Abstract
Background
Acne vulgaris is a common cutaneous disorder. Diet and metabolism, specifically glycemic content and dairy, influence hormones such as insulin, insulin-like growth factor 1, and androgens, which affect acnegenesis.
Objective
To systematically review high-quality evidence regarding the association of dietary glycemic and dairy intake with acnegenesis.
Methods
A comprehensive literature search, without timeline restriction, of MEDLINE (completed between October and November 2021) for English-language papers that examined the association between diet and acne was conducted. The evidence quality was assessed using the Ottawa quality assessment scale.
Results
The literature search yielded 410 articles, of which 34 articles met the inclusion criteria. The literature on whether dairy product intake is associated with acnegenesis is mixed and may be dependent on sex, ethnicity, and cultural dietary habits. High glycemic index and increased daily glycemic load intake were positively associated with acnegenesis and acne severity, an observation supported by randomized controlled trials.
Conclusion
High glycemic index, increased glycemic load, and carbohydrate intake have a modest yet significant proacnegenic effect. Increased dairy consumption may have been proacnegenic in select populations, such as those in which a Western diet is prevalent. The impact of diet on acnegenesis is likely dependent on sex and ethnicity. Further randomized trials are necessary to fully characterize the potential associations.
Key words: acne, diet, dairy, glycemic index, randomized control trial, sugar
Abbreviations used: IGF-1, insulin-like growth factor 1; GI, glycemic index; LOE, level of evidence; RCT, randomized control trial
Capsule Summary.
-
•
The effect of diet on acne has been of interest for many years, and we present an update of the evidence of the effect of glycemic index/load and dairy on acne.
-
•
We provide a succinct reference by which providers can advise their patients with acne on the effect of dietary habits.
Introduction
Acne vulgaris is a common, puberty-associated, cutaneous disorder, with a lifetime incidence of nearly 100%, that is defined by lesions that result from the inflammation of plugged pilosebaceous units.1,2 Lesion pathogenesis is multifactorial and includes contributions from the hormonal activation of sebaceous glands and Cutibacterium acnes bacteria-associated follicle inflammation.
Consistent with the association between acne and puberty, hormones influence both acne development and severity.3 The exogenous ingestion of either androgens or growth hormone often causes acne.4,5 Similarly, when these hormone levels are endogenously elevated, such as androgens in patients with polycystic ovarian syndrome or growth hormone in patients with acromegaly, acne occurs more commonly.6 In the inverse scenario, a small cohort study of patients with Laron syndrome, a recessive disease characterized by primary growth hormone insensitivity, found that the patients had a lower incidence of acne (1 case of mild acne out of 13 patients).5
A Western diet, characterized by increased dairy intake and high glycemic index (GI) content, has been shown to affect the levels of hormones implicated in acne pathogenesis. GI is a 1-100 scoring system used to determine how quickly carbohydrate content is digested, absorbed, and metabolized.7 In multiple clinical trials, high-GI diets (>55) have been associated with worse glycemic control, higher postprandial insulin levels,7,8 and elevated insulin-like growth factor 1 (IGF-1) levels,7 whereas low-GI diets have been shown to decrease fasting IGF-1 concentrations.9, 10, 11 Similarly, frequent dairy consumers have higher serum levels of IGF-1 and insulin compared with nondairy consumers,12,13 and the ingestion of either whey or casein, protein dairy components, has been associated with increased levels of IGF-1 and insulin.14, 15, 16, 17 Notably, a 2-year randomized control trial (RCT) examining whey protein ingestion and bone health in postmenopausal women determined that high whey consumption resulted in higher serum IGF-1 levels, 7.3% at 1 year and 8% at 2 years.18
Numerous ethnographic observational studies have noted lower acne prevalence among non-Westernized peoples.7, 8, 9, 10, 11 In 1 notable study, the members of 2 South American indigenous peoples, the Kitavans of Papua New Guinea and the Aché of Paraguay, were followed-up. In contrast to Western diets, both the groups adhered to low-GI diets consisting of fish, wild game, tubers, and foraged foods and consumed almost no dairy, alcohol, coffee, tea, oils, sugar, and salt. After many months of observation, the researchers found no cases of acne among adolescents or adults.19 Interestingly, genetically similar Pacific Islanders and South American Indians who have adopted a more Westernized lifestyle have much higher incidences of acne. Similar trends were observed among other peoples, such as Okinawans and the Inuit, in whom the acne prevalence increased after the adoption of a Western lifestyle.9,12 These comparisons suggest that environmental factors present in a rural, nonindustrialized lifestyle protect people from the development of acne. Alternatively, an industrialized lifestyle, specifically, the adoption of a Western diet, high in sugar/glycemic and dairy content, may be proacnegenic.
Based on these observations, many research groups have considered whether the intake of dairy or high-GI foods has a role in modulating acne development and severity; moreover, patients themselves often ask whether changes in diet can improve their acne. Accordingly, the interest in the topic has increased in recent years, and new studies are constantly being added to the literature.13,14 Here, we present a review of the high-quality evidence of the relationship between acne and dairy and between acne and sugar/glycemic content to draw more accurate conclusions from a variety of data. Dozens of dietary components, ranging from specific micronutrients to entire macronutrient classes, have been proposed to both positively and negatively affect acne. In contrast to other excellent, published reviews of these foodstuffs, we limit our focus here to dairy and glycemic content because the biochemical pathways by which they can alter acne-pathogenic hormones have been clearly delineated.
Methods
We conducted a comprehensive literature search, without timeline restriction, of the MEDLINE database according to the 2020 Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines.15 The literature search, article identification and selection, data extraction and analysis, and manuscript screening were performed between October and November 2021. The last literature search was performed on October 4, 2021. Our search terms included “Acne AND dairy,” “Acne AND milk,” “Acne AND whey,” “Acne AND glycemic,” “Acne AND sugar,” and “Acne AND carbohydrate,” yielding a total combined number of 410 articles.
