Abstract
Catalytic nanoparticles with natural enzyme-mimicking properties, known as nanozymes, have emerged as excellent candidate materials for cancer immunotherapy. Owing to their enzymatic activities, artificial nanozymes not only serve as responsive carriers to load drugs and therapeutic molecules for cancer treatment, but also act as enzymes for modulating the immunosuppression of the tumor microenvironment (TME) via the catalytic activities of catalase, peroxidase, superoxide dismutase, and oxidase. The immunosuppressive pro-tumor TME can be reversed to the immunoactive anti-tumor TME by utilizing both reactive oxygen species (ROS)-generating and ROS-scavenging nanozymes, which enhance the efficacy of cancer immunotherapy. In this review, we introduce representative ROS-generating and ROS-scavenging nanozymes and discuss how artificial nanozymes respond to the conditions of the TME. Based on the mutual interaction between nanozymes and TME, recent therapeutic pathways to provoke anti-cancer immune responses using nanozymes are discussed.
Keywords: Cancer immunotherapy, Nanozymes, Reactive oxygen species, Tumor microenvironment, Immunogenic cell death
Introduction
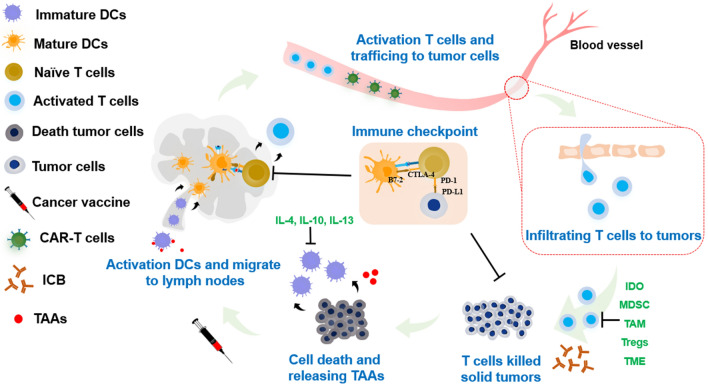
Cancer is one of the leading causes of death worldwide [1]. Thanks to the advanced understanding of cancer immunology and immunosuppressive tumor microenvironment (TME), cancer immunotherapy has opened a new paradigm for cancer treatment by inducing durable antitumor immunity. The goals of cancer immunotherapy include regression of the established tumor, eradication of residual cancer cells, and prevention of metastasis and relapse. A myriad of cancer immunotherapy modalities have been employed to tackle the immunosuppressive barriers in each step of the natural cancer-immunity cycle in the body (Fig. 1): cancer vaccines [2–5], immune checkpoint blockade (ICB) therapies [6–10], and chimeric antigen receptor (CAR)-T cell therapies [11–14]. Cancer vaccines aim to boost the host’s immune system by delivering tumor antigens to immune cells, particularly dendritic cells (DCs), which subsequently induce adaptive immune responses against primary tumors and distant metastases with minimal damage to healthy cells and provoke immune memory for long-term protection [15–18]. Conventionally, cancer vaccines consist of tumor-associated antigens (TAAs) in the form of peptides, proteins, DNA, mRNAs, cancer cell lysates, and the whole cancer cells, along with immune-activating signals for triggering tumor-antigen specific adaptive immune responses against cancer. However, the therapeutic outcomes of conventional cancer vaccines are limited, with them showing a weak antitumor response, established tumor suppression inability, and tumor recurrence [19–26]. Recent findings in past decades have revealed that the immunosuppressive TME is one of the main obstacles to conventional cancer vaccine approaches. The immunosuppressive TME usually accumulates myeloid-derived suppressor cells (MDSCs), tumor-associated macrophages (TAMs) with tumor-promoting phenotype, regulatory T (Treg) cells, and cancer-associated fibroblasts that secrete immunosuppressive cytokines and express immune-inhibitory ligands. These prevent the infiltration of anti-tumor immune cells into the tumor or the impairment of cytotoxicity of activated T cells. Moreover, highly acidic conditions and hypoxia in the TME can facilitate tumor evasion. By utilizing the immunosuppressive features of the TME, cancer cells can evade immune surveillance. Thus, the development of new strategies to reverse the anti-tumor immunosuppressive TME for accelerating cancer immunotherapy is of interest.
Fig. 1.
Schematic of cancer immunity cycle with diverse cancer treatment paradigms. The cells involved in each step of the cancer immunity circle face various conditions including inhibition and stimulation. Tumor-associated antigens (TAAs) are released, followed by their uptake and presentation by professional antigen-presenting cells (e.g., dendritic cells (DCs)), boosting DCs maturation. These processes are inhibited by immunosuppressive cytokines (IL-4, IL-10, and IL-13). Mature DCs subsequently migrate to the lymphoid organs and activate naive T cells. The activated T cells are suppressed by physiological conditions in the tumor microenvironment (TME), indoleamine 2,3-dioxygenase (IDO)-expressing cells, myeloid-derived suppressor cells (MDSCs), Treg cells, tumor-associated macrophages (TAM), and immune checkpoint molecules (PD-1, PD-L1). Thus, CAR-T cells are trained and stimulated ex vivo to enhance the antigen-specific antitumor responses after infusion to patients. ICB therapy results in synergistic effects to enhance the tumor infiltration by cytotoxic T lymphocytes (CTLs) and tumor-killing effects
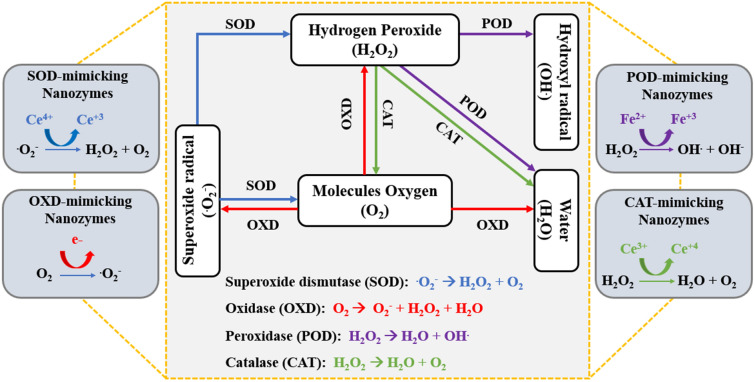
Recently, owing to their intrinsic catalytic properties, enzyme-mimicking nanomaterials (nanozymes) have become potential candidates for modulating the TME for enhanced cancer immunotherapy. Nanozymes are nanomaterials that exhibit an intrinsic enzyme-mimicking activity. Artificial nanozymes have recently been recognized as alternatives to natural enzymes owing to their outstanding properties, such as elevated catalytic efficiency, low cost, high stability, and easy storage [27]. In particular, nanozymes mimicking enzymes with redox activities, including catalase (CAT), peroxidase (POD), superoxide dismutase (SOD), and oxidase (OXD), have the potential to modulate the TME. Reactive oxygen species (ROS) play an important role in balancing the homeostasis of redox reactions and their modulatory effects on cell survival and cell signaling [28]. ROS-modulating nanozymes have been used to control the level of intracellular or extracellular ROS. The catalytic activity of nanozymes can stimulate and interact mutually through five main pillars composed of hydrogen peroxide (H2O2), oxygen (O2), superoxide radicals (.O2−), hydroxyl radical (OH.) and water (H2O), as shown in Fig. 2.
Fig. 2.
Routes of mutual catalytic activity enzyme interaction (POD, SOD, OXD, CAT) through representative products such as H2O2, OH., .O2−, O2, H2O. By mimicking the catalytic activities of enzymes, nanozymes with diverse materials such as SOD- and CAT-mimicking cerium oxide, POD-mimicking iron oxide, or OXD-mimicking electron donors were discovered
Recent approaches using ROS-modulating nanozymes to enhance cancer immunotherapy are based on two mechanisms with responsive TME: (1) modulation of tumor physiological environment including pH, oxygen level, and immunosuppressive cells to reprogram the immunosuppressive TME into tumor-inhibiting anti-tumor TME [29–31]; (2) induction of immunogenic cell death (ICD) of tumor cells, which subsequently releases TAAs which can be captured, processed, and presented by antigen-presenting cells (APCs) such as DCs that prime naïve CD8+ and CD4+ T cells [32, 33]. In this review, we discuss how ROS-modulating nanozymes contribute to enhanced cancer immunotherapy. First, we briefly introduce the recent understanding of immunosuppressive TME and representative nanozymes, focusing on how nanozymes interfere with and manipulate TME towards the promotion of antitumor responses. Next, recent achievements of ROS-modulating nanozymes for inducing adaptive immunity and subsequently, enhanced cancer immunotherapy are described. Finally, our perspectives on the challenges and future directions for advancing the clinical translation of the field are proposed.
