Abstract
Background
The effects of ABO incompatibility on cord blood transplantation (CBT) have not been confirmed. We retrospectively investigated the effect of ABO incompatibility on the clinical outcomes and changes of isoagglutinin titres of 261 consecutive patients who underwent CBT in a single centre.
Material and methods
We studied patients with haematological malignancies undergoing unrelated CBT following myeloablative conditioning. There were 80 matched, 72 major mismatched, 72 minor mismatched, and 37 bidirectional mismatched transplants. Risk factors that could potentially influence the patients’ outcomes were evaluated. Immunoglobulin M (IgM) isohaemagglutinin antibody (IHA) titres were determined 1 day before and 2, 4, 6 and 8 weeks after the transplant.
Results
ABO mismatches did not influence engraftment, transfusion requirements, event-free survival or overall survival following CBT. The anti-donor IgM serum IHA titres fell to ≤1:8 at week 8 after CBT in all patients with ABO major and bidirectional mismatches. The percentages of patients requiring platelet and red blood cell transfusions in the period 31–61 days after CBT were markedly lower than in the period 0–30 days after CBT, being 15 vs 99% for platelets and 23 vs 78% for red blood cells, respectively. Of the 69 recipients of minor mismatched CBT tested, only three with AB blood type developed low titres of anti-recipient IHA after 5 months.
Discussion
In this study ABO incompatibility did not affect clinical outcomes after CBT. A higher number of CD34+ cells infused was correlated with earlier engraftment. Severe acute graft-versus-host disease was associated with poor overall survival. As the IHA titre decreased, so did the number of patients requiring blood transfusion. Rapidly decreasing anti-donor IHA titres and the non-production of donor anti-recipient A and/or B antibodies might contribute to a good outcome of ABO-incompatible CBT with myeloablative conditioning for haematological malignancies.
Keywords: ABO incompatibility, isoagglutinin titres, cord blood transplantation
INTRODUCTION
Allogeneic haematopoietic stem cell transplantation (HSCT) is a curative treatment for a variety of malignant and non-malignant haematological diseases. Human leucocyte antigen (HLA) is a major histocompatibility antigen system that should be taken into consideration in allogeneic HSCT. Since most patients will not have a fully HLA-matched sibling donor, the number of HSCT from alternative donor sources, such as unrelated adults, unrelated cord blood and haploidentical related donors, has increased substantially in recent years and these sources are providing potential donors for almost every candidate HSCT recipient1. The main criterion for unrelated donor selection is HLA compatibility. The patients’ engraftment, clinical outcomes and adverse events following allogeneic HSCT, including graft-versus-host disease (GVHD), non-relapse mortality, and overall survival (OS), are related to the different degrees of HLA incompatibility between donors and recipients2,3. Compared with adult unrelated bone marrow and peripheral blood stem cell grafts, cord blood has the major advantages of rapid availability and markedly reduced stringency of HLA matching. Given the low risks of chronic GVHD and relapse, cord blood transplantation (CBT) is a valid option for patients with haematological diseases, including high-risk malignancies4. In contrast to the situation with adult volunteer donors, in CBT non-HLA factors, including quality of the cord blood units and cell dose, are as critical as HLA matching.
The ABO blood group system is inherited independently of the HLA system, which is coded separately by genes on chromosomes 9 and 65. In contrast to HLA incompatibility, ABO blood group mismatches seem to have less effect on allogeneic HSCT outcomes. Indeed, studies have documented that approximately 25–50% of allogeneic HSCT at present are ABO incompatible6–8. The presence of ABO incompatibility is more common in HLA-mismatched allogeneic HSCT. A large number of studies have identified different complications related to ABO-incompatible transplants, such as pure red cell aplasia9–11, delayed engraftment of red blood cells (RBC), platelets, and neutrophils12,13, delayed haemolysis14, acute GVHD, chronic GVHD, event-free survival (EFS), and overall survival (OS)15–19. ABO antigens are present in multiple tissues as well as erythrocytes. Isohaemagglutinins (IHA) against non-self ABO blood group antigens are produced throughout life, starting shortly after birth. The effect of ABO incompatibility on clinical outcomes following transplantation is most likely due to an immune reaction between ABO blood group antigens and IHA. Therefore, damage to early erythroid precursors, which express ABO blood group antigens, in bone marrow by persistent or recurring recipient-originated IHA could be the reason for the development of pure red cell aplasia or delayed erythroid engraftment and increased transfusion requirements after major ABO-mismatched allogeneic HSCT. Anti-RBC antibodies in passenger lymphocyte syndrome from donors may be the cause of delayed haemolytic complications after minor ABO-mismatched allogeneic HSCT20.
The data on the effect of ABO incompatibility on clinical outcomes are, however, still controversial18–21 because of the heterogeneity of the primary diseases, disease states, conditioning regimens, donor sources, stem cell sources, and other variables. Furthermore, given the limited cell doses in cord blood units, lympho-haematopoietic recovery can be delayed following CBT, as can the production of donor ABO blood group antigens and antibodies. A study by Snell et al. showed that patients undergoing ABO minor-incompatible transplants did not produce donor-derived IHA after CBT22. In this study, we retrospectively investigated transfusion requirements and major clinical outcomes following unrelated CBT with myeloablative conditioning for haematological malignancies, examining the role of ABO incompatibility in particular. The changes in anti-A/B isoagglutinins and their relationship to clinical outcomes were also analysed.
MATERIALS AND METHODS
Patients
From January 2019 to April 2020, 261 consecutive patients with haematological diseases who received unrelated CBT at the First Affiliated Hospital of the University of Science and Technology of China (USTC) were included in this study. HLA typing for patients and unrelated cord blood units was performed by DNA-based methods at high resolution for HLA-A, HLA-B, HLA-C, and HLA-DRB1 loci. Ideally, better than 5/8 HLA loci matching was required and cord blood units against which the recipient had donor-specific antibodies were to be avoided. Of the CBT included in this study, 80 were ABO identical, 72 had major ABO mismatches, 72 had minor ABO mismatches, and 37 had bidirectional ABO incompatibilities. The details are shown in Table I.
Table I.
