Abstract
E5531, a novel synthetic lipid A analogue, antagonizes the toxic effects of lipopolysaccharide, making it a potential intravenously administered therapeutic agent for the treatment of sepsis. This report describes the distribution of E5531 in human blood and its activity when it is associated with different lipoprotein subclasses. After in vitro incubation of [14C]E5531 with blood, the great majority (>92%) of material was found in the plasma fraction. Analysis by size-exclusion and affinity chromatographies and density gradient centrifugation indicates that [14C]E5531 binds to lipoproteins, primarily high-density lipoproteins (HDLs), with distribution into low-density lipoproteins (LDLs) and very low density lipoproteins (VLDLs) being dependent on the plasma LDL or VLDL cholesterol concentration. Similar results were also seen in a limited study of [14C]E5531 administration to human volunteers. The potency of E5531 in freshly drawn human blood directly correlates to increasing LDL cholesterol levels. Finally, preincubation of E5531 with plasma or purified lipoproteins indicated that binding to HDL resulted in a time-dependent loss of drug activity. This loss in activity was not observed with drug binding to LDLs or to VLDLs or chylomicrons. Taken together, these results indicate that E5531 binds to plasma lipoproteins, making its long-term antagonistic potency dependent on the plasma lipoprotein composition.
E5531, an endotoxin antagonist, is being evaluated as a treatment for human sepsis. Endotoxin or lipopolysaccharide (LPS) is the major constituent of the outer membranes of gram-negative bacteria. It is believed that the presence of high levels of endotoxin or its persistent presence, caused by an uncontrolled or blood-borne bacterial infection, stimulates monocytes/macrophages to release cytokines such as tumor necrosis factor alpha (TNF-α), interleukin 1 (IL-1), IL-6, and IL-8, as well as other cellular mediators such as nitric oxide and leukotrienes. When present in excess, these cellular mediators comprise part of a severe pathological response that leads to tissue damage that can result in hypotensive shock, multiorgan failure, and death (3, 5–7, 11, 16, 17, 20, 23, 28, 36).
The toxicity of LPS can be attributed to its lipid A moiety. Nonpathogenic bacteria such as Rhodobacter capsulatus synthesize LPS and lipid A that lack stimulatory activities and that can antagonize activation of cells by more toxic LPS (19). E5531 is a synthetic stabilized analogue of R. capsulatus lipid A and has been described to be a potent in vitro and in vivo antagonist of LPS (8). The amphiphilic nature of E5531 has prompted us to study its disposition in whole blood and to analyze the effects of the interaction of different serum components on E5531 activity. Studies with other drugs have demonstrated that interaction with lipoproteins can alter their activities. For instance, cyclosporin A (CSA) demonstrates enhanced antiproliferative activity when it is bound to low-density lipoproteins (LDLs) but not high-density lipoproteins (HDLs) or very low density lipoproteins (VLDLs) (18, 22). In hypertriglyceridemic patients, this interaction can reduce the activity of CSA (N. De Klippel, J. Sennesael, J. Lamote, G. Ebinger, and J. de Keyser, Lancet 339:1114, 1992, letter; J. Nemunaitis, H. J. Deeg, and G. C. Yee, Lancet ii:744–745, 1986, letter). In addition, it has been suggested that the toxicity as well as activity of CSA can depend upon the class of lipoprotein to which it is bound (18). Taken together, it is possible that changes in plasma lipoprotein profiles can alter the efficacies and pharmacodynamic profiles of lipophilic drugs. This report describes both the in vitro and the in vivo interactions of E5531 with different serum lipoproteins and the correlation of drug activity in blood containing different levels of lipoprotein (LDL or VLDL and HDL) cholesterol.
MATERIALS AND METHODS
Materials.
E5531 was synthesized at Eisai Research Institute and has been described previously (8). [14C]E5531 was synthesized by the same procedure with 14C at the β carbons of both the C-2 and the C-2′ positions. For these studies, E5531 was reconstituted from a powder made from drug dissolved at 2 mg/ml in sterile NaOH (0.003 M), heated to 50°C for 30 min, and then diluted to 100 μg/ml in lactose (100 mg/ml) and sodium phosphate (4.2 mM). After adjusting the pH to 7.1–7.5, the material was sterile filtered and lyophilized. Each assay used freshly reconstituted drug. [14C]E5531 (100 mCi/mmol) was similarly prepared, except that it was retained as a frozen solution and was not lyophilized. LPS from Escherichia coli O111:B4 was purchased from List Biologicals (Campbell, Calif.). LPS was dissolved in sterile water at 1 mg/ml and was stored at −20°C. Prior to use, LPS was sonicated in a bath sonicator (VW-380; Heat Systems-Ultrasonics Inc., Farmingdale, N.Y.) for 1 to 2 min and was then diluted into Ca2+- and Mg2+-free Hanks balanced salt solution (HBSS; Sigma). The levels of cholesterol in plasma, column chromatography fractions, or gradient fractions were assayed with a total cholesterol assay kit and a standard curve with diluted cholesterol calibrators (Sigma Chemical Co.).
TNF-α assays with human whole blood.
Induction of TNF-α in human whole blood has been described previously (24). Briefly, the indicated concentrations of antagonists were added as 10× stocks in 50 μl of 5% dextrose in water, followed by the addition of 50 μl of LPS (final concentration, 10 ng/ml), to 400 μl of heparinized whole blood obtained from healthy volunteers (age, 18 to 51 years; weight, 50 to 105 kg) for a total of 500 μl/well (final concentration of whole blood, 80%). After a 3-h incubation with gentle shaking at 37°C in a 5% CO2 atmosphere, the plates were centrifuged at 1,000 × g for 10 min at 4°C, and then the plasma was drawn off and frozen at −80°C. The plasma samples were analyzed for TNF-α by an enzyme-linked immunosorbent assay (Genzyme Corp., Cambridge, Mass.). Each point represents the mean of triplicate assays. HDL, LDL, and total cholesterol were assayed in freshly drawn blood by a commercial pathology laboratory with an Hitachi 747 analyzer.