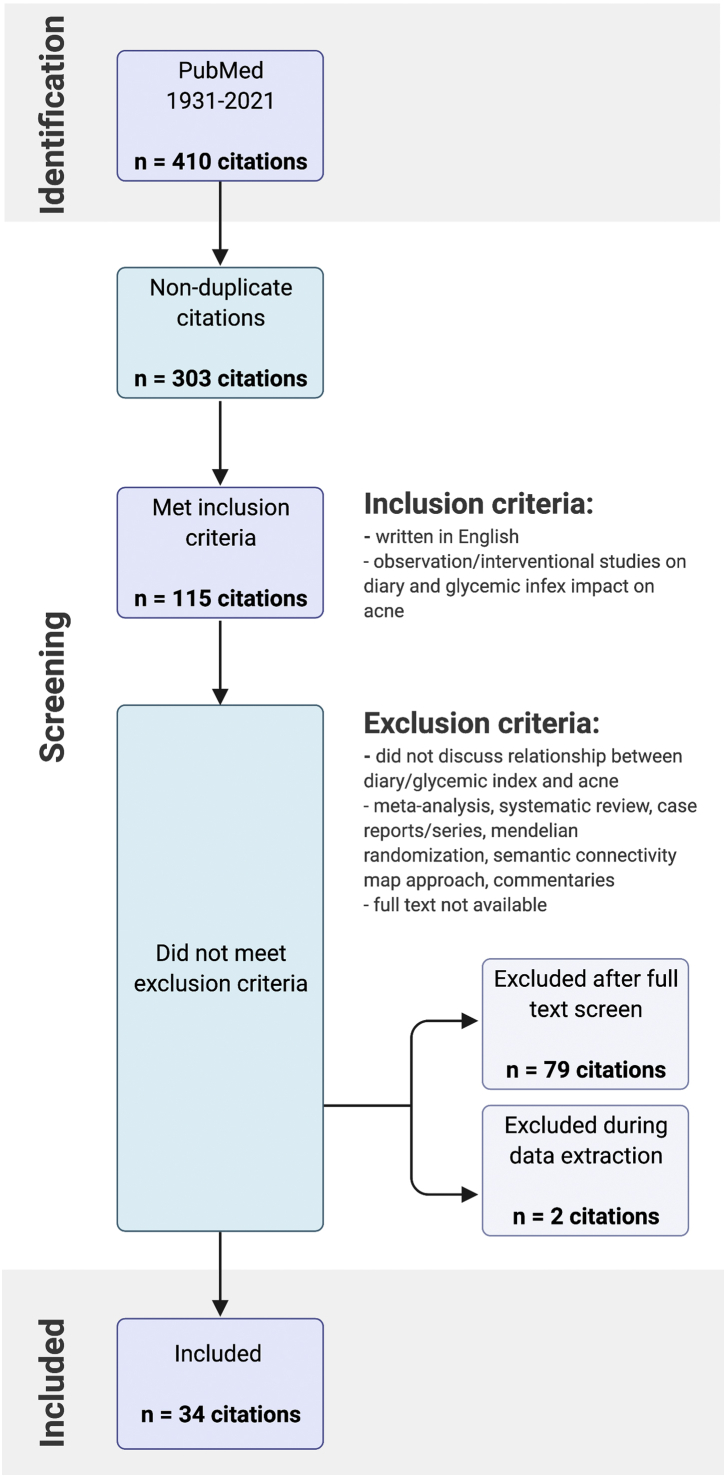
Two reviewers independently screened the titles, abstracts, and full text to determine whether they met the inclusion criteria detailed in Fig 1. The inclusion criteria were as follows: (1) primary observational and interventional studies of participants with acne; (2) a survey/questionnaire/interview assessment of eating habits or specific dietary interventions; and (3) outcomes examining the association of acne with GI, glycemic load, and/or dairy products. We excluded non-English papers, meta-analyses, case reports, case series, systematic and general reviews, Mendelian randomizations, semantic connectivity map approaches, commentaries, and articles that were not accessible for the full-text review.
Fig 1.
Systematic review flow diagram. The PRISMA flow diagram for the systematic review detailing the database searches and studies that met inclusion and exclusion criteria.
Data extraction for pertinent variables (study design, participant characteristics, methods/intervention, outcome, and the level of evidence [LOE]) was conducted by J.M., C.R., C.V., and B.K.H. The LOEs for observational studies were independently assessed using the Newcastle-Ottawa quality assessment scale by J.M., C.R., and B.K.H. based on 3 categories of study group selection (4 points), group comparability (2 points), and the ascertainment of exposure or the outcome of interest (3 points), with a maximum score of 9 (8 for cross-sectional studies). Discrepancies were discussed between 2 reviewers, and in the event of a disagreement, the scores were averaged and rounded up. The strengths and limitations of the studies were synthesized using the scoring process by J.M.. Case selection was categorized as “well-defined” if it was physician directed and not self-reported by patients. “Appropriate” controls were age/sex matched and recruited from the same clinic and not separate locations. “Unbiased” exposure classifications were defined as the utilization of general dietary questionnaires and/or real-time diet diaries, which are less susceptible to a recall bias. Recruiting by advertising on the internet was categorized as “biased.” Finally, the sample size was deemed as “appropriate” if the study provided a power analysis justification in its methods. If such a justification was not provided, then a posthoc power calculation (alpha, 0.05; 80% power) was performed based on the study’s described differences. If the participant number surpassed the calculated threshold, then the study was deemed to have had an appropriate sample size.
Results
Summary of study demographics
The initial search yielded 410 articles. After the removal of duplicates, 303 articles remained. Following title/abstract screening, 113 met the inclusion criteria of being English-written observation/interventional studies related to the topics of interest, ie, dairy, glycemic content, and acne (Fig 1). Of these, 81 articles were excluded during the full-text screen because of their study type and inappropriate content, and 34 articles were included in the final data extracted and the LOE assessment. Overall, 82.4% (28/34) of the included studies were observational, with the remainder being interventional controlled trials (6/34). Among the observational studies, 42.9% (12/28) were case-control studies, 14.3% (4/28) were longitudinal cohort studies, and 42.9% (12/28) were cross-sectional studies. Of the controlled trials, majority (5/6) were RCTs. One trial was nonrandomized.
Overall, 17.9% (5/28) of the observational studies exclusively assessed the association between GI/glycemic load and acne (Table I).19, 20, 21, 22, 23 Overall, 35.7% (10/28) exclusively commented on the association between dairy and acne (Table II),24, 25, 26, 27, 28, 29, 30, 31, 32, 33 and 46.4% (13/28) described the associations of dairy products and GI/glycemic load with acne (Table III).34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46 All the interventional trials included examined the effect of GI/glycemic load on acne (Table IV).47, 48, 49, 50, 51, 52 No interventional trials relating to dairy and acne were identified in our search.
Table I.
Observational studies on the effect of glycemic index/load on acne
| Source; country | Study design; no. of participants | Participant characteristics | Measures | Outcome summary | Strengths | Limitations | Quality of evidence |
|---|---|---|---|---|---|---|---|
| Bett et al,20 1967; United Kingdom | Case-control study; 16 patients with acne, 29 controls | 15-27 y olds recruited from 2 clinics. Age/sex-matched controls recruited from a clinic and a factory/office. | Questionnaire on daily sugar intake. Physician-diagnosed acne. |
No significant difference in daily sugar intake between patients with acne and controls. | Well-defined case selection. Appropriate control selection. | Low sample size. Potential bias in exposure classification | 5 |
| Kaymak et al,19 2007; Turkey | Case-control study; 49 patients with acne, 42 controls | 19-34 y olds recruited from outpatient clinics. | Questionnaire on the frequency of food consumption and dietary intake. Dermatologist-assessed acne severity. | No significant difference in GI between patients with acne and controls. No significant difference in glycemic load between patients with acne and controls. | Well-defined case selection. Appropriate control selection. | Low sample size. Potential bias in exposure classification | 7 |
| Koku Aksu et al,21 2011; Turkey | Cross-sectional study; 2230 | 13-18-y-old adolescents from multiple schools. | Questionnaire on dietary intake and personal history of acne. Dermatologist-evaluated acne severity (Pillsbury diagnostic criteria). | Frequent sugar intake (aOR, 1.3 [1.06-1.82]) and frequent sweet consumption (aOR 1.2 [1.16-1.43]) associated with acne. | Appropriate sample size. Unbiased exposure classification. | Potential biases in outcome classification | 7 |
| Burris et al,22 2017; New York City | Cross-sectional study; 64 | 18-40 y olds with moderate/severe and no acne recruited by advertising. | Dietician-instructed 5-d food and beverage record. Dermatologist-evaluated acne severity. Bloodwork at study conclusion. | Moderate/severe acne group had a higher glucose load than participants without acne (137 ± 41 vs 117 ± 41, respectively; P < .001). No association between GI and acne. | Well-defined outcome classification. Unbiased exposure classification. | Low sample size. Potential bias in recruitment. | 7 |
| Huang et al,23 2019; China | Cross-sectional study; 8197 | 18-19-y-old students from 353 cities. | Questionnaire on dietary intake. Dermatologist-assessed acne history and severity (Pillsbury diagnostic criteria). | Soft drink sugar intake ≥100 g/d significantly associated with moderate-to-severe acne (aOR 3.12 [1.80-5.41]). | Appropriate sample size. Well-defined case selection. | Potential bias in exposure classification. | 7 |
aOR, Adjusted odds ratio; GI, glycemic index.