Immunosuppressive features of TME
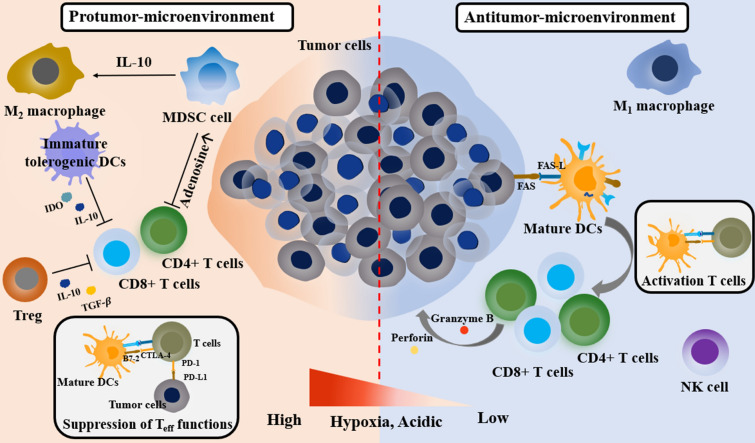
The most important components that contribute to the immunosuppressive TME are immunosuppressive cells, such as TAMs, MDSCs, Tregs, and immunosuppressive cytokines (Fig. 3). In the TME, cancer cells directly interact with non-cancerous cells and transform them into tumor-assistant agents to avoid the immunosurveillance of antitumor cells. TAMs predominantly account for a number of tumor-infiltrating cells, which are classified into M1 and M2 phenotypes depending on the physiological microenvironment. Typically, the M1-phenotype is responsible for the anti-tumor functions by secreting pro-inflammatory cytokines such as interleukin (IL)-12 and tumor necrosis factor (TNF)-α in the anti-TME. In contrast, the M2-phenotype is responsible for the anti-inflammatory functions by secreting IL-10 and transforming growth factor β (TGF-β) in pro-TME. In general, TAMs are mostly M2-phenotypes supporting immunosuppressive pro-TME [34, 35].
Fig. 3.
Physiological conditions and cellular interaction network of the protumor- and antitumor-microenvironment. Pro-tumor microenvironment is composed of immunosuppressive cells (MDSCs, Tregs, and M2-type macrophages), immunosuppressive cytokines (IL-10 and TGF-β), immunomodulatory enzymes (IDO-expressing cells). In addition, PD-L1, PD-1 and therapeutic barriers (high tumor hypoxia, acidic condition) can restrict the function of the effector T cells. In contrast, anti-tumor microenvironment is composed of a high number of innate immune cell (NK cells), effector T cells (CD8+ and CD4+ T cells), and M1-type macrophages for tumor-killing
MDSCs are also immunosuppressive cells in the TME that initiate cancer metastasis and evade immune surveillance. MDSCs prevent the activity of T cells and natural killer (NK) cells by secreting immunosuppressants such as adenosine to inhibit T cell activation. MDSCs can upregulate the activity of immune checkpoint programmed death-ligand 1 (PD-L1) on cancerous cells, and then downregulate the activity of tumor-infiltrating lymphocytes by binding the receptor PD-1 on the surface of infiltrated T cells. MDSCs are an assistive factor for inducing the expression of immunosuppressive cytokines such as TGF-β and IL-10, which elicit the expansion of Tregs and the anergic state of T cells [36].
Tregs play an essential role in hampering T-cell activation in the pro-TME. In TME, due to immunosuppressive microenvironments, immature DCs are unable to undergo the maturation process and thus maintain the tolerogenic phenotypes, displaying fewer co-stimulatory molecules, resulting in the generation of induced Tregs. Tregs and tolerogenic DCs also secrete anti-inflammatory cytokines such as IL-10 and TGF-β and inhibitory factors including indoleamine 2,3-dioxygenase (IDO), which hinder T cell activation and stabilize the tolerogenic and immunosuppressive TME [37–40].
The representative physiological condition supporting the immunosuppressive environment in pro-TME is a high level of hypoxia, which causes a lack of oxygen in the TME and hinders the function of the immune system. Hypoxia can facilitate immunosuppression by preventing the activity of CD4+ T cells and enhancing Treg activity [41–43]. Hypoxia-inducible factors (HIFs) are known to downregulate active CD8+ T cell populations due to inadequate glucose for metabolic reprogramming, resulting in immunosuppression [44, 45]. Moreover, hypoxia exploits the immune checkpoint molecules (PD-L1), thereby hampering the immunological functions of T cells [46].
To enhance the efficacy of cancer immunotherapy, it is necessary to convert these immunosuppressive characteristics of pro-tumor TME into an immunoactive anti-tumor TME (Fig. 3). For this purpose, various strategies such as nanomaterial-based cancer vaccines, ICB therapy, CAR-T cell therapy, and in situ modulation of the TME have been proposed. In the following section, we will focus on recent progress using ROS-modulating nanozymes for enhanced cancer immunotherapy by the in-situ modulation of TME.
Enhanced cancer immunotherapy based on ROS-modulating nanozymes
ROS-scavenging nanozymes can convert reactive oxygen molecules to harmless molecules, including O2 and H2O, thus providing protection against oxidative stress and maintaining cellular functions. CAT and SOD are representative catalytic enzymes that scavenge ROS. On the other hand, ROS-generating nanozymes, such as POD and OXD can induce more reactive oxygen molecules to cause positive oxidative stress in tumor cells, resulting in damage to cellular components such as lipids, membranes, DNA, and proteins [47]. Despite the different roles of the two types of nanozymes, both types of nanozymes can be utilized to activate anti-tumor immunity cascades through the modulation of TME.
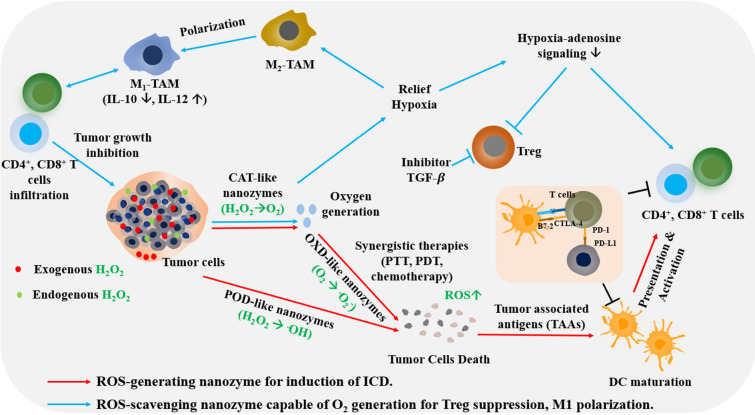
To alleviate the immunosuppressive conditions in TME, ROS-modulating nanozymes can be used to enhance the therapeutic efficiency of cancer immunotherapy. There are two main strategies (Fig. 4): (1) induction of ICD-mediated activation of effector T cells via cytotoxic ROS generation and (2) Treg suppression and M1 macrophage polarization via O2 generation (ROS scavenging). The former strategy is favorable to ROS-generating nanozymes, in which POD and OXD-mimicking nanozymes convert endogenous or exogenous H2O2 into ROS such as OH. and .O2− that facilitate high oxidative stress, thereby inducing ICD and resulting in the subsequent immune responses. The latter approach enables the nanozymes to relieve the TME based on controllable ROS levels and hypoxia, provoking the infiltration of immune cells and secretion of pro-inflammatory cytokines to elicit innate and adaptive immunity, such as M2 to M1 macrophage polarization. In addition to the two main strategies, synergistic effects are observed by combining these strategies with other therapies such as ICB, CAR-T, and anti-inflammation cytokine inhibitors to enhance the immune response and eradicate tumor recurrence. Recent advances of artificial ROS-modulating nanozymes for cancer immunotherapy, which is discussed in this review, are summarized in Table 1.
Fig. 4.
Therapeutic pathways of TME-responsive nanozymes for cancer immunotherapy. Red arrows indicate a strategy based on the catalytic activities of ROS-generating nanozymes (OXD- and POD-like activities) which induce high oxidative stress, resulting in the induction of immunogenic cell death (ICD). Death of tumor cells releases TAAs, subsequently be engulfed, and presented to the naïve T cells by DCs, leading to the activation of CD4+ and CD8+ T cells. Blue arrows indicate a strategy for the control of immune responses by modulating the TME. Treatment with ROS-scavenging nanozymes (SOD- and CAT-like activities) can relieve hypoxia and control ROS levels, leading to the modification of subsequent immunological cascades such as the reprogramming of M2 to M1 macrophages, decrease in hypoxia-adenosine signaling, suppression of Treg cells, and expression of effector T cells. In addition, other therapies are applied to synergistically enhance the adaptive immune responses such as TGF-β inhibitor, anti-PD-L1, anti-PD-1, and anti-CTLA-4 antibodies.
Table 1.
