Abstract
PTEN is a well-known tumor suppressor with various functions that depend on its intracellular localization. Green fluorescent protein (GFP)-tagged live-cell images clarified the crucial amino acids needed to regulate the localization of PTEN in cells. However, it currently remains unknown whether GFP itself affects the intracellular localization of PTEN and its mutants, and the establishment of fixed-cell imaging is important for identifying the exact location of PTEN in cells. I herein investigated a number of immunofluorescence strategies for cell fixation, membrane permeabilization, and antigen retrieval. Permeabilization by detergents was necessary to observe nuclear and cytosolic PTEN in paraformaldehyde (PFA)-fixed cells; however, this permeabilization was not always valid. On the other hand, antigen retrieval by the pre-boiled EDTA treatment was useful for detecting plasma membranous PTEN in PFA-fixed cells in the same manner as in in vivo studies. Furthermore, methanol-fixed images of PTEN were consistent with GFP-tagged live-cell images. Two immunofluorescence methods (the PFA-fixed/pre-boiled EDTA treatment and methanol fixation) are applicable to investigations of the intracellular localization of PTEN without a GFP tag in cultured cells. In conclusion, live-cell imaging and appropriate immunofluorescence including a novel antigen retrieval treatment were both useful for detecting the cellular localization of PTEN, particularly at the plasma membrane.
Keywords: immunofluorescence, nucleus, plasma membrane, PTEN
Introduction
PTEN is an important tumor suppressor protein in various tissues. A PTEN deficiency in cells and tissues leads to disorders of cell proliferation, apoptosis, migration, protein synthesis, and metabolism.1–3 In contrast, PTEN overexpression decreases cell proliferation and migration and suppresses cancer metastasis.4–7 Key resides for the intracellular localization of PTEN have been characterized from green fluorescent protein (GFP)-tagged live-cell imaging.8–12 C-terminal phosphorylation is expected to cover the regulatory membrane-binding motif and, thus, maintains the localization of wild-type PTEN in the cytoplasm. A PTENA4 mutant in which C-terminal phosphorylation sites (Ser380, Thr382, Thr383, and Ser385) is replaced with alanine and certain nuclear proteins and lipids may be involved in the nuclear transport of PTENA4. 10 On the other hand, lysine 13 is an essential residue for nuclear translocation, and its arginine mutant (K13R) does not accumulate in the nuclei.8,10,13,14 Therefore, the combination of K13R and A4 mutations recruits PTENK13R,A4 to the plasma membrane. 10 It currently remains unclear whether a GFP tag itself affects the intracellular localization of PTEN. Moreover, the establishment of immunofluorescence methods is indispensable for accurately identifying PTEN when some PTEN-associating proteins are characterized from merged immunostaining and proximity ligation assays along the plasma membrane, even in paraformaldehyde (PFA)-fixed cells. Therefore, it is necessary to examine both live-cell images of and immunoassays on fixed cells with the objective of visualizing PTEN. The present study demonstrates the applicability of methanol fixation and antigen retrieval after PFA fixation to the immunodetection of PTEN in cultured cells.
Materials and Methods
Cell Culture
The human breast epithelial cell line, MCF10A and its PTEN knockout (KO) cells were kindly provided from Dr. Michele Vitolo (Maryland University) 15 and were maintained in DMEM/F12 (10565-018, Gibco, MA) containing 5% horse serum (26050088, Gibco), 1% penicillin/streptomycin (15140-122, Gibco), 0.5 mg/ml hydrocortisone (H0888, Sigma-Aldrich, MO), 0.1 mg/ml cholera toxin (C8052, Sigma-Aldrich), 4 µg/ml insulin (12585-014, Invitrogen, MA), and 0.4 µg/ml epidermal growth factor (EGF; E-9644, Sigma-Aldrich). DLD-1 PTEN KO cells were kindly provided from Dr. Todd Waldman (Georgetown University School of Medicine). 16 PC-3 cells from human prostate cancer and DLD-1 PTEN KO cells from human colon cancer were maintained in RPMI1640 medium (11875-093, Gibco) containing 10% FBS and 1% penicillin/streptomycin.
