Abstract
Background
Since the introduction of imiquimod cream, patients have reported treatment-emergent symptoms that mimic influenza. However, clear relationships between the onset of symptoms from topical imiquimod and various variables (eg, patient’s age) remain unclear.
Objective
To evaluate potential relationships between the onset of visceral symptoms that mimic influenza and variables including patient age, the severity of local cutaneous reactions, the amount of surface area, areas of the body being treated, and serum levels of inflammatory cytokines present during 2 cycles of 14-day treatment of imiquimod 3.75% cream.
Methods
In this single-center, open-label, investigator-initiated trial, 22 patients with 5-20 actinic keratosis were stratified into 1 of 2 age groups: ages 30-59 and ages 60-89.
Results
Although the occurrence of systemic symptoms was infrequent during the treatment period, the majority of patients who reported local skin reactions preceding systemic symptoms developed them within 7-11 days of the treatment cycle. Levels of circulating cytokines had no predictive value.
Limitations
This study was limited by its small sample size as well as the small number of cytokines evaluated. Chemokines and cytokines beyond those evaluated may contribute to influenza symptoms and/or systemic responses to imiquimod.
Conclusion
The onset of local skin reactions may serve as a predictor for the potential onset of systemic symptoms that mimic those of influenza and could be used as a talking point for patients, though further research is needed.
Key words: actinic keratosis, cytokines, drug response, flu, imiquimod, medical dermatology, myalgias, oncology, symptoms
Abbreviations used: AE, adverse event; AK, actinic keratosis; IL, interleukin; LSR, local skin reaction
Capsule Summary.
-
•
This report is from a single-center, open-label trial measuring the predictive value of serological quantification of cytokines with the onset of influenza-like signs and symptoms that are induced by topical application of imiquimod 3.75% cream.
-
•
Younger patients treated on the face and scalp may have a higher susceptibility for systemic symptoms.
Introduction
Imiquimod is an immune response modifier approved by the US Food and Drug Administration for the treatment of actinic keratosis (AK), external genital warts, and superficial basal cell carcinoma. The mechanism of action for imiquimod is based on the promotion of immune mechanisms that involve Th1 immunity, with multiple cytokines responsible for the clinical effects.1 Imiquimod binds to toll-like receptors 7, 8 of antigen-presenting cells and stimulates the production of immune modulatory cytokines as well as induction of apoptosis. These effects result in enhanced antigen presentation by Langerhans cells to T cells, which promotes migration to the draining lymph nodes and other adaptive immune responses.1,2
Since the introduction of imiquimod cream, patients have reported treatment-emergent symptoms that mimic influenza, such as myalgias, malaise, headaches, low-grade fever, and fatigue. However, clear relationships between the onset of symptoms from topical imiquimod and variables such as the patient’s age, degree of severity of local skin reactions (LSRs), body parts treated, or the amount of surface area treated have been established.3, 4, 5 A previous study by Hayden et al6 of immune responses to infection by the influenza virus suggests that interleukin (IL) 6 and interferon α play key roles in the formation of symptoms. A poster presentation of a meta-analysis by Freeman et al7 explored the possibility of using the history of the intensity of symptoms related to influenza infection as a predictive tool to determine which patients treated with imiquimod would develop a similar symptom profile, which in their analysis was of important historical value.8 The results of this analysis showed that there was a higher risk of visceral events associated with treatment with imiquimod cream based on the number of days the patients had been bedridden if they had influenza in the past, such that those spending greater than 9 days in bed had the most severe reactions, as opposed to those with no days in bed most likely experiencing ineffective treatment.7
In this study, we attempted to explore the relationship between symptoms and elevation of serum cytokine levels, which may provide insight into which patients may experience dose-limiting symptoms during imiquimod therapy, potentially allowing clinicians to anticipate the need for dose adjustment and adjunctive treatments to maximize compliance and efficacy outcomes (Supplementary Material, available via Mendeley at https://data.mendeley.com/datasets/xxxfxrwkg3/1).
