Abstract
Duck circovirus (DuCV) infection occurs frequently in ducks in China and is generally believed to lead to immunosuppression and secondary infection, though there has been a lack of detailed research and direct evidence. In this study, one-day-old Cherry Valley ducklings were artificially infected with DuCV alone and co-infected with DuCV and Avian Pathogenic Escherichia coli (APEC). The immune indexes at 32 d old were systematically monitored, including immune organ weight, lymphocyte transformation rate, IL-10, IL-12, soluble CD4 (sCD4), soluble CD8 (sCD8), IFN-γ, viral loads in each organ, APEC colonization, and so on. The results showed the development of immune organs in ducklings was affected, resulting in a decrease in the lymphocyte transformation rate (LTR), IL-12, sCD4, sCD8, IFN-γ and an increase in IL-10 content at 8 to 32 d postinfection (dpi). In the detection of virus loads in some organs, it was found that 8 dpi, DuCV existed stably in various organs, suggesting the importance of preventing and controlling the virus in the early stage of culture. The results of exploring the DuCV infection that shows some influence on secondary infection by APEC. The results showed that DuCV infection could significantly enhance the pathogenicity of APEC and the colonization ability of APEC in vivo. DuCV can induce more serious APEC infection in 24 dpi than in 14 dpi. Based on the above results, it can be concluded that DuCV infection will affect the immune system, cause immunosuppression, and lead to more serious secondary infection.
Key words: duck circovirus, Avian pathogenic Escherichia Coli, immunosuppression, secondary infection
Introduction
Duck industry has produced five billion ducks in China, accounting for more than 80% of the worldwide quantity, and duck has become the third-largest meat food in China after pork and chicken. However, the current situation of duck breeding is extremely worrying; the breeding model is relatively simple and extensive, and epidemic prevention is extremely difficult. From the analysis of the surveillance results of duck virus, duck circovirus (DuCV) has the highest infection rate among all virus diseases, and we found an obvious rule that if there is DuCV infection, the degree of bacterial infection is often very serious. Although a few routine artificial infection tests have been carried out in the past decade, and some evidence of DuCV infection in vivo and multiple organ infection and damage have been obtained (Yang et al., 2020; Zhu et al., 2019), due to the lack of a suitable culture system in vitro, there are few studies on the infection and pathogenic mechanism of DuCV. In addition, DuCV often shows chronic or subclinical infection in the clinic, and there are no corresponding drugs and vaccines in the clinic (Huang et al., 2018; Li et al., 2020). These problems lead to the lack of awareness of the harm of DuCV throughout the industry, so that prevention and control of DuCV are seriously ignored.
DuCV belongs to circoviridae, circovirus genus, no enveloped. The DNA structure is a single-stranded circular structure, the diameter is small, and there is no significant difference between circovirus and other circoviruses under an electron microscope (Stewart et al., 2006). DuCV was first found on a German farm by Hattermann in 2003 (Hattermann et al., 2003). For more than a decade, the infection rate of DuCV has not decreased, its harm has become more prominent (Cha et al., 2014; Wozniakowski et al., 2014). At first, people thought that DuCV was similar to other species of circovirus, which could infect the corresponding immune organs, resulting in low immunity and immunosuppression, thus, increasing the probability of infection of other pathogenic microorganisms to the body and causing secondary infection or aggravating the clinical manifestations of other diseases (Fringuelli et al., 2005; Liu et al., 2006). According to data obtained from the artificial infection of DuCV in Beijing ducks, the pathogenic characteristics of DuCV include systemic infection (especially immune organs), persistent infection, and horizontal transmission, which makes DuCV more likely to circulate infection and increase susceptibility to other pathogens (Hong et al., 2018). There are a few studies on the infection and pathogenicity of DuCV but there are no studies to systematically evaluate the effect of DuCV infection on the immune system.
At present, bacterial secondary infection is an enormous problem facing duck industry in China. Most meat ducks develop rapidly after 25 d of age, resulting in large-scale acute death, and conventional treatment cannot adequately control the disease. In the ranks of immunosuppressive viruses, DuCV has the characteristics of a wide spread and a high infection rate. Epidemiological investigation found that DuCV infection exists on farms in most areas (Liu et al., 2020a), and there is even evidence that DuCV has the characteristic of vertical transmission (Li et al., 2014). Multichannel transmission and persistent infection may lead to a very high infection rate of DuCV. It has been proved that ducks infected with DuCV have more bacterial or viral infections and a higher pathogen loads than noninfected ducks (Ji et al., 2020; Zhang et al., 2013). The pathogen detection and analysis in our laboratory in the past three years also found that DuCV-positive diseased ducks often have very serious bacterial infections. Combined with the previous research progress and clinical judgment analysis of DuCV (Liu et al., 2020b; Yang et al., 2020), the immunosuppression caused by DuCV infection is likely to play a key role in many factors of bacterial secondary infection. However, this inference lacks the most direct evidence, and so far there is no systematic test to prove the existence of a correlation between DuCV and other pathogenic secondary infections, especially bacterial secondary infections.
