Abstract
Introduction
Physical exercise and cognitive training have the potential to enhance cognitive function and mobility in older adults at risk of Alzheimer’s disease and related dementia (ADRD), but little is known about the feasibility of delivering multidomain interventions in home settings of older adults at risk of ADRD. This study aims to assess the feasibility of home-based delivery of exercise and cognitive interventions, and to evaluate the relationship between participants’ intervention preferences and their subsequent adherence. Secondary objectives include the effect of the interventions on ADRD risk factors, including frailty, mobility, sleep, diet and psychological health.
Methods and analysis
The SYNchronising Exercises, Remedies in GaIt and Cognition at Home (SYNERGIC@Home) feasibility trial is a randomised control trial that follows a 2×2 factorial design, with a 16-week home-based intervention programme (3 sessions per week) of physical exercises and cognitive training. Participants will be randomised in blocks of four to one of the following four arms: (1) combined exercise (aerobic and resistance)+cognitive training (NEUROPEAK); (2) combined exercise+control cognitive training (web searching); (3) control exercise (balance and toning)+cognitive training; and (4) control exercise+control cognitive training. SYNERGIC@Home will be implemented through video conferencing. Baseline and post-intervention assessments at 4-month and 10-month follow-up will include measures of cognition, frailty, mobility, sleep, diet and psychological health. Primary feasibility outcome is adherence to the interventions. Primary analytic outcome is the relationship between pre-allocation preference for a given intervention and subsequent adherence to the allocated intervention. A series of secondary analytic outcomes examining the potential effect of the individual and combined interventions on cognitive, mobility and general well-being will be measured at baseline and follow-up.
Ethics and dissemination
Ethics approval was granted by the relevant research ethics boards. Findings of the study will be presented to stakeholders and published in peer-reviewed journals and at provincial, national and international conferences.
Trial registration number
NCT04997681, Pre-results.
Keywords: Dementia, Physiology, GERIATRIC MEDICINE, Neuropathology
Strengths and limitations of this study.
This study is one of the first randomised controlled trials (RCTs) in Canada to establish the feasibility of fully remote recruitment, consent, assessment and delivery of bilingual, multi-domain, contactless interventions in the home for preventing dementia in at-risk older adults.
This study will also quantify the relationship between participants’ preferences for intervention type and their subsequent adherence to the interventions they were allocated to, which will provide evidence on whether alternate experimental designs that account for preference are scientifically justified.
Consistent with a feasibility study, the sample is powered for feasibility outcomes rather than cognitive and health outcomes.
The study intervention duration of 16 weeks is short but sufficient for evaluating feasibility and estimating effect sizes of cognitive and mobility outcomes using remote assessments.
Elements of the study design are consistent with a full-scale double-blind RCT, including robust screening, randomisation and allocation, comprehensive pre-assessments and post-assessments with long-term follow-up assessment and semi-structured exit interview.
Introduction
In 2015, over 46 million people lived with Alzheimer’s disease and related dementias (ADRD) worldwide, with 1 new case appearing every 4.1 s.1 The cost associated with these cases is over a trillion Canadian dollars.1–3 There is no cure for dementia.4 Recently, there has been a shift in interventional studies on ADRD to targeting pre-dementia states, such as mild cognitive impairment (MCI).5 6 The SYNchronising Exercises, Remedies in GaIt and Cognition (SYNERGIC) trial implemented a multidomain intervention study for individuals with MCI at sites across Canada7 in both English and in French. The positive results of multidomain trials like SYNERGIC,8–10 and the ensuing COVID-19 pandemic, have warranted investigation of a home-based version of the protocol that can reach a wider population of older adults.
The primary goal of the SYNERGIC at Home (SYNERGIC@Home) feasibility trial is to assess the feasibility of in-home delivery of exercise and cognitive training interventions for improving cognitive and physical functioning in older adults at risk for ADRD. Remote delivery of physical exercise interventions has been of significant interest for decades,11 12 but randomised controlled trials (RCTs) almost always happen in clinical or academic environments. Building capacity for conducting assessments and interventions in the home of older adults is now critical for ensuring safety and accessibility, with the added benefit of reaching a wider and more diverse population of at-risk older adults13 while reducing costs of programme delivery.14 Despite the convenience and lower participant burden (eg, travel to and from clinic), adherence to interventions delivered remotely suffer the same threats to continued participation as traditional delivery methods,15 such as negative outcome expectation16 and time constraints.17 Challenges arising from the use of computer and internet technology may not be significant barrier for younger adults,18 but little is known about how well an older population with or at risk of cognitive decline will adhere to a virtual delivery environment.
There is a growing interest in understanding the impact of preference on clinical trial participation19 and novel designs have been proposed that incorporate preference (practitioner and/or patient)20 21 that could improve accrual rates and generalisability of results. Although the concept of preference trials has been around since the 1990s, these studies have focused on trial designs and randomisation schemes, where preference is a treatment arm and not a measured outcome. Therefore, the analytic aim of this feasibility trial is to assess if participant’s pre-allocation preference for different types of interventions is related to their subsequent adherence to the interventions allocated to them. The landmark Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability10 supports the efficacy of multidomain interventions, but to date no studies have examined if preference plays a role in adherence to those interventions. Our study will inform whether a future preference trial design is warranted for multidomain brain health interventions.
Rationale for the SYNERGIC@HOME interventions
Aerobic exercise (AE) and progressive resistance training (RT) have been shown to improve cognition, physical capacity and mobility in older adults.22–25 Both AE26 and RT27 trials have reported positive results in improving cognitive performance, with effects lasting more than 3 months.22 28 Given the potential benefits of combining both types of exercise, we will deliver a combined (AE+RT) progressive exercise programme as our active exercise intervention. The control exercise will include balance and toning (BAT) exercises with equivalent time exposure but no progression. While evidence exists that BAT exercises can improve gait stability29 and strength,30 their effect on cognition is not demonstrated.31
The rationale for adding cognitive training stems from a plethora of recent research suggesting that improvements in brain plasticity occur after cognitive training,32–34 and from the potential synergistic effect of combining it with physical exercise. Both simultaneous and sequential exercise and cognitive training have been shown efficacious for improving cognition35 in older adults; SYNERGIC@Home adopts a sequential approach. Active cognitive training will be delivered using the NEUROPEAK programme, which consists of a dual-task cognitive training regimen designed by our group. NEUROPEAK has been shown to improve balance,36 mobility33 and cognition37 38 in healthy older adults. The control cognitive training will involve basic web searching and watching videos (WS+V), which is expected to have a minimal effect on cognition or mobility.
Finally, 16-week interventions of exercise and cognitive training has been conducted in previous studies in a clinical environment, which has been shown to give significant and promising results,39 40 however, has not been tested virtually in a home setting.
Primary objectives and research questions
Our primary feasibility objective will measure adherence to interventions to answer the question: will community-dwelling older adults adhere to a 16-week in-home, multidomain, supervised intervention programme to improve their health and reduce their risk of ADRD?
To determine if affinity for any one intervention is an important factor in participants’ adherence to the study interventions, we designed the Intervention Preference Questionnaire (see online supplemental appendix A) that will be used to answer the following questions:
bmjopen-2021-059988supp001.pdf (2.3MB, pdf)
Relation to adherence: is adherence correlated with receiving the active treatment they prefer as indicated by their pre-allocation preference ratings?
