Abstract
Research on optimal relative dose intensity (RDI) of FOLFOX in stage III colon cancer by risk profiles is scarce. Using cancer registries’ data, our study found when the risk of death remained the same, the minimum RDI of FOLFOX was as low as 70% for high-risk stage III colon cancer patients and as low as 45% for low-risk patients.
Background:
The National Comprehensive Cancer Network (NCCN) guidelines have recommended tailored chemotherapy for stage III high-risk (T4 and/or N2) and low-risk (T1-T3 and N1) colon cancer since 2018. Studies have investigated the effect of relative dose intensity (RDI) of FOLFOX on stage III colon cancer survival, however, none has performed a stratified analysis by risk profiles. This study aims to identify the FOLFOX optimal RDI for high-risk and low-risk stage III colon cancer patients.
Methods:
Data on 407 eligible patients, diagnosed with stage III colon cancer in 2011 who received FOLFOX, were collected by 8 population-based cancer registries. Multivariable Cox model and Fine-Gray competing risks model were employed to explore Optimal RDI defined as the lowest RDI administered without significant differences in either overall or cause-specific death.
Results:
Among the 168 high-risk patients, the optimal RDI cut-off was 70% (HR = 1.59 with 95% CI: 0.69-3.66 in overall mortality; HR = 1.24 with 95% CI: 0.42-3.64 in cause-specific mortality when RDI < 70% vs. RDI ≥ 70%). Among the 239 low-risk patients, none of the evaluated cut-offs were associated with significant differences in risk of death between comparison groups. The lowest assessed RDI was 45%, HR = 0.80; 95% CI: 0.24 to 2.73 for overall mortality and HR = 0.53; 95% CI: 0.06 to 4.95 for cause-specific mortality, when RDI <45% versus RDI ≥45%.
Conclusions:
There is no significant harm on the risk of death when reducing RDI by <30% for high-risk patients. For the low-risk patients, we found that RDI as low as 45% did not significantly affect the risk of death.
Keywords: Chemotherapy toxicity, High-risk stage III, Low-risk stage III, Mortality, Tailored chemotherapy
Background
Colorectal cancer (CRC) is the fourth most commonly diagnosed cancer, and the second leading cause of cancer death for males and females combined in the United States (US).1 For patients with stage III colon cancer, the National Comprehensive Cancer Network (NCCN) has recommended adjuvant chemotherapy with fluoropyrimidines and oxaliplatin by risk profiles.2, 3 FOLFOX (Oxaliplatin, fluorouracil aka 5-FU, and leucovorin) is one of the standard adjuvant therapies commonly administered.2, 4, 5
Despite the efficacy of FOLFOX, this regimen usually results in significant toxicities.6–9 Six randomized phase III clinical trials have been conducted in 12 countries to evaluate the noninferiority of adjuvant therapy with either FOLFOX or CAPOX (capecitabine and oxaliplatin) administered for 3 months, as compared with 6 months.10 The meta-analysis of these trials showed that for stage III colon cancer patients, 3-month FOLFOX therapy resulted in a lower disease-free survival rate, particularly for the high-risk subgroup (patients with T4, N2, or both) (definitions of T and N are in Appendix 1), whereas among patients with low-risk cancers (T1, T2, or T3 and N1), the noninferiority with the 3-month chemotherapy use was not proven.10
Previous observational studies have also found that patients who failed to complete the standard cycles of 5-FU chemotherapy had worse colon cancer-specific survival,11, 12 or overall survival.11 These studies, however, didn’t consider dose intensity or cumulative dose. Dose intensity is defined as the amount of drug delivered per unit time per square meter of body surface area, and relative dose intensity (RDI) is the ratio of delivered dose intensity (DDI) to the standard dose intensity (SDI).13, 14 Aspinall et al investigated the effect of RDI of multiple chemotherapy regimens (FOLFOX included) on survival among 367 stage III colon cancer patients and found that those who received >70% of SDI had higher 5-year overall survival.15 A study based on data from Korea found that for patients with stage II/III CRC receiving adjuvant FOLFOX,16 >60% of the standard dose of oxaliplatin was necessary to achieve similar 5-year disease-free survival or overall survival to the standard dose group. Both studies used data from healthcare facilities with limited generalizability, and neither of these studies considered the effect of RDI by the tumor risk, as defined in clinical trial studies.
Hence, we used data collected by population-based cancer registries covering all patients in selected US states to identify the optimal RDI of FOLFOX for high-risk and low-risk stage III colon cancer, respectively, regarding the effect of FOLFOX RDI on overall and cause-specific mortality in these 2 patient populations.
Methods
Data Source and Study Population
Data were obtained from 8 state central cancer registries (AK, CO, FL, ID, LA, NC, NH, RI) participating in the Enhancing Cancer Registry Data for Comparative Effectiveness Research (CER) Project, funded by the Centers for Disease Control and Prevention (CDC) National Program of Cancer Registries (NPCR). Details of the CER study have been described elsewhere.17
Patients diagnosed with stage III colon cancers in 2011 who received colon resection and chemotherapy of FOLFOX4 or FOLFOX6 were eligible for this study. Colon cancer was defined according to the SEER site recode of the International Classification of Diseases for Oncology third edition codes of C18.0, C18.2-C18.9 (https://seer.cancer.gov/siterecode/icdo3_dwhoheme/index.html) We further excluded patients who had unknown risk groups, unknown number of lymph nodes, died within 30 days of colon resection, received neoadjuvant chemotherapy, or had missing data on vital status, date of last contact, or RDI related variables. A flow chart of patient selection was presented in Appendix 2.
Outcomes
Two outcomes were defined in this study: all-cause and cause-specific mortality. State cancer registries linked their data with state death certificate data files and the National Death Index (NDI) database to obtain data on vital status and causes of death. We used the Surveillance, Epidemiology, and End Results Program (SEER) cause-specific death classification to define the death due to colon cancer, which took into account causes of death in conjunction with tumor sequence, the primary site of the cancer diagnosis, and comorbidities.18
The survival time was measured in months from colon cancer diagnosis to the date of death or last follow-up if a participant was still alive. The observations were censored if patients were alive during the time of clinical follow-up.
Exposure
Chemotherapy dose intensity was defined as the amount of drug administered per unit time (week or day) per square meter of body surface area (BSA).13, 14, 19 Chemotherapy RDI was the ratio of DDI to the SDI.2 The details of the RDI calculation are described in Appendix 2.
Based on previous research that optimal RDI of adjuvant chemotherapy was 70%15 for patients with stage III colon cancer, and clinical trials suggesting that a high dose of chemotherapy (6 months of FOLFOX other than 3 months) was needed for high-risk stage III colon cancer patients10 (see Appendix 1 for the definition of risk groups), we defined the low and high RDI groups by using the cut-off points of 55%, 60%, 65%, 70%, 75%, and 80% for high-risk patients. For low-risk patients, we used the cut-off points of 45%, 50%, 55%, 60%, 65%, and 70%. For each cut-off point, patients receiving RDI greater than or equal to the predefined cut-off point were compared with patients receiving RDI lower than the predefined cut-off point, on their overall and cause-specific mortality. The optimal RDI was defined as the lowest RDI administered without a significant increase in either overall mortality or cause-specific mortality.
Covariates
Covariates included age at diagnosis, race/ethnicity,20 health insurance, census-tract residence, poverty, education,21, 22 marital status,23 tumor size,24,25 lymph nodes retrieved,26 tumor grade, Charlson comorbidity index (0, ≥1),27 anatomic subsites,28 colon cancer classification,29 number of positive lymph nodes, and delayed chemotherapy.30
Statistical Analysis
Socio-demographic and clinical characteristics of the patients were summarized using descriptive statistics.
The overall survival probability was estimated using the Kaplan-Meier method and log rank test. The probabilities of cause-specific mortalities were estimated using the cumulative incidence function (CIF), which were compared among the various RDI groups using the Gray’s test.31 Cox proportional hazards model and the competing risks model developed by Fine and Gray were applied to estimate the hazard ratio (HR) for overall mortality and cause-specific mortality, respectively. The proportional hazards assumption was tested using the Schoenfeld residuals.
