Abstract
Purpose
The abscopal effect is defined when a form of local therapy causes tumor regression of both the target lesion and any untreated tumors. Herein cases of the abscopal effect were systematically reviewed and a patient-level data analysis was performed for clinical predictors of both duration of response and survival.
Methods and Materials
The Population, Intervention, Control, Outcome, Study (PICOS) design approach, Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) literature selection process, and Meta-analysis of Observational Studies in Epidemiology (MOOSE) were used to find articles published before September 2019 in MEDLINE/PubMed and Google Scholar. Inclusion criteria were (1) population: patients with reported abscopal response; (2) intervention: documented treatment(s); (3) control: none; (4) outcomes: overall and progression-free survival; and (5) setting: retrospective case reports. Time from treatment until abscopal response and time from abscopal response until progression/death were calculated. Univariate and multivariate analyses were conducted for survival outcomes.
Results
Fifty studies (n = 55 patients) were included. Median age was 65 years (interquartile range [IQR], 58-70) and 62% were male. Fifty-four (98%) patients received radiation therapy, 34 (62%) received radiation therapy alone, 5 (9.1%) underwent surgery, 4 (7.3%) received chemotherapy, and 11 (20%) received immunotherapy. Median total dose was 32 Gy (IQR, 25.5-48 Gy) and median dose per fraction was 3 Gy (IQR, 2-7.2). Median time until abscopal response was 4 months (IQR, 1-5; min 0.5, max 24). At 5 years, overall survival was 63% and distant progression-free survival was 45%. No variables had statistical significance in predicting duration of response or survival.
Conclusions
Almost all reported cases of the abscopal response are after radiation therapy; however, there are no known predictors of duration of response or survival in this population.
Introduction
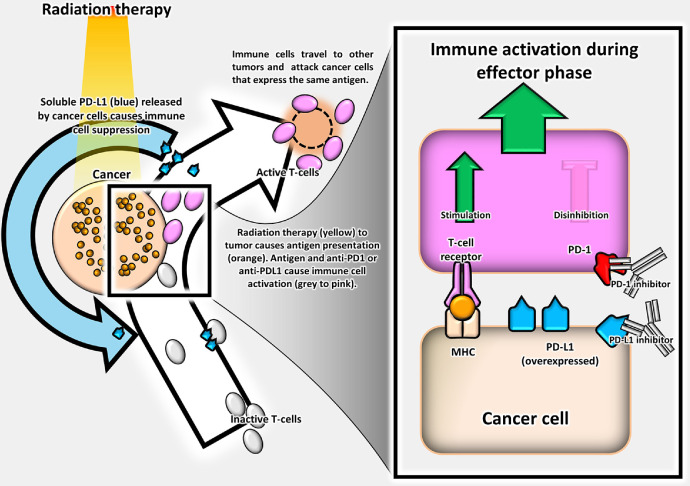
The abscopal effect is defined when a form of local therapy (eg, radiation therapy [RT]) causes tumor regression of both the target lesion and any untreated tumors. Precise biological mechanisms are unknown, but the immune system may be integral in abscopal responses (Fig. 1).1, 2, 3 Owing to promising preclinical data, interest exists in combining immunotherapy with hypofractionated RT to stimulate abscopal responses4, 5, 6, 7 and improve patient outcomes.1,8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19
Figure 1.
Abscopal effect defined.
Various mechanisms have been proposed to explain how radiation interacts with the immune system.10, 11, 12,20 Synergistic RT and immunotherapy have been shown to promote local immune responses4, 5, 6, 7,13 and have been hypothesized to improve the probability of tumor control.11 As a brief review, high dose per fraction RT has been shown to stimulate tumor associated antigen presentation and causes an increased ratio of immunologic cell death to tolerogenic responses, which stimulates CD8+ T lymphocytes, dendritic cells, and natural killer cells.11 Immune checkpoint inhibitors prevent immune tolerance of tumor cells by blocking tumor cell escape from immune surveillance via cellular targets (ie, Programmed Cell Death-1 and Cytotoxic T-Lymphocyte Antigen-4), which are expressed by tumor suppressor cells (CD4+ T lymphocytes, CD8+ T lymphocytes, dendritic cells, and natural killer cells).4, 5, 6, 7,11,13 These synergistic effects were thought to prime the immune system and induce an abscopal response.
Previous systematic reviews have examined the abscopal effect2,9; however, data on progression-free survival, distant metastasis, and overall survival were not reported; use of systemic therapies (including immune checkpoint inhibitors) were not routinely mentioned; and analyses for predictors of response were not performed. The hypothesis was that certain clinical covariates might predict for survival of patients with an abscopal response. Thus, we performed the first patient-level data meta-analysis for predictors of response.
Methods and Materials
Literature selection
The Population, Intervention, Control, Outcome, Study (PICOS) design approach was used to define the inclusion criteria (Table 1). A systematic search was performed using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) literature selection process (Fig. 2) of studies in MEDLINE (PubMed) and Google Scholar. Inclusion criteria were (1) population: patients with reported abscopal response, which was defined by any form of local therapy causing regression of both the target lesion and any untreated tumors; (2) intervention: documented treatment(s); (3) control: none; (4) outcomes: overall and progression-free survival; and (5) setting: retrospective case reports.
Table 1.
PICOS inclusion criteria
| Population | Case reports of the abscopal effect published before September 26, 2019. |
| Intervention | Clearly defined cancer therapy at time of abscopal response and prior courses of treatment (eg, radiation therapy, immunotherapy, chemotherapy, target therapy). |
| Control | None. |
| Outcomes | Overall and progression-free survival. Time from treatment until abscopal response and time from abscopal response until progression or death were calculated. For overall and progression-free survival, the start time was calculated from the time of abscopal effect. |
| Study design | Case reports and case series published in the English literature. |
Abbreviation: PICOS = Population, Intervention, Control, Outcome, Study.
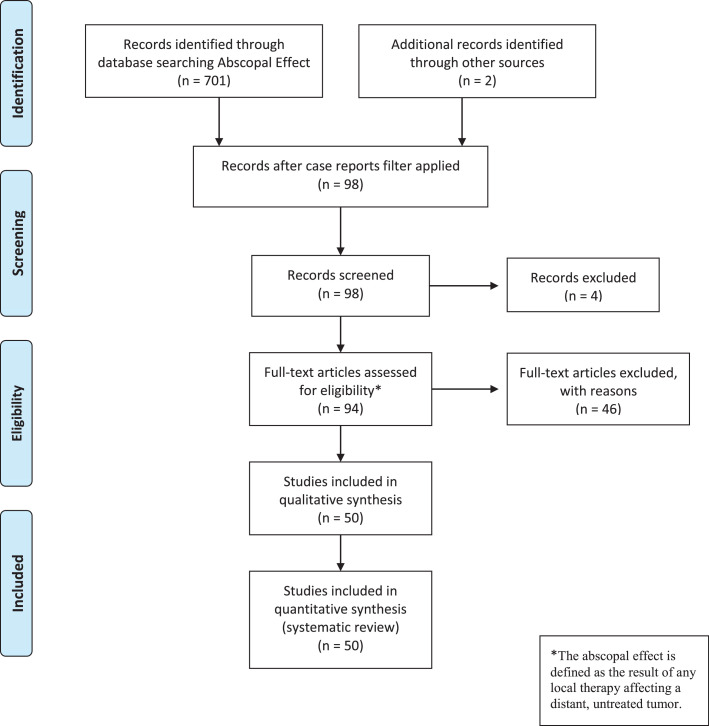
Figure 2.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram.