Recent advances of nanozyme-based enhanced cancer immunotherapy
| Nanozyme | Catalytic activity | Synergistic therapy | Therapeutic effects | References |
|---|---|---|---|---|
| Hyaluronic- and polyethylene glycol-decorated Cu-based nanozymes | POD-like activity | Photothermal therapy (PTT) | Induction of immunogenic death (ICD) to boost the immunological function of effector CAR-T cells | [51] |
| Glucose oxidase (GOx), hemin, dihydroartemisinic (DHA) enveloped in ZIF-8 framework | GOx activity, POD-like activity | Anti-PD-L1 | Formation of elevated ROS to cause tumor cell death, to release TAAs, to enhance infiltration of tumor-specific T cells into tumor site | [52] |
| Core–shell gold nanocage@manganese dioxide (AuNC@MnO2) nanozyme | CAT-like activity, NIR-mediated conversion of O2 to .O2− | Photodynamic therapy (PDT) | Induction of ICD, release of damage-associated molecular patterns (DAMPs), activation of effector T cells | [53] |
| Phospholipid-coated Na2S2O8 nanoparticles | Generation of sulfate (.SO4−) and hydroxyl (OH.) radical by dissociation of persulfate | Anti-CTLA-4 | High ROS level to induce ICD, release of DAMPs, activation of tumor-specific T cells | [55] |
| Co-loading of Ce6 and DOX into hollow manganese dioxide (H-MnO2) | CAT-like activity | PDT, chemotherapy, anti-PD-L1 | O2 supplement for relief of tumor hypoxia, M2 macrophage polarization, induction of ICD, cytotoxic T lymphocyte CTL) activation | [61] |
| Combination of CaO2- and MnO2-based nanozymes | H2O2-generating activity, CAT-like activity, | Anti-CTLA-4, chemotherapy | Relief of tumor hypoxia, suppression of Treg activity, activation of CTLs | [63] |
| Iron- and manganese-based nanozymes encapsuled into mesoporous silica nanoparticles (MSN) | CAT- and POD-like activity | Delivery of TGF-β inhibitor | Relief of tumor hypoxia, M2 macrophage polarization, suppression of Tregs, induction of ICD, activation of effector T cells | [62] |
ROS-generating nanozyme for the induction of ICD
POD- and OXD-mimicking nanozymes generate ROS, such as H2O2, .O2−, and OH., by peroxidation and oxidation, respectively. The resulting high oxidative stress causes ICD of tumor cells which releases tumor antigens and damage-associated molecular patterns (DAMPs) into the surroundings, that are capable of boosting antigen-specific anti-cancer immune responses. Various POD- and OXD-mimicking nanozymes can be used to produce .O2−, OH. using H2O2 in the TME. The most representative POD-like activity is the Fenton or Fenton-like reaction mediated by Fe3O4 nanoparticles [48, 49] that produce OH. radicals. To enhance the radical generation capacity, glucose oxidase (GOx) can be added to generate H2O2 over the amounts of radicals generated by POD activity. In addition, some nanomaterials without catalytic-mimicking activities are used as photosensitizing substances for photodynamic therapy (PDT) or photothermal therapy (PTT), which show a strong capacity to produce ROS from O2 in the surrounding tissue [50]. ROS generation by PDT or PTT can be further enhanced by combining with oxygen-supplying components such as oxygen-storing CaO2 or CAT-mimicking MnO2, which produce oxygen which can be converted into ROS (singlet oxygen (1O2), .O2−, and OH.) under light irradiation. Another strategy for ROS generation is via degradation; these materials can directly generate toxic radical species (sulfate radicals, alkylating carbon-centered radicals) after being decomposed under specific conditions.
Based on these diverse options for ROS-generating components such as POD- and OXD-mimicking nanoparticles, PDT/PTT agents, and toxic radical-generating molecules via degradation, the resulting ROS-generating nanozymes have been used to induce the ICD of cancer cells and the subsequent release of TAAs for enhanced anti-cancer adaptive immune responses.
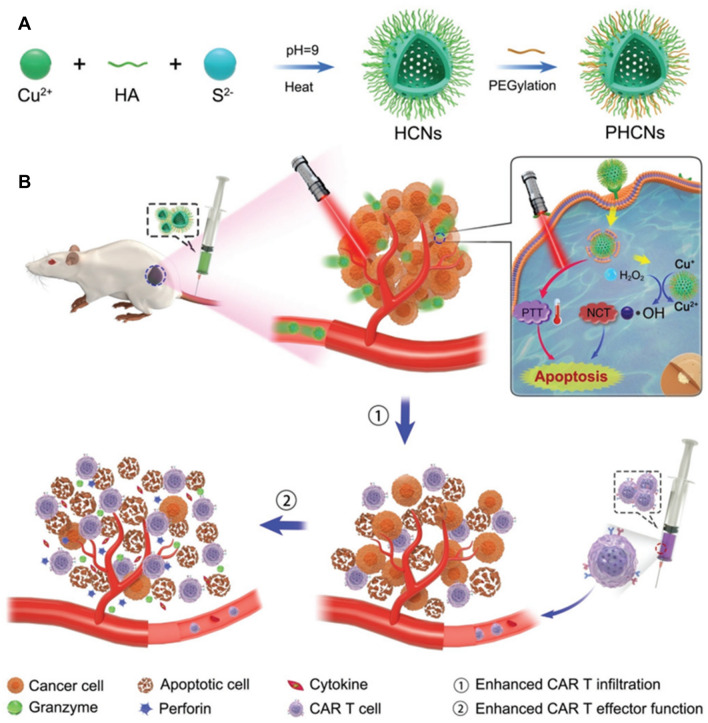
The use of copper (Cu) nanoparticle-based POD-mimicking nanozymes has been demonstrated to enhance cancer therapy of solid tumors via ICD-mediated strong adaptive immune responses combined with CAR-T cell therapy (Fig. 5) [51]. Cu nanoparticles were synthesized by reacting copper (II) chloride with sodium sulfate (Na2S) using a capping agent as a stabilizer (hyaluronic acid, HA), and then further modifying using polyethylene glycol (PEG) to allow high colloidal stability for intravenous injections. The resulting HA-stabilized Cu-based nanozymes were targeted to CD44 (HA receptor) overexpressed on tumor cells after intravenous injection. Copper-based nanozymes with intrinsic POD-like activity were able to convert high levels of H2O2 in the TME to toxic OH. radicals. In addition, the eradication of tumor cells was elicited by PTT via near-infrared (NIR) irradiation of Cu nanoparticles. This enhanced toxic ROS generation and local heating led to ICD of the tumor cells. The apoptosis of tumor cells released TAAs, which enhanced the activation of DCs and effector T cells, and tumor infiltration of CAR-T cells. Because of the expanded blood perfusion and degradation of the tumor extracellular matrix by PTT and the catalytic activity of nanozymes, the CAR-T cells easily infiltrated the tumor lesions. Nanozymes promoted the infiltration of a high proportion of CD8+ CAR-T cells and CD4+ CAR-T cells compared to that induced by non-nanozymes both in vitro and in vivo. Consistently, CAR-T cell-induced nanozymes secreted more inflammatory cytokines (TNF-α and IFN-γ) than CAR-T cells alone. Hence, the combined synergistic effects of ROS-generating nanozymes, PTT, and CAR-T cells substantially enhanced the efficacy of immunotherapy against solid tumors.
Fig. 5.
Hyaluronic acid (HA) and polyethylene glycol-decorated copper-based nanozymes (Cu2−xS) enhanced CAR-T cell therapy. Copper-based nanozymes with HA modification on the surface are targeted to tumor cells and react with endogenous H2O2 which can be transformed to OH. through POD-like activity. High local ROS levels caused apoptosis, leading to the release of TAAs. CAR-T cells were engineered to recognize tumor-specific antigens; thus, the combination of nanozyme-based therapy and photothermal therapy (PTT) with CAR-T cells enhanced the tumor infiltration of and function of CAR-T cells.
