Abstract
Cytokines, the hallmarks of infectious and inflammatory diseases, modify phagocyte activities and thus may interfere with the immunomodulating properties of antibacterial agents. We have investigated whether various proinflammatory cytokines (interleukin 1 [IL-1], IL-6, IL-8, gamma interferon, tumor necrosis factor alpha [TNF-α], and granulocyte-macrophage colony-stimulating factor [GM-CSF]) modify two macrolide properties, i.e., inhibition of oxidant production by polymorphonuclear neutrophils (PMN) and cellular uptake. Roxithromycin and two ketolides, HMR 3647 and HMR 3004, were chosen as the test agents. TNF-α and GM-CSF (but not the other cytokines) decreased the inhibitory effect of HMR 3647 only on oxidant production by PMN. Fifty percent inhibitory concentrations were, however, in the same range in control and cytokine-treated cells (about 60 to 70 μg/ml), suggesting that HMR 3647 acts downstream of the priming effect of cytokines. In contrast, the impairment of oxidant production by roxithromycin and HMR 3004 was unchanged (or increased) in cytokine-treated cells. This result suggests that HMR 3004 (the strongest inhibitory drug, likely owing to its quinoline side chain) and roxithromycin act on a cellular target upstream of cytokine action. In addition, TNF-α and GM-CSF significantly (albeit moderately) impaired (by about 20%) the uptake of the three molecules by PMN. The inhibitory effect of these two cytokines seems to be related to activation of the p38 mitogen-activated protein kinase. Our data also illuminate the mechanism underlying macrolide uptake: protein kinase A- and tyrosine kinase-dependent phosphorylation seems to be necessary for optimal uptake, while protein kinase C activation impairs it. The relevance of our data to the clinical setting requires further investigations, owing to the complexity of the cytokine cascade during infection and inflammation.
Cytokines are polypeptide mediators involved in the communication network of immune cells (26). They regulate both the initiation and the maintenance of the immune response and select the effector mechanisms that mediate resistance to pathogens. However, certain cytokines, particularly when produced in excess, can be pathogenic (10, 37). During the course of infection, a cascade of cytokines is produced which may have both beneficial and detrimental effects by activating the phagocytes which are involved in bacterial destruction and inflammation. For instance, various proinflammatory cytokines (interleukin 1 [IL-1], IL-6, IL-8, and tumor necrosis factor alpha [TNF-α]) have been recognized as pathophysiological markers in septic shock and other consequences of severe infections (34, 36–38).
Some antibacterial agents given to cure infection can also modify the host immune response (17). In particular, macrolides have potential “nonantibiotic” antiinflammatory activity (18, 20, 22, 23). Macrolides are strongly accumulated within phagocytes (the necessary basis for their intracellular bioactivity), and this cellular concentration (>10- to 200-fold the extracellular concentration) can modify cell activities (19, 21). We and others have observed that erythromycin A-derived macrolides impair oxidant production by phagocytes in a time- and concentration-dependent manner (1). This property seems to be related to the presence of l-cladinose (a neutral sugar) at position 3 of the lactone ring (1), although this notion does not exclude the possibility that other chemical structures linked to the erythronolide ring may also interact with cell functions (M. T. Labro, H. Abdelghaffar, and H. Kirst, Abstr. 37th Intersci. Conf. Antimicrob. Agents Chemother., abstr. F-268, 1997). In addition, we have found that l-cladinose-bearing macrolides directly stimulate the exocytosis of polymorphonuclear neutrophils (PMN) (1). The modulation of these two PMN responses was due to interference of these molecules with the phospholipase D-phosphatidate phosphohydrolase pathway (1).
There are few studies on the possible modulation of macrolide-induced alterations of phagocyte functions by cytokines. Bermudez and Young have reported antimycobacterial synergy between TNF-α and azithromycin or roxithromycin in a model of human macrophages (4). The same group observed increased uptake of azithromycin by macrophages in the presence of TNF-α or gamma interferon (IFN-γ) (5). Similarly, Quadrhiri et al. have shown that IFN-γ enhances the cellular accumulation of azithromycin in the human myelomonocytic cell line THP-1 (31). In vivo, the combination of granulocyte colony-stimulating factor (G-CSF) and clarithromycin was more effective than the macrolide alone (G-CSF being ineffective) (25). Lastly, Kadota et al. have recently reported that low concentrations of erythromycin A markedly suppress superoxide anion production by G-CSF-primed PMN stimulated with formylmethionyl-leucyl-phenylalanine (FMLP) (14). The paucity of results in this context, together with the demonstrated anti-inflammatory activity of macrolides in vitro and in vivo, prompted us to analyze whether the priming effect of various proinflammatory cytokines could antagonize (or act in synergy with) the inhibitory effect of erythromycin A-derived macrolides on the PMN oxidative burst. As intracellular accumulation of macrolides is the basis for their immunomodulating properties, we also analyzed the effect of cytokines on the accumulation of these drugs in human PMN.
We compared roxithromycin and two other antibacterial agents, HMR 3004 and HMR 3647, which belong to a new class of semisynthetic erythromycin A derivatives, the ketolides, characterized by a 3-keto group in place of the l-cladinose moiety at position C-3 of the lactone ring (7). HMR 3004 (formerly RU 64004) possesses a quinoline side chain linked to position C-11–C-12 of the lactone ring by a cyclic hydrazonocarbamate function. It is strongly accumulated by PMN (39) and seems to reduce inflammation in vitro and in some animal models (9). HMR 3647 differs from HMR 3004 by having two azolium moieties (imidazolium and pyridinium) linked by an alkyl chain to the C-11–C-12 carbamate ring. It is strongly accumulated by PMN in a time-dependent manner and impairs the oxidative capacity of PMN (40).
(These results have been presented in part at the 36th Interscience Conference on Antimicrobial Agents and Chemotherapy [H. Abdelghaffar and M. T. Labro, Abstr. 36th Intersci. Conf. Antimicrob. Agents Chemother., abstr. F-225, 1996] and at the 38th Interscience Conference on Antimicrobial Agents and Chemotherapy [M. T. Labro and D. Vazifeh, Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother., abstr. A-113, 1998].)
MATERIALS AND METHODS
Antimicrobial agents.
HMR 3647, HMR 3004, roxithromycin, and the radiolabeled drugs [3H]HMR 3647 (35.4 Ci/mmol), [3H]HMR 3004 (25.2 Ci/mmol), and [3H]roxithromycin (21.9 Ci/mmol) in ethanol-water (7:3, vol/vol) were provided by Hoechst-Marion-Roussel (Romainville, France). The tritiated drugs (2.5 μl, about 30 μg/ml) were mixed with 25 μl of unlabeled drugs (1,000 μg/ml of Hanks buffered salt solution [HBSS]; Sigma, St. Louis, Mo.) and 222.5 μl of HBSS. Stock solutions were further diluted in HBSS to the desired concentrations. Chloroquine diphosphate was obtained from Sigma.
Cytokines.
The following sterile, endotoxin-free cytokines (purity, >98%) were obtained from Genzyme Corporation (Cambridge, Mass.): (IL-1α (9.32 × 107 U/mg), endothelial IL-8 (8.5 ng/ml), IL-6 (105 U/5 μg), TNF-α (5.88 × 107 U/mg), granulocyte-macrophage colony-stimulating factor (GM-CSF) (1.82 × 108 U/mg), and IFN-γ (4.75 × 106 U/100 mg). All cytokines were prepared in 0.1% human serum albumin (HSA; Laboratoire Français de Fonctionnement et de Biotechnologie, Les Ulis, France).
Human PMN.
