Abstract
Interspecific hybridization has been suggested to occur frequently in Rumex (Polygonaceae). Several hypothesized combinations of parental species of hybrids based on their intermediate morphology have been suggested in the genus, but few of them have been phylogenetically tested. We analyzed nuclear and chloroplast DNA sequence data of a putative natural hybrid between Rumex crispus and Rumex obtusifolius from Korea to confirm its hybrid status and to determine the maternal parent. Analysis of the nuclear DNA pgiC region revealed that R. crispus and R. obtusifolius have contributed to the nuclear genome of the putative hybrids. The haplotype distribution pattern inferred from the combined sequence data set of five chloroplast DNA regions (matK, rbcL-accD IGS, trnK-rps16 IGS, ycf6-psbM IGS and psbA-trnH IGS) indicated bidirectional hybridization events between R. crispus and R. obtusifolius. This paper provides the first molecular evidence for interspecific hybridization between R. crispus and R. obtusifolius. In addition, our findings strongly suggested that Korean populations of Rumex japonicus have a hybrid origin, and R. crispus may represent one of the parental taxa.
Subject terms: Evolution, Plant sciences
Introduction
The widespread occurrence of interspecific hybridization within Rumex L. (Polygonaceae) suggests that it may play a significant role in speciation and evolution in the genus1–4. Rumex comprises approximately 200 species commonly known as docks and sorrels in colloquial English5–7. It has a nearly worldwide distribution, but most species occur in temperate regions of both hemispheres, and some have become naturalized beyond their native range3,6. The genus has been classified into four subgenera, Rumex, Acetosa Raf., Acetosella Raf., and Platypodium (Willk.) Rech.f. Although interspecific hybrids have been reported frequently in subgenus Rumex3,8, hybridization has not been recorded between species in different subgenera3. Hybrids are often morphologically intermediate between the putative parents and generally exhibit partial or almost complete sterility. Spontaneous hybrids between species of Rumex are usually less fertile and ecologically successful than the parental species6.
Among the species of Rumex, R. crispus L. is the most widespread and ecologically most successful. It is native to Europe and southwestern Asia, but occurs almost worldwide as a fully naturalized and sometimes invasive alien6,8. Rumex obtusifolius L. usually coexists with R. crispus and likewise has a global distribution. Rumex obtusifolius is also native to Europe and southwestern Asia, but it is also naturalized in many other parts of the world, including southern Africa, South and North America, and south and northeast Asia and Australia6,8,9. Naturally occurring hybrids between R. crispus and R. obtusifolius are the most common in the genus, at least in Europe, and occur frequently wherever the parental species grow together, even in their naturalized ranges, including Australia and North America1,2,6,8. Rumex japonicus Houtt., which is distributed across northeast Asia including China, Korea, Japan and Far East Russia, frequently cooccurs with either R. crispus or R. obtusifolius separately or occasionally together.
Based on field observations and measurements, previous studies have indicated the occurrence of bidirectional introgression between R. crispus and R. obtusifolius1,2. In those studies, the hybrids exhibited characteristics of both parents in widely varying proportions ranging from individuals morphologically more similar to R. crispus to individuals that are nearly indistinguishable from R. obtusifolius. Backcrossing occurs with both parents, but is likely more common with R. crispus than with R. obtusifolius1. Backcrosses with R. crispus have been produced experimentally but have not been confirmed with R. obtusifolius3. In addition, hybrids are less fertile than the parents2.
Both R. crispus and R. obtusifolius are considered invasive alien species in Korea7,10,11. Such invasive plants can have significant negative impacts on the abundance and diversity of native species12,13. Plant invasions may also occur through hybridization. Invasive species can reduce genetic diversity and cause the extirpation of locally adapted populations of native species through hybridization, which is of particular concern for rare and threatened native species. Further, introgression with native relatives may give rise to more aggressive hybrid types, and the spread of aggressive hybrid taxa can reduce the fitness of, or replace, native species14.
Hybrid assessment in plants has been traditionally determined by using several criteria based on morphology, distribution, and crossing experiments15–17. More recently, various molecular methods have been used in determining hybridization events in higher plants18–21. Chloroplast DNA (cpDNA) is usually maternally inherited in angiosperms, so that it can be used to identify the maternal parent of putative hybrids22–24. The application of biparentally inherited, single- or low-copy nuclear DNA (nDNA) regions in combination with appropriate cpDNA regions has become an efficient way to validate hybridization events in plants25–27. By utilizing such methods, hybrids have been proposed and validated in many genera, including Melastoma L., Eriobotrya Lindl., Ilex L., and Microsorum Link28–31. However, only a few attempts have been made to characterize hybrids in Rumex using molecular methods4.
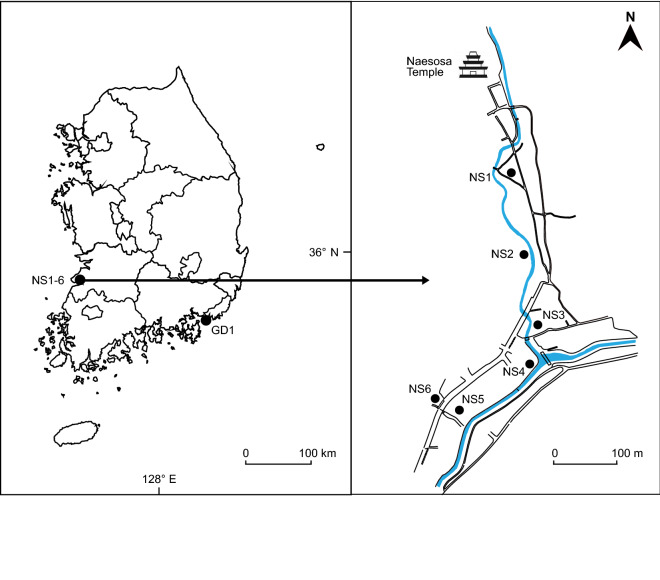
In Korea, 12 species of Rumex comprising three subgenera, Rumex (10 species), Acetosa (one species) and Acetosella (one species), have been reported7. In our investigation of natural hybridization among the species of subgenus Rumex across their entire range in South Korea, we found several plants with intermediate morphology in mixed populations of R. crispus and R. obtusifolius in seven localities (Fig. 1). Those observations prompted us to investigate further by employing low copy nDNA pgiC gene and five cpDNA regions (matK, rbcL-accD IGS, trnK-rps16 IGS, ycf6-psbM IGS and psbA-trnH IGS) to assess their hybrid status and potential introgression.
Figure 1.
Geographic location of seven mixed populations of species of Rumex and putative hybrids in Korea sampled for this study. Six populations (NS1–NS6) were in the Naesosa area of Jeon-buk Province. One population (GD1) was on Gadeok Island, Busan. Naesosa and Gadeok Island are separated by over 200 km.
