamo, Italy). The procedures were performed in accordance with the Declaration of Helsinki of 1975, as revised in 2000.
Blood collection and blood cell count
Blood samples were drawn early in the morning, before any therapy, with a 21-gauge butterfly needle after applying a light tourniquet. After discarding the first 3 mL, peripheral blood samples were collected into BD Vacutainer® tubes (Becton Dickinson, Franklin Lakes, NJ, USA) containing trisodium citrate (0.109 M, 9:1 vol:vol) for platelet adhesion assays, or in K3-ethylenediaminetetraacetic acid (K3-EDTA), for blood cell counts, which were performed with a Cell-Dyn Ruby analyser (Abbott, Rome, Italy).
Whole-blood microfluidic perfusion over collagen
Whole blood thrombus formation on collagen was induced as previously described13. Briefly, blood was perfused over a collagen-coated surface for 4 min at a shear rate of 1,000 s−1 in a parallel-plate flow chamber, designed and produced by Maastricht University, under an EVOS® fluorescence microscope (AMG, Mill Creek, WA, USA). Adherent platelets were then stained for 2 min with an anti-CD62P (P-selectin)-FITC antibody (1:40; clone AK4; BioLegend; San Diego, CA, USA) to evaluate P-selectin as an index of platelet activation, and with annexin V-AlexaFluor647 (1:200; 0.25 μg/mL; Invitrogen, Carlsbad, CA, USA) to measure procoagulant phosphatidylserine exposure. After staining, brightfield images and fluorescence images were taken in random fields using an EVOS system (total magnification: 60X). These images were analysed using MetaMorph® NX 2.0 software (Microscopy Automation & Image Analysis Software; Molecular Devices, Downingtown, PA, USA). Results are expressed as the percentage of area covered by all platelets (“coverage”), or by either P-selectin-positive platelets or phosphatidylserine-positive platelets. All samples from study subjects were assayed twice by performing two separate runs each.
Statistical analysis
The results are reported in the text as mean ± standard error and/or ranges (minimum - maximum values). The Student’s t-test was used for the determination of levels of statistical significance between groups. Correlation and linear regression analyses between values were assessed by means of the Pearson’s correlation test. All tests for statistical significance were two-tailed and p values less than 0.05 were considered statistically significant. All analyses were performed with SPSS® Statistics version 21.0 software (IBM, Chicago, IL, USA).
Results
Characteristics of the study subjects
The demographic and clinical characteristics of the PV patients enrolled into the study are shown in Table I. The patient’s median age was 65 years (range, 38–83) and 56% were males. The JAK2V617F mutation was detected in 37/39 patients (95%), 25 (64%) with a <50% JAK2V617F allele burden and 12 (31%) with a >50% allele burden. A JAK2 exon 12 mutation was found in one patient (2.5%), while the remaining patient was negative for any known JAK2 mutation. In comparison to healthy subjects, PV patients displayed significantly higher leucocyte, platelet, erythrocyte, and plateletcrit values, and lower mean platelet volume. No statistically significant differences in the haematological parameters were observed in relation to JAK2V617F burden (Table I).
Table I.
Characteristics of study subjects
| Characteristics | Healthy controls | All PV patients | PV JAK2V617F heterozygous | PV JAK2V617F homozygous |
|---|---|---|---|---|
| N, gender (males/females) | 30 (15/15) | 39 (22/17) | 25 (15/10) | 12 (6/6) |
| Age, years (range) | 54 (37–71) | 65 (38–83) | 62 (38–79) | 66 (40–83) |
| Platelets (10 9 /L) | 243 (165–349) | 521 (142–1,325)** | 511 (189–1,089) | 597 (299–1,325) |
| Leucocytes (10 9 /L) | 6.8 (4.7–10.2) | 9.8 (5.7–20.8)* | 9.5 (5.7–20.8) | 10.73 (8.3–15.4) |
| Erythrocytes (10 12 /L) | 4.9 (4.1–5.6) | 5.4 (2.9–7.6)** | 5.1 (2.9–7.1) | 5.9 (4.1–7.6) |
| MPV (fL) | 9.1 (7.5–11.4) | 8.3 (6.3–13.6)* | 8.2 (6.3–13.6) | 8.4 (6.8–10.2) |
| Plateletcrit (%) | 0.21 (0.15–0.30) | 0.38 (0.15–0.94)** | 0.41 (0.15–0.82) | 0.48 (0.27–0.94) |
| Haematocrit (%) | 43 (37–51) | 44 (30–51) | 44 (30–51) | 45 (38–49) |
| Haemoglobin (g/dL) | 14.4 (12.9–16) | 14.1 (5.1–16.9) | 14.1 (5.1–16.9) | 14.2 (12.4–15.6) |
Data are median (range).
p<0.01,
p<0.001.