Transfection
Wild-type and mutant PTEN-expressing PC-3 cells were generated by lentivirus infection. Lentiviruses were produced as previously described.12,13 The expression of PTEN was induced by 0.1 µg/ml doxycycline (D9851, Sigma-Aldrich) overnight in lentivirus-infected cells. Wild-type and mutant PTEN expression plasmids were transfected using lipofectamine 3000 (L3000015, Invitrogen) in DLD-1 PTEN KO cells.
Antibodies
Anti-PTEN rabbit IgG (clone 138G6) (9559, Cell Signaling Technology: CST, MA), anti-PTEN mouse IgG (clone 6H2.1) (04-035, Millipore, MA, US), and anti-GAPDH mouse IgG (MA5-15738, Thermo, MA) were used. The following fluorescent-labeled secondary antibodies were obtained from Invitrogen: Alexa488 anti-rabbit IgG (A21206), Alexa647 anti-rabbit IgG (A31573), Alexa488 anti-mouse IgG (A21202), and Alexa647 anti-mouse IgG (A31571).
Immunoblotting
MCF10A cells and their PTEN KO cells were homogenized in radioimmunoprecipitation assay (RIPA) buffer (9806, CST) containing a protease inhibitor cocktail (1697498001, Roche, Basel, Switzerland). Cell lysates were centrifuged at 16,000 × g at 4°C for 10 min and the supernatants were collected. Proteins were separated using SDS-PAGE and transferred onto PVDF membranes (IPFL00010, EMD Millipore, MA). After blocking in PBS containing 0.05% Tween 20 supplemented with 3% BSA at room temperature for 1 hr, the membranes were incubated with the primary antibodies at 4°C overnight. Immunocomplexes were visualized using fluorescent-labeled secondary antibodies and detected by a molecular imager (PharosFX Plus, Bio-Rad, CA).
Live-cell Imaging
PC-3 cells overexpressing PTEN were seeded on LabTek 8-well chambered coverglass (Nalge Nunc, Rochester, NY, US). After an overnight incubation in 0.1 µg/ml doxycycline, cells were observed under an LSM800 GaAsP laser scanning confocal microscope with Zen software (Zeiss, Oberkochen, Germany). The objective was 63×, the scan mode was framed, and the scan direction was bidirectional. Images were obtained from a single slice with an average of four scans.
Immunofluorescence
Lentivirus-infected PC-3 or DLD-1 PTEN KO cells were seeded on LabTek 8-well chambered coverglass (Nalge Nunc) and treated with 0.1 µg/ml doxycycline or transfection by Lipofectamine 3000, respectively. The next day, cells were fixed with pre-warmed 4% PFA or cold methanol for 20 min. After PFA fixation, cells were treated with 0.1% Triton X-100 in PBS for 8 min (PFA/Triton X-100) (Table 1). 1 mM EDTA solution was prepared in the medium bottle and boiled in a microwave (used as pre-boiled solution). The pre-boiled EDTA solution was instantly added to the PFA-fixed cells in LabTek 8-well chambered coverglass. The samples were kept in this EDTA solution at room temperature for 10 min. These processes were repeated three times (PFA/EDTA) (Table 1). Cells were blocked with 3% BSA in PBS (3% BSA/PBS) at room temperature for 30 min and then incubated with anti-PTEN antibodies (1:500 in 3% BSA/PBS) at 4°C overnight. After washing with PBS, cells were incubated with appropriate fluorescent-labeled secondary antibodies (1:500 in 3% BSA/PBS) at room temperature for 1 hr. Nuclear DNA was stained with 1 µg/ml DAPI (10236276001, Roche). Samples were observed on an LSM800 GaAsP laser scanning confocal microscope with Zen software (Zeiss). The fluorescence intensities of nuclei, the cytoplasm, and plasma membrane were quantified by ImageJ.