Results
Chloroplast DNA
The sequence characteristics of the examined cpDNA regions, matK, rbcL-accD IGS, trnK-rps16 IGS, ycf6-psbM IGS and psbA-trnH IGS, are summarized in Table 1. The combined cpDNA data set was 4007 base pairs (bp) in length after alignment (Table 1). There were 259 (6.5%) variable characters, 108 (2.7%) of which were parsimony informative (Table 1).
Table 1.
Statistics for cpDNA and nDNA sequence data sets used in this study. Numbers in parentheses represent comparisons of ingroup taxa only.
| cpDNA | nDNA | ||||||
|---|---|---|---|---|---|---|---|
| matK | rbcL-accD IGS | trnK-rps16 IGS | ycf6-psbM IGS | psbA-trnH IGS | Combined cpDNA | pgiC | |
| Sequence length (bp) | 961–967 | 463–475 | 765–895 | 918–1094 | 328–369 | 3623–3646 | 962–1249 |
| Aligned length (bp) | 967 | 508 | 967 | 1147 | 418 | 4007 | 1387 |
| No. of variable sites | 37 (2) | 28 (0) | 79 (3) | 79 (3) | 36 (6) | 259 (14) | 284 (107) |
| No. of parsimony informative characters | 23 (2) | 6 (0) | 28 (3) | 34 (3) | 17 (6) | 108 (14) | 132 (86) |
| GC ratio (%) | 33.6–33.9 | 31.9–32.5 | 26.4–27.4 | 30.8–32.5 | 28.7–33.2 | 30.5–31.6 | 34.9–37.7 |
| Optimal model of sequence evolution | GTR + I | HKY | GTR + G | GTR | F81 + I | GTR + G | HKY + I |
Based on the combined data set, six cpDNA haplotypes were identified from 70 accessions of R. crispus, R. obtusifolius, R. japonicus and the putative hybrids (Table 2, Supplementary Table S1). Four haplotypes (C1, C3–C5) were recovered from 17 accessions of R. crispus sampled for this study. Among those haplotypes, haplotypes C3–C5 were also detected in accessions from pure, non-mixed populations of R. crispus, but haplotype C1 was restricted to those from mixed populations in the Naesosa area (Table 2, Supplementary Table S1). In R. crispus, haplotype C5 was detected in one accession and haplotypes C3 and C4 were detected in three and four accessions, respectively. In contrast to R. crispus, a single haplotype (C2) was detected in all accessions of R. obtusifolius. A total of five haplotypes (C1–C5) were recovered from the putative hybrids. In R. japonicus, two haplotypes (C1 and C6) were detected (Table 2, Supplementary Table S1).
Table 2.
Variable nucleotide sites in five cpDNA regions of Rumex crispus, R. obtusifolius, R. japonicus and putative hybrids.
| Haplotype | Taxon | Variable nucleotide sites in alignment | ||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| matK | rbcL-accD IGS | trnK-rps16 IGS | ycf6-psbM IGS | psbA-trnH IGS | ||||||||||||||||||||||||||||||||||||||
| 0 5 4 4 |
0 6 3 2 |
1 4 1 1 |
2 4 1 1 |
3 4 1 1 |
4 4 1 1 |
5 4 1 1 |
1 9 2 1 |
6 4 6 1 |
1 9 0 2 |
6 1 2 2 |
1 9 0 3 |
2 9 0 3 |
3 9 0 3 |
4 9 0 3 |
5 9 0 3 |
6 9 0 3 |
3 8 1 3 |
8 1 3 3 |
6 2 3 3 |
6 5 5 3 |
7 5 5 3 |
8 5 5 3 |
9 5 5 3 |
0 6 5 3 |
6 6 7 3 |
8 6 7 3 |
9 6 7 3 |
0 7 7 3 |
1 7 7 3 |
3 7 7 2 |
3 7 7 3 |
4 7 7 3 |
5 7 7 3 |
6 7 7 3 |
3 1 8 3 |
8 1 8 3 |
2 6 8 3 |
4 8 8 3 |
5 0 9 3 |
7 0 9 3 |
||
| C1 | R. crispus, R. japonicus, R. crispus x R. obtusifolius | T | G | – | – | – | – | – | – | G | T | T | – | – | – | – | – | – | T | A | A | C | A | T | T | A | A | – | – | – | – | – | – | – | – | – | A | T | G | T | T | A |
| C2 | R. obtusifolius, R. crispus x R. obtusifolius | T | T | T | T | G | C | G | – | A | T | C | A | T | A | T | G | T | C | G | G | C | A | T | T | A | A | – | – | – | – | – | – | – | – | – | G | – | G | G | T | A |
| C3 | R. crispus, R. crispus x R. obtusifolius | T | T | – | – | – | – | – | – | A | T | C | A | T | A | T | G | T | C | G | G | C | A | T | T | A | C | – | – | – | – | – | – | – | – | – | G | – | G | G | T | A |
| C4 | R. crispus, R. crispus x R. obtusifolius | T | T | – | – | – | – | – | – | A | T | C | A | T | A | T | G | T | C | G | G | C | A | T | T | A | A | – | – | – | – | – | – | – | – | – | G | – | G | G | T | A |
| C5 | R. crispus, R. crispus x R. obtusifolius | A | G | – | – | – | – | – | T | A | G | T | A | T | A | T | G | T | C | G | G | – | – | – | – | – | A | T | G | T | T | A | T | G | A | G | G | – | A | G | G | G |
| C6 | R. japonicus | A | G | – | – | – | – | – | T | A | G | T | A | T | A | T | G | T | C | G | G | C | A | T | T | A | A | T | G | T | T | A | T | G | A | G | G | – | A | G | G | G |
The distribution of cpDNA haplotypes in six populations in the Naesosa (NS1–NS6) area and one population on Gadeok Island (GD1) was examined (Supplementary Table S1). In populations NS1 and NS6, R. crispus contained haplotype C1, whereas the putative hybrids exhibited either haplotype C2 (three accessions) or C5 (five accessions). Rumex japonicus in population NS1 contained haplotype C1. In population NS2, R. crispus possessed either haplotype C1 (three accessions) or C4 (one accession), whereas all seven accessions of the putative hybrids exhibited haplotype C2. Rumex japonicus possessed either haplotype C1 (one accession) or C6 (two accessions). Two accessions of R. crispus sampled from population NS3 possessed haplotype C1. Haplotype C2 occurred in two putative hybrid individuals sampled from the same population. Rumex japonicus in this population exhibited haplotype C6 (Supplementary Table S1).