PV: polycythaemia vera; MPV: mean platelet volume.
At enrolment, all patients were receiving antiplatelet and/or cytoreductive therapies, i.e., low-dose aspirin alone (ASA, 13 patients) or low-dose ASA plus hydroxyurea (ASA+HU, 26 patients).
Statistically significant differences in the haematological parameters were observed among PV patients in relation to pharmacological therapy. In particular, PV patients on ASA, compared to PV patients on ASA+HU, had statistically significant higher levels of platelet count (710±351×109/L vs 423±184×109/L; p<0.014), plateletcrit (0.55±0.23% vs 0.37±0.15%; p<0.02) and red blood cell count (6.3±1.05×1012/L vs 5.0±1.03×1012/L; p<0.001).
Nine patients (22.5%) had a positive history of thrombotic events, in three cases (7.5%) venous thromboembolism and in six cases (15%) arterial thrombosis, while 25 (64.1%) had experienced microcirculatory disturbances. Two patients (5%) had reported minor bleeds (epistaxis for both). No differences in the history of thrombosis were found on the basis of JAK2V617F mutational status.
Thrombus formation
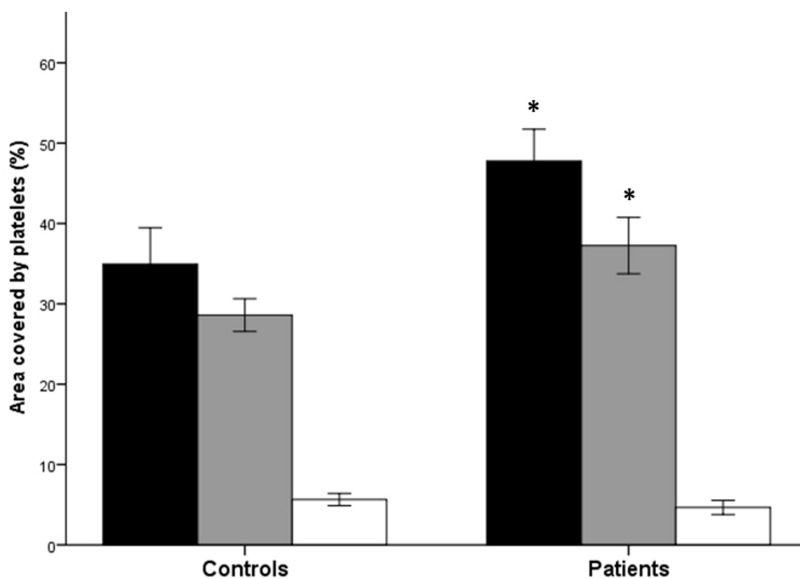
The quantification of platelet adhesion, obtained from brightfield images acquired at the end of whole blood perfusion on collagen, is shown in Figure 1.
Figure 1.
Platelet adhesion to collagen, and P-selectin and phosphatidylserine expression by adherent platelets, in patients with polycythaemia vera and in controls
The graph shows the total platelet adhesion to collagen (black bars), and the percentage of adherent platelets positive for either P-selectin (grey bars) or phosphatidylserine (white bars), in patients with polycythaemia vera and healthy controls. Data are expressed as the mean ± standard error of the percentage of area covered by platelets. *p<0.0001 PV patients’ values vs respective control values.
The area covered by platelets (i.e. % coverage) was significantly greater for PV samples (47.4±1.94%) compared to those from control subjects (34.9±2.21%; p<0.0001). This difference was still significant after multivariable analysis adjusted for age and gender (β=0.433, p=0.005). The morphological evaluation also showed differences in the adhesion/thrombus patterns between samples from PV patients and controls. Specifically, thrombi from PV patients were usually larger and more often interconnected, while thrombi from controls were more frequently smaller and well isolated one from another (Figure 2A, B).
