Abstract
Background and objectives
Little is known about the efficacy and durability of anti-RBD IgG antibodies induced by certain SARS-CoV-2 vaccines. It has been shown that neutralizing antibodies are associated with the protection against re-infection. This study aims to compare the mean titers, duration, and efficacy of generating protective anti-RBD IgG antibody response among recipients of Pfizer/BioNTech, AstraZeneca, Sputnik V, Johnson & Johnson, Moderna, and Sinopharm COVID-19 vaccines. In addition, we aimed to compare the susceptibility of getting COVID-19 breakthrough infections after various types of vaccines.
Materials and methods
Samples from 2065 blood bank donors and healthcare workers at King Hussein Cancer Center (KHCC) were collected between February and September 2021. Anti-Spike/RBD IgG levels were measured using Chemiluminescent microparticle-immunoassay (CMIA) (ARCHITECT IgG II Quant test, Abbott, USA).
Results
The mean titer of anti-RBD IgG levels was significantly diverse among different types of vaccines. The highest titer level was seen in participants who took a third booster vaccine shot, followed by Pfizer/BioNTech, AstraZeneca, and Sinopharm vaccine. The mean titer levels of anti-RBD IgG antibodies in the Pfizer vaccinated group was the highest after vaccination but started to drop after 60 days from vaccination unlike AstraZeneca and Sinopharm vaccine-induced antibodies where the mean titers continued to be stable until 120 days but their levels were significantly lower. Most of the breakthrough infections were among the Sinopharm vaccinated group and these breakthroughs happened at random times for the three main types of vaccines.
Conclusions
Our data demonstrate that the mean-titer of anti-RBD IgG levels drop after four months which is the best time to take the additional booster shot from a more potent vaccine type such as mRNA vaccines that might be needed in Jordan and worldwide.
Keywords: COVID-19; SARS-CoV-2; Antibody titer; Anti-RBD antibody; IgG; Healthcare workers; Blood bank donors, vaccine
1. Introduction
A newly discovered coronavirus is the causative agent of the current coronavirus disease and the virus is called Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) [1]. The major immunogenic components of SARS-CoV-2 are the spike and nucleocapsid proteins which are mainly produced in large quantities during infection. The interaction between the SARS-receptor binding domain (RBD) and the receptor–neutralizing antibody provides opportunities for predicting and analyzing the function of mutations in cross-species transmission and immunity. Furthermore, monoclonal antibodies against the S protein could neutralize viral infectivity. Neutralizing antibodies are a subset of antibodies able to inactivate viruses and are associated with protective immunity against re-infection for many infectious pathogens [2].
The control over the COVID-19 disease transmission in Jordan during the first ten months of the pandemic depended on the public health measures implemented by the government which can be classified into three phases [3]. The first phase began in the last week of February 2020, where the government applied strict control measures which resulted in flattening the epidemiological infection curve and prolonging virus transmission in the community [4]. The second phase started in August 2020 and was marked by the loosening of strict measures [5]. This resulted in a dramatic increase of cases up to 8000 positive PCR cases in a single day during November 2020 [6] and Jordan was placed among the countries that had the highest number of cases in the world during that time. The third phase of the pandemic showed a steady and continuous decline of the epidemiological curve with an average of 2000 cases per day over the last week of December 2020 [3], [6].
The Clinical manifestations of the disease involve both respiratory and extra-respiratory symptoms that can range from asymptomatic mild disease to acute respiratory tract infections. Hence, misdiagnosis of COVID-19 infection in asymptomatic patients especially when molecular testing is not available is possible. These challenges limited the understanding of the epidemiology and the real extent of SARS-CoV-2 infection and thus adversely affected the implementation of infection control and prevention policies [7].
On August 11, 2020, several companies and academic bodies such as Moderna, CanSino, the University of Oxford, BioNTech, Sinovac, Sinopharm, Anhui Zhifei Longcom, Inovio, Novavax, Vaxine, Zydus Cadila, Institute of Medical Biology, and the Gamaleya Research Institute embarked on developing numerous clinical trials on proposed vaccines to test its initial safety and immunogenicity [8]. Jordan Food & Drug Administration (JFDA) approved the use of the following vaccines; Pfizer/BioNTech, Sinopharm, AstraZeneca, Johnson & Johnson, and Sputnik V [9]. Up to date, the government managed to administer (7, 354, 7747) doses of COVID vaccines [10], and almost (3, 898, 463) received one dose while (3, 479, 694) received two doses [11]. Despite all the vaccination regimens and forced laws to implement strict protective measures it remains unclear, however, is to what degree the immune system’s safeguards that protect vaccinated people against COVID-19 infection.
