Abstract
Background
Real-world evidence supporting vaccination against COVID-19 in individuals who have recovered from a previous SARS-CoV-2 infection is sparse. We aimed to investigate the long-term protection from a previous infection (natural immunity) and whether natural immunity plus vaccination (hybrid immunity) was associated with additional protection.
Methods
In this retrospective cohort study, we formed three cohorts using Swedish nationwide registers managed by the Public Health Agency of Sweden, the National Board of Health and Welfare, and Statistics Sweden. Cohort 1 included unvaccinated individuals with natural immunity matched pairwise on birth year and sex to unvaccinated individuals without natural immunity at baseline. Cohort 2 and cohort 3 included individuals vaccinated with one dose (one-dose hybrid immunity) or two doses (two-dose hybrid immunity) of a COVID-19 vaccine, respectively, after a previous infection, matched pairwise on birth year and sex to individuals with natural immunity at baseline. Outcomes of this study were documented SARS-CoV-2 infection from March 20, 2020, until Oct 4, 2021, and inpatient hospitalisation with COVID-19 as main diagnosis from March 30, 2020, until Sept 5, 2021.
Findings
Cohort 1 was comprised of 2 039 106 individuals, cohort 2 of 962 318 individuals, and cohort 3 of 567 810 individuals. During a mean follow-up of 164 days (SD 100), 34 090 individuals with natural immunity in cohort 1 were registered as having had a SARS-CoV-2 reinfection compared with 99 168 infections in non-immune individuals; the numbers of hospitalisations were 3195 and 1976, respectively. After the first 3 months, natural immunity was associated with a 95% lower risk of SARS-CoV-2 infection (adjusted hazard ratio [aHR] 0·05 [95% CI 0·05–0·05] p<0·001) and an 87% (0·13 [0·11–0·16]; p<0·001) lower risk of COVID-19 hospitalisation for up to 20 months of follow-up. During a mean follow-up of 52 days (SD 38) in cohort 2, 639 individuals with one-dose hybrid immunity were registered with a SARS-CoV-2 reinfection, compared with 1662 individuals with natural immunity (numbers of hospitalisations were eight and 113, respectively). One-dose hybrid immunity was associated with a 58% lower risk of SARS-CoV-2 reinfection (aHR 0·42 [95% CI 0·38–0·47]; p<0·001) than natural immunity up to the first 2 months, with evidence of attenuation thereafter up to 9 months (p<0·001) of follow-up. During a mean follow-up of 66 days (SD 53) in cohort 3, 438 individuals with two-dose hybrid immunity were registered as having had a SARS-CoV-2 reinfection, compared with 808 individuals with natural immunity (numbers of hospitalisations were six and 40, respectively). Two-dose hybrid immunity was associated with a 66% lower risk of SARS-CoV-2 reinfection (aHR 0·34 [95% CI 0·31–0·39]; p<0·001) than natural immunity, with no significant attenuation up to 9 months (p=0·07). To prevent one reinfection in the natural immunity cohort during follow-up, 767 individuals needed to be vaccinated with two doses. Both one-dose (HR adjusted for age and baseline date 0·06 [95% CI 0·03–0·12]; p<0·001) and two-dose (HR adjusted for age and baseline date 0·10 [0·04–0·22]; p<0·001) hybrid immunity were associated with a lower risk of COVID-19 hospitalisation than natural immunity.
Interpretation
The risk of SARS-CoV-2 reinfection and COVID-19 hospitalisation in individuals who have survived and recovered from a previous infection remained low for up to 20 months. Vaccination seemed to further decrease the risk of both outcomes for up to 9 months, although the differences in absolute numbers, especially in hospitalisations, were small. These findings suggest that if passports are used for societal restrictions, they should acknowledge either a previous infection or vaccination as proof of immunity, as opposed to vaccination only.
Funding
None.
Introduction
Evidence from clinical trials and real-world observational studies conclusively shows that vaccines against COVID-19 induce an immunity that effectively reduces the risk of SARS-CoV-2 infection1, 2, 3, 4, 5, 6, 7 and severe COVID-19 disease including hospitalisation and death.4, 7, 8, 9, 10, 11, 12 Research also shows that individuals who have recovered from an infection can develop naturally acquired immunity, which seems to be at least as protective as vaccine-induced immunity.13 Although some countries acknowledge a recent documented infection as sufficient proof of immunity, others do not unless the natural immunity has been supplemented by vaccination,14 so-called hybrid immunity. In general, national health-care authorities and government institutions recommend that individuals who have recovered from an infection should receive primary series and booster vaccination.15 There are several lines of evidence underpinning these recommendations and regulations. For example, not all individuals develop detectable concentrations of antibodies following a SARS-CoV-2 infection, especially if the infection is asymptomatic.16 Research also indicates a vaccine-induced immune response in individuals with a documented previous infection,17 suggesting that vaccines in people with natural immunity provide additional benefits, with some support also from published18 and preliminary data.19 Yet, the strongest argument for the immunisation of people with natural immunity is the scarcity of studies investigating the long-term protection from natural immunity and its protection against severe disease, hospitalisation, and death.13, 15, 18, 20, 21
Research in context.
Evidence before this study
We did not do a formal literature search; however, we searched standard databases such as PubMed, and used search engines such as Google, to identify relevant literature until Jan 4, 2022, using the key words ”natural immunity”, ”hybrid immunity”, ”immunity”, ”infection”, ”vaccination”, ”SARS-CoV-2,” and ”COVID-19”. A meta-analysis of 15 cohort studies published on Sept 15, 2021, showed that naturally acquired immunity following recovery from a SARS-CoV-2 infection was associated with an 87% reduced risk of reinfection for up to 1 year. Yet, most national health-care authorities and government institutions recommend that individuals who have recovered from a documented previous infection should also be vaccinated. One reason for this might be evidence suggesting enhanced immunogenicity from vaccination in people with natural immunity, resulting in what is known as hybrid immunity. However, the primary reason for vaccinating people with natural immunity is probably the scarcity of evidence of the long-term protection against reinfection beyond 1 year.
Added value of this study
This registry-based study based on the total population of Sweden showed that natural immunity was associated with a 95% lower risk of SARS-CoV-2 reinfection and an 87% lower risk of COVID-19 hospitalisation than no immunity, for up to 20 months. In head-to-head comparisons, hybrid immunity induced by either one or two doses of a COVID-19 vaccine was associated with an additional risk reduction of SARS-CoV-2 reinfection compared with natural immunity for up to 9 months, although with small absolute differences. Furthermore, one-dose hybrid immunity was associated with an additional 94% lower risk of COVID-19 hospitalisation, and two-dose hybrid immunity with an additional 90% lower risk of COVID-19 hospitalisation, than natural immunity, although the number of hospitalisations were few.