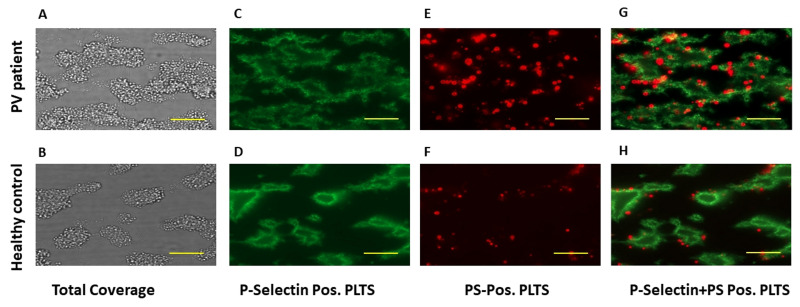
Figure 2.
Representative pictures from platelet adhesion assays
The panels show representative photographs of adhesion assays with platelets from a patient with polycythaemia vera or a healthy subject. The green fluorescence represents platelets expressing P-selectin on the surface, while the red fluorescence represents platelets expressing phosphatidylserine on the surface. Actual dimensions of each photographed area are 142 μm (height) × 107 μm (width): the horizontal yellow bars represent a 20-μm length. PV: polycythaemia vera; PS: phosphatidylserine; Pos: positive; PLTS: platelets
To evaluate the activation status of platelets that formed the thrombi, at the end of blood perfusion, a fluorescent antibody against P-selectin was used to stain the adherent platelets and fluorescence images were recorded and analysed. As shown in Figure 1, the area covered by P-selectin positive platelets was significantly greater for the samples from PV patients than for samples from control subjects (37.3±1.73% vs 28.6±1.25%; p<0.0001). To understand whether the increased P-selectin expression by thrombi in PV patients was related to increased activation of platelets as compared to those from controls, a ratio between the values of area covered by P-selectin-positive platelets and the total platelet coverage (from brightfield images) was calculated for each sample. According to this calculation, no significant differences were found in the relative proportion of adherent platelets expressing P-selectin between PV patients and controls (78.4±2.0% vs 75.2±1.93%; p=n.s.). This observation was also sustained by a significant correlation between percentage of coverage in brightfield images and percentage of coverage by P-selectin-positive platelets (R=0.841, p=0.0001).
To identify procoagulant platelets, i.e. those expressing phosphatidylserine on their surface, the specimens were stained with fluorescent annexin V. As shown in Figure 1, the area covered by phosphatidylserine-positive platelets was not different for the samples from PV patients compared to those from control subjects (4.65±0.43% vs 5.64±0.46%; p=n.s.). However, after correcting these values for the total coverage, the relative proportion of phosphatidylserine-positive platelets was significantly lower for PV samples compared to control samples (9.24±0.8% vs 15.9±1.55%; p<0.01). No significant correlation was found between the percentage of coverage by phosphatidylserine-positive platelets (R=0.105, p=n.s.) and the percentage of total coverage or percentage of coverage by P-selectin-positive platelets (R=0.263, p=n.s.). Images with P-selectin-stained platelets (in green) and phosphatidylserine-stained platelets (in red) were merged. Qualitative analysis of merged images showed a similar distribution of P-selectin and phosphatidylserine in thrombi of PV patients and control subjects, with no co-localisation of the two markers. Indeed, P-selectin-positive platelets were situated at the core of the thrombi (Figure 2C, D), while phosphatidylserine-positive platelets were all located at the border of the thrombi (Figure 2E–H).