Reproduced from [51] with permission from Wiley–VCH. Copyright 2021
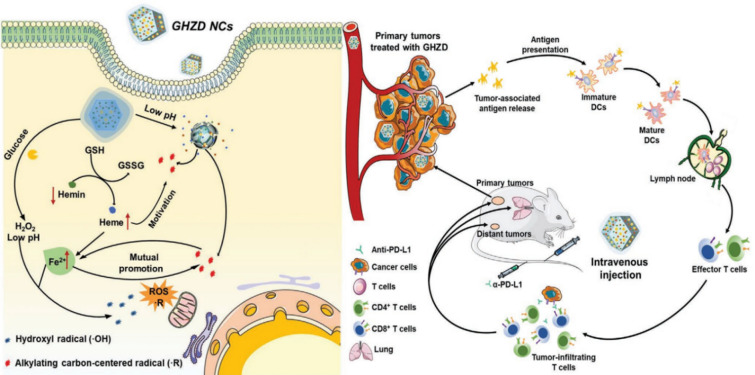
Lin et al. introduced a novel nanozyme with multienzyme-mimicking activities, including GOx, POD, and glutathione (GSH) peroxidase (GPx) for tumor ICD induction (Fig. 6) [52]. GOx, hemin nanozyme, and lactone endoperoxide-derived dihydroartemisinic acid (DHA) were encapsulated in the zeolitic imidazolate framework (ZIF-8). The resulting multienzyme mimics were injected intravenously into tumor-bearing mice to induce passive targeting into the tumor via the enhanced permeability and retention (EPR) effect. In the TME, GOx elevated H2O2 levels during the glucose oxidation process, and the subsequent in situ cascade reaction by POD-like heme (Fe2+) converted H2O2 to OH. radicals. In addition, GPx-mimicking hemin (Fe3+) was converted to heme (Fe2+), consuming abundant antioxidant GSH, and the resulting heme (Fe2+) provoked the co-delivered DHA to induce C-centered free radicals. Inexhaustible endogenous free radicals (C. and OH.) are generated in an accelerated manner, which amplifies the high oxidative stress and triggers ICD to release danger signals (CGT, HMGB-1). Immature DCs capture, engulf, and present danger signals for DC maturation. The mature DCs migrate to the lymphoid organs where they interact and activate antigen-specific effector T cells. Through blood vessels, the effector T cells are systemically trafficked and infiltrated solid tumors, resulting in the elimination of primary as well as distant tumors. The cellular immune response indicated that this multienzyme-mimicking framework showed a high percentage of DC maturation through the expression of CD86+ and CD80+ compared to control groups. In parallel, the secretion levels of inflammatory cytokines (TNF-α, IFN-γ, and IL-6) increased consistently from the supernatant of splenocytes. High expression of mature DCs led to an increased number of tumor-infiltrating effector T cells (CD4+ and CD8+ T cells) in tumors. When anti-PD-L1 antibody was combined with the catalytic activity of nanozymes, eradication of the primary and distant tumors was achieved by systemically activated effector T cells.
Fig. 6.
Complex of glucose oxidase (GOx), hemin, and dihydroartemisinic acid (DHA) enveloped in ZIF-8 framework for cancer immunotherapy. Alkylating carbon-centered radical (R.) and hydroxyl radical (OH.) were generated by utilizing ROS-generating complexed framework through GOx and Fenton reaction. Elevated ROS levels caused tumor cell death and subsequent release of TAAs which are captured and presented on mature DCs. DCs travel to the lymph node for priming tumor-specific T cells, leading to their infiltration into tumor sites. The combination of the nanozyme platform and anti-PD-L1 therapy increased the efficacy of cancer treatment against primary and distant tumors.
Reproduced from [52] with permission from Wiley–VCH. Copyright 2020
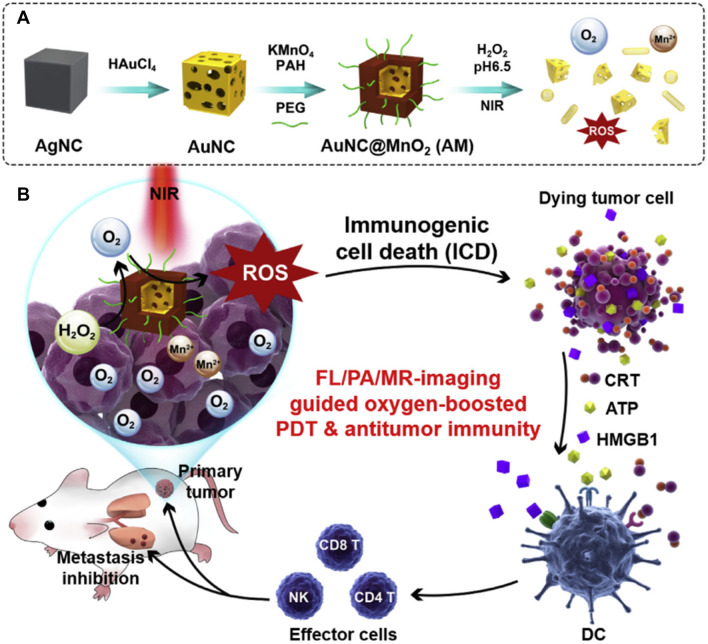
Enhanced ROS generation by combining PTT and O2 supplying a CAT-mimicking nanozyme was proposed for synergistic photothermal immunotherapy. Liang et al. fabricated ROS-generating nanocomposites by developing a core–shell structure (AuNC@MnO2) with gold nanocages (AuNCs) as the core and manganese dioxide (MnO2) as a shell for the treatment of metastatic triple-negative breast cancer (mTNBC) (Fig. 7) [53]. AuNC@MnO2 was injected intravenously into tumor-bearing mice. The MnO2 shell could catalyze the breakdown of intratumoral H2O2 to H2O and O2 under acidic conditions in the TME through the reaction MnO2 + H2O2 + 2H+ → Mn2+ + 2H2O + O2. Then, the plasmonic Au nanocage worked as a photosensitizer under NIR one/two-photon illumination for PDT, which converts O2 into diverse toxic ROS (1O2, O2−, and OH.) [54]. Excessive levels of ROS induce ICD, which subsequently secrete DAMPs, including adenosine triphosphate (ATP), calreticulin (CRT), and high-mobility group box 1 protein (HMGB-1). The exposure of DAMPs, which act as danger signals, could provoke the maturation and antigen presentation of DCs for activation. Mature DCs activate antigen-specific T cells and the subsequent infiltration of effector immune cells to the tumor, leading to the eradication of primary and metastatic tumors. Notably, gold nanocages (AuNCs) are not GOx-mimicking nanozymes in this case, but only act as photocatalytic agents in boosting PDT. Administration of AuNC@MnO2 following NIR irradiation-induced CD83+ and CD86+ expression on tumor-infiltrated DCs in vivo. The mature DCs secreted increased levels of IL-12 than AuNCs alone, with or without laser irradiation. The tumor antigen-exposed mature DCs strongly elicited the expansion of CD4+ T cells, CD8+ T cells, and NK cells in both tumor and tumor-draining lymph nodes. High antitumor immunity led to the dramatic diminishment of primary tumor as well as the number of metastatic lesions.
Fig. 7.
Core–shell gold nanocage@manganese dioxide (AuNC@MnO2) nanozymes for cancer immunotherapy. Under highly acidic condition in the TME, the complex structure was degraded. AuNC@MnO2 nanozymes are ROS-scavenging nanozymes that produce elevated O2 levels. Meanwhile, under near-infrared (NIR) irradiation, O2 was converted into .O2−, increasing ROS levels and leading to the induction of ICD. Subsequently, damage-associated molecular patterns (DAMPs; CRT, ATP, HMGB1) released due to ICD trigger DCs maturation and tumor antigen presentation for activating effector T cells against primary tumor and its metastasis.
Reproduced from [53] with permission from Elsevier. Copyright 2018
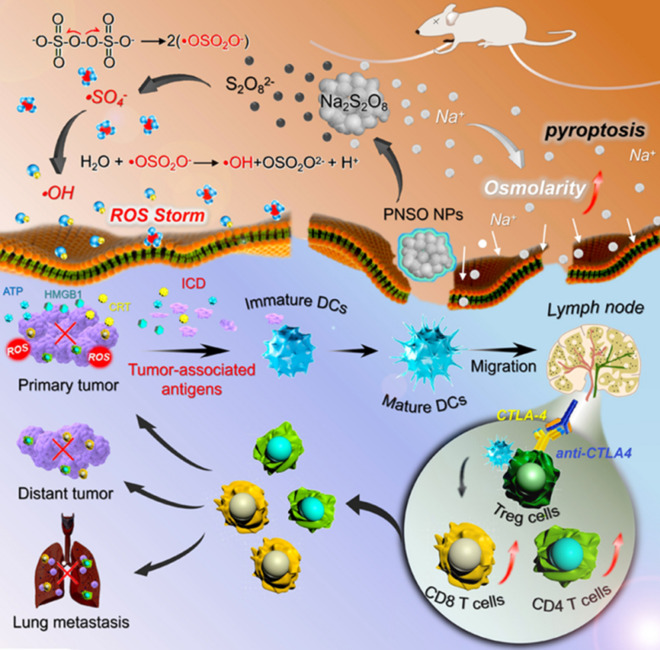
Phospholipid-coated Na2S2O8 nanoparticles (PNSO NPs) were utilized as ROS-generating nanozymes for enhanced cancer immunotherapy (Fig. 8) [55]. PNSO NPs were degraded to generate Na+ and S2O82− under physiological conditions in the TME. S2O82− can be converted into a sulfate radical (.SO4−), which subsequently reacts with water to produce OH.. This conversion amplified oxidative stress, thus inducing ICD of tumor cells. In addition, it provoked anti-cancer adaptive immune responses and enhanced the intratumoral infiltration of CD8+ and CD4+ T cells. PNSO NP-treated cancer cells released higher levels of DAMPs (including ATP and HMGB-1), which elevated the maturation of DCs (high expression of CD80+ and CD86+), as well as the levels of pro-inflammatory cytokines (TNF-α, IL-6, IL-10) in vitro and in vivo. As CTLA-4 hinders the immunological functions of activated T cells [56, 57], combining PNSO NPs with anti-CTLA-4 antibodies showed enhanced antitumor response and simultaneously prevented the growth of distant tumors and metastatic lung tumors.
