Abstract
Rationale
Pulse glucocorticoid therapy is used in hyperinflammation related to coronavirus disease 2019 (COVID-19). We evaluated the efficacy and safety of pulse intravenous methylprednisolone in addition to standard treatment in COVID-19 pneumonia.
Methods
In this multicentre, randomised, double-blind, placebo-controlled trial, 304 hospitalised patients with COVID-19 pneumonia were randomised to receive 1 g of methylprednisolone intravenously for three consecutive days or placebo in addition to standard dexamethasone. The primary outcome was the duration of patient hospitalisation, calculated as the time interval between randomisation and hospital discharge without the need for supplementary oxygen. The key secondary outcomes were survival free from invasive ventilation with orotracheal intubation and overall survival.
Results
Overall, 112 (75.4%) out of 151 patients in the pulse methylprednisolone arm and 111 (75.2%) of 150 in the placebo arm were discharged from hospital without oxygen within 30 days from randomisation. Median time to discharge was similar in both groups (15 days, 95% CI 13.0–17.0 days and 16 days, 95% CI 13.8–18.2 days, respectively; hazard ratio (HR) 0.92, 95% CI 0.71–1.20; p=0.528). No significant differences between pulse methylprednisolone and placebo arms were observed in terms of admission to intensive care unit with orotracheal intubation or death (20.0% versus 16.1%; HR 1.26, 95% CI 0.74–2.16; p=0.176) or overall mortality (10.0% versus 12.2%; HR 0.83, 95% CI 0.42–1.64; p=0.584). Serious adverse events occurred with similar frequency in the two groups.
Conclusions
Methylprenisolone pulse therapy added to dexamethasone was not of benefit in patients with COVID-19 pneumonia.
Short abstract
The quick and strong anti-inflammatory effect of pulse glucocorticoid therapy seems to be of no benefit in COVID-19 pneumonia https://bit.ly/3IkUmSn
Introduction
Effective pharmacological treatments for coronavirus disease 2019 (COVID-19) are needed [1]. Glucocorticoids, for their broad and rapid anti-inflammatory effects, are ideal candidates for the treatment of hyperinflammation in COVID-19 [2–4]. Interestingly, in the 1970s Fauci et al. [5] showed that glucocorticoids block inflammation by inhibiting the efflux of neutrophils and monocytes to inflammatory sites. Both cell types play an important role in COVID-19-induced lesions, in both the peripheral blood and lungs [3, 6]. The Randomised Evaluation of COVID-19 Therapy (RECOVERY) trial showed that low-dose dexamethasone reduced mortality in hospitalised patients with COVID-19 requiring respiratory support [7]. This observation was confirmed by a prospective meta-analysis of seven randomised trials in severe COVID-19 patients which showed that the administration of systemic glucocorticoids, compared with usual care or placebo, was associated with lower 28-day all-cause mortality, and that it was safe [8].
Pulse glucocorticoid therapy (>250 mg of prednisone equivalent per day for 1 day or a few days) has the most relevant genomic and nongenomic actions, which are responsible for the anti-inflammatory and rapid effects of glucocorticoids; therefore, this treatment modality is used for particularly severe and/or life-threatening immuno-inflammatory diseases as initial therapy [9–12]. In COVID-19, the cytokine storm represents the acme of the inflammatory process, suggesting the need for a prompt and strong anti-inflammatory effect. Therefore, the addition of pulse glucocorticoid therapy to the standard low dose of dexamethasone scheme can suppresses the hyperinflammatory processes in COVID-19 more effectively than dexamethasone alone and may represent a potential treatment option for patients with severe and critical COVID-19, where pulse glucocorticoid therapy can provide a better alternative to non-pulse treatment.
No double-blind randomised trials have been conducted to assess the efficacy of the addition of steroid pulse therapy to the available usual care for COVID-19 pneumonia. To evaluate the efficacy and safety of pulse intravenous methylprednisolone therapy in hospitalised patients with COVID-19 pneumonia, we conducted a multicentre, randomised, double-blind, placebo-controlled trial.
Methods
Study design and participants
This is a multicentre, randomised, double-blind, placebo-controlled trial coordinated by the Azienda Unità Sanitaria Locale-IRCCS of Reggio Emilia, Italy. 19 Italian centres participated to this trial.
The trial was submitted and approved on 20 November 2020, by the Italian Medicines Agency (Agenzia Italiana del Farmaco (AIFA)) (code 130662) and on 25 November 2020, by the COVID-19 ethics committee established at the Lazzaro Spallanzani National Institute for Infectious Diseases (Rome, Italy) (code 217). The trial was conducted in accordance with the principles of the good clinical practice guidelines of the International Conference on Harmonisation. The trial was overseen by an independent data safety and monitoring committee. Written informed consent was obtained from each patient or from the patient's legally authorised representative if the patient was unable to provide consent. Full details of the trial design, conduct, oversight, analyses, protocol and statistical analysis plan can be found in the supplementary material.
