Abstract
Background
COVID-19 vaccines have proven highly effective among individuals without a previous SARS-CoV-2 infection, but their effectiveness in preventing symptomatic infection and severe outcomes among individuals with previous infection is less clear. We aimed to estimate the effectiveness of four COVID-19 vaccines against symptomatic infection, hospitalisation, and death for individuals with laboratory-confirmed previous SARS-CoV-2 infection.
Methods
Using national COVID-19 notification, hospitalisation, and vaccination datasets from Brazil, we did a test-negative, case-control study to assess the effectiveness of four vaccines (CoronaVac [Sinovac], ChAdOx1 nCoV-19 [AstraZeneca], Ad26.COV2.S [Janssen], and BNT162b2 [Pfizer-BioNtech]) for individuals with laboratory-confirmed previous SARS-CoV-2 infection. We matched cases with RT-PCR positive, symptomatic COVID-19 with up to ten controls with negative RT-PCR tests who presented with symptomatic illnesses, restricting both groups to tests done at least 90 days after an initial infection. We used multivariable conditional logistic regression to compare the odds of test positivity and the odds of hospitalisation or death due to COVID-19, according to vaccination status and time since first or second dose of vaccines.
Findings
Between Feb 24, 2020, and Nov 11, 2021, we identified 213 457 individuals who had a subsequent, symptomatic illness with RT-PCR testing done at least 90 days after their initial SARS-CoV-2 infection and after the vaccination programme started. Among these, 30 910 (14·5%) had a positive RT-PCR test consistent with reinfection, and we matched 22 566 of these cases with 145 055 negative RT-PCR tests from 68 426 individuals as controls. Among individuals with previous SARS-CoV-2 infection, vaccine effectiveness against symptomatic infection 14 or more days from vaccine series completion was 39·4% (95% CI 36·1–42·6) for CoronaVac, 56·0% (51·4–60·2) for ChAdOx1 nCoV-19, 44·0% (31·5–54·2) for Ad26.COV2.S, and 64·8% (54·9–72·4) for BNT162b2. For the two-dose vaccine series (CoronaVac, ChAdOx1 nCoV-19, and BNT162b2), effectiveness against symptomatic infection was significantly greater after the second dose than after the first dose. Effectiveness against hospitalisation or death 14 or more days from vaccine series completion was 81·3% (75·3–85·8) for CoronaVac, 89·9% (83·5–93·8) for ChAdOx1 nCoV-19, 57·7% (−2·6 to 82·5) for Ad26.COV2.S, and 89·7% (54·3–97·7) for BNT162b2.
Interpretation
All four vaccines conferred additional protection against symptomatic infections and severe outcomes among individuals with previous SARS-CoV-2 infection. The provision of a full vaccine series to individuals after recovery from COVID-19 might reduce morbidity and mortality.
Funding
Brazilian National Research Council, Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro, Oswaldo Cruz Foundation, JBS, Instituto de Salud Carlos III, Spanish Ministry of Science and Innovation, and Generalitat de Catalunya.
Introduction
As of March 11, 2022, over 450 million confirmed cases of COVID-19 have been reported since the start of the pandemic,1 and the true cumulative incidence has probably been several times greater.2 Within a year of the identification of SARS-CoV-2, multiple vaccines were developed, found to be highly efficacious among seronegative individuals in clinical trials, and introduced into national vaccination programmes.3, 4 Coverage of COVID-19 vaccination has varied across populations due to inequalities in access and public hesitancy. Additionally, public debate has emerged about the need for vaccination among people who have had a previous SARS-CoV-2 infection5 and, if so, whether a single dose is sufficient.6, 7 The emergence of more transmissible variants with enhanced immune escape, and the resulting waves of infection and reinfection, have renewed questions about the importance of vaccination in individuals who have had COVID-19.8, 9
Research in context.
Evidence before this study
We searched PubMed, medRxiv, and SSRN for articles published from Jan 1, 2020, to Feb 14, 2022, with no language restrictions, using the search terms “vaccine effectiveness” AND “previous*” AND (“SARS-CoV-2” OR “COVID-19”). We found several studies evaluating ChAdOx1 nCoV-19 (AstraZeneca) and BNT162b2 (tozinameran; Pfizer-BioNtech), and one additionally reporting on mRNA-1273 (elasomeran; Moderna) and Ad26.COV2.S (Janssen), which found that individuals who were previously infected and were vaccinated had lower risk of symptomatic SARS-CoV-2 infection than those who were unvaccinated. One study found that for individuals who were previously infected, the risk of hospitalisation was lower after a full series of BNT162b2 or mRNA-1273 than for those who were unvaccinated. One study reported on effectiveness of an inactivated virus vaccine (BBV152; Bharat Biotech International) against reinfection, and no studies reported on effectiveness of CoronaVac among individuals who were previously infected. Scarce evidence is available comparing effectiveness of one dose versus two doses of vaccine among individuals with previous infection.
Added value of this study
We used national databases of COVID-19 case surveillance, laboratory testing, and vaccination from Brazil to investigate the effectiveness of CoronaVac, ChAdOx1 nCoV-19, Ad26.COV2.S, and BNT162b2 among individuals with a previous, laboratory-confirmed SARS-CoV-2 infection. We matched more than 22 000 RT-PCR-confirmed re-infections with more than 145 000 RT-PCR-negative controls, using a test-negative design. All four vaccines were effective against symptomatic SARS-CoV-2 infection, with effectiveness from 14 days after series completion ranging from 39·4% (95% CI 36·1–42·6) for CoronaVac to 64·8% (54·9–72·4) for BNT162b2. For vaccines with two-dose regimens, the second dose provided significantly increased effectiveness compared with one dose alone. Effectiveness against COVID-19-associated hospitalisation or death from 14 days after series completion was over 80% for CoronaVac, ChAdOx1 nCoV-19, and BNT162b2.
Implications of all the available evidence
We found evidence that these four vaccines, using three different platforms, all provide protection against symptomatic SARS-CoV-2 infection and severe outcomes to individuals who were previously infected, with a second dose conferring significant additional benefits. These results support the provision of a full vaccine series among individuals with previous SARS-CoV-2 infection.