Fig. 8.
Phospholipid-coated Na2S2O8 nanoparticles (PNSO NPs) are nanozymes merged with anti-CTLA-4 antibodies for treating cancer. Sulfate radicals (.SO4−) and OH. were generated by activating the persulfate of PNSO NPs, resulting in high ROS levels, tumor cell death, and DAMPs release (ATP, CRT, and HMGB 1). Then, immature DCs capture and present TAAs and became mature DCs. The tumor antigen-exposed mature DCs migrate to the lymph node and prime tumor-specific T cells, leading to the attack of CTLs against cancer. Combination with anti-CTLA-4 antibodies suppresses the Treg activity and upregulates the expression of effector T cells, thus preventing the evasion of primary and distant tumors as well as lung metastasis.
Reproduced from [55] with permission from the American Chemical Society (ACS). Copyright 2020
ROS-scavenging nanozymes are capable of O2 generation for Treg suppression and M1 macrophage polarization
Typically, SOD and CAT are representative catalytic enzymes that scavenge and convert ROS to harmless molecules such as water and oxygen. In detail, SOD transforms O2− to H2O2 and O2, and CAT converts H2O2 into H2O and O2. Once O2 is generated by the SOD- and CAT-mimicking catalytic activities of nanozymes, tumor hypoxia is relieved, and the immunosuppressive TME is reversed. This causes dramatic transitions in the TME, including polarization of tumor-advocating M2 macrophages to anti-tumor inflammatory M1 macrophages, reduction of hypoxia and acidic conditions which favorably improve the tumor infiltration of effector T cells and hamper Treg activity.
The representative mechanism of ROS-scavenging nanozymes to produce O2 is an autocatalytic redox reaction mediated by metal species with different oxidation states on the surface of metal oxide nanoparticles such as MnO2 and CeO2. For example, because of their reversible surface Mn2+/Mn3+ double oxidation state, manganese-based nanozymes act as radical scavengers that show CAT-like activity that converts H2O2 into O2 [58–60]. To enhance O2 generation through CAT-like nanozymes, O2/H2O2-generating molecules can be used to provide more O2 or H2O2. For example, CaO2 is capable of releasing O2 and H2O2 upon reaction with water under acidic conditions in the TME, which could synergistically increase the level of O2 in the TME in the presence of SOD- or CAT-mimicking nanozymes.
Recently, TME modulation for enhanced immunotherapy through local O2 generation has been combined with other cancer therapeutic modalities (chemotherapy, PTT, PDT) or other ROS-generating nanozymes to enhance the efficacy of cancer treatment. The combination of different therapies not only alleviates the TME, triggering favorable presentation and activation of effector cells, but also provokes antigen-specific T cells by providing substantial TAAs through synergistic therapies. For more practical applications, ROS-scavenging and O2-producing nanozymes were combined with anticancer drugs [61], inhibitors of immunosuppressants [62], and PDT agents [63] to induce synergistic efficacy in cancer immunotherapy.
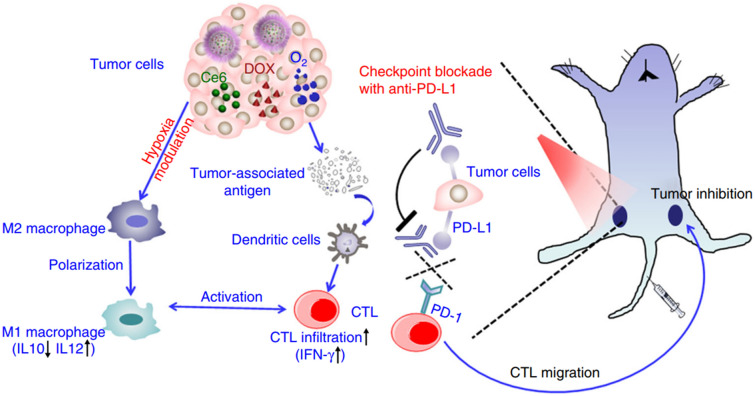
Yang and colleagues designed a ROS-scavenging, O2-generating nanozyme based on hollow manganese oxide (H-MnO2) nanoparticles for TME modulation in hot tumors (Fig. 9). [61] H-MnO2 was synthesized by utilizing silica nanoparticles as a core, followed by encapsulation by a mesoporous MnO2 shell, and the subsequent removal of SiO2 core. The surface of H-MnO2 was subsequently PEGylated to enhance the colloidal stability under physiological conditions for cancer targeting. Co-loading of doxorubicin (DOX) and photosensitizer Ce6 into the hollow interior space of H-MnO2 was optimized to enhance antitumor immunity. In the TME, CAT-mimicking H-MnO2 reacts with endogenous H2O2 under acidic conditions to produce O2. The generated O2 not only relieved tumor hypoxia and modulated the TME but was also converted into .O2− by Ce6 photosensitizer under light irradiation. Additionally, chemotherapy was simultaneously performed by releasing DOX for the induction of chemotherapy-based ICD. H-MnO2-PEG-Ce6-Dox under light illumination led to a dramatic increase in the ratio of M1/M2 macrophages and the recruitment of macrophages in the tumor, as well as an increase in the levels of infiltrated CTLs and inflammatory cytokines (TNF-α and IFN-γ). As a result, tumor growth was greatly inhibited in animals treated with H-MnO2-PEG-Ce6-Dox nanoparticles combined with chemotherapy, PDT, and nanozyme-based immunotherapy.
Fig. 9.
Co-loading of Ce6 and DOX into hollow manganese dioxide (H-MnO2) decorated by PEG enhanced antitumor responses when combined with anti-PD-L1 therapy. ROS-scavenging nanozymes based on H-MnO2 could generate elevated O2 levels, leading to the relieving tumor hypoxia. O2 was converted into .O2− under light illumination, which induced the tumors to release TAAs. TAAs were then engulfed and presented to naïve T cells by DCs, resulting in CTL activation and tumor infiltration. The combination of photosensitizer (Ce6) and chemotherapeutic agents (DOX) accelerate the tumor-killing effects by triggering tumor cell death. Anti-PD-L1 antibody was employed to block the immune checkpoint PD-L1 on tumor cells, unleashing the cytotoxic function of the activated CTLs against tumors.
Reproduced from [61] with permission from Nature. Copyright 2017
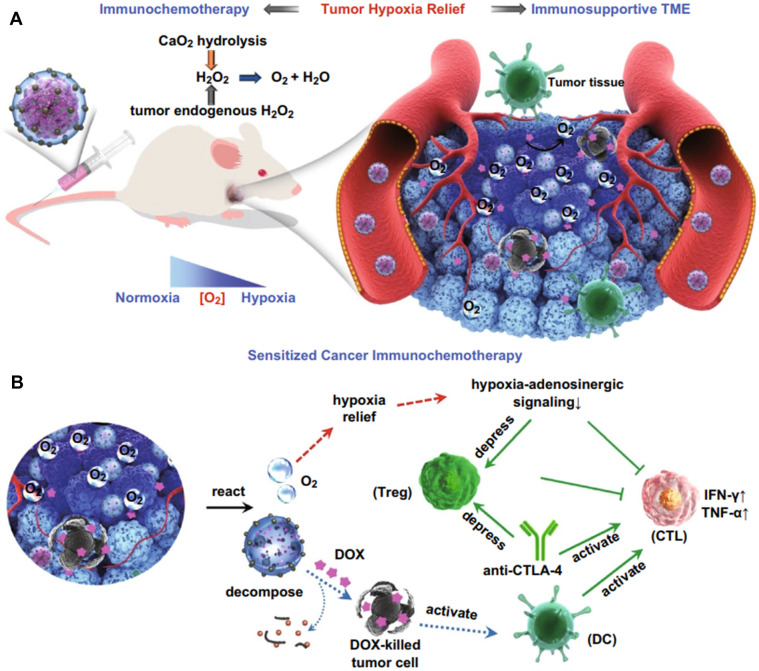
Wang et al. [63] introduced a core–shell structure composed of a DOX core and silica shell decorated with CAT-mimicking MnO2 nanoparticles for cancer immunotherapy, which was assisted by H2O2-releasing calcium peroxide nanoparticles (CaO2 NPs) (Fig. 10). CaO2 was used to produce H2O2 by reacting with water to supplement H2O2 levels for CAT-mimicking MnO2 nanoparticles to produce more O2. This process could continuously supply long-term O2, reversing the immunosuppressive TME from pro-tumor to anti-tumor through local hypoxia relief. Once hypoxic conditions are relieved, hypoxia-adenosine signaling regulates immune cell function and decreases IFN-γ production, which suppresses the activity of Tregs and elicits anti-tumor immune responses mediated by CD8+ and CD4+ T cells. Additionally, DOX is a chemotherapeutic drug that causes tumor cell death and induces ICD for T-cell activation mediated by DCs. Therefore, the systemic administration of nanoparticles with dual catalytic-chemotherapeutic properties simultaneously reversed the immunosuppressive TME and induced CTL responses. The combination of anti-CTLA-4 antibody with nanozyme could unleash the immunological function of the CTLs and suppress Treg population, resulting in a synergistic antitumor effect against established melanoma.