Table II.
Observational studies on the effect of dairy on acne∗
| Source; country | Study design; no. of participants | Participant characteristics | Measures | Outcome summary | Strengths | Limitations | Quality of evidence |
|---|---|---|---|---|---|---|---|
| Adebamowo et al,24 2005; United States | Retrospective cohort study; 47,355 | 25-42-y-old women enrolled in the Nurses’ Health Study II. | Questionnaire on “physician-diagnosed severe teenage acne” (1989) and high school diet (1998). | Total milk (PR 1.22 [1.03-1.44]), skim milk (PR 1.44 [1.21-1.72]), and cottage cheese (PR 1.63 [1.22-2.20]) consumption associated with severe acne. Whole milk and low-fat milk not associated. | Appropriate sample size. Generalized exposure classification. | Potential bias in exposure and outcome classification. | 4 |
| Adebamowo et al,25 2006; United States | Retrospective cohort study; 6094 | 9-15-y-old girls from GUTS. | Questionnaires on food consumption frequency and the self-assessment of the presence and severity of acne. | Whole, low-fat, skim, or chocolate milk associated with acne in 1996. PRs ranged from 1.17 (1.04-1.31) to 1.29 (1.08-1.53). | Appropriate sample size. Longitudinal, generalized exposure classification. | Potential bias in outcome classification. | 5 |
| Adebamowo et al,26 2008; United States | Retrospective cohort study; 4273 | 9-15-y-old boys from GUTS. | Questionnaires on food consumption frequency and the self-assessment of the presence and severity of acne. | Skim milk associated with acne (PR 1.19 [1.01-1.40]). Total milk, whole milk, 2% milk, and low-fat milk not associated. | Appropriate sample size. Longitudinal, generalized exposure classification. | Potential bias in outcome classification. Multiple testing bias. | 4 |
| Di Landro et al,27 2012; Italy | Case-control study; 205 patients with acne, 358 controls | 10-24 y olds from 15 different outpatient hospital clinics. | Questionnaire on the frequency of dietary intake. Dermatologist-evaluated acne severity (global acne assessment scale). | Milk consumption (>3 portions per wk) associated with moderate-to-severe acne (OR 1.78 [1.22-2.59]). | Appropriate sample size. Multiple institutions. Well-defined outcome classification. Unbiased study recruitment. Generalized exposure classification. | None. | 9 |
| Pontes et al,28 2013; Brazil | Longitudinal cohort study; 30 | 18-30 y olds with recent protein calorie supplementation use from dermatology clinics and gyms. | Dermatologist-assessed acne (Leeds) before, after 30 d, and after 60 d of protein-calorie supplementation. | Protein calorie supplementation significantly increased comedo and acne lesion count. | Well-defined exposure and outcome classification. | Low sample size. Potential recruitment bias. | 6 |
| Semedo et al,29 2016; Portugal | Cross-sectional study; 1055 | 20-60 y olds from 5 different clinics. | Questionnaire on dietary intake. Dermatologist-evaluated acne (Pillsbury). | Reduced-fat milk and whole milk consumption associated with acne (OR 1.33 [1.03-1.70]). | Appropriate sample size. Multiple institutions. Well-defined outcome classification. Unbiased study recruitment. Generalized exposure classification. | None. | 8 |
| Duquia et al,30 2017; Brazil | Cross-sectional study; 2201 | 18-y-old enlisted men. | Questionnaire (Y/N) on daily cheese, whole milk, low-fat milk, and yogurt consumption. Dermatologist-evaluated acne. | No significant association between acne and milk was found. | Appropriate sample size. Unbiased recruitment. | Generalizability. Limited exposure classification in Y/N format. No severity grading. | 6 |
| Ulvestad et al,31 2017; Norway | Longitudinal cohort study; 2489 | 15-16-y-old (10th grade) Norwegian students. | Questionnaire on dairy consumption at baseline and the presence of acne after 3 y. | High intake of full-fat dairy associated with acne (OR 1.56 [1.02-2.39]). Total milk intake in girls only associated with acne (OR 1.80 [1.02-3.16]). | Appropriate sample size. Unbiased recruitment. | Potential bias in exposure and outcome classification. Generalizability. | 6 |
| Heng et al,32 2022; Singapore | Cross-sectional study; 2090 cases and 1798 controls | 17-71 y olds from a clinic. | Questionnaire on diet. Trained personnel evaluated the presence, severity, and scarring of acne lesions. | Frequent milk consumption associated with a lower likelihood of moderate-to-severe acne (OR 0.572 [0.360-0.910]). | Appropriate sample size. Well-defined outcome classification. | Potential bias in exposure classification. | 7 |
| Say et al,33 2021; Singapore | Cross-sectional study; 1117 patients with acne and 723 controls | 17-77 y olds from 2 different hospital sites. | Questionnaire on diet. Medical staff evaluated the presence, severity, and scarring of acne lesions. | No association between the frequency of milk consumption and acne severity. Increased milk consumption associated with a lower risk of scarring. | Appropriate sample size. Well-defined outcome classification. | Potential bias in exposure classification. | 7 |
GUTS, Growing Up Today Study; OR, odds ratio; PR, prevalence ratio.
Negative studies - Say et al58 and Heng et al56 specifically asked about dairy and, therefore, had a potential bias in exposure classification. However, their results appear well supported, with hints that different populations and cultural attitudes toward food consumption influence the role of diet and acne.
Table III.
