Abstract
Context:
After completion of treatment, a proportion of pulmonary tuberculosis (TB) (PTB) patients experience lung function impairment (LFI) which can influence their quality of life.
Aim:
This study was aimed to determine the prevalence of LFI in patients treated for PTB and the associated factors.
Settings and Design:
A cross-sectional study was conducted among patients treated for PTB in eight primary health centers in Puducherry.
Subjects and Methods:
The study was carried out among 118 patients. Those aged 18 and above whose PTB treatment outcomes were declared as cured or completed between 2018 and 2019 were included. Demographic data, respiratory symptoms before TB diagnosis, comorbidities, and chest radiography findings before TB treatment were collected. All participants underwent spirometric tests before and after dilatation with salbutamol nebulization.
Statistical Analysis:
Multivariable analysis identified smear-negative TB and indoor exposure to biomass for cooking as significant independent risk factors for LFI.
Results:
Of 118 participants interviewed, 70.3% were male and the median age of the participants was 47.7 years. The prevalence of LFI was 62.7% (95% confidence interval: 53.3–71.4).
Conclusion:
LFI was frequent in patients treated previously for TB. Creating awareness about the possible LFI among these patients along with the awareness for seeking health care for this condition is the need of the hour.
Keywords: Lung function impairment, pulmonary tuberculosis, spirometer
INTRODUCTION
Pulmonary tuberculosis (TB) (PTB) is one of the top ten causes of mortality worldwide killing about five thousand people daily. Among 30 high TB burden countries, India ranks first followed by Russia and South Africa.[1] Lung function impairment (LFI) following PTB is reported in more than half of microbiologically cured patients, with approximately 10% having already lost half of their lung function.[2] Very little is known about the prevalence of LFI among the patients treated for TB, in particular from countries like India where the incidence of the disease is more. Hence, this study was conducted to know the prevalence of LFI and to determine these associated factors in “previously treated” and “cured” patients (PTB) because completion of Anti-tubercular Treatment (ATT) is just initiation but not an end of their illness.
SUBJECTS AND METHODS
A cross-sectional analytical study was done among adults aged 18 years and above from January–December 2019 in primary health centers (PHCs) at Puducherry, South India. There are 27 PHCs in Puducherry and about 10% of the TB patients are availing treatment from the eight PHCs around Jawaharlal Institute of Postgraduate Medical Education and Research (JIPMER). The remaining 90% of the patients are receiving treatment, either from the remaining 19 PHCs or the government hospitals and medical colleges in Puducherry. The PHCs were selected around JIPMER based on convenience.
All adults with PTB (new or previously treated) from the eight PHCs, who were declared as “cured” or “treatment completed” under the National TB Program, and whose treatment outcomes were declared within 12 months before the date of interview (September 2019 to October 2019) were included. Multidrug-resistant TB patients, patients who were declared cured or treatment completed and again diagnosed as “smear-positive TB” during the study period, and patients with a history of asthma and chronic obstructive pulmonary disease were excluded from the study.
Assuming the expected proportion of PTB patients with LFI as 45.4%,[3] by using OpenEpi software version 3.03, a sample size of 185 was arrived. Eight primary health centers near to the hospital were selected conveniently, and a total of 200 patients who were declared as “cured” or “treatment completed” under these eight PHCs were approached for the study. However, results of 118 patients were taken as they only had performed the complete spirometry procedure [Figure 1].
Figure 1.
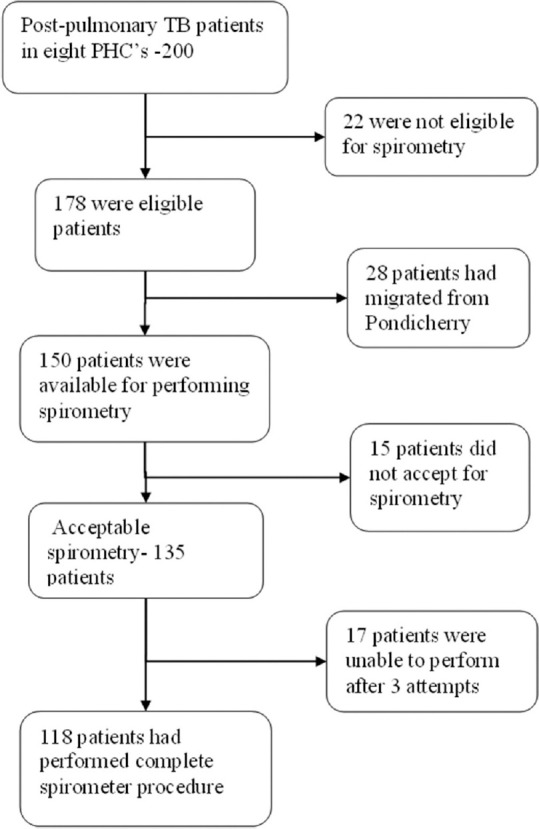
Sampling of post pulmonary TB participants
A list of eligible study participants was prepared from the TB registers/NIKSHAY database maintained at the State TB Office.