PMN were obtained from venous blood of healthy volunteers by Ficoll-Paque centrifugation followed by 2% Dextran sedimentation and osmotic lysis of residual erythrocytes. The viability and purity of the PMN preparation, assessed by trypan blue exclusion, were both greater than 96%.
PMN viability.
PMN were incubated in the presence of HMR 3004 or chloroquine (10 to 100 μg/ml) at 37°C for 5 to 60 min. PMN viability was assessed by measuring the amount of the cytoplasmic enzyme marker lactic dehydrogenase released in the supernatant (3). Only high concentrations of HMR 3004 and not of chloroquine significantly impaired PMN viability in a time- and concentration-dependent manner. The concentrations which resulted in 50% lactate dehydrogenase release, calculated from regression curves, were largely irrelevant to clinically achievable concentrations: at 30 and 60 min, they were 204 μg/ml (256 μM) (P <0.001; r, 0.901) and 120 μg/ml (151 μM) (P <0.001; r, 0.888), respectively.
Superoxide anion production.
Superoxide anion production was measured in terms of superoxide dismutase-inhibitable cytochrome c reduction as previously described (1). Formyl-methionyl-leucyl-phenylalanine (FMLP; 10−6 M) plus cytochalasin B (5 μg/ml) or phorbol myristate acetate (PMA; 100 ng/ml) was used as the stimulating agent. Results were expressed as nanomoles of superoxide anion produced by 106 PMN over a 5-min period (overall production) and as nanomoles of superoxide anion produced by 106 PMN per min when the rate of production was maximal (initial rate). A value of 21,100 M−1 · cm−1 was used for the extinction coefficient of cytochrome c.
Effect of cytokines on macrolide-induced inhibition of superoxide anion production by PMN.
PMN were pretreated for 30 min with cytokines or HSA (0.1%) and further incubated in the presence of ketolides, roxithromycin, or the corresponding buffers before stimulation with PMA or FMLP plus cytochalasin B. An incubation time of 30 min was necessary for roxithromycin to exert a significant inhibitory effect (1), whereas 5 min was sufficient for the ketolides to impair the PMN oxidative burst (40; Abdelghaffar and Labro, 36th ICAAC).
Macrolide uptake.
A radiometric assay was used to measure macrolide uptake (39, 40). Briefly, 2.5 × 106 PMN were incubated at 37°C with the radiolabeled drugs and then centrifuged at 12,000 × g for 3 min at 22°C through a water-impermeable silicone-paraffin oil (86%:14%, vol/vol) barrier. The pellet was solubilized in Hionic Fluor (Packard), and cell-associated radioactivity was quantified by liquid scintillation counting (LS-6000-S apparatus; Beckman). Standard dilution curves were used to determine the amounts of cell-associated drug. The results were expressed as nanograms/2.5 × 106 PMN. The concentration of macrolide used in the assays was 2.5 μg/ml, unless otherwise stated.
Effect of cytokines on macrolide or ketolide uptake by PMN.
PMN were preincubated for 5 to 120 min in the presence of the cytokines at various concentrations or 0.1% HSA (control). Then, the radiolabeled drugs were added for 5 to 120 min before cellular uptake was measured as described above.
Effect of cytokines on macrolide and ketolide efflux.
Aliquots of macrolide-loaded PMN (10 μg/ml for 30 min) were centrifuged through the silicone-paraffin oil barrier; one aliquot was used to quantify cell-associated macrolides (total associated drug). The other cell pellets were placed in drug-free HBSS containing the cytokines or HSA and, at various times, were centrifuged again through the oil barrier. Radioactivity in the cell pellet and supernatant was measured. We checked that the sum of the radioactivity (that in the pellet plus that in the supernatant) did not significantly differ from the total load. Macrolide efflux was expressed as the percentage of drug associated with the cell pellet compared to the sum of the radioactivity (pellet plus supernatant).
Statistical analysis.
Results are expressed as means ± standard errors of the means for n experiments conducted with PMN from different volunteers. Analysis of variance, regression analysis, and Student's t test for paired data were used to determine statistical significance. All tests were run on the Statworks program, version 1.2 (Cricket Software).
RESULTS
Effect of HMR 3004 and chloroquine on PMN superoxide anion production.
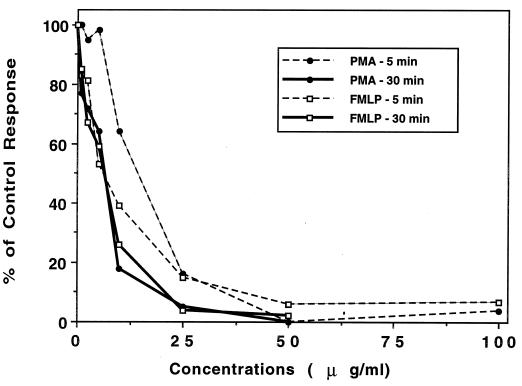
HMR 3004 exerted a strong inhibitory effect on the PMN oxidative burst, whatever the stimulus (PMA or FMLP) (Fig. 1), at concentrations that did not affect cell viability during incubation periods of 5 and 30 min. The inhibitory effects on initial rate and overall production (data not shown) were similar. The concentrations which inhibited 50% of the control PMN response (IC50) were calculated from logarithmic regression curves after 5 and 30 min of incubation. They were, respectively, 9.6 and 2.7 μg/ml (12 and 3.4 μM) (P, <0.001; r, 0.738 and 0.789) (PMA stimulation) and 7.3 and 4.7 μg/ml (9 and 5.9 μM) (P, <0.001; r, 0.910 and 0.859) (FMLP stimulation). In agreement with our previously published data (24), we found that chloroquine also had a strong inhibitory effect on PMN superoxide anion production, with IC50 in the same range as those of HMR 3004: 13 and 8 μg/ml (25 and 15.5 μM) (P, <0.001; r, 0.901 and 0.847) (PMA, 5 and 30 min) and 5.9 and 1.9 μg/ml (11.4 and 3.7 μM) (P, <0.001; r, 0.858 and 0.918) (FMLP, 5 and 30 min). The inhibitory effects of these two drugs were far stronger than those of roxithromycin (IC50, about 49 μg/ml [58 μM] with both stimuli at 30 min [Abdelghaffar and Labro, 36th ICAAC]) and HMR 3647 (IC50, 117 and 44 μg/ml [143 and 54 μM] for PMA at 5 and 30 min and 55 and 30 μg/ml [67 and 36 μM] for FMLP at 5 and 30 min [39]). With all the drugs, the inhibition was significantly stronger at 30 min than at 5 min (P, <0.05).
FIG. 1.
Effect of HMR 3004 on superoxide anion production by PMN. PMN were pretreated with HMR 3004 or control buffer for 5 or 30 min before stimulation with FMLP plus cytochalasin B or PMA. Results are expressed as percent control response (initial rate) (means of 3 to 11 experiments. For HMR 3004 ≥ 10 μg/ml (5 min) and ≥ 2.5 μg/ml (30 min), the P value was <0.05 for all data. Control responses for superoxide anion production (nanomoles/106 PMN/min) were as follows: FMLP at 5 min, 8 ± 0.4, and at 30 min, 4 ± 0.5; and PMA at 5 min, 4 ± 0.3, and at 30 min, 2 ± 0.1.
Effect of cytokines on the antioxidant effect of macrolides. (i) Priming effect of cytokines.