Observational studies on the effect of both glycemic index/glycemic load and dairy on acne
| Source; country | Study design; no. of participants | Participant characteristics | Measures | Outcome summary | Strengths | Limitations | Quality of evidence |
|---|---|---|---|---|---|---|---|
| Ismail et al,34 2012; Malaysia | Case-control study; 44 patients with acne, 44 controls | 18-30 y olds from a dermatology clinic (with acne) and a university campus (control). | Interview on the frequency of milk consumption and 3-d food diary. Dermatologist-evaluated acne severity (comprehensive acne severity scale). | High glycemic load (175 ± 35 in patients with acne vs 122 ± 28 in controls), milk (OR 3.99 [1.39-11.43]), and ice cream (OR 4.47 [1.77-11.266]) consumption associated with acne. | Appropriate sample size. Well-defined case selection and exposure classification. | Unrepresentative control selection (university student). | 6 |
| Burris et al,35 2014; New York City | Cross-sectional study; 248 | 18-25 y olds from “public locations” in New York. | Questionnaire on dietary intake. Self-reported acne severity. | Higher GI (51.8 ± 3 vs 48.9 ± 4.6) and daily milk servings (0.7 ± 0.7 vs 0.3 ± 0.5) associated with acne severity. | None. | Low sample size. Potential recruitment bias. Potential biases in exposure (limited) and outcome classification (self-report). | 4 |
| Wolkenstein et al,36 2015; France | Cross-sectional study; 1375 patients with acne, 891 control patients | 15-24 y olds from a national French database who self-reported acne status. | Questionnaire on dietary habits and acne severity. | Daily consumption of chocolate and sweets associated with acne (OR 2.38 [1.31-4.31]). Dairy and sugary drink consumption not associated. | Appropriate sample size. Generalized exposure classification. | Potential recruitment bias. Potential bias in outcome classification (self-report). | 7 |
| LaRosa et al,37 2016; United States | Case-control study; 120 patients with moderate facial acne, 105 controls | 14-19 y olds from dermatology and pediatric clinics. | Structured telephone interviews assessing 24-h dietary recall. Dermatologist-evaluated facial acne (global acne assessment scale). | Increased servings of low-fat/skim milk (0.61 vs 0.41) in moderate acne cases compared with those in acne-free controls. Glycemic load not associated. | Appropriate case and control selection. Well-defined exposure and outcome classification. | Low sample size. | 6 |
| Çerman et al,38 2016; Turkey | Case-control study; 50 patients with acne, 36 controls | Participants (mean age: 18.8 y) recruited from a dermatology outpatient clinic. | Self-reported dietary intake over 1 wk. GL and GI calculated using a dietary analysis software. Dermatologist-assessed acne severity. | GI (47.24 ± 6.6 for patients with acne and 44.52 ± 6.58 for controls) and GL increased in patients with acne. Milk consumption not associated. | Well-defined exposure and outcome classification. | Low sample size. Low effect size. Unrepresentative control selection (hospital volunteer). | 5 |
| Okoro et al,39 2016; Nigeria | Cross-sectional study; 464 | Secondary school students (mean age: 13.6 y) recruited from 4 sites. | Interview on dietary habits and questionnaire on food frequency. Dermatologists evaluated the presence of acne. | Daily milk consumption (72.6% cases vs 62.0% controls) and cake (77.8% cases vs 62.3% controls) associated with acne. | Well-defined exposure and outcome classification. | Low sample size. | 8 |
| Suppiah et al,40 2018; Malaysia | Case-control study; 57 patients with acne, 57 control patients | 14 y or older recruited from a single hospital clinic. | Questionnaires on dietary habits. Dermatologist-assessed acne (comprehensive acne severity scale). | Milk (OR 2.19 [1.04-4.65]) and chocolate (OR 2.4 [1.08-5.33]) consumption associated with acne. | Appropriate sample size. Appropriate case and control selection. Well-defined outcome classification. | Limited exposure classification (Y/N format). | 5 |
| Aalemi et al,41 2019; Afghanistan | Case-control study; 279 patients with acne, 279 controls | 10-24 y olds from a single dermatology clinic. | Questionnaire on food consumption. Dermatologist-evaluated facial acne (global acne assessment scale). | Whole milk (OR 2.36 [1.39-4.01]) and low-fat milk consumption (OR 1.95 [1.10-3.45]) associated with acne severity. Chocolate associated with acne. | Appropriate sample size. Appropriate case and control selection. Well-defined outcome and exposure classification. | None. | 8 |
| Karadağ et al,42 2019; Turkey | Case-control study; 3826 patients with acne, 759 controls | 12-31 y olds from 26 different clinics. Control subjects had no record of past or present acne. | Questionnaire on eating frequency and habits. Dermatologist-evaluated acne severity (global acne assessment scale) | Chocolate associated with acne (OR 1.48 [1.24-1.76]). Milk and cheese not associated (OR 1.13 [0.94-1.36]). | Appropriate sample size. Multiple institutions. Appropriate case and control selection. Well-defined exposure and outcome classification. | None. | 9 |
| Akpinar Kara and Ozdemir,43 2020; Turkey | Case-control study; 53 patients with acne, 53 controls | 13-44 y olds from dermatology, nutrition, and dietetics clinics at a single hospital. | Interview on the frequency and quantity of food consumed over 3 d. Dermatologist-evaluated facial acne (global acne assessment scale). | Cheese associated with acne (P < .05). No association found with other dairy products. Increased carbohydrates correlated with acne severity (correlation coefficient 0.36; P < .01). | Appropriate case and control selection. Well-defined exposure and outcome classification. | Low sample size. | 7 |
| Dreno et al,44 2020; France, Germany, Italy, Brazil, Canada, and Russia | Cross-sectional study; 2826 participants with acne and 3853 control patients. | 15-39 y olds recruited from the internet. | Questionnaire on personal nutritional habits and the presence of clinically confirmed acne. | Dairy (OR 1.21 [1.1-1.35]), whey protein (OR 3.94 [3.29-4.71]), and high-GI food consumption associated with acne. | Appropriate sample size. Appropriate case and control selection. | Potential recruitment bias. Limited exposure classification (Y/N). Potential bias in outcome classification (self-report). | 6 |
| Penso et al,45 2020; France | Cross-sectional study; 24,452 | Participants (mean age: 57 y); 75% women and 25% men) from the French NutriNet-Santé study. | Questionnaire on food intake at baseline and every 6 mo. Self-reported acne presence and severity. | Fatty and sugary products (aOR 1.54 [1.09-2.16]), sugary beverages (aOR 1.18; [1.01-1.38]), and milk products (aOR 1.12; [1.00-1.25]) associated with acne. | Appropriate sample size. Longitudinal exposure classification. | Potential recruitment bias. Potential biases in outcome classification (self-report). Significant demographic differences in comparison groups. | 5 |
| Anaba et al,46 2021; Nigeria | Case-control study; 56 cases, 56 controls | ≥25-y-old women recruited from an outpatient clinic. | Questionnaire on dietary frequency and intake. Dermatologist-evaluated acne presence and severity (comprehensive acne severity scale). | Milk consumption, cakes, sweets, and starchy foods not associated with acne presence, severity, or frequency. | Appropriate case and control selection. Well-defined exposure and outcome classification. | Low sample size. Generalizability. | 4 |
aOR, Adjusted odds ratio; GI, glycemic index; GL, glycemic load; OR, odds ratio.
Table IV.
Interventional studies on the effect of dairy and glycemic index on acne
| Source; country | Design | Study population | Intervention | Control | Outcome summary | Strengths | Limitations |
|---|---|---|---|---|---|---|---|
| Smith et al,47 2007; Australia | Randomized control trial | 15-25-y-old males (n = 43) with mild-to-moderate acne for >6 mo recruited from a university. Excluded if consuming acne or glucose metabolism medications. Encouraged the use of skin cleansers. | 12-wk LGL or control diet. | Control group instructed to eat carbohydrate-dense foods (moderate-to-high GL) daily but were not informed about GI. | LGL diet decreased total lesion count (−22.0 ± 3.5 for LGL vs −10.9 ± 2.9 for control, P = .02) and inflammatory lesion count (−16.2 ± 2.9 for LGL vs −5.6 ± 2.5 for control, P = .01). | Appropriate sample size. Rigorous dietary compliance measures (phone calls, daily intake, urea samples, and food weights). Significant exposure differences achieved for both GI (low GI 43 vs high GI 56) and GL (low GI 101 vs high GI 171). Well-defined outcome classification (dermatologist-assessed acne). | Generalizability (young adult males). |
| Smith et al,48 2007; Australia | Randomized control trial | See above. | See above. | See above. | Reduced inflammatory lesion counts in LGL diet (−16.0 [−20.6 to −11.4]) vs control (−8.5 [−13.4 to −3.5]), P = .04, and did not reduce total acne lesion count after adjusting for BMI. | See above. | Generalizability (young adult males). Appears to be reanalyzed data from above trial. |
| Smith et al,49 2008; Australia | Randomized control trial | 15-25-y-old males (n = 31) with mild-to-moderate facial acne. Remainder above. | See above. | See above. | LGL diet had decreased acne lesion counts (−59%) compared with control diet (−38%), P = .046), and decreased skin oiliness mean response score, P = .013. | See above. | Generalizability (young adult males). Appears to be reanalyzed data from above 2 trials. |
| Reynolds et al,50 2010; Australia | Nonrandomized controlled trial | Teenage boys (n = 43; average age: 16.5 y) recruited from boarding schools. | 8 wk of high-GI diet. | Low-GI diet. | Trend toward significant association between acne severity and low-GI diet (score −0.65 ± 0.14) vs high-GI diet (−0.35 ± 0.15), P = .15. | Appropriate sample size. Significant exposure differences achieved for both GI (low GI 51 ± 1, high GI 61 ± 2) and GL (low GI 102 ± 9, high GI 157 ± 18). Well-defined outcome classification (dermatologist-graded acne). | Generalizability (teenage boys), more stringent exclusion criteria (smoking/drinking, final examinations, “dark” skin). Less rigorous dietary compliance measures (weekly assessments of weight and diet). |
| Kwon et al.51 2012; Korea | Randomized controlled trial | 20-27 y olds (24 men, 8 women) with mild/moderate acne recruited from a clinic. | 10 wk of an LGL diet. | Control group asked to maintain regular diet. | LGL diet significantly improved the number and severity of acne lesions compared with those at baseline (−70.9%) and in controls. | Significant exposure differences achieved for both GI (low GI 50.1 ± 6.3, high GI 69.5 ± 2.4) and GL (low GI 129.5 ± 22.2, high GI 207.2 ± 23.2). Well-defined outcome classification (dermatologist-graded acne severity). Investigator blinding. | Unclear sample size justification. Less rigorous dietary compliance measure (GL and GI recorded at wk 2, 5, and 10). |
| Pavithra et al,52 2019; India | Randomized controlled investigator-blinded trial. | 14-29 y olds (26 males, 58 females) recruited from dermatology clinics. | 12 wk of an LGL diet. | Control group asked to continue usual diet. Topical benzoyl peroxide gel 2.5% and noncomedogenic cleanser recommended for all participants. | No significant association between acne severity and LGL diet. All members of the intervention and control groups showed acne reduction at 12 wk. | Appropriate sample size. Well-defined outcome classification (dermatologist-graded acne severity longitudinally). | No data available to judge the success of dietary intervention. Less rigorous dietary compliance measure (GL and GI recorded at wk 4, 8, and 12). 100% improvement among all trial participants. |
BMI, Body mass index; GI, glycemic index; GL, glycemic load; LGL, low glucose load.
