Abstract
Reactive oxygen species (ROS) are omnipresent in the ocean, originating from both biological (e.g., unbalanced metabolism or stress) and non-biological processes (e.g. photooxidation of colored dissolved organic matter). ROS can directly affect the growth of marine organisms, and can also influence marine biogeochemistry, thus indirectly impacting the availability of nutrients and food sources. Microbial communities and evolution are shaped by marine ROS, and in turn microorganisms influence steady-state ROS concentrations by acting as the predominant sink for marine ROS. Through their interactions with trace metals and organic matter, ROS can enhance microbial growth, but ROS can also attack biological macromolecules, causing extensive modifications with deleterious results. Several biogeochemically important taxa are vulnerable to very low ROS concentrations within the ranges measured in situ, including the globally distributed marine cyanobacterium Prochlorococcus and ammonia-oxidizing archaea of the phylum Thaumarchaeota. Finally, climate change may increase the amount of ROS in the ocean, especially in the most productive surface layers. In this review, we explore the sources of ROS and their roles in the oceans, how the dynamics of ROS might change in the future, and how this change might impact the ecology and chemistry of the future ocean.
Keywords: Reactive oxygen species, Marine biogeochemistry, Invertebrates, Prochlorococcus, Ammonia oxidizing archaea, Hydrogen peroxide treatment
1. Introduction
The distribution, abundance, and productivity of organisms in the World Ocean is intimately tied to biogeochemical cycles. Most marine ecosystems are dependent on primary production by aquatic photoautotrophs such as diatoms, cyanobacteria, seaweeds and seagrasses, and the growth of these organisms is in turn limited by the availability of nitrogen and/or phosphorus, or in some cases trace nutrients such as iron or vitamins. These molecules exist in a variety of chemical forms in the ocean, and because of their foundational importance in marine ecology, the enzymatic and abiotic reactions that shuttle them between their various states have been studied extensively.
Despite its ubiquity in both the ocean environment and in living biomass, the cycling of oxygen has received comparatively little attention from marine ecologists. The oxygen cycle is routinely imagined as a simple back-and-forth between the release of O2 as a by-product of photosynthetic water-splitting and the reduction of O2 to water by aerobic heterotrophic metabolism. But this formulation underestimates the complexity of environmental reactions involving oxygen, which in fact cycles continually through a variety of oxygen-containing intermediates known as reactive oxygen species (ROS) (see Ref. [1] for an overview). Sometimes these ROS act as potent toxins; sometimes they are created by organisms as part of their natural growth processes; and in many cases they interact with other biogeochemical cycles in critical ways [[1], [2], [3]].
In this review, we will explore the roles played by ROS in the oceans. We will begin with a description of the chemistry of ROS and a survey of the biotic and abiotic mechanisms that generate them, follow up with an exploration of how ROS interact with marine biogeochemical cycles, and then consider some specific positive and negative interactions ROS have with marine organisms. Finally, we will close with a consideration of how the dynamics of ROS might change in the future due to human activity, and how this might impact the ecology and chemistry of the future ocean.
2. Aquatic chemistry of ROS
ROS are oxygen-containing molecules in which the redox state of oxygen is intermediate between that of O2 and H2O, which form the dominant redox couple in most oxic natural waters. The co-existence of O2 and H2O, despite their very different redox potentials, is a thermodynamically unfavorable situation that is enabled by two primary factors [4]:
-
1.
O2 is relatively unreactive because its most energetically favorable state (the ground state) is a triplet state with two unpaired electrons; and
-
2.
The continuous input of free energy into the global aquatic system, primarily due to sunlight-driven photo(bio)chemical reactions, prevents the system from reaching a thermodynamic endpoint in which O2 is fully consumed.
The triplet ground state of O2 (denoted 3O2) means that it is typically only able to react with other ground state molecules containing unpaired electrons, which forces the reduction of O2 to occur via a series of one-electron transfer steps [4]. Intracellularly, these one-electron transfer steps are often mediated by enzymes such that they occur in quick succession and the intermediate reaction products are not released [5]. In contrast, in the extracellular milieu of natural aquatic systems the sequence of one-electron transfer steps during the reduction of O2 can give rise to a range of ROS in free solution.
2.1. Thermodynamics
In oxic waters the overall thermodynamic drive is primarily for reduction of O2 (and ROS) to H2O, coupled to oxidation of other chemical species [3]. In other words, ROS are predominantly oxidants. Nonetheless, there are circumstances in which some ROS can also function as reductants and drive redox cycling of various biogeochemically important elements [3]. While ROS may often be found in free solution in the aquatic milieu as noted above, there are circumstances in which they are bound to other species, either because they are not released as free species during formation, or due to additional reactions with other suitable substrates [4]. In this case, the resulting bound species may also function as oxidants or facilitate redox cycling.
This stepwise reduction of O2 to H2O occurs via the following series of reactions [4]:
| (1) |
| (2) |
| (3) |
| (4) |
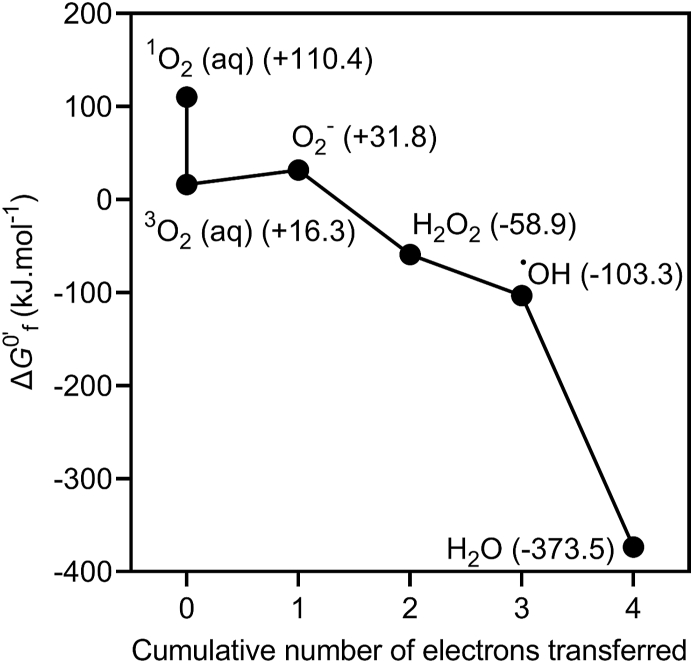
The thermodynamics of this sequence of redox reactions is illustrated in Fig. 1 under standard reaction conditions (unit activity and a temperature of 298 K) at pH 7.
Fig. 1.
Gibbs free energy of formation of ROS under standard conditions (1 M activity) in aqueous solution at pH 7.1O2 refers to the 1Δg excited state of dioxygen. Values were calculated from thermodynamic data reported in Sawyer (1991) [4].
As illustrated in Fig. 1, O2− is mildly reducing under standard reaction conditions at pH 7. In contrast, H2O2 is a relatively powerful two-electron oxidant from a thermodynamic perspective, and is not constrained to one-electron reactions in the same way as 3O2, but typically must overcome a large activation energy barrier [6]. Consequently, H2O2 reacts mostly as a one electron oxidant. One of the most well-known such reactions is the so-called Fenton reaction with Fe(II):
| (5) |
where Mn+ is Fe(II) in the case of the Fenton reaction. This reaction, and similar “Fenton-like” reactions with other trace metals and certain non-metals, produce •OH or high-valent metal ions [6], both of which are very strong and highly reactive oxidants.
In addition to these species formed during reduction of O2, the electronically excited 1Δg singlet state of dioxygen is typically also considered as one of the ROS. While two electronically excited states of dioxygen are possible, the highest energy 1Σg+ singlet state relaxes back to the 1Δg state faster than it can undergo bimolecular reaction; hence the common notation 1O2 is used in this and other work in reference to the latter. While 1O2 is a relatively powerful oxidant, and not subject to the one-electron transfer constraints of 3O2, in practice it is highly selective as an oxidant because it readily relaxes back to the 3O2 ground state through either energy transfer (particularly to water in aqueous environments), or by partial charge transfer in which formation of an intermediate charge-transfer complex results in relaxation of 1O2 back to 3O2 without completion of electron transfer [7].
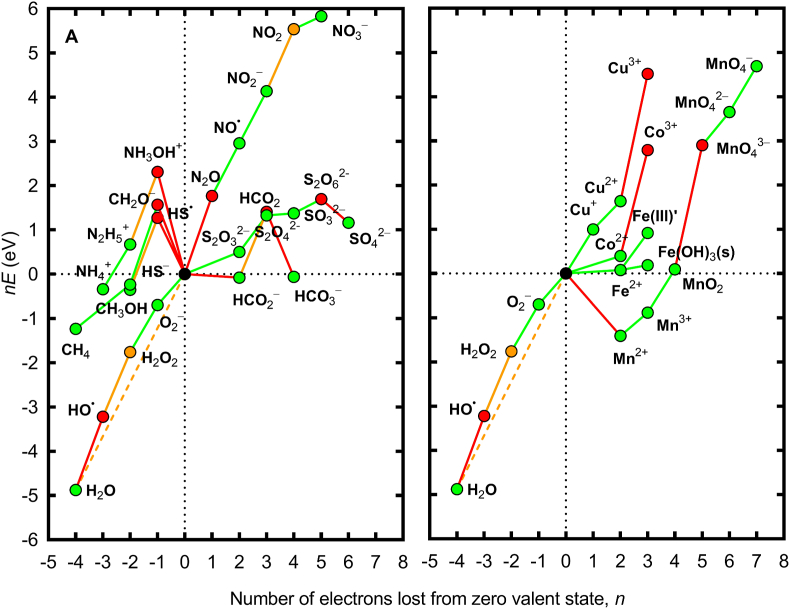
When electron transfer is required to occur in single electron transfer steps, additional thermodynamic constraints are imposed on the system: not only must the overall reaction be thermodynamically favorable, but so too must each one-electron transfer step, else the reaction will be unable to proceed beyond the thermodynamic barrier imposed by a particular electron transfer step. Consequently, there are a limited set of redox reactions that are thermodynamically possible in oxic marine waters [3], as illustrated in Fig. 2. From a thermodynamic perspective, this explains why some intermediates in the redox reactions of various biogeochemically important species are only ever transient, and present at exceedingly low concentrations. While Fig. 2 does not provide an exhaustive examination of all possible redox couples that may be influenced by ROS, it does give an indication of some of the most important.
Fig. 2.
Frost diagrams for some common redox active elements in natural waters at pH 8.1 accounting for typical activities of oxygen redox species in natural waters. (A) Representative species for the major elements C, N and S. (B) Trace metals Co, Cu, Fe and Mn. Red lines indicate redox couples that are absolutely unstable with respect to one-electron transfer processes involving the H2O/•OH couple (lines with positive slope) or the H2/H+ couple (lines with negative slope); orange lines indicate redox couples that are conditionally unstable with respect to multi-electron transfer processes involving the H2O/O2 couple; and green lines indicate redox couples that are thermodynamically permissible in aqueous solution at pH 8.1. The dashed orange line represents the redox potential associated with the four-electron transfer for complete reduction of O2 to H2O. Red symbols represent species that are thermodynamically unable to exist due to absolute instability with respect to one-electron transfer processes involving the H2O/•OH couple (lines with positive slope) or the H2/H+ couple; orange symbols represent species that are thermodynamically able to exist in the absence of processes to catalyze multi-electron transfer processes involving the H2O/O2 couple; and green symbols represent species that are thermodynamically able to coexist with both the H2/H+ and H2O/O2 couples. See Ref. [3] for details of concentrations and thermodynamic data used. Reproduced from Ref. [3] with permission.
2.2. Reaction kinetics
Although thermodynamics is a critical control on the energetic favorability of potential reactions involving ROS, marine systems are dynamic and in many cases equilibrium is never reached. As such, the biogeochemistry of ROS is strongly influenced by reaction kinetics, which control their lifetimes and hence steady-state concentrations. The reactivity of the different ROS varies considerably. At the pH of seawater, O2•−exists predominantly in the deprotonated form, which possesses a resonance-stabilized form with a “three-electron bond”, and exhibits very little free radical character [5]. As such, it is relatively stable in marine waters and only reacts with other species possessing unpaired electrons in the valence shell, resulting in half-lives on the order of a few seconds to minutes in marine waters [1]. The reactivity of H2O2 is usually kinetically limited due to the high activation energy barrier noted previously, with the overall reaction kinetics often controlled by rate of the formation of reaction intermediates that lower the activation energy barrier [6]. For example, the Fenton reaction is thought to involve formation of an inner sphere complex between Fe(II) and H2O2 as the initial step, resulting in an overall reaction rate constant many orders of magnitude less than diffusion-controlled [6]. Consequently, typical half-lives of H2O2 in marine waters are considerably longer, on the order of hours to days [1]. In contrast, •OH reacts at diffusion-controlled rates with many organic and inorganic compounds, and as such typically has a very short lifetime in natural waters (half-life ∼250 ns, computed using data from Ref. [8]). Similarly, 1O2 is highly reactive in aqueous environments with a half-life of around 4 μs due to physical relaxation by solvent water [9].
Short half-lives and localized sources (as discussed further in Section III) may also result in spatially heterogenous distributions of ROS. This is particularly the case for •OH and 1O2, but also to some extent for O2• −, which may be formed through highly localized processes (e.g. in cells or at particle surfaces) and unable to diffuse far from the site of production due to their short half-life. This can result in significant spatial variability in steady-state concentrations, with the potential for concentrations near the site of ROS production that are many orders of magnitude greater than in the bulk solution, and associated differences in reaction kinetics.
3. Sources and sinks of ROS in the ocean
Naturally occurring H2O2 in the ocean was first reported in the 1960's [10], and even earlier than that, researchers were aware that H2O2 added to seawater was not stable, but disappeared with enzyme-like kinetics [11]. Nevertheless, the ability to study the distribution, sources, and sinks of ROS in the ocean awaited the development of sufficiently sensitive detection methods which would not occur until the 1980's. For instance, classical titration-based methods for quantifying H2O2 had limits of detection in the millimolar range, but the development of fluorescence methods in the 1980s [12] and chemiluminescence methods in the 2000s [13] made it possible to accurately measure the nanomolar H2O2 commonly found in natural waters (Fig. 4). In this section we first give an overview of the major ROS production and destruction pathways along with their impact on steady-state ROS concentrations, and finally we describe some of the most common methods used for assessing seawater ROS concentrations in the field.
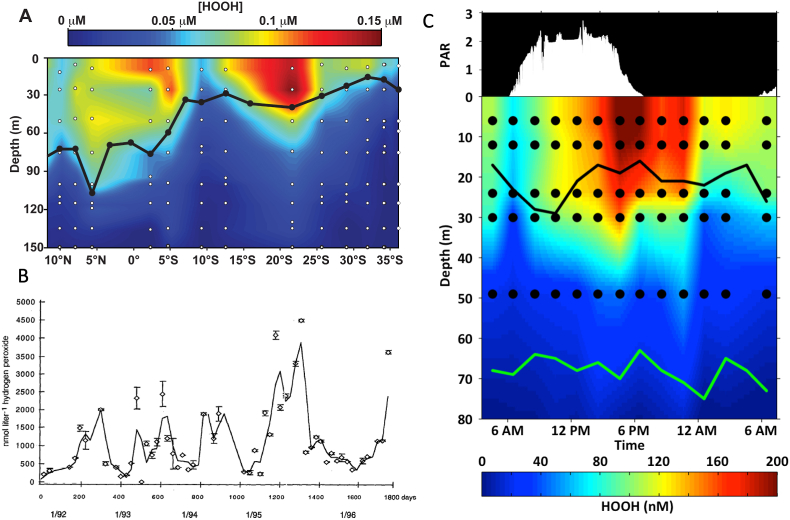
Fig. 4.
