Abstract
Tissue engineering (TE) is a therapeutic option within regenerative medicine that allows to mimic the original cell environment and functional organization of the cell types necessary for the recovery or regeneration of damaged tissue using cell sources, scaffolds, and bioreactors. Among the cell sources, the utilization of mesenchymal cells (MSCs) has gained great interest because these multipotent cells are capable of differentiating into diverse tissues, in addition to their self-renewal capacity to maintain their cell population, thus representing a therapeutic alternative for those diseases that can only be controlled with palliative treatments. This review aimed to summarize the state of the art of the main sources of MSCs as well as particular characteristics of each subtype and applications of MSCs in TE in seven different areas (neural, osseous, epithelial, cartilage, osteochondral, muscle, and cardiac) with a systemic revision of advances made in the last 10 years. It was observed that bone marrow-derived MSCs are the principal type of MSCs used in TE, and the most commonly employed techniques for MSCs characterization are immunodetection techniques. Moreover, the utilization of natural biomaterials is higher (41.96%) than that of synthetic biomaterials (18.75%) for the construction of the scaffolds in which cells are seeded. Further, this review shows alternatives of MSCs derived from other tissues and diverse strategies that can improve this area of regenerative medicine.
Keywords: Tissue engineering, Mesenchymal stem cells, Regenerative medicine, Biocompatible materials, Tissue therapy
Introduction
There are certain limitations within the therapeutic area focused on the treatment of diseases in which the reparation or replacement of tissue and organs are needed because of the limited capacity of the human body to restore most tissues and organs to their original state [1]. The two principal issues are the lack of donors and transplant rejection by the immune system [2–4]; therefore, and considering that the pharmacological therapy currently available improves the patient’s quality of life but fails to restore the functionality of damaged cells, it is necessary to find therapeutic alternatives that allows the reparation and recovery of the damaged tissues.
In the last decades, an alternative known as regenerative medicine, a new branch of medicine dedicated to replace, regenerate, and repair cells, tissues, and organs damaged owing to factors such as age, chronic illness or congenital defects, has emerged [5, 6]. Currently, this term encompasses diverse areas and technologies, mainly cell therapy (CT), in which stem, progenitor, and primary cells are used to reconstruct structures and restore tissue and organ functions [7–9]; gene therapy, which entails a group of techniques to introduce genetic material and modify the expression of a gene product or alter the biological properties of cells to treat diseases [10]; and tissue engineering (TE), which involves the use of cell sources, scaffolds (or cell supports), and bioreactors to regenerate damaged tissue [11].
The following three basic pillars are necessary for the application of regenerative medicine in TE: cell sources, biomaterials, and culture stimulation. It is important to highlight that the last two pillars provide support for the cell type used, allowing proliferation and differentiation into the tissue of interest and promoting its migration and anchoring [12] to either natural (e.g., derived from components of the extracellular matrix) or synthetic biomaterials [13], along with the creation of an appropriate environment that mimics in vivo conditions to enhance tissue development through chemical and electrical mechanisms [14, 15]. Overall, the use of biomaterials has been reported as an option for delivery systems of differentiation and conditioning to induce a desire-specific cell lineage of mesenchymal cells (MSCs). Within this type of signals, it is important to emphasize those known as growth factors, whose function is to signal cells during their development and tissue repair [16], such as some cytokines, which requires support to prolong their half-life in normal physiological conditions, as they are prone to proteolysis, and can be applied for therapeutic purposes. Among this kind of molecules reported in regenerative medicine, especially to TE for various applications, are the following [16]. Transforming growth factor (TGF) for the regulation of cell growth and immune function; vascular endothelial growth factor (VEGF) induces of angiogenesis; Insulin-like growth factor (IGF) acts in endocrine regulation of the somatic growth, induces extracellular matrix synthesis and differentiation of MSCs to different phases in bone tissue repair [17]. Bone morphogenic protein (BMP) is used for promotion of cell regeneration and proliferation as well as metabolic homeostasis and the reduction of pro-inflammatory factors [18]. Fibroblast growth factor (FGF) has an important role in the promotion of angiogenesis and epithelization besides the migration and proliferation of keratinocytes and fibroblast. Epidermal growth factor (EGF) increases the deposition of extracellular matrix and the stimulation of the proliferation of chondrocytes and MSCs as a potent mitogen [19]. Platelet-derived growth factor (PDGF) controls the differentiation of neurons. Neurotrophic factors (NF) and those factors that are used to stimulate cellular proliferation, morphogenesis, and angiogenesis have been widely used in regenerative medicine. Hepatocyte growth factor (HGF) can be used in combination, such as platelet-rich plasma (PPP), to obtain a platelet lysate (PL) in a singular solution with high concentration of growth factors such as TGB-β, PDGF-AA, PDGF-AB, PDGF-BB, EGF, IGF-1, HGF [20, 21], or independently according to the application of interest. Regarding the first pillar, adult stem cells are one of the principal cell sources in regenerative medicine, and sufficient evidence on their safety has been reported [22]. These cells are responsible for the development, maintenance, and repair of tissues, and numerous clinical trials have demonstrated their high potential in the treatment of diseases; however, until 2018, only three trials had completed phase III [23–25]. Nevertheless, a wide variety of studies in the early stages of clinical trials have used this cell type [26]. MSCs, multipotent stem cells, are considered a key option for tissue repair therapy because they have been studied in clinical trials, and evidence supports their great tissue repair potential in the treatment of numerous diseases, including orthopedic diseases (pseudoarthrosis and craniofacial trauma); degenerative diseases of the skeletal system (osteonecrosis and osteogenesis imperfecta); ocular (glaucoma and macular degeneration); cardiac (ischemic cardiomyopathy); renal, hepatic, and pulmonary diseases; and autoimmune diseases such as rheumatoid arthritis, and multiple sclerosis [24, 27, 28]. Important advances have been made in the TE field using MSCs, for example, the co-culture of these cells with human auricular chondrocytes seeded on collagen have been used to generate cartilage [29], and adipose derived-mesenchymal stem cells (AD-MSCs) have been combined with hyaluronic acid in rat models to regenerate osseous tissue [30]. In addition, the use of same type of MSCs in recombinant human tropoelastin scaffolds resulted in epithelium recovery in a murine model [31]. Apart from the applications of MSCs mentioned previously, these cells have also been used in the cardiac area of TE because cardiac and cardiovascular diseases (CVD) represent the major public health problem, according to the WHO, of which myocardial infarction affects a large part of the population. The WHO estimates that by 2030, almost 23.6 million individuals will die from a CVD and that this type of disease will continue being the main cause of death worldwide [32]. Although there are diverse therapeutic options, these are limited because they can only control the symptoms or prevent disease progress, but without tissue recovery. This review focused on the description of stem cells, specifically MSCs, because they comprise an important cell source in TE, and analyzed published studies that employed these cells in the principal applications of TE.
The most promising cell source for TE: mesenchymal/stromal stem cells
Stem cells
Stem cells (SCs) are defined as non-specialized cells with unlimited or extended self-renewal potential, capable of differentiating into various cell types, and thus, of serving as a reservoir to produce, maintain, repair, and regenerate tissues, while also maintaining their own population [26, 33, 34].
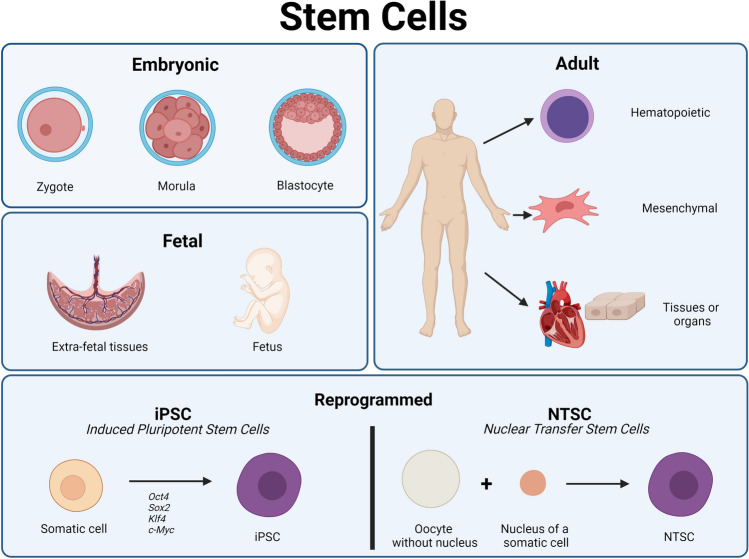
SCs are generally divided into six main categories based on the biology of the source from which they were obtained, which correspond to some stages of the development of a complete organism (Figure 1). These categories include embryonic SCs (ESCs) found in the first stages of embryonic development [35], fetal SCs (FSC) or FSCs from extraembryonic tissues, which can be obtained in the fetal stage, from the tenth week post-fertilization [36]; adult SCs (ASCs), including mesenchymal, hematopoietic, and stromal cells [37]; very small embryonic-like SCs (VSEL), found both in the adult stage and early stages of embryonic development [38]; and finally, those obtained by reprogramming, the induced pluripotent SCs (iPSCs); and nuclear transfer SCs (NTSC) [39].
Fig. 1.
Stem cell sources in the different stages of human development and stem cell extraction by reprogramming
Table 1 shows the summarized information of studies on the particular biologic characteristic of each SC, published in the last 5 years.
Table 1.
General characteristics of stem cells according to their biology
| Characteristic | Embryonic stem cells (ESC) | Fetal Stem cells | Very small embryonic-like stem cells (VSEL) | Adult stem cells (ASC) | Induced pluripotent stem cells (iPSC) | Nuclear transfer stem cells (NTSC) |
|---|---|---|---|---|---|---|
| Definition | Stem cells derived from the internal mass of the blastocyst or cells available from the morula stage, besides zygote | Pluripotent and multipotent stem cells derived directly from the fetus or extra fetal tissues | Non-hematopoietic pluripotent stem cells that are negative to the lineage marker CD133 that come from adult and fetal tissues | Quiescent stem cells that are present in an adult organism that responds to specific stimuli from the tissues to produce specialized cells | Pluripotent stem cells generated by in vitro reprogramming of somatic adult cells by simultaneous expression to inductor genes | A single cell is produced by the insertion of a nucleus from a somatic cell in an enucleated oocyte |
| Potential | Totipotent (Zygote) Pluripotent (Morula, blastocyst) | Pluripotent Multipotent | Pluripotent | Pluripotent Multipotent | Pluripotent | Totipotent |
| Source | Zygote Morula Blastocyst | Fetus Extra fetal tissues (umbilical cord, amniotic fluid, placenta) | Adult bone marrow, umbilical cord blood, peripheral blood | Bone marrow, adipose tissue, skin, skeletal muscle, heart, liver, blood, nerve tissue | Somatic cells | Enucleated oocytes and nucleus from somatic cells |
| Ref. | [36, 39, 40] | [36] | [37–39] | [34, 39, 41] | [39, 42] | [39, 42] |
SCs can also be classified based on their cellular potential, in other words, their ability to differentiate. (Table 1) Thus, SCs are classified into totipotent stem cells, which are the least specialized and thus can divide and differentiate into all cell types necessary to form a complete organism [40], pluripotent stem cells, which can form cells of any of the germ layers, with ESCs and iPSC belonging to this group, and multipotent stem cells, which possess one level of specialization lower than the pluripotent SCs and whose function is to generate cells of specific lineages, such as the hematopoietic SCs, which can develop into several types of blood cells [40].
Mesenchymal cells
MSCs are an in vivo multipotent cell population present in adult and neonatal tissues of mesodermal origin as well as in the neural crest; they possess differentiation and self-renewal functions as progenitor cells, and their role is to maintain homeostasis and provide de novo specialized cells of mesodermal lineage [14, 28, 43].
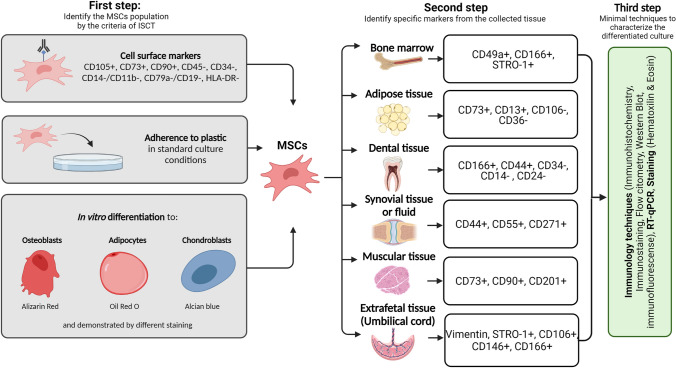
According to the International Society for Cellular Therapy (ISCT), this cell type presents three main characteristics: adherence to plastic under culture conditions, expression of specific surface markers (Table 2), and the ability to differentiate in vitro into osteoblasts, chondrocytes, and adipocytes [22]. Moreover, it has been observed that they maintain a fibroblast-like morphology and do not generate an immune response [15].
Table 2.
MSCs surface markers according to the ISCT
| Surface markers [44] | |
|---|---|
| Positive | Negative |
| CD105 | CD45 |
| CD73 | CD34 |
| CD90 | CD14 o CD11b |
| CD79α o CD19 | |
| HLA-DR | |
Two of the criteria for the expression of surface antigens is that ≥ 95% of the total population must express positive markers and only ≤ 2% can express negative markers
However, there is controversy regarding the classification criteria that must be considered to guarantee homogeneity. One of the main controversies is the use of the acronym “MSCs,” as some authors use it to describe mesenchymal stem cells and others to refer to mesenchymal stromal cells, but without mentioning the source of extraction, which is the main difference between these cell types. The former population is directly isolated from bone marrow, whereas the latter correspond to any structural or nonstructural tissue [45]. This has led to differences in the expression of the surface cell markers used to differentiate MSCs, one of them being the negative expression of CD34. In this regard, it has been reported that the CD34 expression depends on the donor and type of tissue, e.g., a percentage of adipose tissue-derived SCs are CD34 positive [43]. Moreover, although other surface markers have been suggested to characterize MSCs (Stro-1, CD271, SSEA-4, and CD146), no consensus has been reached as their expression varies depending on the cell source [25, 46]. Therefore, it is mandatory to know the markers of MSCs obtained from a particular tissue besides those established by the ISCT. Table 3 summarizes the different surface markers reported in the last five years to characterize MSCs based on their cell source.
Table 3.
Surface markers present in MSCs based on the source of isolation. Markers presented in bold are reported as tissue-specific markers from the tissue of isolation in various sources
| MSCs | Surface Markers | Ref. |
|---|---|---|
| BM-MSCs Bone marrow mesenchymal stem cells | CD11a-, CD11b-, CD13-, CD14-, CD19-, CD29 + , CD31 + , CD34 ± , CD45-, CD49a + , CD49b + , CD49c + , CD49d + , CD49e + , CD51 + , CD54 + , CD58 + , CD61 + , CD71 + , CD73 + , CD90 + , CD102 + , CD104 + , CD105 + , CD106 + , CD120a + , CD120b + , CD121a + , CD124 + , CD133-, CD140a + , CD140b + , CD146 + , CD166 + , CD200 + , CD221 + , CD271 + , SSEA-4 + , STRO-1 + , Nestin + | [25, 47–49] |
| AD-MSCs Adipose tissue mesenchymal stromal cells |
CD73 + , CD90 + , CD105 + , CD45-, CD34 ± , CD14- o CD11b-, CD79a- o CD19-, HLA-DR- CD13 + , CD29 + , CD10 + , CD49 + , CD26 + , CD3-, CD11b-, CD106-, CD36 + y CD44 + in > 80% of the surface of the AD − MSCs y CD31-, CD45- y CD235a- in less than 2% of the surface. Other: CD271 + , CD200 + , CD273 + , CD274 + , CD146 + , CD248 + , CD140b + , CD163-, CD166 + , CD59 + |
[50–53] |
| D-MSCs Dental tissue mesenchymal stromal cells | CD90 + , CD166 + , CD44 + , CD29 + , Cd73 + , CD146 + , CD106 + , CD105 + , STRO1 + , SSEA4 + , Nanog + , Oct4 + , Nestin + , Notch1 + , CD34-, CD45-, CD271-, CD71-, CD14-, CD49-, CD11b-, CD133-, HLA-DR-, CXCR4-, CD40 + , CD133-, CD24- | [54] |
| UC-MSCs Umbilical cord mesenchymal stromal cells | CD29 + , CD44 + , CD73 + , CD90 + , CD105 + , HLA-ABC + , HLA-DR-, Vimentin + , Stro1 + , CD106 + , CD146 + , CD166 + | [55–57] |
| S-MSCs Synovial tissue or fluid mesenchymal stem cells synovial | CD73 + , CD90 + , STRO-1 + , CD105 + , CD34-, CD45-, HLA-DR-, CD19-, CD40-, CD80-, CD86-, CD11b-, CD14 ± , CD44 + , CD55 + , CD271-, CD55-, CD271 + (according to the region of isolation) | [58–60] |
| Mu-MSCs Muscle tissue mesenchymal stem cells | CD45-, CD19-, CD14-, CD34-, CD73 + , CD90 + , CD105 + , PDGRFα, CD146 + , CD56 + , CD201 + , CD82 + , CD318 + | [61–63] |
Cell source, isolation, and culture
MSCs are present in nearly all the tissues of the body, are located in a perivascular niche [28], and can be obtained from diverse sources, including adult tissue (peripheral blood, adipose tissue, bone marrow, skeletal muscle, brain, dermis, dental pulp, synovial fluid, and tendons) and neonatal tissue (umbilical cord, umbilical cord blood, and placenta) [28, 64].
The proportion of MSCs varies depending on the tissue; they represent approximately 0.00001% of the bone marrow cells and ≥1% of the cells in the adipose tissue. Although the frequency of umbilical cord-derived MSCs is similar or even lower than that previously mentioned, they have a better in vivo expansion capacity owing to their fetal nature [64].
Table 4 presents the most important characteristics of MSCs based on their isolation source because heterogeneity has been reported in their cellular yield as well as their advantages and disadvantages, which represent the principal criteria for their use in regenerative medicine.
Table 4.
