Abstract
Objectives:
To assess efficacy and safety of the new Dextroamphetamine Transdermal System (d-ATS) to treat children and adolescents (aged 6–17 years) with attention-deficit/hyperactivity disorder (ADHD).
Methods:
In this phase 2, randomized, placebo-controlled study, 4 d-ATS patches of differing doses (5, 10, 15, and 20 mg) were evaluated. Patients began a 5-week, open-label, stepwise dose-optimization period in which they received a 5-mg d-ATS patch (applied to hip) for 9 hours. During weekly visits, patients were evaluated for possible adjustments to the next dose level based on efficacy and safety. Once at the optimal dose, that dose was maintained during a 2-week, crossover double-blind treatment period. Primary endpoint was to assess efficacy of d-ATS versus placebo as measured by Swanson, Kotkin, Agler, M-Flynn, and Pelham Scale (SKAMP) total score; key secondary endpoints included assessing onset and duration of efficacy by SKAMP total score, and additional secondary endpoints included Permanent Product Measure of Performance (PERMP) scores. Safety was assessed throughout.
Results:
d-ATS treatment resulted in significant improvements versus placebo in ADHD symptoms as measured by SKAMP total score, with overall least-squares mean difference (95% confidence interval) versus placebo of −5.87 (6.76, −4.97; p < 0.001) over the 12-hour assessment period. Onset of efficacy was observed at 2 hours postdose (p < 0.001), and duration of effect continued through 12 hours (patch removed at 9 hours), with significant differences between d-ATS and placebo at all time points from 2 hours onward (all p ≤ 0.003). Significant improvements versus placebo in PERMP-A and PERMP-C scores were also observed from 2 to 12 hours postdose with d-ATS treatment. d-ATS was safe and well-tolerated, with a systemic safety profile similar to that observed with oral amphetamines.
Conclusions:
This study demonstrates that d-ATS is an effective and well-tolerated treatment for children and adolescents with ADHD. These data indicate that d-ATS can deliver sustained levels of efficacy along with the advantages of transdermal drug delivery, making it a beneficial new treatment option.
Clinical Trial Registration no.: NCT01711021.
Keywords: ADHD, dextroamphetamine, transdermal, clinical trial
Introduction
Attention-deficit/hyperactivity disorder (ADHD) is a neurodevelopmental disorder characterized by a persistent pattern of inattention and/or hyperactivity-impulsivity that interferes with function or development and negatively impacts social and academic activities (American Psychiatric Association 2013; Bélanger et al. 2018). ADHD is one of the most common mental health disorders of childhood and has been diagnosed in ∼7.2% of children and adolescents worldwide aged 18 years and younger (Polanczyk et al. 2007; Thomas et al. 2015). Although the exact level of persistence of ADHD into adulthood remains undetermined, it is clear that the majority of people diagnosed with ADHD in childhood have continued impairments as adults (Caye et al. 2016).
As many as one-third to two-thirds of children with ADHD have ≥1 coexisting condition(s), such as learning disabilities, oppositional defiant disorder, conduct disorder, anxiety disorder, mood disorders, tics, and sleep disorders (Green et al. 1999; Pliszka 2003; Barbaresi et al. 2020). ADHD-related symptoms have significant negative effects on quality of life and general functioning, affecting various psychosocial and achievement domains, including issues with academics, emotional volatility, dissatisfaction with family life, and increased risk of drug and alcohol abuse (Danckaerts et al. 2010; Mattingly et al. 2017). The disorder has been associated with a 30%–140% increased risk of morbidity and an 85%–110% increase in mortality; thus, it is critical to treat patients as quickly as possible after diagnosis to mitigate negative outcomes (Dalsgaard et al. 2015a, 2015b; London and Landes 2016; Chien et al. 2017).
Recommended first-line treatments for children and adolescents with ADHD include pharmacotherapy with stimulants such as methylphenidate and amphetamines (Huss et al. 2017; Mattingly et al. 2017; Wolraich et al. 2019). Stimulants are highly effective in reducing ADHD symptoms in elementary school children and adolescents. However, rates of response to methylphenidate versus amphetamines are idiosyncratic, with ∼40% of patients responding to either drug and ∼40% responding to only one of the two (Wolraich et al. 2019). Thus, there is a need to provide additional treatment options, including various formulations of each of these medications, for patients with ADHD.
The use of transdermal treatments for nervous system disorders has grown significantly, as numerous advantages exist for administering medications through transdermal formulations (Findling and Dinh 2014). Transdermal delivery can improve adherence to treatment for various reasons. Caregivers and patients can confirm administration of medication by visually observing the patch, transdermal formulations often reduce dosing frequency, and patch removal enables secure termination of drug delivery, thus offering the potential to tailor the duration of therapy to an individual patient's needs (Citrome et al. 2019). Transdermal drugs allow the delivery of medication without swallowing of tablets, which can be a hurdle for some children and adolescents (Findling and Dinh 2014; Daytrana Prescribing Information 2019). Additionally, the transdermal method of delivery minimizes first-pass metabolism as well as potential hepatic side effects and may reduce the risk of drug–drug interactions, gastrointestinal effects, and abuse liability.
At present, there is only one FDA-approved transdermal treatment for ADHD in children and adolescents, a methylphenidate patch (Findling and Dinh 2014; Daytrana Prescribing Information 2019). The need for an amphetamine transdermal treatment option for ADHD exists for those patients who respond better to amphetamines or need to transition from methylphenidate to amphetamine treatment (Newcorn et al. 2017).
Dextroamphetamine is commonly used to treat ADHD in children and adults and is available as immediate-release, sustained-release, and extended-release oral medications. The novel Dextroamphetamine Transdermal System (d-ATS) was designed as an alternative to oral extended-release amphetamines and as a method to provide sustained levels of dextroamphetamine throughout the day. This study was conducted to assess the efficacy and safety of d-ATS in the treatment of children and adolescents 6–17 years of age with ADHD in a randomized laboratory classroom study.