This method of a patient-level data meta-analysis has been described previously in the literature.21,22 Relevant case reports were systematically identified through a search of PubMed/MEDLINE and Google Scholar with the broad term “abscopal effect.” The initial search yielded 701 articles. Reports that were cited or linked from any review articles and the individual case reports were included. After identifying 98 articles, 4 were rejected because they were not case reports. The remaining articles were screened by the first author (S.J.H.) and were included based upon the aforementioned criteria. Patients could receive any combination of treatment: surgery, RT, immunotherapy, chemotherapy, or targeted therapy for any malignancy. Finally, 50 studies including 55 patients were included.
Data abstraction and analysis
The definition for the start time of an abscopal effect was marked by any regression at an untreated tumor site as noted by imaging, per the report of primary authors from each case report. Individual case reports were reviewed by authors and information was manually extracted and coded into a database. The following data were coded from each case report: characteristics about patients (ie, age, sex), cancer (ie, type [per National Comprehensive Cancer Network guidelines], histology, mutation, stage), treatments received at any point before an abscopal response (ie, surgery, radiation, chemotherapy, immunotherapy), radiation dose (ie, Gy, dose/Gy, biologically effective dose with an α/β of 10), treatment received during the abscopal response (eg, radiation, chemotherapy, immunotherapy, target therapy), time from radiation to an abscopal effect, interval of follow- up/recurrence, and outcomes (ie, overall survival and distant progression-free survival at last known contact date). Unknown/missing variables were coded as missing in the database.
Results
The data of both patient and cancer characteristics are in Table 2. Fifty studies (n = 55 patients) published from 1954 to 2019 met inclusion criteria and were used.23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76 The median patient age was 65 years (interquartile range [IQR], 57-70), and there were 34 (62%) men and 21 (38%) women. Although all patients had metastatic disease at the time of the abscopal effect, only 17 (31%) patients initially presented with a metastatic tumor, and all others had recurrences after initially localized disease. Sixty-seven percent of patients had 5 cancer types: non-small cell lung cancer (NSCLC) (10, 18%),25,32,33,40, 41, 42,49,60,68,72 kidney (9, 16%),24,45,58,64,66,75 melanoma (7, 13%),28,38,48,54,65,70,71 lymphoma (6, 11%),37,51,59,63,73 and hepatobiliary (5, 9.1%).35,44,55, 56, 57
Table 2.
Included articles demonstrating abscopal effect
| Study | Year | True defined* | Sex | Age | Cancer type | RT to primary | RT to metastasis | RT at multiple sites | RT total dose (Gy) | RT fx | Average RT dose/fx (Gy) | BED | RT only | RT + surgery | RT + chemotherapy | RT + IT | Follow- up time (mo) | Outcome | New met |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cotter et al74 | 2011 | Y | M | 70 | 39 | N | Y | N | 12 | 2 | 6 | 19.2 | Y | N | N | N | 25 | Alive | Y |
| Ebner et al69 | 2017 | Y | M | 75 | 13 | N | Y | N | 73.6 | 16 | 4.6 | 107 | Y | N | N | N | 46 | Dead | Y |
| Y | M | 85 | 13 | N | Y | N | 50.4 | 12 | 4.2 | 71.5 | Y | N | N | N | 92 | Alive | N | ||
| Fairlamb66 | 1981 | Y | F | 73 | 23 | N | Y | N | 40 | 15 | 2.7 | 50.6 | Y | N | N | N | 56 | Alive | Y |
| Golden et al72 | 2013 | Y | M | 64 | 41 | N | Y | N | 30 | 5 | 6 | 48 | N | N | N | Y | 10 | Alive | Y |
| Antoniades et al73 | 1977 | Y | M | 44 | 55 | Y | N | N | 30 | 20 | 1.5 | 34.5 | Y | N | N | N | - | Alive | N |
| Y | M | 40 | 55 | Y | N | N | 30 | 20 | 1.5 | 34.5 | Y | N | N | N | - | Alive | N | ||
| Cong et al68 | 2017 | Y | F | 64 | 41 | Y | N | N | 37.5 | 5 | 7.5 | 65.6 | N | N | N | Y | - | Alive | N |
| Desar et al67 | 2016 | Y | M | 19 | 47 | N | Y | N | 30 | 10 | 3 | 39 | Y | N | N | N | 6 | Dead | Y |
| Joe et al61 | 2017 | Y | F | 57 | 14 | Y | Y | Y | 54 | 30 | 1.8 | 63.7 | N | N | Y | N | 48 | Alive | N |
| Lakshmanagowda et al59 | 2009 | Y | F | 65 | 55 | Y | N | N | 24 | 12 | 2 | 28.8 | Y | N | N | N | 6 | Alive | N |
| MacManus et al58 | 1994 | Y | M | 58 | 23 | Y | N | N | 20 | 10 | 2 | 24 | Y | N | N | N | 9 | Dead | N |
| Nam et al44 | 2005 | Y | M | 65 | 21 | N | Y | N | 30 | - | - | - | Y | N | N | N | 15 | Alive | N |
| Ohba et al56 | 1998 | Y | M | 76 | 21 | N | Y | N | 36 | - | - | - | Y | N | N | N | 25 | Alive | N |