Information on morbidity and treatment characteristics was extracted from the TB register and individual patient card. Participant general information and respiratory symptoms present before diagnosis of TB and at present, biomass exposure for cooking or heating, and dust exposure (workplace) were also obtained through interviews after obtaining the consent.
Lung function tests were performed with the patient in a sitting position using a portable spirometer (RMS Helios 401) at a place convenient to the patient (home or PHC) as per standard guidelines for performing spirometry. To prevent air leakage, a nose clip was used. About 7–8 spirometry tests were performed for every subject. The values within 5% of the three tests were acceptable. Forced expiratory volume in 1 s (FEV1), forced vital capacity (FVC), and FEV1/FVC ratio were recorded. A bronchodilation test was conducted by using salbutamol nebulisation by positioning the patient in a sitting posture. Nebulization was given for 10–15 min with a mixture of 1 ml of salbutamol (100 mg) and 3 ml of normal saline. Three additional acceptable tests were recorded 15 min later. Before the study, the investigator was trained for a 1-week duration at the department of pulmonary medicine in performing lung function tests using the portable spirometer. Those who were found to have impaired lung function test during spirometric measurements were referred to the department of pulmonary medicine for further evaluation and management.
Operational definitions
Airflow obstruction was defined as FEV1/FVC <70% with FVC >80% predicted. Restrictive defects were defined as an FEV1/FVC ratio of >70% with an FVC <80% predicted. Mixed defects were defined as FVC of <80% predicted and an FEV1/FVC ratio of <70%. LFI is defined as the presence of at least any one of these three abnormalities.[4]
Statistical analysis
Data were entered using EpiData version 4.4.2.1 and data analysis was done using SPSS version 22. SPSS software name originally stood for Statistical Package for the Social Sciences (SPSS) but later changed to Statistical Product and Service Solutions. Its manufacturer is SPSS Inc, IBM and has its headquarter at Chicago, USA. LFI and pattern are expressed as percentages with a 95% confidence interval (CI). Multivariable regression analysis was done to identify the independent risk factors and adjusted prevalence ratios (aPRs) with 95% CI were calculated. P < 0.05 was considered statistically significant.
RESULTS
Of the 185 participants invited to participate, 67 were excluded (22 – not eligible, 28 – migrated, 15 – contraindication for performing spirometry, and 17 – unacceptable spirometry curves). The mean age of the participants was 48 (±15) and the male-to-female ratio was found to be 3:1.
The overall proportion of patients with LFI (n = 74) was 62.7% (95% CI: 53.3–71.4). The restrictive pattern was more predominant (60.1%) followed by a mixed (2.6%). Based on FEV1 values, about half (48.6%) had severe LFI, followed by mild and moderate with 25.7% each. More than half of the participants with restrictive form had moderate LFI (51%). Participants, who were previously treated, smear-negative and those with dyspnea and tobacco use had significantly severe LFI [Tables 1 and 2].
Table 1.