Methods
Study design
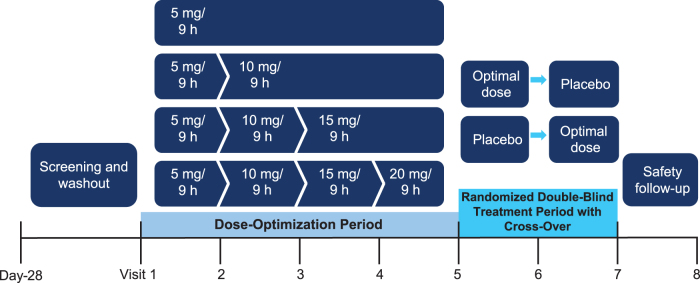
This was a phase 2, randomized, double-blind, crossover, placebo-controlled study conducted in two periods (Fig. 1). This study was approved by the Western Institutional Review Board and the UC Irvine Institutional Review Board. Four d-ATS patches, delivering a range of dextroamphetamine doses (5 mg/4.76 cm2, 10 mg/9.52 cm2, 15 mg/14.29 cm2, and 20 mg/19.05 cm2), were evaluated. Eligible patients were enrolled in a 5-week, open-label, stepwise dose-optimization period. All patients started the dose-optimization period with daily administration of a 5 mg d-ATS patch applied to the hip for 9 hours on alternating sides. Patients returned for weekly clinic visits to be evaluated for possible adjustments to the next dose level based on efficacy and safety—if unacceptable tolerability was observed, one dose reduction was permitted through Visit 4.
FIG. 1.
Study design. Visit numbers also correspond to week number.
Once the optimal dose was reached, it was maintained throughout the dose-optimization period. Patients who achieved dose optimization then entered a 2-week, crossover, double-blind treatment period. Patients were randomized to either double-blind treatment with the optimal dose of d-ATS or placebo, and patients crossed over to the other treatment sequence after 1 week.
Patients
Key inclusion criteria
Male and female patients 6–17 years of age with a DSM-IV-TR primary diagnosis of ADHD combined, hyperactive/impulsive subtype, or predominately inattentive subtype were included (American Psychiatric Association 2000). Patients were required to have a screening and baseline visit Attention-Deficit/Hyperactivity Disorder Rating Scale IV (ADHD-RS-IV) score ≥90% of the general population of children by age and gender. Patients had to be able to wear the transdermal formulation for 9 hours with caregivers present to apply and remove the patch as necessary. An intelligence quotient score ≥80 and the ability to complete the Permanent Product Measure of Performance (PERMP) assessment were also required (Wigal 2019).
Key exclusion criteria
Patients were excluded if they were currently taking an ADHD medication that provided adequate symptom control or were a known nonresponder to amphetamine treatment. If patients had a documented allergy, intolerance, or hypersensitivity to amphetamine or a recent history (≤6 months from screening) of suspected substance abuse or dependence, they were also excluded. In addition, patients with any psychiatric disorder that could interfere with study participation or with their own safety or that of other participants, such as conduct disorder or oppositional defiant disorder with a history of prominent aggressive outbursts, were excluded.
Objectives
The primary efficacy objective was to assess the efficacy of d-ATS versus placebo as measured by the Swanson, Kotkin, Agler, M-Flynn, and Pelham Scale (SKAMP) total score (Wigal et al. 1998). Key secondary endpoints included assessing the onset and duration of efficacy of d-ATS versus placebo using the SKAMP total score. Additional efficacy assessments included the PERMP score (Wigal and Wigal 2006). Safety was assessed throughout.
Assessments
SKAMP assessments were conducted on days 35, 42, and 49; PERMP assessments were performed at baseline and on days 7, 14, 21, 28, 35, 42, and 49. Application-site inspections were conducted on every study day, and patch adhesion was assessed on days 7, 14, 21, 28, 35, 42, and 49. Assessments of irritation, discomfort, and adhesive residue were conducted by investigators on days 7, 14, 21, 28, 35, 42, and 49 and by caregivers/patients on all other days of the study. Safety was assessed through treatment-emergent adverse events (TEAEs), ECGs, vital signs, labs, and assessments of dermal safety.
Statistical analyses
Primary efficacy analysis was performed for SKAMP total scores on the Full Analysis Set (FAS), which included all randomized patients who received at least one dose of study medication. A mixed model for repeated measures (MMRM) was used to assess efficacy in the FAS, with evaluation of carryover effect. To preserve the type I error rate in analyzing the secondary endpoints of onset and duration of efficacy, a closed-test procedure was used. The closed-test procedure indicated that the order of testing endpoints mattered, and if the primary efficacy endpoint was statistically significant (p < 0.05), then the secondary variables of onset and duration of efficacy were tested.
The onset of efficacy, based on the SKAMP total scores, was defined as the first assessment time showing a statistically significant difference between d-ATS and placebo. If no significant difference was found at any time point for the onset of efficacy, no onset of efficacy was deemed to have occurred, and the onset of efficacy was defined as “none.” Continuous summary statistics were presented for the SKAMP total score at each time point for Visits 6 and 7. The duration of efficacy, based on the SKAMP total scores, was defined as the time point at which there was a nonsignificant difference between the two treatment groups after a time point in which there was a significant difference between the two treatment groups.
Continuous summary statistics were presented for PERMP scores, and the number of items attempted and number of items correct at each time point after dosing for Visits 6 and 7 were analyzed in the same way as the SKAMP primary analysis. Safety outcomes were analyzed for the Safety Population, which included all patients who took ≥1 dose of study medication and had ≥1 postdose safety measurement (including dermal safety).