Studies from 18 countries were included in the data extracted. In the observational studies, 46.4% (13/28) were conducted in Western countries (the United States, Europe, and Australia), with the remainder being conducted in East Asia (17.9%, 5/28), Western Asia (21.4%,6/28), South America (7.1%, 2/28), and Africa (7.1%, 2/28). Among the interventional studies, 4 trials were conducted in Australia. One was conducted in India and the other in Korea.
Summary of patient characteristics
The observational studies examining GI/glycemic load and acne reported data from 22,671 cases (30.6% men and 69.4% women, 69.6% from the United States and Europe) and 27,951 controls (39.7% men and 60.3% women, 65.2% from the United States and Europe) (Tables I and III). Articles that assessed the association between dairy and acne included 26,782 cases (36.1% men and 63.9% women, 63.2% from the United States and Europe) and 25,262 controls (34.3% men and 65.7% women, 83.2% from the United States and Europe) (Tables II and III). The majority of the described participants being from the United States and Europe was largely because of 2 large-scale cross-sectional studies, ie, studies by Dreno et al44 and Penso et al.45
Among the observational studies, 67.9% (19/28) reported the participant age for the cases and controls as a continuous variable. The remainder reported the participant age as discrete ranges. Studies that reported the extractable age and examined the relationship between GI/glycemic load and acne (Tables I and III) included 7139 cases, with a mean age of 20.7 years, and 13,684 controls, with a mean age of 20.3 years. Similarly, studies that reported the definable age and examined the association between dairy products and acne (Tables II and III) included 10,601 cases, with a mean age of 21.0 years, and 10,594 controls, with a mean age of 21.7 years. The study by Dreno et al44 was excluded from this analysis because they reported age as a discrete variable, notably, skewed, older age, with a mean age of 55.2 years for cases.
In the interventional trials, there were a total of 276 participants. Three of the trials, ie, those by Smith et al,47 Smith et al,48 and Smith et al,49 seemed to use data, reported differently, from the same trial participants. After excluding potential duplicates, there were a total of 202 unique participants (67.3% men and 32.7% women, 42.6% from a Western nation [Australia]), with a mean age of 20.2 years.
Summary of study findings
Of 18 observational studies that commented on GI/glycemic load, sweets, and carbohydrate intake and acne, 77% (14/18) reported at least 1 item to be positively associated with acnegenesis or acne severity. Studies that linked glycemic content and acne had an average LOE score of 6.5 (Tables I and III). Articles that did not find an association had an average LOE score of 5.5 (Tables I and III). Overall, 50% (2/4) of studies were conducted in nations with a predominant Western diet (the United States and Europe).
Among articles that examined the association between dairy and acne, 70% (16/23) linked at least 1 dairy food item with acnegenesis or acne severity and had an average LOE score of 6.1 (Tables II and III). Those that did not find an association had an average LOE score of 6.4 (Tables II and III). Overall, 14% (1/7) of negative studies were conducted in either Western-diet–predominant United States or Europe.
Based on our search criteria, 6 trials relating GI to acne were identified. No interventional studies linking whey or casein ingestion with acne severity or prevalence were uncovered. In 2 articles, 43 male patients with acne, treated with a topical cleanser, were randomized to 12 weeks of either a low-GI diet (GI, 43) or a control diet (GI, 56).47,48 Although both the groups experienced significant improvements in lesion count, the low-GI diet group experienced a significantly greater improvement compared with the control-diet group (inflammatory lesion counts: −16.0 with low GI vs −8.5 with control, P = .04; total lesion counts: −22.0 ± 3.5 with low GI vs −10.9 ± 2.9 with control, P = .02).47,48 Similarly, a low-GI diet (GI, 50) decreased acne severity and lesion count (mean decrease: 70.9% of baseline) in a 10-week trial conducted on 32 patients with mild-to-moderate acne compared with a control diet (GI, 70).51 In contrast, an 8-week trial of a low-GI diet (GI of 51) conducted on 58 adolescent participants failed to demonstrate a significant improvement in facial acne severity compared with a control diet (GI, 61); however, there was a nonsignificant trend toward a greater benefit with the low-GI diet (acne severity score change: low GI mean ± SEM, n = 23, −0.65 ± 0.14 vs high GI mean ± SEM, n = 20, −0.35 ± 0.15; P = .15).45 Two additional RCTs examined whether glucose load can affect acne lesion count and severity. In 1, the low-glucose-load diet group had significantly decreased lesion counts (−59%) compared with the control-diet group (−38%) (P = .046),48 but in the other, which was a 12-week trial of a low-glucose-load diet, no significant differences were found between the intervention and control.52
Discussion
Glycemic content and acne
In this review, we conducted a focused literature search for articles examining the relationship between dairy and acne and between glycemic content and acne and assigned observational studies an LOE score based on the Newcastle-Ottawa scale. In terms of whether glycemic content (GI, load, carbohydrates, chocolate, soda, sweets, etc) affects acne, a majority (77%) of the identified observational studies, independent of prevailing culinary traditions in the country of origin, supported an association between dietary glycemic content and acne. These studies had a higher assigned strength of evidence (6.5 vs 5.5), and their conclusions were backed by numerous RCTs that demonstrated either significant improvement in acne with the adoption of a low-GI diet or a trend toward significance (Table I, Table II, Table III, Table IV). Pavithra et al,52 in their sole negative trial, did not show significant improvement with a low-GI diet, likely in part due to substantial improvement among all participants from baseline because of their participation in the trial and associated counseling. Importantly, because most identified studies focused on young men and women in their teens and twenties, conclusions must be limited to this age bracket. Based on this evidence, we concluded that despite conflicting articles, high-GI and increased-glycemic content diets can exacerbate acnegenesis and acne severity, whereas the adoption of a low-GI diet may benefit acnegenesis, decrease the number of lesions, and alleviate acne severity, irrespective of the geographic region or country.
