Abstract
Previous studies have shown that a cationic water-soluble pyridinium zinc phthalocyanine (PPC) is a powerful photosensitizer that is able to inactivate Escherichia coli. In the current work incubation of E. coli cells with PPC in the dark caused alterations in the outer membrane permeability barrier of the cells, rendering the bacteria much more sensitive to hydrophobic compounds, with little effect seen with hydrophilic compounds. Addition of Mg2+ to the medium prior to incubation of the cells with PPC prevented these alterations in the outer membrane permeability barrier. The presence of Mg2+ in the medium also prevented the photoinactivation of E. coli cells with PPC. These results are consistent with the hypothesis that PPC gains access across the outer membrane of E. coli cells via the self-promoted uptake pathway, a mechanism of uptake postulated for the uptake of other cationic compounds across the outer membranes of gram-negative bacteria.
Whereas gram-positive bacteria have generally been sensitive to photoinactivation with porphyrin and phthalocyanine photosensitizers (1, 3, 4, 15), gram-negative bacteria have been reported (1–4, 15, 27) to be relatively insensitive unless the photosensitizers are administered in the presence of additional substances, for example, CaCl2, Tris-EDTA, or polymyxin B nonapeptide (PMBN), that alter the permeability of the outer membrane. The insensitivity of gram-negative bacteria to photoinactivation was attributed to the presence of the outer membrane, which markedly decreases the permeability of the membrane for large or hydrophobic compounds compared to that of usual biological membranes (9, 15, 21, 24, 28, 31). In addition, the presence of multidrug efflux pumps, such as AcrAB, further enhances the level of intrinsic resistance to compounds which are hydrophobic in nature (21, 22). This membrane has an asymmetric structure with an inner leaflet of phospholipid and an outer leaflet of lipopolysaccharide (LPS). Embedded within the membrane are proteins called porins which allow the influx of low-molecular-mass compounds (<600 to 700 Da for Escherichia coli) but which exclude compounds above this molecular mass from crossing the outer membrane (24). Within the LPS are many negatively charged groups which endow the surface of gram-negative bacteria with a strong negative charge. The binding of divalent cations, such as Mg2+, prevents electrostatic repulsion of adjacent LPS molecules and is important in preserving the structure and integrity of the outer membrane (24).
The insensitivity of gram-negative bacteria to photoinactivation can be overcome with the use of cationic porphyrin or phthalocyanine photosensitizers (16–18). Our recent discovery that a cationic hydrophilic pyridinium zinc phthalocyanine (PPC) photoinactivates gram-negative bacteria as well as gram-positive bacteria has posed an interesting question. How does PPC cross the outer membrane of the gram-negative bacterial cell? The size of PPC means that it will be too large to pass through the outer membrane of E. coli by the porin pathway. An alternative mechanism postulated for the uptake of some compounds, many of which are cationic in nature, is the self-promoted uptake pathway (7, 9–11). Passage across the outer membrane via the self-promoted uptake pathway involves interaction of the compounds at the sites at which divalent cations cross-link adjacent LPS molecules (7, 9–11). Compounds which interact at these sites by either chelating or displacing the divalent cation profoundly affect the structure and function of the outer membrane (7, 9–11, 24, 31). The size of the interacting compounds causes disorganization of the outer membrane and an increase in outer membrane permeability (7, 9–11). This increase in outer membrane permeability renders the bacterial cells more sensitive to compounds, generally hydrophobic in nature, such as various antibacterial agents, detergents, dyes, and steroid probes (7, 9–11, 13, 24, 31, 33–36). Little effect is seen on the sensitivity of the cells to hydrophilic compounds which cross the outer membrane via the porin pathway (9, 24, 31). This increase in outer membrane permeability also allows an increase in the uptake of the interacting compound itself (7, 9–11). Since the self-promoted uptake pathway involves interaction of the compounds at divalent cation binding sites, the presence of excess divalent cations, such as Mg2+, causes a competitive inhibition of binding and subsequent activity of the compounds (9–11, 13, 24, 31). The purpose of this study was to determine if the uptake of PPC into E. coli cells rendered the bacterial cells more sensitive to relatively hydrophobic compounds and also to determine if the uptake and subsequent activity of PPC could be altered in the presence of Mg2+.
MATERIALS AND METHODS
The antibacterial agents used in this study were obtained from Sigma, Poole, United Kingdom. Ampicillin and cloxacillin (sodium salt) were dissolved in 0.1 M phosphate buffer (pH 7.0). Novobiocin (sodium salt), erythromycin, tetracycline (hydrochloride), and fusidic acid were dissolved in ethanol. Rifampin was dissolved in a minimal volume of dimethyl sulfoxide and was then diluted to the appropriate concentration with buffer.
Photosensitizer solutions.
Figure 1 depicts the structure of the cationic water-soluble zinc phthalocyanine used in this study. The compound was synthesized at the University of Leeds, United Kingdom. It contains material with different degrees of substitution, with the disubstituted derivative predominating (8). Samples were dissolved in water to a concentration of 1 mg/ml, passed through a 0.22-μm-pore-size filter, and stored at −20°C until required.
