Abstract
Many scoring systems for predicting the outcomes of patients with acute coronary syndrome (ACS) have been proposed. In some populations, a significant reduction in length of hospital stay may be achieved without compromising patient prognoses. However, the use of such scoring systems in clinical practice is limited. The aim of this study was to propose a universal list of predictors that can identify low-risk ACS patients who may be eligible for an earlier hospital discharge without increased short-term risk for major adverse cardiac events. A cohort of 1420 patients diagnosed with ACS were enrolled into a single-centre registry between October 2018 and December 2020. Clinical, laboratory, echocardiographic, and angiographic measurements were taken for each patient and entered into the study database. Using retrospective univariant analyses of patients treated with percutaneous coronary intervention (PCI) (n = 932), we compared each predictor to 30-day mortality rate using the Czech national registry of dead people. Eleven predictors correlate significantly with 30-day survival: age <80 years, ejection fraction >50%, no cardiopulmonary resuscitation, no mechanical ventilation needed, Killip class I at admission, haemoglobin levels >110 g/L while hospitalized, successful PCI procedure(s), no residual stenosis over 90%, Thrombolysis in Myocardial Infarction 3 flow after PCI, no left main stem disease, and no triple-vessel coronary artery disease. In all, presence of all predictors applies to 328 patients (35.2% of the cohort), who maintained a 100% survival rate at 30 days. A combination of clinical, echocardiographic, and angiographic findings provides valuable information for predicting the outcomes of patients with all types of ACS. We created a simple, useful tool for selecting low-risk patients eligible for early discharge.
Keywords: Acute coronary syndrome, Prognosis, Predictors, Low risk, Early discharge
Introduction
Attempts to define risk factors correlated with adverse events in patients with acute coronary syndrome (ACS) have been made for decades. Since Califf et al.1 first presented the Angina Score in the 1980s, many scoring systems and calculators using biomarkers to predict the probability of major adverse cardiac events (MACEs) have been proposed.
Current guidelines support the Thrombolysis in Myocardial Infarction (TIMI) and Global Registry of Acute Coronary Events (GRACE) scoring systems, although the latter is based on patients treated mainly by thrombolysis.2–6 As percutaneous coronary intervention (PCI) has become a golden standard in the treatment of ACS, new scoring systems have emerged. However, their clinical use remains very limited, perhaps due to complicated assessment, need of special calculators and further diversification based on subtypes of ACS.
An ability to predict patient outcome gives professionals a tool for adjusting their therapeutic approach. Patients at low risk for MACEs may benefit from earlier rehabilitation and a subsequently shorter hospitalization time without increasing risk of death or recurrent ACS.7 Another benefit is efficient utilization of hospital resources.8,9 We hypothesize that precisely selected clinical parameters adjusted to modern therapeutical approaches could be used as predictors to identify low-risk patients.
To this end, we propose a list of predictors associated with favourable outcome based on data from a high-volume cardiovascular centre in central Europe. Reflecting current clinical practice, we were able to select low-risk ACS patients, who may be eligible for early discharge without a risk for premature death or recurrent ACS.
Methods
We created a prospective registry of patients with ACS admitted to the University Hospital Královské Vinohrady Cardiocentre, Prague, Czech Republic, in September 2018. The registry consists of all consecutively admitted patients with ACS since October 2018. We defined ACS types based on the European guidelines for defining acute myocardial infarction (MI) with ST-segment elevation MI (STEMI) and guidelines for ACS without ST elevation [non-ST-elevation-acute coronary syndrome (NSTE-ACS)].4,5 When the time of symptom onset to first medical contact including electrocardiogram results with typical features exceeded 24 h, patients were characterized as having subacute STEMI. Patients with takotsubo cardiomyopathy were not assigned to either the STEMI group or the NSTE-ACS group. There were no exclusion criteria for patients admitted with a final diagnosis of ACS. For each patient, we collected data on clinical characteristics and angiographic, laboratory, and therapeutic findings. Medical documentation and reports served as the foundation for clinical data and in-hospital outcomes. Angiographic data were exported from dedicated software for angiography and from medical PCI descriptions. Data were validated by cross-controls between data sources and random monitoring of 100 patients by experienced physicians/researchers. The registry was approved by the local ethics committee.