Characteristics of the population studied
| Variables | Total | Identical | Major ABO incompatible | Minor ABO incompatible | Bidirectional incompatibility | p |
|---|---|---|---|---|---|---|
|
| ||||||
| N. of patients | 261 | 80 | 72 | 72 | 37 | |
|
| ||||||
| Patients’ age [median years (range)] | 15 (1–65) | 14 (1–65) | 15 (1–54) | 17 (2–55) | 14 (2–56) | 0.742 |
|
| ||||||
| Patients’ sex | ||||||
| Male (%) | 150 (57.5) | 47 (58.8) | 41 (56.9) | 40 (55.6) | 22 (59.5) | 0.973 |
| Female (%) | 111 (42.5) | 33 (42.2) | 31 (43.1) | 32 (44.4) | 15 (40.5) | |
|
| ||||||
| Weight of the patients [median kg (range)] | 48 (7–102) | 45 (8.4–84) | 48.5 (7–102) | 51.5 (11–90) | 45 (8–80) | 0.676 |
|
| ||||||
| Donor-recipient sex (%) | ||||||
| Male-male | 74 (28.3) | 28 (35.0) | 21 (28.2) | 18 (25.0) | 7 (18.9) | |
| Female-female | 62 (23.8) | 19 (23.8) | 16 (22.5) | 19 (26.4) | 8 (21.6) | 0.754 |
| Male-female | 49 (18.8) | 14 (17.4) | 15 (21.1) | 13 (18.1) | 7 (18.9) | |
| Female-male | 76 (29.1) | 19 (23.8) | 20 (28.2) | 22 (30.5) | 15 (40.6) | |
|
| ||||||
| HLA match (%) | ||||||
| 4/8 | 2 (7.7) | 0 (0) | 0 (0) | 2 (2.8) | 0 (0) | |
| 5/8 | 104 (39.9) | 31 (38.8) | 26 (36.1) | 30 (41.7) | 17 (46.0) | 0.657 |
| 6/8 | 100 (38.3) | 34 (42.5) | 32 (44.4) | 23 (31.9) | 11 (29.7) | |
| 7/8 | 44 (16.9) | 13 (16.2) | 9 (12.5) | 14 (19.4) | 8 (21.6) | |
| 8/8 | 11 (4.2) | 2 (2.5) | 5 (7.0) | 3 (4.2) | 1 (2.7) | |
|
| ||||||
| Diagnosis (%) | ||||||
| ALL | 85 (32.6) | 32 (40.0) | 27 (37.5) | 15 (20.8) | 11 (29.7) | |
| AML | 137 (52.5) | 41 (51.2) | 35 (48.6) | 40 (55.5) | 21 (56.8) | 0.201 |
| MDS | 26 (10.0) | 4 (5.0) | 6 (8.3) | 12 (16.7) | 4 (10.8) | |
| MPAL | 4 (1.5) | 1 (1.3) | 2 (2.8) | 1 (1.4) | 0 (0) | |
| Other | 9 (3.4) | 2 (2.5) | 2 (2.8) | 4 (5.6) | 1 (2.7) | |
|
| ||||||
| TNC (×107/kg) median (range) | 3.00 (0.11–30.80) | 3.28 (0.51–19.53) | 2.76 (0.11–14.02) | 3.02 (0.18–30.80) | 2.85 (1.35–11.13) | 0.596 |
|
| ||||||
| Infused CD34+ cells (×105/kg), median (range) | 1.94 (0.10–20.91) | 2.04 (0.24–8.76) | 1.83 (0.10–7.15) | 1.93 (0.17–20.91) | 1.83 (0.39–8.09) | 0.863 |
|
| ||||||
| Conditioning regimen (%) | ||||||
| Flu/Bu/Cy | 249 (95.4) | 76 (95.0) | 67 (93.0) | 70 (97.2) | 36 (97.3) | 0.619 |
| Flu/Cy+TBI | 12 (4.6) | 4 (5.0) | 5 (7.0) | 2 (2.8) | 1 (2.7) | |
HLA: human leucocyte antigen; ALL: acute lymphocytic leukaemia; AML: acute myeloid leukaemia: MDS: myelodysplastic syndrome; MPAL, mixed phenotype acute leukaemia; TNC: total nucleated cells; Flu: fludarabine; BU: busulphan; Cy: cyclophosphamide; TBI: total body irradiation.
The study was approved by the Ethics Committee of the First Affiliated Hospital of USTC, and written informed consent was obtained from all subjects in accordance with the principles of the Declaration of Helsinki23.
Haemagglutinin titre monitoring
Serum IgM antibodies to the recipients’ RBC ABO antigens were tested using standard blood-banking procedures. The unrelated CBT patients with major (± minor) ABO incompatibility had anti-A/B titres, which were determined by incubating a 5% suspension of standard type A and B RBC in saline with two-fold serial dilutions of the patients’ serum at room temperature followed by centrifugation at 1,000 × g for 15 s. The titres were scored for agglutination and the last dilution causing macroscopic agglutination by the tube method was recorded. The titres were determined 1 day before the transplant (day −1), as well as 2 (day +14 titre), 4 (day +28 titre), 6 (day +42 titre), and 8 weeks (day +56 titre) after transplantation.
Conditioning and graft-versus-host disease prophylaxis regimens
The conditioning regimens were fludarabine 120 mg/m2 combined with cyclophosphamide at a total dose of 120 mg/kg, and busulphan at a dose of 16 mg/kg in 249 patients, and fluradabine plus cyclophosphamide and total marrow irradiation 12–13.5 Gy in 12 patients, with or without carmustine (BCNU) 250 mg/m2. All patients received cyclosporine A and mycophenolate mofetil for GVHD prophylaxis, as previously described24.
Blood transfusion policy
In the cases of ABO-identical transplants, patients were transfused with the same type of blood. In cases of major ABO incompatibility, patients were transfused with recipient RBC type components and donor type plasma and platelets until isoagglutinins against donor-type ABO antigens had disappeared. In cases of minor incompatibility, donor-type RBC, and recipient-type plasma and platelets were transfused after transplantation until recipient-type RBC disappeared. In the group of patients with bidirectional ABO incompatibility, O-type RBC were transfused until anti-donor antibodies were no longer detectable, or AB-type plasma and platelets were used until recipient-type RBC disappeared. Pre-storage filtration was used to separate RBC from leucocytes and plasma. All transfused packed RBC were washed with 0.9% saline. RBC and platelets were irradiated with 25 Gy before transfusion. RBC were transfused to patients if their haemoglobin levels were <60 g/L or if the patients showed clinical signs of anaemia25. Platelets were transfused to non-bleeding and non-febrile patients at platelet counts <10×109/L26. In our local blood bank, 200 mL of blood were generally collected from blood donors and then separated into blood components. One unit of RBC was derived from 200 mL of whole blood. One unit of platelets was derived from 200 mL of whole blood and contained ≥2.5×1010 platelets. One unit of apheresis platelets was collected from one healthy donor and contained ≥2.5×1011 platelets, so one unit of apheresis platelets was equivalent to ten units of platelets.
Definitions
ABO-identical transplants were defined as those between recipients and donors who had the same ABO blood type. Major ABO incompatibility was defined as the recipient having isohaemagglutinins directed against the donor’s RBC antigens. Minor ABO incompatibility was defined as serological evidence of the donor having isohaemagglutinins directed against the recipient’s RBC antigens, while bidirectional incompatibility was defined as isohaemagglutinins against both recipient and donor RBC antigens. Erythrocyte engraftment was defined as reticulocytes ≥1% on 3 consecutive days, while platelet engraftment was determined by a platelet count ≥20×109/L on 3 consecutive days without any platelet transfusion support for more than 7 days. Neutrophil engraftment was considered to have occurred when the absolute neutrophil count was ≥0.5×109/L for 3 consecutive days17. EFS was defined as the time after CBT without relapse or death during the follow-up. OS was determined as the time from CBT to death from any cause, and surviving patients were censored at the date of last follow-up. Acute GVHD was classified and graded according to international criteria27,28.