H2O2concentrations in different environments and their biological impacts on various organisms.
3.1. Sources of marine ROS
3.1.1. Photochemical production
Many studies in a wide variety of waters have supported the hypothesis that most ROS in bulk surface waters arise by photochemistry, especially via the photooxidation of dissolved organic matter (DOM) [[14], [15], [16], [17]]. Colored DOM can absorb photons and enter an excited triplet state capable of reducing O2 to O2•−, which can then undergo spontaneous dismutation to H2O2 and O2. Because of its relatively long residence time, H2O2 is the most easily measured ROS in the field and has been used as a proxy for the relative abundance of other ROS such as O2•−and •OH. The rate of H2O2 accumulation is generally correlated with DOM concentration [18], and therefore steady-state H2O2 concentrations are generally greater in terrestrially-impacted waters like coasts and estuaries, as opposed to the oligotrophic ocean gyres (Table 1 and references therein). ROS fluxes in bulk water and H2O2 steady-state concentrations also decrease rapidly with depth and light level, dropping to near or below the limit of detection below the euphotic zone (Fig. 3a, Table 1). Because of the influence of sunlight, steady state H2O2 concentrations in surface waters tend to exhibit a diel cycle with afternoon maxima and early morning minima (Fig. 3c) [[19], [20], [21]], and also a seasonal cycle peaking in the summer (Fig. 3b) [22,23]. Such trends have not been widely observed for other ROS, however.
Table 1.
H2O2concentrations in various waters.
| Type | Location | [H2O2] | Reference |
|---|---|---|---|
| Freshwater | Jacks Lake, Ontario, surface | 10–800 nM | [19] |
| Swedish Highland Lakes, surface | 132–984 nM | [247] | |
| Lake Erie, surface | 50–175 nM | [248] | |
| Brackish | Patuxent Estuary, surface | 12–350 nM | [22] |
| Chesapeake Bay, surface | 3–1700 nM | [15] | |
| Chesapeake Bay, surface | 40–80 nM | [248] | |
| Coastal Germany, Intertidal salt flat | 0.1–4.5 μM | [23] | |
| Southern Ocean, tidepool | 2 μM | [249] | |
| Coastal | Southern Ocean | 1.5 μM | [249] |
| Antarctica | 9–25 nM | [250] | |
| Peru | 80–500 nM | [251] | |
| Southern California | 44–370 nM | [252] | |
| Baja California | 50–125 nM | [248] | |
| Texas | 14–170 nM | [10] | |
| Florida | 80–210 nM | [15] | |
| Gulf of Mexico | 100–240 nM | [253] | |
| Bahamas | 50–190 nM | [15] | |
| Amazon River plume | 25–165 nM | [21] | |
| Open ocean surface | Tropical Pacific | 50–150 nM | [200] |
| Tropical Pacific, during rain | 100–300 nM | [28] | |
| North Pacific | 25–120 nM | [254] | |
| South and Central Atlantic | 16–68 nM | [21] | |
| Subtropical Atlantic | 50–220 nM | [18] | |
| Equatorial Atlantic ITCZ | 31–236 nM | [255] | |
| Caribbean Sea | 50–100 nM | [256] | |
| Gulf of Mexico | 90–140 nM | [253] | |
| Sargasso Sea | 95–175 nM | [257] | |
| Western Atlantic | 40–175 nM | [248] | |
| Eastern Atlantic | 30–80 nM | [258] | |
| North Atlantic | 135–483 nM | [32] | |
| Bermuda Atlantic Time Series | 20–200 nM | [31] | |
| Mediterranean Sea | 100–140 nM | [259] | |
| Southern Ocean Indian Sector | 5–20 nM | [260] | |
| Open ocean depths | North Pacific, >1000 m | <6 nM | [261] |
| Mediterranean, South Atlantic and Pacific, >1000 m | <3 nM | [262] | |
| South and Central Atlantic, below euphotic zone | <1 nM | [21] | |
| Bermuda Atlantic Time Series, 150 m | <2 nM | [31] | |
| Precipitation | South and Central Atlantic, rain | 3.5–71 μM | [27] |
| Equatorial Atlantic ITCZ | 1.5–22.8 μM | [255] | |
| Gulf of Mexico, rain | 40.2 μM | [263] | |
| Florida Keys, rain | 28.4 μM | [263] | |
| Miami/Bahamas, rain | 30.7 μM | [264] | |
| West Atlantic, rain | 12.7 μM | [263] | |
| Bermuda Atlantic Time Series, rain | 11–40 μM | [31] | |
| Bermuda Atlantic Time Series, rain | 5–85 μM | [26] | |
| New Zealand, rain | 10–30 μM | [30] | |
| Equatorial Pacific, rain | 8.5 μM | [28] | |
| North Pacific ITCZ, rain | 10.4 μM | [28] | |
| Jacks Lake, Ontario, rain | 1.3–34 μM | [19] | |
| Southern Ocean, snow | 10–14 μM | [249] | |
Fig. 3.
H2O2profiles across time and space. A) H2O2 depth profiles from samples across a Pacific transect in Jan–Feb 2007. B) Monthly H2O2 measurements of a tide pool in Germany from 1991 to 1996. C) H2O2 depth profiles and light intensity from a station in the South Pacific (Feb 2007) across a 24-h period; PAR, photosynthetically active radiation, with white bars indicating light intensity. In A and C dots represent sampled depths/times, with H2O2 values interpolated between the points; black lines represent the depth of the surface mixed layer; and green line in C represents the deep chlorophyll maximum. Reprinted with permission from Ref. [200] (A) [273], (B), and [20] (C).
H2O2 production is stimulated by light in the visual range but is much faster under UV irradiation. While representing only a small fraction of the irradiance impacting natural seawater, photons in the UV-B range cause the majority of H2O2 accumulation [24]. H2O2 can also form in the atmosphere by the photolysis of water into hydrogen atoms and •OH [25]. These radicals can then join to form H2O2 in the gas phase, which can subsequently be recruited into rain droplets, resulting in H2O2 concentrations that are typically two orders of magnitude greater than that found in surface seawater [26]. Rain events have been shown to temporarily increase the in situ H2O2 of seawater [[26], [27], [28], [29], [30], [31], [32]].
3.1.2. Stress production by organisms
In addition to direct photochemical ROS production, marine organisms can release H2O2 into the environment, often due to stress. Many enzymes capable of single-electron transfer to a substrate are also capable of reducing O2, and the ubiquity of O2 in oxic environments ensures that some H2O2 will escape. Common metabolic pathways that produce H2O2 as a by-product even under ideal conditions include the Mehler reaction (aka the water-water cycle) and photorespiration in photosynthetic organisms [33] as well as the electron transfer reactions of aerobic oxidative phosphorylation [34]. O2 reduction is enhanced when a primary electron transport carrier is not available in sufficient quantities due either to excess electron flow, nutrient deficiency, or other problems. For instance, in Fe-starved cyanobacteria, ROS buildup occurs due to a lack of ferredoxin, the Fe-containing primary cytoplasmic electron acceptor for photosystem I [35]. When more electrons flow through the photosynthetic electron transport chain than can be accepted by ferredoxin, O2 reduction becomes practically inevitable [33], and therefore excess ROS production and leakage of H2O2 into the environment is likely to be common in low-Fe marine habitats, particularly near the ocean surface under conditions of high light [36]. Release of H2O2 has also been observed in Antarctic diatoms when suddenly exposed to light following the long periods of uninterrupted darkness characteristic of the polar winter [37]. Importantly, H2O2 release by light-stressed photoautotrophs may contribute to the diel cycle in H2O2 observed at the ocean's surface, leading to overestimates of the role of DOM photooxidation in ROS production. ROS buildup from unbalanced metabolism could also explain the toxicity of high-nutrient culture media to marine microorganisms adapted to oligotrophic lifestyles [38].
Low levels of ROS are continuously produced by many organisms for signaling purposes and as normal, short-lived metabolic intermediates. However, intracellular ROS accumulation – a condition generally referred to as oxidative stress – can occur as a general consequence of diverse stresses such as temperature, osmotic, and dehydration stress [[39], [40], [41], [42], [43], [44]]. Exposure to anthropogenic pollutants can also induce oxidative stress in marine organisms. For example, silver nanoparticles and microplastics like polyacrylonitrile induced lethal oxidative stress in the photosynthetic microorganisms Prochlorococcus and Chlorella pyrenoidosa, respectively [45,46]. Exposure to heavy metals, elevated temperatures, acidification, organic pollutants, allelochemicals, and other stressors lead to ROS accumulation and other biomarkers of oxidative stress (e.g. DNA strand breaks) in a wide variety of marine animals, plants, and microbes [[45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57]]. However, it is not clear how much of this intracellular ROS is able to escape the organism and contribute to bulk ROS concentrations in the environment. O2• −, for instance, is incapable of crossing cellular membranes due to its charge, while highly reactive ROS such as •OH have such short lifespans that they are unlikely to move far from their point of origin.
3.1.3. Extracellular ROS production by marine organisms
ROS are also released into the environment by healthy organisms as a normal part of their metabolism. For example, animals use H2O2 at low concentrations as a signaling compound [58] and in high concentrations as an antimicrobial defense [59,60]. The rapid production of H2O2 bursts, often facilitated by NADPH-dependent oxidases, is an important component of the innate immune system of land plants and is also observed in aquatic phototrophs, where oxidative bursts have been observed after wounding or exposure to grazers or pathogens in a wide variety of macroalgae [[61], [62], [63], [64], [65], [66]].
H2O2 may also be produced as a weapon against prey or parasites. For instance, the coral Stylophora pistillata responded to physical stimulation (e.g. by pushing against its tissue) by releasing H2O2 from the site of stimulation, and the amount of H2O2 released was proportional to the intensity of the stimulus [67]. Contact with prey organisms also stimulated H2O2 release. The production of H2O2 was unaffected by the presence or absence of zooxanthellae, indicating it was a native function of the cnidarian organism, possibly serving as a protection against pathogens or a way to stun and capture prey more effectively. Similarly, H2O2 released as a response to wounding in the Antarctic seaweeds Ascoseira mirabilis and Saccharina latissima was able to inhibit grazing by amphipods, the primary herbivore feeding on these organisms in their native habitat [68,69].
Over the past decade, a variety of studies have also shown that extracellular production of O2•−by marine microbes is nearly ubiquitous (e.g. Ref. [70]). This body of work has been summarized in detail in several excellent recent reviews [1,2,71] and will not be repeated here. However, it is worth emphasizing that many of these studies have suggested that this process may be “intentional”, in the sense that the process may be exploited and even tightly regulated [72] to create or maintain locally favorable biogeochemical conditions. For example, one of the most significant and potentially widespread uses of ROS in the marine environment is the facilitation of iron acquisition by extracellular O2•−produced by microorganisms. The effectiveness of this process depends strongly on the concentration and speciation of iron but appears to be exploited by marine microbes under some conditions at least, as discussed in detail in Rose (2012) [73].
Algal blooms represent conspicuous hot spots for gross ROS production. Blooms are common in freshwater systems, but also occur in marine environments where they are often associated with the production of harmful toxins. H2O2 accumulation has been documented in the extracellular environment for a number of toxic species, albeit with substantial variation in production rates even between different strains of the same species [[74], [75], [76]]. It remains an open question how much of the ROS generation associated with blooms represents passive release or active production of ROS by the algae, and how much is passive photochemical generation caused by the high DOM conditions created by the bloom. For instance, production of O2− and H2O2 was observed in filtered supernatants of cultures of several toxic algae [74], consistent with the same photochemical or other abiotic processes acting on DOM. On the other hand, the contribution of factors such as colony morphology [77], nutrient deficiency [78], CO2 depletion [79], and lectin induction [76] to O2•−production all suggest active processes by the algae involved. Importantly however, some of these experiments were performed with axenic cultures whereas others also had undefined populations of associated bacteria; therefore it is not clear how much of the observed ROS production was caused by the algae and how much by the bacterial component, or how the presence of different groups of bacteria may have altered the metabolism or ROS dynamics of the cultures during the experiments.
Regardless of the mechanisms underlying ROS production by algal blooms, they are capable of dramatically increasing ROS fluxes and steady-state H2O2 concentrations compared to normal conditions. For example, the bloom-forming seaweed Ulva secretes sufficient H2O2 to more than double the ambient concentration of H2O2 in coastal ecosystems where it dominates [80]. High concentrations of superoxide (∼10 nM) were detected on the surface of coral Porites astreoides in the dark, independent of photosynthesis, and its symbionts also contributed extracellular superoxide in the dark and at low light levels [81]. ROS concentrations in a number of harmful algal blooms are so high that they have been considered a possible source of ichthyotoxicity [[82], [83], [84], [85], [86]] (although other studies such as [87] have argued against this conclusion). One possible factor that may exacerbate the impact of blooms on the prevailing H2O2 concentration is the fact that blooms have mechanisms for remaining at the sea surface, forming thin, very dense films at the air-water interface. One experiment using a recently developed microelectrode method for H2O2 determination found H2O2 concentrations 3–5 times greater in the upper few millimeters of the water column as compared to water a few centimeters deep during a freshwater Microcystis bloom that would have eluded older measurement protocols [88]; it is possible that such fine-scale measurements may reveal more high-ROS microenvironments in marine systems as well.
3.1.4. ROS production by non-photochemical abiotic processes
The final major source of ROS is via non-photochemical, abiotic redox processes, predominantly through the oxidation of reactive reduced species by O2. Such processes can potentially result in significant fluxes of ROS whenever there is a continuous supply of these reduced species to oxygenated waters, such as at the interface of reducing sediments with oxygenated waters. Murphy et al. demonstrated that, in the presence of hydrous ferric oxides, S2− undergoes net oxidation by O2 to produce O2•−and H2O2 [89]. More recently, Shaw et al. demonstrated a similar process for ROS production in hydrothermal vents [90] and calculated that the resulting flux of H2O2 to the global ocean via this pathway is of a similar magnitude to that produced by photochemical processes in the surface ocean. Non-photochemical, abiotic ROS production could also occur at the interface of reducing microenvironments within the oxic water column, such as at the surfaces of abiotic particles (e.g. minerals). However, studies examining dark, particle-associated ROS production in the marine water column have primarily found this source to be biological (e.g. Refs. [81,91,92]).
3.2. Microorganisms: the predominant sink for marine H2O2
As has been previously mentioned, most ROS have very short residence times, and are rapidly eliminated by reaction with other molecules near their point of generation. Those reactions involving DOM or metals, or involved in toxic attacks on biomolecules, will be considered in later sections. Here we exclusively consider the role of microorganisms in the removal of H2O2 from their environment. Microbes are generally considered the primary agents of active H2O2 quenching in the ocean [1] and indeed in freshwater systems as well. Cooper and Zepp [93] found that H2O2 decomposition in freshwater was arrested in chemically sterilized samples. Similarly, Petasne and Zika [94] found that 0.2 μm filtration of samples greatly reduced H2O2 decay rates, and that most of the H2O2 degradation ability was contained within the 0.2–1 μm size fraction, implicating bacteria as the predominant players in H2O2 removal. Both these studies found that re-seeding sterilized samples with cultured bacteria restored H2O2 degradation, confirming the important role played by microbes as the predominant sink for H2O2 in natural waters.