Impact of JAK2V617F mutational status and concomitant disease-specific therapies on thrombus formation
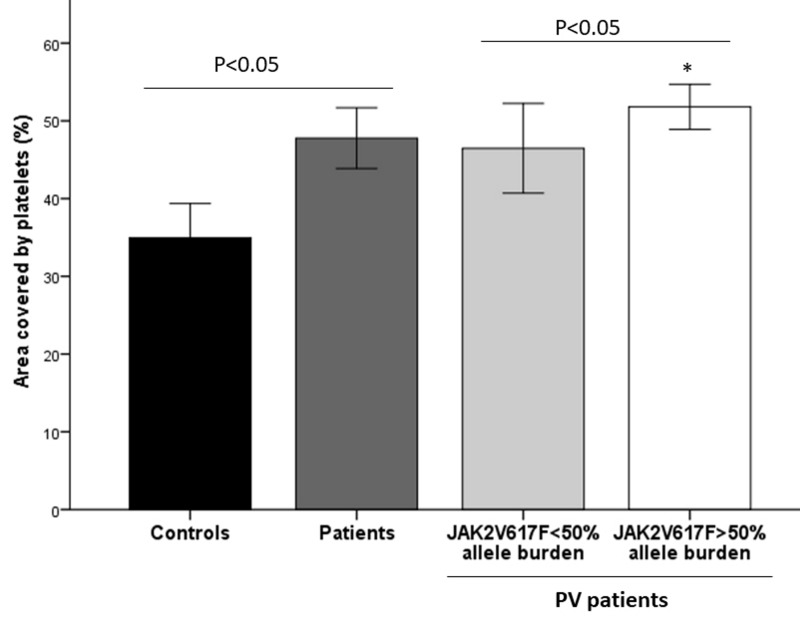
The JAK2V617F mutation was expressed in 25 patients in a heterozygous state (i.e. <50% allele burden) and in 12 patients in a homozygous state (i.e. >50% allele burden). The total platelet coverage of samples from PV patients with >50% JAK2V617F allele burden was significantly greater total than that of samples from patients with <50% JAK2V617F allele burden (51.8±1.44% vs 45.9±2.83%, p<0.05) (Figure 3). The percentage of platelets positive for P-selectin (77.8±2.3% vs 78.5±4.46%) or phosphatidylserine (9.23±1.05% vs 9.49±1.45%) was not statistically different between these two subgroups of patients, also after corrections of the values for the total coverage.
Figure 3.
Platelet adhesion to collagen according to JAK2V617F mutational status
The figure shows platelet adhesion data (mean ± standard error) in patients with polycythaemia vera (PV) divided according to JAK2V617F mutational status compared to the overall group of patients or controls. *p<0.0001 vs controls.
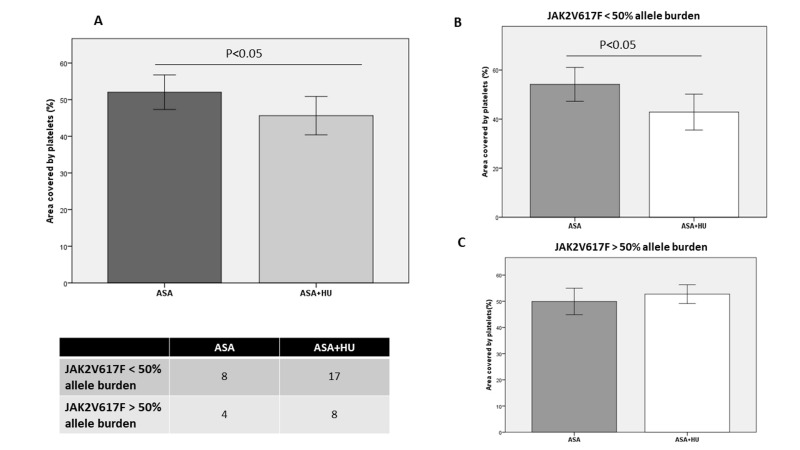
In the overall group of PV patients, the analysis according to pharmacological therapy showed that the total platelet coverage was significantly lower in patients on ASA+HU (45.6±2.61% vs 52.0±2.36%; p<0.05) compared to that of patients on ASA alone (Figure 4). In particular, in patients with <50% JAK2V617F allele burden, platelet adhesion was statistically significantly lower in patients receiving ASA+HU treatment than in those being treated with ASA alone (Figure 4B), while, in patients with >50% JAK2V617F allele burden there was no statistical difference between the two treatment subgroups (Figure 4C).
Figure 4.
Platelet adhesion to collagen in relation to pharmacological therapy for polycythaemia vera
(A) Platelet adhesion data (mean ± standard error) according to therapy. (B, C) Patients’ platelet adhesion data (mean ± standard error) according to therapy and JAK2V617F mutational status. ASA: aspirin; ASA+HU: aspirin plus hydroxyurea.