Table 1.
Cell Treatments for an Immunofluorescence Assay of PTEN in Cultured Cells.
| Method | Fixation | Permeabilization/Antigen Retrieval |
|---|---|---|
| PFA | Pre-warmed 4% PFA at room temp. for 20 min | Not done |
| PFA/Triton | Pre-warmed 4% PFA at room temp. for 20 min | 0.1% Triotn X-100 at room temp. for 8 min |
| PFA/EDTA | Pre-warmed 4% PFA at room temp. for 20 min | Pre-boiled 1 mM EDTA for 5 min at three times |
| MeOH | Cold methanol at -20°C for 20 min | Not done |
Results
Intracellular Localization of PTEN, PTENA4, and PTENK13R,A4
Three doxycycline-inducible lentivirus vectors (GFP-fused to PTEN, PTRENA4, and PTENK13R,A4) expressed in PC-3 cells that lack endogenous PTEN were prepared. The intracellular localization of PTEN and its mutants were observed in PC-3 cells using GFP-tagged live imaging. As shown in Fig. 1, PTEN localized to the nucleus and cytoplasm. PTENA4 was also mainly incorporated into nuclei, while PTENK13R,A4 localized along the plasma membrane. These images revealed the characteristic localization of PTEN, PTENA4, and PTENK13R,A4 in PC-3 cells.
Figure 1.
Live-cell imaging of PTEN-GFP and mutant versions of PTEN-GFP showing specific intracellular localization in PC-3 cells. Exogenous PTEN-GFP, PTENA4-GFP, and PTENK13R,A4-GFP localize in whole cells, nuclei, and the plasma membrane, respectively. Representative images of one or two PC-3 cells expressing PTEN-GFP. Scale bar: 20 µm.
Immunofluorescence Methods for PTEN
To demonstrate that the immunofluorescence assay is not dependent on specific antibodies, two different antibodies were used in the present study. Rabbit monoclonal IgG (clone 138G6) and mouse monoclonal IgG (clone 6H2.1) (that recognize the C-tail of PTEN) were used to test the specificity of antibodies. To investigate whether PTEN antibodies exhibit a single band in a Western blot analysis of the cell lysates of a human cell line, but not PTEN KO cells, MCF10A cells, and MCF10A PTEN KO cells were used in the present study. MCF10A and MCF10A PTEN KO cells were examined as genetically established cell lines. Western blotting using both antibodies revealed a PTEN band (expected size: 54–55 kDa) in the lysate of wild-type MCF10A cells, but not in that of MCF10A PTEN KO cells (Fig. 2). Moreover, Western blotting showed single bands for wild-type PTEN and its mutant from the lysate of PC-3 cells transfected with the GFP tag (expected size: 80 kDa) (Supplemental Fig. 1). Accordingly, these two antibodies were used in immunological studies.
Figure 2.

Western blotting with two antibodies that detect PTEN. (A-C) Western blotting of whole cell lysates prepared from wild-type and PTEN KO MCF10A cells was performed using anti-PTEN rabbit monoclonal antibodies (clone 138G6) (A), anti-PTEN mouse monoclonal antibodies (clone 6H2.1) (B), and anti-GAPDH antibodies (C).