Sources of mesenchymal cells and their characteristics
| Source | Location | Isolation method | Culture | Cellular yield | Advantages | Disadvantages | Cell formation efficiency | Ref. |
|---|---|---|---|---|---|---|---|---|
| Bone marrow | Endostal surface of the iliac crest, trabecular and compact bone, femoral head, humeral head, and vertebral body | Aspiration in the posterior iliac crests. In animal models, it can also be obtained directly from long bones of freshly animals. MSCs are isolated by gradient density centrifugation (Ficoll or Percoll method) | Cell culture in DMEM medium with 10% fetal calf serum (FCS) | Dependent on the characteristics of the donor (e.g., Age). Approximately 100–1000 cells/mL or 10 FCE per 105 cells | Easy to isolate within a surgical procedure.High cell growth | Chronic pain at the site of isolation.High risk of infection | 52% | [65–67] |
| Adipose tissue | Adipose tissue | Liposuction or removed fat. Cells are isolated from the white adipose tissue | Cell culture in DMEM containing 10% fetal bovine serum (FBS) | 5000 SCs per 1 gr of fat | Easily obtained High initial cell yields. Robust proliferative capacity in vitro | Do not show chondrogenic or myogenic differentiation in all culture conditions. Do not present lineage markers: CD31, CD34, and CD45 | 60% | [66–68] |
| Dental tissue | Dental pulp, periodontal ligament, dental follicle, gingiva | Isolation of the tissue from non-invasive surgical tooth extractions | α-MEM with 10% FBS and antibiotic and antimycotic solutions | The frequency of colony formation is 22–77 colonies per 104 cells per plate | Easily obtained if the isolation is scheduled.Higher osteogenic differentiation because the memory of its origin. Depending of the source, there is higher or lower generation of mineral compounds | Samples must weigh more than 0.2 gr It cannot survive more than 24 h after extraction Higher restriction of differentiation in vivo | 80.4% | [50, 54, 69–72] |
| Fetal and extra fetal tissues | Umbilical cord, placenta, Wharton’s jelly (WJ), amniotic fluid, fetal tissues from the heart, liver, pancreas, and others | Umbilical cord is the most reported: harvest 10 cm of umbilical cord in DMEM medium. The most common methods of isolation are enzymatic and explants | DMEM medium with 10% human serum/FBS, antibiotic solution (0.1% gentamycin, 0.2% streptomycin and 0.12% penicillin) | Source dependent: blood from the umbilical cord has 1 UFC (Unit forming colonies) per 108 cells and WJ have a higher frequency | Does not have ethics limitations.Easy access Colony duplication is faster than BM-MSCs | Current isolation methods generate low cell count. Low capacity of adipogenicity differentiation | Varies with source, from 15% (amniotic fluid) to 80% (WJ) | [55, 67, 73, 74] |
| Synovial | Membrane and synovial fluids Knee articulations | Arthroscopy with biopsy |
MesenGro medium with 10% FBS and 1% penicillin–streptomycin At the third passage, cells can be used for in vitro assays |
37 cells/mL in the synovial fluid in patients with osteoarthritis, the number increases with a knee lesion. In healthy persons, cell numbers are 7 times lower to the mentioned above |
Higher cell count per colony than other sources Extensive proliferation in culture Maintains its cellular potential in vitro Higher chondrogenic potential |
Limited number of cells in the tissue No standard procedure for purifying MSCs from synovial fluid |
55%–75% depending on the culture medium (with autologous human serum or FBS respectively) | [58, 66, 75, 76] |
| Muscle | Postnatal skeletal muscle, below the basal lamina of muscular fibers | Small biopsies. Obtained by isolation of a single fiber or enzymatic digestion of the entire muscle | αMEM 1 × , + L-glutamine, + 4 ribonucleosides, + deoxyribonucleosides, -ascorbic acid, SH, insulin y 1% penicillin/streptomycin | The yield of viable cells depends on the source of the tissue | Promote angiogenesis Long-term proliferation | Satellite cells are already committed to muscle lineage Passage limit is 10 | ~ 45% | [66, 77–80] |
Therapeutic applications of MSCs in different tissues
According to previous researches, six of the more studied applications of mesenchymal cells in TE were selected, including those in neural, osseous, muscle, cartilaginous, epithelial, and cardiac tissues.
This review covers the use of six sources of MSCs in each of the TE applications. To that end, boolean expressions obtained from the PubMed database over the last 10 years were used. In addition, because more than 500 results were found for some of the applications, the search was limited to the last 5 years in those cases.
Currently, several therapies are under development in the TE field which use MSCs extracted from different sources to treat problems associated with diverse tissues; therefore, a search of the applications with great number of studies published in recent years was conducted, and the most frequently used cell sources were identified.
Therapies have emerged as an alternative to treat disorders that can no longer be treated with drugs or surgery because the damaged tissue is not able to recover owing to the limited capacity of the organism. The most studied areas include health issues related to the joints, bones, muscle, skin, and cardiac and neurodegenerative diseases, considering their incidence in the global population.
Applications of MSCs for cartilage and osteochondral diseases
An example of these disorders is osteoarthritis, one of the most common illnesses in middle aged and older individuals, which affects over 250 million people worldwide and is caused by age-related degeneration of the articular cartilage. Although an early intervention can help to prevent the damage from spreading to the healthy cartilage and current treatments are quite promising, there is no definitive treatment to completely restore the auricular cartilage to its normal state [81, 82]. To overcome these limitations, TE has emerged as an alternative to treat this disease. Rahman et al. (2015) created a BM-MSCs rabbit-derived composite construct of poly(lactic-co-glycolic acid) (PLGA)/fibrin scaffold, cultured in a commercial medium for chondrocyte differentiation, and observed that the construct was capable of regenerating tissue in an in vitro environment [81], thus proving its potential in cartilage TE. In addition, Yin et al. (2016) formed a compound with goat cartilage extracellular matrix (ECM); particles of the fresh cartilage (cartilage ECM-derived particles; CEDPs) were produced through a series of steps; rat BM-MSCs were seeded on the surface of CEDPs and the construct was implanted in rats with defects in the cartilage of the trochlear groove. It was found that the animals showed a good and fast recovery in their joint function [83]. Furthermore, in other congenital diseases associated with cartilage, such as microtia, infants have only few options for atrial reconstruction. Thus, Cohen et al. (2018) generated constructs of human auricular chondrocytes and human MSCs with type I collagen and subcutaneously implanted them in athymic mice, and a cartilage generation equivalent to that of the native auricular cartilage was observed [29].
Applications of MSCs for musculoskeletal diseases
Another example are the musculoskeletal pathologies, such as fractures, lumbago, and osteoporosis, which have increased owing to a longer life expectancy and the aging global population [84]; thus, it is necessary to find options to treat these disorders. Boeckel et al. (2019) implanted a graft of AD-MSCs with hyaluronic acid (HA) in rats with a critical osseous defect in each femur and observed an increase in the osseous tissue [30]. Moreover, Toosi et al. (2016) created collagen sponges reinforced with biodegradable polyglycolic acid (PGA) fibers, and in vitro experiments using these sponges demonstrated that the incorporation of PGA fibers increase cell adherence as well as proliferation and differentiation of BM-MSCs, which make them useful for the treatment of pseudoarthrosis [85]. Zhang et al. (2017) intramuscularly implanted hydroxyapatite/PLGA nanocomposites scaffolds with BM-MSCs and bone morphogenetic protein-2 (BMP-2) in rabbits and found that hydroxyapatite promotes an improvement in bone mineralization and formation [86].
Applications of MSCs for epithelial covering in traumas
TE has also shown important advances in the field of epithelial tissue regeneration as it is most exposed tissue to damage caused by burns, irritators, traumas, or tumor resection [87]. In addition, when the damage encompasses a large surface, there are limitations in the performance of a skin autograft, leading to complications; thus, researchers have looked for alternatives in graft creation [87, 88]. In preclinical stages, Mashiko et al. (2018) implanted a scaffold composed of recombinant human collagen peptide (rhCP), together with the combination of human AD-MSCs and endothelial cells from umbilical veins, in an ulcer model of irradiated mice and detected improved cicatrization compared with the groups that had only received the biomaterials or AD-MSCs and the controls (without scaffold or cells) [89]. Kellar et al. (2016) also reported the use of AD-MSCs with human tropoelastin in a murine model of excisional wound, in which the scaffold-treated wounds showed a closure rate of 94% as well as a recovery of normal epithelium of 75%, which was higher than that of the controls [31].
Applications of MSCs for muscle pathologies
Furthermore, TE is a great alternative for the treatment of certain muscle pathologies, such as muscular dystrophy or diseases caused by lesions or congenital defects [90]. Ansari et al. (2016) created RGD microspheres (common peptide motif of cell adhesion: arginine-glycine-aspartic acid) coupled to alginate and used them to encapsulate gingival mesenchymal stem cells (GMSCs). This construct was subcutaneously implanted in immunocompromised mice, together with a cocktail for myogenic differentiation, showing that after 4 weeks of in vitro differentiation, the GMSCs exhibited morphology similar to that of muscle cells and that the RGD-alginate-coupled scaffold regulated their myogenic differentiation. Furthermore, in in vivo experiments, the generation of small muscle-like structures was observed through hematoxylin-eosin (H&E) staining [90].
Applications of MSCs for regenerations of nervous tissue
Owing to the specific properties of nervous tissue-derived cells (excitability and conductivity) and their low regenerative capacity, it is highly complicated to administer treatments to cure disorders of the central nervous system, such as neurodegenerative diseases, traumatic lesions of the brain or spine, and cerebral infarction. Therefore, cell replacement through regenerative medicine has been an innovation in neuronal engineering, in which only devices to treat nervous system dysfunctions had been designed [91, 92]. The viability and neurogenesis of MSC have been examined in vitro in different scaffolds. In this regard, Quintiliano et al. (2016) studied a 15% PLGA scaffold with D-MSCs obtained from dental pulp and reported a fast degradation, 41% by day 28, which represents an advantage as this attenuates the progressive damage sustained by the nervous fibers after a lesion and provides sufficient time for the cells to appropriately proliferate, migrate, and differentiate into mature nervous tissue; these results support the execution of preclinical tests in animal models [93]. For neurodegenerative diseases, Jamali et al. (2017) proposed the use of a poly-L-lactic acid scaffold with MSCs collected from the trabecular meshwork to obtain dopaminergic neurons, which constitute an appropriate source for the treatment of Parkinson’s disease [94].
Applications of MSCs for mechanical cardiac pathologies
Finally, another area of interest for TE is the treatment of cardiac diseases, such as myocardial infarction, as the currently available treatments have diverse limitations. Nali et al. (2017) created a construct using UC-MSCs over a scaffold comprising decellularized umbilical artery, implanted it in a myocardial infarction rat model, and observed an improvement in the heart function [95]. Chen et al. (2018) created nanofiber patches of chitosan/silk-fibroin-modified cellulose with mice AD-MSCs, implanted them in rats with myocardial infarction, and later observed myocardial fibrosis attenuation [96]. Moreover, Prat-Vidal et al. (2020) created a scaffold called PeriCord comprising a decellularized pericardial matrix colonized with WJ-MSCs, which was implanted on a non-revascularizable scar in the inferior wall of a 63-year-old male patient. The three-month follow up showed optimal results, and the magnetic resonance indicated a ~9% reduction in the scar mass of the treated area [97]. Currently, the PeriCord scaffold is commercialized for its use in similar researches.
The tables below summarize the most recent studies on TE regarding osseous (Table 5), cartilaginous (Table 6), osteochondral (Table 7), muscle (Table 8), dermal (Table 9), nervous (Table 10), and cardiac (Table 11) tissues. These tables provide detailed information on the cell source, extraction tissues, and type of isolated MSCs, in addition to the analyses performed, markers used for cell characterization by different techniques (Immunofluorescence (IF), Flow cytometry (FC), Immunohistochemistry (IHC), Immunostaining (IS), Western-Blot (WB), Enzyme-linked immunosorbent assay (ELISA) and Polymerase chain reaction (PCR)), cellularized scaffolds, type of scaffold used, and findings.
Table 5.
Applications of MSCs in bone tissue engineering
| Source | Type of MSCs | Extraction tissue | Type of analysis and markers | Scaffold | Discovery | Year | Ref. | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Immunodetection techniques | PCR | Histology | Spectrometry | |||||||
| Human | BM-MSCs | Unspecified | COL I, IBSP, BMP2, OCN | H&E staining, Masson trichrome staining | ALP activity assay (alkaline phosphatase) | Silk fibroin (SF) with bioactive mesoporous glass (MBG) | MBG/SF scaffolds had superior strength and good biocompatibility and stimulated bone formation ability superior to a polycaprolactone/MBG scaffold. In vivo gene expression was common to osteogenic markers on this scaffold | 2019 | [98] | |
| BM-MSCs | Unspecified | ALP activity assay | Silk fibroin scaffolding and bioactive glass of nanoparticle silk fibroin | 50% more fixation of MSCs compared with scaffolding with microparticles with greater promotion of ALP activity | 2019 | [99] | ||||
| UC-MSCs + hUVEC | Lonza | IF: PECAM1 | ALP, OCN, COL I. VEGF, CDH5, vWF | H&E staining, Alizarin-red stain | Calcium phosphate cement scaffolding (CPC) | The scaffold was implanted in a rat model with cranial defect. Excellent osteogenic and angiogenic capacity were observed | 2018 | [100] | ||
| UC-MSCs | Umbilical cord | ALP, OCN | H&E staining, Alizarin-red stain | Films of 3-hydroxybutarate, 3-hydroxyhexanoate and silk fibroin (P(3HB-co-3HHx)/SF) | The union of these biomaterials promotes osteogenic proliferation and differentiation which makes it a candidate in bone tissue engineering | 2020 | [101] | |||
| D-MSCs | Dental pulp and dental follicle | FC: CD44+, CD90+, CD73+, CD34−CD45− | RUNX2, SPP1, BMP2, OCN | Masson’s trichrome stain, Alizarin-red stain | Collagen-nanohydroxyapatite/phosphoserine cryogel with osteogenic factors in the culture medium | The construct was implanted subcutaneously in a mouse model and a bone-like structure was observed at the periphery of the scaffold (with both DP-MSCs and DF-MSCs) | 2020 | [102] | ||
| D-MSCs | Dental pulp | FC: CD73+, CD105+, CD90+, CD34+, CD45+ | SPP1, ALP, RUNX2, COL I | In a rabbit model with bilateral mandibular critical size defect, the construct was implanted, and new bone formation and angiogenesis were observed | 2020 | [103] | ||||
| AD-MSCs | Lipoaspirate | IHC: OCN | Alizarin-red stain | ALP activity assay | Collagen hydrogel, elastin-like polypeptide (ELP) and bioglass | In vitro, there was an increase in ALP activity, osteocalcin content. Formation of calcium and phosphorus mineral deposits were observed | 2020 | [104] | ||
| AD-MSCs + endothelial cells from the umbilical cord | Abdominal fat tissue | IS: CD105, PECAM1, ACTA2 | H&E staining, Masson’s trichrome stain, Alizarin-red stain | ALP activity assay | Hydroxyapatite granules with fibrin | Confirmed bone formation, with a complete vascular network in vitro | 2020 | [105] | ||
| SF-MSCs | Synovial fluid | FC: CD44+, CD90+, CD105+, CD73+, CD45−CD34−, CD11b−, CD19−, HLA-DR- | SPP1, OCN, COL1A1, RUNX2 | Ketone–ketone polyether (PEKK) | A rabbit model with calota’s skeletal damage had the construct implanted and increased osteogenic ability was observed | 2019 | [106] | |||
| Rat | BM-MSCs | Bilateral Femur | ALP, SPP1, OCN, RUNX2 | H&E staining, Masson’s trichrome staining, Alizarin-red, Von Kossa stain | ALP activity assay | Scaffold composed of a bovine serum polyvinylpyrrolidone/albumin/BMM2-derived peptide nucleus with a polycaprolactone shell with collagen I | In vitro scaffolding was favorable for cell adhesion and survival. In vivo, they promoted bone formation and locking of the calvary defect in rat model | 2019 | [107] | |
| BM-MSCs | Femur | IF: VCL and OCN. WB: ERK1/2, p-ERK1/2, HIF1A, PECAM1 and EMCN | ACTB, ALP, OCN, BMP2, SP7, HIF1A, VEGFA, CXCR4 | Alizarin-red staining, Calcein stain | Mesoporous active glass scaffolding (MBG) of coglycolic lactic acid pole (PLGA) particles with bioactive lipid FTY720 using supercritical CO2 foaming technique | In vitro facilitated osteogenic differentiation with an overexpression of Hif-1a: osteogenic and proangiogenic effects. In vivo promoted vascularized bone regeneration | 2019 | [108] | ||
| D-MSCs | Dental pulp from incisor teeth | IF: SPP1 | SPP1, OCN, COL I | Self-assembled peptide hydrogel (SPG-178-Gel) | Osteogenic differentiation was achieved in the hydrogel | 2017 | [109] | |||
| D-MSCs | Dental pulp | FC: CD31−, CD45−, CD73+, CD90+ | Sirius-red stain and toluidine blue, TRAP stain | ALP activity assay, | Heavy collagen gel | Rat model with bone defect in calota to which the construct was implanted demonstrating an improvement in the healing of cranioencephalic bone | 2016 | [110] | ||
| AD-MSCs | Inguinal adipose tissue | H&E stain, picrosirius red | Decellularized human amniotic membrane | In vitro osteoinduction promoted bone-like matrix deposition mineralized by AD-MSCs. In vivo, the stimulation of bone deposition compared with controls, in addition to the healing of calvary defects with large graft incorporation | 2021 | [111] | ||||
| Mu-MSCs | Hind limb and back muscles | OCN, COL I, TGFB1, RUNX2, ACTB | H&E stain, alizarin-red | ALP activity assay | 2D alginate hydrogel with platelet-rich plasma | Osteoblastic phenotype was induced in MSCs in vitro and in vivo (scaffolding with MSCs was implanted subcutaneously in mice) | 2012 | [112] | ||
| Mice | AD-MSCs | Inguinal fat pads | H&E stain, Masson’s Trichrome staining | Macroporous hydrogel of gelatin micro ribbons and encapsulation of BMP-2 | Using low doses of BMP2 accelerated bone mineralization regeneration | 2020 | [113] | |||
| Pig | UC-MSCs | Umbilical cord | H&E staining | Electrospinning of poly(lactic-coglycholic) copolymer (PLGA) seeded with green fluorescent protein (GFP) | In a model of alveolar cleft of pork, the construct was implanted and proved to be a good candidate for the repair of this condition; UC-MSCs in vitro may contribute to bone regeneration | 2017 | [114] | |||
| AD-MSCs | Unspecified |
WB: ACTB, ERK1/2, p-ERK1/2 IHC: SPP1 |
COL1A2, OCN, BMP2, SP7, CXCL8, RUNX2 | H&E staining, Alizarin red, Von Kossa stain | AlamarBlue Trial | Mineralized collagen scaffold/amniotic membrane | Promotes osteogenesis without inflammatory response | 2020 | [115] | |
| Rabbit | BM-MSCs | Femur | IHC: SPP1 | Rhodamine B | Fibrous mesh of polycaprolactone and lactic acid | Increased capacity for biomineralization, infiltration, and cell proliferation. In vitro osteoblastic differentiation | 2020 | [116] | ||
| BM-MSCs | Bone marrow | ALP activity test | Collagen sponges derived from duck skin with hydroxyapatite with silymarin | The scaffold with concentration of 100 μM of Smn had greater efficiency for the adhesion of MSCs, growth and expression of osteogenic markers | 2019 | [117] | ||||
| AD-MSCs | Adipose tissue | H&E staining | Gelatin and tricalcium phosphate (TCP) scaffolds | Average mineral density higher than that of the control group in in vivo model. Scaffold osteoconductivity managed to fill in and cure the model’s host defect in mice | 2021 | [118] | ||||
. Immunodetection techniques markers: PECAM1 = Platelet and endothelial cell adhesion molecule 1, ACTA2 = Actin alpha 2, smooth muscle, aorta, VCL = Vinculin. OCN = OCN/BGLAP (Osteocalcin), ERK1/2 = Mitogen-activated protein kinase, p-ERK1/2 = Mitogen-activated protein kinase phosphorylate, HIF1A = Hypoxia inducible factor 1 subunit alpha, EMCN = Endomucin, SPP1 = Secreted phosphoprotein 1. PCR markers: COL I = Collagen type I, IBSP = integrin binding sialoprotein, BMP2 = Bone Morphogenetic Protein 2, ALP = Alkaline Phosphatase, VEGF = Vascular endothelial growth factor, CDH5 = cadherin 5, vWF = von Willebrand factor, RUNX2 = RUNX family transcription factor 2, COL1A1 = Collagen type I alpha 1 chain, ACTB = Actin beta, SP7 = SP7/OSX (Sp7 transcription factor), VEGFA = Vascular endothelial growth factor A, CXCR4 = C-X-C motif chemokine receptor 4, TGFB1 = Transforming growth factor beta 1, COL1A2 = Collagen type I alpha 2 chain, CXCL8 = C-X-C motif chemokine ligand 8
Table 6.