In population NS4, four haplotypes (C1, C3, C4 and C5) were detected in six accessions of R. crispus (Supplementary Table S1). Those same haplotypes were shared by nine accessions of the putative hybrids sampled from the same population. Three other accessions of the putative hybrids had haplotype C2. In particular, haplotype C3 was not found in any other populations in the Naesosa area, but was detected in accessions of R. crispus and the putative hybrids from the distantly located population on Gadeok Island (GD1). In population NS5, R. crispus possessed haplotype C1, whereas the putative hybrids possessed haplotypes C1 (three accessions), C2 (one accession) or C5 (two accessions). In population NS6, R. crispus possessed haplotype C1, whereas the putative hybrids possessed haplotypes C2 (two accessions) or C5 (two accessions). In population GD1 of Gadeok Island, accessions of both R. crispus and the putative hybrids shared haplotype C3; no putative hybrids shared a cpDNA haplotype with R. obtusifolius (Supplementary Table S1).
Nuclear DNA pgiC
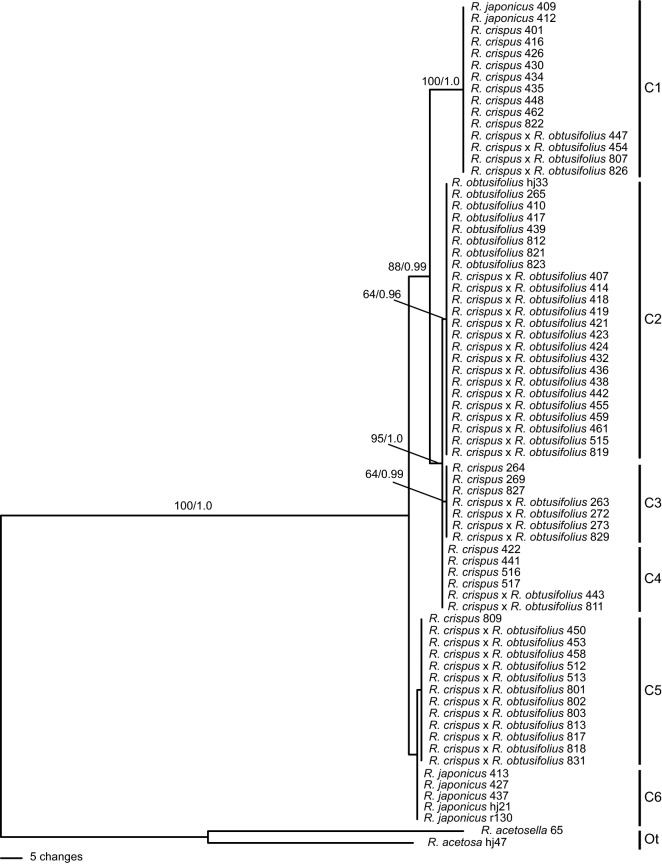
A total of 85 sequences of the nDNA pgiC region was recovered from 57 accessions representing R. crispus, R. obtusifolius, R. japonicus and the putative hybrids (Fig. 3). In addition, we examined the pgiC sequences of nine accessions of R. crispus and six of R. obtusifolius obtained from pure, non-mixed populations of each species (Supplementary Table S1). The length of pgiC was 978 bp in R. crispus, 996 bp in R. obtusifolius, 968–1249 bp in R. japonicus, and 978–996 bp in the putative hybrids. The length of the pgiC data set after alignment was 1387 bp (Table 1). There were 284 (20.5%) variable characters, 132 (9.5%) of which were parsimony informative (Table 1).
Figure 3.
Most parsimonious tree from the maximum parsimony analysis of the nDNA pgiC sequence data set for Rumex crispus, R. obtusifolius, R. japonicus and putative hybrids. Numbers above branches indicate MP bootstrap values (BS ≥ 50) and Bayesian posterior probabilities (PP ≥ 0.7). Accession numbers correspond to those in Supplementary Table S1. Numbers following accession numbers are clone numbers. N1–N5 = nDNA pgiC haplotypes; Ot = Outgroup.
All accessions of R. crispus and R. obtusifolius and eight accessions of the putative hybrids had a single pgiC sequence type, but 19 accessions of the putative hybrids had two sequence types. Accessions of R. japonicus had two or three sequence types (Fig. 3). Five pgiC haplotypes (N1–N5) were identified from 57 accessions of R. crispus, R. obtusifolius, R. japonicus, and the putative hybrids (Fig. 3, Supplementary Table S1). All accessions of R. crispus exhibited haplotype N2, whereas those of R. obtusifolius possessed haplotype N3. Four haplotypes (N1, N2, N4 and N5) were detected in R. japonicus. Accessions of the putative hybrids possessed either haplotype N2 or N3 only, or both N2 and N3 pgiC haplotypes (Fig. 3).
Phylogenetic analyses
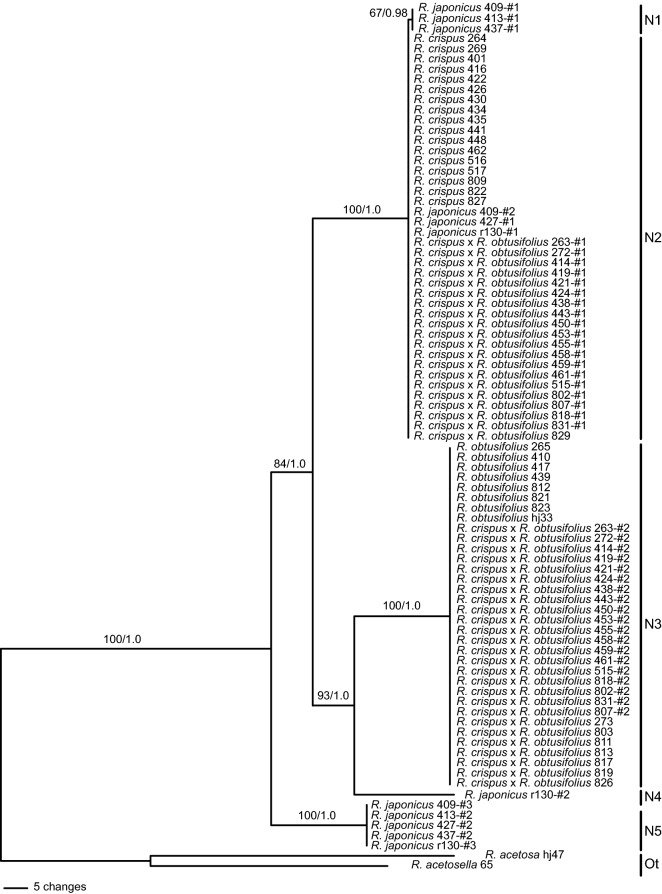
The maximum parsimony (MP) analysis of the combined cpDNA data set resulted in a single most parsimonious tree with 278 steps (CI = 0.989, RI = 0.994) (Fig. 2). The majority-rule consensus tree obtained from Bayesian inference (BI) analysis of the same data set was basically identical to the MP tree in topology and groupings (Supplementary Fig. S1). In the cpDNA tree (Fig. 2), none of the species included in this study were resolved as monophyletic. Accessions of R. obtusifolius formed a weakly supported clade (BS = 64, PP = 0.96) with 16 accessions of the putative hybrids; these accessions shared haplotype C2. Also, accessions of R. crispus were not resolved as monophyletic; they formed widely separated clades either with accessions of the putative hybrids (clades with haplotype C3 or C5) or with those of the putative hybrids and R. japonicus (clade with haplotype C1). Four accessions of R. crispus and two accessions of the putative hybrids with haplotype C4 remained unresolved within the clade comprising accessions with haplotype C2 or C3. The accessions of the putative hybrids grouped with either R. crispus or R. obtusifolius, or with a clade including R. crispus and R. japonicus (Fig. 2). The results strongly suggested that hybridization has occurred in the populations sampled.