Statistical analysis
Analyses were performed using SPSS version 20.0 (IBM Corp., Armonk, NY, USA). The cumulative incidences of neutrophil, platelet, and RBC engraftment were evaluated by competing risks, with relapse and death being considered as competing events. The recipients’ packs of blood transfused were compared across groups using analysis of variance (ANOVA) and Kruskal-Wallis tests. In the multivariable analysis, a logistic regression model was used to estimate the risk factors for engraftment and transfusion requirements after CBT, through calculation of the odds ratios (OR) with 95% confidence intervals (95% CI). Factors that significantly affected EFS and OS were assessed by multivariate Cox proportional hazard ratios (HR) with 95% confidence intervals. The outcomes in relation to ABO incompatibility and isoagglutinins were analysed using GraphPad Prism 8 software (GraphPad Software, Inc. San Diego, CA, USA). Statistical significance was defined as P<0.05.
RESULTS
Patients and cord blood characteristics
A total of 261 patients with haematological malignancies were included in this research. Based on ABO compatibility between donors and recipients, there were four groups of transplants: identical (n=80, 30.7%), those with major incompatibility (n=72, 27.6%), those with minor incompatibility (n=72, 27.6%), and those with bidirectional incompatibility (n=37, 14.1%). The study population consisted of 150 (57.5%) males and 111 (42.5%) females with a median weight of 48 kg (range, 7–102). The median age of patients at the time of CBT was 15 years (range, 1–65 years). The median number of total nucleated cells infused was 3.00×107/kg (range, 0.11–30.8×107/kg). The median number of CD34+ cells infused was 1.94×105/kg (range, 0.10–20.91×105/kg). The donor-recipient sex distribution was male-male in 74 (28.3%) cases, female-female in 62 (23.8%), male-female in 49 (18.8%), and female-male in 76 (29.1%) cases. The underlying diagnosis was acute lymphoblastic leukaemia in 85 (32.6%) cases, acute myeloid leukaemia in 137 (52.5%), myelodysplastic syndrome in 26 (10.0%), mixed phenotype acute leukaemia in four (1.5%), and various other haematological diseases in nine (3.4%) cases. There were no statistically significant differences between the aggregated patients’ data and those of the individual ABO incompatibility groups (identical, major, minor and bidirectional). The basic characteristics of the patients and cord blood units are shown in Table I.
Engraftment and transfusions
Neutrophil engraftment
Of the 261 consecutive patients with haematological malignancies who received CBT, three patients died of pre-engraftment syndrome and infection before neutrophil recovery, and nine patients developed primary graft failure. Overall, 249 patients successfully achieved neutrophil engraftment at a median of 17 (range, 10 to 42) days after CBT. The cumulative incidences of neutrophil engraftment at 42 days after transplantion were 94% (95% CI: 88–100%), 97% (95% CI: 93–100%), 96% (95% CI: 92–100%), and 95% (95% CI: 82–100%) in the ABO-identical, major, minor, and bidirectional ABO incompatibility groups, respectively (Table II and Online Supplementary Content, Figure S1). The median time that neutrophil engraftment occurred in the four groups was 18 (range, 11–42), 16 (range, 11–39), 16 (range, 10–28), and 16 (range, 11–30) days, respectively, after CBT. There were no statistically significant differences in neutrophil engraftment among the four groups (p=0.489). Likewise, when we combined the identical/minor-incompatible groups and compared this group with the combination of the major/bidirectional ABO-incompatible groups, neutrophil engraftment in the two groups was not statistically different(median days, 17 vs 16, p=0.455) (Online Supplementary Content, Figure S1). In the multivariable analysis of risk factors for neutrophil engraftment after transplantation, the results showed that a higher number of CD34+ cells infused was associated with a higher rate of neutrophil engraftment at 42 days (OR=0.391, 95% CI=0.214–0.715, p=0.002) (Table III). In this research, the absolute count of CD34+ cells referred to that at the time of processing the cord blood unit. The number of total nucleated cells infused did not influence neutrophil engraftment at 42 days (OR=1.442, 95% CI=0.784–2.653, p=0.239).
Table II.
Engraftment and transfusion data according to group of ABO compatibility
| Identical | Major incompatibility | Minor incompatibility | Bidirectional incompatibility | |
|---|---|---|---|---|
|
| ||||
| Engraftment (n) | 80 | 72 | 72 | 37 |
|
| ||||
| Neutrophils | ||||
| Cumulative incidence (%) | 94 | 97 | 96 | 95 |
| 95% CI | 88–100 | 93–100 | 92–100 | 82–100 |
| Median days (range) | 18 (11–42) | 16 (11–39) | 16 (10–28) | 16 (11–30) |
|
| ||||
| Platelets | ||||
| Cumulative incidence (%) | 89 | 94 | 89 | 89 |
| 95% CI | 83–95 | 88–100 | 81–97 | 79–99 |
| Median days (range) | 36 (13–126) | 32 (9–108) | 34 (11–211) | 34 (16–137) |
|
| ||||
| Red blood cells | ||||
| Cumulative incidence (%) | 93 | 97 | 94 | 95 |
| 95% CI | 87–99 | 93–100 | 88–100 | 82–100 |
| Median days (range) | 20 (11–110) | 20 (12–76) | 19 (0–55) | 20 (0–82) |
|
| ||||
| Transfusion requirements in 60 days | ||||
|
| ||||
| Red blood cells | ||||
| Median (units/kg) | 0.080 | 0.070 | 0.089 | 0.069 |
| Range | 0–2.200 | 0–0.714 | 0–0.833 | 0–0.673 |
|
| ||||
| Platelets | ||||
| Median (units/kg) | 1.67 | 1.30 | 1.15 | 1.36 |
| Range | 0–16.20 | 0.22–5.71 | 0–4.89 | 0.20–4.00 |
95% CI: 95% confidence interval.
Table III.