SARS-CoV-2 infection induces robust T-cell and B-cell responses,10 and the risk of symptomatic infection and severe outcomes is lower among people with previous SARS-CoV-2 infection than among naive individuals.11 Emerging evidence suggests that vaccination with ChAdOx1 nCoV-19 (AstraZeneca), Ad26.COV2.S (Janssen), BNT162b2 (tozinameran; Pfizer-BioNtech), or mRNA-1273 (elasomeran; Moderna) confers additional protection against symptomatic reinfection among individuals with previous SARS-CoV-2 infection.12, 13, 14, 15, 16, 17, 18 However, only one study has assessed protection against severe outcomes in previously infected individuals, with just 75 hospital admissions and two deaths.18 Moreover, data for inactivated vaccines, which account for almost half of all doses given globally, are still needed.19
Brazil has recorded more than 22 million SARS-CoV-2 infections and 600 000 deaths as of Nov 15, 2021. On Jan 18, 2021, a national COVID-19 immunisation programme was initiated, which has used four vaccines of three different classes: inactivated virus (CoronaVac; Sinovac), viral vector (ChAdOx1 nCoV-19 and Ad26.COV2.S), and mRNA (BNT162b2). We used national disease surveillance and vaccination databases to estimate the effectiveness of these four vaccines among individuals with laboratory-confirmed previous SARS-CoV-2 infection against symptomatic infection, hospitalisation, and death.
Methods
Study design, population, and data sources
We did a test-negative, case-control study to evaluate the effectiveness of four vaccines (CoronaVac, ChAdOx1 nCoV-19, Ad26.COV2.S, and BNT162b2) in individuals with previous SARS-CoV-2 infection in Brazil. The study population included individuals with a previous positive RT-PCR or rapid antigen test for SARS-CoV-2 who presented again to health facilities with symptomatic illness and were tested for SARS-CoV-2 at least 90 days after their first positive test.20 We matched positive tests (cases) to negative tests (controls).
We used data from several national data sources: a deterministically linked dataset comprised of the Programa Nacional de Imunizações, which contains records of all vaccines administered in Brazil; the e-SUS Notifica, which contains records of suspected and confirmed COVID-19 cases in outpatient clinics; and the Sistema de Informação da Vigilância Epidemiológica da Gripe, which contains records of severe acute respiratory illnesses, including COVID-19 hospitalisations and deaths.21, 22, 23, 24, 25 All data were pseudo-anonymised with a common unique identifier provided by the Brazilian Ministry of Health. The research protocol was approved by the Brazilian National Commission in Research Ethics (4.921.308).
Brazil's national COVID-19 immunisation programme commenced on Jan 18, 2021. Rollout plans were determined at the state and local level; health-care workers and older individuals were the first groups to be eligible, with age criteria for eligibility decreasing over time. Four vaccines have been offered in immunisation programmes in Brazil: CoronaVac, provided as a two-dose series with a 4-week interval between doses; ChAdOx1 nCoV-19, provided as a two-dose series with a 12-week interval between doses that was subsequently reduced to 8 weeks in some states; Ad26.COV2.S, provided as a single dose series; and BNT162b2, provided as a two-dose series with an initial 12-week interval that was subsequently reduced to 3 weeks in some states. Brazil's national guidelines recommend that individuals who were previously infected be vaccinated 4 weeks or more after infection, and this recommendation did not change during the study period.
Eligibility and selection of cases and controls
Inclusion criteria for this study included age 18 years or older, previous SARS-CoV-2 infection confirmed by RT-PCR or rapid antigen test, and a second exam (RT-PCR test) fulfilling the following criteria: being associated with an event of acute respiratory symptomatic illness and occurring within 10 days of symptom onset, being done at least 90 days after the individual's first positive test, and occurring after the vaccination programme began in Brazil (Jan 18, 2021). We included individuals whose first infection occurred between Feb 24, 2020, and Aug 13, 2021, and with a subsequent RT-PCR test being done between Jan 18, 2021, and Nov 11, 2021.
We excluded individuals for whom data were incomplete on age, sex, location of residence, vaccination status, or testing status or dates; those who received different vaccines for their first and second dose; those whose time interval between the first and second doses was less than 14 days; and those vaccinated before the first infection or less than 14 days after the first infection. For tests, we excluded negative tests that were followed by a positive test within 7 days (to avoid misclassification of cases as controls), tests done after the second positive test, tests for which the individual's symptom onset date occurred after notification of the suspected case in the surveillance system (to exclude individuals without symptoms at the time of testing), tests done in individuals without symptoms, and tests done after a third vaccine dose, as this analysis was not powered to examine effectiveness of third doses. In some cases, more than one negative test from one individual was available for matching, and we included these as candidates for matching if they met the described eligibility criteria.
We matched cases, defined as positive SARS-CoV-2 RT-PCR tests from previously infected, symptomatic individuals, with controls, defined as negative SARS-CoV-2 RT-PCR tests from previously infected, symptomatic individuals. We did not attempt to ascertain causality between SARS-CoV-2 infection and hospitalisation or death as this information was not available. Instead, we defined hospitalisation or death related to COVID-19 using a commonly used, temporally defined surveillance case definition for COVID-19-related outcomes: a positive SARS-CoV-2 RT-PCR test accompanied by hospital admission or death occurring within 28 days of the sample collection date. For the analysis of hospitalisation or death, we selected matched sets from the overall matched dataset in which cases were positive tests from patients admitted to hospital or who died, and we fitted the model described to each subset. For severe outcomes, controls thus represented negative tests from patients in ambulatory or hospital settings who had RT-PCR testing, to reflect the population at risk for that outcome. We did not require controls for the severe outcomes analysis to be negative tests from patients admitted to hospital or who died, as the goal was to estimate overall effectiveness against severe outcomes. We matched one case to a maximum of ten controls, with replacement, by date of RT-PCR testing (±10 days), age (±5 years), sex, and municipality of residence. Individuals who were selected as cases could also serve as controls if they had negative tests that were collected more than 7 days before their positive test.
Statistical analyses
We calculated standardised differences for demographic characteristics of matched cases and controls, considering a difference higher than 0·1 for variables not included in the exact match to be significant;26, 27 for exact matched variables, no differences exist within each stratum of the analysis. The primary exposure of interest was vaccination status, which was categorised by vaccine and according to the vaccination status of the individual at the time of RT-PCR test collection as unvaccinated, 0–13 days after the first dose, 14 days or more after the first dose, 0–13 days after the second dose, or 14 days or more after the second dose. Post-second dose status is not applicable to Ad26.COV2.S. We considered vaccine effectiveness against symptomatic SARS-CoV-2 infection and against COVID-19-related hospitalisation or death among individuals with previous confirmed SARS-CoV-2 infection 14 days or more after vaccine series completion (two doses for CoronaVac, ChAdOx1 nCoV-19, and BNT162b2 and one dose for Ad26.COV2.S) to be the primary estimands of interest. We considered effectiveness in the 6 days after the first vaccine dose to be an indicator of bias, because we expected protection to be minimal during this time and substantial differences in risk could reflect residual confounding between the vaccinated and unvaccinated populations.28
We estimated vaccine effectiveness (1–odds ratio) using conditional logistic regression, accounting for the matched design, with vaccination status (including number of doses and time period since dose) as the predictor and adjusting for the number of reported chronic comorbidities (diabetes, cardiovascular disease, obesity, chronic kidney disease, and immunosupression, categorised as none, one, and at least two), pregnancy, postpartum period, self-reported race, days elapsed between the first positive test and the second test (as a restricted cubic spline), and whether the individual was admitted to hospital during their first SARS-CoV-2 infection. For severe outcomes, age (as a continuous variable) was also included due to anticipated residual confounding and observed improved model fit and Bayesian Information Criterion.