Microorganisms that inhabit oxic waters have evolved many strategies for protecting themselves from ubiquitous H2O2. The most conspicuous of these defenses are ROS-scavenging enzymes, including both catalases and peroxidases. These enzymes are widespread in both microbes and macroscopic life and have traditionally been thought to be essential for survival in the presence of atmospheric concentrations of O2, although recent evidence suggests that they are actually present in only a fraction of marine microbes [20,95]. Both catalase and peroxidase are phylogenetically diverse – there are multiple, independently evolved enzyme groups that catalyze these reactions – and many use redox-active metal cofactors containing Fe or Mn to facilitate the removal of H2O2 [96]. Canonical catalase disproportionates H2O2 directly into water and O2, but other catalases as well as metal-cofactor peroxidases are also capable of reducing H2O2 using electrons from other metabolites such as ascorbate. Peroxiredoxins, another group of peroxidases common in marine bacteria, do not have metal cofactors, but rather use redox-competent cysteine residues to reduce peroxides (e.g., H2O2 as well as lipid and other organic peroxides) using electrons from small molecules (e.g., glutathione and thioredoxin) that are common intermediaries in cellular redox processes [97]. While some peroxiredoxins are clearly front-line defenses against H2O2 (e.g., AhpC in Escherichia coli [98] and the recently-discovered 2-Cys peroxiredoxin from Chattonella marina [[99], [100], [101]]), the function of others appears to be directed more toward scavenging of lipid or other organic peroxides [102].
In the ocean, removal of H2O2 appears to occur primarily through the action of microbial catalases and peroxidases, although the relative importance of the two enzymes as well as the identities of the organisms expressing them remain incompletely understood. In a study of H2O2 decomposition using 18O-labelled H2O2, Moffett and Zafiriou [103] determined that the majority of H2O2 was converted to O2, indicating that most of the H2O2 was broken down using catalase. These results were recently revisited with improved methods, revealing that H2O2 removal processes proceed from catalase-like to peroxidase-like redox profiles with increasing depth [104]. Interestingly this same study also revealed that some intermediate depth samples exhibited unexpectedly high oxidation:reduction ratios, suggesting the existence of unknown powerful oxidants capable of scavenging H2O2 from the water column.
While catalase appears to be the predominant sink for H2O2, at least at the ocean's surface, only a minority of marine microorganisms produce it. In a re-examination of several diel metatranscriptomic studies from the surface ocean, the bacterial enzyme catalase-peroxidase (KatG) was much more abundant than the monofunctional catalase found in both bacteria and eukaryotes in all samples [20]. Algal ascorbate peroxidases were also widespread, and the expression of both enzymes was found to oscillate over the diel cycle, with higher expression during the day than at night, corresponding with increased rates of H2O2 removal during the day than at night. The taxonomic composition of the two enzymes varied between stations, but KatG sequences like those from heterotrophic bacterial clades Alteromonodales, SAR11, and SAR116 and green algal ascorbate peroxidase sequences were always abundant and over-represented relative to their share of the overall microbial population. Thus, a small subset of total microbial diversity in all these samples appeared to be responsible for the majority of the effort required to remove H2O2 from the environment.
If only a fraction of the total microbial community is engaged in ROS removal and most of the removal is performed by intracellular enzymes, how do the other organisms survive? First, it is possible that some ROS scavenging enzymes are actually secreted into the environment; extracellular SODs are common in many bacteria and fungi [105], and a few organisms also express extracellular catalases [[106], [107], [108]]. Cellular contents may also be released into the environment as a result of sloppy feeding or viral lysis [109]. Residual H2O2 degradation has been detected in sterile-filtered seawater in a variety of studies as well [94,110]. More likely, however, is that intracellular H2O2 degradation unavoidably also reduces the extracellular H2O2 concentration due to the fact that H2O2 is freely membrane permeable [111]. Thus, it is possible that the entire microbial community can be protected from H2O2 by a minority of H2O2-scavenging organisms. This phenomenon creates a feedback mechanism that may be responsible for the evolution of complex, market-like interdependencies in marine microbial communities wherein H2O2-removing “helpers” protect their neighbors who in some cases “pay” them with other products, such as photosynthate or vitamins, a process described by the Black Queen Hypothesis [95,112].
4. The impact of ROS on marine biogeochemistry
As summarized previously, each of the various ROS exhibit quite different characteristics in terms of their reactivity. While all can act as oxidants, some are also able to act as reductants under different circumstances. Thus, ROS impact the biogeochemical cycling of a wide range of elements. Some of the major mechanisms by which ROS react with various elements, and the impact on their biogeochemical cycles, are discussed in further detail below.
4.1. ROS and organic carbon
Organic carbon (OC) molecules in their ground state are relatively stable against direct oxidation by 3O2, despite the thermodynamic favorability of the overall reaction with respect to the O2/H2O couple, due to the barrier imposed by the one-electron transfer requirement. This constraint is overcome when OC molecules are in an excited (typically singlet) state [4], as can occur by photochemical excitation of CDOM (although the majority of excited CDOM molecules transfer energy rather than electrons to 3O2, yielding 1O2 [9]). While 1O2 may itself oxidize certain OC compounds, the effectiveness of this reaction is limited by its very short lifetime due to relaxation by solvent water [9]. Consequently, 1O2 is unable to diffuse far from the site of its formation, and the highest concentrations are typically found in hydrophobic microenvironments within large CDOM molecules (e.g. humic acids) [113]. In these localized environments, oxidation by 1O2 may represent a significant pathway for degradation of certain OC compounds, including organic pollutants [9].
OC is also readily oxidized by •OH. Due to its high reactivity, •OH is typically short-lived and thus this process is only significant where there is a continuous flux of •OH production, usually from photochemical processes in marine systems. •OH can also be formed though the reduction of H2O2 via Fenton or Fenton-like reactions, but these are unlikely to occur at biogeochemically significant rates in most marine systems unless there is a continuous source of reduced trace metals. This can occur in the photic zone though photochemical reduction of some metals, such as Fe, giving rise to the so-called photo-Fenton reaction. The photo-Fenton reaction may be a major pathway for OC degradation under conditions like those found in marine waters [114], although evidence suggests that the primary oxidant may not necessarily be •OH. This is consistent with the fact that the Fenton reaction does not necessarily produce free •OH, but may instead yield other oxidants such as Fe(IV) (ferryl iron), depending on the complex speciation of iron [115]. While the reactivity of Fe(IV) towards OC may differ from that of free •OH, it is still a powerful oxidant [116]. This may also be true for the Fenton-like system involving Cu(I) and H2O2 [117].
The reaction between •OH and OC may be a significant sink for OC in marine waters [118]. This reaction initially produces carbon-centered radicals, which (unlike bulk OC) may subsequently react with 3O2. This process typically involves addition of O2 to the carbon-centered radical to yield a peroxyl radical:
| (6) |
where R represents an organic group and ROO• the peroxyl radical. Peroxyl radicals are typically far more stable than carbon-centered radicals under oxic conditions, since the latter typically react with 3O2 at near diffusion-controlled rates [119], but may undergo further reaction with or other peroxyl radicals to yield organic peroxides:
| (7) |
| (8) |
where ROOH and ROOR′ are organic peroxides. The fate of these organic peroxides is discussed further below.
In contrast to both 1O2 and •OH, is highly selective in its reactions due to its unusual electronic structure, such that it readily reacts only with molecules possessing unpaired valence shell electrons, including a range of redox-active trace metals and organic radicals. is also unique among the ROS in that it is thermodynamically more favorable as a reductant than as an oxidant under typical conditions found in oxic marine waters (Fig. 1). As such, does not readily react with bulk OC, but may facilitate redox cycling of stable radical groups, such as semiquinones [120] and phenoxyl radicals [121,122], which are thought to be present in natural organic matter [122,123]. Goldstone and Voelker [124] first reported a non-metallic sink for in natural waters associated with the presence of humic and fulvic substances. Heller and Croot [125] showed that reactions with CDOM represented a significant sink of in tropical Atlantic surface waters, but that such a pathway was not a major sink for in the Southern Ocean [126]. King et al. [127] also showed that unidentified antioxidant compounds able to consume were present at concentrations of 100–400 pM in seawater samples from the South Atlantic Ocean, but only during daylight hours.
While H2O2 is a relatively powerful oxidant from a thermodynamic perspective [4], it does not readily oxidize OC under ambient conditions due to a high activation energy barrier for the reaction [6]. The oxidation of semiquinone-type radicals via a “metal-independent Fenton reaction” has been reported in aqueous solutions [128] and the formation of •OH by such a pathway has been reported in solutions of irradiated humic acids [129]. However, this process has not been widely examined in marine waters.
Organic peroxides may reach concentrations comparable to H2O2 of up to several hundred nM in surface nearshore marine waters [130], suggesting that they are similarly relatively unreactive. Nonetheless, relatively little is known about the fate of organic peroxides in the ocean, or their influence on biogeochemical cycles.
4.2. ROS and trace metals
ROS can potentially react with, and thus influence the redox speciation, of a range of redox-active trace metals in seawater. Among the biogeochemically relevant trace metals found in marine waters, Fe, Cu, Mn, Mo and V are particularly notable in terms of their ability to cycle between metastable redox states, at least in part because of the thermodynamic constraints imposed by the one electron transfer requirement in systems dominated by the O2/H2O redox couple [3]. This section will thus focus on these five trace metals as examples of trace metals with particular biological significance whose redox cycling in marine waters is well known to be influenced by ROS. This list is not intended to be exhaustive, however; there are a range of other redox active trace metals that are known to react with ROS under aqueous conditions (see, for example, compilations of reactions of [131] and •OH [132] with various trace metals and other compounds), including in seawater (for example oxidation of Cr(III) by H2O2 [133] or the suggested involvement of ROS in Hg cycling [134]).
As stated previously, H2O2 and •OH are predominantly oxidizing in nature at the pH of typical marine waters, with the notable exception of the reduction of Cu(II) by H2O2 (discussed further below). In contrast, is capable of functioning as both an oxidant and reductant and, as such, plays a somewhat special role in relation to the redox cycling of trace metals. The role of 1O2 in redox cycling of trace metals is likely to be limited due to its low concentrations and localization near the site of its predominantly photochemical generation, but due to their unpaired valence shell electrons the trace metals discussed above may all react directly with 3O2, albeit at varying rates.
The general sequence of reactions between ROS and the trace metals may be described as follows:
| (9) |
| (10) |
| (11) |
| (12) |
where M represents the metal and Mn+ and M(n+1)+ are reduced and oxidized redox states, respectively. (The first reaction is shown as a two-way reaction due to the commonly reducing behavior of , in contrast to the other reactions). A critical control on the nature of ROS-induced redox cycling of trace metals is the relative rates of each of these reactions, leading to three general scenarios:
Scenario 1 – The forward reactions in eqs. (9), (10), (11) and/or 12 are fast relative to the backward reaction in eq. (9): the trace metal will exist primarily in its oxidized state and does not readily undergo ROS-induced redox cycling. The relative importance of 3O2 compared to other ROS as oxidants of the trace metals will depend on the relative rates of the forward reactions in eqs. (9), (10), (11), (12)).
Scenario 2 – The forward reactions in eqs. (9), (10), (11), (12)) are all slow compared to backward reaction in eq. (9): the trace metal will exist primarily in its reduced state and does not readily undergo ROS-induced redox cycling.
Scenario 3 – The forward and backward reactions in eq. (9) are both fast relative to the reactions in eqs. (10), (11), (12): the trace metal will be rapidly cycled between the reduced and oxidized states and reaches a steady-state redox equilibrium with the O2/ redox couple.
It is important to note that the relative reaction rates depend on both the rate constant, which is an intrinsic property of the reacting species, and the concentrations of 3O2 and the various other ROS. Thus, the applicability of each of these scenarios will depend on both the chemistry of the trace metal involved and local conditions and concentrations.
4.2.1. Iron
The reactions of ROS with Fe are perhaps the most well-studied among these trace metals. In typical oxic marine waters, Fe falls into scenario 1 above. While Fe(III) is the thermodynamically stable redox state of free Fe in oxic marine waters, Fe(II) has been measured in a range of surface and deep ocean environments even under fully oxygenated conditions (e.g. Refs. [135,136]). The existence of measurable Fe(II) in these environments can result from photochemical and biological processes, in addition to physical transport from more reducing waters (e.g. in oxygen deficient zones [137], or potentially in reducing microenvironments in sinking particles [138]). The biogeochemical cycling of Fe in these oxic waters is tightly coupled to the cycling of O2 and [73] via the two-way reaction:
| (13) |
The tight coupling between these redox pairs means that it is often difficult to resolve whether the redox cycling is initiated by the formation of Fe(II) or [73], although arguably this may be unimportant in terms of the overall biogeochemistry. The dynamics of this system additionally depends on the chemical speciation of Fe, which is strongly influenced by the complexation of both Fe(II) and Fe(III) by organic matter [e.g. Refs. [[139], [140], [141]]]. This influence of iron speciation on reactivity with the O2/ couple has been examined in detail previously [73], and as such will not be explored further here.
Fe(II) may also be oxidized by H2O2 and •OH according to the series of reactions shown in eqs. (10), (11), (12)). Both the rates and mechanisms of these reactions depend strongly on the speciation of Fe(II), in addition to the relative concentrations of the various ROS. For example [142], showed that humic-type organic matter can inhibit oxidation of Fe(II) by H2O2 [115]. additionally showed that the oxidation of Fe(II) by H2O2 yields •OH when the Fe(II) is complexed by certain organic ligands, but in the absence of organic complexation at circumneutral pH appears to yield a different (but still highly oxidizing) species. In lake waters, the reduction of organically complexed Fe(III) by was observed to generate an “autocatalytic Fenton reaction” [143], resulting in significant •OH production due to redox cycling of Fe(II), and subsequent oxidation of OC by the generated •OH. Such processes are also likely to occur in marine waters.
4.2.2. Copper
Many of the reactions of Cu with ROS are analogous to those of Fe with ROS, although with some important differences. Like Fe, Cu may exist in both reduced and oxidized states in marine waters, with the oxidized Cu(II) state the most thermodynamically stable. Unlike Fe(II), the reduced Cu(I) state reacts at near diffusion-limited rates with 3O2 except when complexed to certain stabilizing ligands, notably including Cl−, which is an important factor in why Cu(I) is able to persist at measurable concentrations in marine waters in some circumstances [144,145]. However, Cu(I) and Cu(II), including their organic complexes, react at near-diffusion controlled rates with [146]. As such Cu also tends to fall into scenario 1 above, in which both Cu(I) and Cu(II) may coexist at biogeochemically significant concentrations particularly in surface waters [147,148]. Also similarly to Fe, both Cu(I) and Cu(II) may be complexed by organic matter in marine waters (e.g. Refs. [149,150]), which influences the kinetics of the various reactions shown in eqs (1), (2), (3), (4)) and hence its overall redox cycling dynamics [151]. However, unlike Fe(III), Cu(II) can be reduced by H2O2 at biogeochemically significant rates according to the reaction [152]:
| (14) |
This reaction introduces an additional mechanism by which ROS can induce redox cycling of Cu between the Cu(I) and Cu(II) states. Cu can also participate in Fenton-like processes under marine conditions, but as with Fe the formation of •OH through this process depends on factors such as pH and complex speciation of Cu [117,153].
4.2.3. Manganese
Manganese has been measured to exist in three redox states in marine systems: Mn(II), Mn(III) and Mn(IV). The most oxidized Mn(IV) state is thermodynamically favored under oxic conditions with respect to the overall O2/H2O redox couple, but the first electron transfer step in the oxidation of Mn(II) by 3O2 is thermodynamically unfavorable (i.e. with respect to the O2/ redox couple) [154]. Hence, the oxidation of free, dissolved Mn(II) by 3O2 under conditions typical of marine waters is negligibly slow [155], but can be accelerated by microbes, organic complexation or adsorption to mineral surfaces (see Refs. [156,157] for detailed reviews). Mn(II) is nonetheless often the most abundant redox state measured in oxic marine waters [157].