Association of sociodemographic, morbidity, and behavioral characteristics with lung function impairment among post pulmonary tuberculosis participants in selected primary health centers, Puducherry, 2019 (n=118)
| Variables | Total (n=118) | LFI (n=74; 63%), n (%) | Crude PR (95% CI) | P |
|---|---|---|---|---|
| Age group (years) | ||||
| <45 | 51 | 28 (54.9) | 1 | 0.2 |
| 45-60 | 49 | 35 (71.4) | 1.3 (0.9-1.7) | |
| >60 | 18 | 11 (61.1) | 1.1 (0.7-1.7) | |
| Gender | ||||
| Male | 87 | 59 (67.8) | 1.4 (0.9–2.0) | 0.05 |
| Female | 31 | 15 (48.4) | 1 | |
| Occupation | ||||
| Unemployed | 45 | 24 (53.3) | 1 | |
| Employed | 73 | 50 (68.4) | 0.7 (0.5-1.0) | 0.06 |
| Diabetes | 59 | 40 (67.7) | 1.1 (0.8-1.5) | 0.25 |
| Hypertension | 30 | 22 (73.3) | 0.9 (0.7-1.2) | 0.8 |
| Type of treatment category† | ||||
| New | 90 | 53 (58.8) | 1 | 0.03 |
| Previously | 28 | 21 (75.0) | 1.4 (1.1-1.8) | |
| Type of pulmonary TB‡ | ||||
| Smear positive | 106 | 63 (59.4) | 1 | 0.02 |
| Smear negative | 12 | 11 (91.6) | 1.5 (1.2-1.3) | |
| Cavitations in X-ray (diagnosis) | ||||
| Yes | 25 | 16 (64.0) | 1.02 (0.7-1.4) | 0.89 |
| No | 72 | 45 (62.5) | 1 | |
| Lung infiltrates at the time of diagnosis | ||||
| Yes | 55 | 35 (63.6) | 1.02 (0.7-1.3) | 0.84 |
| No | 63 | 39 (70) | 1 | |
| The extent of lung infiltrates at the time of diagnosis (n=55) | ||||
| Unilateral | 28 | 15 (53.5) | 1 | 0.1 |
| Bilateral | 27 | 20 (74.0) | 1.3 (0.9-2.0) |
‡Fisher’s exact, †Statistically significant with P<0.05. LFI: Lung function impairment, CI: Confidence interval, PR: Prevalence ratio, TB: Tuberculosis
Table 2.
Association of respiratory symptoms and behavioral characteristics with lung function impairment among post pulmonary tuberculosis participants in selected primary health centers, Puducherry, 2019 (n=118)
| Variables | Total (n=118) | LFI (n=74; 63%), n (%) | Crude PR (95% CI) | P |
|---|---|---|---|---|
| Respiratory symptoms | ||||
| Chest pain | ||||
| Yes | 33 | 19 (57.6) | 1.12 (0.8-1.5) | 0.47 |
| No | 85 | 55 (64.7) | 1 | |
| Cough | ||||
| Yes | 109 | 70 (64.2) | 1.4 (0.6-3.0) | 0.2 |
| No | 9 | 4 (44.4) | 1 | |
| Hemoptysis | ||||
| Yes | 53 | 35 (66.0) | 1.1 (0.8-1.4) | 0.49 |
| No | 65 | 39 (60) | 1 | |
| Dyspnea† | ||||
| Yes | 65 | 47 (71.2) | 1.3 (1.01-1.85) | 0.03 |
| No | 53 | 27 (51.9) | 1 | |
| Ever-smoked tobacco† | ||||
| Yes | 72 | 51 (70.8) | 1.4 (1.0-1.9) | 0.02 |
| No | 46 | 23 (50) | 1 | |
| Alcohol use‡ | ||||
| Yes | 53 | 31 (58.4) | 0.8 (0.6-1.1) | 0.39 |
| No | 65 | 43 (66.1) | 1 | |
| Indoor exposure to biomass for cooking or heating | ||||
| Yes | 15 | 12 (80.0) | 1.3 (0.9-1.7) | 0.13 |
| No | 103 | 62 (60) | 1 | |
| Exposure to dust at the workplace | ||||
| Yes | 39 | 22 (56.4) | (0.7-1.2) | 0.8 |
| No | 79 | 52 (65.8) | 1 |
†Statistically significant with P<0.05, ‡History of alcohol use in the past year. LFI: Lung function impairment, CI: Confidence interval, PR: Prevalence ratio
In adjusted analysis, smear-negative patients (aPR = 1.2 [95% CI: 1.2–2.0]; P = 0.002) and indoor exposure to biomass (aPR = 1.4 (95% CI: 1.1–1.8]; P = 0.02) were found significant [Table 3].
Table 3.