FIG. 1.
Structure of the cationic water-soluble PPC. The structural details are explained in the Materials and Methods section.
14C-labeled protoporphyrin IX (14C-PpIX), a hydrophobic porphyrin, at a specific activity of 34 Ci/mol (prepared as described previously [38]), was dissolved in 1 M ammonium hydroxide, and cold PpIX (obtained from Sigma) was added to a final concentration of 10 μg/ml (specific activity, 30 nCi/μg of PpIX).
Standard preparation of E. coli cells.
E. coli DH5α [genotype K-12 F′ φ80dlacZΔM15 recA1 endA1 gyrA26 thi-1 hsdR17 supE44 relA1 deoR Δ(lacZYA-argF)U169] (1) was grown in nutrient broth (1% tryptone, 0.5% yeast extract [wt/vol]) overnight. A total of 10 ml of the culture was aseptically transferred to 200 ml of fresh medium and was grown at 37°C to the mid-logarithmic phase in a shaking incubator. The cells were harvested by centrifugation (5,000 × g for 10 min) and washed once in 0.1 M phosphate buffer (pH 7.0). The cells were then resuspended in the same buffer to give a turbidity (optical density at 650 nm [OD650]) of 0.87, which corresponds to 3.2 × 108 CFU/ml for E. coli. This was used as a standard preparation.
Uptake of 14C-PpIX into PPC-treated E. coli.
Experiments were carried out to determine if the presence of PPC affected the uptake of the hydrophobic sensitizer PpIX. One milliliter of the standard preparation of E. coli described above was incubated with 0, 1, 5, or 10 μg of PPC per ml for 30 min at room temperature. 14C-PpIX was added at 1 μg/ml, and the preparation was incubated for a further 30 min at room temperature. A 200-μl sample was then taken and was placed in a scintillation vial containing 10 ml of emulsifier-safe liquid scintillation cocktail (Canberra Packard Ltd.). To determine the amount of 14C-PpIX taken up by the cells, washing experiments were carried out. The cells were centrifuged with a bench microcentrifuge (11,600 × g for 2 min), the supernatant was discarded, and the cell pellet was resuspended in buffer with a volume equal to the volume of the supernatant that was discarded. A 200-μl sample was taken and was placed in a separate scintillation vial containing 10 ml of emulsifier-safe liquid scintillation cocktail. The cells were washed and were resuspended up to three times, with samples taken after each wash to determine the amount of bound 14C-PpIX. The amount of radioactivity was measured by liquid scintillation counting (Packard 1900 TR scintillation counter).
To determine if the PPC in the external buffer had an effect on the uptake of 14C-PpIX, further washing experiments were carried out. The washing experiment involved a standard preparation of E. coli treated with 10 μg of PPC per ml for 30 min at room temperature. The cells were then washed, i.e., centrifuged and resuspended in fresh phosphate buffer (0.1 M; pH 7.0) up to three times to remove free PPC. The cells were then incubated with 1 μg of 14C-PpIX (30 nCi/μg) per ml for 30 min at room temperature. The cells were centrifuged and the pellet was resuspended and washed up to three times, with a 200-μl aliquot of the cell suspension taken after each step to determine the amount of bound 14C-PpIX as described earlier.
Cell killing experiment with PPC and PpIX.
The standard assay used to determine the ability of a photosensitizer to photoinactivate bacterial cells was a slight modification of the assay of Bertoloni and coworkers (2). Samples (25 ml) of a standard cell suspension were incubated in the dark at 37°C in foil-covered 250-ml conical flasks with either 5 μg of PPC per ml for 30 min, 10 μg of PpIX per ml for 30 min, or 5 μg of PPC per ml for 30 min and then with 10 μg of PpIX per ml for a further 30 min. The cell suspensions were then placed 70 cm below two 400-W metal halide spectral lamps for 0, 15, or 30 min. The lamps gave a light intensity of 10 mW/cm2 (with approximately 1 mW/cm2 in the range of 600 to 700 nm). Control experiments were carried out with illumination of the cells in the absence of photosensitizer and cells incubated in the dark with photosensitizer. Irradiated and unirradiated samples were assayed for cell viability by serial dilution in 0.1 M phosphate buffer (pH 7.0) and were then plated in triplicate on nutrient broth supplemented with 2% (wt/vol) agar. Experiments were designed to obtain between 30 and 300 colonies per plate (37°C, 24 h).
Influence of antibacterial agents on growth of PPC-treated E. coli.