Modeling and simulation
Similar to other stimulants, pharmacodynamic (PD) effects of amphetamines are tightly linked to their pharmacokinetic (PK) profile (Center for Drug Evaluation and Research 2019). To investigate the d-ATS exposure-response relationship, a post hoc analysis was conducted in which a series of population PD and PK/PD analyses were performed using an established PK model built with d-ATS phase 1 PK data and d-ATS efficacy data (SKAMP scores) from this efficacy trial and a previous PK/PD study (data on file).
The objectives of these analyses were to describe the d-ATS exposure–response relationship with SKAMP scores as the efficacy measure and to characterize the onset and duration of effect in response to d-ATS application. Simulations examining the effect of early patch removal (4 to <9 hours postapplication) on efficacy were also performed. Patch removal simulations were conducted under several assumptions. The nonlinear absorption of dextroamphetamine necessitated the use of a parameter “βremove” in simulations to account for possible effects of a skin depot on PK. When βremove was set to zero, it represented no effect, whereas higher nonzero values represented a larger depot effect and, thus, stronger impacts on systemic exposure upon patch removal.
Results
Patient disposition, baseline demographics, and clinical characteristics
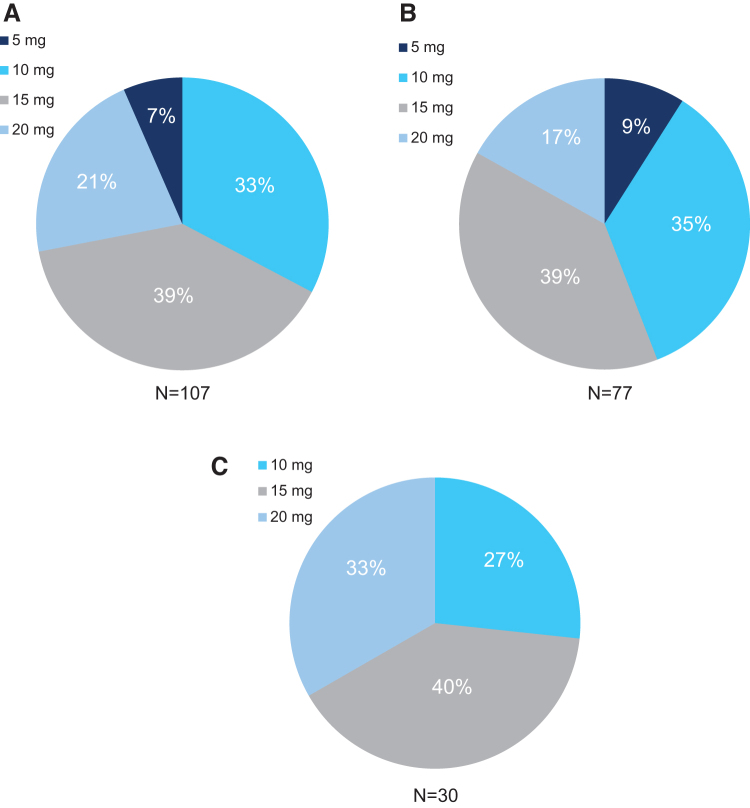
A total of 110 patients were enrolled in the dose-optimization phase, and 4 patients discontinued during this phase (n = 3, owing to adverse events and n = 1, owing to withdrawal of consent). Baseline weight, height, and body mass index were similar across treatment sequence groups, and most patients were men and white (Table 1). Optimized doses were achieved for n = 107, all of whom were randomized into the double-blind treatment period. The number (%) of patients optimized to each d-ATS dose was 7 (6.5) for 5 mg/9 hours, 35 (32.7) for 10 mg/9 hours, 42 (39.3) for 15 mg/9 hours, and 23 (21.5) for 20 mg/9 hours (Fig. 2). One patient withdrew consent after having achieved their optimal dose; thus, 106 patients entered the double-blind treatment period.
Table 1.
Demographics and Baseline Characteristics
| Safety population (N = 110) | |
|---|---|
| Age, years, mean (SD) | 10.5 (3.1) |
| 6–12 years, n (%) | 80 (72.7) |
| 13–17 years, n (%) | 30 (27.3) |
| Male, n (%) | 76 (69.1) |
| BMI, kg/m2, mean (SD) | 18.7 (3.4) |
| ADHD-RS-IV total score, mean (SD) | 38.3 (8.6) |
ADHD-RS-IV, Attention-Deficit/Hyperactivity Disorder Rating Scale IV; BMI, body mass index; SD, standard deviation.
FIG. 2.
Optimized d-ATS doses for (A) all randomized patients, (B) those aged 6–12 years, and (C) those aged 13–17 years (dose-optimization period). d-ATS, dextroamphetamine transdermal system.
Efficacy
SKAMP total scores
Treatment with d-ATS resulted in significant improvements versus placebo in ADHD symptoms, as measured by SKAMP total score, with an overall least-squares (LS) mean difference (95% confidence interval [CI]) for d-ATS over placebo of −5.87 (6.76 to −4.97; p < 0.001). Because a significant carryover/sequence effect was detected (p = 0.009), a permutation test was performed on data from Visit 6 to validate the results of the treatment effect. The permutation test was significant (p = 0.008), confirming a treatment effect (mean SKAMP total score was significantly lower in the d-ATS group vs. placebo). Sensitivity analyses were also conducted for the primary efficacy assessment to explore the effect of missing data, and results supported the conclusions from the main analyses. Thus, treatment with d-ATS resulted in significant improvements in SKAMP total score compared with placebo.