In this study, the model of DuCV infection was constructed, the growth performance index was tracked and recorded, and the effect of DuCV infection on immune function was systematically evaluated. In addition, the models of secondary infection of Avian Pathogenic Escherichia coli (APEC) in different time periods were established to record the bacterial colonization, morbidity, and death after secondary infection of APEC, and to compare the effects of different periods of DuCV infection on the secondary infection of APEC. Studies have shown that DuCV has an obvious inhibitory effect on the immune system and can aggravate the secondary infection of bacteria, which should cause widespread concern.
MATERIALS AND METHODS
Virus Strains and Bacterial Strain
DuCV as a strain was isolated in 2018 from a duck in Shandong Province (A/Duck/Shandong/08/2018). The DuCV virus was titrated in Cherry Valley duck by determining TCID50 (50% tissue culture infective dose). It has been prepared into virus suspension. APEC SD1 strain were isolated in our laboratory. APEC was grown on Luria-Bertani (LB) agar and eosinmethylene blue medium for bacterial isolation and identification, respectively. Moreover, APEC was identified by gene sequencing of 16SrDNA (Ma et al., 2018).
Establishment of standard curve of DuCV qPCR method
DuCV DNA from livers was extracted using the virus DNA Extraction Kit (Bioer Technology, Hangzhou, China) and subsequent PCR amplification of 362-bp fragment was carried out using a specific pair of primers (DuCV-F − 5′-AATACACAGACCCACCGGCC-3′; DuCV-R − 5′-CGTACCTTCACCCGCTCCTT-3′) designed by Primer Premier 6.0. After agarose gel electrophoresis, the amplified products were recovered using the Recovery Kit (CWBIO, Beijing, China). The amplified PCR product was cloned into pMD18-T EasyVector (Bao Bio Engineering, Dalian, China) to obtain the recombinant plasmid. Ten-fold serial dilutions of the vector construct were used to generate a standard curve for the qPCR assay. A pair of primers (DuCVqPCR-F − 5′-AAGGAGCGGGTGAAGGTACG-3′; DuCVqPCR-R − 5′-CACCTGTTGTGTTGTCCGGC-3′) for a 138-bp amplicon was redesigned with Primer Premier 6.0. The ChamQ Universal SYBR qPCR Master Mix (Vazyme, Nanjing, China) was used according to the manufacturer's recommendations, with the following reaction conditions: predenaturation at 95°C for 30 s, followed by 40 cycles of denaturation at 95°C for 10 s, annealing at 60°C for 15 s. Each experiment was repeated 3 times.
Animal experiment
Two hundred one-day-old Cherry Valley duck were randomly assigned into four groups (Group Ⅰ–Ⅳ) and maintained in 4 independent positive pressure isolators. Two trials were designed to evaluate the effects of DuCV on immune system (Exp-1, group Ⅰ–Ⅱ) and secondary infection of APEC (Exp-2, group Ⅲ–Ⅳ). In Exp-1, at the 1 d posthatching (dph), each duck in group I was infected with 0.5 mL 102 TCID50/mL of the DuCV virus through intramuscular injection and group Ⅱ was injected with 0.5 mL PBS intramuscularly. In Exp-1, at 8, 16, 24, and 32 dpi, 3 ducks in each group were selected to record and observe the pathological changes, body weight and immune organ index. Fresh anticoagulant blood was collected and peripheral blood lymphocytes were collected to detect the lymphocyte transformation rate (LTR). The concentrations of cytokines, soluble CD4 (sCD4) and soluble CD8 (sCD8) in serum were detected by ELISA, and the virus load in some organs was detected by qPCR. In Exp-2, at the 1 dph, each duck in group Ⅲ was intramuscularly injected with 0.5 mL 102 TCID50/mL of the DuCV virus and group Ⅳ was injected with 0.5 mL PBS intramuscularly. At 8, 14, and 24 dpi, 3 ducks were killed randomly and the viral load was detected by qPCR in groups Ⅲ. It was confirmed that DuCV has been infected. Both group Ⅲ and group Ⅳ received intramuscular injection of 0.5 mL APEC (108 CFU/mL) at 14 and 24dpi. After inoculated with APEC, the mortality in 60 h and bacterial colonization in 12 h and 24 h were recorded and observed respectively.