Preference attitudes: which intervention type (physical exercise or cognitive training) do most participants prefer over the other? What proportion of participants have no particular preference for either intervention?
Our secondary feasibility objectives will measure recruitment rate, retention rate, trial experience, adverse events (AEs) and data loss to answer the questions, respectively: ‘how efficient is recruitment?’, ‘Do participants stay in the trial for its duration?’, ‘How satisfied are participants with the interventions?’, ‘What AEs are related to the intervention(s)?’ and ‘What is the rate of data loss when doing remote assessments?’.
Methods and analysis
Study design
SYNERGIC@Home is a home-based, double-blind, RCT, with a 4-arm full-factorial (2×2) design. It will be administered virtually through a secure online video conferencing platform. Block randomisation by 4 will be used to allocate enrolled participants into one of 4 arms, with 16 participants in each arm (experimental conditions are in bold):
Arm 1: combined exercise (AE+RT)+cognitive training (NEUROPEAK).
Arm 2: combined exercise (AE+RT)+control cognitive training (WS+V).
Arm 3: control exercise (BAT) +cognitive training (NEUROPEAK).
Arm 4: control exercise (BAT) +control cognitive training (WS+V).
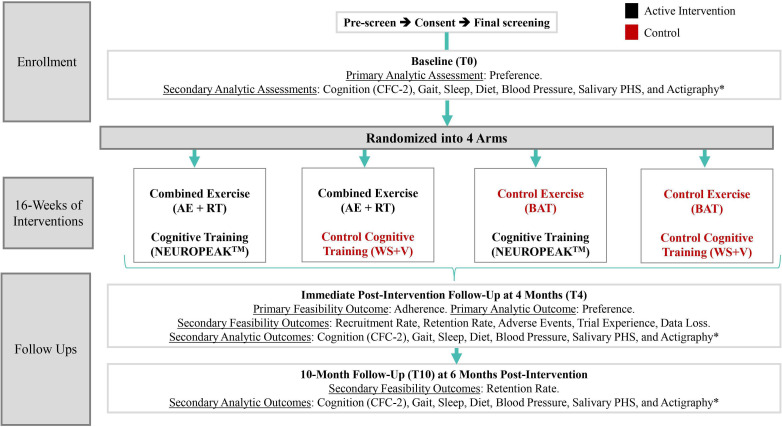
The experimental design is shown in figure 1.
Figure 1.
Design of the SYNERGIC@Home feasibility trial. *Using ActiGraph GT9X. AE, aerobic exercise; BAT, balance and toning; CFC2, Cognitive Functional Composite 2; PHS, Polygenic Hazard Score; RT, resistance training; SYNERGIC@Home, SYNchronising Exercises, Remedies in GaIt and Cognition at Home; T0, baseline; T4, 4-month follow-up; T10, 10-month follow-up; WS+V, web searching and watching videos.
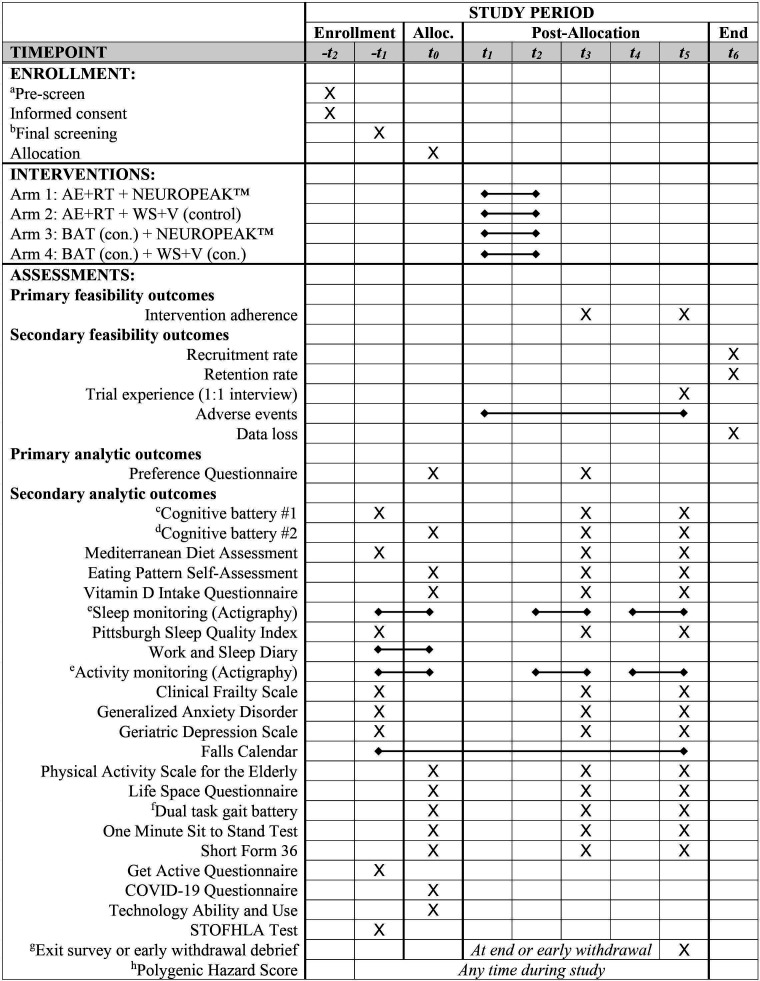
Assessments will occur at baseline (T0), 4-month (T4) and at 10-month follow-up (T10). The Standard Protocol Items: Recommendations for Interventional Trials schedule of enrolment, interventions and assessments is shown in figure 2.
Figure 2.
SPIRIT schedule of enrolment, interventions and assessments. Time points are: −t2=4 weeks prior to allocation; −t1=2 weeks prior to allocation; t0=baseline testing and allocation (T0); t1=first week of interventions; t2=last week of interventions; t3=4-month follow-up assessment (T4); t4=2 weeks prior to 10-month follow-up; t5=10-month follow-up assessment (T10). Interventions are 3× per week for 16 weeks (t1−t2). aPre-screening at –t2 consists of exclusion screening and inclusion screening not requiring assessment, such as clinical dementia status and risk. bFinal screening at –t1 consist cognitive battery #1, diet, sleep and functional risk factors used to designate participants as not demented but having MCI, SCI or CI with 2 or more risk factors. cCognitive battery #1 (–t1, t3 and t5) consists of: TCogS; full MoCA via audio–visual conference; Lawton-Brody IADL; CFC-2 consisting of ADAS-Cog 3 immediate word recall, delayed word recall, and orientation, Logical Memory I and II; CDR Scale and Cognitive Functional Activities Questionnaire. dCognitive battery #2 (t0, t3 and t5) consists of: Oral Trail Making Test (Parts A and B); Boston Naming Test; ADAS-Cog Word Recognition; DKEFS Phonemic Fluency Test and Semantic Fluency Test; Wechsler Adult Intelligence Scale (WAIS) III Digit Span Test; Digit Symbol Modalities Test–Oral Version. eSleep and activity monitoring for 10 days prior to assessment time points (−t1−t0, t2–t3 and t4–t5) using wrist worn actigraph (GT9X) monitor. fDual task gait battery (–t1, t3 and t5) consists of: usual gait, seated dual task and dual task gait counting backwards by ones, naming animals and counting backwards by sevens. gExit survey completed at the end of study or on early withdrawal when possible. hPHS biomarkers assessed via saliva sample at any time point during study. ADAS-Cog, Alzheimer’s Disease Assessment Scale-Cognitive; AE, aerobic exercise; BAT, balance and toning; CDR, Clinical Dementia Rating; CFC2, Cognitive Functional Composite 2; CI, cognitively intact; DKEFS, Delis-Kaplan Executive Function System; IADL, Instrumental Activities of Daily Living; MCI, mild cognitive impairment; MoCA, Montreal Cognitive Assessment; PHS, Polygenic Hazard Score; RT, resistance training; SCI, subjective cognitive impairment; SPIRIT, Standard Protocol Items: Recommendations for Interventional Trials; SYNERGIC@Home, SYNchronising Exercises, Remedies in GaIt and Cognition at Home; T0, baseline; T4, 4-month follow-up; T10, 10-month follow-up; TCogS, telephone cognitive screen; WS+V, web searching and watching videos.