Fig. 10.
Combination of oxygen-generating nanoparticles, chemotherapy, and anti-CTLA-4 therapy boosts cancer immunotherapy. O2 is generated through the catalytic activity of CaO2-based and MnO2-based nanozymes, resulting in the relief of tumor hypoxia and suppression of Treg activity. The release of DOX from the nanoparticle core kills tumor cells and the resulting tumor cell death activates the surrounding immature DCs, which primes CTLs against the tumors. In combination with anti-CTLA-4 antibody, the Treg activity is suppressed and CTL response is upregulated.
Reproduced from [63] with permission from Springer. Copyright 2019
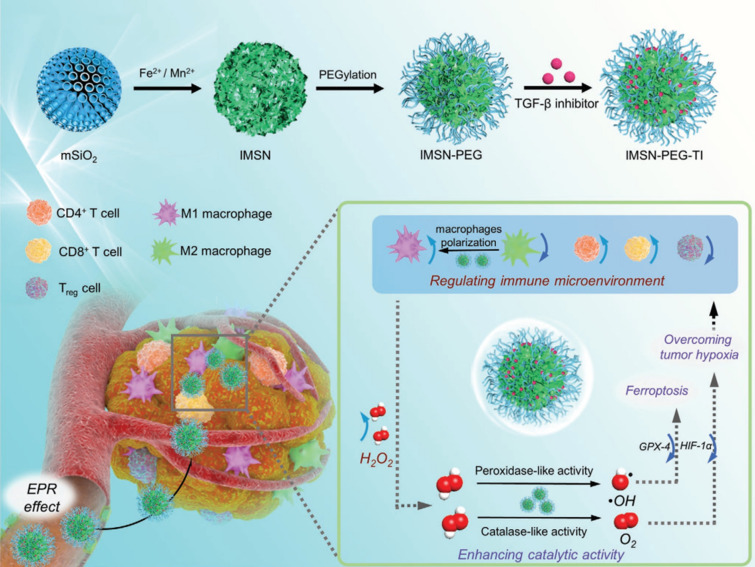
Liu et al. proposed the use of ROS-scavenging, O2-generating nanozymes. These nanozymes were constructed by co-loading iron oxide and manganese oxide nanoparticles on mesoporous silica nanoparticles, which were then modified by PEG for M1 polarization by the supplementation of O2 and ferroptosis by providing OH radicals (Fig. 11) [62]. Intrinsic CAT-like activities of manganese-based NPs can convert endogenous and exogenous H2O2 to O2. O2 production relieves tumor hypoxia and alleviates the TME via regulation of TAMs, in which M2 macrophages are polarized into the M1 phenotype. In addition, the TGF-β inhibitor loaded in the mesopores could prevent Treg activity and induce adaptive T-cell responses. Furthermore, POD-like activities of iron oxide nanoparticles that worked as ROS-generating materials via the Fenton reaction increased ROS levels and accumulated ferrous (Fe2+) ions in the TME, resulting in Fe2+-mediated ferroptosis. Ferroptosis is a programmed cell death process dependent on iron levels and occurs because of the failure to check lipid peroxidation. This could efficiently enhance the activity of immune cells due to the degradation of dead tumor cells, releasing TAAs. These results demonstrated that MSNs containing catalytic-nanozymes and TGF-β inhibitor dramatically decrease tumor hypoxia through the upregulation of transcription factor hypoxia-inducible factors-1α (HIF-1α), enhance ferroptosis through the downregulation of glutathione peroxidase 4 (GPX4), significantly increasing the ratio of M1/M2 macrophages and effector T cells/Treg cells, leading to the suppression of tumor growth.
Fig. 11.
Iron-based (ROS-generating) and manganese-based (ROS-scavenging) components are encapsuled into mesoporous silica nanoparticles (MSN) loaded with TGF-β inhibitor. Systemic administration led to the accumulation of nanoparticles in the tumor site through the enhanced permeability and retention (EPR) effect. O2 generation from the ROS-scavenging nanozymes relieve tumor hypoxia and regulate the TME through macrophage polarization. ROS-generating nanozymes increase local ROS levels through the Fenton-reaction, causing ferroptosis. Subsequently, tumor cell death releases TAAs which are captured, processed, and presented by DCs which activate effector T cells. TGF-β inhibitor was introduced to restrict the activation of Tregs.
Reproduced from [62] with permission from Wiley–VCH. Copyright 2020
Conclusion and perspectives
Currently, conventional cancer therapies have certain limitations, such as low therapeutic efficiency, high dose administration, invasive intervention, and severe side effects. Fortunately, the appearance of artificial nanozymes is a savior of the cancer immunotherapy field because of its numerous benefits such as low cost, strong stability, ease of modification, tumor penetration, high catalytic activity, and intense enhancement of innate and adaptive immunity. Based on their catalytic functions, both ROS-scavenging and ROS-generating nanozymes can enhance the efficacy of cancer immunotherapy. ROS-scavenging and O2-generating nanozymes could reverse the immunosuppression of TME by generating O2 through catalytic enzymes such as SOD or CAT, thereby relieving hypoxia and improving the efficiency of the immune response. One favorable aspect of ROS-scavenging nanozymes is their ability to synergize with other therapies (PTT, PDT, sonotherapy, radiotherapy) to inflate the therapeutic immune efficiency based on the catalytic conversion of endogenous H2O2 to produce ROS, which induces ICD to awake the CTL responses. On the other hand, ROS-generating nanozymes have intrinsic catalytic properties for producing toxic radicals such as OH radicals through POD- and OXD-like activity. The resulting high ROS levels in the TME induce ICD in tumor cells, leading to the release of TAAs and DAMPs, which induce antigen-specific immune responses.
However, recent approaches based on nanozymes for cancer immunotherapy have certain limitations. First, the off-target effect of nanozymes after systemic administration causes damage to normal cells in the other organs. For a more practical approach, intratumoral injection of the nanozymes into solid tumors that are accessible through the needle injection or the endoscope would be beneficial to its safe delivery to tumors without undesired accumulation in other organs. However, for the most cancer with less accessibility through intratumoral injection, innovative strategy enabling high targeting efficiency after intravenous injection needs to be developed. Second, a myriad of nanozymes simultaneously exhibit two different catalytic activities, ROS-scavenging, and ROS-generating; thus, controlling ROS levels in the TME are challenging missions and affect the ability to modulate TME. Depending on immuno-activating strategies, for example, ROS-induced ICD and supply of O2 to the TME and ROS-scavenging and ROS-generating activities have been combined simultaneously for utilizing both activities for different immuno-activating purposes. In this case, an appropriate design of nanozymes is needed to balance their catalytic reactions so as not to significantly compromise the activity of the other. Finally, most nanozyme-based strategies have focused on the response to physiological conditions of the TME, including endogenous ROS such as H2O2, high oxidative stress, hypoxia, and acidic conditions. Novel strategies inducing the modulation of immunosuppressive cells (MDSCs, TAMs, and Tregs) can be combined; for example, CRISPR gene editing/deleting to suppress the metabolism of these cells to suppress immune checkpoints or enhancing immunoactive signals such as pro-inflammatory cytokine and co-stimulatory molecules can be simultaneously applied. Taken together, nanozyme use for cancer immunotherapy is still challenging and has room for further improvement. Nevertheless, there is no doubt that nanozymes play an expansive role in cancer immunotherapy. In the future, we contend that nanozymes with transcendental improvements will contribute to the eradication of cancer in clinics.
Acknowledgements
This research was supported by grant from the National Research Foundation (NRF) of Korea (No. 2020M3A9D3039720) funded by the Korean government (MSIT) and the R&D Program for Forest Science Technology (No. 2020209B10-2222-BA01) provided by the Korea Forest Service (KFS) of the Korea Forestry Promotion Institute. Ngoc Man Phan and Thanh Loc Nguyen contributed equally to this work. All authors approved the final version of the manuscript. Correspondence should be addressed to Jaeyun Kim.