Applications of MSCs in cartilage tissue engineering
| Source | Type of MSCs | Extraction tissue | Type of analysis and markers | Scaffold | Discovery | Year | Ref. | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Immunodetection techniques | PCR | Histology | Spectrometry | |||||||
| Human | BM-MSCs | Knee joints | FC: CD90+, CD44+, CD45−. IF: COLII | COLI, COLII, ACAN, POU5F1, SOX2, NANOG, ZFP42 | DMMB assay and Blyscan assay | Gelatin microspheres in bioreactor with F12: DMEM + 10% FBS and chondrogenic induction medium | Increased chondrogenic differentiation in dynamic culture and 3D culture compared with a 2D culture | 2020 | [119] | |
| BM-MSCs | Femoral head | ACAN, COL2A1, SOX9, COL10A1, MMP13, RPL13A | Alcian blue stain/fast green | Blyscan assay and hydroxyproline assay | Graphene oxide nanosheets with L-lactic/polyethylene glycol poly-D-acid hydrogel | Continuous release of TGF-β3. In vitro: increased chondrogenesis, dependent on TGF-B3. In vivo: greater production of cartilaginous matrix than scaffolds without graphene nanosheets | 2020 | [120] | ||
| UC-MSCs | Umbilical cord | IF: Calcein assay AM | H&E stain | ECM biomimetic scaffolding of adipose tissue with hydroxyapatite gradients (HA-G) | A rat model with rotator cuff defect was implanted with the construct and an improvement in the repair of the lesion was noted | 2020 | [121] | |||
| WJ-MSCs | Wharton ‘s jelly | H&E stain, and toluidine blue | Extracellular matrix of acellular cartilage | Model of goat with defect in the articular cartilage of the femoral condyle to which the scaffold was inserted with the cells showing repair and regeneration of the cartilage | 2018 | [122] | ||||
| DP-MSCs | Third molar | FC: CD44+, CD90+, CD106+, CD11b−, CD45−.IHC: COLII | ACAN, COL10A1, COLII | H&E stain | Nanocellulose-based thermosensitive hydrogel (NC) by adhesion of beta-glycerophosphate (GP) and chitosan (CS) (NC-CS/GP-21) | THE DPSC implanted in the NC-CS/GP-21 hydrogel showed proliferative capacity in addition to chondrogenesis | 2020 | [123] | ||
| AD-MSCs | ATCC | ELISA: COLII | COLIA1, SOX9, ACAN | Alcian blue stain, Sirius red and Safranin-O | DMMB assay | Rosette nanotubes functionalized with lysine and covered with gelatin methacrylate and polyethylene glycol diacrylate | Increased expression of chondrogenic markers significantly (59%, 71%, and 60% of Col II, GAG, collagen synthesis) | 2020 | [124] | |
| AD-MSCs | Infrapatellar fat pad | ELISA: COLII | COL1, COL2, ACAN, SOX9 | Masson’s trichrome stain and toluidine blue | Bioprinted scaffold of polyurethane elastomer 1,4-butanediol thermoplastic | They maintained the proliferative potential of MSCs, maintaining chondrogenesis. In vivo, extracellular matrix deposition and integration with adjacent tissue | 2020 | [125] | ||
| SF-MSCs | Synovial liquid | FC: CD73+, CD90+, CD105+, CD34−, CD45−, HLA-DR− IHC: COL2A1 | ACAN, SOX9, COL2A1, COMP, COL2B | H&E stain | Collagen sponge | SF-MSCs seeded in collagen sponges demonstrated good chondrogenic capacity | 2018 | [126] | ||
| SF-MSCs | Synovial liquid |
FC: CD13+, CD29+, CD44+, CD49C+, CD73+, CD90+, CD105+, CD151+, CD34−, CD45−, CD49f−, CD184− IF: COLI, COLII, ACAN |
ACAN, COL1A2, COL2A1, SOX9, COL10A1 | Safranin-O | Meniscus-derived decellularized matrix | SF-MSCs seeded on the scaffold with TGF-beta3 and IGF-1 have a beneficial effect on fibro chondrogenesis of cells | 2018 | [127] | ||
| Rat | BM-MSCs | Femur | IHC: COLII | COLII, COLI, COL10A1, SOX9, ACAN | H&E stain, Alcian blue, Safranin-O | Blyscan assay | Gelatin methacrylate hydrogel bioprints (GelMA) | In vitro and in vivo regeneration of mature cartilage | 2020 | [128] |
| BM-MSCs | bone marrow | IHC: COL II, ACAN, COL10A1 | COLI, COLII, GAG, SOX9| | Toluidine blue and Safranin-O | Glycerol poly-sebacate scaffold with poly (1,3- propylene sebacate) and carthagen | In vitro they promoted chondrocyte differentiation and inhibited osteogenic differentiation | 2020 | [129] | ||
| Rabbit | BM-MSCs | Femur and tibia | IHC: PRG4, CILP, COLII, COLI, SOX9. ELISA: COLII. IF: COLII, COLI, PRG4, SOX9 | PRG4, CILP, COL2A1, COL1A1, SOX9, ACTB | Sirius red | Printed polycaprolactone (PCL) or PCL/hydroxyapatite (HA) scaffolds with cytosine-containing microspheres | Heterogeneity of chondrocytes and secreted proteins that promote the regeneration of functional cartilage in vivo and in vitro | 2020 | [130] | |
| BM-MSCs | Femur and tibia | IF: COLII, ACAN | COLII, COLI, SOX9, ACAN, COMP | Safranin O/red Picrosirius y de F-actin | DMMB assay and hydroxyproline assay | Poly-ε-caprolactone fibers coated with extracellular matrix derived from decellularize chondrocytes | MSCs tended to spread more on scaffolding with PCL than scaffolding without this cover. The expression of chondrogenesis markers was higher | 2021 | [131] | |
| AD-MSCs | Adipose tissue of the abdomen | IF: COLII | COL2A1, ACTB | H&E stain, Safranin-O | Scaffolding of soluble poly-L-lysine/carthagegenine nanoparticles with polylactic-coglycolic acid/methacrylate hyaluronic acid | In vivo, a condroncial defect was recovered with the presence of intact and smooth cartilaginous tissue. Results greater than just using NP or scaffolding separately | 2020 | [132] | ||
| SF-MSCs | Synovial fluid | IHC: COLII | Toluidine blue | Chitosan-based hydrogel | The construct was implanted in a rabbit model with cartilage defect in femoral patellar grooves and cartilage regeneration was observed | 2019 | [133] | |||
| Goat | BM-MSCs | Nape | IHC: COLI, COLII | COLI, COLII, SOX9 | H&E stain, Safranin-O/Fast Green | Two-layered scaffold: polyethylene glycol (PEG) with poly (glutamic L acid)-g-polycaprolactone (PLGA-g-PCL) | After 2 months in vivo in goat, there is neocartilage formation in the model of defect in the temporomandibular junction | 2021 | [134] | |
Immunodetection techniques markers: COLII = Collagen type II, COL2A1 = Collagen type II alpha 1 chain, ACAN = Aggrecan COL10A1 = Collagen type X alpha 1 chain, PRG4 = Proteoglycan 4, CILP = Cartilage intermediate layer protein, SOX9 = SRY-box transcription factor 9. PCR markers: POU5F1 = POU class 5 homeobox 1, SOX2 = SRY-box transcription factor 2, NANOG = Nanog homeobox, ZFP42 = ZFP42 zinc finger protein/REX1, MMP13 = Matrix metallopeptidase 13, RPL13A = Ribosomal protein L13a, COMP = Cartilage oligomeric matrix protein, COL2B = Collagen type II alpha 1 chain isoform B, GAG = Glycosaminoglycan
Table 7.
Applications of MSCs in osteochondral tissue engineering. Immunodetection techniques markers: IL1 = Interleukin 1, TNF-alpha = Tumor necrosis alpha. PCR markers: CDH2 = Cadherin 2, ITGA5 = Integrin subunit alpha 5
| Source | Type of MSCs | Extraction tissue | Type of analysis and markers | Scaffold | Discovery | Year | Ref. | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Immunodetection techniques | PCR | Histology | Spectrometry | |||||||
| Human | BM-MSCs | Lonza | IF: COLII, SOX9, COLI, RUNX2 | Alizarin-red stain | Poly (lactic-coglycolic acid) with porous gradient and hydrogel of hyaluronic acid and hydroxyapatite with BMP-2 and TGF-B1 | Spatial differentiation facilitated to osteocytes and chondrocytes with the use of growth factors in the scaffold | 2020 | [135] | ||
| BM-MSCs | Cyagen | IHC: COLII, GAG, COLI, OCN. ELISA: IL1, TNF-alpha | ALP, OCN, RUNX2, COL II, ACAN, SOX9, COL1 | Toluidine blue | ALP activity assay | Poly hydrogel (N-acryloyl 2-glycine) and methacrylate gelatin | Increases the expression of genes related to chondrogenesis and osteogenesis in hBM-MSCs, with concurrent regeneration of cartilage and subchondral bone in rat model | 2019 | [136] | |
| UC-MSCs | Umbilical cord | FC: CD73+, CD90+, CD105+, CD44+, CD29+, HLA-ABC+ CD34−, HLA-DR−. IHC: SOX9, COLII | Hyaff-11 (FIDIA Advanced Biopolymers, Italy) o Chondro-gide (Geistlich Biomaterials, Italy S.r.l.) | The study shows the commitment of UC-MSCs seeded on the scaffold to chondrogenic differentiation after 4 weeks and to osteogenesis after 30 days | 2017 | [137] | ||||
| AD-MSCs | Lipoaspirate | IHC: COLII, COLI | COLI, CDH2, ITGA5 | Toluidine blue | DMMB assay | Bilayer scaffold: poly-L-glutamic acid and chitosan cartilage layer and PLGA bone layer with nanohydroxyapatite/chitosan | Chondrogenic differentiation (expression of glycosaminoglycans and COL II). AD-MSCs migrated from the chondrogenic layer through the scaffold to the osteogenic zone and differentiated | 2020 | [138] | |
| AD-MSCs | Subcutaneous adipose tissue | IS: COLI | ACAN, SOX9, COL2A1 | H&E stain, Masson’s trichrome stain, Safranin-O/Fast Green, toluidine blue | Decellularized extracellular matrix of bovine cartilage with Poly-ε-caprolactone nanofibers | Increased chondrogenic expression; in addition, they managed to fill osteochondral defects in vivo in rats, obtaining a long-term regeneration of cartilage | 2021 | [139] | ||
| Rat | BM-MSCs | Unspecified | IF: F-actin, SOX9, COLII, ACAN, RUNX2, OCN | ALP, OCN, RUNX2, SOX9, COLII, ACAN | Alizarin-red stain, toluidine blue and Safranin-O | ALP activity assay | Two layers: emulsions of osteogenic peptide/B-tricalcium/polyphosphate (lactic-coglycolic acid) and water in pole oil (D,L-lactic-co-trimethylene carbonate acid) in a thermosensitive cartilage frame with another layer of Cabbage I hydrogel with TGF-B1 | The scaffold allowed the formation of three layers: osteogenic, subchondral, and cartilaginous; high viability and proliferation in the subchondral and cartilage layer, but also in the osteogenic layer | 2020 | [140] |
| BM-MSCs + chondrocytes | Femur and tibia | IHC: COLI, COLII | H&E stain y Masson’s trichrome stain | Chitosan bilayer scaffold with chitosan/phosphate-B-Tricalcium | Chondrocytes maintained their lineage while MSCs differentiated to the osteogenic lineage. In vivo study showed that the scaffold had characteristics similar to native tissue: hyaline cartilage and subchondral bone | 2019 | [141] | |||
| Rabbit | AD-MSCs | Subcutaneous adipose tissue of the neck | IHC: COLII. ELISA: GAG, COLII | H&E stain, Toluidine blue | Silk fibroin and hydroxyapatite scaffolding with a layer of calcified cartilage | In vivo model in osteochondral defects in rabbit knees, the scaffolded group with AD-MSCs had greater integrity and rigidity. There was cartilage and bone formation | 2020 | [142] | ||
Table 8.
Applications of MSCs in muscle tissue engineering
| Source | Type of MSCs | Extraction tissue | Type of analysis and markers | Scaffold | Discovery | Year | Ref. | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Immunodetection techniques | PCR | Histology | Spectrometry | |||||||
| Human | BM-MSCs | Bone marrow | ACTA2, CNN1, SMNT, PECAM1, vWF, KDR | Pectin hydrogel nanofibers with 25% and 50% oxidations | They allow the differentiation of MSCs into vascular smooth muscle cells and endothelial cells | 2019 | [143] | |||
| BM-MSCs + células troncales hematopoyéticas | Lonza | IHC: CD34 | H&E stain y Masson’s trichrome stain | Poly-(1,8 octamethylene citrate) with small intestine submucosa | Increasing cell density with coculture allowed to increase the regeneration of bladder muscle tissue | 2021 | [144] | |||
| UC-MSCs | Wharton’s jelly | IS: ACTA2, α-actinin, MYH1, ACTA1 | MYH1, ACTN3 | Fibrin hydrogel with rapidly degrading microbeads | MSCs planted on the scaffold gave excellent cell viability and proliferation as well as good myogenic differentiation | 2012 | [145] | |||
| UC-MSCs | Wharton’s jelly | IHC: α-actinin, MYH1 | ACTN3, MYH1 | Alginate-fibrin microbeaters packed in an alginate matrix modified with Arg-Gly-Asp (RGD) | The use of UC-MSCs in the scaffold demonstrated an improvement in cell viability and myogenic differentiation | 2012 | [146] | |||
| G-MSCs | Unspecified | FC: CD73+, CD105+, CD146+, CD34−, CD45−. IF: MYH2, MYOD, MYF5 | MYOD1, MYF5, MYOG | H&E stain | Alginate scaffold coupled to RGD | An immunocompromised mouse model was implanted with the construct subcutaneously and the formation of muscle-like structures was observed | 2016 | [90] | ||
| G-MSCs | Unspecified | FC: CD29+, CD44+, CD73+, CD90+. IF: MYOD, MYF5, PAX7. WB: MYOD, PAX7, MYF5 | H&E stain y Masson’s trichrome stain | Acellular extracellular matrix of porcine intestinal mucosa (SIS-ECM) | In a rat model with a myomucosal defect of the tongue, tissue regeneration was observed with the use of the construct | 2017 | [147] | |||
| AD-MSCs | Abdominal adipose tissue | IHC: ACTA2 | H&E stain | PLGA Triple Layer Sheet | In in vivo model of rats with subtotal bladder resection, those who received the cellularized implant with smooth muscle cells derived from AD-MSCs, the bladder was completely regenerated with a full recovery of function | 2020 | [148] | |||
| AD-MSCs | Liposuction of abdominal subcutaneous fat | FC: CD29, CD44, CD73, CD90, CD105, HLA-ABC. IHC: DES, ACTA2. SMMHC. IB: ACTA2, CNN1, TGFB1 | H&E stain, Masson’s trichrome stain | Poly-L-lactic-co-ε-caprolactone multilayer scaffold | Biocompatibility with scaffolding. In the rat model, there was an improvement in contractibility, suggesting an improvement in bladder function. Uniform distribution between the leaves as well as differentiation of the AD-MSCs to smooth muscle | 2019 | [149] | |||
| Mu-MSCs | Hind limb | IS: MHC, PECAM1,COLI, alpha-Btx, laminin, SYP, NF-M, LAMA1 e integrin | Extracellular muscle matrix of lower limbs decellularized from mice in perfusion bioreactor | The scaffolds planted with Mu-MSCs showed growth and survival in vivo as well as myofiber formation and neovascularization in a model of volumetric muscle loss. Ex vivo analysis showed restoration of muscle strength treated | 2017 | [150] | ||||
| Mu-MSCs | Muscle biopsies of children | FC: CD34, CD56, 7-aminoactinominD WB: laminin, Ki67, fibronectin, PAX7, MYOD, MHC, MYH3, ACTA2, TE7, laminin, | PAX7, MYF5, MYOD, MYH1, DMD, LAMA1, B2M | Fluo-4 AM | Mouse diaphragm decellularized extracellular matrix | The cellularized scaffold was tested with cardiotoxin and the in vitro regenerative response of self-renewal and remodeling of the extracellular matrix was activated | 2019 | [151] | ||
| Rat | BM-MSCs | Femur and tibia | IHC: cTnI | H&E stain | Polylactic acid and polyglycolic acid scaffolding (50:50) | Adhesion to the scaffolding at 24 h. It contains normal arrangements of myofilaments, desmosomes, gap slits, and Z-lines | 2019 | [152] | ||
| BM-MSCs | Femur | NKX2.5, TNNI2, MYH7 | Polyvinyl alcohol (PVA), chitosan (CS) and carbon nanotube (CNT1) at different concentrations | Voltage stimulation was performed, with the PVA-CS-CNT1 scaffold being the most viable (PVA 66%, CS 25%, 8% CNT) | 2019 | [153] | ||||
| AD-MSCs | Inguinal region | IHC: ACTA2. IF: ACTA2 | H&E stain | Poly-ε-caprolactone/chitosan scaffold | In in vivo rat model, there was smooth muscle of a strong bladder regeneration, with a large capacity and a greater number of veins | 2018 | [154] | |||
| AD-MSCs | Adipose tissue of inguinal or subcutaneous region | IHC: MYOG, MYOD1, DBS. PECAM1, MHC, SYP | BDNF, NTF3, NGF | H&E stain and Masson’s trichrome stain | Decellularized human amniotic membrane | In in vivo model, combined with interval-based high-intensity training, there was increased muscle regeneration and improved tissue remodeling regeneration after volumetric muscle loss in rats | 2020 | [155] | ||
| Mu-MSCs | Hind limb | IHC: ACTA2, VEGF | PAX7, MYOG, FGF2 y VEGFB | H&E stain and Masson’s trichrome stain | Biodegradable elastomer of urea poly (urethane ester) | Scaffolds planted with Mu-MSCs had a higher number of blood vessels and smaller residual scaffolding area and multinucleated giant cells | 2017 | [156] | ||
| Mouse | BM-MSCs | Femur | H&E stain, Alizarin red, Oil I, Safranin-I | Pluronic F-127 | Increased the number of myofibers in regeneration and improved muscle strength in a mouse muscle contusion model | 2020 | [157] | |||
| BM-MSCs | Unspecified |
IHC: VEGF, SFC, IL6 WB: MYOD, DES, MYOG, ACTA1, iNOS, Arginase, TGFR-2, SPP1 |
Gelatin and decellularized extracellular matrix of skeletal muscle | The scaffold supports survival, growth, and production of trophic factors and expression of myogenic proteins of MSCs in vitro | 2019 | [158] | ||||
| AD-MSCs | Abdominal adipose tissue | FISH: chromosome Y in transplanted cells. BCA Protein Assay. Mouse Cytokine Array | H&E stain | Hybrid 3D polypropylene fumarate resin (PPF) scaffolds: diethyl fumarate (DEF) with gold nanoparticles | In vivo, there was low presence of immunomodulators and increased tissue regeneration of muscle | 2017 | [159] | |||
| Mu-MSCs | Neonatal stage skeletal muscle (satellite cells) | IS: TnI y CDH15 | PAX 7, MYOD, ACTA2, MYH, CDH15, CD34 | Polyaniline nanofibers (PANI) and polyacrylateanitrile (PAN) with graphene and graphene oxide | Cells cultured on PANI-CSA/G scaffolds had greater proliferation and differentiation than other scaffolds | 2016 | [160] | |||
| Mu-MSCs | Skeletal muscle of the front and back legs (satellite cells) | PAX7, MYOD, ACTA2, MYH, CDH15, CD34 | Polyacrylonitrile and polyaniline membrane | They showed greater cell proliferation and differentiation, and due to the conductivity of the material, the cells were induced to a more mature state | 2016 | [161] | ||||
| Pig | AD-MSCs | Adipose tissue | IHC. ACTA2, elastin, CD3, CD25, c-Kit, MEF2, cTnI NKX2.5, cTnT, vWF, PECAM1, Ki67 | Masson’s trichrome stain, Picrosiruis red and Gallego stain | Decellularized human pericardium | In animals with scaffold in in vivo model of myocardial infarction, the area of infarction at one month was lower than the controls, and there was an improvement in cardiac function | 2017 | [162] | ||
Immunodetection techniques markers: MYH1 = Myosin heavy chain 1, ACTA1 = Alpha actin 1, MYH2 = Myosin heavy chain 2, MYOD = Myoblast determination protein 1, MYF5 = Myogenic factor 5, PAX7 = Paired box 7, DES = Desmin, SMMHC = Myosin heavy chain, smooth muscle isoform, CNN1 = Calponin 1, MHC = Myosin heavy chain, muscle, alpha-Btx = Alpha-bungarotoxin, SYP = Synaptophysin, NF-M = Neurofilament medium polypeptide, LAMA1 = Laminin subunit alpha 1, Ki67 = Proliferation marker protein Ki-67, MYH3 = Myosin heavy chain 3, TE7 = Fiobroblast marker TE7, cTnI = Cardiac troponin I, DBS = Guanine nucleotide exchange factor DBS, SFC = Stem cell factor, IL6 = Interleukin 6, iNOS = Nitric oxide synthase, inducible, TGFR-2 = TGF-beta receptor type-2, TnI = Troponin I, CDH15 = Cadherin 15, c-Kit = Mast/stem cell growth factor receptor Kit, MEF2 = Myocyte-specific enhancer factor 2, cTnT = Troponin T, cardiac muscle. PCR markers: SMNT = Survival of motor neuron 1, telomeric, KDR = kinase insert domain receptor, ACTN3 = Actinin alpha 3, MYOD1 = Myogenic differentiation 1, MYOG = Myogenin, DMD = Dystrophin, B2M = Beta-2-microglobulin, TNNI2 = Troponin I2, fast skeletal type, MYH7 = Homo sapiens myosin heavy chain 7, BDNF = Brain derived neurotrophic factor, NTF3 = Neurotrophin 3, NGF = Nerve growth factor, FGF2 = Fibroblast growth factor 2, VEGFB = Vascular endothelial growth factor B, CD34 = CD34 molecule
Table 9.