In contrast, the oxidation of Mn(II) to Mn(III) by is thermodynamically favorable under conditions found in marine waters [154], and has been shown to occur experimentally under similar conditions via either direct addition of [ 158,159 ] or by microbial production [160]. However, oxidation of Mn(II) via this pathway appears to be readily reversible, such that net oxidation of Mn(II) to Mn(IV) may not necessarily occur, due to the reverse reaction in which intermediate Mn(III) is reduced back to Mn(II) by H2O2 [157,158,161]:
| (15) |
As such, for net oxidation of Mn(II) to Mn(III) to occur via this pathway, H2O2 produced by the reaction must be rapidly consumed via other processes.
Mn(III) may also be able to persist in marine waters when complexed [157], and has been shown to represent a substantial proportion of the total dissolved Mn even under oxic conditions [162]; however there is currently only limited evidence for this. The major fate of Mn(III) is thought to be disproportionation:
| (16) |
Mn(IV) is highly insoluble in marine waters, and predominantly exists as Mn(IV), or in mixed valence Mn(III)/Mn(IV), oxides. Reductive dissolution of these Mn oxides has been shown to be induced by reactions with both and H2O2 [163]. The ranges of potential reactions of Mn(II), Mn(III) and Mn(IV) with and H2O2 are consistent with observations of dynamic redox cycling of Mn in marine systems (e.g. Ref. [164]), and the suggestion that redox cycling of Mn may be a potential mechanism for the observed decay of H2O2 in marine waters [104].
4.2.4. Vanadium
Vanadium can exist in the V(III), V(IV) or V(V) redox states in aqueous systems; however, the V(III) state is only thermodynamically favored under strongly reducing conditions [165]. In oxic marine systems, V(V) is the most thermodynamically stable state, but both the V(IV) or V(V) states have been measured at abundances of >10% of total dissolved V in oxic marine waters (e.g. Refs. [166,167]).
The rate constant for oxidation of VO(OH)+, the dominant V(IV) species at circumneutral pH, by 3O2 is around 1.1 M−1s−1 [168], which is only slightly lower than that for inorganic Fe(II) under similar conditions. However, as with many other trace metals discussed here, both V(IV) and V(V) form complexes with organic matter ([169] and references therein), which likely influences V redox dynamics. Given that oxidation by 3O2 proceeds via one-electron transfer steps, this reaction would be expected to yield , but this has not been experimentally confirmed. V(IV) has been reported to react with HO2 (the conjugate acid of ) under highly acidic conditions in aqueous solution [170]. It is also oxidized by H2O2 under circumneutral conditions [171]. However, there remains a considerable knowledge gap around the dynamics of ROS-induced vanadium redox cycling in marine waters, including its reactions with and H2O2, which have not been well studied under these conditions.
4.2.5. Molybdenum
Like vanadium, molybdenum can exist in three redox states in aqueous systems: Mo(IV), Mo(V) and Mo(VI) [172]. Of these, Mo(IV) is known to occur only under highly reducing conditions, with Mo(VI) being the most thermodynamically stable and dominant form under oxic conditions, and Mo(V) typically found in mildly reducing microenvironments [172]. Relatively few studies have examined Mo redox speciation in oxic waters. Wang et al. observed the coexistence of both Mo(V) and Mo(VI) in both fully oxic and low oxygen (<10 μM dissolved O2) conditions in the Peconic River Estuary, with the reduced Mo(V) present as a greater proportion (up to 15%) of total dissolved Mo under the lower oxygen conditions but nonetheless present at measurable concentrations in some fully oxygenated surface waters. However, some doubts have been raised about the existence of Mo(V) in the oxygenated water column [173]. In any case, the diffusion of Mo(V) from reducing environments (e.g. sediments) into oxygenated waters would likely result in oxidation of Mo(V) to Mo(VI) by 3O2, given that reaction is thermodynamically favorable to proceed via one-electron steps [3]. This would suggest the involvement of ROS in redox cycling between Mo(V) and Mo(VI); however we are unaware of any experimental evidence for such processes in the literature at this time.
4.3. ROS and other inorganic species
ROS have been implicated in the biogeochemical cycling of a range of other elements in marine systems. In the case of iodine, I− reacts exceedingly slowly with 3O2, but reacts readily with [174], H2O2 [175] and organic peroxides [176]. Consistent with these observations, Luther [177] demonstrated that the oxidation of halides under conditions typical of seawater is thermodynamically feasible by a range of ROS including 1O2, , H2O2 and •OH, with the effectiveness of each depending on the exact pathway.
ROS are additionally known to react with transient inorganic species that are found in seawater, including nitrogen radicals such as NO• and sulfur radicals such as thiols [178]. While the reaction of with NO• to yield peroxynitrite in alkaline solutions has been known for decades [179], recent work has shown this reaction is a major sink for photochemically generated NO• in marine waters [180]. Where these radicals exist in oxygenated waters, often as transient species, reactions with ROS are likely through so-called ‘cryptic cycles’ in which the rapid turnover and low concentrations of the species involved renders them largely undetectable [181].
5. ROS impacts in marine ecosystems
In addition to the roles that ROS play in biogeochemical cycling, they also can create profound biological effects. ROS have predominantly been considered to have negative impacts on living organisms due to their ability to react, often indiscriminately, with biological macromolecules. However, increasing evidence indicates that ROS can also play beneficial roles for individual organisms and at the ecosystem level, including through potentially favorable moderation of the local redox environment (e.g. by increasing the bioavailability of micronutrients like iron [73]), but also through biological processes such as regulating growth and resisting pathogens. The beneficial effects of extracellular ROS have recently been reviewed by Hansel and Diaz [2], so will not be considered in further detail here. This section will therefore focus primarily on the potential negative impacts of ROS in marine ecosystems.
Steady-state concentrations and fluxes of ROS in the ocean are generally well below levels that cause toxicity in laboratory studies. For instance, the bacterium Escherichia coli was able to tolerate acute exposure to 15 mM H2O2 with only a moderate loss of viable counts and a short lag period [182]; this value is almost five orders of magnitude higher than any in situ concentration measured in the surface ocean. Animals easily tolerate exposure to even higher concentrations of H2O2, with 3% (∼100 mM) H2O2 routinely used as a topical first-aid antiseptic in humans, for example. Environmental concentrations of free and •OH are even lower, and orders of magnitude less than concentrations measured in healthy animal cells. Despite this, research over the past decade has revealed that some important marine microbial groups are vulnerable to even these low ROS concentrations. Moreover, some marine microenvironments, such as saltmarsh pools and certain kinds of algal blooms, may accumulate high concentrations of ROS capable of damaging even relatively well-protected organisms. In this section, we review the cellular targets of ROS attack and consider several key organisms that are particularly at risk.
5.1. Targets of ROS attack in marine organisms
ROS, especially •OH, can attack all classes of biological macromolecules. •OH may attack the backbone deoxyribose sugars in DNA, fragmenting them in a variety of ways leading to single- or double-strand breaks [105,183,184]. Also, the nucleotide bases themselves may be modified, either before or after incorporation into a DNA molecule. 8-Hydroxyguanine, one result of •OH attack on guanine, base pairs with thymine rather than cytosine, and if not repaired may lead to transversion mutations. RNA, including relatively long-lived rRNA and tRNA molecules, is even more vulnerable to oxidation than DNA [185], and oxidative mRNA damage predictably correlates with translational errors [186].
•OH is also capable of many non-specific reactions on proteins, oxidizing both side chains and polypeptide backbones. The typical result of such oxidation is the formation of carbonyl groups, and the detection of this “carbonylation” using antibodies is a common method for measuring oxidative protein damage in vitro [105]. Compared to •OH, other ROS have more well-defined protein targets. degrades proteins that bind iron, such as ferritin, catalase, and Fe–S cluster enzymes [105,187,188], causing the release of free Fe into the cytoplasm, allowing destructive Fenton chemistry. H2O2, while more stable than either or •OH, attacks certain proteins even in the absence of free Fe, including important, constitutively expressed proteins such as the β-subunit of ATPase and prokaryotic translation elongation factor G [189,190].
Lipids are also prone to attack by •OH, and oxidized lipids are capable of dramatically magnifying the effects of ROS attack via radical propagation. In a typical scenario, •OH attacks a double bond in an unsaturated lipid and abstracts a hydrogen atom, leaving a carbon radical and water. This new lipid radical may react with any other radical with no energy barrier. One possible reaction is with a second lipid radical, quenching the radical character of the system and forming a stable, cross-linked species that terminates radical propagation. This reaction is rare in oxic systems, however, compared to reaction with 3O2 to form a lipid hydroperoxide and a new lipid radical. This propagation step may be carried out many times before the original lipid radical is quenched by a termination reaction. Lipid hydroperoxides are capable of attacking membrane proteins as well as facilitating the transition of 3O2 to 1O2 in the cytoplasm [191].
5.2. Major ROS-vulnerable marine populations
There are two ways that an organism living in the ocean may experience oxidative stress. First, the stress may arise intracellularly because of unbalanced metabolism, increased temperature or pH stress, or exposure to toxic substances in the environment such as xenobiotics and heavy metals. Some of these stressors were described above in section II. Second, the stress may arise through direct exposure to ROS in the environment. As described above, extracellular ROS fluxes in marine systems tend to be substantially lower than doses shown to negatively impact organisms in the laboratory. However, these observations may underestimate the direct risk organisms face from ROS in their native habitats. For instance, an E. coli population in natural river water exposed to light experienced ∼99% mortality after 3 days unless catalase or sodium pyruvate (a •OH trap) was added to the water prior to illumination [192]. Based on other studies, the likely H2O2 concentration of the river water was no greater than 10 μM (Table 1), more than 1000-fold less than the concentration necessary to kill laboratory populations of E. coli [182]. Laboratory culture media often contain hidden sources of ROS [[193], [194], [195], [196]] which may select for disproportionately high levels of resistance over time, obscuring the sensitivity of native populations. More broadly, Xenopoulus and Bird [197] reported >50% reduction of bacterial productivity in a lake following exposure to 100 nM H2O2 as compared to a catalase-treated control. Similar to these freshwater observations, a study in a coastal macrophyte-dominated ecosystem found that additions of as little as 20 nM H2O2 significantly reduced bacterial productivity, with 1 μM completely eliminating productivity over a 1 h timescale [80]; importantly however, other studies have shown no effect on bacterial production with H2O2 addition [198].
Among the first marine organisms to be conclusively demonstrated to have high sensitivity to H2O2 inhibition was Prochlorococcus, the numerically dominant phytoplankton genus throughout the temperate and tropical oligotrophic oceans. When grown in pure culture, Prochlorococcus is completely inhibited by 800 nM H2O2, but when grown in co-culture with a wide variety of heterotrophic bacteria, it can tolerate much higher exposures [199,200]. In the absence of bacteria, open ocean seawater from areas that are rich in Prochlorococcus rapidly accumulates H2O2 concentrations at least this high when exposed to sunlight, suggesting that Prochlorococcus is obligately dependent on other members of its community to survive. This sensitivity likely exists because Prochlorococcus has an extensively streamlined genome, which contains no genes for catalase or any heme peroxidases, essentially eliminating its capacity to defend itself from exogenous H2O2 [201]. This lack of intrinsic H2O2 resistance is reflected in conspicuous vulnerability of pure Prochlorococcus cultures to a wide variety of stresses, including dark exposure [202,203] and suboptimal temperatures [204]. While Prochlorococcus is more vulnerable than most, cyanobacteria in general appear to be vulnerable to lower doses of H2O2 than most other studied organisms. Both unicellular cyanobacteria and filamentous forms experienced high mortality when exposed to ∼10 μM H2O2, a value that was consistent across numerous experiments in different laboratories [[205], [206], [207], [208]]. On the other hand, eukaryotic microalgae (green algae, diatoms) survived doses as high as 1 mM [206,207].
H2O2 can also inhibit phytoplankton growth by altering the availability of limiting nutrients. The primary source of Fe in surface waters of the Atlantic ocean is atmospheric deposition, e.g. via dust from the Sahara Desert. Much of this Fe enters the ocean in the form of rainwater, which can also carry H2O2 at concentrations hundreds of times higher than surface seawater (Table 1). Experiments in March 2000 at the Bermuda Atlantic Time Series stations showed that additions of either FeCl2 or FeCl3 to seawater increased chlorophyll-a concentrations over a three-day incubation, indicating Fe limitation of primary production in the absence of seasonal dust deposition [29]. However, when the Fe was added as a synthetic rainwater mixture also containing H2O2, the increase in phytoplankton growth compared to unamended controls was completely eliminated. The presence of H2O2 effectively made the Fe unavailable, presumably through Fenton chemistry and precipitation of insoluble Fe(III) complexes. It is also possible that the increase in H2O2 (to a final concentration of approximately 400 nM) may have directly damaged or killed phytoplankton in this experiment.
Another ecologically important group of organisms, the ammonia-oxidizing archaea (AOA) of the phylum Thaumarchaeota, are also catalase and peroxidase deficient and conspicuously vulnerable to H2O2 [209]. AOA are important regulators of the speciation of bioavailable nitrogen in the ocean, representing up to 40% of the total bacterioplankton in some parts of the World Ocean and directly or indirectly contributing significant fractions of the greenhouse gases N2O and CH4 to the atmosphere [210]. Some AOA strains are also among the most H2O2-vulnerable aerobic organisms known. For instance, the growth of one Southern Ocean strain was inhibited by 10 nM H2O2, nearly two orders of magnitude lower concentration than the lethal dose for Prochlorococcus, and the addition of only 6 nM H2O2 to a field population completely eliminated nitrification while not affecting overall bacterial production at all [211]. Inhibition of AOA by nanomolar H2O2 was also observed in isolates from coastal temperate seas and the deep ocean [212,213], and enhancement of growth was frequently seen after addition of H2O2-scavenging pyruvate or catalase, or by co-culture with helper bacteria [[213], [214], [215]]. AOA isolates have been observed to secrete H2O2 into their growth media in proportion to the amount of ammonia oxidized, suggesting that their vulnerability may arise as a side effect of their primary metabolism [215]. If true, this may represent a difficult-to-overcome constraint on AOA in the ocean, and could account for the fact that they are primarily active in deep water, only thriving at the surface during polar winters when photochemically generated H2O2 is at a minimum [211].
In general, macroscopic organisms, and even multicellular microscopic organisms such as fish and invertebrate larvae, are much less vulnerable to H2O2 in the nanomolar-micromolar range than these bacteria, although a number of them are inhibited by doses low enough to be of concern in the presence of some human activities (see section VI below). However, there are exceptions. For instance, one study demonstrated substantial variability in H2O2 tolerance in different clonal populations of the starlet sea anemone Nematostella vectensis, which inhabits coastal salt marshes where natural H2O2 concentrations can reach the 100 μM range. When anemones were sampled from South Carolina, New Jersey, and Nova Scotia, a clear north-south gradient in tolerance for acute H2O2 exposure was observed, supporting the hypothesis that elevated temperatures correlate with greater exposure to H2O2 [216]. Interestingly, when the same researchers sampled anemones from a variety of saltmarsh ponds in Massachusetts, they found substantial variation in H2O2 tolerance between populations. Notably, these organisms were severely impacted by H2O2 concentrations within the range observed in the estuarine waters they inhabit (Fig. 4), so this high variation over a short spatial scale suggests that H2O2 in these ranges represents a sufficiently potent stress to affect local adaptation, at least in this species.