The initial dataset consisted of 1420 consecutive patients with ACS admitted to our cardiology department between 1 October 2018 and 31 December 2020. Importantly, we included all patients admitted between these dates, and no exclusion criteria were applied. Therefore, our register truly reflects clinical practice.
Based on previous research and clinical experience, we defined 27 clinical, echocardiographic, and angiographic variables. 2,3,9–14 The main goal was to determine which variables were best correlated with 30-day survival and to select low-risk patients. Selective coronarography with subsequent PCI and the availability of data for all 27 variables were necessary for further evaluation of each patient.
Cardiopulmonary resuscitation (CPR) and mechanical ventilation were documented at admission. A Killip class was assigned to each patient based on clinical status at admission. Regarding laboratory results such as glycaemia (<15 mmol/L) and haemoglobin (≥110 g/L), we evaluated a single value during hospitalization (highest and lowest, respectively) to meet the criterion. Results of glomerular filtration were based on laboratory results before discharge for accurate prognostic value. For ejection fraction, we collected the lowest echocardiography value during hospitalization. Angiography variables were collected based on selective coronarography and subsequent PCI results. The requirement for a successful PCI was TIMI 3 flow post-PCI, adequate stent expansion, no dissection, and no occlusion of the side branch.
In 932 ACS patients, all of the above-mentioned criteria were available. We evaluated the 30-day survival rate of these patients in cooperation with the Czech statistical office. Using the univariant analyses, we compared each variable to 30-day outcome. For each variable, P < 0.05 was considered statistically significant. Of note, in this article, survival rate always refers to the 30-day follow-up period.
Results
Patient characteristics
Of 932 patients eligible for further evaluation, 401 (43.2%) were admitted for STEMI and 528 (56.6%) for NSTE-ACS. In the NSTE-ACS subgroup, there were 142 (15.3%) unstable angina pectoris and 386 (41.5%) non-ST-elevation MI (NSTEMI) patients. Detailed characteristics of the population are shown in Table 1. Interestingly, for both types of ACS, there was a very similar proportion of patients who required mechanical ventilation (6.2% in STEMI and 4.7% in NSTE-ACS) and CPR (7.0% in STEMI and 4.5% in NSTE-ACS).
Table 1.
Patients’ characteristic
| NSTE-ACS (n = 528) | STEMI (n = 401) | P-Value | |
|---|---|---|---|
| Age | 69.4 ± 12.1 | 65.2 ± 12.6 | <0.0001 |
| Sex | 0.07 | ||
| Men | 360 (68.2%) | 254 (63.3%) | |
| Women | 168 (31.8%) | 147 (36.7%) | |
| History of PCI | 150 (28.4%) | 53 (13.2%) | <0.0001 |
| History of MI | 163 (30.9%) | 56 (14.0%) | <0.0001 |
| History of CABG | 88 (16.7%) | 12 (3.0%) | <0.0001 |
| History of stroke | 57 (10.8%) | 31 (7.7%) | 0.125 |
| Hypertension | 403 (76.3%) | 228 (56.9%) | <0.0001 |
| Hyperlipidemia | 240 (45.5%) | 128 (31.9%) | <0.0001 |
| Diabetes | 0.176 | ||
| Diet | 26 (4.9%) | 26 (6.5%) | |
| Oral antidiabetic drugs | 104 (19.7%) | 64 (16.0%) | |
| Insulin dependent | 53 (10.0%) | 27 (6.7%) | |
| Peripheral artery disease | 68 (12.9%) | 26 (6.5%) | 0.001 |
| ECG—rhytm at admission | 0.001 | ||
| Sinus rhytm | 438 (83.0%) | 359 (89.5%) | |
| Atrial fibrilation/flutter | 59 (11.2%) | 27 (6.7%) | |
| Other rhytm | 11 (2.1%) | 4 (1.0%) | |
| Pacemaker | 19 (3.6%) | 5 (1.2%) | |
| Killip | <0.0001 | ||
| I | 437 (82.8%) | 320 (79.8%) | |
| II | 42 (8.0%) | 32 (8.0%) | |
| III | 25 (4.7%) | 9 (2.2%) | |
| IV | 24 (4.5%) | 40 (10.0%) | |
| Mechanical ventilation | 25 (4.7%) | 25 (6.2%) | 0.602 |
| Cardiopulmonary resuscitation | 24 (4.5%) | 28 (7.0%) | 0.271 |
| Ejection fraction (%) | 50.0 ± 11.8 | 43.5 ± 10.8 | <0.0001 |
| Number of vessel diseased | 0.039 | ||
| Single-vessel disease | 167 (31.6%) | 164 (40.9%) | |
| Two-vessel disease | 159 (30.1%) | 114 (28.4%) | |
| Three-vessel disease | 199 (37.7%) | 122 (30.4%) | |
| TIMI 3 flow post-PCI | 483 (91.5%) | 342 (85.3%) | <0.0001 |
General characteristic of patients with ACS eligible for further evaluation.
