Abstract
Background
In partial response to the coronavirus disease 2019 (COVID-19) pandemic, countries around the world are conducting large-scale vaccination campaigns. Real-world estimates of vaccine effectiveness (VE) against the B.1.617.2 (Delta) variant are still limited. An outbreak in Ruili city of China provided an opportunity to evaluate VE against the Delta variant of two types of COVID-19 vaccines in use in China and globally – inactivated (CoronaVac and BBIBP-CorV) and adenovirus type 5 vectored (Convidecia) vaccines.
Methods
We estimated VE using a retrospective cohort study two months after the Ruili vaccination campaign (median: 63 days). Close contacts of infected people (Chinese nationality, 18 years and above) were included to assess VE against symptomatic Covid-19, COVID-19 pneumonia, and severe COVID-19. We calculated the relative risks (RR) of the outcomes for unvaccinated compared with fully vaccinated individuals. We used logistic regression analyses to estimate adjusted VEs, controlling for gender and age group (18–59 years and 60 years and over).We compared unvaccinated and fully vaccinated individuals on duration of RT-PCR positivity and Ct value.
Findings
There were 686 close contacts eligible for VE estimates. Adjusted VE of ad5-vectored vaccine was 61.5% (95% CI, 9.5–83.6) against symptomatic COVID-19, 67.9% (95%CI: 1.7–89.9) against pneumonia, and 100% (95%CI: 36.6–100) against severe/critical illness. For the two inactivated vaccines, combined VE was 74.6% (95% CI, 36.0–90.0) against symptomatic COVID-19, 76.7% (95% CI: 19.3–93.3) against pneumonia, and 100% (95% CI: 47.6–100) against severe/critical COVID-19. There were no statistically significant differences in VE between two inactivated vaccines for symptomatic COVID-19 and for pneumonia, nor were there statistically significant differences between inactivated and ad5-vectored VE in any of the three outcomes. The median durations of RT-PCR positivity were 17 days for fifteen people vaccinated with an inactivated vaccine, 18 days for forty-four people vaccinated with the Ad5 vectored vaccine, and 26 days for eleven unvaccinated individuals.
Interpretation
These results provide reassuring evidence that the three vaccines are effective at preventing Delta-variant COVID-19 in short term following vaccination campaign, and are most effective at preventing more serious illness. The findings of reduced duration of RT-PCR positivity and length of hospital stay associated with full vaccination suggests potential saving of health-care system resources.
Keywords: COVID-19 vaccine, Effectiveness, Delta variant, China
1. Introduction
The COVID-19 pandemic is entering its third year, with 281 million confirmed cases and 5.4 million COVID-19-related deaths as of 29 December 2021 [1]. Although the Omicron variant is rising in prominence, the Delta variant continues to circulate globally. In China, effective implementation of the policy of “zero tolerance for local transmission” [2] eliminated SARS-CoV-2 by 8 April 2020, with only small importation-related outbreaks occurring since containment. These outbreaks have all been stopped by non-pharmaceutical interventions (NPIs), especially the strategy of finding and quarantining close contacts of infected individuals [3]. The initial post-containment outbreaks in China were with ancestral strains, but with the rise of the Delta variant globally, since May 2021, the outbreaks in China have become Delta-strain outbreaks [4].
Due to the rapid and sustained elimination of indigenous transmission of SARS-CoV-2 in China, essentially all of China’s population immunity against SARS-CoV-2 comes from vaccine-induced immunity. Hybrid immunity is virtually absent. The real-world performance of vaccines used in China is therefore critically important for determining the quality of population immunity in terms of the ability to prevent the more severe forms COVID-19, and in anticipation of lifting the “zero tolerance” policy, after which the virus will become endemic. However, under “zero tolerance,” due to a lack of ongoing local transmission, measuring vaccine effectiveness (VE) of the China-produced vaccines has to rely on investigations of importation related outbreaks in China and real-world studies conducted overseas.
An outbreak in a border city in Yunnan province provided an opportunity to evaluate VE of two types of COVID-19 vaccines in use in China and globally – two inactivated, whole-virus vaccines and an adenovirus type 5 vectored vaccine that have been used in a combined total of 153 countries – against the Delta variant. We report an evaluation of VE for these vaccines and analyses of vaccine impact on duration of SARS-CoV-2 RT-PCR positivity and RT-PCR Ct values.
