Abstract
Physical activity and diet are recommended lifestyle strategies to improve human health. Consequently, this study aimed to investigate the impact of aerobic exercise combined with dietary restriction on hormonal, metabolic, and psychological variables in postmenopausal women. Eligible 60 women were enrolled and assigned into two equal groups; the experimental group performed aerobic exercise three times per week for 12 weeks with diet restriction, and the control group only received the same diet program. Serum levels of sex hormones, insulin resistance, and depression scores were measured before and after the intervention.
Results
All measured variables were significantly changed in the experimental group compared to the control group (P = 0.001).
Conclusion
Changes in sex hormones are a biological marker of metabolic complications such as insulin resistance, which can be reduced with exercise and diet. In addition, they are effective therapeutic interventions in the treatment of mild depression.
Trial registration
Registration identifier number of this study is: NCT05136742 on https://register.ClinicalTrials.gov.
Keywords: Biomarkers, Body weight, Humans, Female, Gonadal steroid hormones, Obesity
Biomarkers; Body weight; Humans; Female; Gonadal steroid hormones; Obesity.
1. Introduction
Postmenopausal women's health is of international concern because it has a significant social and economic influence in developed and underdeveloped countries (Moratalla-Cecilia et al., 2016). In Egypt, the average age of women at menopause is 46.7, with a higher incidence of menopausal symptoms (Elbahrawe et al., 2020). After menopause, approximately 80% of women experience various disorders that negatively impact their quality of life as a result of hormonal changes (Moratalla-Cecilia et al., 2016), such as increased weight gain and central abdominal fat accumulation, which leads to increased hepatic production of atherogenic lipids, elevated blood glucose levels, and an increased risk of insulin resistance (IR) (Golden et al., 2007), and a high incidence of diabetes rather than premenopausal women (Sharma et al., 2016).
In premenopausal, estradiol promotes glucose uptake both directly and indirectly by acting on insulin-sensitive target tissues (Tamakoshi et al., 2007) and indirectly by lowering factors such as oxidative stress, which is inversely correlated with IR (Sharma et al., 2016). While in postmenopausal women, estradiol inhibits the action of insulin-stimulated glucose oxidation in body myocytes and increases IR (Golden et al., 2007).
In addition to stimulating glucose uptake in the liver, muscles, and adipose tissues, testosterone hormone has a positive effect on bone mass, mood, and well-being in premenopausal women (Matsui et al., 2013). After menopause, testosterone increases basal fatty acids uptake, impairs lipolysis, causing visceral fat accumulation, has an adverse effect on IR (Maturana et al., 2011), and is negatively correlated with high-density lipoprotein cholesterol and sex hormone-binding globulin (SHBG), a protein produced by the liver that transports the sex-steroid hormones, testosterone, and estradiol in the blood (Golden et al., 2007). Therefore, low levels of SHBG resulted in increased levels of estrogen and androgen that further increased the risk of diabetes (Muka et al., 2017).
The second most common postmenopausal changes are related to psychological status (Bashar et al., 2017). The prevalence of depression and anxiety symptoms among peri- and postmenopausal women is 7%–25% and 18%–41.8%, respectively (Ahlawat et al., 2019). Depression is one of the significant issues affecting postmenopausal women in many countries (Bashar et al., 2017). It has a negative impact on an individual's social relations and workability, and it is a risk factor for suicide and self-harm (Ahlawat et al., 2019).
Many studies have found a significant correlation between depression and excessive body weight gain and IR (Bashar et al., 2017; Ahlawat et al., 2019). Everson–Rose et al. (2004) revealed that depression is correlated to IR and diabetes in postmenopausal women. Preventive strategies for those at high risk for IR, depression, and its sequelae before pathological insult (Golden et al., 2004); thus, a balanced lifestyle (exercise and diet) is recommended to avoid those complications (Keshel and Coker, 2015).
Aerobic exercise combined with calorie restriction reduces body fat, which is linked to the development of chronic diseases such as cardiovascular disease, Type 2 diabetes, and higher mortality (Aghamohammadi et al., 2013). Furthermore, it is a predictor of IR, myocardial infarction, and hypertension (Despres, 1998). Regular physical activity and a healthy diet contribute to alleviating depression symptoms by inducing brain neuroplasticity, blood flow, neurotransmitter release, antioxidant, anti-inflammatory substances, and improving body image (Meeusen, 2014).
The purpose of this study was to investigate the combined effect of aerobic exercise and a balanced, restricted diet on IR and depression, as well as their relationship to sex hormones, which are thought to play a role in metabolic and psychological disorders in postmenopausal women but have not been demonstrated in previous studies. We hypothesized that combining aerobic exercise with diet induces hormonal, metabolic, and psychological improvements among postmenopausal women.
2. Materials and methods
2.1. Study design
The study was planned as a randomized controlled trial. Before the study began, the research ethics committee was obtained from the Faculty of Physical Therapy, Cairo University, Egypt (no: P.T.REC/012/002774). All procedures were in accordance with the Helsinki Declaration on the Conduct of Human Research. After a thorough explanation of the purpose and procedures, each woman provided informed consent to participate. The study was conducted from January 2021 to October 2021.