In this study, we aim to compare the mean titers, duration, and efficacy in generating protective anti-RBD IgG antibody response among different types of COVID-19 vaccines. We also aim to compare the susceptibility of getting COVID-19 breakthrough infections after the various types of vaccines.
2. Methods
This study was a prospective cross-sectional seroepidemiological study conducted from February to September 2021 at King Hussein Cancer Center (KHCC) with a total number of 2065 participants of healthy blood bank donors and healthcare workers (HCW) at KHCC regardless of their vaccination status or type of vaccine taken. Participants were randomly recruited among the blood bank donors and HCWs who were willing and interested in participating in the study. Thereafter, the participants were requested to fill out the official blood bank donor questionnaire based on World Health Organization recommendations [12]. If the HCW was deferred from donating blood for any reason but he/she was still interested in participating in the study, they were requested to fill out another consent form which included gathering information related to the study. This information was then collected in a data collection sheet approved by the Research Ethics Committee (IRB) of King Hussein Cancer Center. All participants provided written informed consent approved by the IRB committee at KHCC.
Using Raosoft calculator the recommended sample size should be 372 participants, based on the following assumption: alpha = 0.05, Power 95%, using a population size of 11,566 (9269 blood bank donors during the study period donated blood + 2297 eligible medical staff workers = total of 11566) and response rate 50%; however, we screened 2065 participants to increase the power of the study.
2.1. Sampling and antibody titer quantification
Serum samples collected during the process of blood or apheresis platelet donations were used for the study for blood bank donors and they were also taken from healthcare workers after signing the consent forms. Blood samples (3.5 mL) were drawn from participants and the serum was collected by centrifuging the samples at 4300 relative centrifugal force (rcf) for 5 min. Serum samples were stored at −80 degrees Celsius until analyzed. Frozen samples were thawed gently by transferring the samples to 4 degrees Celsius refrigerator for 24 h before analysis. The samples were then vortexed before sample processing and analysis. Samples were tested using the SARS-CoV-2 IgG II Quant assay. The test is a chemiluminescent microparticle immunoassay for the quantitative determination of IgG antibodies to SARS-CoV-2, including neutralizing antibodies, to the receptor-binding domain (RBD) of the S1 subunit of the spike protein of SARS-CoV-2 in serum and plasma from individuals who are suspected to have had coronavirus disease (COVID-19) or in serum and plasma of individuals that may have been infected by or vaccinated against SARS-CoV-2 (Abbott Architect SARS-CoV-2 IgG with ARCHITECT i1000SR analyzer; Abbott Laboratories, USA) according to the manufacturer’s instructions [13]. The Abbott SARS-CoV-2 IgG II Quant assay has a measuring range of 21.0–40,000.0 AU/mL, with ≥50 AU/mL considered seropositive. The assay has 99.35% sensitivity and 99.6% specificity with 100% (86/86) positive agreement with the Plaque reduction neutralization test (PRNT); 95% CI = 95.72. Plaque reduction neutralization tests (PRNT) are used to quantify the titer of neutralizing antibodies for a virus [13], [14], [15], [16].
2.2. Types of vaccines
Vaccinated participants took the COVID-19 vaccine which was provided by the Jordanian Ministry of health and had no control over choosing the type of vaccine administered to them. All vaccinated individuals received standard two doses of the same vaccine type (all before adopting the booster dose policy). A small number (3 participants) took multiple types of vaccines for personal reasons such as traveling requirements. Two of the participants who took multiple types, had two shots of Sinopharm followed by one Pfizer/BioNTech shot, the third participant took the first vaccine shot from Sinopharm, the second vaccine shot from AstraZeneca, followed by the third vaccine shot from Pfizer/BioNTech depending on the availability of the vaccines. The Ministry of Health provided four types of vaccines. These types are as follows: Pfizer/BioNTech /BioNTech: BNT162b2 (vaccine type: RNA, USA/Germany) (Pfizer/BioNTech), Sinopharm (Beijing) BBIBP-CorV (Vero Cells) (vaccine type: inactivated virus; China) (Sinopharm), Oxford/AstraZeneca AZD1222 (vaccine type: None replicating viral vector; UK) (AstraZeneca), and the Gamaleya Sputnik V (vaccine type: None replicating viral vector; Russia) (Sputnik) vaccine [17].
