Abstract
Efforts to end the HIV and hepatitis C virus (HCV) epidemics begin with ascertainment of a person's infection status through screening. Despite its importance as a site of testing, missed opportunities for screening in the Emergency Department (ED) are common. We describe the impact of implementing an individualized provider feedback intervention on HIV and HCV testing in a quaternary ED. We conducted an interrupted time series analysis to evaluate the impact of the intervention on weekly HIV and HCV screening in an observational cohort of patients seeking care in the ED. The intervention included a physician champion individualized feedback with peer comparisons to all providers in the ED and an existing HIV/HCV testing and response team. Data were abstracted from the electronic medical record (EMR) for 30 weeks before, during, and after implementing the intervention. We used Poisson regression analysis to estimate changes in the weekly counts and rates of HIV and HCV testing. The incidence rate ratios (IRRs) of HIV testing were 1.94 [95% confidence interval (CI) 1.85–2.04] and 1.38 (95% CI 1.31–1.45) times higher for the intervention and post-intervention period compared with the pre-intervention period. The IRRs of HCV testing was 6.96 (95% CI 6.40–7.58) and 4.70 (95% CI 4.31–5.13) for the intervention and post-intervention periods. There were no meaningful differences in demographic characteristics during the observation period. The intervention meaningfully increased HIV and HCV testing volume and positive case detection, including testing in high-risk groups like young adults and individuals without prior testing. Although diminished, the intervention effect sustained in the 30-week period following implementation.
Keywords: HIV testing, HCV testing, End-the-Epidemic, feedback intervention, audit and feedback
Introduction
All efforts to end the HIV and hepatitis C virus (HCV) epidemics begin with ascertainment of a person's infection status. Screening is the critical first step as HIV testing provides potential entry into both the primary and secondary HIV prevention cascades. Multiple federal, state, and local jurisdiction plans for ending the HIV epidemic (EHE) have been proposed, all with testing as a critical step. Many states, including New York State, have laws requiring an offer of routine HIV testing as part of medical care.1 Fewer states have HCV testing laws; while New York was the first to pass a law mandating an offer of HCV testing to individuals born between 1945 and 1965, with recent guidelines suggesting that wider routine testing is needed.2 Despite the significance of routine screening for both HIV and HCV, missed opportunities for earlier diagnosis remain common throughout the health care system.3–10
Urban emergency rooms are an important, and cost-effective, location for HIV and HCV screening programs because they serve as an access point for people who do not have access to routine care and may be at heightened risk for HIV. There are multiple barriers to effective implementation in this complex and busy environment.11,12 Strategies like EMR alerts (with and without hard stops), roving HIV testing services, and triage and nursing testing offers have all been attempted to increase screening in the Emergency Department (ED) with variable success.7,13,14
In patients testing positive for HIV, linkage to care, successful initiation of antiretroviral therapy, and achievement of undetectable viral load status results in the inability to sexually transmit the virus to others. For individuals testing negative for HIV, sexual health screening and referral to effective, proven biomedical HIV prevention services, like HIV pre-exposure prophylaxis, can reduce the risk of HIV infection by 99%.15 While the ED is a cost-effective location for HIV screening, and many ED providers feel that HIV testing is a responsibility, location-specific barriers exist. These include higher priority issues, time constraints, inadequate resources, burdensome consent processes, and concerns about following up laboratory results and obtaining follow-up visits for positive results.16 Enhancing ED HIV testing requires addressing these specific barriers.
While less visible at the national level, many states have similarly developed ending the HCV epidemic campaigns. Ending the HCV epidemic through testing, linkage and treatment has considerable potential since the advent of direct-acting antiviral agents that can cure almost all cases of HCV in 8 to 12 weeks. However, the critical first step remains screening and identification of individuals living with HCV. Recent United States Preventive Services Task Force (USPTF) recommendations have broadened HCV screening recommendations to include one-time HCV testing for adults 18–79 years of age as well as repeat periodic testing for people with ongoing risk factors.2 While EDs have been shown to be a cost-effective location for HCV screening, there remain numerous barriers to the implementation of HCV screening in the ED setting. Unlike HIV, where screening in the ED is frequently required by law, fewer ED providers recognize HCV screening as part of their scope of practice or that EDs are an effective location for HCV screening programs.17 Additional barriers to HCV screening are similar to those noted for HIV screening above, and include arranging care engagement for positive results.17
Increasing HIV and HCV screening rates in EDs could leverage clinicians’ overall motivation to provide care that improves patient health, a Hippocratic tenet that is largely reflected in studies that assess general attitudes regarding HIV and HCV screening in a variety of clinical settings. Various strategies to improve HIV and HCV screening rates in high-value practices utilizing both audit, feedback and peer comparisons have produced mixed results.18–22 Meta-analysis of both manually and computer-generated reminders delivered on paper lead to small-to-moderate increases in quality outcomes.23,24 While a meta-analysis of audit and feedback concluded that it leads to small improvements in practice overall, with effectiveness dependent upon how the feedback is provided.21 Combined interventions that combine audit and feedback with other strategies have been shown to increase adherence to infection prevention and control guidelines.25
Feedback intervention theory (FIT), a hybrid theory which integrates multiple behavioral theories to explain the effect of feedback interventions on performance, can serve to inform intervention development.26 FIT contains three processes that affect an individual's response to feedback; meta-tasks that direct attention, focal tasks that aim to increase motivation, and task details that aim to improve performance through learning.26,27 Actionable feedback that is timely, individualized, nonpunitive, and customizable have been shown to increase performance.28–30
We describe the effect of implementing individualized provider feedback, including peer comparisons, in the setting of a physician champion and an HIV/HCV testing response tea in a quaternary ED that had opt-out HIV screening and a robust linkage to care program.