Applications of MSCs in dermal tissue engineering
| Source | Type of MSCs | Extraction tissue | Type of analysis and markers | Scaffold | Discovery | Year | Ref. | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Immunodetection techniques | PCR | Histology | Spectrometry | |||||||
| Human | BM-MSCs | Lonza | IHC: IVL and laminin | VEGF, PECAM1, ACTA2. RT-PCR: ACTB, VEGF, HIF1A | H&E stain, Masson’s trichrome stain | AuFe nanoparticles with conditioned medium | Injection in mouse model with skin wound presented angiogenesis, re-epithelialization, and tissue remodeling | 2019 | [163] | |
| WJMSCs | Wharton’s jelly | FC: CD73+, CD90+, CD105+, CD14−, CD20−, CD34−, CD45−, HLA-DR−, CD80−, CD86−. IHC: ACTA2, DES | H&E stain, Masson’s trichrome stain | Silk fibroin (SF) scaffolding | In a mouse model of splinting excision wounds, the construct was implanted. Re- epithelialization and a reduced formation of scar tissue in the healing process were observed | 2019 | [164] | |||
| GMSCs | Gum tissue | FC: CD90+, CD105+, CD73+, CD34−, CD45− | KRT18, KRT14, KRT5, IVL, FLG, SFN | HyStem®-HP in addition to the use of Acalypha indica | The use of scaffolding and A. indica promotes epidermal differentiation of G-MSCs | 2018 | [165] | |||
| AD-MSCs | Unspecified | IHC; KRT10, KRT14 | KRT10, KRT14, ACTB | Silk bio-complex/hyaluronic acid modified with polyethylene glycol/chitosan/poly-ε-caprolactone (PCP) | Scaffolding with 20% HA demonstrated better interactions in adhesion, response, proliferation, and cell differentiation in the absence of growth factors | 2021 | [166] | |||
| Rat | BM-MSCs | Bone Marrow | IHC: KRT10, KRT12 | H&E stain, Masson’s trichrome stain | Silk fibroin film with polydopamine | In vitro promoted adhesion and migration of MSCs; in vivo the wound healed completely with the formation of new skin and hair 14 days after trauma, without generating significant inflammation. There was epithelialization and good organization of collagen deposition | 2019 | [167] | ||
| AD-MSCs | Abdominal adipose tissue | IHC: PECAM1 | H&E stain, Masson’s trichrome stain | Alginate hydrogel | Improved wound healing in vivo, collagen synthesis and angiogenesis in the affected area | 2019 | [168] | |||
| Mouse | BM-MSCs | Bone marrow | IF: ACTA2, VEGF y TGFB1 | H&E stain, Masson’s trichrome stain | Graphene oxide nanoparticles with a dermal acellular matrix | Diabetic murine model of total thickness skin wound in the back, where there was angiogenesis and collagen deposition as well as rapid re-epithelialization | 2019 | [169] | ||
| AD-MSCs | Subcutaneous adipose tissue |
IHC: GFP IF: PECAM1 |
VEFGA, PDGFRB, FGF2, HGF, TGFB1, GAPDH | Masson’s trichrome stain | Glycosaminoglycan liquid collagen scaffold (GAG) | In vivo increased capillary formation, collagen content, epidermal thickness, and expression of essential growth factors | 2019 | [169] | ||
| Miniature pigs | BM-MSCs | Bone marrow | IHC: ACTA2 | H&E stain, picrosirius red | Electrolyzing nanofibers of poly-ε-caprolactone and polyvinyl alcohol (PVA) | The scaffold supports proliferation of MSCs, human fibroblasts, and keratinocytes. Improved wound healing in in vivo model | 2019 | [170] | ||
| Yorkshire Pigs | AD-MSCs | Subcutaneous fat | IHC: ACTA2, PECAM1, VEGFA. WB: PECAM1, VEGFA | Picrosiruis red | Hidroxiproline assay | Pegilated Fibrin Hydrogel (FPEG) | In porcine burn model, scaffold with AD-MSCs showed increased angiogenesis and avoided contraction of adjacent tissue, minimizing scarring | 2018 | [171] | |
| Rabbit | AD-MSCs + mouse skin fibroblasts | Subcutaneous fat | AM Calcein | Extracellular matrix gel derived from culture of stem cells derived from adipose tissue | Minimal cytotoxicity, the scaffold showed high concentration of proteins such as collagen, fibronectin, biglicane, TGF-β | 2020 | [172] | |||
Immunodetection techniques markers: IVL = Involucrin, KRT10 = Keratin 10, KRT14 = Keratin 14, KRT12 = Keratin 12, GFP = Green fluorescent protein. PCR markers: KRT18 = Keratin 18, KRT5 = Keratin 5, FLG = Filaggrin, SFN = Stratifin, PDGFRB = Platelet derived growth factor receptor beta, HGF = Hepatocyte growth factor, GAPDH = Glyceraldehyde-3-phosphate dehydrogenase
Table 10.
Applications of MSCs in neural tissue engineering
| Source | Type of MSCs | Extraction tissue | Type of analysis and markers | Scaffold | Discovery | Year | Ref. | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Immunodetection techniques | PCR | Histology | Spectrometry | |||||||
| Human | BM-MSCs | Unspecified | ICC: MAP2 | MAP2, B2M | Electrolyzed nanofibers of polyvinyl alcohol and sulfated alginate | Biocompatible scaffolds that support cell proliferation and neurogenesis of hBM-MSCs in cell culture without using growth factors. Neuroinductive effects without addition of materials for differentiation. They also support the growth of Schwann cells | 2020 | [173] | ||
| UC-MSCs | umbilical cordon | IF: vWF, MBP, NF-M, MAP2, Syn | Masson’s trichrome stain | Silk collagen and fibroin scaffolding | The construct was implanted in a hemiplegic model of traumatic brain injury in canines and it was seen that in addition to repairing the anatomical structure of the lesions, it also restores the gait of the extremities after a brain injury due to trauma | 2021 | [174] | |||
| DPSCs | Third molar |
FC: CD73+, CD90+, CD166+, CD14−, CD19−, HLA-DR− IF: Nes, NeuN, GFAP, TUBB3 WB: Bcl2, Bax, CASP3, MBP, GAP-43 |
H&E stain | Thermosensitive hydrogel heparin-poloxamer | Rat model to which the construct was implanted in addition to fibroblast growth factor (bFGF) promoting neuronal regeneration | 2018 | [175] | |||
| AD-MSCs | Lipoaspirate from abdominal fat |
FC: CD73 CD105. IF: Nes, Ki67, SOX2, CD105, CD73. WB: Nes y SOX2 |
Decellularized dermal matrix | Neuronal differentiation by the expression of nestin and SOX2 in scaffold | 2019 | [176] | ||||
| Rat | BM-MSCs | Femur and tibia | IS: GFP, NF-H, CASP3, NeuN, TUBB3, GFAP, Claudin-11 | CASP3, CSPG4, MMP2, NEFH, VEGFA, GAPDH | H&E stain, NeuN | Polypyrrole/polylactic acid nanofibers | Recovery of the spine injury model with a decrease in scar tissue compared with the control group. Abundant NF and NeuN in stains as well as myelination | 2019 | [177] | |
| AD-MSCs | Perirenal adipose tissue | IHC: CD105, CD90, CD49d, CD31, CD106, CD45, Nes, NF-L, SOX2, Islet-1, HB9, ChAT, SYP | FM1-43 and RH 795 stain | BD™PurMatriz™ peptide hydrogel (BD Biosciences) | Increased expression of Islet1 (immature) in 3D cultures. HB9, ChAT, and synaptophysin increased in 2D (mature); therefore, 3D demonstrates more proliferation for neuron progenitor cells | 2020 | [178] | |||
| Mouse | BM-MSCs | Cell Bank, Chinese Academy of Sciences, China | WB: NSE, DCX, MAP2, BDNF, NTF3, Bax, Bcl-2 | MAP2, ENO2, DCX, BDNF, NGF, NTF3 | H&E stain | Chitosan hydrogel, hydroxyethyl cellulose, collagen and B-phosphoglycerate | In a mouse model of spinal cord injury, the hydrogel had a thermosensitive behavior similar to neural tissue with biocompatibility. Promoted the recovery of motor function of the hind limbs probably by suppression of apoptosis and increased neurotrophy of nerve cells | 2020 | [179] | |
Immunodetection techniques markers: MAP2 = Microtubule associated protein 2, MBP = Myelin basic protein, Syn = Synapsine, Nes = Nestin, NeuN = RNA binding protein fox-1 homolog 3, GFAP = Glial fibrillary acidic protein, TUBB3 = Tubulin beta-3 chain, Bcl2 = Apoptosis regulator Bcl-2, Bax = Apoptosis regulator BAX, CASP3 = Caspase 3, GAP-43 = Neuromodulin, NF-H = Neurofilament heavy polypeptide, NF-L = Neurofilament light polypeptide, Islet-1 = Insulin gene enhancer protein ISL-1, HB9 = Motor neuron and pancreas homeobox protein 1, ChAT = Choline acetyltransferase, NSE = Enolase, DCX = Doublecortin. PCR markers: B2M = Beta-2-microglobulin, CSPG4 = Chondroitin sulfate proteoglycan 4, MMP2 = Matrix metallopeptidase 2, NEFH = Neurofilament heavy chain, ENO2 = Enolase 2
Table 11.
Applications of MSCs in cardiac tissue engineering
| Source | Type of MSCs | Extraction tissue | Type of analysis and markers | Scaffold | Discovery | Year | Ref. | ||
|---|---|---|---|---|---|---|---|---|---|
| Immunodetection techniques | PCR | Histology | |||||||
| Human | BM-MSCs | Unspecified |
FC: CD73+, CD90+, CD105+, CD11b−, CD14−, CD19−, CD34− CD45- y HLA-DR2− IHC: MYH3, cTnT |
ACTA2, BMP4, CCL5, COL1A1, CCN2, FN1, HGF, IDO1, IL6, IL8, IL10, LIF, NFKBIA, PDGFA, PTGS2, TNFAIP6, VEGF | Collagen scaffold | The recovery of cardiac function may not be due to the direct contractional contribution of the cells but to the fact that other cellular pathways that promote the repair process by the MSCs can be activated | 2017 | [180] | |
| BM-MSCs | Unspecified | IF: CD90, GATA4, cTnT, cTnI, Cx43, MHC, ACTA2 | Mat of type I collagen nanofibers, oriented at random | Cells cultured in scaffolds show a time-dependent evolution of cardiomyogenesis, an improvement in the expression of cardiac markers and a decrease in cell stem markers | 2018 | [181] | |||
| hMSCs | PT-2501; Lonza | IF: cTnT, ACTA2, PECAM1 | Poly (glycerol sebacate)/gelatin fibers | The scaffold has the mechanical strength to support the MSCs seeded as well as ability of differentiation into cardiomyocytes to repair the myocardium and improve cardiac function. It is shown to be biocompatible and have mechanical properties for myocardial restoration | 2011 | [182] | |||
| hMSCs | PT-2501; Lonza | IF: ACTA2, TnT, CD44 | Poly(ε-caprolactone)-gelatin nanofibers coupled with VEGF | The incorporation of VEGF showed an improvement in the proliferation of MSCs as well as an increase in the expression of specific cardiac proteins | 2017 | [183] | |||
| hMSCs | PT-2501; Lonza | GATA4, MEF2C, NKX2.5, CACNA1C | Elastic polyurethane nanofibers | Construct stimulates cardiac differentiation of MSCs | 2011 | [184] | |||
| hMSCs + cardiomyocytes | PT-2501; Lonza | IF: actinin | Polycaprolactone scaffolds with gold nanoparticles | An increase in proliferation and differentiation of MSCs with gold nanoparticles on the scaffold was shown | 2015 | [185] | |||
| UCB-MSCs | Umbilical cord blood | FC: ACTA2, Tn, Tm, ANP | Fibrin/PLGA-based electrolyzing scaffolding | The scaffold supports cell anchorage, viability, and proliferation as well as provides a favorable environment for the differentiation of cells to a cardiac phenotype | 2013 | [186] | |||
| UC-MSCs | umbilical cord | IHC: PECAM1 | H&E stain | Decellularized umbilical artery | Scaffold seeded with cells showed an improvement in cardiac function in a rat MI model | 2017 | [95] | ||
| WJ-MSCs | Wharton’s jelly | FC: CD73+, CD90+, CD105+, CD31, CD45− y HLA-DR− | PeriCord, decellularized pericardial matrix | PeriCord was tested in a first implantation in humans, and magnetic resonance imaging after 3 months showed a reduction of ~ 9% of scar mass in the treated area | 2020 | [97] | |||
| UC-MSCs + cardiomyocytes | umbilical cord | IF: ACTA2, cTnI | Graphene oxide/alginate microgel | Implanted scaffolding in rats showed decreased infarcted area and improved cardiac function | 2019 | [187] | |||
| WJ-MSCs | Wharton’s jelly | IHC: PECAM1, ACTA2, MHC | MYH6, MYH7, TNNT2 y NKX2.5 | Polyethylene glycol, hyaluronic acid and chitosan | hWJ-MSCs were mixed with the scaffold and injected into rabbits with MI. Angiogenesis and cardiogenesis were observed | 2017 | [188] | ||
| Rat | BM-MSCs | Tibia |
FC: CD44+, CD90+, CD34−, CD45− IHC: DES y ACTA2 |
Collagen-I scaffold | MSCs patch can promote reverse remodeling of the infarcted area | 2012 | [189] | ||
| BM-MSCs | Unspecified | Sirius red stain | Alginate and chitosan anti-charge polyelectrolyte complexes | The scaffolds with the cells were implanted in rats with MI. Increase in ejection fraction, improvement in neovascularization, and attenuation of fibrosis were seen | 2014 | [190] | |||
| BM-MSCs | Unspecified |
FC: CD90+, CD29+, CD44−, CD106−, CD25−, CD11b−, CD45− IHC: DES, cTnT |
Decellularized cardiac tissue | MSCs isolated from rats can be used to generate bioartificial hearts along with isolated endothelial and cardiac cells on a decellularized cardiac scaffold | 2019 | [191] | |||
| BM-MSCs | Unspecified | IF: PECAM, cTnT, Cx43 | H&E stain, Masson’s trichrome stain | Poly(ε-caprolactone/gelatin) nanofibers (PG) | In a rat model with myocardial infarction, the construct was implanted and demonstrated to provide mechanical support to induce angiogenesis and accelerate cardiac repair | 2014 | [192] | ||
| AD-MSCs | Cervical adipose tissue | IHC: DES, CD68, CD45, CD34 | H&E stain, Masson’s trichrome stain y Sirius red | Acellular pericardium | MI was induced in rats and the patch was implanted and neovascularization was observed | 2018 | [193] | ||
| AD-MSCs | Edpididymis | IHC: ACTA2, CD31+, Sca-1, CD117 | Platelet-rich fibrin (PRF) | AD-MSCs grafted into PRF implanted in rats with MI has a benefit in preserving left ventricle function | 2015 | [194] | |||
| Mouse | BM-MSCs | Femur and tibia |
FC: CD34−, CD45−, CD90+ y CD73+ IF: Cx43, cTnT |
Polyurethane, 3-hydroxybutyrate-co-4-hydroxybutyrate (P(3HB-co-4HB) or polypropylene carbonate | Cells were more abundant in polyurethane and p(3HB-co-4HB) | 2013 | [195] | ||
| AD-MSCs | Unspecified |
FC: CD44+, CD90+, CD34−, CD45− IHC: PECAM1 |
Masson’s trichrome stain | Patches of cellulose nanofibers modified with chitosan/silk fibroin | Patches with AD-MSCs were implanted in rats with MI which demonstrated survival of grafted cells as well as attenuation of myocardial fibrosis | 2018 | [96] | ||
| Pig | AD-MSCs | Unspecified | Movat stain, Galician stain | A myocardial decellularized scaffold and a pericardial scaffold | Scaffolds with the cells were implanted in pigs and both grafts showed a recovery of ventricular function; however, the pericardial scaffold showed greater structural integrity as well as greater penetration and retention of cells | 2018 | [196] | ||
| Rabbit | AD-MSCs | Adipose tissue of the nape | IHC: DES, CD34 | H&E stain, Masson’s trichrome stain | Decellularized pericardium | The scaffold with the cells was implanted in rabbits with MI and was shown to improve the contractile function of the left ventricle | 2017 | [197] | |
Immunodetection techniques markers: GATA4 = GATA binding protein 4, Cx43 = Gap junction alpha-1 protein, TnT = Troponin T, Tn = Troponin, Tm = Tropomyosin, ANP = Atrial natriuretic peptide, Sca-1 = Complex scaffold protein scaA, CD117 = Mast/stem cell growth factor receptor Kit. PCR markers: BMP4 = Homo sapiens bone morphogenetic protein 4 (BMP4), transcript variant 1, CCL5 = Homo sapiens C–C motif chemokine ligand 5 (CCL5), transcript variant 2, CCN2 = Homo sapiens cellular communication network factor 2, FN1 = Fibronectin 1, IDO1 = Indoleamine 2,3-dioxygenase 1, IL8 = C-X-C motif chemokine ligand 8, IL10 = Interleukin 10, LIF = LIF interleukin 6 family cytokine, NFKBIA = , NFKB inhibitor alpha PDGFA = Platelet derived growth factor subunit A, PTGS2 = Prostaglandin-endoperoxide synthase 2, TNFAIP6 = TNF alpha induced protein 6, MEF2C = Myocyte enhancer factor 2C, CACNA1C = Calcium voltage-gated channel subunit alpha1 C, MYH6 = Myosin heavy chain 6, TNNT2 = , Troponin T2, cardiac type
Clinical trials in TE with MSCs
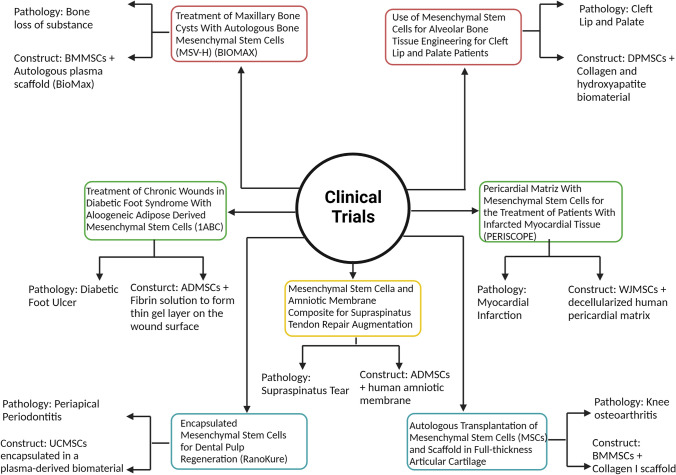
There are several clinical trials in the TE field where MSCs are used, as shown in Figure 2. Some of the clinical trials are focused on treating cartilage and osteochondral diseases. One example is the clinical trial that seeks to treat knee osteoarthritis (NCT00850187) using BMMSCs on a collagen I scaffold [198]. Another one is using ADMSCs seed on a human amniotic membrane (NCT04670302) as a treatment for supraspinatus tear [199].