5.3. Impacts of ROS on marine biology experiments
Along with the discovery that some marine organisms are negatively affected by naturally occurring concentrations of ROS comes the possibility that cryptic ROS production caused by experimental procedures may impact results. For example, in a series of in situ mesocosm experiments in the Canary Islands in 2016 designed to test the effects of different degrees of ocean acidification on the structure and function of natural plankton assemblages, H2O2 concentrations within the mesocosm enclosures were observed to increase significantly (>200 nM) in comparison to ambient conditions (∼50 nM) after several days [198,217]. The authors concluded that these increases were caused by the mesocosm enclosures inhibiting vertical mixture of surface and deep waters, causing H2O2 formed near the surface to remain concentrated relative to conditions outside the enclosures. Elevated H2O2 correlated with decreased bacterial and phytoplankton abundances in these experiments, possibly indicating H2O2 toxicity that may have killed or otherwise inhibited these cells, but also confirming that reduced bacterial concentrations exerted a positive feedback on H2O2 concentration by removing the primary H2O2 sink in the mesocosms. Further, the elevated H2O2 concentrations likely impacted the lability of DOM and trace metals and, as a consequence, the availability of important growth substrates.
Unexpected ROS production can also influence laboratory experiments with marine microorganisms. For example, HEPES and other zwitterionic pH buffers commonly used in culture media for algae and bacteria generate H2O2 continuously when exposed to light, and can produce lethal concentrations for some of the more sensitive species (e.g. Prochlorococcus) in less than 24 h [194]. Collecting Thalassiosira weissflogii diatoms by syringe instead of peristaltic pump increased their release of H2O2, which could influence transcriptomic studies of these organisms by artifactually increasing oxidative stress gene expression, for example [75]. Based on these observations, researchers working in these systems need to consider H2O2 concentrations, and the effect of experimental procedures on them, in the same manner they measure light, temperature, and other physicochemical properties that may be important confounding variables, and should also consider the possibility that laboratory strains are substantially more H2O2 resistant than their relatives in the ocean.
6. Impacts of human activity on marine ROS
There are several reasons to expect that the threat of oxidative stress will increase for marine organisms in the future as secondary effects of anthropogenic changes, including both the introduction of pollutants into marine systems as well as the broader impacts of CO2 emissions on the physicochemical environment of the ocean. In this section we consider two vectors for increased oxidative stress, specifically the natural increase in ROS due to anthropogenic CO2 increase, and secondarily human activities that may introduce or concentrate novel ROS sources in the environment.
6.1. Global CO2 increase and ROS
The CO2 concentration of Earth's atmosphere is increasing at an unprecedented pace due to human consumption of fossil fuels. The two most profound sequelae of this change are i) an increase in global average temperature, both in the atmosphere and the ocean, and ii) an imbalance in the carbonate buffering system of aquatic ecosystems, leading to acidification. Both conditions could lead directly to increased ROS exposure for marine organisms, as well as creating secondary changes to marine environments that may exacerbate oxidative stresses.
First, higher temperatures are likely to lead to greater rates of biological H2O2 production due to temperature stress on marine organisms, at least in the short term before local populations either adapt or are outcompeted by more temperature-tolerant competitors. When West Antarctic Peninsula seaweeds were exposed to 2-8 °C of warming they increased their rate of H2O2 release into the surrounding seawater as well as exhibiting metabolic signs of oxidative stress [49]. Similarly, the microscopic gametophytes of three Arctic kelp species released greater amounts of into the surrounding medium when exposed to elevated temperatures [218]. In both cases, the seaweeds themselves appeared to be unharmed by the conditions, but the possibility of enhanced ROS release negatively impacting the surrounding community was not explored.
Increased H2O2 release is also observed in sessile animals. For example, 16 different Symbiodinium coral symbionts exposed to heat stress showed substantial variability in signs of oxidative stress. Some strains exhibited severe electron transport chain damage resulting in production of large amounts of H2O2, whereas others avoided H2O2 accumulation by downregulating PSII [219]. Like the anemones described earlier [216], this observation suggests a substantial amount of intraspecific variation in ROS tolerance that may increase the rate of evolution in a changing ROS environment, but perhaps at the cost of reduced photosynthesis or other unforeseen physiological trade-offs.
Increased temperature may also lead to higher ROS fluxes in future waters. Studies across time and space under present conditions reveal that shallower, hotter waters accumulate greater H2O2 concentrations (Table 1, Fig. 3). One predicted effect of global warming on the ocean is the shoaling of the mixed layer, where higher temperatures strengthen thermal stratification of the ocean and yield shallower thermoclines [220]. Fig. 3 shows clearly that H2O2 concentrations drop off rapidly below the thermocline, suggesting that most production occurs near the surface and only accumulates near the surface because H2O2 diffuses slowly across this barrier. Thus, shallower thermoclines may yield greater steady-state H2O2 concentrations at the ocean's surface, which may negatively impact ROS-sensitive organisms such as Prochlorococcus.
Ocean acidification may additionally enhance the susceptibility of marine organisms to environmental ROS. Most acidification research has focused on its interference with calcification in corals and other shelled organisms, but acidification also creates oxidative stress in a wide variety of both calcified and non-calcified species by imbalancing intracellular redox states, resulting in oxidative stress (e.g. Refs. [52,54,221]) and enhanced vulnerability of some keystone species to a wide variety of both biotic and abiotic challenges [48,50,56]. In some cases, acidification may disrupt important symbiotic interactions as well. For instance, the synergistic “helper” interaction between Prochlorococcus and the bacterium Alteromonas appeared to shift from a commensal or mutualistic state under present-day conditions to an exploitative state under elevated CO2, with Alteromonas significantly downregulating its catalase genes and causing a dramatic increase in oxidative stress and cell death for Prochlorococcus [222]. Moreover, rates of hydroxyl radical formation by the Fenton reaction increase with H+ concentration [223] as well as with temperature, suggesting that not just the concentrations, but also the lethality of ROS will be enhanced in future oceans, and that oxidative stress enhancement may underlie some of the synergistic effects of “multiple stressors” on marine life.
We must note, however, that much remains unknown about the impact of CO2 increase on the World Ocean in general, and other effects of acidification or temperature increase may reduce ROS stress on marine organisms. For instance, higher temperatures will increase the rates of all chemical, and most enzymatic, reactions, which will simultaneously increase both ROS production and removal processes and may also increase organismal maximum growth rates. All biogeochemical cycles, including both the biotic and abiotic cycling of DOM and other C compounds, will speed up, potentially increasing the bioavailability of many nutrients, which could reduce nutrient limitation stresses and/or improve the ability of organisms to protect themselves from ROS. Much more research into these processes will be necessary before we can draw any confident conclusions about ROS exposure will affect life in future oceans.
6.2. Pollution and marine ROS
In addition to the global impacts of CO2 release, human activity is increasing the exposure of marine life to a wide variety of compounds that may increase oxidative stress. We have already discussed the effects of allelochemicals such as microplastics and heavy metals on oxidative stress in invertebrates, but in this section we will focus specifically on pollutants that may lead directly to increased ROS fluxes, particularly in coastal ecosystems.
6.2.1. Use of H2O2 in aquaculture
Since 1993, H2O2 has been used to control the growth of parasitic “sea lice” copepods and other ectoparasites in salmon aquaculture operations in Norway and elsewhere, with usage peaking in 2015 and remaining common today [224]. H2O2 has been popular because of its relatively low toxicity profile compared to other effective pesticides and the fact that it is removed from water bodies after a few days by microbes as described above [225]. Relatively high H2O2 dosages are necessary for complete lice control, however, with approximately 100 mM necessary to achieve 98% reduction in parasite load (Fig. 4). Because water from salmon farms is released directly into coastal seas, there is concern that these concentrations, which are orders of magnitude higher than naturally occurring aquatic H2O2 concentrations, may have toxic effects on off-target organisms. Indeed, studies have shown that several marine invertebrates and fish can be killed by lesser exposures, particularly in their immature forms (Table 2). For example, the LC50 for a 1-h H2O2 exposure for European lobster Homarus gammarus larvae increased from 5 mM to 22 mM as the larvae progressed through stage I-IV molts [226], although one study showed that adult Calanus copepods were killed by significantly lower H2O2 doses than adults [227]. Notably, assays that utilized longer exposures typically reported lower LC50 values, indicating that chronic exposure to lower doses of H2O2 can cumulatively increase damage even to complex multicellular organisms. For many of these species, lethal H2O2 doses were substantially lower than the recommended dosing for delousing of salmon enclosures, but it is unclear whether concentrations of H2O2 released from these ponds into the ocean is sufficiently high to kill significant numbers of animals, or if instead dilution by the bulk seawater is sufficient to eliminate their negative impacts. Importantly, however, much lower sublethal doses of H2O2 contribute to other syndromes for these organisms, including impairing their ability to find refuge or otherwise avoid predation [226,227], reducing their feeding rate ([228], Japanese language paper cited in Ref. [216]), or slowing healing processes [216]. These impediments may indirectly contribute to increased mortality for species living in the vicinity of aquaculture operations or other environments where humans use H2O2. Combined with the facts that repeated H2O2 treatment has negative impacts on salmon health [229] and appears to select for H2O2-resistant sea louse varieties [230], H2O2 has lost popularity in recent years in favor of mechanical and biocontrol delousing methods [225], but the practice remains widespread and worthy of further attention regarding its impacts on the surrounding natural environment.
Table 2.
Lethal limits for H2O2exposure in various organisms.
| Species | Type of assay | Effective dosage | Reference | |
|---|---|---|---|---|
| Cnidarians | Nematostella sp. | 14-d 100% mortality | 163 μM | [216] |
| Crustaceans | Mysis sp. | 1-h LC50 | 29 mM | [265] |
| Calanus finmarchicus | 1-h LC50 | 180 μM | [266] | |
| Calanus spp. Stage V larvae | 1-h LC50 | 6.3 mM | [227] | |
| Calanus spp. Stage V larvae | 25-h LC50 | 2.3 mM | [227] | |
| Calanus spp. Adult | 1-h LC50 | 1.4 mM | [227] | |
| Calanus spp. Adult | 25-h LC50 | 900 μM | [227] | |
| Crangon septemspinosa | 1-h LC50 | 94 mM | [265] | |
| Atemia salina | 24-h LC50 | 24 mM | [267] | |
| Crophium volutator | 96-h LC50 | 1.4 mM | [268] | |
| Homarus americanus, stage I larva | 1-h LC50 | 48 mM | [265] | |
| Homarus gammarus, larvae stages I-IV | 1-h LC50 | 5.2–22 mM | [226] | |
| Molluscs | Dreissena polymorpha | 72-h LC50 | 890 μM | [55] |
| Fish | Siganus fuscescens | 24-h LC50 | 6.6 mM | [269] |
| Trachurus japonicus | 24-h LC50 | 2.6 mM | [269] | |
| Oncorhynchus mykiss juveniles | 3-h 90% mortality | ∼9 μMa | [270] | |
| Phytoplankton | Microcystis aeruginosa | 3-h LC50 | 7.9–195 μM | [207] |
| Selenastrum capricornutum | 3-h LC50 | 119–625.3 μM | [207] | |
| Raphidocelis subcapitata | 3-h LC50 | 359–2088 μM | [207] | |
| Prochlorococcus | 24-h 100% mortality | 800 nM | [200] | |
| Zooplankton | Daphnia sp. | 48-h LC50 | 164 μM | [271] |
| Moina sp | 48-h LC50 | 58.8 μM | [271] | |
| Bacteria | Escherichia coli | 3 h-LC50 | 7.8 mM | [272] |
| Escherichia coli | 6 h 100% mortality | 15 mM | [182] | |
| Vibrio coralliilyticus | 0.5 h- LC50 | ∼15 μM | [67] |
Measured in the presence of harmful alga Heterosigma carterae; algal toxins likely enhanced ROS lethality.
6.2.2. Use of H2O2 to combat harmful algal blooms
Harmful cyanobacterial blooms are increasing globally, and this trend may continue in the next decades [231]. One study showed that in 100 lakes investigated in North America and Europe, 60% have observed a substantial increase in cyanobacteria since the industrial revolution [232], and recently, the model of Chapra et al. [233] predicted that the average days per year with cyanobacterial blooms in USA will increase from ∼7 d to 18–39 d by 2090. Rising temperatures and atmospheric CO2 as well as the increasing frequency of extreme hydrologic events increase the growth rates of algae and alter critical nutrient thresholds for the development of algal blooms [234,235]. The expansion of agriculture in developing countries also accelerates the input of nitrogen and phosphorus into coast area, which also promotes the occurrence of algal blooms.
A large body of work from Russia and the former Soviet Union, summarized in English by Skurlatov and Ernestova [236], suggested that environmental H2O2 has the positive effect of reducing the frequency and severity of toxic algal blooms. Consequently, toxin-producing freshwater cyanobacteria were rare in waters where the concentration of H2O2 exceeded 0.3 μM. When waters became loaded with ROS “traps” from wastewater effluents, however, toxic blooms frequently formed. As H2O2 is generally toxic at lower concentrations to microorganisms than macroorganisms, it has been proposed as a very promising algicide in the control of cyanobacterial blooms [231,234,235]. Researchers in 1986 discovered that as little as 50 μM H2O2 strongly suppressed photosynthesis and growth in the cyanobacterium Oscillatoria rubescens [237]. Afterwards, laboratory and field tests [206,238,239] proved the effectiveness of H2O2 in selectively eliminating cyanobacterial blooms and preventing bloom-forming species from surviving during winter [214]. Both high light and blue light were found to enhance the inhibition of H2O2 to Microcystis aeruginosa PCC 7806 [240]. A range of authors observed a dose-effect response between H2O2 and their effectiveness in the inhibition of different cyanobacteria. Considering the cost and ecological risk, a maximum dose of 5 mg L−1 (∼150 μM) was recommended for use in freshwater systems [241].
One major concern of using H2O2 to control Microcystis blooms was the release of the toxin microcystin from the lysing cells. However, a field experiment showed that the particulate and water soluble microcystin degraded 90% in less than 3 days. The decomposition of microcystin may be related to microbial degradation [242] but also to •OH catalyzation due to Fenton chemistry involving the added H2O2 [243]. Though H2O2 could not completely eliminate the harmful cyanobacterial bloom, it created a possibility for the rapid succession of phytoplankton populations from HAB species to non-harmful species, which could be readily consumed in classic microbial food chains [244].
Most applications of H2O2 to control algal blooms have been in freshwater systems, but recent efforts have also shown promise in marine systems. In the Norfolk Broads, a series of shallow saltwater lakes in Great Britain, the eukaryotic chrysophyte Prymnesium parvum is a toxic eukaryotic alga that has been responsible for massive fish kills. Administration of approximately 1.5 mM H2O2 to the Broads during a period between blooms reduced the ambient P. parvum levels by >90%, with effects starting only 2 h after exposure [245]. The H2O2 treatment also led to sizable changes in the bacterial community, with especially conspicuous losses of unicellular cyanobacterial taxa. Interestingly, the community rebounded within 4 d after treatment, suggesting that H2O2 did not cause a permanent shift in community structure although it could possibly help to reduce the magnitude of toxic taxa during a bloom. Similar concentrations were applied to a bloom of the toxic dinoflagellate Alexandrium in a brackish creek in the Netherlands, resulting in 99.8% reduction of algal biomass as well as elimination of the algal toxin from the water column [246]. The dosage necessary to destroy Alexandrium also caused severe reduction in zooplankton as well as altering the macroinvertebrate and even fish communities, however, reflecting the concerns raised in the previous section on the trade-offs in using H2O2 as a biocontrol agent.