Implications of all the available evidence
The risk of SARS-CoV-2 reinfection and COVID-19 hospitalisation is low in individuals with a previous infection. Vaccination after recovering from a previous infection might result in additional risk reduction against reinfection and hospitalisation for up to 9 months, but the differences in absolute risk appear small. In relation to determining proof of immunity, distinguishing between immune and non-immune individuals could be more appropriate than the frequently used terminology of vaccinated and unvaccinated.
In this retrospective cohort study based on the total population of Sweden, we investigated the association between natural immunity and risk of SARS-CoV-2 reinfection and COVID-19 hospitalisation for up to 20 months of follow-up. To investigate whether individuals with natural immunity would benefit further from vaccination, we also did head-to-head comparisons between people with hybrid immunity and people with natural immunity for up to 9 months of follow-up.
Methods
Study design and cohort construction
This retrospective cohort study was based on registry data covering the total population of Sweden. Vaccination in Sweden began on Dec 27, 2020, with older, frail individuals and individuals with specific comorbidities initially prioritised for vaccination.22 For the specific time period and data underlying the present study, Sweden had three large pandemic waves: the first was from March to June, 2020; the second from October, 2020, to January, 2021; and the third from February to May, 2021. There was also a small wave that started in August, 2021.
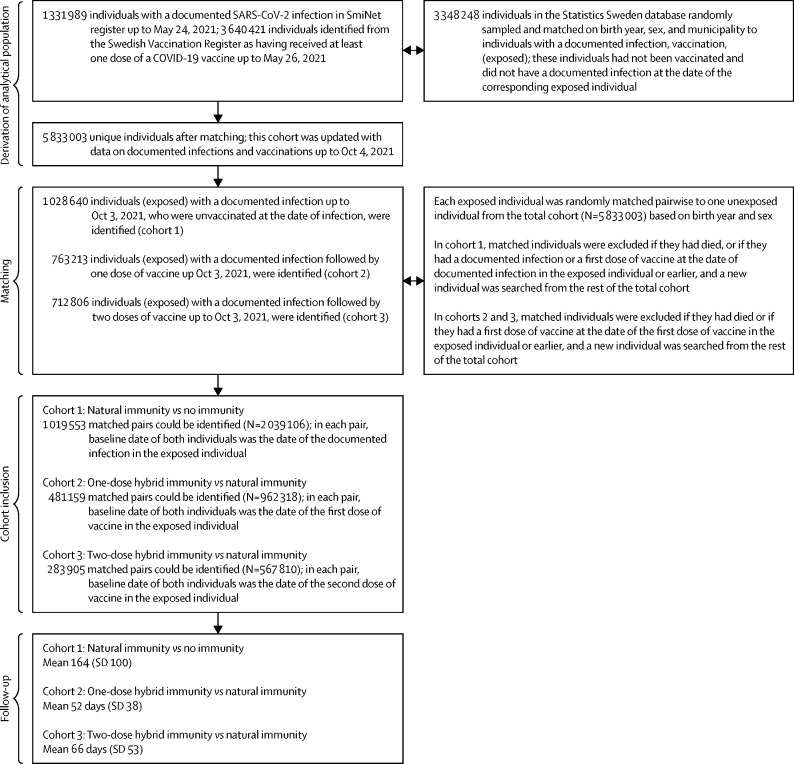
Individuals considered for inclusion were all people who had received at least one dose of any vaccine up until May 26, 2021 (N=3 640 421), and all individuals with a documented SARS-CoV-2 infection up until May 24, 2021 (N=1 331 989). Data on individuals vaccinated against COVID-19, including the type of vaccine received, were collected from the Swedish Vaccination Register and data on documented SARS-CoV-2 infections were collected from the SmiNet register; both registers are managed by the Public Health Agency of Sweden.23, 24 All health-care providers in Sweden are obliged to report to these registers according to Swedish law, with an expected 100% coverage. For each of these individuals, we randomly sampled one individual from the general population using the Statistics Sweden database. Individuals were matched (1:1) on birth year, sex, and municipality, resulting in a total cohort of 5 833 003 unique individuals (figure 1 ), from Sweden's total population of about 10·5 million individuals in June, 2021. This cohort was updated with respect to data on documented infections and vaccinations up to Oct 4, 2021. From this cohort, three study cohorts were formed. Cohort 1 was formed to compare natural immunity (exposed) to no immunity (unexposed). Here, all individuals with natural immunity with no previous vaccination (N=1 028 640) were randomly matched pairwise on birth year and sex to an individual from the total cohort. The matched individual was required to be alive at baseline, uninfected and without previous infection, and unvaccinated, otherwise a new match was sought from the remaining total cohort. A total of 1 019 553 exposed individuals could be pairwise matched to unexposed individuals, resulting in a total cohort size of 2 039 106 individuals. Baseline date for both individuals within each pair was the date of the documented previous infection in the exposed individual. The second and third cohorts were formed to do head-to-head comparisons of one-dose and two-dose hybrid immunity (exposed) versus natural immunity (unexposed). All individuals with one-dose hybrid immunity (N=763 213) or two-dose hybrid immunity (N=712 806) were randomly matched pairwise to an individual from the total cohort with natural immunity (N=1,028,640). Using the same principles for matching as in the first cohort, 481 159 matched pairs were identified in the second cohort (N=962 318), and 283 905 matched pairs were identified in the third cohort (N=567 810). Baseline date for both individuals within each pair in the second and third cohorts was the date of first dose of vaccine and second dose of vaccine in the exposed individual, respectively. The present study was approved by the Swedish Ethical Review Authority (495/2021), who waived the requirement of obtaining informed consent given the retrospective study design.
Figure 1.
Selection of the cohort
Study definitions
No immunity was defined as being unvaccinated and not having a documented previous SARS-CoV-2 infection at baseline. Natural immunity was defined as having a documented previous infection but being unvaccinated at baseline. One-dose hybrid immunity was defined as having a documented previous infection and having received a single dose of either ChAdOx1 nCoV-19 (Oxford-AstraZeneca), BNT162b2 (Pfizer-BioNTech), or mRNA-1273 (Moderna) either before or after infection at baseline. Two-dose hybrid immunity was defined as having a documented previous infection and having received two doses of any of the vaccines at baseline, with at least the second dose given after the infection.