We did subgroup analyses in which we assessed vaccine effectiveness by age (18–49 years vs ≥50 years), time since vaccine series completion (14–90 days vs >90 days; to assess for possible waning), and time from initial positive test to vaccination (91–180 days vs 181–613 days). We used generalised linear hypothesis tests for comparisons across different vaccination status, and the confidence intervals and p values were not adjusted for multiple comparisons. All data processing and analyses were done in R (version 4.1.1), using the packages tidyverse, multcomp, MatchIt, and survival.
Role of the funding source
Julio Croda is affiliated with Oswaldo Cruz and received support from the Oswaldo Cruz Foundation for this work. The Oswaldo Cruz Foundation and the other funders of the study did not have any further role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
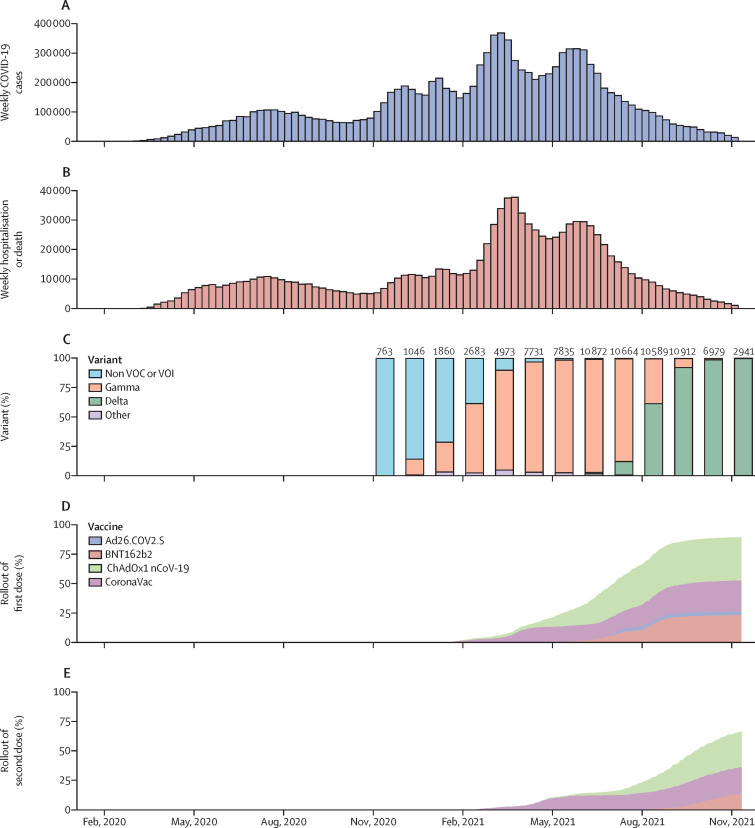
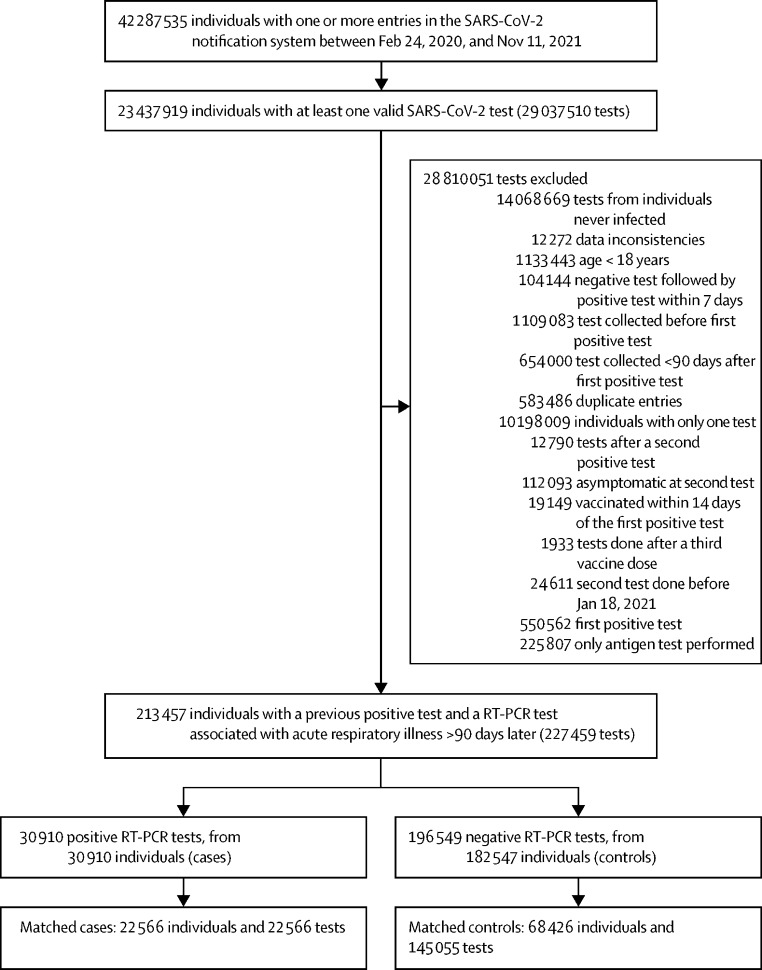
Brazil has had two COVID-19 epidemic waves up to the end of 2021, with the first occurring between July and September 2020, and the second between February and June 2021, during which the gamma (P.1) variant was dominant (figure 1 ). Brazil's national vaccination programme commenced on Jan 18, 2021; 50% of the adult population (83 million individuals) had received a first vaccine dose by July 7, 2021. Between Feb 24, 2020, and Nov 11, 2021, more than 23 million individuals had valid SARS-CoV-2 tests and 11 million were confirmed cases (figure 2 ). Among these, we identified 213 457 individuals who had a subsequent, symptomatic illness with RT-PCR testing done at least 90 days after their initial SARS-CoV-2 infection and after the vaccination programme commenced. Among these, 30 910 (14·5%) had a positive RT-PCR test consistent with reinfection. We matched 22 566 of these cases with 145 055 negative RT-PCR tests from 68 426 individuals as controls. Among cases, 1545 (6·8%) were admitted to hospital and 290 (1·3%) died within 28 days of a positive SARS-CoV-2 RT-PCR; 1564 (6·9%) were admitted to hospital or died (table ).