7. Conclusions
The oxygen cycle in the ocean is considerably more complex than the simple shuttling of O between water and organic matter, with reactive intermediates engaged in a wide variety of reactions that have critical importance for the biogeochemistry of the sea. Improvements in our technological capability to measure ROS fluxes have led to an improved understanding of the cycling of DOM and trace metals in the ocean and have revealed previously unsuspected mortality pathways for microorganisms as well as potential confounding factors for oceanographic experiments. However, we still know comparably little about the mechanistic reasons for why some organisms are so much more susceptible to ROS damage than others, or how changing environmental conditions impact ROS fluxes and their effect on marine life. Critically, we also do not understand fully how human activity will affect ROS fluxes, nor how our use of ROS such as H2O2 for biocontrol may affect ecosystems. Because many stress pathways involve ROS, future efforts to mitigate harm to the environment from human activity must involve a deeper understanding of the pathways of formation and degradation of these enigmatic molecules.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
This work was partially funded by a grant to JJM from the US National Science Foundation (OCE-1851085) and an Early Career Investigator in Marine Microbial Ecology and Evolution award from the Simons Foundation to JJM.
Contributor Information
J. Jeffrey Morris, Email: evolve@uab.edu.
Zhiying Lu, Email: luzy05111036@yahoo.com.
References
- 1.Zinser E.R. The microbial contribution to reactive oxygen species dynamics in marine ecosystems. Environmental Microbiology Reports. 2018;10(4):412–427. doi: 10.1111/1758-2229.12626. [DOI] [PubMed] [Google Scholar]
- 2.Hansel C.M., Diaz J.M. Production of extracellular reactive oxygen species by marine biota. Ann. Rev. Mar. Sci. 2021;13:177–200. doi: 10.1146/annurev-marine-041320-102550. [DOI] [PubMed] [Google Scholar]
- 3.Rose A.L. The influence of reactive oxygen species on local redox conditions in oxygenated natural waters. Front. Earth Sci. 2016;4 [Google Scholar]
- 4.Sawyer D.T. Oxford University Press; Oxford: 1991. Oxygen Chemistry; p. 223. [Google Scholar]
- 5.Fridovich I. Oxygen toxicity: a radical explanation. J. Exp. Biol. 1998;201(8):1203–1209. doi: 10.1242/jeb.201.8.1203. [DOI] [PubMed] [Google Scholar]
- 6.Winterbourn C.C. The biological chemistry of hydrogen peroxide. Methods Enzymol. 2013;528:3–25. doi: 10.1016/B978-0-12-405881-1.00001-X. [DOI] [PubMed] [Google Scholar]
- 7.Foote C.S., Clennan E.L. Active Oxygen in Chemistry. Springer; 1995. Properties and reactions of singlet dioxygen; pp. 105–140. [Google Scholar]
- 8.Mopper K., Zhou X. Hydroxyl radical photoproduction in the sea and its potential impact on marine processes. Science. 1990;250:661–664. doi: 10.1126/science.250.4981.661. [DOI] [PubMed] [Google Scholar]
- 9.Vione D., et al. Indirect photochemistry in sunlit surface waters: photoinduced production of reactive transient species. Chem. Eur J. 2014;20(34):10590–10606. doi: 10.1002/chem.201400413. [DOI] [PubMed] [Google Scholar]
- 10.Van Baalen C., Marler J.E. Occurrence of hydrogen peroxide in sea water. Nature. 1966;211(5052):951. [Google Scholar]
- 11.Harvey H.W. Oxidation in sea water. J. Mar. Biol. Assoc. U. K. 1925;13(4):953–969. [Google Scholar]
- 12.Miller W.L., Kester D.R. Hydrogen peroxide measurement in seawater by (p-hydroxyphenyl)acetic acid dimerization. Anal. Chem. 1988;60:2711–2715. [Google Scholar]
- 13.King D.W., et al. Flow injection analysis of H2O2 in natural waters using acridinium ester chemiluminescence: method development and optimization using a kinetic model. Anal. Chem. 2007;79:4169–4176. doi: 10.1021/ac062228w. [DOI] [PubMed] [Google Scholar]
- 14.Draper W.M., Crosby D.G. The photochemical generation of hydrogen peroxide in natural waters. Arch. Environ. Contam. Toxicol. 1983;12:121–126. [Google Scholar]
- 15.Cooper W.J., et al. Photochemical formation of H2O2 in natural waters exposed to sunlight. Environ. Sci. Technol. 1988;22:1156–1160. doi: 10.1021/es00175a004. [DOI] [PubMed] [Google Scholar]
- 16.Cooper W.J., Zika R.G. Photochemical formation of hydrogen peroxide in surface and ground waters exposed to sunlight. Science. 1983;220:711–712. doi: 10.1126/science.220.4598.711. [DOI] [PubMed] [Google Scholar]
- 17.Wilson C.L., Hinman N.W., Sheridan R.P. Hydrogen peroxide formation and decay in iron-rich geothermal waters: the relative roles of abiotic and biotic mechanisms. Photochem. Photobiol. 2000;71(6):691–699. doi: 10.1562/0031-8655(2000)071<0691:hpfadi>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 18.Obernosterer I., Ruardij P., Herndl G.J. Spatial and diurnal dynamics of dissolved organic matter (DOM) fluorescence and H2O2 and the photochemical oxygen demand of surface water DOM across the subtropical Atlantic Ocean. Limnol. Oceanogr. 2001;46(3):632–643. [Google Scholar]
- 19.Cooper W.J., Lean D.R.S. Hydrogen peroxide concentration in a northern lake: photochemical formation and diel variability. Environ. Sci. Technol. 1989;23:1425–1428. [Google Scholar]
- 20.Morris J.J., et al. Diel regulation of hydrogen peroxide defenses by open ocean microbial communities. J. Plankton Res. 2016;38(4):1103–1114. [Google Scholar]
- 21.Yuan J., Shiller A.M. The distribution of hydrogen peroxide in the southern and central Atlantic ocean. Deep-Sea Res. 2001;48:2947–2970. [Google Scholar]
- 22.Kieber R.J., Helz G.R. Temporal and seasonal variations of hydrogen peroxide levels in estuarine waters. Estuar. Coast Shelf Sci. 1995;40:495–503. [Google Scholar]
- 23.Abele-Oeschger D., Tug H., Rottgers R. Dynamics of UV-driven hydrogen peroxide formation on an intertidal sandflat. Limnol. Oceanogr. 1997;42(6):1406–1415. [Google Scholar]
- 24.Gerringa L.J.A., et al. The influence of solar ultraviolet radiation on the photochemical production of H2O2 in the equatorial Atlantic Ocean. J. Sea Res. 2004;51:3–10. [Google Scholar]
- 25.Kasting J.F., Holland H.D., Pinto J.P. Oxidant abundances in rainwater and the evolution of atmospheric oxygen. J. Geophys. Res. Atmos. 1985;90(ND6):10497–10510. doi: 10.1029/jd090id06p10497. [DOI] [PubMed] [Google Scholar]
- 26.Kieber R.J., et al. Hydrogen peroxide at the Bermuda Atlantic Time Series Station. Part 1: temporal variability of atmospheric hydrogen peroxide and its influence on seawater concentrations. J. Atmos. Chem. 2001;39:1–13. [Google Scholar]
- 27.Yuan J., Shiller A.M. The variation of hydrogen peroxide in rainwater over the South and Central Atlantic Ocean. Atmos. Environ. 2000;34:3973–3980. [Google Scholar]
- 28.Hanson A.K., Tindale N.W., Abdel-Moati M.A.R. An equatorial Pacific rain event: influence on the distribution of iron and hydrogen peroxide in surface waters. Mar. Chem. 2001;75:69–88. [Google Scholar]
- 29.Willey J.D., Kieber R.J., Avery G.B., Jr. Effects of rainwater iron and hydrogen peroxide on iron speciation and phytoplankton growth in seawater near Bermuda. J. Atmos. Chem. 2004;47:209–222. [Google Scholar]
- 30.Kieber R.J., et al. Iron speciation and hydrogen peroxide concentrations in New Zealand rainwater. Atmos. Environ. 2001;35:6041–6048. [Google Scholar]
- 31.Avery G.B.J., et al. Hydrogen peroxide at the Bermuda Atlantic Time Series Station: temporal variability of seawater hydrogen peroxide. Mar. Chem. 2005;97:236–244. [Google Scholar]
- 32.Willey J.D., Paerl H.W., Go M. Impact of rainwater hydrogen peroxide on chlorophyll a content of surface Gulf Stream seawater off North Carolina, USA. Mar. Ecol. Prog. Ser. 1999;178:145–150. [Google Scholar]
- 33.Asada K. Production and scavenging of reactive oxygen species in chloroplasts and their functions. Plant Physiol. 2006;141(2):391–396. doi: 10.1104/pp.106.082040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Imlay J.A. The molecular mechanisms and physiological consequences of oxidative stress: lessons from a model bacterium. Nat. Rev. Microbiol. 2013;11(7):443–454. doi: 10.1038/nrmicro3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Latifi A., et al. Iron starvation leads to oxidative stress in Anabaena sp. strain PCC 7120. J. Bacteriol. 2005;187(18):6596–6598. doi: 10.1128/JB.187.18.6596-6598.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schoffman H., et al. Iron-nutrient interactions within phytoplankton. Front. Plant Sci. 2016;7 doi: 10.3389/fpls.2016.01223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kennedy F., Martin A., McMinn A. Rapid changes in spectral composition after darkness influences nitric oxide, glucose and hydrogen peroxide production in the Antarctic diatom Fragilariopsis cylindrus. Polar Biol. 2021;44(7):1289–1303. [Google Scholar]
- 38.Kirchman D.L., editor. Microbial Ecology of the Oceans. second ed. Wiley; Hoboken, NJ: 2008. [Google Scholar]
- 39.Ross C., Alstyne K.L.V. Intraspecific variation in stress-induced hydrogen peroxide scavenging by the ulvoid macroalga Ulva lactuca. J. Phycol. 2007;43(3):466–474. [Google Scholar]
- 40.Prasad T.K., et al. Evidence for chilling-induced oxidative stress in maize seedlings and a regulatory role for hydrogen peroxide. Plant Cell. 1994;6:65–74. doi: 10.1105/tpc.6.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kong I.S., et al. Role of catalase and oxyR in the viable but nonculturable state of Vibrio vulnificus. FEMS (Fed. Eur. Microbiol. Soc.) Microbiol. Ecol. 2004;50(3):133–142. doi: 10.1016/j.femsec.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 42.Higuchi T., et al. The synergistic effects of hydrogen peroxide and elevated seawater temperature on the metabolic activity of the coral Galaxea fascicularis. Mar. Biol. 2009;156(4):589–596. [Google Scholar]
- 43.Suggett D.J., et al. Photosynthesis and production of hydrogen peroxide by Symbiodinium (Pyrrhophyta) phylotypes with different thermal tolerances. J. Phycol. 2008;44(4):948–956. doi: 10.1111/j.1529-8817.2008.00537.x. [DOI] [PubMed] [Google Scholar]
- 44.Franca M.B., Panek A.D., Eleutherio E.C.A. Oxidative stress and its effects during dehydration. Comparative Biochemistry and Physiology A - Molecular & Integrative Physiology. 2007;146(4):621–631. doi: 10.1016/j.cbpa.2006.02.030. [DOI] [PubMed] [Google Scholar]
- 45.Dedman C.J., et al. Mechanisms of silver nanoparticle toxicity on the marine cyanobacterium Prochlorococcus under environmentally-relevant conditions. Sci. Total Environ. 2020;747 doi: 10.1016/j.scitotenv.2020.141229. [DOI] [PubMed] [Google Scholar]
- 46.Lin W., et al. Effect of microplastics PAN polymer and/or Cu2+ pollution on the growth of Chlorella pyrenoidosa. Environ. Pollut. 2020;265(Pt A) doi: 10.1016/j.envpol.2020.114985. [DOI] [PubMed] [Google Scholar]
- 47.Barboza L.G.A., et al. Microplastics in wild fish from North East Atlantic Ocean and its potential for causing neurotoxic effects, lipid oxidative damage, and human health risks associated with ingestion exposure. Sci. Total Environ. 2020;717 doi: 10.1016/j.scitotenv.2019.134625. [DOI] [PubMed] [Google Scholar]
- 48.Cao R., et al. Seawater acidification increases copper toxicity: a multi-biomarker approach with a key marine invertebrate, the Pacific Oyster Crassostrea gigas. Aquat. Toxicol. 2019;210:167–178. doi: 10.1016/j.aquatox.2019.03.002. [DOI] [PubMed] [Google Scholar]
- 49.Celis-Plá P.S.M., et al. Antarctic intertidal macroalgae under predicted increased temperatures mediated by global climate change: would they cope? Sci. Total Environ. 2020;740 doi: 10.1016/j.scitotenv.2020.140379. [DOI] [PubMed] [Google Scholar]
- 50.Engström-Öst J., et al. Eco-physiological responses of copepods and pteropods to ocean warming and acidification. Sci. Rep. 2019;9(1):4748. doi: 10.1038/s41598-019-41213-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fu D., et al. Aged microplastics polyvinyl chloride interact with copper and cause oxidative stress towards microalgae Chlorella vulgaris. Aquat. Toxicol. 2019;216 doi: 10.1016/j.aquatox.2019.105319. [DOI] [PubMed] [Google Scholar]
- 52.Lee Y.H., et al. Effects of ocean acidification on life parameters and antioxidant system in the marine copepod Tigriopus japonicus. Aquat. Toxicol. 2019;212:186–193. doi: 10.1016/j.aquatox.2019.05.007. [DOI] [PubMed] [Google Scholar]
- 53.Liu Q., et al. ROS changes are responsible for tributyl phosphate (TBP)-induced toxicity in the alga Phaeodactylum tricornutum. Aquat. Toxicol. 2019;208:168–178. doi: 10.1016/j.aquatox.2019.01.012. [DOI] [PubMed] [Google Scholar]
- 54.Luz D.C., et al. Oxidative stress in the hydrocoral Millepora alcicornis exposed to CO2-driven seawater acidification. Coral Reefs. 2018;37(2):571–579. [Google Scholar]
- 55.Martin I.D., Mackie G.L., Baker M.A. Acute toxicity tests and pulsed-dose delayed mortality at 12 °C and 22 °C in the zebra mussel (Dreissena polymorpha) Arch. Environ. Contam. Toxicol. 1993;24(3):389–398. [Google Scholar]
- 56.Nardi A., et al. Oxidative and interactive challenge of cadmium and ocean acidification on the smooth scallop Flexopecten glaber. Aquat. Toxicol. 2018;196:53–60. doi: 10.1016/j.aquatox.2018.01.008. [DOI] [PubMed] [Google Scholar]
- 57.Zhang Y.M., et al. Nitrogen starvation induced oxidative stress in an oil-producing green alga Chlorella sorokiniana C3. PLoS One. 2013;8(7) doi: 10.1371/journal.pone.0069225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rhee S.G. H2O2, a necessary evil for cell signaling. Science. 2006;312(5782):1882–1883. doi: 10.1126/science.1130481. [DOI] [PubMed] [Google Scholar]
- 59.Visick K.L., Ruby E.G. The periplasmic, group III catalase of Vibrio fischeri is required for normal symbiotic competence and is induced both by oxidative stress and by approach to stationary phase. J. Bacteriol. 1998;180(8):2087–2092. doi: 10.1128/jb.180.8.2087-2092.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Babior B.M. Phagocytes and oxidative stress. Am. J. Med. 2000;109(1):33–44. doi: 10.1016/s0002-9343(00)00481-2. [DOI] [PubMed] [Google Scholar]