2. Methods
2.1. Setting
Ruili city is located in the western Yunnan province, sharing a relatively porous border with Myanmar in a part of Myanmar that had continuous community circulation of SARS-CoV-2 throughout 2021. In March 2021, a 116-case outbreak of COVID-19, caused by the B.1.36.16 variant, occurred in Ruili that was stopped by non-pharmaceutical interventions (NPIs) alone. This outbreak occurred at the very beginning of China’s COVID-19 vaccination campaign. In March through May 2021, following containment of the March Ruili outbreak, adults in Ruili were vaccinated with either of the three conditional licensed COVID-19 vaccines in China – Convidecia (Cansino’s Ad5-vectored vaccine), BBIBP-CorV (Sinopharm’s inactivated vaccine), or CoronaVac (Sinovac’s inactivated vaccine).
In July 2021, two months after completion of the Ruili vaccination campaign, another Covid-19 outbreak occurred in Ruili. The July outbreak was caused by the Delta variant. The total number of infections in this outbreak was 117, and among people infected, 59 were Chinese nationals and 58 were Myanmar nationals. Most (72.6% of cases) of the outbreak was centered in JG community which borders with Myanmar and connects to the Ruili urban area by a single bridge over a river. Yunnan province implemented the National Health Commission’s Prevention and Control of COVID-19 guidance, 8th edition to stop the outbreak. Key measures included: all cases received isolated medical care; all cases’ close contacts were traced, identified, and quarantined in managed quarantine facilities with daily SARS-CoV-2 RT-PCR testing (Reagents were from Shengxiang Biotechnology and Shanghai ZJ Bio-tech Co., LTD); institution of travel restrictions; performing environmental disinfection; conducting population-wide periodic, regular RT-PCR screening; and genome sequencing of the viruses.
2.2. Study design and participants
We conducted three related studies – evaluating the vaccine effectiveness among cases and their close contacts, analyzing the association of vaccination with RT-PCR Ct values among infected individuals, and analyzing duration of RT-PCR positivity and hospital stay among infected individuals. Ct values and hospital stay durations were obtained from medical records at the designated COVID-19 isolation hospital.
2.3. Vaccine effectiveness
We used a retrospective cohort design among close contacts of infected individuals to determine VE. Close contacts were defined as individuals living in the same residence, having close contact in the same room such as a restaurant, being in the same confined space, or being exposed to an environment contaminated by an infected person during the four days before and after symptom onset for a confirmed case, or four days before and after an RT-PCR positive swab was obtained in an asymptomatically-infected person.
The outbreak was caused by simultaneous importations from multiple locations in the border area, with more than 10 independent transmission chains that were concentrated in JG community. We included infected persons diagnosed by RT-PCR and their close contacts in JG community who were Chinese nationals, aged ≥18 years, and identified between July 4 and September 3, 2021. Sporadic cases outside JG community and their close contacts, and cases with unknown vaccination history were excluded from the VE estimations.
VE was determined in this cohort of quarantined close contacts by comparing vaccination status of quarantined individuals who ultimately tested positive for SARS-Cov-2 with those who remained RT-PCR negative.
2.4. RT-PCR Ct values
We obtained initial Ct values of 80 infected patients, including Myanmar nationals, who tested positive between July 4 and July 30. Among these, 70 were included in the analysis of the association of vaccination with Ct values early in the course of infection. Inclusion criteria were being ≥18 years old and having known vaccination status and RT-PCR results. We determined the proportion of individuals with nucleic acid Ct values below 30 and ≥30 by vaccination status. We also determined the length of time that individuals remained RT-PCR-positive by vaccination status.
2.5. Duration of hospital stay
Under the Protocol for Prevention and Control of COVID-19, infected individuals (RT-PCR positive) are required to be in isolated medical management until turning RT-PCR-negative with respiratory symptoms resolved. We obtained admission and discharge dates of 80 cases diagnosed between July 4 and July 30. Length of stay was the difference in days between admission and discharge. We analyzed the length of hospitalisation by vaccination status. We included 71 cases who were ≥18 years old and had complete information at the time of hospital discharge. We excluded individuals younger than 18 years old and individuals without complete information.
2.6. Vaccination status
Vaccination history was obtained by interviewing individuals and assessing vaccination records from national and provincial immunization information systems. We considered vaccinations to be valid only if documented in either the national or the provincial immunization information system.