Onset, duration, and effects of early patch removal
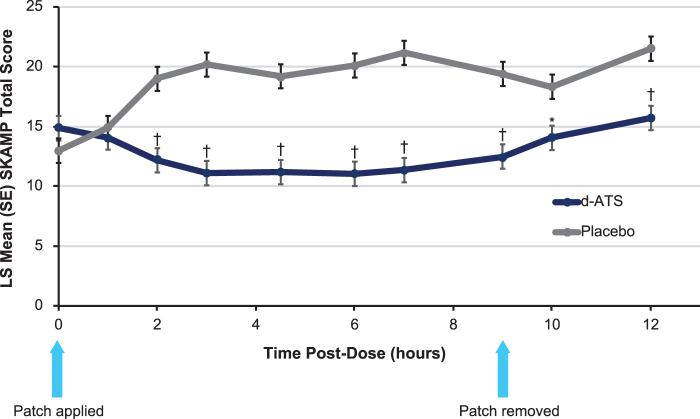
Analyses of Visits 6 and 7 indicated that the initial onset of efficacy of d-ATS was observed at 2 hours postdose (p < 0.001), where mean (SD) SKAMP total scores were 12.2 (8.4) and 19.0 (12.2) for d-ATS and placebo, respectively (Fig. 3). The LS mean difference (95% CI) between d-ATS and placebo for these visits was −6.8 (−9.6 to −4.0; p < 0.001). There was a significant difference between d-ATS and placebo at all time points after 2 hours (p ≤ 0.003).
FIG. 3.
Time course of SKAMP total scores during laboratory classroom assessment (full analysis set). *p < 0.05; †p < 0.001. CI, confidence interval; LS, least-squares; SE, standard error; SKAMP, Swanson, Kotkin, Agler, M-Flynn, and Pelham Scale.
Analyses of duration of effect indicated a significant effect with d-ATS over placebo from 2 hours postdose through all time points up to 12 hours. Mean (SD) SKAMP total scores from 2 hours postdose through 12 hours were 15.7 (11.8) for d-ATS and 21.5 (11.6) for placebo, with an LS mean difference (95% CI) of −5.8 (−8.6 to −3.0; p < 0.001).
PERMP scores
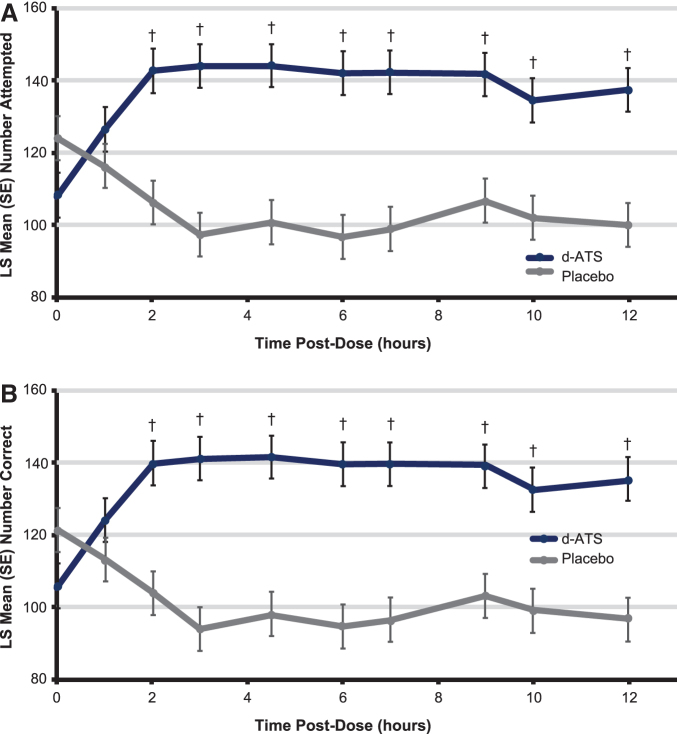
Significantly greater improvements in PERMP-A score with d-ATS treatment versus placebo were observed from 2 hours postdose onward (Fig. 4A and Table 2). Similarly, from the 2-hour time point onward, treatment with d-ATS resulted in significantly greater improvements in PERMP-C compared with placebo (Fig. 4B and Table 2). For both the PERMP number attempted and number correct, significant improvements with d-ATS over placebo were sustained up to 12 hours after patch application (patch removed at 9 hours).
FIG. 4.
PERMP (A) number of problems attempted by time and (B) number of problems correct by time during the double-blind treatment period (full analysis set). †p < 0.001. LS, least squares; PERMP, Permanent Product Measure of Performance; SE, standard error.
Table 2.
Change in Predose Permanent Product Measure of Performance Scores by Time Point for Double-Blind Phase (Full Analysis Set)
| Time postdose (hours) | Change from predose score, mean (SD) |
LS mean differencea | 95% CI | p | |
|---|---|---|---|---|---|
| d-ATS (N = 106) | Placebo (N = 106) | ||||
| No. attempted | |||||
| 1 | 17.5 (34.8) | −9.2 (33.0) | 10.3 | −6.6 to 27.2 | 0.231 |
| 2 | 34.4 (42.2) | −19.5 (38.0) | 36.4 | 19.5 to 53.2 | <0.001 |
| 3 | 35.7 (44.8) | −28.2 (43.7) | 46.6 | 29.7 to 63.4 | <0.001 |
| 4.5 | 35.6 (43.0) | −25.2 (37.9) | 43.1 | 26.2 to 59.9 | <0.001 |
| 6 | 33.5 (43.4) | −29.0 (47.0) | 45.2 | 28.4 to 62.1 | <0.001 |
| 7 | 34.1 (45.3) | −27.0 (39.3) | 43.5 | 26.6 to 60.4 | <0.001 |
| 9 | 32.2 (46.3) | −18.8 (36.4) | 34.9 | 18.0 to 51.8 | <0.001 |
| 10 | 24.9 (52.6) | −23.7 (43.8) | 32.5 | 15.6 to 49.4 | <0.001 |
| 12 | 28.0 (48.6) | −25.7 (43.4) | 37.3 | 20.4 to 54.2 | <0.001 |
| No. correct | |||||
| 1 | 17.3 (34.2) | −9.4 (32.7) | 10.9 | −5.9 to 27.7 | 0.204 |
| 2 | 33.9 (42.0) | −19.0 (37.5) | 36.0 | 19.2 to 52.8 | <0.001 |
| 3 | 35.2 (44.9) | −29.0 (40.8) | 47.5 | 30.8 to 64.3 | <0.001 |
| 4.5 | 35.5 (42.9) | −25.3 (37.8) | 43.6 | 26.8 to 60.3 | <0.001 |
| 6 | 33.6 (42.8) | −28.5 (46.1) | 45.4 | 28.6 to 62.2 | <0.001 |
| 7 | 33.7 (45.2) | −26.9 (38.9) | 43.5 | 26.7 to 60.4 | <0.001 |
| 9 | 31.8 (45.6) | −19.7 (35.0) | 36.1 | 19.2 to 52.9 | <0.001 |
| 10 | 25.0 (51.0) | −24.0 (42.1) | 33.5 | 16.6 to 50.3 | <0.001 |
| 12 | 28.2 (47.4) | −26.5 (42.1) | 38.9 | 22.0 to 55.7 | <0.001 |
Difference does not equal to that of the mean for d-ATS versus placebo, as those are mean values rather than LS mean values.