- 61.McDowell R.E., et al. Reactive oxygen species and the antarctic macroalgal wound response. J. Phycol. 2014;50(1):71–80. doi: 10.1111/jpy.12127. [DOI] [PubMed] [Google Scholar]
- 62.Collen J., Pedersen M. A stress-induced oxidative burst in Eucheuma platycladum (Rhodophyta) Physiol. Plantarum. 1994;92(3):417–422. [Google Scholar]
- 63.Kupper F.C., et al. Oligoalginate recognition and oxidative burst play a key role in natural and induced resistance of sporophytes of Laminariales. J. Chem. Ecol. 2002;28(10):2057–2081. doi: 10.1023/a:1020706129624. [DOI] [PubMed] [Google Scholar]
- 64.Bouarab K., et al. Sulfated oligosaccharides mediate the interaction between a marine red alga and its green algal pathogenic endophyte. Plant Cell. 1999;11(9):1635–1650. doi: 10.1105/tpc.11.9.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Weinberger F., Friedlander M., Hoppe H.G. Oligoagars elicit a physiological response in Gracilaria conferta (Rhodophyta) J. Phycol. 1999;35(4):747–755. [Google Scholar]
- 66.Ross C., et al. Evidence of a latent oxidative burst in relation to wound repair in the giant unicellular chlorophyte Dasycladus vermicularis. J. Phycol. 2005;41(3):531–541. [Google Scholar]
- 67.Armoza-Zvuloni R., et al. Rapid hydrogen peroxide release from the coral Stylophora pistillata during feeding and in response to chemical and physical stimuli. Sci. Rep. 2016;6 doi: 10.1038/srep21000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.McDowell R.E., et al. Reactive oxygen species as a marine grazing defense: H2O2 and wounded Ascoseira mirabilis both inhibit feeding by an amphipod grazer. J. Exp. Mar. Biol. Ecol. 2014;458:34–38. [Google Scholar]
- 69.McDowell R.E., et al. Control of grazing by light availability via light-dependent, wound-induced metabolites: the role of reactive oxygen species. J. Exp. Mar. Biol. Ecol. 2016;477:86–91. [Google Scholar]
- 70.Sutherland K.M., et al. Extracellular superoxide production by key microbes in the global ocean. Limnol. Oceanogr. 2019;64(6):2679–2693. [Google Scholar]
- 71.Diaz J.M., Plummer S. Production of extracellular reactive oxygen species by phytoplankton: past and future directions. J. Plankton Res. 2018;40(6):655–666. doi: 10.1093/plankt/fby039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hansel C.M., Diaz J.M., Plummer S. Tight regulation of extracellular superoxide points to its vital role in the physiology of the globally relevant Roseobacter clade. mBio. 2019;10(2) doi: 10.1128/mBio.02668-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rose A.L. The influence of extracellular superoxide on iron redox chemistry and bioavailability to aquatic microorganisms. Front. Microbiol. 2012;3 doi: 10.3389/fmicb.2012.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Diaz J.M., et al. Production of extracellular superoxide and hydrogen peroxide by five marine species of harmful bloom-forming algae. J. Plankton Res. 2018;40(6):667–677. doi: 10.1093/plankt/fby043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schneider R.J., et al. Species-level variability in extracellular production rates of reactive oxygen species by diatoms. Front. Chem. 2016;4 doi: 10.3389/fchem.2016.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kim D., et al. Extremely high level of reactive oxygen species (ROS) production in a newly isolated strain of the dinoflagellate Karenia mikimotoi. Eur. J. Phycol. 2019;54(4):632–640. [Google Scholar]
- 77.Cho K., et al. Haemolytic activity and reactive oxygen species production of four harmful algal bloom species. Eur. J. Phycol. 2017;52(3):311–319. [Google Scholar]
- 78.Yuasa K., et al. Nutrient deficiency stimulates the production of superoxide in the noxious red-tide-forming raphidophyte Chattonella antiqua. Harmful Algae. 2020;99 doi: 10.1016/j.hal.2020.101938. [DOI] [PubMed] [Google Scholar]
- 79.Vardi A., et al. Programmed cell death of the dinoflagellate Peridinium gatunense is mediated by CO2 limitation and oxidative stress. Curr. Biol. 1999;9(18):1061–1064. doi: 10.1016/s0960-9822(99)80459-x. [DOI] [PubMed] [Google Scholar]
- 80.Twigg I.M., et al. Revealing hydrogen peroxide as an external stressor in macrophyte-dominated coastal ecosystems. Oecologia. 2020;193(3):583–591. doi: 10.1007/s00442-020-04690-0. [DOI] [PubMed] [Google Scholar]
- 81.Zhang T., et al. Extensive dark biological production of reactive oxygen species in brackish and freshwater ponds. Environ. Sci. Technol. 2016;50(6):2983–2993. doi: 10.1021/acs.est.5b03906. [DOI] [PubMed] [Google Scholar]
- 82.Twiner M.J., Trick C.G. Possible physiological mechanisms for production of hydrogen peroxide by the ichthyotoxic flagellate Heterosigma akashiwo. J. Plankton Res. 2000;22(10):1961–1975. [Google Scholar]
- 83.Oda T., et al. Hydroxyl radical generation by red tide algae. Arch. Biochem. Biophys. 1992;294(1):38–43. doi: 10.1016/0003-9861(92)90133-h. [DOI] [PubMed] [Google Scholar]
- 84.Oda T., et al. Generation of reactive oxygen species by raphidophycean phytoplankton. Biosc. Biotech. Biochem. 1997;61(10):1658–1662. doi: 10.1271/bbb.61.1658. [DOI] [PubMed] [Google Scholar]
- 85.Yang C., Albright L., Yousif A. Oxygen-radical-mediated effects of the toxic phytoplankter Heterosigma carterae on juvenile rainbow trout Oncorhynchus mykiss. Dis. Aquat. Org. 1995;23(2):101–108. [Google Scholar]
- 86.Cho K., et al. Generation of reactive oxygen species (ROS) by harmful algal bloom (HAB)-Forming phytoplankton and their potential impact on surrounding living organisms. Antioxidants. 2022;11(2):206. doi: 10.3390/antiox11020206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dorantes-Aranda J.J., et al. Progress in understanding algal bloom-mediated fish kills: the role of superoxide radicals, phycotoxins and fatty acids. PLoS One. 2015;10(7) doi: 10.1371/journal.pone.0133549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Urakawa H., et al. Subtropical freshwater cyanobacterial blooms as hydrogen peroxide hot spots. Environ. Sci. Technol. Lett. 2021;8(10):911–917. [Google Scholar]
- 89.Murphy S.A., et al. Hydrous ferric oxides in sediment catalyze formation of reactive oxygen species during sulfide oxidation. Front. Mar. Sci. 2016;3 [Google Scholar]
- 90.Shaw T.J., et al. Fe-catalyzed sulfide oxidation in hydrothermal plumes is a source of reactive oxygen species to the ocean. Proc. Nat. Acad. Sci. U.S.A. 2021;vol. 118(40) doi: 10.1073/pnas.2026654118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rose A.L., et al. Dynamics of nonphotochemical superoxide production and decay in the Great Barrier Reef lagoon. Limnol. Oceanogr. 2010;55(4):1521–1536. [Google Scholar]
- 92.Sutherland K.M., et al. Spatial heterogeneity in particle-associated, light-independent superoxide production within productive coastal waters. Journal of Geophysical Research-Oceans. 2020;125(10) doi: 10.1029/2020JC016747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cooper W.J., Zepp R.G. Hydrogen peroxide decay in waters with suspended soils: evidence for biologically mediated processes. Can. J. Fish. Aquat. Sci. 1990;47:888–893. [Google Scholar]
- 94.Petasne R.G., Zika R.G. Hydrogen peroxide lifetimes in south Florida coastal and offshore waters. Mar. Chem. 1997;56:215–225. [Google Scholar]
- 95.Morris J.J., Lenski R.E., Zinser E.R. The Black Queen Hypothesis: evolution of dependencies through adaptive gene loss. mBio. 2012;3(2) doi: 10.1128/mBio.00036-12. e00036-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Regelsberger G., et al. Occurrence and biochemistry of hydroperoxidases in oxygenic phototrophic prokaryotes (cyanobacteria) Plant Physiol. Biochem. 2002;40:479–490. [Google Scholar]
- 97.Hofmann B., Hecht H.J., Flohe L. Peroxiredoxins. Biol. Chem. 2002;383(3–4):347–364. doi: 10.1515/BC.2002.040. [DOI] [PubMed] [Google Scholar]
- 98.Seaver L.C., Imlay J.A. Alkyl hydroperoxide reductase is the primary scavenger of endogenous hydrogen peroxide in Escherichia coli. J. Bacteriol. 2001;183(24):7173–7181. doi: 10.1128/JB.183.24.7173-7181.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mukai K., et al. Effects of light and hydrogen peroxide on gene expression of newly identified antioxidant enzymes in the harmful algal bloom species Chattonella marina. Eur. J. Phycol. 2019;54(3):393–403. [Google Scholar]
- 100.Mukai K., et al. Gene structure and cDNA sequence of 2-Cys peroxiredoxin in the harmful algal bloom species Chattonella marina and its gene transcription under different light intensities. Eur. J. Phycol. 2017;53(1):29–38. [Google Scholar]
- 101.Qiu X., et al. Time-series responses in photosynthetic activity, 2-cysteine peroxiredoxin gene expression, and proteomics of Chattonella marina var. antiqua under different oxidative stress. Harmful Algae. 2020;94 doi: 10.1016/j.hal.2020.101808. [DOI] [PubMed] [Google Scholar]
- 102.Perez-Perez M.E., et al. A comprehensive analysis of the peroxiredoxin reduction system in the cyanobacterium Synechocystis sp. strain PCC 6803 reveals that all five peroxiredoxins are thioredoxin dependent. J. Bacteriol. 2009;191(24):7477–7489. doi: 10.1128/JB.00831-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Moffett J.W., Zafiriou O.C. An investigation of hydrogen peroxide chemistry in surface waters of Vineyard Sound with H218O2. Limnol. Oceanogr. 1990;35(6):1221–1229. [Google Scholar]
- 104.Sutherland K.M., et al. The redox fate of hydrogen peroxide in the marine water column. Limnol. Oceanogr. 2021;66(10) 3828-2841. [Google Scholar]
- 105.Halliwell B., Gutteridge J.M.C. fourth ed. Oxford University Press; New York: 2007. Free Radicals in Biology and Medicine; p. 851. [Google Scholar]
- 106.Naclerio G., et al. Bacillus subtilis vegetative catalase is an extracellular enzyme. Appl. Environ. Microbiol. 1995;61(12):4471–4473. doi: 10.1128/aem.61.12.4471-4473.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hasset D.J., et al. A protease-resistant catalase, KatA, released upon cell lysis during stationary phase is essential for aerobic survival of a Pseudomonas aeruginosa oxyR mutant at low cell densities. J. Bacteriol. 2000;182(16):4557–4563. doi: 10.1128/jb.182.16.4557-4563.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Eremin A.N., Mikhailova R.V., Metelitsa D.I. Comparative kinetic characterization of catalases of the genus Penicillum. Appl. Biochem. Microbiol. 2000;36(3):221–226. [Google Scholar]
- 109.Brum J.R., et al. In: Eco-DAS IX Symposium Proceedings. Kemp P.F., editor. ASLO; 2014. Mortality in the oceans: causes and consequences; pp. 16–48. [Google Scholar]
- 110.Angel D.L., et al. Catalase activity in macro- and microorganisms as an indicator of biotic stress in coastal waters of the eastern Mediterranean Sea. Helgol. Mar. Res. 1999;53:209–218. [Google Scholar]
- 111.Seaver L.C., Imlay J.A. Hydrogen peroxide fluxes and compartmentalization inside growing Escherichia coli. J. Bacteriol. 2001;183(24):7182–7189. doi: 10.1128/JB.183.24.7182-7189.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Morris J.J. Black Queen evolution: the role of leakiness in structuring microbial communities. Trends Genet. 2015;31(8):475–482. doi: 10.1016/j.tig.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 113.Latch D.E., McNeill K. Microheterogeneity of singlet oxygen distributions in irradiated humic acid solutions. Science. 2006;311(5768):1743–1747. doi: 10.1126/science.1121636. [DOI] [PubMed] [Google Scholar]
- 114.Vermilyea A.W., Dixon T.C., Voelker B.M. Use of H2O2-18O to measure absolute rates of dark H2O2 production in freshwater systems. Environ. Sci. Technol. 2010;44(8):3066–3072. doi: 10.1021/es100209h. [DOI] [PubMed] [Google Scholar]
- 115.Miller C.J., Rose A.L., Waite T.D. Importance of iron complexation for fenton-mediated hydroxyl radical production at circumneutral pH. Front. Mar. Sci. 2016;3(134) [Google Scholar]
- 116.Koppenol W.H., Liebman J.F. The oxidizing nature of the hydroxyl radical. A comparison with the ferryl ion (FeO2+) J. Phys. Chem. 1984;88(1):99–101. [Google Scholar]
- 117.Pham A.N., et al. Fenton-like copper redox chemistry revisited: hydrogen peroxide and superoxide mediation of copper-catalyzed oxidant production. J. Catal. 2013;301:54–64. 0. [Google Scholar]
- 118.Page S.E., et al. Evidence for dissolved organic matter as the primary source and sink of photochemically produced hydroxyl radical in arctic surface waters. Environ. Sci.: Processes & Impacts. 2014;16(4):807–822. doi: 10.1039/c3em00596h. [DOI] [PubMed] [Google Scholar]
- 119.von Sonntag C., et al. The fate of peroxyl radicals in aqueous solution. Water Sci. Technol. 1997;35(4):9–15. [Google Scholar]
- 120.Garg S., Rose A.L., Waite T.D. Production of reactive oxygen species on photolysis of dilute aqueous quinone solutions. Photochem. Photobiol. 2007;83(4):904–913. doi: 10.1111/j.1751-1097.2007.00075.x. [DOI] [PubMed] [Google Scholar]
- 121.d'Alessandro N., et al. Reaction of superoxide with phenoxyl-type radicals. J. Chem. Soc. Perkin Transac. 2000;2(9):1862–1867. [Google Scholar]
- 122.Le Roux D.M., Powers L.C., Blough N.V. Photoproduction rates of one-electron reductants by chromophoric dissolved organic matter via fluorescence spectroscopy: comparison with superoxide and hydrogen peroxide rates. Environ. Sci. Technol. 2021;55(17):12095–12105. doi: 10.1021/acs.est.1c04043. [DOI] [PubMed] [Google Scholar]
- 123.Cory R.M., McKnight D.M. Fluorescence spectroscopy reveals ubiquitous presence of oxidized and reduced quinones in dissolved organic matter. Environ. Sci. Technol. 2005;39(21):8142–8149. doi: 10.1021/es0506962. [DOI] [PubMed] [Google Scholar]
- 124.Goldstone J.V., Voelker B.M. Chemistry of superoxide radical in seawater: CDOM associated sink of superoxide in coastal waters. Environ. Sci. Technol. 2000;34:1043–1048. [Google Scholar]
- 125.Heller M.I., Croot P.L. Kinetics of superoxide reactions with dissolved organic matter in tropical Atlantic surface waters near Cape Verde (TENATSO) J. Geophys. Res. 2010;115(C12) [Google Scholar]