Fig. 2.
Clinical trials from the last ten years in TE using MSCs as the principal cell source for different pathologies
On the other hand, there are clinical trials focused on bone regeneration. One of them consists of treating bone loss using BMMSCs on a scaffold called BioMax (NCT01389661). It was reported that in the nine patients who participated in the study, there was bone formation in the space where maxillary cyst was located, which had already been extracted and there were no severe adverse reactions reported [200, 201]. Another clinical trial attempted to treat the cleft lip and palate condition using DPMSCs seeded on a collagen and hydroxyapatite scaffold (NCT01932164). The study reports that bone formation was quantified in five patients with this condition using TC. Six months after the intervention, final completion of the alveolar defect, with an 89.5% mean bone height was detected [202]. Furthermore, another clinical trial is based on treating periapical periodontitis using UCMSCs encapsulated in a plasma-derived biomaterial (NCT03102879). The study was reported as an efficacy trial. One year after the intervention, the treated tooth remained in the mouth without pain [203].
Other clinical trials are focused on the recovery of epithelial tissue. One example is a clinical trial that consists of treating diabetic foot ulcer (NCT03865394) using ADMSCs suspended in a fibrin solution [204]; however, no results were presented.
Finally, there is a clinical trial that consists of evaluating patch with WJMSCs seeded on a decellularized human pericardial matrix to treat myocardial infarction (NCT03798353). The study reported that the construct with the scaffold called Pericord and the WJMSCs was implanted on a non-revascularizable scar in the inferior wall of a 63-year-old man. The three-month follow up showed optimal results, and magnetic resonance indicated ~9% reduction in the scar mass of the treated area [97, 205]. Currently, the PeriCord scaffold is commercialized for its use in similar research.
As can be seen, TE using MSCs seeks to treat a wide variety of pathologies and uses various types of MSCs according to the purpose of the clinical trial.
By November 2021, 15 clinical studies had reported the use of MSC in TE in accordance with the CrinicalTrials.gov web page of the United States. Among those 15 results, eight have been already completed, two are in the recruitment phase, two are in an unknown state, three aren´t in recruitment phase yet and one clinical trial doesn´t have specific information about the phase of the study Among all of these results, only nine are examining the use of MSCs with scaffolds, showing that this therapeutic option represents a great area of opportunity.
Discussion
The use of TE in the treatment of several diseases has proved to be a promising alternative for many patients. This review is particularly based on the analysis of recent studies that use MSCs in TE and also considers the type of scaffold employed and the application in TE.
MSCs terminology
However, there is a lack of homogeneity in the use of terminology in this research field. As previously described, the ISCT proposed three minimal criteria to define MSCs, a subtype of SCs, for consistency purposes. Nevertheless, its statement also mentions that one of the main issues found is that the “MSCs” acronym has been used to refer to mesenchymal stem cells as well as mesenchymal stromal cells. To avoid confusion, the offered solution was to specify the cell source from which the cell culture was obtained, making it possible to continue using the “MSCs” acronym to describe either of the two populations without having to directly indicate the differences between them [44]. Despite this effort, there is still controversy on how to characterize and name MSCs, which hinders the search of information in databases. Consequently, given the extended use of the acronyms without mentioning the biologic origin of the cells, it is considered important to highlight the differences between both concepts. The term “mesenchymal stem cells” refers to the fraction of SCs derived from bone marrow with in vivo multipotent ability, and thus would be equivalent to the term “BM-MSCs,” whereas “mesenchymal stromal cells” are found in tissues different from the bone marrow, also showing in vitro multipotent features [45], and thus encompassing, by definition, all the other tissues in which SCs can be found. Although both populations present the same proposed basic characteristics, defining the acronyms helps to restrict the search of information. Additionally, this lack of specificity creates a problem on one of the proposed identification criteria of MSCs, the surface markers, as the ISCT has only accepted a few markers to describe MSCs based on the premise that positive markers should be present in 95% of the population; however, as shown in Table 4, surface markers tend to change, and their specificity depends on the cell source or even the donor. Therefore, it is necessary to explain whether one is referring to a mesenchymal stem cell or a mesenchymal stromal cell, and if appropriate, the source of isolation.
Another factor to consider is the notorious lack of standardization and characterization of the cell cultures of mesenchymal cells, as there are several studies in which the surface markers are not verified by immunodetection techniques, but through a test of differentiation into the three main linages (osteoblasts, chondrocytes, and adipocytes), which is verified through histological staining and without confirming the homogeneity of the culture. Figure 3 shows the methodology proposed to work with the MSCs populations using the minimum markers necessary to characterize them based on their extraction source.
Fig. 3.
Methodology proposed to characterize mesenchymal and stromal cell cultures based on their site of collection (specific surface markers according to the literature) and the three minimum general techniques to characterize MSCs after differentiation into the target cell population
Limitation between TE and CT
Moreover, the search of TE applications showed that consensus on the information encompassed by the TE and CT fields and maybe even on the implant of biomaterials is still necessary probably because TE possesses the same fundamentals as CT, but it is characterized by the use of biocompatible scaffolds and bioreactors or physical and chemical stimuli that improve the differentiation of the cell cultures. This issue was evinced after the first search filter, in which the results obtained classified the exclusive use of MSC differentiated in special culture media such as TE only because of the use of a special inductor media. In this particular research, frequent use of scaffolds from different origins derived from tissues derived from MSCs, known as MSCs-derived decellularized extracellular matrices (MSCs-ECM), serve as cell support. During the publication review, some of the studies were found to classify the use of MSCs-ECM scaffolds implanted in animal models as TE [206], but no culture of MSCs was performed in the scaffold; thus, these studies were regarded as regenerative medicine therapies and were not included as part of the specific TE field.
First pillar of TE: Cell source
Furthermore, it was observed that most studies address osseous, cartilaginous, and osteochondral TE (Tables 5, 6, and 7); the last being a combination of the first two. In addition, a higher use of MSCs derived from bone marrow can be observed, as shown in the tables previously mentioned, followed by those derived from adipose tissue, and lastly those derived from umbilical cord. This is because the bone marrow possesses a better differentiation capacity, and it was also the first source from which MSCs were isolated and identified. Nonetheless, its use for cell collection is limited as it involves performing a procedure that is dangerous for the patient, which makes bone marrow a complex source for the isolation of MSCs. In contrast, the adipose tissue, which is an accessible cell source and is largely present in the organism, is the second most used tissue for the collection of MSCs and has the highest extraction yield of MSCs; thus, it represents an autologous transplant alternative for patients (Table 4).
In the case of the umbilical cord, although it represents an easily accessible source and the MSCs are closer to the first stages of development, presenting more primitive properties [207], there is not much research on this subject because it is not a common practice to save this type of extraembryonic tissue at the moment of birth, particularly owing to the associated costs, and the genetic and chromosomal studies required to verify that the donor (the neonate) will develop normally [208].
Second pillar of TE: Biomaterials
In accordance with TE’s second pillar, including biomaterials, it can be observed that the use of scaffold synthesized using natural biomaterials or of those with a combination of natural and synthetic biomaterials, predominates in most studies, with the synthetic biomaterials the least used ones. Of the total 112 studies analyzed (Tables 5–11), 41.96% used natural biomaterials, 32.14% employed a combination of natural and synthetic biomaterials, and only 18.75% used exclusively synthetic biomaterials for the construction of scaffolds. This is because the use of natural or combined materials allows us to better mimic the natural environment of cells, favoring their differentiation and adhesion to the scaffold. The five biomaterials most widely used are collagen, chitosan, gelatin, hydroxyapatite, and silk fibroin; however, alginate, HA, and fibrin, among others, are also employed. Decellularized extracellular matrices have also been reported as scaffolds for the seeding of MSCs [111, 122, 127] as well as the use of other compounds, such as growth factors or functionalization with nanoparticles to improve the scaffolds’ functionality [183, 209].
Third pillar of TE: Culture stimulation
Finally, the last pillar addressed by TE, external stimuli are applied to improve cellular growth and differentiation, which constitutes an important aspect to successfully mimic the tissue of interest through the use of bioreactors. The use of bioreactors has been reported in the different applications discussed in this revision, either with scaffolds or cell cultures only; the most relevant studies are discussed in the following text. Quarta et al. (2017) employed Mu-MSCs and other muscle-resident cells and a perfusion bioreactor as an effective treatment for muscle volume loss, which enhanced the efficacy of the Mu-MSCs in the constructs [96]; Sulaiman et al. (2020) observed that the culture of BM-MSCs in gelatin microspheres in a dynamic reactor increased the differentiation of chondrocytes in vitro, along with cell proliferation as well as RNA and protein expression when compared with the static cultures [97]. Moreover, Xu et al. (2019) reported that the use of a bioreactor in a 3D culture of human BM-MSCs with alginate and gelatin microspheres presented higher proliferation than 2D cultures using only alginate or alginate and gelatin for the generation of cartilage [210]. In osseous TE, Zhang et al. (2021) demonstrated that the mechanical stimulation of human UC-MSCs cultured in a porous hydroxyapatite scaffold that was created using a bioreactor led to the modulation of the inflammatory response and osteogenic differentiation [211]. Finally, Agabalyan et al. (2017) used dermal SCs (DSCs) from hair follicles in parallel DASGIP (Eppendorf) reactors to produce skin-derived precursors and regenerate the dermis and reported that the expansion in the bioreactor cultures was five times higher than that of the static cultures [212]. Although the use of bioreactors is associated with better differentiation results and larger cultures, it is used in a reduced percentage of the applications of MSCs in TE.
For the MSCs to be stable during the culture in bioreactors, they have to be taken care of and stimulated right from the moment of isolation from the tissue to increase their survival, differentiation efficiency, and homing to the damaged tissue [213]. Growth factors are important for this conditioning because during this phase, the use of biomaterials avoids the degradation of these inductor molecules, maintains their stability and bioactivity, and ensures the release of a low and continuous concentration of these factors without causing toxic damage to the organism [214].
Conclusion
MSCs can be considered the main cell source in regenerative medicine and TE as they are easy to obtain (multiple cell sources) and possess multipotentiality; especially AD-MSCs, which are obtained through procedures that are safer and less painful for the patient, present a high cell yield, facilitating their application in therapy.
As a perspective, this review proposes the need to homogenize the terminologies related to regenerative medicine, including the concepts of CT and TE as well as the use of the acronym “MSCs” to facilitate bibliographic search. In addition, it is necessary that the markers–morphology–differentiation correlation proposed by the ISCT becomes a basic requirement to characterize MSCs and achieve the homogenization goal. Furthermore, standardization for the selection of tissue-specific markers that allow a more specific cell characterization is necessary.
Acknowledgements
The authors thank the support of Children’s Hospital of Mexico Federico Gomez. The authors thank Crimson Interactive Pvt. Ltd. (Enago)—https://www.enago.com/es/ for their assistance in manuscript translation and editing.
Author contribution
R.A.G.V. and A.P.R. Undergraduate student. conceptualization, methodology, writing-original draft preparation, writing-review and editing. C.C.P.M. PhD. Conceptualization, writing-review and editing, final approval. C.S.G. PhD. Writing-review and editing, funding acquisition, final approval. N.E.B.V. PhD. conceptualization, methodology, writing-review and editing, supervision, funding acquisition, final approval.
Declarations
Conflict of interest
The authors have no potential conflicts of interest with respect to the research, authorship, and / or publication of this article.
Ethical statement
We do not report ethical issues for human and animal right.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Barnaby JR. Fisiología del ejercicio físico y del entrenamiento. 2nd ed. 2002, Barcelona: Paidotribo. 192
- 2.Boons MC. Automatism, compulsion: statements and restatements. Rev Fr Psychanal. 1970;34(3):541–570. [PubMed] [Google Scholar]
- 3.Fleischer S, Feiner R, Dvir T. Cardiac tissue engineering: from matrix design to the engineering of bionic hearts. Regen Med. 2017;12(3):275–284. doi: 10.2217/rme-2016-0150. [DOI] [PubMed] [Google Scholar]
- 4.Santini MP, Forte E, Harvey RP, Kovacic JC. Developmental origin and lineage plasticity of endogenous cardiac stem cells. Development. 2016;143(8):1242–58. doi: 10.1242/dev.111591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Appasani K, Appasani RK. Stem cells and regenerative medicine: from molecular embryology to tissue engineering. Springer Science & Business Media; 2010. [DOI] [PubMed]
- 6.Edgar L, Pu T, Porter B, Aziz JM, La Pointe C, Asthana A, et al. Regenerative medicine, organ bioengineering and transplantation. Br J Surg. 2020;107(7):793–800. doi: 10.1002/bjs.11686. [DOI] [PubMed] [Google Scholar]
- 7.Buzhor E, Leshansky L, Blumenthal J, Barash H, Warshawsky D, Mazor Y, et al. Cell-based therapy approaches: the hope for incurable diseases. Regen Med. 2014;9(5):649–672. doi: 10.2217/rme.14.35. [DOI] [PubMed] [Google Scholar]
- 8.Castilho L, Moraes A, Augusto E, Butler M. Animal cell technology: from biopharmaceuticals to gene therapy. Taylor & Francis; 2008.
- 9.Kim I. A brief overview of cell therapy and its product. J Korean Assoc Oral Maxillofac Surg. 2013;39(5):201–202. doi: 10.5125/jkaoms.2013.39.5.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.U.S. Food and Drug Administration. What is Gene Therapy? Cellular & Gene Therapy Products. 2018. https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/what-gene-therapy. Accessed 25 July 2018.
- 11.Wahid F, Khan T, Hussain Z, Ullah H. Nanocomposite scaffolds for tissue engineering; properties, preparation and applications. In: Inamuddin AMA, Mohammad A, editors. Applications of nanocomposite materials in drug delivery. Woodhead Publishing Series in Biomaterials; 2018. p. 701–35.
- 12.Nguyen AH, et al. Cardiac tissue engineering: state-of-the-art methods and outlook. J Biol Eng. 2019;13(1):57. doi: 10.1186/s13036-019-0185-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Theus AS, et al. Biomaterial approaches for cardiovascular tissue engineering. Emergent Mater. 2019;2(2):193–207. [Google Scholar]
- 14.Kangari P, et al. Mesenchymal stem cells: amazing remedies for bone and cartilage defects. Stem Cell Res Ther. 2020;11(1):492. doi: 10.1186/s13287-020-02001-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sheng G. The developmental basis of mesenchymal stem/stromal cells (MSCs) BMC Dev Biol. 2015;15:44. doi: 10.1186/s12861-015-0094-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ren X, et al. Growth factor engineering strategies for regenerative medicine applications. Front. Bioeng. Biotechnol. 2020;7:469. doi: 10.3389/fbioe.2019.00469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Casanova MR, et al. Spatial immobilization of endogenous growth factors to control vascularization in bone tissue engineering. Biomater Sci. 2020;8(9):2577–2589. doi: 10.1039/d0bm00087f. [DOI] [PubMed] [Google Scholar]
- 18.Ding T, et al. An in situ tissue engineering scaffold with growth factors combining angiogenesis and osteoimmunomodulatory functions for advanced periodontal bone regeneration. J Nanobiotechnology. 2021;19(1):247. doi: 10.1186/s12951-021-00992-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Subbiah R, Guldberg RE. Materials science and design principles of growth factor delivery systems in tissue engineering and regenerative medicine. Adv Healthc Mater. 2019;8(1):e1801000. doi: 10.1002/adhm.201801000. [DOI] [PubMed] [Google Scholar]
- 20.Zhao H-Y, et al. Research advances in tissue engineering materials for sustained release of growth factors. BioMed Res Int. 2015;2015:808202. doi: 10.1155/2015/808202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen L, et al. Growth factor and its polymer scaffold-based delivery system for cartilage tissue engineering. Int J Nanomedicine. 2020;15:6097–6111. doi: 10.2147/IJN.S249829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suman S, et al. Potential clinical applications of stem cells in regenerative medicine. Adv Exp Med Biol. 2019;1201:1–22. doi: 10.1007/978-3-030-31206-0_1. [DOI] [PubMed] [Google Scholar]
- 23.Galipeau J, Sensébé L. Mesenchymal stromal cells: clinical challenges and therapeutic opportunities. Cell Stem Cell. 2018;22(6):824–833. doi: 10.1016/j.stem.2018.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hosseini S, et al. Regenerative medicine applications of mesenchymal stem cells. Adv Exp Med Biol. 2018;1089:115–141. doi: 10.1007/5584_2018_213. [DOI] [PubMed] [Google Scholar]
- 25.Samsonraj RM, Raghunath M, Nurcombe V, Hui JH, van Wijnen AJ, Cool SM. Concise review: multifaceted characterization of human mesenchymal stem cells for use in regenerative medicine. Stem Cells Transl Med. 2017;6(12):2173–2185. doi: 10.1002/sctm.17-0129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tonelli FMP, De Cássia Oliveira Paiva N, De Medeiros RVB, Pinto MCX, Tonelli FCP, Resende RR. Tissue engineering: the use of stem cells in regenerative medicine. In: Soccol VT, Pandey A, Resende RR, editors. Current developments in biotechnology and bioengineering: human and animal health applications. Elsevier; 2016. p. 315-24.
- 27.Keshtkar S, Azarpira N, Ghahremani MH. Mesenchymal stem cell-derived extracellular vesicles: novel frontiers in regenerative medicine. Stem Cell Res Ther. 2018;9(1):63. doi: 10.1186/s13287-018-0791-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Naji A, Eitoku M, Favier B, Deschaseaux F, Rouas-Freiss N, Suganuma N. Biological functions of mesenchymal stem cells and clinical implications. Cell Mol Life Sci. 2019;76(17):3323–3348. doi: 10.1007/s00018-019-03125-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cohen BP, Bernstein JL, Morrison KA, Spector JA, Bonassar LJ. Tissue engineering the human auricle by auricular chondrocyte-mesenchymal stem cell co-implantation. PLoS One. 2018;13(10):e0202356. doi: 10.1371/journal.pone.0202356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boeckel DG, Sesterheim P, Peres TR, Augustin AH, Wartchow KM, Machado DC, et al. Adipogenic mesenchymal stem cells and hyaluronic acid as a cellular compound for bone tissue engineering. J Craniofac Surg. 2019;30(3):777–783. doi: 10.1097/SCS.0000000000005392. [DOI] [PubMed] [Google Scholar]
- 31.Kellar R, Diller RB, Machula H, Muller J, Ensley B. Biomimetic skin substitutes created from tropoelastin help to promote wound healing. Front Bioeng Biotechnol. Conference Abstract: 10th World Biomaterials Congress. https://www.frontiersin.org/10.3389/conf.FBIOE.2016.01.00174/event_abstract
- 32.World Health Organization. World No Tobacco Day 2018: Tobacco breaks hearts–choose health, not tobacco (No. WHO/NMH/PND/18.4). World Health Organization. 2018.
