Abstract
The in vitro activities of the novel 1β-methylcarbapenems J-111,225, J-114,870, and J-114,871, which have a structurally unique side chain that consists of a trans-3,5-disubstituted 5-arylpyrrolidin-3-ylthio moiety at the C-2 position, were compared with those of reference antibiotics. Among isolates of both methicillin-resistant Staphylococcus aureus (MRSA) and methicillin-resistant coagulase-negative staphylococci (MRCoNS), 90% were inhibited by J-111,347 (prototype), J-111,225, J-114,870, and J-114,871 at concentrations of 2, 4, 4, and 4 μg/ml (MICs at which 90% of isolates are inhibited [MIC90s]), respectively, indicating that these agents were 32- to 64-fold more potent than imipenem, which has an MIC90 of 128 μg/ml. Although these drugs were less active in vitro than vancomycin, which had MIC90s of 1 and 2 μg/ml for MRSA and MRCoNS, respectively, the new carbapenems displayed better killing kinetics than vancomycin. The potent anti-MRSA activity was ascribed to the excellent affinities of the new carbapenems for penicillin-binding protein 2a of MRSA. Since the new carbapenems also exhibited good activity against gram-positive and -negative bacteria including clinically important pathogens such as penicillin-resistant Streptococcus pneumoniae, Haemophilus influenzae, members of the family Enterobacteriaceae, Pseudomonas aeruginosa, and Clostridium difficile, as well as MRSA, the novel carbapenems are worthy of further evaluation.
The emergence of multidrug-resistant microorganisms has caused serious concern about infectious diseases worldwide. Although more than three decades have passed since the first report of methicillin-resistant Staphylococcus aureus (MRSA), MRSA still presents a serious problem worldwide as a cause of nosocomial infections (7, 12). Vancomycin, a cyclic glycopeptide antibiotic, has been extensively used in the clinic to treat MRSA infections. However, it is not an ideal antibiotic because of the slow clinical response (6) and potential adverse effects (3). Furthermore, the emergence of MRSA strains with reduced susceptibility to vancomycin accelerated an urgent need for new chemotherapeutic agents for the treatment of MRSA infections (5).
Previously reported β-lactam antibiotics with activity against methicillin-resistant staphylococci (MRS) such as L-695,256 (2), SM-17466 (13), BO-3482 (8), TOC-39 (4), MC-02,479/RWJ-54428 (F. Malouin, C. Chan, S. Bond, S. Chamberland, and V. J. Lee, Abstr. 37th Intersci. Conf. Antimicrob. Agents Chemother., abstr. F-177, p. 176, 1997), Ro 63-9141 (P. Hohl, P. Angehrn, R. L. Then, P. Hebeisen, and I. Heinze-Krauss, Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother., abstr. F-24, p. 239, 1998), and L-786,392 (J. Huber, K. L. Dorso, J. Koheler, H. Kropp, H. Rosen, and L. L. Silver, Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother., abstr. F-30, p. 240, 1998) all had weak activities against gram-negative organisms and/or a lack of antipseudomonal activity.
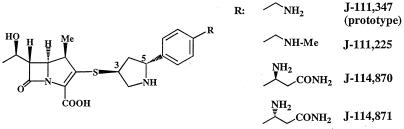
In the course of our derivatization study of 1β-methylcarbapenems, a novel trans-3,5-pyrrolidinylthio-1β-methylcarbapenem, J-111,347, was identified as a broad-spectrum agent with activity against MRS, as well as gram-positive and -negative organisms, including Pseudomonas aeruginosa, that are usually covered by the marketed broad-spectrum carbapenems (A. Shimizu, Y. Sugimoto, S. Sakuraba, H. Imamura, H. Sato, N. Ohtake, R. Ushijima, S. Nakagawa, C. Suzuki, T. Hashizume, and S. Morishima, Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother., abstr. F-52, p. 246, 1998). Subsequently, we synthesized J-111,225, J-114,870, and J-114,871 (Fig. 1) (10). The stereochemistry of the side chains in these compounds is novel; known pyrrolidinylthio-1β-carbapenems like meropenem (14), S-4661 (S. Sasaki, K. Murakami, Y. Nishitani, and S. Kuwahara, Abstr. 34th Intersci. Conf. Antimicrob. Agents Chemother., abstr. F-33, p. 32, 1994), BO-2727 (9), MK-826 (formerly L-749,345) (L. Pelak, S. Gerckens, P. M. Scott, C. Gill, C. Pacholok, L. Lynch, K. Dorso, J. Kohler, D. Shungu, and H. Kropp, Abstr. 36th Intersci. Conf. Antimicrob. Agents Chemother., abstr. F-119, p. 120, 1996), and E1010 (formerly ER-35786) (11) share a cis-counterpart at the side chains.
FIG. 1.
Chemical structures of the trans-3,5-disubstituted 1β-methylcarbapenems J-111,347, J-111,225, J-114,870, and J-114,871. Me, methyl.
In this paper, we describe the in vitro evaluation of the novel trans-3,5-disubstituted pyrrolidinylthio-1β-methylcarbapenems J-111,347 (the prototype), J-111,225, J-114,870, and J-114,871.
(This paper was presented in part at the 38th Interscience Conference on Antimicrobial Agents and Chemotherapy, San Diego, Calif., 24 to 27 September 1998.)
MATERIALS AND METHODS
Antibiotics.