Analysis of E5531 activity after HDL or plasma pretreatment.
E5531 (31 μl of 64 μM E5531) was added to 169 μl of test material (e.g., HDL or plasma) or HBSS in triplicate. Subsequent 10-fold dilutions were made by using test material as diluent, resulting in drug concentrations of 10, 1, 0.1, and 0.01 μM. After 18 h of incubation on an orbital shaker at 37°C, 100 μl of these samples was further diluted fivefold into freshly drawn human whole blood. LPS (final concentration, 10 ng/ml) was then added as an agonist. This reaction mixture was incubated for 3 h and was assayed for TNF-α release as described above for human whole blood.
Analysis of [14C]E5531 binding.
For size-exclusion chromatography, [14C]E5531 (750,000 dpm) was added to 5 ml of plasma (final E5531 concentration, 0.68 μM; 1.06 μg/ml), incubated for 0.5 to 18 h at 37°C, and fractionated by size-exclusion chromatography with Sephacryl S300 (2.5 by 110 cm) developed in endotoxin-free (tissue culture-grade) HBSS containing penicillin (80 U), streptomycin (100 μg/ml), and EDTA (0.1 mM). Fractions were collected after elution of 150 ml of buffer (prevoid volume elution). Extensive analysis by size-exclusion chromatography was difficult as control experiments that tested for elution of [14C]E5531 alone indicated that it did not elute from the column but remained on the top and was excluded from the column bed. [14C]E5531 did not nonspecifically adsorb to the agarose matrix.
Density gradient centrifugation was performed with self-forming iodixanol gradients (Optiprep; Gibco BRL, Gaithersburg, Md.). For in vitro analysis of E5531 binding to plasma components, 300,000 dpm of [14C]E5531 was added to 4 ml of freshly prepared plasma, and the mixture was incubated at 37°C for 18 h. This mixture was diluted to 9 ml with buffered saline (0.9% NaCl, 60 mM HEPES [pH 7.4]) and was combined with 3 ml of iodixanol working solution (50% working solution prepared from a 60% stock of iodixanol [Optiprep; Gibco BRL] by addition of HEPES-buffered saline). The samples were then mixed by vortexing, transferred to 12-ml Quick-Seal polyallomer centrifuge tubes (Beckman), and centrifuged in an NVT65 rotor in Beckman L8-M centrifuge (350,000 × g, 4 h, 16°C). The tubes were then harvested with a density gradient fractionator (model 185; ISCO) set at 25 drops per tube (approximately 700 μl per fraction). The presence of radiolabeled drug in each fraction was determined by obtaining the counts in 200-μl samples in a scintillation counter (Beckman), and density was determined from the refractive index with standard curves supplied by the manufacturer of iodixanol.
For analysis by affinity chromatography, [14C]E5531 (0.64 μM) was incubated in human plasma for 5 min at 37°C, and following the removal of plasma proteins by centrifugation (33), plasma lipoproteins were separated into the HDL and VLDL or LDL fractions with the LDL-Direct cholesterol chromatographic column (Isolab [32]). All steps were performed at 37°C. Cholesterol determinations for the results described in Table 1 were done as described previously (33).
TABLE 1.
Distribution of [14C]E5531 in plasma samples from five human subjectsa
| Subject | Total cholesterol level (mg/dl) | % of added drug recovered in:
|
% Recovery of total drug | ||
|---|---|---|---|---|---|
| HDL fraction | LDL-VLDL fraction | LPDP fraction | |||
| I | 83 | 81.1 ± 0.3 | 8.9 ± 0.1 | 0.8 ± 0.2 | 90.8 ± 3.5 |
| II | 161 | 71.4 ± 1.3 | 18.0 ± 0.3 | 2.2 ± 1.1 | 91.6 ± 4.1 |
| III | 190 | 73.8 ± 0.3 | 20.1 ± 0.3 | 0.8 ± 0.2 | 94.7 ± 7.4 |
| IV | 206 | 59.8 ± 2.5 | 25.5 ± 0.5 | 5.9 ± 1.4 | 91.2 ± 4.4 |
| V | 236 | 66.3 ± 1.9 | 33.1 ± 1.2 | 6.3 ± 0.3 | 105.7 ± 0.8 |
Plasma was incubated for 5 min at 37°C with 0.64 μM [14C]E5531, separated into lipoprotein and lipoprotein-deficient fractions by affinity chromatography and centrifugation, and assayed for radioactivity as described in the Materials and Methods section. Data are expressed as means ± standard deviations; n equals 3 individual replicates for subjects I to III, n equals 5 individual replicates for subject IV, and n equals 6 individual replicates for subject V. LPDP, lipoprotein-deficient plasma which contains albumin and alpha-1-glycoprotein; VLDL includes chylomicrons. Binding in the HDL fraction demonstrates an inverse trend with the total cholesterol level (r = 0.819; P = 0.0902), while the amount of drug recovered in the LDL-VLDL fraction increased as a function of increasing total cholesterol level (r = 0.965;P = 0.0078).
Human infusion studies with [14C]E5531 were done at Harris Laboratories, Inc. (Lincoln, Nebr.). Informed consent was obtained from all participants, and the human experimental guidelines of the U.S. Department of Health and Human Services and those of the author's institutions and Harris Laboratories, Inc., were followed in the conduct of this clinical research. Intravenous infusion of [14C]E5531 was at 1 μCi/250 μg of E5531/h. Heparinized blood samples drawn from the antecubital vein were reduced to plasma, stored on ice, and shipped overnight on ice. Density gradient centrifugation analysis of drug binding in these plasma samples was done as described above, except that a 2-ml sample of plasma was subjected to density gradient centrifugation, fractionated, and assayed for its 14C content. Control experiments with drug added to heparinized plasma in vitro, the results of which are presented in Fig. 1 and 2, indicated that the storage and shipping conditions used here had no effect on the distribution of drug in plasma lipoproteins for up to several days. In addition, repeated assays of several of the samples obtained from the infusion study over a period of several days showed no significant changes when the samples were stored in this manner. Plasma cholesterol levels were determined by the standard clinical method at Harris Laboratories.