CI, confidence interval; d-ATS, dextroamphetamine transdermal system; LS, least squares.
PK/PD modeling and simulation results
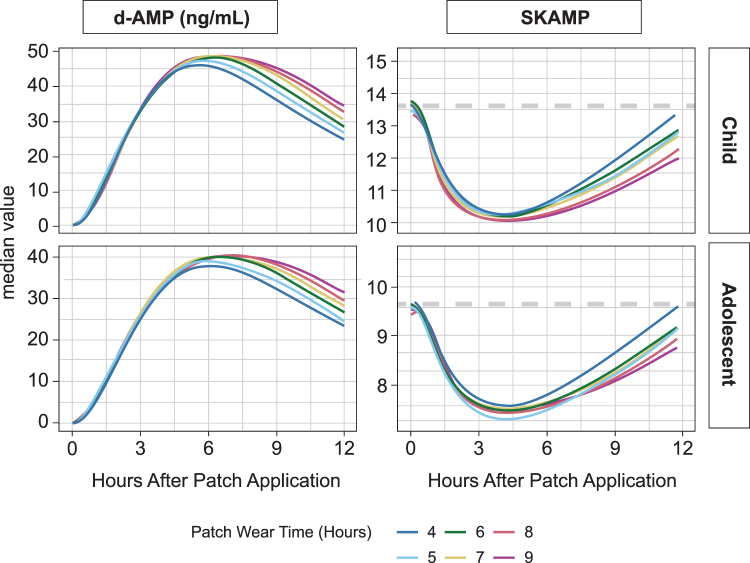
Overall, modeling results indicated that with a 9-hour wear time of d-ATS, SKAMP scores declined and subsequently increased back toward baseline values over a 12-hour window, consistent with the known Emax relationship with amphetamine concentrations (Fig. 5). The initial decline in SKAMP scores was observed around 2 hours after patch application, with a maximum decline occurring at around 4 hours after patch application.
FIG. 5.
Patch wear time simulation: median PK and SKAMP score over time in children and adolescents. PK, pharmacokinetic; SKAMP, Swanson, Kotkin, Agler, M-Flynn, and Pelham Scale.
Under the several skin depot assumptions investigated, the model-based SKAMP scores in simulated children and adolescents returned to within 90% of the baseline value in 41% to 53% of patients by 12 hours, in 48% to 71% of patients by 14 hours, and in 57% to 88% of patients by 16 hours when d-ATS was removed 9 hours after application. Earlier patch removal times were associated with reduced systemic exposure and earlier return to baseline SKAMP values across the various skin depot effect assumptions. Figure 4 shows patch wear time simulations with patch assumption βremove = 1, as these simulations most closely matched the SKAMP score changes observed across studies.
Safety
Approximately 96% of patients reported TEAEs during both the dose-optimization and double-blind treatment periods; for the double-blind period, 40% of patients reported TEAEs during treatment with d-ATS at any dose and 41% with placebo treatment (Table 3). Most TEAEs were mild or moderate in severity, with severe TEAEs occurring in 3 patients receiving d-ATS during the dose-optimization period and 3 patients during the double-blind treatment period (n = 2, d-ATS; n = 1, placebo). The most common TEAEs were decreased appetite, insomnia, and headache during both dose-optimization period and double-blind treatment with d-ATS. Discontinuations caused by TEAEs occurred in 3 patients during dose optimization (n = 1 each: irritated mood, appetite loss, and abdominal pain) and none during double-blind treatment with d-ATS or placebo. No deaths occurred throughout the study.
Table 3.
Summary of Safety During Double-Blind Treatment Period (Safety Population)
| d-ATS (all doses) (N = 105a), n (%) | Placebo (N = 105b), n (%) | |
|---|---|---|
| TEAEs | 44 (41.9) | 43 (41.0) |
| Severe TEAEs | 2 (1.9) | 1 (1.0) |
| Serious TEAEs | 0 (0.0) | 0 (0.0) |
| TEAEs leading to discontinuation | 0 (0.0) | 0 (0.0) |
| Preferred term | ||
| Decreased appetite | 13 (12.3) | 2 (1.9) |
| Insomnia | 6 (5.7) | 5 (4.8) |
| Headache | 6 (5.7) | 4 (3.8) |
| Hyperkalemia | 5 (4.8) | 4 (3.8) |
| Vomiting | 4 (3.8) | 0 (0.0) |
| Nasopharyngitis | 3 (2.9) | 2 (1.9) |
| Abdominal pain upper | 3 (2.9) | 1 (1.0) |
| Nausea | 3 (2.9) | 1 (1.0) |
| Affect lability | 3 (2.9) | 0 (0.0) |
| Tic | 2 (1.9) | 0 (0.0) |
| Irritability | 2 (1.9) | 1 (1.0) |
One patient discontinued the study before crossing over to d-ATS.