Various proinflammatory cytokines amplify the oxidative burst when PMN are activated by suboptimal concentrations of stimulating agents. Here we investigated whether cytokines could still increase the PMN response when stimuli were used at their optimal concentrations, i.e., PMA at 100 ng/ml and FMLP at 10−6 M plus cytochalasin B, as these concentrations are used to explore the inhibitory effect of macrolides. Despite a strong basal PMN response, three cytokines (TNF-α, 100 U/ml; GM-CSF, 125 pM; and, to a lesser extent, IL-8 at 5 × 10−8 M) still induced the cells to produce more superoxide anion. The maximal stimulatory effect was obtained with GM-CSF (P value versus TNF-α, and IL-8 <0.05), whatever the stimulus, FMLP or PMA. The priming effect was stronger on overall superoxide production over 5 min than on the initial rate of production (P, <0.05). The percentages of the control response for PMA and FMLP, respectively, were 218 ± 16 and 236 ± 8 (GM-CSF), 167 ± 15 and 162 ± 8 (TNF-α), and 118 ± 6 and 120 ± 5 (IL-8). It should be noted that IL-8 and GM-CSF also suppressed the lag time normally observed with PMA stimulation.
We further investigated whether PMN priming with cytokines for 30 min modified the inhibitory effect of macrolides on oxidant production.
We first verified that pretreatment of PMN with 0.1% HSA did not modify the inhibitory effects of the drugs. IC50 calculated from regression curves with PMA and FMLP, respectively, were as follows: HMR 3004, 5 min of incubation, 24 and 13 μg/ml; HMR 3647, 5 min of incubation, 71 and 54 μg/ml; and roxithromycin, 30 min of incubation, 53 and 49 μg/ml. These values did not differ from those obtained with control PMN.
TNF and GM-CSF but not IL-1, IL-6, IL-8, or IFN-γ modified the inhibitory potencies of the three drugs (data not shown). Since the results differed between HMR 3647 on the one hand and roxithromycin and HMR 3004 on the other hand, they are presented separately.
(ii) Effect of TNF-α priming on the inhibitory effect of HMR 3647 on the PMN oxidative burst.
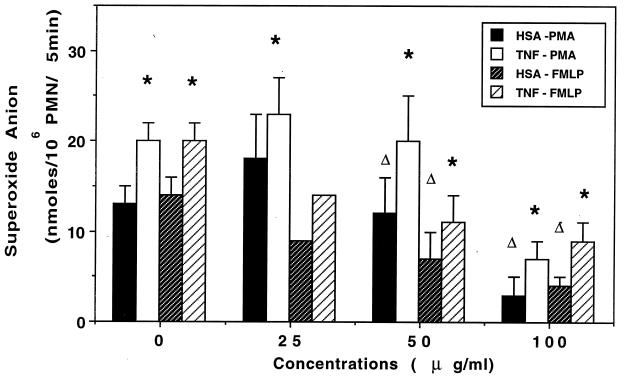
PMN were pretreated with 100 U of TNF-α per ml for 30 min and further incubated with HMR 3647 for 5 min before triggering of the oxidative burst. TNF-α restored the capacity of HMR 3647-treated PMN to produce superoxide anion (Fig. 2). The IC50 calculated on the basis of the respective controls (i.e., HSA-treated or TNF-α-treated PMN) were slightly but not significantly higher in TNF-α-treated cells: 72 versus 82 μg/ml (P, <0.001; r, 0.875 and 0.955) (PMA stimulation) and 63 versus 81 μg/ml (P, <0.001; r, 0.954 and 0.873) (FMLP stimulation). These results suggest that HMR 3647 acts downstream of the priming action of TNF-α. We further investigated whether the priming and restoring effect of TNF-α still occurred when PMN were simultaneously treated with TNF-α and HMR 3647 and when PMN were treated with HMR 3647 before TNF-α treatment. When PMN were treated for 35 min with HSA and HMR 3647 simultaneously, strong concentration-dependent inhibition of the PMN oxidative response was obtained, with IC50 of 46 μg/ml (P, <0.001; r, 0.937) (PMA stimulation) and 40 μg/ml (P, <0.001; r, 0.935) (FMLP stimulation). When TNF-α was present during the incubation period, HMR 3647 was less inhibitory, except at the very high concentration of 100 μg/ml, which almost suppressed the PMN response in either condition (with or without TNF-α). The IC50 were similar to those obtained with HSA-treated PMN (45 μg/ml with PMA; P, <0.001; r, 0.958; and 49 μg/ml with FMLP; P, <0.001; r, 0.992). In an additional experiment, PMN were pretreated with HMR 3647 for 30 min and then incubated with TNF-α for 30 min before stimulation. HMR 3647 did not impair the priming effect of TNF-α: the percentages of the basal response (HSA) were 191 ± 24.6 (FMLP stimulation, control cells, three experiments); 396, 267, and 234 for TNF-α-treated PMN incubated with 50, 25, and 10 mg of HMR 3647 per liter, respectively (one experiment); 134 ± 6.1 (PMA stimulation, control cells, two experiments); and 154 and 121 for PMN incubated with 50 and 25 mg of HMR 3647 per liter, respectively (one experiment).
FIG. 2.
Effect of TNF-α pretreatment on the inhibitory effect of HMR 3647 on oxidant production by PMN. PMN were pretreated with HSA (0.1%) or TNF-α (100 U/ml) for 30 min and then incubated with HMR 3647 or HBSS for 5 min before stimulation. Results are expressed as overall production of superoxide anion (mean ± standard error of the mean for three or four experiments). ∗, P < 0.05 for TNF-α versus HSA; ▵, P < 0.05 for HMR 3647- versus HBSS-treated cells.
(iii) Effect of GM-CSF priming on the inhibitory effect of HMR 3647 on the PMN oxidative burst.
Similar results were obtained with GM-CSF-treated PMN. In particular, PMN pretreatment with 125 pM GM-CSF for 30 min restored a normal response in PMN incubated with HMR 3647 for 5 min (Fig. 3). IC50 were similar in HSA- and GM-CSF-treated cells: 58 and 72 μg/ml for FMLP stimulation (P, <0.001; r, 0.908 and 0.939) and 56 and 64 μg/ml for PMA stimulation (P, <0.001; r, 0.943 and 0.929). Coincubation of PMN with HMR 3647 and GM-CSF also restored overall superoxide anion production. Pretreatment of PMN with HMR 3647 for 15 min did not impair the priming effect of GM-CSF, with increases of 224% ± 22.4% relative to the basal response (FMLP stimulation) versus 284, 273, and 261%, respectively, after pretreatment with 100, 50, and 25 mg of HMR 3647 per liter and of 147% ± 6.1% (PMA stimulation) for the control versus 169 and 132%, respectively, with HMR 3647 at 100 and 50 mg/liter.
FIG. 3.
Effect of GM-CSF pretreatment on the inhibitory effect of HMR 3647 on oxidant production by PMN. Conditions and symbols are the same as those in the legend to Fig. 2, except for the replacement of TNF-α by GM-CSF at 125 pM (three or four experiments).
(iv) Effect of TNF-α and GM-CSF on the inhibitory effect of HMR 3004 and roxithromycin on the PMN oxidative burst.
Contrary to the results obtained with HMR 3647, the strong inhibitory effect of HMR 3004 and the moderate effect of roxithromycin were never restored by pretreatment with TNF or GM-CSF (data not shown). On the contrary, TNF pretreatment tended to increase the suppressive effect of both drugs, which was significant with FMLP stimulation and HMR 3004 at 25 μg/ml (percentages of respective control values: 60 ± 21.5 [HSA-treated PMN] and 14 ± 8.2 [TNF-α-treated PMN]; P, <0.05). Accordingly, the IC50 was lower in TNF-α-treated cells (12 μg/ml [TNF-α] versus 24 μg/ml [HSA]; P, <0.001; r, 0.791 and 0.713). Coincubation of PMN with cytokines and these two drugs and pretreatment of PMN with either drug followed by cytokine treatment also impaired the PMN response (data not shown).
