Abstract
Aspergillus flavus and Aspergillus fumigatus were derived and identified from the ducks infected with fungi. In order to investigate the effectiveness of Chinese herbal medicines against Aspergillus flavus and Aspergillus fumigatus, in vitro antibacterial test and animal infection control test were conducted to study the antibacterial activity of the Chinese medicine mixture which was compatible with Acorus gramineus, Phellodendron chinensis, and Cassia obtusifolia. According to the results, the liver of chickens infected with Aspergillus flavus and Aspergillus fumigatus displayed granulomatous lesions, indicating that the isolation of pathogen from the lungs of sick ducks is also pathogenic to chickens. As suggested by the results of in vitro drug sensitivity test, the mixture 1 MIC80 was the minimum, the MIC80 of Aspergillus flavus was 16 μg/μL, and the MIC80 of Aspergillus fumigatus was 4 μg/μL. In a petri dish of the same concentration, the colony diameter of Aspergillus flavus and Aspergillus fumigatus in Mixture 1 was the minimum. Besides, Aspergillus flavus colonies grew when the concentration was 64 μg/μL, and Aspergillus fumigatus colonies grew when the concentration was 4 μg/μL, which suggests the more significant inhibitory effect of Mixture 1 on Aspergillus flavus and Aspergillus fumigatus. According to the results of animal experiments, there was a significantly lower activity level of Glutamic oxaloacetic transaminase (GOT) and Glutamate pyruvic transaminase (GPT) in the protection group and the treatment group than in the bacterial infection group. As indicated by the blood smear results, there were more neutrophils in the infected group than in the prevention group and the treatment group. Thus, it can be seen from that the Mixture 1 produced preventive and therapeutic effects on the chickens infected with Aspergillus flavus and Aspergillus fumigatus.
Key words: Aspergillus fumigatus, Aspergillus flavus, Chinese herbal medicine, MIC80, colony diameter, GOT, GPT
INTRODUCTION
Poultry production can be affected by a variety of fungal diseases, including aspergillosis, candidiasis, finger mycosis, flavor, mucormycosis, histoplasmosis, and cryptococcosis. Among them, aspergillosis and candidiasis present the most significant risk. Aspergillosis is known as a fungal infection mainly caused by Aspergillus fumigatus and Aspergillus flavus, which is an airborne saprophytic fungus. Currently, there are an increasing number of cases of poultry infected with mold. The most significant contributors to fungal diseases in poultry include the environment and the feed. For aspergillus flavus, the main target is animal liver, which can turn yellow, resembling tofu. The liver necrosis of ducks and geese is particularly serious. Aspergillus fumigatus mainly affects animal air sacs and then the lungs, thus causing chest tightness, coughing and other symptoms. At this stage, there are about two thiords of the layer farms with varying degrees of mold infection, more than half of broiler farms with mold infections, and more than one third of waterfowl farms with mold infections. According to the statistics sourced from the Food and Agriculture Organization of the United Nations, there is an average of 2% of the world's food rendered inedible by mildew each year, with billions of export commodities contaminated with mycotoxins, thus causing the loss of billions of dollars for agricultural and industrial production (Takahashi et al., 1982). The long-term use of animal feed containing mold is detrimental to the liver, kidneys and other organs, which affects the immune system and reproductive system, thus causing cancer and deformity. At this stage, ketoconazole, itraconazole, and fluconazole are most commonly used to treat the systemic infections of poultry aspergillosis. Despite inhibiting fungal growth and control mycotoxin synthesis, the excessive use of antibacterial agents can cause serious side effects, thus threatening the health of both humans and animals. In recent years, there has been continued emergence of drug-resistant strains and super strains due to the abuse of antibacterial medicines. In order to ensure the safety of animal-derived food and protect human health, the Ministry of Agriculture and Rural Affairs has imposed a ban on the use of high-residue antifungal, antiviral, and β-stimulant drugs in animal-derived food. Aside from the side effects of antifungal drugs, the high cost has also hindered the use of antifungal drugs to a significant extent, which makes it imperative to develop the natural and economical Chinese herbal medicines which are effective against fungal infections. Experimental studies have demonstrated that the Chinese herbal medicines including Acorus gramineus, Phellodendron chinensis, and Cassia obtusifolia have inhibitory effects on fungi, and that the compatibility of Chinese herbal medicine can be effective in enhancing the efficacy, expanding the scope of treatment, and reducing drug toxicity (Shuang et al., 2021). Therefore, the experiment was purposed to explore the inhibitory effects of four Chinese herbal mixtures composed of Acorus gramineus, Phellodendron chinensis, and Cassia obtusifolia on Aspergillus flavus and Aspergillus fumigatus, thus contributing new ideas to the development of anti-aspergillus drugs.
