TO THE EDITOR:
The incidence of prostate cancer and its associated mortality vary widely according to race or ethnic background.1 This association is probably due to the interplay of socioeconomic factors, environmental exposures, and biologic and epigenetic phenomena.1,2 Nevertheless, precision oncologic studies have underrepresented Black and other non-White patients, thereby limiting our comprehensive understanding of disparities in the diagnosis and prognosis of prostate cancer among these populations.3 Therefore, we examined tumor genomic profiles across race in a diverse cohort of patients.
First, we reviewed tumor genomic data obtained with the use of next-generation sequencing from patients who had been treated for prostate cancer at either Memorial Sloan Kettering Cancer Center or Dana–Farber Cancer Institute. These data were extracted from the registry of the American Association for Cancer Research Project GENIE (Genomics Evidence Neoplasia Information Exchange), version 7.0.4 Mutational profiles of 474 genes were examined according to race (White, Black, or Asian) and tumor stage (primary or metastatic). We used the Benjamini–Hochberg method to control for the false discovery rate. (Details regarding methods are provided in the Supplementary Appendix, available with the full text of this letter at NEJM.org.)
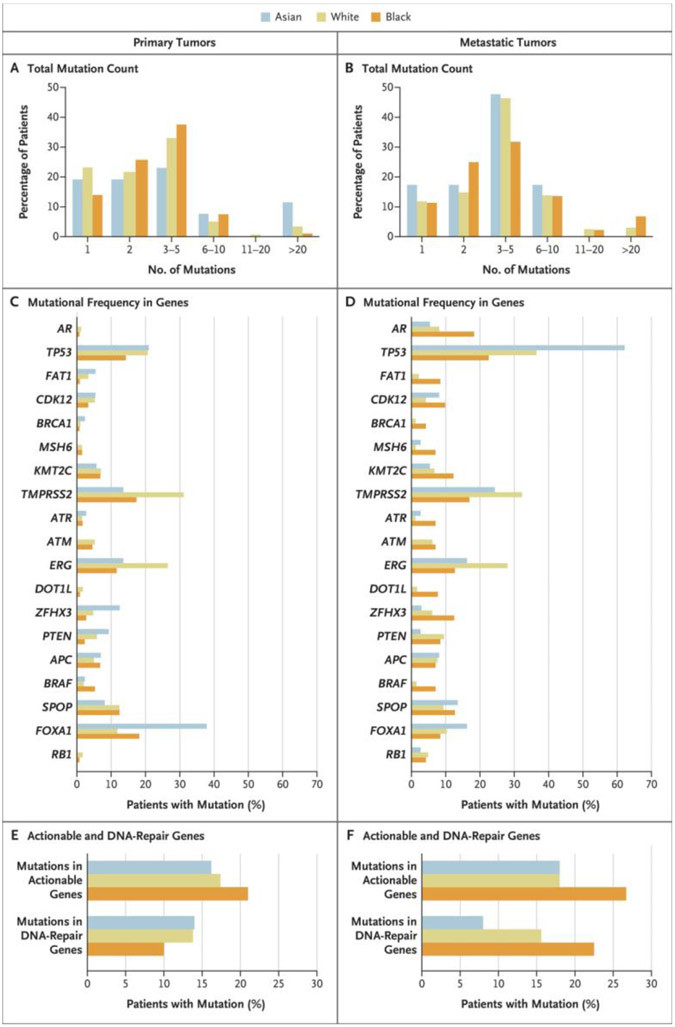
Among 2393 patients (2109 White, 204 Black, and 80 Asian), 1484 had primary disease (1308 White, 133 Black, and 43 Asian) and 909 had metastatic disease (801 White, 71 Black, and 37 Asian). The mutational profiles of the patients are shown in Figure 1; the relative difference in mutations in primary disease and in metastatic disease according to race are shown in Figure S1 in the Supplementary Appendix.
Figure 1. Tumor-Mutation Profiles in the 2393 Study Patients.
Shown are tumor-mutation profiles according to race among men with primary prostate cancer (Panels A, C, and E) or metastatic prostate cancer (Panels B, D, and F). The data were obtained from targeted tumor-sequencing testing available to patients at the Memorial Sloan Kettering (MSK) Cancer Center (Integrated Mutation Profiling of Actionable Cancer Targets [IMPACT]) and from the Dana–Farber Cancer Institute (Oncopanel next-generation sequencing). (Details regarding testing methods are provided in the Supplementary Appendix.) Among the 2393 patients who were included in the study, 1484 patients had primary tumors, and 909 patients had metastatic tumors. The total mutation count in the two subgroups (Panels A and B) was calculated in the MSK-468 cohort (the largest subgroup in the study), in which patients were evaluated on a next-generation sequencing panel of 468 unique genes. Mutational frequency is shown for the genes that were most commonly altered in the study (Panels C and D). Actionable mutations are alterations that are the intended targets of precision-oncology drugs. Genes with actionable mutations (Panels E and F) include ABL1, EGFR, ERBB2, BRAF, BRCA1/2, FGFR2/3, KIT, NTRK1/2/3, PDGFRA, RET, ROS1, ALK, and PIK3CA. Among the patients with metastatic prostate cancer, genes with actionable mutations occurred more often in Black men than in White men (26.7% vs. 18.0%, P=0.05). DNA-repair genes include ERCC5, MRE11, TP53BP1, POLE, RAD21, MSH2, MSH6, BRCA1/2, ATR, and ATM. Mutations in DNA-repair genes occurred more often in Black men than in White men (22.5% vs. 15.6%, P=0.05) among those with metastatic disease.