Participants and setting
Sixty-four older adults (aged 60–90 years) at risk of developing ADRD, who live in the province of New Brunswick, Canada, and meet the inclusion and exclusion criteria will be recruited by study staff not involved in the participant’s ongoing care. Participants will include francophone and anglophone and geographical recruitment areas will be both rural and urban. All intervention activity will take place in the participant’s home.
Inclusion criteria
Aged 60–90 years.
Has a family physician/nurse practitioner.
Has internet access and basic technology ability (able to send and receive emails).
Resides in their own home/apartment.
Has access to a home computer and/or a laptop computer device.
Self-reported levels of proficiency in English and/or French for reading, speaking and writing.
Able to comply with scheduled home-based assessments and interventions.
Able to ambulate at least 10 months independently with or without a walking aid.
At risk of developing dementia (see table 1 and online supplemental appendix B): (1) MCI, (2) subjective cognitive impairment (SCI), (3) cognitively intact (CI) with two or more of the following risk factors: obesity, hypertension, diabetes, cardiovascular disease, physical inactivity, first-degree family history of dementia, dyslipidaemia, poor sleep and poor diet
Deemed safe by the study physician to participate in exercise.31
Preserved activities of daily living (score of >14/23 on the Lawton-Brody Instrumental Activities of Daily Living (IADL) Scale.41
Table 1.
CCNA criteria for CI with risk factors, and SCI and MCI from COMPASS-ND58
| Group | Core diagnostic criteria | Operationalised as |
| CI with risk factors | The absence of SCI and/or MCI based on below definitions, with two or more known risk factors for dementia | Not having SCI or MCI, and having at least 2 of the following risk factors:
|
| SCI59 | Self-experienced persistent decline in cognitive capacity in comparison with a previously normal status and unrelated to an acute event | Answer ‘yes’ to both of the following questions: ‘Do you feel like your memory or thinking is becoming worse?’ and ‘Does this concern you?’ |
| Normal age-adjusted, sex-adjusted and education-adjusted performance on standardised cognitive tests, which are used to classify MCI or prodromal AD | Global CDR scale=0, Logical Memory II above ADNI education-adjusted cutoffs (≥9 for 16+ years of education, ≥5 for 8–15 years of education and ≥3 for 0–7 years of education); ADAS-Cog word list recall score >5; MoCA total score ≥25 | |
| MCI5 | Concern regarding a change in cognition | Report from patient and/or informant of such |
| Impairment in one or more cognitive domains | One or more of the following:
|
|
| Preservation of independence in functional abilities | Score >14/23 on the Lawton-Brody IADL Scale | |
| Not demented | Global CDR ≤0.5 |
AD, Alzheimer’s disease; ADAS-Cog, Alzheimer’s Disease Assessment Scale-Cognitive; ADNI, Alzheimer’s Disease Neuroimaging Initiative; CCNA, Canadian Consortium on Neurodegeneration in Aging; CDR, Clinical Dementia Rating; CI, cognitively intact; COMPASS-ND, Comprehensive Assessment of Neurodegeneration and Dementia; IADL, Instrumental Activities of Daily Living; MCI, mild cognitive impairment; MoCA, Montreal Cognitive Assessment; SCI, subjective cognitive impairment.
Exclusion criteria
Diagnosis of dementia.
Living in nursing homes or adult residential facilities.
Serious underlying disease, which, in the opinion of the study physician, would compromise the participant’s safety.
Surgery within the last 2 months or in the coming 12 months.
History of intracranial surgery.
Regularly takes benzodiazepines that would interfere with participation.
The presence of major depression, schizophrenia, severe anxiety or drug/alcohol abuse or other medical illness that would prohibit safe participation
Current Parkinsonism or any neurological disorder, active musculoskeletal disorders or history of knee/hip replacement that affects gait
Severe visual and/or auditory impairment
Intention to enrol in other clinical trials during the same period
Active participation in an organised and planned exercise programme involving aerobic and/or RT regimen in previous 6 months.
Recruitment and screening
Recruitment procedures
Recruitment will include posters and posts on community and healthcare provider websites, public and social media, physician offices, and paid newspaper advertisements.
Screening and consenting procedures
Consent will be obtained (see online supplemental appendix C) before any screening activities occur. The screening visit will be done virtually using a secure online platform. Following the screening visit, a virtual meeting with the study physician will occur for diagnostic validation and determination of inclusion and exclusion criteria. Participants will then be enrolled and randomised. Participants will indicate on the consent form if acquisition and retention of their saliva sample is permitted for the Polygenic Hazard Score analysis.42 43
Study care partners
Each participant will be asked to identify a care partner (someone who knows them well) who can assist with some of the cognitive tests and assessments as needed. A care partner is not mandatory unless the participant has MCI or SCI. The care partner will be asked to provide informed consent as well (see online supplemental appendix D).
Randomisation and allocation
Randomisation will be conducted by research personnel not involved in screening, assessments or interventions using a simple excel formula that generates a random number within a sequence. A block randomisation by four will be applied to ensure an appropriate balance between treatment arms. Permuted blocks will be employed to ensure balance over time.
Blinding and debriefing
To minimise bias, the study will be double blinded. Research personnel performing the outcome assessments will be blinded to group allocation. Participants will also be blinded to which intervention they received and to study hypotheses. Only the designated research personnel delivering the interventions will know the treatment group that participants belong to and will not reveal the participants’ allocation (unless it is medically necessary to do so) until the end of the trial.
Early withdrawals
Participants will be withdrawn from the study if they: (1) no longer wish to continue their participation in the study (voluntary withdrawal) or (2) in the opinion of one of the study physicians, it is medically necessary to withdraw the participant (medically necessary withdrawal).
Voluntary withdrawal
Participants who inform their intervention research assistant (RA) that they wish to voluntarily withdraw will be asked by the intervention coordinator (to protect blinding) if they would be willing to continue their participation in either intervention on its own and return for their follow-up assessments. In this scenario, they will not be withdrawn from the study provided they agreed to at least the T4 assessment. Voluntary non-compliance will be captured by entering 0 values in their intervention logs for the remainder of the weekly session(s) they withdrew from.