Multivariable analysis showing association between lung function impairment and morbidity and behavioral characteristics among post pulmonary tuberculosis participants in selected primary health centers, Puducherry, 2019 (n=118)
| Variables | Unadjusted PR (95%CI) | Adjusted PR (95%CI) | P |
|---|---|---|---|
| Type of treatment category | |||
| Previously treated TB | 1.4 (1.1-1.8) | 1.12 (0.8-1.4) | 0.3 |
| New diagnosed | 1 | 1 | |
| Type of pulmonary TB | |||
| Smear negative | 1.5 (1.2-1.9) | 1.2 (1.2-2.0) | 0.002 |
| Smear positive | 1 | 1 | |
| Dyspnea | |||
| Yes | 1.3 (1.01-1.85) | 1.2 (0.9-1.6) | 0.1 |
| No | 1 | 1 | |
| Indoor exposure to biomass | |||
| Yes | 1.3 (0.9-1.7) | 1.4 (1.1-1.8) | 0.02 |
| No | 1 | 1 | |
| Ever smoked tobacco | |||
| Yes | 1.4 (1.0-1.9) | 1.3 (0.9-1.8) | 0.06 |
| No | 1 | 1 |
CI: Confidence interval, PR: Prevalence ratio, TB: Tuberculosis
DISCUSSION
The study found that close to two-thirds of treated TB patients had LFI. Studies across the globe had reported varied prevalence ranging from 30.7% to 76.8%.[2,3,5,6,7,8] Even though a standard diagnostic definition was used by all other studies similar to this study, the observed difference could be due to difference in study setting as most of the studies were facility based. Furthermore, the present study included recurrent TB patients, which could have resulted in a higher prevalence.
The pattern of impairment in the present study was restrictive (60.1%), followed by the mixed type. This result is consistent with the other studies conducted across the globe.[2,3] The restrictive pattern is explained by the fact that there will be the destruction of lung parenchyma following TB infection. It could either be due to bronchial stenosis as a result of pressure from the peribronchial lymph nodes or may be due to increased metalloproteinase activity which contributes to pulmonary damage. However, these are not as common as a restrictive type of damage. Gothi et al.[9] demonstrated in one of their studies that the airflow obstruction could be a consequence of obliterative bronchiolitis.
The moderate form of LFI was observed in most of the patients with LFI in our study both among the patients with restrictive and overall functional impairment. It could be argued that the degree of lung damage varies proportionately with the duration of the disease manifestation, which could not be established in our study. A study conducted in the USA had shown that the severity of impairment ranges from moderate to severe.[10]
LFI was found to be significantly higher among PTB participants who had dyspnea (71.2%) (51.9%; PR – 1.3 [95% CI: 1.0–1.8]) at the time of diagnosis. A few studies showed a similar significant association of dyspnea with LFI.[6,11] However, a study conducted by Ngahane et al.[3] had contradictory results. Dyspnea occurs mainly due to parenchymal lung disease which reduces gas exchange. Other major respiratory symptoms in lung dysfunction such as cough, hemoptysis, and chest pain were not significantly associated with LFI. The Fiogbe[6] study showed a significant association of cough and expectoration at the time of diagnosis with LFI. Ngahane et al.[3] also reported an insignificant association with these variables. There is a possibility of recall bias which could have resulted in inconsistent findings across the studies.
Similarly, LFI was significantly higher among the previously treated patients (PR – 1.4 [95% CI: 1.1–1.8]) as compared to newly diagnosed patients. This could be attributed to the fact that the increase in the number of episodes of TB increases lung damage and this finding was consistent with the studies conducted by Verma et al.[12] and Manji.[5]
The present study found that the prevalence of LFI was significantly higher among smear-negative PTB participants when compared with smear-positive cases. There is always a higher chance of delayed diagnosis and, in turn, delayed initiation of treatment in patients with smear-negative PTB. Moreover, the probability of recurrence is higher when compared to the smear-positive TB patients and hence this could explain the significant association. Regarding the highest sputum smear grading at the time of diagnosis, smear-negative PTB patients had more LFI (PR – 1.7 [95% CI: 1.2–2.3]). A study by Chung found that smear-positive cases were significantly associated with LFI.[13] The presence of a higher microbiological load increases lung damage in multiple ways, but the present study did not reveal such association with smear-positive TB and LFI. Again, this may be due to a smaller sample size of the present study compared to other studies.
Our study reported a significant difference in the prevalence of LFI among tobacco users when compared with nonsmokers. Smoking causes changes in metalloproteinase enzymes and lung scarring which leads to lung function damage. A few other studies also showed a significant association with smoking and LFI.[7,8]
To date, very few studies had documented LFI among post TB patients, that too in the community setting. The study was conducted among the patients enrolled from a district TB center, which in turn makes the study more generalizable. The spirometer was used to measure the LFI which added more objectiveness to the study. A pretested questionnaire and dedicated software for statistical analysis were used which reduced the chances of error. Total lung capacity, which is the best method to measure LFI, could not be carried out and the information about the patient's lung function before the diagnosis was not available and hence the chance of bias in classification could not be ruled out.
According to the National TB Program, after treatment completion the patient should be followed up in every 6 months, up to 2 years to see for appearance of any clinical symptoms. This is done for early detection of recurrent TB by doing cough and sputum microscopy.[14] These follow-up visits shall include screening for lung function by spirometry to detect LFI. Further research is required to assess the utility of spirometry at the end of TB treatment when TB patients are still under TB care.