| Okuma et al55 | 2011 | Y | M | 63 | 21 | N | Y | N | 60.8 | 27 | 2.2 | 74.4 | Y | N | N | N | 54 | Alive | N |
| Postow et al70 | 2012 | Y | F | 33 | 25 | N | Y | N | 28.5 | 3 | 9.5 | 55.5 | N | N | N | Y | 10 | Alive | N |
| Rees and Ross52 | 1983 | Y | M | 49 | 16 | Y | N | N | 40 | 20 | 2 | 48 | Y | N | N | N | 20 | Dead | - |
| Robins et al51 | 1981 | Y | F | 59 | 55 | N | Y | N | 20 | 10 | 2 | 24 | Y | N | N | N | 4 | Dead | Y |
| Stamell et al71 | 2012 | Y | M | 67 | 25 | Y | N | N | 24 | 3 | 8 | 43.2 | Y | N | N | N | 84 | Alive | Y |
| Takaya et al46 | 2007 | Y | F | 69 | 10 | Y | N | Y | 22 | 10 | 2.2 | 26.8 | Y | N | N | N | 12 | Alive | N |
| Wersäll et al45 | 2009 | Y | F | 83 | 23 | Y | N | N | 32 | 4 | 8 | 57.6 | Y | N | N | N | - | Alive | N |
| Y | F | 64 | 23 | N | Y | N | - | - | - | - | N | N | Y | N | 54 | Alive | N | ||
| Y | M | 69 | 23 | N | Y | N | 30 | 2 | 15 | 75 | Y | N | N | N | 24 | Alive | Y | ||
| Y | F | 55 | 23 | Y | N | N | 32 | 4 | 8 | 57.6 | Y | N | N | N | - | Alive | N | ||
| Sullivan et al48 | 2013 | Y | M | 68 | 25 | N | Y | N | - | - | - | - | N | N | N | N | 13 | Alive | N |
| Hiniker et al65 | 2012 | Y | - | - | 25 | N | Y | N | - | - | - | - | N | N | N | Y | - | Alive | N |
| Isobe et al63 | 2009 | N | F | 65 | 55 | N | N | N | 40 | - | - | - | N | N | N | N | 60 | Alive | N |
| Joe et al61 | 2018 | Y | M | 74 | 16 | Y | Y | Y | 30 | 10 | 3 | 39 | Y | N | N | N | 14 | Alive | N |
| Kodama et al60 | 2013 | Y | M | 74 | 41 | N | Y | Y | 48 | 24 | 2 | 57.6 | N | N | N | Y | 61 | Dead | Y |
| Nakanishi et al57 | 2008 | Y | M | 79 | 21 | Y | N | N | 48 | 1 | 48 | 278 | Y | N | N | N | - | Alive | N |
| Okwan-Duodu et al54 | 2015 | Y | F | 50 | 25 | N | Y | N | - | - | - | - | N | Y | N | Y | 20 | Alive | Y |
| Hamilton et al25 | 2018 | Y | M | 47 | 41 | N | Y | N | 25 | 5 | 5 | 37.5 | N | Y | N | N | 7 | Alive | N |
| Gutkin et al38 | 2018 | Y | M | 57 | 25 | N | Y | N | 54 | 3 | 18 | 151 | N | N | N | Y | 78 | Alive | Y |
| Chino et al40 | 2018 | Y | M | 58 | 41 | N | Y | N | 60 | 8 | 7.5 | 105 | Y | N | N | N | 18 | Alive | N |
| Leung et al34 | 2018 | Y | F | 65 | 7 | Y | Y | Y | 225 | 15 | 15 | 562 | Y | N | N | N | 60 | Alive | N |
| Sperduto et al28 | 2017 | Y | F | 36 | 25 | N | Y | Y | 25 | 5 | 5 | 37.5 | N | Y | Y | N | 120 | Alive | N |
| Van de Walle et al24 | 2016 | Y | F | 66 | 23 | N | Y | N | 39 | 13 | 3 | 50.7 | Y | N | N | N | 17 | Alive | Y |
| Zhao et al23 | 2018 | Y | M | 65 | 16 | N | Y | N | 42 | 6 | 7 | 71.4 | Y | N | N | N | 15 | Alive | N |
| Katayama et al33 | 2017 | Y | M | 63 | 41 | N | Y | Y | 45 | 15 | 3 | 58.5 | Y | N | N | N | 9 | Alive | N |
| Britschgi et al41 | 2018 | Y | M | 47 | 41 | N | Y | N | 18 | 3 | 6 | 28.8 | N | N | N | Y | 24 | Alive | N |
| Kim and Kim35 | 2019 | Y | M | 70 | 21 | Y | N | N | 48 | 4 | 12 | 105 | N | Y | N | N | 21 | Alive | N |
| Bitran42 | 2019 | Y | F | 62 | 41 | Y | N | N | 27 | 9 | 3 | 35.1 | N | N | N | Y | 54 | Alive | N |
| Shinde et al29 | 2019 | Y | M | 75 | 20 | Y | N | Y | 14.8 | 2 | 7.4 | 25.7 | N | N | N | Y | 10 | Alive | N |
| Lin et al32 | 2019 | Y | M | 71 | 41 | N | Y | N | 48 | 8 | 6 | 76.8 | Y | N | N | N | 19 | Alive | Y |
| Brenneman et al36 | 2019 | Y | F | 67 | 47 | Y | N | N | 50 | 25 | 2 | 60 | Y | N | N | N | 18 | Alive | N |
| Sato et al31 | 2016 | Y | M | 54 | 18 | Y | N | N | 48 | 24 | 2 | 57.6 | N | N | N | Y | 5 | Dead | Y |
| Shi et al27 | 2017 | Y | F | 67 | 43 | Y | N | N | 45 | 15 | 3 | 58.5 | N | Y | N | N | 1 | Dead | N |
| Hidaka et al37 | 2017 | Y | M | 88 | 55 | Y | N | Y | 32 | 8 | 4 | 44.8 | Y | N | N | N | 6 | Dead | N |
| Barsky et al39 | 2019 | Y | M | 67 | 24 | Y | N | N | 30 | 10 | 3 | 39 | N | N | Y | N | 7 | Dead | N |
| Siva et al49 | 2013 | Y | F | 78 | 41 | Y | N | Y | 60 | 30 | 2 | 72 | Y | N | N | N | 2 | Alive | Y |
| Ishiyama et al64 | 2012 | Y | M | 61 | 23 | N | Y | Y | 18 | 1 | 18 | 50.4 | Y | N | N | N | 34 | Alive | Y |
| Poon and Wong53 | 2017 | Y | M | 79 | 45 | N | Y | Y | 24 | 4 | 6 | 38.4 | Y | N | N | N | 6 | Alive | N |
| Azami et al43 | 2018 | Y | F | 64 | 7 | Y | Y | Y | 60 | 30 | 2 | 72 | Y | N | N | N | 21 | Alive | N |
| Masue et al75 | 2007 | N | M | 58 | 23 | N | N | N | - | - | - | - | N | N | N | N | 46 | Alive | N |
| Agyeman et al76 | 2019 | Y | M | 56 | 47 | Y | N | N | 40 | 20 | 2 | 48 | Y | N | N | N | 17 | Alive | N |
Abbreviations: BED = biologically effective dose, assuming an α:β ratio of 10; fx = fraction; IT = immunotherapy; met = metastasis; RT = radiation therapy.
Refers to whether or not the article demonstrates a true abscopal response (as defined by the result of any local therapy affecting a distant, untreated tumor).