All of the carbapenems used in this study except imipenem and vancomycin were synthesized at Banyu Tsukuba Research Institute, Tsukuba, Japan; imipenem was a product of Banyu Pharmaceutical Co., Ltd., Tokyo, Japan, and vancomycin was purchased from Sigma Chemical Co., St. Louis, Mo. The antibiotics were dissolved in 10 mM 3-(N-morpholino)propanesulfonic acid (MOPS) buffer (pH 7.0) on the day of use.
Organisms.
The clinical isolates used in this study were collected from several hospitals in Japan over the past several years. All isolates were maintained in glycerol broth at −80°C.
Determination of MICs.
MICs were determined by the twofold serial broth microdilution method with Mueller-Hinton broth (Difco Laboratories, Detroit, Mich.) unless stated otherwise. Susceptibility testing for streptococci was performed with Todd-Hewitt broth (Difco) supplemented with 5% hemolyzed horse blood. Brain heart infusion broth (Difco), brain heart infusion broth (Difco) supplemented with 5% Fildes enrichment (Difco), and GAM broth (Nissui Seiyaku Co., Ltd., Tokyo, Japan) were used for enterococci, Haemophilus influenzae, and anaerobes, respectively. The inoculum sizes of gram-positive or -negative bacteria and anaerobes were 105 and 106 CFU/ml, respectively. The MIC was defined as the lowest antibiotic concentration that completely prevented visible growth after incubation at 37°C for 20 h.
Killing kinetics.
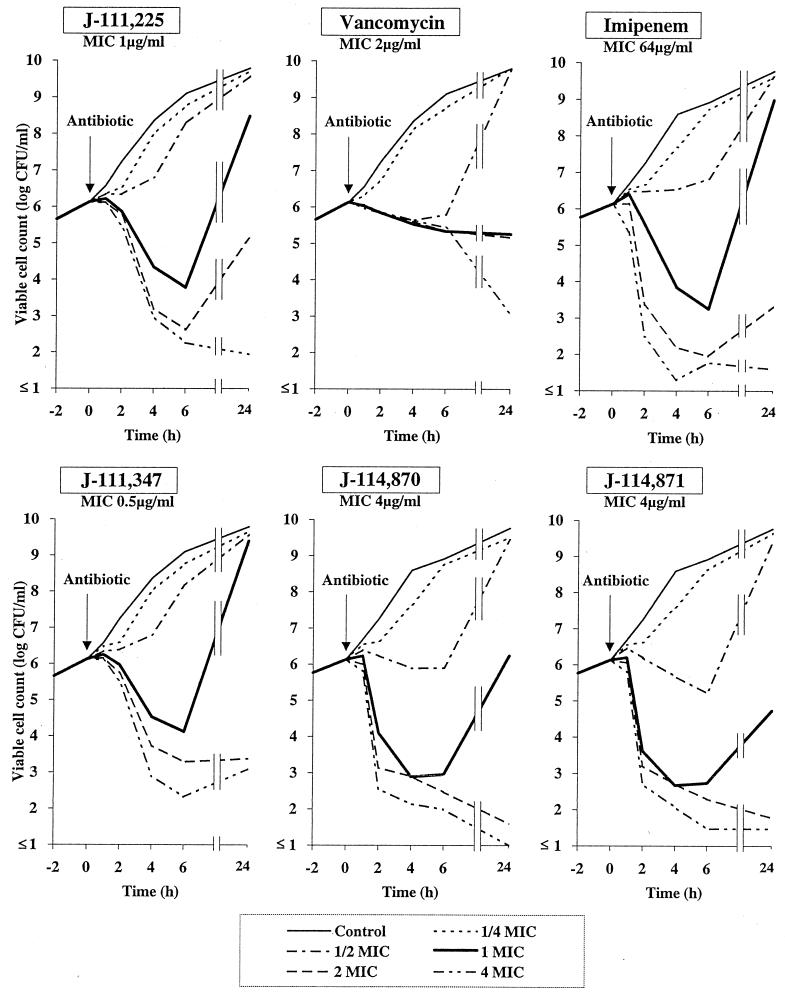
MRSA strain BB6226, a β-lactamase-negative homogeneously resistant strain, was used. After preincubation at 37°C for 2 h, an antibiotic was added and the test tubes were incubated in a water bath at 37°C with gentle shaking. The viable cells were counted on a Mueller-Hinton medium (Difco) plate after an aliquot of the culture was taken at the times indicated in Fig. 2. The limit of detection of viable counts was 10 CFU/ml.
FIG. 2.
Killing kinetic curves for trans-3,5-disubstituted 1β-methyl carbapenems, imipenem, and vancomycin against homogeneous MRSA.
Affinity of PBP 2a of MRSA.
The affinity of PBP 2a of MRSA was determined by a competition assay with [14C]benzylpenicillin (15). Briefly, membrane fractions were preincubated at 30°C for 10 min with nonlabeled antibiotics and were postincubated with [14C]benzylpenicillin for 10 min. Binding affinity was expressed as the concentration of nonlabeled antibiotic that inhibited radiolabeling with [14C]benzylpenicillin by 50% (IC50) compared with the control in the absence of nonlabeled antibiotics.
RESULTS
In vitro activity.