Declarations
Conflicts of interest
The authors declare no competing financial interest.
Ethical Statement
There are no animal experiments carried out for this article.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71:7–33. doi: 10.3322/caac.21654. [DOI] [PubMed] [Google Scholar]
- 2.Zhu G, Lynn GM, Jacobson O, Chen K, Liu Y, Zhang H, et al. Albumin/vaccine nanocomplexes that assemble in vivo for combination cancer immunotherapy. Nat Commun. 2017;8:1954. doi: 10.1038/s41467-017-02191-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuai R, Ochyl LJ, Bahjat KS, Schwendeman A, Moon JJ. Designer vaccine nanodiscs for personalized cancer immunotherapy. Nat Mater. 2017;16:489–496. doi: 10.1038/nmat4822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim J, Li WA, Choi Y, Lewin SA, Verbeke CS, Dranoff G, et al. Injectable, spontaneously assembling, inorganic scaffolds modulate immune cells in vivo and increase vaccine efficacy. Nat Biotechnol. 2015;33:64–72. doi: 10.1038/nbt.3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sahin U, Türeci Ö. Personalized vaccines for cancer immunotherapy. Science. 2018;359:1355–1360. doi: 10.1126/science.aar7112. [DOI] [PubMed] [Google Scholar]
- 6.Gubin MM, Zhang X, Schuster H, Caron E, Ward JP, Noguchi T, et al. Checkpoint blockade cancer immunotherapy targets tumour-specific mutant antigens. Nature. 2014;515:577–581. doi: 10.1038/nature13988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koyama S, Akbay EA, Li YY, Herter-Sprie GS, Buczkowski KA, Richards WG, et al. Adaptive resistance to therapeutic PD-1 blockade is associated with upregulation of alternative immune checkpoints. Nat Commun. 2016;7:10501. doi: 10.1038/ncomms10501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim K, Skora AD, Li Z, Liu Q, Tam AJ, Blosser RL, et al. Eradication of metastatic mouse cancers resistant to immune checkpoint blockade by suppression of myeloid-derived cells. Proc Natl Acad Sci. 2014;111:11774. doi: 10.1073/pnas.1410626111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Topalian Suzanne L, Drake Charles G, Pardoll DM. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell. 2015;27:450–461. doi: 10.1016/j.ccell.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao J, Wen X, Tian L, Li T, Xu C, Wen X, et al. Irreversible electroporation reverses resistance to immune checkpoint blockade in pancreatic cancer. Nat Commun. 2019;10:899. doi: 10.1038/s41467-019-08782-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen Q, Hu Q, Dukhovlinova E, Chen G, Ahn S, Wang C, et al. Photothermal therapy promotes tumor infiltration and antitumor activity of CAR T cells. Adv Mater. 2019;31:e1900192. doi: 10.1002/adma.201900192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mohammed S, Sukumaran S, Bajgain P, Watanabe N, Heslop HE, Rooney CM, et al. Improving chimeric antigen receptor-modified T cell function by reversing the immunosuppressive tumor microenvironment of pancreatic cancer. Mol Ther. 2017;25:249–258. doi: 10.1016/j.ymthe.2016.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maus MV, June CH. Making better chimeric antigen receptors for adoptive T-cell therapy. Clin Cancer Res. 2016;22:1875. doi: 10.1158/1078-0432.CCR-15-1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brown CE, Alizadeh D, Starr R, Weng L, Wagner JR, Naranjo A, et al. Regression of Glioblastoma after chimeric antigen receptor T-cell therapy. N Engl J Med. 2016;375:2561–2569. doi: 10.1056/NEJMoa1610497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nguyen TL, Yin Y, Choi Y, Jeong JH, Kim J. Enhanced cancer DNA vaccine via direct transfection to host dendritic cells recruited in injectable scaffolds. ACS Nano. 2020;14:11623–11636. doi: 10.1021/acsnano.0c04188. [DOI] [PubMed] [Google Scholar]
- 16.Cha BG, Jeong JH, Kim J. Extra-large pore mesoporous silica nanoparticles enabling co-delivery of high amounts of protein antigen and toll-like receptor 9 agonist for enhanced cancer vaccine efficacy. ACS Cent Sci. 2018;4:484–492. doi: 10.1021/acscentsci.8b00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nguyen TL, Cha BG, Choi Y, Im J, Kim J. Injectable dual-scale mesoporous silica cancer vaccine enabling efficient delivery of antigen/adjuvant-loaded nanoparticles to dendritic cells recruited in local macroporous scaffold. Biomaterials. 2020;239:119859. doi: 10.1016/j.biomaterials.2020.119859. [DOI] [PubMed] [Google Scholar]
- 18.Lee JY, Kim MK, Nguyen TL, Kim J. Hollow mesoporous silica nanoparticles with extra-large mesopores for enhanced cancer vaccine. ACS Appl Mater Interfaces. 2020;12:34658–34666. doi: 10.1021/acsami.0c09484. [DOI] [PubMed] [Google Scholar]
- 19.Kerr MD, McBride DA, Chumber AK, Shah NJ. Combining therapeutic vaccines with chemo- and immunotherapies in the treatment of cancer. Expert Opin Drug Discov. 2021;16:89–99. doi: 10.1080/17460441.2020.1811673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guo C, Manjili MH, Subjeck JR, Sarkar D, Fisher PB, Wang X-Y. Chapter seven - therapeutic cancer vaccines: past, present, and future. In: Tew KD, Fisher PB, editors. Advances in Cancer Research. Cambridge: Academic Press; 2013. pp. 421–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Farkona S, Diamandis EP, Blasutig IM. Cancer immunotherapy: the beginning of the end of cancer? BMC Med. 2016;14:73. doi: 10.1186/s12916-016-0623-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duan X, Chan C, Lin W. Nanoparticle-mediated immunogenic cell death enables and potentiates cancer immunotherapy. Angew Chem Int Ed. 2019;58:670–680. doi: 10.1002/anie.201804882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vanneman M, Dranoff G. Combining immunotherapy and targeted therapies in cancer treatment. Nat Rev Cancer. 2012;12:237–251. doi: 10.1038/nrc3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Palucka K, Banchereau J. Cancer immunotherapy via dendritic cells. Nat Rev Cancer. 2012;12:265–277. doi: 10.1038/nrc3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hu Z, Ott PA, Wu CJ. Towards personalized, tumour-specific, therapeutic vaccines for cancer. Nat Rev Immunol. 2018;18:168–182. doi: 10.1038/nri.2017.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Melero I, Gaudernack G, Gerritsen W, Huber C, Parmiani G, Scholl S, et al. Therapeutic vaccines for cancer: an overview of clinical trials. Nat Rev Clin Oncol. 2014;11:509–524. doi: 10.1038/nrclinonc.2014.111. [DOI] [PubMed] [Google Scholar]
- 27.Ma J, Qiu J, Wang S. Nanozymes for catalytic cancer immunotherapy. ACS Appl Nano Mater. 2020;3:4925–4943. [Google Scholar]
- 28.Ying JF, Lu ZB, Fu LQ, Tong Y, Wang Z, Li WF, et al. The role of iron homeostasis and iron-mediated ROS in cancer. Am J Cancer Res. 2021;11:1895–912. [PMC free article] [PubMed]
- 29.Alizadeh D, Trad M, Hanke NT, Larmonier CB, Janikashvili N, Bonnotte B, et al. Doxorubicin eliminates myeloid-derived suppressor cells and enhances the efficacy of adoptive T-cell transfer in breast cancer. Can Res. 2014;74:104. doi: 10.1158/0008-5472.CAN-13-1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Solito S, Marigo I, Pinton L, Damuzzo V, Mandruzzato S, Bronte V. Myeloid-derived suppressor cell heterogeneity in human cancers. Ann N Y Acad Sci. 2014;1319:47–65. doi: 10.1111/nyas.12469. [DOI] [PubMed] [Google Scholar]
- 31.Gabitass RF, Annels NE, Stocken DD, Pandha HA, Middleton GW. Elevated myeloid-derived suppressor cells in pancreatic, esophageal and gastric cancer are an independent prognostic factor and are associated with significant elevation of the Th2 cytokine interleukin-13. Cancer Immunol Immunother. 2011;60:1419–30. [DOI] [PMC free article] [PubMed]