Detection of pathological changes, body weight and immune organ index
Started from 8 dpi, the changing trend of body weight was recorded. At 8, 16, 24, and 32 dpi, 3 ducks were randomly selected for dissection. The hearts, livers, spleens, thymuses, bursa of Fabricius were taken and their pathological changes were observed. The weight of immune organs (spleens, thymus, bursa of Fabricius) was weighed, and the index of immune organs was calculated by the formula: Immune organ index (g/Kg) = Immune organ weight (g)/Body weight (Kg).
Detection of sCD4, sCD8 and cytokines in serum by ELISA
Fresh blood was collected from group I and group Ⅱ to prepare serum, cytokines were detected by duck IL-10, IL-12, IFN-γ, sCD4, sCD8 ELISA Kit (Lengton Bioscience, Shanghai, China; Zajkowska et al., 2001).
Detection of LTR
Anticoagulant blood was collected to isolate peripheral blood lymphocytes in group Ⅰ and group Ⅱ, respectively. Ninty-six-well cell culture plate was taken, 100 μL cell suspension (1 × 106 cell/mL) was added to each well, 6 terms holes were repeated in each sample, and Roswell Park Memorial Institute-1640 (RPMI-1640; Gibco, Shanghai, China) marginal control terms were added around. 25 μL Concanavalin A (ConA; NEW Cell & Molecular biotech, China; 10 μg/mL) was added into 3 of the 6 terms. The culture plate was placed in 37°C incubator for 36 to 48 h. After the end of culture, 10 μL Cell Counting Kit-8 (CCK-8; NEW Cell & Molecular biotech, China) was added to each well, and then incubated for 2 to 4 h. The cell culture plate was taken out and its absorbance at 490 nm was measured. The LTR was calculated according to the formula, LTR = (mean value of OD490nm stimulated by ConA-mean value of OD490nm without ConA stimulation) / mean value of OD490nm in the control group (Yu et al., 2017).
Detection of viral load in organs by qPCR
QPCR was also performed to detect differences in viral loads between days of infection on the basis of an established standard curve. The viral load of Exp-1 was detected at 8, 16, 24, 32 dpi, and the viral load of Exp-2 was detected at 8, 14, 24 dpi.
Statistical analysis
The data from 3 independent experiments were expressed as mean ± SD, and SPSS 17.0 software was used for statistical calculations. A one-way analysis of variance (ANOVA) followed by an LSD mean separation was used to determine the significance of differences among groups, with P < 0.05 used as the threshold for statistical significance. Figures were exported using GraphPad Prism software.
RESULTS
Changes in clinical symptoms and pathological changes and body weight and immune organ index
After DuCV infection, the appearance of symptoms of 8 to 16 dpi was not obvious, but at 16 to 32 dpi, it was obvious that the ducks infected with DuCV were thinner and dispirited, and that they had rougher feathers and more shedding than those of the PBS group. The pathological changes in each organ were not obvious. Due to the slow development of the disease, obvious pathological changes and no death occurred in 24 dpi. As shown in Figure 1A, the overall organ size of the DuCV group was slightly smaller than that of the PBS group at the times of 24 dpi and 32 dpi. In view of this, the body weights of both the DuCV group and the PBS group were recorded, as shown in Figure 1B. The results showed that during the 32 d recording period, the body weights of infected ducks in the DuCV group gradually increased, but were always significantly (P < 0.05) lower than those of normal ducks in the PBS group. As shown in Figure 1C, D, and E, it was found that the immune organ (thymus and spleens) index level in the DuCV group was significantly (P < 0.05) lower than that in the PBS group. The index of immune organs in the PBS group showed a stable trend, while the index of the spleen, thymus, and bursa of Fabricius in the DuCV group decreased at 8 to 24 dpi and increased after 24 dpi, though it was still lower than that in the PBS group.
Figure 1.
Effects of DuCV infection on body weight and immune organ index of ducklings. The immune organs were dissected at 8, 16, 24, and 32 dpi to observe the histopathological changes (A), body weight (B), relative thymus index (C), relative spleen index (D) and relative bursa index (E). *P < 0.05.