In sensitivity analyses, we restricted our sample to patients with colon cancer as the only or the first primary tumor, to exclude the effects of previously diagnosed cancers on survival or on chemotherapy use.
The multivariable models testing whether risk profile modifies the association of RDI with the overall and cause-specific mortalities were employed.
All analyses were conducted using SAS, version 9.4 (SAS Institute, Inc., Cary, NC). A P value<.05 was considered statistically significant.
Results
Patient Characteristics
168 patients with high risk and 239 with low risk stage III colon cancer were included in this study (Table 1). In the high-risk group, patients aged ≥ 60 years accounted for more than half of the cases. Most patients were male, non-Hispanic whites (67%), and privately insured (56%). 60% of patients resided in urban areas, 71% lived in low poverty areas, 78% were in high education areas, 51% were in high married census tracts, and more than 70% were from the states of Florida, Louisiana, and North Carolina. More than half of patients had tumor size greater than 4 cm (57%), had 12 or greater lymph nodes examined (92%), well or moderately differentiated tumors (65%), no comorbid conditions (67%), cancer located in proximal colon (62%), colon cancer as the only cancer (82%), and started chemotherapy within 8 weeks after surgery (77%). The mean number of positive lymph nodes was 6. About 80% of the patients were alive at the end of follow up, with the mean/median follow up time of 35/40 months. Among deceased patients with a known cause of death, 16% died from noncolon cancer causes.
Table 1.
Demographic and Clinical Characteristics of Patients With Stage III Colon Cancer by Different Risk Groups
| Characteristics | High Risk (T4 and/or N2) | Low Risk (T1-3/N1) |
|---|---|---|
| N (%) | N (%) | |
| All | 168 | 239 |
| Age at diagnosis | ||
| <50 | 31 (18.5) | 48 (20.1) |
| 50-59 | 37 (22.0) | 60 (25.1) |
| 60-69 | 56 (33.3) | 75 (31.4) |
| ≥70 | 44 (26.2) | 56 (23.4) |
| Sex | ||
| Male | 92 (54.8) | 126 (52.7) |
| Female | 76 (45.2) | 113 (47.3) |
| Race/Ethnicity | ||
| Non-Hispanic white | 113 (67.3) | 161 (67.4) |
| Non-Hispanic black | 31 (18.5) | 44 (18.4) |
| Hispanics | 21 (12.5) | 29 (12.1) |
| Other | 3 (1.8) | 5 (2.1) |
| Insurance coverage | ||
| Private | 94 (56.0) | 130 (54.4) |
| Medicare/Other public | 48 (28.6) | 71 (29.7) |
| Medicaid | 15 (8.9) | 21 (8.8) |
| Not insured | 9 (5.4) | 16 (6.7) |
| Unknown | 2 (1.2) | 1 (0.4) |
| Census tract residence | ||
| 100% Urban | 101 (60.1) | 126 (52.7) |
| 100% Rural | 10 (6.0) | 16 (6.7) |
| Mixed | 55 (32.7) | 95 (39.8) |
| Unknown | 2 (1.2) | 2 (0.8) |
| Census tract poverty | ||
| <20% | 120 (71.4) | 184 (77.0) |
| ≥20% | 45 (26.8) | 53 (22.2) |
| Unknown | 3 (1.8) | 2 (0.8) |
| Census tract education | ||
| <25% (high) | 131 (78.0) | 186 (77.8) |
| ≥25% (low) | 35 (20.8) | 51 (21.3) |
| Unknown | 2 (1.2) | 2 (0.8) |
| Census tract marital status | ||
| ≤50% married | 81 (48.2) | 104 (43.5) |
| >50% married | 85 (50.6) | 133 (55.7) |
| Unknown | 2 (1.2) | 2 (0.8) |
| State of residence | ||
| AK | 2 (1.2) | 1 (0.4) |
| CO | 21 (12.5) | 42 (17.6) |
| FL | 48 (28.6) | 54 (22.6) |
| ID | 7 (4.2) | 15 (6.3) |
| LA | 45 (26.8) | 64 (26.8) |
| NC | 38 (22.6) | 58 (24.3) |
| NH | 6 (3.6) | 4 (1.7) |
| RI | 1 (0.6) | 1 (0.4) |
| Tumor size (cm) | ||
| ≤4 | 69 (41.1) | 130 (54.4) |
| >4 | 96 (57.1) | 97 (40.6) |
| Unknown | 3 (1.8) | 12 (5.0) |
| Lymph nodes examined | ||
| <12 | 13 (7.7) | 34 (14.2) |
| ≥12 | 155 (92.3) | 205 (85.8) |
| Tumor grade | ||
| Well/moderately differentiated | 109 (64.9) | 194 (81.2) |
| Poor/Undifferentiated | 58 (34.5) | 39 (16.3) |
| Unknown | 1 (0.6) | 6 (2.5) |
| Charlson Comorbidity Index | ||
| 0 | 113 (67.3) | 176 (73.6) |
| ≥1 | 55 (32.7) | 63 (26.4) |
| Anatomic subsite | ||
| Proximal colon (C18.0, C18.2-C18.5) | 104 (61.9) | 141 (59.0) |
| Distal colon (C18.6-C18.7) | 63 (37.5) | 92 (38.5) |
| Other (C18.8-C18.9) | 1 (0.6) | 6 (2.5) |
| Colon cancer classification | ||
| Only with colon cancer | 138 (82.1) | 197 (82.4) |
| Multiple cancers, first primary colon | 12 (7.1) | 16 (6.7) |
| Multiple cancers, nonfirst colon | 18 (10.7) | 26 (10.9) |
| Number of positive lymph nodes | 6.4 ± 4.5 | 2.0 ± 1.7 |
| Delayed chemotherapy | ||
| Yes (>8 weeks after surgery) | 36 (21.4%) | 56 (23.4%) |
| No (≤8 weeks after surgery) | 132 (78.6%) | 183 (76.6%) |
| Vital status | ||
| Alive | 135 (80.4) | 210 (87.9) |
| Death from colon cancer | 21 (12.5) | 15 (6.3) |
| Death from other causes | 4 (2.4) | 8 (3.3) |
| Death reasons unknown | 8 (4.8) | 6 (2.5) |
| Follow up time among alive patients (mean± standard deviation) | 34.6± 21.9 | 35.0± 22.8 |
| Median (interquartile range) | 40.3 (10.5-54.8) | 43.9 (10.6-57.1) |
The low-risk stage III colon cancer cases had similar distributions of sociodemographic characteristics as those with high-risk stage III colon cancer. However, their clinical characteristics were less advanced and aggressive compared to high-risk cases. There were 88% of the patients alive at the end of follow up, with the mean/median follow up time of 35/44 months. Among deceased patients with a known cause of death, 35% died from competing risks.
Regression Analysis for All-Cause and Cause-Specific Mortality in High-Risk Stage III Colon Cancer Patients
Kaplan-Meier curves and CIF plots showed that among the high-risk group, those receiving lower RDI had statistically significantly (borderline significant in CIF plots for cutoff point of 65%) lower overall survival probability and a higher risk of colon-cancer death than those receiving higher RDI, when the cutoff points were 55%, 60%, 65% (Figures 1 and 2). The survival probability and risk of death were not statistically significantly different for other RDI comparisons. The univariable analysis showed consistent results with hazard ratios (Appendix 4).
Figure 1.
