Abstract
Objective
Increased epicardial adipose tissue (EAT) has been identified as a risk factor for the development of coronary artery disease (CAD). However, the exact role of EAT in the development of CAD is unclear. This study aims to compare EAT volumes between healthy controls and individuals with stable CAD and a history of myocardial infarction (MI). Furthermore, associations between clinical and biochemical parameters with EAT volumes are examined.
Methods
This retrospective cross-sectional study included 171 participants from the United Kingdom Biobank (56 healthy controls; 60 stable CAD; 55 post MI), whom were balanced for age, sex and body mass index (BMI). EAT volumes were quantified on end-diastolic cardiac magnetic resonance (CMR) imaging short-axis slices along the left and right ventricle and indexed for body surface area (iEAT) and iEAT volumes were compared between groups.
Results
iEAT volumes were comparable between control, CAD and MI cases (median [IQR]: 66.1[54.4–77.0] vs. 70.9[55.8–85.5] vs. 67.6[58.6–82.3] mL/m2, respectively (p > 0.005 for all). Increased HDL-cholesterol was associated with decreased iEAT volume (β = -14.8, CI = -24.6 to −4.97, p = 0.003) and suggestive associations (P-value < 0.05 and ≥ 0.005) were observed between iEAT and triglycerides (β = 3.26, CI = 0.42 to 6.09, p = 0.02), Apo-lipoprotein A (β = -16.3, CI = -30.3 to −2.24, p = 0.02) and LDL-cholesterol (β = 3.99, CI = -7.15 to −0.84, p = 0.01).
Conclusions
No significant differences in iEAT volumes were observed between patients with CAD, MI and healthy controls. Our results indicate the importance of correcting for confounding by CVD risk factors, including circulating lipid levels, when studying the relationship between EAT volume and CAD. Further mechanistic studies on causal pathways and the role of EAT composition are warranted.
Keywords: Cardiovascular magnetic resonance, Epicardial adipose tissue, Lipids, Coronary artery disease, Myocardial infarction
1. Introduction
Cardiovascular diseases (CVD) are the leading cause of mortality globally [1]. CVD preventive management is important in reducing progression of coronary artery disease (CAD) and is based on individual CVD risk estimates [2]. Obesity has been shown to be one such independent risk factor for CVD [1], [3], [4], [5]. The exact mechanism through which obesity contributes to the onset and progression of CVD is unknown and several theories have been proposed, involving different pathways and levels of risk [6]. In the last decades, research has focused on the contribution of local visceral adipose tissue (VAT) deposits, as they appear to be more directly linked to CVD compared to obesity [5], [7], [8]. Epicardial adipose tissue (EAT) is the metabolically active VAT deposit in the epicardial space that is in direct contact with the heart. EAT and the myocardium share the same microcirculation, and functional paracrine and vasocrine interactions have been suggested to foster the development of CVD [9], [10], [11], [12]. Growing evidence suggests that EAT releases adipokines that promote atherosclerosis, suggesting that EAT might be involved in the development of CAD [3], [13], [14], [15], [16].
Previous studies have established a significant association between EAT and CAD, assessed by echocardiography and computed tomography [17], [18], [19], [20], [21], [22], [23], [24], [25]. Even though CT can objectively assess total fat volume surrounding the heart, Cardiac Magnetic Resonance (CMR) imaging is superior for quantifying EAT, due to its excellent soft tissue contrast, making it possible to separate EAT from the different fat layers surrounding the heart [26], [27]. So far, only one study investigated associations of EAT volumes in regard to the severity of CAD, measured on CMR images. Doesch et al. reported that decreased EAT volumes in severe CAD patients when compared to healthy controls were dependent of left ventricular function (LVF), suggesting a multifactorial relationship between EAT and CAD severity [19]. Although several studies suggest a significant association between EAT and CAD severity, the observed differences in EAT volume between patients with different CAD stages might be susceptible to confounding by group-related patient characteristics and traditional cardiovascular risk factors.
The aim of this study is therefore to compare EAT volumes on CMR between different stages of CAD, corrected for confounding. Furthermore, we aim to explore the associations of EAT volumes with genetic and non-genetic risk factors, to obtain more insights in potential causal pathways.
2. Methods
2.1. Study design
Data for this retrospective cross-sectional analysis were obtained from the United Kingdom (UK) Biobank, of which the study protocol was described in detail previously [28]. Briefly, the UK Biobank is a cohort study that recruited over 500,000 volunteers aged 40–69 years from the general population at 22 assessment centers throughout the UK. The present study was performed under application number 12,010 of the UK Biobank resource.
2.2. Study population
Individuals with available CMR short-axis cine images in the UK Biobank sub study, were selected for analysis. Subjects having non-caucasian ethnicity, active smoking, systolic blood pressure (SBP) > 160 mmHg, diastolic blood pressure (DBP) > 100 mmHg, BMI > 35 kg/m2, history of diabetes mellitus, cardiomyopathy, cardiac surgery, pacemaker/implantable cardioverter-defibrillator, history of cancer, or subjects who underwent radiotherapy were excluded for analyses, because of potential confounding. Diagnoses were captured by consulting Hospital Episode Statistics records using ICD codes and self-reported answers from questionnaires. CAD was defined according to the International Classification of Diseases, Ninth revision (ICD-9) codes 414 and Tenth Revision (ICD-10) codes I24, I25 and Z95.5, covering acute and chronic ischemic heart diseases including replacement, transluminal balloon angioplasty, and other therapeutic transluminal operations on coronary artery, but not angina pectoris [29], [30]. Myocardial infarction covering acute or with a stated duration of 28 days or less from onset was defined according to ICD-9 codes 410, 412 and ICD-10 codes I21-I23 and I252 [29], [30]. Hyperlipidemia was defined according to ICD-10 codes E78.4 and E78.5 [29]. UK Biobank ICD-codes derived from linkage to hospital inpatient records. The identified individuals were divided into three groups: healthy controls, participants with stable CAD, and participants with a history of myocardial infarction (MI). All individuals with MI available in UK Biobank were randomly linked to individuals with CAD and healthy controls in a 1:1:1 fashion, balanced for age, sex and BMI. Age was rounded to the nearest whole number, BMI was rounded to the nearest even number. If no exact link could be made, MI cases were excluded.
2.3. Cardiac magnetic resonance imaging
All CMR studies were performed on a clinical wide bore 1.5 Tesla MRI scanner (MAGNETOM Aera, Syngo Platform VD13A, Siemens Healthcare, Erlangen, Germany). No pharmacological stressor or contrast agent was administered [31]. A standard protocol for cardiac function was used during a 20-minute CMR [32]. A full short-axis stack of balanced steady state free precession (bSSFP) cines was acquired, planned for covering the left and right ventricle. A flip angle of 80° was used, slice thickness was 8 mm with a 2 mm slice gap, pixel size was 1.8 × 1.8 mm, and temporal resolution was 32 ms interpolated to 50 phases per cardiac cycle. TR and TE were 2.6 and 1.10 ms, respectively.