MATERIALS AND METHODS
This study was approved by the Experimental Animal Ethics Committee of Hebei Agricultural University.
Extraction of Chinese Herbal Medicine
Acorus gramineus, Phellodendron chinensis, and Cassia obtusifolia were provided by Anguo Medicinal Materials Wholesale Market in Baoding City, Hebei Province. At the ratio of 1:1, Acorus gramineus and Phellodendron chinensis were prepared into Chinese medicine mixture 1, Acorus gramineus and Cassia obtusifolia were prepared into Chinese medicine mixture 2, Phellodendron chinensis and Cassia obtusifolia were prepared into Chinese medicine mixture 3, while Acorus gramineus, Phellodendron chinensis, and Cassia obtusifolia were prepared into Chinese medicine mixture 4.
First, the massive Chinese medicinal materials were crushed to a weight of 100 g. Second, it was soaked in a 60% ethanol solution at a ratio of 1:10 and placed at ambient temperature for 24 h. Third, it was arranged in an ultrasonic extractor for ultrasonic extraction to be performed 3 times at 45°C for 15 min. Fourth, the residue was placed into a vacuum filter bottle before being sucked and filtered twice. Finally, the supernatant was poured into 80°C rotary evaporator, with the concentration up to 2 g/mL, and then placed at 4°C for use.
Animal Regression Test
The research on live animals complies with the guidelines approved by the institutional animal care and use committee. A total of 20 healthy 10-day-old Hy-Line gray layers were used for animal regression test, with a mixture of Aspergillus flavus and Aspergillus fumigatus applied to the nasal cavity of laying hens for 7 d for subsequent dissection. By observing whether the organs are diseased, the pathological sections of the diseased parts were made for histological observation.
Determination of MIC80
The MIC80 of Chinese herbal medicines were determined with the M27-E4 (Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yegots: approved standard – Fourth Edition) standard compiled by the Clinical and Laboratory Standards Institute. A 96-well plate microdilution method was adopted to determine the value of MIC80. In addition, the concentration of Aspergillus fumigatus and Aspergillus flavus was reduced to 102 CFU/mL for later use. With 2 g/mL Chinese medicine diluted in 8 concentrations, that is, 1024 μg/μL, 512 μg/μL, 256 μg/μL, 128 μg/μL, 64 μg/μL, 32 μg/μL, 16 μg/μL, 8 μg/μL, there were 3 replicates produced for the respective concentration. Furthermore, 100 μL Chinese medicinal liquid and 100-μL bacterial liquid were added to the traditional Chinese herbal medicine tested wells, whereas Chinese herbal medicine liquid was not added to the positive control and negative control wells. Besides, 100-μL SDA liquid medium and 100-μL bacterial liquid were added to the positive control well, and 200-μL SDA liquid medium was added to the negative control. The produce was placed into a spectrophotometer to measure its OD630 value. Then, its OD630 value was measured after 48 h of incubation.