Among the patients with primary prostate cancers, 11.5% of Asian men had more than 20 mutations (Fig. 1A). FOXA1 mutations occurred more often in Black men than in White men (18.6% vs. 11.9%; unadjusted between-group difference [referred to as difference throughout], 6.7 percentage points; 95% confidence interval [CI], 0.8 to 13.5; P = 0.03); such mutations also occurred more frequently in Asian men (37.8% vs. 11.9%; difference, 25.9 percentage points; 95% CI, 10.2 to 41.7; P<0.001) (Fig. 1C). TP53 mutations occurred less frequently in Black men than in White men (14.2% vs. 20.6%; difference, −6.4 percentage points; 95% CI, −12.7 to 0; P = 0.045). Mutations in the gene encoding androgen receptor (AR) were rare, regardless of race. The frequencies of genes with actionable mutations (i.e., alterations that are the intended targets of precision-oncology drugs) and mutations in DNA-repair genes did not differ substantially among the racial groups (Fig. 1E).
Among the patients with metastatic prostate cancer, 6.8% of Black men had more than 20 mutations (Fig. 1B). AR mutations occurred more often in Black men than in White men (18.3% vs. 8.1%; difference, 10.2 percentage points; 95% CI, 1.0 to 19.4; P = 0.004) (Fig. 1D). TP53 mutations occurred more often in Asian men than in either Black men (62.0% vs. 22.5%; difference, 49.5 percentage points; 95% CI, 21.2 to 58.0; P = 0.008) or White men (62.0% vs. 36.4%; difference, 25.6 percentage points; 95% CI, 9.7 to 41.7; P = 0.004). Genes with actionable mutations occurred more often in Black men than in White men (26.7% vs. 18.0%; difference, 8.7 percentage points; 95% CI, 0 to 19.5; P = 0.05), as did mutations in DNA-repair genes (22.5% vs. 15.6%; difference, 6.9 percentage points; 95% CI, 0.5 to 18.1; P = 0.05) (Fig. 1F). In addition, BRAF mutations, which are rare in prostate cancer and are considered to be actionable in many tumor sites, were also more frequent in Black men than in White men (7.0% vs. 1.5%; difference, 5.5 percentage points; 95% CI, 0.4 to 11.5; P = 0.002).
Clinically significant alterations may occur at different frequencies across races. Notably, Black men with metastatic prostate cancer were more likely than either White or Asian men to have tumor mutations in AR, along with mutations in DNA-repair genes and actionable genetic mutations. This finding could have implications for prognosis, response to therapy, and enrollment of minority populations in clinical trials and precision oncology studies.3,5 Our study was limited by its use of confidence intervals that were not adjusted for multiple comparisons. To support and further explore the implications of these findings, we will need studies involving a larger number of non-White men for whom data are available regarding the effects of treatment and results from histopathological analysis on outcomes. The evaluation of such data could help to prevent a worsening of racial disparities in the diagnosis and treatment of prostate cancer.3
Supplementary Material
Acknowledgments
Supported by the American Society for Radiation Oncology, the Prostate Cancer Foundation, the U.S. Department of Defense, and the National Institutes of Health.
Footnotes
Disclosure forms provided by the authors are available with the full text of this letter at NEJM.org.
Contributor Information
Brandon A. Mahal, University of Miami Sylvester Comprehensive Cancer Center, Miami, FL
Mohammed Alshalalfa, Dana–Farber Cancer Institute, Boston, MA
Kevin H. Kensler, Dana–Farber Cancer Institute, Boston, MA
Ilkania Chowdhury-Paulino, Harvard T.H. Chan School of Public Health, Boston, MA
Philip Kantoff, Memorial Sloan Kettering Cancer Center, New York, NY
Lorelei A. Mucci, Harvard T.H. Chan School of Public Health, Boston, MA
Edward M. Schaeffer, Northwestern University Feinberg School of Medicine, Chicago, IL
Daniel Spratt, University of Michigan, Ann Arbor, MI
Kosj Yamoah, H. Lee Moffitt Cancer Center and Research Institute, Tampa, FL
Paul L. Nguyen, Dana–Farber Cancer Institute, Boston, MA
Timothy R. Rebbeck, Dana–Farber Cancer Institute, Boston, MA
References
- 1.Dess RT, Hartman HE, Mahal BA, et al. Association of black race with prostate cancer-specific and other-cause mortality. JAMA Oncol 2019;5:975–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mahal BA, Alshalalfa M, Spratt DE, et al. Prostate cancer genomic-risk differences between African-American and white men across Gleason scores. Eur Urol 2019;75:1038–40. [DOI] [PubMed] [Google Scholar]
- 3.Martin AR, Kanai M, Kamatani Y, Okada Y, Neale BM, Daly MJ. Clinical use of current polygenic risk scores may exacerbate health disparities. Nat Genet 2019;51:584–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.AACR Project GENIE Consortium. AACR Project GENIE: powering precision medicine through an international consortium. Cancer Discov 2017;7:818–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spratt DE, Osborne JR. Disparities in castration-resistant prostate cancer trials. J Clin Oncol 2015;33:1101–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.