2.4. CMR analysis
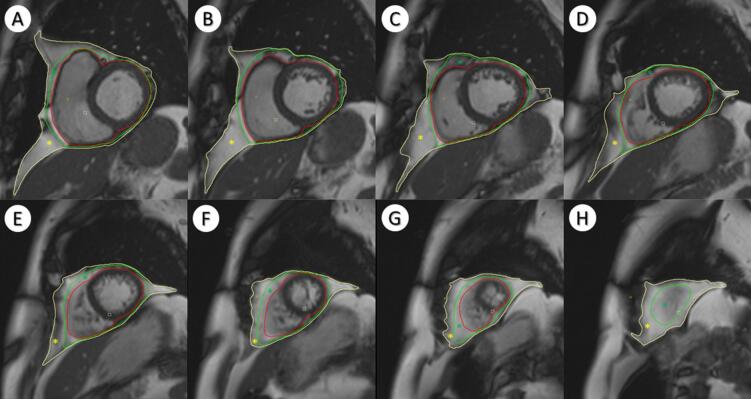
One observer analyzed all CMR short axis images using dedicated post-processing software (Circle Cardiovascular Imaging (CVi42) version 5.10.1, Calgary, Alberta, Canada), according to a standardized protocol (Supplement 1). Cardiac adipose tissue was quantified on all short axis slices (base to apex) in end-diastolic phase (Fig. 1). EAT was defined as the volume between the outer wall of the myocardium and the visceral pericardium. Paracardial adipose tissue (PAT) was defined as the volume between the fibrosa of the parietal pericardium and the mediastinal parietal pleura. Pericardial adipose tissue (PeAT) was defined as the sum of EAT and PAT. Left ventricular end-diastolic volume (EDV) and end-systolic volume (ESV) were determined by contouring endocardial and epicardial borders in the left ventricle from the mitral valve to the apex. Papillary muscles were excluded from endocardial volume. The analyst determining the cardiac fat volumes was blinded to other patient characteristics. Interobserver variability was assessed by re-analysis of random set of 29 CMR images by a second observer blinded to other patient characteristics. EAT volumes measurements had excellent intra-rater reliability (ICC = 0.98) and good inter-rater reliability (ICC = 0.82).
Fig. 1.
Short-axis MR slices for different levels (basal to apical). Red: myocardium, Green: Visceral pericardium, Yellow: Mediastinal parietal pleura. Green star: Epicardial Adipose Tissue; Yellow star: Paracardial adipose tissue. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
2.5. Biomarkers
A wide range of blood and urine biomarkers from all 500.000 UK Biobank participants have been determined at baseline visit, following a strict sample handling and storage protocol [33]. For this study, all available blood and urine biomarkers were studied in relation to EAT volume, corrected for group effects.
2.6. Genetics
Genome-wide genotyping data was available for all participants in the UK Biobank database. The genotyping process has been described in more detail previously [34]. Genetic Risk Scores (GRSs) constructed from single-nucleotide polymorphisms (SNPs) were used as a proxy measure for BMI, waist-hip ratio (WHR), total cholesterol and triglyceride levels, in order to assess whether GRSs based on known variants were associated with cardiac adipose tissue volume.
2.7. Statistical analysis
Continuous variables were presented as mean ± standard deviation (SD) or median and interquartile range (IQR), depending on distribution. Discrete and dichotomous variables were reported as frequencies with the respective percentages. Mean and median differences in baseline characteristics and cardiac fat volumes between groups were compared using one-way ANOVA and Kruskal-Wallis tests, depending on distribution. To compare cardiac fat volumes in each group with the controls, Mann-Whitney U test or Student’s T-test was used, depending on distribution. The Chi-squared test was used to evaluate differences in categorical variables between the groups. Cardiac adipose tissue volumes were indexed for body surface area (BSA) (iEAT, iPAT, iPeAT), which is widely used for indexing cardiovascular parameters to reduce body size related variation [35], [36]. Intra- and interrater reliability was assessed using intra-class correlation coefficients (ICCs). In order to investigate the influence of genetic variances on EAT and iEAT, genetic risk scores (GRSs) based on BMI, WHR, total cholesterol and triglycerides-related SNPs were constructed for analysis. To study the relationship between clinical, biochemical, and genetic parameters with cardiac fat volumes, linear multilevel mixed-effects analysis were performed, in order to correct for group effects and predictors defined at group level. The model was adjusted for age and sex. For genetic associations, the first five principal components and the chip used for genotyping were added to the model. According to Benjamin et al. a P-value < 0.005 was considered statistically significant [37]. A P-value between 0.05 and 0.005 was considered suggestive of statistical significance. Estimated sample size to show a minimal difference of 19 mL EAT between the groups was n = 60, assuming 77% power and alpha 0.05 [38]. Statistical analyses were conducted with STATA version 15.0 (StataCorp. 2017, Stata Statistical Software: Release 15. College Station, TX: StataCorp LLC).
3. Results
3.1. Baseline characteristics
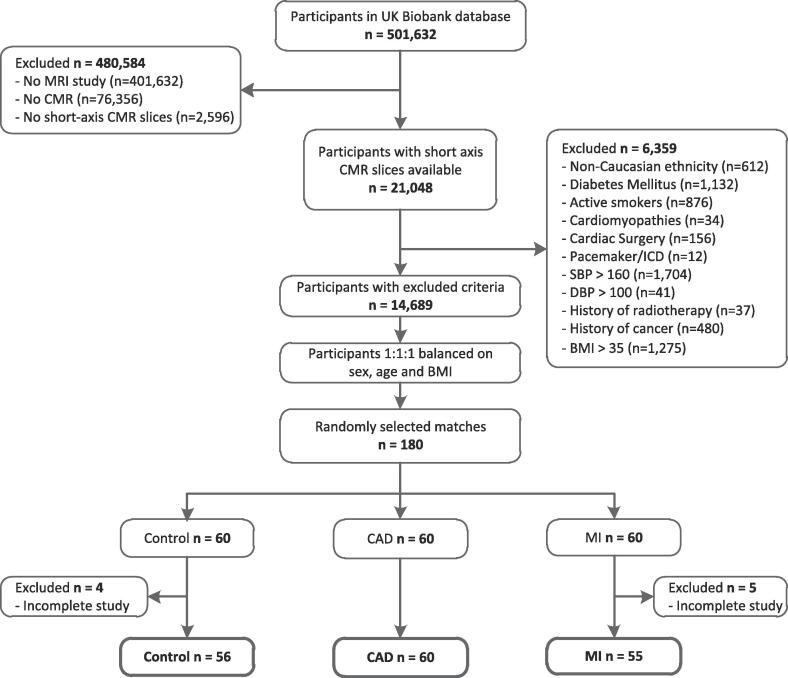
A total of 180 age-, sex-, and BMI-balanced individuals with available short-axis cine images were identified in the UK Biobank database (60 healthy controls, 60 MI, and 60 CAD). 9 participants were excluded due to incomplete CMR studies and 171 subjects remained in final analysis (Fig. 2), with 56 controls, 60 CAD and 55 MI. All anthropometric measurements were comparable between control, CAD and MI group (Table 1). Hyperlipidemia and the use of statins, ACE-inhibitors and calcium channel blockers were more common in CAD and MI groups compared to controls. LDL-cholesterol (LDL-C), total cholesterol and Apolipoprotein B (Apo-B) levels were increased in controls compared to CAD and MI cases.