Drug Sensitivity Test of Each Herbal Medicine
The Chinese herbal medicines of different concentrations were mixed with SDA medium, while the diameter and number of colony growth were recorded to determine the antibacterial effect of Chinese herbal medicine on Aspergillus flavus and Aspergillus fumigatus. Before being poured into a petri dish to cool, the Chinese herbal medicine solution was diluted at a concentration of 2 g/mL with SDA medium into a Chinese herbal medicine mixture at 256 μg/μL, 128 μg/Μl, and 64 μg/μl. The bacterial solution was diluted to 101 CFU/mL with normal saline, and 100 μL of the bacterial solution was drawn into the Chinese herbal medicine mixed medium. Then, the colony growth status was observed at 24 h, 48 h, 72 h and 96 h, respective, with the number and diameter of the colony recorded.
Animal Infection and Treatment Test
The laying hens were divided into 5 groups. To be specific, the first one was a blank control group, the second one was a protection group fed with Chinese herbal medicine before the infection test, the third one was a test group only with infection, the fourth one was fed with Chinese herbal medicine after the infection, and the last one was the treatment group fed with the western medicine fluconazole after the infection. Except for the first group comprised of 6 chicks, there were 11 chicks in each of other groups. Prior to the infection test, the third group of laying hens were fed with Chinese medicine mixture 1 according to the daily feed amount × 1.5%, while those in the other groups were fed as normal. After 7 d, all chicks except those in the first group were tested for infection by wiping the fungal liquid in the nasal cavity with cotton swabs. The infection persisted for 7 d until the chicks became symptomatic. Finally, the fourth group of chicks was fed with Chinese medicine mixture 1 according to the daily feeding amount × 1.5%, the fifth group of chicks was fed fluconazole, and the other groups were fed as normal. After 7 d, all laying hens were dissected to observe the diseased organs. Then, serum and liver tissue were collected, while GOT and GPT were detected according to the kit instructions. Finally, blood smears were prepared.
Data Analysis
All of the experiments were repeated at least three times, with the results expressed as means ± SE. Statistical analyses were conducted using the SPSS soft-ware package V11.5 (SPSS Inc., Chicago, IL). All data were processed through one-way analysis of variance (ANOVA) to determine the differences between these groups. In this study, P < 0.01 is treated as extremely significant and P < 0.05 is treated as significantly different.