To assess the effects that incubation of PPC in the dark had on the MICs of some hydrophobic and hydrophilic antibacterial agents for E. coli, a modified method of Vaara and Vaara (34) was used. An overnight culture of E. coli grown in Davis minimal medium (DMM) (25) supplemented with 2 mg of glucose per ml, 1 mg of tryptone per ml, and 20 μg of l-tryptophan per ml was centrifuged (5,000 × g for 10 min) and was resuspended in the same medium to an absorbance (OD650) of 0.87, which corresponds to 3.2 × 108 CFU/ml. Four rows of 10 Eppendorf tubes containing final concentrations of 0, 1, 5, or 10 μg of PPC per ml in supplemented DMM were set up. Each row of tubes also contained increasing concentrations of the antibacterial agents to be tested in a final volume of 0.9 ml. A total of 100 μl of the E. coli suspension was then added (final number, 3.2 × 107 CFU/ml), and the suspension was vigorously shaken for 6 h at 37°C in the dark. To assess the amount of growth, the A650 was monitored by using a UV-visible light spectrophotometer. Initial experiments were carried out to determine the MIC of each antibacterial agent for untreated cells. The appropriate concentration range of antibacterial agent was then used for both PPC-treated and untreated cells.
Determination of octanol:buffer partition coefficients.
The octanol:buffer partition coefficients of the antibacterial compounds used to assess the changes in outer membrane permeability were determined by using a modified method of Nikaido (20). A total of 0.3 ml of the compound at a concentration of 300 μg/ml was added to 1.35 ml of 2-octanol-saturated 0.1 M phosphate buffer (pH 7.0) and 1.35 ml of 0.1 M phosphate buffer (pH 7.0)-saturated 2-octanol. After vortexing, the samples were centrifuged (2,000 × g for 10 min), and the concentration of the compound in each phase was determined spectrophotometrically by using a UV-visible light spectrophotometer. This was done by interpolation of a standard curve obtained with a known concentration of compound in both the organic and aqueous phases.
Effect of Mg2+ on uptake of PPC into E. coli cells.
One milliliter of a standard E. coli cell suspension was centrifuged (11,600 × g for 2 min), the supernatant was discarded, and the pellet was resuspended to an OD650 of 0.87 in 10 mM phosphate buffer (pH 7.0) (unless stated otherwise) supplemented with up to 50 mM of Mg2+. The phosphate buffer strength was reduced to eliminate the precipitation observed at higher concentrations after the addition of Mg2+. The suspension was incubated with 0, 1, 5, or 10 μg of PPC per ml for 30 min and was washed with 10 mM phosphate buffer supplemented with the appropriate Mg2+ concentration. Samples were taken after each wash to determine the amount of cell-bound phthalocyanine.
Fluorimetric estimation of cell-bound phthalocyanine.
The amount of cell-bound photosensitizer was determined by the fluorimetric approach of Bertoloni and coworkers (2). Samples were treated with 2% (wt/vol) aqueous sodium dodecyl sulfate (SDS) overnight, and the fluorescence emission was measured at 683 nm (excitation at 600 nm) with a Kontron SFM 25 spectrofluorimeter. The amount of phthalocyanine present was determined by interpolation from a standard curve of known concentrations in 2% (wt/vol) SDS.
Growth delay experiment.
E. coli cells were grown overnight in DMM supplemented with 2 mg of glucose per ml, 1 mg of tryptone per ml, and 20 μg of l-tryptophan per ml. Two milliliters of the culture was transferred to 25 ml of fresh minimal medium supplemented with 0, 10, 30, or 50 mM MgCl2, and the culture was grown at 37°C on an orbital shaker. At various times, 1-ml samples of the cultures were taken and the turbidity at 550 nm was measured. When the cultures reached the logarithmic phase the appropriate concentration of PPC was added and the flasks were irradiated as described earlier. The turbidity was then monitored for a further 300 min.
RESULTS
Uptake of 14C-PpIX into PPC-treated E. coli cells.
Experiments were performed to determine the percent uptake of 14C-PpIX into PPC-treated E. coli cells after washing up to three times with 0.1 M phosphate buffer. E. coli cells preincubated with 1, 5, or 10 μg of PPC per ml and then exposed to 1 μg of 14C-PpIX (30 nCi/μg of PpIX) per ml showed an increase in the uptake of 14C-PpIX compared to that for the untreated controls. For example, when determined after two washes, 3.3, 5.4, 16.5, and 32% of the added 14C-PpIX were bound to the cells in the presence of 0, 1, 5, and 10 μg of PPC per ml, respectively. Determination of uptake without washing or with one or three washes produced results with the same tendency. Analysis of the data by the Student t test showed that for E. coli cells preincubated with 5 or 10 μg of PPC per ml, the increased uptake of 14C-PpIX was highly significant (P < 0.001).
Further experiments were then performed to determine the effects that washing of PPC from the external buffer had on the uptake of 14C-PpIX. For example, if the cells were washed twice after exposure to 10 μg of PPC per ml, incubated with 14C-PpIX, and then washed once more, 49.1% of the added 14C-PpIX was still bound to the cells. If the cells were not washed prior to addition of the 14C-PpIX, 54.5% was still bound to the cells. Similar statistically insignificant behavior was observed without washing or with one or three washes. This indicated that PPC appeared to have an effect on the outer membrane permeability to 14C-PpIX that was not reversible upon removal of PPC from the external buffer.