Figure 2.
Most parsimonious tree from the maximum parsimony analysis of the combined cpDNA sequence data set for Rumex crispus, R. obtusifolius, R. japonicus and putative hybrids. Numbers above branches indicate MP bootstrap values (BS ≥ 50) and Bayesian posterior probabilities (PP ≥ 0.7). Accession numbers correspond to those in Supplementary Table S1. C1–C6 = cpDNA haplotypes; Ot = Outgroup.
The MP analysis of the nDNA pgiC data set resulted in a single most parsimonious tree with 345 steps (CI = 0.939, RI = 0.990) (Fig. 3). The majority-rule consensus tree obtained from BI analysis of the same data set was identical to the MP tree in topology and groupings (Supplementary Fig. S2). In the nDNA tree (Fig. 3), four strongly supported clades (BS ≥ 93, PP = 1.0) were resolved; a clade consisting of haplotypes N1 from R. japonicus (three cloned accessions) and N2 from R. crispus, the putative hybrids (20 accessions; 19 cloned) and R. japonicus (three cloned accessions), (2) a clade representing haplotype N3 from R. obtusifolius and the putative hybrids (26 accessions; 19 cloned), (3) clade representing haplotype N4 from R. japonicus (one cloned accession), and (4) clade representing haplotype N5 from R. japonicus (five cloned accessions). Especially noteworthy is that all 19 cloned accessions of the putative hybrids recovered two haplotypes, N2 and N3, which were present in all accessions of R. crispus and R. obtusifolius, respectively. Eight directly sequenced accessions of the putative hybrids recovered either haplotype N2 or N3 (Fig. 3).
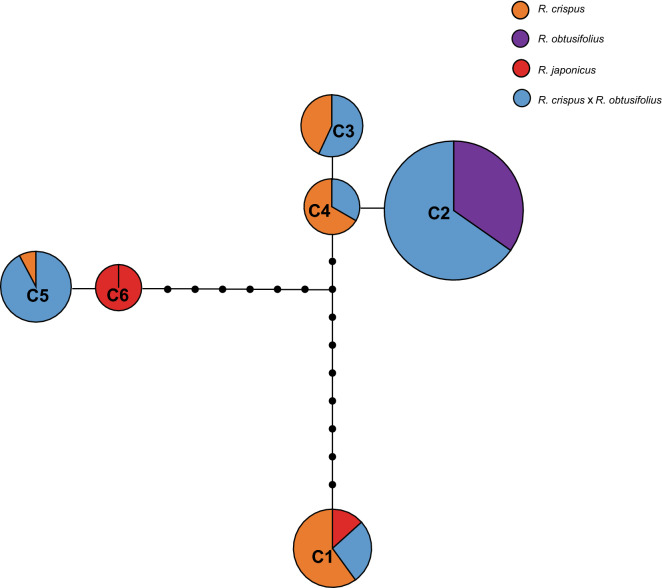
The statistical parsimony analysis of the combined cpDNA sequences as implemented in TCS resulted in a network of six haplotypes (Fig. 4). Those haplotypes were separated by one to 16 mutations. Fifteen haplotypes inferred by TCS were not found in the analyzed individuals and occurred as missing haplotypes in the network. Four haplotypes (C1, C3, C4, and C5) belonged to R. crispus; all those haplotypes were shared with hybrid accessions. The haplotypes in R. crispus were separated by one (C3 and C4) to 16 (C1 and C5) mutation steps. Only one haplotype (C2) was found in R. obtusifolius, and it was shared with hybrid accessions. Haplotype C6 was found only in R. japonicus.
Figure 4.
Statistical parsimony network of cpDNA sequences from Rumex crispus, R. obtusifolius, R. japonicus and putative hybrids. Each circle represents a haplotype; circle size is approximately proportional to haplotype frequency. Lines represent single mutation steps; small black circles represent inferred haplotypes but not present in samples.
Discussion
In the present study, we detected putative hybrid individuals with intermediate morphology from mixed populations of R. crispus and R. obtusifolius in seven localities in Korea (Fig. 1). The cloned nDNA pgiC sequences of the 19 accessions of the putative hybrids sampled from those populations revealed two types of sequences in each accession, haplotypes N2 and N3. Haplotypes N2 and N3 were exhibited by R. crispus and R. obtusifolius, respectively. Haplotype N2 differed from haplotype N3 by 54 bp substitutions, and there were no shared pgiC haplotypes between the two species (Fig. 3, Supplementary Table S2). The intermediate morphology and co-occurrence of two divergent pgiC haplotypes corresponding to R. crispus and R. obtusifolius indicated that those individuals were F1 hybrids between them. The putative hybrid accessions shared cpDNA haplotypes with either R. crispus or R. obtusifolius, suggesting that both species served as the maternal parent.
In comparison, eight directly sequenced accessions of the putative hybrids with intermediate morphology recovered either nDNA pgiC haplotype N2 (one accession) or N3 (seven accessions) (Fig. 3). Since these putative hybrid individuals occur only in mixed populations of the presumed parental species, and most of their flowers failed to set fruit, became dry and fell before any appreciable enlargement of the valves, it is highly likely that they represent backcross generation of F1 hybrids. Repeated backcrossing to either one of parental species may lead to elimination of one of the two copies initially present in the nuclear genome of F1 hybrids32. In particular, six putative hybrid accessions (273, 803, 811, 813, 817, 826) shared nDNA pgiC haplotype N3 with R. obtusifolius, but shared cpDNA haplotypes (C1, C3–C5) with C. crispus (Figs. 2, 3). The findings strongly suggested that backcrossing or introgression has occurred in these populations to at least some extent.
Maternal inheritance of chloroplasts, which is typical in angiosperms, has been experimentally demonstrated in the Polygonaceae18. If maternal chloroplast inheritance is also assumed to occur in Rumex, then the pattern of haplotype distribution revealed in our study suggests bidirectional hybridization events between R. crispus and R. obtusifolius. In population NS4, for example, nine accessions of the putative hybrids comprised four cpDNA haplotypes (C1, C3–C5), all of which were detected in R. crispus accessions from the same population. On the other hand, the other three accessions of the putative hybrids contained haplotype C2 detected in R. obtusifolius accessions also from the same population (Supplementary Table S1). The results strongly indicated that hybridization between the two species was bidirectional, and both R. crispus and R. obtusifolius served as either paternal or maternal parent. Bidirectional hybridization between R. crispus and R. obtusifolius has been suggested in previous studies1,2. Bidirectional hybridization in plants is relatively common and has been reported in several other plant taxa18,33–35.