Figure 1.
Temporal trends in COVID-19 cases, hospitalisation or deaths, variants, and vaccination coverage from national databases in Brazil
Weekly numbers of symptomatic COVID-19 cases (A); COVID-19-associated hospitalisations or deaths reported in national databases (B); monthly proportions of variants among sequenced SARS-CoV-2 samples, with the number of sequenced viruses shown above each bar (C); and cumulative proportion of the population older than 11 years who received a first (D) or second (E) dose of each vaccine. VOC=variant of concern. VOI=variant of interest.
Figure 2.
Flowchart of the study population from surveillance databases and selection of matched cases and controls
Cases and controls were matched on age (±5 years), sex, municipality, and date of test (±10 days).
Table.
Characteristics, vaccination status, and outcomes of individuals eligible for and matched into case-control sets
|
Eligible population |
Matched sets |
Standardised difference | ||||
|---|---|---|---|---|---|---|
| Cases | Controls | Cases | Controls | |||
| Individuals | 30 910 | 182 547 | 22 566 | 68 426 | .. | |
| Tests | 30 910 | 196 549 | 22 566 | 145 055 | .. | |
| Age, years | 38 (29–47) | 37 (28–47) | 37 (29–46) | 36 (29–44) | 0·066 | |
| Sex | .. | .. | .. | .. | 0·047 | |
| Female | 18 106 (58·6%) | 119 134 (60·6%) | 13 631 (60·4%) | 90 931 (62·7%) | .. | |
| Male | 12 804 (41·4%) | 77 415 (39·4%) | 8935 (39·6%) | 54 124 (37·3%) | .. | |
| Race | .. | .. | .. | .. | 0·039 | |
| White | 13 841 (44·8%) | 109 923 (55·9%) | 10 302 (45·7%) | 67 403 (46·5%) | .. | |
| Mixed | 11 363 (36·8%) | 53 401 (27·2%) | 7998 (35·4%) | 50 788 (35·0%) | .. | |
| Black | 1420 (4·6%) | 9034 (4·6%) | 1052 (4·7%) | 7572 (5·2%) | .. | |
| Indigenous or Asian | 2081 (6·7%) | 9305 (4·7%) | 1437 (6·4%) | 8751 (6·0%) | .. | |
| Missing | 2205 (7·1%) | 14 886 (7·6%) | 1777 (7·9%) | 10 541 (7·3%) | .. | |
| Region of residence | .. | .. | .. | .. | 0·085 | |
| Central west | 3260 (10·5%) | 46 968 (23·9%) | 2302 (10·2%) | 12 997 (9·0%) | .. | |
| North | 2406 (7·8%) | 9724 (4·9%) | 1870 (8·3%) | 12 372 (8·5%) | .. | |
| Northeast | 8268 (26·7%) | 30 027 (15·3%) | 5297 (23·5%) | 30 489 (21·0%) | .. | |
| South | 2823 (9·1%) | 16 251 (8·3%) | 1991 (8·8%) | 14 745 (10·2%) | .. | |
| Southeast | 14 153 (45·8%) | 93 579 (47·6%) | 11 106 (49·2%) | 74 452 (51·3%) | .. | |
| Residence in state capital | 9250 (29·9%) | 51 128 (26·0%) | 8982 (39·8%) | 77 198 (53·2%) | 0·271 | |
| Medical comorbidities | .. | .. | .. | .. | .. | |
| None | 25 988 (84·1%) | 166 655 (84·8%) | 19 271 (85·4%) | 124 964 (86·1%) | 0·027 | |
| One | 3552 (11·5%) | 22 178 (11·3%) | 2459 (10·9%) | 15 360 (10·6%) | .. | |
| Two or more | 1370 (4·4%) | 7716 (3·9%) | 836 (3·7%) | 4731 (3·3%) | .. | |
| Days from first positive test to second test | 210 (144–285) | 217 (154–293) | 216 (146–291) | 223 (154–295) | 0·060 | |
| Hospitalised during first infection | 1220 (3·9%) | 9481 (4·8%) | 781 (3·5%) | 6507 (4·5%) | 0·052 | |
| Hospitalisation (up to 28 days) | 2508 (8·1%) | 3770 (1·9%) | 1545 (6·8%) | 2196 (1·5%) | .. | |
| Death (up to 28 days) | 559 (1·8%) | 663 (0·3%) | 290 (1·3%) | 386 (0·3%) | .. | |
| Hospitalisation or death | 2554 (8·3%) | 3829 (1·9%) | 1564 (6·9%) | 2238 (1·5%) | .. | |
Data are n, n (%), or median (IQR). Percentages were calculated using number of tests as the denominator. Matching was based on tests rather than individuals, with up to ten controls matched, with replacement, per case.
Demographics and clinical characteristics of eligible and matched sets are presented in the table. The median age of the matched population was 36 years (IQR 29–44), approximately 60% of cases and controls were women, and the median time between first infection and the subsequent RT-PCR test was similar between cases (216 days, IQR 146–291) and controls (223 days, 154–295). The southeast region of Brazil, which includes São Paulo and Rio de Janeiro and is the most populous region, accounted for 49·2% of matched cases and 51·3% of controls. This was followed by the northeast region, which is the second most populous region, and then the central-west, south, and north regions (table). 39·8% of cases and 53·2% of controls resided in a state capital; due to exact matching on city, we observed no differences within each stratum of analysis.
The majority of cases (14 566 [64·5%] of 22 566) and controls (83 290 [57·4%] of 145 055) were unvaccinated at the time of the test. Among vaccinated individuals (39 717), 17 008 (42·8%) received CoronaVac, 15 897 (40·0%) received ChAdOx1 nCoV-19, 5 935 (14·9%) received BNT162b2, and 877 (2·2%) received Ad26.COV2.S. Demographic characteristics were similar among vaccine recipients included in the analysis, but recipients of ChAdOx1 nCoV-19 tended to be older (p<0·0001) and have more comorbidities (p<0·0001; appendix pp 2–3). The median time between vaccination and testing was 34 days (IQR 17–61) for individuals who received only one dose and 59 days (27–105) for individuals who received two doses, which differed by each vaccine (appendix p 12).