Outcomes
This study had two outcomes. The first outcome was SARS-CoV-2 infection as documented in the SmiNet register from March 20, 2020, until Oct 4, 2021. Given that all confirmed infections are documented in this register, this outcome was defined as a SARS-CoV-2 infection of any severity for the present study. The second outcome was inpatient hospitalisation with COVID-19 as the main diagnosis and reason for admission, traced using the National Inpatient Register and the International Classification of Disease (ICD) version 10 code U071. This register is managed by the National Board of Health and Welfare. Hospitalisations in the cohorts could be tracked from March 30, 2020, until Sept 5, 2021. Only hospitalisations and infections that occurred more than 14 days after baseline were evaluated. Planned follow-up for assessment of outcomes was 20 months for cohort 1 and was 9 months for cohorts 2 and 3, and follow-up time in days was counted until the date of either a confirmed SARS-CoV-2 infection or COVID-19 hospitalisation, a vaccination after baseline (for unvaccinated individuals and individuals with one-dose hybrid immunity), death, or end of possible follow-up time (Oct 4, 2021, for the infection outcome and Sept 5, 2021, for the hospitalisation outcome), whichever occurred first.
Statistical analysis
Hazards over time for the outcome of SARS-CoV-2 infection based on immunity status were illustrated using proportional hazards models with 95% CIs and restricted cubic splines. The knots were placed in default position. To compare the risk of both outcomes (SARS-CoV-2 infection and COVID-19 hospitalisation) on the basis of immunity status, Cox regression was used to calculate hazard ratios (HR). To adjust for the matched samples, 95% CIs were estimated using robust standard errors by the variance–covariance matrix (VCE) command and robust option in Stata. To formally test whether the associations were time dependent, Schoenfeld's residuals were evaluated using estat phtest command in Stata. Because the test indicated that the proportional hazard assumption was violated (p<0·05) for some of the main exposures, the associations were also evaluated in time intervals in the cohorts. In all cohorts, the first model was adjusted for age and baseline date, to account for variations in infection pressure during follow-up (reported as HR). The second model included the additional covariates sex, homemaker service (yes or no), education (six categories), marital status (five categories), whether the individual was born in Sweden or not, and nine diagnoses at baseline (yes or no; reported as adjusted [a]HR). To investigate whether there was effect measure modification of the associations between the exposure and outcome by any of the covariates, interaction analyses were done using product terms created by multiplying the variable coding for immunity status at baseline by each respective covariate, which were added to the fully adjusted Cox model. Given that the interaction terms were significant (p<0·001) for many covariates, associations were investigated in subgroups according to these covariates, including type of vaccine for two-dose hybrid immunity compared with natural immunity. Other subgroup analyses were by age, sex, comorbidity status (compared with the total population), and homemaker service (compared with the total population).
The covariates were selected a priori on the basis of a previous study in a similar population.25 For the diagnoses of interest, the National Inpatient Register was used to obtain information about inpatient care since the beginning of 1998, and the National Outpatient Register was used to obtain information on outpatient specialist care since the beginning of 2001. Information about prescribed drugs of interest at baseline and death during follow up was obtained using the Prescribed Drug Register from the beginning of 2014 onwards and the Cause of Death Register, respectively, which is also managed by the National Board of Health and Welfare. Information about homemaker services (ie, help from the community with housekeeping tasks that older people can no longer perform) was also obtained from the National Board of Health and Welfare. Birth year and month, country of birth, marital status, level of highest education, and sex for all individuals in the cohort was obtained from Statistics Sweden. Definitions of comorbidities are provided in appendix p 2.
All analyses were done using SPSS version 27.0 for Mac and Stata version 16·1 for Mac. A two-sided p-value of less than 0·05 or a HR with a 95% CI not crossing 1 was considered significant. In the two-dose hybrid immunity versus natural immunity cohort, the number needed to vaccinate to prevent one reinfection in individuals with natural immunity was estimated as the difference in event rate between the two groups, inverted.
Role of the funding source
There was no funding source for this study.
Results
According to the SmiNet register, 94·4% of infections were confirmed using PCR, 4·8% through sequencing, and the remaining through a combination of methods. In cohort 1 (natural immunity vs no immunity), the mean baseline date was Jan 1, 2021, and the median age was 39·2 years (IQR 25·5–53·0; table 1 ). Individuals in cohort 2 (one-dose hybrid immunity vs natural immunity) had a median age of 39·9 years (28·3–52·4) at baseline and the mean baseline date was June 7, 2021, more than 6 months later than the baseline date in cohort 1, with a lower resulting infection pressure during follow-up, since individuals in cohort 2 and 3 missed the first wave, and shorter follow-up time. Individuals in cohort 3 (two-dose hybrid immunity vs natural immunity) had a median age of 37·6 years (27·8–50·3) with a baseline date of July 9, 2021. Individuals with natural immunity in the second and third cohorts were more often born outside of Sweden than those with natural immunity in the first cohort. Participant characteristics at different time intervals during follow-up in the cohorts are in the appendix (pp 3–4).