The study population included patients hospitalised for recent-onset COVID-19 pneumonia documented by radiological imaging, with >5 days since initial symptoms of infection, requiring supplemental oxygen in any delivery mode, except invasive mechanical ventilation, with arterial oxygen tension (PaO2)/inspiratory oxygen fraction (FiO2) between 100 mmHg and 300 mmHg, and C-reactive protein (CRP) >5 mg·dL−1. This CRP value was selected because this cut-off on admission is an indicator of COVID-19 disease severity associated with increased mortality in hospitalised patients [13].
Cases of COVID-19 were confirmed by PCR with nasopharyngeal swab.
Patients were excluded if they required invasive mechanical ventilation or in the presence of shock or concomitant organ failure requiring intensive care unit (ICU) admittance. Other exclusion criteria were pregnancy or breastfeeding, severe cardiac or renal failure, diabetes not in good metabolic control based on physician's clinical judgment and other clinical conditions which contraindicate methylprednisolone and which cannot be treated or resolved based on physician's clinical judgement. Therapy with steroid high-dose pulses in the week preceding the enrolment in the study and enrolment in another clinical trial were also exclusion criteria.
Randomisation
Eligible patients were randomly assigned in 1:1 ratio to receive either methylprednisolone pulse therapy or placebo, in addition to standard treatment with dexamethasone in both arms. Random assignment was performed centrally at the trials centre of the Policlinico San Martino-IRCCS (Genova, Italy), stratified by participating centres and type of noninvasive respiratory support (standard oxygen versus high-flow nasal cannula (HFNC) or noninvasive ventilation (NIV)). Computer-generated random lists were prepared using permuted balanced blocks of four in a random sequence. An internet-based randomisation system ensured concealment of the treatment assignment until the patient had been registered in the system. Treatment allocation was communicated electronically only to the study centre's pharmacy to allow local preparation of the methylprednisolone or placebo bag for the intravenous administration of the treatment.
Treatment
Patients in the experimental arm received three boluses of 1 g methylprednisolone i.v. on days 1, 2 and 3 from randomisation in 100 mL physiological solution in addition to standard treatment. Patients in the control arm received 100 mL saline (placebo) administered on days 1, 2 and 3 from randomisation in addition to standard treatment. Standard treatment included dexamethasone (6 mg·day−1 oral or i.v. for 10 days), according to the RECOVERY study protocol [2]. The standard therapy was allowed to change during the study in accordance with the indications of AIFA regarding the choice of drugs, dosages or duration of treatment.
Procedures
Patients were evaluated daily during their hospitalisation, from randomisation (day 1) through day 30. The type of noninvasive respiratory support was recorded daily. Medical evaluations on days 21, 28 and end of study (30 days) could be limited to telephone contact if the patient was at home. The compilation of the end-of-study visit was mandatory and it could coincide with any clinical visit or telephone contact occurring during the 30 days’ observation window. All severe and nonsevere adverse events observed during hospitalisation and in the 30 days from randomisation were recorded in the clinical database. Adverse events were classified and graded according to the Common Terminology Criteria for Adverse Events (CTCAE; version 5.0). For each adverse event the maximum grade of toxicity was identified. Trial participants, investigators and study team care providers were unaware of the trial-group assignments until after all data queries were resolved and the database was locked. Only the hospital pharmacies were aware of the treatment allocation.
Outcomes
The primary end-point was duration of patient hospitalisation, calculated as the interval of time between randomisation and hospital discharge without the need for supplementary oxygen during the first 30 days following randomisation. The patients who were transferred to other hospital wards at different intensity of care were considered as still hospitalised. Patients who were transferred to other institutions outside the hospital (e.g. COVID-hotels) because of the impossibility of transferring the patient home were considered as discharged from the hospital.
Patients who died within 30 days were considered as never discharged.
Patients discharged at home with supplementary oxygen were censored at the date of discharge, because no further follow-up information was available. This choice implied the questionable assumption that their prognosis was similar to that of patients still in hospital: the effects of this assumption were evaluated in two post hoc sensitivity analyses in which these patients were considered either discharged without oxygen, or never discharged. The results of these analyses closely resembled those of the primary analysis.
The secondary end-points of efficacy were 1) survival, free from invasive ventilation with orotracheal intubation, defined as the interval between the randomisation and the first use of invasive ventilation with orotracheal intubation or death; 2) overall survival, defined as the interval between randomisation and death for any cause.