Fig. 2.
Study population selection. BMI: body mass index; CAD: coronary artery disease; CMRI: cardiac magnetic resonance imaging; DBP: diastolic blood pressure; MI: myocardial infarction; MRI: magnetic resonance imaging; SBP: systolic blood pressure; UK: United Kingdom.
Table 1.
Baseline characteristics.
|
Baseline characteristics |
p-value | ||||||
|---|---|---|---|---|---|---|---|
| Control (n = 56) | CAD (n = 60) | MI (n = 55) | |||||
| Age, mean (SD) | 60.6 | (4.7) | 60.9 | (4.6) | 60.7 | (4.5) | 0.96 |
| Sex (Male), n (%) | 46 | (82) | 49 | (82) | 44 | (80) | 0.95 |
| BMI(kg/m2), median (IQR) | 27.0 | (24.9–28.3) | 26.9 | (24.5–28.9) | 27.0 | (24.7–28.7) | 0.93 |
| Weight(kg), mean (SD) | 80.0 | (10.5) | 81.0 | (10.9) | 77.8 | (11.3) | 0.28 |
| Height(cm), mean (SD) | 173 | (8.2) | 173 | (7.6) | 170 | (9.4) | 0.12 |
| LBM (kg), median (IQR) | 58.8 | (54.2–63.9) | 60.0 | (53.0–65.3) | 58.9 | (53.7–65.0) | 0.67 |
| WC (cm), mean (SD) | 92.1 | (9.4) | 92.3 | (9.5) | 90.9 | (8.9) | 0.71 |
| WHR | 0.92 | (0.08) | 0.92 | (0.07) | 0.91 | (0.07) | 0.63 |
| CVD risk factors, n(%) | |||||||
| Hypertension | 34 | (61) | 24 | (40) | 24 | (44) | 0.06 |
| Hyperlipidemia | 18 | (32) | 56 | (93) | 51 | (93) | 0.001 |
| Familiy history – Heart Disease | 25 | (45) | 44 | (73) | 34 | (62) | 0.007 |
| Past smokers | |||||||
| Smoked on most or all days | 16 | (29) | 23 | (38) | 23 | (42) | 0.32 |
| Smoked occasionally | 5 | (9) | 3 | (5) | 5 | (9) | 0.64 |
| Just tried once or twice | 11 | (20) | 7 | (12) | 9 | (16) | 0.50 |
| Never smoked | 24 | (43) | 26 | (43) | 18 | (33) | 0.43 |
| Medication, n(%) | |||||||
| Statins | 19 | (34) | 54 | (90) | 50 | (91) | <0.001 |
| Beta-blockers | 1 | (2) | 9 | (15) | 11 | (20) | 0.01 |
| ACE-inhibitors | 3 | (5) | 18 | (30) | 35 | (64) | <0.001 |
| Diuretics | 6 | (11) | 7 | (12) | 8 | (15) | 0.81 |
| Calcium channel blockers | 7 | (13) | 19 | (32) | 5 | (9) | 0.003 |
| Biomarkers | |||||||
| TG (mmol/L), median (IQR) | 0.02 | (0.02 – 0.03) | 0.02 | (0.01 – 0.03) | 0.02 | (0.01 – 0.03) | 0.23 |
| LDL-C (mmol/L), mean (SD) | 0.10 | (0.02) | 0.08 | (0.02) | 0.08 | (0.02) | <0.001 |
| HDL-C (mmol/L), mean (SD) | 0.04 | (0.01) | 0.03 | (0.01) | 0.03 | (0.01) | 0.07 |
| Total cholesterol (mmol/L), mean (SD) | 0.15 | (0.02) | 0.13 | (0.03) | 0.13 | (0.03) | <0.001 |
| Apo-A (mmol/L), mean (SD) | 0.05 | (0.01) | 0.05 | (0.01) | 0.05 | (0.01) | 0.39 |
| Apo-B (mmol/L), mean (SD) | 0.03 | (0.01) | 0.03 | (0.01) | 0.03 | (0.01) | 0.002 |
| HbA1c (mmol/L), mean (SD) | 35.2 | (3.57) | 36.1 | (3.60) | 35.85 | (3.30) | 0.37 |
| Cr (µmol/L), mean (SD) | 81.5 | (13.3) | 79.4 | (12.0) | 81.4 | (14.9) | 0.64 |
| CRP (mg/dL), median (IQR) | 1.28 | (0.66 – 2.26) | 1.21 | (0.62 – 2.36) | 1.04 | (0.63 – 2.20) | 0.88 |
| Hb (g/dL), mean (SD) | 14.7 | (1.06) | 14.9 | (1.09) | 14.7 | (0.88) | 0.47 |
| Haemodynamics, mean (SD) | |||||||
| SBP (mmHg) | 138 | (12.6) | 139 | (13.4) | 136 | (12.6) | 0.42 |
| DBP (mmHg) | 111 | (8.9) | 110 | (9.6) | 108 | (8.9) | 0.30 |
| MAP (mmHg) | 100 | (7.6) | 97 | (8.2) | 96 | (8.3) | 0.016 |
| HR (BPM) | 67 | (10) | 65 | (12) | 63.4 | (11) | 0.13 |
P-values for differences between the three groups were obtained using the one-way ANOVA and Kruskall-Wallis tests, depending on distribution. ACE: angiotensin-converting enzyme; Apo: Apolipoprotein; BMI: body mass index; Cr: creatinine; CRP: c-reactive protein; DBP: diastolic blood pressure; HbA1c: Hemoglobin A1c; Hb: hemoglobin; HDL-C: high-density lipoprotein cholesterol; HR: heart rate; LDL-C: low-density lipoprotein cholesterol; MAP: mean arterial pressure; TG: triglycerides.
Strong positive associations were found between unindexed EAT volumes and anthropometric parameters including BMI, BSA, LBM, and WHR (Supplementary Table 1), and fat volumes were therefore indexed for BSA in further analysis.