2.3. Statistical analysis
Participants’ characteristics and vaccine information were presented as counts and percentages; such as vaccine type, in addition, to mean and range to describe age and other continuous factors. Comparison between vaccination rates, types, and outcomes according to all factors; were carried out using T-test, Mann-Whitney, Kruskal-Wallis, Fisher’s exact or Chi-square test as appropriate. Logistic regression was used to determine the adjusted and unadjusted odds ratios. Time to event data was assessed using the Kaplan Meier method and log-rank test. A significance criterion of p ≤ 0.05 was used in the analysis. All analysis was performed using SAS version 9.4 (SAS Institute Inc, Cary, NC) and Microsoft Excel data analysis tools.
3. Results
3.1. Study population
The age of the studied population ranged from 17.6 to 70.4 years old with a mean of 31.7 years. Of a total of 2065 individuals, there were 1235 (59.8%) vaccinated individuals and 830 (40.2%) participants who were not vaccinated yet. Of the vaccinated participants, 1,144 (92.9%) tested positive for the anti-RBD IgG antibody and 87 (7.1%) were negative. In the non-vaccinated group, there were 364 (44.6%) who tested positive and 452 (55.4%) who were negative for the anti-RBD IgG antibodies (p-value < 0.0001) (Table 1 ).
Table 1.
Number of vaccinated and non-vaccinated participants.
| Anti-RBD IgG antibody | Seronegative | Seropositive | p-value |
|---|---|---|---|
| Total | 539 (26.3%) | 1508 (73.7%) | <0.0001 |
| Vaccinated | 87 (7.1%) | 1144 (92.9%) | |
| Non-vaccinated | 452 (55.4%) | 364 (44.6%) |
There were 954 males (77.2%) who got the COVID-19 vaccination compared to 281 (22.8%) females. The mean titer value of IgG anti-RBD antibodies among males was 6,191.7 AU/ml compared to 5,465.2 AU/ml in females (p-value 0.042) (see Table 2 ).
Table 2.
Number of participants and mean anti-RBD IgG antibody titer divided by gender.
| label | Total | Vaccinated (n = 1235, 59.8%) | Not vaccinated (n = 830, 40.2%) | Mean titer (Range) |
|---|---|---|---|---|
| Females | 384 (18.6%) | 281 (22.8%) | 103 (12.4%) | 5465.2 (0.0, 40000) |
| Males | 1681 (81.4%) | 954 (77.2%) | 727 (87.6%) | 6191.7 (0.0, 40000) |
3.2. Comparison between different types of vaccines in generating protective anti-RBD IgG antibody response
Among the studied population, 179 subjects received the AstraZeneca vaccine (mean age 36.6 years, range (19.9–67.3 years)), 516 took Pfizer/BioNTech vaccine (mean age 33.6 years, range (17.7–70.4 years)), 510 were vaccinated with Sinopharm (mean age 32.9 years, range (18.5–62 years)). One individual took Johnson & Johnson and four took Moderna outside Jordan, while seven were vaccinated with Sputnic-V vaccine and three took multiple vaccine types. The number and percentage of the seropositive and seronegative individuals according to the 3 main vaccine types are listed in Table 3 . The mean duration in days between the second vaccine dose and sampling for anti-RBD IgG antibody titer in AstraZeneca vaccinated participants was 72.5 (27.8) days. In Pfizer/BioNTech and Sinopharm vaccinated subjects the mean duration was 75.2 (52.2) days and 74.1 (52.3) days respectively.
Table 3.
Number and percent of seropositive and seronegative participants for the three main vaccine types given in Jordan.
| Vaccine type (N) | Seronegative for anti-RBD IgG | Seropositive for anti-RBD IgG |
|---|---|---|
| AstraZeneca (1 7 9) | 7 (3.9%) | 172 (96.1%) |
| Pfizer/BioNTech (5 1 6) | 12 (2.3%) | 504 (97.7%) |
| Sinopharm (5 1 0) | 67 (13.1%) | 443 (86.9%) |
| p-value | <0.0001 | <0.0001 |
The mean titer of anti-RBD IgG level is illustrated in Table 4 . The results show that participants who took multiple vaccine types (three vaccine shots) reported the highest mean titer of 15,832 AU/ml. All other vaccinated participants took two vaccine shots. The mRNA vaccines Pfizer/BioNTech and the Moderna vaccine generated the highest titers among this group of vaccinated participants. The mean titer was 11,478 AU/ml and 7,730.2 AU/ml respectively. As for the non-replicating viral vector vaccines, the Sputnic-V vaccine mean-titer generated was 5,315.9 AU/ml followed by AstraZeneca mean titer of 3,576.5AU/ml. There was only one participant who took the Johnson & Johnson vaccine and the titer was 41.6 AU/ml. Finally, the lowest mean titer was in the inactivated virus vaccine Sinopharm group of 1,385.9 AU/ml (p-value < 0.0001).