Materials and methods
In this single-center, open-label, investigator-initiated trial, 22 enrolled patients with 5-20 AK were stratified by treatment area (face/scalp and trunk/upper extremities) and by age (30-59 years and 60-89 years) (Table I). The patients were screened for any prior use of imiquimod (exclusionary), previous history of malignancy other than skin cancer, and any other systemic illnesses. Patients had to be washed out of topical and systemic immunosuppressive agents as well as screened for previous treatments for actinic keratoses. During the trial, moisturizers, anti-itch therapies, and healing ointments to minimize anticipated cutaneous responses were permitted for symptomatic relief.
Table I.
Patients demographics and stratification of patients by age and treatment sites
| Demographic and baseline characteristics statistics | N = 22 |
|---|---|
| Age (y) | |
| Mean (SD) | 62.8 (8.99) |
| Median | 59 |
| Age group, n (%) | |
| ≥30 years to ≤59 years | 12 (54.5) |
| ≥60 years to ≤ 89 years | 10 (45.5) |
| Gender, n (%) | |
| Female | 7 (31.8) |
| Male | 15 (68.2) |
| Area of treatment n (%) | |
| Entire face or balding scalp | 11 (50.0%) |
| Chest or upper extremities | 11 (50.0%) |
| Group no. | Age | Number of patients | Treatment area |
|---|---|---|---|
| Group 1 | Ages 30-59 | 6 | Entire face or balding scalp |
| Group 2 | Ages 30-59 | 5 | Chest or upper extremities |
| Group 3 | Ages 60-89 | 5 | Entire face or balding scalp |
| Group 4 | Ages 60-89 | 6 | Chest or upper extremities |
| One screening failure patient |
Three assessments were conducted at each visit (baseline, day 8, day 22, day 43, and day 57 end of study) (1) documentation of LSRs; (2) review of any signs and symptoms similar to those of influenza; and (3) obtaining a serological panel (circulating IL-6, IL-8, IL-12, tumor necrosis factor α) to assess immune responses to treatment that might be related to the occurrence of systemic symptoms (Fig 1 and Table II).
Fig 1.
Study Design—patients were treated with imiquimod 3.75% cream for 2 14-day cycles.
Table II.
Evaluation of local and systemic AEs
| Local skin reactions | 5-point scale | Systemic symptoms | 4-point scale |
|---|---|---|---|
| Erythema | Clear | Fever | None |
| Scabbing/crusting | Almost clear | Headache | Mild |
| Edema | Mild | Fatigue | Moderate |
| Erosions/ulcerations | Moderate | Malaise | Severe |
| Exudate | Severe | GI symptoms | |
| Flaking/scaling/dryness | Dizziness | ||
| Pruritus | Myalgias | ||
| Arthralgias | |||
| LAD |
The designated treatment area was cleansed with an approved cleanser and allowed to dry for 5 minutes before application of imiquimod 3.75% cream. Per the approved label, patients were treated to cover up to 200 cm2 total body surface area. The treatment plan was reviewed with patients to ensure appropriate daily treatment use for 14 days, a holiday for 14 days, and a second 14-day treatment cycle followed by a 14-day holiday before the end of the study, consistent with the approved treatment regimen for the commercially available imiquimod 3.75% cream (Fig 1).
Special considerations were made for unscheduled visits as soon as possible for assessments and local site reactions and serology laboratory evaluations if at any time during the study a patient developed the onset of signs or symptoms.
Patients were also given a diary to record any systemic health changes indicative of a flu-like response. In addition, laboratory values were assessed to measure circulating cytokine levels at the time of these events. Scheduling of follow-up evaluations was based on the milestones surrounding conventional treatment with imiquimod 3.75% cream: visit 2 was scheduled on day 8 of treatment cycle 1 to assess for mid-cycle reactions; visit 3 was scheduled on day 22 to assess reactions during the treatment holiday; and visit 4 was scheduled on day 43, after the end of the second treatment cycle. This timing was based on the potential peaks and resolution of the recruited immune responses from the effects of imiquimod 3.75% cream.2,8
Each patient was evaluated for 7 selected LSRs on a 5-point scale from clear (0) to severe (4), and 9 selected systemic symptoms on a 4-point scale from none (0) to severe (3) (Table II). The composite score for LSRs and systemic symptoms was calculated by multiplying the average severity of adverse events (AEs) by the number of AEs present (maximum 7 local and 9 systemic). Only AEs reported as moderate to severe are included in the composite score; mild AEs were not included. For example, at visit 1, the patient had 3 LSRs: erythema (mild, 2); pruritus (severe, 4); and dryness (moderate, 3). The composite score for LSRs at visit 1 for this patient is calculated as 2 events × 3.5 (average severity of events) = 7; note that mild events were not included in the composite score. The composite score at each visit is graphed to represent the overall AE profile for patients as they progressed through the study.