Based on vaccination history and the time of exposure, subjects were categorized into one of three groups: unvaccinated, partially vaccinated, and fully vaccinated. The unvaccinated group consisted of individuals who did not receive any COVID-19 vaccine before exposure. The partially vaccination group consisted of individuals who had received either one dose of a COVID-19 inactivated vaccine or had received two doses of inactivated vaccine with receipt of the second dose less than 14 days of exposure, or had received one doses of Ad5 vaccine less than 14 days before exposure. The full-vaccination group consisted of individuals who completed two doses of inactivated vaccine or one dose of Ad5 vaccine 14 days or more before exposure to an infected individual. For an infected person whose exposure date was unknown, we used seven days prior to initial PCR positivity as the exposure date. For close contacts whose exposure date was unknown, most of whom lived with a case in the same residence, we used the start of quarantine as the last exposure date.
2.7. Outcomes
We evaluated three outcomes: symptomatic COVID-19, COVID-19 pneumonia, and severe illness from COVID-19. Case classifications were based on the COVID-19 Diagnosis and Treatment Protocol (Trial eighth edition) [5] and the COVID-19 Prevention and Control Protocol (eighth edition) [3]: asymptomatic, mild, moderate, severe, and critically severe. Mild cases had mild clinical symptoms without evidence of pneumonia on imaging studies; moderate cases had fever and respiratory symptoms with radiologic findings of pneumonia; severe cases among adults had at least one of the following: respiratory distress with a respiration rate of 30 or greater, resting oxygen saturation of 93% or lower, or PaO2)/FiO2 of 300 mmHg; critical cases had at least one of the following: respiratory failure requiring mechanical ventilation, shock, or other condition requiring admission to an ICU. We categorized symptomatic COVID-19 to include mild, moderate, severe, and critically-severe cases; COVID-19 pneumonia included moderate, severe, and critically severe cases with evidence of pneumonia; and severe COVID-19 included severe and critically severe cases. We intended to evaluate all COVID-19 infections, however, there were no asymptomatic cases in this outbreak, therefore, VE against symptomatic COVID-19 equals VE against all infections in this study.
2.8. Statistical analyses
We used generalized linear logistic regression to calculate the relative risk (RR = the ratio of the incidence of the vaccinated group to that of the unvaccinated group) and 95% confidence intervals, unadjusted and adjusted for gender and age group (18–59 years and 60 years and older). VE was calculated as: VE = (1 − RR) * 100%. Quantitative data (Ct values and times) were described by maxima, minima, medians, and inter-quartile ranges (IQR). We calculated odds ratios to compare Ct values of the first nucleic acid detection by vaccination status. We used Mann-Whitney U Rank tests for comparisons between groups; p < 0.05 was considered statistically significant. All analyses were conducted using Excel 2016, SPSS, or SAS software.
2.9. Ethical considerations
Our study used only routinely-collected data required by the Protocol for Prevention and Control of COVID-19. Individual-identifying information was not retained in analytic data sets. As an analysis of routinely collected data, the study was exempt from Ethical Review Committee review.
3. Results
3.1. Subjects (close contacts)
In total, 1058 close contacts in JG community were potentially eligible for the study. Among potentially eligible subjects, 116 had incomplete baseline information, 249 were less than 18 years old, and seven had unclear vaccination histories and were excluded. Consequently, there were 686 close contacts who met all inclusion criteria and met no exclusion criteria for the VE component of the study. Four-hundred-eighty-one (70.1%) were men and 205 (29.9%) were women; 651 were 18–59 years old and 35 were 60 years or older. All had been quarantined, as per the National Health Commission’s Protocol. Thirty-four of the close contacts became RT-PCR positive: 15 mild, 17 moderate, one severe, and one critical in severity.
3.2. Vaccine effectiveness
Sixty-six (9.6%) of the close contacts were unvaccinated; seven (1.0%) were partially vaccinated (three with one dose of inactive vaccine, two for whom less than 14 days elapsed since their second dose of inactivated vaccine, and two for whom less than 14 days elapsed since their dose of Ad5 vectored vaccine); 331 (48.3%) were fully vaccinated with two doses of inactivated vaccine and 282 (41.1%) were fully vaccinated with one dose of Ad5 vectored vaccine. Among the 331 individuals vaccinated with inactivated vaccine, 170 received homologous CoronaVac, 92 received homologous BBIBP-CorV, and 69 received one dose of each vaccine (Table 1 ).