The comparative antibacterial activities of J-111,347, J-111,225, J-114,870, J-114,871, and the reference antibiotics against the clinical isolates are shown in Table 1. The new carbapenems showed clearly improved activities against the isolates of MRSA and methicillin-resistant coagulase-negative staphylococci (MRCoNS) compared with those of the older carbapenems imipenem, meropenem, and biapenem. J-111,347, J-111,225, J-114,870, and J-114,871 inhibited 90% of the MRSA isolates at concentrations of 2, 4, 4, and 4 μg/ml (MICs at which 90% of isolates are inhibited [MIC90]), respectively, representing activity 32 to 64 times more potent than that of imipenem, which has an MIC90 of 128 μg/ml. The activity of J-111,347 was about half as potent as or equivalent to that of vancomycin against MRSA and MRCoNS in terms of the MIC90. In general, J-111,225, J-114,870, and J-114,871 were almost as active or were slightly less active than J-111,347 against the MRS isolates. Thus, the new carbapenems are definitely differentiated from conventional carbapenems in terms of their anti-MRS activities.
TABLE 1.
Comparative in vitro activities against clinical isolates
| Organism (no. of isolates) and antibiotic | MIC (μg/ml)a
|
|||
|---|---|---|---|---|
| MRSA (26) | ||||
| J-111,347 | 0.5–4 | 2 | 2 | |
| J-111,225 | 0.5–4 | 2 | 4 | |
| J-114,870 | 1–4 | 2 | 4 | |
| J-114,871 | 0.5–4 | 2 | 4 | |
| Imipenem | 16–128 | 64 | 128 | |
| Meropenem | 8–64 | 32 | 64 | |
| Biapenem | 16–128 | 64 | 64 | |
| Vancomycin | 0.5–2 | 1 | 1 | |
| Staphylococcus aureus, methicillin susceptible (21) | ||||
| J-111,347 | 0.008–0.016 | ≤0.008 | 0.016 | |
| J-111,225 | 0.008–0.016 | 0.016 | 0.016 | |
| J-114,870 | 0.008–0.016 | 0.016 | 0.016 | |
| J-114,871 | 0.008–0.032 | 0.016 | 0.016 | |
| Imipenem | 0.008–0.016 | 0.016 | 0.016 | |
| Meropenem | 0.032–0.125 | 0.063 | 0.125 | |
| Biapenem | 0.016–0.063 | 0.032 | 0.063 | |
| Ceftazidime | 4–8 | 4 | 8 | |
| Vancomycin | 0.5–1 | 0.5 | 0.5 | |
| Coagulase-negative staphylococci, methicillin resistant (25) | ||||
| J-111,347 | 0.125–4 | 0.5 | 2 | |
| J-111,225 | 0.125–4 | 1 | 4 | |
| J-114,870 | 0.125–4 | 1 | 4 | |
| J-114,871 | 0.125–4 | 1 | 4 | |
| Imipenem | 0.125–128 | 16 | 128 | |
| Meropenem | 1–128 | 16 | 64 | |
| Biapenem | 0.5–128 | 16 | 128 | |
| Ceftazidime | 8–>128 | 64 | >128 | |
| Vancomycin | 0.5–2 | 1 | 2 | |
| Streptococcus pyogenes (30) | ||||
| J-111,347 | ≤0.008 | ≤0.008 | ≤0.008 | |
| J-111,225 | ≤0.008 | ≤0.008 | ≤0.008 | |
| J-114,870 | ≤0.008 | ≤0.008 | ≤0.008 | |
| J-114,871 | ≤0.008 | ≤0.008 | ≤0.008 | |
| Imipenem | ≤0.008 | ≤0.008 | ≤0.008 | |
| Meropenem | ≤0.008 | ≤0.008 | ≤0.008 | |
| Biapenem | ≤0.008 | ≤0.008 | ≤0.008 | |
| Ceftazidime | 0.063–0.125 | 0.063 | 0.125 | |
| Penicillin G | ≤0.008 | ≤0.008 | ≤0.008 | |
| Vancomycin | 0.5 | 0.5 | 0.5 | |
| Streptococcus agalactiae (24) | ||||
| J-111,347 | 0.008–0.016 | ≤0.008 | 0.016 | |
| J-111,225 | 0.008–0.016 | ≤0.008 | 0.016 | |
| J-114,870 | 0.008–0.016 | 0.016 | 0.016 | |
| J-114,871 | 0.008–0.032 | 0.016 | 0.016 | |
| Imipenem | 0.008–0.016 | ≤0.008 | 0.016 | |
| Meropenem | 0.016–0.063 | 0.032 | 0.032 | |
| Biapenem | 0.008–0.032 | 0.016 | 0.032 | |
| Ceftazidime | 0.5–1 | 0.5 | 0.5 | |
| Penicillin G | 0.032–0.125 | 0.032 | 0.063 | |
| Vancomycin | 0.5–1 | 1 | 1 | |
| Streptococcus pneumoniae, including penicillin-resistant organisms (32) | ||||
| J-111,347 | 0.004–0.25 | 0.008 | 0.25 | |
| J-111,225 | 0.004–0.25 | 0.016 | 0.25 | |
| J-114,870 | 0.004–0.25 | 0.016 | 0.25 | |
| J-114,871 | 0.004–0.25 | 0.016 | 0.25 | |