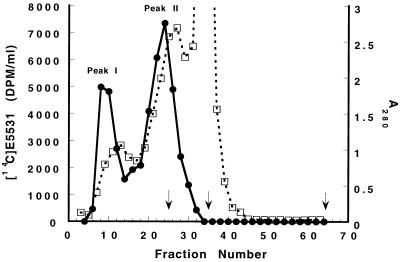
FIG. 1.
Size-exclusion chromatography analysis of [14C]E5531 binding to plasma components. [14C]E5531 (680 nM), prepared as described in the Materials and Methods section, was added to 5 ml of plasma, and the mixture was incubated at 37°C for 18 h, fractionated by size-exclusion chromatography with Sephacryl S300, and assayed for radioactivity (●) and protein (A280; □) as described in the Materials and Methods section. In other column analyses, the elutions of β-amylase (200 kDa; peak fraction 25), bovine serum albumin (67 kDa; peak fraction 35), and [3H]leucine (Vi; peak fraction 65) were determined. These are marked with vertical arrows on the x axis.
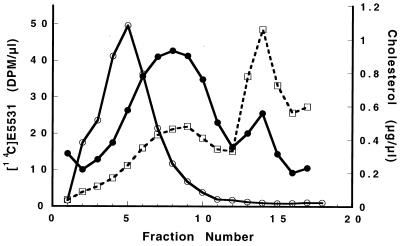
FIG. 2.
Density gradient analysis of [14C]E5531 binding to plasma components. [14C]E5531 (341 nM), prepared as described in the Materials and Methods section, was incubated at 37°C for 18 h in HBSS or freshly prepared plasma and was fractionated by density gradient analysis with self-forming iodixanol gradients. Analysis of the radioactivity in the gradients containing drug incubated with HBSS (○) or with plasma (●), as well as analysis of the cholesterol content of the fractionated plasma (□), was performed as described in the Materials and Methods section.
Statistics.
Linear regression analysis was performed with the Graphpad Prism program (Graphpad Software, San Diego, Calif.). Except where noted, experiments were performed a minimum of three times with determinations in triplicate. Statistical significance was assessed by Student's t test, by use of the Pearson correlation, or by use of the Spearman correlation, as noted in the text.
RESULTS
E5531 activity decreases in the presence of blood or plasma.
E5531 antagonizes the toxic activity of LPS in human blood (8); however, the potency of E5531 activity diminishes with time of incubation in blood or plasma. This can be observed by preincubating E5531 in blood prior to the addition of LPS under the identical conditions used for the LPS activation experiments. On average, in seven experiments with blood from a variety of donors, preincubation for 3 to 6 h resulted in increases in the apparent 50% inhibitory concentration (IC50) from 10 ± 1.8 nM (when it was assayed immediately after E5531 addition) to 29 ± 8 nM (when it was assayed after 3 h of preincubation) and 50 ± 16 nM (when it was assayed after 6 h of preincubation). Similar losses in activity were readily detected by endpoint bioassays done by first incubating E5531 in plasma or plasma fractions overnight and then assaying for apparent IC50. This endpoint bioassay detects decreases in the activity of E5531 of up to 100-fold in plasma and has allowed us to readily screen a large number of samples and fractions for their ability to inhibit the activity of E5531 while studying the association of drug with various plasma fractions in parallel assays.
E5531 binds to plasma lipoproteins. (i) In vitro analysis of E5531 interaction with plasma lipoproteins.
In order to determine whether E5531 remains free in plasma or is rapidly associated with the surface of cells, we have studied the distribution of [14C]E5531 after incubation in human whole blood. As detected by the distribution of 14C after separation of plasma from cells, >92% of added drug was found in the plasma fraction when testing was done under a wide variety of incubation conditions, even after 2 h of incubation. For this reason, drug binding in the plasma fraction was further evaluated. As shown in Fig. 1, Sephacryl S300 size-exclusion fractionation of [14C]E5531 after incubation in plasma indicated that radiolabel was recovered in two fractions. These fractions corresponded to the void fraction of the column (fraction I) and to components of approximately 280 kDa (fraction II). Analysis of material in both of these high-molecular-mass fractions by extraction into chloroform-methanol (4) and high-pressure liquid chromatography (HPLC) indicated that both fractions contained unmodified drug as well as large amounts of lipid and cholesterol. This observation indicated that [14C]E5531 was adsorbing to lipid components of plasma and was coeluting with these components during size-exclusion chromatography.
To further study the drug-lipoprotein interaction, plasma was treated as described above and was analyzed by density gradient centrifugation. As shown in Fig. 2, the majority of [14C]E5531 was found at two densities. Most of this [14C]E5531 was found in fractions 6 to 9 (ρ = 1.063 to 1.096 g/ml), while the balance of 14C-labeled material was found at a density of 1.026 to 1.040 g/ml) in fractions 13 to 15. These densities are characteristic of the broad range of densities described (27) for HDL (ρ = 1.063 to 1.209 g/ml) and LDL (ρ = 1.007 to 1.063 g/ml). [14C]E5531 alone (in HEPES-buffered saline) sedimented to a buoyant density of 1.096 to 1.130 g/ml (fractions 4 to 6; Fig. 2).
To further confirm that drug was interacting with HDLs and LDLs, [14C]E5531 binding to plasma lipoproteins was determined by heparin-manganese affinity chromatography with plasma samples obtained from five human subjects (Table 1). In these assays, plasma samples were incubated for 5 min at 37°C with 0.64 μM [14C]E5531, and lipoproteins were fractionated by affinity chromatography as described previously (32). Under these conditions, binding is predominantly to HDLs. In addition, there appears to be an inverse trend between binding to the HDL fraction and the level of total cholesterol (r = 0.819; P = 0.0902), while the amount of drug recovered in the LDL or VLDL fraction increased as a function of increasing levels of total cholesterol (r = 0.965; P = 0.0078). These results indicate that the level of fractional binding to LDL and VLDL is relatively low but becomes more apparent when concentrations of LDL and VLDL in plasma are elevated.