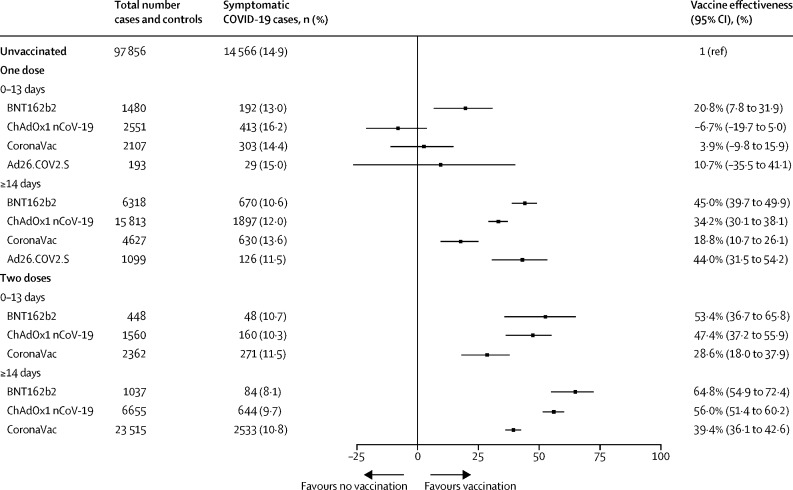
Effectiveness against symptomatic SARS-CoV-2 reinfection 14 days or more from vaccine series completion was 39·4% (95% CI 36·1–42·6) for CoronaVac, 56·0% (51·4–60·2) for ChAdOx1 nCoV-19, 44·0% (31·5–54·2) for Ad26.COV2.S, and 64·8% (54·9–72·4) for BNT162b2 (figure 3 ). The two-dose vaccines (CoronaVac, ChAdOx1 nCoV-19, and BNT162b2) all showed a significant increase in protection from 14 days or more after the first dose to 14 days or more after the second dose. For CoronaVac, effectiveness was twice as high in the period of 14 days or more after the second dose compared with that in 14 days or more after the first (p<0·0001). Only CoronaVac showed protection (21·0%, 2·3–36·1) against symptomatic infection within 6 days of the first dose, which we used as a test of bias (appendix p 4).
Figure 3.
Effectiveness of BNT162b2, ChAdOx1 nCoV-19, CoronaVac, and Ad26.COV2.S vaccines against symptomatic COVID-19 among individuals with previous SARS-CoV-2 infection
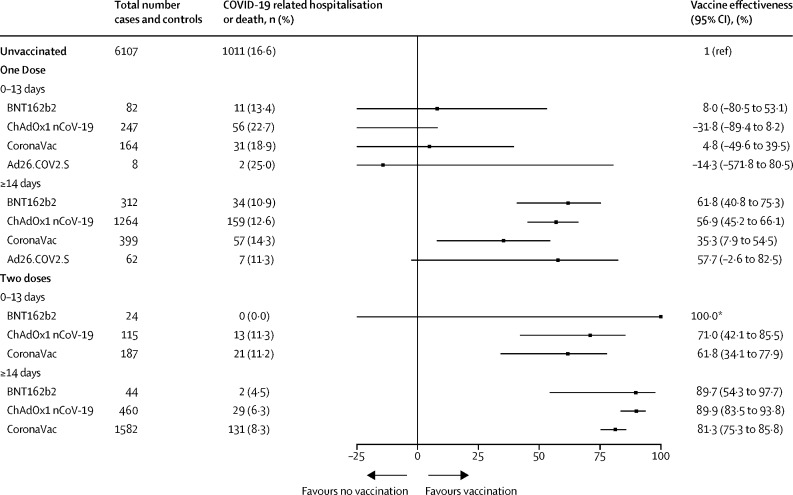
From 14 days after completion of the vaccine series, effectiveness against COVID-19-related hospitalisation or death was 81·3% (75·3–85·8) for CoronaVac, 89·9% (83·5–93·8) for ChAdOx1 nCoV-19, 57·7% (−2·6 to 82·5) for Ad26.COV2.S, and 89·7% (54·3–97·7) for BNT162b2 (figure 4 ). Effectiveness 14 days or more after a single dose was lowest for CoronaVac (35·3%, 7·9–54·5). Effectiveness against hospitalisation or death was significantly greater 14 days or more after two doses than 14 days or more after one dose for CoronaVac (p<0·0001) and ChAdOx1 nCoV-19 (p<0·0001), whereas for BNT162b2, the increase was not significant (p=0·091). We found no evidence of protection for all four vaccines against COVID-19-related hospitalisation or death within 6 days of the first dose (appendix p 4).
Figure 4.
Effectiveness of BNT162b2, ChAdOx1 nCoV-19, CoronaVac, and Ad26.COV2.S vaccines against COVID-19-associated hospitalisation or death among individuals with previous SARS-CoV-2 infection
*95% CI could not be estimated owing to zero events in this group.
For the primary estimands of vaccine effectiveness against symptomatic SARS-CoV-2 infection and against COVID-19-related hospitalisation or death 14 days or more after vaccine series completion, we found no differences between age groups (≥50 years vs 18–49 years; appendix p 5). For three of the vaccines, we saw a non-significant increase in effectiveness against symptomatic infection for vaccination given more than 180 days after previous infection compared with 91–180 days, whereas we observed a significant increase for BNT162b2 (35·3% vs 70·7%, p=0·011; appendix p 5). We found no differences in effectiveness against symptomatic infection when comparing the periods of 14–90 days and more than 90 days after vaccine series completion. For hospitalisation and death, effectiveness of ChAdOx1 nCoV-19 was greater at more than 90 days after completion compared with that at 14–90 days (95·1% vs 86·6%; p=0·007), whereas effectiveness was lower for CoronaVac at more than 90 days than at 14–90 days (74·4% vs 86·6%, p=0·012; appendix p 5).
Discussion
In this nationwide, population-based study among individuals with confirmed previous SARS-CoV-2 infection, we observed a high degree of additional protection of four vaccines against symptomatic COVID-19 and severe outcomes. For the three vaccines with two doses (CoronaVac, ChAdOx1 nCoV-19, and BNT162b2), additional protection against symptomatic infection was observed after the second dose, reaching 39% to 65%, and protection against hospitalisation or death exceeded 80% 14 days or more after the second dose. These results support vaccination, including the full vaccine series, among individuals with previous SARS-CoV-2 infection.