Correlation analyses between platelet adhesion and haematological parameters
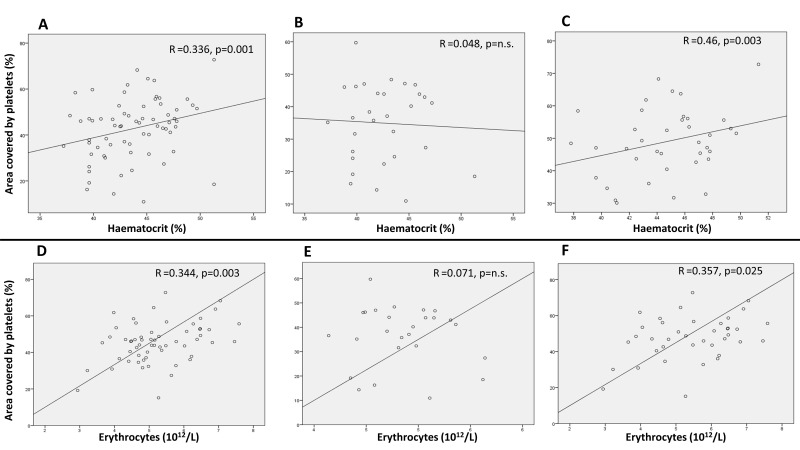
The effects of individual haematological parameters on platelet adhesion were investigated. No correlations were found between platelet adhesion and leucocyte count, either in patients or controls. In the overall study group, platelet adhesion correlated significantly with platelet count and plateletcrit (R=0.422, p=0.0001 and R=0.381, p=0.001, respectively), although these correlations did not remain statistically significant when the patient and control groups were analysed separately. However, in the group of patients, platelet adhesion correlated significantly with both haematocrit levels (R=0.46, p=0.003) (Figure 5C) and red blood cell count (R=0.357, p=0.025) (Figure 5F). These correlations remained statistically significant also after multivariable analysis adjusted for gender, age, HU therapy and JAK2V617F mutational status (R=0.389; p=0.024 for haematocrit; R=0.383; p=0.024 for red blood cell count). Moreover, the percentage of coverage by P-selectin positive platelets correlated significantly with the haematocrit (R=0.463, p=0.001).
Figure 5.
Correlation analyses between platelet adhesion and haematocrit, or between platelet adhesion and RBC count
Panel A, B and C show data of platelet adhesion according to haematocrit in the overall study group (i.e patients+controls) (A), in healthy controls (B), or in PV patients (C). Panel D, E and F show data of platelet adhesion according to RBC count in the overall study group (D), in healthy controls (E), or in PV patients (F). Each panel shows the R of the trend line and the p value of the statistical correlation. n.s.: not significant; PV: polycythaemia vera.
On the basis of the haematocrit target for phlebotomy used in clinics (i.e. 45%), patients were divided into two subgroups: those with a haematocrit below and those with a haematocrit above 45%. Interestingly, patients with a haematocrit >45% showed significantly higher platelet adhesion values than subjects with a haematocrit <45% (51±2.3% vs 44±2.9%; p<0.05): these two subgroups did not differ significantly with regard to platelet count (452±47×109/L vs 598±78×109/L, respectively; p=n.s.).
Discussion
Parallel-plate flow chambers are used to study platelet adhesion and thrombus formation in whole blood at defined shear rates of blood flow13. We implemented one of these systems in our laboratory in Bergamo, combining a parallel-plate flow chamber designed in Maastricht with a brightfield/fluorescent microscope. Fibrillar collagen was used as an adhesive surface15, since it is considered the primary platelet-activating substance in the damaged vessel wall16.
Applying this model to our study population, we observed that platelet adhesion to collagen/thrombus formation is significantly greater for platelets from PV patients than for platelets from controls. Moreover, as illustrated in our sample images, adhesion/thrombus patterns of PV patients were morphologically different from those of controls. In particular, thrombi of patients were more often interconnected forming a continuous network, while thrombi from controls were smaller and isolated.
In our study we also analysed the influence of the JAK2V617F mutation, typical of this disease, on platelet adhesion. Almost all PV patients possess an activating mutation in either exon 12 or 14 of the tyrosine kinase JAK2 gene, with V617F the most common, being present in around 96% of PV patients2. The JAK2V617F mutation plays a key pathogenic role in the onset and progression of PV17. Although clinical data available so far on a positive correlation between JAK2V617F allele burden and level of thrombotic risk are inconclusive, laboratory research shows that a higher mutational load is correlated with higher activation of haemostatic cells, including platelets. In this setting, we recently showed, in a group of patients with myeloproliferative neoplasms (i.e. PV and essential thrombocythaemia), that the JAK2V617F allele burden correlates directly with the platelet-associated thrombin generation potential18. In the present study, the analysis of platelet adhesion according to JAK2V617F mutational status shows that patients with >50% JAK2V617F allele burden have a significantly higher platelet adhesion capacity compared to subjects with <50% JAK2V617F allele burden. Data on the effect of JAK2V617F mutation on platelet adhesion in patients with myeloproliferative neoplasms are very scarce. One study conducted in a mouse model of essential thrombocythaemia19 showed that the presence of the JAK2V617F mutation leads to intrinsic changes in platelet reactivity. Among other findings, the authors, employing the same assay as that used in our study, found that platelet adhesion was increased in the JAK2V617F-mutated mice compared to the non-mutated ones19. We show here that an increased JAK2V617F allele burden further increases the platelet adhesive potential in this kind of diseases.