Multivariable analyses of risk factors for engraftment after cord blood transplantation
| Variables | Neutrophil engraftment at 42 days | Platelet engraftment at 90 days | RBC engraftment at 90 days | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| OR | 95% CI | p | OR | 95% CI | p | OR | 95% CI | p | |
|
| |||||||||
| ABO match | |||||||||
| Identical/minor incompatibility | 1 | 1 | 1 | ||||||
| Major/bidirectional incompatibility | 0.665 | 0.395–1.119 | 0.125 | 0.721 | 0.421–1.233 | 0.232 | 1.010 | 0.591–1.728 | 0.970 |
|
| |||||||||
| Grade of acute GVHD | |||||||||
| 0–II | 1 | 1 | 1 | ||||||
| III–IV | 1.113 | 0.629–1.969 | 0.713 | 1.630 | 0.899–2.956 | 0.107 | 0.734 | 0.406–1.328 | 0.307 |
|
| |||||||||
| HLA match | |||||||||
| 4–5/8 | 1 | 1 | 1 | ||||||
| 6–8/8 | 0.961 | 0.569–1.625 | 0.883 | 0.751 | 0.435–1.297 | 0.304 | 0.612 | 0.357–1.052 | 0.075 |
|
| |||||||||
| CD34+ cells infused | |||||||||
| <median 1.94×105/kg | 1 | 1 | 1 | ||||||
| ≥median 1.94×105/kg | 0.391 | 0.214–0.715 | 0.002 | 0.262 | 0.137–0.501 | <0.001 | 0.381 | 0.209–0.697 | 0.002 |
|
| |||||||||
| TNC infused | |||||||||
| <median 3.00×107/kg | 1 | 1 | 1 | ||||||
| ≥median 3.00×107/kg | 1.442 | 0.784–2.653 | 0.239 | 2.088 | 1.084–4.020 | 0.028 | 0.727 | 0.397–1.331 | 0.302 |
|
| |||||||||
| Diseases | |||||||||
| ALL | 1 | 1 | 1 | ||||||
| AML | 0.847 | 0.481–1.491 | 0.564 | 0.540 | 0.300–0.973 | 0.040 | 1.014 | 0.569–1.806 | 0.962 |
| Other | 1.623 | 0.705–3.733 | 0.255 | 0.892 | 0.376–2.115 | 0.795 | 2.884 | 1.170–7.110 | 0.021 |
OR: odds ratio; 95% CI: 95% confidence interval; GVHD: graft-versus-host disease; HLA: human leucocyte antigen; TNC: total nucleated cells; ALL: acute lymphocytic leukaemia; AML: acute myeloid leukaemia.
Platelet engraftment and transfusion requirements
Among the 249 patients who achieved neutrophil engraftment, one died of an infection, and two died of GVHD before platelet engraftment. Overall, 246 patients successfully achieved platelet engraftment, at a median of 34 (range, 9 to 211) days after CBT. The cumulative incidences of platelet engraftment in the identical, major, minor, and bidirectional groups at 90 days were 89% (95% CI: 83–95%), 94% (95% CI: 88–100%), 89% (95% CI: 81–97%), and 89% (95% CI: 79–99%), respectively (Table II and Online Supplementary Content, Figure S2). The median time of platelet engraftment in the four groups was 36 (range, 13–126), 32 (range, 9–108), 34 (range, 11–211), and 34 (range, 16–137) days after CBT. There were no significant differences in platelet engraftment when comparing the four groups or when comparing the groups combined into two groups, as for the neutrophil engraftment analysis described above (p=0.663 and p=0.185, respectively). When the results were adjusted for patients’ body weight, the median units of platelets transfused in the first 60 days after transplant were 1.67, 1.30, 1.15, and 1.36 U/kg, respectively, for patients with ABO-identical, major, minor, and bidirectional ABO-incompatible transplants (Table II). The transfusion requirements were not statistically significantly different in the major/bidirectional incompatibility groups when compared to the ABO identical/minor incompatibility groups (p=0.188). There were also no statistically significant differences when we analysed the total platelet transfusion requirements in the 60 days after CBT (Online Supplementary Content, Table SII). Multivariable analyses of platelet engraftment showed that a higher dosage of CD34+ cells infused was associated with a higher rate of platelet engraftment at day 90 after CBT (≥median 1.94×105/kg, OR=0.262, 95% CI: 0.137–0.501, p<0.001) but did not influence transfusion requirements (Tables III and IV). Infusion of a higher number of total nucleated cells was beneficial with regard to platelet engraftment after transplantation (≥median 3.00×107/kg, OR=0.479, 95% CI: 0.249–0.922, p=0.028). Severe acute GVHD was not associated with platelet transfusion requirements (OR=1.272, p=0.424).
Table IV.
Multivariable analyses of risk factors for platelet and red blood cell transfusion requirements in 60 days after cord blood transplantation
| Variables | Platelet transfusion requirements | RBC transfusion requirements | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| OR | 95% CI | p | OR | 95% CI | p | |
|
| ||||||
| ABO match | ||||||
| Identical/minor incompatibility | 1 | 1 | ||||
| Major/bidirectional incompatibility | 0.643 | 0.379–1.091 | 0.102 | 0.612 | 0.354–1.058 | 0.079 |
|
| ||||||
| Age (years) | ||||||
| <18 | 1 | 1 | ||||
| ≥18 | 1.254 | 0.669–2.352 | 0.481 | 1.305 | 0.684–2.488 | 0.419 |
|
| ||||||
| HLA match | ||||||
| 4–5/8 | 1 | 1 | ||||
| 6–8/8 | 0.914 | 0.530–1.578 | 0.747 | 0.610 | 0.349–1.069 | 0.084 |
|
| ||||||
| Grade of acute GVHD | ||||||
| 0–II | 1 | 1 | ||||
| III–IV | 1.272 | 0.705–2.293 | 0.424 | 2.699 | 1.463–4.977 | 0.001 |
|
| ||||||
| CD34+ cells infused | ||||||
| <median 1.94×105/kg | 1 | 1 | ||||
| ≥median 1.94×105/kg | 0.560 | 0.308–1.018 | 0.057 | 0.386 | 0.207–0.719 | 0.003 |
|
| ||||||
| TNC infused | ||||||
| <median 3.00×107/kg | 1 | 1 | ||||
| ≥median 3.00×107/kg | 0.837 | 0.425–1.649 | 0.607 | 1.328 | 0.653–2.702 | 0.434 |
|
| ||||||
| Diseases | ||||||
| ALL | 1 | 1 | ||||
| AML | 0.795 | 0.448–1.409 | 0.431 | 0.711 | 0.394–1.283 | 0.257 |
| Other | 1.429 | 0.583–3.501 | 0.435 | 1.914 | 0.787–4.653 | 0.152 |
RBC: red blood cells; OR: odds ratio; 95% CI: 95% confidence interval; HLA: human leucocyte antigen; GVHD: graft-versus-host disease; TNC: total nucleated cells; ALL: acute lymphocytic leukaemia; AML: acute myeloid leukaemia.
Red blood cell engraftment and transfusion requirements
Among the 249 patients who achieved neutrophil engraftment, one patient died before RBC engraftment. Overall, 248 patients achieved erythrocyte engraftment, at a median of 20 (range, 0–110) days after transplantation. The cumulative incidences of RBC engraftment at 90 days were 93% (95% CI: 87–99%) in the ABO identical group, 97% (95% CI: 93–100%) in those undergoing major ABO-incompatible CBT, 94% (95% CI: 88–100%) in those undergoing minor ABO-incompatible CBT, and 95% (95% CI: 82–100%) in those undergoing bidirectional ABO-incompatible CBT (Table II and Online Supplementary Content, Figure S3). The median time of RBC engraftment in the identical, major, minor, and bidirectional transplant groups was 20 (range, 11–110), 20 (range, 12–76), 19 (range, 0–55), and 20 (range, 0–82) days after CBT, respectively. There were no significant differences in RBC engraftment among the four groups (p=0.854). RBC engraftment in patients undergoing ABO-identical/minor-incompatible transplants was not significantly different from that in patients undergoing major/bidirectional ABO-incompatible transplants (median days, 20 vs 20, p=0.854) (Online Supplementary Content, Table SI). ABO mismatch did not influence transfusion requirements when adjusted for the patients’ weight and total amount of RBC units transfused after the transplant (Table II and III and Online Supplementary Content, Table SII). CD34+ cell dosage was an advantageous factor for RBC engraftment in the multivariable analyses (≥median 1.94×105/kg, OR=0.381, 95% CI: 0.209–0.697, p<0.002). Severe acute GVHD was associated with higher RBC transfusion requirements (grade III–IV acute GVHD, OR=2.699, 95% CI: 1.463–4.977, p=0.001) (Table IV). No patients in this study developed pure red cell aplasia or haemolytic reactions after CBT.