ACS, acute coronary syndrome; CABG, coronary artery bypass graft; ECG, electrocardiogram; MI, myocardial infarction; NSTE, non-ST elevation; PCI, percutaneous coronary intervention; STEMI, ST-segment elevation myocardial infarction; TIMI, thrombolysis in myocardial infarction.
Subsequently, we evaluated the survival rate of 932 patients. At 30-day follow-up, the survival rate was 94.5% (n = 872). Overall, 91.3% (n = 366) survived in the STEMI subgroup and 95.3% (n = 503) survived in the NSTE-ACS subgroup.
Survival predictors
We used simple univariant analyses without distinguishing among ACS types to evaluate the correlation between each variable and 30-day survival. Out of 27 variables, 13 showed significant associations with patient outcome (Table 2). Presence of single variable predicted the 30-day survival in >94% of patients. This is strong evidence of a correlation with prognosis.
Table 2.
Predictors with significant association to 30-day survival
| Criteria | Incidence | 30-Day survival rate | P-Value |
|---|---|---|---|
| Age <80 years | 81.0% (755) | 95.0% (717) | <0.0001 |
| No CPR necessary | 91.7% (855) | 95.4% (816) | <0.0001 |
| No mechanical ventilation necessary | 92.3% (860) | 95.8% (824) | <0.0001 |
| Killip class I | 81.4% (759) | 97.4% (739) | <0.0001 |
| Ejection fraction ≥50% | 76.4% (712) | 96.9% (690) | <0.0001 |
| Successful PCI | 90.5% (843) | 94.2% (794) | 0.021 |
| TIMI 3 after PCI | 88.8% (828) | 94.8% (785) | <0.0001 |
| No left main stem disease | 93.8% (874) | 94.3% (824) | 0.003 |
| Glycaemia <15 mmol/L | 89.8% (837) | 95.8% (802) | <0.0001 |
| Haemoglobin >110 g/L during hospitalization | 86.5% (806) | 94.7% (763) | 0.001 |
| Glomerular filtration >60 mL/s | 83.9% (782) | 94.8% (741) | 0.001 |
| No significant residual stenosis (>90%) | 91.1% (849) | 95.5% (798) | 0.076 |
| Single- or two-vessel disease | 64.9% (605) | 96% (581) | 0.0001 |
Each criterium was applied to subgroup of 932 patients. Univariant analysis shows percentage of patients meeting particular criterium, association to 30-day survival, and statistical significance.
CPR, cardiopulmonary resuscitation; PCI, percutaneous coronary intervention; TIMI, thrombolysis in myocardial infarction.
Low-risk patient selection
Patients who fulfilled criteria for all 13 clinical predictors associated with 30-day survival were identified as low risk. Of 932 patients, we identified 328 (35.2%) low-risk patients (Figure 1); these results suggest that assessment of these predictors in clinical practice would identify a significant proportion of low-risk patients. Retrospective analyses indicated that the presence of all criteria was associated with a 100% survival rate at 30 days (Table 3).
Figure 1.
