Highlights
-
•
Melatonin treatment ameliorated chilling injury in cold-stored guava fruit.
-
•
Melatonin treatment reduced cell membrane permeability and weight loss in guava fruit.
-
•
Melatonin treatment retained higher TSS, total sugar, sucrose and vitamin C in guava.
-
•
The optimal concentration of melatonin for postharvest guava fruit was 100 μmol/L.
-
•
Melatonin displayed a great potential application for postharvest guava fruit.
Keywords: Guava fruit, Cold storage, Chilling injury, Quality properties, Cold resistance, Melatonin
Abstract
The influence of melatonin treatment on the quality and chilling injury of guavas during storage at 4 ± 1 °C were evaluated. Compared with control group, fruit of guava cv. Xiguahong exposed to various concentrations (50, 100, 150, and 200 μmol/L) of melatonin showed a significantly lower fruit respiration rate, weight loss, cell membrane permeability, and chilling injury index, but a higher commercially acceptable fruit rate, higher peel L*, h° value, and chlorophyll content. Melatonin treatment also delayed the decreases of fruit firmness, sucrose, total soluble sugar, vitamin C, titratable acidity, and total soluble solids. These data indicate that melatonin treatment could increase chilling tolerance and retain quality of cold-stored guavas. Among various concentrations of melatonin treatment, 100 μmol/L melatonin-treated guavas showed the preferable quality properties and lowest chilling injury index. Thus, melatonin may be a novel method of postharvest handling to enhance cold resistance and extend storage-life of cold-stored guava fruit.
1. Introduction
Guava is a commercial fruit tree in many subtropical and tropical areas, including South China, India, Pakistan, Mexico, and Brazil. During the process of maturation, guava exhibits colors of green to bright yellow, sometimes red. Depending on the type, its flesh is white or orange-pink and contains many small seeds. Guava has considerable amounts of vitamins, antioxidants, and dietary fiber, making it a desirable fruit by many consumers (Formiga et al., 2019, Hong et al., 2012, Nair et al., 2018). However, the storage life and marketing of postharvest fresh guavas are limited by its high perishability and intolerance to diseases, chilling injury, and physical damage. Particularly, guava’s susceptibility to chilling disorder has limited its long-distance transportation and export. Storage of guava fruit below 8 °C may cause chilling injury (Singh & Pal, 2008a). The most prevalent signs and symptoms of chilling injury are surface pitting and browning, water-soaking lesions, inability to ripen, and rapid decay (Murmu & Mishra, 2018). Postharvest treatments, such as chemical substances, controlled atmospheric storage, and edible coating, have been implemented to decrease chilling sensitivity of cold-stored guavas (Murmu and Mishra, 2018, Singh and Pal, 2008a). Although, to some extent, these measures can alleviate the chilling injury of guava fruit, however, due to their defects in cost, operability and efficiency, these measures have still restricted its large-scale commercial application. Thus, there is a need to explore more reliable methods to enhance the cold tolerance and alleviate the chilling injury of guava fruit.
Melatonin, an indole hormone, is found in various fruit, such as cherry, strawberry, and tomato (Arnao and Hernández-Ruiz, 2015, Feng et al., 2014). Melatonin participates in important plant life activities, such as growth, development, maturation, and senescence. It also has a protective effect on the abiotic stresses of plants, including drought, high, and low temperatures (Zhang et al., 2015). Previous studies indicate that postharvest treatment of exogenous melatonin can enhance chilling tolerance and keep better qualities of fresh produces under cold storage, such as peach (Gao et al., 2018), pomegranate (Aghdam et al., 2020), strawberry (Aghdam & Fard, 2017), sweet cherry (Miranda et al., 2020), and tomato (Aghdam et al., 2019). Thus, melatonin is regard as a potential and beneficial postharvest treating agent to mitigate chilling injury of cold-stored fruit. However, the influences of melatonin on chilling injury and quality properties of guava fruit during cold storage have not been documented. The purposes of this study are to evaluate the influences of postharvest treatment with exogenic melatonin on the cold-resistance and quality properties of cold-stored guava fruit, determine the optimum concentration of melatonin treatment, and develop a novel postharvest handling approach to enhance chilling tolerance and lengthen storage-life of postharvest guavas.
2. Materials and methods
2.1. Materials and treatment
Melatonin was obtained from Aladdin Reagent (Shanghai) Co., Ltd.
Fruit of guava cv. Xiguahong, at the status of commercial maturity with a light-green of guava fruit appearance and with 7.34 of total soluble solids (TSS) in guava flesh, were gathered from a well-managed orchard in Zhangzhou, Fujian, China. Then, the guavas were packaged in corrugated boxes and shipped to the lab at Quanzhou city within 4 h. The gathered guava fruit were handpicked based on consistency of maturity, size, and color, while those with mechanical damage, visual defects, or diseases were removed. Then, the distilled water was applied to wash the handpicked guavas, and the washed guavas were separated into two batches. One batch (50 fruit) was chosen to appraise fruit qualities on the day of harvesting. The other batch (2000 fruit) were randomly categorized into five groups (400 guavas per group) for control and melatonin treatments. The fruit (in each group) was submerged in deionized water (control) or melatonin solution (50, 100, 150, and 200 μmol/L) for 20 min, separately. After air-drying for 60 min at 25 °C, the guavas were separately packed in polyethylene bags (five guavas per bag, 80 bags per treatment), then stored at 4 °C (85–90% RH) for 42 d. During cold storage, ten bags with 50 guavas from each group were taken randomly at a 7-day interval to assess fruit chilling injury and quality properties. Additionally, during storage, 3 bags (15 guavas in total) from each group were set to assess weight loss of guava fruit at a 7-storage day interval.
2.2. Evaluation of chilling injury and commercially acceptable fruit rate
Fifteen guava fruit were taken to assess chilling injury based on the following five levels, which evaluated the pitting or browning scale on the surface of guava fruit: 0, represents no browning; while 1, 2, 3, and 4 shows 1–10%, 11–25%, 26–50%, and > 51% browning, respectively (Table 1). The chilling injury index was computed based on calculation formula: ∑(Chilling injury level / The highest level of chilling injury × The ratio of corresponding guavas in each level of chilling injury).