Table 1.
Baseline characteristics of the three study cohorts
|
Cohort 1* |
Cohort 2† |
Cohort 3‡ |
|||||
|---|---|---|---|---|---|---|---|
| Natural immunity (n=1 019 553) | No immunity (n=1 019 553) | One-dose hybrid immunity (n=481 159) | Natural immunity (n=481 159) | Two-dose hybrid immunity (n=283 905) | Natural immunity (n=283 905) | ||
| Age, years | 39·2 (25·5–53·0) | 39·2 (25·5–53·0) | 39·9 (28·3–52·4) | 39·9 (28·3–52·4) | 37·6 (27·8–50·3) | 37·6 (27·8–50·3) | |
| Sex | |||||||
| Female | 518 515 (50·9%) | 538 766 (52·8%) | 249 461 (51·8%) | 236 717 (49·2%) | 155 634 (54·8%) | 142 285 (50·1%) | |
| Male | 501 038 (49·1%) | 480 787 (47·2%) | 231 698 (48·2%) | 244 442 (50·8%) | 128 271 (45·2%) | 141 620 (49·9%) | |
| Homemaker service recipient§ | 29 381 (2·9%) | 17 582 (1·7%) | 9918 (2·1%) | 7160 (1·5%) | 7881 (2·8%) | 5026 (1·8%) | |
| Born in Sweden | 783 373 (76·8%) | 784 810 (77·0%) | 385 140 (80·0%) | 342 936 (71·3%) | 226 898 (79·9%) | 184 893 (65·1%) | |
| Marital status | |||||||
| Married | 366 339 (35·9%) | 343 165 (33·7%) | 184 948 (38·4%) | 182 237 (37·9%) | 101 445 (35·7%) | 100 150 (35·3%) | |
| Not married | 526 479 (51·6%) | 550 797 (54·0%) | 245 855 (51·1%) | 241 689 (50·2%) | 153 522 (54·1%) | 148 319 (52·2%) | |
| Divorced | 92 143 (9·0%) | 96 537 (9·5%) | 39 973 (8·3%) | 45 249 (9·4%) | 22 323 (7·9%) | 27 293 (9·7%) | |
| Widow or widower | 21 481 (2·1%) | 24 578 (2·4%) | 7715 (1·6%) | 7797 (1·6%) | 5013 (1·8%) | 4995 (1·8%) | |
| Other | 13 111 (1·3%) | 4 476 (0·4%) | 171 (< 0·1%) | 124 (< 0·1%) | 1602 (0·6%) | 3148 (1·1%) | |
| Education | |||||||
| Elementary school for <9 years | 40 311 (4·0%) | 42 264 (4·1%) | 14 197 (3·0%) | 20 180 (4·2%) | 8402 (3·0%) | 13 902 (4·9%) | |
| Elementary school for 9 years | 111 606 (10·9%) | 121 185 (11·9%) | 55 717 (11·6%) | 64 194 (13·3%) | 31 578 (11·1%) | 40 430 (14·2%) | |
| Secondary school for 2 years | 153 336 (15·0%) | 152 925 (15·0%) | 68 252 (14·2%) | 76 798 (16·0%) | 37 324 (13·1%) | 44 952 (15·8%) | |
| Secondary school for >2 years | 243 207 (23·9%) | 226 828 (22·2%) | 125 153 (26·0%) | 126 711 (26·3%) | 77 835 (27·4%) | 78 572 (28·7%) | |
| University | 355 077 (34·8%) | 350 063 (34·3%) | 192 321 (40·0%) | 163 866 (34·1%) | 114 261 (40·2%) | 87 778 (30·9%) | |
| Unknown | 116 016 (11·4%) | 126 288 (12·4%) | 25 519 (5·3%) | 29 410 (6·1%) | 14 505 (5·1%) | 18 281 (6·4%) | |
| Comorbidities | |||||||
| Myocardial infarction | 11 213 (1·1%) | 10 190 (1·0%) | 4358 (0·9%) | 4342 (0·9%) | 2371 (0·8%) | 2433 (0·9%) | |
| Stroke | 11 775 (1·2%) | 9 233 (0·9%) | 4522 (0·9%) | 3659 (0·8%) | 2953 (1·0%) | 2203 (0·8%) | |
| Diabetes | 46 234 (4·5%) | 51 901 (5·1%) | 25 365 (5·3%) | 20 994 (4·4%) | 15 078 (5·3%) | 12 242 (4·3%) | |
| Hypertension | 141 376 (13·9%) | 145 880 (14·3%) | 68 487 (14·2%) | 58 712 (12·2%) | 37 800 (13·3%) | 32 299 (11·4%) | |
| Kidney failure | 9614 (0·9%) | 7950 (0·8%) | 4155 (0·9%) | 3458 (0·7%) | 2531 (0·9%) | 2181 (0·8%) | |
| COPD | 8056 (0·8%) | 6642 (0·7%) | 3098 (0·6%) | 3942 (0·8%) | 1779 (0·6%) | 1809 (0·6%) | |
| Asthma | 67 440 (6·6%) | 65 240 (6·4%) | 31 538 (6·6%) | 27 803 (5·8%) | 19 215 (6·8%) | 16 218 (5·7%) | |
| Depression | 150 177 (14·7%) | 159 270 (15·6%) | 79 151 (16·5%) | 74 956 (15·6%) | 49 747 (17·5%) | 45 273 (15·9%) | |
| Cancer | 24 838 (2·4%) | 24 662 (2·4%) | 10 703 (2·2%) | 9932 (2·1%) | 6036 (2·1%) | 5480 (1·9%) | |
Data are n (%) or median (IQR).
Mean baseline date was Jan 1, 2021 (range Jan 25, 2020, to Oct 3, 2021).
Mean baseline date was June 7, 2021 (Dec 27, 2020, to Sept 27, 2021).
Mean baseline date was July 9, 2021 (Dec 30, 2020, to Oct 3, 2021).
Homemaker services include domestic services provided to individuals (primarily older individuals) who live at home but need help with shopping, cleaning, meal preparation, and similar tasks. COPD=chronic obstructive pulmonary disease.
The number of confirmed SARS-CoV-2 infections in Sweden during follow-up, and SARS-CoV-2 variants sequenced in Sweden during the study period, are shown in the appendix (pp 5, 8). During the study period there were three large waves of COVID-19. The first two waves (in March–June, 2020, and October, 2020, to January, 2021) occurred before the sequencing data were available, and before the alpha variant became dominant in Sweden. Based on the sequencing data, the alpha variant dominated during the third wave (in February–May, 2021) and the delta variant dominated from July, 2021, onwards (thus including the fourth wave that started in August, 2021).
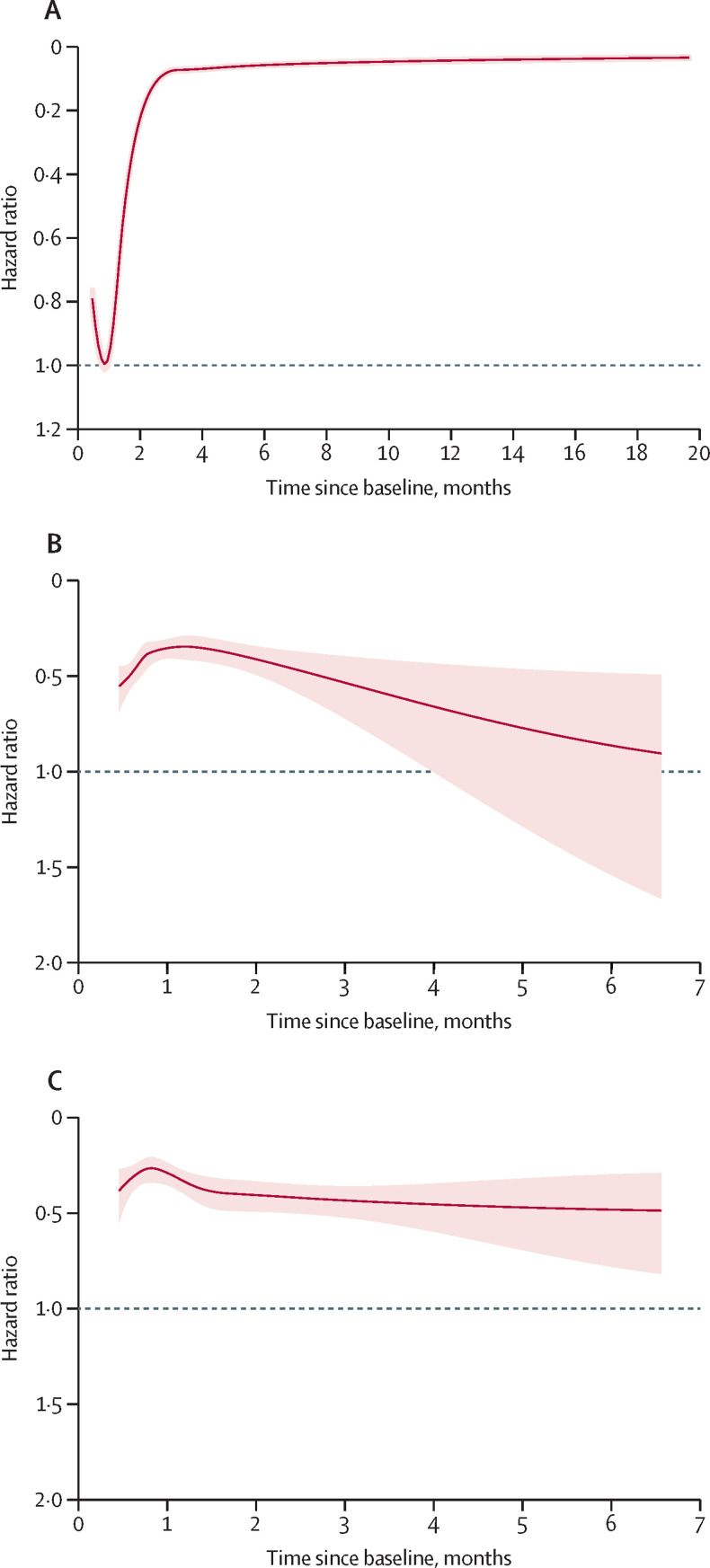
During a mean follow-up of 164 days (SD 100), 34 090 individuals with natural immunity were registered as having had a SARS-CoV-2 reinfection compared with 99 168 infections in non-immune individuals. Compared with no immunity, natural immunity was associated with a gradually reduced risk of reinfection during the first 3 months of follow-up (figure 2A ). After 3 months, the associated risk reduction was 95% (aHR 0·05 [95% CI 0·05–0·05]; p<0·001), with no signs of attenuation for up to 20 months of follow-up (figure 2A; table 2 ). The associations appeared to attenuate with increasing age (table 2), in people born outside Sweden (data not shown), with increasing education level (data not shown), and in people receiving a homemaker service (table 2; p<0·001 for all).