Table 1.
Description of study subjects by vaccination status.
| Variable | Unvaccinated | Partially vaccinated | Fully vaccinated inactive vaccine | Fully vaccinated Adenovirus 5 vectored vaccine | Total |
|---|---|---|---|---|---|
| Clinical outcome | |||||
| Uninfected | 58(87.8) | 6(85.7) | 320(96.7) | 268(95.0) | 652(95.0) |
| Asymptomatic | 0(0) | 0(0) | 0(0) | 0(0) | 0(0) |
| Mild | 3(4.6) | 0(0) | 5(1.5) | 7(2.5) | 15(2.2) |
| Moderate | 3(4.6) | 1(14.3) | 6(1.8) | 7(2.5) | 17(2.4) |
| Severe | 1(1.5) | 0(0) | 0(0) | 0(0) | 1(0.2) |
| Critical | 1(1.5) | 0(0) | 0(0) | 0(0) | 1(0.2) |
| Sex | |||||
| Male | 29(43.9) | 5(71.4) | 267(80.7) | 180(63.8) | 481(70.1) |
| Female | 37(56.1) | 2(28.6) | 64(19.3) | 102(36.2) | 205(29.9) |
| Age group (years) | |||||
| 18–59 | 52(78.8) | 6(85.7) | 325(98.2) | 268(95.0) | 651(94.9) |
| ≥60 | 14(21.2) | 1(14.3) | 6(1.8) | 14(5.0) | 35(5.1) |
| Vaccine history | |||||
| unvaccinated | 66(100) | NA | NA | NA | 66(100) |
| One dose | |||||
| Corona Vac | NA | 2(40) | NA | NA | 2(0.7) |
| BBIBP-CorV | NA | 1(20) | NA | NA | 1(0.3) |
| Ad5-nCoV | NA | 2(40) | NA | 282(100) | 284(99.0) |
| Two doses | |||||
| Corona Vac | NA | 0(0) | 170(51.4) | NA | 170(51.1) |
| BBIBP-CorV | NA | 2(100) | 92(27.8) | NA | 94(28.2) |
| CoronaVac and BBIBP-CorV* | NA | 0(0) | 69(20.8) | NA | 69(20.7) |
| Total | 66(100) | 7(100) | 331(100) | 282(100) | 686(100) |
Means a dose of BBIBP-CorV followed by a dose of CoronaVac or vice versa.
Among the 620 subjects with documented vaccination histories, the median and quartile intervals (by days) from their last dose of vaccine to their exposure to SARS-CoV-2 were 11 days (9–12 days) for individuals partially vaccinated, 63 days (46–94) for individuals fully vaccinated with inactivated vaccine, and 64 days (61–66) for individuals fully vaccinated with Ad5 vectored vaccine. The intervals between vaccination and exposure are consistent with the timing of the vaccination campaign conducted in Ruili after the March 2021 outbreak. Because only 5.1% of the subjects were ≥60 years old, there were too few subjects for stratification by age. Because there were only 7 partially vaccinated individuals, there were too few subjects to estimate VE for partial vaccination.
Combined adjusted vaccine effectiveness for full vaccination with either inactivated vaccine was 74.6% (36.0–90.0) against symptomatic COVID-19, 76.7% (19.3–93.3) against COVID-19 pneumonia, and 100% (95% CI: 47.6–100) against severe/critical cases.
Unadjusted and adjusted VE estimates against the three outcomes stratified by vaccine manufacturer are shown in Table 2 . There were no statistically significant differences in VE estimates against symptomatic COVID-19 or pneumonia between CoronaVac and BBIBP-CorV vaccines: p = 1.0 for symptomatic COVID-19 and p = 0.481 for pneumonia.
Table 2.