The pharmacological treatment of PV is currently based on the administration of low-dose ASA, with the addition of a cytoreductive agent (namely HU) in high-risk patients2. In our study, the analysis of platelet adhesion according to pharmacological therapy shows a lower platelet adhesion in patients treated with ASA+HU as compared to that in patients treated with ASA alone. Thus it appears that a possible anti-adhesive effect of HU might be involved. Indeed, platelet adhesive potential is influenced by different agonists that may also act on pathways that are not all blocked by ASA-induced thromboxane inhibition12. Data on the effect of HU on PV platelets are lacking; however, evidence coming from another haematological disorder, namely sickle cell disease, may support this hypothesis. Indeed, HU is widely used in patients with sickle cell disease as the best pharmacological option to date20. Although preliminary, some in vitro evidence indicates that HU can reduce platelet activation and aggregation responses in patients with sickle cell disease21,22.
As postulated by Heemskerk and collaborators23,24, different platelet populations, characterised by different levels of activation and surface composition, may play different roles in the process of coagulation/thrombus formation. We therefore studied two important platelet markers, namely P-selectin and phosphatidylserine. First, we evaluated the expression of P-selectin on adherent platelets as an index of platelet activation. As shown, the expression of this marker was not different between PV patients and healthy controls. Indeed, thrombi formed by platelets derived from either PV patients or healthy controls contained the same relative proportion of P-selectin-positive platelets (about 80%).
Differently, compared to controls, PV patients showed a significantly lower percentage of procoagulant platelets in the thrombi, as measured by phosphatidylserine expression. However, although the relative proportion (percentage) of phosphatidylserine-positive platelets was lower in patients than in controls, the absolute number of phosphatidylserine-positive platelets was not significantly different in the two groups (Figure 1), so they could still provide an efficient coagulation triggering site.
We analysed platelet adhesion data according to patients’ haematological parameters as well. First, a correlation was found between platelet count and platelet adhesion, although this correlation was weak and became statistically significant only when the entire study group (i.e. patients and controls) was considered in the analysis, while it was not significant in either patients or control subgroups when analysed separately. Platelet count is probably not very relevant in determining the degree of platelet adhesion in our model, and the difference of platelet count between patients and controls may not account for the difference observed in thrombus formation potential. In contrast, it appears that haematocrit has a much greater influence on platelet adhesion. Indeed, in our study, we found a statistically significant correlation between haematocrit levels and platelet adhesion. This correlation was significant in the PV group, and almost absent in the healthy control group. After correction for gender, age, therapy and JAK2V617F mutational burden, the haematocrit values were still significantly associated with platelet coverage. There was a similar correlation between platelet adhesion and red blood cell count.
Currently, the recommended haematocrit target value for patients with PV is below 45%2. Indeed, PV patients with a haematocrit above 45% have a higher risk of thrombosis and a higher rate of deaths of cardiovascular origin compared to patients with a haematocrit below this value25. We therefore divided PV patients into two subgroups, one with haematocrit values above 45% and the other with haematocrit values below 45%. Our results show that PV subjects with a haematocrit >45% had significantly higher platelet adhesion compared to subjects with a haematocrit <45%. The association found in our study may well act as a contribution to this phenomenon. In the last decade, some studies showed that the higher the haematocrit, the greater the presence of platelets on the vessel wall. Recently, the effects of haematocrit on platelet adhesion were demonstrated by in vitro and in vivo studies. In one study26, the absence of red blood cells abolished platelet adhesion to collagen under flow conditions, whereas the increase of haematocrit values caused an exponential increase of platelet adhesion. In another study27, in an experimental model, normal mice transfused to obtain an elevated haematocrit had increased thrombus formation and a shorter vessel occlusion time.