Figure 2.
Risk of SARS-CoV-2 infection in individuals with natural immunity compared with individuals without immunity (A), and risk of these outcomes in individuals with one-dose hybrid immunity (B) and two-dose hybrid immunity (C) compared with individuals with natural immunity
The associations were modelled using restricted cubic splines in default positions. The shaded areas show the 95% CI for the hazard ratios. The associations were adjusted for age, baseline date, sex, marital status, homemaker service, place of birth, education, and comorbidities according to table 1.
Table 2.
Risk of SARS-CoV-2 infection for individuals with natural immunity compared with individuals with no immunity
|
Natural immunity (n=1 019 553) |
No immunity (n=1 019 553) |
Hazard ratio (95% CI) |
|||||
|---|---|---|---|---|---|---|---|
| Number of events | IR per 100 000 person-days | Number of events | IR per 100 000 person-days | Adjusted for age and baseline date | Fully adjusted* | ||
| Follow-up time in total cohort | |||||||
| 14 days to 3 months (n=2 039 106) | 31 272 | 17·9 | 54 368 | 34·2 | 0·54 (0·53–0·55) | 0·52 (0·52–0·53) | |
| 3–6 months (n=1 477 887) | 1814 | 1·4 | 33 014 | 29·3 | 0·05 (0·05–0·05) | 0·04 (0·04–0·05) | |
| 6–9 months (n=783 767) | 700 | 0·7 | 7588 | 8·9 | 0·08 (0·08–0·09) | 0·08 (0·07–0·09) | |
| ≥9 months (n=303 236) | 304 | 0·6 | 4198 | 8·5 | 0·07 (0·06–0·08) | 0·07 (0·06–0·08) | |
| ≥3 months (n=1 477 887) | 2818 | 1·7 | 44 800 | 31·2 | 0·06 (0·05–0·06) | 0·05 (0·05–0·05) | |
| Events after ≥3 months follow-up, by sex | |||||||
| Male (n=722 657) | 1151 | 1·4 | 22 790 | 32·9 | 0·05 (0·04–0·05) | 0·04 (0·04–0·04) | |
| Female (n=755 230) | 1667 | 2·0 | 22 010 | 29·6 | 0·07 (0·06–0·07) | 0·06 (0·06–0·07) | |
| Events after ≥3 months follow-up, by age | |||||||
| <50 years (n=1 102 189) | 2070 | 1·6 | 37 775 | 35·3 | 0·05 (0·05–0·06) | 0·04 (0·04–0·05) | |
| 50–64 years (n=274 315) | 547 | 2·0 | 6139 | 23·4 | 0·08 (0·08–0·09) | 0·08 (0·07–0·09) | |
| 65–79 years (n=74 090) | 103 | 1·5 | 563 | 7·8 | 0·20 (0·16–0·25) | 0·18 (0·15–0·23) | |
| ≥80 years (n=27 305) | 98 | 4·3 | 323 | 8·4 | 0·55 (0·44–0·70) | 0·42 (0·33–0·53) | |
| Events after ≥3 months follow-up in those receiving homemaker service (N=19 324)† | 104 | 4·3 | 258 | 12·9 | 0·33 (0·26–0·41) | 0·32 (0·25–0·40) | |
| Events after ≥3 months follow-up in those with any comorbidity (n=441 752)† | 974 | 2·1 | 11 551 | 26·9 | 0·08 (0·07–0·08) | 0·07 (0·07–0·08) | |
In each matched pair, the baseline date for both individuals was set as the date of the first documented previous infection in the individual with natural immunity. Outcome events were traced from 14 days after baseline until a maximum follow-up of 613 days (mean 164 days [SD 100]). IR=incidence rate.
Adjusted for age, baseline date, sex, marital status, homemaker service, place of birth, education, and comorbidities according to table 1.
Comparison is with the total population.
For the outcome of COVID-19 hospitalisation (table 3 ), 3195 people with natural immunity were hospitalised and 1976 people with no natural immunity were hospitalised; natural immunity was associated with increased risk during the first 3 months of follow-up, but from 3 months onwards there was an associated 87% lower risk of COVID-19 hospitalisation in people with natural immunity than in people with no immunity (aHR 0·13, 95% CI 0·11–0·16, p<0·001) for up to 19 months of follow-up. The associations were weaker with increasing age and in individuals receiving a homemaker service (both p<0·001).
Table 3.