Unadjusted and adjusted* vaccine effectiveness of full vaccination against symptomatic COVID-19, COVID-19 pneumonia, and severe/critical COVID-19.
| Outcome | Vaccination status | N (%) | RR | VE,% (95 %CI) | aVE,% (95 %CI) |
|---|---|---|---|---|---|
| Inactivated vaccine | |||||
| Symptomatic | Unvaccinated(n = 66) | 8 (12) | Ref | – | – |
| CoronaVac(n = 170) | 6 (3.5) | 0.291 | 70.9(19.3 ∼ 89.5) | 73.0(22.3 ∼ 90.6) | |
| BBIBP-CorV(n = 92) | 3 (3.3) | 0.269 | 73.1 (2.4 ∼ 92.6) | 75.5 (6.3 ∼ 93.6) | |
| CoronaVac and BBIBP-CorV*(n = 69) | 2 (2.9) | 0.239 | 76.1(−8.5 ∼ 94.7) | 78.1(−3.3 ∼ 95.3) | |
| Subtotal(n = 331) | 11(3.3) | 0.274 | 72.6 (34.5 ∼ 88.5) | 74.6 (36.0 ∼ 90.0) | |
| Pneumonia | Unvaccinated(n = 66) | 5(7.6) | Ref | – | – |
| CoronaVac(n = 170) | 2(1.2) | 0.155 | 84.5(21.9 ∼ 96.9) | 84.6(18.8 ∼ 97.1) | |
| BBIBP-CorV(n = 92) | 3(3.3) | 0.430 | 57.0(−73.8 ∼ 89.3) | 56.5(−95.9 ∼ 90.4) | |
| CoronaVac and BBIBP-CorV*(n = 69) | 1(1.5) | 0.191 | 80.9(−59.4 ∼ 97.7) | 80.8(−69.3 ∼ 97.8) | |
| Subtotal(n = 331) | 6(1.8) | 0.239 | 76.1 (23.9 ∼ 92.5) | 76.7(19.3 ∼ 93.3) | |
| Severe/Critical | Unvaccinated(n = 66) | 2(3.0) | Ref | – | – |
| CoronaVac(n = 170) | 0(0) | 0.00 | 100 (−1.6 ∼ 100) | 100 | |
| BBIBP-CorV(n = 92) | 0(0) | 0.00 | 100(−87.2 ∼ 100) | 100 | |
| CoronaVac and BBIBP-CorV*(n = 69) | 0(0) | 0.00 | 100(−149.0 ∼ 100) | 100 | |
| Subtotal(n = 331) | 0(0) | 0.00 | 100(47.6 ∼ 100) | 100 | |
| Ad5-nCoV vaccine | |||||
| Symptomatic | Unvaccinated(n = 66) | 8 (12) | Ref | – | – |
| Ad5-nCoV(n = 282) | 14(5.0) | 0.410 | 59.0(6.4 ∼ 82.1) | 61.5(9.5 ∼ 83.6) | |
| Pneumonia | Unvaccinated(n = 66) | 5(7.6) | Ref | – | – |
| Ad5-nCoV(n = 282) | 7(2.5) | 0.328 | 67.2(0 ∼ 89.3) | 67.9(1.7 ∼ 89.9) | |
| Severe/Critical | Unvaccinated(n = 66) | 2(3.0) | Ref | – | – |
| Ad5-nCoV(n = 282) | 0(0) | 0.00 | 100(36.6 ∼ 100) | 100 | |
*Adjusted for gender and age group (18–59 years old; 60 years and older); adjusted VE is designated as aVE in the column header.
**means a dose of BBIBP-CorV followed by a dose of CoronaVac or vice versa.
Adjusted vaccine effectiveness for full, one-dose vaccination with Ad5 vectored vaccine were 61.5% (95 %CI: 9.5–83.6) against symptomatic COVID-19, 67.9% (95 %CI: 1.7–89.9) against COVID-19 pneumonia, and 100% (95 %CI: 36.6–100) against severe/critical COVID-19 (Table 2). There were no statistically significant differences in VE estimates between the inactivated and the ad5-vectored vaccines.