If the participant wishes to completely withdraw from the study, s/he will be asked to complete the exit survey and will subsequently be withdrawn from the study.
Medically necessary withdrawal
Medically necessary withdrawals may be required if participants experience unanticipated AEs or changes in medication or health status, that in the judgement of a study physician, places the participant at risk of harm.
If it is deemed medically necessary to withdraw the participant, the clinical research coordinator and/or study physician will meet with the participant to explain the reason(s) for being withdrawn from the study, and to inquire about the elements of the study that may have led to their change in health status (if applicable). If willing, the participant will be asked to complete the exit survey and will subsequently be withdrawn from the study. These participants will not be included in the adherence analysis.
Interventions
The interventions in this study were adapted from the original SYNERGIC trial,7 and represent sequentially applied cognitive training and physical exercise. All participants will receive home-based intervention sessions of 90 min each 3 times per week for 16 weeks (48 sessions). Intervention RAs trained and certified by the Canadian Society for Exercise Physiology will remotely supervise all sessions via a secure online video conferencing platform. Each participant will be assigned an RA that remains with them throughout the trial. Each session will consist of 20–25 min of cognitive training (NEUROPEAK) or the control cognitive training (WS+V), followed by 50–60 min of exercise intervention (AE+RT) or control exercise (BAT). RAs will maintain an intervention log for each participant, documenting start and end times for each activity.
Active exercise intervention: AE+RT
Participants receiving the AE+RT intervention will have home-based AE+RT exercise (table 2). The RA trainers will coach participants throughout the entire session and document their progress. The level of difficulty and progression for the AE+RT exercise will be tailored to their individual level with constant monitoring.
Table 2.
General overview of active intervention exercise regimen structure
| Section | Type of exercise | Duration (min) |
| Warm up | Marching in one place with arm swings for 1 min | 1 |
| Dynamic hamstring stretching: 15 per side | 1 | |
| Shoulder circles: 15 per direction | 1 | |
| 15 arm reaches | 0.5 | |
| Torso twists: 15 per direction | 1 | |
| Ankle circles: 15 per direction per side | 2 | |
| Side stepping for 1 min | 1 | |
| 15 quarter squats | 1 | |
| Total warm up duration | 8 | |
| Break | 1 | |
| 7 strength training exercises | Chest | 5 |
| Upper back | 5 | |
| Bicep curls | 2.5 | |
| Abdominals | 2.5 | |
| Mid/lower back | 5 | |
| Quadriceps | 5 | |
| Hamstrings | 5 | |
| Total strength training duration | 30 | |
| Break | 3 | |
| AE | Alternating video for participants | 15 |
| Total AE duration | 15 | |
| Break | 3 | |
| Cool down | Quadriceps stretch | 0.5 |
| Hamstring stretch | 0.5 | |
| Calf stretch | 0.5 | |
| 2 hip stretches | 0.5 | |
| Static torso rotation | 0.5 | |
| Seated side bend | 0.5 | |
| Back and shoulder stretch | 0.5 | |
| Chest stretch | 0.5 | |
| Triceps stretch | 0.5 | |
| Neck stretch | 0.5 | |
| Total cool down duration | 5 | |
| Total time | Approximately 65 | |
AE, aerobic exercise.
Control exercise intervention: BAT
Participants receiving the BAT control exercise will have home-based BAT exercises (table 3). The format of the BAT session, including the duration of activities and the amount of coaching, will mirror that of the AE+RT session except the exercises will be devoted to improving muscle tone, balance and flexibility. Resistant load and number of repetitions will not progress during the trial.
Table 3.
General overview of control BAT regimen structure
| Section | Type of exercise | Duration (min) |
| Warm up | Marching in one place with arm swings for 1 min | 1 |
| Dynamic hamstring stretching: 15 per side | 1 | |
| Shoulder circles: 15 per direction | 1 | |
| 15 arm reaches | 0.5 | |
| Torso twists: 15 per direction | 1 | |
| Ankle circles: 15 per direction per side | 2 | |
| Side stepping for 1 min | 1 | |
| 15 quarter squats | 1 | |
| Total warm up duration | 8 | |
| Break | 1 | |
| 7 BAT activities | Standing with feet together+tandem+single leg stand | 10 |
| Core contractions+core and arm raises | 8 | |
| Shoulder retractions |
3 | |
| Isometric quadriceps strength | 3 | |
| Seated hamstring curls | 3 | |
| Seated arm shake | 3 | |
| Total BAT duration | 30 | |
| Break | 3 | |
| Stretching exercise | Alternating video for participants | 15 |
| Total stretching duration | 15 | |
| Break | 3 | |
| Cool down | Quadriceps stretch | 0.5 |
| Hamstring stretch | 0.5 | |
| Calf stretch | 0.5 | |
| 2 hip stretches | 0.5 | |
| Static torso rotation | 0.5 | |
| Seated side bend | 0.5 | |
| Back and shoulder stretch | 0.5 | |
| Chest stretch | 0.5 | |
| Triceps stretch | 0.5 | |
| Neck stretch | 0.5 | |
| Total cool down duration | 5 | |
| Total time | Approximately 65 | |
BAT, balance and toning.
Cognitive training intervention: NEUROPEAK
Participants assigned to the active cognitive intervention will first receive training on how to use NEUROPEAK on a tablet computer provided by the study (for uniformity). For this study, a custom-written programme consisting of a dual-task training programme will be used44–46 that requires participants to maintain and prepare for many response alternatives (working memory) and to share attention between two concurrent tasks (divided attention). Difficulty and progression of cognitive training are tailored to their individual functioning level and performance.
Control cognitive intervention: WS+V
Participants assigned to the control cognitive training will received home-based sessions that alternate between two different tasks: web searching for tourist sites and video watching. For the touristic web searching, participants will be required to find hotels, touristic places and restaurants of their own preference in a city assigned by the RA (a new city will be selected each session). For the video watching, participants will view an educational video about nature and will be asked several questions about it.
Assessment outcomes
All feasibility objectives are consistent with current recommendations on conducting feasibility trials.47
Primary feasibility outcome
Intervention adherence: defined as the percent of all intervention sessions attended of the total planned sessions per participant (48–2=46 allowing for 2 missed sessions). To account for partial sessions, each intervention session will be treated as a fractional measure: the number of minutes training/scheduled session minutes, where scheduled minutes are 50 min for exercise interventions and 20 min for cognitive interventions.
Secondary feasibility outcomes
Recruitment rate: defined as the total percent of enrolled participants relative to number of people screened for eligibility.
Retention rate: defined as the total per cent of enrolled participants who continue throughout the trial and participate in outcomes assessments. Enrolment retention is the per cent of enrolled participants who complete T4 assessment, and follow-up retention is the per cent of those who complete the follow-up T10 assessment.
Trial experience: a mixed methods approach will be used to explore participant experience after the trial using one-on-one interviews with a subsample (purposive sampling, up to 5 per arm=20 to reach saturation). All participants will be invited to complete an exit survey about their experience.
AEs: relationship between AEs severity and relation to trial.
Data loss: defined as technical failures resulting in data loss include problems with electronic equipment or internet communications, personnel errors such as issuing improperly configured equipment, scheduling errors, and omitting assessments, and participant non-compliance such as omitting responses on surveys or declining assessments.