One patient was dispensed placebo patches for Visit 6 of the double-blind phase but did not return for any safety assessments.
TEAE, treatment-emergent adverse event.
During the dose-optimization phase, at Visit 1, 1 patient who received d-ATS had at least 1 occurrence of suicidal ideation. The patient reported wishing to be dead a few times after being aggravated by their sibling, with the goal of getting the sibling to stop bothering them. No patients exhibited suicidal ideation or behavior during Visits 2–5. During the double-blind treatment period (Visits 6 and 7 combined), 1 patient on d-ATS had 1 occurrence of suicidal ideation. This patient was 1 of 4 patients at baseline who had at least one occurrence of nonexclusionary suicidal ideation or behavior recorded in their medical history. The patient reported that they wished to be dead one time when they were frustrated with their phone not working. No patients on placebo exhibited suicidal ideation or behavior.
A meaningful degree of skin irritation (combined skin irritation score ≥3) occurred in 2 (2%) patients, but no patches were removed early owing to irritation during the treatment period. In total, <10% of patients reported severe discomfort or pain associated with patch application (d-ATS or placebo) at any point during both study periods (Table 4). Application-site discomfort/pain typically resolved spontaneously 2–4 hours postapplication.
Table 4.
Patch Application-Site Treatment-Emergent Adverse Events (Safety Population)
| Dose-optimization period, n (%) |
Double-blind period, n (%) |
||
|---|---|---|---|
| d-ATS (all doses) (N = 110) | d-ATS (all doses) (N = 105a) | Placebo (N = 105b) | |
| Pain | 11 (10.0) | 0 (0.0) | 0 (0.0) |
| Pruritus | 8 (7.3) | 0 (0.0) | 0 (0.0) |
| Burn | 3 (2.7) | 0 (0.0) | 0 (0.0) |
| Erythema | 2 (1.8) | 1 (1.0) | 0 (0.0) |
| Discomfort | 1 (0.9) | 0 (0.0) | 0 (0.0) |
| Edema | 1 (0.9) | 0 (0.0) | 0 (0.0) |
| Swelling | 1 (0.9) | 1 (1.0) | 0 (0.0) |
One patient discontinued the study before crossing over to d-ATS.
One patient was dispensed placebo patches for Visit 6 of the double-blind phase but did not return for any safety assessments.
Vital sign changes were consistent with those expected for amphetamine. After 5 weeks of open-label d-ATS treatment during the dose-optimization phase, mean (SD) changes from baseline to Visit 5 were 1.7 (14.0) beats per minute (bpm) for pulse, 1.4 (10.4) mmHg for systolic blood pressure, and 2.3 (8.6) mmHg for diastolic blood pressure. During the double-blind treatment phase, mean (SD) changes in d-ATS versus placebo groups from baseline to Visit 6/7 were 13.4 (13.7) bmp versus 12.8 (12.9) bmp for pulse, 3.3 (11.0) mmHg versus 3.1 (10.8) mmHg for systolic blood pressure, and 3.4 (9.9) mmHg versus 1.8 (9.9) mmHg for diastolic blood pressure.
Discussion
In this study, d-ATS was effective in the treatment of ADHD in children and adolescents, with significantly greater improvements in SKAMP total score, PERMP-A, and PERMP-C observed with d-ATS treatment over placebo. Significantly greater improvements in SKAMP and PERMP scores with d-ATS versus placebo were observed from 2 hours postdose through 12 hours postdose (3 hours after patch removal).
Onset of efficacy, as measured by SKAMP score, was detected as early as 2 hours postapplication, and a duration of effect up to 12 hours was observed. Modeling/simulation data confirmed the PK/PD relationship of amphetamines, with an initial decline in SKAMP scores observed ∼2 hours after patch application and maximum declines observed at ∼4 hours after patch application. Scores subsequently returned to near baseline values within 4 hours after patch removal following a 9-hour wear time. Simulated early patch removal (by assessing patch removal times of 4, 6, 8, and 9 hours postpatch application) was associated with reduced systemic exposure and earlier return to baseline SKAMP values, indicating that early patch removal may result in reduced plasma concentrations of drug and thus control overtreatment duration and late-day side effects.
All patients were optimized to a dose of d-ATS based on prespecified protocol criteria, and no patients discontinued the study owing to lack of efficacy. d-ATS was safe and well-tolerated throughout the current study, and the systemic safety profile of d-ATS was consistent with that observed with oral amphetamines. Application-site reactions were generally mild to moderate, with no application-site reactions leading to study discontinuation.
The overall efficacy and safety of d-ATS demonstrated in this study indicate that this novel dextroamphetamine patch could be a valuable treatment option for children and adolescents with ADHD. d-ATS may be of particular value to patients who are interested in a transdermal stimulant but do not respond to treatment with methylphenidate, who need to transition away from methylphenidate-based treatment, or who cannot or will not swallow pills. Transdermal formulations offer many advantages, especially for patients with psychiatric/nervous system disorders. Transdermal delivery has been shown to improve adherence in various patient populations (Findling and Dinh 2014).
Patients' and caregivers' management of treatment is made easier with transdermal formulations through visual confirmation of dosing, reduced dosing frequency, and the option for early patch removal, which enables individualized duration of therapy. Control of treatment duration in patients with ADHD is of great importance, as this capability can provide opportunities to lessen adverse effects, decrease overall medication burden, and allow timed treatment. The transdermal route also minimizes first-pass metabolism and may reduce hepatic side effects, potentially reducing the risk of drug–drug interactions and gastrointestinal effects. Moreover, with d-ATS, abuse liability may be reduced, as >90% of the medication is depleted by 9 hours after patch application.