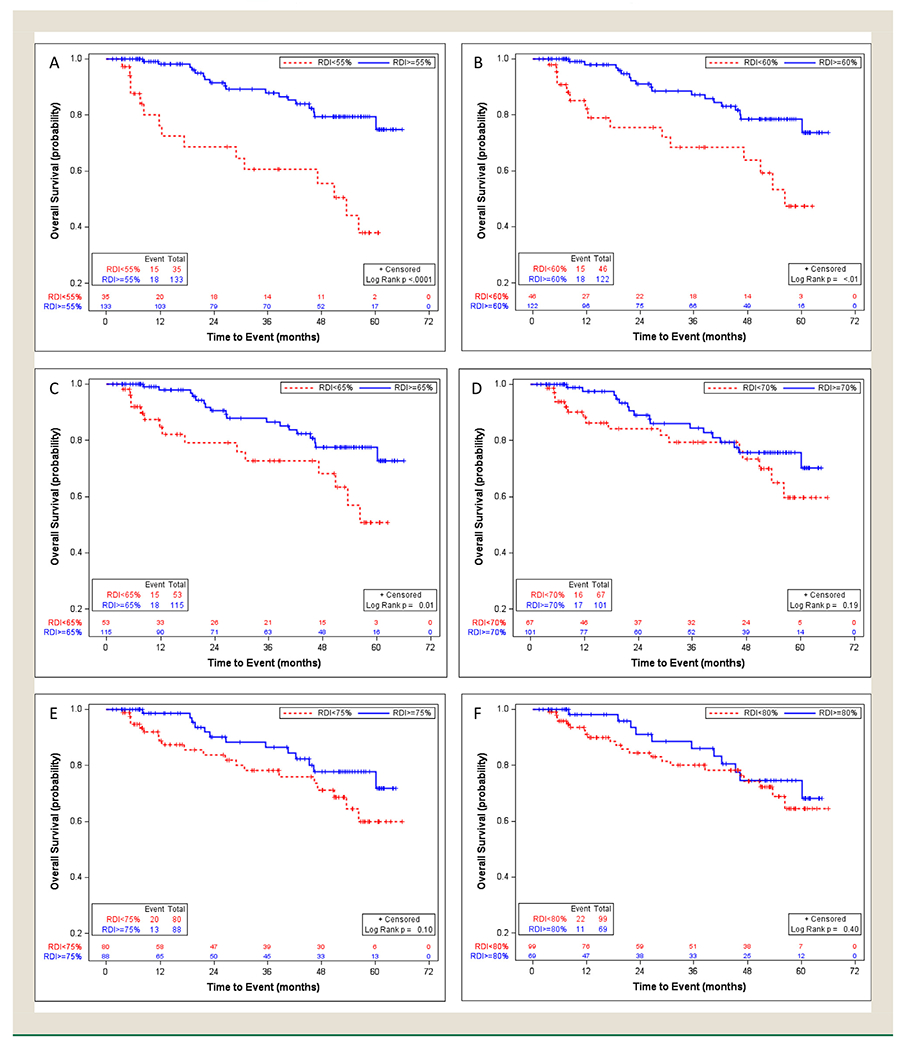
Overall survival in the high-risk stage III colon cancer patients with different relative dose intensity cut-off points of 55% (A), 60% (B), 65% (C), 70% (D), 75% (E), and 80% (F), using Kaplan-Meier method
Figure 2.
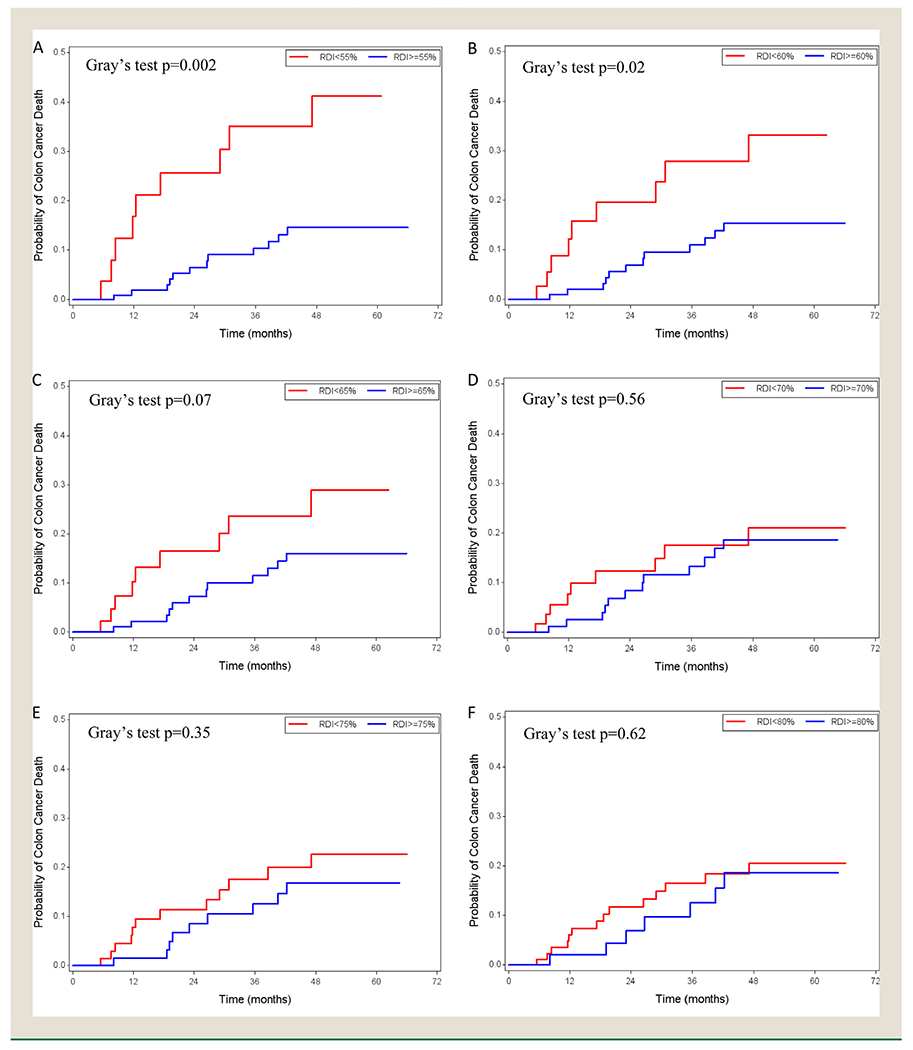
Cumulative Incidence Function plots in the high-risk stage III colon cancer patients with relative dose intensity cut-off points of 55% (A), 60% (B), 65% (C), 70% (D), 75% (E), and 80% (F)
In multivariable analysis, age, sex, race/ethnicity, insurance coverage (private insurance vs. nonprivate insurance), number of positive lymph nodes, tumor grade, Charlson comorbidity index, anatomic subsite, colon cancer classification (colon as only cancer vs. multiple primary cancers) and delayed chemotherapy were included as confounders or significant factors for cancer survival (Table 2). Compared to the higher RDI groups, the lower RDI groups had a higher risk of overall mortality: In the sensitivity analysis which included cases with colon cancer as the only tumor, results followed a similar pattern (data not shown). the all-cause and cause-specific mortalities showed no statistically significant difference for RDI comparisons: RDI< 70% versus RDI ≥70%, RDI< 75% versus RDI ≥75%, and RDI< 80% versus RDI ≥80%.
Table 2.
Impact of RDI Levels of FOLFOX Chemotherapy on All-Cause Mortality and Cause-Specific Mortality in High-Risk Stage III Colon Cancer— Multivariable Analysis
| RDI | All-Cause Mortalityb (N = 161) | Cause-Specific Mortality (N = 153) | |||
|---|---|---|---|---|---|
| Low RDI% (RDI<Selected Cutoffs | Adjusted HRa (95% CI) | P | Adjusted HRa (95% CI) | P | |
| RD< 55% vs. RDI≥ 55% | 20.5 | 3.61 (1.53, 8.49) | .003 | 3.38 (1.18, 9.70) | .02 |
| RDI< 60% vs. RDI≥ 60% | 26.7 | 2.54 (1.13, 5.71) | .02 | 2.28 (0.88, 5.86) | .09 |
| RDI< 65% vs. RDI≥ 65% | 31.1 | 2.39 (1.06, 5.39) | .04 | 2.07 (0.80, 5.33) | .13 |
| RD< 70% vs. RDI≥ 70% | 39.8 | 1.59 (0.69, 3.66) | .27 | 1.24 (0.42, 3.64) | .69 |
| RDI< 75% vs. RDI≥ 75% | 47.8 | 1.86 (0.85, 4.07) | .12 | 1.74 (0.71, 4.27) | .23 |
| RD< 80% vs. RDI≥ 80% | 59.6 | 1.41 (0.60, 3.32) | .44 | 1.39 (0.53, 3.69) | .50 |
Models adjusted for age, sex, race/ethnicity, insurance coverage, number of positive lymph nodes, tumor grade, Charlson comorbidity index, anatomic subsite, colon cancer classification (colon as the only cancer vs. multiple primary cancers), and delayed chemotherapy.