Risk of COVID-19 hospitalisation for individuals with natural immunity compared with individuals with no immunity
|
Natural immunity (n=1 018 636) |
No immunity (n=1 018 636) |
Hazard ratio (95% CI) |
|||||
|---|---|---|---|---|---|---|---|
| Number of events | IR per 100 000 person-days | Number of events | IR per 100 000 person-days | Adjusted for age and baseline date | Fully adjusted* | ||
| Follow-up time in total cohort | |||||||
| 14 days to 3 months (n=2 037 272) | 3065 | 3·6 | 1030 | 1·3 | 3·21 (2·98–3·45) | 3·02 (2·80–3·26) | |
| 3–6 months (n=1 546 582) | 77 | 0·06 | 632 | 0·53 | 0·12 (0·09–0·15) | 0·11 (0·09–0·14) | |
| 6–9 months (n=773 806) | 28 | 0·03 | 190 | 0·21 | 0·18 (0·12–0·26) | 0·15 (0·10–0·22) | |
| ≥9 months (N=260 984) | 25 | 0·06 | 124 | 0·27 | 0·28 (0·18–0·42) | 0·22 (0·15–0·34) | |
| ≥3 months (n=1 546 582) | 130 | 0·08 | 946 | 0·63 | 0·14 (0·12–0·17) | 0·13 (0·11–0·16) | |
| Events after ≥3 months follow-up, by sex | |||||||
| Male (n=756 942) | 80 | 0·10 | 548 | 0·75 | 0·15 (0·12–0·19) | 0·14 (0·11–0·17) | |
| Female (n=789 640) | 50 | 0·06 | 398 | 0·51 | 0·13 (0·10–0·18) | 0·12 (0·09–0·16) | |
| Events after ≥3 months follow-up, by age | |||||||
| <50 years (N=1 160 812) | 27 | 0·02 | 420 | 0·37 | 0·06 (0·04–0·09) | 0·06 (0·04–0·09) | |
| 50–64 years (n=283 569) | 46 | 0·17 | 338 | 1·3 | 0·13 (0·10–0·18) | 0·12 (0·09–0·17) | |
| 65–79 years (n=74 643) | 43 | 0·65 | 92 | 1·3 | 0·50 (0·35–0·71) | 0·43 (0·29–0·63) | |
| ≥80 years (n=27 558) | 14 | 0·62 | 96 | 2·5 | 0·25 (0·14–0·45) | 0·17 (0·10–0·31) | |
| Events after ≥3 months follow-up in those receiving homemaker service (n=19 626)† | 27 | 1·0 | 77 | 3·5 | 0·26 (0·16–0·43) | 0·22 (0·13–0·36) | |
| Events after ≥3 months follow-up in those with any comorbidity (n=459 097)† | 87 | 0·19 | 615 | 1·2 | 0·18 (0·17–0·93) | 0·16 (0·13–0·20) | |
In each matched pair, the baseline date for both individuals was set as the date of the first documented previous infection in the individual with natural immunity. Outcome events were traced from 14 days after baseline until a maximum follow-up of 588 days (mean 165 days [SD 95]).
Adjusted for age, baseline date, sex, marital status, homemaker service, place of birth, education, and comorbidities according to table 1.
Comparison is with the total population. IR=incidence rate.
During a mean follow-up of 52 days (SD 38), 639 individuals with one-dose hybrid immunity were registered with a SARS-CoV-2 reinfection, compared with 1662 individuals with natural immunity (appendix p 6). The associations attenuated with increasing follow-up time (figure 2B, p<0·001). Thus, during the first 2 months of follow-up, compared with natural immunity, one-dose hybrid immunity was associated with a 58% lower risk of reinfection (aHR 0·42 [95% CI 0·38–0·47]; p<0·001), which was reduced to 45% (0·55 [0·39–0·76]; p<0·001) from 2 months onwards. Overall, the associations were weaker in older individuals, in individuals with homemaker service (p<0·001), and in individuals with comorbidities (p<0·001; appendix p 6). With respect to COVID-19 hospitalisations, eight individuals were hospitalised among individuals with one-dose hybrid immunity (incidence rate [IR] 0·04) compared with 113 individuals with natural immunity (IR 0·56; HR 0·06 [95% CI 0·03–0·12]; p<0·001).
During a mean follow-up of 66 days (SD 53), 438 individuals with two-dose hybrid immunity were registered as having had a SARS-CoV-2 reinfection, compared with 808 individuals with natural immunity (appendix p 7). Correspondingly, the number of individuals with natural immunity needed to be double vaccinated to prevent one reinfection during follow-up was 767. Overall, two-dose hybrid immunity was associated with a 66% lower risk of reinfection than natural immunity (aHR 0·34 [95% CI 0·31–0·39]; p<0·001). During the first 2 months of follow-up, two-dose hybrid immunity was associated with a 69% (0·31 [0·26–0·36]; p<0·001) lower risk of reinfection than natural immunity. From 2 months onwards, the associated risk reduction was 56% (0·44 [0·35–0·56]; p<0·001), with no significant attenuation up to 9 months (p=0·07; figure 2C). Overall, the associations were slightly weaker in older individuals, in individuals with homemaker service (p<0·001), and in individuals with comorbidities (p<0·001; (appendix p 7). With respect to vaccine types, a significant association was observed for mRNA vaccines (aHR 0·32 [95% CI 0·28–0·37]; p<0·001) but not ChAdOx1 nCoV-19 (0·75 [0·41–1·37]; p=0·35; appendix p 7).
With respect to COVID-19 hospitalisations, six individuals with two-dose hybrid immunity were hospitalised (IR 0·04) compared with 40 individuals with natural immunity (IR 0·44; HR 0·10 [95% CI 0·04–0·22]; p<0·001).
Discussion
In this nationwide study, immunity acquired from a previous infection was associated with a low risk of SARS-CoV-2 reinfection and COVID-19 hospitalisation for up to 20 months. In head-to-head comparisons, immunity acquired from a previous infection plus either one or two doses of a COVID-19 vaccine was associated with a greater reduced risk of SARS-CoV-2 reinfection and COVID-19 hospitalisation for up to 9 months than previous infection only, although with small differences in absolute numbers during follow-up.