(ii) Analysis of intravenously infused drug in humans.
Plasma samples from volunteers infused with [14C]E5531 were analyzed for drug distribution by density gradient centrifugation. As shown in Fig. 3, two to three peaks of [14C]E5531 were identified. The greatest fraction of material (45 to 65%) was found in high-density fractions 2 to 7 (ρ = 1.07 to 1.18 g/ml), while the balance of the 14C-labeled material was found at a density of 1.026 to 1.038 g/ml (fractions 12 to 14) and in the lowest-density fractions, fractions 16 and 17 (≤1.02 g/ml). The densities of these fractions are characteristic of HDL (ρ = 1.063 to 1.21 g/ml), LDL (ρ = 1.021 to 1.063 g/ml), and VLDL and chylomicrons (ρ < 1.02 g/ml).
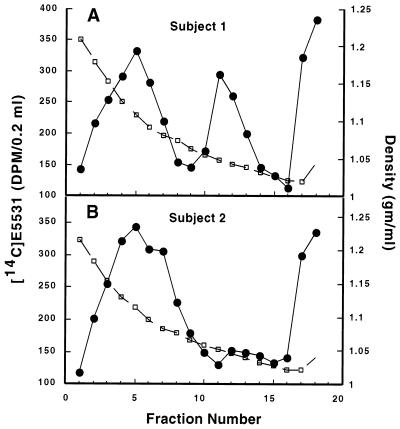
FIG. 3.
Density gradient analysis of [14C]E5531 binding to plasma components after intravenous infusion. A 2-ml plasma sample was prepared from blood 74 h after onset of [14C]E5531 infusion into two human subjects, subject 1 (A) and subject 2 (B), and was fractionated by density gradient analysis with self-forming iodixanol gradients. Analysis of radioactivity (●) and density (□) was performed as described in the Materials and Methods section. Cholesterol levels obtained 2 h before and 72 and 312 h after the beginning of infusion were reported to be 130.7 ± 17.6 mg/dl (LDL) and 39 ± 3 mg/dl (HDL) for subject 1 and 59.3 ± 7.7 mg/dl (LDL) and 39 ± 2.7 mg/dl (HDL) for subject 2.
Differences in the distribution of [14C]E5531 are apparent when plasma binding is evaluated for subjects with different cholesterol contents (compare panels A and B of Fig. 3). In both subjects, the greatest amounts of 14C were detected in the HDL fraction (47.8% of total 14C in subject 1 and 57% of total 14C in subject 2). However, elevated LDL cholesterol levels in subject 1 (131 mg/dl) compared to those in subject 2 (59 mg/dl) were associated with an increased association of drug with the LDL fraction; the proportion of LDL-associated E5531 was more than doubled in subject 1 (24.2% in subject 1 versus 11.3% in subject 2). Roughly equal amounts of material were recovered in the VLDL-chylomicron fractions in the two subjects (11.5% in subject 1 versus 14.3% in subject 2). These results indicate that changes in the LDL cholesterol content of plasma affect the in vivo distribution of binding of [14C]E5531 in the lipoprotein fraction.
Lipoprotein affects in vitro activity of E5531. (i) E5531 inhibition of LPS-induced release of TNF-α is dependent on lipoprotein concentration.
We have established assays to measure LPS-induced cytokine release in order to evaluate the antagonistic activity of E5531 in fresh human whole blood. Without adding LPS, TNF-α release was undetectable (<15 pg/ml). Saturation of endotoxin-mediated stimulation occurred at concentrations greater than 10 ng/ml. As shown in Table 2, the response of whole blood after 3 h of incubation with 10 ng of LPS per ml was robust and reproducible (release of TNF-α = 2,151 ± 140 pg/ml; n = 31). The mean IC50 for inhibition of 10 ng of E. coli LPS per ml in human whole blood was 12.4 ± 1.6 nM, but variability in the potency of E5531 (IC50s varied from 1.5 to 30.6 nM) has led us to investigate whether interaction with plasma components can affect E5531 activity. Analysis of IC50 variability as a function of lipoprotein composition was investigated in side-by-side analyses of samples from several groups of volunteers with different levels of lipoproteins. Analysis of the data in Table 2 indicates that the potency of E5531 increased (i.e., IC50s decreased) as a function of increases in total and LDL cholesterol levels. No significant correlation (Spearman analysis) was observed for different levels of HDL in these plasma samples over a range of 28 to 100 mg of HDL cholesterol per dl. In addition, no statistically significant correlation was found for potency (IC50) and plasma triglyceride content. The best-fit slope of a line fit by Spearman correlation analysis predicts IC50 changes of ∼15 nM for the studied range of total cholesterol, but this correlation was not significant. A significant correlation for potency and LDL concentration which predicted a decrease in the IC50 of ∼25 nM was found for the range of LDL cholesterol concentrations studied (r = −0.415; P < 0.05); a rather small change occurred over a large range of LDL cholesterol concentrations.
TABLE 2.