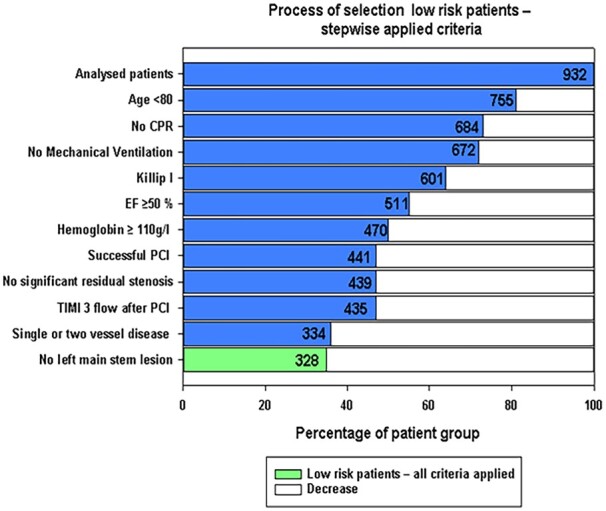
Process of selection low risk patients - stepwise applied criteria.
Table 3.
Suggested protocol for selecting low-risk patients
| ✓ | Age <80 years old |
| ✓ | Killip I at admission |
| ✓ | No cardiopulmonary resuscitation and no mechanical ventilation needed |
| ✓ | Successful PCI without complication |
| ✓ | TIMI 3 flow post-PCI without significant (90%) residual stenosis |
| ✓ | No left main stem lesion and/or three-vessel disease |
| ✓ | Without presence of nonsustained ventricular tachycardia >24 h from revascularization |
| ✓ | Ejection fraction ≥50% |
| ✓ | Haemoglobin >110 g/L during hospitalization |
| ✓ | Fully self-sufficient patient with stable social background. Preferably personal contact once a day with another person |
PCI, percutaneous coronary intervention; TIMI, thrombolysis in myocardial infarction.
Discussion
Although patients with all types of ACS are treated using invasive strategies, the risks for death and/or severe complications vary. Several prognostic scores (PSs) for risk stratification of patients with ACS have been introduced; however, they cannot be universally applied. In addition, the majority of registries focus on specific subtype of ACS, which complicates further risk stratification. 2,3,10–12,14
From a prognostic point of view, current European Society of Cardiology (ESC) guidelines recommend the GRACE risk score for estimating the outcomes of patients with ACS.5,6 Additional prognostic information may provide high sensitive troponin and B-type natriuretic peptide or N-terminal-pro hormone B-type natriuretic peptide.6 Nevertheless, there are few notable PSs that evaluate patient outcome and reflect modern therapeutic strategies including PCI.
The Zwolle risk score, which was developed using data from 1791 STE-ACS patients who underwent primary coronary angioplasty, is able to define low-risk patients.12 In that study, six variables were associated with a very low mortality rate: Killip class, age, postprocedural TIMI flow, presence or absence of three-vessel disease, anterior MI, and ischaemic time over 4 h. Cohort patients (n = 1315, 73.4%) had a low mortality rate at 2 days (0.1%) and between 3 and 10 days (0.2%), as well as a low malignant arrhythmia risk (0.2% risk VT/VF) at 48 h. Although the results were promising for selecting patients suited for early discharge, severe contraindications were found in 16.6% (n = 218) of patients (heart failure, malignant arrhythmia or AV block, pericardial effusion, cardiac surgery, intra-aortic balloon pump, fever, reocclusion, renal insufficiency), which prolonged hospitalization.