Table 1.
Types of chilling injury in guava fruit.
| Fruit chilling injury type | The ratio of the pitting or browning area on the surface of fruit to the total area of one guava fruit | Picture |
|---|---|---|
| 0 | No browning |  |
| 1 | Browning area ≤ 10% |  |
|
2 |
11%< Browning area ≤ 25% |
 |
|
3 |
26%< Browning area ≤ 50% |
 |
| 4 | Browning area >51% |  |
Based on the above five levels of assessing chilling injury for guava fruit, the level of chilling injury<3 and no pathogenic infection on the surface of guavas was regarded as commercially acceptable fruit. Commercially acceptable fruit rate was calculated using the following formula: Commercially acceptable fruit rate (%) = (Number of commercially acceptable fruit / Total fruit number) × 100%.
2.3. Assessment of fruit weight loss
Three bags (15 guavas in total) were applied to assess weight loss of guavas at a 7-storage day interval. The weight loss of guavas in each bag was assayed through comparing the weight of guava fruit at the storage day 0.
2.4. Assay of cell membrane permeability
Based on the assaying procedures of Lin et al., 2016, Chen et al., 2020, five guava fruits were applied to measure the electrolyte leakage. The percentage rate of relative leakage was calculated and the result was adopted to indicate the unit of cell membrane permeability (Lin et al., 2016).
2.5. Assay of fruit respiration rate and firmness
Five guava fruits were used for assaying respiration rate referring to the procedure of Lin et al. (2020), and the unit was mg CO2 kg-1h−1.
Texture analyzer (TA.XT Plus, Stable Micro System, UK) with a 2-mm cylindrical probe was used to determine the firmness of guava fruit. On opposite sides, firmness was determined along the equatorial region (in five fruit) and punctured at the rate of 2 mm/s (for 10 mm). The average force value of the probe (3–7 mm) was documented on the fruit and represented as Newton (N).
2.6. Assay of color characteristics and chlorophyll amount in peel of guava fruit
The lightness L* value and hue angle (h°) of two spots on opposite side (equatorial area) in five different guava fruit were assayed using Chroma meter (Minolta CR 400, Japan), which was based on the procedures of Farcuh et al., 2020, Chen et al., 2015.
Two grams of peel from five guavas was used to assay chlorophyll amount referring to the approach of Lin et al. (2020), and the unit was mg kg−1.
2.7. Assaying vitamin C, sugar, TA (titratable acidity), and TSS amounts
Referring to the approach of Liu et al. (2021), one gram of flesh from five guavas was applied to assay vitamin C amount. Additionally, 2 g of flesh from five guavas was sampled to estimate the contents of reducing sugar, sucrose, and total soluble sugar according to the procedure of Jiang et al. (2018). Furthermore, the flesh juice extracting from five guava (equatorial regions) was applied for assaying TSS amount with a digital pocket refractometer (Atago PAL-1, Japan) and TA content with an acid-base titrator (Mettler ET18, Switzerland). The content of TA and TSS were indicated as a percentage, while those of vitamin C, reducing sugar, sucrose, and total soluble sugar were presented with g kg−1 as their unit.
2.8. Statistical analysis
Evaluation of above parameters was conducted thrice. The data in the figures were presented as the mean ± standard error. The experimental data were statistically analyzed using statistical software SPSS 22.0 (Chicago, IL, USA).
3. Results and discussion
3.1. Chilling injury symptoms, chilling injury index and cell membrane permeability
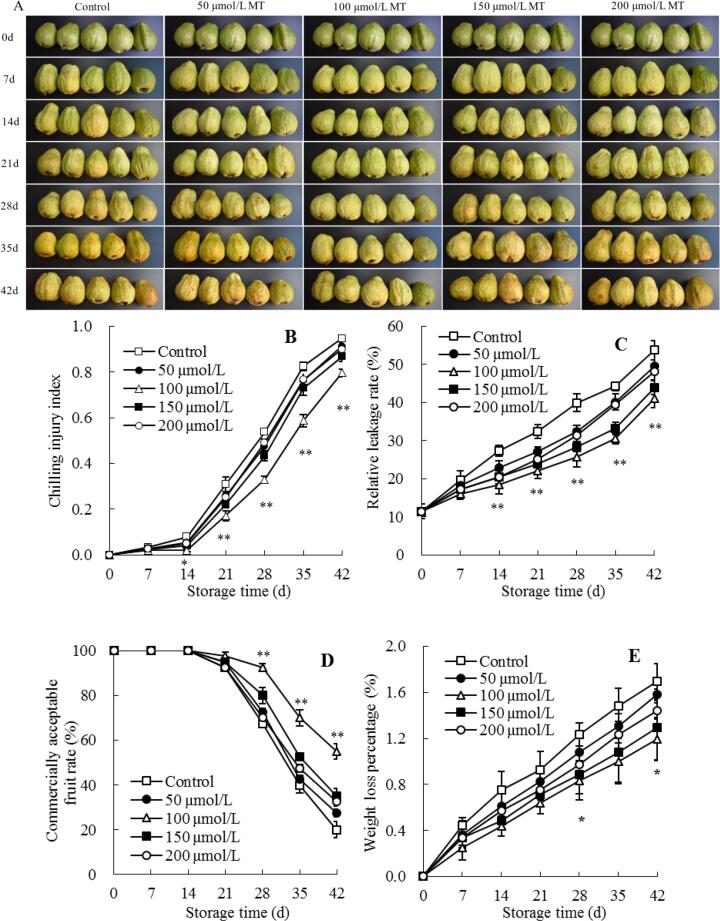
Surface pitting and browning are the main symptoms of chilling injury for guavas under low-temperature storage, which seriously affect their edible quality, commodity value, storage period, and limited distribution of fruit to distant markets (Singh & Pal, 2008a). In this study, after 7 d of cold storage, the control guava fruit displayed chilling injury symptoms (Fig. 1A-B). Additionally, chilling injury index in control guava gradually raised at storage day 0–14, then increased sharply. During storage, chilling tolerance of guava fruit at 4 °C was caused by melatonin treatment, but the different inhibitory effects of chilling injury were found in different concentrations of melatonin-treated guava (Fig. 1A-B). No obvious differences of guava chilling injury were observed among the control, the treatment of melatonin at 50 or 200 μmol/L concentration. In contrast, melatonin at the concentrations of 100 μmol/L showed considerable effects in decreasing the chilling injury of cold-stored guava fruit. After storage 28 d, compared with control guava, the chilling injury index in 50, 100, 150, and 200 μmol/L melatonin-treated guava were reduced by 11.6%, 38.8%, 19.4%, and 8.5%, respectively (Fig. 1B). Further comparison revealed that, the mitigated chilling injury symptoms and a considerably (P < 0.05) lower index of chilling injury were found in 100 μmol/L melatonin-treated guava than control guava within storage day 14 to 42 (Fig. 1A-B).