Effects of DuCV infection on Lymphocyte Proliferation and cytokine secretion
From the previous results, it is concluded that DuCV infection can indeed cause damage to immune organs, so we further detected lymphocyte proliferation and cytokine secretion. The result is shown in Figure 2A. In the DuCV group, the LTR of 8 to 24 dpi decreased gradually and the LTR of 24 dpi reached the lowest. Twenty-four to 32 dpi began to increase, but it was still lower than that of the PBS group. The LTR of the DuCV group was significantly (P < 0.05) lower than that of the PBS group. Determination of sCD4 and sCD8 useful in the evaluation of the state of immune activation. As shown in Figure 2B and C, the molecular contents of sCD4 and sCD8 in the DuCV group at 8 to 16 dpi were highly significant (P < 0.01) lower than those in the PBS group, 24 to 32 dpi was extremely significant (P < 0.001) lower than that in the PBS group, the content of sCD8 in the 8 dpi PBS group was highly significant (P < 0.01) higher than that in the DuCV group, and 16 to 32 dpi was extremely significant (P < 0.001) higher than that in the DuCV group. From the overall trend, the molecular concentration of sCD4 and sCD8 in the DuCV group decreased continuously at 8 to 24 dpi, while at 24 to 32 dpi it increased gradually. As shown in Figure 2D, E, F, the contents of IL-12 and IFN-γ in the DuCV group decreased at 8 to 24 dpi and increased gradually 24 to 32 dpi, and the overall levels were lower than those in the PBS group and were extremely significant (P < 0.001) lower than those in the PBS group at 24 dpi while the trend of IL-10 was opposite.
Figure 2.
Effect of DuCV infection on cytokine secretion and LTR. The lymphocytes isolated from duck peripheral blood were collected, the OD value was measured by CCK-8, and the transformation rate (A) was calculated according to the formula. The concentrations of sCD4 molecule (B), sCD8 molecule (C), IFN-γ (D), IL-10 (E) and IL-12 (F) in serum at 8, 16, 24, and 32 dpi were detected by enzyme linked immunosorbent assay (ELISA). *P < 0.05; **P < 0.01;***P < 0.001.
Determination of viral loads
Previous studies have shown that DuCV can cause persistent infection, and the amount of virus in various organs is relatively stable 8 d after infection (Liu et al., 2020b). Similarly, we also detected the viral load in some organs, especially in immune organs. The result is shown in Figure 3. The viral loads of the thymus (Figure 3A), spleens (Figure 3B), and bursa of Fabricius (Figure 3C) increased gradually in 8 to 24 dpi, and reached the peak in 24 dpi, then decreased slightly, but there was no significant difference Figure 3.D and E are the viral loads of hearts and livers, respectively. The viral loads of hearts and livers are not significantly different but show an upward trend.
Figure 3.
The viral loads of each organ changed after DuCV infection. The viral loads of livers (A), hearts (B), spleens (C), thymus (D) and bursa of Fabricius (E) were detected by qPCR at 8, 16, 24, and 32 dpi, and the virus copy number was calculated according to the standard curve.
Statistics of death and colonization in vivo after inoculation with APEC
Immunosuppression induced by virus infection is a crucial mechanism of secondary bacterial infection. The suppression of immune function inevitably increases the susceptibility of poultry to a variety of bacteria (Subler et al., 2006). Therefore, we speculated that duck circovirus will also increase the susceptibility of ducks to bacteria, so we tested the survival rate and colonization rate of ducks infected with DuCV and APEC.
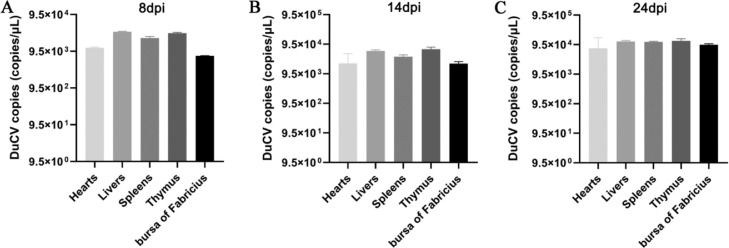
We tested the DuCV viral load in various organs before the bacteria inoculation. As shown in Figure 4A, B, and C, viral loads were detected in each organ at 8, 14, and 24 dpi. The 24 dpi compared to the 8 and 14 dpi, the viral loads of the livers, spleens, thymus, exceeded 9.51 × 104 copies/μL. This shows that DuCV grows and multiplies in vivo when 24 dpi is inoculated with bacteria; the results of this viral loads detection are consistent with those detected in the previous study of the immunosuppressive effect and proved that the subsequent test is DuCV secondary APEC infection.
Figure 4.
The viral loads of each organ changed after DuCV infection and before APEC inoculation. When 8 dpi (A), 14 dpi (B) and 24 dpi (C) were detected by qPCR, the viral loads in thymus, spleens, bursa of Fabricius, hearts and livers were detected, and the virus copy number was calculated according to the standard curve.