3.2. Epicardial adipose tissue volumes in controls versus coronary artery disease and myocardial infarction
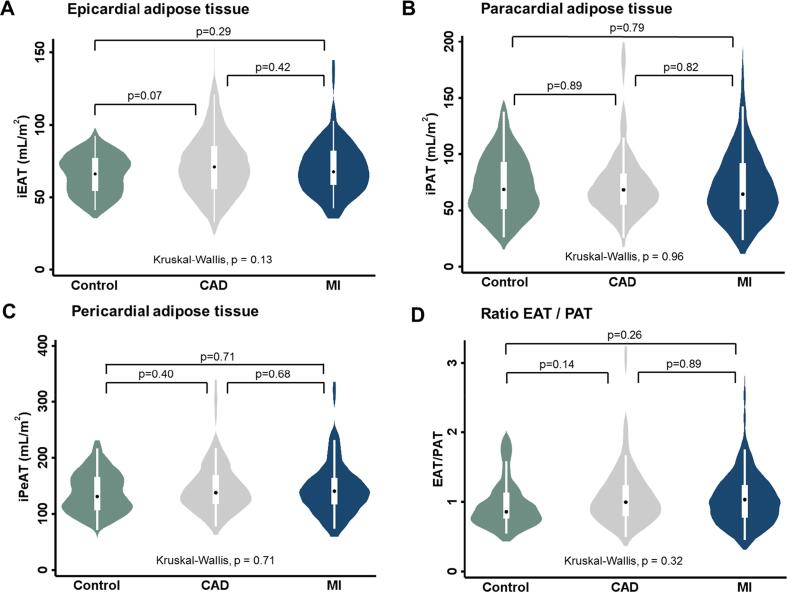
Median iEAT volumes were 66.1 mL/m2 (IQR: 54.4 – 77.0), 70.9 mL/m2 (IQR: 55.8 – 85.5) and 67.6 mL/m2 (IQR: 58.6 – 82.3) in control, CAD and MI cases, respectively (Fig. 3A). No significant differences were found in iEAT volume among the three groups (p = 0.13). Also, iPAT (Fig. 3B), iPeAT (Fig. 3C) volumes and EAT/PAT ratio (Fig. 3D) were not statistically different between the three groups.
Fig. 3.
Cardiac adipose tissue volumes and EAT/PAT ratio in CAD and MI versus controls. Violinplots showing iEAT (A), iPAT (B), iPeAT (C) volumes (mL/m2) and EAT/PAT ratio in controls, CAD and MI study groups. Inter-group differences in cardiac adipose tissue volume were tested using the Mann-Whitney U tests. The Kruskal-Wallis test was used to test overall differences in cardiac adipose tissue volume between the three groups. CAD: coronary artery disease; MI: myocardial infarction; iEAT: indexed epicardial adipose tissue; iPAT: indexed paracardial adipose tissue; iPeAT: indexed pericardial adipose tissue.
3.3. Multilevel mixed-effects analysis of biomarkers on epicardial adipose tissue volume
Increased HDL-Cholesterol (HDL-C) levels were associated with decreased iEAT volume (β = -14.8, 95% CI −24.6 - −4.97, p = 0.003) when correcting for age, sex and group (Table 3). Increased triglyceride levels were suggestively associated with increased iEAT volume (β = 3.26, 95% CI 0.42–6.09, p = 0.02), while increased levels of Apo-lipoprotein A (β = -16.3, 95% CI −30.3 - −2.24, p = 0.02) and LDL-C (β = -3.99, 95% CI −7.15 - −0.84, p = 0.01) were suggestively associated with decreased iEAT volume. The negative association between HDL-C and iEAT volume was suggestively larger in combination with statins after interaction analysis (β = -11.7, 95% −21.7 - −1.73, p = 0.02). (Table 3).
Table 3.
Multilevel mixed-effects analysis of biomarkers and left ventricle parameters on iEAT volumes.
|
Associations of biomarkers and left ventricle parameters on iEAT volume | |||
|---|---|---|---|
|
Total study group (n = 171) |
|||
| β | 95% CI | p-value | |
| Blood cells | |||
| Leukocytes, 109 cells/L | 0.62 | −1.07 – 2.30 | 0.48 |
| Monocytes, 109 cells/L | 8.36 | −10.1 – 26.8 | 0.38 |
| Monocytes, % | 0.53 | −1.12 – 1.96 | 0.59 |
| Basophill, 109 cells/L | −42.5 | −141.8–56.9 | 0.40 |
| Basophill, % | −0.86 | −10.5 – 8.80 | 0.86 |
| Neutrophill, 109 cells/L | 0.35 | −1.85 – 2.56 | 0.75 |
| Neutrophill, % | −0.05 | −0.41 – 0.31 | 0.79 |
| Eosinophill, 109 cells/L | 9.19 | −15.4 – 33.8 | 0.46 |
| Eosinophill, % | 0.40 | −1.38 – 2.18 | 0.66 |
| Lymphocytes, 109 cells/L | 2.15 | −2.49 – 6.78 | 0.36 |
| Lymphocytes, % | 0.02 | −0.40 – 0.43 | 0.94 |
| Platelets, 109 cells/L | −0.02 | −0.07 – 0.02 | 0.34 |
| Biomarkers | |||
| TG (mg/dL) | 3.26 | 0.42–6.09 | 0.02 |
| CRP (mg/dL) | 0.17 | −0.07 – 0.41 | 0.17 |
| Haemoglobin | −1.99 | −5.31 – 1.33 | 0.24 |
| Creatinine (umol/L) | 0.14 | −0.62 – 0.90 | 0.72 |
| HbA1c (mmol/L) | 0.80 | −0.03 – 1.62 | 0.06 |
| Apo-A (g/L) | −16.3 | −30.3 – (-2.24) | 0.02 |
| Apo-B (g/L) | −11.9 | –23.5 – (-0.23) | 0.05 |
| HDL-C (mg/dL) | −14.8 | −24.6 – (-4.97) | 0.003 |
| LDL-C (mg/dL) | −3.99 | −7.15 – (-0.84) | 0.01 |
| Total cholesterol (mg/dL) | −3.14 | −5.56 – (-0.71) | 0.01 |
| Use of medication | |||
| Statins (n,%) | 8.32 | 2.31 – 14.3 | 0.007 |
| Beta-blockers (n, %) | 11.3 | 3.11 – 19.4 | 0.007 |
| Interaction analysis | |||
| Statins#HDL-C | −11.7 | −21.7 – (-1.73) | 0.02 |
| Statins#LDL-C | −3.04 | −6.45 – 0.38 | 0.08 |
| CVD risk factors | |||
| Family history | 2.73 | −2.90 – 8.35 | 0.34 |
| β | 95% CI | p-value | |
| Past smokers | |||
| Smoked on most or all days | 2.46 | −3.22 – 8.14 | 0.40 |
| Smoked occasionally | 2.59 | −7.68 – 12.9 | 0.62 |
| Just tried once or twice | −0.49 | −7.97 – 6.99 | 0.90 |
| Never smoked | −3.36 | −8.88 – 2.16 | 0.23 |
| SBP | 0.15 | −0.07 – 0.37 | 0.17 |
| Left ventricle parameters | |||
| EDV (mL) | 0.03 | −0.07 – 0.12 | 0.61 |
| ESV (mL) | 0.04 | −0.13 – 0.21 | 0.65 |
| LVEF (%) | 0.05 | −0.30 – 0.40 | 0.78 |
Adjusted for age, sex and group. Apo: Apolipoprotein; Cr: creatinine; CRP: c-reactive protein; EDV: end-diastolic volume; ESV: end-systolic volume; HbA1c: Hemoglobin A1c; HDL-C: high-density lipoprotein cholesterol; LDL-C: low-density lipoprotein cholesterol; LVEF: left ventricle ejection fraction; TG: triglycerides. SBP: systolic blood pressure.