Conclusions
PV patients’ whole blood samples showed increased platelet adhesion under flow conditions, with the highest values observed in subjects with >50% JAK2V617F allele burden. However, platelet adhesion is reduced in PV patients under combined treatment with HU and ASA. Among haematological parameters, the patients’ haematocrit and red blood cell count were significant determinants of increased platelet adhesion.
These data support evidence that increased platelet adhesion may participate in the thrombotic diathesis of patients with PV, and that the haematocrit can influence the adhesive properties of platelets.
Footnotes
Funding
This study was supported by grants from the AIRC (Associazione Italiana Ricerca sul Cancro), Milan, Italy (n. IG14505 and n. 12237 AIRC “5xMILLE”).
Authors’ contributions
AV and SG contributed equally to this manuscript as co-first Author. AV, SG, MM, HTC, JWMH and AF helped with the conception and design of the study. AV, SG, PEJVDM, MM, LR, ST, CG, FS, HTC, GF, JWMH and AF provided study material or patients. AV, SG, MM, LR, ST, CG, FS and AF contributed to this manuscript by collecting and/or assembling data. AV, SG and MM analysed and interpreted data. All Authors contributed to writing the manuscript and approved its final version.
The Authors declare no conflicts of interest.
References
- 1.Falanga A, Marchetti M. Thrombosis in myeloproliferative neoplasms. Semin Thromb Hemost. 2014;40:348–58. doi: 10.1055/s-0034-1370794. [DOI] [PubMed] [Google Scholar]
- 2.Tefferi A, Barbui T. Polycythemia vera and essential thrombocythemia: 2019 update on diagnosis, risk-stratification and management. American journal of hematology. 2019;94:133–43. doi: 10.1002/ajh.25303. [DOI] [PubMed] [Google Scholar]
- 3.De Grandis M, Cambot M, Wautier MP, et al. JAK2V617F activate Lu/BCAM-mediated red cell adhesion in polycythemia vera through an EpoR-independent Rap1/Akt pathway. Blood. 2013;121:658–65. doi: 10.1182/blood-2012-07-440487. [DOI] [PubMed] [Google Scholar]
- 4.Guy A, Gourdou-Latyszenok V, Le Lay N, et al. Vascular endothelial cell expression of JAK2(V617F) is sufficient to promote a pro-thrombotic state due to increased P-selectin expression. Haematologica. 2019;104:70–81. doi: 10.3324/haematol.2018.195321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wolach O, Sellar RS, Martinod K, et al. Increased neutrophil extracellular trap formation promotes thrombosis in myeloproliferative neoplasms. Sci Transl Med. 2018;10:eaan8292. doi: 10.1126/scitranslmed.aan8292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Falanga A, Marchetti M, Vignoli A, et al. Leukocyte-platelet interaction in patients with essential thrombocythemia and polycythemia vera. Experimental hematology. 2005;33:523–30. doi: 10.1016/j.exphem.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 7.Patrono C, Rocca B, De Stefano V. Platelet activation and inhibition in polycythemia vera and essential thrombocythemia. Blood. 2013;121:1701–11. doi: 10.1182/blood-2012-10-429134. [DOI] [PubMed] [Google Scholar]
- 8.Frenette PS, Denis CV, Weiss L, et al. P-Selectin glycoprotein ligand 1 (PSGL-1) is expressed on platelets and can mediate platelet-endothelial interactions in vivo. The Journal of experimental medicine. 2000;191:1413–22. doi: 10.1084/jem.191.8.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Evangelista V, Manarini S, Rotondo S, et al. Platelet/polymorphonuclear leukocyte interaction in dynamic conditions: evidence of adhesion cascade and cross talk between P-selectin and the beta 2 integrin CD11b/ CD18. Blood. 1996;88:4183–94. [PubMed] [Google Scholar]
- 10.Dole VS, Bergmeier W, Patten IS, et al. PSGL-1 regulates platelet P-selectin-mediated endothelial activation and shedding of P-selectin from activated platelets. Thromb Haemost. 2007;98:806–12. [PubMed] [Google Scholar]