Methods
Study design and setting
Using an observational cohort, we conducted an interrupted time series analysis to evaluate the impact of the intervention on weekly HIV and HCV testing levels in two related academic EDs (one academic and one community) between March 1, 2018 and November 20, 2019. We chose to combine both EDs as they were staffed from the same provider pool. We selected this study period because it included a 30-week pre-implementation time, 30-week program implementation time, and 30-week post-implementation time period.
Intervention periods
Pre-Intervention
This project was part of hospital-wide initiatives that focused upon improving HIV and HCV testing, linkage to care, and treatment. For several years before the individualized provider intervention, the ED had an EMR “hard-stop” that required documentation of an offer of HIV testing to all patients coming to the ED, consistent with New York State law. During the pre-intervention period, institutional HIV and HCV testing dashboards were developed that provided the HIV/HCV testing and response team with near real-time HIV and HCV testing results. The HIV/HCV testing and response team was led by a care coordinator who followed up positive cases and assigned them to care coordinators for contact and linkage to care. Multiple clinical care members were available for consultation as needed.
Furthermore, during the pre-intervention period, an ED physician champion was identified who met regularly with the HIV/HCV testing and response team to design the intervention and provided input on messaging. On September 19, 2018, the physician champion provided a single educational update to ED providers during their monthly faculty meeting; this included information about the local epidemiology, stressing the importance of HIV and HCV screening in the ED, and an introduction to the upcoming proposed study.
Intervention
At the start of the intervention period, individualized feedback was sent to all clinical staff, such as physicians, resident physicians, physician assistants, and nurse practitioners, who could offer an HIV or HCV test in the ED. Feedback to all providers consisted of an e-mail from the physician champion with an attachment showing their preceding 1- and 6-month individual and peer HIV and HCV screening counts and rates, the overall HIV and HCV screening rate for the prior month, and a target goal (“Our goal is to increase the overall ED screening rate to 70%”). All faculty physicians and physician assistants were also provided simultaneously an e-mail and a monthly text message feedback on the performance. Monthly text messages were sent on September 28th, October 30th, November 27th, December 27th, January 29th, February 26th, and March 28th. Text messages included individual screening percentages and peer comparisons. (“Over the past month you screened X% of your patients for HIV and X% for HCV compared with the ED averages of Y% and Y%”).
Post-intervention
The intervention was considered complete on April 26th, 4 weeks after the final feedback was sent. The HIV/HCV testing and response team remained in place but there were no further educational sessions and feedback provided by the physician champion and no changes to order sets or screening practices throughout the 18-month study.
Data sources and linkage
Data were abstracted from the EMR retrospectively for all patients with an ED visit from March 1, 2018, through November 30, 2019. Data acquired for each ED visit included patient demographics (age, sex at birth, race and/or ethnicity, visit date, visit location), current and historical HIV and HCV testing, and visit disposition. Historical HIV and HCV testing were available back to 2012. This study was approved by the Institutional Review Board with a waiver of informed consent.
Outcomes
The primary outcome was the number of patients receiving an HIV or HCV test during their ED visit per week. Secondary outcomes included the percent of patients receiving an HIV or HCV test in the ED, percent of patients screened for HIV and HCV since 2012, the number of positive tests, and the positivity rate.