Effect of cytokines on macrolide accumulation.
We next investigated whether cytokines altered the accumulation of the macrolides or ketolides by PMN.
(i) Effect of TNF-α on macrolide uptake.
We first verified that PMN pretreatment with HSA (0.1%) did not modify the cellular accumulation of the drugs compared to control uptake in HBSS alone. The amounts of cell-associated drug (nanograms/2.5 × 106 PMN) after 5 min of incubation did not differ significantly in control and HSA-treated cells: respectively, 455 ± 26.5 and 380 ± 63.2 (HMR 3004); 69 ± 13.9 and 71 ± 23.6 (HMR 3647); and 63 ± 5.0 and 75 ± 12.6 (roxithromycin).
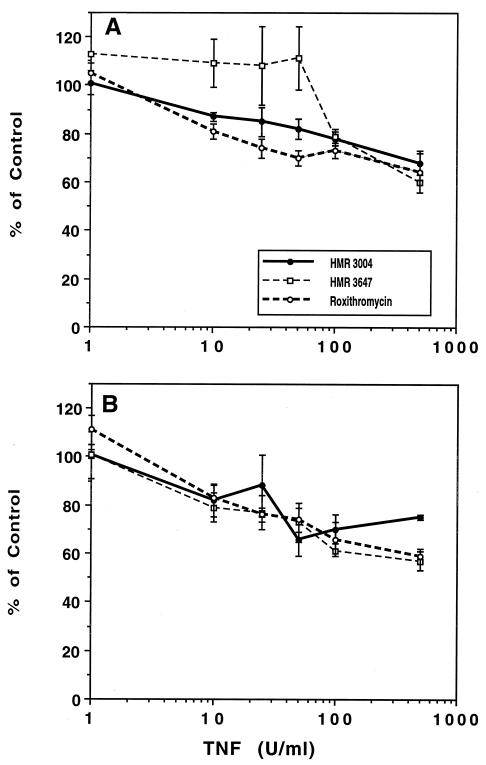
TNF-α was tested at a wide range of concentrations and preincubation times (10 to 120 min) (Fig. 4). TNF-α had a moderate but significant effect on the accumulation of the three drugs. The effect was significant from 10 U/ml for HMR 3004 and roxithromycin (30-min pretreatment) and from 100 U/ml for HMR 3647. After 60 min of preincubation, TNF-α had a concentration-dependent inhibitory effect on the three drugs, although the IC50 were very high: 815 U/ml for HMR 3647 (P, <0.001; r, 0.727), 725 U/ml for roxithromycin (P, <0.001; r, 0.852), and 3,958 U/ml for HMR 3004 (P, 0.005; r, 0.572). The inhibitory effect of TNF-α was rapid (10 min of incubation) and did not increase with time (P, >0.05) for HMR 3004 and roxithromycin. When macrolide-loaded PMN were incubated in drug-free medium supplemented with TNF-α at 100 U/ml, there was no difference in efflux between these cells and cells incubated in HSA-supplemented medium for 2 h (data not shown), suggesting that the inhibitory effect of TNF-α on drug accumulation is due to a modification of drug entry into cells.
FIG. 4.
Effect of TNF-α on macrolide or ketolide uptake. PMN were pretreated with TNF-α or HSA for 30 min (A) or 60 min (B), and the drugs were added at a final concentration of 2.5 μg/ml for 5 min. Results are expressed as percent control uptake (mean ± standard error of the mean for 3 to 15 experiments). Control values (nanograms/2.5 × 106 PMN/5 min) were as follows: for HMR 3004 at 30 min, 354 ± 19.7, and at 60 min, 334 ± 34.7; for HMR 3647 at 30 min, 59 ± 4.5, and at 60 min, 63 ± 10.6; and for roxithromycin at 30 min, 69 ± 4.9, and at 60 min, 61 ± 8.8.
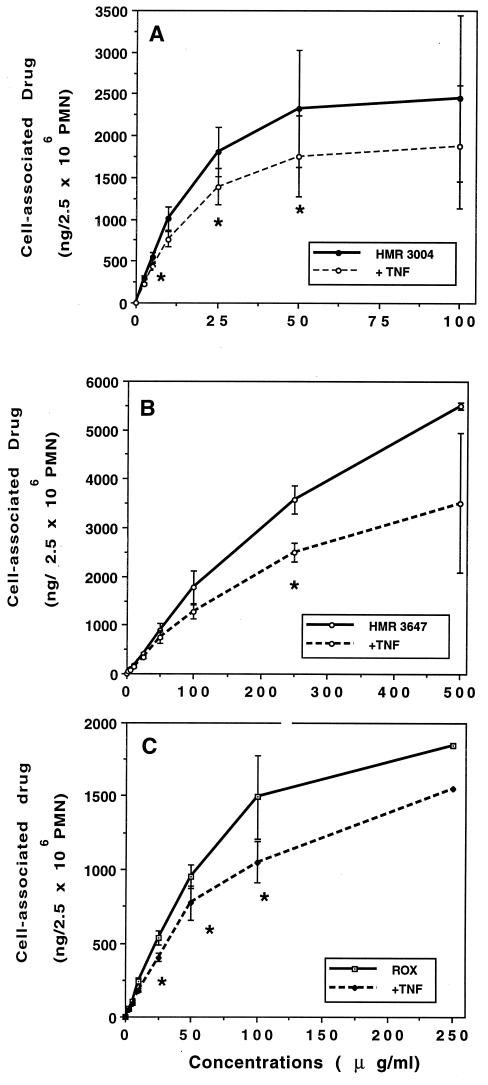
We have previously postulated that macrolide entry into PMN is mediated by a common active membrane transport system with saturable Michaelis-Menten kinetics (39, 40). We thus investigated whether TNF-α modified the activity of this carrier. PMN were pretreated with TNF-α at 100 U/ml and further incubated with the macrolides (1 to 500 μg/ml) (Fig. 5). Vmax was significantly decreased for the three drugs: 79% ± 5.2% for HMR 3004 (P, <0.05), 52% ± 7.2% for HMR 3647 (P, 0.007), and 75% ± 1.6% for roxithromycin (P, 0.006). In contrast, Km values were not significantly modified: 18 ± 7.5 (HSA) versus 20 ± 8.3 (TNF-α) μg/ml for HMR 3004; 140 ± 43.5 versus 86 ± 25.6 μg/ml for HMR 3647; and 97 ± 40.9 versus 110 ± 45.7 μg/ml for roxithromycin.
FIG. 5.
Effect of TNF-α on saturation kinetics of HMR 3004 (A), HMR 3647 (B), and roxithromycin (C) accumulation. Experimental conditions were the same as those in the legend to Fig. 4, except for the drug concentrations. Results are expressed as the absolute amount of cell-associated drugs (mean ± standard error of the mean for four to six experiments). ∗, P < 0.05 versus control (HSA-treated PMN).
(ii) Effect of GM-CSF on macrolide uptake.
GM-CSF also impaired macrolide or ketolide uptake to an extent (about 20%) similar to that induced by TNF-α, although this inhibition was observed at a later incubation time (data not shown). No concentration dependence was observed in the range of 100 to 500 pM. No modification of drug efflux was obtained during a 2-h incubation period (data not shown).
(iii) Effect of other cytokines on macrolide uptake.
None of the other cytokines assessed here impaired drug uptake or efflux at a wide range of concentrations and incubation times (10 to 120 min of pretreatment) (data not shown).