The cpDNA haplotype C3 detected in population NS4 was not recovered from any other populations in the Naesosa area, but was detected in accessions of R. crispus and putative hybrids from the distantly located Gadeok Island population (GD1); populations NS4 and GD1 are separated by over 200 km. It is possible that we were unable to detect this haplotype in other populations in the Naesosa area due to the relatively small sample size. Conversely, the results may suggest the occurrence of long-distance seed dispersal. In Rumex, achenes are dispersed by wind or occasionally through excreta of mammals and birds36. Birds occasionally eat seeds of R. crispus when other quality food is not available, and seedlings have been raised from the excreta of various birds36,37.
The detection of four cpDNA haplotypes (C1, C3–C5) in only six accessions of R. crispus in population NS4 indicated that R. crispus is highly polymorphic for cpDNA haplotypes (Supplementary Tabel S1). The haplotype network revealed that haplotypes C1 and C5 possessed by the accessions of R. crispus in population NS4 were markedly different from each other and separated by 16 mutation steps (Fig. 4); this strongly suggested that haplotypes C1 and C5 probably diverged long ago. In contrast, all accessions of R. crispus from all populations had identical nuclear haplotypes. Since R. crispus invaded Korea relatively recently, the co-occurrence of highly divergent cpDNA haplotypes in populations of R. crispus may suggest repeated invasions from multiple sources with different genetic backgrounds10,38.
Although cpDNA haplotype C1 and nDNA pgiC haplotype N1 were shared by R. japonicus, R. crispus and some putative hybrid accessions, it is unlikely that R. japonicus participated in the formation of those hybrid individuals, as one of the two cpDNA haplotypes and three of the four nDNA pgiC haplotypes exhibited by R. japonicus were unique and not detected in any putative hybrid accessions (Figs. 2, 3, 4). In contrast, all the cpDNA and nDNA pgiC haplotypes of R. crispus were detected in the putative hybrids (Figs. 2, 3, 4). The results strongly suggested that R. crispus, rather than R. japonicus, served as one of the parental species of the putative hybrids.
The cloning of five accessions of R. japonicus recovered multiple pgiC sequence types; two accessions (r130, 409) had three sequence types (haplotypes N2, N5 and N1 or N4), and three (413, 427, 437) had two sequence types (haplotypes N1 or N2 and N5) (Fig. 3). Among the multiple pgiC haplotypes recovered from R. japonicus, haplotype N2 was shared by R. crispus (Fig. 3). Rumex japonicus possessed two cpDNA haplotypes, one of which was shared with R. crispus. Our findings suggested that Korean populations of R. japonicus have a hybrid origin and that R. crispus may represent one of the parental taxa. However, the hybrid status and parentage of the Korean populations of R. japonicus were beyond the scope of this study. Further studies are needed to validate the hybrid origin and parental taxa involved.
Putative hybrid individuals in all populations bore a few fruit containing potentially viable seeds. Molecular data supported the F1 hybrid or backcross nature of these individuals. The fertility of F1 hybrids and backcrosses between R. crispus and R. obtusifolius have been reported previously1,2,39. As such, putative hybrids and backcrosses may lead to the formation of hybrid swarms. Hybrid individuals between R. crispus and R. obtusifolius reported from Europe, Australia and North America have been referred to as R. x pratensis Mert. & W. D. J. Koch1–3,8,40.
Analyses of chloroplast and nuclear DNA sequence data in the present study provided compelling evidence for the occurrence of natural bidirectional hybridization between R. crispus and R. obtusifolius. The hybrids arise frequently in mixed populations of the two parental species in Korea. Confirmation of interspecific hybridization between recently introduced alien species such as R. crispus and R. obtusifolius would be significant because of the possible ecological consequences of hybrids and hybrid swarms. The hybrid populations we sampled were located in disturbed sites including roadsides, abandoned fields, forest clearings, and riparian zones. Thus, it appears that habitat disturbance may favor the formation of interspecific hybrids in Rumex. Frequent hybridization between species of Rumex in disturbed habitats has been reported previously1,2,4. The possible existence of F2 progeny and backcross generations in Rumex have been reported in previous studies2,4. The fertility of backcross generations usually increases as compared to that of the F1 generation in Rumex and in several other angiosperms, including Trifolium L. and Helianthus L.2,41,42 The increased fertility of backcross generations in hybrid swarms may result in the loss of genetic integrity of the parental species. In addition, it is possible that F1 hybrids and backcross plants are more invasive than their parental species due to hybrid vigor. Consequently, those plants may negatively impact native biodiversity, especially native plants of grasslands and forest margins, by occupying similar habitats and competing for resources. We recommend further study to understand the ecological consequences of the hybrids and to clarify whether the genetic integrity of the parental species is maintained. In addition, further studies are needed to validate the hybrid origin of the Korean populations of R. japonicus.
Materials and methods
Taxon sampling
We sampled 17 individuals of R. crispus, eight individuals of R. obtusifolius, seven individuals of R. japonicus and 38 individuals of putative hybrids from six populations (NS1–NS6) in the Naesosa region, Jeon-buk Province, and one population (GD1) on Gadeok Island of Busan, Korea (Fig. 1, Supplementary Table S1). We also examined nine accessions of R. crispus and six of R. obtusifolius obtained from pure, non-mixed populations of each species to validate species-specific haplotypes of those species (Supplementary Table S1). Because it occurs in some mixed populations of R. crispus and R. obtusifolius, we included R. japonicus in our study to evaluate its potential contribution to the nuclear and/or chloroplast genome of the putative hybrids. The individuals were collected in July 2020 and June-July 2021. Naesosa (35° 37′ N 126° 35′ E) refers to the site of an ancient Buddhist temple in Byeonsanbando National Park on the west coast of South Korea (Fig. 1). It is located at the base of a mountain at an altitude of 30 m above sea level. The study sites in Naesosa were disturbed areas, including roadsides, abandoned agricultural fields, forest clearings and riparian zones. Gadeok (35° 02′ N 128° 49′ E) is an island in Busan, South Korea (Fig. 1). The sampled population on the island was on a roadside at the base of a mountain, at an altitude of about 40 m above sea level. Permission for collecting plant samples from the Naesosa area was obtained from Byeonsanbando National Park, Korea. The collection locations of the other plant samples were neither in protected areas nor on private land, and thus no permission was required for collections from these locations. Rumex acetosa L. (subgenus Acetosa) and R. acetosella L. (subgenus Acetosella) were selected as outgroups.