According to the study protocol, the following explorative end-points were also evaluated in selected populations. 1) Survival, free from clinical worsening, defined as the interval between randomisation and the first episode in which the PaO2/FiO2 ratio drops below 150 mmHg, or death. Only patients with PaO2/FiO2 >200 mmHg at enrolment were included in this analysis. 2) Survival, free from NIV or HFNC, defined as the interval between randomisation and the first use of NIV or the first administration of HFNC, or death. Only patients who did not receive oxygen in this modality at randomisation were included in this analysis.
Statistical analysis
Assuming a median time to hospital discharge of 14 days in the control arm, the experimental treatment was considered effective if it reduced time to discharge by 37.5% in relative terms (hazard ratio (HR) 1.60). This corresponds to an absolute increase in the cumulative probability of discharge at 14 days from 50% to 67%, i.e. a decrease in the median time to discharge from 14 to <9 days. Assuming a statistical power of 90% and a 5% error rate α, for a two-tailed log rank test ≥198 hospital discharges needed to be observed. The sample size required to observe 198 hospital discharges, assuming a 5% overall dropout rate, was estimated to be ≥260 patients: 130 per arm.
Statistical analysis is detailed in the statistical analysis plan. The cumulative probabilities of survival free from discharge in the two treatment groups were estimated according to the Kaplan–Meier method and compared with a nonstratified log-rank test. The hazard ratio and its 95% confidence interval were computed by fitting a univariate Cox's model. All secondary efficacy analyses were performed with the same approach as described for the primary efficacy analysis. Primary and secondary efficacy analyses were performed on all patients included in the intention-to-treat population. Explorative end-point analyses were carried out on the appropriate subpopulations, identified from the intention-to-treat population.
We reported the p-value (two-tailed; significance defined as p<0.05) and bilateral 95% confidence interval not adjusted for multiplicity.
We performed some subgroup analyses not planned in the statistical analysis plan, reported in the supplementary material. The subgroups were defined according to PaO2/FiO2 values at randomisation (PaO2/FiO2 100–200 mmHg versus PaO2/FiO2 201–300 mmHg), the modality of oxygen administration (supplemental standard oxygen versus NIV or high-flow oxygen), CRP values at randomisation (stratified as >5 and ≤10 mg·dL−1, >10 and ≤15 mg·dL−1 and >15 mg·dL−1), age (≤60 years and >60 years) and body mass index (BMI) (stratified in quartiles: first quartile ≤24.6 kg·m−2, second quartile 24.7–27.6 kg·m−2, third quartile 27.7–30.9 kg·m−2, fourth quartile ≥31.0 kg·m−2). Hazard ratio estimates obtained in the Cox model were reported within each subgroup. The presence of a significant variation of the hazard ratios across the strata of each factor was assessed by means of the treatment-by-factor interaction tests.
Statistical analyses were performed using SAS (version 9.4; SAS Institute) and SPSS (version 23; IBM Corporation). The trial is registered with EudraCT (2020-004323-16) and ClinicalTrials.gov (NCT04673162).
Results
Patients
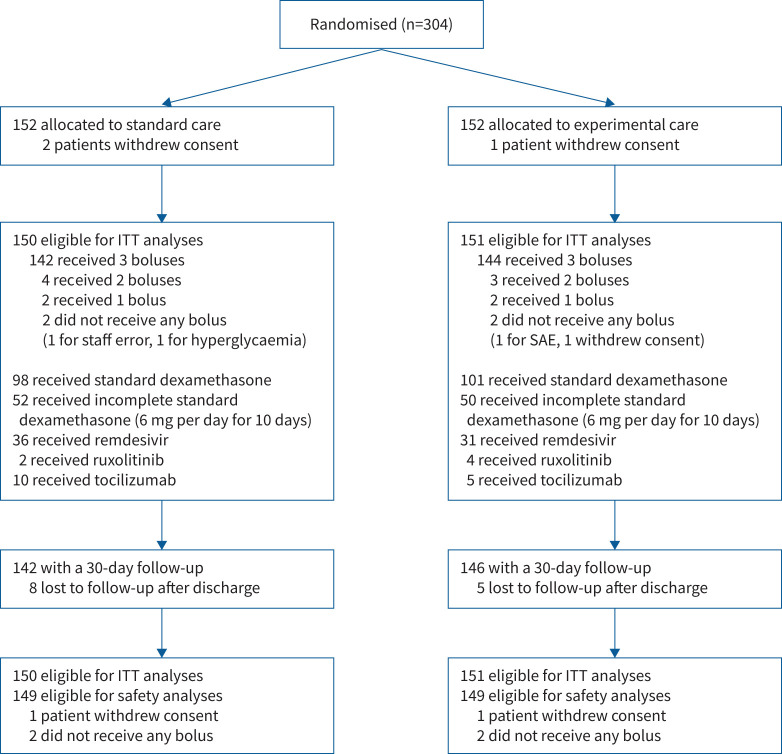
Between 21 December 2020 and 10 March 2021, the 19 centres randomised 304 patients; 152 in the methylprednisolone pulses arm and 152 in the placebo and standard therapy arm. Three patients (one in the experimental arm and two in the control arm) withdrew consent, leaving 301 patients eligible for the intention-to-treat analysis (figure 1).