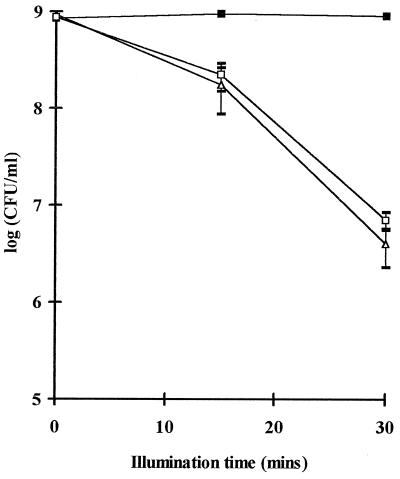
Since PPC caused an increase in the uptake of 14C-PpIX into E. coli cells, experiments were carried out to determine if this caused a subsequent increase in the amount of cell killing. PpIX itself is a photosensitizer, but it is not effective against gram-negative bacteria due to its inability to cross the outer membrane (15). The killing curves for E. coli cells treated with PpIX alone, PPC alone, and a mixture of both PPC and PpIX are shown in Fig. 2. Figure 2 illustrates that, as expected, PpIX alone had no cell-killing effect, whereas PPC alone had a significant effect. An increase in the illumination time for the cells incubated with 5 μg of PPC per ml caused an increase in the level of cell killing. In our system, after 30 min of illumination there was greater than 2 log10 of cell killing (i.e., greater than 99% of the E. coli cells were killed). However, Fig. 2 also illustrates that addition of both PPC and PpIX to the system does not increase the amount of cell killing over that achieved with PPC alone. Thus, PPC and PpIX do not work in a synergistic manner to enhance the killing of E. coli cells.
FIG. 2.
Killing of E. coli cells with PPC and PpIX. E. coli cells were incubated in the dark with 10 μg of PpIX per ml alone (■), 5 μg of PPC per ml alone (▵), or 5 μg of PPC per ml for 30 min and then 10 μg of PpIX per ml for a further 30 min (□). The cells were then illuminated for 0, 15, or 30 min and were then assayed for cell survival as described in Materials and Methods. Each point is the mean ± standard deviation of three experiments.
Effect of preincubation with PPC on outer membrane permeability of E. coli cells to antibacterial agents.
The MICs of various antibacterial agents for E. coli preincubated with 0 or 10 μg of PPC per ml are shown in Table 1. The MIC is defined as the lowest concentration of the antibacterial agent tested which resulted in no growth of the cells (34). The antibacterial agents tested have been placed into two groups depending on their relative hydrophobicities (as measured by the octanol:buffer partition coefficient) (20). Group I antibacterial agents are relatively hydrophobic compounds which have octanol:buffer partition coefficients greater than 0.13. Group II antibacterial agents are relatively hydrophilic in nature, having octanol:buffer partition coefficients lower than 0.03.
TABLE 1.
MICs of antibacterial agents for E. coli cells preincubated with various concentrations of PPCa
| Group and antibacterial agent | MIC (μg/ml) for E. coli preincubated with PPC at:
|
Fold increase in sensitivity | Partition coefficient | |
|---|---|---|---|---|
| 0 μg/ml | 10 μg/ml | |||
| Group I | ||||
| Cloxacillin | >2,000 | 750 | >2.7 | 0.13 |
| Erythromycin | >200 | 50 | >4 | 0.79b |
| Fusidic Acid | >50 | 12 | >4.1 | >20 |
| Novobiocin | >100 | 2 | >50 | >20 |
| Rifampin | >3 | 0.3 | >10 | 9.1 |
| Group II | ||||
| Ampicillin | 2 | 2 | 0 | <0.01 |
| Tetracycline | 3 | 5 | 0 | 0.03 |
The MICs of several antibacterial agents for E. coli cells preincubated with PPC were determined as described in Materials and Methods. The antibacterial agents were placed into two groups depending on their relative hydrophobicities (as measured by the octanol:buffer partition coefficient). The increase in sensitivity is fold increase in the MIC for cells preincubated with PPC at 0 μg/ml compared to that for cells preincubated with PPC at 10 μg/ml. The partition coefficients of the antibacterial agents were determined as described in Materials and Methods.
The partition coefficient was taken from reference 6.
Analysis of the data for the group I antibacterial agents showed that there was no effect on the MICs of the antibacterial agents for E. coli cells when the cells were preincubated with 1 μg of PPC per ml compared to the effect for control cells. However, preincubation with 5 μg of PPC per ml caused a decrease in the MICs of rifampin, novobiocin, erythromycin, and fusidic acid to 0.3, 32, 100, and 12 μg/ml, respectively. No decrease in the MIC of cloxacillin was observed with preincubation at this concentration of PPC. Preincubation with 10 μg of PPC per ml caused a decrease in the MICs of all five of these group I antibacterial agents (Table 1). The increase in the sensitivity of E. coli to these relatively hydrophobic group I antibacterial agents was in the range of at least 2.7 times for cloxacillin to greater than 50 times for novobiocin. Analysis of the data for the group II antibacterial agents showed that there was no decrease in the MICs for E. coli cells when the cells were treated with 1, 5, or 10 μg of PPC per ml compared to the effect for control cells. The data shown in Table 1 provide strong evidence that preincubation with PPC makes E. coli cells more sensitive to the relatively hydrophobic group I antibacterial agents but not to the relatively hydrophilic group II antibacterial agents.