E5531 activity in whole blood and lipid levels in plasmaa
| Concn (pg/ml) of TNF-α releasedb | IC50 (nM) of E5531 | Cholesterol level (mg/dl)
|
Triglyceride level (mg/dl) | ||
|---|---|---|---|---|---|
| Total | LDL | HDL | |||
| 2,347 | 15.7 | 187 | 105 | 59 | 115 |
| 2,714 | 14.1 | 91 | 108 | 71 | 61 |
| 3,106 | 15.7 | 233 | 133 | 57 | 215 |
| 1,768 | 9.9 | 243 | 164 | 47 | 159 |
| 2,929 | 13.3 | 171 | 66 | 90 | 75 |
| 1,425 | 28.3 | 169 | 97 | 58 | 69 |
| 3,735 | 30.6 | 138 | 77 | 38 | 115 |
| 823 | 22.2 | 189 | 82 | 100 | 37 |
| 2,422 | 21.6 | 201 | 122 | 50 | 143 |
| 2,262 | 21.6 | 146 | 80 | 57 | 45 |
| 2,609 | 22.2 | 136 | 72 | 46 | 89 |
| 2,677 | 2.5 | 181 | 109 | 56 | 81 |
| 2,299 | 15.7 | 198 | 129 | 28 | 205 |
| 1,735 | 21.9 | 198 | 127 | 45 | 129 |
| 1,047 | 3.8 | 233 | 174 | 38 | 105 |
| 3,823 | 25.2 | 180 | 112 | 29 | 196 |
| 2,487 | 11.9 | 202 | 129 | 60 | 63 |
| 2,436 | 17.9 | 223 | 95 | 91 | 183 |
| 1,557 | 5.1 | 191 | 114 | 59 | 90 |
| 1,704 | 1.8 | 253 | 183 | 42 | 141 |
| 1,748 | 1.2 | 178 | 121 | 48 | 46 |
| 788 | 2.6 | 219 | 126 | 85 | 39 |
| 1,773 | 3.9 | 260 | 187 | 43 | 148 |
| 2,382 | 7.0 | 278 | 149 | 89 | 198 |
| 918 | 1.5 | 165 | 88 | 63 | 69 |
| 1,626 | 3.6 | 168 | 97 | 47 | 122 |
| 1,989 | 4.9 | 242 | 148 | 81 | 65 |
| 1,751 | 16.5 | 191 | 131 | 52 | 41 |
| 1,733 | 1.7 | 198 | 137 | 35 | 128 |
| 3,212 | 12 | 171 | 100 | 44 | 137 |
| 2,858 | 10.2 | 158 | 57 | 91 | 51 |
Potency of E5531 (IC50), measurement of TNF-α release, and cholesterol and triglyceride levels were determined as described in the Methods and Materials section.
E5531 activity demonstrated a trend toward the level of response of blood; stronger responders to LPS were slightly less sensitive to drug inhibition (the IC50 changed 25-fold with a 2-fold change in the amount of TNF-α released; r2 = 0.196), but this correlation was not significant.
(ii) E5531 activity is inhibited by HDLs.
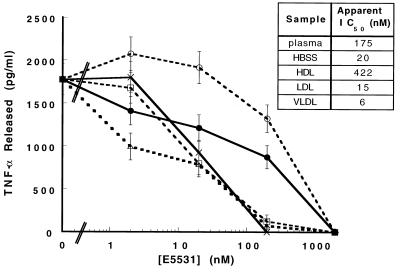
VLDL-chylomicron, LDL, and HDL fractions were partially purified from plasma by the centrifugation or size-exclusion chromatography methods described above. After addition of E5531 to these lipoprotein fractions or plasma, the activity of E5531 was then tested in our standard human blood assay. Immediately after addition of E5531 to these fractions, we were unable to detect significant changes in activity compared to that for drug diluted in HBSS (data not shown). However, as shown in Fig. 4, E5531 incubated overnight in plasma or partially purified HDL was significantly less active (apparent IC50s, 175 and 422 nM, respectively) than E5531 incubated overnight in either HBSS (IC50 = 20 nM), VLDL-chylomicron (IC50 = 6 nM), or LDL (IC50 = 15 nM). HPLC analysis indicated that no significant measurable degradation of E5531 occurred in blood or plasma over this period of time (data not shown), indicating that E5531 is somehow “inactivated.”
FIG. 4.
HDL inhibition of E5531 activity. E5531 at 0, 100, 1,000, and 10,000 nM (final concentrations) was incubated overnight in HBSS (×), plasma (●), HDL (○; 40 mg of cholesterol per dl), LDL (□; 245 mg of cholesterol per dl), or VLDL (▵; 82 mg of cholesterol per dl) and was then added by fivefold dilution to human whole blood followed by the addition of 10 ng of LPS per ml (final concentration). This reaction mixture was incubated for 3 h, and plasma samples were analyzed for TNF-α as described in the Materials and Methods section.
DISCUSSION
As reported previously (15, 25), the in vitro activity of E5531 decreased in the presence of high serum concentrations. An approximately 20-fold difference in potency is observed when E5531 was tested in in vitro assays with monocytes (1% serum; mean IC50, 0.11 nM) and whole-blood assays (80% serum; mean IC50, 2.3 nM). This report describes a possible means by which the activity of E5531 is modulated by plasma lipoproteins. Analysis of the activity of formulated material now reveals small but detectable differences in IC50s with blood from different donors. More dramatically, however, the activity of E5531 is compromised with increased time of exposure to blood (unpublished data) or plasma (Fig. 4).
LPS has been shown to bind to a variety of serum proteins, including transferrin (2), lactoferrin (1), lysozyme (29, 30), bactericidal permeability-increasing protein (14), albumin (37), and the cell surface LPS-binding protein CD14 (26, 38). It has also become clear that LPS is moved into serum lipoproteins by lipid transferases, resulting in a loss of agonistic potency (9, 10, 13, 21, 31, 35). E5531 is a lipophilic molecule that lacks the complex polysaccharide of LPS. When [14C]E5531 was incubated with plasma from heparinized blood (Fig. 1 and 2) or was infused intravenously into humans (Fig. 3), it demonstrated a clear propensity for binding to high-molecular-mass entities, none of which could be purified to single proteins. In addition, stoichiometric calculations on the amount of protein present compared to the amount of drug bound suggested that binding had to occur at ratios far greater than 1:1 E5531 molecule/protein molecule. Cholesterol and apolipoproteins (data not shown) were present in all plasma fractions containing bound E5531, as determined by size-exclusion chromatography, density gradient centrifugation, and affinity chromatography. Similar results were found in all analyses; [14C]E5531 bound to plasma fractions characteristic of HDLs and LDLs with no measurable association of 14C-labeled drug with any common “bulk” plasma proteins. This observation does not, however, rule out the possibility that [14C]E5531 may bind specifically to a protein that is associated with the lipid component(s).