FIGURE 1.
The Consolidated Standards of Reporting Trials flow diagram of the study. ITT: intention to treat; SAE: serious adverse event.
149 (98.7%) patients assigned to receive methylprednisolone pulses and 148 (98.7%) assigned to receive placebo received the therapy as assigned. Most patients, 144 (95.4%) out of 151 in the active treatment arm and 142 (94.7%) out of 150 in the placebo arm, received all three pulses. Demographic and baseline clinical characteristics are summarised in table 1.
TABLE 1.
Demographic and clinical characteristics of patients at randomisation
| Placebo + standard | Methylprednisolone pulses + standard | |
| Patients | 150 | 151 |
| Age, years | 64.0 (55.0–72.2) | 64.0 (54.0–74.0) |
| Male | 106 (70.7) | 111 (73.5) |
| Days from symptom onset to randomisation | 8.0 (6.0–10.0) | 9.0 (6.0–11.0) |
| Days from pneumonia diagnosis to randomisation | 1.0 (1.0–2.0) | 1.0 (1.0–2.0) |
| PaO2/FiO2, mmHg | 204.0 (158.0–243.0) | 208.0 (158.0–248.0) |
| Respiratory rate, breaths·min −1 | 20.0 (18.0–24.0) | 20.5 (18.0–24.0) |
| CRP, mg·dL−1 | 10.9 (7.6–14.6) | 10.6 (7.3–14.9) |
| Modality of oxygen administration | ||
| Supplemental standard oxygen | 101 (67.3) | 104 (68.9) |
| NIV or high-flow oxygen | 49 (32.7) | 47 (31.1) |
| Coexisting conditions | ||
| Diabetes mellitus | 19 (12.7) | 26 (17.2) |
| Hypertension | 74 (49.3) | 83 (55.0) |
| COPD | 8 (5.3) | 5 (3.3) |
| Heart failure | 7 (4.7) | 9 (6.0) |
| Obesity (BMI ≥30 kg·m−2) | 35/112 (31.3) | 34/121 (28.1) |
| Other treatments # | ||
| Glucocorticoids | 135 (90.0) | 131 (86.8) |
| Remdesivir | 27 (18.0) | 19 (12.6) |
| Antibiotics | 41 (27.3) | 40 (26.5) |
| LMWH | 117 (78.0) | 120 (79.5) |
Data are presented as n, median (interquartile range) or n (%). PaO2: arterial oxygen tension; FiO2: inspiratory oxygen fraction; CRP: C-reactive protein; NIV: noninvasive ventilation; BMI: body mass index; LMWH: low-molecular-weight heparin. #: any treatments administered during the period from the onset of symptoms until randomisation.
Primary outcome
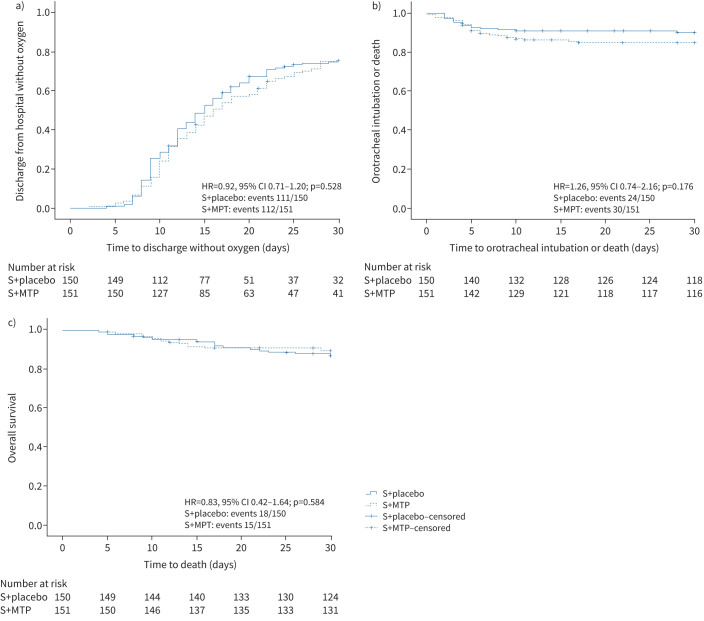
112 (75.4%) out of 151 patients in the pulse methylprednisolone arm and 111 (75.2%) out of 150 patients in the placebo arm were discharged from hospital without oxygen within 30 days. No difference in time to discharge without oxygen was observed between the two groups: the median was 15 days (95% CI 13.0–17.0 days) in the methylprednisolone arm versus 16 days (95% CI 13.8–18.2 days) in the placebo arm (HR 0.92, 95% CI 0.71–1.20; p=0.528) (table 2 and figure 2a).