Table 4.
Mean titer of anti-RBD IgG level among all types of vaccines and main three types.
| Mean Titer (Range) AU/ml | p-value | ||
|---|---|---|---|
| Covid-19 Vaccine types | Johnson | 41.6 (41.6, 41.6) | <0.0001 |
| Moderna | 7730.2 (1477.2, 12786) | ||
| Sputnic | 5315.9 (250.5, 13695) | ||
| multiple | 15,832 (8307.0, 21010) | ||
| Covid-19 main vaccine types | AstraZeneca | 3576.5 (0.3, 40,000) | <0.0001 |
| Pfizer/BioNTech | 11,478 (0.0, 40,000) | ||
| Sinopharm | 1385.9 (0.0, 40,000) | ||
3.3. The decline of anti-RBD IgG antibody titer produced in response to different COVID-19 vaccines as a function of time
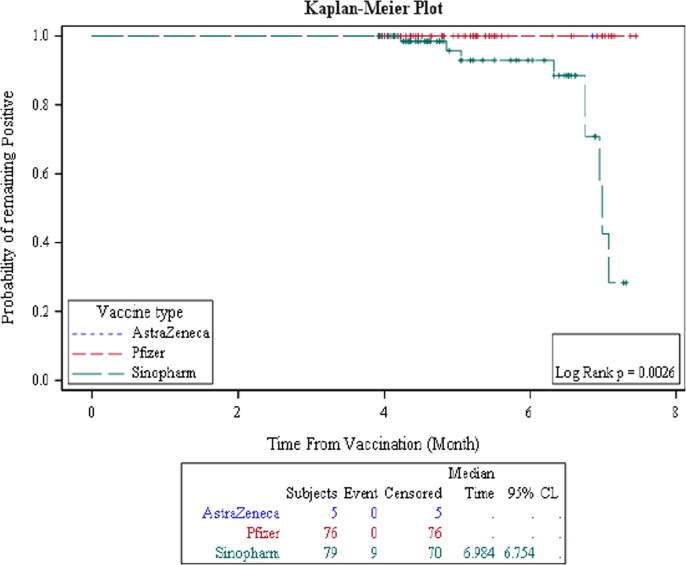
The duration from the date of the second dose of COVID-19 vaccine to the date of sample acquisition (this period shall be called “Duration”) was analyzed. The percent of seroconversion; which is according to the manufacturer's recommendation interpretation of the test result is considered seropositive/immune or seronegative/non-immune, for the three main types of vaccines was evaluated during the elapsed time since the second dose of vaccine. We found that all three main vaccine types (AstraZeneca, Pfizer/BioNTech, and Sinopharm) had the same seropositive status among the participants during the first four months after vaccination. Thereafter, a drop in the number of seropositive participants vaccinated with Sinopharm, compared to individuals vaccinated with Pfizer/BioNTech or AstraZeneca is observed. Fig. 1 shows that the drop in the probability of being seropositive for Sinopharm vaccinated individuals exceeded 50% after the sixth month from the second vaccine dose (Table 5 ). On the other hand, the probability of being seropositive for anti-RBD IgG against SARS-Cov2 virus in AstraZeneca and Pfizer/BioNTech vaccinated individuals remained steady after 6 months of vaccination (Fig. 1 and Table 5).
Fig. 1.
Comparing the probability of being seropositive for anti-RBD IgG antibodies among the three main vaccine types.
Table 5.