Effect of Mg2+ on uptake of PPC into E. coli.
The uptake of PPC was then further studied by determining the effects that increasing Mg2+ concentrations had on PPC uptake into E. coli. For example, 56.9, 54.4, 37, 28.1, 20.8, and 16% of the added PPC was bound to the E. coli cells after one wash when the incubation was done in the presence of 0, 10, 20, 30, 40, and 50 mM Mg2+, respectively. Similar trends were seen when the cells were unwashed or when they were washed twice. These data illustrated that an increase in the external concentration of Mg2+ caused a decrease in the amount of PPC uptake by E. coli. Analysis of the amount of bound PPC on resuspension by the Student t test showed no significant decrease in uptake when 10 mM Mg2+ was present (P > 0.05). However, addition of 20 mM Mg2+ caused a statistically significant decrease in PPC uptake (P < 0.01). Furthermore, upon the addition of 30, 40, or 50 mM Mg2+, the decrease in uptake of PPC on resuspension becomes highly significant (P < 0.001).
Effects of Mg2+ on growth delay of E. coli treated with PPC.
The growth curves for E. coli in DMM supplemented with increasing concentrations of Mg2+ are shown in Fig. 3. At the point marked D, 10 μg of PPC per ml was added to the medium and the cells were illuminated in the light system described earlier. When no excess Mg2+ was added, growth (as measured by turbidity) stopped. Assaying for the numbers of CFU shows that this growth arrest occurs because PPC has a bactericidal action rather than a bacteriostatic action on the cells (18). However, Fig. 3 shows that the action of PPC upon growth delay can be affected if Mg2+ is added to the medium. Addition of 10 mM Mg2+ to the medium partially protects the cells from the bactericidal action of PPC. When 30 and 50 mM Mg2+ were added to the medium, this “protective” effect was more striking. At these concentrations of Mg2+ the no growth delay appeared to occur compared with that for the control cells.
FIG. 3.
Effect of Mg2+ on the growth delay of E. coli treated with PPC. Growth delay experiments were carried out with E. coli cells as described in Materials and Methods. The medium was supplemented with 0 mM Mg2+ (⧫), 10 mM Mg2+ (■), 30 mM Mg2+ (○), or 50 mM Mg2+ (□). The control contained 50 mM Mg2+ and no PPC (▵). D marks the time point at which 10 μg of PPC per ml was added to the appropriate cultures.
DISCUSSION
Alteration in outer membrane permeability barrier of E. coli cells treated with PPC.
The uptake experiments performed show that preincubation with PPC allows an increase in the uptake of 14C-PpIX. Nitzan et al. (27) reported that the use of the membrane-disorganizing agent PMBN, which formed a complex with the photosensitizer deuteroporphyrin, caused a change in outer membrane permeability that allowed greater uptake of the photosensitizer into E. coli cells. Further experiments revealed that removal of PPC which was not bound to E. coli cells, by washing, did not alter the amount of 14C-PpIX bound to the cells, indicating that the increased uptake of 14C-PpIX into E. coli cells was not due to the formation of a 14C-PpIX–PPC complex which was better able to permeate the outer membrane. Rather, the uptake of PPC has probably altered the outer membrane permeability barrier, allowing an increase in the uptake of 14C-PpIX. Even so, there was no enhanced cell-killing effect when both PPC and PpIX were used in combination (Fig. 2). This may indicate not only that the uptake of the photosensitizer into the cells is an important factor but also that other factors such as the actual subcellular localization or the rapid photobleaching of the PpIX may play a critical role in the efficacy of the cell-killing process (18).
Preincubation of E. coli cells with PPC in the dark also had marked effects on the sensitivities of the cells to various antibacterial agents (Table 1). E. coli cells which were treated with PPC became more sensitive to relatively hydrophobic antibacterial agents. This again suggests that PPC uptake has altered the outer membrane permeability barrier to these relatively hydrophobic antibacterial agents, causing an increase in their level of uptake and a subsequent decrease in the MICs of the antibacterial agents for the cells. In contrast, little difference was observed with relatively hydrophilic antibacterial agents (Table 1). Since ampicillin and tetracycline (as the magnesium complex) are thought to enter the cell through the porins (5), any compound which crosses the outer membrane via the self-promoted uptake pathway will have only slight effects upon the uptake of these compounds. Hancock and coworkers (7, 9–11, 13) have shown extensively that many cationic compounds cause an increase in the level of uptake of a hydrophobic fluorophore, 1-N-phenylnaphthylamine. Crystal violet uptake and the detergent action of SDS are also enhanced by cationic compounds which are thought to enter the cell by the self-promoted uptake pathway (12, 33, 35). In addition, Plesiat and Nikaido (28) have shown that deacylpolymyxin treatment, which perturbs the LPS structure, or the introduction of mutations that lead to the production of deep rough LPS caused a marked increase in the rates of permeation of some hydrophobic steroid probes.