TABLE 2.
Clinical outcomes in the intention-to-treat population
| Placebo + standard | Methylprednisolone pulses + standard | Hazard ratio (95% CI) | p-value | |
| Primary end-point | ||||
| Discharge without oxygen# | 111/150 (75.2) | 112/151 (75.4) | 0.92 (0.71–1.20) | 0.528 |
| Time to discharge within 30 days | 16 (13.8–18.2) | 15 (13.0–17.0) | ||
| Secondary end-points | ||||
| Admission to ICU or death¶ | 24/150 (16.1) | 30/151 (20.0) | 1.26 (0.74–2.16) | 0.176 |
| Deaths | 18/150 (12.2) | 15/151 (10.0) | 0.83 (0.42–1.64) | 0.584 |
| Explorative end-points | ||||
| Clinical worsening or death+ | 31/77 (40.3) | 37/81 (45.7) | 1.17 (0.73–1.89) | 0.430 |
| Use of high-flow oxygen or NIV, or death§ | 51/101 (50.6) | 55/104 (52.9) | 1.16 (0.79–1.70) | 0.430 |
Data are presented as n (% at 30 days) or median (95% confidence interval), unless otherwise stated. All proportions were cumulative probabilities estimated using the Kaplan–Meier product limit estimator and compared using the log-rank test. ICU: intensive care unit; NIV: noninvasive ventilation. #: one patient in the placebo arm and three patients in the methylprednisolone arm had been admitted to ICU, but had a full recovery and were discharged without oxygen within 30 days; ¶: eight patients in the placebo arm and seven patients in the methylprednisolone arm died after admission to ICU, while 10 patients and eight patients, respectively, died before being admitted to the ICU; +: in the 158 patients with arterial oxygen tension/inspiratory oxygen fraction ratio >200 mmHg at randomisation; §: in the 205 patients who received standard supplementary oxygen at randomisation.
FIGURE 2.
Kaplan–Meier estimates of a) hospital discharge without oxygen; b) orotracheal intubation or death; and c) death. S: standard treatment; MTP: methylprednisolone; HR: hazard ratio.
Secondary outcomes
54 patients were admitted to the ICU and received invasive ventilation with orotracheal intubation or died, with no significant differences between the pulse methylprednisolone and placebo arms (20.0% versus 16.1%; HR 1.26, 95% CI 0.74–2.16) (table 2 and figure 2b).
33 deaths occurred within 30 days since randomisation, and mortality was comparable in the two arms (10.0% versus 12.2%; HR 0.83, 95% CI 0.42–1.64) (table 2 and figure 2c).
Explorative end-points and subgroup analyses
At randomisation, 158 patients had PaO2/FiO2 ratio >200 mmHg. In these patients, the incidence of clinical worsening defined as progression to a PaO2/FiO2 ratio <150 mmHg or death was similar in methylprednisolone pulse and placebo arms (45.7% versus 40.3%; HR 1.17, 95% CI 0.73–1.89) (table 2).
At randomisation, 205 patients received standard supplementary oxygen. In these patients, the incidence of progression to the use of HFNC or NIV, mechanical ventilation or death was not different in methylprednisolone pulse and placebo arms (52.9% versus 50.6%; HR 1.16, 95% CI 0.79–1.70).
No differences were observed between the two arms in subgroup analyses, where subgroups were defined according to PaO2/FiO2 values, different modalities of oxygen therapy, CRP values, age and BMI at randomisation (supplementary material).
Safety outcomes
86 adverse events were reported in 51 (34.2%) out of 149 patients treated with placebo and 90 in 54 (36.2%) out of 149 patients treated with pulses of methylprednisolone. 28 (32.6%) grade 3 or 4 adverse events occurred in placebo group versus 35 (38.9%) in the pulse methylprednisolone group (table 3). 48 adverse events were judged to be treatment related by the principal investigators: 18 (20.9%) and 30 (33.3%) in the placebo and methylprednisolone arms, respectively. Adverse events by Medical Dictionary for Regulatory Activities system organ class (version 22.0) are listed in the supplementary material.
TABLE 3.