Comparing the persistence of the percent seropositive rate for anti-RBD IgG antibodies among the three main vaccine types.
| Months | % seropositive rate |
||
|---|---|---|---|
| AstraZeneca | Pfizer/BioNTech | Sinopharm | |
| 5 | 100% | 100% | 95.8% |
| 6 | 100% | 100% | 92.9% |
| 7 | 100% | Not reached | 42.5% |
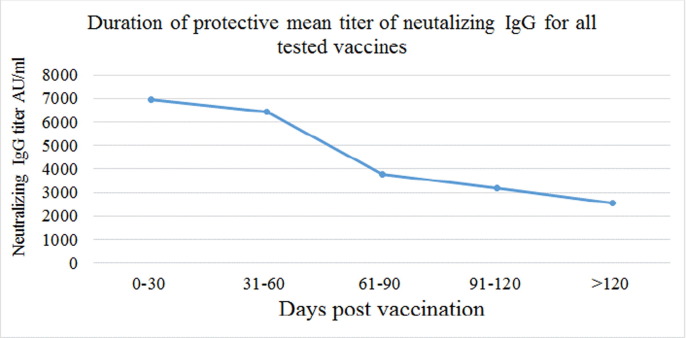
The duration period was further divided into five intervals to make the groups as homogenous as possible for accurate statistical comparison. The antibody titer for all the three main vaccine types showed a decline with elapsed time from the date of vaccination to the date of sampling (p-value < 0.005) as shown in Fig. 2 .
Fig. 2.
Titer of anti-RBD IgG in all vaccinated participants vs post vaccinations period p-value < 0.005.
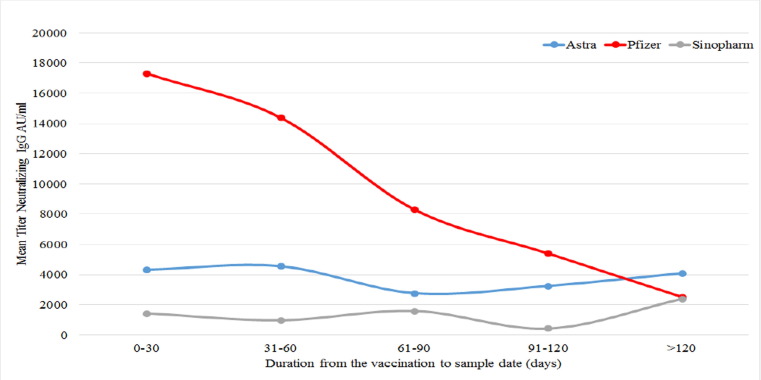
Fig. 3 represents the mean titer level for each vaccine plotted against the predefined intervals post-vaccination. Data shows that the Pfizer/BioNTech vaccine induced a high titer level in the first period (0–30 days) after vaccination unlike the other two vaccines AstraZeneca and Sinopharm (p < 0.05) then the titer dropped to the level of the other vaccines after four months (Fig. 3).
Fig. 3.
Comparison of the mean titer of anti-RBD IgG in vaccinated participants during the five-time intervals for the three main anti-COVID-19 vaccines.
3.4. Comparison of the susceptibility of breakthrough COVID-19 infection after vaccination
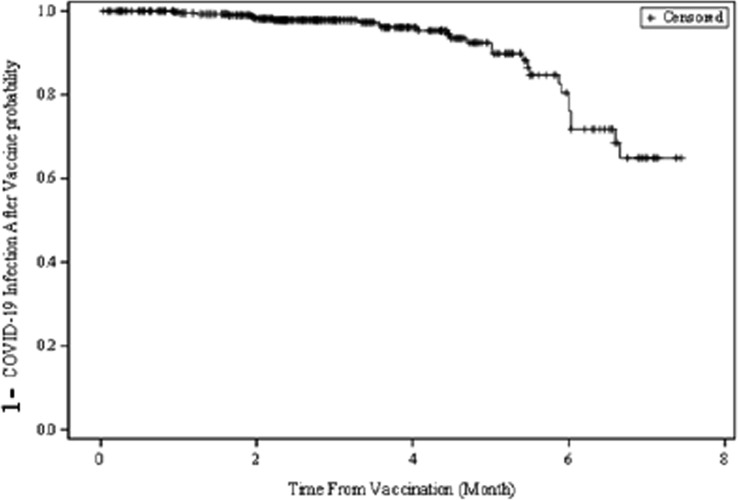
The probability of the occurrence of a breakthrough infection with the SARS-Cov2 virus after vaccination (regardless of vaccine type) was estimated to be around 20% after an average of 6 months from vaccination (Fig. 4 ).
Fig. 4.
The probability of a possible breakthrough infection with the SARS-Cov2 virus after vaccination regardless of the type of vaccine administered.