Effects of Mg2+ and PPC on outer membrane permeability.
Consistent with the fact that PPC uptake occurs via the self-promoted uptake pathway are the effects observed on the uptake and subsequent action of PPC in the presence of Mg2+. Increasing concentrations of Mg2+ caused a reduction in the amount of PPC bound to E. coli. On the basis of this evidence the effects of Mg2+ on the growth delay of E. coli treated with PPC (Fig. 3) can be explained. Since previous work revealed that it is the cell-bound PPC which is involved in the cell-killing process, the addition of Mg2+ to the medium caused the observed “protective” effect by reducing the amount of the important cell-bound PPC (18). This protective effect also prevents the killing and alterations in outer membrane permeabilities of cells treated with many compounds, including polymyxin B, and causes increases in the MICs of some deglucoteicoplanin amide derivatives (11, 13, 31). The concentration of Mg2+ required to antagonize the action of PPC appears to be slightly high at 10 to 20 mM, but there is no obvious explanation for this.
Uptake of PPC across the outer membrane of E. coli.
The self-promoted uptake pathway mechanism would involve PPC interacting at the sites at which Mg2+ cross-links adjacent LPS molecules. PPC may displace Mg2+ from the binding site by a direct competition. The uptake of the compound could then be similar to that seen with EDTA, in which removal of Mg2+ leads to an increase in the electrostatic repulsion between adjacent LPS molecules, resulting in the release of LPS into the external medium. The void left by the release of LPS could be filled by phospholipids found in the inner leaflet of the outer membrane or from the plasma membrane (31, 35). This phospholipid bilayer would then allow the observed increase in the outer membrane permeability to relatively hydrophobic compounds as well as the phthalocyanine itself. Alternatively, due to the large size of the phthalocyanine, interaction at the Mg2+ binding site could cause a distortion of the outer membrane. As a result of alterations in the interactions between adjacent LPS molecules, the outer membrane would be less ordered, causing an increase in the outer membrane permeability to hydrophobic compounds as well as PPC itself (15, 31).
It should be noted that the nature as well as the concentration of proteins in various bacteriological culture media also has an influence on the level of photosensitization by various photosensitizers. For example, the gram-negative bacterium Acinetobacter baumannii was sensitive to a cationic porphyrin only in a low-protein-concentration nutrient broth (25). This appeared to be caused by the protein capture of a portion of the porphyrin, making it unavailable for interaction with the bacterial cell membranes. Furthermore, the use of PMBN did not influence the level of photosensitization of A. baumannii or E. coli by the same cationic porphyrin (25, 26). In contrast, PMBN does increase the level of photosensitization of deuteroporphyrin to E. coli in such media (15, 27). This may further influence the potential applications of bacterial photosensitization in vitro and in vivo.
Development of resistance to photosensitization.
Photosensitization involves the generation of reactive oxygen species, such as singlet oxygen or hydroxyl radicals, which damage cellular components, leading to cell death. Whether bacteria could develop resistance to these active oxygen species is still under debate (19). However, development of resistance to photosensitization could occur via a different mechanism. One such mechanism could be that the bacteria could reduce or prevent the uptake of the photosensitizer across the outer membrane and hence reduce subsequent cell killing. Indeed, that is what has hampered the progress of bacterial photosensitization as a general means of antisepsis for many years (15). Gram-negative bacteria are resistant to bacterial photosensitization with anionic or neutral porphyrin and phthalocyanine photosensitizers because the outer membrane prevents the uptake of these compounds into the cell. Only with the advent of cationic phthalocyanine and porphyrin photosensitizers has this resistance been overcome (16–18).
This then begs the question of whether the outer membrane of gram-negative bacteria could reduce or prevent the uptake of compounds such as cationic photosensitizers which enter the cell via the self-promoted uptake pathway. Makela et al. (14) isolated a strain of Salmonella enterica serovar Typhimurium which had low-level resistance to polycationic compounds. The LPS of this strain had an overall reduced net negative charge compared with that of the wild-type LPS, which may explain the decreased amount of polymyxin B binding to the mutant LPS (37). In addition, Roland et al. (29) isolated a gene from S. enterica serovar Typhimurium which encoded a protein that conferred resistance to polymyxin B and which is a component of the pathway that leads to the modification of lipid A in the outer membrane. If such bacterial strains show a general increased resistance to compounds which cross the outer membrane via the self-promoted uptake pathway, they may well have a higher threshold of resistance to the cell-killing activity of PPC photosensitization. Furthermore, some bacterial strains already have a high level of intrinsic resistance to such compounds. Differences in the LPS phosphate and sugar contents of species such as Burkholderia cepacia (Pseudomonas cepcia) and Proteus mirabilis lead to decreases in the affinities and subsequent activities of some polycations (10, 31). This intrinsic resistance may well also reduce the effective activity of photosensitization with cationic photosensitizers against these bacteria.