Event-free survival and overall survival
Two hundred and sixty-one patients were evaluated for EFS and OS. Twenty-eight (10.7%) patients had died from transplant-related diseases by the 1-year follow-up; 21 (8.0%) patients died from relapse. In the multivariable risk analyses, ABO major/bidirectional incompatibility was not significantly associated with EFS (HR=0.682, p=0.110) or OS (HR=0.538, p=0.059) when compared to the ABO identical/minor-incompatible group. The cumulative proportions of EFS among patients undergoing identical/minor-incompatible and major/bidirectional-incompatible CBT were 0.678 and 0.780, respectively. The corresponding cumulative proportions for OS were 0.789 and 0.881, respectively (Online Supplementary Content, Figure S4). Seventy-five (28.7%) patients were diagnosed with grade III–IV acute GVHD. In the multivariable analyses, grade III–IV acute GVHD was significantly associated with poor OS (HR=2.793, p=0.001) but not with EFS (HR=1.407, p=0.170) when compared to grade 0–II acute GVHD. The results showed that HLA match, patient’s age, donor-recipient sex, and haematological disease did not have statistically significant effects on 1-year EFS and OS rates. The hazard ratios and 95% CI of the multivariate Cox regression model for the influences on EFS and OS are shown in Table V.
Table V.
Multivariable analyses of risk factors influencing 1-year event-free survival and overall survival after cord blood transplantation
| Factors | EFS | OS | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| HR | 95% CI | p | HR | 95% CI | p | |
|
| ||||||
| ABO match | ||||||
| Identical/minor incompatibility | 1 | 1 | ||||
| Major/bidirectional incompatibility | 0.682 | 0.427–1.091 | 0.110 | 0.538 | 0.282–1.024 | 0.059 |
|
| ||||||
| HLA | ||||||
| 6–8/8 match | 1 | 1 | ||||
| 4–5/8 match | 1.034 | 0.648–1.650 | 0.899 | 1.039 | 0.564–1.915 | 0.902 |
|
| ||||||
| Age | ||||||
| <18 years | 1 | 1 | ||||
| ≥18 years | 1.208 | 0.748–1.952 | 0.440 | 1.501 | 0.799–2.821 | 0.207 |
|
| ||||||
| Donor-recipient sex | ||||||
| Female-male | 1 | 1 | ||||
| Others | 0.869 | 0.535–1.409 | 0.568 | 0.754 | 0.403–1.411 | 0.377 |
|
| ||||||
| Patients’ diseases | ||||||
| ALL | 1 | 1 | ||||
| AML | 0.648 | 0.392–1.070 | 0.090 | 0.720 | 0.361–1.435 | 0.350 |
| Other | 0.970 | 0.488–1.929 | 0.931 | 2.093 | 0.929–4.712 | 0.075 |
|
| ||||||
| Acute GVHD | ||||||
| Grade 0–II | 1 | 1 | ||||
| Grade III–IV | 1.407 | 0.864–2.294 | 0.170 | 2.793 | 1.526–5.110 | 0.001 |
|
| ||||||
| CD34+ cells infused | ||||||
| <median 1.94×105/kg | 1 | 1 | ||||
| ≥median 1.94×105/kg | 0.704 | 0.440–1.126 | 0.143 | 0.686 | 0.368–1.280 | 0.236 |
EFS: event-free survival; OS: overall survival; HR: hazard ratio; 95% CI: 95% confidence interval; HLA: human leucocyte antigen; ALL: acute lymphocytic leukaemia; AML: acute myeloid leukaemia; GVHD: graft-versus-host disease.
Change in isoagglutinin titres
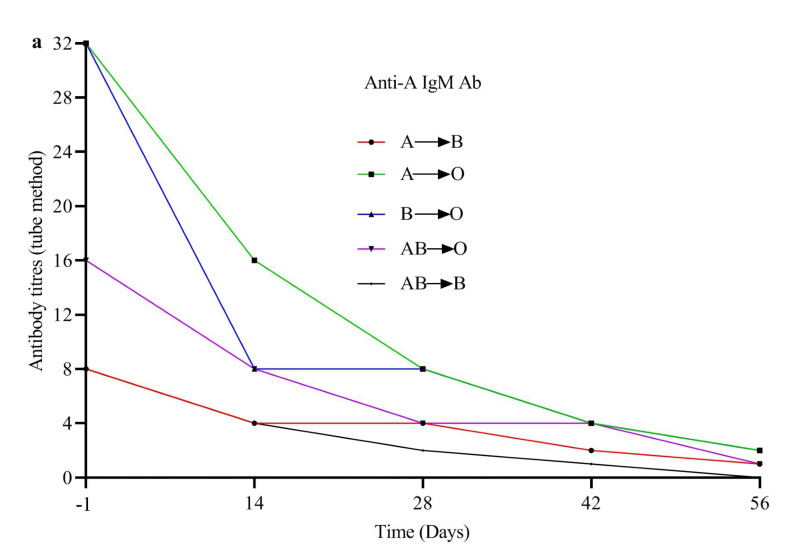
A total of 104 patients, comprising 70 patients with major ABO mismatches and 34 patients with bidirectional mismatches, were evaluated for recipient IgM-type antibody anti-donor RBC. Antibody titres were tested 1 day before the transplant (day −1 titre), as well as 2 (+14 days), 4 (+28 days), 6 (+42 days) and 8 weeks (+56 days) after transplantation. Based on the patient’s blood type, tube IgM titres were determined with A1 and B target reagent cells. Overall, the anti-donor titre was ≤1:8 at day −1 before the transplant and at 2, 4, 6 and 8 weeks after the CBT in 47%, 50%, 77%, 93%, and 100% of the patients, respectively (Figure 1 and Online Supplementary Content, Table SIII).
Figure 1.
Levels of anti-donor isoagglutinins from day −1 before transplantation to day +56 after transplantation divided on the basis of ABO matching
(a) Anti-A IgM antibody titres. (b) Anti-B IgM antibody titres.