Data pertinent to treatment characteristics are in Table 2. Treatment analysis was broken up into 3 phases: (1) prior treatment course, (2) treatment course leading up to an abscopal response, and (3) treatment during or after an abscopal response. All but 1 patient75 received RT either at the time of an abscopal response or in prior courses of therapy. (1) Prior treatment courses were as follows (treatments in this phase were not mutually exclusive): 9 (16%) had RT,28,33,38,41,46,53,63,72,74 26 (47%) had surgery,23,24,28,31,33,38, 39, 40, 41, 42,45,48,53, 54, 55,60,66, 67, 68, 69, 70,74, 75, 76 23 (42%) had chemotherapy,23,27,28,31,32,35,36,38,40, 41, 42,48,49,51,53,56,59,63,67,68,70, 71, 72 and 14 (25%) had immunotherapy.28,29,32,39,41,45,53,54,65,67,68,70,72,76 (2) During the course of treatment leading up to an abscopal response, 34 (62%) patients received RT alone,24,32, 33, 34,36,37,40,43, 44, 45,46,49,51, 52, 53,55,56,57, 58, 59,62,64,66, 67, 68, 69,71,73,74,76 5 (9.1%) underwent surgery with RT,25,27,28,35,54 4 (7.3%) received chemotherapy with RT,28,39,45,61 and 11 (20%) received immunotherapy with RT.29,31,38,41,42,54,60,65,68,70,72 (3) Treatments during or after the abscopal response were as follows: 22 (40%) patients received RT only to their primary tumor27,29,31,35, 36, 37,39,42,43,45,46,49,52,57, 58, 59,68,71,73,76 and 29 (53%) received RT only to a metastasis.23, 24, 25,28,32,33,38,40,41,43, 44, 45,48,51,53, 54, 55, 56,60,64, 65, 66, 67,69,70,72,74 Three (5.5%) patients received RT to both the primary tumor and a metastasis,34,61,62 but still experienced the abscopal response at a distant site from radiation (Table 2). Only 1 (1.8%) patient did not receive RT during the course of treatment leading to an abscopal response, but had a history of prior RT (Table 2).63 Targeted therapy was documented in 8 (15%) cases.24,43,48,53,54,60,67,68 The median reported radiation dose and dose per fraction were 32 Gy (IQR, 25.5-48 Gy; min 12 Gy, max 73.6 Gy) and 3 Gy per fraction (IQR, 2-7.2 Gy per fraction; min 1.5 Gy per fraction, max 48 Gy per fraction).
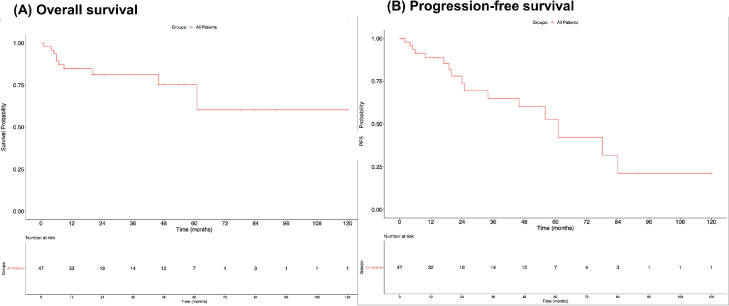
Ninety-six percent of the articles selected in this work demonstrated a clear abscopal effect as defined by the result of any local therapy affecting a distant, untreated tumor (Table 2). The median time until an abscopal effect was 3 months (IQR, 1-5; min 0.5, max 24). Median follow-up time after the abscopal effect was 18.5 months.23, 24, 25, 26, 27,28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56,58, 59, 60, 61, 62, 63, 64, 65, 66, 67,69, 70, 71, 72,74 New metastases occurred in 16 (29%) patients postabscopal effect,24,31,32,38,45,49,51,54,60,64,66,67,69,71,72,74 whereas the rest of the 39 (71%) patients had stable disease during case follow-up Figure 3. depicts Kaplan-Meier curves showing a 5-year overall survival of 63% and a 5-year progression-free survival of 45%. Univariate analysis was performed to explore factors that correlate to patient survival and development of new metastases. No variables had statistical significance in predicting duration of response or survival (Table 3).
Figure 3.
Kaplan-Meier curves. (A) Overall survival at 5 years was 63%. (B) Progression-free survival at 5 years was 45%.
Table 3.
Predictors of overall survival and progression-free survival after abscopal response
| Univariate hazard ratio | 95% CI | P value | Multivariate hazard ratio | 95% CI | P value | |
|---|---|---|---|---|---|---|
| Overall survival | ||||||
| Surgery | ||||||
| No | Ref | Ref | Ref | Ref | Ref | Ref |
| Yes | 0.9 | 0.12-7.60 | .96 | 1.16 | 0.11-12.13 | .9 |
| Chemotherapy | ||||||
| No | Ref | Ref | Ref | Ref | Ref | Ref |
| Yes | 0.63 | 0.08-5.01 | .66 | 0.46 | 0.04-4.79 | .51 |
| Immunotherapy | ||||||
| No | Ref | Ref | Ref | Ref | Ref | Ref |
| Yes | 0.68 | 0.14-3.22 | .63 | 0.6 | 0.12-2.92 | .52 |
| BED 10 Gy increase | 0.99 | 0.96-1.01 | .29 | 0.99 | 0.96-1.01 | .3 |
| Progression-free survival | ||||||
| Surgery | ||||||
| No | Ref | Ref | Ref | Ref | Ref | Ref |
| Yes | 0.46 | 0.06-3.57 | .46 | 3.7 × 10^-9 | 0-Inf | .99 |
| Chemotherapy | ||||||
| No | Ref | Ref | Ref | Ref | Ref | Ref |
| Yes | 0.32 | 0.04-2.42 | .27 | 0.93 | 0.11-7.73 | .95 |
| Immunotherapy | ||||||
| No | Ref | Ref | Ref | Ref | Ref | Ref |
| Yes | 1.97 | 0.70-5.59 | .2 | 1.21 | 0.39-3.80 | .74 |
| BED 10 Gy increase | 0.99 | 0.98-1.01 | .42 | 0.99 | 0.98-1.01 | .36 |
Abbreviations: BED = biologically effective dose; CI = confidence interval; Ref = reference value.
Discussion
This is the first patient-level data meta-analysis of reported abscopal effects. We found that 67% of abscopal responses were reported in NSCLC, kidney cancer, melanoma, lymphomas, and hepatobiliary cancers. Five years after an abscopal response, 55% of patients had disease progression and 63% were alive, which suggested these patients with metastatic cancer had a relatively favorable and indolent biology. All reported cases of the abscopal response were after RT, but not other local treatments like surgery. There were no known predictors of duration of response or survival.