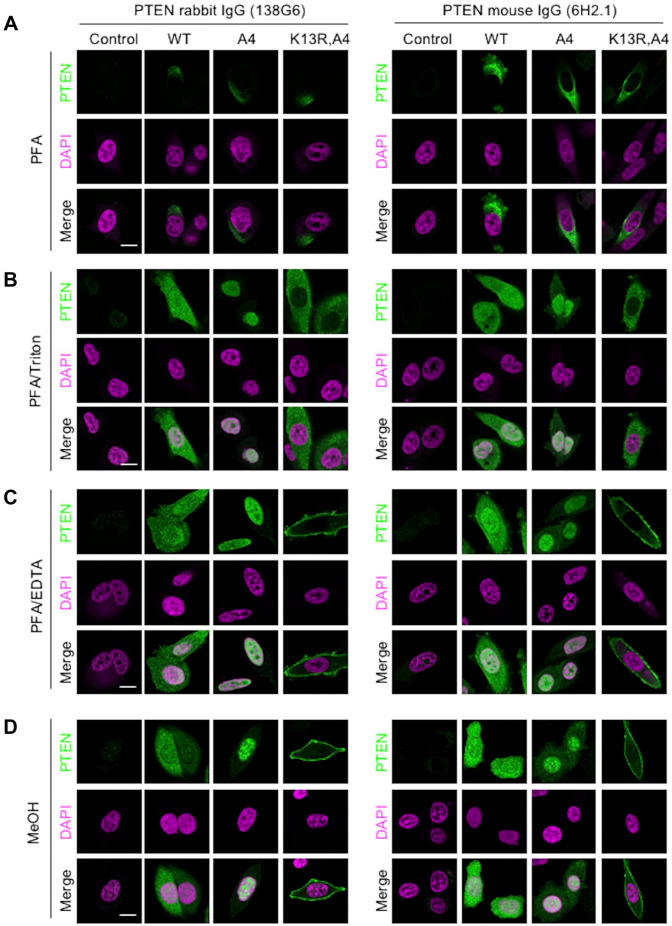
Immunofluorescence assays for PTEN in PC-3 cells transfected with GFP-tagged PTEN utilized Alexa647-conjugated (not Alexa488 or Alexa546) secondary antibodies to prevent the GFP signals from being mixed into the immunofluorescent. Although the non-specific signals were observed, cells did not show PTEN signals after fixation with 4% PFA in PBS (Fig. 3A). Because the antibodies non-specifically bound to the irrelevant proteins and/or lipids fixed at the plasma membrane, these fluorescence signals were entirely different from the live-cell images (Fig. 1). As a standard protocol for the immunofluorescence assay, cells were incubated with 0.1% Triton X-100 in PBS to permeabilize both the plasma membrane and organelle membranes. PTEN and PTENA4 showed cytosolic and nuclear distributions, respectively (Fig. 3B). However, the immunolabeling of PTENK13R,A4 was cytosolic, not along the plasma membrane (Fig. 3B) in contrast to live-cell imaging (Fig. 1). To develop a new protocol that captures the distribution of PTEN to the plasma membrane, antigen retrieval was performed on PFA-fixed cells with pre-boiled 1 mM EDTA solution. This treatment has been used in the immunofluorescence microscopy of tissue sections to remove chemical cross-links created by 4% PFA.17,18 As shown in Fig. 3C, this method revealed PTENK13R,A4 at the plasma membrane in PFA-fixed cells. On the other hand, PTEN and its mutants showed the same intracellular localization as that in methanol-fixed cells on GFP-tagged cell images (Fig. 3D). In comparisons of live-cell images, the ratio of the nuclear/cytosolic signals of PTENA4 was significantly reduced after methanol and PFA fixation (Fig. 4A). Methanol-fixed cells exhibited higher nuclear signals of PTENA4 than PFA-fixed/Triton X-100-treated cells (Fig. 4A). On the other hand, to investigate the amount of PTENK13R,A4 at the plasma membrane, the signal intensities were measured over 40 µm across the cells (Fig. 4B). By adjusting total signal intensities to 100, relative signal intensities were calculated at each point. Two peak signals were observed in live-cell images of PFA-fixed/EDTA-treated and methanol-fixed cells, whereas peaks were not clearly detected in PFA-fixed/Triton X-100-treated cells (Fig. 4B and C). Further analyses showed the merged images of GFP and immunofluorescence signals of PTENK13R,A4. As shown in Fig. 5, GFP fluorescence tagged at PTENK13R,A4 was observed after PFA and methanol fixation, although its signal intensity at the plasma membrane was weaker than that in live-cell images (3.03 +/- 0.50 in Fig. 4). The Triton X-100 treatment lowered GFP signal intensities at the plasma membrane (Fig. 5). PTENK13R,A4-GFP protein that was associated with the plasma membrane was removed with the Triton X-100 treatment, while the cytosolic protein was not removed, at least not the same rate as the plasma membranous. To elucidate the intracellular localization of PTEN, PTENA4, and PTENK13R,A4 mutants without the GFP tag, these expression vectors were transfected into DLD-1 PTEN KO cells. The immunofluorescence signals of PTEN and its mutants were not detected after PFA fixation (Fig. 6A), and permeabilization with 0.1% Triton X-100/PBS resulted in the detection of PTEN and PTENA4 signals (Fig. 6B). However, these methods did not identify the PTENK13R,A4 signal along the plasma membrane (Fig. 6B). On the other hand, it was observed plasma membranous PTENK13R,A4 by antigen retrieval using pre-boiled EDTA solution after PFA fixation (Fig. 6C) or methanol fixation (Fig. 6D).