RESULTS AND DISCUSSION
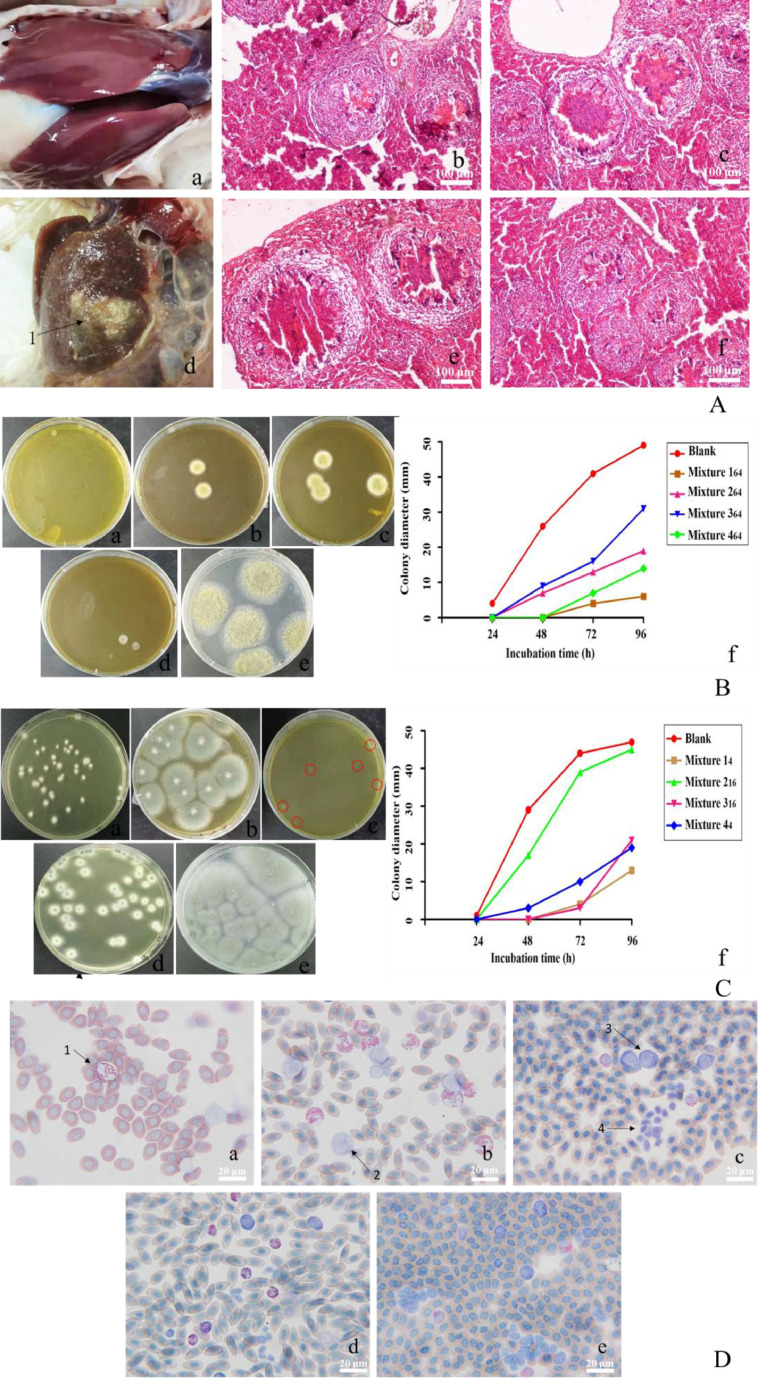
Aspergillus infection is still posing a major threat to the health of both animals and humans. The inhalation of Aspergillus spores can lead to colonization, allergic manifestations, or invasive infections, the severity of which is determined by such factors as the immunity of the host, long-term neutropenia, allogeneic hematopoietic stem cell transplantation, solid organ transplantation, genetic or acquired (Patterson et al., 2016). Aspergillus flavus and Aspergillus fumigatus have different pathogenicity to animals, poultry are very sensitive. After infection, the chicks exhibited such symptoms as low spirits, the loss of appetite, light green and loose stools, and the instability to stand. According to histological observation, there was evident granulomatous lesions in the liver of the chicks (Figure 1A). Studies have revealed that granulomas resulted from the macrophage infiltration caused by various pathogen infections, which are the typical symptoms of fungal infections (María et al., 2003). Aspergillus flavus and Aspergillus fumigatus can take different forms in different media. Aspergillus flavus grows fastest in SDA medium. The hyphae are long and thin. The culture starts to turn yellow and then yellow-green. It is dark green, resembling fluffy filaments. Therefore, SDA medium was adopted for in vitro drug sensitivity test.
Figure 1.
Animal regression test, in vitro drug susceptibility test of Aspergillus flavus and Aspergillus fumigatus, as well as animal infection control test. (A) Normal liver; a, Chicks infected with pathogenic bacteria; b, c, e, f, Pathological observation of diseased liver; d, Liver disease in chicks; 1, Lesion; (B) the colony growth status and diameter of Aspergillus flavus in a medium with a concentration of 64 μg/μL for 72; a, Mixture 1; b, Mixture 2; c, Mixture 3; d, Mixture 4; e, Blank; f, Colony diameter chart; (C) the colony growth status and diameter of Aspergillus fumigatus in a medium; a, Mixture 1 petri dish with a concentration of 4 μg/μL; b, Mixture 2 petri dish with a concentration of 16 μg/μL; c, Mixture 3 petri dish with a concentration of 16 μg/μL; d, Mixture 4 petri dish with a concentration of 4 μg/μL; e, Blank; f, Colony diameter chart; (D) results of blood smear test on animal contamination; a, 1, Neutrophils; b, 2, Monocyte; c, 3, Monocyte. 4, Thrombocytes.