| Imipenem | 0.004–0.25 | 0.016 | 0.25 | |
| Meropenem | 0.008–0.5 | 0.032 | 0.5 | |
| Biapenem | 0.008–0.5 | 0.016 | 0.25 | |
| Ceftazidime | 0.125–16 | 2 | 16 | |
| Penicillin G | 0.008–2 | 0.125 | 2 | |
| Vancomycin | 0.25–0.5 | 0.5 | 0.5 | |
| Enterococcus faecalis (26) | ||||
| J-111,347 | 1–8 | 2 | 8 | |
| J-111,225 | 1–16 | 4 | 8 | |
| J-114,870 | 1–8 | 4 | 8 | |
| J-114,871 | 2–16 | 4 | 8 | |
| Imipenem | 0.5–4 | 1 | 4 | |
| Meropenem | 4–32 | 8 | 16 | |
| Biapenem | 2–16 | 8 | 16 | |
| Ceftazidime | >128 | >128 | >128 | |
| Ampicillin | 0.5–4 | 1 | 4 | |
| Vancomycin | 0.5–4 | 2 | 2 | |
| Enterococcus faecium (17) | ||||
| J-111,347 | 2–>128 | 32 | >128 | |
| J-111,225 | 4–>128 | 32 | >128 | |
| J-114,870 | 4–>128 | 64 | >128 | |
| J-114,871 | 4–>128 | 64 | >128 | |
| Imipenem | 4–>128 | 64 | >128 | |
| Meropenem | 16–>128 | 128 | >128 | |
| Biapenem | 16–>128 | >128 | >128 | |
| Ceftazidime | >128 | >128 | >128 | |
| Ampicillin | 1–>128 | 32 | 128 | |
| Vancomycin | 1–4 | 2 | 4 | |
| Escherichia coli (29) | ||||
| J-111,347 | 0.016–0.032 | 0.016 | 0.032 | |
| J-111,225 | 0.016–0.063 | 0.032 | 0.032 | |
| J-114,870 | 0.016–0.032 | 0.016 | 0.016 | |
| J-114,871 | 0.016–0.063 | 0.032 | 0.032 | |
| Imipenem | 0.032–0.125 | 0.063 | 0.125 | |
| Meropenem | 0.016–0.016 | 0.016 | 0.016 | |
| Biapenem | 0.016–0.063 | 0.032 | 0.063 | |
| Ceftazidime | 0.063–16 | 0.063 | 0.125 | |
| Klebsiella pneumoniae (25) | ||||
| J-111,347 | 0.016–0.063 | 0.032 | 0.032 | |
| J-111,225 | 0.016–0.063 | 0.032 | 0.063 | |
| J-114,870 | 0.016–0.063 | 0.032 | 0.063 | |
| J-114,871 | 0.016–0.063 | 0.032 | 0.063 | |
| Imipenem | 0.063–0.5 | 0.125 | 0.25 | |
| Meropenem | ≤0.008–0.032 | 0.016 | 0.032 | |
| Biapenem | 0.032–1 | 0.063 | 0.5 | |
| Ceftazidime | 0.032–4 | 0.063 | 0.25 | |
| Enterobacter cloacae (27) | ||||
| J-111,347 | 0.032–0.5 | 0.063 | 0.5 | |
| J-111,225 | 0.032–1 | 0.063 | 0.5 | |
| J-114,870 | 0.032–2 | 0.063 | 1 | |
| J-114,871 | 0.016–1 | 0.032 | 0.5 | |
| Imipenem | 0.063–1 | 0.5 | 1 | |
| Meropenem | 0.016–4 | 0.032 | 2 | |
| Biapenem | 0.032–2 | 0.125 | 1 | |
| Ceftazidime | 0.125–>128 | 0.5 | >128 | |
| Enterobacter aerogenes (23) | ||||
| J-111,347 | 0.032–0.5 | 0.063 | 0.125 | |
| J-111,225 | 0.032–1 | 0.125 | 0.25 | |
| J-114,870 | 0.016–1 | 0.063 | 0.25 | |
| J-114,871 | 0.032–1 | 0.125 | 0.5 | |
| Imipenem | 0.25–2 | 0.5 | 1 | |
| Meropenem | 0.032–1 | 0.063 | 0.25 | |
| Biapenem | 0.063–1 | 0.25 | 1 | |
| Ceftazidime | 0.063–>128 | 0.5 | 64 | |
| Citrobacter freundii (22) | ||||
| J-111,347 | 0.016–0.25 | 0.032 | 0.25 | |
| J-111,225 | 0.032–0.5 | 0.063 | 0.25 | |
| J-114,870 | 0.016–0.5 | 0.032 | 0.5 | |
| J-114,871 | 0.016–0.5 | 0.032 | 0.5 | |
| Imipenem | 0.063–1 | 0.25 | 1 | |
| Meropenem | ≤0.008–2 | 0.016 | 1 | |
| Biapenem | 0.032–1 | 0.125 | 0.5 | |
| Ceftazidime | 0.063–>128 | 0.5 | >128 | |
| Citrobacter diversus (17) | ||||
| J-111,347 | 0.016–0.125 | 0.032 | 0.063 | |
| J-111,225 | 0.016–0.25 | 0.032 | 0.063 | |
| J-114,870 | 0.016–0.125 | 0.032 | 0.063 | |
| J-114,871 | 0.016–0.25 | 0.032 | 0.063 | |
| Imipenem | 0.063–0.5 | 0.125 | 0.25 | |
| Meropenem | ≤0.008–0.5 | 0.016 | 0.125 | |
| Biapenem | 0.016–0.125 | 0.032 | 0.125 | |
| Ceftazidime | 0.032–16 | 0.063 | 0.5 | |
| Proteus mirabilis (24) | ||||
| J-111,347 | 0.063–1 | 0.125 | 0.25 | |
| J-111,225 | 0.063–1 | 0.25 | 0.5 | |
| J-114,870 | 0.032–1 | 0.125 | 0.25 | |
| J-114,871 | 0.063–1 | 0.25 | 0.5 | |
| Imipenem | 0.5–4 | 2 | 4 | |
| Meropenem | 0.032–0.125 | 0.032 | 0.063 | |
| Biapenem | 1–4 | 2 | 4 | |
| Ceftazidime | 0.016–0.063 | 0.032 | 0.032 | |