Fig. 1.
Influences of melatonin treatment on fruit chilling injury symptoms (A), chilling injury index (B), cell membrane relative leakage rate (C), commercially acceptable fruit rate (D) and weight loss (E) of guavas during storage at 4 °C. Mean ± standard error (n = 3) was applied to represent each value. Remarkable differences between the 100 μmol/L melatonin-treated guavas and control guavas on the same storage day are indicated by * (P < 0.05) and ** (P < 0.01).
Chilling injury of guavas is accompanied by the damage of cell membrane, which can be reflected by an increased cell membrane permeability (Alba-Jiménez et al., 2018). Relative electrolyte leakage could effectively indicate the damage of plasma membrane (Zhang et al., 2010). As the storages time progressed, the plasma membrane relative leakage rate in control guava fruit raised quickly (Fig. 1C). Melatonin-treated guavas showed a similar increasing tendency, while the group exposed to 100 μmol/L melatonin showed a considerably reduced level of plasma membrane relative leakage rate than control guava. Compared with the control fruit, at storage day 42, approximately 7.6%, 23.7%, 18.1%, and 10.2% decreases in cell membrane relative leakage rate were, respectively, noted for fruit exposed to 50, 100, 150, and 200 μmol/L melatonin. Statistical analysis displayed that the clearly (P < 0.01) lower relative leakage rates of cellular membrane were found in 100 μmol/L melatonin-treated guava than control guava during storage day 14–42 (Fig. 1C).
Additionally, correlation analysis showed the positive correlation between relative leakage rate of cellular membrane (Fig. 1C) and chilling injury index (Fig. 1B) in control guava (r = 0.974, P < 0.01). These findings suggested a continuous elevation in the plasma membrane permeability, which might contribute to the development of chilling injury in guavas.
Hence, melatonin treatment could lower fruit chilling injury index, restrain the enhancement in relative leakage rate of cellular membrane, and moderate chilling injury symptoms of guava within the cold storage.
3.2. Commercially acceptable fruit rate and weight loss
The rate of commercially acceptable fruit is an important indicator of fresh fruit acceptance (Liu et al., 2021). In present work, the commercially acceptable rate of guava showed a decreasing trend during cold storage (Fig. 1D). No considerable variation in commercially acceptable rate was found between the control guava and melatonin-treated groups before storage 21 d. However, the guava exposed to melatonin, particularly at 100 μmol/L (P < 0.01), showed a higher fruit acceptability rate than control guava at storage day 28–42 (Fig. 1D).
Weight loss is a significant indicator for estimating storage-life of fruit during postharvest storage period, since it reflects the transpiration, respiration, and various physiological changes (Jiang et al., 2018). Because of their thin skin, the susceptibility for water loss was increased in guava fruit (Hong et al., 2012). In this work, during storage, the percentage of weight loss in control guava fruit displayed a quick increase (Fig. 1E), while guava fruit exposed to melatonin showed a similar increasing trend of weight loss percentage. However, a considerably (P < 0.05) lower weight loss was observed in 100 μmol/L melatonin-treated guava than control guava on storage day 28 and 42 (Fig. 1E). Following further analyses, we found that there was a negative correlation between commercially acceptable fruit rate (Fig. 1D) and weight loss percentage (Fig. 1E) in control guava (r = –0.994, P < 0.01).
The above data indicate that melatonin treatment could retain a higher rate of fruit acceptability and decrease the rate of weight loss of guava fruit, which might maintain their good quality following cold storage.
3.3. Fruit respiration rate and firmness
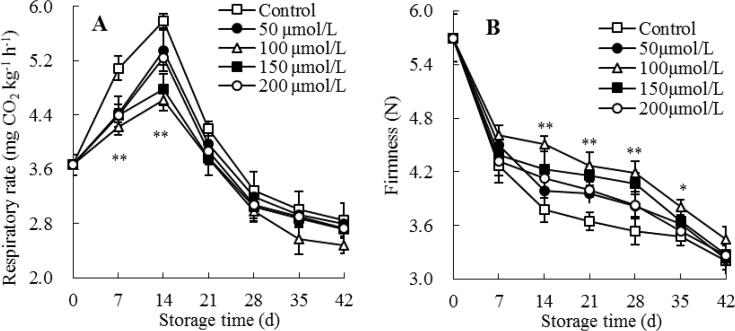
Guava is a climacteric fruit, which has strong respiratory intensity after storage at room temperature (Singh & Pal, 2008b). Suitable low temperatures could inhibit the respiratory intensity and delay the senescence of guavas during postharvest storage (Singh & Pal, 2008a). Fig. 2A depicts that the respiration rate in control guava fruit enhanced rapidly at storage 0–14 d, then dropped quickly at day 14–42. A similar trend of respiration rate was shown in melatonin-treated guavas. Relative to control guavas, the rate of respiration was decreased in guavas exposed to melatonin during the postharvest storage period. The considerable variations were not observed among the different concentrations of melatonin treatments, but the 100 μmol/L melatonin-treated group had a remarkably (P < 0.01) lower respiration rate than control guava from day 7 to 14 of storage.