Compared to the group with titres ≥1:16, patients in the groups undergoing major or bidirectional-incompatible ABO CBT with titres ≤1:8 at day −1 did not have statistically significant differences in RBC transfusion requirements during 30 and 60 days after the transplant (for 30 days, 0.068 ± 0.067 vs 0.086 ± 0.086, p=0.240; for 60 days, 0.101 ± 0.140 vs 0.112 ± 0.138, p=0.684). Platelet transfusion requirements were also not significantly different (for 30 days, 1.430 ± 0.917 vs 1.559 ± 1.140, p=0.531; for 60 days, 1.562 ± 1.112 vs 1.618 ± 1.231, p=0.810). Compared with patients with titres ≤1:8, patients with day −1 titres ≥1:16 did not have statistically significant differences in engraftment (neutrophils p=0.195; platelets p=0.691; and RBC p=0.237). Sixty-nine patients who underwent ABO minor mismatched CBT were evaluated for recipient IgM-type antibodies. At day 56 after CBT, none of the patients had developed antibodies against the recipient’s blood group. After prolonged follow-up, only three of these 69 patients developed donor-derived anti-recipient ABO antibodies. No patients had haemolytic reactions. Two AB-type patients were transplanted from B-type donors.
These two patients produced anti-A titres 260 days after CBT (anti-A IgM=8, grade I skin acute GVHD at +14 days, alive) and 358 days after CBT (anti-A IgM=1, grade IV intestinal acute GVHD at +38 days, alive). The third patient, an AB-type recipient, received a graft from an A-type donor and produced anti-B titres at 155 days after CBT (anti-B IgM=1, no acute GVHD, alive). There were no significant differences in acute GVHD between minor ABO-mismatched patients and patients with major/bidirectional ABO incompatibility (HR=1.001, 95% CI: 0.586–1.712, p=0.996). The patients’ blood transfusion requirements decreased together with the decline in isoagglutinin titres after CBT. Two hundred and forty-three patients required platelet transfusions in the first 30 days after their transplant, whereas only 36 patients required platelet transfusions between 31 and 60 days after CBT (99 vs 15%). The number of patients requiring RBC transfusions was likewise lower in the later period: 193 (78%) up to day 30 vs 56 (23%) from day 31 to day 60.
DISCUSSION
Unrelated CBT has become a standard therapy for patients with high-risk haematological malignancies because of its rapid availability as well as the lower risk of GVHD and relapse. However, limited numbers of haematopoietic stem and progenitor cells in cord blood units delay haematological and immunological recovery and result in suboptimal engraftment after CBT. The selection of optimal cord blood grafts can reduce early post-transplantation morbidity and transplantation-related mortality and improve CBT outcomes. The minimum cell dose is 2.5–3.0×107 total nucleated cells per kilogram of the patient’s body weight and incorporation of CD34+ cell dose is now considered standard practice in the selection of cord blood units. In addition, despite the less stringent HLA matching requirement for CBT than for unrelated adult donor transplantation, the importance of the number of matches for HLA-A, HLA-B, HLA-C, and HLA-DRB1 at the high-resolution level is being increasingly recognised29. Although the CD34+ cell count is a critical factor in selection of the optimal unit, an adequate CD34+ cell dose has remained undefined because the CD34+ cell dose must be considered in unit selection along with the total nucleated cell dose. A study by Wagner et al.30, in 2002, showed that the survival rate was improved when more than 1.7×105 CD34+ cells/kg were infused in 4–6/6 HLA-matched single-unit CBT recipients. This study included a total of 261 patients with malignant haematological diseases who received a CBT following myeloablative conditioning in a single institution. Generally, the total number of nucleated cells and the number of CD34+ cells in the graft are considered important determinants of patients’ recovery after CBT. The multivariate analysis showed that a higher number of total nucleated cells was correlated with earlier platelet engraftment but did not affect other clinical outcomes. The infusion of higher numbers of CD34+ cells correlated with earlier engraftment and lower transfusion requirements, but did not influence EFS or OS. For ABO-incompatible grafts, we do not evaluate the total amount of RBC infused. We do not remove the incompatible RBC before infusion because of the potential destruction of nucleated cells. In our transplant practice we have not found that residual incompatible RBC influence the clinical outcomes. No adverse effects on clinical outcomes were found in the analysis of HLA mismatches in our study. Given that we adopted high-resolution HLA matching and the vast majority of our donor-recipient pairs had better than 5/8 matching, it is possible that an impact of extreme HLA incompatibility on survival was avoided.
There is no doubt that cell dose and HLA match are of prime importance in the selection of cord blood grafts. Since the ABO blood group system is inherited independently of the HLA system, more than 50% of CBT are performed with ABO incompatible donors. In our study, the percentage of blood incompatibilities was as high as 69%. ABO antigens consist of oligosaccharide glycoproteins that are found on the surface of RBC, neutrophils, platelets, vascular endothelial cells and epithelial cells31. ABO-type isohaemogglutinins could target these antigens and mediate immune-haematological complications in ABO-mismatched allogeneic HSCT. ABO compatibility has been considered in the clinical algorithm for haploidentical donor selection32. Hefazi et al.12 showed that major and bidirectional incompatibilities were associated with later RBC engraftment and higher RBC transfusion requirements after HSCT and some other studies made similar findings14,19,33. An individual patient-based meta-analysis of cohort studies revealed that patients with minor ABO mismatching had delayed platelet engraftment compared to the ABO-compatible group34. Kimura and colleauges8 retrospectively analysed 5,549 Japanese patients who underwent bone marrow transplantation. They found that major ABO incompatibility was associated with delayed platelet and neutrophil recovery. However Solves et al.35, in a review of 318 cases from 2000 to 2014 who underwent umbilical CBT in a single institution (La Fe University) did not find a correlation between ABO incompatibility and blood transfusions. Yan-Ru and colleagues17 reported no influence of ABO incompatibility on reticulocyte, platelet or neutrophil engraftment. Some other studies were in line with this research18–36. In our retrospective study, there were no significant differences between ABO-matched and –mismatched patients in terms of baseline disease and clinical characteristics after CBT with myeloablative conditioning for haematological malignancies. The results showed that ABO incompatibility did not influence engraftment, transfusion requirements or OS.
Theoretically, immunohaematological problems may develop from any type of ABO-incompatible allogeneic HSCT. The pathogenic mechanisms underlying the conflicting clinical outcomes may involve the difference in ABO blood group antigens and IHA immune response. Different graft sources, diseases, and transplantation procedures influence the production of donor ABO antigens and anti-recipient IHA and the disappearance of anti-donor IHA after allogeneic HSCT. Persistent high host-IHA titres against donor A and/or B antigens after allogeneic HSCT may be responsible for increased transfusion requirements, pure red cell aplasia, and delayed engraftment. We examined the titres of antibody in the groups with major and bidirectional ABO incompatibility and found that the antibody titre of the recipient against the donor was lower than 1:8 at day +56 in 100% of cases. The low level of antibodies in the recipients may have been the reason for protection against haematopoietic suppression in our major ABO-mismatched CBT population. Pre-transplant chemotherapy and depletion of antibody-producing cells by the myeloablative conditioning may contribute to rapid antibody reduction.
