Abstract
The recanalization effect of large-vessel occlusion (LVO) in anterior circulation is well documented but only some patients benefit from endovascular treatment. We analysed clinical and radiological factors determining clinical outcome after successful mechanical intervention. We included 146 patients from the Prague 16 study enrolled from September 2012 to December 2020, who had initial CT/CTA examination and achieved good recanalization status after mechanical intervention (TICI 2b-3). One hundred and six (73%) patients achieved a good clinical outcome (modified Rankin Scale 0–2 in 3 months). It was associated with age, leptomeningeal collaterals (LC), onset to intervention time, ASPECTS, initial NIHSS, and leukoaraiosis (LA) in univariate analysis. The regression model identified good collateral status [odds ratio (OR) 5.00, 95% confidence interval (CI) 1.91–13.08], late thrombectomy (OR 0.24, 95% CI 0.09–0.65), LA (OR 0.44, 95% CI 0.19–1.00), ASPECTS (OR 1.45, 95% CI 1.08–1.95), and NIHSS score (OR 0.86, 95% CI 0.78–0.95) as independent outcome determinants. In the late thrombectomy subgroup, 14 out of 33 patients (42%) achieved a favourable clinical outcome, none of whom with poor collateral status. The presence of LC and absence of LA predicts a good outcome in acute stroke patients after successful recanalization of LVO in anterior circulation. Late thrombectomy was associated with higher rate of unfavourable clinical outcome. Nevertheless, collateral status in this subgroup was validated as a reliable selection criterion.
Keywords: Stroke, Mechanical thrombectomy, Leptomeningeal collaterals
Introduction
Mechanical thrombectomy became the most effective treatment of arterial occlusion in anterior cerebral circulation.1 Nevertheless, only some patients demonstrate clinical benefit after successful mechanical recanalization.
The actual indication criteria for mechanical thrombectomy take into account symptoms duration and the extent of early ischaemic cerebral tissue changes.2 Patients who were treated in different time points after the onset of stroke symptoms were selected using radiological parameters of tissue viability. Collateral status on CTA was used as a selection criterion in patients treated within 12 h after stroke onset.3 The beneficial effect of mechanical thrombectomy performed within 24 h was proven in patients selected according to CT perfusion or MRI with DWI.4,5
In addition to symptoms duration other factors can potentially determine clinical outcome, including age, radiological parameters ASPECTS or collateral status, clinical signs of cortical deficit or vigilance impairment, serum glucose level or blood pressure.6–8 The presence of leptomeningeal collaterals (LC) on CTA predicts better clinical outcome as well as smaller infarct size after mechanical intervention and intravenous thrombolysis.9 Imaging markers of small vessel disease are risk factors for haemorrhagic transformation after thrombolysis.10 A similar unfavourable relation was described also in mechanical thrombectomy.11
The aim of our analysis was to determine the impact of the initial radiological and clinical parameters on clinical outcome after successful recanalization therapy.
Methods
A cohort of consecutive patients in the Prague 16 study from September 2012 to December 2020 was included in the study. The ethical approval of the study was obtained from the medical ethics committee of University Hospital Kralovske Vinohrady. A total of 390 patients were treated for occlusion of any brain supplying vessel. We used the following criteria for patient selection: no pre-existing severe neurological deficit [modified Rankin Scale (mRS) <3]; complete initial non-contrast CT (NCCT) and CTA examination; proximal intracranial occlusion in anterior circulation (distal ICA or M1) on CTA; successful recanalization (TICI 2b or 3 in final angiogram); a follow-up of at least 3 months. The analysis was made retrospectively.
Demographic data (age, gender) and medical history of arterial hypertension, diabetes, smoking, and atrial fibrillation (AF; known at the admission or detected during the acute phase of stroke) were recorded. Severity of initial stroke symptoms was assessed by NIHSS score. Clinical outcome was evaluated in 3 months (personal contact or telephone inquiry) according to the mRS.