3.3. RT-PCR Ct values
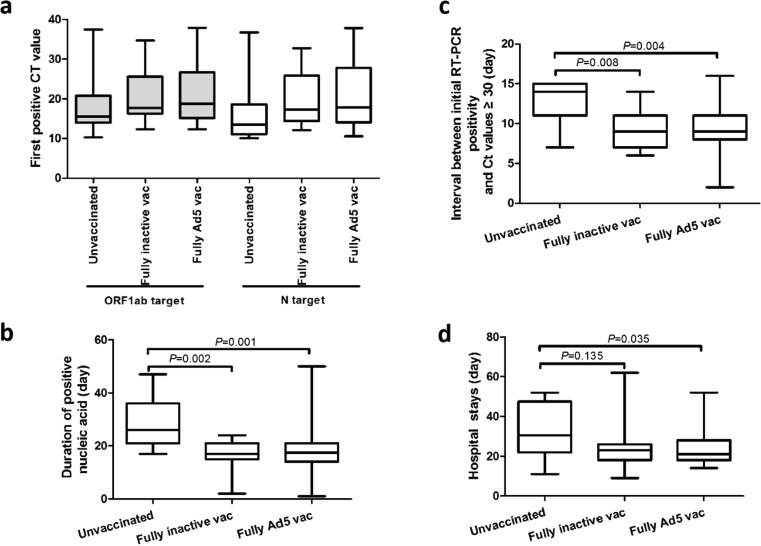
Point estimates of median Ct values of the first positive nucleic acid RT-PCR test for ORF1ab and N targets were slightly but not statistically significantly higher among individuals (n = 15) fully vaccinated with inactivated vaccine or fully vaccinated with Ad5 vectored vaccine (n = 44) compared with unvaccinated individuals (n = 11) (Fig. 1 a).
Fig. 1.
RT-PCR Ct values and duration of RT-PCR positivity. a. Distribution of Ct values of first positive nucleic acid test for ORF1ab and N targets in infected individuals by vaccination status. b. Duration of positive nucleic acid test results in infected persons by vaccination status. c. Interval between initial RT-PCR positivity and Ct values exceeding 30 by vaccination status. d. Hospital stays by vaccination status.
After categorizing the 68 patients with both ORF1ab and N target Ct results as being <30 or ≥30 for both targets, the proportion of vaccinated subjects with Ct values <30 was lower, but not statistically significantly lower and among unvaccinated individuals. Odds ratios by vaccine of higher and lower Ct value groups are shown in Table 3 .
Table 3.
Ct values of first nucleic acid detection by vaccination status.
| Vaccination status | Ct value ≥30 |
Ct value<30 |
OR | 95 %CI | |||
|---|---|---|---|---|---|---|---|
| N | % | N | % | ||||
| Unvaccinated(n = 11) | 1 | 9.09 | 10 | 90.91 | Ref | – | |
| Fully vaccinated inactivated vaccine(n = 15) | 2 | 13.33 | 13 | 86.67 | 0.65 | 0.05–8.23 | |
| Fully vaccinated Ad5 vaccine (n = 42) | 7 | 16.67 | 35 | 83.33 | 0.50 | 0.05–4.56 | |
| Total (n = 68) | 10 | 14.71 | 58 | 85.29 | |||
3.4. Duration of RT-PCR positivity
The median durations of RT-PCR positivity were 17 days for individuals vaccinated with inactivated vaccine (n = 15), 18 days for individuals vaccinated with Ad5 vectored vaccine (n = 44), and 26 days for unvaccinated individuals (n = 11). Mann-Whitney U testing showed statistically significant differences between unvaccinated and inactivated vaccine vaccinated (U = 24, P = 0.002) and unvaccinated and Ad5 vectored vaccine vaccinated (U = 77.50, P = 0.001) individuals (Fig. 1b).
Among unvaccinated individuals, the median time between initial RT-PCR positivity and when the Ct value became greater than 30 was 14 days. Intervals for fully inactivated vaccine vaccinated individuals and fully ad5 vectored vaccinated individuals were both 9 days. Differences in intervals between unvaccinated and inactivated or Ad5 vectored vaccinated individuals were statistically significant (U = 32, P = 0.008 and U = 104, P = 0.004) (Fig. 1c).
3.5. Duration of hospital stay
There were 71 infected individuals meeting criteria for analyzing length of hospital stay. The median hospital stay was 30.5 days for unvaccinated individuals, 23 days for individuals fully vaccinated with inactivated vaccine (p = 0.135), and 21 days for individuals vaccinated with Ad5 vectored vaccine (p = 0.035) (Fig. 1d).