Preclinical data suggested surgery did not boost the abscopal response77 nor did it induce antigen-specific immune responses in patients with prostate cancer, whereas radiation did.78 Furthermore, preclinical data combining focal RT with anti-programmed cell death protein 1 (anti-PD1/PDL1) agents (PD-1 is an immune checkpoint) demonstrated abscopal responses more reliably with higher doses per fraction than without combination.6 In clinical reports, patients with dramatic changes to their T-cell repertoire were more likely to be responders.15 Although these effects were hypothesized, 25% of patients in this work received immunotherapy but did not appear to have improvement in progression-free survival or overall survival, compared with patients who did not receive immunotherapy. Additionally, there was no apparent effect of radiation dose, dose per fraction, or treatment location (primary vs metastasis vs both) on outcomes.
Several case reports and retrospective studies have shown relationships between RT and immunotherapy in certain cancer types.16,17 Prospective trials in metastatic head and neck squamous cell carcinoma by McBride et al79 found the combination of stereotactic body RT (SBRT) and checkpoint blockade did not improve objective response rate in nonirradiated lesions or overall survival in unselected patients with metastatic disease. Yet, this approach was moderately predictive for overall survival in patients based on human papilloma virus status and PD1 status. A study by Theelen et al80 examined the effects of pembrolizumab in activating the tumor microenvironment in NSCLC. They discovered that administering SBRT before pembrolizumab doubled the overall response rate, but did not meet the prespecified endpoint, so larger studies are needed to fully examine this relationship.
Limitations of this analysis are as follows: first, recognizing a true abscopal effect as an example of clear systemic response may be obscured by bias in how the abscopal effect is reported. Distinguishing abscopal effects from spontaneous regression and the bystander effect can be highly subjective, and may cause underreporting or misreporting of abscopal responses by clinicians. There is heterogeneity for how an abscopal response is defined (regression at some untreated lesions vs regression at all untreated lesions). Second, the utilization of second or third line treatment, in addition to RT, may cause difficulty in deciphering the precise treatment that generated the abscopal effect. Finally, the study lacked a control group, so although the abscopal effect is extremely rare, it remains difficult to assess how these patients performed in comparison to patients of a similar cohort that did not exhibit an abscopal effect. In the primary literature, the magnitude of the abscopal response was often not documented. Not every case report of the abscopal effect is being published and not every study reported the same duration of follow-up in the same manner. To keep the data consistent, progression free survival and overall survival were used for gauging abscopal response.
To better study the abscopal effect in the future, there must be an emphasis on standardizing how the abscopal response is reported and monitoring for reporting bias within case reports. It has been reported that the peak PD1 upregulation can occur 4 to 6 days after tumor irradiation. Afterward, the expression of PD1 will decrease gradually.11 More work remains to be done for how to place various immunologic, pharmacologic, and radiotherapeutic mechanisms on a definitive abscopal effect timeline. To completely assess abscopal effects, a full timeline of disease evolution must be determined, and potential confounders must be accounted for in addition to evaluating the type of RT administered (SBRT vs conventionally fractionated RT). Future research should also consider investigating biomarkers, clinical parameters, and other methods to guide studies into the abscopal effect.
Conclusion
This is the first patient-level data meta-analysis of reported abscopal effects. We found that 67% of abscopal responses were reported in NSCLC, kidney cancers, melanomas, lymphomas, and hepatobiliary cancers. Ninety-six percent of the articles selected in this work demonstrated a clear abscopal effect as defined by the result of any local therapy affecting a distant, untreated tumor. Five years after an abscopal response, 55% of patients had disease progression and 63% were alive. Almost every reported case of the abscopal effect was after RT, and only rarely in other local treatments like surgery. There were no known clinical predictors of duration of response or survival.
Footnotes
Sources of support: This work had no specific funding.
Disclosures: Dr Zaorsky is supported by startup funding from the Penn State Cancer Institute and Penn State College of Medicine, and is funded by the National Institutes of Health Grant LRP 1 L30 CA231572-01 and by the American Cancer Society – Tri State CEOs Against Cancer Clinician Scientist Development Grant, CSDG-20-013-01-CCE. Dr Zaorsky and Dr Trifiletti received remuneration from Springer Nature for the textbook, Absolute Clinical Radiation Oncology Review. Dr Siva is supported by the Cancer Council Victoria Colebatch Fellowship and Peter MacCallum Discovery Partner Fellowship. Dr McBride is supported by funding from Genentech, Janssen, and Stand-Up-to-Cancer.
Data sharing statement: All data generated and analyzed during this study are included in this published article (and its supplementary information files).
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.adro.2022.100909.
Appendix. Supplementary materials
References
- 1.Liu Y, Dong Y, Kong L, Shi F, Zhu H, Yu J. Abscopal effect of radiotherapy combined with immune checkpoint inhibitors. J Hematol Oncol. 2018;11:104. doi: 10.1186/s13045-018-0647-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abuodeh Y, Venkat P, Kim S. Systematic review of case reports on the abscopal effect. Curr Probl Cancer. 2016;40:25–37. doi: 10.1016/j.currproblcancer.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 3.Siva S, MacManus MP, Martin RF, Martin OA. Abscopal effects of radiation therapy: A clinical review for the radiobiologist. Cancer Lett. 2015;356:82–90. doi: 10.1016/j.canlet.2013.09.018. [DOI] [PubMed] [Google Scholar]
- 4.Marciscano AE, Haimovitz-Friedman A, Lee P, et al. Immunomodulatory effects of stereotactic body radiation therapy: Preclinical insights and clinical opportunities. Int J Radiat Oncol Biol Phys. 2021;110:35–52. doi: 10.1016/j.ijrobp.2019.02.046. [DOI] [PubMed] [Google Scholar]