Imaging and treatment protocol
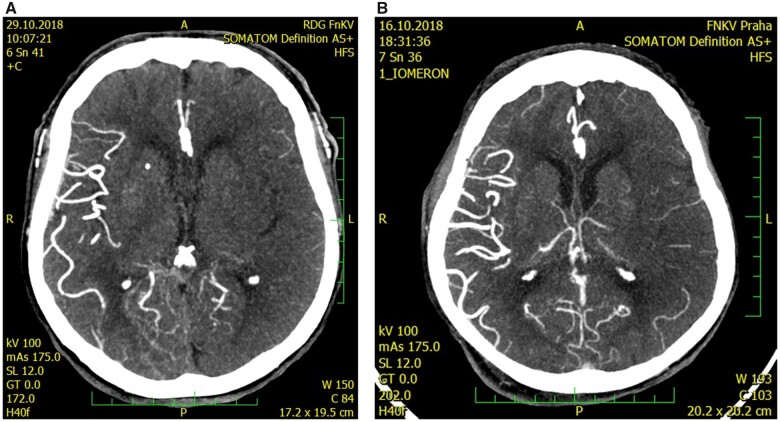
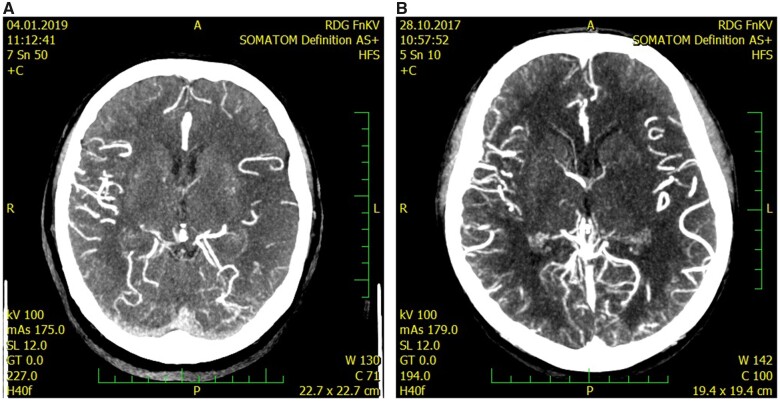
All patients initially underwent a multimodal CT scan. We assessed the presence of early ischaemic changes with ASPECTS on NCCT scan along with chronic leukoaraiotic lesions qualitatively according to van Sweeten’s scoring system (score 1 or 2 in any region was categorized as a positive finding). CTA was performed in helical mode, 0.6 mm slice thickness, 60 mL iodine, with bolus tracking technique. Collateral score was evaluated on maximal intensity projection images using the modified Tan score12 and categorized as good for grades 2–3 and poor for grades 0–1 (Figures 1 and 2).
Figure 1.
CT angiography of the left middle cerebral artery occlusion with poor collateral leptomeningeal status, Tan score—grade 0 (A) and grade 1 (B).
Figure 2.
CT angiography of the left middle cerebral artery occlusion with good collateral leptomeningeal status, Tan score—grade 2 (A) and grade 3 (B).
After the NCCT and CTA realization, the patients were directly transferred to the angio lab. Intravenous thrombolysis was administered in eligible patients when it did not delay mechanical thrombectomy initiation. Any application of thrombolytic therapy was noted, regardless of the administered dose. The recanalization was instrumentally carried through retriever and/or aspiration techniques. We assessed onset to intervention time in a dichotomized way: early thrombectomy within 6 h after the onset and late thrombectomy beyond this time limit or an unknown onset (wake-up strokes).
Statistics
We used Fisher’s exact test for categorical parameters and Pearson’s χ2 test for continual variables to assess their correlation with the clinical outcome defined as mRS at 3 months in a univariate model. Logistic regression analysis with analysis of variance test was applied to determine the multivariate association of the evaluated parameters with the outcome.
Results
Among 390 patients treated for acute ischemic stroke in anterior circulation, 146 met the selection criteria. Their characteristics and the results of univariate analysis are listed in Table 1. We found significant results for age (P < 0.001), good collateral status (P < 0.001), presence of leukoaraiosis (LA; P = 0.002), ASPECTS (P < 0.001), and initial NIHSS (P < 0.01).