All variables met the proportional hazards assumption with P values of the correlation coefficient between Schoenfeld residuals and survival time >.05.
To avoid loss from the risk of death, this study showed a minimum RDI of 70% may be administered.
Regression Analysis for All-Cause and Cause-Specific Mortality in Low-Risk Stage III Colon Cancer Patients
Kaplan-Meier curves and CIF plots were not significantly different at any of the RDI cut-off points from 45% to 70% (Figures 3 and 4). The univariable analysis showed consistent results with hazard ratios (Appendix 5).
Figure 3.
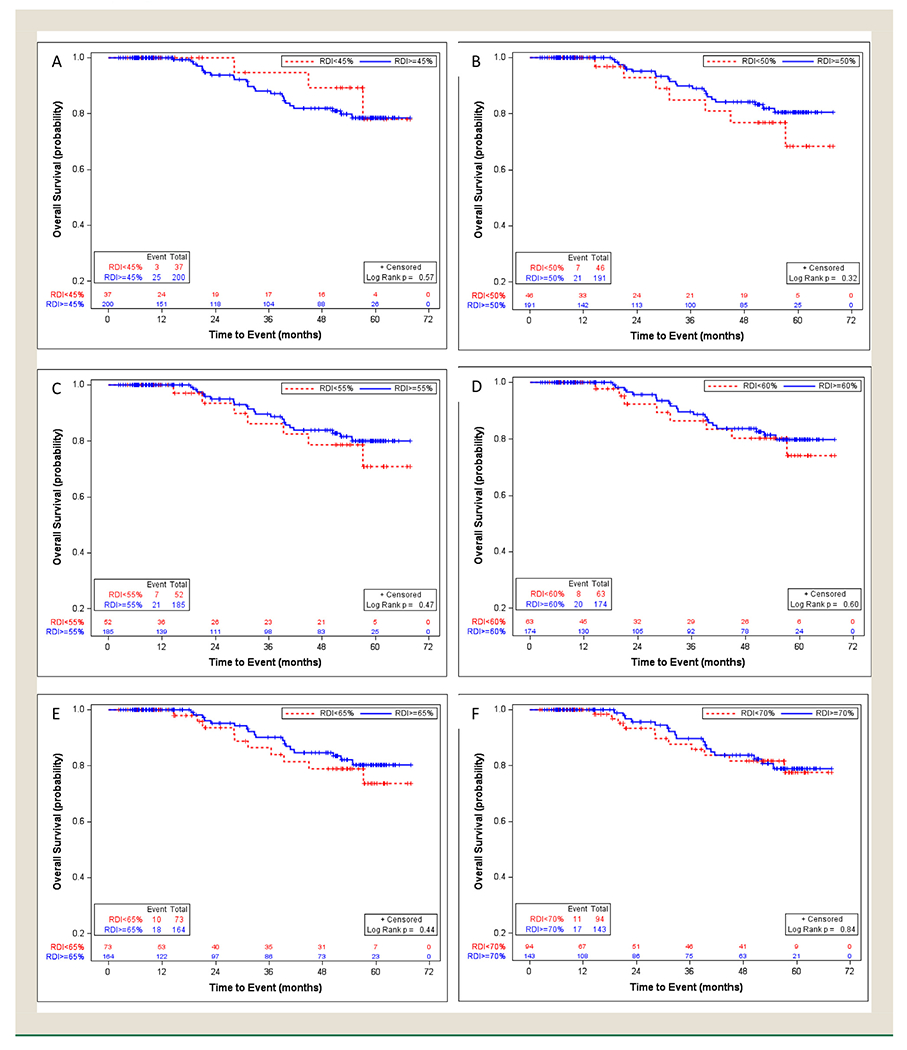
Overall survival in the low-risk stage III colon cancer patients with relative dose intensity cut-off points of 45% (A), 50% (B), 55% (C), 60% (D), 65% (E), and 70% (F), using Kaplan-Meier method
Figure 4.
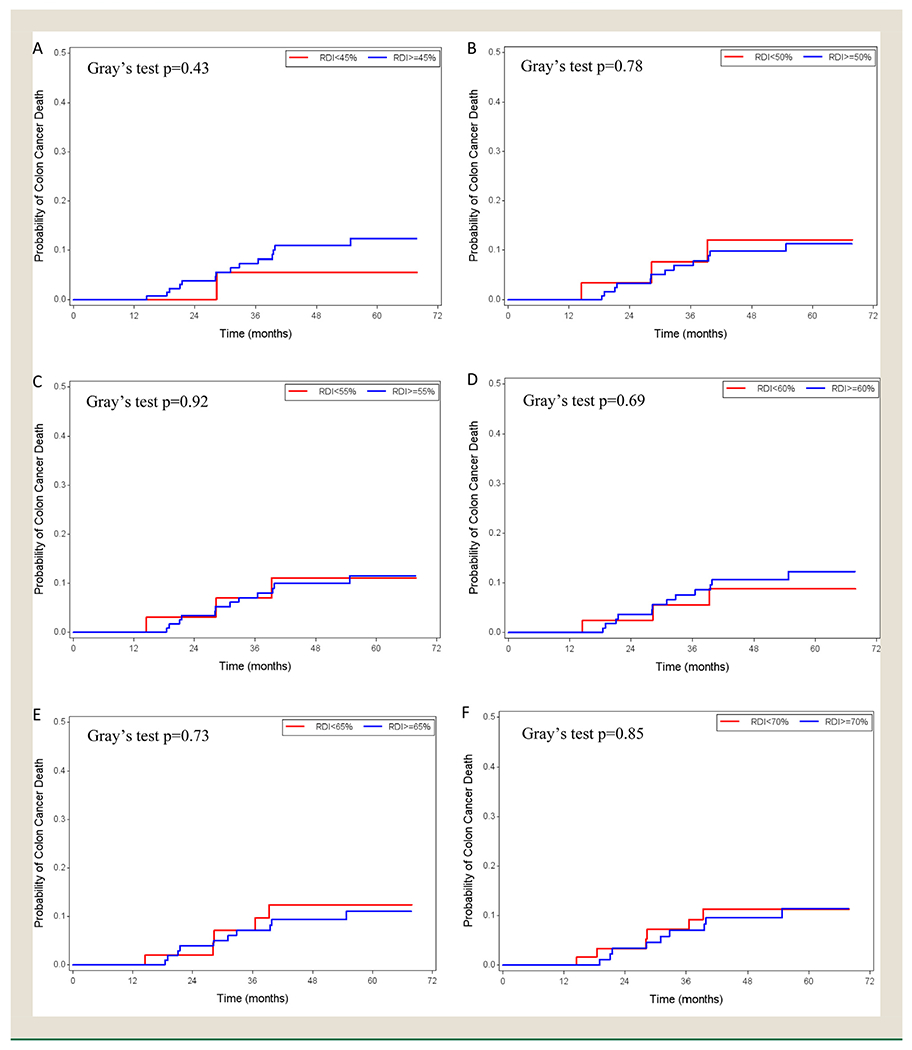
Cumulative Incidence Function plots in the low-risk stage III colon cancer patients with relative dose intensity cut-off points of 45% (A), 50% (B), 55% (C), 60% (D), 65% (E), and 70% (F)
After adjusting for age, sex, race, insurance coverage, number of positive lymph nodes, tumor grade, Charlson comorbidity index, anatomic subsite, and colon cancer classification, and delayed chemotherapy which were confounders or significant factors for cancer survival (Table 3), no cut-off points were significant hazard ratios in either all-cause or cause-specific mortality. Because of the small sample size, we did not explore the mortality influence of RDI<40%. Results were similar in the sensitivity analysis which included cases with colon cancer as the only tumor (data not shown).