2.2. Participants
Participants were recruited from the Departments of Obstetrics and Gynecology and internal medicine outpatient clinics at Cairo University Hospital. Eligible postmenopausal women were enrolled based on the following inclusion criteria: women in the postmenopausal stage, as determined based on sex hormones concentration (estradiol: 26.12 ± 3.44 pg/ml, total testosterone (TT): 0.42 ± 0.11 ng/ml, free testosterone (FT): 1.49 ± 0.62 pg/ml), and the mean of the absence of menstrual cycle was 8.5 ± 6.52 years. Medical and physical examination revealed that all women could safely engage in exercise and diet programs. The mean of their age was: 58.79 ± 2.81 years, body mass index (BMI): 37.67 ± 1.68 kg/m2 and level of physical activity: 11.22 ± 3.46 units according to physical activity questionnaire) (Godin, 2011); participants with mild depression had a score mean: 54.62 ± 2.55 according to the Zung Self-Rating Depression Scale (SDS) (Zung et al., 1965). The mean of family income, education level preparatory education, married, and unemployed were all included in the social demographic data. The last menstrual period date, the time of menopause, and details about daily living activities are all provided.
Also, we excluded women who had a history of any circulatory disease, malignancy diseases, stroke, uncontrolled diabetes mellitus, renal diseases, liver disorders, uncontrolled hypertension, or use of medication for lipid-lowering or weight loss, as well as antidepressants and hormonal replacement.
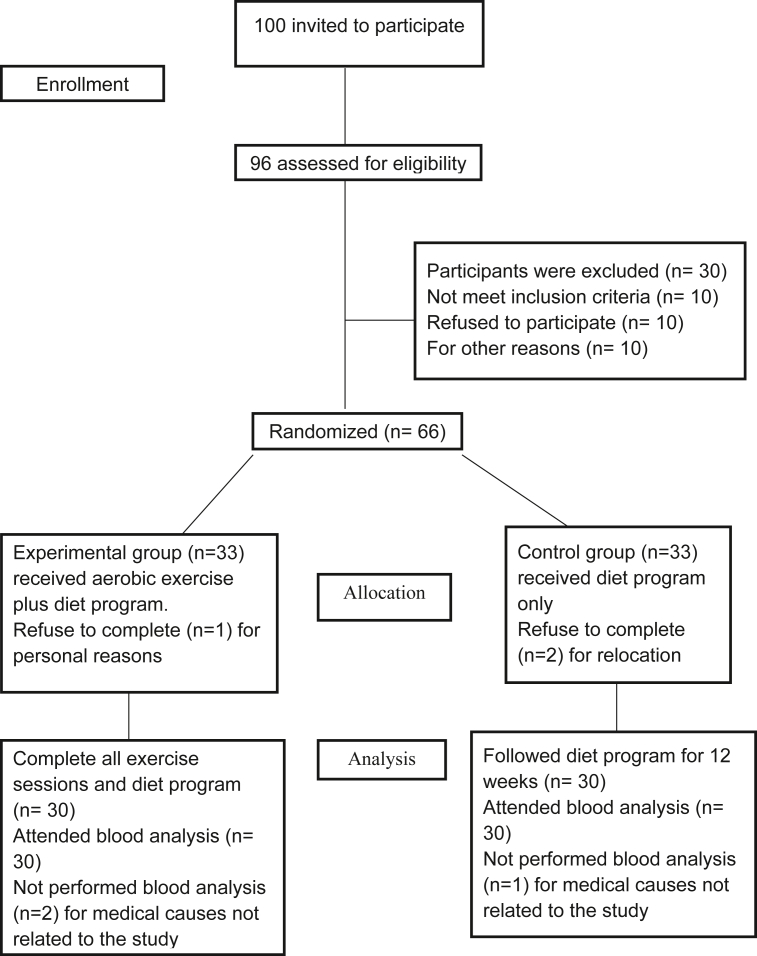
Participants' eligibility: 100 women were invited to participate, 96 women were assessed for eligibility, ten women were omitted because they did not match inclusion criteria, through the evaluation, ten women withdrew from contribution, and ten women did not want to take part due to other reasons, as demonstrated in Figure 1. Finally, 60 women were divided into two equal groups, completed the intervention and the analysis, and were only included in data analysis, as six women declined for different reasons (relocation, personal reasons, and medical causes unrelated to the study).
Figure 1.
Consort Flow diagram of this study.
2.3. Randomization and blinding
Online software generated the random allocation sequence. The randomization sequence was concealed using sequentially numbered, opaque sealed envelopes, and thus neither the researcher (assessor of the outcomes) nor the participant was aware of the upcoming assignment. Each participant was given a unique identification number. These numbers were randomly assigned into two equal-sized groups (n = 30). A blinded and independent research assistant opened the sealed envelopes and gave each participant a hand-picked envelope, and participants were assigned to groups. The experimental group (n = 30) participated in an aerobic exercise program as well as a balanced, restricted diet program, whereas the control group (n = 30) only followed the balanced-restricted diet program.
2.4. Intervention
The type of exercise was an aerobic exercise on the treadmill (Wolf Germany Pro Runner S2 Motorized Treadmill) in accordance with the American College of Sports Medicine (ACSM) recommendations (Donnelly et al., 2009). The target intensity ranged from 60% to 70% of maximum heart rate (MHR). In order to confirm this intensity, women were asked to perform a symptom-limited submaximal treadmill exercise test (Gappmaier, 2012). The modified Bruce treadmill protocol was used to measure peak heart rate (PHR) for each woman (i.e., determining the target exercise intensity) (Kozlov et al., 2020). Participants performed the test and ended it upon their complaints of exertion, breathlessness, fatigue, or discomfort, or reaching 85% of their age-predicted peak heart rate, calculated according to Fox formula, HR max = 220 − age (Fox et al., 1971). HR was assessed by pulse Oximetry (Portable pulse Oximetry DIXION Storm 5000, Germany). Participants exercised three times per week for 12 weeks under the supervision of the study physician and the physiotherapists in the Out Clinics at the Faculty of Physical Therapy. They were instructed to do a warm-up exercise including low-intensity treadmill walking for 10 min, followed by 30 min intensity increased to reach target training heart rate, which was 60% MHR in the first six weeks then progressed to 70% MHR in the past six weeks of intervention, determination progressed intensity based on a reassessment by the symptom-limited submaximal exercise test. Subsequently, at the end of the exercise session, participants cooled down for 10 min, with the intensity of exercise decreasing as in the warm-up phase.