- 33.Chagastelles PC, Nardi NB. Biology of stem cells: an overview. Kidney Int suppl. 2011;1(3):63–67. doi: 10.1038/kisup.2011.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Laplane L, Solary E. Towards a classification of stem cells. ELife. 2019;8:e46563. doi: 10.7554/eLife.46563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gilbert S.F. Developmental Biology. 6a ed. 2000, Sunderland (MA): Sinauer Associates
- 36.Bacakova L, Zarubova J, Travnickova M, Musilkova J, Pajorova J, Slepicka P, et al. Stem cells: their source, potency and use in regenerative therapies with focus on adipose-derived stem cells – a review. Biotechnol Adv. 2018;36(4):1111–1126. doi: 10.1016/j.biotechadv.2018.03.011. [DOI] [PubMed] [Google Scholar]
- 37.Zuba-Surma EK, Wojakowski W, Ratajczak MZ, Dawn B. Very small embryonic-like stem cells: biology and therapeutic potential for heart repair. Antioxid Redox Signal. 2011;15(7):1821–1834. doi: 10.1089/ars.2010.3817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kuruca SE, Çelik DD, Özerkan D, Erdemir G. Characterization and isolation of very small embryonic-like (vsel) stem cells obtained from various human hematopoietic cell sources. Stem Cell Rev. Rep. 2019;15(5):730–742. doi: 10.1007/s12015-019-09896-1. [DOI] [PubMed] [Google Scholar]
- 39.Liu G, David BT, Trawczynski M, Fessler RG. Advances in pluripotent stem cells: history, mechanisms, technologies, and applications. Stem Cell Rev. Rep. 2020;16(1):3–32. doi: 10.1007/s12015-019-09935-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zakrzewski W, Dobrzyński M, Szymonowicz M, Rybk Z. Stem cells: past, present, and future. Stem Cell Res Ther. 2019;10(1):68. doi: 10.1186/s13287-019-1165-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tweedell KS. The adaptability of somatic stem cells: a review. J. Stem Cells Regener. Med. 2017;13(1):3–13. doi: 10.46582/jsrm.1301002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Romito A, Cobellis G. Pluripotent stem cells: current understanding and future directions. Stem Cells Int. 2016;2016:9451492–9451492. doi: 10.1155/2016/9451492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Viswanathan S, Shi Y, Galipeau J, Krampera M, Leblanc K, Martin I, et al. Mesenchymal stem versus stromal cells: international society for cell & gene therapy (ISCT®) mesenchymal stromal cell committee position statement on nomenclature. Cytotherapy. 2019;21(10):1019–1024. doi: 10.1016/j.jcyt.2019.08.002. [DOI] [PubMed] [Google Scholar]
- 44.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, et al. Minimal criteria for defining multipotent mesenchymal stromal cells the international society for cellular therapy position statement. Cytotherapy. 2006;8(4):315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 45.Wilson A, Webster A, Genever P. Nomenclature and heterogeneity: consequences for the use of mesenchymal stem cells in regenerative medicine. Regen Med. 2019;14(6):595–611. doi: 10.2217/rme-2018-0145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lv FJ, Tuan RS, Cheung KM, Leung VY. Concise review: the surface markers and identity of human mesenchymal stem cells. Stem Cells. 2014;32(6):1408–1419. doi: 10.1002/stem.1681. [DOI] [PubMed] [Google Scholar]
- 47.Jones EA, English A, Kinsey SE, Straszynski L, Emery P, Ponchel F, et al. Optimization of a flow cytometry-based protocol for detection and phenotypic characterization of multipotent mesenchymal stromal cells from human bone marrow. Cytometry B Clin Cytom. 2006;70(6):391–399. doi: 10.1002/cyto.b.20118. [DOI] [PubMed] [Google Scholar]
- 48.Halfon S, Abramov N, Grinblat B, Ginis I. Markers distinguishing mesenchymal stem cells from fibroblasts are downregulated with passaging. Stem Cells Dev. 2011;20(1):53–66. doi: 10.1089/scd.2010.0040. [DOI] [PubMed] [Google Scholar]
- 49.Nombela-Arrieta C, Ritz J, Silberstein LE. The elusive nature and function of mesenchymal stem cells. Nat Rev Mol Cell Biol. 2011;12(2):126–131. doi: 10.1038/nrm3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Berebichez-Fridman R, Montero-Olvera PR. Sources and clinical applications of mesenchymal stem cells: state-of-the-art review. Sultan Qaboos Univ Med J. 2018;18(3):e264–e277. doi: 10.18295/squmj.2018.18.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mildmay-White A, Khan W. Cell surface markers on adipose-derived stem cells: a systematic review. Curr Stem Cell Res Ther. 2017;12(6):484–492. doi: 10.2174/1574888X11666160429122133. [DOI] [PubMed] [Google Scholar]
- 52.Varma MJ, Breuls RG, Schouten TE, Jurgens WJ, Bontkes HJ, Schuurhuis GJ, et al. Phenotypical and functional characterization of freshly isolated adipose tissue-derived stem cells. Stem Cells Dev. 2007;16(1):91–104. doi: 10.1089/scd.2006.0026. [DOI] [PubMed] [Google Scholar]
- 53.Bourin P, Bunnell BA, Casteilla L, Dominici M, Katz AJ, March KL, et al. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: a joint statement of the international federation for adipose therapeutics and science (IFATS) and the international society for cellular therapy (ISCT) Cytotherapy. 2013;15(6):641–648. doi: 10.1016/j.jcyt.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dave JR, Tomar GB. Dental tissue-derived mesenchymal stem cells: applications in tissue engineering. Crit Rev Biomed Eng. 2018;46(5):429–468. doi: 10.1615/CritRevBiomedEng.2018027342. [DOI] [PubMed] [Google Scholar]
- 55.Beeravolu N, McKee C, Alamri A, Mikhael S, Brown C, Perez-Cruet M, et al. Isolation and characterization of mesenchymal stromal cells from human umbilical cord and fetal placenta. J Vis Exp. 2017;122:e55224. doi: 10.3791/55224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bharti D, Shivakumar SB, Park JK, Ullh I, Subbarao RB, Park JS, et al. Comparative analysis of human wharton's jelly mesenchymal stem cells derived from different parts of the same umbilical cord. Cell Tissue Res. 2018;372(1):51–65. doi: 10.1007/s00441-017-2699-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Maleki M, Ghanbarvand F, Reza Behvarz M, Ejtemaei M, Ghadirkhomi E, et al. Comparison of mesenchymal stem cell markers in multiple human adult stem cells. Int J Stem Cells. 2014;7(2):118–126. doi: 10.15283/ijsc.2014.7.2.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fang W, Sun Z, Chen X, Han B, Vangsness CT., Jr. Synovial fluid mesenchymal stem cells for knee arthritis and cartilage defects: a review of the literature. J Knee Surg. 2021;34(13):1476–1485. doi: 10.1055/s-0040-1710366. [DOI] [PubMed] [Google Scholar]
- 59.Rahmadian R, et al. Clinical application prospect of human synovial tissue stem cells from osteoarthritis grade iv patients in cartilage regeneration. Open Access Maced J. Med. Sci. 2021;9:52–57. [Google Scholar]
- 60.Mizuno M, Katano H, Mabuchi Y, Ogata Y, Ichinose S, Fujii S, et al. Specific markers and properties of synovial mesenchymal stem cells in the surface, stromal, and perivascular regions. Stem Cell Res Ther. 2018;9(1):123. doi: 10.1186/s13287-018-0870-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Čamernik K, Mihelič A, Mihalič R, Marolt Presen D, Janež A, Trebše R, et al. Skeletal-muscle-derived mesenchymal stem/stromal cells from patients with osteoarthritis show superior biological properties compared to bone-derived cells. Stem Cell Res. 2019;38:101465. doi: 10.1016/j.scr.2019.101465. [DOI] [PubMed] [Google Scholar]
- 62.Klimczak A, Kozlowska U, Kurpisz M. Muscle stem/progenitor cells and mesenchymal stem cells of bone marrow origin for skeletal muscle regeneration in muscular dystrophies. Arch Immunol Ther Exp (Warsz). 2018;66(5):341–354. doi: 10.1007/s00005-018-0509-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Uezumi A, Nakatani M, Ikemoto-Uezumi M, Yamamoto N, Morita M, Yamaguchi A, et al. Cell-surface protein profiling identifies distinctive markers of progenitor cells in human skeletal muscle. Stem Cell Rep. 2016;7(2):263–278. doi: 10.1016/j.stemcr.2016.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Singh A, Singh A, Sen D. Mesenchymal stem cells in cardiac regeneration: a detailed progress report of the last 6 years (2010–2015) Stem Cell Res Ther. 2016;7(1):82. doi: 10.1186/s13287-016-0341-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chu DT, Phuong TNT, Tien NLB, Tran DK, Thanh VV, Quang TL, et al. An update on the progress of isolation, culture, storage, and clinical application of human bone marrow mesenchymal stem/stromal cells. Int J Mol Sci. 2020;21:708. doi: 10.3390/ijms21030708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Via AG, Frizziero A, Oliva F. Biological properties of mesenchymal stem cells from different sources. Muscles Ligaments Tendons J. 2012;2(3):154–162. [PMC free article] [PubMed] [Google Scholar]
- 67.Brown C, McKee C, Bakshi S, Walker K, Hakman E, Halassy S, et al. Mesenchymal stem cells: cell therapy and regeneration potential. J Tissue Eng Regen Med. 2019;13(9):1738–1755. doi: 10.1002/term.2914. [DOI] [PubMed] [Google Scholar]
- 68.Si Z, Wang X, Sun C, Kang Y, Xu J, Wang X, et al. Adipose-derived stem cells: Sources, potency, and implications for regenerative therapies. Biomed. Pharmacother. 2019;114:108765. doi: 10.1016/j.biopha.2019.108765. [DOI] [PubMed] [Google Scholar]
- 69.Murakami M, Horibe H, Iohara K, Hayashi Y, Osako Y, Takei Y, et al. The use of granulocyte-colony stimulating factor induced mobilization for isolation of dental pulp stem cells with high regenerative potential. Biomaterials. 2013;34(36):9036–9047. doi: 10.1016/j.biomaterials.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 70.Noda S, Kawashima N, Yamamoto M, Hashimoto K, Nara S, Sekiya I, et al. Effect of cell culture density on dental pulp-derived mesenchymal stem cells with reference to osteogenic differentiation. Sci Rep. 2019;9(1):5430. doi: 10.1038/s41598-019-41741-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Alsulaimani RS, Ajlan SA, Aldahmash AM, Alnabaheen MS, Ashri NY. Isolation of dental pulp stem cells from a single donor and characterization of their ability to differentiate after 2 years of cryopreservation. Saudi Med J. 2016;37(5):551–560. doi: 10.15537/smj.2016.5.13615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sharpe PT. Dental mesenchymal stem cells. Development. 2016;143(13):2273–2280. doi: 10.1242/dev.134189. [DOI] [PubMed] [Google Scholar]
- 73.Hassan G, Kasem I, Antaki R, Antaki MB, AlKadry R, Aljamali M. Isolation of umbilical cord mesenchymal stem cells using human blood derivatives accompanied with explant method. Stem Cell Investig. 2019;6:28. doi: 10.21037/sci.2019.08.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Troyer DL, Weiss ML. Concise review: wharton's jelly-derived cells are a primitive stromal cell population. Stem Cells. 2008;26(3):591–599. doi: 10.1634/stemcells.2007-0439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jia Z, Liang Y, Xu X, Li X, Liu Q, Ou Y, et al. Isolation and characterization of human mesenchymal stem cells derived from synovial fluid by magnetic-activated cell sorting (MACS) Cell Biol Int. 2018;42(3):262–271. doi: 10.1002/cbin.10903. [DOI] [PubMed] [Google Scholar]
- 76.Li J, Campbell DD, Bal GK, Pei M. Can arthroscopically harvested synovial stem cells be preferentially sorted using stage-specific embryonic antigen 4 antibody for cartilage, bone, and adipose regeneration? Arthroscopy. 2014;30(3):352–361. doi: 10.1016/j.arthro.2013.12.009. [DOI] [PubMed] [Google Scholar]
- 77.Biz C, Crimi A, Fantoni I, Pozzuoli A, Ruggieri P. Muscle stem cells: what's new in orthopedics? Acta Biomed. 2019;90((1–S)):8–13. doi: 10.23750/abm.v90i1-S.8078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Franzin C, Piccoli M, Urbani L, Biz C, Gamba P, De Coppi P, et al. Isolation and expansion of muscle precursor cells from human skeletal muscle biopsies. Methods Mol Biol. 2016;1516:195–204. doi: 10.1007/7651_2016_321. [DOI] [PubMed] [Google Scholar]
- 79.Čamernik K, Marc J, Zupan J. Human skeletal muscle-derived mesenchymal stem/stromal cell isolation and growth kinetics analysis. Methods Mol Biol. 2019;2045:119–129. doi: 10.1007/7651_2018_201. [DOI] [PubMed] [Google Scholar]
- 80.Yoshimura H, Muneta T, Nimura A, Yokoyama A, Koga H, Sekiya I. Comparison of rat mesenchymal stem cells derived from bone marrow, synovium, periosteum, adipose tissue, and muscle. Cell Tissue Res. 2007;327(3):449–462. doi: 10.1007/s00441-006-0308-z. [DOI] [PubMed] [Google Scholar]
- 81.Abdul Rahman R, Mohamad Sukri N, Md Nazir n, Ahmad Radzi MA, Zulkifly AH, Che Ahmad A, et al. The potential of 3-dimensional construct engineered from poly(lactic-co-glycolic acid)/fibrin hybrid scaffold seeded with bone marrow mesenchymal stem cells for in vitro cartilage tissue engineering. Tissue Cell. 2015;47(4):420–430. doi: 10.1016/j.tice.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 82.Li X, Wang M, Jing X, Guo W, Hao C, Zhang Y, et al. Bone marrow- and adipose tissue-derived mesenchymal stem cells: characterization, differentiation, and applications in cartilage tissue engineering. Crit Rev Eukaryot Gene Expr. 2018;28(4):285–310. doi: 10.1615/CritRevEukaryotGeneExpr.2018023572. [DOI] [PubMed] [Google Scholar]
- 83.Yin H, Wang Y, Sun Z, Sun X, Xu Y, Li P, et al. Induction of mesenchymal stem cell chondrogenic differentiation and functional cartilage microtissue formation for in vivo cartilage regeneration by cartilage extracellular matrix-derived particles. Acta Biomater. 2016;33:96–109. doi: 10.1016/j.actbio.2016.01.024. [DOI] [PubMed] [Google Scholar]
- 84.Roseti L, Parisi V, Petretta M, Cavallo C, Desando G, Bartolotti I, et al. Scaffolds for bone tissue engineering: state of the art and new perspectives. Mater Sci Eng C Mater Biol Appl. 2017;78:1246–1262. doi: 10.1016/j.msec.2017.05.017. [DOI] [PubMed] [Google Scholar]
- 85.Toosi S, Naderi-Meshkin H, Kalalinia F, Peivandi MT, HosseinKhani H, Bahrami AR, et al. PGA-incorporated collagen: toward a biodegradable composite scaffold for bone-tissue engineering. J Biomed Mater Res A. 2016;104(8):2020–2028. doi: 10.1002/jbm.a.35736. [DOI] [PubMed] [Google Scholar]
- 86.Zhang B, Zhang PB, Wang ZL, Lyu ZW, Wu H. Tissue-engineered composite scaffold of poly(lactide-co-glycolide) and hydroxyapatite nanoparticles seeded with autologous mesenchymal stem cells for bone regeneration. J Zhejiang Univ Sci B. 2017;18(11):963–976. doi: 10.1631/jzus.B1600412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Klar AS, Zimoch J, Biedermann T. Skin tissue engineering: application of adipose-derived stem cells. Biomed Res Int. 2017;2017:9747010. doi: 10.1155/2017/9747010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kucharzewski M, Rojczyk E, Wilemska-Kucharzewska K, Wilk R, Hudecki J, Los MJ. Novel trends in application of stem cells in skin wound healing. Eur J Pharmacol. 2019;843:307–315. doi: 10.1016/j.ejphar.2018.12.012. [DOI] [PubMed] [Google Scholar]
- 89.Mashiko T, Takada H, Wu SH, Kanayama K, Feng J, Tashiro K, et al. Therapeutic effects of a recombinant human collagen peptide bioscaffold with human adipose-derived stem cells on impaired wound healing after radiotherapy. J Tissue Eng Regen Med. 2018;12(5):1186–1194. doi: 10.1002/term.2647. [DOI] [PubMed] [Google Scholar]
- 90.Ansari S, Chen C, Xu X, Annabi N, Zadeh HH, Wu BM, et al. Muscle tissue engineering using gingival mesenchymal stem cells encapsulated in alginate hydrogels containing multiple growth factors. Ann Biomed Eng. 2016;44(6):1908–1920. doi: 10.1007/s10439-016-1594-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Prochazka A. Neurophysiology and neural engineering: a review. J Neurophysiol. 2017;118(2):1292–1309. doi: 10.1152/jn.00149.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zimmermann JA, Schaffer DV. Engineering biomaterials to control the neural differentiation of stem cells. Brain Res Bull. 2019;150:50–60. doi: 10.1016/j.brainresbull.2019.05.007. [DOI] [PubMed] [Google Scholar]
- 93.Quintiliano K, Crestani T, Silveira D, Helfer VE, Rosa A, Balbueno E, et al. Neural differentiation of mesenchymal stem cells on scaffolds for nerve tissue engineering applications. Cell Reprogram. 2016;18(6):369–381. doi: 10.1089/cell.2016.0024. [DOI] [PubMed] [Google Scholar]
- 94.Jamali S, Mostafavi H, Barati G, Eskandari M, Nadri S. Differentiation of mesenchymal stem cells -derived trabecular meshwork into dopaminergic neuron-like cells on nanofibrous scaffolds. Biologicals. 2017;50:49–54. doi: 10.1016/j.biologicals.2017.09.002. [DOI] [PubMed] [Google Scholar]
- 95.Li N, Huang R, Zhang X, Xin Y, Li J, Huang Y, et al. Stem cells cardiac patch from decellularized umbilical artery improved heart function after myocardium infarction. Biomed Mater Eng. 2017;28(s1):S87–S94. doi: 10.3233/BME-171628. [DOI] [PubMed] [Google Scholar]
- 96.Chen J, Zhang Y, Wang Y, Han D, Tao B, Luo Z, et al. Chitosan/silk fibroin modified nanofibrous patches with mesenchymal stem cells prevent heart remodeling post-myocardial infarction in rats. Acta Biomater. 2018;80:154–168. doi: 10.1016/j.actbio.2018.09.013. [DOI] [PubMed] [Google Scholar]
- 97.Prat-Vidal C, Rodríguez-Gómez L, Aylagas M, Nieto-Nicolau N, Gastelurrutia P, Agustí E, et al. First-in-human PeriCord cardiac bioimplant: scalability and GMP manufacturing of an allogeneic engineered tissue graft. EBioMedicine. 2020;54:102729. doi: 10.1016/j.ebiom.2020.102729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Du X, Wei D, Huang L, Zhu M, Zhang Y, Zhu Y. 3D printing of mesoporous bioactive glass/silk fibroin composite scaffolds for bone tissue engineering. Mater Sci Eng C Mater Biol Appl. 2019;103:109731. doi: 10.1016/j.msec.2019.05.016. [DOI] [PubMed] [Google Scholar]