Results
There were 23 total patients screened for the study, of which there was 1 screen failure. Of the 22 patients who were enrolled, there were 6 patients in groups 1 and 4 and 5 patients in groups 2 and 3. Given the open-label design of the study, all patients received active test articles from the manufacturer without blinding. All except one patient experienced either absolute complete clearance or partial clearance with no more than 3 AKs remaining in the treatment field. No patient discontinued the study due to AEs.
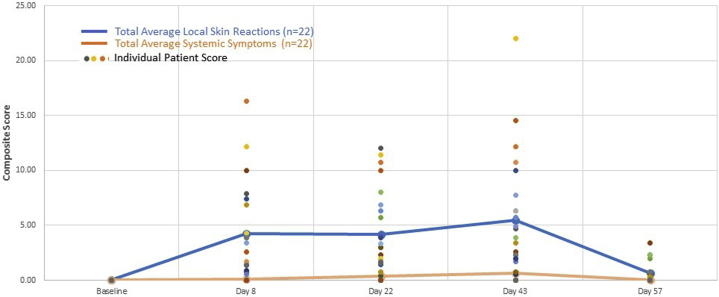
Both LSRs and systemic symptoms increased slightly with continued treatment after the second cycle and returned to normal baseline after holiday (end of study), as represented by the average composite score of all patients (Fig 2). Of the 22 patients, 13 (59%) had composite scores for LSRs at 1 or more visits above the study average score. Seven of these 13 patients also had above-average systemic symptom composite scores at 1 or more study visits. Of the 7 patients with above-average composite scores for both LSRs and systemic symptoms, 5 (71%) were treated on the face or scalp, and 2 were treated on the trunk/arm; 4 (57%) were in the younger age group. None were in the group of older patients treated on trunk/arm (group 4).
Fig 2.
Average composite score for all patients for systemic symptoms and LSRs.
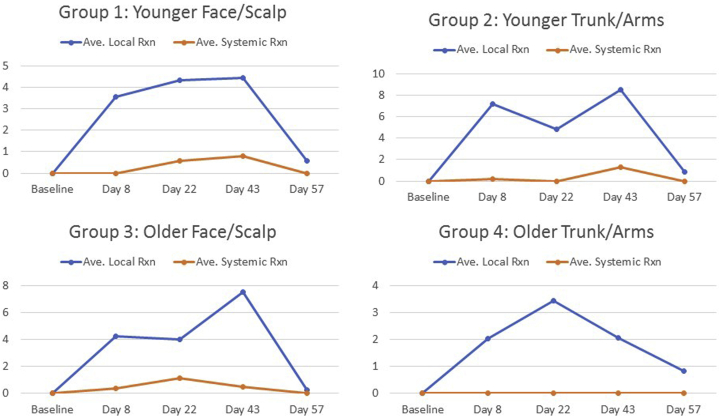
Results for the 4 patient subgroups are summarized in Figure 3. Group 1 (ages 30-59; entire face or balding scalp): None of the patients had an elevated laboratory value at any visit. For 3 patients, LSRs preceded systemic symptoms, in 1 case by 3 visits (no. 14) and in 2 cases by 2 visits (no. 16 and no. 19). Group 2 (ages 30-59; chest or upper extremities): None of the patients had an elevated laboratory value at any visit. For 2 patients, LSRs preceded systemic symptoms, in 1 case by 3 visits (no. 2) and in 1 case by 1 visit (no. 3). Group 3: (ages 60-89; entire face or balding scalp): For patient number 11, LSRs preceded systemic symptoms. Local site reactions and systemic symptoms first appeared at the same visit (visit 2). Patient number 21 had elevated laboratory values at visits 2 and 4. Both the elevated laboratory values and LSRs preceded systemic symptoms. Patient number 23 had an elevated laboratory value at visits 1, 2, 3, 4, and an unscheduled visit. For this patient, both the elevated laboratory values and LSRs preceded systemic symptoms. Group 4 (ages 60-89; chest or upper extremities): patient number 6 reported 1 systemic symptom (mild myalgia) at visit 5 but did not have any elevated laboratory values.