2.5. Diet program
A dietician interviewed all participants in both groups (experimental and control) to determine a personalized balanced energy restriction calorie diet, which was prescribed according to the Dutch National Guidelines for a healthy diet (Health Council of the Netherlands, 2006). The diet included 55% carbohydrate, 15% protein, and 30% fat >25 g of fibers per day and detection of feeding habits, abnormal dietary behavior, and determination of required daily calories depending on the individual basal metabolic rate. These were calculated using Harris & Benedict formula based on ideal adjusted body weight (Roza and Shizgal, 1984), multiplied by an estimate of their physical activity level according to Godin Leisure-Time Exercise questionnaire (Godin, 2011), to prescribe a balanced low-calorie diet that restricts 20% of needed calories/day according to recommendations of ACSM, with a mean energy intake of 1270.65 ± 8.79 kcal/d (E. Donnelly et al., 2009). The control group was advised to maintain their habitual daily level of physical activity for 12 weeks. Dietitians and physiotherapists supervised weight-loss interventions—both groups (experimental and control) self-weighed weekly to monitor body weight. In addition, the dietitian and physiotherapist performed supervised weighing on each visit (weekly). Participants' adherence to the diet program was monitored via 24-hour phone calls.
2.6. Outcome measures
2.6.1. Primary outcomes
2.6.1.1. Serum sex hormones
All blood samples from one individual were analyzed in the same batch after 12 weeks of intervention and at least ten hours of fasting. All hormones were tested using chemiluminescence hormonal analysis, including estradiol, TT, FT, SHBG, and blood collection occurred between 7:00 and 10:00 AM. Samples were analyzed in duplicate batches, with each batch containing both baseline and 12 weeks after samples from each participant, as well as an equal number of participants from the experimental and control groups.
Plasma glucose level was determined using radioimmunoassay [Diagnostic Systems Laboratories, Webster, Texas, TX (DSL-1600)], with a mean intra-assay (CV) of 9%. Serum insulin levels were measured by radioimmunoassay (Asbach Medical Products GmBH, Obrigheim, Germany. The intra- and inter-assay CV were 2.1% and 6.5%, respectively. Homeostatic Model Assessment for Insulin Resistance (HOMA-IR) was used to assess IR, and it was calculated for all subjects using the following formula:
The HOMA-IR index was calculated using fasting plasma glucose (mmol/L) ×fasting plasma insulin (mU/L)/22.5 (Matthews et al., 1985).
2.6.1.2. Depression
SDS: This scale was used to assess depression levels in both groups (experimental and control) before and after the end of the study, with scores falling into four categories: (20–44) normal range, (45–59) mild depression, (60–69) moderate depression, and 70 and over severe depression (Zung et al., 1965).
2.6.2. Secondary outcomes
Weight and height were measured using (Medical Equipment Folding Portable Weight and Height BMI Analyzer Health Measurement Equipment Scale, China), and BMI was calculated using the formula: BMI = body weight in kilograms (kg)/height in meter squared (m2).
2.6.2.1. Sample size determination
Prior to the study, the sample size was calculated using G∗POWER statistical software (version 3.1.9.2; Franz Faul, Universitat Kiel, Germany) [t-tests – Means]: The difference between two independent means (two groups) revealed that the appropriate required sample size for the study was n = 60, assuming a 5% drop out rate, based on the (Cohen's d) effect size of.73 for the outcome of IR, α error prob = 0.05, 1-β error prob = 0.8.
2.7. Statistical analysis
SPSS software, version 25, was used to analyze the data (IBM SPSS, Chicago, Illinois, USA). The Shapiro–Wilk test was used to determine whether the data were normally distributed. The mean ± standard deviation (SD) was used to describe continuous data. The Independent Sample t-test was used to compare baseline characteristics between the two groups. To investigate the changes in variables before and after the intervention, as well as the differences between the experimental and control groups, dependent and independent sample t-tests were used. In addition, Pearson's correlation coefficient test and multiple linear regression models were used to estimate the association between changes in sex hormones, the HOMA-IR index, and depression scores for each group. The level of statistical significance was set at p < 0.05.
3. Results
The study included a total of 60 postmenopausal obese women.
At baseline, there were no statistically significant differences between the experimental and control groups in terms of age, height, BMI, weight, sex hormone concentration, HOMA-IR index, and depression scores (P > 0.05), as depicted in (Table 1). Participants reported no adverse events during the intervention.
Table 1.