- 5.Vanpouille-Box C, Alard A, Aryankalayil MJ, et al. DNA exonuclease Trex1 regulates radiotherapy-induced tumour immunogenicity. Nat Commun. 2017;8:15618. doi: 10.1038/ncomms15618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deng L, Liang H, Burnette B, et al. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest. 2014;124:687–695. doi: 10.1172/JCI67313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lan J, Li R, Yin LM, et al. Targeting myeloid-derived suppressor cells and programmed death ligand 1 confers therapeutic advantage of ablative hypofractionated radiation therapy compared with conventional fractionated radiation therapy. Int J Radiat Oncol Biol Phys. 2018;101:74–87. doi: 10.1016/j.ijrobp.2018.01.071. [DOI] [PubMed] [Google Scholar]
- 8.Wani SQ, Dar IA, Khan T, Lone MM, Afroz F. Radiation therapy and its effects beyond the primary target: an abscopal effect. Cureus. 2019;11:e4100. doi: 10.7759/cureus.4100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dagoglu N, Karaman S, Caglar HB, Oral EN. Abscopal effect of radiotherapy in the immunotherapy era: Systematic review of reported cases. Cureus. 2019;11:e4103. doi: 10.7759/cureus.4103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reynders K, Illidge T, Siva S, Chang JY, De Ruysscher D. The abscopal effect of local radiotherapy: Using immunotherapy to make a rare event clinically relevant. Cancer Treat Rev. 2015;41:503–510. doi: 10.1016/j.ctrv.2015.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ashrafizadeh M, Farhood B, Eleojo Musa A, Taeb S, Rezaeyan A, Najafi M. Abscopal effect in radioimmunotherapy. Int Immunopharmacol. 2020;85 doi: 10.1016/j.intimp.2020.106663. [DOI] [PubMed] [Google Scholar]
- 12.Xing D, Siva S, Hanna GG. The abscopal effect of stereotactic radiotherapy and immunotherapy: Fool's gold or El Dorado? Clin Oncol. 2019;31:432–443. doi: 10.1016/j.clon.2019.04.006. [DOI] [PubMed] [Google Scholar]
- 13.Rodríguez-Ruiz ME, Vanpouille-Box C, Melero I, Formenti SC, Demaria S. Immunological mechanisms responsible for radiation-induced abscopal effect. Trends Immunol. 2018;39:644–655. doi: 10.1016/j.it.2018.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luke JJ, Lemons JM, Karrison TG, et al. Safety and clinical activity of pembrolizumab and multisite stereotactic body radiotherapy in patients with advanced solid tumors. J Clin Oncol. 2018;36:1611–1618. doi: 10.1200/JCO.2017.76.2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Formenti SC, Rudqvist NP, Golden E, et al. Radiotherapy induces responses of lung cancer to CTLA-4 blockade. Nat Med. 2018;24:1845–1851. doi: 10.1038/s41591-018-0232-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shaverdian N, Lisberg AE, Bornazyan K, et al. Previous radiotherapy and the clinical activity and toxicity of pembrolizumab in the treatment of non-small-cell lung cancer: A secondary analysis of the KEYNOTE-001 phase 1 trial. Lancet Oncol. 2017;18:895–903. doi: 10.1016/S1470-2045(17)30380-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koller KM, Mackley HB, Liu J, et al. Improved survival and complete response rates in patients with advanced melanoma treated with concurrent ipilimumab and radiotherapy versus ipilimumab alone. Cancer Biol Ther. 2017;18:36–42. doi: 10.1080/15384047.2016.1264543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parker CC, James ND, Brawley CD, et al. Radiotherapy to the primary tumour for newly diagnosed, metastatic prostate cancer (STAMPEDE): A randomised controlled phase 3 trial. Lancet. 2018;392:2353–2366. doi: 10.1016/S0140-6736(18)32486-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ngwa W, Irabor OC, Schoenfeld JD, Hesser J, Demaria S, Formenti SC. Using immunotherapy to boost the abscopal effect. Nat Rev Cancer. 2018;18:313–322. doi: 10.1038/nrc.2018.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brooks ED, Chang JY. Time to abandon single-site irradiation for inducing abscopal effects. Nat Rev Clin Oncol. 2019;16:123–135. doi: 10.1038/s41571-018-0119-7. [DOI] [PubMed] [Google Scholar]
- 21.Lehrer EJ, Peterson J, Brown PD, et al. Treatment of brain metastases with stereotactic radiosurgery and immune checkpoint inhibitors: An international meta-analysis of individual patient data. Radiother Oncol. 2019;130:104–112. doi: 10.1016/j.radonc.2018.08.025. [DOI] [PubMed] [Google Scholar]
- 22.Cummings M, Lehrer EJ, Drabick JJ, Gusani NJ, Trifiletti DM, Zaorsky NG. Exceptional responders in oncology: A systematic review and meta-analysis of patient level data. Am J Clin Oncol Cancer Clin Trials. 2019;42:624–635. doi: 10.1097/COC.0000000000000572. [DOI] [PubMed] [Google Scholar]
- 23.Zhao X, Kang J, Zhao R. Abscopal effect of radiation on lymph node metastasis in esophageal carcinoma: A case report and literature review. Oncol Lett. 2018;16:3555–3560. doi: 10.3892/ol.2018.9084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van de Walle M, Demol J, Staelens L, Rottey S. Abscopal effect in metastatic renal cell carcinoma. Acta Clin Belgica Int J Clin Lab Med. 2017;72:245–249. doi: 10.1080/17843286.2016.1201614. [DOI] [PubMed] [Google Scholar]
- 25.Hamilton AJ, Seid J, Verdecchia K, Chuba P. Abscopal effect after radiosurgery for solitary brain metastasis from non-small cell lung cancer. Cureus. 2018;10:e3777. doi: 10.7759/cureus.3777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matsushita Y, Nakamura K, Furuse H, Ichinohe K, Miyake H. Marked response to nivolumab combined with external radiation therapy for metastatic renal cell carcinoma: Report of two cases. Int Cancer Conf J. 2019;8:29–32. doi: 10.1007/s13691-018-0349-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shi F, Wang X, Teng F, Kong L, Yu J. Abscopal effect of metastatic pancreatic cancer after local radiotherapy and granulocyte-macrophage colony-stimulating factor therapy. Cancer Biol Ther. 2017;18:137–141. doi: 10.1080/15384047.2016.1276133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sperduto W, King DM, Watanabe Y, Lou E, Sperduto PW. Case report of extended survival and quality of life in a melanoma patient with multiple brain metastases and review of literature. Cureus. 2017;9:e1947. doi: 10.7759/cureus.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shinde A, Novak J, Freeman ML, Glaser S, Amini A. Induction of the abscopal effect with immunotherapy and palliative radiation in metastatic head and neck squamous cell carcinoma: A case report and review of the literature. Cureus. 2019;11:e4201. doi: 10.7759/cureus.4201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.MacManus MP, Hofman MS, Hicks RJ, et al. Abscopal regressions of lymphoma after involved-site radiation therapy confirmed by positron emission tomography. Int J Radiat Oncol Biol Phys. 2020;108:204–211. doi: 10.1016/j.ijrobp.2020.02.636. [DOI] [PubMed] [Google Scholar]