Table 3.
Impact of RDI Levels of FOLFOX Chemotherapy on All-Cause Mortality and Cause-Specific Mortality in Low-Risk Stage III Colon Cancer— Multivariable Analysis
| RDI | All-Cause Mortality (N = 221)b | Cause-Specific Mortality (N = 216) | |||
|---|---|---|---|---|---|
| Low RDI% (RDI<Selected Cutoffs | Adjusted HRa (95% CI) | P | Adjusted HRa (95% CI) | P | |
| RDI< 45% vs. RDI≥ 45% | 16.3 | 0.80 (0.24, 2.73) | .72 | 0.53 (0.06, 4.95) | .58 |
| RDI< 50% vs. RDI≥ 50% | 19.9 | 1.26 (0.47, 3.41) | .65 | 0.75 (0.16, 3.46) | .71 |
| RD< 55% vs. RDI≥ 55% | 22.2 | 1.24 (0.46, 3.33) | .67 | 0.74 (0.16, 3.39) | .70 |
| RDI< 60% vs. RDI≥ 60% | 27.2 | 1.08 (0.41, 2.81) | .88 | 0.40 (0.09, 1.84) | .24 |
| RDI< 65% vs. RDI≥ 65% | 31.7 | 0.96 (0.36, 2.59) | .94 | 0.79 (0.16, 3.96) | .78 |
| RDI< 70% vs. RDI≥ 70% | 40.7 | 0.80 (0.31, 2.02) | .63 | 0.93 (0.21, 4.15) | .92 |
Models adjusted for age, sex, race/ethnicity, insurance coverage, number of positive lymph nodes, tumor grade, Charlson comorbidity index, anatomic subsite, colon cancer classification (colon as the only cancer vs. multiple primary cancers), and delayed chemotherapy.
All variables met the proportional hazards assumption with P values of the correlation coefficient between Schoenfeld residuals and time > .05.
Regression Analysis for All-Cause and Cause-Specific Mortality in Multivariable Models Including the Risk Profile and RDI Interaction
The results from the multivariable models with the above covariates, the risk profile, RDI, and interaction between risk profile and RDI showed that the impact of RDI on overall and cause-specific mortalities varied by the risk profile (Appendix 6). The interaction between risk profile and RDI was statistically significant (P < .05) in models of either overall mortality or cause-specific mortality when RDI cut points were 45%, 50%, 55%, and 60%.
Discussion
To our knowledge, this is the first population-based study to examine the association of FOLFOX RDI with the risk of overall and cause-specific deaths in 2 subgroups of patients diagnosed with stage III colon cancer. We found that the association of RDI on overall and cause-specific mortalities varied significantly by the risk profile. The risk-stratified analysis showed that in high-risk stage III colon cancer patients, lower RDI was related to higher risk of all-cause mortality when the cutoff points were 55%, 60%, or 65%. The risk of deaths was not statistically significantly different for the RDI comparisons of RDI <70% versus RDI ≥70%, RDI< 75% versus RDI ≥75%, and RDI< 80% versus RDI ≥80%. Therefore, to preserve the survival benefits, the lowest RDI from this study is 70%. For low-risk (T1-T3 and N1) stage III colon cancer, we did not find a significant difference in overall and cause-specific mortalities at any predefined RDI cutoff points from 45% to 70%.
Our findings confirmed that higher chemotherapy RDI was needed in high-risk stage III colon cancers, compared with low-risk cancers, and are consistent with the conclusions from 6 randomized, phase 3 clinical trials.5, 10, 32, 33 Results from a pooled analysis of these 6 trials showed that in stage III colon cancer patients treated with FOLFOX, 6-month therapy had a higher rate of disease-free survival than 3-month therapy, particularly in the high-risk group.10 Data from the IDEA France suggested that for FOLFOX6 regimen, patients with high-risk stage III colon cancer need 6-month chemotherapy for a maximal relapse risk reduction, for low-risk cancer patients, the absolute difference in the 3-year disease-free survival rate between 6 and 3 months of chemotherapy was clinically less relevant (2%).33 Older clinical trials have also shown that the efficacy of oxaliplatin is more significant in stage III N2 tumors, compared to stage III N1 tumors34, 35 (10-year overall survival advantage of 12.9% (P = .013) in those with N2 tumors, and 6% (P = .248) in those with N1 tumors, when adding oxaliplatin to 5-FU plus leucovorin35). André et al suggested defining a low-risk subset of patients with stage III colon cancer to spare the toxicity of oxaliplatin.35
Oxaliplatin can induce SPN, which can be long-lasting or even permanent.8, 9, 36 Reduced chemotherapy RDI to a minimal effective dose may spare neurotoxicity and maintain quality of life in cancer survivors. Our results found that the optimal cut-off point of RDI was 70% in the high-risk stage III colon cancer patients, which implied for patients who need dose reductions due to toxicities, a 70% of the preplanned dose does not seem to impact survival outcomes; we didn’t find significant differences in risk of death at predefined RDI cutoff points of 45%, 50%, 55%, 60%, 65%, and 70% for low-risk stage III colon cancer. A few other observational studies have investigated the effect of RDI on colon cancer survival. Aspinall et al found that patients from Veterans medical centers with stage III colon cancer who received >70% RDI adjuvant chemotherapy had improved 5-year overall survival, compared to the ones receiving RDI≤ 70%; and the survival benefits were only seen in the first year after the completion of chemotherapy.15 However, unlike the chemotherapy regime in our study, the regimens in their study included 5-FU/LV (30.8%), FOLFOX (34.3%), oxaliplatin plus capecitabine (5.4%), capecitabine monotherapy (12.5%), and mixed/others chemo (16.9%). Given that the toxicity of oxaliplatin is more severe than 5-FU, the optimal RDI of FOLFOX may be lower than 70% in combined high-risk and low-risk stage III colon cancer as the defined population in Aspinall’s study.15 Another study performed in Korea University Anam Hospital, with patients receiving FOLFOX as the only chemotherapy regimen, suggested that more than 60% of the standard dose of oxaliplatin was necessary to achieve similar 5-year disease-free survival or overall survival to those of the standard dose group, for patients with stage II/III CRC patients.16 Both of the studies from Veterans medical centers and Korea University Anam Hospital had a restricted population, and they didn’t take into consideration risk features in stage III colon cancers. Those are some reasons that may contribute to the discrepancies about optimal RDI cut off points.
There are several strengths in this study. The study cohort is from the CER study, which includes 10 population-based cancer registries covering about 27% of the US population.17 Our analytical data from 8 of the 10 cancer registries with complete chemotherapy data represents patients from different geographic regions and reflects clinical practice in the community. The collection of detailed chemotherapy data, including chemotherapy regimens, dosage, and duration by NPCR cancer registries met CDC’s objective to support CER, such as this RDI study. While the reported survival outcomes in the paper are short-term, this cohort of CER colon patients will continue to be followed by the registries and be able to present longterm survival and recurrence in the future.