Another simple risk scoring system for predicting mortality after MI treated by PCI is the CADILLAC risk score, proposed in 2005.13 It uses seven variables weighted proportionally based on odds ratios (age, Killip class, baseline left ventricular ejection fraction, anaemia, renal insufficiency, triple-vessel disease, and post-procedural TIMI flow grade) and was the first to point out the importance of left ventricular ejection fraction upon presentation, which turns out to be the single most powerful predictor of survival. In the study that proposed the scoring system, more than half (56.5%) of the patients were identified to be low risk, with 0.1% 30-day and 0.8% 1-year mortality rates. However, the trial database had strict exclusion criteria such as symptoms ≤12 h, cardiogenic shock, failed thrombolytic therapy, requirement for multivessel PCI, bleeding diathesis and severe comorbidities with a life expectancy of <1 year. 13
The ACUITY-PCI risk score, published in 2012, is a complex scoring system that combines clinical, laboratory, electrocardiographic, and angiographic findings of NSTE-ACS patients.14 By evaluating the angiographic complexity of lesions (extent of lesions, small/diffuse coronary artery disease, and presence of bifurcation lesions), baseline ST-segment deviation or cardiac troponin elevation, presence of insulin-treated diabetes, and renal insufficiency (characterized as creatinine clearance < 60 mL/min determined using the Cockcroft–Gault equation) patients can be divided into three groups. In the study introducing this score, both derivation (1692 patients) and validation (846 patients) cohorts had patients in the lower tertile with similar outcomes in 1-year mortality/MI (5.3% and 5.1%, respectively). 14
The very promising results of these abovementioned studies are a cornerstone of our analysis. The correct combination of clinical, laboratory, echocardiographic, and interventional data can provide sufficient information for successful selection of low-risk patients. However, some crucial differences need to be settled.
We focused on simplifying the selection process by not differentiating between STE-ACS and NSTE-ACS patients. The only other previous system that included both types of ACS is the CADILLAC score; however, that system applies very strict exclusion criteria, which limits its use in heterogenous populations. Unlike previous systems, we also do not weight criteria differently, which can be confusing and discourage further use. Instead, we use a simple binary system according to the presence or absence of variables.
One of the main strengths of our model is that no ACS patients are excluded, which truly reflects every day practice.
Although our population was composed of fewer patients than in the previously mentioned scores (except PAMI risk score), we report a very promising 30-day survival rate of 100%.
Zwolle risk score identified 73.4% (1315) of patients as a low risk with 98.9% 30-day survival rate.12 There were 56.5% (1176) of low-risk patients in CADILLAC risk score with 30-day survival rate of 99.9% and 99.8% (in derivation and validation set respectively).13 According to PAMI risk score, 68 patients (46%) were defined as low risk. There was 97.1% 30-day survival rate among this subgroup.11
In the most recent one, ACUITY-PCI score, patients in lower tertile (≤12 points) had 97.5–97% 30-day survival rate (derivation and validation cohort respectively).14 Our list of predictors show excellent results in comparison with risk scores designed for population with ACS treated by PCI.
Patients identified as low risk may be eligible for earlier discharge, resulting in numerous benefits. Longer hospitalization stay is associated with increased levels of anxiety,15 which is correlated with worse prognosis (potentially due to impaired vagal control, increased sympathetic outflow, reduced heart rate variability, and baroreflex reactivity16–18). In addition, the economic burden associated with ACS is not negligible.7,8
According to ESC guidelines, low-risk STEMI patients may be discharged earlier (after a minimum of 48 h of monitoring). However, no specifics are described for the process of selecting these patients.5 NSTE-ACS patients with low arrhythmia risk need to be monitored for up to 24 h or until PCI (whichever comes first), and monitoring should be prolonged (>24 h) if the risk for arrhythmia is increased. However, the minimal/recommended hospitalization time is not specified.6
Therefore, we believe that shortening hospitalization stay to 48–72 h in eligible patients with ACS is beneficial, and this list of predictors associated with favourable outcome is useful tool for the proper selection of such patients.
Limitations
This list of predictors is based on a single centre’s retrospectively analysed dataset, containing a limited number of patients. The analyses were performed in patients undergoing invasive therapy and treated by PCI, the most common treatment strategy. The risk scale should be applied to this specific subset of patients. Further prospective studies on larger patient cohorts are needed, preferably in cooperation with multiple centres to acquire more heterogenous data. Although excellent 30-day outcomes were achieved in our study, there is a need for further evaluation of the long-term (1-year) prognosis of these low-risk patients.
Conclusion
We are confident that our list of predictors may be used, in accordance with guidelines, to minimize hospitalization stay in selected patients. Based on our dataset, nearly one-third of patients (32.5%) were suitable for early discharge (within 48–72 h), without compromising short-term prognoses. The great advantage is its simplicity. It is easy to use, no calculators or dedicated programs are needed, yet provides excellent results in comparison to more complicated scoring systems.