Primary analytic outcomes
Intervention preference
The primary analytic goal of SYNERGIC@Home is to assess the relationship between participants’ adherence to the interventions and their affinity for each intervention going into the trial, as well as other questions about preference. All participants will be given the intervention preference questionnaire (IPQ) at T0, prior to randomisation.
The IPQ asks about their affinity for the offered interventions by quantifying interest level and preferences for the interventions. We will explain to participants that their responses on the questionnaire will not in any way influence the intervention group they will be randomly assigned to.
Secondary analytic outcomes
Various cognitive and psychological tests will be administered as part of a neuropsychological test battery, as well as gait, mobility, sleep, diet and biological markers (please see figure 2 for a fuller list).
Safety evaluation
All AEs and serious AEs (SAEs) that occur between consent and completion of the study will be reported. All AEs and SAEs will be monitored to determine the outcome or until the study physician and/or appropriate research personnel considers it justifiable to terminate follow-up. An SAE will be defined as an event that results in death is life threatening, requires hospitalisation or results in persistent significant disability. AEs will be classified as mild, moderate or severe. The relationship of the AE and SAE to study procedure will be determined and classified as not related, unlikely, possible, probable or definite. All AEs and SAEs will be reported to the Safety and Data Monitoring Committee and Research Ethics Boards as required.
Sample size
Power analysis was conducted using G*Power V.3.1 based on our primary analytic goal of assessing the relationship between intervention preference and subsequent adherence to the interventions. Specifically (see the Analytic outcomes section), we plan on examining correlations among continuous variables with one-tailed analyses at α=0.05 for two pairs of variables (equivalent to a two-tailed test at α=0.1, to account for both intervention types). To achieve a power of 0.8, we would require 48 participants. Assuming a 25% loss, a total of 64 participants will be enrolled.
Statistical analysis
All calculations will be made using the SPSS V.23.0 and Stata (Stata Statistical Software: Release 14, StataCorp LP, College Station, Texas, USA).
Descriptive statistics for demographic and baseline characteristics will be provided with means and SD, or medians and the IQR, where appropriate, for continuous characteristics and frequencies and percentages for categorical variables.
Feasibility outcomes
Adherence to the interventions will be analysed using a one-sample t-test that will test the null hypothesis that participants complete 50% of their scheduled intervention time. This test will be used to determine if the adherence is superior to that hypothesised (feasibility target is 75%) or inferior to that hypothesised (questionable feasibility is significantly <50%).
Secondary feasibility outcomes will be analysed using non-parametric χ2 tests. Target enrolment retention (75%) and follow-up retention (56%) will be tested against observed frequencies using a χ2 goodness-of-fit test. This test will be used to determine if the achieved distribution of eligible participants is similar to that hypothesised, superior to that hypothesised or inferior to that hypothesised. AEs will be analysed using a χ2 cross-tabulation analysis between AEs severity and AEs relation to trial. We will use this analysis to test the hypothesis that there is a relationship between AEs severity and being in the trial. Furthermore, we will stratify the sample by treatment arm and use a χ2 goodness-of-fit test to determine if AEs are distributed differently across treatment arms against the null hypothesis of an even distribution (no relation to treatment arm).
Analytic outcomes
Intervention preference will be analysed by transforming a set of variables:
Interest in the interventions: question 1 in the IPQ rates participant’s interest in each intervention independently: exercise (INT_EX) and cognitive training (INT_CT), on a 0–10 scale.
Intervention preference: the second question rates their relative preference for either intervention. This will generate a single variable that gives the relative preference (−2 to 2 scale), PR, where negative scores and positive scores indicate a preference for exercise or cognitive training, respectively.
Intervention allocated: the treatment arms can be represented by two dummy (0,1) variables for exercise (EX_ARM) and cognitive (CT_ARM), where 1=active treatment and 0=control treatment.
Adherence to interventions: adherence to the interventions at the end of the trial, for exercise (AD_EX) and cognitive training (AD_CT), as well as overall AD, are continuous scale variables.
What is the relationship between adherence and intervention interest? We will correlate interest level for each intervention with adherence rates calculated from trial logs, using Pearson correlation coefficient (ρX, Y) with a one-tailed α of 0.05. The intervention is powered for testing this hypothesis (see the Sample size section).
H0: ρX, Y = 0, H1: ρX, Y > 0, where X=INT_EX and Y=AD_EX.
H0: ρX, Y = 0, H1: ρX, Y > 0, where X=INT_CT and Y=AD_CT.
Rejection of the null hypothesis for either test will allow us to conclude that interest level in the intervention type prior to the trial explains a significant amount of variance in adherence to the trial.
Do participants adhere better if they receive the active treatments they prefer? Because some participants will be randomly assigned to the active intervention that matches their preference and others will not, we will transform the PR score into a signed logical PR_MET (−1=preference not met, 0=no preference and +1=preference met) according to what intervention (EX_ARM and/or CT_ARM) they were allocated to. We will test the hypothesis that:
H0: ρX, Y = 0, H1: ρX, Y ≠ 0, where X=PR_MET and Y=AD.
Rejection of the null hypothesis (p<0.05) will allow us to conclude that adherence to the interventions is significantly influenced by receiving the active intervention they prefer.
How do cognitive and mobility outcomes change as a result of the interventions? Finally, intention-to-treat analysis of cognitive and mobility outcomes with a general linear model or linear mixed model approach will be used to measure intervention effects, and we will estimate effect size based on Cohen’s descriptors (0.2=small, 0.5=moderate and 0.8=large) for cognitive and mobility outcomes listed in figure 2.
Data management and monitoring
All electronic data will be stored on a secure platform at the lead university site. Paper copies of assessment forms will be stored in locked cabinets located at the workplaces of remote study research staff, and then transferred to the participating hospital site. Deidentified copies of the data will also be stored on a secure server called Longitudinal Online Research and Imaging System (LORIS) at the McGill Centre for Integrative Neuroscience, McGill University, Montreal, Quebec. All data will be double entered for data quality monitoring. Assessments at T0, T4 and T10 will be video and audio recorded. In addition, a subset of three intervention sessions will be selected to be video recorded per participant for quality control. The video and audio recordings will be deleted once the data have been validated and released by LORIS.
There will be a Data Safety and Monitoring Committee chaired by an independent person not related to the study and will be comprised of the principal investigators, key research staff and researchers, an independent physician and two community representatives (anglophone and francophone). They will review all AEs, SAEs, protocol deviations, progress of the research and audit study procedures if needed. Protocol amendments will be reported to this committee. All information related to AEs, protocol amendments and protocol deviations will be reported to the appropriate research ethics boards.
Access to data
Access to and analyses of study data stored in LORIS may be granted to qualified persons 12 months after the principal paper answering primary research questions are published. Such requests will be made via email to the Canadian Consortium on Neurodegeneration in Aging (ccna.admin@ladydavis.ca) or via the LORIS Data Access Module. The full protocol and relevant statistical code will also be made available through LORIS.
Participant and public involvement
The SYNERGIC@Home feasibility study offers older adults and their families a unique opportunity to participate in a fully remote bilingual (French and English) RCT from their home. Participants will be invited to share their experience through questionnaires on completion of the study as well as through individual semi-structured interviews. Participants will be able to provide direct feedback on trial improvement strategies, which could be implemented in future studies.