Dairy and acne
Regarding the relationship between dairy intake and acne, unfortunately, we could not identify any RCTs on the topic and, instead, had to base our conclusions on the observational studies, which varied widely in terms of exposure assessment and reported results. A majority of the included articles (70%) positively associated at least 1 dairy food item with acnegenesis or acne severity. However, both positive and negative studies had similar LOE scores (6.1 vs 6.4, respectively). Importantly, a majority of the negative studies, including 1 notable study that reported that increased milk consumption decreased the risk of acne scarring,53,54 were conducted in countries outside the United States and Europe. Despite mixed evidence, we concluded that increased dairy intake may exacerbate acne among young people in nations with a prevailing Western diet, such as the United States, Europe, and Australia. In contrast, dairy is unlikely to exacerbate acne among youths from non-Western countries.
Limitations
Over the years, associations between acne and specific foods, such as dairy, chocolate, fats, sweets, and carbonated beverages, have proven controversial,55, 56, 57, 58 with much conflicting evidence on the topic. This is due, in large part, to the fact that epidemiologic and interventional studies involving diet are difficult to conduct. Dietary records and self-reporting are subject to substantial biases, and strict dietary adherence in interventional trials is difficult to achieve. Among observational studies, ideal exposure classification would include externally validated long-term knowledge of dietary intake, which is difficult to achieve in the real-world setting. Some of the reviewed studies, such as those by Burris et al59 and Ismail et al,34 both of which concluded that increased dairy and GI consumption exacerbate acne, hewed closer to this ideal through the use of food diaries and/or telephone interviews in their assessment of participants’ diets. In contrast, articles such as those by Bett et al20 and Adebamowo et al,24 which relied on participants’ recall of diet consumption/habit days, weeks, or even years after the fact, were likely subject to more substantially biases.
The studies identified using our methodology have several additional limitations. Many did not include a prior justification of sample size based on power analyses and, upon a posthoc review, were determined to be underpowered to determine the modest effect sizes that associated diet with acne. In addition, despite the majority of the participants in the observational studies being women, the RCTs described enrolled mostly men. This is problematic, given the known differences in hormones and, likely, the sex-specific effects of diet on hormone levels and, thus, acnegenesis.
A myriad of dietary components, such as micronutrients, fruits, vegetables, fatty foods, eggs, and chocolate, have been linked to acne. In our methodology, we specifically focused on larger dietary categories, such as dairy and glycemic content, and not specific food items. However, many of the included studies did comment on associations between acne and additional foods, which we did not categorize as either “glycemic-related” or dairy. Chocolate bears special mentioning among additional food items because of the widespread patient belief that it exacerbates acne, numerous RCTs and observational studies on the subject,21 and the fact that it represents a high-GI food item, which also oftentimes contains dairy. For our analyses, we strictly categorized chocolate as “glycemic-related,” an imperfect classification.
Most of the observational studies cited gathered data on multiple food items from their cases, controls, or cohorts. One additional limitation of the analyses was our classification of a study as “positive” if a single food item related to glycemic content or dairy was significantly associated, even if many others in the study were not associated, which created biases in our summary statistics toward the overestimation of a potential association. One notable example is that Adebamowo et al26 found a significant association between skim milk and acne but not between total milk, whole milk, 2% milk, or low-fat milk and acne.
Conclusion
Despite the discussed limitations, we believe that the discussed studies suggest that diet can affect acne within select populations. Increased glycemic intake and high-GI diets promote acnegenesis and exacerbate acne severity, whereas increased dairy intake has been consistently shown to promote acnegenesis only among Western populations. Additional RCTs examining dairy intake and acne with respect to ethnicity are required to define the exact relationship.
The effect of diet on acne is highly variable even in an idealized study population. Observational studies examining the relationship between diet and acne should use longitudinal food diaries or daily interviews to appropriately categorize exposure. Future RCTs must be appropriately powered to determine moderate effect sizes.
Conflicts of interest
None disclosed.
Acknowledgments
The authors would like to acknowledge their funding sources from the Johns Hopkins Department of Dermatology, the MSTP training grant, and the critical opinions and discussions of Drs Janelle Ho, Sewon Kang, Shawn Kwatra, Jihad Alhariri, Annie Grossberg, and other faculty members.
Footnotes
Dr Meixiong and Ms Ricco contributed equally to this article.
Funding sources: Supported by NIHT32 GM136577 (to C.V. and J.M.).
IRB approval status: This review was exempt from regulation by the Johns Hopkins School of Medicine Institutional Review Board.
Accepted for publication February 25, 2022.
References
- 1.Collier C.N., Harper J.C., Cantrell W.C., Wang W., Foster K.W., Elewski B.E. The prevalence of acne in adults 20 years and older. J Am Acad Dermatol. 2008;58(1):56–59. doi: 10.1016/j.jaad.2007.06.045. [DOI] [PubMed] [Google Scholar]
- 2.Tan J.K., Bhate K. A global perspective on the epidemiology of acne. Br J Dermatol. 2015;172(suppl 1):3–12. doi: 10.1111/bjd.13462. [DOI] [PubMed] [Google Scholar]
- 3.Thiboutot D. Acne: hormonal concepts and therapy. Clin Dermatol. 2004;22(5):419–428. doi: 10.1016/j.clindermatol.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 4.Smit D.L., de Ronde W. Outpatient clinic for users of anabolic androgenic steroids: an overview. Neth J Med. 2018;76(4):167. [PubMed] [Google Scholar]
- 5.Ben-Amitai D., Laron Z. Effect of insulin-like growth factor-1 deficiency or administration on the occurrence of acne. J Eur Acad Dermatol Venereol. 2011;25(8):950–954. doi: 10.1111/j.1468-3083.2010.03896.x. [DOI] [PubMed] [Google Scholar]
- 6.Lolis M.S., Bowe W.P., Shalita A.R. Acne and systemic disease. Med Clin North Am. 2009;93(6):1161–1181. doi: 10.1016/j.mcna.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 7.Ratnam A.V., Jayaraju K. Skin diseases in Zambia. Br J Dermatol. 1979;101(4):449–453. doi: 10.1111/j.1365-2133.1979.tb00025.x. [DOI] [PubMed] [Google Scholar]