Statistical analysis
We conducted descriptive statistics to summarize the demographic and clinical outcomes of the sample. Time series analysis is a form of forecasting in which past HIV and HCV testing data were modeled to make predictions about the future. We conducted a Poisson regression analysis to estimate changes in the counts and weekly rate of HIV and HCV testing.31,32 Poisson regression approximates the binomial distribution because of the large sample size and few independent predictors. Further, we examined the weekly number of ER visits and did not observe any significant change in patient volume during the observation period. We estimated the Newey–West standard errors to account for autocorrelation and heteroskedasticity because of the correlation between proximal observations. HIV and HCV screening data were grouped into a time unit of 7 days. We categorized the intervention into three intervention stages: pre-intervention, during intervention, and post-intervention as a dummy variable. The model also included a time variable and an interaction between time and intervention period.
We used the following regression parameterization:
Where,
Yt is the number of HIV or HCV tests in week t;
is the number of HIV/HCV tests at the start of the time series;
is the trend (rate of change) in the number of weekly HIV/HCV tests in the pre-intervention period;
is a continuous variable indicating time in weeks at time t from the start of the time series;
is the change in the number of weekly HIV/HCV tests immediately after the start of and during the intervention, which represents the effect of the intervention;
is a dummy variable coded 0 for the pre-intervention period, 1 for the intervention period;
is the change in the trend in the weekly number of HIV/HCV tests during post-intervention phase compared with the pre-intervention period, which represents any sustained effect;
is a dummy variable coded 0 for the pre-intervention period, 1 for the post-intervention period; and is the error term at time t.
Results
Overall, there were 215,622 ED encounters during the study period; 70,568 in the pre-intervention period, 70,532 in the intervention period, and 74,522 in the post-intervention period. Most patients seen in the ED were over age 60 (23%), female (56%), had another or unknown race or ethnicity (76%), and seen at the academic medical center (68%). There were no key differences in age, sex at birth, race/ethnicity, hospital setting among the three time periods, or mean number of patients seen weekly (Table 1).
Table 1.
Demographics of Emergency Department Patients
| Characteristic | Overall, N = 215,622a | Pre, N = 70,568a | Intervention, N = 70,532a | Post, N = 74,522a |
|---|---|---|---|---|
| Age category | ||||
| 18–24 | 27,563 (13%) | 9225 (13%) | 9151 (13%) | 9187 (12%) |
| 25–29 | 29,051 (13%) | 9493 (13%) | 9415 (13%) | 10,143 (14%) |
| 30–39 | 43,706 (20%) | 14,213 (20%) | 14,245 (20%) | 15,248 (20%) |
| 40–49 | 32,391 (15%) | 10,474 (15%) | 10,713 (15%) | 11,204 (15%) |
| 50–59 | 33,489 (16%) | 11,273 (16%) | 10,773 (15%) | 11,443 (15%) |
| >60 | 49,422 (23%) | 15,890 (23%) | 16,235 (23%) | 17,297 (23%) |
| Sex at birth | ||||
| Female | 121,245 (56%) | 39,329 (56%) | 40,241 (57%) | 41,675 (56%) |
| Male | 94,377 (44%) | 31,239 (44%) | 30,291 (43%) | 32,847 (44%) |
| Ethnicity/race | ||||
| Hispanic/Latino | 26,442 (12%) | 9227 (13%) | 8692 (12%) | 8523 (11%) |
| Non-Hispanic Black/African American | 14,215 (6.6%) | 4651 (6.6%) | 4753 (6.7%) | 4811 (6.5%) |
| Non-Hispanic White | 11,583 (5.4%) | 3775 (5.3%) | 3820 (5.4%) | 3988 (5.4%) |
| Other/unknown | 163,382 (76%) | 52,915 (75%) | 53,267 (76%) | 57,200 (77%) |
| Hospital setting | ||||
| Academic | 147,559 (68%) | 48,131 (68%) | 48,147 (68%) | 51,281 (69%) |
| Community | 68,063 (32%) | 22,437 (32%) | 22,385 (32%) | 23,241 (31%) |
| Testing for the first time | ||||
| Yes | 122,694 (57%) | 41,851 (60%) | 39,901 (57%) | 40,942 (55%) |
| No | 91,736 (43%) | 28,375 (40%) | 30,174 (43%) | 33,187 (45%) |
| Average patient visit by week | 2396 | 2352 | 2351 | 2484 |
n (%).
Pre-intervention period: March 1, 2018 through September 26, 2018.
Intervention period: September 27, 2018 through April 24, 2019.
Post-intervention period: April 25, 2019 through November 20, 2019.