Fig. 2.
Influences of melatonin treatment on fruit respiration rate (A) and firmness (B) of guavas during storage at 4 °C. Mean ± standard error (n = 3) was applied to represent each value. Remarkable differences between the 100 μmol/L melatonin-treated guavas and control guavas on the same storage day are indicated by * (P < 0.05) and ** (P < 0.01).
Fruit texture has a great influence on postharvest storage and transportation. Yet, it is usually the first of many main qualities to be judged by consumers, hence effectively contributing to product acceptance (Hong et al., 2012). In this work, the firmness in control guava fruit declined rapidly during storage (Fig. 2B). Whereas, melatonin treatment help retain higher firmness of postharvest guava fruit, especially on storage day 14 (19.31% higher, relative to control group). Following further analyses indicated that 100 μmol/L melatonin-treated guava fruit had a clearly (P < 0.05) higher firmness than control guava at day 14–35 (Fig. 2B).
Thus, exogenous melatonin treatment could lower fruit respiration rate, maintain higher fruit firmness of guava fruit during cold storage.
3.4. Lightness L* value, hue angle, and chlorophyll content
In many fruits, including guava, appearance color is an essential factor for assessing harvest maturity and quality of fruit (Etemadipoor et al., 2019, Farcuh et al., 2020). The preference of consumers is also influenced by the appearance color of fruit. The development of darkening or browning in postharvest fruit is revealed by a dropped L* value (Liu et al., 2021). Also, the deterioration of chlorophyll causes a reduction in hue angle (Li et al., 2019).
In present work, L* value in surface of control guavas reduced quickly during cold storage (Fig. 3A), additionally, a high negative correlation was observed between L* value (Fig. 3A) and chilling injury index (Fig. 1B) in control guava (r = –0.979), indicating that the development of chilling injury led to the decreased peel lightness and the browning peel in guava fruit (Fig. 1A-B, Fig. 3A). Whereas, melatonin treatment could reduce the decrease of L* value and maintain a higher L* value in guava fruit during cold storage. Statistical analysis showed a considerably (P < 0.01) higher L* value in the 100 μmol/L melatonin-treated guava fruit than control guava from storage day 14–42 (Fig. 3A), indicating that 100 μmol/L melatonin treatment delayed peel browning and lightened chilling injury symptoms of guava during cold storage (Fig. 1A-B).
Fig. 3.
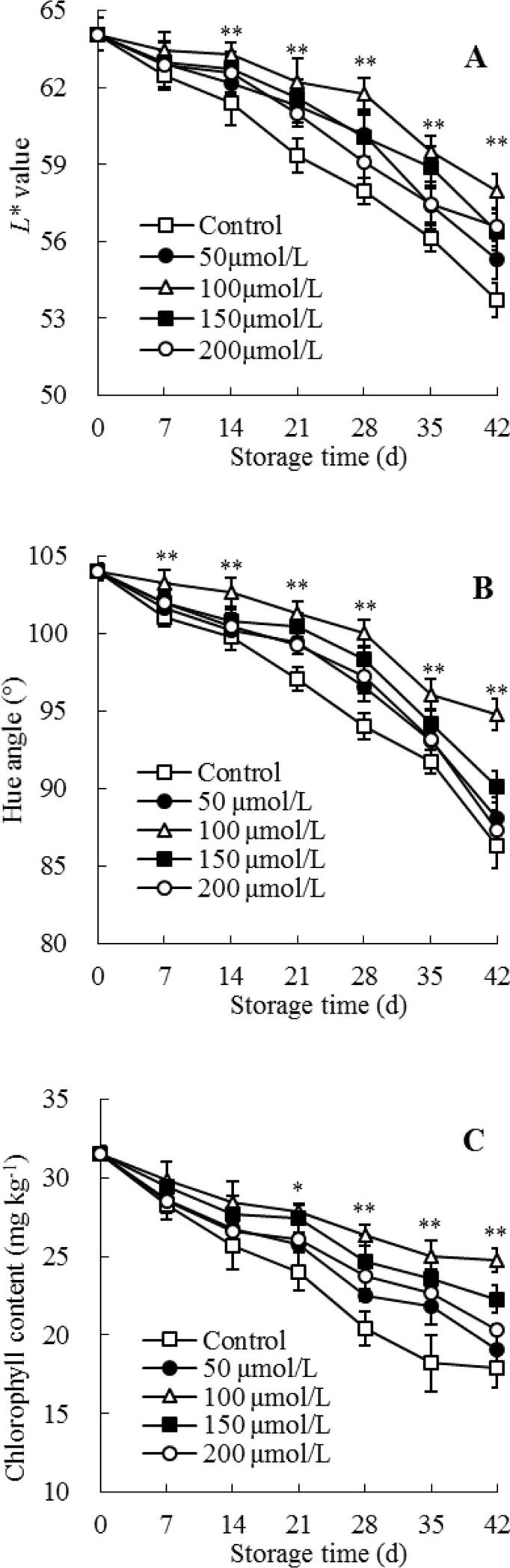
Influences of melatonin treatment on lightness L* value (A), hue angle (B) and chlorophyll content (C) in the peel of guavas during storage at 4 °C. Mean ± standard error (n = 3) was applied to represent each value. Remarkable differences between the 100 μmol/L melatonin-treated guavas and control guavas on the same storage day are indicated by * (P < 0.05) and ** (P < 0.01).
The peel color of guava fruit was light green at harvest, then turned yellow-green on storage day 28 (Fig. 1A), which was revealed by the quick decrease of hue angle values (Fig. 3B). Nevertheless, the treatment of exogenous melatonin retarded the color change of guavas (Fig. 1A, Fig. 3B). When stored for 28 d, the hue angle value in 50, 100, 150, and 200 μmol/L melatonin-treated guava were 96.5, 100, 98.3, and 97.2, respectively, which were higher than the control guava (hue angle value 94). Further analysis revealed that the 100 μmol/L melatonin-treated guava fruit showed a clearly (P < 0.01) higher hue angle than control guava at day 7–42 (Fig. 3B).