- 8.Bechelli L.M., Haddad N., Pimenta W.P., et al. Epidemiological survey of skin diseases in schoolchildren living in the Purus Valley (Acre State, Amazonia, Brazil) Dermatologica. 1981;163(1):78–93. doi: 10.1159/000250144. [DOI] [PubMed] [Google Scholar]
- 9.Cordain L., Lindeberg S., Hurtado M., Hill K., Eaton S.B., Brand-Miller J. Acne vulgaris: a disease of Western civilization. Arch Dermatol. 2002;138(12):1584–1590. doi: 10.1001/archderm.138.12.1584. [DOI] [PubMed] [Google Scholar]
- 10.Park R.G. The age distribution of common skin disorders in the Bantu of Pretoria, Transvaal. Br J Dermatol. 1968;80(11):758–761. doi: 10.1111/j.1365-2133.1968.tb11941.x. [DOI] [PubMed] [Google Scholar]
- 11.Verhagen A.R., Koten J.W., Chaddah V.K., Patel R.I. Skin diseases in Kenya: a clinical and histopathological study of 3,168 patients. Arch Dermatol. 1968;98(6):577–586. doi: 10.1001/archderm.98.6.577. [DOI] [PubMed] [Google Scholar]
- 12.Steiner P.E. Necropsies on Okinawans; anatomic and pathologic observations. Arch Pathol (Chic) 1946;42(4):359–380. [PubMed] [Google Scholar]
- 13.Conforti C, Agozzino M, Emendato G, et al. Acne and diet: a review. Preprint. Posted online August 22, 2021. Int J Dermatol. https://doi.org/10.1111/ijd.15862 [DOI] [PubMed]
- 14.Dall'Oglio F., Nasca M.R., Fiorentini F., Micali G. Diet and acne: review of the evidence from 2009 to 2020. Int J Dermatol. 2021;60(6):672–685. doi: 10.1111/ijd.15390. [DOI] [PubMed] [Google Scholar]
- 15.Page M.J., McKenzie J.E., Bossuyt P.M., et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. J Clin Epidemiol. 2021;134:178–189. doi: 10.1016/j.jclinepi.2021.03.001. [DOI] [PubMed] [Google Scholar]
- 16.Augustin L.S., Kendall C.W., Jenkins D.J., et al. Glycemic index, glycemic load and glycemic response: an International Scientific Consensus Summit from the International Carbohydrate Quality Consortium (ICQC) Nutr Metab Cardiovasc Dis. 2015;25(9):795–815. doi: 10.1016/j.numecd.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 17.Silva F.M., Kramer C.K., Crispim D., Azevedo M.J. A high-glycemic index, low-fiber breakfast affects the postprandial plasma glucose, insulin, and ghrelin responses of patients with type 2 diabetes in a randomized clinical trial. J Nutr. 2015;145(4):736–741. doi: 10.3945/jn.114.195339. [DOI] [PubMed] [Google Scholar]
- 18.Runchey S.S., Pollak M.N., Valsta L.M., et al. Glycemic load effect on fasting and post-prandial serum glucose, insulin, IGF-1 and IGFBP-3 in a randomized, controlled feeding study. Eur J Clin Nutr. 2012;66(10):1146–1152. doi: 10.1038/ejcn.2012.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaymak Y., Adisen E., Ilter N., Bideci A., Gurler D., Celik B. Dietary glycemic index and glucose, insulin, insulin-like growth factor-I, insulin-like growth factor binding protein 3, and leptin levels in patients with acne. J Am Acad Dermatol. 2007;57(5):819–823. doi: 10.1016/j.jaad.2007.06.028. [DOI] [PubMed] [Google Scholar]
- 20.Bett D.G., Morland J., Yudkin J. Sugar consumption in acne vulgaris and seborrhoeic dermatitis. Br Med J. 1967;3(5558):153–155. doi: 10.1136/bmj.3.5558.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koku Aksu A.E., Metintas S.E., Saracoglu Z.N., et al. Acne: prevalence and relationship with dietary habits in Eskisehir, Turkey. J Eur Acad Dermatol Venereol. 2012;26(12):1503–1509. doi: 10.1111/j.1468-3083.2011.04329.x. [DOI] [PubMed] [Google Scholar]
- 22.Burris J., Rietkerk W., Shikany J.M., Woolf K. Differences in dietary glycemic load and hormones in New York city adults with no and moderate/severe acne. J Acad Nutr Diet. 2017;117(9):1375–1383. doi: 10.1016/j.jand.2017.03.024. [DOI] [PubMed] [Google Scholar]
- 23.Huang X., Zhang J., Li J., et al. Daily intake of soft drinks and moderate-to-severe acne vulgaris in Chinese adolescents. J Pediatr. 2019;204:256–262. doi: 10.1016/j.jpeds.2018.08.034. [DOI] [PubMed] [Google Scholar]
- 24.Adebamowo C.A., Spiegelman D., Danby F.W., Frazier A.L., Willett W.C., Holmes M.D. High school dietary dairy intake and teenage acne. J Am Acad Dermatol. 2005;52(2):207–214. doi: 10.1016/j.jaad.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 25.Adebamowo C.A., Spiegelman D., Berkey C.S., et al. Milk consumption and acne in adolescent girls. Dermatol Online J. 2006;12(4):1. [PubMed] [Google Scholar]
- 26.Adebamowo C.A., Spiegelman D., Berkey C.S., et al. Milk consumption and acne in teenaged boys. J Am Acad Dermatol. 2008;58(5):787–793. doi: 10.1016/j.jaad.2007.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Di Landro A., Cazzaniga S., Parazzini F., et al. Family history, body mass index, selected dietary factors, menstrual history, and risk of moderate to severe acne in adolescents and young adults. J Am Acad Dermatol. 2012;67(6):1129–1135. doi: 10.1016/j.jaad.2012.02.018. [DOI] [PubMed] [Google Scholar]
- 28.Pontes T.D., Fernandes Filho G.M., Trindade A.D., Sobral Filho J.F. Incidence of acne vulgaris in young adult users of protein-calorie supplements in the city of Joao Pessoa-PB. An Bras Dermatol. 2013;88(6):907–912. doi: 10.1590/abd1806-4841.20132024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Semedo D., Ladeiro F., Ruivo M., et al. Adult acne: prevalence and portrayal in primary healthcare patients, in the Greater Porto Area, Portugal. Acta Med Port. 2016;29(9):507–513. doi: 10.20344/amp.6626. [DOI] [PubMed] [Google Scholar]
- 30.Duquia R.P., Dos Santos I.D., de Almeida H., Jr., Souza P.R., de Avelar Breunig J., Zouboulis C.C. Epidemiology of acne vulgaris in 18-year-old male army conscripts in a South Brazilian city. Dermatology. 2017;233(2-3):145–154. doi: 10.1159/000475775. [DOI] [PubMed] [Google Scholar]
- 31.Ulvestad M., Bjertness E., Dalgard F., Halvorsen J.A. Acne and dairy products in adolescence: results from a Norwegian longitudinal study. J Eur Acad Dermatol Venereol. 2017;31(3):530–535. doi: 10.1111/jdv.13835. [DOI] [PubMed] [Google Scholar]
- 32.Heng A.H., Say Y.H., Sio Y.Y., Ng Y.T., Chew F.T. Epidemiological risk factors associated with acne vulgaris presentation, severity, and scarring in a Singapore Chinese population: a cross-sectional study. Dermatology. 2022;238(2):226–235. doi: 10.1159/000516232. [DOI] [PubMed] [Google Scholar]
- 33.Say Y.H., Heng A.H., Reginald K., et al. Modifiable and non-modifiable epidemiological risk factors for acne, acne severity and acne scarring among Malaysian Chinese: a cross-sectional study. BMC Public Health. 2021;21(1):1–12. doi: 10.1186/s12889-021-10681-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ismail N.H., Manaf Z.A., Azizan N.Z. High glycemic load diet, milk and ice cream consumption are related to acne vulgaris in Malaysian young adults: a case control study. BMC Dermatol. 2012;12(1):1–8. doi: 10.1186/1471-5945-12-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Burris J., Rietkerk W., Woolf K. Relationships of self-reported dietary factors and perceived acne severity in a cohort of New York young adults. J Acad Nutr Diet. 2014;114(3):384–392. doi: 10.1016/j.jand.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 36.Wolkenstein P., Misery L., Amici J.M., et al. Smoking and dietary factors associated with moderate-to-severe acne in French adolescents and young adults: results of a survey using a representative sample. Dermatology. 2015;230(1):34–39. doi: 10.1159/000366195. [DOI] [PubMed] [Google Scholar]