| Proteus vulgaris (24) | ||||
| J-111,347 | 0.032–0.25 | 0.125 | 0.25 | |
| J-111,225 | 0.063–0.5 | 0.125 | 0.25 | |
| J-114,870 | 0.032–0.25 | 0.125 | 0.25 | |
| J-114,871 | 0.063–0.5 | 0.125 | 0.25 | |
| Imipenem | 0.5–4 | 2 | 2 | |
| Meropenem | 0.032–0.063 | 0.032 | 0.063 | |
| Biapenem | 0.125–2 | 1 | 2 | |
| Ceftazidime | 0.016–16 | 0.063 | 4 | |
| Providencia rettgeri (25) | ||||
| J-111,347 | 0.032–0.25 | 0.125 | 0.25 | |
| J-111,225 | 0.032–0.5 | 0.125 | 0.25 | |
| J-114,870 | 0.032–0.25 | 0.125 | 0.25 | |
| J-114,871 | 0.063–0.5 | 0.125 | 0.25 | |
| Imipenem | 0.25–2 | 1 | 2 | |
| Meropenem | 0.016–0.125 | 0.032 | 0.063 | |
| Biapenem | 0.125–1 | 0.5 | 1 | |
| Ceftazidime | 0.016–0.5 | 0.032 | 0.063 | |
| Morganella morganii (21) | ||||
| J-111,347 | 0.125–1 | 0.25 | 0.5 | |
| J-111,225 | 0.125–1 | 0.5 | 1 | |
| J-114,870 | 0.25–2 | 0.5 | 2 | |
| J-114,871 | 0.125–2 | 0.25 | 1 | |
| Imipenem | 1–4 | 4 | 4 | |
| Meropenem | 0.063–2 | 0.125 | 0.125 | |
| Biapenem | 1–4 | 1 | 4 | |
| Ceftazidime | 0.032–128 | 0.125 | 16 | |
| Serratia marcescens (28) | ||||
| J-111,347 | 0.032–1 | 0.063 | 0.5 | |
| J-111,225 | 0.032–1 | 0.063 | 0.5 | |
| J-114,870 | 0.063–2 | 0.063 | 1 | |
| J-114,871 | 0.032–2 | 0.063 | 1 | |
| Imipenem | 0.125–4 | 0.25 | 0.5 | |
| Meropenem | 0.016–4 | 0.032 | 1 | |
| Biapenem | 0.063–4 | 0.25 | 0.5 | |
| Ceftazidime | 0.063–32 | 0.125 | 4 | |
| Moraxella catarrhalis (25) | ||||
| J-111,347 | ≤0.002–0.008 | ≤0.008 | ≤0.008 | |
| J-111,225 | ≤0.002–0.016 | ≤0.008 | ≤0.008 | |
| J-114,870 | ≤0.002–0.016 | ≤0.008 | ≤0.008 | |
| J-114,871 | ≤0.002–0.016 | ≤0.008 | ≤0.008 | |
| Imipenem | 0.008–0.063 | 0.032 | 0.063 | |
| Meropenem | 0.002–0.004 | ≤0.008 | ≤0.008 | |
| Biapenem | 0.016–0.063 | 0.032 | 0.032 | |
| Ceftazidime | 0.016–0.25 | 0.063 | 0.125 | |
| Haemophilus influenzae (20) | ||||
| J-111,347 | 0.032–0.25 | 0.063 | 0.125 | |
| J-111,225 | 0.063–0.5 | 0.125 | 0.125 | |
| J-114,870 | 0.063–0.5 | 0.125 | 0.125 | |
| J-114,871 | 0.063–0.5 | 0.125 | 0.125 | |
| Imipenem | 0.25–2 | 1 | 1 | |
| Meropenem | 0.032–0.125 | 0.063 | 0.063 | |
| Biapenem | 0.25–4 | 1 | 1 | |
| Ceftazidime | 0.032–0.25 | 0.125 | 0.125 | |
| Ampicillin | 0.125–64 | 0.25 | 1 | |
| Pseudomonas aeruginosa, imipenem susceptible (MIC, ≤4) (34) | ||||
| J-111,347 | 0.125–8 | 0.5 | 4 | |
| J-111,225 | 0.125–16 | 1 | 4 | |
| J-114,870 | 0.125–8 | 1 | 4 | |
| J-114,871 | 0.125–8 | 1 | 4 | |
| Imipenem | 0.5–4 | 2 | 4 | |
| Meropenem | 0.125–16 | 1 | 4 | |
| Biapenem | 0.25–4 | 1 | 2 | |
| Ceftazidime | 0.5–>128 | 1 | 16 | |
| Pseudomonas aeruginosa, imipenem resistant (MIC, ≥8) (38) | ||||
| J-111,347 | 1–16 | 4 | 8 | |
| J-111,225 | 2–32 | 8 | 16 | |
| J-114,870 | 1–32 | 4 | 16 | |
| J-114,871 | 2–64 | 8 | 16 | |
| Imipenem | 8–128 | 16 | 32 | |
| Meropenem | 8–128 | 8 | 16 | |
| Biapenem | 1–>128 | 8 | 16 | |
| Ceftazidime | 1–>128 | 8 | 128 | |
| Acinetobacter spp. (21) | ||||
| J-111,347 | ≤0.008–0.25 | 0.032 | 0.125 | |
| J-111,225 | ≤0.008–0.5 | 0.032 | 0.125 | |
| J-114,870 | 0.032–0.5 | 0.063 | 0.25 | |
| J-114,871 | 0.016–0.5 | 0.032 | 0.125 | |
| Imipenem | 0.016–0.25 | 0.125 | 0.125 | |
| Meropenem | 0.032–0.25 | 0.125 | 0.25 | |
| Biapenem | 0.016–0.25 | 0.125 | 0.25 | |
| Ceftazidime | 0.5–32 | 2 | 8 | |
| Burkholderia cepacia (21) | ||||
| J-111,347 | 0.25–16 | 8 | 16 | |
| J-111,225 | 0.5–32 | 8 | 16 | |
| J-114,870 | 0.5–16 | 8 | 16 | |
| J-114,871 | 0.5–32 | 8 | 32 | |
| Imipenem | 0.5–16 | 4 | 16 | |
| Meropenem | 0.032–8 | 1 | 4 | |
| Biapenem | 0.125–8 | 2 | 8 | |
| Ceftazidime | 0.5–>128 | 2 | 16 | |
| Bacteroides fragilis (22) | ||||