Initial identifications of the individuals collected from the above populations were carried out by the authors based on differences in the major morphological characters of Rumex. Rumex crispus, R. obtusifolius and R. japonicus are distinguished mainly on the basis of differences in the shape of the leaves and the valves of their fruit. The basal and lower cauline leaves of R. crispus are lanceolate or oblong-lanceolate with strongly crisped margins. The valves are broadly ovate with entire margins. In contrast, the basal and lower cauline leaves of R. obtusifolius are usually ovate to elliptic or ovate-oblong with entire margins, and the valves are triangular-ovoid with spinose margins. Rumex japonicus has lanceolate to oblanceolate leaves somewhat similar to those of R. crispus, but it can be distinguished from the other two species by its reniform or broadly pentagonal valves with irregularly and shallowly toothed margins. Hybrid individuals of R. crispus and R. obtusifolius are usually intermediate in leaf shape between the two parental species. Most flowers of hybrid individuals failed to set fruit; they became dry and fell before any appreciable enlargement of the valves. A few flowers appeared to be fertile and set fruit, with the valves enlarging to varying degrees. All voucher specimens were deposited in Seoul National University Herbarium (SNU) with Herbarium IDs: 119,355–119,426. All experiments and methods were performed in accordance with relevant regulations and guidelines.
DNA extraction, amplification, and sequencing
Total genomic DNA was extracted from leaf samples, either fresh or dried with silica gel, using the DNeasy Plant Mini Kit (QIAGEN, Germany). Five cpDNA regions (matK, rbcL-accD IGS, trnK-rps16 IGS, ycf6-psbM IGS and psbA-trnH IGS) and nDNA pgiC were amplified by polymerase chain reaction (PCR). Amplifications were carried out using a Veriti 96-Well Thermal Cycler (Applied Biosystems, USA) in 25 μL total volume containing 10–30 ng of genomic DNA, 1.25 U of EF-Taq DNA Polymerase (SolGent Co., Korea), 10 × EF-Taq Reaction Buffer with 25 mM MgCl2, 0.5 mM of each dNTP, 5% DMSO, and 0.4 μM of each primer. PCR and sequencing primers and PCR cycling conditions used in this study are provided in Table 3. The PCR products were purified using the enzymatic purification method43. Purified PCR products were sequenced using the BigDye Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems, USA) following the manufacturer’s instructions. The sequence products were purified by ethanol precipitation and then run on an ABI 3730xl DNA Analyzer (Applied Biosystems, USA) at Macrogen Inc., Korea.
Table 3.
PCR and sequencing primers and PCR cycling conditions for cpDNA matK, rbcL-accD IGS, trnK–rps16 IGS, ycf6-psbM IGS and psbA-trnH IGS, and nDNA pgiC. Superscripts following primer names denote reference numbers. Primers pgiC_16F (5'-CAC AGC TTT TAC CAA CTG ATT C-3') and pgiC_21R (5'- CCT AAC TCA ACT CCC CAC-3') were designed by the authors.
| Region | Primer | PCR cycling condition (35 cycles) | ||||
|---|---|---|---|---|---|---|
| Predenaturation (3 min) (°C) |
Denaturation (1 min) (°C) |
Annealing (40 s) (°C) |
Extension (1 min) (°C) |
Final extension (7 min) (°C) |
||
| pgiC | pgiC16F | 95 | 95 | 55 | 72 | 72 |
| pgiC21R | ||||||
| matK | 670F, 193F44 | 95 | 95 | 52 | 72 | 72 |
| 1246R44 | ||||||
| rbcL-accD IGS | rbcL50F45 | 95 | 95 | 50 | 72 | 72 |
| accD79R44 | ||||||
| trnK-rps16 IGS | trnKx146 | 95 | 95 | 53 | 72 | 72 |
| rps16 × 2F246 | ||||||
| ycf6-psbM IGS | ycf6F47 | 95 | 95 | 54 | 72 | 72 |
| psbMR47 | ||||||
| psbA-trnH IGS | psbAF48 | 95 | 95 | 55 | 72 | 72 |
| trnHR48 | ||||||
Cloning of nDNA pgiC
Due to the presence of polymorphic nucleotide positions in some direct sequences, PCR products of the nDNA pgiC region for 24 accessions of putative hybrids and R. japonicus were cloned using the pGEM-T Easy Vector System I (Promega, USA) following the manufacturer's instructions. Transformed E. coli cells were spread onto LB agar plates with ampicillin (50 µg/mL), and incubated at 37 °C for 12–16 h. Ten to 15 colonies per plate were randomly selected, and colonies were then screened for inserts by PCR. Temperature and cycling conditions consisted of lysis of cells and initial denaturation for 3 min at 95 °C, followed by 35 cycles of 1 min denaturation at 95 °C, 30 s annealing at 50 °C, and 1 min 15 s elongation at 72 °C, with a 7 min final extension at 72 °C. The PCR products were purified and sequenced using the same procedure described above.
Sequence alignment and analyses
Nucleotide sequences were assembled and edited using Sequencher 5.4.6 (Gene Codes Corporation, USA). Edited sequences were aligned with Clustal X v. 2.0 with final manual adjustment using Se-Al v. 2.0a1149,50. All DNA sequences obtained in this study were deposited in GenBank (Supplementary Table S1). We identified a short inversion of 10 bp in the aligned sequence data set of ycf6-psbM IGS region. The inversion was replaced with the reverse complement of its sequence. As the inversion was assumed to be a single mutation event and appeared to have phylogenetic information, it was subsequently coded as a single binary character following the procedure suggested by Whitlock et al.51 In addition, six indels ranging from 1 to 9 bp in length in the cpDNA sequence data set and one 271-bp indel in the nDNA pgiC sequence data set were coded and added to the data matrices as extra binary characters52. Phylogenetic analyses were performed on the combined cpDNA sequence data sets using maximum parsimony (MP) and Bayesian inference (BI).
MP analyses were performed in PAUP*4.0a16953 using a heuristic search strategy with 100 random sequence additions, tree bisection-reconnection (TBR) branch swapping, ACCTRAN, MULTREES on, MAXTREE set to no limit, and HOLD = 10 in effect. All characters were treated as unordered and equally weighted, and gaps were treated as missing data except for seven indels and one inversion coded as binary characters. Bootstrap (BS) analyses of 1000 replicates were conducted in PAUP* to evaluate support for clades using the same search parameters as in the MP analyses above54. For BI analyses, the optimal model of sequence evolution for each data set was identified using the Akaike information criterion (AIC) in MrModeltest 2.355. The following models of sequence evolution were identified as optimal for the five cpDNA regions examined in this study; GTR + I for matK, HKY for rbcL-accD IGS, GTR + G for trnK-rps16 IGS and the combined cpDNA data set, GTR for ycf6-psbM IGS, F81 + I for psbA-trnH IGS. For nDNA pgiC region, the optimal model of sequence evolution was HKY + I. (Table 1). BI analyses were performed in MrBayes 3.256 using two independent runs of four chains (three heated and one cold) for one million generations. Trees were sampled every 1000 generations, and the first 25% were discarded as burn-in. The remaining trees were used to produce a 50% majority-rule consensus tree and determine posterior probabilities (PP). An unrooted haplotype network was constructed for the cpDNA data set using the parsimony method as implemented in the TCS 1.21 program57. Gaps were treated as single evolutionary events and indels as the fifth state of character. The 95% probability limit of parsimonious connections was applied to produce the network.