As suggested by the in vitro drug susceptibility test, Mixture 1 produced a significant inhibitory effect on Aspergillus flavus and Aspergillus fumigatus. The OD630 value of the 4 Chinese herbal medicine mixtures diminished gradually with an increase in the concentration of Chinese herbal medicine. Mixture 1 inhibited 80% of the colonies of Aspergillus flavus and Aspergillus fumigatus at a lower concentration of 4 μg/μL, and the colony diameter of mixture 1 was the smallest in the petri dish of the same concentration during the medium drug sensitivity test. By comparing the MIC80 of Aspergillus flavus and Aspergillus fumigatus and the colony growth diameter in the culture medium of different concentrations, it was discovered that the MIC80 of Aspergillus fumigatus mixture 1 was smaller than that of Aspergillus flavus, while the concentration of inhibiting the growth of Aspergillus fumigatus colony in the culture medium was lower than that of inhibiting the growth of Aspergillus flavus. As by indicated by the concentration, the inhibitory effect of mixture 1 on Aspergillus fumigatus was higher than that of Aspergillus flavus (Table 1). It has been demonstrated in some studies that Acorus gramineus and Phellodendron chinensis contain volatile oil active ingredients, and that their α-asarone and acateroneare-c synergistically antifungal can exert a significant inhibitory effect on Candida albicans (Wang et al., 2020). It is thus suspected that these 2 chemical components can produce an antibacterial effect on Aspergillus flavus and Aspergillus fumigatus.
Table 1.
MIC80 determination results of Aspergillus flavus and Aspergillus fumigatus (n = 3).
| Mixture 1 | Mixture 2 | Mixture 3 | Mixture 4 | |
|---|---|---|---|---|
| MIC80Aspergillus fumigatus (μg/μL) | 4 | 128 | 16 | 16 |
| MIC80 Aspergillus flavus (μg/μL) | 16 | 64 | 64 | 32 |
Note: MIC80 means comparing with the negative control, the lowest drug concentration with an OD630 value declining more than 80%.
Abbreviation: MIC, minimum inhibitory concentration.
Animal experiments were performed to further explore the preventive and therapeutic effects of Mixture 1 on the chickens infected with Aspergillus flavus and Aspergillus fumigatus. Aspergillus flavus and Aspergillus fumigatus can produce mycotoxins in large amounts, which is detrimental to animals and poultry. It is known that the percentage of hepatocellular carcinoma in the world with a potential link to aflatoxin in the diet ranges from 5 to 28%. The active content of GOT and GPT is considered as an important biochemical indicator used for the detection of liver tissue damage. As suggested by the test results, the active content of GOT and GPT in the liver tissue and serum of the chickens was significantly higher for group 3 than for the other groups, with the mortality rate in group 3 reaching the maximum. In contrast, the level of GOT and GPT activity and mortality of chickens was the lowest for groups 2 and 4 (Table 2). It can be seen that Aspergillus flavus and Aspergillus fumigatus caused liver damage to chickens, while Mixture 1 produced a preventive and therapeutic effect on liver damage.
Table 2.
GOT and GPT activity content and white blood cell count results of chicks in each group.
| Group | Liver tissue GOT (U/L) | Serum GOT (U/L) | Liver tissue GPT (U/L) | Serum GPT (U/L) | White blood cell count |
|---|---|---|---|---|---|
| 1 | 66.33 ± 5.41a | 30.81 ± 2.56a | 8.06 ± 1.89a | 7.66 ± 0.62a | 7.32 ± 0.29a |
| 2 | 134.05 ± 30.57b | 49.98 ± 2.97b | 23.74 ± 0.89c | 17.74 ± 2.36b | 17.6 ± 5.14b |
| 3 | 94.08 ± 7.81a | 37.09 ± 4.75a | 18.81 ± 3.09b | 13.99 ± 4.83b | 9.38 ± 0.84ab |
| 4 | 91.98 ± 2.14a | 37.56 ± 7.38a | 20.85 ± 2.44bc | 14.23 ± 2.30b | 12.6 ± 1.91ab |
| 5 | 89.63 ± 6.86a | 36.67 ± 1.37a | 19.80 ± 0.76bc | 15.66 ± 1.99b | 13.9 ± 1.27ab |
Abbreviations: GOT, glutamic oxaloacetic transaminase; GPT, glutamate pyruvic transaminase.