ACKNOWLEDGMENT
We thank Yorkshire Cancer Research for financial support.
Footnotes
Present address: Department of Pharmacology, University of Cambridge, Cambridge CB2 1QJ, United Kingdom.
REFERENCES
- 1.Bachmann B J. Linkage map of Escherichia coli K-12, edition 8. Microbiol Rev. 1990;54:130–197. doi: 10.1128/mr.54.2.130-197.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bertoloni G, Rossi F, Valduga G, Jori G, Ali H, van Lier J E. Photosensitising activity of water- and lipid-soluble phthalocyanines on prokaryotic and eukaryotic microbial cells. Microbios. 1992;71:33–46. [PubMed] [Google Scholar]
- 3.Bertoloni G, Sacchetto R, Jori G, Vernon D I, Brown S B. Protoporphyrin photosensitisation of Enterococcus hirae and Candida albicans cells. Lasers Life Sci. 1993;5:267–275. [Google Scholar]
- 4.Bertoloni G, Salvato B, Dall' Acqua M, Vazzoler M, Jori G. Hematoporphyrin-sensitised photoinactivation of Streptococcus faecalis. Photochem Photobiol. 1984;39:811–816. doi: 10.1111/j.1751-1097.1984.tb08864.x. [DOI] [PubMed] [Google Scholar]
- 5.Chopra I, Hawkey P M, Minton M. Tetracyclines, molecular and clinical aspects. J Antimicrob Chemother. 1992;29:245–277. doi: 10.1093/jac/29.3.245. [DOI] [PubMed] [Google Scholar]
- 6.Coleman W C, Leive L. Two mutations which affect the barrier function of the Escherichia coli K-12 outer membrane. J Bacteriol. 1979;115:899–910. doi: 10.1128/jb.139.3.899-910.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Falla T J, Karunaratne D N, Hancock R E W. Mode of action of the antimicrobial peptide indolicin. J Biol Chem. 1996;271:19298–19303. doi: 10.1074/jbc.271.32.19298. [DOI] [PubMed] [Google Scholar]
- 8.Griffiths J, Schofield J, Wainwright M, Brown S B. Some observations on the synthesis of polysubstituted zinc phthalocyanine sensitisers for photodynamic therapy. Dyes Pigments. 1997;33:65–78. [Google Scholar]
- 9.Hancock R E W. Alterations in outer membrane permeability. Annu Rev Microbiol. 1984;38:237–264. doi: 10.1146/annurev.mi.38.100184.001321. [DOI] [PubMed] [Google Scholar]
- 10.Hancock R E W. The bacterial outer membrane as a drug barrier. Trends Microbiol. 1997;5:37–42. doi: 10.1016/S0966-842X(97)81773-8. [DOI] [PubMed] [Google Scholar]
- 11.Hancock R E W, Farmer S W. Mechanism of uptake of deglucoteicoplanin amide derivatives across outer membranes of Escherichia coli and Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1993;37:453–456. doi: 10.1128/aac.37.3.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Helander I M, Alakomi H-L, Latva-Kala K, Koski P. Polyethyleneimine is an effective permeabilizer of gram-negative bacteria. Microbiology. 1997;143:3193–3199. doi: 10.1099/00221287-143-10-3193. [DOI] [PubMed] [Google Scholar]
- 13.Loh B, Grant C, Hancock R E W. Use of the fluorescent probe 1-N-phenylnapthylamine to study the interactions of aminoglycoside antibiotics with the outer membrane of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1984;26:546–551. doi: 10.1128/aac.26.4.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Makela P H, Sarvas M, Calcagno S, Lounatmaa K. Isolation and characterization of polymyxin resistant mutants of Salmonella. FEMS Microbiol Lett. 1978;3:323–326. [Google Scholar]
- 15.Malik Z, Ladan H, Nitzan Y. Photodynamic inactivation of gram negative bacteria: problems and possible solutions. J Photochem Photobiol B Biol. 1992;14:262–265. doi: 10.1016/1011-1344(92)85104-3. [DOI] [PubMed] [Google Scholar]
- 16.Merchat M, Bertoloni G, Giacomoni P, Villanueva A, Jori G. Meso-substituted cationic porphyrins as efficient photosensitisers of gram-positive and gram-negative bacteria. J Photochem Photobiol B Biol. 1996;32:153–157. doi: 10.1016/1011-1344(95)07147-4. [DOI] [PubMed] [Google Scholar]
- 17.Merchat M, Spikes J D, Bertoloni G, Jori G. Studies on the mechanism of bacteria photosensitization by meso-substituted cationic porphyrins. J Photochem Photobiol B Biol. 1996;35:149–157. doi: 10.1016/s1011-1344(96)07321-6. [DOI] [PubMed] [Google Scholar]
- 18.Minnock A, Vernon D I, Schofield J, Griffiths J, Parish J H, Brown S B. Photoinactivation of bacteria. Use of a cationic water-soluble zinc phthalocyanine to photoinactivate both gram-positive and gram-negative bacteria. J Photochem Photobiol B Biol. 1996;32:159–164. doi: 10.1016/1011-1344(95)07148-2. [DOI] [PubMed] [Google Scholar]