Primary outcome
Overall, there were 11,562 HIV tests and 2410 HCV tests performed during the study period. During the preintervention period, 2640 HIV and 268 HCV tests were performed, compared with 5129 HIV and 1221 HCV tests during the intervention period and 3793 HIV and 921 HCV tests during the post-intervention period. Individuals tested for HIV were more likely to be age 30–39 (26%), female (56%), seen in the academic medical center (73%), and tested for the first time in our system (58%); these percentages were relatively consistent in the pre, intervention, and post-time periods. Individuals tested for HCV were more likely to be over 60 years of age (28%), female (56%), seen in the academic medical center (72%), and tested for the first time in our system (63%); these percentages were relatively consistent in the pre, intervention, and post-time periods. In the intervention and post-intervention period, there were an additional 545 HIV and an additional 186 HCV tests among those 18–24, an additional 1875 HIV tests, and 947 HCV tests among women and an additional 2143 HIV and 1024 HCV tests among individuals without documentation of prior testing (Table 2).
Table 2.
Demographics of Emergency Department Patients with HIV/HCV Tests on Visit Date
| Characteristic | HIV |
HCV |
||||||
|---|---|---|---|---|---|---|---|---|
| Overall, N = 11,562a | Pre, N = 2640a | Intervention, N = 5129a | Post, N = 3793a | Overall, N = 2410a | Pre, N = 268a | Intervention, N = 1221a | Post, N = 921a | |
| Age category | ||||||||
| 18–24 | 2219 (19%) | 558 (21%) | 970 (19%) | 691 (18%) | 258 (11%) | 24 (9.0%) | 136 (11%) | 98 (11%) |
| 25–29 | 2239 (19%) | 544 (21%) | 984 (19%) | 711 (19%) | 259 (11%) | 32 (12%) | 115 (9.4%) | 112 (12%) |
| 30–39 | 2995 (26%) | 684 (26%) | 1259 (25%) | 1052 (28%) | 462 (19%) | 56 (21%) | 235 (19%) | 171 (19%) |
| 40–49 | 1740 (15%) | 357 (14%) | 800 (16%) | 583 (15%) | 353 (15%) | 46 (17%) | 164 (13%) | 143 (16%) |
| 50–59 | 1300 (11%) | 273 (10%) | 612 (12%) | 415 (11%) | 410 (17%) | 43 (16%) | 207 (17%) | 160 (17%) |
| >60 | 1069 (9.2%) | 224 (8.5%) | 504 (9.8%) | 341 (9.0%) | 668 (28%) | 67 (25%) | 364 (30%) | 237 (26%) |
| Sex at birth | ||||||||
| Female | 6468 (56%) | 1411 (53%) | 2954 (58%) | 2103 (55%) | 1358 (56%) | 137 (51%) | 704 (58%) | 517 (56%) |
| Male | 5094 (44%) | 1229 (47%) | 2175 (42%) | 1690 (45%) | 1052 (44%) | 131 (49%) | 517 (42%) | 404 (44%) |
| Ethnicity/race | ||||||||
| Hispanic/Latino | 1375 (12%) | 316 (12%) | 624 (12%) | 435 (11%) | 243 (10%) | 24 (9.0%) | 141 (12%) | 78 (8.5%) |
| Non-Hispanic Black/African American | 623 (5.4%) | 155 (5.9%) | 297 (5.8%) | 171 (4.5%) | 146 (6.1%) | 24 (9.0%) | 65 (5.3%) | 57 (6.2%) |
| Non-Hispanic White | 408 (3.5%) | 94 (3.6%) | 180 (3.5%) | 134 (3.5%) | 120 (5.0%) | 10 (3.7%) | 69 (5.7%) | 41 (4.5%) |
| Other/unknown | 9156 (79%) | 2075 (79%) | 4028 (79%) | 3053 (80%) | 1901 (79%) | 210 (78%) | 946 (77%) | 745 (81%) |
| Hospital setting | ||||||||
| Academic | 8459 (73%) | 1975 (75%) | 3839 (75%) | 2645 (70%) | 1727 (72%) | 217 (81%) | 844 (69%) | 666 (72%) |
| Community | 3103 (27%) | 665 (25%) | 1290 (25%) | 1148 (30%) | 683 (28%) | 51 (19%) | 377 (31%) | 255 (28%) |
| Testing for the first time | ||||||||
| Yes | 6733 (58%) | 1530 (58%) | 3021 (59%) | 2182 (58%) | 1510 (63%) | 162 (61%) | 755 (62%) | 593 (65%) |
| No | 4810 (42%) | 1110 (42%) | 2093 (41%) | 1607 (42%) | 888 (37%) | 103 (39%) | 464 (38%) | 321 (35%) |
| Positive cases | 93 (0.82%) | 25 (0.96%) | 35 (0.69%) | 33 (0.91%) | 272 (3.5%) | 46 (7.5%) | 121 (2.8%) | 105 (3.5%) |
| Patient screened in ED per week | 127 (5.4%) | 88 (3.8%) | 171 (7.3%) | 122 (5.1%) | 88 (3.7%) | 21 (0.87%) | 144 (6.1%) | 100 (4.2%) |
| Average patient visit by week | 128 | 88 | 171 | 126 | 27 | 9 | 41 | 31 |
n (%).