Baseline characteristics of study participants mean ± standard deviation (SD).
| Characteristics | Experimental group (n = 30) | Control group (n = 30) | P-value | |
|---|---|---|---|---|
| Age (yrs) | Mean ± SD | 58.99 ± 3.22 | 58.58 ± 2.37 | 0.573 |
| Range | 54.10–65.00 | 55.30–64.00 | ||
| Height (cm) | Mean ± SD | 160.37 ± 3.61 | 159.10 ± 3.55 | 0.175 |
| Range | 155.00–168.00 | 155.00–169.00 | ||
| Weight (kg) | Mean ± SD | 97.42 ± 6.18 | 94.87 ± 5.20 | 0.089 |
| Range | 88.30–117.50 | 87.00–103.20 | ||
| BMI (kg/m2) | Mean ± SD | 37.88 ± 1.93 | 37.47 ± 1.39 | 0.353 |
| Range | 35.10–44.80 | 35.20–39.80 | ||
| Estradiol hormone (Pg/ml) | Mean ± SD | 26.02 ± 3.66 | 26.22 ± 3.22 | 0.823 |
| Range | 18.70–32.10 | 20.80–32.10 | ||
| Total testosterone hormone (ng/ml) | Mean ± SD | 0.44 ± 0.10 | 0.40 ± 0.11 | 0.122 |
| Range | 0.23–0.60 | 0.22–0.60 | ||
| Free testosterone hormone (pg/ml) | Mean ± SD | 1.44 ± 0.57 | 1.54 ± 0.66 | 0.531 |
| Range | 0.41–2.65 | 0.45–2.74 | ||
| SHBG (nmol/ml) | Mean ± SD | 40.25 ± 5.06 | 39.39 ± 5.00 | 0.510 |
| Range | 32.60–50.30 | 31.90–47.60 | ||
| HOMA-IR index | Mean ± SD | 2.65 ± 0.59 | 2.63 ± 0.45 | 0.864 |
| Range | 1.12–3.61 | 1.94–3.61 | ||
| Depression scores | Mean ± SD | 54.90 ± 2.64 | 54.33 ± 2.45 | 0.393 |
| Range | 50.00–59.00 | 50.00–59.00 | ||
BMI: Body mass index. SHBG: Sex Hormone Binding Globulin. HOMA-IR index: Homeostatic Model Assessment for Insulin Resistance.
According to the Paired Sample t-test, we found statistically significant differences in weight, BMI, sex hormones concentration, HOMA-IR index, and depression scores in experimental and control groups between pre-intervention and post-intervention (p < 0.001), (p < 0.05), respectively.
According to Table 2, the experimental group had lower levels of weight, BMI, estradiol hormone, TT hormone, and FT hormone than the control group (P = 0.001at 95% CI (−12.79, -0.70), (−2.50, -0.70), (−4.31, -1.23), (−0.15, -0.06), and (−0.88, -0.39), respectively. After a 12-week intervention, SHBG hormone levels in the experimental group were significantly higher than in the control group (P = 0.001 at 95% CI (2.96, 9.11).
Table 2.
Comparison of weight, BMI, sex hormones, IR and depression scores before and after 12 weeks between both groups mean ± standard deviation (SD).
| Variables | Experimental group (n = 30) |
95%Cl (lower, upper) | PPaired t-test –value | Control group (n = 30) |
95%Cl (lower, upper) | PPaired t-test -value | 95%Cl (lower, upper) | PIndep.t-test value | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Before | After | Before | After | |||||||
| Weight (kg) | 97.42 ± 6.18 | 79.22 ± 6.07 | (17.50, 18.92) | <0.001 ∗ | 94.87 ± 5.20 | 85.96 ± 15.52 | (3.19, 14.63) | <0.05 ∗ | (-12.79, -0.70) | 0.001∗∗ |
| BMI (kg/m2) | 37.88 ± 1.93 | 33.02 ± 1.92 | (4.66,5.06) | <0.001 ∗ | 37.47 ± 1.39 | 34.62 ± 1.56 | (2.56,3.15) | <0.05 ∗ | (-2.50, -0.70) | 0.001∗∗ |
| Estradiol hormone (Pg/ml) | 26.02 ± 3.66 | 20.71 ± 3.02 | (5.01,5.61) | <0.001 ∗ | 26.22 ± 3.22 | 23.48 ± 2.94 | (2.53–2.94) | <0.05 ∗ | (-4.31, -1.23) | 0.001∗∗ |
| Total testosterone hormone (ng/ml) | 0.44 ± 0.10 | 0.27 ± 0.07 | (0.15,0.20) | <0.001 ∗ | 0.40 ± 0.11 | 0.37 ± 0.11 | (0.02,0.03) | <0.05 ∗ | (-0.15, -0.06) | 0.001∗∗ |
| Free testosterone hormone (pg/ml) | 1.44 ± 0.57 | 0.77 ± 0.3 | (0.55,0.79) | <0.001 ∗ | 1.54 ± 0.66 | 1.40 ± 0.60 | (0.11,0.16) | <0.05 ∗ | (-0.88, -0.39) | 0.001∗∗ |
| SHBG hormone (nmol/ml) | 40.25 ± 5.06 | 49.36 ± 6.28 | (-9.63, -8.59) | <0.001 ∗ | 39.39 ± 5.00 | 43.32 ± 5.59 | (-4.32, -3.55) | <0.05 ∗ | (2.96,9.11) | 0.001∗∗ |
| HOMA-IR index | 2.65 ± 0.59 | 2.08 ± 0.47 | (0.52,0.63) | <0.001 ∗ | 2.63 ± 0.45 | 2.33 ± 0.41 | (0.27,0.32) | <0.05 ∗ | (-0.48, -0.03) | 0.001∗∗ |
| Depression scores | 54.90 ± 2.64 | 35.10 ± 3.00 | (18.67,20.93) | <0.001 ∗ | 54.33 ± 2.45 | 40.07 ± 1.95 | (13.31,15.22) | <0.05 ∗ | (-6.27, -3.66) | 0.001∗∗ |
BMI: Body mass index, SHBG: Sex Hormone Binding Globulin. HOMA-IR index: Homeostatic Model Assessment for Insulin Resistance.
∗: Statistically significant at P ≤ 0.05 according to Paired Sample t-test.
∗∗: Statistically significant at P ≤ 0.05 according to Independent Sample t-test.