Figure 3.
Immunofluorescence assays identify the specific intracellular localization of PTEN-GFP and mutant versions of PTEN-GFP in methanol-fixed and PFA-fixed/pre-boiled EDTA-treated PC-3 cells. Cells were treated with two anti-PTEN rabbit monoclonal antibodies (clone 138G6) and anti-PTEN mouse monoclonal antibodies (clone 6H2.1) (green). Cells were chemically fixed with 4% PFA (A-C) or cold methanol (D). PFA-fixed cells were either untreated (A) or treated with 0.1% Triton X-100/PBS (B) or treated with pre-boiled 1 mM EDTA (C). DAPI was used as the nuclear stain (magenta). Scale bar: 20 µm.
Figure 4.
PTENA4 and PTENK13R,A4 in methanol-fixed and PFA-fixed/pre-boiled EDTA-treated PC-3 cells, but at different fluorescence intensities to GFP live-cell imaging. (A) Ratio of fluorescence intensities for nuclear and cytosolic PTENA4. Values are represented as box plots (n=10–34). (B) (left panel) The fluorescence signals of PTEN immunostaining were measured along the white lines (40 µm) across the cells. (right) The fluorescence intensities of PTEN immunostaining were calculated relative to 100 of the total signal intensity along the lines. Right graphs show representative images. (C) PTENK13R,A4 at the plasma membrane was quantified as peak fluorescence. Average fluorescence values on both sides were represented as box plots (n=7–12). A statistical analysis was performed using a one-way analysis of variance (ANOVA) with Tukey’s post hoc test. *p<0.05, ***p<0.001. Scale bar: 20 µm.
Figure 5.
Immunofluorescence signals merged with GFP show PTENK13R,A4 in PFA-fixed/pre-boiled EDTA-treated and methanol-fixed PC-3 cells. (A) Cells were detected with GFP (green) and an immunofluorescence assay with anti-PTEN rabbit monoclonal antibodies (clone 138G6) (magenta). Cells were chemically fixed with 4% PFA or cold methanol. Representative images of PC-3 cells expressing PTENK13R,A4-GFP. Scale bar: 20 µm. (B) PTENK13R,A4 at the plasma membrane was quantified as peak fluorescence intensities. Total fluorescence intensities were counted as 100 along the lines (40 µm). Average fluorescence intensities on both sides were represented as a box plot. A statistical analysis was performed using a one-way analysis of variance (ANOVA) with Tukey’s post hoc test. *p<0.05, **p<0.01, ***p<0.001.
Figure 6.
Immunofluorescence assays identify the intracellular localization of PTEN and its mutants without the GFP tag in PFA-fixed/pre-boiled EDTA-treated and methanol-fixed DLD-1 PTEN KO cells. DLD-1 PTEN KO cells transfected with PTEN and its mutant plasmids were subjected to an immunofluorescence assay with anti-PTEN rabbit monoclonal antibodies (clone 138G6) (green). Cells were chemically fixed with 4% PFA (A-C) or cold methanol (D). PFA-fixed cells were either untreated (A) or treated with 0.1% Triton X-100/PBS (B) or treated with pre-boiled 1 mM EDTA (C). DAPI was used as the nuclear stain (magenta). Representative images of one or two DLD-1 PTEN KO cells expressing exogenous PTEN. Scale bar: 20 µm.