Table 1.
Univariate analysis of the observed parameters related to treatment outcome
| Total (n = 146) |
mRS 0–2 (n = 83) |
mRS > 2 (n = 63) |
P-value | |
|---|---|---|---|---|
| Age | 66.5 ± 12.8 | 65.9 ± 13.9 | 72.1 ± 12.9 | <0.01 |
| Male gender, N (%) | 70 (48) | 42 (51) | 28 (44) | 0.46 |
| Hypertension, N (%) | 93 (64) | 49 (59) | 44 (70) | 0.18 |
| Diabetes, N (%) | 30 (21) | 16 (19) | 14 (22) | 0.66 |
| Smoking, N (%) | 29 (20) | 17 (20) | 12 (19) | 0.83 |
| Atrial fibrillation, N (%) | 70 (48) | 34 (41) | 36 (57) | 0.05 |
| Right side, N (%) | 40 (54) | 42 (51) | 35 (56) | 0.55 |
| ASPECTS—median | 8 (4–10) | 8 (5–10) | 7 (4–10) | <0.01 |
| LA, N (%) | 70 (48) | 33 (40) | 37 (59) | 0.02 |
| LC, N (%) | 106 (73) | 73 (88) | 33 (52) | <0.01 |
| IVT, N (%) | 68 (47) | 43 (52) | 25 (40) | 0.14 |
| Late thrombectomy, N (%) | 33 (23) | 14 (17) | 19 (30) | 0.04 |
| NIHSS—mean ±SD | 16 ± 5 | 14 ± 5 | 18 ± 5 | <0.01 |
ASPECTS, alberta stroke program early CT score; IVT, intravenous thrombolysis; LA, leukoaraiosis; LC, leptomeningeal collaterals; mRS, modified Rankin Scale; NIHSS, national institute of health stroke scale; SD, significant difference.
Regression logistic analysis defined LC, late thrombectomy, ASPECTS, NIHSS, and LA as independent outcome predictors (see Table 2). Odds ratio for continuous variables ASPECTS and NIHSS are defined for any score change.
Table 2.
Multivariate logistic regression analysis of good clinical outcome defined as modified Rankin Scale 0–2 at 3 months
| OR | 95% CI | P-value | |
|---|---|---|---|
| LC | 5.00 | 1.91–13.08 | <0.001 |
| LA | 0.44 | 0.19–1.00 | 0.049 |
| ASPECTS | 1.45 | 1.08–1.95 | 0.014 |
| Late thrombectomy | 0.24 | 0.09–0.65 | 0.005 |
| NIHSS | 0.86 | 0.78–0.95 | 0.004 |
ASPECTS, alberta stroke program early CT score; CI, confidence interval; LA, leukoaraiosis; LC, leptomeningeal collaterals; NIHSS, national institute of health stroke scale; OR, odds ratio.
The 33 patients undergoing late thrombectomy were included in the analysis (19 patients with wake-up stroke and 15 patients with symptoms duration >6 h). Low collateral status on the initial CTA was identified in four subjects of this subgroup. None of them achieved a favourable clinical outcome in 3 months. On the contrary, 48% of those with formed LC reached a good clinical outcome.