Pre-intervention period: March 1, 2018 through September 26, 2018.
Intervention period: September 27, 2018 through April 24, 2019.
Post-intervention period: April 25, 2019 through November 20, 2019.
ED, emergency department; HCV, hepatitis C virus.
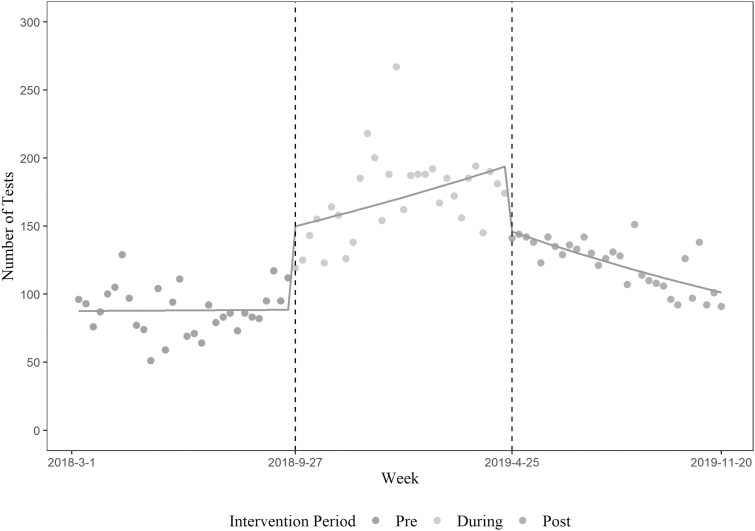
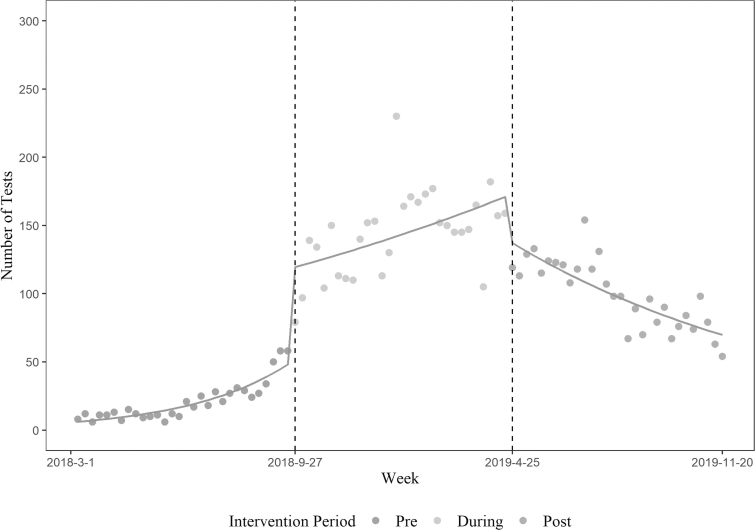
Figures 1 and 2 show trends in HIV and HCV testing in the pre, during, and post-intervention periods, respectively. HIV testing levels were unchanged during the pre-intervention period, increased during the intervention period, and decreased in the period immediately after, but did not return to pre-intervention levels. HCV testing levels began increasing during the pre-intervention, further increased in the intervention period, and declined in the period immediately after but did not return to pre-intervention levels. After adjusting for weekly patient volume, the incidence rate of weekly HIV testing during the intervention and post-intervention periods increased by 1.94 [95% confidence interval (CI) 1.85–2.04] and 1.38 (95% CI 1.31–1.45) folds compared with pre-intervention period, respectively (Table 3). HCV testing rates also increased by 6.96 (95% CI 6.40–7.58) and 4.70 (95% CI 4.31–5.13) folds compared with pre-intervention period, respectively (Table 3). The interaction effects of weekly patient volume and intervention period resulted in statistically unstable estimates, and those were removed from the final model.
FIG. 1.
HIV testing during the pre-intervention, intervention, and post-intervention periods.
FIG. 2.
HCV testing during the pre-intervention, intervention, and post-intervention periods. HCV, hepatitis C virus.
Table 3.