Adverse events by treatment arm, grade and relatedness in the safety population of 297 patients
| Placebo + standard | Methylprednisolone + standard | |
| Adverse events | ||
| Reported | 86 | 90 |
| Grade# | ||
| 1–2 | 51 (59.3) | 52 (57.8) |
| 3–4 | 28 (32.6) | 35 (38.9) |
| 5 | 7 (8.1) | 3 (3.3) |
| Relatedness (yes) | 18 (20.9) | 30 (33.3) |
| Patients with adverse events | 51/149 (34.2) | 54/149 (36.2) |
| SAEs | ||
| Reported | 16 | 9 |
| Grade# | ||
| 1–2 | 1 (6.2) | |
| 3–4 | 8 (50.0) | 7 (77.8) |
| 5 | 7 (43.8) | 2 (22.2) |
| Relatedness (yes) | 3 (18.7) | 2 (22.2) |
| Patients with SAEs | 12/149 (8.0) | 8/149 (5.4) |
Data are presented as n or n (%). SAE: serious adverse event. #: 1=mild, 2=moderate, 3=severe, 4=life-threatening, 5=death.
16 serious adverse events (SAEs) occurred in the placebo arm and nine in the methylprednisolone pulse arm. Eight grade 3 or 4 SAEs occurred in the placebo arm and seven in the methylprednisolone pulse arm. The number of SAEs considered by the investigators as treatment related were similar in the two arms, as well as the frequency of patients with SAEs (table 3). A summary description of SAEs is reported in the supplementary material. Bacterial infections were the most frequently reported SAEs in both arms, and they are detailed in supplementary table S2. In particular, serious infections thought to be related to the treatment were observed in 3.3% of the patients treated with methylprednisolone pulses and in 4% of those treated with placebo. Cases of severe uncontrolled hyperglycaemia were not reported in the two arms.
Discussion
In this double-blind, randomised, placebo-controlled trial no significant difference was observed in time to hospital discharge between the high-dose methylprednisolone pulse group and the standard of care group in patients with COVID-19 pneumonia. In addition, there was no benefit in the secondary outcomes, survival and admission to ICU. The lack of benefit on the primary outcome detected in the intention-to-treat population was also observed in subgroups of patients defined according to different PaO2/FiO2 values, different modalities of oxygen therapy and different CRP values at randomisation. Furthermore, we failed to observe beneficial effects of methylprednisolone pulses on progression of respiratory failure in the subgroups of 158 patients with PaO2/FiO2 ratio >200 mmHg and in the 206 patients who received standard supplementary oxygen at randomisation. Therefore, a rapid and vigorous suppression of inflammation with the addition of methylprednisolone pulses did not provide clinical benefit compared to standard care with dexamethasone according to the RECOVERY trial schedule in COVID-19 hospitalised patients requiring oxygen therapy [7].
Pulse glucocorticoid therapy is used in many immuno-inflammatory conditions to obtain a quick and strong suppression of inflammation in emergency situations [9–12, 14]. However, despite its diffuse use in clinical practice, very few studies, often uncontrolled and underpowered, have evaluated the efficacy and safety of this therapy [10–12, 14–17]. Indeed, a systematic literature review on safety and efficacy of pulse glucocorticoid therapy for severe acute respiratory syndrome coronavirus (SARS-CoV), Middle East respiratory syndrome CoV or SARS-CoV-2 showed that the quality of the evidence is poor and randomised controlled trials are needed [14]. A recent Iranian randomised trial showed a significant pulmonary improvement and a reduced mortality in hospitalised patients with severe COVID-19 treated with pulse methylprednisolone compared to those treated with standard therapy [18]. However, this study had several limitations. It was not double-blind, placebo-controlled, and enrolled only 62 patients. Even more important, differently from our study, standard of care did not include glucocorticoid treatment. A Spanish randomised, open-label, controlled study in hospitalised patients with COVID-19 pneumonia needing oxygen therapy compared low-dose dexamethasone (6 mg once daily for 10 days) to high-dose dexamethasone (20 mg once daily for 5 days, followed by 10 mg once daily for an additional 5 days) [19]. Similar to our study, this trial showed no difference in efficacy between high-dose and low-dose dexamethasone. However, the two studies were not completely comparable, because we used in pulse therapy a 10 times higher steroid dosage (125 mg of prednisone equivalent per day versus 1250 mg of prednisone equivalent per day), maximising the corticosteroid genomic and nongenomic actions [9]. Although all our patients had severe inflammation at enrolment (CRP values had to be >10 times the upper reference), they represented a spectrum of COVID-19 disease, ranging from patients more inflamed and critically ill at the time of randomisation to those with less severe inflammatory disease receiving supplemental oxygen by nasal prongs. We only excluded patients in early symptomatic phases (i.e. interval between initial symptoms of infection and randomisation ≤5 days), as we did not want to prevent functional immune responses in the early stages of the infection, and patients in invasive mechanical ventilation or with presence of shock or concomitant organ failure requiring ICU admittance.