Does E5531 exist in free form in plasma? Size-exclusion analysis of [14C]E5531 binding was complicated by the discovery that free drug did not penetrate into the column matrix, indicating that E5531 aggregates in protein-free salt solution. Survey experiments with protein carriers indicated that drug could be cochromatographed with protein if it was allowed to preequilibrate with a carrier such as albumin. Even with this preadsorbtion, however, the rapid association of [14C]E5531 with the high-molecular-mass fractions (<2 h at 37°C) seen in Fig. 1 still occurred. This observation that free [14C]E5531 does not elute from our columns, combined with our calculated recovery of approximately 100% (when added to plasma), argues that levels of free drug in plasma may be below detection levels. Analysis by affinity chromatography confirmed this result. Is it possible that E5531 activity can be attributed to an immeasurable amount of unbound material that can interact with cells and that can be responsible for the initial activity of E5531? This cell surface-bound fraction could then time-dependently associate with HDLs and become inactivated. This possibility is unlikely because unbound E5531 is immeasurable after overnight incubation in LDLs or VLDLs; however, the apparent IC50 is similar to that of antagonist preincubated overnight in saline, a condition that should leave drug entirely unbound. This leads us to believe that E5531 retains its activity immediately after binding to serum proteins and lipoproteins and then either retains its activity or becomes inactivated with time, depending on the lipoprotein to which it is bound.
For two subjects infused intravenously with [14C]E5531, the distribution into lipoproteins was strikingly different and was dependent on the concentrations of these lipoproteins in plasma. In this case, there appears to be a correlation between increasing LDL and VLDL or chylomicron concentrations and an increased association of drug with these lipoproteins. In subject 1, in whom LDL levels were relatively high, the association of [14C]E5531 with this lipoprotein fraction was enhanced. Subject 2 had lower LDL cholesterol levels and concomitantly lower levels of LDL binding. These results and those from the in vitro binding assays (Fig. 1 to 3) indicate that drug binds to LDL when LDL is present at relatively high concentrations, but when the LDL concentration is decreased, more E5531 binds to VLDLs or chylomicrons and HDLs. This observation makes it unlikely that there is a strict “competition” for drug binding to HDLs and LDLs; instead, it appears that E5531 preferably binds to HDLs, even when some LDLs are present, but increasingly partitions into LDLs or VLDLs as their concentrations increase.
The quantities of [14C]E5531 bound to HDL in the in vitro and in vivo infusion studies were remarkably similar, even though a wide variety of assay and “incubation” conditions were used. In the in vitro binding and centrifugation studies (Fig. 2), the level of recovery of 14C-labeled material in HDLs was roughly 65%, while 35% was found in the LDL fraction. Similar results were obtained with plasma from patients with “normal” cholesterol levels by centrifugation and affinity chromatography (60 to 74%; subjects II to IV in Table 1). This ratio is also similar to that found after analysis of binding by size-exclusion chromatography, as shown in Fig. 2. In that experiment, peak 2, the HDL fraction, contained approximately 67% of the total radioactivity, whereas ∼35% [14C]E5531 bound to LDLs or VLDLs (peak 1). After infusion in vivo, these ratios were approximately 50:50 (HDLs:LDLs-VLDLs).
Association of [14C]E5531 with these lipoprotein fractions is rapid. In contrast to reports on the interaction of LPS with HDLs (12, 39), we describe in a parallel study (34) that we are unable to measure any time dependence for an association of [14C]E5531 to HDLs by density gradient centrifugation or affinity chromatography. Furthermore, no redistribution of E5531 between plasma components after the initial interaction is detectable.
The consequences of E5531 binding to lipoproteins vary with the target lipoprotein acceptor. One consequence of drug binding to these different lipoprotein fractions can be seen in Fig. 4. Long-term (overnight) incubation of therapeutic concentrations of E5531 (0.01 to 1 μM) with plasma results in the inactivation of E5531. This inactivation can be mimicked by addition of physiological concentrations of purified HDLs but not purified LDLs or VLDLs. Such an inactivation may be similar to that described for LPS and HDLs (21). While profound inhibition of drug activity is seen after long-term incubation with HDLs (or plasma), immediate effects on drug potency may be more subtle. The association of [14C]E5531 with LDLs clearly correlates with the LDL cholesterol concentration (Table 1). Data on drug potency as a function of LDL cholesterol levels (Table 2), however, are not as predictive of drug activity as data on direct binding of drug to LDLs, indicating a weaker correlation. However, it must be considered that the potency of a drug in blood is likely to be affected by a wide variety of other factors, possibly including degree of cellular response and a wide variety or combination of other serum factors that could affect the activity of LPS. The result of these variabilities is reflected in the wide range of TNF-α levels produced in response to a standard LPS dose (Table 2).
The means by which E5531 is inactivated by HDLs is unclear, but it is possible that E5531 is sequestered from the outer surface to the lipid core of the particle. We are studying this possibility. If HDL inactivates E5531, then why is there no apparent significant inverse correlation between the HDL cholesterol concentration and activity? In vitro assays that study inactivation as a function of purified HDL concentration indicates that HDL has a high capacity for the inactivation of E5531. In these assays, a 10% solution of “normal” concentrations of HDL can completely inactivate 100 nM E5531 (unpublished data). This makes it likely that physiological HDL concentrations are not a limiting factor for complete inactivation of E5531 at the concentrations used in this study. In light of this ample capacity for HDLs to inactivate E5531 and the apparent affinity of E5531 for HDLs, the association of E5531 to LDLs may be limited to those blood or plasma samples with higher LDL concentrations. At increased LDL concentrations, less E5531 may associate with HDLs and maintain activity, thereby maintaining antagonistic potency over time (Table 2). This result argues that an immediate association of drug with LDLs, as opposed to HDLs, may be beneficial for sustained drug activity.
From our results, we can conclude that the balance of HDLs and other lipoproteins affect the activity of E5531 in plasma. We propose that the interaction of E5531 with HDLs is reflected in the more subtle, weak inhibition of E5531 activity immediately after its addition to serum and the more complete inhibition of antagonistic activity after extended incubation.