Ethics and dissemination
Research ethics approvals
This study is conducted in compliance with International Conference on Harmonisation of Good Clinical Practice and all applicable regulatory requirements. SYNERGIC@Home has undergone review and approval from the research ethics committees/boards of Horizon Health Network (#2020–2954), Vitalité Health Network (#2020–35), University of New Brunswick (#2020–168) and Université de Moncton (#2021–049). Protocol modifications will be approved by all relevant boards prior to implementation of the changes.
Dissemination plan and authorship
Results of the study will be published in peer-reviewed journals, and presented to local stakeholders, and at provincial, national and international conferences. In accordance with the International Committee of Medical Journal Editors’ standards, authorship of publications resulting from this study should accurately reflect the academic contribution of individuals to the design and implementation of the trial, analysis of the data and preparation of the manuscript. No researcher shall include identifiable personal health information in any publication or presentation.
Discussion
Older adults at risk for ADRD have incident rates of related risk factors several times higher than their cognitively healthy counterparts.48 Additionally, these individuals at risk for ADRD have an increased risk of falling and mobility decline.49 50 Physical exercise and cognitive training are emerging as promising non-pharmacological interventions to enhance mobility and cognitive functioning in older adults, especially in pre-dementia states. These interventions have been tested separately, with positive results for physical exercise and cognitive training in improving cognitive function.9 22 24 27 51 The preliminary success of the original SYNERGIC programme and similar combined interventions have illustrated the promising nature of non-pharmacological exercise interventions and cognitive training to enhance cognition for older adults at risk of developing ADRD.7 52–54
To the best of our knowledge, this is the first study investigating the feasibility of conducting an entirely virtual, home-based, combined exercise and cognitive training intervention programme for older adults at risk for ADRD.
Significance of establishing feasibility
Establishing the feasibility of conducting a virtual, home-based, multidomain intervention has the potential to inform other researchers on the logistics of designing remote intervention programmes. If successful, the methodology and procedures tested in this feasibility trial could set the standard for a new platform in which participants are no longer restricted to intervention studies conducted in a common physical space.
Significance of examining intervention preference
Establishing if preference bias plays a role in which interventions older adults at risk of ADRD will adhere to is expected to provide unique insights into multidomain trial adherence, and will inform the design of future larger RCTs if it is found warranted to control for such bias using a preference design.20
Significance of secondary outcomes
We expect that the combined active exercise and cognitive training arms will have the greatest improvement (or least decline) of cognitive and mobility outcomes, followed by those who receive one active treatment, and finally those receiving both control treatments having the least improvement (or greatest decline). If successful, the combined interventions will further demonstrate a delay in their progression to dementia, warranting a larger RCT.
Benefits of interventions
Mechanistically, AE and RT exercises can provoke a cascade of biochemical, physiological and structural changes in the brain, including increases in blood flow, neurotrophic factor release, neurogenesis, immune system efficacy and metabolism. These effects of exercise could combat inflammatory processes and the atrophy of brain structures often associated with ageing and ADRD.32 34 Mechanisms suggested involve modulation of insulin-like growth factor-1 and insulin sensitivity, decreasing inflammation, enhancing release of brain-derived neurotrophic factor pathways and even a decrease in brain amyloid.27 55 56 Combined exercise interventions have also shown increased brain volume and muscle mass in older adults.57 Furthermore, cognitive training has also been shown to improve overall cognition.37 38 Individuals who practiced monitoring of two tasks at the same time on computer devices have presented with improved connectivity between prefrontal and temporal cortices, areas known to be important for executive functioning and memory, when compared with control participants.34
Strengths and concluding remarks
To the best of our knowledge, this fully remote RCT is the first to test the feasibility of implementing, in two official languages, a combined physical exercise programme with cognitive training to improve cognition and mobility in community-dwelling older adults at risk for ADRD. We will also establish the extent to which measuring participant preference for a given intervention is related to subsequent adherence. We believe that this will inform other researchers and scholars on whether the costs and efforts associated with tailoring interventions in future studies to match participant preferences are worthwhile.
In conclusion, SYNERGIC@Home will build capacity for future research RCT designs using home-based interventions in older adults at risk for ADRD.
Supplementary Material
Acknowledgments
We acknowledge the significant contributions of Canadian Consortium on Neurodegeneration in Aging CAN-THUMBS UP Group's co-principal investigators: Howard Chertkow, Sylvie Belleville, Howard Feldman, Manuel Montero-Odasso, Haakon Nygaard; and Steering Committee members: Danielle Alcock, Nicole Anderson, Sarah Banks, Paul Brewster, Senny Chan, Marc Cuesta, Samir Das, Carol Evans, Guylaine Ferland, Tati Herold, Scott Hofer, Inbal Itzhak, Diane Jacobs, Pam Jarrett, Nellie Kamkar, Andrew Lim, Jody-Lynn Lupo, Lisa Madlensky, Chris McGibbon, Karen Messer, Zia Mohades, Carolyn Revta, Julie Robillard, Penny Slack, Eric Smith, Mark Speechley, Jennifer Walker and Jingjing Zou. This programme was made possible by the participation of the Citizen Advisory Group, research students, support staff and other special groups.
Footnotes
Collaborators: Canadian Consortium on Neurodegeneration in Aging, CAN-THUMBS UP Group.
Contributors: All authors have read and approved of the final manuscript. CM and PJ are the co-lead authors and contributed to the conception and development of the protocol and writing the manuscript. GH, DB and CCT contributed to the conception and development of the interventions and writing the manuscript. AMS, LY, BR, SC, NK and LC-W contributed to the development of the protocol and writing the manuscript. MS contributed to the study design and analysis. MMO conceived of the SYNERGIC programme and various elements of the interventions and assessments were developed by TL-A, LEM, QJA, LB and AL.
Funding: This work is supported by the Healthy Seniors Pilot Project (funding application C0042, January 2020 – October 2022), funded through the Government of New Brunswick and the Public Health Agency of Canada. The Canadian Consortium on Neurodegeneration in Aging is supported by a grant from the Canadian Institutes of Health Research with additional funding from several partners. A substantial component of this funding for the CAN-THUMBS UP program derives from the Alzheimer’s Society of Canada CCNA partnership.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Contributor Information
the Canadian Consortium on Neurodegeneration in Aging (CCNA), CAN-THUMBS UP Group:
Howard Chertkow, Sylvie Belleville, Howard Feldman, Manuel Montero-Odasso, Haakon Nygaard, Danielle Alcock, Nicole Anderson, Sarah Banks, Paul Brewster, Senny Chan, Marc Cuesta, Samir Das, Carol Evans, Guylaine Ferland, Tati Herold, Scott Hofer, Inbal Itzhak, Diane Jacobs, Pam Jarrett, Nellie Kamkar, Andrew Lim, Jody-Lynn Lupo, Lisa Madlensky, Chris McGibbon, Karen Messer, Zia Mohades, Carolyn Revta, Julie Robillard, Penny Slack, Eric Smith, Mark Speechley, Jennifer Walker, and Jingjing Zou
Ethics statements
Patient consent for publication
Not applicable.