Acknowledgements
The English in this document has been checked by at least two professional editors, both native speakers of English. For a certificate, please see: http://www.textcheck.com/certificate/W6cW3T. Authors acknowledge Petr Tuma, MD. for his support in data analysis.
Funding
Supported by project Interventional treatment of life-threatening cardiovascular diseases – INTERCARDIS, project EU Nr. CZ.02.1.01/0.0/0.0/16_026/0008388.
Conflict of interest: VK reports consultant contracts, lecture fees from Philips, Medtronic, and BBraun. PT reports consultant contracts, lecture fees from Medtronic and BBraun. All other authors have no potential conflict of interest.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
- 1. Califf RM, Mark DB, Harrell FE, Hlatky MA, Lee KL, Rosati RA, Pryor DB.. Importance of clinical measures of ischemia in the prognosis of patients with documented coronary artery disease. J Am Coll Cardiol 1988;11:20–26. [DOI] [PubMed] [Google Scholar]
- 2. Morrow DA, Antman EM, Charlesworth A, Cairns R, Murphy SA, de Lemos JA, Giugliano RP, McCabe CH, Braunwald E.. TIMI risk score for ST-elevation myocardial infarction: a convenient, bedside, clinical score for risk assessment at presentation: an Intravenous nPA for Treatment of Infarcting Myocardium Early II trial substudy. Circulation 2000;102:2031–2037. [DOI] [PubMed] [Google Scholar]
- 3. Antman EM, Cohen M, Bernink PJLM, McCabe CH, Horacek T, Papuchis G, Mautner B, Corbalan R, Radley D, Braunwald E.. The TIMI risk score for unstable angina/non-ST elevation MI: a method for prognostication and therapeutic decision making. J Am Med Assoc 2000;284:835–842. [DOI] [PubMed] [Google Scholar]
- 4. Tang EW, Wong CK, Herbison P.. Global Registry of Acute Coronary Events (GRACE) hospital discharge risk score accurately predicts long-term mortality post acute coronary syndrome. Am Heart J 2007;153:29–35. [DOI] [PubMed] [Google Scholar]
- 5. Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, Caforio ALP, Crea F, Goudevenos JA, Halvorsen S, Hindricks G, Kastrati A, Lenzen MJ, Prescott E, Roffi M, Valgimigli M, Varenhorst C, Vranckx P, Widimský P; ESC Scientific Document Group. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J 2018;39:119–177.28886621 [Google Scholar]
- 6. Collet J-P, Thiele H, Barbato E, Barthélémy O, Bauersachs J, Bhatt DL, Dendale P, Dorobantu M, Edvardsen T, Folliguet T, Gale CP, Gilard M, Jobs A, Jüni P, Lambrinou E, Lewis BS, Mehilli J, Meliga E, Merkely B, Mueller C, Roffi M, Rutten FH, Sibbing D, Siontis GCM, Kastrati A, Mamas MA, Aboyans V, Angiolillo DJ, Bueno H, Bugiardini R, Byrne RA, Castelletti S, Chieffo A, Cornelissen V, Crea F, Delgado V, Drexel H, Gierlotka M, Halvorsen S, Haugaa KH, Jankowska EA, Katus HA, Kinnaird T, Kluin J, Kunadian V, Landmesser U, Leclercq C, Lettino M, Meinila L, Mylotte D, Ndrepepa G, Omerovic E, Pedretti RFE, Petersen SE, Petronio AS, Pontone G, Popescu BA, Potpara T, Ray KK, Luciano F, Richter DJ, Shlyakhto E, Simpson IA, Sousa-Uva M, Storey RF, Touyz RM, Valgimigli M, Vranckx P, Yeh RW, Barbato E, Barthélémy O, Bauersachs J, Bhatt DL, Dendale P, Dorobantu M, Edvardsen T, Folliguet T, Gale CP, Gilard M, Jobs A, Jüni P, Lambrinou E, Lewis BS, Mehilli J, Meliga E, Merkely B, Mueller C, Roffi M, Rutten FH, Sibbing D, Siontis GCM; ESC Scientific Document Group. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J 2020;42:1289–1367. [DOI] [PubMed] [Google Scholar]