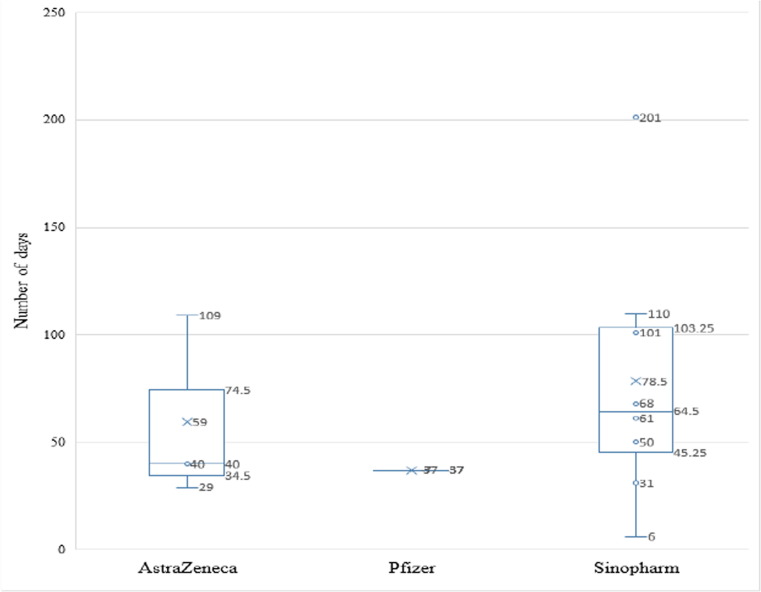
Among the 1210 participants who received the AstraZeneca, Pfizer/BioNTech, or Sinopharm vaccines 27 got a breakthrough infection with the SARS-Cov2 virus after vaccination. Out of these 27 participants, 19 (70.4%) had 2 shots of Sinopharm vaccine while 4 (14.8%) had 2 shots of AstraZeneca and 4 (14.8%) had 2 Pfizer/BioNTech vaccines (Table 6 ). The duration between getting the second dose of the vaccine and encountering the breakthrough infection was available for 12 participants out of the 27. The duration for the breakthrough infection for the AstraZeneca vaccinated participants ranged from 29 to 109 (mean 59.3) days, while for Sinopharm vaccinated participants it ranged from 6 days to 201 (mean 78.5) days, and for the Pfizer/BioNTech vaccinated participant with available data it was 37 days (Fig. 5 ).
Table 6.
Breakthrough infections in vaccinated subjects (N = 27).
| SARS-Cov2 virus infection after vaccination | AstraZeneca | Pfizer/BioNTech | Sinopharm |
|---|---|---|---|
| Count | 4 | 4 | 19 |
| Percentage | 14.81% | 14.81% | 70.37% |
Fig. 5.
Box and Whisker plot for the 12 participants duration period between the second vaccine dose and COVID-19 breakthrough infection for the three main vaccine types.
4. Discussion
Our data show that the seroprevalence rate for anti-RBD IgG antibodies was significantly higher among the SARS-Cov2 virus vaccinated group (92.9%) compared to (55.4%) in the non-vaccinated participants (p-value < 0.0001). Furthermore, the seroconversion rate generated by the mRNA vaccines or the vector type vaccines (Pfizer/BioNTech) 97.7% and AstraZeneca 96.1% was significantly higher compared to the inactivated virus vaccine (Sinopharm 86.9%). The mean anti-RBD IgG antibody titer was significantly higher in those vaccinated with Pfizer/BioNTech (11,478 AU/ml) followed by AstraZeneca (3576 AU/ml) then Sinopharm (1385 AU/ml) p-value < 0.0001. This result supports the finding by Modenese, A. et al that the Pfizer/BioNTech (BNT162b2) vaccine induces high titers of anti-SARS-CoV-2 anti-RBD IgG antibodies [18]. Moreover, since there was no significant difference between the mean duration in days to sample acquisition following the second vaccine dose between AstraZeneca, Pfizer/BioNTech, and Sinopharm vaccinated subjects, it is less likely that there is any effect of vaccination time and sample collection on seroconversion rate or titer measured in the serum of participants.