- 99.Bidgoli MR, Alemzadeh I, Tamjid E, Khafaji M, Vossoughi M. Fabrication of hierarchically porous silk fibroin-bioactive glass composite scaffold via indirect 3D printing: effect of particle size on physico-mechanical properties and in vitro cellular behavior. Mater Sci Eng C Mater Biol Appl. 2019;103:109688. doi: 10.1016/j.msec.2019.04.067. [DOI] [PubMed] [Google Scholar]
- 100.Chen W, Liu X, Chen Q, Bao C, Zhao L, Zhu Z, et al. Angiogenic and osteogenic regeneration in rats via calcium phosphate scaffold and endothelial cell co-culture with human bone marrow mesenchymal stem cells (MSCs), human umbilical cord MSCs, human induced pluripotent stem cell-derived MSCs and human embryonic stem cell-derived MSCs. J Tissue Eng Regen Med. 2018;12(1):191–203. doi: 10.1002/term.2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ang SL, Shaharuddin B, Chuah JA, Sudesh K. Electrospun poly(3-hydroxybutyrate-co-3-hydroxyhexanoate)/silk fibroin film is a promising scaffold for bone tissue engineering. Int J Biol Macromol. 2020;145:173–188. doi: 10.1016/j.ijbiomac.2019.12.149. [DOI] [PubMed] [Google Scholar]
- 102.Salgado CL, Barrias CC, Monteiro FJM. Clarifying the tooth-derived stem cells behavior in a 3D biomimetic scaffold for bone tissue engineering applications. Front Bioeng Biotechnol. 2020;8:724. doi: 10.3389/fbioe.2020.00724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gutierrez-Quintero JG, Durán Riveros JY, Martínez Valbuena CA, Pedraza Alonso S, Munévar JC, Viafara-García SM. Critical-sized mandibular defect reconstruction using human dental pulp stem cells in a xenograft model-clinical, radiological, and histological evaluation. Oral Maxillofac Surg. 2020;24(4):485–493. doi: 10.1007/s10006-020-00862-7. [DOI] [PubMed] [Google Scholar]
- 104.Gurumurthy B, Pal P, Griggs JA, Janorkar AV. Optimization of collagen-elastin-like polypeptide-bioglass scaffold composition for osteogenic differentiation of adipose-derived stem cells. Materialia (Oxf). 2020;9:100572. doi: 10.1016/j.mtla.2019.100572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Winkler S, Mutschall H, Biggemann J, Fey T, Greil P, Körner C, et al. Human umbilical vein endothelial cell support bone formation of adipose-derived stem cell-loaded and 3D-printed osteogenic matrices in the arteriovenous loop model. Tissue Eng Part A. 2021;27(5–6):413–423. doi: 10.1089/ten.TEA.2020.0087. [DOI] [PubMed] [Google Scholar]
- 106.Lin Y, Umebayashi M, Abdallah MN, Dong G, Roskies MG, Zhao YF, et al. Combination of polyetherketoneketone scaffold and human mesenchymal stem cells from temporomandibular joint synovial fluid enhances bone regeneration. Sci Rep. 2019;9(1):472. doi: 10.1038/s41598-018-36778-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chi H, Jiang A, Wang X, Chen HG, Song C, Prajapati RK, et al. Dually optimized polycaprolactone/collagen I microfiber scaffolds with stem cell capture and differentiation-inducing abilities promote bone regeneration. J Mater Chem B. 2019;7(44):7052–7064. doi: 10.1039/c9tb01359h. [DOI] [PubMed] [Google Scholar]
- 108.Li S, Song C, Yang S, Yu W, Zhang W, Zhang G, et al. Supercritical CO2 foamed composite scaffolds incorporating bioactive lipids promote vascularized bone regeneration via Hif-1alpha upregulation and enhanced type H vessel formation. Acta Biomater. 2019;94:253–267. doi: 10.1016/j.actbio.2019.05.066. [DOI] [PubMed] [Google Scholar]
- 109.Tsukamoto J, Naruse K, Nagai Y, Kan S, Nakamura N, Hata M, et al. Efficacy of a self-assembling peptide hydrogel, SPG-178-Gel, for Bone regeneration and three-dimensional osteogenic induction of dental pulp stem cells. Tissue Eng Part A. 2017;23(23–24):1394–1402. doi: 10.1089/ten.TEA.2017.0025. [DOI] [PubMed] [Google Scholar]
- 110.Chamieh F, Collignon AM, Coyac BR, Lesieur J, Ribes S, Sadoine J, et al. Accelerated craniofacial bone regeneration through dense collagen gel scaffolds seeded with dental pulp stem cells. Sci Rep. 2016;6:38814. doi: 10.1038/srep38814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Dziedzic DSM, Francisco JC, Mogharbel BF, Irioda AC, Stricker PEF, Floriano J, et al. Combined biomaterials: amniotic membrane and adipose tissue to restore injured bone as promoter of calcification in bone regeneration: preclinical model. Calcif Tissue Int. 2020;108(5):667–679. doi: 10.1007/s00223-020-00793-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Huang S, Jia S, Liu G, Fang D, Zhang D. Osteogenic differentiation of muscle satellite cells induced by platelet-rich plasma encapsulated in three-dimensional alginate scaffold. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012;114(5 Suppl):S32–40. doi: 10.1016/j.tripleo.2011.07.048. [DOI] [PubMed] [Google Scholar]
- 113.Tang Y, Tong X, Conrad B, Yang F. Injectable and in situ crosslinkable gelatin microribbon hydrogels for stem cell delivery and bone regeneration in vivo. Theranostics. 2020;10(13):6035–6047. doi: 10.7150/thno.41096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Caballero M, Jones DC, Shan Z, Soleimani S, van Aalst JA. Tissue engineering strategies to improve osteogenesis in the juvenile swine alveolar cleft model. Tissue Eng Part C Methods. 2017;23(12):889–899. doi: 10.1089/ten.tec.2017.0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Dewey MJ, Johnson EM, Slater ST, Milner DJ, Wheeler MB, Harley BAC. Mineralized collagen scaffolds fabricated with amniotic membrane matrix increase osteogenesis under inflammatory conditions. Regen Biomater. 2020;7(3):247–258. doi: 10.1093/rb/rbaa005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hwang TI, Kim JI, Lee J, Moon JY, Lee JC, Joshi MK, et al. In situ biological transmutation of catalytic lactic acid waste into calcium lactate in a readily processable three-dimensional fibrillar structure for bone tissue engineering. ACS Appl Mater Interfaces. 2020;12(16):18197–18210. doi: 10.1021/acsami.9b19997. [DOI] [PubMed] [Google Scholar]
- 117.Song JE, Jeon YS, Tian J, Kim WK, Choi MJ, Carlomagno C, et al. Evaluation of silymarin/duck's feet-derived collagen/hydroxyapatite sponges for bone tissue regeneration. Mater Sci Eng C Mater Biol Appl. 2019;97:347–355. doi: 10.1016/j.msec.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 118.Liu J, Zhou P, Smith J, Xu S, Huang C. A Plastic beta-Tricalcium phosphate/gelatine scaffold seeded with allogeneic adipose-derived stem cells for mending rabbit bone defects. Cell Reprogram. 2021;23(1):35–46. doi: 10.1089/cell.2020.0031. [DOI] [PubMed] [Google Scholar]
- 119.Sulaiman S, Chowdhury SR, Fauzi MB, Rani RA, Yahaya NHM, Tabata Y, et al. 3D culture of MSCs on a gelatin microsphere in a dynamic culture system enhances chondrogenesis. Int J Mol Sci. 2020;21(8):2688. doi: 10.3390/ijms21082688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Shen H, Lin H, Sun AX, Song S, Wang B, Yang Y, et al. Acceleration of chondrogenic differentiation of human mesenchymal stem cells by sustained growth factor release in 3D graphene oxide incorporated hydrogels. Acta Biomater. 2020;105:44–55. doi: 10.1016/j.actbio.2020.01.048. [DOI] [PubMed] [Google Scholar]
- 121.Yea JH, Bae TS, Kim BJ, Cho YW, Jo CH, et al. Regeneration of the rotator cuff tendon-to-bone interface using umbilical cord-derived mesenchymal stem cells and gradient extracellular matrix scaffolds from adipose tissue in a rat model. Acta Biomater. 2020;114:104–116. doi: 10.1016/j.actbio.2020.07.020. [DOI] [PubMed] [Google Scholar]
- 122.Zhang Y, Liu S, Guo W, Wang M, Hao C, Gao S, et al. Human umbilical cord Wharton's jelly mesenchymal stem cells combined with an acellular cartilage extracellular matrix scaffold improve cartilage repair compared with microfracture in a caprine model. Osteoarthritis Cartilage. 2018;26(7):954–965. doi: 10.1016/j.joca.2018.01.019. [DOI] [PubMed] [Google Scholar]
- 123.Talaat W, Aryal Ac S, Al Kawas S, Samsudin ABR, Kandile NG, Harding DRK, et al. Nanoscale thermosensitive hydrogel scaffolds promote the chondrogenic differentiation of dental Pulp stem and progenitor cells: a minimally invasive approach for cartilage regeneration. Int J Nanomedicine. 2020;15:7775–7789. doi: 10.2147/IJN.S274418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhou X, Tenaglio S, Esworthy T, Hann SY, Cui H, Webster TJ, et al. Three-dimensional printing biologically inspired dna-based gradient scaffolds for cartilage tissue regeneration. ACS Appl Mater Interfaces. 2020;12(29):33219–33228. doi: 10.1021/acsami.0c07918. [DOI] [PubMed] [Google Scholar]
- 125.Chocarro-Wrona C, de Vicente J, Antich C, Jiménez G, Martínez-Moreno D, Carrillo E, et al. Validation of the 1,4-butanediol thermoplastic polyurethane as a novel material for 3D bioprinting applications. Bioeng Transl Med. 2021;6(1):e10192. doi: 10.1002/btm2.10192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Neybecker P, Henrionnet C, Pape E, Mainard D, Galois L, Loeuille D, et al. In vitro and in vivo potentialities for cartilage repair from human advanced knee osteoarthritis synovial fluid-derived mesenchymal stem cells. Stem Cell Res Ther. 2018;9(1):329. doi: 10.1186/s13287-018-1071-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Liang Y, Idrees E, Szojka ARA, Andrews SHJ, Kunze M, Mulet-Sierra A, et al. Chondrogenic differentiation of synovial fluid mesenchymal stem cells on human meniscus-derived decellularized matrix requires exogenous growth factors. Acta Biomater. 2018;80:131–143. doi: 10.1016/j.actbio.2018.09.038. [DOI] [PubMed] [Google Scholar]
- 128.Luo C, Xie R, Zhang J, Liu Y, Li R, Zhang Y, et al. Low-temperature three-dimensional printing of tissue cartilage engineered with Gelatin Methacrylamide. Tissue Eng Part C Methods. 2020;26(6):306–316. doi: 10.1089/ten.TEC.2020.0053. [DOI] [PubMed] [Google Scholar]
- 129.Xuan H, Hu H, Geng C, Song J, Shen Y, Lei D, et al. Biofunctionalized chondrogenic shape-memory ternary scaffolds for efficient cell-free cartilage regeneration. Acta Biomater. 2020;105:97–110. doi: 10.1016/j.actbio.2020.01.015. [DOI] [PubMed] [Google Scholar]
- 130.Han Y, Lian M, Jia B, Wu Q, Qiao Z, et al. Preparation of high precision multilayer scaffolds based on melt electro-writing to repair cartilage injury. Theranostics. 2020;10(22):10214–10230. doi: 10.7150/thno.47909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Xu J, Fang Q, Liu Y, Zhou Y, Ye Z, Tan WS, et al. In situ ornamenting poly(epsilon-caprolactone) electrospun fibers with different fiber diameters using chondrocyte-derived extracellular matrix for chondrogenesis of mesenchymal stem cells. Colloids Surf B Biointerfaces. 2021;197:111374. doi: 10.1016/j.colsurfb.2020.111374. [DOI] [PubMed] [Google Scholar]
- 132.Hong Y, Liu N, Zhou R, Zhao X, Han Y, Xiag F, et al. Combination therapy using Kartogenin-based chondrogenesis and complex polymer scaffold for cartilage defect regeneration. ACS Biomater Sci Eng. 2020;6(11):6276–6284. doi: 10.1021/acsbiomaterials.0c00724. [DOI] [PubMed] [Google Scholar]
- 133.Jia Z, Zhu F, Li X, Liang Q, Zhuo Z, Huang J, et al. Repair of osteochondral defects using injectable chitosan-based hydrogel encapsulated synovial fluid-derived mesenchymal stem cells in a rabbit model. Mater Sci Eng C Mater Biol Appl. 2019;99:541–551. doi: 10.1016/j.msec.2019.01.115. [DOI] [PubMed] [Google Scholar]
- 134.Yu X, Hu Y, Zou L, Yan S, Zhu H, Zhang K, et al. A bilayered scaffold with segregated hydrophilicity-hydrophobicity enables reconstruction of goat hierarchical temporomandibular joint condyle cartilage. Acta Biomater. 2021;121:288–302. doi: 10.1016/j.actbio.2020.11.031. [DOI] [PubMed] [Google Scholar]
- 135.Dorcemus DL, Kim HS, Nukavarapu SP. Gradient scaffold with spatial growth factor profile for osteochondral interface engineering. Biomed Mater. 2020;16(3):035021. doi: 10.1088/1748-605X/abd1ba. [DOI] [PubMed] [Google Scholar]
- 136.Gao F, Xu Z, Liang Q, Li H, Peng L, Wu M, et al. Osteochondral regeneration with 3D-Printed biodegradable high-strength supramolecular polymer reinforced-Gelatin Hydrogel Scaffolds. Adv Sci (Weinh) 2019;6(15):1900867. doi: 10.1002/advs.201900867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Marmotti A, Mattia S, Castoldi F, Barbero A, Mangiavini L, Bonasia DE, et al. Allogeneic umbilical cord-derived mesenchymal stem cells as a potential source for cartilage and bone regeneration: an in vitro study. Stem Cells Int. 2017;2017:1732094. doi: 10.1155/2017/1732094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Qin Y, Li G, Wang C, Zhang D, Zhang L, Fang H, et al. Biomimetic bilayer scaffold as an incubator to induce sequential Chondrogenesis and Osteogenesis of adipose derived stem cells for construction of Osteochondral tissue. ACS Biomater Sci Eng. 2020;6(5):3070–3080. doi: 10.1021/acsbiomaterials.0c00200. [DOI] [PubMed] [Google Scholar]
- 139.Kim HS, Mandakhbayar N, Kim HW, Leong KW, Yoo HS, et al. Protein-reactive nanofibrils decorated with cartilage-derived decellularized extracellular matrix for osteochondral defects. Biomaterials. 2021;269:120214. doi: 10.1016/j.biomaterials.2020.120214. [DOI] [PubMed] [Google Scholar]
- 140.Wang C, Yue H, Huang W, Lin X, Xie X, He Z, et al. Cryogenic 3D printing of heterogeneous scaffolds with gradient mechanical strengths and spatial delivery of osteogenic peptide/TGF-beta1 for osteochondral tissue regeneration. Biofabrication. 2020;12(2):025030. doi: 10.1088/1758-5090/ab7ab5. [DOI] [PubMed] [Google Scholar]
- 141.Xu D, Cheng G, Dai J, Li Z, et al. Bi-layered composite scaffold for repair of the Osteochondral defects. Adv Wound Care (New Rochelle). 2020;10(8):401–414. doi: 10.1089/wound.2019.1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zhao Y, Ding X, Dong Y, Sun X, Wang L, Ma X, et al. Role of the calcified cartilage layer of an integrated trilayered silk fibroin scaffold used to regenerate osteochondral defects in Rabbit knees. ACS Biomater Sci Eng. 2020;6(2):1208–1216. doi: 10.1021/acsbiomaterials.9b01661. [DOI] [PubMed] [Google Scholar]
- 143.Li N, Xue F, Zhang H, Sanyour HJ, Rickel AP, Uttecht A, et al. Fabrication and characterization of pectin hydrogel nanofiber scaffolds for differentiation of mesenchymal stem cells into vascular cells. ACS Biomater Sci Eng. 2019;5(12):6511–6519. doi: 10.1021/acsbiomaterials.9b01178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Bury MI, Fuller MI, Sturm RM, Rabizadeh RR, Nolan BG, Barac M, et al. The effects of bone marrow stem and progenitor cell seeding on urinary bladder tissue regeneration. Sci Rep. 2021;11(1):2322. doi: 10.1038/s41598-021-81939-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Liu J, Xu HH, Zhou H, Weir MD, Chen Q, Trotman CA, et al. Human umbilical cord stem cell encapsulation in novel macroporous and injectable fibrin for muscle tissue engineering. Acta Biomater. 2013;9(1):4688–4697. doi: 10.1016/j.actbio.2012.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Liu J, Zhou H, Weir MD, Xu HH, Chen Q, Trotman CA, et al. Fast-degradable microbeads encapsulating human umbilical cord stem cells in alginate for muscle tissue engineering. Tissue Eng Part A. 2012;18(21–22):2303–2314. doi: 10.1089/ten.tea.2011.0658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Xu Q, et al. A gingiva-derived mesenchymal stem cell-laden porcine small intestinal submucosa extracellular matrix construct promotes myomucosal regeneration of the tongue. Tissue Eng Part A. 2017;23(7–8):301–312. doi: 10.1089/ten.tea.2016.0342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Salem SA, Rashidbenam Z, Jasman MH, Ho CCK, Sagap I, Singh R, et al. Incorporation of smooth muscle cells derived from human adipose stem cells on poly(Lactic-co-Glycolic Acid) scaffold for the reconstruction of subtotally resected urinary bladder in athymic rats. Tissue Eng Regen Med. 2020;17(4):553–563. doi: 10.1007/s13770-020-00271-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Shrestha KR, Jeon SH, Jung AR, Kim IG, Kim GE, Park YH, et al. Stem cells seeded on multilayered scaffolds implanted into an injured bladder rat model improves bladder function. Tissue Eng Regen Med. 2019;16(2):201–212. doi: 10.1007/s13770-019-00187-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Quarta M, Cromie M, Chacon R, Blonigan I, Garcia V, Akimenko I, et al. Bioengineered constructs combined with exercise enhance stem cell-mediated treatment of volumetric muscle loss. Nat Commun. 2017;8:15613. doi: 10.1038/ncomms15613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Trevisan C, Fallas MEA, Maghin E, Franzin C, Pavan P, Caccin P, et al. Generation of a functioning and self-renewing diaphragmatic muscle construct. Stem Cells Transl Med. 2019;8(8):858–869. doi: 10.1002/sctm.18-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Xing Y, Shi S, Zhang Y, Liu F, Zhu L, Shi B, et al. Construction of engineered myocardial tissues in vitro with cardiomyocytelike cells and a polylacticcoglycolic acid polymer. Mol Med Rep. 2019;20(3):2403–2409. doi: 10.3892/mmr.2019.10434. [DOI] [PubMed] [Google Scholar]
- 153.Mombini S, Mohammadnejad J, Bakhshandeh B, Narmani A, Nourmohammadi J, Vahdat S, et al. Chitosan-PVA-CNT nanofibers as electrically conductive scaffolds for cardiovascular tissue engineering. Int J Biol Macromol. 2019;140:278–287. doi: 10.1016/j.ijbiomac.2019.08.046. [DOI] [PubMed] [Google Scholar]
- 154.Zhou Z, Yan H, Liu Y, Xiao D, Li W, Wang Q, et al. Adipose-derived stem-cell-implanted poly(-caprolactone)/chitosan scaffold improves bladder regeneration in a rat model. Regen Med. 2018;13(3):331–342. doi: 10.2217/rme-2017-0120. [DOI] [PubMed] [Google Scholar]