The amount of chlorophyll in the peel of control guavas tended to decrease as storage time increased (Fig. 3C), additionally, peel chlorophyll amount (Fig. 3C) was positively correlated with its hue angle (Fig. 3B) (r = –0.954). Whereas, the dropped chlorophyll amount was inhibited by melatonin treatment (Fig. 3C). Compared with the storage day 0, chlorophyll amount in the peels of 50, 100, 150, 200 μmol/L melatonin-treated fruit lost 39%, 21%, 29%, and 36% on day 42, respectively, while the control fruit lost 43%. By statistical analysis, it was revealed that the 100 μmol/L melatonin-treated guava fruit exhibited a considerably (P < 0.05) higher peel chlorophyll content than control guava at storage day 21 to 42 (Fig. 3C).
These results revealed that melatonin treatment could considerably reduce color changes, retain higher lightness and hue angle value of guava fruit surface, and inhibit peel chlorophyll degradation in guavas during cold storage.
3.5. Vitamin C, TA, and TSS contents
The vitamin C, TA, and TSS contents are the key nutritional quality index in guava fruit (Silva et al., 2017). During storage, flesh TSS amount in control guavas reduced quickly (Fig. 4A). Whereas, exogenous melatonin treatment restrained the decrease of TSS content in postharvest guava fruit, particularly the 100 μmol/L melatonin-treated guavas preserved the highest content of TSS during cold storage. Following further analyses indicated that 100 μmol/L melatonin-treated guava fruit had a considerably (P < 0.01) higher content of TSS than control guava at storage day 7–42 (Fig. 4A).
Fig. 4.
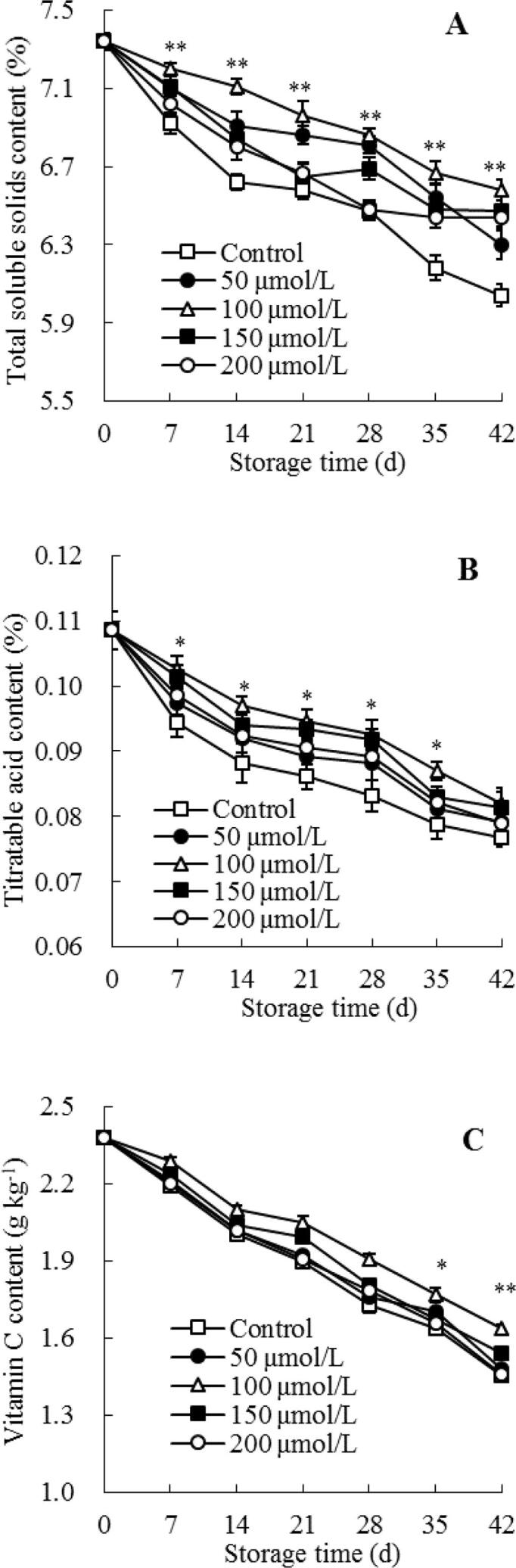
Influences of melatonin treatment on the amounts of TSS (A), TA (B), and vitamin C (C) in flesh of guavas during storage at 4 °C. Mean ± standard error (n = 3) was applied to represent each value. Remarkable differences between the 100 μmol/L melatonin-treated guavas and control guavas on the same storage day are indicated by * (P < 0.05) and ** (P < 0.01).
The TA content in control guavas tended to decrease as the storage time progressed, while TA content of melatonin-treated guavas showed a similar variation tendency (Fig. 4B). Specifically, the highest TA content was detected in 100 μmol/L melatonin-treated guavas during storage. A considerable (P < 0.05) discrepancy in TA amount between the control groups and 100 μmol/L melatonin-treated guavas was displayed at day 7–35, but there were no significant difference on storage day 42 (Fig. 4B).
One of the most important indicators of the nutritional value of fruit is vitamin C, which acts as an excellent antioxidant. As depicted in Fig. 4C, during storage, the vitamin C content in control guavas dropped drastically, and was only 60% on storage day 42 compared to the harvest day. Compared with the control group, a slower decline of vitamin C amount was shown in the melatonin-treated group, revealing that melatonin could inhibit vitamin C degradation in guavas. Particularly, compared to control guavas, the 100 μmol/L melatonin-treated guavas exhibited a considerably (P < 0.05) higher vitamin C amount at day 35–42 (Fig. 4C).