- 31.Sato H, Suzuki Y, Yoshimoto Y, et al. An abscopal effect in a case of concomitant treatment of locally and peritoneally recurrent gastric cancer using adoptive T-cell immunotherapy and radiotherapy. Clin Case Reports. 2017;5:380–384. doi: 10.1002/ccr3.758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin X, Lu T, Xie Z, et al. Extracranial abscopal effect induced by combining immunotherapy with brain radiotherapy in a patient with lung adenocarcinoma: A case report and literature review. Thorac Cancer. 2019;10:1272–1275. doi: 10.1111/1759-7714.13048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Katayama K, Tamiya A, Koba T, Fukuda S, Atagi S. An abscopal response to radiation therapy in a patient with metastatic non-small cell lung cancer: A case report. J Cancer Sci Ther. 2017;9 [Google Scholar]
- 34.Leung HWC, Wang SY, Jin-Jhih H, Chan ALF. Abscopal effect of radiation on bone metastases of breast cancer: A case report. Cancer Biol Ther. 2018;19:20–24. doi: 10.1080/15384047.2017.1394545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim JO, Kim CA. Abscopal resolution of a hepatic metastasis in a patient with metastatic cholangiocarcinoma following radical stereotactic body radiotherapy to a synchronous early stage non-small cell lung cancer. Cureus. 2019;11:e4082. doi: 10.7759/cureus.4082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brenneman RJ, Sharifai N, Fischer-Valuck B, et al. Abscopal effect following proton beam radiotherapy in a patient with inoperable metastatic retroperitoneal sarcoma. Front Oncol. 2019;9:922. doi: 10.3389/fonc.2019.00922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hidaka Y, Takeichi T, Ishikawa Y, Kawamura M, Akiyama M. Abscopal effect of local irradiation treatment for diffuse large B-cell lymphoma. Acta Derm Venereol. 2017;97:1140–1141. doi: 10.2340/00015555-2729. [DOI] [PubMed] [Google Scholar]
- 38.Gutkin PM, Hiniker SM, Swetter SM, Reddy SA, Knox SJ. Complete response of metastatic melanoma to local radiation and immunotherapy: 6.5 year follow-up. Cureus. 2018;10:e3723. doi: 10.7759/cureus.3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barsky AR, Cengel KA, Katz SI, Sterman DH, Simone CB. First-ever abscopal effect after palliative radiotherapy and immuno-gene therapy for malignant pleural mesothelioma. Cureus. 2019;11:e4102. doi: 10.7759/cureus.4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chino F, Pollis KE, Choi S, Salama JK, Palta M. stereotactic body radiation therapy–induced abscopal effect on hepatocellular carcinoma after treatment for lung cancer: A case report. Hepatology. 2018;68:1653–1655. doi: 10.1002/hep.30100. [DOI] [PubMed] [Google Scholar]
- 41.Britschgi C, Riesterer O, Burger IA, Guckenberger M, Curioni-Fontecedro A. Report of an abscopal effect induced by stereotactic body radiotherapy and nivolumab in a patient with metastatic non-small cell lung cancer. Radiat Oncol. 2018;13:102. doi: 10.1186/s13014-018-1049-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bitran J. The abscopal effect exists in non-small cell lung cancer: A case report and review of the literature. Cureus. 2019;11:e4118. doi: 10.7759/cureus.4118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Azami A, Suzuki N, Azami Y, et al. Abscopal effect following radiation monotherapy in breast cancer: A case report. Mol Clin Oncol. 2018;9:283–286. doi: 10.3892/mco.2018.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nam SW, Han JY, Kim JI, et al. Spontaneous regression of a large hepatocellular carcinoma with skull metastasis. J Gastroenterol Hepatol. 2005;20:488–492. doi: 10.1111/j.1440-1746.2005.03243.x. [DOI] [PubMed] [Google Scholar]
- 45.Wersäll PJ, Blomgren H, Pisa P, Lax I, Kälkner K-M, Svedman C. Regression of non-irradiated metastases after extracranial stereotactic radiotherapy in metastatic renal cell carcinoma. Acta Oncol (Madr) 2006;45:493–497. doi: 10.1080/02841860600604611. [DOI] [PubMed] [Google Scholar]
- 46.Takaya M, Niibe Y, Tsunoda S, et al. Abscopal effect of radiation on toruliform para-aortic lymph node metastases of advanced uterine cervical carcinoma - A case report. Anticancer Res. 2007;27:499–503. [PubMed] [Google Scholar]
- 47.Tubin S, Raunik W. Hunting for abscopal and bystander effects: Clinical exploitation of non-targeted effects induced by partial high-single-dose irradiation of the hypoxic tumour segment in oligometastatic patients. Acta Oncol. 2017;56:1333–1339. doi: 10.1080/0284186X.2017.1346385. [DOI] [PubMed] [Google Scholar]
- 48.Sullivan RJ, Lawrence DP, Wargo JA, Oh KS, Gonzalez RG, Piris A. Case 21-2013. N Engl J Med. 2013;369:173–183. doi: 10.1056/NEJMcpc1302332. [DOI] [PubMed] [Google Scholar]
- 49.Siva S, Callahan J, Macmanus MP, Martin O, Hicks RJ, Ball DL. Asbcopal effects after conventional and stereotactic lung irradiation of non-small-cell lung cancer. J Thorac Oncol. 2013;8:e71–e72. doi: 10.1097/JTO.0b013e318292c55a. [DOI] [PubMed] [Google Scholar]
- 50.Sham RL. The abscopal effect and chronic lymphocytic leukemia. Am J Med. 1995;98:307–308. doi: 10.1016/S0002-9343(99)80380-5. [DOI] [PubMed] [Google Scholar]
- 51.Robins HI, Buchon JA, Varanasi VR, Weinstein AB. The abscopal effect: Demonstration in lymphomatous involvement of kidneys. Med Pediatr Oncol. 1981;9:473–476. doi: 10.1002/mpo.2950090510. [DOI] [PubMed] [Google Scholar]
- 52.Rees GJG, Ross CMD. Abscopal regression following radiotherapy for adenocarcinoma. Br J Radiol. 1983;56:63–66. doi: 10.1259/0007-1285-56-661-63. [DOI] [PubMed] [Google Scholar]
- 53.Poon DMC, Wong KCW. Lymph node response in a patient with metastatic castration-resistant prostate cancer treated with radium-223. Clin Genitourin Cancer. 2018;16:e397–e401. doi: 10.1016/j.clgc.2017.10.024. [DOI] [PubMed] [Google Scholar]
- 54.Okwan-Duodu D, Pollack BP, Lawson D, Khan MK. Role of radiation therapy as immune activator in the era of modern immunotherapy for metastatic malignant melanoma. Am J Clin Oncol Cancer Clin Trials. 2015;38:119–125. doi: 10.1097/COC.0b013e3182940dc3. [DOI] [PubMed] [Google Scholar]