Supplementary Information
Acknowledgements
We are grateful to Dr. Jin Hee Park, Heekyeong Jeon and Mi Sun Jun for their help with fieldwork in Korea. We deeply thank Dr. D. E. Boufford for his invaluable suggestions and critical reading of the manuscript. For his assistance in revising the manuscript, we would like to express deep appreciation to Daniel S. Park. We are also grateful to Min Kyung Kim, who provided logistic support. This project was supported by a grant from National Research Foundation of Korea (Grant No. 2019RIA2C1084173) to C.-W. Park.
Author contributions
C.W.P. conceived and designed the study; G.S.B. collected plants and performed the molecular experiments; C.W.P. and G.S.B. analyzed the data; G.S.B. wrote the manuscript.
Data availability
All sequence data have been deposited in GenBank.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-09292-9.
References
- 1.Williams J. Seed polymorphism and germination II. The role of hybridization in the germination polymorphism of Rumexcrispus and R.obtusifolius. Weed Res. 1971;11:12–21. [Google Scholar]
- 2.Ziburski A, Kadereit JW, Leins P. Quantitative aspects of hybridization in mixed populations of Rumexobtusifolius L. and R. crispus L. (Polygonaceae) Flora. 1986;178:233–242. [Google Scholar]
- 3.Akeroyd, J. R. Docks and knotweeds of Britain and Ireland (BSBI Handbook No. 3, Botanical Society of Britain and Ireland, 2014).
- 4.Takahashi K, Hanyu M. Hybridization between alien species Rumex obtusifolius and closely related native vulnerable species R. longifolius in a mountain tourist destination. Sci. Rep. 2015;5:13898. doi: 10.1038/srep13898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li A, et al. et al. Polygonaceae in Flora of China. In: Wu Z, et al.et al., editors. Ulmaceae Through Basellaceae. Science Press and Missouri Botanical Garden Press; 2003. pp. 277–350. [Google Scholar]
- 6.Mosyakin, S. L. Rumex Linnaeus in Flora of North America. Vol. 5. Magnoliophyta: Caryophyllidae, part 2 (ed. Flora of North America Editorial Committee) 489–533 (Oxford University Press, 2005).
- 7.Park, C.-W. et al. Polygonaceae in Flora of Korea Vol. 3. Caryophyllidae (ed. Flora of Korea Editorial Committee) 62–115 (Doohyun Publishing Co., 2018).
- 8.Rechinger KH. Rumex (Polygonaceae) in Australia: A reconsideration. Nuytsia. 1984;5:75–122. [Google Scholar]
- 9.CABI. Rumex obtusifolius. in Invasive Species Compendium https://www.cabi.org/isc/datasheet/48064 (2022).
- 10.Jung, S. Y. et al. Invasive Alien Plants in South Korea. (Korea National Arboretum, 2017).
- 11.Kang ES, et al. Comprehensive review about alien plants in Korea. Korean J. Pl. Taxon. 2020;50:89–119. [Google Scholar]
- 12.Vilà M, et al. Ecological impacts of invasive alien plants: A meta-analysis of their effects on species, communities and ecosystems. Ecol. Lett. 2011;14:702–708. doi: 10.1111/j.1461-0248.2011.01628.x. [DOI] [PubMed] [Google Scholar]
- 13.Pyšek P, et al. A global assessment of invasive plant impacts on resident species, communities and ecosystems: The interaction of impact measures, invading species' traits and environment. Glob. Change Biol. 2012;18:1725–1737. [Google Scholar]
- 14.Vilà M, Weber E, Antonio CM. Conservation implications of invasion by plant hybridization. Biol. Invas. 2000;2:207–217. [Google Scholar]
- 15.Mitchell N, Owens GL, Hovick SM, Rieseberg LH, Whitney KD. Hybridization speeds adaptive evolution in an eight-year field experiment. Sci. Rep. 2019;9:6746. doi: 10.1038/s41598-019-43119-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gottlieb LD. Levels of confidence in the analysis of hybridization in plants. Ann. Missouri Bot. Gard. 1972;59:435–446. [Google Scholar]
- 17.Stace CA. Hybridization and the Flora of the British Isles. Academic Press; 1975. [Google Scholar]
- 18.Hollingsworth ML, Bailey JP, Hollingsworth PM, Ferris C. Chloroplast DNA variation and hybridization between invasive populations of Japanese knotweed and giant knotweed (Fallopia, Polygonaceae) Bot. J. Linn. Soc. 1999;129:139–154. [Google Scholar]
- 19.Laureto PJ, Barkman TJ. Nuclear and chloroplast DNA suggest a complex single origin for the threatened allopolyploid Solidago houghtonii (Asteraceae) involving reticulate evolution and introgression. Syst. Bot. 2011;36:209–226. [Google Scholar]
- 20.Goulet BE, Roda F, Hopkins R. Hybridization in plants: Old ideas, new techniques. Pl. Physiol. 2017;173:65–78. doi: 10.1104/pp.16.01340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Triplett JK, Clark LG. Hybridization in the temperate bamboos (Poaceae: Bambusoideae: Arundinarieae): A phylogenetic study using AFLPs and cpDNA sequence data. Syst. Bot. 2021;46:48–69. [Google Scholar]
- 22.Corriveau JL, Coleman AW. Rapid screening method to detect potential biparental inheritance of plastid DNA and results for over 200 angiosperm species. Am. J. Bot. 1988;75:1443–1458. [Google Scholar]
- 23.Reboud X, Zeyl C. Organelle inheritance in plants. Heredity. 1994;72:132–140. [Google Scholar]
- 24.Birky CW. Uniparental inheritance of mitochondrial and chloroplast genes: Mechanisms and evolution. Proc. Natl. Acad. Sci. U.S.A. 1995;92:11331–11338. doi: 10.1073/pnas.92.25.11331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sang T. Utility of low-copy nuclear gene sequences in plant phylogenetics. Crit. Rev. Biochem. Mol. Biol. 2002;37:121–147. doi: 10.1080/10409230290771474. [DOI] [PubMed] [Google Scholar]
- 26.Small RL, Cronn RC, Wendel JF. Use of nuclear genes for phylogeny reconstruction in plants. Aust. Syst. Bot. 2004;17:145–170. [Google Scholar]