In addition, the experimental group experienced a greater significant decrease in the HOMA-IR index and depression scores than the control group (P = 0.001), with 95% CI (−0.48, -0.03), (−6.27, -3.66), respectively.
Table 3 depicts the correlation between changes in serum sex hormones, the HOMA-IR index, and the depression scores in both groups. In the experimental group, estradiol hormone, TT hormone, and FT hormone levels were positively correlated with the HOMA-IR index (r = 0.423, 0.364, and 0.318, respectively, P < 0.05), whereas SHBG hormone levels were negatively correlated with the HOMA-IR index (r = -0.594, P < 0.05). There was no significant correlation between change in depression scores and sex hormone levels (P > 0.05). With regard to the control group, there was no significant correlation between change in sex hormones, HOMA-IR index, and depression scores (P > 0.05).
Table 3.
Correlation among changes in sex hormones, HOMA-IR index, and depression scores within both experimental and control groups.
| Variables | Experimental group (n = 30) |
Control group (n = 30) |
||||||
|---|---|---|---|---|---|---|---|---|
| HOMA-IR index |
Depression scores |
HOMA-IR index |
Depression scores |
|||||
| R | P –value | R | P –value | R | P –value | R | P –value | |
| Estradiol hormone (Pg/ml) | 0.423 | 0.005∗ | 0.277 | 0.070 | 0.034 | 0.430 | 0.106 | 0.288 |
| Total testosterone hormone (ng/ml) | 0.364 | 0.013∗ | -0.237 | 0.104 | -0.032 | 0.434 | 0.211 | 0.132 |
| Free testosterone hormone (pg/ml) | 0.318 | 0.028∗ | 0.135 | 0.238 | 0.037 | 0.423 | 0.085 | 0.328 |
| SHBG hormone (nmol/ml) | -0.594 | 0.001∗ | -0.349 | 0.069 | 0.002 | 0.495 | -0.078 | 0.342 |
SHBG: Sex Hormone Binding Globulin. HOMA-IR index: Homeostatic Model Assessment for Insulin Resistance.
r: Correlation coefficient.
∗: Correlation is significant at P ≤ 0.05 according to Pearson's correlation coefficient.
Multiple linear regression revealed a significant inverse relationship between SHBG hormone levels and the HOMA-IR index (β = 0.630, p = 0.014) in the experimental group, indicating that changes in SHBG hormone levels may affect the HOMA-IR index. Furthermore, after 12 weeks of exercise and diet restriction, sex hormones had no significant effects on the depression scores (P > 0.05), as illustrated in (Table 4). In the control group, sex hormones were not correlated with the HOMA-IR index and depression scores (P > 0.05).
Table 4.
Multivariate linear regression analysis of the association between the change in assessed variables within both experimental and control groups.
| Variables | Experimental group |
Control group |
||||||
|---|---|---|---|---|---|---|---|---|
| HOMA-IR index |
Depression scores |
HOMA-IR index |
Depression scores |
|||||
| Β | P –value | Β | P –value | Β | P –value | Β | P –value | |
| Estradiol hormone (Pg/ml) | 0.042 | 0.823 | 0.279 | 0.118 | 0.038 | 0.853 | 0.105 | 0.593 |
| Total testosterone hormone (ng/ml) | 0.093 | 0.570 | -0.235 | 0.187 | -0.033 | 0.871 | 0.212 | 0.284 |
| Free testosterone hormone (pg/ml) | -0.151 | 0.447 | -0.094 | .648 | 0.038 | 0.852 | 0.100 | 0.609 |
| SHBG hormone (nmol/ml) | -0.630 | 0.014∗ | -0.414 | 0.062 | -0.001 | 0.995 | -0.101 | 0.606 |
| Regression equation (R2) | 0.377 | 0.264 | 0.004 | 0.072 | ||||
| P –value | 0.004∗ | 0.093 | 0.999 | 0.746 | ||||
β: Standardized regressions Coefficients.
∗: Statistically significant at p < 0.05 according to Multivariate linear regression analysis.
SHBG: Sex Hormone Binding Globulin. HOMA-IR index: Homeostatic Model Assessment for Insulin Resistance.
4. Discussion
In the current study, we discovered that 12 weeks of aerobic exercise combined with a balanced, restricted diet significantly reduced body weight, BMI, serum estradiol concentration, TT, FT, and increased SHBG levels more than diet alone (P = 0.001). This finding is supported by Campbell et al. (2012). Exercise and diet reduce serum estrogen and TT through weight loss and lowering fat tissue, the principal source of estrogen and androstenedione after menopause (Schmitz et al., 2007). Increased levels of SHBG are associated with diminished fat tissues, improved insulin sensitivity, and diminished insulin levels (Birkeland et al., 1993), which enhances the hepatic production of SHBG, and thus lower amounts of unbound estrogens and androgens (Wu et al., 2001).
Therefore, in the current study, there was a decline in IR following the aerobic exercise and diet program rather than diet program alone, which is consistent with Mason et al. (2011) who demonstrated that a combined diet restriction and aerobic exercise program has a significant effect on improving IR among postmenopausal women. The mechanism behind that exercise with diet leads to decreased weight and body fat as the accumulation of subcutaneous and abdominal fat (Campbell et al., 2012) included the decline insulin receptors sensitivity characterized by an inhibited uptake of glucose within skeletal muscle and an impaired ability to suppress endogenous glucose production (Makki et al., 2013). Also, with aerobic exercise action of muscles increases; skeletal muscles play an essential role in regulating whole-body homeostasis. Skeletal muscles are responsible for about 80% of the postprandial clearance of glucose (Mason et al., 2011).