3.4. Multilevel mixed-effects analysis of left ventricle parameters on epicardial adipose tissue volume
Both EDV (135.1 ± 33.9 mL vs. 120.7 ± 23.8 mL, p = 0.02) and ESV (56.2 ± 20.3 mL vs. 45.2 ± 13.4 mL, p = 0.002) were increased in MI cases compared to controls (Table 2). Left ventricle ejection fraction (LVEF) was decreased in MI cases compared to CAD and controls (59.2 ± 7.5 vs. 62.9 ± 6.3 and 63.6 ± 9.1 respectively, both p < 0.003). No significant associations between left ventricle volumetric and functional parameters and iEAT volume were observed (Table 3). Associations between left ventricle parameters and iEAT volume by group are provided in Supplementary Table 2.
Table 2.
CMR characteristics.
|
CMR characteristics |
|||||||
|---|---|---|---|---|---|---|---|
| Control (n = 56) | CAD (n = 60) | MI (n = 55) | |||||
| Cardiac function parameters | |||||||
| EDV (mL) | 120.7 | (23.8) | 128.0 | (34.1) | 135.1 | (33.9) | 0.05 |
| ESV (mL) | 45.2 | (13.4) | 48.1 | (16.3) | 56.2 | (20.3) | 0.002 |
| LVEF (%) | 62.9 | (6.3) | 63.6 | (9.1) | 59.2 | (7.5) | 0.008 |
| Cardiac adipose tissue | |||||||
| EAT (mL), median (IQR) | 127.4 | (100.6–149. 6) | 141.5 | (106.8–170.5) | 124.7 | (108.3–159.3) | 0.13 |
| PAT (mL), median (IQR) | 133.4 | (98.0–183.2) | 126.7 | (103.7–168.0) | 125.9 | (95.2–186.2) | 0.92 |
| PEAT (mL), median (IQR) | 247.7 | (209.7–330.0) | 272.6 | (217.4–334.8) | 267.3 | (213.1–329.3) | 0.65 |
| iEAT (mL/m2), median (IQR) | 66.1 | (54.4 – 77.0) | 70.9 | (55.8 – 85.5) | 67.6 | (58.6 – 82.3) | 0.18 |
| iPAT (mL/m2), median (IQR) | 68.7 | (51.3–92.9) | 68.4 | (55.1 – 82.7) | 64.6 | (51.0 – 91.9) | 0.96 |
| iPEAT (mL/m2), median IQR) | 131.1 | (106.6 – 166.1) | 138.0 | (117.7 – 169.0) | 140.7 | (117.0 – 163.8) | 0.71 |
| Ratio EAT/PEAT, median (IQR) | 0.86 | (0.76 – 1.13) | 1.00 | (0.79 – 1.24) | 1.03 | (0.77 – 1.24) | 0.32 |
P-values for differences between the three groups were obtained using the one-way ANOVA test and Kruskal-Wallis test, depending on distribution. EDV: end-diastolic volume; EAT: epicardial adipose tissue; PAT: paracardial adipose tissue; ESV: end-systolic volume; iEAT: indexed epicardial adipose tissue; iPAT: indexed paracardial adipose tissue; iPeAT: indexed pericardial adipose tissue.
3.5. Multilevel mixed-effects analysis of genetic risk scores on epicardial adipose tissue volume
No relationship between genetic risk, expressed in GRS, between BMI (p = 0.68), WHR (p = 0.67), total cholesterol (p = 0.89) and triglycerides (p = 0.25) and EAT volumes was observed (Table 4).
Table 4.
Multilevel mixed-effects analysis for genetic risk scores and EAT volumes.
| Associations of genetic parameters and EAT volume | |||
|---|---|---|---|
|
Total study group (n = 171) |
|||
| β | 95% CI | p-value | |
| Genetic Risk Scores | |||
| BMI | −4.78 | −27.7 – 18.1 | 0.68 |
| WHR | −8.80 | −48.6 – 31.0 | 0.67 |
| Total cholesterol | 0.55 | –22.0 – 23.1 | 0.89 |
| Triglycerides | 12.9 | −8.97 – 34.8 | 0.25 |
| BMI* | −3.05 | −13.9 – 7.82 | 0.58 |
| WHR* | 5.97 | −12.9 – 24.9 | 0.54 |
| Total cholesterol* | −0.97 | −11.7 – 9.76 | 0.86 |
| Triglycerides* | 7.72 | −2.66 – 18.1 | 0.15 |
Adjusted for age, sex, group, the first five principal components and the chip used for genotyping. BMI: body mass index; WHR: waist hip ratio.
BSA-indexed EAT (iEAT) volume was used in analysis.
4. Discussion
In this retrospective cross-sectional analysis, we did not observe significant differences in EAT volume between the control, CAD, and MI groups. Interestingly, increased HDL-C was associated with decreased EAT volume, independent of age, sex, and group allocation. Together this may suggest that previous associations between EAT and CAD reflected residual confounding rather than a true association.
Contradictory to our findings, previous studies did report significant differences in EAT quantities comparing groups with various CAD stages, and suggested a causal relationship between EAT quantity and CAD severity [17], [18], [19], [20], [21], [22], [23], [39], [40]. However, these studies applied no or poor strategies to protect against confounding. In our study, we minimized confounding by studying an age-, sex- and BMI-balanced population without extremes of several cardiovascular risk predictors. We observed no difference in EAT volumes between patients with stable CAD, previous MI, and healthy controls, suggesting that there is no direct relationship between EAT volume and CAD progression, when adequately correcting for confounding. Our findings are in line with another study, in which the association between EAT and CAD was lost after adjustment for CVD risk predictors [41]. These results illustrate the importance of correcting for confounding and investigating causal pathways of associations. Another explanation for the absence of an association between EAT volume might be the importance of EAT quality rather than EAT volume. Both Pandey et al. and Antonopoulos et al. show that not EAT quantity but EAT quality is an independent predictor of obstructive CAD by using CT-measured attenuation [42], [43]. However, Hell et al. demonstrated that variations in CT attenuation might also occur due to partial volume effects and image interpolation rather than differences in tissue composition or metabolic activity [44]. Given the findings of these studies (Antanopoulos and Pandey et al.) and since it has been established that epicardial adipose tissue interacts with the coronary vasculature as well as the myocardial wall in a bidirectional manner, accurate quantification of true epicardial fat is essential when studying the role of fat in CAD [45]. CMR is the gold standard for soft tissue characterization. We therefore used CMR images to accurately quantify solely EAT in which the coronary arteries are embedded, instead of total pericardial adipose tissue [46]. Most previous CT studies assessing fat volume quantified total pericardial adipose tissue volume, consisting of both the epicardial and paracardial layers, because no distinction can be made between these layers using CT. Pericardial fat was unjustly referred to as epicardial fat by these studies, due to discrepancies and ambiguities in the definition and nomenclature among different authors [47]. In contrast to most CT studies, we quantified true epicardial fat volume, which contains perivascular adipose tissue of the coronary arteries, enabling us to precisely study the role of local fat volume on CAD and to study associations with separate cardiac fat layers, - pericardial, paracardial and epicardial. Local differences in fat composition and inflammation in epicardial fat, that have been linked to adverse events, could not be assessed because no CT-images were available [48].