Discussion
The conventional immunofluorescence method for culturing cells necessarily includes fixation with PFA followed by the permeabilization treatment to label intracellular antigens. In the present study, the immunofluorescence signals were not observed in samples only subjected to PFA fixation, because the PFA-fixed plasma and nuclear membranes may block the antibodies’ penetration into the cytoplasm and nuclei. However, detergents such as Triton X-100 break these PFA-fixed membranes, and PTEN and PTENA4 show representative patterns on GFP-derived live-cell images. However, PTENK13R,A4 was not detected along the plasma membrane after permeabilization by the detergent. Since PTENK13R,A4 closely associates with the lipids of the plasma membrane, the detergent treatment may break this link. The permeabilization step is widely used, but herein novel methods were developed using antigen retrieval with pre-boiled EDTA solution. In many in vivo studies, the heat-induced antigen retrieval method has become widespread for improving the immunolabelling of various human or experimental animal antigens after aldehyde fixation, such as formaldehyde and PFA, since it was initially described by Shi et al. 19 Our group used a heat-induced antigen retrieval process with a microwave in EDTA solution for frozen liver sections against PTEN.13,14,20 The present study demonstrated that the heat-induced antigen retrieval process also improves the immunolabelling of antigens in cultured cells, particularly at the plasma membrane. Although the underlying mechanisms have not yet been elucidated in detail, heat-induced antigen retrieval is generally considered to involve the direct or indirect exposure of epitopes, such as the destruction of formalin-induced cross-linked epitopes. Additionally, pre-boiled EDTA solution may break the membranes without removing their lipid components as permeabilization. On the other hand, organic solvents, such as methanol or acetone, dissolve lipids from the plasma membrane, thereby allowing antibodies to reach intracellular and intra-organelle antigens. At the same time, methanol coagulates proteins for fixation to prevent membranous PTEN diffusing into the cytosol without cross-links. 21 Therefore, methanol fixation provides clearer images of membranous PTEN in cultured cells for a microscopic analysis.
The functions of PTEN depend on its intracellular localization. A well-known function of PTEN is the dephosphorylation of phosphatidylinositol 3,4,5-triphosphate, which is a critical second messenger in the phosphoinositide 3-kinase/Akt pathway.22,23 Although PTEN is mainly present the cytoplasm, it needs to translocate into the plasma membrane to exert this function. Therefore, it is imperative to accurately identify PTEN in the plasma membrane. In an established immunofluorescence method, methanol fixation is recommended for identifying the intracellular localization of PTEN. 24 The present study reinforces this protocol, and also proposes a novel immunofluorescence method using pre-boiled EDTA solution for PFA-fixed cells. However, whether the antigen retrieval exerts additive/synergistic effects on methanol-fixed or PFA-fixed detergent-treated cells and whether other antigen retrieval methods, such as citrate buffer treatment, have significant effects remain unknown. Further studies are needed for the development of more accurate PTEN immunofluorescence.
In addition to GFP-derived live-cell imaging, an immunofluorescence assay is important for accurately assessing the intracellular localization of PTEN. The present results indicate that methanol fixation is suitable for detecting PTEN in the nucleus and plasma membrane. In cases in which PFA fixation is necessary, the antigen retrieval step by pre-boiled EDTA is useful. However, a conventional permeabilization step with detergents needs to be avoided.
Supplemental Material
Supplemental material, sj-pdf-1-jhc-10.1369_00221554221082539 for Immunofluorescence Detection of Plasma Membranous PTEN in Cultured Cells by Takashi Kato in Journal of Histochemistry & Cytochemistry
Acknowledgments
I thank Miho Iijima, Hiromi Sesaki, and the members of the Iijima/Sesaki labs in Johns Hopkins university for their helpful discussions and technical assistance.