(iv) Kinetics of macrolide uptake by TNF-α- or GM-CSF-treated PMN.
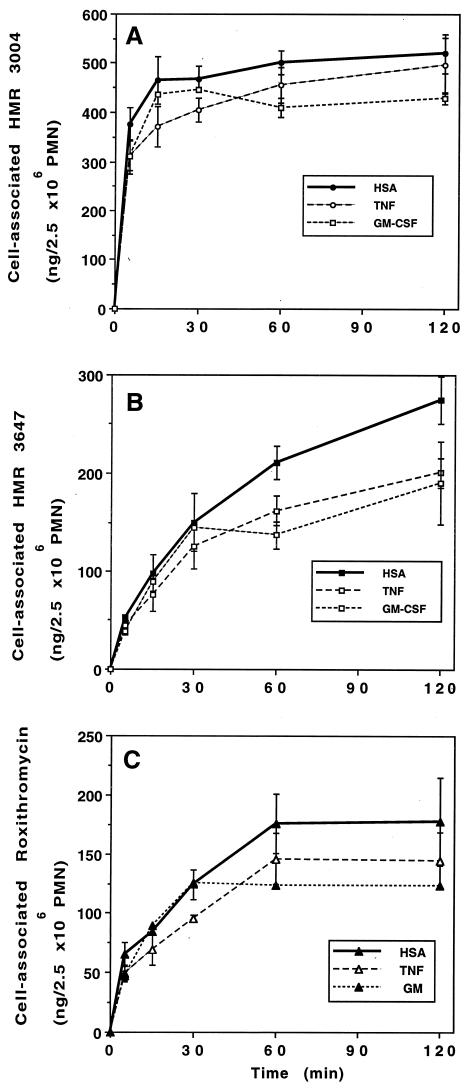
Lastly, we investigated whether the inhibitory effect of TNF-α and GM-CSF on the rapid (5 min) phase of uptake persisted during the incubation period. PMN were first pretreated with TNF-α (100 U/ml) or GM-CSF (125 pM) for 30 min; then, the radiolabeled drugs were added for 5 to 120 min (Fig. 6). The inhibition obtained with TNF-α was significant within the first 5 min, and the 20% decrease persisted up to 60 min (87% ± 4.7% of control [P, 0.038] for HMR 3004; 78% ± 6.7% [P, 0.022] for HMR 3647; and 82% ± 2.1% [P, 0.003] for roxithromycin). At 120 min, the differences were not significant. The inhibitory effect of GM-CSF occurred at 60 min of incubation and persisted at 120 min.
FIG. 6.
Effect of TNF-α and GM-CSF on the kinetics of macrolide or ketolide uptake. PMN were pretreated with TNF-α, GM-CSF, or HSA for 30 min and further incubated with HMR 3004 (A), HMR 3647 (B), or roxithromycin (C) for 5 to 120 min. Results are expressed as the mean ± standard error of the mean for three to six experiments for TNF-α (P < 0.05 from 5 to 60 min) and two experiments for GM-CSF.
(v) Mechanisms of inhibition of macrolide uptake in TNF-α- or GM-CSF-treated PMN.
It was recently reported that PMN pretreatment with various cytokines, including TNF-α and GM-CSF, increased the adhesive properties of the cells, which stuck to the plastic (polypropylene) incubation tubes, resulting in an apparent loss of functional cells of about 20 to 40% (30). We therefore first analyzed whether a similar phenomenon was responsible here for the decrease (about 20%) in drug uptake, even though we used polyethylene tubes. PMN (2.5 × 106/500 μl) were incubated in the presence of HSA, TNF-α (100 U/ml), or GM-CSF (250 pM) for 30 to 60 min, and cell aliquots were counted microscopically in triplicate after Türck staining. In all the conditions, the number of cells did not differ significantly from that in nonincubated controls (2.5 × 106 ± 0.2 × 106 cells/500 μl).
We next investigated whether various agents which alter certain activation mechanisms in PMN also modified the inhibitory effect of TNF-α and GM-CSF. The molecular mechanisms by which cytokines alter PMN functions is still a matter of debate. However, serine or threonine phosphorylation and/or tyrosine phosphorylation are recognized as key pathways in the priming actions of TNF-α and GM-CSF (2, 6, 13, 15, 27, 29, 35). We therefore assessed the effect of the following drugs, which interfere with protein phosphorylation, on the inhibition of macrolide uptake induced by cytokines: PMA, a protein kinase C (PKC) activator; H89, a protein kinase A (PKA) inhibitor; tyrphostin A23, a tyrosine kinase (TK) inhibitor; staurosporin, a not fully specific PKC inhibitor; H7, a nonspecific protein kinase inhibitor; and two inhibitors of the mitogen-activated protein (MAP) kinase pathways, PD 098059 and SB 203580.
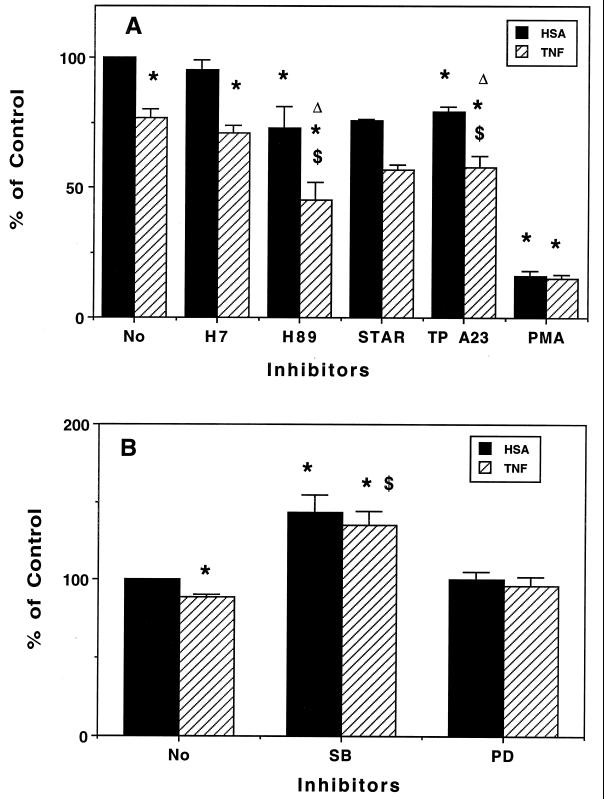
PMN were pretreated with control buffer or the inhibitors for 10 min (45 min for MAP kinase inhibitors) and further incubated with HSA, TNF-α (30 min), or GM-CSF (60 min) before macrolides were added for 5 min. Roxithromycin and TNF-α were chosen to explore the inhibitory pathway (Fig. 7). H89, tyrphostin A23, and staurosporin impaired roxithromycin uptake by HSA-treated PMN; this inhibition was increased by TNF-α pretreatment, and H7 also potentiated the inhibitory effect of TNF-α (Fig. 7A). In contrast, PMA strongly impaired the uptake of roxithromycin (16% ± 2.0% of the control value), and TNF-α did not modify this value (15% ± 0.6% of the control value). PMN pretreatment with staurosporin restored PMA-mediated inhibition (HSA- and TNF-α-treated PMN) to an extent similar to that observed with staurosporin alone (60% ± 0.5% and 51% ± 10.0% of control PMN values, respectively, for PMA- plus HSA-treated PMN and for PMA- plus TNF-α-treated cells). These results further emphasize the potential active mechanism which sustains the accumulation of macrolides in PMN and seems to require phosphorylation of the carrier by PKA- and/or TK-dependent activity. It should be noted that staurosporin is not fully specific for PKC and can inhibit PKA and TK activities, a fact which may explain the 25% inhibition seen with HSA-treated PMN. PKC-mediated phosphorylation of this carrier seems to negatively regulate its activity. That TNF-α acts in synergy with the various protein kinase inhibitors suggests that its inhibitory effect is dependent on complementary mechanisms. The similar inhibition obtained in PMA (with or without TNF-α)-treated PMN could be due either to a down-modulation of TNF-α receptors (32) or to a similar inhibitory pathway (PKC activation).