We showed that increased HDL-C is associated with decreased EAT volume. In addition, we showed that increased triglycerides levels are suggestively associated with increases in EAT volume. In literature, decreased HDL-C and increased triglyceride levels have been reported as markers for the atherogenic dyslipidaemia complex and were independently associated with an increased risk of CAD [49], [50], [51], [52], [53], [54], [55]. This means, for example, that when EAT volume is quantified in individuals with severe CAD who also tend to have decreased HDL-C values, CAD progression in these individuals might be related to decreased HDL-C values rather than increased EAT volumes. The fact that decreased HDL-C and increased triglycerides are associated with both CAD progression and EAT volume underlines the importance of correcting for these factors when studying the relationship of EAT and CAD.
Remarkably, we observed also a negative association between LDL-C and iEAT. A possible explanation might be that 72% of our patients was on statin therapy, and lower LDL-C might reflect patients with more intensive statin therapy at higher cardiometabolic risk which might be linked to higher iEAT. Indeed, in a sensitivity analysis between LDL-C within patients with statin therapy a negative trend between LDL-C and iEAT was observed (β-3.9 95%-CI: −7.8 – 0.11, P = 0.056), whereas in the patients without statins no significant associations were observed (β: 0.91, 95%-CI: −7.0 – 8.8, P = 0.817).
Nevertheless, a causal pathway between EAT and CAD progression through interaction of EAT with blood lipids might still exist, for instance by paracrine and vasocrine effects exerted by EAT on coronary vasculature, potentially under influence of blood lipids. The potential interaction between EAT, blood lipids and CAD has also been reported in the study of Colom et al., in which Apo-A was strongly related to the development of subclinical atherosclerosis and the accumulation of epicardial fat [56]. Furthermore, other studies demonstrated that EAT stores triglycerides, which results in EAT production of free fatty acids and adipokines for the energy supply of the underlying myocardium, suggesting that EAT together with TG are able to exert effects on neighbouring tissues [10], [53]. In addition, inflammatory markers such as interleukin-6 (IL-6), might also take part in the atherosclerotic process in CAD [57], [58]. As EAT is considered as a source of several inflammatory mediators, inflammatory status in EAT might be involved in the development of CAD [59], [60]. Unfortunately, inflammatory markers like IL-6 were unavailable for our study. However, the precise mechanisms through which EAT might promote CAD still remains unclear and further investigation to unravel the extensive web of confounding variables explaining potential relationships between EAT and CAD is needed.
To our knowledge, this is the first study to assess EAT volumes in relation to genetic predisposition for BMI, WHR, cholesterol and triglycerides. We found no significant associations between EAT volumes and genetic risk scores for BMI, WHR, cholesterol and triglycerides, suggesting lifestyle might have a larger effect on EAT volume than genetic predisposition. However, these results should be interpreted with caution because the sample size is relatively small to study genetic predisposition.
4.1. Future perspectives
Large longitudinal studies with extensive follow-up investigating the association between EAT and CAD progression should be conducted to provide a definite conclusion on the exact mechanism of EAT in CAD severity. These studies should minimize confounding by adjusting factors associated both with EAT and CAD. In addition, further studies investigating the potential causal relationship between blood lipids, EAT and CAD progression are warranted.
4.2. Strengths and limitations
We used strict exclusion criteria to rule out confounding by risk predictors related to both EAT and CAD progression. Therefore, external validity and associations between EAT volume and CVD risk factors with removed extreme values might be limited. Since there is a tradeoff between internal and external validity, we chose a strict population enabling us to study of the distribution of EAT volumes among CAD severity groups without confounding. However, residual confounding might have occurred, due to the inclusion of individuals with some risk factor prevalence. The definition of CAD in our study includes a broad spectrum of disease. In literature EAT volume increases gradually in early CAD progression, but decreases in further stages of CAD [19], [61]. This might have leveled out increased EAT volumes of early CAD stages and decreased volumes of late CAD stages in our CAD population. EAT and other fat layers might play a different role in CAD pathophysiology [12].
5. Conclusions
We found no differences in EAT volumes among patients with previous MI, stable CAD and healthy controls in an age-, sex- and BMI-balanced population without extremes of traditional CVD risk factors. Our findings might suggest that there is no independent relationship between EAT volume and CAD progression. We observed that decreased HDL-C and increased TG levels, which are strongly related to CVD, were associated with increased EAT volume. In addition, the results of this study indicate the importance of correcting for confounding by CVD risk factors, such as HDL-C and triglyceride levels, when studying the relationship between EAT volume and CAD. Further mechanistic studies that study in depth-lipid profiles including adipokines and their associations with EAT volumes and quality are warranted.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijcha.2022.101006.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Benjamin E.J., Muntner P., Alonso A., et al. Heart Disease and Stroke Statistics-2019 Update: A Report From the American Heart Association. Circulation. 2019;139(10):e56–e66. doi: 10.1161/CIR.0000000000000659. [DOI] [PubMed] [Google Scholar]
- 2.Conroy R. Estimation of ten-year risk of fatal cardiovascular disease in Europe: the SCORE project. Eur. Heart J. 2003;24(11):987–1003. doi: 10.1016/S0195-668X(03)00114-3. [DOI] [PubMed] [Google Scholar]
- 3.Raggi P., Alakija P. Epicardial adipose tissue: A long-overlooked marker of risk of cardiovascular disease. Atherosclerosis. 2013;229(1):32–33. doi: 10.1016/J.ATHEROSCLEROSIS.2013.02.030. [DOI] [PubMed] [Google Scholar]
- 4.Roth G.A., Johnson C.O., Abate K.H., et al. The burden of cardiovascular diseases among us states, 1990–2016. JAMA Cardiol. 2018;3(5):375–389. doi: 10.1001/jamacardio.2018.0385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Poirier P., Giles T.D., Bray G.A., et al. Obesity and cardiovascular disease: Pathophysiology, evaluation, and effect of weight loss: An update of the 1997 American Heart Association Scientific Statement on obesity and heart disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2006;113(6):898–918. doi: 10.1161/CIRCULATIONAHA.106.171016. [DOI] [PubMed] [Google Scholar]