Minor or bidirectional ABO incompatibility has been associated with an increased incidence of passenger lymphocyte syndrome and GVHD among patients receiving an allogeneic bone marrow or peripheral blood stem cell transplant. However, it has been rarely reported in CBT37. Donor anti-host IHA produced after minor or bidirectional ABO-mismatched allogeneic HSCT can attack host endothelial cells expressing A or B antigens, triggering GVHD. We found no cases of passenger lymphocyte syndrome within 1 month after CBT, and no significant differences were observed between the ABO-matched and ABO-mismatched groups in acute GVHD by multivariate analysis. No anti-host IHA was detected in our study at day 56. In addition, only three patients produced low titres of donor-against-recipient ABO-type antibody in the minor mismatch group after extended follow-up. The presence of naive T and B cells in the cord blood graft and higher frequencies and absolute numbers of regulatory B-cell reconstitution after CBT have been correlated, and no donor-derived IHA against recipients can be produced after minor and bidirectional mismatched CBT38. Severe acute GVHD leads to prolonged thrombocytopenia, gastrointestinal bleeding, increased transfusion requirements, and reduced OS39,40.
Our study had a few shortcomings. The first limitation of this study was that it was a single-centre, retrospective, cohort study. Second, we tested IHA titres but did not identify B-cell reconstitution, which produced IHA. Third, the follow-up period was short. Therefore, a study conducted in multiple centres with a large number of samples, a longer follow-up and with monitoring of B-cell-producing blood group antibodies would help to provide more accurate clinical data.
CONCLUSIONS
In summary, our data suggest that donor-recipient ABO incompatibility does not have a significant impact on transfusion requirements and clinical outcome after myeloablative CBT for haematological malignancies. Rapidly decreasing anti-donor IHA and the non-production of donor anti-recipient A and/or B antibodies might contribute to the good outcome of ABO-incompatible CBT. When there is a choice among several cord blood units, ABO compatibility does not need to be considered as a graft selection criterion.
Supplementary Information
Footnotes
FUNDING
This work was supported by the programme of the Key Research and Development Plan in Anhui Province (grants 1704a0802149) and Fundamental Research Funds for the Central Universities (grants WK9110000001).
AUTHORSHIP CONTRIBUTIONS
YC conceived the main idea of the study, analysed the data and wrote the manuscript. XW, YC, HW, DH and YZ collected the data and performed the statistical analysis. WY, KS, QF, XZ and ZS provided the patients’ transplant data. HL critically revised the data, tables, figures and the manuscript. All the Authors approved the final version of the manuscript and take responsibility for the integrity and accuracy of the data.
The Authors declare that they have no conflicts of interest.
REFERENCES
- 1.Deteix C, Mesnil F, Furst S, et al. Influence of alternative donor type on early survival after hematopoietic stem cell transplantation for acute myeloid leukemia lacking a sibling donor. Bone Marrow Transplant. 2020;55:749–57. doi: 10.1038/s41409-019-0722-y. [DOI] [PubMed] [Google Scholar]
- 2.Booth GS, Gehrie EA, Bolan CD, Savani BN. Clinical guide to ABO-incompatible allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2013;19:1152–8. doi: 10.1016/j.bbmt.2013.03.018. [DOI] [PubMed] [Google Scholar]
- 3.Graczyk-Pol E, Rogatko-Koros M, Nestorowicz K, et al. Role of donor HLA class I mismatch, KIR-ligand mismatch and HLA:KIR pairings in hematological malignancy relapse after unrelated hematopoietic stem cell transplantation. HLA. 2018;92(Suppl 2):42–6. doi: 10.1111/tan.13386. [DOI] [PubMed] [Google Scholar]
- 4.Barker JN, Devlin SM, Naputo KA, et al. High progression-free survival after intermediate intensity double unit cord blood transplantation in adults. Blood Adv. 2020;4:6064–76. doi: 10.1182/bloodadvances.2020003371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shiina T, Hosomichi K, Inoko H, Kulski JK. The HLA genomic loci map: expression, interaction, diversity and disease. J Hum Genet. 2009;54:15–39. doi: 10.1038/jhg.2008.5. [DOI] [PubMed] [Google Scholar]
- 6.Canaani J, Savani BN, Labopin M, et al. ABO incompatibility in mismatched unrelated donor allogeneic hematopoietic cell transplantation for acute myeloid leukemia: a report from the acute leukemia working party of the EBMT. Am J Hematol. 2017;92:789–96. doi: 10.1002/ajh.24771. [DOI] [PubMed] [Google Scholar]
- 7.Akkok CA, Seghatchian J. Immunohematologic issues in ABO-incompatible allogeneic hematopoietic stem cell transplantation. Transfus Apher Sci. 2018;57:812–5. doi: 10.1016/j.transci.2018.10.020. [DOI] [PubMed] [Google Scholar]
- 8.Kimura F, Sato K, Kobayashi S, et al. Impact of AB0-blood group incompatibility on the outcome of recipients of bone marrow transplants from unrelated donors in the Japan Marrow Donor Program. Haematologica. 2008;93:1686–93. doi: 10.3324/haematol.12933. [DOI] [PubMed] [Google Scholar]
- 9.Tomac G, Bojanic I, Mazic S, et al. Haemolysis, pure red cell aplasia and red cell antibody formation associated with major and bidirectional ABO incompatible haematopoietic stem cell transplantation. Blood Transfus. 2018;16:397–404. doi: 10.2450/2017.0322-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aung FM, Lichtiger B, Rondon G, et al. Pure red cell aplasia in major ABO-mismatched allogeneic hematopoietic stem cell transplantation is associated with severe pancytopenia. Biol Blood Marrow Transplant. 2016;22:961–5. doi: 10.1016/j.bbmt.2016.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sackett K, Cohn CS, Fahey-Ahrndt K, et al. Successful treatment of pure red cell aplasia because of ABO major mismatched stem cell transplant. J Clin Apher. 2018;33:108–12. doi: 10.1002/jca.21553. [DOI] [PubMed] [Google Scholar]
- 12.Hefazi M, Litzow M, Hogan W, et al. ABO blood group incompatibility as an adverse risk factor for outcomes in patients with myelodysplastic syndromes and acute myeloid leukemia undergoing HLA-matched peripheral blood hematopoietic cell transplantation after reduced-intensity conditioning. Transfusion. 2016;56:518–27. doi: 10.1111/trf.13353. [DOI] [PubMed] [Google Scholar]
- 13.Vaezi M, Oulad DD, Souri M, et al. ABO incompatibility and hematopoietic stem cell transplantation outcomes. Int J Hematol Oncol Stem Cell Res. 2017;11:139–47. [PMC free article] [PubMed] [Google Scholar]
- 14.Badros A, Tricot G, Toor A, et al. ABO mismatch may affect engraftment in multiple myeloma patients receiving nonmyeloablative conditioning. Transfusion. 2002;42:205–9. doi: 10.1046/j.1537-2995.2002.00027.x. [DOI] [PubMed] [Google Scholar]