Public debate has occurred about whether individuals who were previously infected need to be vaccinated, due to substantial immunity conferred by SARS-CoV-2 infection.5 Additionally, in view of data showing robust immune responses after a first vaccine dose in individuals who were previously infected, some have argued that two doses are not necessary.6, 7 Indeed, several countries recommend that a single vaccine dose is sufficient for individuals who were previously infected.29, 30, 31 We found that a second dose of CoronaVac, ChAdOx1 nCoV-19, and BNT162b2 provided significant additional protection against symptomatic infections and severe disease. A recent study has shown that IgG antibodies to the receptor binding domain in individuals who recovered from COVID-19 declined to about 35% of their individual level by 9 months.32 Additionally, repeated antigen exposures were observed to increase antibody diversity, which might improve protection against emergent variants.32 Taken together, these findings might help explain the additional benefits of a second vaccine dose among individuals who were previously infected, despite robust immune responses to the first dose.33
The results of this analysis are consistent with studies reporting that individuals with previous SARS-CoV-2 infection who received ChAdOx1 nCoV-19 and BNT162b2 had a lower risk of symptomatic COVID-19 than those who were previously infected and unvaccinated.12, 13, 15, 16 Direct comparison with vaccine effectiveness estimates from these studies is challenged by differences in design, with most studies reporting risk in comparison with individuals who were unvaccinated and without a previous SARS-CoV-2 infection. However, inferred protection from those studies ranged from 40% to 94%, consistent with the magnitude of protection against symptomatic infection found for ChAdOx1 nCoV-19 (56·0%) and BNT162b2 (64·8%) in this study. Our analysis also adds new estimates on effectiveness of the CoronaVac and Ad26.COV2.S vaccines among individuals who were previously infected, finding that these vaccines provide more modest levels of protection against symptomatic infection, consistent with their lower effectiveness in naive populations.21, 34 Concerns have been raised about less robust and durable neutralising antibody responses in individuals naive to SARS-CoV-2 who have received CoronaVac compared with other vaccines.35 We found that two doses of CoronaVac provided high levels of protection against severe outcomes (81·3%, 95% CI 75·3–85·8). As CoronaVac is among the most widely used vaccines in the world, these findings have broad implications for many national programmes.19
To our knowledge, only one previous study reported vaccine effectiveness against COVID-19-related hospitalisation or death among individuals who were previously infected; with just 75 outcomes and three vaccines evaluated, the power of that study was limited for assessing vaccine and dose-specific effectiveness, but estimates ranged from 58% (BNT162b2) to 68% (mRNA-1273), with no significant protection from Ad26.COV2.S.18 We found that protection against these severe outcomes, from 14 days after the second dose, was greater than 80% for the three two-dose vaccines (CoronaVac, ChAdOx1 nCoV-19, and BNT162b2). These results are consistent with recent data showing that individuals who were previously infected have even greater increases in T-cell and B-cell responses after vaccination than those without previous infection.36 This high degree of hybrid immunity, from infections and vaccination, might explain why Brazil, despite having similar vaccination coverage as the USA and many European countries, did not have a similar increase in hospitalisations and deaths in the period in which the delta (B.1.617.2) variant become dominant.
Effectiveness against severe outcomes was lower (57·7%) for the single-dose Ad26.COV2.S vaccine than for the vaccines given in two-dose series, although the confidence limits were wide. The Ad26.COV2.S vaccine was used in a more focal rollout from June to July, 2021, and far fewer individuals received this vaccine compared with the others, such that we had modest power to characterise the effectiveness of this vaccine against severe outcomes. Brazil's Ministry of Health now recommends that individuals who received this vaccine receive a second dose after 60 days.
We focused our analyses on individuals who were previously infected to address the question of whether and to what extent vaccines confer additional protection against symptomatic infection and severe outcomes. We did not compare against individuals without a previous infection because their risk of exposure might be different, which could lead to biased estimates in this population-based study. Additionally, the misclassification of individuals who were previously infected as not having been previously infected is a substantial risk, due to incomplete surveillance and asymptomatic infections; restricting vaccine effectiveness analysis to individuals with PCR-confirmed previous infection avoids this bias. Although much discussion has occurred concerning the relative protection conferred by infection-derived and vaccine-derived immunity, from a medical and public health standpoint, the crucial question is understanding whether individuals with previous infection would benefit from vaccination. This study suggests that individuals infected before vaccination benefit from strong protection against severe outcomes with all four vaccines studied.
A major difficulty with observational studies of vaccine effectiveness is the risk of confounding, whereby differences in the vaccinated and unvaccinated populations are associated with the risk of a COVID-19 diagnosis. The matched, test-negative design has been recommended by WHO to mitigate risk of confounding introduced by care-seeking and diagnostic access; nevertheless, residual confounding might occur. We used vaccine effectiveness in the 6 days after the first dose as a bias indicator, in that differences during this period before vaccine-conferred protection is expected could indicate confounding.28 We only observed significant effectiveness in this time interval for one vaccine (CoronaVac) and one outcome (symptomatic infection); over the 7–13-day time window, no effectiveness for this vaccine was observed (appendix p 4). Whether the effectiveness observed over days 0–6 reflects bias or chance among the eight bias indicator tests (4 vaccines with 2 outcomes each) is unclear, but the absence of effects in the 7–13-day window might point away from systematic differences in recipients of CoronaVac regarding SARS-CoV-2 risk. For BNT162b2, we found modest protection in the 7–13-day window (appendix p 4). In clinical trials of BNT162b2, efficacy was apparent from approximately 11 days after the first dose.3 Given the rapid and robust immune responses after first vaccination among individuals who were previously infected, we believe these findings are consistent with early vaccine-conferred immunity.
This study has several limitations. First, we were not powered to assess vaccine effectiveness by age groups. We compared effectiveness in individuals older and younger than 50 years and did not observe major differences. The mean age of our study population was 36 years, with 75% younger than 45 years; these findings might not generalise to older populations. Second, there were differences in the timing of introduction and eligibility for each of the vaccines. This should prompt some caution in the comparison of effectiveness between vaccines, as the calendar period and median duration from second dose differed somewhat between vaccines. For example, if effectiveness wanes over time, vaccines used earlier would have lower effectiveness than those introduced later. Additionally, changes in variant distribution during the study period could alter effectiveness by time since vaccination. We did not have individual-level data on variants, which precluded assessment of variant-specific vaccine effectiveness. Different types and collection methods for RT-PCR tests are used throughout the country, which might have varying accuracy, and specific information about these characteristics are not recorded in the national databases. We used a matched, test-negative design with multivariable regression to reduce non-vaccine-related differences between cases and controls; however, unmeasured differences could exist that lead to confounding.37 In particular, there were differences in the allocation of specific vaccines that might have been associated with unmeasured risk of COVID-19 or severe outcomes, which should prompt caution in the comparison of vaccine effectiveness between vaccines. This study included individuals who presented to health facilities and underwent diagnostic testing who might differ from individuals who did not seek medical care and might not be generalisable to that population. Finally, our study was unable to address the important question of when vaccines should be given to individuals with previous SARS-CoV-2 infection. To avoid misclassification of reinfections, we only considered tests done at least 90 days after the initial infection.