As shown in Figure 5A, the survival rate of 14 dpi inoculated with APEC was 20% in the DuCV + APEC group; this was significantly (P < 0.05) lower than that in the APEC group Figure 5.B shows the survival rate of 24 dpi inoculation with APEC. After 24 h, the survival rate of the DuCV + APEC group was as low as 5%, while that of the APEC group was 15%.
Figure 5.
The mortality of ducklings secondary to APEC infection and bacterial colonization in different time after DuCV infection. Each group was inoculated with APEC at 14 dpi and 24 dpi, and the death of 14 dpi (A) and 24 dpi (B) and the colonization of APEC at 12 h (C), 24 h (D), and 24 dpi at 12 h (E) and 24 h (F) were recorded. *P < 0.05; **P < 0.01;***P < 0.001.
The bacterial loads count in each organ was shown in Figure 5C, D and E, F. As shown in Figure 5C, the number of APEC in livers 12 h after 14 dpi inoculation. The number of APEC in livers and hearts of the DuCV+APEC group was significantly (P < 0.01) higher than that of the APEC group, about twice as much as that of the APEC group. As shown in Figure 5D, the number of APEC in livers and hearts in DuCV + APEC group was extremely significantly (P < 0.001) higher than that in APEC group at 24 h, about three times higher than that in the APEC group. As shown in Figure 5E and F, the number of 24 dpi inoculated with APEC in hearts and livers in the DuCV + APEC group was significantly (P < 0.01) higher than that in the APEC group; the number of APEC inoculated by 14 dpi was about 7 × 107 CFU/mL at 12 h and reached 7 × 108 CFU/mL at 24 h, which was higher than that in APEC inoculated by 14 dpi.
DISCUSSION
DuCV has spread widely all over the world and is one of the viruses with the highest infection rate in waterfowl (Wan et al., 2011; Wang et al., 2021). The harm of DuCV has gradually attracted our attention with the large-scale development of the duck industry in China, nevertheless, not much is known about the pathological mechanism so far.
Most members of the circoviridae family—such as porcine circovirus, chicken infectious anemia, parrot beak feather virus, and so on—will cause immunosuppression. It has been proved that DuCV invades the host immune system and can cause feather disorder, growth retardation, weight loss, and lymphocytopenia (Soike et al., 2004; Wang et al., 2011). Our study also found that compared to the normal group, the DuCV infection group experienced significantly inhibited body weight and immune organ development. LTR refers to the detection of T lymphoid activity and function in vitro, so as to reflect the immune ability of the body (Bertram et al., 1997). The results of our study showed that the LTR in the DuCV group was significantly lower than that in the normal group, indicating that DuCV infection significantly inhibited the proliferation and differentiation of lymphocytes; the decrease in the number of lymphocytes may be an important reason for aggravating bacterial infection. IL-10 is produced by Treg lymphocytes, macrophages, and dendritic cells, which can inhibit the production of proinflammatory cytokines (such as IL-2, IL-12, TNF-γ, etc.), promote the expression and secretion of HLA-G5, and inhibit the expression of costimulatory molecules in dendritic cells and macrophages (Docke et al., 2009). According to our results, the results showed that the content of IL-10 increased rapidly after DuCV invaded the body, which significantly inhibited the secretion of IL-12 and IFN-γ, resulting in immunosuppression. This is consistent with the previous results (Jansky et al., 2003). We speculated that cytokine secretion stimulators or IL-10 blockers may play a role in reducing the harm of the virus; this requires further exploration.