| J-111,347 | 0.125–0.5 | 0.25 | 0.5 | |
| J-111,225 | 0.25–1 | 0.25 | 0.5 | |
| J-114,870 | 0.125–1 | 0.25 | 0.5 | |
| J-114,871 | 0.25–1 | 0.25 | 0.5 | |
| Imipenem | 0.063–2 | 0.5 | 0.5 | |
| Meropenem | 0.063–4 | 0.25 | 0.25 | |
| Biapenem | 0.125–4 | 0.25 | 0.5 | |
| Ceftazidime | 4–>128 | 16 | >128 | |
| Clostridium difficile (21) | ||||
| J-111,347 | 0.5–1 | 1 | 1 | |
| J-111,225 | 1–2 | 2 | 2 | |
| J-114,870 | 1–2 | 2 | 2 | |
| J-114,871 | 1–2 | 2 | 2 | |
| Imipenem | 4–16 | 8 | 8 | |
| Meropenem | 0.5–4 | 1 | 2 | |
| Biapenem | 4–16 | 8 | 16 | |
| Ceftazidime | 32–>128 | 128 | >128 | |
Determined by the broth microdilution method.
The new carbapenems were as active as imipenem against methicillin-susceptible S. aureus, Streptococcus pyogenes, Streptococcus agalactiae, and penicillin-resistant Streptococcus pneumoniae. The MIC90s of the new carbapenems against penicillin-resistant S. pneumoniae were uniformly 0.25 μg/ml, as were those of imipenem and biapenem, while the MIC90 of penicillin G was 16 μg/ml. Against Enterococcus faecalis, the new carbapenems were two to four times less active than imipenem, but they were two to four times more active than meropenem and biapenem. Imipenem-resistant Enterococcus faecium showed cross-resistance to the new carbapenems.
As for gram-negative isolates, J-111,347, J-111,225, J-114,870, and J-114,871 had improved activities compared to those of imipenem and biapenem against Moraxella catarrhalis and H. influenzae, with MIC90s of ≤0.008 and 0.125 μg/ml, respectively (MIC90s of imipenem, 0.063 and 1 μg/ml, respectively); however, their activities against these two gram-negative bacteria were similar to that of meropenem. The activities of the new compounds were similar to or greater than those of imipenem and biapenem against members of the family Enterobacteriaceae such as Escherichia coli, Klebsiella pneumoniae, Enterobacter cloacae, Citrobacter freundii, Citrobacter diversus, Proteus mirabilis, Proteus vulgaris, Providencia rettgeri, Morganella morganii, and Serratia marcescens, but meropenem was the most potent agent against the gram-negative bacteria tested. Some of these enteric bacteria showed high-level resistance to ceftazidime, probably due to the high level of expression of chromosomal AmpC β-lactamase (or extended spectrum β-lactamases). All of the new compounds were two to four times more active than imipenem against imipenem-susceptible P. aeruginosa, with the MIC50s ranging from 0.5 to 1 μg/ml, whereas the MIC50 of imipenem was 2 μg/ml; likewise, the new compounds were more active than imipenem against imipenem-resistant P. aeruginosa.
As a whole, there was no significant difference in the antibacterial activities of J-111,225, J-114,870, and J-114,871, although these compounds were slightly less active than J-111,347. Considering the history of development of β-lactam antibiotics, it is interesting that the new agents have the advantage of dual activity against both MRS and P. aeruginosa.
Bactericidal activity.
Bactericidal activity against MRSA was determined by constructing time-kill curves (Fig. 2). As expected, J-111,225, J-114,870, and J-114,871 showed bactericidal kinetics at concentrations above the MICs of 1 to 4 μg/ml, although substantial regrowth was observed after 24 h of incubation in the presence of two times the MICs of J-111,225, J-111,347, and imipenem. Vancomycin (MIC, 1 μg/ml) showed slow killing kinetics even at four times the MIC. Imipenem required high concentrations (over 64 μg/ml) to kill MRSA.
Activity against β-lactamase producers.