Furthermore, the correlation analyses revealed that the raised index of chilling injury was negatively related to the dropped amounts of TSS, TA, and vitamin C, with the r value of − 0.958, −0.961, and − 0.971, separately, indicating the occurrence of chilling injury in guava fruit could lead to the loss of nutritional qualities such as TA, TSS, and vitamin C. These data indicated that melatonin treatment could keep the higher amounts of vitamin C, TA, and TSS in flesh of guavas within the storage period, and thus significantly maintain the nutritional characteristics of guava fruit.
3.6. Reducing sugar, sucrose, and total soluble sugar amounts
Soluble sugar is critical for good flavor and taste in guava fruit (Anjum, Akram, Zaidi, & Ali, 2020). Total soluble sugar and sucrose amounts in control guavas raised rapidly and attained a maximum on storage day 21, hereafter declined quickly at day 21–42 (Fig. 5A–B). The same trend was shown in melatonin-treated guavas, but they had relatively higher amounts of sucrose and total soluble sugar than control guava during storage, especially in 100 μmol/L melatonin-treated guava (Fig. 5A–B). Further comparison with the control group indicated that the 100 μmol/L melatonin-treated group had a remarkably (P < 0.05) higher content of total soluble sugar from day 28 to 42 of storage (Fig. 5A). Regarding the changes in the sucrose content of guava fruit, the 100 μmol/L melatonin-treated group had a prominently (P < 0.01) higher content than control guavas within the storage 14–42 d (Fig. 5B).
Fig. 5.
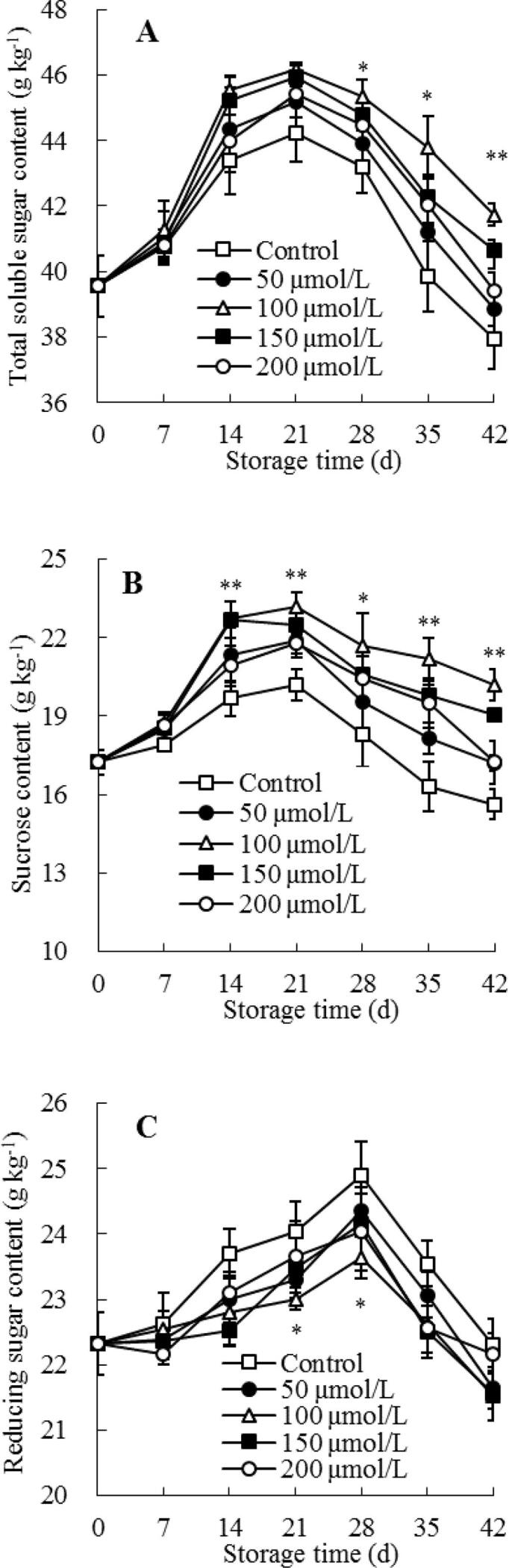
Influences of melatonin treatment on the amounts of total soluble sugar (A), sucrose (B), and reducing sugar (C) in flesh of guavas during storage at 4 °C. Mean ± standard error (n = 3) was applied to represent each value. Remarkable differences between the 100 μmol/L melatonin-treated guavas and control guavas on the same storage day are indicated by * (P < 0.05) and ** (P < 0.01).
According to Fig. 5C, the reducing sugar content in control guava fruit showed a quickly elevating trend within storage day 0–28, then a sharp reduction at day 28–42. A similar trend was observed in melatonin-treated group. In addition to the 100 μmol/L melatonin-treated group, which had remarkably (P < 0.05) lower content of reducing sugar than control guava from day 21 to 28 of storage, there were no considerable variations in reducing sugar content between control guava and melatonin-treated guava within the whole storage time (Fig. 5C).
Soluble sugars, especially sucrose, have a considerable contribution to protect plant cell from injury against chilling stress (Wang et al., 2020). An elevated level of sucrose was linked to an increased chilling resistance in fresh fruit (Zhao et al., 2019, Wang et al., 2019, Fan et al., 2022). In present work, during cold storage, melatonin treatment could enhance chilling tolerance of fresh guava fruit (Fig. 1A-B), especially in 100 μmol/L melatonin-treated guava, which was related to melatonin restraining the degradation of sucrose to reducing sugar, and retaining higher amount of sucrose (Fig. 5B) but a lower reducing sugar amount (Fig. 5C), and thus help to retain an integrity of cellular membrane structures, and display a lower relative leakage rate of cell membranes (Fig. 1C), less chilling injury symptoms (Fig. 1A), and lower index of chilling injury symptoms (Fig. 1B) in melatonin-treated guava during cold storage.