- 6.Hruby A., Hu F.B. The Epidemiology of Obesity: A Big Picture. Pharmacoeconomics. 2015;33(7):673–689. doi: 10.1007/s40273-014-0243-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bastien M., Poirier P., Lemieux I., Després J.-P. Overview of Epidemiology and Contribution of Obesity to Cardiovascular Disease. Prog. Cardiovasc. Dis. 2014;56(4):369–381. doi: 10.1016/J.PCAD.2013.10.016. [DOI] [PubMed] [Google Scholar]
- 8.Alexopoulos N., Katritsis D., Raggi P. Visceral adipose tissue as a source of inflammation and promoter of atherosclerosis. Atherosclerosis. Published online. 2014 doi: 10.1016/j.atherosclerosis.2013.12.023. [DOI] [PubMed] [Google Scholar]
- 9.Aeddula N., Cheungpasitporn W., Thongprayoon C., Pathireddy S. Epicardial Adipose Tissue and Renal Disease. J. Clin. Med. 2019;8(3):299. doi: 10.3390/jcm8030299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sacks H.S., Fain J.N. Human epicardial adipose tissue: A review. Am. Heart J. 2007;153(6):907–917. doi: 10.1016/J.AHJ.2007.03.019. [DOI] [PubMed] [Google Scholar]
- 11.Gaborit B., Sengenes C., Ancel P., Jacquier A., Dutour A. Role of epicardial adipose tissue in health and disease: A matter of fat? Compr. Physiol. 2017;7(3):1051–1082. doi: 10.1002/cphy.c160034. [DOI] [PubMed] [Google Scholar]
- 12.Iacobellis G. Epicardial and Pericardial Fat: Close, but Very Different. Obesity. 2009;17(4):625. doi: 10.1038/oby.2008.575. [DOI] [PubMed] [Google Scholar]
- 13.Gastaldelli A., Basta G. Ectopic fat and cardiovascular disease: What is the link? Nutr. Metab. Cardiovasc. Dis. 2010;20(7):481–490. doi: 10.1016/j.numecd.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 14.Iacobellis G. Epicardial adipose tissue in endocrine and metabolic diseases. Endocrine. Published online. 2013 doi: 10.1007/s12020-013-0099-4. [DOI] [PubMed] [Google Scholar]
- 15.Iacobellis G., Barbaro G. Epicardial adipose tissue feeding and overfeeding the heart. Nutrition. 2019;59:1–6. doi: 10.1016/j.nut.2018.07.002. [DOI] [PubMed] [Google Scholar]
- 16.Yoo H.J. Adipokines as a novel link between obesity and atherosclerosis. World J. Diabetes. 2014;5(3):357. doi: 10.4239/wjd.v5.i3.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ghaderi F., Eshraghi A., Shamloo A.S., Mousavi S. Assosiation of Epicardial and Pericardial Fat Thickness with Coronary Artery Disease. Electron Physician. 2016;8(9):2982–2989. doi: 10.19082/2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Verma B., Katyal D., Patel A., Singh V.R., Kumar S. Relation of systolic and diastolic epicardial adipose tissue thickness with presence and severity of coronary artery disease (The EAT CAD study) J. Fam. Med. Prim care. 2019;8(4):1470–1475. doi: 10.4103/jfmpc.jfmpc_194_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Doesch C., Süselbeck T., Haghi D., et al. The Relationship between the Severity of Coronary Artery Disease and Epicardial Adipose Tissue Depends on The Left Ventricular Function. PLoS ONE. 2012;7(11) doi: 10.1371/journal.pone.0048330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meenakshi K, Rajendran M, Srikumar S, Chidambaram S. Epicardial fat thickness: A surrogate marker of coronary artery disease - Assessment by echocardiography. Indian Heart J. 68(3) 336-341. 10.1016/j.ihj.2015.08.005. [DOI] [PMC free article] [PubMed]
- 21.Parisi V., Petraglia L., Formisano R., et al. Validation of the echocardiographic assessment of epicardial adipose tissue thickness at the Rindfleisch fold for the prediction of coronary artery disease. Nutr Metab Cardiovasc Dis. 2020;30(1):99–105. doi: 10.1016/j.numecd.2019.08.007. [DOI] [PubMed] [Google Scholar]
- 22.Kim S.H., Chung J.H., Kwon B.J., Song S.W., Choi W.S. The associations of epicardial adipose tissue with coronary artery disease and coronary atherosclerosis. Int. Heart J. 2014;55(3):197–203. doi: 10.1536/ihj.13-303. [DOI] [PubMed] [Google Scholar]
- 23.Nagayama Y., Nakamura N., Itatani R., et al. Epicardial fat volume measured on nongated chest CT is a predictor of coronary artery disease. Eur. Radiol. 2019;29(7):3638–3646. doi: 10.1007/s00330-019-06079-x. [DOI] [PubMed] [Google Scholar]
- 24.Zhou J., Chen Y., Zhang Y., et al. Epicardial Fat Volume Improves the Prediction of Obstructive Coronary Artery Disease Above Traditional Risk Factors and Coronary Calcium Score. Circ. Cardiovasc. Imaging. 2019;12(1) doi: 10.1161/CIRCIMAGING.118.008002. [DOI] [PubMed] [Google Scholar]
- 25.Mancio J., Azevedo D., Saraiva F., et al. Epicardial adipose tissue volume assessed by computed tomography and coronary artery disease: A systematic review and meta-analysis. Eur. Heart J. Cardiovasc. Imaging. 2018;19(5):490–497. doi: 10.1093/ehjci/jex314. [DOI] [PubMed] [Google Scholar]
- 26.Mahajan R., Kuklik P., Grover S., et al. Cardiovascular magnetic resonance of total and atrial pericardial adipose tissue: a validation study and development of a 3 dimensional pericardial adipose tissue model. J. Cardiovasc. Magn. Reson. 2013;15(1):73. doi: 10.1186/1532-429X-15-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Flüchter S., Haghi D., Dinter D., et al. Volumetric assessment of epicardial adipose tissue with cardiovascular magnetic resonance imaging. Obesity. 2007;15(4):870–878. doi: 10.1038/oby.2007.591. [DOI] [PubMed] [Google Scholar]
- 28.Sudlow C., Gallacher J., Allen N., Biobank U.K., et al. An Open Access Resource for Identifying the Causes of a Wide Range of Complex Diseases of Middle and Old Age. PLoS Med. 2015;12(3) doi: 10.1371/journal.pmed.1001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.World Health Organization. ICD-10 : International Statistical Classification of Diseases and Related Health Problems: Tenth Revision. 2nd ed. World Health Organization.
- 30.World Health Organization. International Classification of Diseases : [9th] Ninth Revision, Basic Tabulation List with Alphabetic Index. World Health Organization, 1978.
- 31.Petersen S.E., Matthews P.M., Bamberg F., et al. Imaging in population science: cardiovascular magnetic resonance in 100,000 participants of UK Biobank - rationale, challenges and approaches. J. Cardiovasc. Magn. Reson. 2013;15(1):46. doi: 10.1186/1532-429X-15-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.UK Biobank Imaging Modality Cardiovascular Magnetic Resonance (CMR), 2015. Accessed December 6, 2019. http://www.ukbiobank.ac.uk/.
- 33.UK Biobank: Protocol for a Large-Scale Prospective Epidemiological Resource (AMENDMENT ONE FINAL), 2007.
- 34.C. Bycroft, C. Freeman, D. Petkova et al. Genome-wide genetic data on ∼500,000 UK Biobank participants. bioRxiv. Published online 2017:166298. 10.1101/166298.