4. Discussion
Our evaluation of the effectiveness of three COVID-19 vaccines in the setting of a Delta- (B.1.617.2) variant outbreak that occurred two months after a vaccination campaign showed that vaccine effectiveness levels of two inactivated COVID-19 vaccines, analyzed together, were 74.6% against symptomatic COVID-19, 76.7% against COVID-19 pneumonia, and 100% against serious/critical COVID-19 illness, with no statistically significant differences by brand of inactivated vaccine. VEs for the Ad5 vectored vaccine were similar, at 61.5% for symptomatic COVID-19, 67.9% for COVID-19 pneumonia and 100% for serious/critical COVID-19, with no statistically significant differences by vaccine type. These three vaccines are effective at preventing Delta-variant COVID-19, and are most effective at preventing more serious illness, which is the primary goal of the World Health Organization’s COVID-19 strategic vaccination response. These three vaccines therefore merit continued use in China and in other countries.
VEs for the inactivated vaccines measured in our study are similar to VE estimates reported from Chile for CoronaVac vaccine against the Alpha and Gamma variants (65.9% and 66.6%) [6], and in the United Arab Emirates for BBIBP-CorV prevention of hospitalisation due to COVID-19 from Non-VOC and Alpha variants (74%) [7]. In a similar population to our study population, McMenamin and colleagues showed 2-dose CoronaVac VE against severe disease and death among adults 60 years and older in Hong Kong’s 2022 wave of Omicron COVID-19 of 72.2% and 3-dose VE of 98.3% [8]. Our VE estimates for Cansino’s Ad5 vectored vaccine are similar to the efficacy levels demonstrated in a phase 3 clinical trial conducted prior to emergence of the Delta variant of 57.5% against RT-PCR confirmed COVID-19 and 91.7% against severe COVID-19 [9]. Thus, with the caveat that protection levels we measured represent protection approximately two months from time of vaccination and did not address booster doses, the three vaccines retain the effectiveness against Delta-variant SARS-CoV-2 as shown for earlier lineages of the coronavirus.
Secondary findings from our study were that, although vaccination was not associated with Ct values at the early stage of infection, vaccination was associated with reduction in the duration of RT-PCR positivity. Ultimately, this may reduce spread of infection, but with the caveat that RT-PCR positivity imperfectly correlates to infectiousness, since RT-PCR results can be positive for non-infectious virus particles [10]. Reduced duration of SARS-CoV-2 shedding has not been shown for other vaccines. In an incarcerated population in the US [11], duration of positive serial test results was similar for both vaccinated and unvaccinated groups, and infectious virus were cultured in specimen from both groups. Another study also indicated RT-PCR Ct values were similar among specimens from patients who were fully vaccinated and those who were not [12]. Our study is consistent with these findings, indicating that even with high vaccination rates, maintaining multicomponent prevention strategies is crucial. However, the shorter duration of RT-PCR positivity and the length of hospital stay among full vaccinated COVID-19 cases could potentially reduce health-care system resource needs, which is critically important in current and future pandemic situations.
Strengths of our study include that it was conducted in a well-defined and clinically meaningful cohort that was assembled in accordance with national guidance for prevention and control of COVID-19. The cohort members all had known exposure to someone infected with the Delta variant, and they were tested daily for infection. This ensures that the subjects had similar exposure risk without introduction of bias. Second, our real-world study allowed assessment of three epidemiologically and clinically meaningful outcomes: symptomatic COVID-19, COVID-19 pneumonia, and severe/critical COVID-19. Third, the study was able to provide information on duration of RT-PCR positivity, which is likely related to duration of viral shedding. Finally, since the study was conducted in China, well over a year since elimination of indigenous transmission of SARS-CoV-2 virus, it is a study of pure vaccine-induced immunity, as China has almost no hybrid immunity.
The findings in this report are subject to several limitations. First, due to the “zero tolerance local transmission” policy in China, the outbreak was stopped while small, making the sample size too small to evaluate VE among subgroups and partially vaccinated individuals. Second, the study began two months after a vaccination campaign in the study area, so our results reflect only short-term VE. Third, RT-PCR Ct values do not have a linear relation with virus load, which is subject to nasopharyngeal swab sampling technique and procedure tolerance of the individual tested. Fourth, there were some subjects for whom an exact contact date could not be determined. We do not believe that this will compromise vaccination status assessment because there was more than one month between the end of the vaccination campaign and the start of the outbreak. Finally, determining duration of protection, protection against future variants, and vaccine impact on infectivity will require new studies.