Despite having many advantages, transdermal formulations do have some potential issues, such as application-site reactions and time to onset. Although application-site reactions did not result in any discontinuations from this study, they were reported by some patients. Patients starting a transdermal medication should be counseled to rotate the site of application to minimize skin irritation. As with other patches, a gradual onset of efficacy (∼2 hours) was observed with d-ATS. This may be an important consideration for patients and caregivers when selecting a time to apply the patch. For example, with the other available transdermal stimulant (methylphenidate patch), parents are advised to apply the patch to their sleeping child ∼2 hours before the desired wake-up time (Daytrana Prescribing Information 2019).
Stimulants have been shown to be a highly effective treatment option in children and adolescents with ADHD and are the recommended first-line treatments for this patient population (Pliszka 2007; Huss et al. 2017; Mattingly et al. 2017; Wolraich et al. 2019). Because many patients respond better to amphetamines than methylphenidate, having transdermal formulations of both treatments is beneficial. d-ATS will be the first transdermal amphetamine available for the treatment of ADHD and is an important option for patients.
Conclusion
Data from this study indicate that d-ATS treatment significantly improved ADHD symptoms, as measured by SKAMP total score, over the 12-hour assessment period. Onset of efficacy was observed at 2 hours postdose, and duration of effect continued through 12 hours (patch removed at 9 hours), with significant differences between d-ATS and placebo at all time points from 2 hours onward. Significant improvements versus placebo in PERMP-A and PERMP-C scores were also observed with d-ATS treatment from 2 to 12 hours postdose. d-ATS was safe and well-tolerated, with a systemic safety profile similar to that observed with oral amphetamines. These results indicate that d-ATS is an effective and well-tolerated treatment for children and adolescents with ADHD, and its ability to deliver sustained efficacy along with the advantages of transdermal drug delivery makes it a beneficial new treatment option.
Clinical Significance
Stimulants, such as methylphenidate and amphetamine, are the first-line pharmacological treatment for children and adolescents who are diagnosed with ADHD. Rates of response to these treatments can be idiosyncratic; thus, there is a need for treatment options in terms of various medications and formulations to provide more individualized care. Although there are many advantages for transdermal formulations in the treatment of nervous system disorders, only one FDA-approved transdermal treatment (methylphenidate) for ADHD in children and adolescents is currently available.
This study demonstrates that the novel d-ATS is an effective treatment for ADHD in children and adolescents, with significant improvements over placebo in SKAMP and PERMP scores from 2 hours postapplication through 12 hours postdose (patch removed at 9 hours). d-ATS was also safe and well-tolerated throughout the study, with a safety profile similar to that of oral amphetamines. These data indicate that this new patch can deliver sustained levels of dextroamphetamine while also providing the advantages of transdermal drug delivery, such as improved adherence, easier medication management, no need to swallow pills, reduced abuse liability, and the option for early patch removal, allowing an individualized duration of treatment. As the first transdermal amphetamine available for the treatment of ADHD, d-ATS may be a valuable new treatment option.
Acknowledgments
This article was prepared according to the International Society for Medical Publication Professionals' “Good Publication Practice for Communicating Company-Sponsored Medical Research: GPP3.”
Disclosures
A.J.C. reports grants from Aevi Genomic Medicine, Akili Interactive, Allergan, Arbor, Axsome, Biohaven, Ironshore, KemPharm, Neos Therapeutics, Novartis, Noven, Otsuka, Purdue, Rhodes, Sage Therapeutics, Sunovion, Supernus, and Tris Pharma; personal fees from AbbVie, Acadia, Akili Interactive, Alfasigma, Alkermes, Allergan, Atentiv, Cingulate, Corium, Intra-Cellular Therapies, Ironshore, Janssen, Jazz, Lundbeck, MedAvante-ProPhase, Neurocrine, Neuroscience Education Institute, Noven, Otsuka, Sage Therapeutics, Sunovion, Supernus, Takeda, Teva, and Tris Pharma; and other relationship(s) with Cognitive Research (Data and Safety Monitoring Board) and the Neuroscience Education Institute (employee and board member).
K.S., B.S., K.B., M.K., M.C., and S.M. report employment with Noven Pharmaceuticals, Inc.
A.C.C. reports grants from Aevi Genomic Medicine, Akili Interactive, Allergan, Arbor, Ironshore, KemPharm, Neos Therapeutics, Noven, Otsuka, Purdue, Rhodes, Sunovion, Supernus, Takeda, Tris Pharma, Tulex, and the U.S. Food and Drug Administration; consulting for Arbor, Corium, Ironshore, Jazz, KemPharm, Lumos, Neos Therapeutics, Purdue, Rhodes, Sunovion, Supernus, Takeda, and Tulex; serving on an advisory board for Adlon, Akili Interactive, Arbor, Cingulate, Corium, Ironshore, Neos Therapeutics, Noven, Otsuka, Purdue, Rhodes, Sunovion, Supernus, Takeda, and Tris Pharma; serving as a speaker for Arbor, Ironshore, Neos Therapeutics, Supernus, Takeda, and Tris Pharma; and other relationship(s) with Arbor (writing support), Ironshore (writing support), Neos Therapeutics (writing support), Noven (writing support), Purdue (writing support), Rhodes (writing support), Sunovion (writing support), Takeda (writing support), and Tris Pharma (writing support).