Although our study was comprehensive in its evaluation of acceptable RDI to maintain efficacy in a relatively homogenous population, there are potential limitations. First, the small number of patients could restrict the power to achieve the statistical significance in mortality differences for certain RDI cut-off points, especially for low-risk cancer patients. Lack of multiple comparisons in the analysis increased Type I error. The results need to be interpreted with caution and with considerations of the retrospective study design. Second, there were substantial differences in the survival time or follow up time for different states, which resulted in a low percentage of events in the survival analysis and short-term survival outcome. Third, the data may under-estimate the comorbid conditions, since patients with unknown comorbidity were categorized as no comorbidity according to the Facility Oncology Registry Data Standard (FORDS) coding manual.20, 37 In addition, data about comorbidity severity, postoperative complications, and reasons for stopping chemotherapy were not available. Furthermore, our study had incomplete data on microsatellite instability (MSI) status and did not collect BRAF mutations, and those molecular factors may affect the adjuvant therapy benefits and survival outcomes in stage II or III colon cancer patients.38–40 Given our study limitations, a single observational study is not sufficient to determine optimal RDI for high-risk or low-risk stage III colon cancer patients, but we provided some real-world evidence of individualized chemotherapy for patients with stage III colon cancer. Further studies with a larger number of patients and more completed data are needed to validate our findings.
Conclusion
In conclusion, among patients with stage III colon cancer who were receiving adjuvant therapy of FOLFOX, the acceptable RDI was 70% for high-risk stage III colon cancer patients, and we did not find a significant difference in the risk of death at any predefined RDI cutoff points from 45% to 70% for low-risk group. These results can be considered with the findings from clinical trials performed by the IDEA collaboration. Our findings add new evidence on the quality of cancer therapy, including how to administer FOLFOX to reduce chemotherapy toxicity and the use of health care resources if efficacy were maintained.
Clinical Practice Points.
To reduce the toxicity, it is imperative to identify the lowest dosage to preserve the same survival outcomes. In 2018, the NCCN guidelines recommended tailored chemotherapy for stage III high-risk (T4 and/or N2) and low-risk (T1-T3 and N1) colon cancer.
We used detailed data on chemotherapy extracted from medical records by cancer registries to identify the FOLFOX optimal RDI based on the stage III colon cancer risk profiles. The results show in high-risk patients, there does not appear to be significant harm from a reduction as long as it is 30% or less; in low-risk patients, we found that RDI as low as 45% did not significantly affect the risk of death.
Our study provided population-based evidence of individualized chemotherapy for patients with stage III colon cancer. More research is warranted.
Acknowledgments
We would like to acknowledge the project investigators at the participating central cancer registries, as well as other organizations, and individuals, including the registrars, that supported the collection of the data to enhance NPCR for Comparative Effectiveness Research: Alaska Cancer Registry (Judy Brockhouse); Cancer Registry of Greater California (Dee W West); Colorado Central Cancer Registry (Randi K. Rycroft); Cancer Data Registry of Idaho (Christopher J. Johnson); Florida Cancer Data System (Monique N. Hernandez); Division of Cancer Prevention and Control, National Center for Chronic Disease Prevention and Health Promotion, Centers for Disease Control and Prevention (Christie R. Eheman, David Butterworth); ICF International (Kevin B. Zhang); Louisiana Tumor Registry and Epidemiology Program (Xiao-Cheng Wu); Rhode Island Cancer Registry (David Rousseau); New Hampshire State Cancer Registry (Maria O. Celaya); CDC-NPCR Contractor, DB Consulting (Jennifer M. Wike); North Carolina Cancer Registry (Melissa Pearson); and Texas Cancer Registry (Anne M. Hakenewerth).
The data collection was supported in part under CDC Cooperative Agreements of the National Program of Cancer Registries: #U58/DP000792 in conjunction with the participating states and a CDC Comparative Effectiveness Research contract to ICF: #200-2008-27957.
The work of this manuscript was supported by the National Cancer Institute’s contract to the LSU Health Sciences Center New Orleans (LSUHSC-NO) for the Surveillance, Epidemiology, and End Results program (Grant #: HHSN261201300016I/HHSN26100006 and HHSN261201800007I/HHSN26100002) and the CDC-NPCR Cooperative Agreement of the National Program of Cancer Registries to the LSUHSC-NO (1 NU58DP006332-01-00).
Abbreviations:
- 5-FU
Fluorouracil
- AK
Alaska
- BSA
Body Surface Area
- CAPOX
Capecitabine and Oxaliplatin
- CDC
Centers for Disease Control and Prevention
- CER
Enhancing Cancer Registry Data for Comparative Effectiveness Research
- CI
Confidence Interval
- CIF
Cumulative Incidence Function
- CO
Colorado
- CRC
Colorectal Cancer
- DDI
Delivered Dose Intensity
- FL
Florida
- FOLFOX
Oxaliplatin, 5-FU, and Leucovorin
- FORDS
Facility Oncology Registry Data Standard
- HR
Hazard Ratio
- ID
Idaho
- IDEA
International Duration Evaluation of Adjuvant Therapy
- MSI
Microsatellite Instability
- LA
Louisiana
- NC
North Carolina
- NCCN
National Comprehensive Cancer Network
- NH
New Hampshire
- NPCR
National Program of Cancer Registries
- RDI
Relative Dose Intensity
- RI
Rhode Island
- SDI
Standard Dose Intensity
- SEER
Surveillance, Epidemiology, and End Results Program
- SPN
Sensory Peripheral Neuropathy
- US
United States
Appendix 1.
Definition of Tumor Risks in Colon Cancer*
| Primary tumor (T) | |
| T1 | Tumor invades submucosa |
| T2 | Tumor invades muscularis propria |
| T3 | Tumor invades through the muscularis propria into pericolorectal tissues |
| T4 | Tumor invades the visceral peritoneum or invades or is adherent to other organs or structures |
| Regional lymph nodes (N) | |
| N1 | Metastasis in 1-3 regional lymph nodes |
| N2 | Metastasis in 4 or more regional lymph nodes |
Appendix 2.
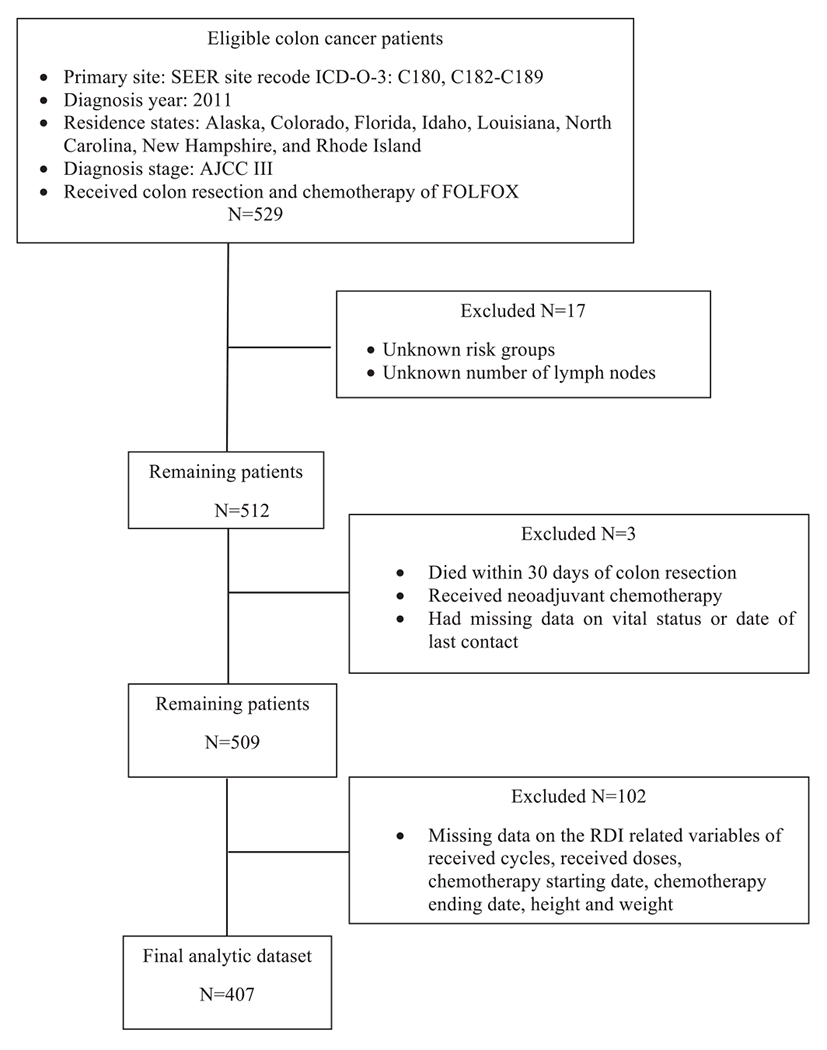
Flowchart of patient selection
Appendix 3. Calculation of FOLFOX RDI
Chemotherapy relative dose intensity (RDI) was the ratio of delivered dose intensity (DDI) to the standard dose intensity (SDI). If the patient received fewer cycles than recommend standard cycles, we assumed the drug dosage is zero for the missing cycles, and the corresponding duration of cycles was based on standard values. According to the 2011 National Comprehensive Cancer Network (NCCN) guidelines, which applies to the diagnosis year for colon cancer cases included in this study, the SDI is based on the following: FOLFOX 4 was administered every 2 weeks: Oxaliplatin 85 mg/m2 IV infusion on day 1 and leucovorin 200 mg/m2 IV infusion followed by fluorouracil (5-FU) 400 mg/m2 IV bolus and 600 mg/m2 IV over 22 hours on days 1 and 2.3 FOLFOX 6 was given every 2 weeks as oxaliplatin 85 mg/m2 IV infusion on day 1, leucovorin 400 mg/m2 IV followed by 5-FU 400 mg/m2 IV bolus on day 1, then 1200 mg/m2/day for 2 days (total 2400 mg/m2 over 46-48 hours) continuous infusion.2 The standard chemotherapy course for both regimens were 12 cycles. Leucovorin is a reduced folic acid, which is used in combination with 5-FU to improve the tumor response.25 The RDI of the regimens were the mean of the individual RDIs for Oxaliplatin and 5-FU. The formulas were as follows:
RDI = (DDI/SDI) x100%, where:
Appendix 4.