While the lipoprotein concentration may affect E5531 activity, it is likely that use of higher doses or extended administration of E5531 can be used to overcome the effects of varying lipoprotein concentration. However, in a therapeutic setting it is also possible that dramatic disease state alterations in plasma lipoprotein levels may need to be considered in dosing calculations. Finally, these observations also indicate that altering the drug distribution in plasma may increase its potency or long-term efficacy. It remains to be determined what such manipulations might have on the efficacy of E5531.
REFERENCES
- 1.Appelmelk B J, An Y Q, Geerts M, Thijs B G, de Boer H A, MacLaren D M, de Graaff J, Nuijens J H. Lactoferrin is a lipid A-binding protein. Infect Immun. 1994;62:2628–2632. doi: 10.1128/iai.62.6.2628-2632.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berger D, Schleich S, Seidelmann M, Beger H G. Demonstration of an interaction between transferrin and lipopolysaccharide—an in vitro study. Eur Surg Res. 1991;23:309–316. doi: 10.1159/000129169. [DOI] [PubMed] [Google Scholar]
- 3.Billiau A, Vandekerckhove F. Cytokines and their interactions with other inflammatory mediators in the pathogenesis of sepsis and septic shock. Eur J Clin Invest. 1991;21:559–573. doi: 10.1111/j.1365-2362.1991.tb01410.x. [DOI] [PubMed] [Google Scholar]
- 4.Bligh E G, Dyer W J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 5.Bone R C. Modulators of coagulation. A critical appraisal of their role in sepsis. Arch Intern Med. 1992;152:1381–1389. doi: 10.1001/archinte.152.7.1381. [DOI] [PubMed] [Google Scholar]
- 6.Bone R C. The pathogenesis of sepsis. Ann Intern Med. 1991;115:457–469. doi: 10.7326/0003-4819-115-6-457. [DOI] [PubMed] [Google Scholar]
- 7.Brown J M, Grosso M A, Harken A H. Cytokines, sepsis and the surgeon. Surg Gynecol Obstet. 1989;169:568–575. [PubMed] [Google Scholar]
- 8.Christ W J, Asano O, Robidoux A L C, Perez M, Wang Y A, Dubuc G R, Gavin W E, Hawkins L D, Mcguinness P D, Mullarkey M A, Lewis M D, Kishi Y, Kawata T, Bristol J R, Rose J R, Rossignol D P, Kobayashi S, Hishinuma L, Kimura A, Asakawa N, Katayama K, Yamatsu I. E5531, a pure endotoxin antagonist of high potency. Science. 1995;268:80–83. doi: 10.1126/science.7701344. [DOI] [PubMed] [Google Scholar]
- 9.Emancipator K, Csako G, Elin R J. In vitro inactivation of bacterial endotoxin by human lipoproteins and apolipoproteins. Infect Immun. 1992;60:596–601. doi: 10.1128/iai.60.2.596-601.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flegel W A, Baumstark M W, Weinstock C, Berg A, Northoff H. Prevention of endotoxin-induced monokine release by human low- and high-density lipoproteins and by apolipoprotein A-I. Infect Immun. 1993;61:5140–5146. doi: 10.1128/iai.61.12.5140-5146.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fong Y, Lowry S F. Tumor necrosis factor in the pathophysiology of infection and sepsis. Clin Immunol Immunopathol. 1990;55:157–170. doi: 10.1016/0090-1229(90)90094-7. [DOI] [PubMed] [Google Scholar]
- 12.Hailman E, Albers J J, Wolfbauer G, Tu A Y, Wright S D. Neutralization and transfer of lipopolysaccharide by phospholipid transfer protein. J Biol Chem. 1996;271:12172–12178. doi: 10.1074/jbc.271.21.12172. [DOI] [PubMed] [Google Scholar]
- 13.Harris H W, Grunfeld C, Feingold K R, Rapp J H. Human very low density lipoproteins and chylomicrons can protect against endotoxin-induced death in mice. J Clin Invest. 1990;86:696–702. doi: 10.1172/JCI114765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jin H K, Yang R H, Marsters S, Ashkenazi A, Bunting S, Marra M N, Scott R W, Baker J B. Protection against endotoxic shock by bactericidal/permeability-increasing protein in rats. J Clin Invest. 1995;95:1947–1952. doi: 10.1172/JCI117877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kawata T, Bristol J R, Rossignol D P, Rose J R, Kobayashi S, Yokohama H, Ishibashi A, Christ W J, Katayama K, Yamatsu I, Kishi Y. E5531, a synthetic non-toxic lipid A derivative blocks the immunobiological activities of lipopolysaccharide. Br J Pharmacol. 1999;127:853–862. doi: 10.1038/sj.bjp.0702596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kilbourn R G, Gross S S, Jubran A, Adams J, Griffith O W, Levi R, Lodato R F. NG-methyl-l-arginine inhibits tumor necrosis factor-induced hypotension: implications for the involvement of nitric oxide. Proc Natl Acad Sci USA. 1990;87:3629–3632. doi: 10.1073/pnas.87.9.3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kilbourn R G, Jubran A, Gross S S, Griffith O W, Levi R, Adams J, Lodato R F. Reversal of endotoxin-mediated shock by NG-methyl-l-arginine, an inhibitor of nitric oxide synthesis. Biochem Biophys Res Commun. 1990;172:1132–1138. doi: 10.1016/0006-291x(90)91565-a. [DOI] [PubMed] [Google Scholar]
- 18.Lemaire M, Pardridge W M, Chaudhuri G. Influence of blood components on the tissue uptake indices of cyclosporin in rats. J Pharmacol Exp Ther. 1988;244:740–743. [PubMed] [Google Scholar]