FIG. 7.
Effect of activators or inhibitors of protein kinases on roxithromycin uptake by HSA- or TNF-α-treated PMN. PMN were pretreated with various agents which alter protein phosphorylation for 10 min (A) or 45 min (B); they were then incubated with TNF-α or HSA for 30 min before roxithromycin was added for 5 min. Results are expressed as percent control uptake (mean ± standard error of the mean for 3 to 11 experiments [2 experiments for staurosporin {STAR}]). TP, tyrphostin; SB, SB 203580; PD, PD 098059. Control values (nanograms/2.5 × 106 PMN/5 min) were 63 ± 5.7 (A) and 43 ± 5.6 (B). ∗, P < 0.05 versus control (HSA); $, P < 0.05 versus TNF-α alone; ▵, P < 0.05 for inhibitor versus inhibitor plus TNF-α.
Various studies have demonstrated that TNF-α and GM-CSF prime PMN by activating MAP kinase pathways, particularly p38 and ERK1/2 (extracellular signal-regulated kinases [ERK]) (12, 29, 35). We thus investigated the effects of SB 203580 (an inhibitor of p38 MAP kinase) and PD 098059 (an inhibitor of ERK activation) on TNF-α-mediated inhibition of uptake (Fig. 7B). PMN pretreatment for 45 min in HBSS resulted in a significant decrease in roxithromycin uptake by HSA-treated PMN compared to pretreatment for 10 min (43 ± 5.6 versus 63 ± 5.7 ng/2.5 × 106 PMN/5 min; P, 0.026; 11 experiments). Also, the inhibitory effect of TNF-α was significantly less pronounced in these PMN (11% ± 2.1% inhibition versus 23% ± 3.1% inhibition; P, 0.006). Interestingly, SB 203580 but not PD 098059 increased roxithromycin uptake by HSA- and TNF-treated PMN (143% ± 11.6% and 135% ± 9.2% of control values, respectively; P, 0.014 and 0.012, respectively). The amount of cell-associated roxithromycin (nanograms/2.5 × 106 PMN) in SB 203580-treated PMN was similar to that in control cells (treated for 10 min): 67 ± 12.0 (SB 203580 plus HSA) and 64 ± 10.0 (SB 203580 plus TNF-α). These data suggest that, during a long (45-min) preincubation, PMN are preactivated, possibly via a p38 MAP kinase pathway, and that TNF-α-mediated inhibition of uptake also involves this pathway. Similar data were obtained with GM-CSF and SB 203580 (data not shown).
DISCUSSION
The nonantibiotic anti-inflammatory potential of macrolides has triggered increased interest worldwide. Some of these antibiotics display anti-inflammatory activities in vitro and in vivo (16, 22, 23). Among the many known interactions between macrolides and effectors of the inflammatory response, the inhibitory effect of erythromycin A derivatives on the oxidative capacity of phagocytes is widely acknowledged (1, 18). This property is likely dependent on the marked intracellular accumulation of these drugs (19, 21). Despite reports suggesting an active macrolide entry process (39, 40), the underlying mechanisms are still poorly understood. Furthermore, macrolide accumulation is generally assessed in vitro, in conditions which cannot reflect the in vivo situation. Cytokines, the hallmarks of infectious and inflammatory diseases, modify phagocyte activities and may interfere with the modulation of phagocyte functions by macrolides. Few studies have explored the consequences of phagocyte activation (priming) by cytokines (4, 5, 14, 25, 31).
Here we investigated whether various proinflammatory cytokines could modify two possibly related macrolide characteristics, i.e., cellular uptake and inhibition of oxidant production by PMN. Three cytokines (TNF-α, GM-CSF, and, to a lesser extent, IL-8) were able to enhance the oxidative response of optimally stimulated PMN, and only the former two cytokines interfered with the macrolide properties studied here. With regard to the inhibition of the PMN oxidative burst, we first demonstrated that the ketolide HMR 3004 strongly impaired the PMN oxidative burst at concentrations similar to those therapeutically achievable in serum or tissues for various macrolides. The quinoline substituent of HMR 3004 is likely responsible for the inhibitory effect of this drug and could possibly also explain the rapid and marked accumulation of HMR 3004 (39), as a similar accumulation has been reported with chloroquine (33). The inhibitory effects of HMR 3004 and roxithromycin on the PMN oxidative burst were unchanged (or even seemed to be increased) by the pretreatment of PMN with TNF-α or GM-CSF. These data are in line with the report by Kadota et al. (14) that erythromycin A-induced inhibition is stronger in GM-CSF-primed PMN than in control PMN. Our results thus suggest that roxithromycin and HMR 3004 act upstream of cytokine action. Similar data were obtained with other erythromycin A derivatives (clarithromycin and azithromycin) (data not shown) which have been shown to interfere with the same activation pathway in PMN (1). In contrast, the inhibitory effect of HMR 3647 was offset by PMN pretreatment with TNF-α or GM-CSF (Fig. 2 and 3). As demonstrated by IC50, HMR 3647 had similar inhibitory effects on control and cytokine-primed PMN but, owing to the enhancement of the PMN response by the two cytokines, the overall result was an apparent restoration of the oxidative capacity of the cells. In addition, HMR 3647 did not impair the priming effect of TNF-α or GM-CSF during coincubation or when added to PMN before the cytokines. These data suggest that HMR 3647 acts downstream of the activation pathway used by cytokines.
The relevance of our data to the clinical situation is unclear. The concentrations of TNF-α used in this study (100 U/ml; about 1,700 pg/ml) are compatible with those observed in clinical settings; in septic patients with fatal diseases, serum TNF-α levels have been reported to reach 495 to 1,800 pg/ml (8, 37), and even higher values are obtained in cerebrospinal fluid (11). The observation that proinflammatory cytokines do not interfere with the inhibitory effects of roxithromycin and HMR 3004 on oxidant production by phagocytes is in agreement with their demonstrated anti-inflammatory properties in vivo (9, 22, 23) and suggests that HMR 3647 would not display the same activity, although no data are yet available for this drug in this context.
The second set of data that we present here concerns the modification of macrolide uptake by cytokine-primed PMN. It is interesting to note that the same cytokines (TNF-α and GM-CSF) that interfered with the oxidative response of PMN also decreased the accumulation of HMR 3004, HMR 3647, and roxithromycin (Fig. 4 and 6). This result suggests that these two cytokines interfere with a common transductional pathway in PMN which is important both for their priming effect and for the optimal activity of the macrolide carrier. The impairment of drug uptake does not explain the decreased inhibition of oxidant production by HMR 3647-treated PMN; indeed, whereas the cytokine-induced impairment of uptake was observed with all the drugs tested, only the inhibition induced by HMR 3647 was restored. In addition, GM-CSF (30 min) restored the inhibition induced by HMR 3647, whereas an incubation time of 60 to 120 min was necessary to impair HMR 3647 uptake. The effect of TNF-α was rapid (particularly with HMR 3004 and roxithromycin), maximal after 60 min of incubation with all the drugs (Fig. 4), and concentration dependent, but the IC50 (>500 to 1,000 U/ml) were much higher than those observed in human disease (8, 11, 37). These data are at variance with those reported by Bermudez et al. (5) and Ouadrhiri et al. (31), who observed an increase in azithromycin uptake in IFN-γ, TNF-α, or IL-1-treated human macrophages (5) and IFN-γ-treated TH-P1 cells (31). Either the macrolide carrier present at the PMN membrane differs from that of mononucleated cells or the cytokine-induced transductional mechanisms differ in phagocytes of different lineages.