Furthermore, our findings revealed a negative correlation between SHBG and IR and a positive correlation between TT, FT, and estradiol with IR that appears only in the experimental group due to highly significant weight loss compared to the control group reported by Kim and Kim (2012); van Gemert et al. (2015); Matsui et al. (2013). Low estradiol levels increased glucose uptake while high estradiol levels decreased basal and insulin-stimulated glucose oxidation in postmenopausal women (Inada et al., 2016). Also, it was found that exogenous androgens increase visceral adiposity in obese postmenopausal women by elevating testosterone levels (Lovejoy et al., 1996). In consequence, visceral obesity is thought to impair hepatic insulin clearance and increase hepatic glucose production (Marin and Arver, 1998), and it has been correlated with clinical signs of hyperandrogenemia and low serum SHBG levels (Despres, 1998), which were found to be significant predictors of diabetes development in premenopausal and postmenopausal women (Birkeland et al., 1993).
The current study revealed that the experimental group showed a significant reduction in depression scores than the control group, which aligns with many randomized controlled trials that demonstrated that regular aerobic exercise and diet could help adults with mild-moderate depression (Blumenthal et al., 1999; Craft and Perna, 2004) due to reducing body weight and improving positive body image and self-esteem (Dimeo et al., 2001). Furthermore, aerobic exercise alters the levels of many biologically active particles (e.g., catecholamines, opioid peptides, cytokines, adrenocorticotrophic hormone, cortisol, and retroactive substances) (Stathopoulou et al., 2006). In addition, a healthy diet contains antioxidants and anti-inflammatory substances that activate microglia in the central nervous system (Meeusen, 2014), as well as synaptic plasticity, hippocampal neurogenesis, neurotransmitter production, and inflammation reduction (Lee and Giuliani, 2019). Depression and anxiety disorders were linked to more disruptions in brain function (Meeusen, 2014).
Moreover, we found no correlation between sex hormones and depression in both groups, as reported by Ryan et al. (2009). Many studies have demonstrated that stressors such as verbal abuse, physical abuse, caregiving, social strain, adverse life events, financial stress, low income, acute pain, and several chronic medical conditions are all associated with higher levels of depression symptoms in women after menopause. This finding denotes that depression modulation requires the integration of many factors (Uebelacker et al., 2013; Ahlawat et al., 2019).
4.1. Strengths and limitations of this study
The present study demonstrated the effect of combined aerobic exercise with a balanced, restricted calorie diet on sex hormones, IR, and depression. Previous studies demonstrated the effect of exercise and diet on IR only, sex hormones only, or depression. Therefore, we added aerobic exercise with diet declines the metabolic and psychological complications resulting from hormonal changes occurring among postmenopausal women.
Despite our positive outcomes in this study, it is essential to consider the limitations to the current study where women with cardiovascular, uncontrolled medical disorders, and receive hormonal therapy were excluded, which may limit variations in the results of the study, using one scale to investigate the psychological status of the participants. The control group showed statistically significant differences between pre-intervention and post-intervention. Finally, we investigated a specific exercise type, intensity, and diet program. Consequently, we could not attribute the results to different exercise types, intensities, or diet programs.
In conclusion, our study demonstrated that aerobic training with a balanced, restricted diet affects sex hormone levels and reduces postmenopausal women's IR and depression scoring.
Declarations
Author contribution statement
Marwa Mahmoud Elsayed, Ghada Ebrahim Elrefaye, and Ahmed Rabiee: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Sameh Abouzeid: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Hany Farid Elsisi: Conceived and designed the experiments; Performed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data will be made available on request.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
The clinical trial described in this paper was registered at ClinicalTrials.gov under the registration number NCT05136742.
Acknowledgements
The authors thank all individuals who supported this study; the authors are grateful to Ebthall Mohamed for her assistance in data analysis and interpretation.
References
- Aghamohammadi A., Rajabi A., Zafari M., Aghamohammadi A. Effect of regular aerobic exercise on depression in postmenopausal women. Eur. Psychiatr. 2013;28(1):1. [Google Scholar]
- Ahlawat P., Singh M.M., Garg S., Mala Y.M. Prevalence of depression and its association with sociodemographic factors in postmenopausal women in an urban resettlement colony of Delhi. J. Midlife Health. 2019;10(1):33–36. doi: 10.4103/jmh.JMH_66_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashar M., Ahmed K., Uddin M.S., Ahmed F., Emran A.A., Chakraborty A. Depression and quality of life among postmenopausal women in Bangladesh: a cross-sectional study. J. Menopausal. Med. 2017;23(3):172–181. doi: 10.6118/jmm.2017.23.3.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkeland K.I., Hanssen K.F., Torjesen P.A., Vaaler S. Level of sex hormonebinding globulin is positively correlated with insulin sensitivity in men with type 2 diabetes. J. Clin. Endocrinol. Metab. 1993;76:275–278. doi: 10.1210/jcem.76.2.8432768. [DOI] [PubMed] [Google Scholar]