Footnotes
Author Contributions: TK confirms the sole responsibility for the following: study conception and design, experimental performance, analysis and interpretation of results, and manuscript preparation.
Competing Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by grants to Miho Iijima (NIH, GM131768) and Hiromi Sesaki (NIH, GM123266).
ORCID iD: Takashi Kato  https://orcid.org/0000-0002-1417-3813
https://orcid.org/0000-0002-1417-3813
Literature Cited
- 1. Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet. 2006;7(8):606–19. doi: 10.1038/nrg1879. [DOI] [PubMed] [Google Scholar]
- 2. Milella M, Falcone I, Conciatori F, Cesta Incani U, Del Curatolo A, Inzerilli N, Nuzzo CM, Vaccaro V, Vari S, Cognetti F, Ciuffreda L. PTEN: multiple functions in human malignant tumors. Front Oncol. 2015;5:24. doi: 10.3389/fonc.2015.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chen CY, Chen J, He L, Stiles BL. PTEN: tumor suppressor and metabolic regulator. Front Endocrinol (Lausanne). 2018;9:338. doi: 10.3389/fendo.2018.00338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Davies MA, Koul D, Dhesi H, Berman R, McDonnell TJ, McConkey D, Yung WK, Steck PA. Regulation of Akt/PKB activity, cellular growth, and apoptosis in prostate carcinoma cells by MMAC/PTEN. Cancer Res. 1999;59(11):2551–6. [PubMed] [Google Scholar]
- 5. Liu JL, Sheng X, Hortobagyi ZK, Mao Z, Gallick GE, Yung WK. Nuclear PTEN-mediated growth suppression is independent of Akt down-regulation. Mol Cell Biol. 2005;25(14):6211–24. doi: 10.1128/MCB.25.14.6211-6224.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lu Y, Lin YZ, LaPushin R, Cuevas B, Fang X, Yu SX, Davies MA, Khan H, Furui T, Mao M, Zinner R, Hung MC, Steck P, Siminovitch K, Mills GB. The PTEN/MMAC1/TEP tumor suppressor gene decreases cell growth and induces apoptosis and anoikis in breast cancer cells. Oncogene. 1999;18(50):7034–45. doi: 10.1038/sj.onc.1203183. [DOI] [PubMed] [Google Scholar]
- 7. Davies MA, Kim SJ, Parikh NU, Dong Z, Bucana CD, Gallick GE. Adenoviral-mediated expression of MMAC/PTEN inhibits proliferation and metastasis of human prostate cancer cells. Clin Cancer Res. 2002;8(6):1904–14. [PubMed] [Google Scholar]
- 8. Nguyen HN, Afkari Y, Senoo H, Sesaki H, Devreotes PN, Iijima M. Mechanism of human PTEN localization revealed by heterologous expression in Dictyostelium. Oncogene. 2014;33(50):5688–96. doi: 10.1038/onc.2013.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nguyen HN, Yang JM, Afkari Y, Park BH, Sesaki H, Devreotes PN, Iijima M. Engineering ePTEN, an enhanced PTEN with increased tumor suppressor activities. Proc Natl Acad Sci USA. 2014;111(26):E2684–93. doi: 10.1073/pnas.1409433111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nguyen HN, Yang JM, Miyamoto T, Itoh K, Rho E, Zhang Q, Inoue T, Devreotes PN, Sesaki H, Iijima M. Opening the conformation is a master switch for the dual localization and phosphatase activity of PTEN. Sci Rep. 2015;5:12600. doi: 10.1038/srep12600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nguyen HN, Yang JM, Jr, Rahdar M, Keniry M, Swaney KF, Parsons R, Park BH, Sesaki H, Devreotes PN, Iijima M. A new class of cancer-associated PTEN mutations defined by membrane translocation defects. Oncogene. 2015;34(28):3737–43. doi: 10.1038/onc.2014.