The statistically significant but moderate impairment of macrolide uptake by cytokine-treated PMN likely has few consequences for the intracellular bioactivity of these drugs. First, this inhibitory effect has not been shown for macrophages (5, 31), the main phagocytic cells harboring intracellular bacteria; second, the decreased uptake is observed in the first 5 min but is gradually restored with longer incubation times (Fig. 6). The mechanism responsible for the decrease in macrolide or ketolide uptake is unclear. We found that TNF-α pretreatment of PMN decreased the activity (not the affinity) of the macrolide carrier (Fig. 5); Vmax was decreased by about 20 to 50%, and Km was unchanged. How TNF-α and GM-CSF modify the macrolide carrier system in PMN was not elucidated. Both cytokines stimulate various kinases, including PKC and TK. TNF-α-mediated inhibition was increased by PKA and TK inhibitors but was not modified by PMA, suggesting that this inhibition is secondary to PKC activation. PKC activation by TNF-α is likely a first step in the priming effect of TNF-α and GM-CSF, as MAP kinase activation is recognized as a common effector mechanism in the modulation of PMN functions by these cytokines (15, 29). That SB 203580, the p38 MAP kinase inhibitor, was able to reverse the inhibitory action of TNF-α and GM-CSF and even to restore roxithromycin uptake in resting PMN incubated for long periods suggests that the p38 MAP kinase pathway is involved in both cytokine-mediated and spontaneous inactivation of the PMN macrolide carrier.
Although we have not identified the macrolide carrier, we have obtained data suggesting that, in resting PMN, the macrolide carrier is phosphorylated by PKA (M. T. Labro et al., Abstr. 7th Int. Congr. Infect. Dis., abstr. 110-018, 1996) and TK. Indeed, inhibition of PKA or TK (by H89, H7, staurosporin, or tyrphostin A23) impairs the optimal functioning of the carrier (Fig. 7); conversely, PKC activation by PMA leads directly (PKC-dependent phosphorylation) or indirectly (PKC-dependent activation of p38 MAP kinase [28]) to inappropriately phosphorylated forms of the carrier which are less efficient at accumulating macrolides (Fig. 7). During long incubation times, similar activation of protein kinases may result in decreased accumulation of macrolides, which is restored by pretreatment with SB 203580. TNF-α and GM-CSF also seem to interfere with macrolide uptake by activating PKC or p38 MAP kinase, as demonstrated by the restoration of roxithromycin accumulation in SB 203580-treated PMN (Fig. 7B).
In summary, we observed a complex interplay among macrolides, cytokines, and phagocytes in vitro. The situation in vivo is certainly even more complex, as not one cytokine but a cascade of cytokines is sequentially produced; these cytokines may act in synergy or in an antagonistic manner to alter phagocyte functions.
ACKNOWLEDGMENTS
This work was supported in part by a grant from Hoechst-Marion-Roussel.
REFERENCES
- 1.Abdelghaffar H, Vazifeh D, Labro M T. Erythromycin A-derived macrolides modify the functional activities of human neutrophils by altering the phospholipase D-phosphatidate phosphohydrolase transduction pathway. J Immunol. 1997;159:3995–4005. [PubMed] [Google Scholar]
- 2.Bereta J, Bereta M, Cohen S, Cohen M C. Studies on the role of protein kinases in the TNF-mediated enhancement of murine tumor cell-endothelial cell interactions. J Cell Biochem. 1991;47:62–78. doi: 10.1002/jcb.240470109. [DOI] [PubMed] [Google Scholar]
- 3.Bergmeyer H U, Bernt E. Lactate dehydrogenase. In: Bergmeyer H U, editor. Methods in enzymatic analysis. New York, N.Y: Academic Press, Inc.; 1963. pp. 737–739. [Google Scholar]
- 4.Bermudez L E, Young L S. Activities of amikacin, roxithromycin, and azithromycin alone or in combination with tumor necrosis factor against Mycobacterium avium complex. Antimicrob Agents Chemother. 1988;32:1149–1153. doi: 10.1128/aac.32.8.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bermudez L E, Inderlied C, Young L S. Stimulation with cytokines enhances penetration of azithromycin into human macrophages. Antimicrob Agents Chemother. 1991;35:2625–2629. doi: 10.1128/aac.35.12.2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bourgoin S, Poubelle P E, Liao N W, Umezawa K, Borgeat P, Naccache P H. Granulocyte-macrophage colony-stimulating factor primes phospholipase D activity in human neutrophils in vitro: role of calcium, G-proteins, and tyrosine kinases. Cell Signal. 1992;4:487–500. doi: 10.1016/0898-6568(92)90018-4. [DOI] [PubMed] [Google Scholar]
- 7.Bryskier A, Agouridas C, Chantot J F. Ketolides: new semisynthetic 14-membered-ring macrolides. In: Zinner S H, Young L S, Acar J F, Neu H C, editors. Expanding indications for the new macrolides, azalides, and streptogramins. New York, N.Y: Marcel Dekker, Inc.; 1997. pp. 39–50. [Google Scholar]
- 8.De Bont E S J M, Martens A, Van Raan J, Samson G, Fetter W P F, Okken A, de Leij L H F M. Tumor necrosis factor α, interleukin-1β and interleukin-6 plasma levels in neonatal sepsis. Pediatr Res. 1993;33:380–383. doi: 10.1203/00006450-199304000-00013. [DOI] [PubMed] [Google Scholar]
- 9.Duong M, Simard M, Bergeron Y, Ouellet N, Côté-Richier M, Bergeron M G. Immunomodulating effects of HMR 3004 on pulmonary inflammation caused by heat-killed Streptococcus pneumoniae in mice. Antimicrob Agents Chemother. 1998;42:3309–3312. doi: 10.1128/aac.42.12.3309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fresno M, Kopf M, Rivas L. Cytokines and infectious diseases. Immunol Today. 1997;18:56–58. doi: 10.1016/s0167-5699(96)30069-8. [DOI] [PubMed] [Google Scholar]
- 11.Glimaker M, Kragsberg P, Forsgren M, Olcén P. Tumor necrosis factor-α (TNF-α) in cerebrospinal fluid from patients with meningitis of different etiologies. High levels of TNF-α indicate bacterial meningitis. J Infect Dis. 1993;167:882–889. doi: 10.1093/infdis/167.4.882. [DOI] [PubMed] [Google Scholar]
- 12.Gomez-Cambronero J, Colasanto J M, Huang C K, Sha'afi R I. Direct stimulation by tyrosine phosphorylation of microtubule-associated protein (MAP) kinase activity by granulocyte-macrophage colony-stimulating factor in human neutrophils. Biochem J. 1993;291:211–217. doi: 10.1042/bj2910211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson S E, Baglioni C. Positive and negative regulation of a tumor necrosis factor response in melanoma cells. J Biol Chem. 1990;265:6642–6649. [PubMed] [Google Scholar]