Fig 3.
Subgroup analysis of the intensity of LSRs and onset of systemic symptoms among groups 1-4.
Discussion
Study results indicated no clear relationships between the levels of the cytokines assessed and the timing or occurrence of any influenza-like symptoms. The onset and severity of LSRs associated with the application of imiquimod cream were also unrelated to cytokine levels. Previous pharmacokinetic studies of imiquimod have demonstrated that serum levels of active drugs typically range from very low to undetectable.9 However, as demonstrated, laboratory assessments were of no predictive value at baseline, as elevations were seen in a few patients in group 3 only. Although there was no direct relationship demonstrated with the timeline of symptoms to treatment applications, the most predictable time course for onset was between days 8-14 and days 35-43. Most importantly, there was no reporting of systemic symptoms when there was an absence of noticeable LSRs. These findings should assist dermatologists as they address potential obstacles to adherence and counseling on pertinent negative findings during treatment.
Patients in groups 1 and 3 had LSRs preceding the systemic symptoms, which developed within 7-11 days of the treatment cycle, primarily during cycle 1 but less often during the second cycle of treatment. By contrast, none of the patients in group 4 experienced systemic symptoms, any measurable LSRs, or elevated laboratory values, suggesting that older patients treated on the trunk or upper extremities may be less susceptible to systemic responses. One patient achieved complete clearance of AKs compared to 3 with partial clearance and 1 with poor clearance, suggesting that efficacy was not influenced by the degree of LSRs or evidence of visceral symptoms.
There is a presumption that patients over age 60 experience variable degrees of immune senescence and therefore diminished cellular responses that mediate the development of systemic symptoms.10 In addition, the variability in the reactions of the various surface areas of the head compared to on the body is not clearly understood. The differences among the 4 groups were not influenced by skin color, sex, or weight. As expected, nearly all patients achieved clearance of the actinic keratoses in the treatment areas.
The assessments of this study were scheduled at milestones based on the mechanisms of action, recruitment of cytokines, and cellular responses. At 72 hours, most cellular immune responses, analogous to type IV delayed-type hypersensitivity, tend to reach a peak.11 However, the results of previous studies suggested that cellular, cytokine, and dendritic cell reactions could occur as late as the eighth day of application,7,8 which could account for the timing of several patients reporting influenza-like symptoms around those days. A case report published by Heikkinen et al3 discussed how inflamed skin, particularly of the head, can facilitate higher absorption of imiquimod. Based on these observations, it is possible that the mechanisms that resulted in accelerated LSRs from immune responses had a closer relationship with the reported systemic symptoms. However, the question arises about the time interval between the onset of cutaneous and visceral responses and if they can be linked to sustained effects of interleukins.
Overall, the patterns from the 4 groups of patients treated with imiquimod cream point to the onset of systemic symptoms being more likely during the first cycle of treatment in patients treated on the head and neck compared to the body, independent of age and circulating levels of cytokines measured at time courses during treatment. Analysis of the 4 different groups stratified by age and treatment areas shows that measuring cytokine levels at peak times of the course, or in conjunction with LSRs, did not serve as potential markers or predictors for the development of systemic symptoms. Nevertheless, the onset of LSRs may serve as a predictor for the potential onset of systemic symptoms that mimic those of influenza and could be used as a talking point for patients. By counseling the patient on these possible relationships, adherence to a complete course of therapy can be optimized to maximize clearance regardless of the disease being treated. Studies with larger sample sizes could provide more information on the clinical presentation of dose-limiting symptoms that coincide with the level of LSRs, also demonstrating that serological testing would most likely not provide any value, especially given the expense of the tests. In addition, the lack of significance of the patient’s age and the amount of surface area would make the patient counseling more focused on anticipated LSRs.