- 37.LaRosa C.L., Quach K.A., Koons K., et al. Consumption of dairy in teenagers with and without acne. J Am Acad Dermatol. 2016;75(2):318–322. doi: 10.1016/j.jaad.2016.04.030. [DOI] [PubMed] [Google Scholar]
- 38.Çerman A.A., Aktaş E., Altunay İ.K., Arıcı J.E., Tulunay A., Ozturk F.Y. Dietary glycemic factors, insulin resistance, and adiponectin levels in acne vulgaris. J Am Acad Dermatol. 2016;75(1):155–162. doi: 10.1016/j.jaad.2016.02.1220. [DOI] [PubMed] [Google Scholar]
- 39.Okoro E.O., Ogunbiyi A.O., George A.O., Subulade M.O. Association of diet with acne vulgaris among adolescents in Ibadan, southwest Nigeria. Int J Dermatol. 2016;55(9):982–988. doi: 10.1111/ijd.13166. [DOI] [PubMed] [Google Scholar]
- 40.Suppiah T.S., Sundram T.K., Tan E.S., Lee C.K., Bustami N.A., Tan C.K. Acne vulgaris and its association with dietary intake: a Malaysian perspective. Asia Pac J Clin Nutr. 2018;27(5):1141–1145. doi: 10.6133/apjcn.072018.01. [DOI] [PubMed] [Google Scholar]
- 41.Aalemi A.K., Anwar I., Chen H. Dairy consumption and acne: a case control study in Kabul, Afghanistan. Clin Cosmet Investig Dermatol. 2019;12:481–487. doi: 10.2147/CCID.S195191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Karadağ A.S., Balta I., Saricaoğlu H., et al. The effect of personal, familial, and environmental characteristics on acne vulgaris: a prospective, multicenter, case controlled study. G Ital Dermatol Venereol. 2019;154(2):177–185. doi: 10.23736/S0392-0488.17.05532-8. [DOI] [PubMed] [Google Scholar]
- 43.Akpinar Kara Y., Ozdemir D. Evaluation of food consumption in patients with acne vulgaris and its relationship with acne severity. J Cosmet Dermatol. 2020;19(8):2109–2113. doi: 10.1111/jocd.13255. [DOI] [PubMed] [Google Scholar]
- 44.Dreno B., Shourick J., Kerob D., Bouloc A., Taieb C. The role of exposome in acne: results from an international patient survey. J Eur Acad Dermatol Venereol. 2020;34(5):1057–1064. doi: 10.1111/jdv.16119. [DOI] [PubMed] [Google Scholar]
- 45.Penso L., Touvier M., Deschasaux M., Hercberg S., Ezzedine K., Sbidian E. Association between adult acne and dietary behaviors: findings from the NutriNet-Santé Prospective Cohort Study. JAMA Dermatol. 2020;156(8):854–862. doi: 10.1001/jamadermatol.2020.1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Anaba E.L., Oaku I.R. Adult female acne: a cross-sectional study of diet, family history, body mass index, and premenstrual flare as risk factors and contributors to severity. Int J Womens Dermatol. 2021;7(3):265–269. doi: 10.1016/j.ijwd.2020.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Smith R.N., Mann N.J., Braue A., Makelainen H., Varigos G.A. A low-glycemic-load diet improves symptoms in acne vulgaris patients: a randomized controlled trial. Am J Clin Nutr. 2007;86(1):107–115. doi: 10.1093/ajcn/86.1.107. [DOI] [PubMed] [Google Scholar]
- 48.Smith R.N., Mann N.J., Braue A., Makelainen H., Varigos G.A. The effect of a high-protein, low glycemic-load diet versus a conventional, high glycemic-load diet on biochemical parameters associated with acne vulgaris: a randomized, investigator-masked, controlled trial. J Am Acad Dermatol. 2007;57(2):247–256. doi: 10.1016/j.jaad.2007.01.046. [DOI] [PubMed] [Google Scholar]
- 49.Smith R., Mann N., Mäkeläinen H., Roper J., Braue A., Varigos G. A pilot study to determine the short-term effects of a low glycemic load diet on hormonal markers of acne: a nonrandomized, parallel, controlled feeding trial. Mol Nutr Food Res. 2008;52(6):718–726. doi: 10.1002/mnfr.200700307. [DOI] [PubMed] [Google Scholar]
- 50.Reynolds R.C., Lee S., Choi J.Y., et al. Effect of the glycemic index of carbohydrates on acne vulgaris. Nutrients. 2010;2(10):1060–1072. doi: 10.3390/nu2101060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kwon H.H., Yoon J.Y., Hong J.S., Jung J., Park M.S., Suh D.H. Clinical and histological effect of a low glycaemic load diet in treatment of acne vulgaris in Korean patients: a randomized, controlled trial. Acta Derm Venereol. 2012;92(3):241–246. doi: 10.2340/00015555-1346. [DOI] [PubMed] [Google Scholar]
- 52.Pavithra G., Upadya G.M., Rukmini M.S. A randomized controlled trial of topical benzoyl peroxide 2.5% gel with a low glycemic load diet versus topical benzoyl peroxide 2.5% gel with a normal diet in acne (grades 1-3) Indian J Dermatol Venereol Leprol. 2019;85(5):486–490. doi: 10.4103/ijdvl.IJDVL_109_17. [DOI] [PubMed] [Google Scholar]
- 53.Danby F.W. Acne, dairy and cancer: the 5alpha-P link. Dermatoendocrinology. 2009;1(1):12–16. doi: 10.4161/derm.1.1.7124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ma J., Giovannucci E., Pollak M., et al. Milk intake, circulating levels of insulin-like growth factor-I, and risk of colorectal cancer in men. J Natl Cancer Inst. 2001;93(17):1330–1336. doi: 10.1093/jnci/93.17.1330. [DOI] [PubMed] [Google Scholar]
- 55.King D.G., Walker M., Campbell M.D., Breen L., Stevenson E.J., West D.J. A small dose of whey protein co-ingested with mixed-macronutrient breakfast and lunch meals improves postprandial glycemia and suppresses appetite in men with type 2 diabetes: a randomized controlled trial. Am J Clin Nutr. 2018;107(4):550–557. doi: 10.1093/ajcn/nqy019. [DOI] [PubMed] [Google Scholar]
- 56.Giezenaar C., Hutchison A.T., Luscombe-Marsh N.D., Chapman I., Horowitz M., Soenen S. Effect of age on blood glucose and plasma insulin, glucagon, ghrelin, CCK, GIP, and GLP-1 responses to whey protein ingestion. Nutrients. 2017;10(1):2. doi: 10.3390/nu10010002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Larnkjaer A., Arnberg K., Michaelsen K.F., Jensen S.M., Molgaard C. Effect of milk proteins on linear growth and IGF variables in overweight adolescents. Growth Horm IGF Res. 2014;24(2-3):54–59. doi: 10.1016/j.ghir.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 58.Hoppe C., Molgaard C., Dalum C., Vaag A., Michaelsen K.F. Differential effects of casein versus whey on fasting plasma levels of insulin, IGF-1 and IGF-1/IGFBP-3: results from a randomized 7-day supplementation study in prepubertal boys. Eur J Clin Nutr. 2009;63(9):1076–1083. doi: 10.1038/ejcn.2009.34. [DOI] [PubMed] [Google Scholar]
- 59.Burris J., Shikany J.M., Rietkerk W., Woolf K. A low glycemic index and glycemic load diet decreases insulin-like growth factor-1 among adults with moderate and severe acne: a short-duration, 2-week randomized controlled trial. J Acad Nutr Diet. 2018;118(10):1874–1885. doi: 10.1016/j.jand.2018.02.009. [DOI] [PubMed] [Google Scholar]