- 35.Le Ven F., Bibeau K., De Larochellière É., et al. Cardiac morphology and function reference values derived from a large subset of healthy young Caucasian adults by magnetic resonance imaging. Eur. Hear J. – Cardiovasc. Imaging. 2016;17(9):981–990. doi: 10.1093/ehjci/jev217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Petersen S.E., Aung N., Sanghvi M.M., et al. Reference ranges for cardiac structure and function using cardiovascular magnetic resonance (CMR) in Caucasians from the UK Biobank population cohort. J. Cardiovasc. Magn. Reson. 2017;19(1):18. doi: 10.1186/s12968-017-0327-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Benjamin D.J., Berger J.O., Johannesson M., et al. Redefine statistical significance. Nat. Hum. Behav. 2018;2(1):6–10. doi: 10.1038/s41562-017-0189-z. [DOI] [PubMed] [Google Scholar]
- 38.Tamarappoo B., Dey D., Shmilovich H., et al. Increased Pericardial Fat Volume Measured From Noncontrast CT Predicts Myocardial Ischemia by SPECT. JACC Cardiovasc. Imaging. 2010;3(11):1104–1112. doi: 10.1016/j.jcmg.2010.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Di Cesare M., Bentham J., Stevens G.A., et al. Trends in adult body-mass index in 200 countries from 1975 to 2014: A pooled analysis of 1698 population-based measurement studies with 19.2 million participants. Lancet. 2016;387(10026):1377–1396. doi: 10.1016/S0140-6736(16)30054-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Spearman J.V., Renker M., Schoepf U.J., et al. Prognostic value of epicardial fat volume measurements by computed tomography: a systematic review of the literature. Eur. Radiol. 2015;25(11):3372–3381. doi: 10.1007/s00330-015-3765-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gianluca M., Mario S., Eufrasia L.R., et al. Validity of epicardial fat volume as biomarker of coronary artery disease in symptomatic individuals: Results from the ALTER-BIO registry. Int. J. Cardiol. 2020 doi: 10.1016/j.ijcard.2020.04.031. [DOI] [PubMed] [Google Scholar]
- 42.Pandey N.N., Sharma S., Jagia P., Kumar S. Epicardial fat attenuation, not volume, predicts obstructive coronary artery disease and high risk plaque features in patients with atypical chest pain. Br. J. Radiol. 2020;93(1114) doi: 10.1259/BJR.20200540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Antonopoulos A.S., Sanna F., Sabharwal N., et al. Detecting human coronary inflammation by imaging perivascular fat. Sci. Transl. Med. 2017;9(398) doi: 10.1126/SCITRANSLMED.AAL2658. [DOI] [PubMed] [Google Scholar]
- 44.Hell M.M., Ding X., Rubeaux M., et al. Epicardial adipose tissue volume but not density is an independent predictor for myocardial ischemia. J. Cardiovasc. Comput. Tomogr. 2016;10(2):141–149. doi: 10.1016/j.jcct.2016.01.009. [DOI] [PubMed] [Google Scholar]
- 45.Lin A., Dey D., Wong D.T.L., Nerlekar N. Perivascular Adipose Tissue and Coronary Atherosclerosis: from Biology to Imaging Phenotyping. Curr Atheroscler Rep. 2019;21(12):47. doi: 10.1007/S11883-019-0817-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.K. Britton, C. Fox, Clinical Lipidology Perivascular adipose tissue and vascular disease, 2017. 10.2217/clp.10.89. [DOI] [PMC free article] [PubMed]
- 47.Bertaso A.G., Bertol D., Duncan B.B., Foppa M. Epicardial fat: definition, measurements and systematic review of main outcomes. Arq. Bras. Cardiol. 2013;101(1):e18–e28. doi: 10.5935/abc.20130138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Oikonomou E.K., Marwan M., Desai M.Y., et al. Non-invasive detection of coronary inflammation using computed tomography and prediction of residual cardiovascular risk (the CRISP CT study): a post-hoc analysis of prospective outcome data. Lancet (London, England). 2018;392(10151):929–939. doi: 10.1016/S0140-6736(18)31114-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xiao C., Dash S., Morgantini C., Hegele R.A., Lewis G.F. Pharmacological targeting of the atherogenic dyslipidemia complex: The next frontier in CVD prevention beyond lowering LDL cholesterol. Diabetes. 2016;65(7):1767–1778. doi: 10.2337/db16-0046. [DOI] [PubMed] [Google Scholar]
- 50.J.S. Dron, R.A. Hegele, Genetics of Triglycerides and the Risk of Atherosclerosis 2017. 10.1007/s11883-017-0667-9. [DOI] [PMC free article] [PubMed]
- 51.Talayero B.G., Sacks F.M. The role of triglycerides in atherosclerosis. Curr. Cardiol. Rep. 2011;13(6):544–552. doi: 10.1007/s11886-011-0220-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Reiner Ž. Hypertriglyceridaemia and risk of coronary artery disease. Nat. Rev. Cardiol. 2017;14(7):401–411. doi: 10.1038/nrcardio.2017.31. [DOI] [PubMed] [Google Scholar]
- 53.Miller M., Stone N.J., Ballantyne C., et al. Triglycerides and cardiovascular disease: A scientific statement from the American Heart Association. Circulation. 2011;123(20):2292–2333. doi: 10.1161/CIR.0b013e3182160726. [DOI] [PubMed] [Google Scholar]
- 54.Barter P., Gotto A.M., LaRosa J.C., et al. HDL Cholesterol, Very Low Levels of LDL Cholesterol, and Cardiovascular Events. N. Engl. J. Med. 2007;357(13):1301–1310. doi: 10.1056/NEJMoa064278. [DOI] [PubMed] [Google Scholar]
- 55.Gordon D.J., Probstfield J.L., Garrison R.J., et al. High-density lipoprotein cholesterol and cardiovascular disease. Four prospective American studies. Circulation. 1989;79(1):8–15. doi: 10.1161/01.CIR.79.1.8. [DOI] [PubMed] [Google Scholar]
- 56.Colom C., Viladés D., Pérez-Cuellar M., et al. Associations between epicardial adipose tissue, subclinical atherosclerosis and high-density lipoprotein composition in type 1 diabetes. Cardiovasc. Diabetol. 2018;17(1):156. doi: 10.1186/s12933-018-0794-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sarwar N., Butterworth A.S., Freitag D.F., et al. Interleukin-6 receptor pathways in coronary heart disease: A collaborative meta-analysis of 82 studies. Lancet. 2012;379(9822):1205–1213. doi: 10.1016/S0140-6736(11)61931-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ouwens D.M., Sell H., Greulich S., Eckel J. The role of epicardial and perivascular adipose tissue in the pathophysiology of cardiovascular disease. J. Cell Mol. Med. 2010;14(9):2223–2234. doi: 10.1111/j.1582-4934.2010.01141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mazurek T., Zhang L., Zalewski A., et al. Human epicardial adipose tissue is a source of inflammatory mediators. Circulation. 2003;108(20):2460–2466. doi: 10.1161/01.CIR.0000099542.57313.C5. [DOI] [PubMed] [Google Scholar]
- 60.Baker A.R., Harte A.L., Howell N., et al. Epicardial adipose tissue as a source of nuclear factor-κB and c-Jun N-terminal kinase mediated inflammation in patients with coronary artery disease. J. Clin. Endocrinol. Metab. 2009;94(1):261–267. doi: 10.1210/jc.2007-2579. [DOI] [PubMed] [Google Scholar]
- 61.Djaberi R., Schuijf J.D., van Werkhoven J.M., Nucifora G., Jukema J.W., Bax J.J. Relation of Epicardial Adipose Tissue to Coronary Atherosclerosis. Am. J. Cardiol. 2008;102(12):1602–1607. doi: 10.1016/j.amjcard.2008.08.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.