References
- American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, Text Revision, 4th ed. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013. [Google Scholar]
- Barbaresi WJ, Campbell L, Diekroger EA, Froehlich TE, Liu YH, O'Malley E, Pelham WE Jr., Power TJ, Zinner SH, Chan E: Society for Developmental and Behavioral Pediatrics Clinical Practice Guideline for the assessment and treatment of children and adolescents with complex attention-deficit/hyperactivity disorder. J Dev Behav Pediatr 41:S35–S57, 2020. [DOI] [PubMed] [Google Scholar]
- Bélanger SA, Andrews D, Gray C, Korczak D: ADHD in children and youth: Part 1—Etiology, diagnosis, and comorbidity. Paediatr Child Health 23:447–453, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caye A, Spadini AV, Karam RG, Grevet EH, Rovaris DL, Bau CH, Rohde LA, Kieling C: Predictors of persistence of ADHD into adulthood: A systematic review of the literature and meta-analysis. Eur Child Adolesc Psychiatry 25:1151–1159, 2016. [DOI] [PubMed] [Google Scholar]
- Center for Drug Evaluation and Research: Attention Deficit Hyperactivity Disorder: Developing Stimulant Drugs for Treatment: Guidance for Industry. Rockville, MD: Center for Drug Evaluation and Research; 2019. [Google Scholar]
- Chien WC, Chung CH, Lin FH, Yeh CB, Huang SY, Lu RB, Chang HA, Kao YC, Chiang WS, Chou YC, Tsao CH, Wu YF, Tzeng NS: The risk of injury in adults with attention-deficit hyperactivity disorder: A nationwide, matched-cohort, population-based study in Taiwan. Res Dev Disabil 65:57–73, 2017. [DOI] [PubMed] [Google Scholar]
- Citrome L, Zeni CM, Correll CU: Patches: Established and emerging transdermal treatments in psychiatry. J Clin Psychiatry 80:e1–e10, 2019. [DOI] [PubMed] [Google Scholar]
- Dalsgaard S, Leckman JF, Mortensen PB, Nielsen HS, Simonsen M: Effect of drugs on the risk of injuries in children with attention deficit hyperactivity disorder: A prospective cohort study. Lancet Psychiatry 2:702–709, 2015a. [DOI] [PubMed] [Google Scholar]
- Dalsgaard S, Østergaard SD, Leckman JF, Mortensen PB, Pedersen MG: Mortality in children, adolescents, and adults with attention deficit hyperactivity disorder: A nationwide cohort study. Lancet 385:2190–2196, 2015b. [DOI] [PubMed] [Google Scholar]
- Danckaerts M, Sonuga-Barke EJ, Banaschewski T, Buitelaar J, Döpfner M, Hollis C, Santosh P, Rothenberger A, Sergeant J, Steinhausen HC, Taylor E, Zuddas A, Coghill D: The quality of life of children with attention deficit/hyperactivity disorder: A systematic review. Eur Child Adolesc Psychiatry 19:83–105, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daytrana Prescribing Information: Daytrana® (methylphenidate transdermal system), CII. 2019. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=2c312c31-3198-4775-91ab-294e0b4b9e7f (Accessed October 28, 2019).
- Findling RL, Dinh S: Transdermal therapy for attention-deficit hyperactivity disorder with the methylphenidate patch (MTS). CNS Drugs 28:217–228, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M, Wong M, Atkins D, Taylor J, Feinleib M: AHRQ Technical Reviews: Diagnosis of Attention-Deficit/Hyperactivity Disorder. Rockville, MD: Agency for Health Care Policy and Research; 1999. [PubMed] [Google Scholar]
- Huss M, Duhan P, Gandhi P, Chen CW, Spannhuth C, Kumar V: Methylphenidate dose optimization for ADHD treatment: Review of safety, efficacy, and clinical necessity. Neuropsychiatr Dis Treat 13:1741–1751, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London AS, Landes SD: Attention deficit hyperactivity disorder and adult mortality. Prev Med 90:8–10, 2016. [DOI] [PubMed] [Google Scholar]
- Mattingly GW, Wilson J, Rostain AL: A clinician's guide to ADHD treatment options. Postgrad Med 129:657–666, 2017. [DOI] [PubMed] [Google Scholar]
- Newcorn JH, Nagy P, Childress AC, Frick G, Yan B, Pliszka S: Randomized, double-blind, placebo-controlled acute comparator trials of lisdexamfetamine and extended-release methylphenidate in adolescents with attention-deficit/hyperactivity disorder. CNS Drugs 31:999–1014, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pliszka S: Practice parameter for the assessment and treatment of children and adolescents with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 46:894–921, 2007. [DOI] [PubMed] [Google Scholar]
- Pliszka SR: Psychiatric comorbidities in children with attention deficit hyperactivity disorder: Implications for management. Paediatr Drugs 5:741–750, 2003. [DOI] [PubMed] [Google Scholar]
- Polanczyk G, de Lima MS, Horta BL, Biederman J, Rohde LA: The worldwide prevalence of ADHD: A systematic review and metaregression analysis. Am J Psychiatry 164:942–948, 2007. [DOI] [PubMed] [Google Scholar]
- Thomas R, Sanders S, Doust J, Beller E, Glasziou P: Prevalence of attention-deficit/hyperactivity disorder: A systematic review and meta-analysis. Pediatrics 135:e994–e1001, 2015. [DOI] [PubMed] [Google Scholar]
- Wigal SB, Gupta S, Guinta D, Swanson JM: Reliability and validity of the SKAMP rating scale in a laboratory school setting. Psychopharmacol Bull 34:47–53, 1998. [PubMed] [Google Scholar]
- Wigal SB, Wigal TL: The laboratory school protocol: Its origin, use, and new applications. J Atten Disord 10:92–111, 2006. [DOI] [PubMed] [Google Scholar]
- Wigal SB: Laboratory school protocol mini-review: Use of direct observational and objective measures to assess ADHD treatment response across the lifespan. Front Psychol 10:1796, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolraich ML, Hagan JF Jr., Allan C, Chan E, Davison D, Earls M, Evans SW, Flinn SK, Froehlich T, Frost J, Holbrook JR, Lehmann CU, Lessin HR, Okechukwu K, Pierce KL, Winner JD, Zurhellen W: Clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Pediatrics 144:e20192528, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]