T cells are the main effector cells of acquired immunity and are widely involved in many immune responses, such as signal recognition, antigen presentation, the release of inflammatory factors, activation and chemotaxis of other immune cells, and so on (Farber et al., 2014). SCD4 molecule is the main surface marker of helper T lymphocytes and an important immune cell in the immune system, which can regulate or assist other lymphocytes to function in the immune response. SCD8 molecule is the main surface marker of cytotoxic T lymphocytes and is immunized by class Ⅰ histocompatibility on the surface. SCD4 and sCD8 monitoring is considered an indicator of lymphatic system reconstruction (Ho et al., 1994). Many authors believe that sCD4, sCD8 are markers of T lymphocyte activation (De Rie et al., 1996; Sato et al., 1996). The increase in T lymphocyte soluble receptor concentrations would confirm the activation and proliferation of T lymphocytes, thus maintaining the inflammatory process (Kuryliszyn-Moskal, 1998). Our study found that, from the overall trend, the concentration of sCD4 and sCD8 molecules in the DuCV infection group decreased continuously at 8 to 24 dpi, while 24 to 32 dpi increased, though it was still significantly lower than that in the normal group. This indicates that DuCV infection significantly reduced T cell-mediated immune function, which had a great impact on humoral and cellular immunity. In addition, we found that the immune organ index, LTR, IL-12, sCD4, sCD8, IFN-γ, and other concentrations in the infected group reached the lowest in 24 dpi, and then gradually increased. In the detection of viral loads in some tissues and organs, it was found that the viral loads of the thymus, spleen, bursa of Fabricius, and other organs reached the peak at 24 dpi. It can be seen that with the increasing or decreasing trend of viral loads, the contents of IL-12, sCD4, sCD8, and IFN-γ are also positively correlated with the change of viral loads, and it has been reported that IFN-γ is secreted by CD4 and CD8 (Liu et al., 2015), which also confirms that the content of IFN-γ is positively correlated with the content of sCD4 and sCD8 molecules in this study. Therefore, the immunosuppression caused by DuCV infection is a continuous process, especially in 24 dpi, which may be related to the clinical incidence of meat duck breeding at about 25 d old. This suggests that we should pay special attention to the improvement of immune function by drugs before the age of 25. In addition, we found that 8 dpi DuCV has been stable in various organs, which is consistent with previous studies (Liu et al., 2020b). Therefore, the relationship between viral changes and immune system damage in the first 8 d after infection must be further studied, as this is very important to understanding the pathogenic mechanism of DuCV.
In this study, we found that DuCV infection significantly promoted the pathogenicity and colonization ability of APEC, which was reflected in mortality, onset time, bacterial loads, and other indicators. In addition, the infection of APEC in 24 dpi after DuCV infection was more serious than that in 14 dpi, which was consistent with the above immunosuppression results. Therefore, our study shows that immunosuppression caused by DuCV infection is the key factor causing bacterial secondary infection, and the degree of immunosuppression is positively correlated with the degree of bacterial secondary infection.
In short, this study proved that DuCV infection causes serious damage to the immune system and used direct evidence to prove that DuCV infection promoted the pathogenicity and colonization of APEC infection. Our findings are essential to helping the industry fully understand the dangers of DuCV. At the same time, our data play a positive role in guiding the clinical use of drugs and promoting the establishment of an integrated prevention and control system for DuCV and the development of vaccines and drugs. However, we recognize that the more in-depth infection and pathogenesis of DuCV, especially the molecular mechanism, are not clear and require further study.
Acknowledgments
Acknowledgments
We thank the staff of College of Veterinary Medicine, Shandong Agricultural University, in Tai'an, Shandong Province, China. This work is supported by the development and industrialization of anti-inflammatory and anti-virus “T” egg yolk antibody (YDZX20203700004857).
Ethics statement: The animal study was reviewed and approved by Animal Q8 Protection and Utilization Committee of Shandong Agricultural University (Permit number: 20010510).