The new carbapenems were active against various β-lactamase-producing bacteria except for Burkholderia cepacia and metallo-β-lactamase producers (Table 2). With the exceptions of B. cepacia and metallo-β-lactamase producers, the MICs of the new carbapenems for ampicillin- and/or cefazolin-resistant β-lactamase producers were within the susceptible range for other carbapenems, as were those of the reference carbapenems. In E. coli, there seemed to be no appreciable influence of β-lactamase producing strain on the activity since basal AmpC β-lactamase production had similar susceptibility to the respective new carbapenems (MIC, 0.031 μg/ml). It is noteworthy that the new drugs had greater activities than the marketed carbapenems against the IMP-1 β-lactamase-producing organism.
TABLE 2.
Comparative antibacterial activities against β-lactamase producers
| Enzyme produced and organism | MIC (μg/ml)a
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| J-111,347 | J-111,225 | J-114,870 | J-114,871 | Imipenem | Biapenem | Meropenem | Aztreonam | Ceftazidime | Cefazolin | Ampicillin | |
| Penicillinase | |||||||||||
| E. coli ML4901 (TEM-1) | 0.031 | 0.031 | 0.063 | 0.031 | 0.125 | 0.063 | 0.013 | 0.063 | 0.125 | 2 | >128 |
| E. coli ML4901 (PSE-1) | 0.016 | 0.031 | 0.031 | 0.031 | 0.125 | 0.031 | 0.016 | 0.125 | 0.25 | 2 | >128 |
| E. coli ML4901 (OXA-1) | 0.031 | 0.063 | 0.063 | 0.063 | 0.25 | 0.125 | 0.031 | 0.25 | 0.25 | 2 | 128 |
| Cephalosporinase | |||||||||||
| E. coli GN5482 | 0.016 | 0.031 | 0.031 | 0.031 | 0.125 | 0.031 | 0.016 | 2 | 2 | 64 | 64 |
| M. morganii GN5407 | 0.125 | 0.125 | 0.125 | 0.063 | 2 | 0.5 | 0.031 | 0.008 | 0.125 | 128 | 128 |
| C. freundii GN346 | 0.063 | 0.125 | 0.125 | 0.063 | 0.25 | 0.125 | 0.063 | 32 | 64 | >128 | >128 |
| E. cloacae GN7471 | 0.063 | 0.125 | 0.125 | 0.125 | 0.5 | 0.5 | 0.063 | 8 | 32 | >128 | >128 |
| P. aeruginosa GN10362 | 0.5 | 0.5 | 0.5 | 1 | 1 | 1 | 1 | 4 | 1 | >128 | >128 |
| Oxyiminocephalosporinase | |||||||||||
| Klebsiella oxytoca GN10650 | 0.063 | 0.063 | 0.063 | 0.063 | 0.25 | 0.063 | 0.031 | 8 | 0.125 | >128 | 128 |
| P. vulgaris GN7919 | 0.125 | 0.125 | 0.125 | 0.125 | 0.5 | 0.25 | 0.031 | 1 | 2 | 64 | 64 |
| B. cepacia GN11164 | 2 | 4 | 8 | 8 | 8 | 2 | 4 | 2 | 0.5 | >128 | >128 |
| Carbapenemase | |||||||||||
| P. aeruginosa GN17203 (IMP-1) | 16 | 32 | 32 | 64 | 128 | >128 | 128 | 2 | >128 | >128 | >128 |
| Stenotrophomonas maltophilia GN12873 (L1, L2) | 32 | 128 | 128 | 64 | 128 | >128 | 32 | >128 | 16 | >128 | 128 |
Determined by the broth microdilution method.
Mechanism of action against MRSA.
Since PBP 2a, which has a low affinity for β-lactams, is the resistance determinant for β-lactam antibiotics in MRS, the binding affinities of J-111,347 and J-111,225 for PBP 2a were investigated. These two carbapenems had improved IC50s of 2.6 and 2.5 μg/ml, respectively, compared with the IC50 of 85 μg/ml of imipenem in a competition assay with benzylpenicillin. There was a good correlation between anti-MRSA activity and the binding affinity for PBP 2a of the MRSA strain tested, indicating that the mechanism of anti-MRSA activity could be ascribed to the inhibition of PBP 2a.
DISCUSSION
MRS and gram-negative organisms including P. aeruginosa are common pathogens in serious infections, and vancomycin has been the agent of choice for the treatment of infections due to MRS, despite its association with a variety of drug-related side effects. Although a number of antibiotics that target MRSA and other resistant gram-positive organisms have been reported, no antibiotic so far provides potent anti-MRS activity while also providing the excellent broad-spectrum activity offered by carbapenems.
We conducted chemical modification studies with 1β-methylcarbapenem on the basis of the concept that a new analogue should provide coverage against MRS as well as other gram-negative organisms including P. aeruginosa. J-111,225, J-114,870, and J-114,871 are crystalline forms, and their structural features include a unique stereochemistry of the side chain, i.e., a phenyl ring directly attached to the pyrrolidine ring with a 3,5-trans configuration at the C-2 position of the carbapenem nucleus.
Since previously reported anti-MRS agents have limited activity against gram-negative bacteria, it was noted that the newly synthesized carbapenems with activity against MRSA showed improved activity against gram-negative organisms including P. aeruginosa and metallo-β-lactamase producers compared with the activity of imipenem. Metallo-β-lactamase or carbapenemase is known to confer high-level resistance to penicillins and cephems as well as to carbapenems (16). A recent problem is the spread of the transferable plasmid-mediated IMP-1 enzyme, especially in Japan (1). The mechanism underlying improved activity against IMP-1-producing organisms was explained by resistance to hydrolysis by this enzyme (R. Nagano, Y. Adachi, T. Hashizume, and S. Morishima, Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother., abstr. F-55, p. 247, 1998).