The probability of being seropositive for the anti-RBD IgG antibodies after vaccination remained constant until the seventh month in the mRNA vaccine and the vector vaccine, while it dropped gradually after the fourth month in the inactivated virus vaccine (p-value 0.0015). We also noticed that the mean titer levels of anti-RBD IgG antibodies in the Pfizer/BioNTech vaccinated group was the highest after vaccination at 17,325.5 AU/ml, then started to drop to 8317.5 AU/ml between day 61 and 90 after vaccination then dropped to 2521.1 AU/ml after 120 days. This drop in the antibody response to the S1 antigen in the mRNA vaccinated individuals was also documented by other researchers [19], [20], [21]. On the other hand, AstraZeneca and Sinopharm vaccine mean titers continued to be stable until 120 days but their levels were significantly lower (4095.5 AU/ml, 2381.7 AU/ml respectively) after day 120 (p-value < 0.05). Our data further, indirectly support the study by Saban et al. which demonstrated that taking a third-booster vaccine reduced hospitalizations and mortality rates [22]. Here we demonstrate that a booster dose from a different type of vaccine induced a significantly higher titer of anti-RBD IgG antibodies (15,832 AU/ml) compared to all types of vaccines including the mRNA type of vaccines.
Finally, although a recent study showed that there was no COVID-19 breakthrough infection 1–2 months post-vaccination with Pfizer/BioNTech vaccine in Japan [23], our data showed that the duration for the breakthrough infection for the Pfizer/BioNTech vaccinated participant was 37 days compared to the duration for the breakthrough infection in the AstraZeneca vaccinated participants which ranged from 29 to 109 days (mean 59.3), while for Sinopharm vaccinated participants the duration ranged from 6 to 201 days (mean 78.5).
It is noteworthy that during the period of this study there were two SARS-CoV-2 virus strains (alpha and delta) circulating in Jordan and this should be taken into consideration as one of the limitations. We could not determine the strain that was causing the observed breakthrough infection in the latter part of the study period. However, we suspect that the cause of the breakthrough infections was probably due to the delta variant.
This study is one of the very few studies that directly compare the humoral response of three vaccine types. This may help shed light and improve vaccination strategies.
This is the first study in the region comparing the efficacy and seroconversion duration generated by the three main types of vaccines implemented by the ministry of health in Jordan in generating IgG antibodies to SARS-CoV-2. We suggest that further prospective follow-up studies must be done regarding the breakthrough infections.
5. Ethics statement
Written informed consent was obtained from all participants and the institutional review board of King Hussein Cancer Center (IRB # 20 KHCC 174F) approved the study.
Funding
Abdul Hameed Shoman Foundation Innovation Award (AHSF-IA) – 2nd edition in response for COVID-19 with grant number 2/2020, funded this study.
Contributors
LS conceived and designed the study. LS, MMAA, SA, MO, and TAA recruited the study participants and collected clinical specimens. LS, MMAA, SA, TAA, and MS carried out the laboratory work. DAR, LJ, LS, and MAS analyzed the data. LJ helped in the scientific editing of the manuscript, LS acquired the funding and wrote the first draft of the manuscript. All authors have read and agreed to the published version of the manuscript.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgments
The authors thankfully acknowledge the study participants for their contributions to this research. We thank Mr. Nedal Edeinat, Mr. Raed Almousa, Ms. Alaa Al-Shorman, and Ms. Diana Al-Srihin, for facilitating participant recruitment and financial management of the project. Special thanks to Drs. Imad and Laila Jarjour for helpful discussions and English editing.
Implications of all the available evidence
Exploring the protective capacity and the duration of anti-RBD IgG after COVID-19 vaccination is critical for managing the pandemic and would also provide more evidence about the efficacy of SARS-CoV-2 vaccines. We anticipate that the data from our study would help in improving vaccination strategies and public health decisions.
References
- 1.Wu A., Peng Y., Huang B., Ding X., Wang X., Niu P., et al. Genome composition and divergence of the novel coronavirus (2019-nCoV) originating in China. Cell Host Microbe. 2020;27(3):325–328. doi: 10.1016/j.chom.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chia W.N., Tan C.W., Foo R., Kang A.E.Z., Peng Y., Sivalingam V., et al. Serological differentiation between COVID-19 and SARS infections. Emerg Microbes Infect. 2020;9(1):1497–1505. doi: 10.1080/22221751.2020.1780951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bellizzi S., Alsawalha L., Sheikh Ali S., Sharkas G., Muthu N., Ghazo M., et al. A three-phase population based sero-epidemiological study: Assessing the trend in prevalence of SARS-CoV-2 during COVID-19 pandemic in Jordan. One Health. 2021;13 doi: 10.1016/j.onehlt.2021.100292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Al-Tamimi M., Qaisieh R., Tadros R.E., Shalabi M., Alhasoun A., Kilani M.M. Successful flattening of COVID-19 epidemiological curve in Jordan. J Glob Health. 2020;10:020361. doi: 10.7189/jogh.10.020361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Center B.D. Brookings Doha Center; Jordan. Doha, Qatar: 2021. Policy and institutional responses to COVID-19 in the Middle East and North Africa. [Google Scholar]
- 6.health JMo. COVID-19 Statistical report – Jordan. Jordan: Jordanian Ministry of health; 2021.