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yang JM, Schiapparelli P, Nguyen HN, Igarashi A, Zhang Q, Abbadi S, Amzel LM, Sesaki H, Quiñones-Hinojosa A, Iijima M. Characterization of PTEN mutations in brain cancer reveals that pten mono-ubiquitination promotes protein stability and nuclear localization. Oncogene. 2017;36(26):3673–3685. doi: 10.1038/onc.2016.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kato T, Yamada T, Nakamura H, Igarashi A, Anders RA, Sesaki H, Iijima M. The loss of nuclear PTEN increases tumorigenesis in a preclinical mouse model for hepatocellular carcinoma. iScience. 2020;23(10):101548. doi: 10.1016/j.isci.2020.101548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kato T, Murata D, Anders RA, Sesaki H, Iijima M. Nuclear PTEN and p53 suppress stress-induced liver cancer through distinct mechanisms. Biochem Biophys Res Commun. 2021;549:83–90. doi: 10.1016/j.bbrc.2021.02.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Vitolo MI, Weiss MB, Szmacinski M, Tahir K, Waldman T, Park BH, Martin SS, Weber DJ, Bachman KE. Deletion of PTEN promotes tumorigenic signaling, resistance to anoikis, and altered response to chemotherapeutic agents in human mammary epithelial cells. Cancer Res. 2009;69:8275–83. doi: 10.1158/0008-5472.CAN-09-1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lee C, Kim JS, Waldman T. PTEN gene targeting reveals a radiation-induced size checkpoint in human cancer cells. Cancer Res. 2004;64:6906–14. doi:10.1158/0008-5472.CAN-04-1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yamashita S, Katsumata O. Heat-induced antigen retrieval in immunohistochemistry: mechanisms and applications. Methods Mol Biol. 2017;1560:147–61. doi: 10.1007/978-1-4939-6788-9_10. [DOI] [PubMed] [Google Scholar]
- 18. Pileri SA, Roncador G, Ceccarelli C, Piccioli M, Briskomatis A, Sabattini E, Ascani S, Santini D, Piccaluga PP, Leone O, Damiani S, Ercolessi C, Sandri F, Pieri F, Leoncini L, Falini B. Antigen retrieval techniques in immunohistochemistry: comparison of different methods. J Pathol. 1997;183(1):116–23. doi: [DOI] [PubMed] [Google Scholar]
- 19. Shi SR, Key ME, Kalra KL. Antigen retrieval in formalin-fixed, paraffin-embedded tissues: an enhancement method for immunohistochemical staining based on microwave oven heating of tissue sections. J Histochem Cytochem. 1991;39(6):741–8. doi: 10.1177/39.6.1709656. [DOI] [PubMed] [Google Scholar]
- 20. Kato T, Igarashi A, Sesaki H, Iijima M. Generating a new mouse model for nuclear PTEN deficiency by a single K13R mutation. Genes Cells. 2021;26:1014–1022. doi: 10.1111/gtc.12902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jamur MC, Oliver C. Permeabilization of cell membranes. Methods Mol Biol. 2010;588:63–6. doi: 10.1007/978-1-59745-324-0_9. [DOI] [PubMed] [Google Scholar]
- 22. Maehama T, Dixon JE. The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem. 1998;273(22):13375–8. doi: 10.1074/jbc.273.22.13375. [DOI] [PubMed] [Google Scholar]
- 23. Maehama T, Dixon JE. PTEN: a tumour suppressor that functions as a phospholipid phosphatase. Trends Cell Biol. 1999;9(4):125–8. doi: 10.1016/s0962-8924(99)01519-6. [DOI] [PubMed] [Google Scholar]
- 24. Gil A, López JI, Pulido R. Assessing PTEN subcellular localization. Methods Mol Biol. 2016;1388:169–86. doi: 10.1007/978-1-4939-3299-3_12. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-jhc-10.1369_00221554221082539 for Immunofluorescence Detection of Plasma Membranous PTEN in Cultured Cells by Takashi Kato in Journal of Histochemistry & Cytochemistry