4. Conclusions
Melatonin treatment could mitigate chilling injury and retain qualities of cold-stored ‘Xiguahong’ guavas at 4 °C, it appeared that the melatonin treated-guavas displayed the lower fruit respiration rate, weight loss, cell membrane permeability, and chilling injury index, but maintained the higher rate of commercially acceptable fruit, higher fruit firmness, higher value of pericarp L*, h° and chlorophyll amount, and higher levels of sucrose, total soluble sugar, vitamin C, TA, and TSS in in guavas. Hence, postharvest melatonin treatment, especially at 100 μmol/L melatonin, could be a potential alternative to enhance cold resistance and lengthen storage-life of fresh guava during cold storage.
CRediT authorship contribution statement
Hongbin Chen: Conceptualization, Investigation, Data curation, Formal analysis, Writing – original draft. Hetong Lin: Supervision, Resources, Conceptualization, Project administration, Writing – review & editing. Xuanjing Jiang: Investigation. Mengshi Lin: Writing – original draft. Zhongqi Fan: Supervision, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the Fujian Provincial Natural Science Foundation of China (Grant Nos. 2021 J01976 and 2017 J01455), and the Science and Technology Innovation Foundation at Fujian Agriculture and Forestry University of China (Grant No. CXZX2020115A).
Contributor Information
Hetong Lin, Email: hetonglin@163.com.
Zhongqi Fan, Email: ffanzqi@qq.com.
References
- Aghdam M.S., Fard J.R. Melatonin treatment attenuates postharvest decay and maintains nutritional quality of strawberry fruits (Fragaria × anannasa cv. Selva) by enhancing GABA shunt activity. Food Chemistry. 2017;221:1650–1657. doi: 10.1016/j.foodchem.2016.10.123. [DOI] [PubMed] [Google Scholar]
- Aghdam M.S., Luo Z., Jannatizadeh A., Sheikh-Assadi M., Sharafi Y., Farmani B.…Razavi F. Employing exogenous melatonin applying confers chilling tolerance in tomato fruit by upregulating ZAT2/6/12 giving rise to promoting endogenous polyamines, proline, and nitric oxide accumulation by triggering arginine pathway activity. Food Chemistry. 2019;275:549–556. doi: 10.1016/j.foodchem.2018.09.157. [DOI] [PubMed] [Google Scholar]
- Aghdam M.S., Luo Z., Li L., Jannatizadeh A., Fard J.R., Pirzad F. Melatonin treatment maintains nutraceutical properties of pomegranate fruit during cold storage. Food Chemistry. 2020;303 doi: 10.1016/j.foodchem.2019.125385. [DOI] [PubMed] [Google Scholar]
- Alba-Jiménez J.E., Benito-Bautista P., Nava G.M., Rivera-Pastrana D.M., Vázquez-Barrios M.E., Mercado-Silva E.M. Chilling injury is associated with changes in microsomal membrane lipids in guava fruit (Psidium guajava L.) and the use of controlled atmospheres reduce these effects. Scientia Horticulturae. 2018;240:94–101. doi: 10.1016/j.scienta.2018.05.026. [DOI] [Google Scholar]
- Anjum M.A., Akram H., Zaidi M., Ali S. Effect of gum arabic and Aloe vera gel based edible coatings in combination with plant extracts on postharvest quality and storability of ‘Gola’ guava fruits. Scientia Horticulturae. 2020;271 doi: 10.1016/j.scienta.2020.109506. [DOI] [Google Scholar]
- Arnao M.B., Hernández-Ruiz J. Functions of melatonin in plants: A review. Journal of Pineal Research. 2015;59(2):133–150. doi: 10.1111/jpi.12253. [DOI] [PubMed] [Google Scholar]
- Chen Y., Lin H., Shi J., Zhang S., Lin Y., Lin T. Effects of a feasible 1-methylcyclopropene postharvest treatment on senescence and quality maintenance of harvested Huanghua pears during storage at ambient temperature. LWT - Food Science and Technology. 2015;64:6–13. doi: 10.1016/j.lwt.2015.05.021. [DOI] [Google Scholar]
- Chen Y., Xie H., Tang J., Lin M., Hung Y.-C., Lin H. Effects of acidic electrolyzed water treatment on storability, quality attributes and nutritive properties of longan fruit during storage. Food Chemistry. 2020;320 doi: 10.1016/j.foodchem.2020.126641. [DOI] [PubMed] [Google Scholar]
- Etemadipoor R., Ramezanian A., Dastjerdi A.M., Shamili M. The potential of gum arabic enriched with cinnamon essential oil for improving the qualitative characteristics and storability of guava (Psidium guajava L.) fruit. Scientia Horticulturae. 2019;251:101–107. doi: 10.1016/j.scienta.2019.03.021. [DOI] [Google Scholar]
- Fan Z., Lin B., Lin H., Lin M., Chen J., Lin Y. γ-Aminobutyric acid treatment reduces chilling injury and improves quality maintenance of cold-stored Chinese olive fruit. Food Chemistry: X. 2022;13 doi: 10.1016/j.fochx.2022.100208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farcuh, M., Copes, B., Le-Navenec, G., Marroquin, J., Cantu, D., Bradford, K. J., …Deynze, A., V. (2020). Sensory, physicochemical and volatile compound analysis of short and long shelf-life melon (Cucumis melo L.) genotypes at harvest and after postharvest storage. Food Chemistry: X, 8, 100107. 10.1016/j.fochx.2020.100107. [DOI] [PMC free article] [PubMed]
- Feng X., Wang M., Zhao Y., Han P., Dai Y. Melatonin from different fruit sources, functional roles, and analytical methods. Trends in Food Science and Technology. 2014;37(1):21–31. doi: 10.1016/j.tifs.2014.02.001. [DOI] [Google Scholar]