The accelerated development of effective vaccines against COVID-19 has been a remarkable scientific achievement but, as of March 11, 2022, 37·4% of the world's population has yet to receive a first dose, and a substantial proportion of these individuals have already been infected with SARS-CoV-2.1 The results of this study provide evidence for the benefits of vaccination among individuals who have already been infected with SARS-CoV-2, with all four studied vaccines conferring substantial reductions in hospitalisation and death due to COVID-19. Ensuring vaccine access to individuals with previous infection might be particularly important amid reports of the omicron (B.1.1.529) variant, which suggest that immunity conferred by previous infection is reduced.9, 10, 38 The expanded, equitable rollout of vaccines for all individuals remains crucial for mitigating the continued threat posed by SARS-CoV-2.
Data sharing
One of the study coordinators (MB-N) signed a term of responsibility on using each database made available by the Brazilian Ministry of Health. Each member of the research team signed a term of confidentiality before accessing the data. Data were manipulated in a secure computing environment, ensuring protection against data leakage. The Brazilian National Commission in Research Ethics approved the research protocol (CONEP approval number 4.921.308). Our agreement with the Ministry of Health for accessing the databases patently denies authorisation of access to a third party. Any request for assessing the databases must be addressed to the Brazilian Ministry of Health. We used anonymised secondary data following the Brazilian Personal Data Protection General Law, but they are vulnerable to re-identification by third parties as they contain dates of relevant health events regarding the same person. To protect the research participants' privacy, the approved research protocol authorises only the dissemination of aggregated data, such as the ones presented here.
Declaration of interests
MB-N reports grants from the Fazer o Bem Faz Bem programme from JBS. AIK reports grants from Bristol Myers Squibb, Regeneron, and Serimmune; and grants and personal fees from Tata Medical Devices, outside the submitted work. VdAO, VSB, MLB, JC, and MB-N are employees of Fiocruz, a federal public institution, which manufactures Vaxzevria (ChAdOx1 nCoV-19 vaccine) in Brazil through a full technology transfer agreement with AstraZeneca. Fiocruz allocates all its manufactured products to the Ministry of Health for public health use. All other authors declare no competing interests.
Acknowledgments
Acknowledgments
This study was partly supported by a donation from the Fazer o Bem Faz Bem programme from JBS. GLW, MLB, JC, and MB-N are research fellows from the Brazilian National Research Council. GLW acknowledges the Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (E-26/210.180/2020). JC is supported by the Oswaldo Cruz Foundation (Edital COVID-19—resposta rápida 48111668950485). OTR is funded by a Sara Borrell fellowship (CD19/00110) from the Instituto de Salud Carlos III. OTR acknowledges support from the Spanish Ministry of Science and Innovation through the Centro de Excelencia Severo Ochoa 2019–2023 programme and from the Generalitat de Catalunya through the Centres de Recerca de Catalunya programme.
Acknowledgments
Contributors
JRA, JC, and MB-N conceived the idea for the study. All authors contributed to the study design. TC-S, JRA, and OTR developed the statistical analysis plan and wrote the code for statistical analyses. TC-S, VdAO, JBJ, and MB-N had access to the raw data, and TC-S and MB-N verified the underlying data. TC-S, MB-N, VdAO, and MLB organised the data linkage. All authors contributed to interpretation of the study findings. JRA and TC-S drafted the manuscript. All authors critically revised the manuscript and approved the final version for submission.
Supplementary Material
References
- 1.WHO WHO coronavirus (COVID-19) dashboard. 2021. https://covid19.who.int/
- 2.Chen X, Chen Z, Azman AS, et al. Serological evidence of human infection with SARS-CoV-2: a systematic review and meta-analysis. Lancet Glob Health. 2021;9:e598–e609. doi: 10.1016/S2214-109X(21)00026-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thomas SJ, Moreira ED, Jr, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine through 6 months. N Engl J Med. 2021;385:1761–1773. doi: 10.1056/NEJMoa2110345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Voysey M, Clemens SAC, Madhi SA, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Block J. Vaccinating people who have had COVID-19: why doesn't natural immunity count in the US? BMJ. 2021;374 doi: 10.1136/bmj.n2101. [DOI] [PubMed] [Google Scholar]
- 6.Dolgin E. Is one vaccine dose enough if you've had COVID? What the science says. Nature. 2021;595:161–162. doi: 10.1038/d41586-021-01609-4. [DOI] [PubMed] [Google Scholar]
- 7.Frieman M, Harris AD, Herati RS, et al. SARS-CoV-2 vaccines for all but a single dose for COVID-19 survivors. EBioMedicine. 2021;68 doi: 10.1016/j.ebiom.2021.103401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cele S, Jackson L, Khoury DS, et al. Omicron extensively but incompletely escapes Pfizer BNT162b2 neutralization. Nature. 2021 doi: 10.1038/s41586-021-04387-1. published online Dec 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pulliam JRC, van Schalkwyk C, Govender N, et al. Increased risk of SARS-CoV-2 reinfection associated with emergence of the omicron variant in South Africa. medRxiv. 2021 doi: 10.1101/2021.11.11.21266068. published online Dec 2. (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dan JM, Mateus J, Kato Y, et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science. 2021;371 doi: 10.1126/science.abf4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abu-Raddad LJ, Chemaitelly H, Bertollini R. Severity of SARS-CoV-2 reinfections as compared with primary infections. N Engl J Med. 2021;385:2487–2489. doi: 10.1056/NEJMc2108120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cavanaugh AM, Spicer KB, Thoroughman D, Glick C, Winter K. Reduced risk of reinfection with SARS-CoV-2 after COVID-19 vaccination—Kentucky, May–June 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1081–1083. doi: 10.15585/mmwr.mm7032e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gazit S, Shlezinger R, Perez G, et al. Comparing SARS-CoV-2 natural immunity to vaccine-induced immunity: reinfections versus breakthrough infections. medRxiv. 2021 doi: 10.1101/2021.08.24.21262415. published online Aug 25. (preprint). [DOI] [Google Scholar]
- 14.Abu-Raddad LJ, Chemaitelly H, Ayoub HH, et al. Association of prior SARS-CoV-2 infection with risk of breakthrough infection following mRNA vaccination in Qatar. JAMA. 2021;326:1930–1939. doi: 10.1001/jama.2021.19623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hall V, Foulkes S, Insalata F, et al. Effectiveness and durability of protection against future SARS-CoV-2 infection conferred by COVID-19 vaccination and previous infection; findings from the UK SIREN prospective cohort study of healthcare workers March 2020 to September 2021. medRxiv. 2021 http://www.medrxiv.org/content/10.1101/2021.11.29.21267006v1 published online Dec 1. (preprint). [Google Scholar]