- 7. Yndigegn T, Gilje P, Dankiewicz J, Mokhtari A, Isma N, Holmqvist J, Schiopu A, Ravn-Fischer A, Hofmann R, Szummer K, Jernberg T, James S, Gale CG, Fröbert O, Mohammad MM.. Safety of Early hospital discharge following admission with ST-elevation myocardial infarction treated with percutaneous coronary intervention: a nationwide cohort study. EuroIntervention 2022;17:1091–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhao Z, Winget M.. Economic burden of illness of acute coronary syndromes: medical and productivity costs. BMC Health Serv Res 2011;11:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wasfy JH, Kennedy KF, Masoudi FA, Ferris TG, Arnold SV, Kini V, Peterson P, Curtis JP, Amin AP, Bradley SM, French WJ, Messenger J, Ho PM, Spertus JA.. Predicting length of stay and the need for postacute care after acute myocardial infarction to improve healthcare efficiency. Circ Cardiovasc Qual Outcomes 2018;11:e004635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Boersma E, Pieper KS, Steyerberg EW, Wilcox RG, Chang WC, Lee KL, Akkerhuis KM, Harrington RA, Deckers JW, Armstrong PW, Lincoff AM, Califf RM, Topol EJ, Simoons ML.. Predictors of outcome in patients with acute coronary syndromes without persistent ST-segment elevation: results from an international trial of 9461 patients. Circulation 2000;101:2557–2567. [DOI] [PubMed] [Google Scholar]
- 11. Addala S, Grines CL, Dixon SR, Stone GW, Boura JA, Ochoa AB, Pellizzon G, O'Neill WW, Kahn JK.. Predicting mortality in patients with ST-elevation myocardial infarction treated with primary percutaneous coronary intervention (PAMI risk score). Am J Cardiol 2004;93:629–632. [DOI] [PubMed] [Google Scholar]
- 12. De Luca G, Suryapranata H, van’t Hof AWJ, de Boer M-J, Hoorntje JCA, Dambrink J-HE, Gosselink ATM, Ottervanger JP, Zijlstra F.. Prognostic assessment of patients with acute myocardial infarction treated with primary angioplasty: implications for early discharge. Circulation 2004;109:2737–2743. [DOI] [PubMed] [Google Scholar]
- 13. Halkin A, Singh M, Nikolsky E, Grines CL, Tcheng JE, Garcia E, Cox DA, Turco M, Stuckey TD, Na Y, Lansky AJ, Gersh BJ, O’Neill WW, Mehran R, Stone GW.. Prediction of mortality after primary percutaneous coronary intervention for acute myocardial infarction: the CADILLAC risk score. J Am Coll Cardiol 2005;45:1397–1405. [DOI] [PubMed] [Google Scholar]
- 14. Palmerini T, Genereux P, Caixeta A, Cristea E, Lansky A, Mehran R, Della Riva D, Fahy M, Xu K, Stone GW.. A new score for risk stratification of patients with acute coronary syndromes undergoing percutaneous coronary intervention: the ACUITY-PCI (Acute Catheterization and Urgent Intervention Triage Strategy-Percutaneous Coronary Intervention) risk score. JACC: Cardiovasc Interv 2012;5:1108–1116. [DOI] [PubMed] [Google Scholar]
- 15. Shoar S, Naderan M, Aghajani M, Sahimi-Izadian E, Hosseini-Araghi N, Khorgami Z.. Prevalence and determinants of depression and anxiety symptoms in surgical patients. Oman Medi J 2016;31:176–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kawachi I, Sparrow D, Vokonas PS, Weiss ST.. Decreased heart rate variability in men with phobic anxiety (data from the normative aging study). Am J Cardiol 1995;75:882–885. [DOI] [PubMed] [Google Scholar]
- 17. Strik JJMH, Denollet J, Lousberg R, Honig A.. Comparing symptoms of depression and anxiety as predictors of cardiac events and increased health care consumption after myocardial infarction. J Am Coll Cardiol 2003;42:1801–1807. [DOI] [PubMed] [Google Scholar]
- 18. Shibeshi WA, Young-Xu Y, Blatt CM.. Anxiety worsens prognosis in patients with coronary artery disease. J Am Coll Cardiol 2007;49:2021–2027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.