- 14.Kadota J-I, Iwashita T, Matsubara Y, Ishimatsu Y, Yoshinaga M, Abe K, Kohno S. Inhibitory effect of erythromycin on superoxide anion production by human neutrophils primed with granulocyte colony-stimulating factor. Antimicrob Agents Chemother. 1998;42:1866–1867. doi: 10.1128/aac.42.7.1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kanakura Y, Druker B, Cannistra S A, Furukawa Y, Torimoto Y, Griffin J D. Signal transduction of the human granulocyte-macrophage-stimulating factor and interleukin-3 receptor involves tyrosine phosphorylation of a common set of cytoplasmic proteins. Blood. 1990;76:706–715. [PubMed] [Google Scholar]
- 16.Khan A A, Slifer T R, Araujo F G, Remington J S. Effect of clarithromycin and azithromycin on production of cytokines by human monocytes. Int J Antimicrob Agents. 1999;11:121–132. doi: 10.1016/s0924-8579(98)00091-0. [DOI] [PubMed] [Google Scholar]
- 17.Labro M T. Immunomodulation by antibacterial agents. Is it clinically relevant? Drugs. 1993;45:319–328. doi: 10.2165/00003495-199345030-00001. [DOI] [PubMed] [Google Scholar]
- 18.Labro M T. Effect of macrolides on host natural defenses. In: Bryskier A, Butzler J P, Neu H C, Tulkens P M, editors. Macrolides: chemistry, pharmacology and clinical use. Paris, France: Arnette-Blackwell; 1993. pp. 389–408. [Google Scholar]
- 19.Labro M T. Intraphagocytic penetration of macrolide antibiotics. In: Bryskier A, Butzler J P, Neu H C, Tulkens P M, editors. Macrolides: chemistry, pharmacology and clinical use. Paris, France: Arnette-Blackwell; 1993. pp. 379–388. [Google Scholar]
- 20.Labro M T. Immunomodulatory action of antibacterial agents. Clin Immunother. 1996;6:454–464. [Google Scholar]
- 21.Labro M T. Effects of macrolides on leukocytes and inflammation. In: Zinner S H, Young L S, Acar J F, Neu H C, editors. Expanding indications for the new macrolides, azalides, and streptogramins. New York, N.Y: Marcel Dekker, Inc.; 1997. pp. 101–116. [Google Scholar]
- 22.Labro M T. Anti-inflammatory activity of macrolides: a new therapeutic potential? J Antimicrob Chemother. 1998;41(Suppl. B):37–46. doi: 10.1093/jac/41.suppl_2.37. [DOI] [PubMed] [Google Scholar]
- 23.Labro M T. Immunological effects of macrolides. Curr Opin Infect Dis. 1998;11:681–688. doi: 10.1097/00001432-199812000-00006. [DOI] [PubMed] [Google Scholar]
- 24.Labro M T, Babin-Chevaye C. Effects of amodiaquine, chloroquine, and mefloquine on human polymorphonuclear neutrophil function in vitro. Antimicrob Agents Chemother. 1988;32:1124–1130. doi: 10.1128/aac.32.8.1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lazard T, Perronne C, Cohen Y, Grosset J, Vilde J-L, Pocidalo J-J. Efficacy of granulocyte colony-stimulating factor and RU-40555 in combination with clarithromycin against Mycobacterium avium complex infection in C57BL/6 mice. Antimicrob Agents Chemother. 1993;37:692–695. doi: 10.1128/aac.37.4.692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liles W C, Van Voorhis W C. Review: nomenclature and biologic significance of cytokines involved in inflammation and the host immune response. J Infect Dis. 1995;172:1573–1580. doi: 10.1093/infdis/172.6.1573. [DOI] [PubMed] [Google Scholar]
- 27.McColl S R, DiPersio J F, Caon A C, Naccache P H. Involvement of tyrosine kinases in the activation of human peripheral blood neutrophils by granulocyte-macrophage colony-stimulating factor. Blood. 1991;78:1842–1852. [PubMed] [Google Scholar]
- 28.McLeish K R, Klein J B, Coxon P Y, Head K Z, Ward R A. Bacterial phagocytosis activates extracellular signal-regulated kinase and p38 mitogen-activated protein kinase cascades in human neutrophils. J Leukoc Biol. 1998;64:835–844. [PubMed] [Google Scholar]
- 29.McLeish K R, Knall C, Ward R A, Gerwins P, Coxon P Y, Klein J B, Johnson G L. Activation of mitogen-activated protein kinase cascades during priming of human neutrophils by TNF-α and GM-CSF. J Leukoc Biol. 1998;64:537–545. [PubMed] [Google Scholar]
- 30.Ogle J D, Noel J G, Sramkoski R M, Ogle C K. Adhesive effect of certain cytokines and other perturbants on human neutrophils. Inflammation. 1992;16:603–612. doi: 10.1007/BF00919343. [DOI] [PubMed] [Google Scholar]
- 31.Ouadrhiri Y, Scorneaux B, Sibille Y, Tulkens P M. Mechanism of the intracellular killing and modulation of antibiotic susceptibility of Listeria monocytogenes in THP-1 macrophages activated by gamma interferon. Antimicrob Agents Chemother. 1999;43:1342–1351. doi: 10.1128/aac.43.5.1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Porteu F, Nathan C F. Shedding of tumor necrosis factor receptors by activated human neutrophils. J Exp Med. 1990;172:599–607. doi: 10.1084/jem.172.2.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Raghoebar M, Van der Berg W B, Van Ginneken C A M. Alteration of chloroquine accumulation in human polymorphonuclear leucocytes under inflammatory conditions. Int J Tissue React. 1987;IX:255–261. [PubMed] [Google Scholar]
- 34.Spooner C E, Markowitz N P, Saravolatz L D. The role of tumor necrosis factor in sepsis. Clin Immunol Immunopathol. 1992;62:S11–S17. doi: 10.1016/0090-1229(92)90036-n. [DOI] [PubMed] [Google Scholar]
- 35.Suzuki K, Hino M, Hato F, Tatsumi N, Kitayama S. Cytokine-specific activation of distinct mitogen-activated protein kinase subtype cascades in human neutrophils stimulated by granulocyte colony-stimulating factor, granulocyte-macrophage colony-stimulating factor, and tumor necrosis factor α. Blood. 1999;93:341–349. [PubMed] [Google Scholar]
- 36.Van der Poll T, Sauerwein H P. Tumour necrosis factor-α: its role in the metabolic response to sepsis. Clin Sci. 1993;84:247–256. doi: 10.1042/cs0840247. [DOI] [PubMed] [Google Scholar]
- 37.Van Deuren M, van der Ven-Jongekrijg J, Bartelink A K M, van Dalen R, Sauerwein R W, van der Meer J W M. Correlation between proinflammatory cytokines and antiinflammatory mediators and the severity of disease in meningococcal infections. J Infect Dis. 1995;172:433–439. doi: 10.1093/infdis/172.2.433. [DOI] [PubMed] [Google Scholar]
- 38.Vassalli P. The pathophysiology of tumor necrosis factors. Annu Rev Immunol. 1992;10:411–452. doi: 10.1146/annurev.iy.10.040192.002211. [DOI] [PubMed] [Google Scholar]
- 39.Vazifeh D, Abdelghaffar H, Labro M T. Cellular accumulation of the new ketolide RU 64004 by human neutrophils: comparison with that of azithromycin and roxithromycin. Antimicrob Agents Chemother. 1997;41:2099–2107. doi: 10.1128/aac.41.10.2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vazifeh D, Preira A, Bryskier A, Labro M T. Interactions between HMR 3647, a new ketolide, and human polymorphonuclear neutrophils. Antimicrob Agents Chemother. 1998;42:1944–1951. doi: 10.1128/aac.42.8.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]