Discussion
The role of LC in the progress of ischaemic deterioration was documented previously. Visual assessment of collaterals using a modified Tan score was significantly linked with the clinical outcome in our cohort. A rate of good leptomeningeal collateral flow status was reached by 73% of patients and was comparable with previous observations. We did not include patients with distal MCA occlusion, since the visual Tan score would underestimate low collateral flow in occlusion of the M2 or M3 segment. Therefore, the cohort is less heterogeneous regarding temporal variations of collateral leptomeningeal supply. The patency of LC declines with increased age but we did not find any significant relation either to conventional vascular risk factors (arterial hypertension, diabetes, and smoking) or to a presence of leukoaraiotic lesions. Our results support the hypothesis that exposure to factors promoting atherosclerosis is not a major determinant for recruitment of LC in acute ischaemic stroke.8
Leukoaraiosis as a radiological hallmark of subcortical vascular disease was significantly associated with final outcome in univariate as well as in multivariate analysis. This finding corresponds with previous observation in a model with semi-quantitative differentiation of LA extent.13 Interestingly, some models incorporating the LA volume did not display a significant association with the clinical outcome.11 A significant relationship with unfavourable outcome was demonstrated in the severe degree of LA lesions.14
Age was significantly linked with clinical outcome in our univariate analysis but its impact decreased after comprising of other variables in the regression model. Sex and vascular risk factors in our cohort affect neither the collateral status nor the clinical outcome. Atrial fibrillation (AF) found out on admission or detected during acute phase of stroke was negatively associated with the collateral status. This observation demonstrates the fact that LC tend to develop rather in chronic ischaemic conditions like atherotrombosis than in an acute embolic occlusion of cerebral arteries. However, AF had no significant impact on final clinical outcome. This finding may be limited as we did not include results of later Holter ECG monitoring in the analysis.
Initial NIHSS and ASPECTS were significantly related to the final outcome. Moreover, the association of ASPECTS with the collateral status displays an interconnection in the sense that these parameters measure the same aspect of ischaemic pathophysiology. Our findings correspond with previous observations7 and agree with the pathophysiological model.15
Thirty-three patients with symptoms lasting >6 h or with unknown onset were comprised into the analysis. Although the outcome in these patients was generally worse than in the group treated with early thrombectomy, the preserved LC were in this subgroup a sensitive parameter for good outcome prediction. Our conclusion corresponds with previous findings. The Dutch acute stroke study indicates that most wake-up stroke patients without severe ischaemic changes on NCCT (ASPECT <5) and good collateral filling may be eligible candidates for mechanical thrombectomy.16 The analysis of the Korean stroke register6 suggests that the collateral status is a potential simple tool for selection of favourable outcome candidates after successful thrombectomy. Two recent retrospective works, based exclusively on NCCT imaging with ASPECTS assessment, demonstrate the benefit of more permissive criteria for identification of late thrombectomy aspirants.17,18
The limitations of the study include single-centre patients’ enrolment and its retrospective design. The accuracy of the visual rating scale of LC may be inferior to automated evaluation programmes. Single-phase CTA has a limited temporal resolution though the image acquisition was triggered by tracking bolus technique19 and the evaluated cohort was homogenous given the proximal site of occlusion in the anterior circulation.
Conclusion
Our study showed that good collateral status, absence of LA, early thrombectomy, higher ASPECTS, and lower NIHSS are related to clinical favourable outcome. Assessment of LC is a simple way to identify the patients who may benefit from revascularization therapy in acute ischaemic stroke even in later timepoints. Negative predictive value of LA lesions on clinical outcome in our analysis warrants further observation including the stratification of leukoaraiotic degree.
Funding
Supported by project Interventional treatment of life-threatening cardiovascular diseases – INTERCARDIS, project EU Nr. CZ.02.1.01/0.0/0.0/16_026/0008388.
Conflict of interest: The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Data availability
Additional data are available from the corresponding author on reasonable request.