Time Series Analysis of HIV & HCV Testing by Intervention Stage
| HIV |
HCV |
|||||
|---|---|---|---|---|---|---|
| IRR | 95% CI | p | IRR | 95% CI | p | |
| Weekly visits | 1.00 | 1.00–1.00 | 0.003 | 1.00 | 1.00–1.00 | <0.001 |
| Stage | ||||||
| Pre | — | — | ||||
| Intervention | 1.94 | 1.85–2.04 | <0.001 | 6.96 | 6.40–7.58 | <0.001 |
| Post | 1.38 | 1.31–1.45 | <0.001 | 4.70 | 4.31–5.13 | <0.001 |
CI, confidence interval; IRR, incidence rate ratio.
Pre, intervention period: March 1, 2018 through September 26, 2018.
Intervention period: September 27, 2018 through April 24, 2019.
Post, intervention period: April 25, 2019 through November 20, 2019.
Secondary outcomes
Across all ED patients, 3.8% of patients were screened for HIV per week in the pre-intervention period; this increased to 7.3% during the intervention period and decreased to 5.1% in the post-intervention period. In the pre-intervention period, 0.9% of ED patients were screened for HCV; this increased to 6.1% during the intervention period and decreased to 4.2% in the post-intervention period. Overall, 41.80% of patients had documented HIV testing and 33.46% had documented HCV testing since 2012 in the pre-intervention period. This increased to 44.04% for HIV and 37.87% for HCV during the intervention period and decreased to 41.10% for HIV and 35.79% for HCV in the post-intervention period.
In the pre-intervention period, there were 25 patients diagnosed with HIV (0.96% positivity) and 46 with HCV (7.5% positivity). During the intervention period, 35 patients were diagnosed with HIV (0.69% positivity) and 121 with HCV (2.8% positivity). In the post-intervention period, 33 patients were diagnosed with HIV (0.91% positivity) and 105 were diagnosed with HCV (3.5% positivity).
Discussion
This study provides information on HIV and HCV testing in a busy urban ED before and after a FIT-informed HIV and HCV testing intervention. The study demonstrates the objective of increasing HIV and HCV testing during the intervention period. We observed an absolute increase in tests, an increase in the number of patients screened for the first time in our EMR, and an impressive increase in the number of patients testing positive for HIV and HCV. We did not observe an increase in percent of patients screened positive during the intervention period compared with the pre-intervention period. This may be attributed to HIV and HCV risk ascertainment differences used by providers to offer a test, resulting in those at highest risk being tested, rather than all those who are in need of HIV and HCV testing. Although, some effect was sustained immediately after implementation of the intervention was removed, testing decreased significantly.
In considering the success of this intervention, numerous design characteristics were deemed important. Surveys of providers about HIV and HCV testing identified higher priority issues, time constraints, inadequate resources, and concerns about following up laboratory results, and navigating care engagement for patients who were identified as positive.16,17 This intervention was designed in conjunction with our physician champion to address each of these concerns. The physician champion, a member of the ED, reviewed with providers the importance of testing for EtE goals as well as the emerging data on rising local HCV rates, highlighting the importance of this work and demonstrating that it should be considered a priority.33 Secondarily, there was an HIV/HCV testing and response team that took responsibility for following up all positive test results and arranging for linkage to care, reducing the amount of ED time and resources dedicated to this often difficult task. This was messaged to providers both during the physician champion kick-off session and in notes left in the EMR for providers to see confirming that the service did receive the positive result and follow-up with the patient.
Further, this intervention was designed using FIT. The monthly e-mails and text messages directed attention to the meta-task of HIV and HCV testing regularly. Given that laboratories are already frequently ordered in the ED, adding additional laboratories does not require significant processing and is thus more amenable to shifting focus and attention. The individual feedback of both 1- and 6-month screening rates can improve motivation by showing improvement over time. Peer comparisons were also utilized having been shown to enhance these interventions. Finally, by making this feedback timely (monthly), non-punitive (not shared with administration), and personalized, it was accepted by ED staff, actionable with minimal additional effort, and understood to be effective.
During this study, numerous providers were both surprised by, and did not at first believe their personal testing rates were so low. To address these concerns, a testing quality check (QC) dashboard was developed and introduced in November that allowed providers to see granular provider-specific patient-level testing data, including HIV and HCV results. This ability to review their own performance and understand gaps in care appeared to reduce provider concerns regarding overall accuracy of the feedback and likely further helped drive adoption.
This study was performed in two Emergency Rooms associated with a single academic medical center using Emergency Medicine staff working at both facilities. It is unclear if this intervention would work as well in a non-urban ED with lower rates of HIV and HCV, the nonacademic settings, or nonemergency settings. All positive tests were new diagnosis at the institution; however, we have no way of ensuring that patients were not diagnosed at other facilities, and did not disclose their diagnosis. However, we used the same definition in each time period to create comparable cohorts. We did not disaggregate testing by type of provider since we did not have sufficient power to examine provider-specific testing effects. Therefore, it is unclear if the intervention worked the same across provider groups (physicians, residents, nurse practitioners, physician assistants) or if the intervention had a differential effect by provider group, and if those providers have a greater effect on our findings. Future studies should consider evaluating at the provider level, as different provider groups may require further individualized interventions.