References
- 1. Alzheimer Disease International . The Global Impact of Dementia: An analysis of prevalence, incidence, cost and trends. In: The world Alzheimer report, 2016. [Google Scholar]
- 2. Alzheimer Society of Canada . Report summary prevalence and monetary costs of dementia in Canada (2016): a report by the Alzheimer Society of Canada. Health Promot Chronic Dis Prev Can 2016;36:231–2. [PMC free article] [PubMed] [Google Scholar]
- 3. Ostbye T, Crosse E. Net economic costs of dementia in Canada. CMAJ 1994;151:1457–64. [PMC free article] [PubMed] [Google Scholar]
- 4. Takeda A, Loveman E, Clegg A, et al. A systematic review of the clinical effectiveness of donepezil, rivastigmine and galantamine on cognition, quality of life and adverse events in Alzheimer's disease. Int J Geriatr Psychiatry 2006;21:17–28. 10.1002/gps.1402 [DOI] [PubMed] [Google Scholar]
- 5. Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement 2011;7:270–9. 10.1016/j.jalz.2011.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Petersen RC. Clinical practice. mild cognitive impairment. N Engl J Med 2011;364:2227–34. 10.1056/NEJMcp0910237 [DOI] [PubMed] [Google Scholar]
- 7. Montero-Odasso M, Almeida QJ, Burhan AM, et al. Synergic trial (SYNchronizing exercises, remedies in gait and cognition) a multi-centre randomized controlled double blind trial to improve gait and cognition in mild cognitive impairment. BMC Geriatr 2018;18:93. 10.1186/s12877-018-0782-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Teixeira CVL, Ribeiro de Rezende TJ, Weiler M, et al. Cognitive and structural cerebral changes in amnestic mild cognitive impairment due to Alzheimer's disease after multicomponent training. Alzheimers Dement 2018;4:473–80. 10.1016/j.trci.2018.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ballesteros S, Voelcker-Rehage C, Bherer L. Editorial: cognitive and brain plasticity induced by physical exercise, cognitive training, video games, and combined interventions. Front Hum Neurosci 2018;12:169. 10.3389/fnhum.2018.00169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ngandu T, Lehtisalo J, Solomon A, et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (finger): a randomised controlled trial. Lancet 2015;385:2255–63. 10.1016/S0140-6736(15)60461-5 [DOI] [PubMed] [Google Scholar]
- 11. Norman GJ, Zabinski MF, Adams MA, et al. A review of eHealth interventions for physical activity and dietary behavior change. Am J Prev Med 2007;33:336–45. 10.1016/j.amepre.2007.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hillsdon M, Foster C, Thorogood M. Interventions for promoting physical activity. Cochrane Database Syst Rev 2005;1:CD003180. 10.1002/14651858.CD003180.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jong M, Mendez I, Jong R. Enhancing access to care in northern rural communities via telehealth. Int J Circumpolar Health 2019;78:1554174. 10.1080/22423982.2018.1554174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Maddison R, Rawstorn JC, Stewart RAH, et al. Effects and costs of real-time cardiac telerehabilitation: randomised controlled non-inferiority trial. Heart 2019;105:122–9. 10.1136/heartjnl-2018-313189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Johnson NA, Ewald B, Plotnikoff RC, et al. Predictors of adherence to a physical activity counseling intervention delivered by exercise physiologists: secondary analysis of the NewCOACH trial data. Patient Prefer Adherence 2018;12:2537–43. 10.2147/PPA.S183938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Clark RE, McArthur C, Papaioannou A, et al. "I do not have time. Is there a handout I can use?": combining physicians' needs and behavior change theory to put physical activity evidence into practice. Osteoporos Int 2017;28:1953–63. 10.1007/s00198-017-3975-6 [DOI] [PubMed] [Google Scholar]
- 17. Choi J, Lee M, Lee J-K, et al. Correlates associated with participation in physical activity among adults: a systematic review of reviews and update. BMC Public Health 2017;17:356. 10.1186/s12889-017-4255-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Torriani-Pasin C, Palma GCDS, Makhoul MP, et al. Adherence rate, barriers to attend, safety, and overall experience of a remote physical exercise program during the COVID-19 pandemic for individuals after stroke. Front Psychol 2021;12:647883. 10.3389/fpsyg.2021.647883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Corbett MS, Watson J, Eastwood A. Randomised trials comparing different healthcare settings: an exploratory review of the impact of pre-trial preferences on participation, and discussion of other methodological challenges. BMC Health Serv Res 2016;16:589. 10.1186/s12913-016-1823-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Walter SD, Turner RM, Macaskill P, et al. Estimation of treatment preference effects in clinical trials when some participants are indifferent to treatment choice. BMC Med Res Methodol 2017;17:29. 10.1186/s12874-017-0304-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sessler DI, Myles PS. Novel clinical trial designs to improve the efficiency of research. Anesthesiology 2020;132:69–81. 10.1097/ALN.0000000000002989 [DOI] [PubMed] [Google Scholar]
- 22. Bherer L, Erickson KI, Liu-Ambrose T. Physical exercise and brain functions in older adults. J Aging Res 2013;2013:197326. 10.1155/2013/197326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Liu-Ambrose T, Nagamatsu LS, Voss MW, et al. Resistance training and functional plasticity of the aging brain: a 12-month randomized controlled trial. Neurobiol Aging 2012;33:1690–8. 10.1016/j.neurobiolaging.2011.05.010 [DOI] [PubMed] [Google Scholar]
- 24. Öhman H, Savikko N, Strandberg TE, et al. Effect of physical exercise on cognitive performance in older adults with mild cognitive impairment or dementia: a systematic review. Dement Geriatr Cogn Disord 2014;38:347–65. 10.1159/000365388 [DOI] [PubMed] [Google Scholar]
- 25. Lautenschlager NT, Cox KL, Flicker L, et al. Effect of physical activity on cognitive function in older adults at risk for Alzheimer disease: a randomized trial. JAMA 2008;300:1027–37. 10.1001/jama.300.9.1027 [DOI] [PubMed] [Google Scholar]
- 26. Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults: a meta-analytic study. Psychol Sci 2003;14:125–30. 10.1111/1467-9280.t01-1-01430 [DOI] [PubMed] [Google Scholar]
- 27. Langlois F, Vu TTM, Chassé K, et al. Benefits of physical exercise training on cognition and quality of life in frail older adults. J Gerontol B Psychol Sci Soc Sci 2013;68:400–4. 10.1093/geronb/gbs069 [DOI] [PubMed] [Google Scholar]
- 28. Gates N, Fiatarone Singh MA, Sachdev PS, et al. The effect of exercise training on cognitive function in older adults with mild cognitive impairment: a meta-analysis of randomized controlled trials. Am J Geriatr Psychiatry 2013;21:1086–97. 10.1016/j.jagp.2013.02.018 [DOI] [PubMed] [Google Scholar]
- 29. Choi J-H, Kim N-J. The effects of balance training and ankle training on the gait of elderly people who have fallen. J Phys Ther Sci 2015;27:139–42. 10.1589/jpts.27.139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. McAuley E, Wójcicki TR, Learmonth YC, et al. Effects of a DVD-delivered exercise intervention on physical function in older adults with multiple sclerosis: a pilot randomized controlled trial. Mult Scler J Exp Transl Clin 2015;1:2055217315584838. 10.1177/2055217315584838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yogev-Seligmann G, Eisenstein T, Ash E, et al. Neurocognitive plasticity is associated with cardiorespiratory fitness following physical exercise in older adults with amnestic mild cognitive impairment. J Alzheimers Dis 2021;81:91–112. 10.3233/JAD-201429 [DOI] [PubMed] [Google Scholar]