Conflicts of interest
Dr Bhatia is the Vice President of the American Academy of Dermatology, 2021-2022, and is an advisor, consultant, and investigator for Ortho Dermatologics.
Acknowledgments
This was an investigator-initiated study written by the author and supported by a research grant from Ortho Dermatologics. The author would like to acknowledge and thank the following individuals for their contributions and assistance with the execution of the study and publication of the article: The entire research team from Therapeutics Clinical Research and Therapeutics Inc. as well as the patients who participated in the trial; Nelly Gilyadov, Dr Tina Lin and Dr Naveen Anbalagan from Ortho Dermatologics; Dr Andrew Korotzer and Dr Jason Olin (formerly of Valeant Pharmaceuticals); Tom Prunty from Aramed Strategies; and Suren, Kiran, and Sangita for inspiration.
Footnotes
Funding sources: Research Grant provided by Ortho Dermatologics.
IRB approval status: Reviewed and approved by Western IRB; approval no. 472920. Clinicaltrials.gov (or equivalent) listing (if applicable): NCT24435875.
References
- 1.Bubna A.K. Imiquimod - Its role in the treatment of cutaneous malignancies. Indian J Pharmacol. 2015;47(4):354–359. doi: 10.4103/0253-7613.161249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dockrell D.H., Kinghorn G.R. Imiquimod and resiquimod as novel immunomodulators. J Antimicrob Chemother. 2001;48(6):751–755. doi: 10.1093/jac/48.6.751. [DOI] [PubMed] [Google Scholar]
- 3.Heikkinen A.K., Susitaival P. Severe systemic reaction to topical imiquimod. Acta Derm Venereol. 2011;91(5):594–595. doi: 10.2340/00015555-1121. [DOI] [PubMed] [Google Scholar]
- 4.Hanger C., Dalrymple J., Hepburn D. Systemic side effects from topical imiquimod. N Z Med J. 2005;118:U1682. [PubMed] [Google Scholar]
- 5.Del Rosso J.Q. The use of topical imiquimod for the treatment of actinic keratosis: a status report. Cutis. 2005;76(4):241–248. [PubMed] [Google Scholar]
- 6.Hayden F.G., Fritz R., Lobo M.C., Alvord W., Strober W., Straus S.E. Local and systemic cytokine responses during experimental human influenza A virus infection: relation to symptom formation and host defense. J Clin Invest. 1998;101(3):643–649. doi: 10.1172/JCI1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Freeman A., Freeman M. Predicting success and side effects with imiquimod therapy. J Am Acad Dermatol. 2012;66(4):AB157. Poster. [Google Scholar]
- 8.Zhang J.M., An J. Cytokines, inflammation, and pain. Int Anesthesiol Clin. 2007;45(2):27–37. doi: 10.1097/AIA.0b013e318034194e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harrison L.I., Skinner S.L., Marbury T.C., et al. Pharmacokinetics and safety of imiquimod 5% cream in the treatment of actinic keratoses of the face, scalp, or hands and arms. Arch Dermatol Res. 2004;296:6–11. doi: 10.1007/s00403-004-0465-4. [DOI] [PubMed] [Google Scholar]
- 10.Mahler V., Geier J., Schnuch A. Current trends in patch testing—new data from the German Contact Dermatitis Research Group (DKG) and the Information Network of Departments of Dermatology (IVDK) J Dtsch Dermatol Ges. 2014;12(7):583–592. doi: 10.1111/ddg.12371. [DOI] [PubMed] [Google Scholar]
- 11.Ventura M.T., Casciaro M., Gangemi S., Buquicchio R. Immunosenescence in aging: between immune cells depletion and cytokines up-regulation. Clin Mol Allergy. 2017;15(21):21. doi: 10.1186/s12948-017-0077-0. [DOI] [PMC free article] [PubMed] [Google Scholar]