- Blumenthal J.A., Babyak M.A., Moore K.A., Craighead W.E., Herman S., Khatri P., Waugh R., Napolitano M.A., Forman L.M., Appelbaum M., Doraiswamy P.M., Krishnan K.R. Effects of exercise training on older patients with major depression. Arch. Intern. Med. 1999;159(19):2349–2356. doi: 10.1001/archinte.159.19.2349. [DOI] [PubMed] [Google Scholar]
- Campbell K.L., Foster-Schubert K.E., Alfano C.M., Wang C.C., Wang C.Y., Duggan C.R., Mason C., Imayama I., Kong A., Xiao L., Bain C.E., Blackburn G.L., Stanczyk F.Z., McTiernan A.A. Reduced-calorie dietary weight loss, exercise, and sex-hormones in postmenopausal women: randomized controlled trial. J. Clin. Oncol. 2012;30(19):2314–2326. doi: 10.1200/JCO.2011.37.9792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craft L.L., Perna F.M. The benefits of exercise for the clinically depressed. Prim. Care Companion J. Clin. Psychiatry. 2004;6(3):104–111. doi: 10.4088/pcc.v06n0301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Despres J.P. The insulin resistance-dyslipidemic syndrome of visceral obesity: effect on patients risk. Obes. Res. 1998;6:8–17. doi: 10.1002/j.1550-8528.1998.tb00683.x. [DOI] [PubMed] [Google Scholar]
- Dimeo F., Bauer M., Varahram I., Proest G., Halter U. Benefits from aerobic exercise in patients with major depression: a pilot study. Br. J. Sports Med. 2001;35(2):114–117. doi: 10.1136/bjsm.35.2.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly E., Blair S.N., Jakicic J.M., Manore M.M., Rankin J.W., Smith B.K. Smith American College of Sports Medicine Position Stand. Appropriate PA intervention strategies for weight loss and prevention of weight regain for adults. Med. Sci. Sports Exerc. 2009;41:459–471. doi: 10.1249/MSS.0b013e3181949333. [DOI] [PubMed] [Google Scholar]
- Elbahrawe R., Fahim A., Abdou L., Abbas A., Saleh L. Quality of life among postmenopausal Egyptian women: a cross-sectional study. J. Family Commun. Med. 2020;3:42–49. [Google Scholar]
- Everson-Rose S.A., Meyer P.M., Powell L.H., Pandey D., Torrens J.I., Kravitz H.M., Bromberger J.T., Matthews K.A. Depressive symptoms, insulin resistance, and risk of diabetes in women at midlife. Diabetes Care. 2004;27(12):2856–2862. doi: 10.2337/diacare.27.12.2856. [DOI] [PubMed] [Google Scholar]
- Fox S.M., III., Naughton J.P., Haskell W.L. Physical activity and the prevention of coronary heart disease. Ann. Clin. Res. 1971;3:404–432. [PubMed] [Google Scholar]
- Gappmaier E. The submaximal clinical exercise tolerance test (SXTT) to establish safe exercise prescription parameters for patients with chronic disease and disability. Cardiopulm. Phys. Ther. J. 2012;23(2):19–29. [PMC free article] [PubMed] [Google Scholar]
- Godin G. The Godin-Shephard leisure-time physical activity questionnaire. Health & Fitness Journal of Canada. 2011;4(1):18–22. [Google Scholar]
- Golden S.H., Dobs A.S., Vaidya D., Szklo M., Gapstur S., Gapstur P., Liu k., Ouyang P. Endogenous sex hormones and glucose tolerance status in postmenopausal women. J. Clin. Endocrinol. Metab. 2007;92(4):1289–1295. doi: 10.1210/jc.2006-1895. [DOI] [PubMed] [Google Scholar]
- Golden S.H., Williams J.E., Ford D.E., Yeh H.C., Sanford C.P., Nieto F.J., Nieto F.J., Nieto F.L. Depressive symptoms and the risk of type 2 diabetes: the Atherosclerosis Risk in Communities study. Diabetes Care. 2004;27(2):429–435. doi: 10.2337/diacare.27.2.429. [DOI] [PubMed] [Google Scholar]
- Health Council of the Netherlands . The Hague; 2006. Guidelines for a Healthy Diet. publication no. 2006/21E. [Google Scholar]
- Inada A., Fujii N.L., Inada O., Higaki Y., Furuichi Y., Nabeshima Y. Effects of 17β-estradiol and androgen on glucose metabolism in skeletal muscle. Endocrinology. 2016;157(12):4691–4705. doi: 10.1210/en.2016-1261. [DOI] [PubMed] [Google Scholar]
- Keshel T.E., Coker R.H. Exercise training and insulin resistance: a current review. J. Obes. Weight Loss Ther. 2015;5(Suppl 5) doi: 10.4172/2165-7904.S5-003. S5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.W., Kim D.Y. Effects of aerobic exercise training on serum sex hormone binding globulin, body fat index, and metabolic syndrome factors in obese postmenopausal women. Metab. Syndr. Relat. Disord. 2012;10(6):452–457. doi: 10.1089/met.2012.0036. [DOI] [PubMed] [Google Scholar]
- Kozlov S., Caprnda M., Chernova O., Matveeva M., Alekseeva I., Gazdikova K., Gaspar L., Kruzliak P., Filipova S., Gabbasov Z. Peak responses during exercise treadmill testing using individualized ramp protocol and modified Bruce protocol in elderly patients. Folia Med. 2020;62(1):76–81. doi: 10.3897/folmed.62.e49809. [DOI] [PubMed] [Google Scholar]
- Lee C.H., Giuliani F. The role of inflammation in depression and fatigue. Front. Immunol. 2019;10:1696. doi: 10.3389/fimmu.2019.01696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovejoy J.C., Bray G.A., Bourgeois M.O., Macchiavelli R., Rood J.C., Greeson C., Partington C. Exogenous androgens influence body composition and regional body fat distribution in obese postmenopausal women: a clinical research center study. J. Clin. Endocrinol. Metab. 1996;81:2198–2203. doi: 10.1210/jcem.81.6.8964851. [DOI] [PubMed] [Google Scholar]