- 32.Meng X, Li D, Chen L, He H, Wang Q, Hong C, et al. High-performance self-cascade pyrite nanozymes for apoptosis-ferroptosis synergistic tumor therapy. ACS Nano. 2021;15(3):5735–5751. doi: 10.1021/acsnano.1c01248. [DOI] [PubMed] [Google Scholar]
- 33.Wang Q, Niu D, Shi J, Wang L. A three-in-one ZIFs-derived CuCo(O)/GOx@PCNs hybrid cascade nanozyme for immunotherapy/enhanced starvation/photothermal therapy. ACS Appl Mater Interfaces. 2021;13:11683–11695. doi: 10.1021/acsami.1c01006. [DOI] [PubMed] [Google Scholar]
- 34.Zhou K, Cheng T, Zhan J, Peng X, Zhang Y, Wen J, et al. Targeting tumor-associated macrophages in the tumor microenvironment (Review) Oncol Lett. 2020;20(5):234. doi: 10.3892/ol.2020.12097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pan Y, Yu Y, Wang X, Zhang T. Tumor-associated macrophages in tumor immunity. Front Immunol. 2020;11:3151. doi: 10.3389/fimmu.2020.583084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Umansky V, Blattner C, Fleming V, Hu X, Gebhardt C, Altevogt P, et al. Myeloid-derived suppressor cells and tumor escape from immune surveillance. Semin Immunopathol. 2017;39:295–305. doi: 10.1007/s00281-016-0597-6. [DOI] [PubMed] [Google Scholar]
- 37.Wei T, Zhong W, Li Q. Role of heterogeneous regulatory T cells in the tumor microenvironment. Pharmacol Res. 2020;153:104659. doi: 10.1016/j.phrs.2020.104659. [DOI] [PubMed] [Google Scholar]
- 38.Chaudhary B, Elkord E. Regulatory T cells in the tumor microenvironment and cancer progression: role and therapeutic targeting. Vaccines. 2016;4:28. doi: 10.3390/vaccines4030028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu C, Workman CJ, Vignali DAA. Targeting regulatory T cells in tumors. FEBS J. 2016;283:2731–2748. doi: 10.1111/febs.13656. [DOI] [PubMed] [Google Scholar]
- 40.Beyer M, Schultze LJ. Regulatory T cells: major players in the tumor microenvironment. Curr Pharm Des. 2009;15:1879–1892. doi: 10.2174/138161209788453211. [DOI] [PubMed] [Google Scholar]
- 41.Zhang L, Wang S, Wang Y, Zhao W, Zhang Y, Zhang N, et al. Effects of hypoxia in intestinal tumors on immune cell behavior in the tumor microenvironment. Front Immunol. 2021;12:252. doi: 10.3389/fimmu.2021.645320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li Y, Zhao L, Li X-F. Hypoxia and the Tumor microenvironment. Technol Cancer Res Treat. 2021;20:15330338211036304. doi: 10.1177/15330338211036304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vito A, El-Sayes N, Mossman K. Hypoxia-driven immune escape in the tumor microenvironment. Cells. 2020;9:992. doi: 10.3390/cells9040992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bohme I, Bosserhoff AK. Acidic tumor microenvironment in human melanoma. Pigment Cell Melanoma Res. 2016;29:508–523. doi: 10.1111/pcmr.12495. [DOI] [PubMed] [Google Scholar]
- 45.Sitkovsky MV, Hatfield S, Abbott R, Belikoff B, Lukashev D, Ohta A. Hostile, hypoxia–A2-adenosinergic tumor biology as the next Barrier to overcome for tumor immunologists. Cancer Immunol Res. 2014;2:598. doi: 10.1158/2326-6066.CIR-14-0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barsoum IB, Smallwood CA, Siemens DR, Graham CH. A mechanism of hypoxia-mediated escape from adaptive immunity in cancer cells. Cancer Res. 2014;74:665–674. doi: 10.1158/0008-5472.CAN-13-0992. [DOI] [PubMed] [Google Scholar]
- 47.Aboelella NS, Brandle C, Kim T, Ding Z-C, Zhou G. Oxidative stress in the tumor microenvironment and its relevance to cancer immunotherapy. Cancers. 2021;13:986. doi: 10.3390/cancers13050986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang H, Cheng L, Ma S, Ding L, Zhang W, Xu Z, et al. Self-assembled multiple-enzyme composites for enhanced synergistic cancer starving-catalytic therapy. ACS Appl Mater Interfaces. 2020;12:20191–20201. doi: 10.1021/acsami.0c02006. [DOI] [PubMed] [Google Scholar]
- 49.Chen M, Deng G, He Y, Li X, Liu W, Wang W, et al. Ultrasound-enhanced generation of reactive oxygen species for MRI-guided tumor therapy by the Fe@Fe3O4-based peroxidase-mimicking nanozyme. ACS Appl Bio Mater. 2019;3:639–647. doi: 10.1021/acsabm.9b01006. [DOI] [PubMed] [Google Scholar]
- 50.Lan M, Zhao S, Liu W, Lee CS, Zhang W, Wang P. Photosensitizers for photodynamic therapy. Adv Healthc Mater. 2019;8:1900132. doi: 10.1002/adhm.201900132. [DOI] [PubMed] [Google Scholar]
- 51.Zhu L, Liu J, Zhou G, Liu TM, Dai Y, Nie G, et al. Remodeling of tumor microenvironment by tumor-targeting Nanozymes enhances immune activation of CAR T cells for combination therapy. Small. 2021;17:e2102624. doi: 10.1002/smll.202102624. [DOI] [PubMed] [Google Scholar]
- 52.Zhao Y, Xiao X, Zou M, Ding B, Xiao H, Wang M, et al. Nanozyme-initiated in situ cascade reactions for self-amplified biocatalytic immunotherapy. Adv Mater. 2021;33:e2006363. doi: 10.1002/adma.202006363. [DOI] [PubMed] [Google Scholar]
- 53.Liang R, Liu L, He H, Chen Z, Han Z, Luo Z, et al. Oxygen-boosted immunogenic photodynamic therapy with gold nanocages@manganese dioxide to inhibit tumor growth and metastases. Biomaterials. 2018;177:149–160. doi: 10.1016/j.biomaterials.2018.05.051. [DOI] [PubMed] [Google Scholar]
- 54.Gao L, Liu R, Gao F, Wang Y, Jiang X, Gao X. Plasmon-mediated generation of reactive oxygen species from near-infrared light excited gold nanocages for photodynamic therapy in vitro. ACS Nano. 2014;8:7260–7271. doi: 10.1021/nn502325j. [DOI] [PubMed] [Google Scholar]
- 55.Liu Y, Zhen W, Wang Y, Song S, Zhang H. Na2S2O8 nanoparticles trigger antitumor immunotherapy through reactive oxygen species storm and surge of tumor osmolarity. J Am Chem Soc. 2020;142:21751–21757. doi: 10.1021/jacs.0c09482. [DOI] [PubMed] [Google Scholar]
- 56.Tang F, Du X, Liu M, Zheng P, Liu Y. Anti-CTLA-4 antibodies in cancer immunotherapy: selective depletion of intratumoral regulatory T cells or checkpoint blockade? Cell Biosci. 2018;8:30. doi: 10.1186/s13578-018-0229-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen H, Luan X, Paholak HJ, Burnett JP, Stevers NO, Sansanaphongpricha K, et al. Depleting tumor-associated Tregs via nanoparticle-mediated hyperthermia to enhance anti-CTLA-4 immunotherapy. Nanomedicine. 2019;15:77–92. doi: 10.2217/nnm-2019-0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yao J, Cheng Y, Zhou M, Zhao S, Lin S, Wang X, et al. ROS scavenging Mn3O4 nanozymes for in vivo anti-inflammation. Chem Sci. 2018;9:2927–2933. doi: 10.1039/c7sc05476a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kaizer J, Csay T, Kővári P, Speier G, Párkányi L. Catalase mimics of a manganese(II) complex: the effect of axial ligands and pH. J Mol Catal A Chem. 2008;280:203–209. [Google Scholar]
- 60.Singh N, Savanur MA, Srivastava S, D'Silva P, Mugesh G. A manganese oxide nanozyme prevents the oxidative damage of biomolecules without affecting the endogenous antioxidant system. Nanoscale. 2019;11:3855–3863. doi: 10.1039/c8nr09397k. [DOI] [PubMed] [Google Scholar]
- 61.Yang G, Xu L, Chao Y, Xu J, Sun X, Wu Y, et al. Hollow MnO2 as a tumor-microenvironment-responsive biodegradable nano-platform for combination therapy favoring antitumor immune responses. Nat Commun. 2017;8:902. doi: 10.1038/s41467-017-01050-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xu B, Cui Y, Wang W, Li S, Lyu C, Wang S, et al. Immunomodulation-enhanced nanozyme-based tumor catalytic therapy. Adv Mater. 2020;32:e2003563. doi: 10.1002/adma.202003563. [DOI] [PubMed] [Google Scholar]
- 63.Wang J, Fang L, Li P, Ma L, Na W, Cheng C, et al. Inorganic nanozyme with combined self-oxygenation/degradable capabilities for sensitized cancer immunochemotherapy. Nanomicro Lett. 2019;11:74. doi: 10.1007/s40820-019-0305-x. [DOI] [PMC free article] [PubMed] [Google Scholar]