We recognize that provider feedback and peer comparisons were provided in the context of a larger package of supportive services, including the use of a testing dashboard, testing service response team, and patient navigation. For the purposes of this study, linkage to care was not a specific outcome; however, a robust HIV and HCV linkage to care service was available that provided follow-ups for all positive results, scheduled appointments, and provided navigation services to clients. Historically the HIV testing response team has a greater than 98% linkage to care rate for individuals testing positive for HIV. Robust follow-up, linkage, and navigation services require significant resources and it is unclear if the increase in HIV and HCV screening would have been as large without these services.
Similarly, without the application of a novel information technology intervention that allowed for testing and results notification through a near “real-time” dashboard, as well as the QC dashboard, providers may have lacked confidence in the overall intervention. The study also benefited from a physician champion, or popular opinion leader, to drive dissemination and adoption. Finally, the intervention was in place for only 30 weeks, and it is unclear if further fatigue would have occurred had the intervention continued. The study ended after 30 weeks post-intervention, while average testing is up in the post-intervention period, the general trend was a decline in testing and it is unclear if HIV and HCV testing rates would have returned to baseline over time.
In the setting of a HIV/HCV testing response team and a physician champion, provider feedback and peer comparisons can significantly increase HIV and HCV testing, including testing in high-risk groups, such as adolescents and young adults, and individuals without prior testing. Further study is needed to see if this process could be further automated, applied to additional settings and screenings, and if post-intervention testing fatigue can be mitigated with intermittent feedback.
Author Disclosure Statement
S.O.—Gilead investigator-initiated study funding during 2019–2021. No other authors report any conflicts of interest.
Funding Information
Research reported in this publication was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under award nos. K23AI150378 (J.Z.), L30AI133789 (J.Z.), and T32AI114398 (L.P.).
References
- 1. State HIV Laws | Law | Policies | HIV/AIDS | CDC [Internet]. https://www.cdc.gov/hiv/policies/law/states/index.html. Available at: https://www.cdc.gov/hiv/policies/law/states/index.html (Last accessed September 17, 2021).
- 2. Chou R, Dana T, Fu R, et al. Screening for hepatitis C virus infection in adolescents and adults: Updated evidence report and systematic review for the US Preventive Services Task Force. JAMA 2020;323:976–991. [DOI] [PubMed] [Google Scholar]
- 3. Levy I, Maor Y, Mahroum N, et al. Missed opportunities for earlier diagnosis of HIV in patients who presented with advanced HIV disease: A retrospective cohort study. BMJ Open 2016;6:e012721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Klein PW, Martin IBK, Quinlivan EB, et al. Missed opportunities for concurrent HIV-STD testing in an academic emergency department. Public Health Rep 2014;129(Suppl 1):12–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zucker J, Patterson B, Ellman T, et al. Missed opportunities for engagement in the prevention continuum in a predominantly Black and Latino community in New York City. AIDS Patient Care STDS 2018;32:432–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Duffus W, Davis HT, Byrd MD, et al. HIV testing in women: Missed opportunities. J Womens Health 2012;21:170–178. [DOI] [PubMed] [Google Scholar]
- 7. Zucker J, Cennimo D, Sugalski G, Swaminathan S. Identifying areas for improvement in the HIV screening process of a high-prevalence emergency department. AIDS Patient Care STDS 2016;30:247–253. [DOI] [PubMed] [Google Scholar]
- 8. Torian LV, Felsen UR, Xia Q, et al. Undiagnosed HIV and HCV infection in a New York City Emergency Department, 2015. Am J Public Health 2018;108:e1–e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gutman CK, Middlebrooks L, Camacho-Gonzalez A, et al. Asymptomatic adolescent HIV: Identifying a role for universal HIV screening in the pediatric emergency department. AIDS Patient Care STDS 2020;34:373–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Magel T, Conway B. Targeted outreach, education, and point-of-care testing for HIV and hepatitis C: Strategies to address HIV infection in marginalized communities in vancouver. AIDS Patient Care STDS 2020;34:281–283. [DOI] [PubMed] [Google Scholar]
- 11. Mendlowitz AB, Naimark D, Wong WWL, et al. The emergency department as a setting-specific opportunity for population-based hepatitis C screening: An economic evaluation. Liver Int 2020;40:1282–1291. [DOI] [PubMed] [Google Scholar]
- 12. Mwachofi A, Fadul NA, Dortche C, Collins C. Cost-effectiveness of HIV screening in emergency departments: A systematic review. AIDS Care 2021;33:1243–1254. [DOI] [PubMed] [Google Scholar]
- 13. Romero RA, Klausner JD, Marsch LA, Young SD. Technology-delivered intervention strategies to bolster HIV testing. Curr HIV/AIDS Rep 2021;18:391–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Walensky RP, Reichmann WM, Arbelaez C, et al. Counselor- versus provider-based HIV screening in the emergency department: Results from the universal screening for HIV infection in the emergency room (USHER) randomized controlled trial. Ann Emerg Med 2011;58(1 Suppl 1):S126–S132.e1–e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pre-Exposure Prophylaxis (PrEP) | HIV Risk and Prevention | HIV/AIDS | CDC [Internet]. Available at: https://www.cdc.gov/hiv/risk/prep/index.html (Last accessed September 17, 2021).