- Makki K., Froguel P., Wolowczuk I. Adipose tissue in obesity-related inflammation and insulin resistance: cells, cytokines, and chemokines. ISRN Inflamm. 2013:139239. doi: 10.1155/2013/139239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin P., Arver S. Androgens and abdominal obesity. Baillieres Clin. Endocrinol. Metab. 1998;12:441–451. doi: 10.1016/s0950-351x(98)80191-2. [DOI] [PubMed] [Google Scholar]
- Mason C., Foster-Schubert K.E., Imayama I., Kong A., Xiao L., Bain C., Campbell K.L., Wang C.Y., Duggan C.R., Ulrich C.M., Alfano C.M., Blackburn G.L., McTiernan A. Dietary weight loss and exercise effects on insulin resistance in postmenopausal women. Am. J. Prev. Med. 2011;41(4):366–375. doi: 10.1016/j.amepre.2011.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui S., Yasui T., Tani A., Kunimi K., Uemura H., Yamamoto S., Kuwahara A., Matsuzaki T., Irahara M. Associations of estrogen and testosterone with insulin resistance in pre- and postmenopausal women with and without hormone therapy. Int. J. Endocrinol. Metabol. 2013;11(2):65–70. doi: 10.5812/ijem.5333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews D.R., Hosker J.P., Rudenski A.S., Naylor B.A., Treacher D.F., Turner R.C. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- Maturana M.A., Moreira R.M., Spritzer P.M. Lipid accumulation product (LAP) is related to androgenicity and cardiovascular risk factors in postmenopausal women. Maturitas. 2011;70:395–399. doi: 10.1016/j.maturitas.2011.09.012. [DOI] [PubMed] [Google Scholar]
- Meeusen R. Exercise, nutrition and the brain. Sports Med. 2014;44:47–56. doi: 10.1007/s40279-014-0150-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moratalla-Cecilia N., Soriano-Maldonado A., Ruiz-Cabello P., Fernández M.M., Gregorio-Arenas E., Aranda P., Aparicio V.A. Association of physical fitness with health-related quality of life in early postmenopause. Qual. Life Res. 2016;25(10):2675–2681. doi: 10.1007/s11136-016-1294-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muka T., Nano J., Jaspers L., Meun C., Bramer W.M., Hofman A., Dehghan A., Kavousi M., Laven L.S.E., Franco O.H. Associations of steroid sex hormones and sex hormone-binding globulin with the risk of type 2 diabetes in women: a population-based cohort study and meta-analysis. Diabetes. 2017;66(3):577–586. doi: 10.2337/db16-0473. [DOI] [PubMed] [Google Scholar]
- Roza A.M., Shizgal H.M. The Harris Benedict equation reevaluated: resting energy requirements and the body cell mass. Am. J. Clin. Nutr. 1984;40:168–182. doi: 10.1093/ajcn/40.1.168. [DOI] [PubMed] [Google Scholar]
- Ryan J., Burger H.G., Szoeke C., Lehert P., Ancelin M.L., Henderson V.W., Dennerstein L. A prospective study of the association between endogenous hormones and depressive symptoms in postmenopausal women. Menopause. 2009;16(3):509–517. doi: 10.1097/gme.0b013e31818d635f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz K.H., Lin H., Sammel M.D., Gracia C.R., Nelson D.B., Kapoor S., DeBlasis T.L., Freeman E.W. Association of physical activity with reproductive hormones: the penn ovarian aging study. Cancer Epidemiol. Biomarkers Prev. 2007;16(10):2042–2047. doi: 10.1158/1055-9965.EPI-07-0061. [DOI] [PubMed] [Google Scholar]
- Sharma S., Aggarwal N., Joshi B., Suri V., Badada S. Prevalence of metabolic syndrome in pre- and post-menopausal women: a prospective study from apex institute of North India. J. Midlife Health. 2016;7(4):169–174. doi: 10.4103/0976-7800.195695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stathopoulou M.B., Powers A.C., Berry J., Smits A.J., Otto M.W. Exercise interventions for mental health: a quantitative and qualitative review. Clin. Psychol. 2006;13(2):179–193. [Google Scholar]
- Tamakoshi K., Yatsuya H., Wada K., Matsushita K., Otsuka R., Yang P.O., Sugiura K., Hotta Y., Mitsuhashi H., Takefuji S., Kondo T. The transition to menopause reinforces adiponectin production and its contribution to improvement of insulin-resistant state. Clin. Endocrinol. 2007;66:65–71. doi: 10.1111/j.1365-2265.2006.02687.x. [DOI] [PubMed] [Google Scholar]
- Uebelacker L.A., Eaton C.B., Weisberg R., Sands M., Williams C., Calhoun D., Manson J.E., Denburg N.L., Taylor T. Social support and physical activity as moderators of life stress in predicting baseline depression and change in depression over time in the Women's Health Initiative. Soc. Psychiatr. Psychiatr. Epidemiol. 2013;48(12):1971–1982. doi: 10.1007/s00127-013-0693-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gemert W.A., Schuit A.J., van der Palen J., May A.M., Iestra J.A., Wittink H., Peeters P.H., Monninkhof E.M. Effect of weight loss, with or without exercise, on body composition and sex hormones in postmenopausal women: the SHAPE-2 trial. Breast Cancer Res. 2015;17(1):120. doi: 10.1186/s13058-015-0633-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F., Ames R., Evans M.C., France J.T., Reid I.R. Determinants of sex hormone-binding globulin in normal postmenopausal women. Clin. Endocrinol. 2001;54(1):81–87. doi: 10.1046/j.1365-2265.2001.01183.x. [DOI] [PubMed] [Google Scholar]
- Zung W.W., Richards C.B., Short M.J. Self-rating depression scale in an outpatient clinic. Further validation of the SDS. Arch. Gen. Psychiatr. 1965;13(6):508–515. doi: 10.1001/archpsyc.1965.01730060026004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.