CONCLUSION
Two in three post TB persons were suffering from LFI. In India, almost half of the individuals who have a history of PTB are at a risk of developing LFI and these changes are increasing day by day. The risk factors such as smoking, air pollution, and indoor exposure to biomass further deteriorate lung function. Hence, there is a need for assessment of lung function in post PTB participants.
Financial support and sponsorship
This study was financially supported by JIPMER intramural funding.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We thank Dr. Pritam and Dr. Sneha masters of Public Health Scholar, JIPMER, and eight primary health center staff, Puducherry, for their support in the conduct of the study.
REFERENCES
- 1.WHO | Chronic Respiratory Diseases (CRDs) World Health Organization; [Last accessed on 2021 Feb 25]. Available from: http://www.who.int/respiratory/en/ [Google Scholar]
- 2.Pasipanodya JG, Miller TL, Vecino M, Munguia G, Garmon R, Bae S, et al. Pulmonary impairment after tuberculosis. Chest. 131:1817–24. doi: 10.1378/chest.06-2949. [DOI] [PubMed] [Google Scholar]
- 3.Ngahane BH, Nouyep J, Motto MN, Njankouo YM, Wandji A, Endale M, et al. Post-tuberculous lung function impairment in a tuberculosis reference clinic in Cameroon. Respir Med. 2016;114:67–71. doi: 10.1016/j.rmed.2016.03.007. [DOI] [PubMed] [Google Scholar]
- 4.Aggarwal A, Aggarwal R, Dhooria S, Prasad K, Sehgal I, Muthu V, et al. Joint Indian chest society-National College of Physicians (India) guidelines for spirometry. Lung India. 2019;36:1–35. doi: 10.4103/lungindia.lungindia_300_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Manji M, Shayo G, Mamuya S, Mpembeni R, Jusabani A, Mugusi F. Lung functions among patients with pulmonary tuberculosis in Dar es Salaam – A cross-sectional study. BMC Pulm Med. 2016;16:58. doi: 10.1186/s12890-016-0213-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fiogbe AA, Agodokpessi G, Tessier JF, Affolabi D, Zannou DM, Adé G, et al. Prevalence of lung function impairment in cured pulmonary tuberculosis patients in Cotonou, Benin. Int J Tuberc Lung Dis. 2019;23:195–202. doi: 10.5588/ijtld.18.0234. [DOI] [PubMed] [Google Scholar]
- 7.Ralph AP, Kenangalem E, Waramori G, Pontororing GJ, Sandjaja, Tjitra E, et al. High morbidity during treatment and residual pulmonary disability in pulmonary tuberculosis: Under recognised phenomena. PLoS One. 2013;8:e80302. doi: 10.1371/journal.pone.0080302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rhee CK, Yoo KH, Lee JH, Park MJ, Kim WJ, Park YB, et al. Clinical characteristics of patients with tuberculosis destroyed lung. Int J Tuberc Lung Dis. 2013;17:67–75. doi: 10.5588/ijtld.12.0351. [DOI] [PubMed] [Google Scholar]
- 9.Gothi D, Shah D, Joshi J. Clinical profile of disease-causing chronic airflow limitation in a tertiary care centre in India. J Assoc Physicians India. 2007;55:551–5. [PubMed] [Google Scholar]
- 10.Mannino D, Buist A, Petty T. Lung function and mortality in the united states: Data from the first national health and nutrition examination survey follow up study. Thorax. 2003;58:388–93. doi: 10.1136/thorax.58.5.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singh SK, Naaraayan A, Acharya P, Menon B, Bansal V, Jesmajian S. Pulmonary rehabilitation in patients with chronic lung impairment from pulmonary tuberculosis. Cureus. 2018;10:e3664. doi: 10.7759/cureus.3664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Verma SK, Kumar S, Narayan KV, Sodhi R. Post tubercular obstructive airway impairment. Indian J Allergy Asthma Immunol. 2009;23:95–9. [Google Scholar]
- 13.Chung KP, Chen JY, Chih Hsin L, Wu HD, Wang JY, Lee LN, et al. Trends and predictors of changes in pulmonary function after treatment for pulmonary tuberculosis. Clinics (Sao Paulo) 2011;66:549 56. doi: 10.1590/S1807-59322011000400005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Technical and Operational Guidelines for TB Control in India 2016: Central TB Division. Available from: https://tbcindia.gov.in/index1 .