Impact of RDI Levels of FOLFOX Chemotherapy on All-Cause Mortality and Cancer-Specific Mortality in High-Risk Stage III Colon Cancer—Univariable Analysis.
| All-cause mortality (N=168) |
Cancer-specific mortality (N=160) |
||||
|---|---|---|---|---|---|
| RDI | Low RDI% (RDI<selected cutoffs | Crude HR (95% CI) | P | Crude HR (95% CI) | P |
| RDI< 55% vs. RDI≥ 55% | 20.8 | 3.91 (1.97, 7.78) | <.0001 | 3.79 (1.59, 9.03) | 0.003 |
|
| |||||
| RDI< 60% vs. RDI≥ 60% | 27.4 | 2.86 (1.44, 5.69) | 0.003 | 2.73 (1.16, 6.42) | 0.02 |
|
| |||||
| RDI< 65% vs. RDI≥ 65% | 31.6 | 2.39 (1.20, 4.76) | 0.01 | 2.18 (0.93, 5.11) | 0.07 |
|
| |||||
| RDI< 70% vs. RDI≥ 70% | 39.9 | 1.57 (0.79, 3.11) | 0.2 | 1.29 (0.55, 3.03) | 0.56 |
|
| |||||
| RDI< 75% vs. RDI≥ 75% | 47.6 | 1.78 (0.88, 3.58) | 0.11 | 1.51 (0.65, 3.53) | 0.34 |
|
| |||||
| RDI< 80% vs. RDI≥ 80% | 58.9 | 1.37 (0.66, 2.82) | 0.4 | 1.26 (0.52, 3.06) | 0.62 |
Appendix 5.
Impact of RDI Levels of FOLFOX Chemotherapy on All-Cause Mortality and Cancer-Specific Mortality in Low-Risk Stage III Colon Cancer—Univariable Analysis.
| All-cause mortality (N=237) |
Cancer-specific mortality (N=232) |
||||
|---|---|---|---|---|---|
| RDI | Low RDI% (RDI<selected cutoffs | Crude HR (95% CI) | P | Crude HR (95% CI) | P |
| RDI< 45% vs. RDI≥ 45% | 15.6 | 0.71 (0.21, 2.35) | 0.58 | 0.45 (0.06, 3.42) | 0.44 |
|
| |||||
| RDI< 50% vs. RDI≥ 50% | 19.4 | 1.54 (0.65, 3.62) | 0.32 | 1.20 (0.34, 4.21) | 0.78 |
|
| |||||
| RDI< 55% vs. RDI≥ 55% | 21.9 | 1.37 (0.58, 3.23) | 0.47 | 1.06 (0.30, 3.74) | 0.92 |
|
| |||||
| RDI< 60% vs. RDI≥ 60% | 26.6 | 1.24 (0.55, 2.83) | 0.6 | 0.77 (0.22, 2.72) | 0.68 |
|
| |||||
| RDI< 65% vs. RDI≥ 65% | 30.8 | 1.36 (0.63, 2.94) | 0.44 | 1.21 (0.42, 3.50) | 0.73 |
|
| |||||
| RDI< 70% vs. RDI≥ 70% | 39.7 | 1.08 (0.51, 2.31) | 0.84 | 1.11 (0.40, 3.08) | 0.85 |
Appendix 6.
Statistical significance of interaction between risk profile and RDI in multivariable analysis for all-cause and cause-specific mortality.
| For overall mortality (n=382) | |
|---|---|
| RDI category | P values of interaction (RDI*risk group) |
| RDI< 45% vs. RDI≥ 45% | 0.009 |
| RDI< 50% vs. RDI≥ 50% | 0.04 |
| RDI< 55% vs. RDI≥ 55% | 0.07 |
| RDI< 60% vs. RDI≥ 60% | 0.14 |
| RDI< 65% vs. RDI≥ 65% | 0.11 |
| RDI< 70% vs. RDI≥ 70% | 0.14 |
| RDI< 75% vs. RDI≥ 75% | 0.06 |
| RDI< 80% vs. RDI≥ 80% | 0.07 |
| For cause-specific mortality (n=369) | |
| RDI category | P values of interaction (RDI*risk group) |
| RDI< 45% vs. RDI≥ 45% | 0.03 |
| RDI< 50% vs. RDI≥ 50% | 0.03 |
| RDI< 55% vs. RDI≥ 55% | 0.04 |
| RDI< 60% vs. RDI≥ 60% | 0.03 |
| RDI< 65% vs. RDI≥ 65% | 0.08 |
| RDI< 70% vs. RDI≥ 70% | 0.23 |
| RDI< 75% vs. RDI≥ 75% | 0.17 |
| RDI< 80% vs. RDI> 80% | 0.09 |
Footnotes
Publisher's Disclaimer: Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Ethics approval and consent to participate
The Institutional Review Board (IRB) Expedited Review was approved by the Louisiana State University Health Sciences Center-New Orleans.
Disclosure
The authors have stated that they have no conflicts of interest.
REFERENCES
- 1.U.S. Cancer Statistics Working Group. U.S. Cancer Statistics Data Visualizations Tool, based on November 2018 submission data (1999–2016): U.S. Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute; 2019. Available at: www.cdc.gov/cancer/dataviz (Accessed June 2019). [Google Scholar]
- 2.National Comprehensive Cancer Network. Archive NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Colon V.2011.