- 16. Zucker J, Carnevale C, Theodore D, et al. Attitudes and perceived barriers to routine HIV screening and provision and linkage of postexposure prophylaxis and pre-exposure prophylaxis among graduate medical trainees. AIDS Patient Care STDS 2021;35:180–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Winetsky D, Zucker J, Carnevale C, et al. Attitudes, practices and perceived barriers to hepatitis C screening among medical residents at a large urban academic medical center. J Viral Hepat 2019;26:1355–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gerber JS, Prasad PA, Fiks AG, et al. Effect of an outpatient antimicrobial stewardship intervention on broad-spectrum antibiotic prescribing by primary care pediatricians: A randomized trial. JAMA 2013;309:2345–2352. [DOI] [PubMed] [Google Scholar]
- 19. Meeker D, Linder JA, Fox CR, et al. Effect of behavioral interventions on inappropriate antibiotic prescribing among primary care practices: A randomized clinical trial. JAMA 2016;315:562–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tuti T, Nzinga J, Njoroge M, et al. A systematic review of electronic audit and feedback: Intervention effectiveness and use of behaviour change theory. Implement Sci 2017;12:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ivers N, Jamtvedt G, Flottorp S, et al. Audit and feedback: Effects on professional practice and healthcare outcomes. Cochrane Database Syst Rev 2012;2012:CD000259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hysong SJ. Meta-analysis: Audit and feedback features impact effectiveness on care quality. Med Care 2009;47:356–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Arditi C, Rège-Walther M, Durieux P, Burnand B. Computer-generated reminders delivered on paper to healthcare professionals: Effects on professional practice and healthcare outcomes. Cochrane Database Syst Rev 2017;7:CD001175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pantoja T, Grimshaw JM, Colomer N, et al. Manually-generated reminders delivered on paper: Effects on professional practice and patient outcomes. Cochrane Database Syst Rev 2019;12:CD001174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Silva MT, Galvao TF, Chapman E, et al. Dissemination interventions to improve healthcare workers’ adherence with infection prevention and control guidelines: A systematic review and meta-analysis. Implement Sci 2021;16:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kluger AN, DeNisi A. The effects of feedback interventions on performance: A historical review, a meta-analysis, and a preliminary feedback intervention theory. Psychol Bull 1996;119:254–284. [Google Scholar]
- 27. Dowding D, Merrill J, Russell D. Using feedback intervention theory to guide clinical dashboard design. AMIA Annu Symp Proc 2018;2018:395. [PMC free article] [PubMed] [Google Scholar]
- 28. Sinuff T, Muscedere J, Rozmovits L, et al. A qualitative study of the variable effects of audit and feedback in the ICU. BMJ Qual Saf 2015;24:393–399. [DOI] [PubMed] [Google Scholar]
- 29. Lu C-YE, Vinci LM, Quinn MT, et al. Using feedback to change primary care physician behavior. J Ambul Care Manage 2015;38:118–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hysong SJ, Best RG, Pugh JA. Audit and feedback and clinical practice guideline adherence: Making feedback actionable. Implement Sci 2006;1:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bernal JL, Cummins S, Gasparrini A. Interrupted time series regression for the evaluation of public health interventions: A tutorial. Int J Epidemiol 2017;46:348–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wagner AK, Soumerai SB, Zhang F, Ross-Degnan D. Segmented regression analysis of interrupted time series studies in medication use research. J Clin Pharm Ther 2002;27:299–309. [DOI] [PubMed] [Google Scholar]
- 33. Winetsky D, Zucker J, Slowikowski J, et al. Preliminary screening results outside the 1945–1965 birth cohort: A forgotten population for hepatitis C? Open Forum Infect Dis 2019;6:ofz178. [DOI] [PMC free article] [PubMed] [Google Scholar]