References
- 1. Goyal M, Menon BK, van Zwam WH, Dippel DWJ, Mitchell PJ, Demchuk AM, Dávalos A, Majoie CBLM, van der Lugt A, de Miquel MA, Donnan GA, Roos YBWEM, Bonafe A, Jahan R, Diener H-C, van den Berg LA, Levy EI, Berkhemer OA, Pereira VM, Rempel J, Millán M, Davis SM, Roy D, Thornton J, Román LS, Ribó M, Beumer D, Stouch B, Brown S, Campbell BCV, van Oostenbrugge RJ, Saver JL, Hill MD, Jovin TG; HERMES Collaborators. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet 2016;387:1723–1731. [DOI] [PubMed] [Google Scholar]
- 2. Turc G, Bhogal P, Fischer U, Khatri P, Lobotesis K, Mazighi M, Schellinger PD, Toni D, de Vries J, White P, Fiehler J.. European Stroke Organisation (ESO)—European Society for Minimally Invasive Neurological Therapy (ESMINT) Guidelines on mechanical thrombectomy in acute ischaemic StrokeEndorsed by Stroke Alliance for Europe (SAFE). Eur Stroke J 2019;4:6–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Goyal M, Demchuk AM, Menon BK, Eesa M, Rempel JL, Thornton J, Roy D, Jovin TG, Willinsky RA, Sapkota BL, Dowlatshahi D, Frei DF, Kamal NR, Montanera WJ, Poppe AY, Ryckborst KJ, Silver FL, Shuaib A, Tampieri D, Williams D, Bang OY, Baxter BW, Burns PA, Choe H, Heo J-H, Holmstedt CA, Jankowitz B, Kelly M, Linares G, Mandzia JL, Shankar J, Sohn S-I, Swartz RH, Barber PA, Coutts SB, Smith EE, Morrish WF, Weill A, Subramaniam S, Mitha AP, Wong JH, Lowerison MW, Sajobi TT, Hill MD; ESCAPE Trial Investigators. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med 2015;372:1019–1030. [DOI] [PubMed] [Google Scholar]
- 4. Nogueira RG, Jadhav AP, Haussen DC, Bonafe A, Budzik RF, Bhuva P, Yavagal DR, Ribo M, Cognard C, Hanel RA, Sila CA, Hassan AE, Millan M, Levy EI, Mitchell P, Chen M, English JD, Shah QA, Silver FL, Pereira VM, Mehta BP, Baxter BW, Abraham MG, Cardona P, Veznedaroglu E, Hellinger FR, Feng L, Kirmani JF, Lopes DK, Jankowitz BT, Frankel MR, Costalat V, Vora NA, Yoo AJ, Malik AM, Furlan AJ, Rubiera M, Aghaebrahim A, Olivot J-M, Tekle WG, Shields R, Graves T, Lewis RJ, Smith WS, Liebeskind DS, Saver JL, Jovin TG; DAWN Trial Investigators. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med 2018;378:11–21. [DOI] [PubMed] [Google Scholar]
- 5. Albers GW, Lansberg MG, Kemp S, Tsai JP, Lavori P, Christensen S, Mlynash M, Kim S, Hamilton S, Yeatts SD, Palesch Y, Bammer R, Broderick J, Marks MP.. A multicenter randomized controlled trial of endovascular therapy following imaging evaluation for ischemic stroke (DEFUSE 3). Int J Stroke 2017;12:896–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Menon BK, Smith EE, Coutts SB, Welsh DG, Faber JE, Goyal M, Hill MD, Demchuk AM, Damani Z, Cho K-H, Chang H-W, Hong J-H, Sohn SI.. Leptomeningeal collaterals are associated with modifiable metabolic risk factors. Ann Neurol 2013;74:241–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nannoni S, Sirimarco G, Cereda CW, Lambrou D, Strambo D, Eskandari A, Mosimann PJ, Wintermark M, Michel P.. Determining factors of better leptomeningeal collaterals: a study of 857 consecutive acute ischemic stroke patients. J Neurol 2019;266:582–588. [DOI] [PubMed] [Google Scholar]
- 8. van Seeters T, Biessels GJ, Kappelle LJ, van der Graaf Y, Velthuis BK; Dutch Acute Stroke Study (DUST) Investigators. Determinants of leptomeningeal collateral flow in stroke patients with a middle cerebral artery occlusion. Neuroradiology 2016;58:969–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nambiar V, Sohn SI, Almekhlafi MA, Chang HW, Mishra S, Qazi E, Eesa M, Demchuk AM, Goyal M, Hill MD, Menon BK.. CTA collateral status and response to recanalization in patients with acute ischemic stroke. AJNR Am J Neuroradiol 2014;35:884–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kongbunkiat