- 27.Roy T, Cole LW, Chang TH, Lindqvist C. Untangling reticulate evolutionary relationships among New World and Hawaiian mints (Stachydeae, Lamiaceae) Mol. Phylogen. Evol. 2015;89:46–62. doi: 10.1016/j.ympev.2015.03.023. [DOI] [PubMed] [Google Scholar]
- 28.Dai S, et al. Molecular evidence for hybrid origin of Melastoma intermedium. Biochem. Syst. Ecol. 2012;41:136–141. [Google Scholar]
- 29.Fan Q, et al. Molecular evidence for natural hybridization between wild loquat (Eriobotryajaponica) and its relative E. prinoides. BMC Pl. Biol. 2014;14:1–7. doi: 10.1186/s12870-014-0275-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shi L, et al. Molecular evidence for the hybrid origin of Ilex dabieshanensis (Aquifoliaceae) PLoS ONE. 2016;11:e0147825. doi: 10.1371/journal.pone.0147825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nitta JH, Amer S, Davis CC. Microsorum × tohieaense (Polypodiaceae), a new hybrid fern from French Polynesia, with implications for the taxonomy of Microsorum. Syst. Bot. 2018;43:397–413. [Google Scholar]
- 32.Fuertes Aguilar J, Rosselló J, Nieto Feliner G. Nuclear ribosomal DNA (nrDNA) concerted evolution in natural and artificial hybrids of Armeria (Plumbaginaceae) Mol. Ecol. 1999;8:1341–1346. doi: 10.1046/j.1365-294x.1999.00690.x. [DOI] [PubMed] [Google Scholar]
- 33.Ning H, Yu J, Gong X. Bidirectional natural hybridization between sympatric Ligulariavellerea and L. subspicata. Plant Divers. 2017;39:214–220. doi: 10.1016/j.pld.2017.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carine MA, Robba L, Little R, Russell S, Guerra AS. Molecular and morphological evidence for hybridization between endemic Canary Island Convolvulus. Bot. J. Linn. Soc. 2007;154:187–204. [Google Scholar]
- 35.Yan L-J, Gao L-M, Li D-Z. Molecular evidence for natural hybridization between Rhododendronspiciferum and R. spinuliferum (Ericaceae) J. Syst. Evol. 2013;51:426–434. [Google Scholar]
- 36.Salisbury EJ. Weeds and Aliens. Collins Publishers; 1961. [Google Scholar]
- 37.Cavers P, Harper J. Rumexobtusifolius L. and R. crispus L. J. Ecol. 1964;52:737–766. [Google Scholar]
- 38.Gammon MA, Kesseli R. Haplotypes of Fallopia introduced into the US. Biol. Invas. 2009;12:421–427. [Google Scholar]
- 39.Lousley, J. & Williams, J. Rumex L. in Hybridization and the Flora of the British Isles (ed. Stace, C. A.) 278–292 (Academic Press, 1975).
- 40.Rechinger KH. Die australischen und neuseeländischen Arten der Gattung Rumex: Vorarbeiten zu einer Monographie der Gattung Rumex, IV. Oesterr. Bot. Z. 1935;84:31–52. [Google Scholar]
- 41.Isobe S, Sawai A, Yamaguchi H, Gau M, Uchiyama K. Breeding potential of the backcross progenies of a hybrid between Trifoliummedium x T. pratense to T. pratense. Can. J. Pl. Sci. 2002;82:395–399. [Google Scholar]
- 42.Rieseberg LH, Sinervo B, Linder CR, Ungerer MC, Arias DM. Role of gene interactions in hybrid speciation: Evidence from ancient and experimental hybrids. Science. 1996;272:741–745. doi: 10.1126/science.272.5262.741. [DOI] [PubMed] [Google Scholar]
- 43.Werle E, Schneider C, Renner M, Volker M, Fiehn W. Convenient single-step, one tube purification of PCR products for direct sequencing. Nucl. Acids Res. 1994;22:4354–4355. doi: 10.1093/nar/22.20.4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim MH. Molecular Phylogeny and Taxonomy of the Polygonaceae. Seoul National University; 2007. [Google Scholar]
- 45.Yasui Y, Ohnishi O. Interspecific relationships in Fagopyrum (Polygonaceae) revealed by the nucleotide sequences of the rbcL and accD genes and their intergenic region. Am. J. Bot. 1998;85:1134–1142. [PubMed] [Google Scholar]
- 46.Shaw J, Lickey EB, Schilling EE, Small RL. Comparison of whole chloroplast genome sequences to choose noncoding regions for phylogenetic studies in angiosperms: The tortoise and the hare III. Am. J. Bot. 2007;94:275–288. doi: 10.3732/ajb.94.3.275. [DOI] [PubMed] [Google Scholar]
- 47.Shaw J, et al. The tortoise and the hare II: Relative utility of 21 noncoding chloroplast DNA sequences for phylogenetic analysis. Am. J. Bot. 2005;92:142–166. doi: 10.3732/ajb.92.1.142. [DOI] [PubMed] [Google Scholar]
- 48.Sang T, Crawford DJ, Stuessy TF. Chloroplast DNA phylogeny, reticulate evolution, and biogeography of Paeonia (Paeoniaceae) Am. J. Bot. 1997;84:1120–1136. [PubMed] [Google Scholar]
- 49.Larkin MA, et al. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23:2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- 50.Rambaut, A. Se–Al: A Manual Sequence Alignment Editor. Version 2.0a11. (University of Oxford, 2002).
- 51.Whitlock BA, Hale AM, Groff PA. Intraspecific inversions pose a challenge for the trnH-psbA plant DNA barcode. PLoS ONE. 2010;5:e11533. doi: 10.1371/journal.pone.0011533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Simmons MP, Ochoterena H. Gaps as characters in sequence-based phylogenetic analyses. Syst. Biol. 2000;49:369–381. [PubMed] [Google Scholar]
- 53.Swofford, D. L. Phylogenetic analysis using parsimony (* and other methods) Version 4.0a169. http://phylosolutions.com/paup-test/ (2020).
- 54.Felsenstein J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution. 1985;39:783–791. doi: 10.1111/j.1558-5646.1985.tb00420.x. [DOI] [PubMed] [Google Scholar]
- 55.Nylander, J. A. A. MrModeltest, version 2.3. Program distributed by the author. (Evolutionary Biology Centre, Uppsala University, 2004).
- 56.Ronquist F, et al. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012;61:539–542. doi: 10.1093/sysbio/sys029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Clement M, Posada D, Crandall K. TCS: A computer program to estimate gene genealogies. Mol. Ecol. 2000;9:1657–1660. doi: 10.1046/j.1365-294x.2000.01020.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All sequence data have been deposited in GenBank.