Different lowercase letters in the same column of data indicate significant differences (P < 0.05), and the same lowercase letters indicate insignificant differences (P > 0.05).
Playing an important role in immunity for the body, white blood cells can swallow foreign bodies, which is significant to resisting the invasion of pathogenic bacteria and maintaining immunity to diseases. As one of the important components of the body's nonspecific immunity, neutrophils can not only eliminate pathogenic microorganisms, but also aggravate the damage caused to ischemia-reperfusion tissues or organs (Alex et al., 11AD). According to the results of white blood cell count, the number of white blood cells in the chicks from the infection group was the highest, with no significant difference shown between the Chinese medicine protection group and the control group (Table 2). After the chickens were infected in the test, neutrophils exhibited an increasing trend in the blood smear, with the immune system of the chickens stimulated by Aspergillus flavus and Aspergillus fumigatus.
In some studies, it has been suggested that Chinese herbal medicine is effective in promoting the development of the immune organs of poultry, enhancing the phagocytic function of macrophages, regulating specific immune responses, and improving mucosal immune function. Therefore, it is necessary to study the mechanism of action in depth. To sum up, this research provides an experimental basis for the development of natural residue-free prevention and treatment of Aspergillus flavus and Aspergillus fumigatus.
ACKNOWLEDGMENTS
This project was supported by Fund for Hebei Province Agricultural Quality Development Key Generic Technology, Targeted Project: 21326617D
DISCLOSURES
The authors declare that there were no conflicts of interest regarding the publication of this manuscript.
REFERENCES
- Alex H., Allison S., Samantha K., Michael S.A., Christa S.Z., Mary C.D., Michael K.M., Daniel I., Correction Publisher. Neutrophil swarming delays the growth of clusters of pathogenic fungi. Nat. Commun. 2020;11:2492. doi: 10.1038/s41467-020-16446-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- María T.D., Jesús D.P., María T.Y., María G.C., María J.P., María A.C., Josep G. Cutaneous infection caused by Ulocladium chartarum in a heart transplant recipient: case report and review. Acta Dermato-Venereol. 2003;83:218–221. doi: 10.1080/00015550310007256. [DOI] [PubMed] [Google Scholar]
- Patterson T.F., Thompson G.R., Denning D.W., Fishman J.A., Hadley S., Herbrecht R., Kontoyiannis D.P., Marr K.A., Morrison V.A., Nguyen M.H., Segal B.H., Steinbach W.J., Stevens D.A., Walsh T.J., Wingard J.R., Young J.H., Bennett J.E. Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2016;63:e1–e60. doi: 10.1093/cid/ciw326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuang Z., Hui Z.Q., Hui X.W., Rong L.Y., Yu G., Jun W.X., Ying H.S., Yong L., Yao L.C. The isolation and identification of Candida glabrata from avian species and a study of the antibacterial activities of Chinese herbal medicine in-vitro. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H., Yasaki H., Manabe M., Matsuura S. Scanning electron microscopic observation of surface and surface layer of fungus damaged brown rice grains. JSM Mycotoxins. 1982;16:18–19. 1982. [Google Scholar]
- Wang Z.W., Zhu Y.Y., Yi X., Zhou Z.S., He Y.J., Zhou Y., Qi Z.H., Jin D.N., Zhao L.X., Luo X.D. Bioguided isolation, identification and activity evaluation of antifungal compounds from Acorus tatarinowii Schott. J. Ethnopharmacol. 2020;261 doi: 10.1016/j.jep.2020.113119. [DOI] [PubMed] [Google Scholar]