- 32. Reijnders J, van Heugten C, van Boxtel M. Cognitive interventions in healthy older adults and people with mild cognitive impairment: a systematic review. Ageing Res Rev 2013;12:263–75. 10.1016/j.arr.2012.07.003 [DOI] [PubMed] [Google Scholar]
- 33. Ballesteros S, Prieto A, Mayas J, et al. Brain training with non-action video games enhances aspects of cognition in older adults: a randomized controlled trial. Front Aging Neurosci 2014;6:277. 10.3389/fnagi.2014.00277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chapman SB, Aslan S, Spence JS, et al. Neural mechanisms of brain plasticity with complex cognitive training in healthy seniors. Cereb Cortex 2015;25:396–405. 10.1093/cercor/bht234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gavelin HM, Dong C, Minkov R, et al. Combined physical and cognitive training for older adults with and without cognitive impairment: a systematic review and network meta-analysis of randomized controlled trials. Ageing Res Rev 2021;66:101232. 10.1016/j.arr.2020.101232 [DOI] [PubMed] [Google Scholar]
- 36. Li KZH, Roudaia E, Lussier M, et al. Benefits of cognitive Dual-task training on balance performance in healthy older adults. J Gerontol A Biol Sci Med Sci 2010;65:1344–52. 10.1093/gerona/glq151 [DOI] [PubMed] [Google Scholar]
- 37. Lampit A, Hallock H, Valenzuela M. Computerized cognitive training in cognitively healthy older adults: a systematic review and meta-analysis of effect modifiers. PLoS Med 2014;11:e1001756. 10.1371/journal.pmed.1001756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Weng W, Liang J, Xue J, et al. The transfer effects of cognitive training on working memory among Chinese older adults with mild cognitive impairment: a randomized controlled trial. Front Aging Neurosci 2019;11:212. 10.3389/fnagi.2019.00212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Legault C, Jennings JM, Katula JA, et al. Designing clinical trials for assessing the effects of cognitive training and physical activity interventions on cognitive outcomes: the seniors health and activity research program pilot (SHARP-P) study, a randomized controlled trial. BMC Geriatr 2011;11:27. 10.1186/1471-2318-11-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Coelho FGdeM, Andrade LP, Pedroso RV, et al. Multimodal exercise intervention improves frontal cognitive functions and gait in Alzheimer's disease: a controlled trial. Geriatr Gerontol Int 2013;13:198–203. 10.1111/j.1447-0594.2012.00887.x [DOI] [PubMed] [Google Scholar]
- 41. Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist 1969;9:179–86. [PubMed] [Google Scholar]
- 42. Desikan RS, Fan CC, Wang Y, et al. Genetic assessment of age-associated Alzheimer disease risk: development and validation of a polygenic hazard score. PLoS Med 2017;14:e1002258. 10.1371/journal.pmed.1002258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tan CH, Bonham LW, Fan CC, et al. Polygenic hazard score, amyloid deposition and Alzheimer's neurodegeneration. Brain 2019;142:460–70. 10.1093/brain/awy327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bherer L, Kramer AF, Peterson MS, et al. Training effects on Dual-task performance: are there age-related differences in plasticity of attentional control? Psychol Aging 2005;20:695–709. 10.1037/0882-7974.20.4.695 [DOI] [PubMed] [Google Scholar]
- 45. Bherer L, Kramer AF, Peterson MS, et al. Testing the limits of cognitive plasticity in older adults: application to attentional control. Acta Psychol 2006;123:261–78. 10.1016/j.actpsy.2006.01.005 [DOI] [PubMed] [Google Scholar]
- 46. Bherer L, Kramer AF, Peterson MS, et al. Transfer effects in task-set cost and Dual-task cost after Dual-task training in older and younger adults: further evidence for cognitive plasticity in attentional control in late adulthood. Exp Aging Res 2008;34:188–219. 10.1080/03610730802070068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. El-Kotob R, Giangregorio LM. Pilot and feasibility studies in exercise, physical activity, or rehabilitation research. Pilot Feasibility Stud 2018;4:137. 10.1186/s40814-018-0326-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Petersen RC, Smith GE, Waring SC, et al. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol 1999;56:303–8. 10.1001/archneur.56.3.303 [DOI] [PubMed] [Google Scholar]
- 49. Camicioli R, Majumdar SR. Relationship between mild cognitive impairment and falls in older people with and without Parkinson's disease: 1-year prospective cohort study. Gait Posture 2010;32:87–91. 10.1016/j.gaitpost.2010.03.013 [DOI] [PubMed] [Google Scholar]
- 50. Davis JC, Best J, Hsu CL, et al. Examining the effect of the relationship between falls and mild cognitive impairment on mobility and executive functions in community-dwelling older adults. J Am Geriatr Soc 2015;63:590–3. 10.1111/jgs.13290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Pothier K, Gagnon C, Fraser SA, et al. A comparison of the impact of physical exercise, cognitive training and combined intervention on spontaneous walking speed in older adults. Aging Clin Exp Res 2018;30:921–5. 10.1007/s40520-017-0878-5 [DOI] [PubMed] [Google Scholar]
- 52. Montero-Odasso M, Ismail Z, Livingston G. One third of dementia cases can be prevented within the next 25 years by tackling risk factors. The case "for" and "against". Alzheimers Res Ther 2020;12:81. 10.1186/s13195-020-00646-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Montero Odasso M, Almeida Q, Camicioli R, et al. Preliminary results from the synergic trial: a multimodal intervention for mild cognitive impairment. Innov Aging 2018;2:439–40. 10.1093/geroni/igy023.1646 [DOI] [Google Scholar]
- 54. Cheng S-T, Chow PK, Song Y-Q, et al. Mental and physical activities delay cognitive decline in older persons with dementia. Am J Geriatr Psychiatry 2014;22:63–74. 10.1016/j.jagp.2013.01.060 [DOI] [PubMed] [Google Scholar]
- 55. Sage MD, Almeida QJ. Symptom and gait changes after sensory attention focused exercise vs aerobic training in Parkinson's disease. Mov Disord 2009;24:1132–8. 10.1002/mds.22469 [DOI] [PubMed] [Google Scholar]
- 56. Sage MD, Almeida QJ. A positive influence of vision on motor symptoms during sensory attention focused exercise for Parkinson's disease. Mov Disord 2010;25:64–9. 10.1002/mds.22886 [DOI] [PubMed] [Google Scholar]
- 57. Nagamatsu LS, Handy TC, Hsu CL, et al. Resistance training promotes cognitive and functional brain plasticity in seniors with probable mild cognitive impairment. Arch Intern Med 2012;172:666–8. 10.1001/archinternmed.2012.379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. The comprehensive assessment of neurodegeneration and dementia (COMPASS-ND) study, 2020. Available: https://ccna-ccnv.ca/compass-nd-study/
- 59. Jessen F, Amariglio RE, van Boxtel M, et al. A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer's disease. Alzheimers Dement 2014;10:844–52. 10.1016/j.jalz.2014.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2021-059988supp001.pdf (2.3MB, pdf)