- 15.Zaimoku Y, Takami A, Sato H, et al. IgM anti-recipient ABO antibodies predict acute graft-versus-host disease following allogeneic hematopoietic stem cell transplantation. Int J Hematol. 2013;98:96–101. doi: 10.1007/s12185-013-1360-6. [DOI] [PubMed] [Google Scholar]
- 16.Ciftciler R, Goker H, Buyukasik Y, et al. Impact of ABO blood group incompatibility on the outcomes of allogeneic hematopoietic stem cell transplantation. Transfus Apher Sci. 2020;59:102597. doi: 10.1016/j.transci.2019.06.024. [DOI] [PubMed] [Google Scholar]
- 17.Ma YR, Wang WJ, Cheng YF, et al. Impact of ABO incompatibility on outcomes after haploidentical hematopoietic stem cell transplantation for severe aplastic anemia. Bone Marrow Transplant. 2020;55:1068–75. doi: 10.1038/s41409-020-0779-7. [DOI] [PubMed] [Google Scholar]
- 18.Kudek MR, Shanley R, Zantek ND, et al. Impact of graft-recipient ABO compatibility on outcomes after umbilical cord blood transplant for nonmalignant disease. Biol Blood Marrow Transplant. 2016;22:2019–24. doi: 10.1016/j.bbmt.2016.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Griffith LM, VanRaden M, Barrett AJ, et al. Transfusion support for matched sibling allogeneic hematopoietic stem cell transplantation (1993–2010): factors that predict intensity and time to transfusion independence. Transfusion. 2019;59:303–15. doi: 10.1111/trf.14966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Worel N. ABO-mismatched allogeneic hematopoietic stem cell transplantation. Transfus Med Hemother. 2016;43:3–12. doi: 10.1159/000441507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Logan AC, Wang Z, Alimoghaddam K, et al. ABO mismatch is associated with increased nonrelapse mortality after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2015;21:746–54. doi: 10.1016/j.bbmt.2014.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Snell M, Chau C, Hendrix D, et al. Lack of isohemagglutinin production following minor ABO incompatible unrelated HLA mismatched umbilical cord blood transplantation. Bone Marrow Transplant. 2006;38:135–40. doi: 10.1038/sj.bmt.1705409. [DOI] [PubMed] [Google Scholar]
- 23.World Medical Association. Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310:2191–4. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- 24.Liu HL, Sun ZM, Geng LQ, et al. Similar survival, but better quality of life after myeloablative transplantation using unrelated cord blood vs matched sibling donors in adults with hematologic malignancies. Bone Marrow Transplant. 2014;49:1063–9. doi: 10.1038/bmt.2014.102. [DOI] [PubMed] [Google Scholar]
- 25.Stussi G, Halter J, Bucheli E, et al. Prevention of pure red cell aplasia after major or bidirectional ABO blood group incompatible hematopoietic stem cell transplantation by pretransplant reduction of host anti-donor isoagglutinins. Haematologica. 2009;94:239–48. doi: 10.3324/haematol.13356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parkhideh S, Chegeni R, Mehdizadeh M, et al. Effects of ABO incompatibility on the outcome of allogeneic hematopoietic stem cell transplantation. Transfus Apher Sci. 2020;59:102696. doi: 10.1016/j.transci.2019.102696. [DOI] [PubMed] [Google Scholar]
- 27.Sullivan KM, Shulman HM, Storb R, et al. Chronic graft-versus-host disease in 52 patients: adverse natural course and successful treatment with combination immunosuppression. Blood. 1981;57:267–76. [PubMed] [Google Scholar]
- 28.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15:825–8. [PubMed] [Google Scholar]
- 29.Barker JN, Kurtzberg J, Ballen K, et al. Optimal practices in unrelated donor cord blood transplantation for hematologic malignancies. Biol Blood Marrow Transplant. 2017;23:882–96. doi: 10.1016/j.bbmt.2017.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wagner JE, Barker JN, DeFor TE, et al. Transplantation of unrelated donor umbilical cord blood in 102 patients with malignant and nonmalignant diseases: influence of CD34 cell dose and HLA disparity on treatment-related mortality and survival. Blood. 2002;100:1611–8. doi: 10.1182/blood-2002-01-0294. [DOI] [PubMed] [Google Scholar]
- 31.Huang C, Sun S, Yan J, et al. Identification of carbohydrate peripheral epitopes important for recognition by positive-ion MALDI multistage mass spectrometry. Carbohydr Polym. 2020;229:115528. doi: 10.1016/j.carbpol.2019.115528. [DOI] [PubMed] [Google Scholar]
- 32.Wang Y, Wu DP, Liu QF, et al. Donor and recipient age, gender and ABO incompatibility regardless of donor source: validated criteria for donor selection for haematopoietic transplants. Leukemia. 2018;32:492–8. doi: 10.1038/leu.2017.199. [DOI] [PubMed] [Google Scholar]
- 33.Canals C, Muniz-Diaz E, Martinez C, et al. Impact of ABO incompatibility on allogeneic peripheral blood progenitor cell transplantation after reduced intensity conditioning. Transfusion. 2004;44:1603–11. doi: 10.1111/j.1537-2995.2004.04106.x. [DOI] [PubMed] [Google Scholar]
- 34.Kanda J, Ichinohe T, Matsuo K, et al. Impact of ABO mismatching on the outcomes of allogeneic related and unrelated blood and marrow stem cell transplantations for hematologic malignancies: IPD-based meta-analysis of cohort studies. Transfusion. 2009;49:624–35. doi: 10.1111/j.1537-2995.2008.02043.x. [DOI] [PubMed] [Google Scholar]
- 35.Solves P, Carpio N, Carretero C, et al. ABO incompatibility does not influence transfusion requirements in patients undergoing single-unit umbilical cord blood transplantation. Bone Marrow Transplant. 2017;52:394–9. doi: 10.1038/bmt.2016.264. [DOI] [PubMed] [Google Scholar]
- 36.Blin N, Traineau R, Houssin S, et al. Impact of donor-recipient major ABO mismatch on allogeneic transplantation outcome according to stem cell source. Biol Blood Marrow Transplant. 2010;16:1315–23. doi: 10.1016/j.bbmt.2010.03.021. [DOI] [PubMed] [Google Scholar]
- 37.Berglund S, Le Blanc K, Remberger M, et al. Factors with an impact on chimerism development and long-term survival after umbilical cord blood transplantation. Transplantation. 2012;94:1066–74. doi: 10.1097/TP.0b013e31826c39b2. [DOI] [PubMed] [Google Scholar]
- 38.Sarvaria A, Basar R, Mehta RS, et al. IL-10+ regulatory B cells are enriched in cord blood and may protect against cGVHD after cord blood transplantation. Blood. 2016;128:1346–61. doi: 10.1182/blood-2016-01-695122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dulery R, Bastos J, Paviglianiti A, et al. Thiotepa, busulfan, and fludarabine conditioning regimen in T cell-replete HLA-haploidentical hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2019;25:1407–15. doi: 10.1016/j.bbmt.2019.02.025. [DOI] [PubMed] [Google Scholar]
- 40.Salas MQ, Lam W, Law AD, et al. Reduced-intensity conditioning allogeneic transplant with dual T-cell depletion in myelofibrosis. Eur J Haematol. 2019;103:597–606. doi: 10.1111/ejh.13327. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.