Many authorities recommend that all individuals should receive both primary series vaccination and booster vaccination irrespective of whether they have previously been infected. The strongest argument behind this recommendation might be the scarcity of evidence on long-term protection from natural immunity. A meta-analysis of 15 observational studies showed that natural immunity was associated with an 87% lower risk of reinfection than non-immunity for up to 1 year.13 Our study extends the body of evidence with up to 20 months of follow-up and more than 130 000 documented SARS-CoV-2 infections, and our results showed that individuals with natural immunity had an associated 95% protection against SARS-CoV-2 reinfection during follow-up (from 3 months after initial infection until 20 months), with no signs of waning. These results indicate that natural immunity might be better maintained than immunity induced by vaccination only, as suggested also by preliminary data from an Israeli study.19 In further support of our findings, we recently reported waning vaccine effectiveness against SARS-CoV-2 infection within a few months in a similar study based on the total population of Sweden.26 Another important finding of the present study is the association between natural immunity and later hospitalisation for a reinfection, which has not previously been reported in the literature. As expected, there was an increased risk of hospitalisation during the first 3 months after the first infection, but for those who survived, natural immunity was associated with 87% protection against COVID-19 hospitalisation during the rest of follow-up. The associated level of protection remained high (78%) even from 9 up to 19 months of follow-up, altogether indicating long-lasting protection, including against severe disease, from natural immunity. Together, these findings might suggest that any passports or documents used to identify whether a person is immune or not, and used for societal restrictions, should acknowledge either a previous infection or vaccination as proof of immunity, as opposed to vaccination only. This policy could have social and equity implications, especially for individuals in countries with low vaccine coverage, considering also that the dominant omicron variant is less sensitive to current vaccines than previous variants.27
The associated protection from natural immunity was lower in older individuals and in individuals receiving a homemaker service, as previously reported in a nationwide study from Denmark.28 Because these individuals have higher risk of critical illness and death from COVID-19,24, 29 boosting their level of protection is important. Thus, as further shown through head-to-head comparisons, individuals who had recovered from a previous infection had an additional associated protection against SARS-CoV-2 reinfection if they had also been vaccinated, although the associations appeared slightly weaker in older individuals. The associated benefit seemed slightly stronger and more stable for those given two doses rather than one dose of vaccine, and it was more clearly detectable among those vaccinated with mRNA vaccines than ChAdOx1 nCoV-19 (in whom the association was weaker and not significant, although the number of participants contributing to this analysis was small). This finding is supported by a systematic review and meta-regression of vaccine effectiveness studies, which suggested faster waning of immunity with ChAdOx1 nCoV-19 than with BNT162b2.30 In the present study, there was also evidence of waning protection, especially for those with one-dose hybrid immunity. Waning protection from hybrid immunity was observed also in a recent Israeli preprint study of 5·7 million individuals.19 Additionally, the absolute risk reduction associated with hybrid immunity in the present study was small, indicating that to prevent one SARS-CoV-2 reinfection among those with natural immunity, 767 individuals would need to be vaccinated with two doses. Similar results were reported in an Israeli preprint study including 14 029 matched pairs,31 in which one additional dose of vaccine in those with natural immunity was associated with seven fewer cases of symptomatic reinfection, suggesting that about 2000 individuals needed to be vaccinated to prevent one reinfection. If these associations are causal, the overall clinical relevance of these effects appears uncertain. In the present study, both one-dose and two-dose hybrid immunity were associated with protection against COVID-19 hospitalisation that was above the level of protection afforded by natural immunity. Because the primary aim of COVID-19 vaccination is to prevent severe disease, this finding is important. However, hospitalisations were rare, and because these analyses were based on a small number of cases, further studies should seek to assess the duration of protection of hybrid immunity against severe COVID-19.
This study has limitations that should be considered. First, the observational nature of the study limits the possibility to draw causal inferences, and there might be unknown confounding or bias not accounted for. For example, there is a risk of selection bias in individuals without a previous infection because they might be less inclined to take a PCR test than individuals with a documented previous infection, although this bias would not affect the estimates for the outcome of COVID-19 hospitalisation. Furthermore, as individuals with a previous infection were censored upon vaccination, the remaining cohort might have become less representative as time passed, introducing another selection bias. Although the associations were stable after adjustment for a rather rich set of covariates, the possibility of unmeasured confounding or bias remains. Moreover, in the analysis of hybrid immunity versus natural immunity, it is possible that vaccinated individuals with symptoms of infection are more prone to self-testing than individuals that remain unvaccinated after a documented infection. If so, this behaviour would attenuate the associations for the outcome of infection; however, it would not affect the associations with hospitalisation. Second, mean baseline date was not the same in all cohorts, which could mean that variations in infection pressure and dominating SARS-CoV-2 variants during follow-up influenced the results, although we adjusted all models for baseline date. Third, we could not evaluate how different variants of SARS-CoV-2 influenced the associations, as we did not have access to such data on an individual level. Fourth, although individuals with a documented previous infection were excluded from the non-immune cohort, individuals with a previous asymptomatic infection might still have been included. Similarly, there is a risk of misclassification bias (ie, false-positive and false-negative tests, which could potentially result in underestimated associated benefits of natural immunity vs no immunity). However, the PCR test has been estimated to have a 97·1% sensitivity and 99·9% specificilty.32 Fifth, it cannot be determined to what extent the results apply to the omicron variant. However, a study found that previous infection was associated with about 60% protection against symptomatic reinfection and about 90% protection against severe reinfection (hospitalisation or death) with the omicron variant.33
Strengths of the present study include the use of registers with 100% nationwide coverage and a follow-up time up of 20 months for the exposure of natural immunity and up to 9 months for hybrid immunity. Another strength is the head-to-head comparisons of hybrid immunity versus natural immunity, in which individuals in each pair were matched on birth year, had similar baseline characteristics, and started follow-up on the same date, and in which several covariates were adjusted for. Together, these methods reduce the risk of confounding when the aim is to perform direct comparisons. Finally, the large, population-based sample size increases the generalisability of the results to other countries with similar population structures.
This online publication has been corrected. The corrected version first appeared at thelancet.com/infection on April 8, 2022
Data sharing
The data files used for the present study are publicly unavailable according to regulations under Swedish law. However, access to all the data used for the present study can be requested from the National Board of Health and Welfare, Statistics Sweden, and the Public Health Agency of Sweden.
Declaration of interests
We declare no competing interests.
Acknowledgments
Contributors
All authors conceived and designed the study. PN acquired the data and did the statistical analyses. PN and MB accessed and verified the underlying data and drafted the manuscript. PN and AN supervised the work. All authors interpreted the data, critically revised the manuscript for intellectual content, gave final approval of the version to be published, had full access to all the data, and had final responsibility for the decision to submit for publication.