- 55.Okuma K, Yamashita H, Niibe Y, Hayakawa K, Nakagawa K. Abscopal effect of radiation on lung metastases of hepatocellular carcinoma: A case report. J Med Case Rep. 2011;5:111. doi: 10.1186/1752-1947-5-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ohba K, Omagari K, Nakamura T, et al. Abscopal regression of hepatocellular carcinoma after radiotherapy for bone metastasis. Gut. 1998;43:575–577. doi: 10.1136/gut.43.4.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nakanishi M, Chuma M, Hige S, Asaka M. Abscopal effect on hepatocellular carcinoma. Am J Gastroenterol. 2008;103:1320–1321. doi: 10.1111/j.1572-0241.2007.01782_13.x. [DOI] [PubMed] [Google Scholar]
- 58.MacManus MP, Harte RJ, Stranex S. Spontaneous regression of metastatic renal cell carcinoma following palliative irradiation of the primary tumour. Ir J Med Sci. 1994;163:461–463. doi: 10.1007/BF02940567. [DOI] [PubMed] [Google Scholar]
- 59.Lakshmanagowda PB, Viswanath L, Thimmaiah N, Dasappa L, Supe SS, Kallur P. Abscopal effect in a patient with chronic lymphocytic leukemia during radiation therapy: A case report. Cases J. 2009;2:204. doi: 10.1186/1757-1626-2-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kodama K, Higashiyama M, Okami J, et al. A possible abscopal effect of post-irradiation immunotherapy in two patients with metastatic lung tumors. Int Cancer Conf J. 2014;3:122–127. [Google Scholar]
- 61.Joe MB, Lum JJ, Watson PH, Tonseth RP, McGhie JP, Truong PT. Radiation generates an abscopal response and complete resolution of metastatic squamous cell carcinoma of the anal canal: A case report. J Gastrointest Oncol. 2017;8:E84–E89. doi: 10.21037/jgo.2017.06.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bruton Joe M, Truong PT. Abscopal effect after palliative radiation therapy for metastatic adenocarcinoma of the esophagus. Cureus. 2018;10:e3089. doi: 10.7759/cureus.3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Isobe Y, Aritaka N, Sasaki M, Oshimi K, Sugimoto K. Spontaneous regression of natural killer cell lymphoma. J Clin Pathol. 2009;62:647–650. doi: 10.1136/jcp.2008.062976. [DOI] [PubMed] [Google Scholar]
- 64.Ishiyama H, Teh BS, Ren H, et al. Spontaneous regression of thoracic metastases while progression of brain metastases after stereotactic radiosurgery and stereotactic body radiotherapy for metastatic renal cell carcinoma: Abscopal effect prevented by the blood-brain barrier? Clin Genitourin Cancer. 2012;10:196–198. doi: 10.1016/j.clgc.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 65.Hiniker SM, Chen DS, Knox SJ. Abscopal effect in a patient with melanoma. N Engl J Med. 2012;366:2035–2036. doi: 10.1056/NEJMc1203984. [DOI] [PubMed] [Google Scholar]
- 66.Fairlamb DJ. Spontaneous regression of metastases of renal cancer: A report of two cases including the first recorded regression following irradiation of a dominant metastasis and review of the world literature. Cancer. 1981;47:2102–2106. doi: 10.1002/1097-0142(19810415)47:8<2102::aid-cncr2820470833>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 67.Desar IME, Braam PM, Kaal SEJ, Gerritsen WR, Oyen WJG, van der Graaf WTA. Abscopal effect of radiotherapy in a patient with metastatic diffuse-type giant cell tumor. Acta Oncol. 2016;55:1510–1512. doi: 10.1080/0284186X.2016.1243805. [DOI] [PubMed] [Google Scholar]
- 68.Cong Y, Shen G, Wu S, Hao R. Abscopal regression following SABR for non-small-cell-lung cancer: A case report. Cancer Biol Ther. 2017;18:1–3. doi: 10.1080/15384047.2016.1264541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ebner DK, Kamada T, Yamada S. Abscopal effect in recurrent colorectal cancer treated with carbon-ion radiation therapy: 2 case reports. Adv Radiat Oncol. 2017;2:333–338. doi: 10.1016/j.adro.2017.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Postow MA, Callahan MK, Barker CA, et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med. 2012;366:925–931. doi: 10.1056/NEJMoa1112824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stamell EF, Wolchok JD, Gnjatic S, Lee NY, Brownell I. The abscopal effect associated with a systemic anti-melanoma immune response. Int J Radiat Oncol Biol Phys. 2013;85:293–295. doi: 10.1016/j.ijrobp.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Golden EB, Demaria S, Schiff PB, Chachoua A, Formenti SC. An abscopal response to radiation and ipilimumab in a patient with metastatic non-small cell lung cancer. Cancer Immunol Res. 2013;1:365–372. doi: 10.1158/2326-6066.CIR-13-0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Antoniades J, Brady LW, Lightfoot DA. Lymphangiographic demonstration of the abscopal effect in patients with malignant lymphomas. Int J Radiat Oncol Biol Phys. 1977;2:141–147. doi: 10.1016/0360-3016(77)90020-7. [DOI] [PubMed] [Google Scholar]
- 74.Cotter SE, Dunn GP, Collins KM, et al. Abscopal effect in a patient with metastatic Merkel cell carcinoma following radiation therapy: Potential role of induced antitumor immunity. Arch Dermatol. 2011;147:870–872. doi: 10.1001/archdermatol.2011.176. [DOI] [PubMed] [Google Scholar]
- 75.Masue N, Hasegawa Y, Moriyama Y, Ikeda Y, Gotoh T, Deguchi T. Spontaneous disappearance of multiple lung metastases after nephroureterectomy from sarcomatoid carcinoma of the renal pelvis: A case report. Int J Urol. 2007;14:75–78. doi: 10.1111/j.1442-2042.2006.01603.x. [DOI] [PubMed] [Google Scholar]
- 76.Agyeman MB, Vanderpuye VD, Yarney J. Abscopal effect of radiotherapy in imatinib-resistant dermatofibrosarcoma protuberans. Cureus. 2019;11:1–7. doi: 10.7759/cureus.3857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Krall JA, Reinhardt F, Mercury OA, et al. The systemic response to surgery triggers the outgrowth of distant immune-controlled tumors in mouse models of dormancy. Sci Transl Med. 2018;10:eaan3464. doi: 10.1126/scitranslmed.aan3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nesslinger NJ, Sahota RA, Stone B, et al. Standard treatments induce antigen-specific immune responses in prostate cancer. Clin Cancer Res. 2007;13:1493–1502. doi: 10.1158/1078-0432.CCR-06-1772. [DOI] [PubMed] [Google Scholar]
- 79.McBride SM, Lee NY, Pfister DG, et al. Biomarker predictors of outcome from a randomized trial of nivolumab +/- stereotactic body radiotherapy (SBRT) in metastatic (M1) head and neck squamous cell carcinoma (HNSCC) J Clin Oncol. 2019;37(15 suppl):6063. [Google Scholar]
- 80.Theelen WSME, Peulen HMU, Lalezari F, et al. Effect of pembrolizumab after stereotactic body radiotherapy vs pembrolizumab alone on tumor response in patients with advanced non-small cell lung cancer: Results of the PEMBRO-RT phase 2 randomized clinical trial. JAMA Oncol. 2019;5:1276–1282. doi: 10.1001/jamaoncol.2019.1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.