- 11.Kissova J, Ovesna P, Bulikova A, et al. Increasing procoagulant activity of circulating microparticles in patients with Philadelphia-negative myeloproliferative neoplasms: a single-centre experience. Blood Coagul Fibrinolysis. 2015;26:448–53. doi: 10.1097/MBC.0000000000000293. [DOI] [PubMed] [Google Scholar]
- 12.Panova-Noeva M, Marchetti M, Russo L, et al. ADP-induced platelet aggregation and thrombin generation are increased in essential thrombocythemia and polycythemia vera. Thromb Res. 2013;132:88–93. doi: 10.1016/j.thromres.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 13.Van Kruchten R, Cosemans JM, Heemskerk JW. Measurement of whole blood thrombus formation using parallel-plate flow chambers - a practical guide. Platelets. 2012;23:229–42. doi: 10.3109/09537104.2011.630848. [DOI] [PubMed] [Google Scholar]
- 14.Tefferi A, Thiele J, Orazi A, et al. Proposals and rationale for revision of the World Health Organization diagnostic criteria for polycythemia vera, essential thrombocythemia, and primary myelofibrosis: recommendations from an ad hoc international expert panel. Blood. 2007;110:1092–7. doi: 10.1182/blood-2007-04-083501. [DOI] [PubMed] [Google Scholar]
- 15.Auger JM, Kuijpers MJ, Senis YA, et al. Adhesion of human and mouse platelets to collagen under shear: a unifying model. FASEB J. 2005;19:825–7. doi: 10.1096/fj.04-1940fje. [DOI] [PubMed] [Google Scholar]
- 16.de Witt SM, Swieringa F, Cavill R, et al. Identification of platelet function defects by multi-parameter assessment of thrombus formation. Nat Commun. 2014;5:4257. doi: 10.1038/ncomms5257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vannucchi AM, Guglielmelli P. JAK2 mutation-related disease and thrombosis. Semin Thromb Hemost. 2013;39:496–506. doi: 10.1055/s-0033-1343890. [DOI] [PubMed] [Google Scholar]
- 18.Panova-Noeva M, Marchetti M, Spronk HM, et al. Platelet-induced thrombin generation by the calibrated automated thrombogram assay is increased in patients with essential thrombocythemia and polycythemia vera. Am J Hematol. 2011;86:337–42. doi: 10.1002/ajh.21974. [DOI] [PubMed] [Google Scholar]
- 19.Hobbs CM, Manning H, Bennett C, et al. JAK2V617F leads to intrinsic changes in platelet formation and reactivity in a knock-in mouse model of essential thrombocythemia. Blood. 2013;122:3787–97. doi: 10.1182/blood-2013-06-501452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meier ER. Treatment options for sickle cell disease. Pediatr Clin North Am. 2018;65:427–43. doi: 10.1016/j.pcl.2018.01.005. [DOI] [PubMed] [Google Scholar]
- 21.Proença-Ferreira R, Machado TFGS, Traina F, et al. Hydroxyurea therapy is associated with decreased platelet aggregation responses and activation in sickle cell disease. Blood. 2009;114:2565. [Google Scholar]
- 22.Kim K, Li J, Barazia A, et al. ARQ 092, an orally-available, selective AKT inhibitor, attenuates neutrophil-platelet interactions in sickle cell disease. Haematologica. 2017;102:246–59. doi: 10.3324/haematol.2016.151159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heemskerk JW, Mattheij NJ, Cosemans JM. Platelet-based coagulation: different populations, different functions. J Thromb Haemost. 2013;11:2–16. doi: 10.1111/jth.12045. [DOI] [PubMed] [Google Scholar]
- 24.de Witt SM, Verdoold R, Cosemans JM, et al. Insights into platelet-based control of coagulation. Thromb Res. 2014;133(Suppl 2):S139–48. doi: 10.1016/S0049-3848(14)50024-2. [DOI] [PubMed] [Google Scholar]
- 25.Marchioli R, Finazzi G, Specchia G, et al. Cardiovascular events and intensity of treatment in polycythemia vera. N Engl J Med. 2013;368:22–33. doi: 10.1056/NEJMoa1208500. [DOI] [PubMed] [Google Scholar]
- 26.Spann AP, Campbell JE, Fitzgibbon SR, et al. The effect of hematocrit on platelet adhesion: experiments and simulations. Biophys J. 2016;111:577–88. doi: 10.1016/j.bpj.2016.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Walton BL, Lehmann M, Skorczewski T, et al. Elevated hematocrit enhances platelet accumulation following vascular injury. Blood. 2017;129:2537–46. doi: 10.1182/blood-2016-10-746479. [DOI] [PMC free article] [PubMed] [Google Scholar]