K, Wilson D, Kasemsap N, Tiamkao S, Jichi F, Palumbo V, Hill MD, Buchan AM, Jung S, Mattle HP, Henninger N, Werring DJ.. Leukoaraiosis, intracerebral hemorrhage, and functional outcome after acute stroke thrombolysis. Neurology 2017;88:638–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Giurgiutiu D-V, Yoo AJ, Fitzpatrick K, Chaudhry Z, Leslie-Mazwi T, Schwamm LH, Rost NS.. Severity of leukoaraiosis, leptomeningeal collaterals, and clinical outcomes after intra-arterial therapy in patients with acute ischemic stroke. J Neurointerv Surg 2015;7:326–330. [DOI] [PubMed] [Google Scholar]
- 12. Tan IYL, Demchuk AM, Hopyan J, Zhang L, Gladstone D, Wong K, Martin M, Symons SP, Fox AJ, Aviv RI, et al. CT angiography clot burden score and collateral score: correlation with clinical and radiologic outcomes in acute middle cerebral artery infarct. AJNR Am J Neuroradiol 2009;30:525–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Liu X, Zhang J, Tian C, Wang J.. The relationship of leukoaraiosis, haemorrhagic transformation and prognosis at 3 months after intravenous thrombolysis in elderly patients aged ≥ 60 years with acute cerebral infarction. Neurol Sci 2020;41:3195–3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mutzenbach JS, Müller-Thies-Broussalis E, Killer-Oberpfalzer M, Griessenauer CJ, Hecker C, Moscote-Salazar LR, Paradaiser P, Pikija S.. Severe leukoaraiosis is associated with poor outcome after successful recanalization of M1 middle cerebral artery occlusion strokes. Cerebrovasc Dis 2020;49:253–261. [DOI] [PubMed] [Google Scholar]
- 15. Jung S, Wiest R, Gralla J, McKinley R, Mattle H, Liebeskind D.. Relevance of the cerebral collateral circulation in ischaemic stroke: time is brain, but collaterals set the pace. Swiss Med Wkly 2017;147:w14538. [DOI] [PubMed] [Google Scholar]
- 16. Dankbaar JW, Bienfait HP, van den Berg C, Bennink E, Horsch AD, van Seeters T, van der Schaaf IC, Kappelle LJ, Velthuis BK; on behalf of the DUST Investigators. Wake-up stroke versus stroke with known onset time: clinical and multimodality CT imaging characteristics. Cerebrovasc Dis 2018;45:236–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nannoni S, Strambo D, Sirimarco G, Amiguet M, Vanacker P, Eskandari A, Saliou G, Wintermark M, Dunet V, Michel P.. Eligibility for late endovascular treatment using DAWN, DEFUSE-3, and more liberal selection criteria in a stroke center. J Neurointerv Surg 2020;12:842–847. [DOI] [PubMed] [Google Scholar]
- 18. Nguyen TN, Abdalkader M, Nagel S, Qureshi MM, Ribo M, Caparros F, Haussen DC, Mohammaden MH, Sheth SA, Ortega-Gutierrez S, Siegler JE, Zaidi S, Olive-Gadea M, Henon H, Möhlenbruch MA, Castonguay AC, Nannoni S, Kaesmacher J, Puri AS, Seker F, Farooqui M, Salazar-Marioni S, Kuhn AL, Kaliaev A, Farzin B, Boisseau W, Masoud HE, Lopez CY, Rana A, Kareem SA, Sathya A, Klein P, Kassem MW, Ringleb PA, Cordonnier C, Gralla J, Fischer U, Michel P, Jovin TG, Raymond J, Zaidat OO, Nogueira RG.. Noncontrast computed tomography vs computed tomography perfusion or magnetic resonance imaging selection in late presentation of stroke with large-vessel occlusion. JAMA Neurol 2021;79:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. García-Tornel A, Carvalho V, Boned S, Flores A, Rodríguez-Luna D, Pagola J, Muchada M, Sanjuan E, Coscojuela P, Juega J, Rodriguez-Villatoro N, Menon B, Goyal M, Ribó M, Tomasello A, Molina CA, Rubiera M.. Improving the evaluation of collateral circulation by multiphase computed tomography angiography in acute stroke patients treated with endovascular reperfusion therapies. Interv Neurol 2016;5:209–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Additional data are available from the corresponding author on reasonable request.