Despite the overall negative results of our study, we cannot exclude potential benefit in specific subgroups of patients with more severe disease and more severe inflammatory response. Also, our study cannot rule out a role for pulse glucocorticoid therapy as a rescue therapy in patients who failed to improve after dexamethasone and that a gradual tapering of pulse methylprednisolone would have better controlled the inflammation, avoiding possible rebound. Of interest, our study did not show an increased risk of glucocorticoid side-effects, particularly infections or hyperglycaemia, in the patients treated with methylprednisolone pulses. Previous studies observed only an increased frequency of mild acute adverse events (e.g. sleep disturbance, mood change) in patients treated with pulse glucocorticoids compared to those treated with placebo [10, 12]. Therefore, our results confirm that pulses of methylprednisolone are safe also in the inflammatory phase of patients with COVID-19.
Our trial has some strengths, but also some limitations. One of the strengths is that the trial was multicentre, double-blind, placebo-controlled. Our trial population was intentionally chosen to have a severe inflammatory status as reflected by the elevated CRP values at baseline which were similar in the pulse methylprednisolone and placebo patients. These patients were presumably those with better chance of responding to methylprednisolone pulses. The two groups were balanced in terms of their baseline characteristics, including demographics, days from symptom onset to randomisation, PaO2/FiO2 value and modality of noninvasive support at randomisation, coexisting conditions and concurrent treatment. The selected primary outcome, duration of patient hospitalisation, may have important limitations, particularly regarding differences in local clinical practice. However, in Italy, during the study period, the treatment of hospitalised patients with COVID-19 was standardised by AIFA and the treatments were similar across all trial sites.
The main limitation of this trial derived from its limited sample size, which implies that the observed results do not allow the ruling out of the hypothesis that pulses of methylprednisolone are associated with a modest beneficial effect, e.g. a 15–16% reduction in time to discharge. More importantly, we cannot exclude possible benefits in specific subpopulations of patients that were underpowered to detect clinically relevant differences. Platform trials enrolling large numbers of patients could better evaluate the efficacy of pulse glucocorticoid therapy in these specific subgroups of patients.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERJ-00025-2022.SUPPLEMENT (404.6KB, pdf)
Shareable PDF
Acknowledgements
We would like to thank all patients for their participation in this important research. A special thank goes to the members of the Italian Medicines Agency (AIFA) Scientific Committee and COVID-19 Crisis Unit for their support.
Footnotes
This article has an editorial commentary: https://doi.org/10.1183/13993003.01220-2022
This study is registered with EudraCT, 2020-004323-16, and at ClinicalTrials.gov with identifier NCT04673162. De-identified participant data including data dictionaries will be made available within 3 months of publication. The proposed use of the data and analyses must be approved by the scientific committee before to have access to the data. The scientific committee will have the right to review and comment any draft manuscripts prior to publication. Those wishing to request access should write to the corresponding author.
Author contributions: C. Salvarani, M. Costantini, D.F. Merlo, G.L. Mariani, L. Boni, P. Bruzzi, C. Turrà and S. Cavuto had access to the raw data; C. Salvarani, M. Massari, M. Costantini, D.F. Merlo, G.L. Mariani, P. Viale, S. Nava, G. Dolci, L. Boni, L. Savoldi, P. Bruzzi, C. Turrà and N. Facciolongo contributed to the study design, data collection, data review, interpretation, writing and approval of the manuscript, and the decision to submit; M. Costantini, D.F. Merlo, G.L. Mariani, L. Boni, L. Savoldi and S. Cavuto accessed and verified the data and did the statistical analysis; G. Guaraldi, M. Codeluppi, A.M. Marata, C. Barbieri, A. Valcavi, F. Franzoni, G. Mazzi, R. Corsini, F. Trapani, A. Bartoloni, E. Barisione, C. Barbieri, G.J. Burastero, A. Pan, W. Inojosa, R. Scala, C. Burattini, F. Luppi, M. Catanoso, K.E. Tarek, G. Cenderello, M. Salio, G. Foti, R. Dongilli, G. Bajocchi, E.A. Negri, G. Ciusa, G. Fornaro, I. Bassi, L. Zammarchi and T. Aloè contributed to collection, verification, and interpretation of the data, and critical review and revision of the manuscript; C. Salvarani, M. Massari and M. Costantini had final responsibility for the decision to submit for publication.
Conflict of interest: The authors declare no competing interests.
Support statement: This research received no external funding. The coordinator centre and participating centres used local resources to conduct the trial.