Although further evaluation of J-111,347 was suspended due to its epileptogenicity, this undesirable adverse effect was clearly eliminated by N methylation (J-111,225) or the introduction of carbamoylmethyl substituents (J-114,870 and J-114,871) at the α position of the benzylamino group.
The new carbapenems could be suited for use as monotherapy for serious polymicrobial infections associated with MRS. Furthermore, monotherapy with the new carbapenems would avoid unexpected adverse reactions due to combination therapy and the emergence of vancomycin-resistant MRS due to the intensive use of vancomycin. In conclusion, the novel trans-3,5-disubstituted pyrrolidinylthio-1β-methylcarbapenems J-111,347 (prototype), J-111,225, J-114,870, and J-114,871 possess broad spectra of activity, with coverage of MRSA and P. aeruginosa, and are worthy of further evaluation.
REFERENCES
- 1.Arakawa Y, Murakami M, Suzuki K, Ito H, Wacharotayankun R, Ohsuka S, Kato N, Ohta M. A novel integron-like element carrying the metallo-β-lactamase gene blaIMP. Antimicrob Agents Chemother. 1995;39:1612–1615. doi: 10.1128/aac.39.7.1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chambers H F. In vitro and in vivo antistaphylococcal activities of L-695,256, a carbapenem with high affinity for penicillin-binding protein PBP2a. Antimicrob Agents Chemother. 1995;39:462–466. doi: 10.1128/aac.39.2.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Duffull S B, Begg E J. Vancomycin toxicity. What is the evidence for dose dependency? Adverse Drug Reactions Toxicol Rev. 1994;13:103–114. [PubMed] [Google Scholar]
- 4.Hanaki H, Akagi H, Yasui M, Otani T, Hyodo A, Hiramatsu K. TOC-39, a novel parenteral broad-spectrum cepharosporin with excellent activity against methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 1995;39:1120–1126. doi: 10.1128/aac.39.5.1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hiramatsu K, Hanaki H, Ino T, Yabuta K, Oguri T, Tenover F C. Methicillin-resistant Staphylococcus aureus clinical strain with reduced vancomycin susceptibility. J Antimicrob Chemother. 1997;40:135–136. doi: 10.1093/jac/40.1.135. [DOI] [PubMed] [Google Scholar]
- 6.Levine D P, Fromm B S, Reddy B R. Slow response to vancomycin plus rifampin in methicillin-resistant Staphylococcus aureus endocarditis. Ann Intern Med. 1991;115:674–680. doi: 10.7326/0003-4819-115-9-674. [DOI] [PubMed] [Google Scholar]
- 7.Lyon B R, Skurray R. Antimicrobial resistance of Staphylococcus aureus: genetic basis. Microbiol Rev. 1987;51:88–134. doi: 10.1128/mr.51.1.88-134.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nagano R, Shibata K, Naito T, Fuse A, Asano K, Hashizume T, Nakagawa S. Therapeutic efficacy of BO-3482, a novel dithiocarbamate carbapenem in mice infected with methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 1997;41:2278–2281. doi: 10.1128/aac.41.10.2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nakagawa S, Hashizume T, Matsuda K, Sanada M, Okamoto O, Fukatsu H, Tanaka N. In vitro activity of a new carbapenem antibiotic, BO-2727, with potent antipseudomonal activity. Antimicrob Agents Chemother. 1993;37:2756–2759. doi: 10.1128/aac.37.12.2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakano, M., H. Kiyonaga, H. Imamura, A. Shimizu, H. Sato, Y. Sugimoto, S. Sakuraba, S. Nakagawa, H. Fukatsu, R. Ushijima, T. Hashizume, and R. Nagano. June 1999. World (PCT) patent WO9931106-A1.
- 11.Ohba F, Nakamura-Kamijo M, Watanabe N, Katsu K. In vitro and in vivo activity of ER-35786, a new antipseudomonal carbapenem. Antimicrob Agents Chemother. 1997;41:298–307. doi: 10.1128/aac.41.2.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saravolatz D L, Pohlod D J, Arking L M. Community-acquired methicillin-resistant Staphylococcus aureus infections: a new source for nosocomial outbreaks. Ann Intern Med. 1982;97:325–329. doi: 10.7326/0003-4819-97-3-325. [DOI] [PubMed] [Google Scholar]
- 13.Sumita Y, Nouda H, Kanazawa K, Fukasawa M. Antimicrobial activity of SM-17466, a novel carbapenem antibiotic with potent activity against methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 1995;39:910–916. doi: 10.1128/aac.39.4.910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sunagawa M, Matsumura H, Inoue T, Fukasawa M, Kato M. A novel carbapenem antibiotic, SM-7338: structure-activity relationships. J Antibiot. 1990;43:519–532. doi: 10.7164/antibiotics.43.519. [DOI] [PubMed] [Google Scholar]
- 15.Utsui Y, Yokota T. Role of an altered penicillin-binding protein in methicillin- and cephem-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 1985;28:397–403. doi: 10.1128/aac.28.3.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Watanabe M, Iyobe S, Inoue M, Mitsuhashi S. Transferable imipenem resistance in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1991;35:147–151. doi: 10.1128/aac.35.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]