Supplementary Material
References
- 1.Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Voysey M, Clemens SAC, Madhi SA, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chemaitelly H, Yassine HM, Benslimane FM, et al. mRNA-1273 COVID-19 vaccine effectiveness against the B.1.1.7 and B.1.351 variants and severe COVID-19 disease in Qatar. Nat Med. 2021;27:1614–1621. doi: 10.1038/s41591-021-01446-y. [DOI] [PubMed] [Google Scholar]
- 5.Lopez Bernal J, Andrews N, Gower C, et al. Effectiveness of COVID-19 vaccines against the B.1.617.2 (delta) variant. N Engl J Med. 2021;385:585–594. doi: 10.1056/NEJMoa2108891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nordström P, Ballin M, Nordström A. Effectiveness of heterologous ChAdOx1 nCoV-19 and mRNA prime–boost vaccination against symptomatic COVID-19 infection in Sweden: a nationwide cohort study. Lancet Reg Health Eur. 2021;11 doi: 10.1016/j.lanepe.2021.100249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chung H, He S, Nasreen S, et al. Effectiveness of BNT162b2 and mRNA-1273 COVID-19 vaccines against symptomatic SARS-CoV-2 infection and severe COVID-19 outcomes in Ontario, Canada: test negative design study. BMJ. 2021;374 doi: 10.1136/bmj.n1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thomas SJ, Moreira ED, Jr, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine through 6 months. N Engl J Med. 2021;385:1761–1773. doi: 10.1056/NEJMoa2110345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.El Sahly HM, Baden LR, Essink B, et al. Efficacy of the mRNA-1273 SARS-CoV-2 vaccine at completion of blinded phase. N Engl J Med. 2021;385:1774–1785. doi: 10.1056/NEJMoa2113017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Falsey AR, Sobieszczyk ME, Hirsch I, et al. Phase 3 safety and efficacy of AZD1222 (ChAdOx1 nCoV-19) COVID-19 vaccine. N Engl J Med. 2021;385:2348–2360. doi: 10.1056/NEJMoa2105290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haas EJ, Angulo FJ, McLaughlin JM, et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet. 2021;397:1819–1829. doi: 10.1016/S0140-6736(21)00947-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hyams C, Marlow R, Maseko Z, et al. Effectiveness of BNT162b2 and ChAdOx1 nCoV-19 COVID-19 vaccination at preventing hospitalisations in people aged at least 80 years: a test-negative, case-control study. Lancet Infect Dis. 2021;21:1539–1548. doi: 10.1016/S1473-3099(21)00330-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Petráš M. Highly effective naturally acquired protection against COVID-19 persists for at least 1 year: a meta-analysis. J Am Med Dir Assoc. 2021;22:2263–2265. doi: 10.1016/j.jamda.2021.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Block J. Vaccinating people who have had COVID-19: why doesn't natural immunity count in the US? BMJ. 2021;374 doi: 10.1136/bmj.n2101. [DOI] [PubMed] [Google Scholar]
- 15.US Centers for Disease Control and Prevention Interim clinical considerations for use of COVID-19 vaccines currently approved or authorized in the United States. Feb 22, 2022. https://www.cdc.gov/vaccines/covid-19/clinical-considerations/covid-19-vaccines-us.html
- 16.Wei J, Matthews PC, Stoesser N, et al. Anti-spike antibody response to natural SARS-CoV-2 infection in the general population. Nat Commun. 2021;12 doi: 10.1038/s41467-021-26479-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goel RR, Painter MM, Apostolidis SA, et al. mRNA vaccines induce durable immune memory to SARS-CoV-2 and variants of concern. Science. 2021;374 doi: 10.1126/science.abm0829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abu-Raddad LJ, Chemaitelly H, Ayoub HH, et al. Association of prior SARS-CoV-2 infection with risk of breakthrough infection following mRNA vaccination in Qatar. JAMA. 2021;326:1930–1939. doi: 10.1001/jama.2021.19623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goldberg Y, Mandel M, Bar-On YM, et al. Protection and waning of natural and hybrid COVID-19 immunity. medRxiv. 2021 doi: 10.1101/2021.12.04.21267114. published online Dec 5. (preprint). [DOI] [Google Scholar]
- 20.Chemaitelly H, Bertollini R, Abu-Raddad LJ. Efficacy of natural immunity against SARS-CoV-2 reinfection with the beta variant. N Engl J Med. 2021;385:2585–2586. doi: 10.1056/NEJMc2110300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kojima N, Klausner JD. Protective immunity after recovery from SARS-CoV-2 infection. Lancet Infect Dis. 2022;22:12–14. doi: 10.1016/S1473-3099(21)00676-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Public Health Agency of Sweden Recommendations on order of priority of vaccination against COVID-19. https://www.folkhalsomyndigheten.se/smittskydd-beredskap/utbrott/aktuella-utbrott/covid-19/vaccination-mot-covid-19/rekommendationer-for-vaccination-mot-covid-19/
- 23.Public Health Agency of Sweden National Vaccination Register. https://www.folkhalsomyndigheten.se/smittskydd-beredskap/vaccinationer/nationella-vaccinationsregistret/
- 24.Public Health Agency of Sweden SmiNet. https://www.folkhalsomyndigheten.se/smittskydd-beredskap/overvakning-och-rapportering/sminet/
- 25.Bergman J, Ballin M, Nordström A, Nordström P. Risk factors for COVID-19 diagnosis, hospitalization, and subsequent all-cause mortality in Sweden: a nationwide study. Eur J Epidemiol. 2021;36:287–298. doi: 10.1007/s10654-021-00732-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nordström P, Ballin M, Nordström A. Risk of infection, hospitalisation, and death up to 9 months after a second dose of COVID-19 vaccine: a retrospective, total population cohort study in Sweden. Lancet. 2022;399:814–823. doi: 10.1016/S0140-6736(22)00089-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.UK Health Security Agency COVID-19 vaccine surveillance report: week 4. Jan 27, 2022. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1050721/Vaccine-surveillance-report-week-4.pdf
- 28.Hansen CH, Michlmayr D, Gubbels SM, Mølbak K, Ethelberg S. Assessment of protection against reinfection with SARS-CoV-2 among 4 million PCR-tested individuals in Denmark in 2020: a population-level observational study. Lancet. 2021;397:1204–1212. doi: 10.1016/S0140-6736(21)00575-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ballin M, Bergman J, Kivipelto M, Nordström A, Nordström P. Excess mortality after COVID-19 in Swedish long-term care facilities. J Am Med Dir Assoc. 2021;22:1574. doi: 10.1016/j.jamda.2021.06.010. 80.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Feikin D, Higdon MM, Abu-Raddad LJ, et al. Duration of effectiveness of vaccines against SARS-CoV-2 infection and COVID-19 disease: results of a systematic review and meta-regression. Lancet. 2022;399:924–944. doi: 10.1016/S0140-6736(22)00152-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gazit S, Shlezinger R, Perez G, et al. Comparing SARS-CoV-2 natural immunity to vaccine-induced immunity: reinfections versus breakthrough infections. medRxiv. 2021 doi: 10.1101/2021.08.24.21262415. published online Aug 25, 2021. (preprint). [DOI] [Google Scholar]
- 32.Lorentzen HF, Schmidt SA, Sandholdt H, Benfield T. Estimation of the diagnostic accuracy of real-time reverse transcription quantitative polymerase chain reaction for SARS-CoV-2 using re-analysis of published data. Dan Med J. 2020;67:67. [PubMed] [Google Scholar]
- 33.Altarawneh H, Chemaitelly H, Tang P, et al. Protection against the omicron variant from previous SARS-CoV-2 infection. N Engl J Med. 2022 doi: 10.1056/NEJMc2200133. published online Feb 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data files used for the present study are publicly unavailable according to regulations under Swedish law. However, access to all the data used for the present study can be requested from the National Board of Health and Welfare, Statistics Sweden, and the Public Health Agency of Sweden.