References
- 1.Welte T, Ambrose LJ, Sibbring GC, et al. . Current evidence for COVID-19 therapies: a systematic literature review. Eur Respir Rev 2021; 30: 200384. doi: 10.1183/16000617.0384-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rhen T, Cidlowski JA. Antiinflammatory action of glucocorticoids – new mechanisms for old drugs. N Engl J Med 2005; 353: 1711–1723. doi: 10.1056/NEJMra050541 [DOI] [PubMed] [Google Scholar]
- 3.Gustine JN, Jones D. Immunopathology of hyperinflammation in COVID-19. Am J Pathol 2021; 191: 4–17. doi: 10.1016/j.ajpath.2020.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cassone G, Dolci G, Besutti G, et al. . Acute-phase reactants during tocilizumab therapy for severe COVID-19 pneumonia. Clin Exp Rheumatol 2020; 38: 1215–1222. [PubMed] [Google Scholar]
- 5.Fauci AS, Dale DC, Balow JE. Glucocorticosteroid therapy: mechanisms of action and clinical considerations. Ann Intern Med 1976; 84: 304–315. doi: 10.7326/0003-4819-84-3-304 [DOI] [PubMed] [Google Scholar]
- 6.Gibellini L, De Biasi S, Paolini A, et al. . Altered bioenergetics and mitochondrial dysfunction of monocytes in patients with COVID-19 pneumonia. EMBO Mol Med 2020; 12: e13001. doi: 10.15252/emmm.202013001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.RECOVERY Collaborative Group, Horby P, Lim WS, et al. . Dexamethasone in hospitalized patients with Covid-19. N Engl J Med 2021; 384: 693–704. doi: 10.1056/NEJMoa2021436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group , Sterne JAC, Murthy S, et al. . Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis. JAMA 2020; 324: 1330–1341. doi: 10.1001/jama.2020.17023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buttgereit F, Straub RH, Wehling M, et al. . Glucocorticoids in the treatment of rheumatic diseases: an update on the mechanisms of action. Arthritis Rheum 2004; 50: 3408–3417. doi: 10.1002/art.20583 [DOI] [PubMed] [Google Scholar]
- 10.Roujeau JC. Pulse glucocorticoid therapy. The ‘big shot’ revisited. Arch Dermatol 1996; 132: 1499–1502. doi: 10.1001/archderm.1996.03890360091015 [DOI] [PubMed] [Google Scholar]
- 11.The big shot. Lancet 1977; 309: 633–634. doi: 10.1016/S0140-6736(77)92065-7 [DOI] [PubMed] [Google Scholar]
- 12.Sinha A, Bagga A. Pulse steroid therapy. Indian J Pediatr 2008; 75: 1057–1066. doi: 10.1007/s12098-008-0210-7 [DOI] [PubMed] [Google Scholar]
- 13.Stringer D, Braude P, Myint PK, et al. . The role of C-reactive protein as a prognostic marker in COVID-19. Int J Epidemiol 2021; 50: 420–429. doi: 10.1093/ije/dyab012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dolci G, Cassone G, Venturelli F, et al. . High-dose glucocorticoids pulse-therapy for beta-coronaviridae pneumonia: a systematic literature review and case-series of coronavirus disease-2019. Clin Exp Rheumatol 2021; 39: 1119–1125. [DOI] [PubMed] [Google Scholar]
- 15.Woods JE, Anderson CF, DeWeerd JH, et al. . High-dosage intravenously administered methylprednisolone in renal transplantation. A preliminary report. JAMA 1973; 223: 896–899. doi: 10.1001/jama.1973.03220080028007 [DOI] [PubMed] [Google Scholar]
- 16.Mazlumzadeh M, Hunder GG, Easley KA, et al. . Treatment of giant cell arteritis using induction therapy with high-dose glucocorticoids: a double-blind, placebo-controlled, randomized prospective clinical trial. Arthritis Rheum 2006; 54: 3310–3318. doi: 10.1002/art.22163 [DOI] [PubMed] [Google Scholar]
- 17.La Mantia L, Eoli M, Milanese C, et al. . Double-blind trial of dexamethasone versus methylprednisolone in multiple sclerosis acute relapses. Eur Neurol 1994; 34: 199–203. doi: 10.1159/000117038 [DOI] [PubMed] [Google Scholar]
- 18.Edalatifard M, Akhtari M, Salehi M, et al. . Intravenous methylprednisolone pulse as a treatment for hospitalised severe COVID-19 patients: results from a randomised controlled clinical trial. Eur Respir J 2020; 56: 2002808. doi: 10.1183/13993003.02808-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taboada M, Rodríguez N, Varela PM, et al. . Effect of high versus low dose of dexamethasone on clinical worsening in patients hospitalised with moderate or severe COVID-19 pneumonia: an open-label, randomised clinical trial. Eur Respir J 2022; 60: 2102518. doi: 10.1183/13993003.02518-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERJ-00025-2022.SUPPLEMENT (404.6KB, pdf)
This one-page PDF can be shared freely online.
Shareable PDF ERJ-00025-2022.Shareable (391.1KB, pdf)