In conclusion, our study showed that full vaccination with Cansino’s Ad5 vectored COVID-19 vaccine or the two whole-virus, Vero-cell grown, alum-adjuvanted inactivated COVID-19 vaccines – CoronaVac and BBIBP-Cor – effectively prevent symptomatic COVID-19, COVID-19 pneumonia, and severe illness caused by the SARS-CoV-2 Delta variant. VE was greatest for the more severe forms of COVID-19. VE estimates of these vaccines are similar to VE estimates against earlier lineages of SARS-CoV-2 – well within the desirable VE range in the World Health Organization’s COVID-19 vaccines target product profile. Continued use of these vaccines in China and in other countries is therefore warranted, as is continued VE assessment when new variants cause local transmission.
Funding
China CDC: The Emergency Response Mechanism Operation Program (131031001000200001).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.WHO. WHO Coronavirus (COVID-19) Dashboard. https://covid19.who.int/2021.
- 2.Tang J.L., Abbasi K. What can the world learn from China's response to covid-19? BMJ (Clinical research ed) 2021;375 doi: 10.1136/bmj.n2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu F., Zheng C., Wang L., et al. Interpretation of the Protocol for Prevention and Control of COVID-19 in China (Edition 8) China CDC Wkly. 2021;3(25):527–530. doi: 10.46234/ccdcw2021.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li X.-N., Huang Y., Wang W., Jing Q.-L., Zhang C.-H., Qin P.-Z., et al. Effectiveness of inactivated SARS-CoV-2 vaccines against the Delta variant infection in Guangzhou: a test-negative case-control real-world study. Emerging Microbes Infect. 2021;10(1):1751–1759. doi: 10.1080/22221751.2021.1969291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.National Health Commission of China. Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia (Trial Version 8). http://www.gov.cn/zhengce/zhengceku/2021-04/15/content_5599795.htm 2021.
- 6.Jara A., Undurraga E.A., González C., Paredes F., Fontecilla T., Jara G., et al. Effectiveness of an Inactivated SARS-CoV-2 Vaccine in Chile. New Engl J Med. 2021;385(10):875–884. doi: 10.1056/NEJMoa2107715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.AlHosani1 FI, Stanciole AE, Bashir Aden AT, et al. Sinopharm’s BBIBP-CorV vaccine effectiveness on preventing hospital admission and deaths: results from a retrospective study in the Emirate of Abu Dhabi, United Arab Emirates (UAE). https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3951143 2021. [DOI] [PMC free article] [PubMed]
- 8.McMenamin ME, Nealon J, Lin Y, et al. Vaccine effectiveness of two and three doses of BNT162b2 and CoronaVac against COVID-19 in Hong Kong. MedRxiv preprint doi: 10.1101/2022.03.22.22272769. [DOI] [PMC free article] [PubMed]
- 9.Halperin S.A., Ye L., MacKinnon-Cameron D., Smith B., Cahn P.E., Ruiz-Palacios G.M., et al. Final efficacy analysis, interim safety analysis, and immunogenicity of a single dose of recombinant novel coronavirus vaccine (adenovirus type 5 vector) in adults 18 years and older: an international, multicentre, randomised, double-blinded, placebo-controlled phase 3 trial. The Lancet. 2022;399(10321):237–248. doi: 10.1016/S0140-6736(21)02753-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bullard J., Dust K., Funk D., Strong J.E., Alexander D., Garnett L., et al. Predicting Infectious Severe Acute Respiratory Syndrome Coronavirus 2 From Diagnostic Samples. Clin Infect Dis : Off Publ Infect Dis Soc Am. 2020;71(10):2663–2666. doi: 10.1093/cid/ciaa638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hagan LM, McCormick DW, Lee C, et al. Outbreak of SARS-CoV-2 B.1.617.2 (Delta) Variant Infections Among Incarcerated Persons in a Federal Prison - Texas, July-August 2021. MMWR Morb Mortal Wkly Rep 2021; 70(38): 1349–54. [DOI] [PMC free article] [PubMed]
- 12.Brown C.M., Vostok J., Johnson H., Burns M., Gharpure R., Sami S., et al. Outbreak of SARS-CoV-2 Infections, Including COVID-19 Vaccine Breakthrough Infections, Associated with Large Public Gatherings - Barnstable County, Massachusetts, July 2021. MMWR Morb Mortal Wkly Rep. 2021;70(31):1059–1062. doi: 10.15585/mmwr.mm7031e2. [DOI] [PMC free article] [PubMed] [Google Scholar]