- 19.Moore P, Crystall B. Lethal weapon. New Scientist. 1998;158(2130):40–43. [Google Scholar]
- 20.Nikaido H. Outer membrane of Salmonella typhimurium. Transmembrane diffusion of some hydrophobic substances. Biochim Biophys Acta. 1976;433:118–132. doi: 10.1016/0005-2736(76)90182-6. [DOI] [PubMed] [Google Scholar]
- 21.Nikaido H. Multidrug efflux pumps of gram-negative bacteria. J Bacteriol. 1996;178:5853–5859. doi: 10.1128/jb.178.20.5853-5859.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nikaido H, Basina M, Nguyen V, Rosenberg E Y. Multidrug efflux pump AcrAB of Salmonella typhimurium excretes only those β-lactam antibiotics containing lipophilic side chains. J Bacteriol. 1998;180:4686–4692. doi: 10.1128/jb.180.17.4686-4692.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nikaido H, Nakae T. The outer membrane of gram-negative bacteria. Adv Microb Physiol. 1979;20:163–250. doi: 10.1016/s0065-2911(08)60208-8. [DOI] [PubMed] [Google Scholar]
- 24.Nikaido H, Vaara M. Molecular basis of bacterial outer membrane permeability. Microbiol Rev. 1985;49:1–32. doi: 10.1128/mr.49.1.1-32.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nitzan Y, Balzam-Sudakevitz A, Ashkenazi H. Eradication of Acinetobacter baumannii by photosensitized agents in vitro. J Photochem Photobiol B Biol. 1998;42:211–218. doi: 10.1016/s1011-1344(98)00073-6. [DOI] [PubMed] [Google Scholar]
- 26.Nitzan Y, Dror R, Ladan H, Malik Z, Kimel S, Gottfried V. Structure-activity relationship of porphines for photoinactivation of bacteria. Photochem Photobiol. 1995;62:342–347. doi: 10.1111/j.1751-1097.1995.tb05279.x. [DOI] [PubMed] [Google Scholar]
- 27.Nitzan Y, Gutterman M, Malik Z, Ehrenberg B. Inactivation of gram-negative bacteria by photosensitised porphyrins. Photochem Photobiol. 1992;55:89–96. doi: 10.1111/j.1751-1097.1992.tb04213.x. [DOI] [PubMed] [Google Scholar]
- 28.Plesiat P, Nikaido H. Outer membranes of gram-negative bacteria are permeable to steroid probes. Mol Microbiol. 1992;6:1323–1333. doi: 10.1111/j.1365-2958.1992.tb00853.x. [DOI] [PubMed] [Google Scholar]
- 29.Roland K L, Esther C R, Spitznagel J K. Isolation and characterization of a gene, pmrD, from Salmonella typhimurium that confers resistance to polymyxin when expressed in multiple copies. J Bacteriol. 1994;176:3589–3597. doi: 10.1128/jb.176.12.3589-3597.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1982. [Google Scholar]
- 31.Vaara M. Agents that increase the permeability of the outer membrane. Microbiol Rev. 1992;56:395–411. doi: 10.1128/mr.56.3.395-411.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vaara M. Increased outer membrane resistance to ethylenediaminetetraacetate and cations in novel lipid A mutants. J Bacteriol. 1981;148:426–434. doi: 10.1128/jb.148.2.426-434.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vaara M, Vaara T. Outer membrane permeability barrier disruption by polymyxin in polymyxin-susceptible and polymyxin-resistant Salmonella typhimurium. Antimicrob Agents Chemother. 1981;19:578–583. doi: 10.1128/aac.19.4.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vaara M, Vaara T. Sensitisation of gram-negative bacteria to antibiotics and complement by a nontoxic oligopeptide. Nature. 1983;303:526–528. doi: 10.1038/303526a0. [DOI] [PubMed] [Google Scholar]
- 35.Vaara M, Vaara T. Polycations as outer membrane-disorganizing agents. Antimicrob Agents Chemother. 1983;24:114–122. doi: 10.1128/aac.24.1.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vaara M, Vaara T. Polycations sensitize enteric bacteria to antibiotics. Antimicrob Agents Chemother. 1983;24:107–113. doi: 10.1128/aac.24.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vaara M, Vaara T, Jensen M, Helander I, Nurminen M, Rietshel E T, Makela P H. Characterization of the lipopolysaccharide from the polymyxin-resistant pmrA mutants of Salmonella typhimurium. FEBS Lett. 1981;129:145–149. doi: 10.1016/0014-5793(81)80777-6. [DOI] [PubMed] [Google Scholar]
- 38.Vernon D I, Brown S B. The preparation of radiolabelled porphyrins and their use in studies of photodynamic therapy. Photochem Photobiol. 1987;45:581–586. doi: 10.1111/j.1751-1097.1987.tb04817.x. [DOI] [PubMed] [Google Scholar]