- 16.Pouwels KB, Pritchard E, Matthews PC, et al. Effect of delta variant on viral burden and vaccine effectiveness against new SARS-CoV-2 infections in the UK. Nat Med. 2021;27:2127–2135. doi: 10.1038/s41591-021-01548-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Monge S, Olmedo C, Alejos B, Lapeña MF, Sierra MJ, Limia A. Direct and indirect effectiveness of mRNA vaccination against severe acute respiratory syndrome coronavirus 2 in long-term care facilities, Spain. Emerg Infect Dis. 2021;27:2595–2603. doi: 10.3201/eid2710.211184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levin-Rector A, Firestein L, McGibbon E, et al. Reduced odds of SARS-CoV-2 reinfection after vaccination among New York City adults, June–August 2021. medRxiv. 2021 doi: 10.1093/cid/ciac380. http://www.medrxiv.org/content/10.1101/2021.12.09.21267203v1 published online Dec 11. (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mallapaty S. China's COVID vaccines have been crucial—now immunity is waning. Nature. 2021;598:398–399. doi: 10.1038/d41586-021-02796-w. [DOI] [PubMed] [Google Scholar]
- 20.WHO Enhancing response to omicron SARS-CoV-2 variant: technical brief and priority actions for member states. 2022. https://www.who.int/docs/default-source/coronaviruse/2022-01-07-global-technical-brief-and-priority-action-on-omicron-corr2.pdf?sfvrsn=918b09d_23&download=true
- 21.Ranzani OT, Hitchings MDT, Dorion M, et al. Effectiveness of the CoronaVac vaccine in older adults during a gamma variant associated epidemic of COVID-19 in Brazil: test negative case-control study. BMJ. 2021;374 doi: 10.1136/bmj.n2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ranzani OT, Bastos LSL, Gelli JGM, et al. Characterisation of the first 250 000 hospital admissions for COVID-19 in Brazil: a retrospective analysis of nationwide data. Lancet Respir Med. 2021;9:407–418. doi: 10.1016/S2213-2600(20)30560-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oliveira EA, Colosimo EA, Simões E Silva AC, et al. Clinical characteristics and risk factors for death among hospitalised children and adolescents with COVID-19 in Brazil: an analysis of a nationwide database. Lancet Child Adolesc Health. 2021;5:559–568. doi: 10.1016/S2352-4642(21)00134-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cerqueira-Silva T, Oliveira VA, Boaventura VS, et al. Influence of age on the effectiveness and duration of protection of Vaxzevria and CoronaVac vaccines: a population-based study. Lancet Reg Health Am. 2022;6 doi: 10.1016/j.lana.2021.100154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Katikireddi SV, Cerqueira-Silva T, Vasileiou E, et al. Two-dose ChAdOx1 nCoV-19 vaccine protection against COVID-19 hospital admissions and deaths over time: a retrospective, population-based cohort study in Scotland and Brazil. Lancet. 2022;399:25–35. doi: 10.1016/S0140-6736(21)02754-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Flury BK, Riedwyl H. Standard distance in univariate and multivariate analysis. Am Stat. 1986;40:249–251. [Google Scholar]
- 27.Austin PC. Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research. Commun Stat Simul Comput. 2009;38:1228–1234. [Google Scholar]
- 28.Hitchings MDT, Lewnard JA, Dean NE, et al. Use of recently vaccinated individuals to detect bias in test-negative case-control studies of COVID-19 vaccine effectiveness. medRxiv. 2021 doi: 10.1101/2021.06.23.21259415. published online July 2. (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Federal Office of Public Health Switzerland. Coronavirus: vaccination. 2021. https://www.bag.admin.ch/bag/en/home/krankheiten/ausbrueche-epidemien-pandemien/aktuelle-ausbrueche-epidemien/novel-cov/impfen.html#-995735508
- 30.German Federal Ministry of Health Proof of vaccination as defined in the COVID-19 protective measures exemption directive and the directive on coronavirus entry regulations. 2021. https://www.pei.de/EN/newsroom/dossier/coronavirus/coronavirus-content.html?cms_pos=3
- 31.Haute Autorité de Santé Stratégie de vaccination contre le SARS-CoV-2—vaccination des personnes ayant un antécédent de COVID-19. 2021. https://www.has-sante.fr/jcms/p_3237271/en/strategie-de-vaccination-contre-le-sars-cov-2-vaccination-des-personnes-ayant-un-antecedent-de-covid-19
- 32.Li C, Yu D, Wu X, et al. Twelve-month specific IgG response to SARS-CoV-2 receptor-binding domain among COVID-19 convalescent plasma donors in Wuhan. Nat Commun. 2021;12 doi: 10.1038/s41467-021-24230-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Muecksch F, Weisblum Y, Barnes CO, et al. Affinity maturation of SARS-CoV-2 neutralizing antibodies confers potency, breadth, and resilience to viral escape mutations. Immunity. 2021;54:1853. doi: 10.1016/j.immuni.2021.07.008. 68.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zheng C, Shao W, Chen X, Zhang B, Wang G, Zhang W. Real-world effectiveness of COVID-19 vaccines: a literature review and meta-analysis. Int J Infect Dis. 2022;114:252–260. doi: 10.1016/j.ijid.2021.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lim WW, Mak L, Leung GM, Cowling BJ, Peiris M. Comparative immunogenicity of mRNA and inactivated vaccines against COVID-19. Lancet Microbe. 2021;2:e423. doi: 10.1016/S2666-5247(21)00177-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Angyal A, Longet S, Moore SC, et al. T-cell and antibody responses to first BNT162b2 vaccine dose in previously infected and SARS-CoV-2-naive UK health-care workers: a multicentre prospective cohort study. Lancet Microbe. 2021;3:e21–e31. doi: 10.1016/S2666-5247(21)00275-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lewnard JA, Patel MM, Jewell NP, et al. Theoretical framework for retrospective studies of the effectiveness of SARS-CoV-2 vaccines. Epidemiology. 2021;32:508–517. doi: 10.1097/EDE.0000000000001366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Andrews N, Stowe J, Kirsebom F, et al. COVID-19 vaccine effectiveness against the omicron (B.1.1.529) variant. N Engl J Med. 2022 doi: 10.1056/NEJMoa2119451. published online March 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
One of the study coordinators (MB-N) signed a term of responsibility on using each database made available by the Brazilian Ministry of Health. Each member of the research team signed a term of confidentiality before accessing the data. Data were manipulated in a secure computing environment, ensuring protection against data leakage. The Brazilian National Commission in Research Ethics approved the research protocol (CONEP approval number 4.921.308). Our agreement with the Ministry of Health for accessing the databases patently denies authorisation of access to a third party. Any request for assessing the databases must be addressed to the Brazilian Ministry of Health. We used anonymised secondary data following the Brazilian Personal Data Protection General Law, but they are vulnerable to re-identification by third parties as they contain dates of relevant health events regarding the same person. To protect the research participants' privacy, the approved research protocol authorises only the dissemination of aggregated data, such as the ones presented here.