- 19.Loppnow H, Libby P, Freudenberg M, Krauss J H, Weckesser J, Mayer H. Cytokine induction by lipopolysaccharide (LPS) corresponds to lethal toxicity and is inhibited by nontoxic Rhodobacter capsulatus LPS. Infect Immun. 1990;58:3743–3750. doi: 10.1128/iai.58.11.3743-3750.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oettinger W, Berger D, Beger H G. The clinical significance of prostaglandins and thromboxane as mediators of septic shock. Klin Wochenschr. 1987;65:61–68. doi: 10.1007/BF01745474. [DOI] [PubMed] [Google Scholar]
- 21.Pajkrt D, Doran J E, Koster F, Lerch P G, Arnet B, van der Poll T, ten Cate J W, van Deventer S J. Antiinflammatory effects of reconstituted high-density lipoprotein during human endotoxemia. J Exp Med. 1996;184:1601–1608. doi: 10.1084/jem.184.5.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pardridge W M, Mietus L J. Transport of steroid hormones through the rat blood-brain barrier. Primary role of albumin-bound hormone. J Clin Invest. 1979;64:145–154. doi: 10.1172/JCI109433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Petros A, Bennett D, Vallance P. Effect of nitric oxide synthase inhibitors on hypotension in patients with septic shock. Lancet. 1991;338:1557–1558. doi: 10.1016/0140-6736(91)92376-d. [DOI] [PubMed] [Google Scholar]
- 24.Rose J R, Christ W J, Bristol J R, Kawata T, Rossignol D P. Agonistic and antagonistic activities of bacterially derived Rhodobacter sphaeroides lipid A: comparison with activities of synthetic material of the proposed structure and analogs. Infect Immun. 1995;63:833–839. doi: 10.1128/iai.63.3.833-839.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rossignol D P, Christ W J, Hawkins L D, Kobayashi S, Kawata T, Lynn M, Yamatsu I, Kishi Y. Synthetic endotoxin antagonists. In: Brade H, Morrison D, Opal S, Vogel S, editors. Endotoxin in health and disease. New York, N.Y: Marcel Dekker, Inc.; 1998. [Google Scholar]
- 26.Schumann R R, Leong S R, Flaggs G W, Gray P W, Wright S D, Mathison J C, Tobias P S, Ulevitch R J. Structure and function of lipopolysaccharide binding protein. Science. 1990;249:1429–1431. doi: 10.1126/science.2402637. [DOI] [PubMed] [Google Scholar]
- 27.Segrest J, Albers J, editors. Comparative analysis of mammalian plasma lipoproteins. Methods Enzymol. 1986;128:70–143. doi: 10.1016/0076-6879(86)28063-5. [DOI] [PubMed] [Google Scholar]
- 28.Spooner C E, Markowitz N P, Saravolatz L D. The role of tumor necrosis factor in sepsis. Clin Immunol Immunopathol. 1992;62(1 Pt 2):S11–S17. doi: 10.1016/0090-1229(92)90036-n. [DOI] [PubMed] [Google Scholar]
- 29.Takada K, Ohno N, Yadomae T. Binding of lysozyme to lipopolysaccharide suppresses tumor necrosis factor production in vivo. Infect Immun. 1994;62:1171–1175. doi: 10.1128/iai.62.4.1171-1175.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takada K, Ohno N, Yadomae T. Lysozyme regulates LPS-induced interleukin-6 release in mice. Circulatory Shock. 1994;44:169–174. [PubMed] [Google Scholar]
- 31.Vosbeck K, Tobias P, Mueller H, Allen R A, Arfors K E, Ulevitch R J, Sklar L A. Priming of polymorphonuclear granulocytes by lipopolysaccharides and its complexes with lipopolysaccharide binding protein and high density lipoprotein. J Leukoc Biol. 1990;47:97–104. doi: 10.1002/jlb.47.2.97. [DOI] [PubMed] [Google Scholar]
- 32.Wasan K M, Brazeau G A, Keyhani A, Hayman A C, Lopez-Berestein G. Roles of liposome composition and temperature in distribution of amphotericin B in serum lipoproteins. Antimicrob Agents Chemother. 1993;37:246–250. doi: 10.1128/aac.37.2.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wasan K M, Pritchard P H, Ramaswamy M, Wong W, Donnachie E M, Brunner L J. Differences in lipoprotein lipid concentration and composition modify the plasma distribution of cyclosporine. Pharm Res. 1997;14:1613–1620. doi: 10.1023/a:1012190620854. [DOI] [PubMed] [Google Scholar]
- 34.Wasan K M, Strobel F W, Parrott S C, Lynn M, Christ W J, Hawkins L D, Rossignol D P. Lipoprotein distribution of a novel endotoxin antagonist, E5531, in plasma from human subjects with various lipid levels. Antimicrob Agents Chemother. 1999;43:2562–2564. doi: 10.1128/aac.43.10.2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weinstock C, Ullrich H, Hohe R, Berg A, Baumstark M W, Frey I, Northoff H, Flegel W A. Low density lipoproteins inhibit endotoxin activation of monocytes. Arterioscler Thromb. 1992;12:341–347. doi: 10.1161/01.atv.12.3.341. [DOI] [PubMed] [Google Scholar]
- 36.Welbourn C R, Young Y. Endotoxin, septic shock and acute lung injury: neutrophils, macrophages and inflammatory mediators. Br J Surg. 1992;79:998–1003. doi: 10.1002/bjs.1800791006. [DOI] [PubMed] [Google Scholar]
- 37.Wollenweber H W, Morrison D C. Synthesis and biochemical characterization of a photoactivatable, iodinatable, cleavable bacterial lipopolysaccharide derivative. J Biol Chem. 1985;260:15068–15074. [PubMed] [Google Scholar]
- 38.Wright S D, Ramos R A, Tobias P S, Ulevitch R J, Mathison J C. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science. 1990;249:1431–1433. doi: 10.1126/science.1698311. [DOI] [PubMed] [Google Scholar]
- 39.Yu B, Hailman E, Wright S D. Lipopolysaccharide binding protein and soluble CD14 catalyze exchange of phospholipids. J Clin Invest. 1997;99:315–324. doi: 10.1172/JCI119160. [DOI] [PMC free article] [PubMed] [Google Scholar]