Disclosures
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Bertram E.M., Jilbert A.R., Kotlarski I. Optimization of an in vitro assay which measures the proliferation of duck T lymphocytes from peripheral blood in response to stimulation with PHA and ConA. Dev. Compar. Immunol. 1997;21:299–310. doi: 10.1016/s0145-305x(97)00005-0. [DOI] [PubMed] [Google Scholar]
- Cha S.-Y., Song E.-T., Kang M., Wei B., Seo H.-S., Roh J.-H., Yoon R.-H., Moon O.-K., Jang H.-K. Prevalence of Duck Circovirus infection of subclinical pekin ducks in South Korea. J. Vet. Med. Sci. 2014;76:597–599. doi: 10.1292/jvms.13-0447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Rie M.A., Zonneveld I.M., Witkamp L., Van Lier R.A., Out T.A., Bos J.D. Soluble interleukin-2 receptor (sIL-2R) is a marker of disease activity in psoriasis: a comparison of sIL-2R, sCD27, sCD4, sCD8 and sICAM-1. Acta Derm Venereol. 1996;76:357–360. doi: 10.2340/0001555576357360. [DOI] [PubMed] [Google Scholar]
- Docke W.-D., Asadullah K., Belbe G., Ebeling M., Hoflich C., Friedrich M., Sterry W., Volk H.-D. Comprehensive biomarker monitoring in cytokine therapy: Heterogeneous, time-dependent, and persisting immune effects of interleukin-10 application in psoriasis. J. Leukocyte Biol. 2009;85:582–593. doi: 10.1189/jlb.0408249. [DOI] [PubMed] [Google Scholar]
- Farber D.L., Yudanin N.A., Restifo N.P. Human memory T cells: generation, compartmentalization and homeostasis. Nat. Rev. Immunol. 2014;14:24–35. doi: 10.1038/nri3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fringuelli E., Scott A.N.J., Beckett A., McKillen J., Smyth J.A., Palya V., Glavits R., Ivanics E., Mankertz A., Franciosini M.P., Todd D. Diagnosis of duck circovirus infections by conventional and real-time polymerase chain reaction tests. Avian Pathol. 2005;34:495–500. doi: 10.1080/03079450500368334. [DOI] [PubMed] [Google Scholar]
- Hattermann K., Schmitt C., Soike D., Mankertz A. Cloning and sequencing of Duck circovirus (DuCV) Arch. Virol. 2003;148:2471–2480. doi: 10.1007/s00705-003-0181-y. [DOI] [PubMed] [Google Scholar]
- Ho A.D., Maruyama M., Maghazachi A., Mason J.R., Glück S., Corringham R.E. Soluble CD4, soluble CD8, soluble CD25, lymphopoieitic recovery, and endogenous cytokines after high-dose chemotherapy and blood stem cell transplantation. Blood. 1994;84:3550–3557. [PubMed] [Google Scholar]
- Hong Y.T., Kang M., Jang H.K. Pathogenesis of duck circovirus genotype 1 in experimentally infected Pekin ducks. Poultry Sci. 2018;97:3050–3057. doi: 10.3382/ps/pey177. [DOI] [PubMed] [Google Scholar]
- Huang J., Yang C., Jia R., Wang M., Chen S., Liu M., Zhu D., Zhao X., Yang Q., Wu Y., Zhang L., Yin Z., Jing B., Cheng A. Induction of a protective response in ducks vaccinated with a DNA vaccine encoding engineered duck circovirus Capsid protein. Vet. Microbiol. 2018;225:40–47. doi: 10.1016/j.vetmic.2018.09.002. [DOI] [PubMed] [Google Scholar]
- Jansky L., Reymanova P., Kopecky J. Dynamics of cytokine production in human peripheral blood mononuclear cells stimulated by LPS or infected by Borrelia. Physiol. Res. 2003;52:593–598. [PubMed] [Google Scholar]
- Ji J., Chen Q., Sui C., Yu Z., Xu X., Yao L., Kan Y., Bi Y., Xie Q. Novel genotype definition and genome characteristics of duck circovirus in central and Eastern China. Transbound. Emerg. Dis. 2020;67:2993–3004. doi: 10.1111/tbed.13676. [DOI] [PubMed] [Google Scholar]
- Kuryliszyn-Moskal A. Cytokines and soluble CD4 and CD8 molecules in rheumatoid arthritis: relationship to systematic vasculitis and microvascular capillaroscopic abnormalities. Clin. Rheumatol. 1998;17:489–495. doi: 10.1007/BF01451285. [DOI] [PubMed] [Google Scholar]
- Li Z., Fu G., Feng Z., Chen J., Shi S., Liu R., Cheng L., Chen H., Wan C., Huang Y. Evaluation of a novel inactivated vaccine against duck circovirus in muscovy ducks. Vet. Microbiol. 2020;241:108574. doi: 10.1016/j.vetmic.2019.108574. [DOI] [PubMed] [Google Scholar]
- Li Z., Wang X., Zhang R., Chen J., Xia L., Lin S., Xie Z., Jiang S. Evidence of possible vertical transmission of duck circovirus. Vet. Microbiol. 2014;174:229–232. doi: 10.1016/j.vetmic.2014.09.001. [DOI] [PubMed] [Google Scholar]