- 155.Izadi MR, Habibi A, Khodabandeh Z, Nikbakht M, et al. Synergistic effect of high-intensity interval training and stem cell transplantation with amniotic membrane scaffold on repair and rehabilitation after volumetric muscle loss injury. Cell Tissue Res. 2021;383(2):765–779. doi: 10.1007/s00441-020-03304-8. [DOI] [PubMed] [Google Scholar]
- 156.Takanari K, Hashizume R, Hong Y, Amoroso NJ, Yoshizumi T, Gharaibeh B, et al. Skeletal muscle derived stem cells microintegrated into a biodegradable elastomer for reconstruction of the abdominal wall. Biomaterials. 2017;113:31–41. doi: 10.1016/j.biomaterials.2016.10.029. [DOI] [PubMed] [Google Scholar]
- 157.Chiu CH, Chang TH, Chang SS, Chang GJ, Chen AC, Cheng CY, et al. Application of bone marrow-derived mesenchymal stem cells for muscle healing after contusion injury in mice. Am J Sports Med. 2020;48(5):1226–1235. doi: 10.1177/0363546520905853. [DOI] [PubMed] [Google Scholar]
- 158.Talovic M, Patel K, Schwartz M, Madsen J, Garg K, et al. Decellularized extracellular matrix gelloids support mesenchymal stem cell growth and function in vitro. J Tissue Eng Regen Med. 2019;13(10):1830–1842. doi: 10.1002/term.2933. [DOI] [PubMed] [Google Scholar]
- 159.Zsedenyi A, Farkas B, Abdelrasoul GN, Romano I, Gyukity-Sebestyen E, Nagy K, et al. Gold nanoparticle-filled biodegradable photopolymer scaffolds induced muscle remodeling: in vitro and in vivo findings. Mater Sci Eng C Mater Biol Appl. 2017;72:625–630. doi: 10.1016/j.msec.2016.11.124. [DOI] [PubMed] [Google Scholar]
- 160.Mahmoudifard M, Soleimani M, Hatamie S, Zamanlui S, Ranjbarvan P, Vossoughi M, et al. The different fate of satellite cells on conductive composite electrospun nanofibers with graphene and graphene oxide nanosheets. Biomed Mater. 2016;11(2):025006. doi: 10.1088/1748-6041/11/2/025006. [DOI] [PubMed] [Google Scholar]
- 161.Hosseinzadeh S, Mahmoudifard M, Mohamadyar-Toupkanlou F, Dodel M, Hajarizadeh A, Adabi M, et al. The nanofibrous PAN-PANi scaffold as an efficient substrate for skeletal muscle differentiation using satellite cells. Bioprocess Biosyst Eng. 2016;39(7):1163–1172. doi: 10.1007/s00449-016-1592-y. [DOI] [PubMed] [Google Scholar]
- 162.Galvez-Monton C, Bragós R, Soler-Botija C, Díaz-Güemes I, Prat-Vidal C, Crisóstomo V, et al. Noninvasive assessment of an engineered bioactive graft in myocardial infarction: impact on cardiac function and scar healing. Stem Cells Transl Med. 2017;6(2):647–655. doi: 10.5966/sctm.2016-0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Im GB, Kim YH, Kim YJ, Kim SW, Jung E, Jeong GJ, et al. Enhancing the wound healing effect of conditioned medium collected from mesenchymal stem cells with high passage number using bioreducible nanoparticles. Int J Mol Sci. 2019;20(19):4835. doi: 10.3390/ijms20194835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Millan-Rivero JE, Martínez CM, Romecín PA, Aznar-Cervantes SD, Carpes-Ruiz M, Cenis JL, et al. Silk fibroin scaffolds seeded with Wharton's jelly mesenchymal stem cells enhance re-epithelialization and reduce formation of scar tissue after cutaneous wound healing. Stem Cell Res Ther. 2019;10(1):126. doi: 10.1186/s13287-019-1229-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Murugan Girija D, Kalachaveedu M, Ranga Rao S, Subbarayan R, et al. Transdifferentiation of human gingival mesenchymal stem cells into functional keratinocytes by Acalypha indica in three-dimensional microenvironment. J Cell Physiol. 2018;233(11):8450–8457. doi: 10.1002/jcp.26807. [DOI] [PubMed] [Google Scholar]
- 166.Izadyari Aghmiuni A, Heidari Keshel S, Sefat F, AkbarzadehKhiyavi A, et al. Fabrication of 3D hybrid scaffold by combination technique of electrospinning-like and freeze-drying to create mechanotransduction signals and mimic extracellular matrix function of skin. Mater Sci Eng C Mater Biol Appl. 2021;120:111752. doi: 10.1016/j.msec.2020.111752. [DOI] [PubMed] [Google Scholar]
- 167.Wang J, Chen Y, Zhou G, Chen Y, Mao C, Yang M, et al. Polydopamine coated antheraea pernyi (a. pernyi) silk fibroin films promote cell adhesion and wound healing in skin tissue repair. ACS Appl Mater Interfaces. 2019;11(38):34736–34743. doi: 10.1021/acsami.9b12643. [DOI] [PubMed] [Google Scholar]
- 168.Shafei S, Khanmohammadi M, Heidari R, Ghanbari H, Taghdiri Nooshabadi V, Farzamfar S, et al. Exosome loaded alginate hydrogel promotes tissue regeneration in full-thickness skin wounds: an in vivo study. J Biomed Mater Res A. 2020;108(3):545–556. doi: 10.1002/jbm.a.36835. [DOI] [PubMed] [Google Scholar]
- 169.Forbes D, Russ B, Kilani R, Ghahary A, Jalili R. Liquid dermal scaffold with adipose-derived stem cells improve tissue quality in a murine model of impaired wound healing. J Burn Care Res. 2019;40(5):550–557. doi: 10.1093/jbcr/irz099. [DOI] [PubMed] [Google Scholar]
- 170.Buzgo M, Plencner M, Rampichova M, Litvinec A, Prosecka E, Staffa A, et al. Poly-epsilon-caprolactone and polyvinyl alcohol electrospun wound dressings: adhesion properties and wound management of skin defects in rabbits. Regen Med. 2019;14(5):423–445. doi: 10.2217/rme-2018-0072. [DOI] [PubMed] [Google Scholar]
- 171.Burmeister DM, Stone R, Wrice N, Laborde A, Becerra SC, Natesan S, et al. Delivery of allogeneic adipose stem cells in polyethylene glycol-fibrin hydrogels as an adjunct to meshed autografts after sharp debridement of deep partial thickness burns. Stem Cells Transl Med. 2018;7(4):360–372. doi: 10.1002/sctm.17-0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Nyambat B, Manga YB, Chen CH, Gankhuyag U, Pratomo WP A, Kumar Satapathy M, et al. New insight into natural extracellular matrix: genipin cross-linked adipose-derived stem cell extracellular matrix gel for tissue engineering. Int J Mol Sci. 2020;21(14):4864. doi: 10.3390/ijms21144864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Hazeri Y, Irani S, Zandi M, Pezeshki-Modaress M, et al. Polyvinyl alcohol/sulfated alginate nanofibers induced the neuronal differentiation of human bone marrow stem cells. Int J Biol Macromol. 2020;147:946–953. doi: 10.1016/j.ijbiomac.2019.10.061. [DOI] [PubMed] [Google Scholar]
- 174.Jiang J, Dai C, Liu X, Dai L, Li R, Ma K, et al. Implantation of regenerative complexes in traumatic brain injury canine models enhances the reconstruction of neural networks and motor function recovery. Theranostics. 2021;11(2):768–788. doi: 10.7150/thno.50540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Luo L, Albashari AA, Wang X, Jin L, Zhang Y, Zheng L, et al. Effects of transplanted heparin-poloxamer hydrogel combining dental pulp stem cells and bFGF on spinal cord injury repair. Stem Cells Int. 2018;2018:2398521. doi: 10.1155/2018/2398521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Syu WZ, Chen SG, Chan TJ, Wang CH, Dai NT, Huang SM, et al. The potential of acellular dermal matrix combined with neural stem cells induced from human adipose-derived stem cells in nerve tissue engineering. Ann Plast Surg. 2019;82((1S Suppl 1)):S108–S118. doi: 10.1097/SAP.0000000000001731. [DOI] [PubMed] [Google Scholar]
- 177.Raynald,, Shu B, Liu XB, Zhou JF, Huang H, Wang JY, et al. Polypyrrole/polylactic acid nanofibrous scaffold cotransplanted with bone marrow stromal cells promotes the functional recovery of spinal cord injury in rats. CNS Neurosci Ther. 2019;25(9):951–964. doi: 10.1111/cns.13135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Darvishi M, Ghasemi Hamidabadi H, Sahab Negah S, Moayeri A, Tiraihi T, Mirnajafi-Zadeh J, et al. PuraMatrix hydrogel enhances the expression of motor neuron progenitor marker and improves adhesion and proliferation of motor neuron-like cells. Iran J Basic Med Sci. 2020;23(4):431–438. doi: 10.22038/ijbms.2020.39797.9434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Zhang J, Cheng T, Chen Y, Gao F, Guan F, Yao M. A chitosan-based thermosensitive scaffold loaded with bone marrow-derived mesenchymal stem cells promotes motor function recovery in spinal cord injured mice. Biomed Mater. 2020;15(3):035020. doi: 10.1088/1748-605X/ab785f. [DOI] [PubMed] [Google Scholar]
- 180.Rashedi I, Talele N, Wang XH, Hinz B, Radisic M, Keating A, et al. Collagen scaffold enhances the regenerative properties of mesenchymal stromal cells. PLoS One. 2017;12(10):e0187348. doi: 10.1371/journal.pone.0187348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Joshi J, Brennan D, Beachley V, Kothapalli CR. Cardiomyogenic differentiation of human bone marrow-derived mesenchymal stem cell spheroids within electrospun collagen nanofiber mats. J Biomed Mater Res A. 2018;106(12):3303–3312. doi: 10.1002/jbm.a.36530. [DOI] [PubMed] [Google Scholar]
- 182.Ravichandran R, Venugopal JR, Sundarrajan S, Mukherjee S, Ramakrishna S. Poly(Glycerol sebacate)/gelatin core/shell fibrous structure for regeneration of myocardial infarction. Tissue Eng Part A. 2011;17(9–10):1363–1373. doi: 10.1089/ten.TEA.2010.0441. [DOI] [PubMed] [Google Scholar]
- 183.Kai D, Prabhakaran MP, Jin G, Tian L, Ramakrishna S. Potential of VEGF-encapsulated electrospun nanofibers for in vitro cardiomyogenic differentiation of human mesenchymal stem cells. J Tissue Eng Regen Med. 2017;11(4):1002–1010. doi: 10.1002/term.1999. [DOI] [PubMed] [Google Scholar]
- 184.Guan J, Wang F, Li Z, Chen J, Guo X, Liao J, et al. The stimulation of the cardiac differentiation of mesenchymal stem cells in tissue constructs that mimic myocardium structure and biomechanics. Biomaterials. 2011;32(24):5568–5580. doi: 10.1016/j.biomaterials.2011.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Sridhar S, Venugopal JR, Sridhar R, Ramakrishna S. Cardiogenic differentiation of mesenchymal stem cells with gold nanoparticle loaded functionalized nanofibers. Colloids Surf B Biointerfaces. 2015;134:346–354. doi: 10.1016/j.colsurfb.2015.07.019. [DOI] [PubMed] [Google Scholar]
- 186.Sreerekha PR, Menon D, Nair SV, Chennazhi KP. Fabrication of electrospun poly (lactide-co-glycolide)-fibrin multiscale scaffold for myocardial regeneration in vitro. Tissue Eng Part A. 2013;19(7–8):849–859. doi: 10.1089/ten.TEA.2012.0374. [DOI] [PubMed] [Google Scholar]
- 187.Choe G, Kim SW, Park J, Park J, Kim S, Kim YS, et al. Anti-oxidant activity reinforced reduced graphene oxide/alginate microgels: Mesenchymal stem cell encapsulation and regeneration of infarcted hearts. Biomaterials. 2019;225:119513. doi: 10.1016/j.biomaterials.2019.119513. [DOI] [PubMed] [Google Scholar]
- 188.Rabbani S, Soleimani M, Imani M, Sahebjam M, Ghiaseddin A, Nassiri SM, et al. Regenerating heart using a novel compound and human wharton jelly mesenchymal stem cells. Arch Med Res. 2017;48(3):228–237. doi: 10.1016/j.arcmed.2017.03.019. [DOI] [PubMed] [Google Scholar]
- 189.Maureira P, Marie PY, Yu F, Poussier S, Liu Y, Groubatch F, et al. Repairing chronic myocardial infarction with autologous mesenchymal stem cells engineered tissue in rat promotes angiogenesis and limits ventricular remodeling. J Biomed Sci. 2012;19:93. doi: 10.1186/1423-0127-19-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Ceccaldi C, et al. Evaluation of polyelectrolyte complex-based scaffolds for mesenchymal stem cell therapy in cardiac ischemia treatment. Acta Biomater. 2014;10(2):901–911. doi: 10.1016/j.actbio.2013.10.027. [DOI] [PubMed] [Google Scholar]
- 191.Tong C, Li C, Xie B, Li M, Li M, Qi Z, et al. Generation of bioartificial hearts using decellularized scaffolds and mixed cells. Biomed Eng Online. 2019;18(1):71. doi: 10.1186/s12938-019-0691-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Kai D, Wang QL, Wang HJ, Prabhakaran MP, Zhang Y, Tan YZ, et al. Stem cell-loaded nanofibrous patch promotes the regeneration of infarcted myocardium with functional improvement in rat model. Acta Biomater. 2014;10(6):2727–2738. doi: 10.1016/j.actbio.2014.02.030. [DOI] [PubMed] [Google Scholar]
- 193.Kameli SM, Khorramirouz R, Eftekharzadeh S, Fendereski K, Daryabari SS, Tavangar SM, et al. Application of tissue-engineered pericardial patch in rat models of myocardial infarction. J Biomed Mater Res A. 2018;106(10):2670–2678. doi: 10.1002/jbm.a.36464. [DOI] [PubMed] [Google Scholar]
- 194.Chen YL, Sun CK, Tsai TH, Chang LT, Leu S, Zhen YY, et al. Adipose-derived mesenchymal stem cells embedded in platelet-rich fibrin scaffolds promote angiogenesis, preserve heart function, and reduce left ventricular remodeling in rat acute myocardial infarction. Am J Transl Res. 2015;7(5):781–803. [PMC free article] [PubMed] [Google Scholar]
- 195.Niu H, Mu J, Zhang J, Hu P, Bo P, Wang Y, et al. Comparative study of three types of polymer materials co-cultured with bone marrow mesenchymal stem cells for use as a myocardial patch in cardiomyocyte regeneration. J Mater Sci Mater Med. 2013;24(6):1535–1542. doi: 10.1007/s10856-012-4842-9. [DOI] [PubMed] [Google Scholar]
- 196.Perea-Gil I, Gálvez-Montón C, Prat-Vidal C, Jorba I, Segú-Vergés C, Roura S, et al. Head-to-head comparison of two engineered cardiac grafts for myocardial repair: From scaffold characterization to pre-clinical testing. Sci Rep. 2018;8(1):6708. doi: 10.1038/s41598-018-25115-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Kajbafzadeh AM, Tafti SHA, Khorramirouz R, Sabetkish S, Kameli SM, Orangian S, et al. Evaluating the role of autologous mesenchymal stem cell seeded on decellularized pericardium in the treatment of myocardial infarction: an animal study. Cell Tissue Bank. 2017;18(4):527–538. doi: 10.1007/s10561-017-9629-2. [DOI] [PubMed] [Google Scholar]
- 198.Taghiyar L, Gourabi H, Eslaminejad MB. Autologous transplantation of mesenchymal stem cells (MSCs) and scaffold in full-thickness articular cartilage. ClinicalTrials.gov. 2010. https://clinicaltrials.gov/ct2/show/NCT00850187.
- 199.Suroto H. Mesenchymal stem cells and amniotic membrane composite for supraspinatus tendon repair augmentation. ClinicalTrials.gov. 2020. https://clinicaltrials.gov/ct2/show/NCT04670302
- 200.Regeneration of maxillary bone cystic cavities by bio implant of MSHV-H cells associated to a cross-linked serum scaffold. ClinicalTrials.gov. 2016
- 201.Redondo LM, García V, Peral B, Verrier A, Becerra J, Sánchez A, et al. Repair of maxillary cystic bone defects with mesenchymal stem cells seeded on a cross-linked serum scaffold. J Craniomaxillofac Surg. 2018;46(2):222–229. doi: 10.1016/j.jcms.2017.11.004. [DOI] [PubMed] [Google Scholar]
- 202.Bueno DF. Use of mesenchymal stem cells for alveolar bone tissue engineering for cleft lip and palate patients. ClinicalTrials.gov. 2020. https://clinicaltrials.gov/ct2/show/NCT01932164
- 203.Brizuel C. Encapsulated mesenchymal stem cells for dental pulp regeneration. (RanoKure). ClinicalTrials.gov. 2020. https://clinicaltrials.gov/ct2/show/NCT03102879
- 204.Mrozikiewicz-Rakowska B. Treatment of chronic wounds in diabetic foot syndrome with allogeneic adipose derived mesenchymal stem cells (1ABC). ClinicalTrials.gov. 2021. https://clinicaltrials.gov/ct2/show/NCT03865394
- 205.Bayes-Genís A. Pericardial matrix With mesenchymal stem cells for the treatment of patients With infarcted myocardial tissue (PERISCOPE). ClinicalTrials.gov. 2021. https://clinicaltrials.gov/ct2/show/NCT03798353
- 206.Tang C, Jin C, Li X, Li J, Du X, Yan C, et al. Evaluation of an Autologous Bone Mesenchymal Stem Cell-Derived Extracellular Matrix Scaffold in a Rabbit and Minipig Model of Cartilage Repair. Med Sci Monit. 2019;25:7342–7350. doi: 10.12659/MSM.916481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Gil P, Alonso-Bedate M, Barja de Quiroga G. Different levels of hyperoxia reversibly induce catalase activity in amphibian tadpoles. Free Radic Biol Med. 1987;3(2):137–146. doi: 10.1016/s0891-5849(87)80009-6. [DOI] [PubMed] [Google Scholar]
- 208.Nagamura-Inoue T, He H. Umbilical cord-derived mesenchymal stem cells: Their advantages and potential clinical utility. World J Stem Cells. 2014;6(2):195–202. doi: 10.4252/wjsc.v6.i2.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Sridhar S, Venugopal JR, Sridhar R, Ramakrishna S. Cardiogenic differentiation of mesenchymal stem cells with gold nanoparticle loaded functionalized nanofibers. Colloids Surf B Biointerfaces. 2015;134:346–354. doi: 10.1016/j.colsurfb.2015.07.019. [DOI] [PubMed] [Google Scholar]
- 210.Xu Y, Peng J, Richards G, Lu S, Eglin D. Optimization of electrospray fabrication of stem cell-embedded alginate-gelatin microspheres and their assembly in 3D-printed poly(epsilon-caprolactone) scaffold for cartilage tissue engineering. J Orthop Translat. 2019;18:128–141. doi: 10.1016/j.jot.2019.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Zhang P, Liu X, Guo P, Li X, He Z, Li Z, et al. Effect of cyclic mechanical loading on immunoinflammatory microenvironment in biofabricating hydroxyapatite scaffold for bone regeneration. Bioact Mater. 2021;6(10):3097–3108. doi: 10.1016/j.bioactmat.2021.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Agabalyan NA, Borys BS, Sparks HD, Boon K, Raharjo EW, Abbasi S, et al. Enhanced expansion and sustained inductive function of skin-derived precursor cells in computer-controlled stirred suspension bioreactors. Stem Cells Transl Med. 2017;6(2):434–443. doi: 10.5966/sctm.2016-0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Hu C, Li L. Preconditioning influences mesenchymal stem cell properties in vitro and in vivo. J Cell Mol Med. 2018;22(3):1428–1442. doi: 10.1111/jcmm.13492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Wang Z, Wang Z, Lu WW, Yang D, Peng S. Novel biomaterial strategies for controlled growth factor delivery for biomedical applications. NPG Asia Mat. 2017;9(10):e435. [Google Scholar]