- 3.Benson AB, Venook AP, Al-Hawary MM, et al. NCCN Guidelines Insights: Colon Cancer, Version 2.2018. 2018;16 359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.André T, Boni C, Mounedji-Boudiaf L. et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Eng J Med. 2004;350:2343–2351. [DOI] [PubMed] [Google Scholar]
- 5.Sobrero A, Lonardi S, Rosati G, et al. FOLFOX or CAPOX in stage II to III colon cancer: efficacy results of the italian three or six colon adjuvant trial. J Clin Oncol. 2018;36:1478–1485. [DOI] [PubMed] [Google Scholar]
- 6.Kuebler JP, Colangelo L, O’Connell MJ, et al. Severe enteropathy among patients with stage II/III colon cancer treated on a randomized trial of bolus 5-fluorouracil/leucovorin plus or minus oxaliplatin: a prospective analysis. Cancer. 2007;110:1945–1950. [DOI] [PubMed] [Google Scholar]
- 7.Fata F, Ron IG, Kemeny N. et al. 5-fluorouracil-induced small bowel toxicity in patients with colorectal carcinoma. Cancer. 1999;86:1129–1134. [DOI] [PubMed] [Google Scholar]
- 8.Grothey A Clinical management of oxaliplatin-associated neurotoxicity. Clin Colorectal Cancer. 2005;5(suppl 1):S38–S46. [DOI] [PubMed] [Google Scholar]
- 9.Argyriou AA. Updates on oxaliplatin-induced peripheral neurotoxicity (OXAIPN). Toxics. 2015;3:187–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grothey A Sobrero AF, Shields AF. et al. Duration of adjuvant chemotherapy for stage III colon cancer. N Engl J Med. 2018;378:1177–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Neugut AI, Matasar M, Wang X, et al. Duration of adjuvant chemotherapy for colon cancer and survival among the elderly. J Clin Oncol. 2006;24:2368–2375. [DOI] [PubMed] [Google Scholar]
- 12.Morris M, Platell C, Fritschi L, Iacopetta B. Failure to complete adjuvant chemotherapy is associated with adverse survival in stage III colon cancer patients. Br J Cancer. 2007;96:701–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hryniuk W, Levine MN. Analysis of dose intensity for adjuvant chemotherapy trials in stage II breast cancer. J Clin Oncol. 1986;4:1162–1170. [DOI] [PubMed] [Google Scholar]
- 14.Longo DL, Duffey PL, DeVita VT Jr. et al. The calculation of actual or received dose intensity: a comparison of published methods. J Clin Oncol. 1991;9:2042–2051. [DOI] [PubMed] [Google Scholar]
- 15.Aspinall SL, Good CB, Zhao X. et al. Adjuvant chemotherapy for stage III colon cancer: relative dose intensity and survival among veterans. BMC Cancer. 2015;15:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park D, Baek SJ, Kwak JM. et al. Analysis of reduced-dose administration of oxaliplatin as adjuvant FOLFOX chemotherapy for colorectal cancer. Ann Surg Treat Res. 2018;94:196–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen VW, Eheman CR, Johnson CJ. et al. Enhancing cancer registry data for comparative effectiveness research (CER) project: overview and methodology. J Registry Manag. 2014;41:103–112. [PMC free article] [PubMed] [Google Scholar]
- 18.SEER cause-specific death classification. Vol. 2018. [Google Scholar]
- 19.Weycker D, Barron R, Edelsberg J, et al. Incidence of reduced chemotherapy relative dose intensity among women with early stage breast cancer in US clinical practice. Breast Cancer Res Treat. 2012;133:301–310. [DOI] [PubMed] [Google Scholar]
- 20.Hsieh MC, Thompson T, Wu XC, et al. The effect of comorbidity on the use of adjuvant chemotherapy and type of regimen for curatively resected stage III colon cancer patients. Cancer Med. 2016;5:871–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krieger N, Chen JT, Waterman PD, et al. Race/ethnicity, gender, and monitoring socioeconomic gradients in health: a comparison of area-based socioeconomic measures–the public health disparities geocoding project. Am J Public Health. 2003;93:1655–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu XC, Lund MJ, Kimmick GG. et al. Influence of race, insurance, socioeconomic status, and hospital type on receipt of guideline-concordant adjuvant systemic therapy for locoregional breast cancers. J Clin Oncol. 2012;30:142–150. [DOI] [PubMed] [Google Scholar]
- 23.Marital Status: 2000. Vol. 2018. [Google Scholar]
- 24.Huang B, Feng Y, Mo S-B. et al. Smaller tumor size is associated with poor survival in T4b colon cancer. World J Gastroenterol. 2016;22:6726–6735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mejri N, Dridi M, El Benna H, et al. Prognostic value of tumor size in stage II and III colorectal cancer in Tunisian population. Colorectal Cancer. 2017;6:113–119. [Google Scholar]
- 26.Dillman RO, Aaron K, Heinemann FS, McClure SE. Identification of 12 or more lymph nodes in resected colon cancer specimens as an indicator of quality performance. Cancer. 2009;115:1840–1848. [DOI] [PubMed] [Google Scholar]
- 27.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43:1130–1139. [DOI] [PubMed] [Google Scholar]
- 28.Wu X, Chen VW, Martin J, et al. Subsite-specific colorectal cancer incidence rates and stage distributions among Asians and Pacific Islanders in the United States, 1995 to 1999. Cancer Epidemiol Biomarkers Prev. 2004;13:1215–1222. [PubMed] [Google Scholar]
- 29.Vogt A, Schmid S, Heinimann K, et al. Multiple primary tumours: challenges and approaches, a review. ESMO Open. 2017;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bos AC, van Erning FN, van Gestel YR, et al. Timing of adjuvant chemotherapy and its relation to survival among patients with stage III colon cancer. Eur J Cancer. 2015;51:2553–2561. [DOI] [PubMed] [Google Scholar]
- 31.Dignam JJ, Kocherginsky MN. Choice and interpretation of statistical tests used when competing risks are present. J Clin Oncol. 2008;26:4027–4034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Iveson TJ, Kerr RS, Saunders MP, et al. 3 versus 6 months of adjuvant oxaliplatin-fluoropyrimidine combination therapy for colorectal cancer (SCOT): an international, randomised, phase 3, non-inferiority trial. Lancet Oncol. 2018;19:562–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Andre T, Vernerey D, Mineur L, et al. Three versus 6 months of oxaliplatin-based adjuvant chemotherapy for patients with stage III colon cancer: disease-free survival results from a randomized, open-label, International Duration Evaluation of Adjuvant (IDEA) France, Phase III Trial. J Clin Oncol. 2018;36:1469–1477. [DOI] [PubMed] [Google Scholar]
- 34.Shah MA, Renfro LA, Allegra CJ. et al. Impact of patient factors on recurrence risk and time dependency of oxaliplatin benefit in patients with colon cancer: analysis from modern-era adjuvant studies in the adjuvant colon cancer end points (ACCENT) database. J Clin Oncol. 2016;34:843–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Andre T, de Gramont A, Vernerey D, et al. Adjuvant fluorouracil, leucovorin, and oxaliplatin in stage II to III colon cancer: updated 10-year survival and outcomes according to BRAF mutation and mismatch repair status of the MOSAIC study. J Clin Oncol. 2015;33:4176–4187. [DOI] [PubMed] [Google Scholar]
- 36.Cavaletti G, Marmiroli P. Chemotherapy-induced peripheral neurotoxicity. Nat Rev Neurol. 2010;6:657–666. [DOI] [PubMed] [Google Scholar]
- 37.Facility Oncology Registry Data Standards (FORD) manual. 2016. Available at: https://www.facs.org/quality-programs/cancer/ncdb/registrymanuals/cocmanuals/fordsmanual (Accessed on October 2018), Vol. 2018. [Google Scholar]
- 38.Sargent DJ, Marsoni S, Monges G, et al. Defective mismatch repair as a predictive marker for lack of efficacy of fluorouracil-based adjuvant therapy in colon cancer. J Clin Oncol. 2010;28:3219–3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ogino S, Shima K, Meyerhardt JA, et al. Predictive and prognostic roles of BRAF mutation in stage III colon cancer: results from intergroup trial CALGB 89803. Clin Cancer Res. 2012;18:890–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roth AD, Tejpar S, Delorenzi M, et al. Prognostic role of KRAS and BRAF in stage II and III resected colon cancer: results of the translational study on the PETACC-3, EORTC 40993, SAKK 60-00 trial. J Clin Oncol. 2010;28:466–474. [DOI] [PubMed] [Google Scholar]