- Liu G.G., Sun D.L., Wu H., Zhang M.S., Huan H.X., Xu J.J., Zhang X.Q., Zhou H., Shi M.Q. Distinct Contributions of CD4(+) and CD8(+) T Cells to Pathogenesis of Trypanosoma brucei Infection in the Context of Gamma Interferon and Interleukin-10. Infect. Immun. 2015;83:2785–2795. doi: 10.1128/IAI.00357-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H., Li L.X., Sun W.C., Shi N., Sun X.T., Jin N.Y., Si X.K. Molecular survey of duck circovirus infection in poultry in southern and southwestern China during 2018 and 2019. BMC Vet. Res. 2020:16. doi: 10.1186/s12917-020-02301-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Chen I., Du Q., Chua H., Kwang J. The ORF3 protein of porcine circovirus type 2 is involved in viral pathogenesis in vivo. J. Virol. 2006;80:5065–5073. doi: 10.1128/JVI.80.10.5065-5073.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Yang X., Hao X., Feng Y., Zhang Y., Cheng Z. Effect of goose parvovirus and duck circovirus coinfection in ducks. J. Vet. Res. 2020;64:355–361. doi: 10.2478/jvetres-2020-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L.L., Sun Z.H., Xu Y.L., Wang S.J., Wang H.N., Zhang H., Hu L.P., Sun X.M., Zhu L., Shang H.Q., Zhu R.L., Wei K. Screening host proteins required for bacterial adherence after H9N2 virus infection. Vet. Microbiol. 2018;213:5–14. doi: 10.1016/j.vetmic.2017.11.003. [DOI] [PubMed] [Google Scholar]
- Sato S., Fujimoto M., Kikuchi K., Ihn H., Tamaki K., Takehara K. Soluble CD4 and CD8 in serum from patients with localized scleroderma. Arch. Dermatol. Res. 1996;288:358–362. doi: 10.1007/BF02507103. [DOI] [PubMed] [Google Scholar]
- Soike D., Albrecht K., Hattermann K., Schmitt C., Mankertz A. Novel circovirus in mulard ducks with developmental and feathering disorders. Vet. Rec. 2004;154:792–793. doi: 10.1136/vr.154.25.792. [DOI] [PubMed] [Google Scholar]
- Stewart M.E., Perry R., Raidal S.R. Identification of a novel circovirus in Australian ravens (Corvus coronoides) with feather disease. Avian Pathol. 2006;35:86–92. doi: 10.1080/03079450600597345. [DOI] [PubMed] [Google Scholar]
- Subler K.A., Mickael C.S., Jackwood D.J. Infectious bursal disease virus-induced immunosuppression exacerbates Campylobacter jejuni colonization and shedding in chickens. Avian Dis. 2006;50:179–184. doi: 10.1637/7434-090705R.1. [DOI] [PubMed] [Google Scholar]
- Wan C.-H., Fu G.-H., Shi S.-H., Cheng L.-F., Chen H.-M., Peng C.-X., Lin S., Huang Y. Epidemiological investigation and genome analysis of duck circovirus in Southern China. Virologica Sinica. 2011;26:289–296. doi: 10.1007/s12250-011-3192-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Xie X., Zhang D., Ma G., Wang X., Zhang D. Detection of duck circovirus in China: a proposal on genotype classification. Vet. Microbiol. 2011;147:410–415. doi: 10.1016/j.vetmic.2010.07.014. [DOI] [PubMed] [Google Scholar]
- Wang Y., Zhang D., Bai C.-x., Guo X., Gao W.-h., Li M.-l., Wang J., Li Y.-d. Molecular characteristics of a novel duck circovirus subtype 1d emerging in Anhui. China. Virus Res. 2021;295:198216. doi: 10.1016/j.virusres.2020.198216. [DOI] [PubMed] [Google Scholar]
- Wozniakowski G., Samorek-Salamonowicz E., Gawel A. Occurrence of reovirus infection in Muscovy ducks (Cairina moschata) in south western Poland. Polish J. Vet. Sci. 2014;17:299–305. doi: 10.2478/pjvs-2014-0041. [DOI] [PubMed] [Google Scholar]
- Yang Y., Sui N., Zhang R., Lan J., Li P., Lian C., Li H., Xie Z., Jiang S. Coinfection of novel goose parvovirus-associated virus and duck circovirus in feather sacs of Cherry Valley ducks with feather shedding syndrome. Poultry Sci. 2020;99:4227–4234. doi: 10.1016/j.psj.2020.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C., Wei K., Liu L., Yang S., Hu L., Zhao P., Meng X., Shao M., Wang C., Zhu L., Zhang H., Li Y., Zhu R. Taishan Pinus massoniana pollen polysaccharide inhibits subgroup J avian leucosis virus infection by directly blocking virus infection and improving immunity. Scient. Rep. 2017;7:44353. doi: 10.1038/srep44353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zajkowska J., Hermanowska-Szpakowicz T., Swierzbinska R. Concentration of soluble CD4, CD8 and CD25 receptors in early localized and early disseminated Lyme borreliosis. Infection. 2001;29:71–74. doi: 10.1007/s15010-001-1078-x. [DOI] [PubMed] [Google Scholar]
- Zhang Z., Jia R., Lu Y., Wang M., Zhu D., Chen S., Yin Z., Chen X., Cheng A. Identification, genotyping, and molecular evolution analysis of duck circovirus. Gene. 2013;529:288–295. doi: 10.1016/j.gene.2013.07.028. [DOI] [PubMed] [Google Scholar]
- Zhu D., Zhou D., Liu J., Hao X., Cheng Z. Duck circovirus induces a new pathogenetic characteristic, primary sclerosing cholangitis. Compar. Immunol. Microbiol. Infect. Dis. 2019;63:31–36. doi: 10.1016/j.cimid.2018.12.009. [DOI] [PubMed] [Google Scholar]