- 7.Lai C.C., Wang J.H., Hsueh P.R. Population-based seroprevalence surveys of anti-SARS-CoV-2 antibody: an up-to-date review. Int J Infect Dis. 2020;101:314–322. doi: 10.1016/j.ijid.2020.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chung Y.H., Beiss V., Fiering S.N., Steinmetz N.F. COVID-19 vaccine frontrunners and their nanotechnology design. ACS Nano. 2020;14(10):12522–12537. doi: 10.1021/acsnano.0c07197. [DOI] [PubMed] [Google Scholar]
- 9.Health JMo. Vaccines and medicines for corona virus. Jordan Food and Drug Administration; 2021
- 10.Reuters. REUTERS, COVID19 Tracker; 2021.
- 11.Health JMo. COVID-19 Statistical Report – Jordan; 2021.
- 12.Dhingra N. Blood donor selection: guidelines on assessing donor suitability for blood donation. In: WHO, editor. Geneva, Switzerland World Health Organization; 2012. [PubMed]
- 13.ABBOTT. AdviseDx SARS-CoV-2 IgG II. Abbott Ireland: ABBOTT; March 2021.
- 14.Gallais F., Gantner P., Bruel T., Velay A., Planas D., Wendling M.J., et al. Evolution of antibody responses up to 13 months after SARS-CoV-2 infection and risk of reinfection. EBioMedicine. 2021;71:103561. doi: 10.1016/j.ebiom.2021.103561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kung Y-A, Huang C-G, Huang S-Y, Liu K-T, Huang P-N, Yu K-Y, et al. Antibody titers measured by commercial assays are correlated with neutralizing antibody titers calibrated by international standards. medRxiv. 2021:2021.07.16.21260618.
- 16.Servellita V, Syed AM, Brazer N, Saldhi P, Garcia-Knight M, Sreekumar B, et al. Neutralizing immunity in vaccine breakthrough infections from the SARS-CoV-2 Omicron and Delta variants. medRxiv. 2022:2022.01.25.22269794. [DOI] [PMC free article] [PubMed]
- 17.Team MCVT. COVID-19 vaccine tracker. JORDAN: 4 vaccines approved for use in Jordan. McGill University McGill University Interdisciplinary Initiative in Infection and Immunity (MI4); 2021.
- 18.Modenese A., Paduano S., Bargellini A., Bellucci R., Marchetti S., Bruno F., et al. Neutralizing anti-SARS-CoV-2 antibody titer and reported adverse effects, in a sample of italian nursing home personnel after two doses of the BNT162b2 vaccine administered four weeks apart. Vaccines (Basel) 2021;9(6):652. doi: 10.3390/vaccines9060652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wheeler S.E., Shurin G.V., Yost M., Anderson A., Pinto L., Wells A., et al. Differential antibody response to mRNA COVID-19 vaccines in healthy subjects. Microbiol Spectr. 2021;9:e0034121. doi: 10.1128/spectrum.00341-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Malavazos AE, Basilico S, Iacobellis G, Milani V, Cardani R, Boniardi F, et al. Antibody responses to BNT162b2 mRNA vaccine: infection-naive individuals with abdominal obesity warrant attention. Obesity (Silver Spring); 2021 [DOI] [PubMed]
- 21.Levin E.G., Lustig Y., Cohen C., Fluss R., Indenbaum V., Amit S., et al. Waning immune humoral response to BNT162b2 Covid-19 vaccine over 6 months. N Engl J Med. 2021;385(24):e84. doi: 10.1056/NEJMoa2114583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saban M, Myers V, Wilf-Miron R. Changes in infectivity, severity and vaccine effectiveness against delta COVID-19 variant ten months into the vaccination program: The Israeli case. Prev Med. 2022;154:106890. [DOI] [PMC free article] [PubMed]
- 23.Yamamoto S., Maeda K., Matsuda K., Tanaka A., Horii K., Okudera K., et al. COVID-19 breakthrough infection and post-vaccination neutralizing antibody among healthcare workers in a referral hospital in Tokyo: a case-control matching study. Clin Infect Dis. 2021 doi: 10.1093/cid/ciab1048. [DOI] [PMC free article] [PubMed] [Google Scholar]