- Formiga A.S., Pinsetta Junior J.S., Pereira E.M., Cordeiro I.N.F., Mattiuz B.H. Use of edible coatings based on hydroxypropyl methylcellulose and beeswax in the conservation of red guava ‘Pedro Sato’. Food Chemistry. 2019;290:144–151. doi: 10.1016/j.foodchem.2019.03.142. [DOI] [PubMed] [Google Scholar]
- Gao H., Lu Z., Yang Y., Wang D., Yang T., Cao M., Cao W. Melatonin treatment reduces chilling injury in peach fruit through its regulation of membrane fatty acid contents and phenolic metabolism. Food Chemistry. 2018;245:659–666. doi: 10.1016/j.foodchem.2017.10.008. [DOI] [PubMed] [Google Scholar]
- Hong K., Xie J., Zhang L., Sun D., Gong D. Effects of chitosan coating on postharvest life and quality of guava (Psidium guajava L.) fruit during cold storage. Scientia Horticulturae. 2012;144:172–178. doi: 10.1016/j.scienta.2012.07.002. [DOI] [Google Scholar]
- Jiang X., Lin H., Shi J., Neethirajan S., Lin Y., Chen Y.…Lin Y. Effects of a novel chitosan formulation treatment on quality attributes and storage behavior of harvested litchi fruit. Food Chemistry. 2018;252:134–141. doi: 10.1016/j.foodchem.2018.01.095. [DOI] [PubMed] [Google Scholar]
- Li D., Zhang X., Li L., Aghdam M.S., Wei X., Liu J.…Luo Z. Elevated CO2 delayed the chlorophyll degradation and anthocyanin accumulation in postharvest strawberry fruit. Food Chemistry. 2019;285:163–170. doi: 10.1016/j.foodchem.2019.01.150. [DOI] [PubMed] [Google Scholar]
- Lin Y., Li N., Lin H., Lin M., Chen Y., Wang H.…Lin Y. Effects of chitosan treatment on the storability and quality properties of longan fruit during storage. Food Chemistry. 2020;306 doi: 10.1016/j.foodchem.2019.125627. [DOI] [PubMed] [Google Scholar]
- Lin Y., Lin H., Lin Y., Zhang S., Chen Y., Jiang X. The roles of metabolism of membrane lipids and phenolics in hydrogen peroxide-induced pericarp browning of harvested longan fruit. Postharvest Biology and Technology. 2016;111:53–61. doi: 10.1016/j.postharvbio.2015.07.030. [DOI] [Google Scholar]
- Liu J., Lin Y., Lin H., Lin M., Fan Z. Impacts of exogenous ROS scavenger ascorbic acid on the storability and quality attributes of fresh longan fruit. Food Chemistry: X. 2021;12 doi: 10.1016/j.fochx.2021.100167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda S., Vilches P., Suazo M., Pavez L., García K., Méndez M.A.…Pozo T. Melatonin triggers metabolic and gene expression changes leading to improved quality traits of two sweet cherry cultivars during cold storage. Food Chemistry. 2020;319 doi: 10.1016/j.foodchem.2020.126360. [DOI] [PubMed] [Google Scholar]
- Murmu S.B., Mishra H.N. Selection of the best active modified atmosphere packaging with ethylene and moisture scavengers to maintain quality of guava during low-temperature storage. Food Chemistry. 2018;253:55–62. doi: 10.1016/j.foodchem.2018.01.134. [DOI] [PubMed] [Google Scholar]
- Nair M.S., Saxena A., Kaur C. Effect of chitosan and alginate based coatings enriched with pomegranate peel extract to extend the postharvest quality of guava (Psidium guajava L.) Food Chemistry. 2018;240:245–252. doi: 10.1016/j.foodchem.2017.07.122. [DOI] [PubMed] [Google Scholar]
- Silva W.B., Silva G.M.C., Santana D.B., Salvador A.R., Medeiros D.B., Belghith I.…Misobutsi G.P. Chitosan delays ripening and ROS production in guava (Psidium guajava L.) fruit. Food Chemistry. 2017;242:232–238. doi: 10.1016/j.foodchem.2017.09.052. [DOI] [PubMed] [Google Scholar]
- Singh S.P., Pal R.K. Controlled atmosphere storage of guava (Psidium guajava L.) fruit. Postharvest Biology and Technology. 2008;47(3):296–306. doi: 10.1016/j.postharvbio.2007.08.009. [DOI] [Google Scholar]
- Singh S.P., Pal R.K. Response of climacteric-type guava (Psidium guajava L.) to postharvest treatment with 1-MCP. Postharvest Biology and Technology. 2008;47(3):307–314. doi: 10.1016/j.postharvbio.2007.08.010. [DOI] [Google Scholar]
- Wang L., Shan T., Xie B., Ling C., Shao S., Jin P., Zheng Y. Glycine betaine reduces chilling injury in peach fruit by enhancing phenolic and sugar metabolisms. Food Chemistry. 2019;272:530–538. doi: 10.1016/j.foodchem.2018.08.085. [DOI] [PubMed] [Google Scholar]
- Wang X., Chen Y., Jiang S., Xu F., Wang H., Wei Y., Shao X. PpINH1, an invertase inhibitor, interacts with vacuolar invertase PpVIN2 in regulating the chilling tolerance of peach fruit. Horticulture Research. 2020;7:168. doi: 10.1038/s41438-020-00389-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C., Ding Z., Xu X., Wang Q., Qin G., Tian S. Crucial roles of membrane stability and its related proteins in the tolerance of peach fruit to chilling injury. Amino Acids. 2010;39(1):181–194. doi: 10.1007/s00726-009-0397-6. [DOI] [PubMed] [Google Scholar]
- Zhang N., Sun Q., Zhang H., Cao Y., Weeda S., Ren S., Guo Y. Roles of melatonin in abiotic stress resistance in plants. Journal of Experimental Botany. 2015;66(3):647–656. doi: 10.1093/jxb/eru336. [DOI] [PubMed] [Google Scholar]
- Zhao H., Jiao W., Cui K., Fan X., Shu C., Zhang W.…Jiang W. Near-freezing temperature storage enhances chilling tolerance in nectarine fruit through its regulation of soluble sugars and energy metabolism. Food Chemistry. 2019;289:426–435. doi: 10.1016/j.foodchem.2019.03.088. [DOI] [PubMed] [Google Scholar]