- 126.Heller M.I., Croot P.L. Superoxide decay kinetics in the Southern Ocean. Environ. Sci. Technol. 2010;44(1):191–196. doi: 10.1021/es901766r. [DOI] [PubMed] [Google Scholar]
- 127.King D.W., et al. Measurement of antioxidant activity toward superoxide in natural waters. Front. Mar. Sci. 2016;3 [Google Scholar]
- 128.Sanchez-Cruz P., et al. Metal-independent reduction of hydrogen peroxide by semiquinones. Chem. Res. Toxicol. 2014;27(8):1380–1386. doi: 10.1021/tx500089x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Page S.E., et al. Hydroxyl radical formation upon oxidation of reduced humic acids by oxygen in the dark. Environ. Sci. Technol. 2012;46(3):1590–1597. doi: 10.1021/es203836f. [DOI] [PubMed] [Google Scholar]
- 130.Mostofa K.M.G., Sakugawa H. Spatial and temporal variations and factors controlling the concentrations of hydrogen peroxide and organic peroxides in rivers. Environ. Chem. 2009;6(6):524–534. [Google Scholar]
- 131.Bielski B.H.J., et al. Reactivity of HO2/O−2 radicals in aqueous solution. J. Phys. Chem. Ref. Data. 1985;14(4):1041–1100. [Google Scholar]
- 132.Buxton G.V., et al. Critical review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals (⋅OH/⋅O−) in aqueous solution. J. Phys. Chem. Ref. Data. 1988;17(2):513–886. [Google Scholar]
- 133.Pettine M. In: Chemical Processes in Marine Environments. Gaianguzza A., Pelizetti E., Sammartano S., editors. Springer; Berlin: 2000. Redox processes of chromium in sea water; pp. 281–296. [Google Scholar]
- 134.Lamborg C.H., et al. Dark reduction drives evasion of mercury from the ocean. Front. Environ. Chem. 2021;2 [Google Scholar]
- 135.Hansard S.P., et al. Dissolved iron(II) in the Pacific Ocean: Measurements from the PO2 and P16N CLIVAR/CO2 repeat hydrography expeditions. Deep Sea Res. Oceanogr. Res. Pap. 2009;56(7):1117–1129. [Google Scholar]
- 136.Roy E.G., Wells M.L., King D.W. Persistence of iron(II) in surface waters of the western subarctic Pacific. Limnol. Oceanogr. 2008;53(1):89–98. [Google Scholar]
- 137.Moffett J.W. Iron(II) in the world's oxygen deficient zones. Chem. Geol. 2021;580 [Google Scholar]
- 138.Bianchi D., et al. Global niche of marine anaerobic metabolisms expanded by particle microenvironments. Nat. Geosci. 2018;11(4):263–+. [Google Scholar]
- 139.Hunter K.A., Boyd P.W. Iron-binding ligands and their role in the ocean biogeochemistry of iron. Environ. Chem. 2007;4(4):221–232. [Google Scholar]
- 140.Boyd P.W., Ellwood M.J. The biogeochemical cycle of iron in the ocean. Nat. Geosci. 2010;3(10):675–682. [Google Scholar]
- 141.Hopwood M.J., et al. Dissolved iron(II) ligands in river and estuarine water. Mar. Chem. 2015;173:173–182. [Google Scholar]
- 142.Miller C.J., Rose A.L., Waite T.D. Hydroxyl radical production by H2O2-mediated oxidation of Fe(II) complexed by Suwannee River fulvic acid under circumneutral freshwater conditions. Environ. Sci. Technol. 2013;47(2):829–835. doi: 10.1021/es303876h. [DOI] [PubMed] [Google Scholar]
- 143.Xiao Y., et al. Superoxide-driven autocatalytic dark production of hydroxyl radicals in the presence of complexes of natural dissolved organic matter and iron. Water Res. 2020;177 doi: 10.1016/j.watres.2020.115782. [DOI] [PubMed] [Google Scholar]
- 144.Moffett J.W., Zika R.G. Oxidation kinetics of Cu(I) in seawater: implications for its existence in the marine environment. Mar. Chem. 1983;13(3):239–251. [Google Scholar]
- 145.González-Dávila M., et al. Oxidation of copper(I) in seawater at nanomolar levels. Mar. Chem. 2009;115(1–2):118–124. [Google Scholar]
- 146.Voelker B.M., Sedlak D.L., Zafiriou O.C. Chemistry of superoxide radical in seawater: reactions with organic Cu complexes. Environ. Sci. Technol. 2000;34(6):1036–1042. [Google Scholar]
- 147.Moffett J.W., Zika R.G. Measurement of copper(I) in surface waters of the subtropical Atlantic and Gulf of Mexico. Geochem. Cosmochim. Acta. 1988;52(7):1849–1857. [Google Scholar]
- 148.Buerge-Weirich D., Sulzberger B. formation of Cu(I) in estuarine and marine waters: application of a new solid-phase extraction method to measure Cu(I) Environ. Sci. Technol. 2004;38(6):1843–1848. doi: 10.1021/es034845x. [DOI] [PubMed] [Google Scholar]
- 149.Heller M.I., Croot P.L. Copper speciation and distribution in the atlantic sector of the Southern Ocean. Mar. Chem. 2015;173:253–268. [Google Scholar]
- 150.Wiwit, et al. Wide-range detection of Cu-binding organic ligands in seawater using reverse titration. Mar. Chem. 2021;230 [Google Scholar]
- 151.Xing G., et al. Effect of chloride and suwannee river fulvic acid on Cu speciation: implications to Cu redox transformations in simulated natural waters. Environ. Sci. Technol. 2020;54(4):2334–2343. doi: 10.1021/acs.est.9b06789. [DOI] [PubMed] [Google Scholar]
- 152.Millero F.J., Sharma V.K., Karn B. The rate of reduction of copper(II) with hydrogen peroxide in seawater. Mar. Chem. 1991;36(1–4):71–83. [Google Scholar]
- 153.Xing G., et al. pH-dependence of production of oxidants (Cu(III) and/or HO•) by copper-catalyzed decomposition of hydrogen peroxide under conditions typical of natural saline waters. Geochem. Cosmochim. Acta. 2018;232:30–47. [Google Scholar]
- 154.Luther G.W., III The role of one- and two-electron transfer reactions in forming thermodynamically unstable intermediates as barriers in multi-electron redox reactions. Aquat. Geochem. 2010;16(3):395–420. [Google Scholar]
- 155.Morgan J.J. Kinetics of reaction between O2 and Mn(II) species in aqueous solutions. Geochem. Cosmochim. Acta. 2005;69(1):35–48. [Google Scholar]
- 156.Tebo B.M., et al. Geomicrobiology of manganese(II) oxidation. Trends Microbiol. 2005;13(9):421–428. doi: 10.1016/j.tim.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 157.Hansel C.M. In: Advances in Microbial Physiology. Poole R.K., editor. Academic Press; 2017. Chapter two - manganese in marine microbiology; pp. 37–83. [DOI] [PubMed] [Google Scholar]
- 158.Learman D., et al. Constraints on superoxide mediated formation of manganese oxides. Front. Microbiol. 2013;4(262) doi: 10.3389/fmicb.2013.00262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Hansard S.P., Easter H.D., Voelker B.M. Rapid reaction of nanomolar Mn(II) with superoxide radical in seawater and simulated freshwater. Environ. Sci. Technol. 2011;45(7):2811–2817. doi: 10.1021/es104014s. [DOI] [PubMed] [Google Scholar]
- 160.Learman D.R., et al. Formation of manganese oxides by bacterially generated superoxide. Nat. Geosci. 2011;4(2):95–98. [Google Scholar]
- 161.Wuttig K., Heller M.I., Croot P.L. Reactivity of inorganic Mn and Mn desferrioxamine B with O2, O2–, and H2O2 in seawater. Environ. Sci. Technol. 2013;47(18):10257–10265. doi: 10.1021/es4016603. [DOI] [PubMed] [Google Scholar]
- 162.Oldham V.E., et al. Soluble Mn(III)–L complexes are abundant in oxygenated waters and stabilized by humic ligands. Geochem. Cosmochim. Acta. 2017;199:238–246. [Google Scholar]
- 163.Sunda W.G., Huntsman S.A. Photoreduction of manganese oxides in seawater. Mar. Chem. 1994;46(1–2):133–152. [Google Scholar]
- 164.Oldham V.E., et al. Oxidative and reductive processes contributing to manganese cycling at oxic-anoxic interfaces. Mar. Chem. 2017;195:122–128. [Google Scholar]
- 165.Gustafsson J.P. Vanadium geochemistry in the biogeosphere –speciation, solid-solution interactions, and ecotoxicity. Appl. Geochem. 2019;102:1–25. [Google Scholar]
- 166.Wang D., Sañudo Wilhelmy S.A. Vanadium speciation and cycling in coastal waters. Mar. Chem. 2009;117(1–4):52–58. [Google Scholar]
- 167.Knežević L., et al. Redox speciation of vanadium in estuarine waters using improved methodology based on anion exchange chromatography coupled to HR ICP-ms system. Molecules. 2021;26(9) doi: 10.3390/molecules26092436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Wehrli B., Stumm W. Oxygenation of vanadyl(IV). Effect of coordinated surface hydroxyl groups and hydroxide ion. Langmuir. 1988;4(3):753–758. [Google Scholar]
- 169.Huang J.-H., et al. Vanadium: global (bio)geochemistry. Chem. Geol. 2015;417:68–89. [Google Scholar]
- 170.Bielski B.H.J., et al. Reactivity of HO2/O2- radicals in aqueous solution. J. Phys. Chem. Ref. Data. 1985;14(4):1041–1100. [Google Scholar]
- 171.Shankar H.N.R., Ramasarma T. Multiple reactions in vanadyl-V(IV) oxidation by H2O2. Mol. Cell. Biochem. 1993;129(1):9–29. doi: 10.1007/BF00926572. [DOI] [PubMed] [Google Scholar]
- 172.Wang D. Redox chemistry of molybdenum in natural waters and its involvement in biological evolution. Front. Microbiol. 2012;3:427. doi: 10.3389/fmicb.2012.00427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Smedley P.L., Kinniburgh D.G. Molybdenum in natural waters: a review of occurrence, distributions and controls. Appl. Geochem. 2017;84:387–432. [Google Scholar]
- 174.Li H.-P., et al. Superoxide production by a manganese-oxidizing bacterium facilitates iodide oxidation. Appl. Environ. Microbiol. 2014;80(9):2693–2699. doi: 10.1128/AEM.00400-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Wong G.T.F., Zhang L.-S. The kinetics of the reactions between iodide and hydrogen peroxide in seawater. Mar. Chem. 2008;111(1):22–29. [Google Scholar]
- 176.Li H.-P., et al. Bacterial production of organic acids enhances H2O2-dependent iodide oxidation. Environ. Sci. Technol. 2012;46(9):4837–4844. doi: 10.1021/es203683v. [DOI] [PubMed] [Google Scholar]
- 177.Luther G.W. Aquatic Redox Chemistry. American Chemical Society; 2011. Thermodynamic redox calculations for one and two electron transfer steps: implications for halide oxidation and halogen environmental cycling; pp. 15–35. [Google Scholar]
- 178.Winterbourn C.C., Metodiewa D. Reactivity of biologically important thiol compounds with superoxide and hydrogen peroxide. Free Radic. Biol. Med. 1999;27(3–4):322–328. doi: 10.1016/s0891-5849(99)00051-9. [DOI] [PubMed] [Google Scholar]
- 179.Blough N.V., Zafiriou O.C. Reaction of superoxide with nitric oxide to form peroxonitrite in alkaline aqueous solution. Inorg. Chem. 1985;24(22):3502–3504. [Google Scholar]
- 180.Adesina A.O., Sakugawa H. Photochemically generated nitric oxide in seawater: the peroxynitrite sink and its implications for daytime air quality. Sci. Total Environ. 2021;781 doi: 10.1016/j.scitotenv.2021.146683. [DOI] [PubMed] [Google Scholar]
- 181.Hansel C.M., Ferdelman T.G., Tebo B.M. Cryptic cross-linkages among biogeochemical cycles: novel insights from reactive intermediates. Elements. 2015;11(6):409–414. [Google Scholar]
- 182.Yoshpepurer Y., Henis Y. Factors affecting catalase level and sensitivity to hydrogen peroxide in Escherichia coli. Appl. Environ. Microbiol. 1976;32(4):465–469. doi: 10.1128/aem.32.4.465-469.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Gutteridge J.M.C. Reactivity of hydroxyl and hydroxyl-like radicals discriminated by release of thiobarbituric acid-reactive material from deoxy sugars, nucleosides and benzoate. Biochem. J. 1984;224:761–767. doi: 10.1042/bj2240761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Lu Z., et al. Effects of pyrogallic acid on Microcystis aeruginosa: oxidative stress related toxicity. Ecotoxicol. Environ. Saf. 2016;132:413–419. doi: 10.1016/j.ecoenv.2016.06.039. [DOI] [PubMed] [Google Scholar]
- 185.Hofer T., et al. Hydrogen peroxide causes greater oxidation in cellular RNA than in DNA. Biol. Chem. 2005;386(4):333–337. doi: 10.1515/BC.2005.040. [DOI] [PubMed] [Google Scholar]
- 186.Tanaka M., Chock P.B., Stadtman E.R. Oxidized messenger RNA induces translation errors. Proc. Nat. Acad. Sci. U.S.A. 2007;vol. 104(1):66–71. doi: 10.1073/pnas.0609737104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Keyer K., Imlay J.A. Superoxide accelerates DNA damage by elevating free iron levels. Proc. Natl. Acad. Sci. U.S.A. 1996;93:13635–13640. doi: 10.1073/pnas.93.24.13635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Bolann B.J., Ulvik R.J. Release of iron from ferritin by xanthine oxidase. Biochem. J. 1987;243:55–59. doi: 10.1042/bj2430055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Kojima K., et al. Oxidation of elongation factor G inhibits the synthesis of the D1 protein of photosystem II. Mol. Microbiol. 2007;65(4):936–947. doi: 10.1111/j.1365-2958.2007.05836.x. [DOI] [PubMed] [Google Scholar]
- 190.Tamarit J., Cabiscol E., Ros J. Identification of the major oxidatively damaged proteins in Escherichia coli cells exposed to oxidative stress. J. Biol. Chem. 1998;273(5):3027–3032. doi: 10.1074/jbc.273.5.3027. [DOI] [PubMed] [Google Scholar]
- 191.Gutteridge J.M.C., Halliwell B. The measurement and mechanism of lipid peroxidation in biological systems. Trends Biochem. Sci. 1990;15(4):129–135. doi: 10.1016/0968-0004(90)90206-q. [DOI] [PubMed] [Google Scholar]
- 192.Arana I., et al. Role of hydrogen peroxide in loss of culturability mediated by visible light in Escherichia coli in a fresh water ecosystem. Appl. Environ. Microbiol. 1992;58(12):3903–3907. doi: 10.1128/aem.58.12.3903-3907.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Tanaka T., et al. A hidden pitfall in the preparation of agar media undermines microorganism cultivability. Appl. Environ. Microbiol. 2014;80(24):7659–7666. doi: 10.1128/AEM.02741-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Morris J.J., Zinser E.R. Continuous hydrogen peroxide production by organic buffers in phytoplankton culture media. J. Phycol. 2013;49(6):1223–1228. doi: 10.1111/jpy.12123. [DOI] [PubMed] [Google Scholar]
- 195.Barry V.C., et al. Peroxide formation in bacteriological media. Nature. 1956;178(4533):596–597. doi: 10.1038/178596a0. [DOI] [PubMed] [Google Scholar]
- 196.McDonald L.C., Hackney C.R., Ray B. Enhanced recovery of injured Escherichia coli by compounds that degrade hydrogen peroxide or block its formation. Appl. Environ. Microbiol. 1983;45(2):360–365. doi: 10.1128/aem.45.2.360-365.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Xenopoulos M.A., Bird D.F. Effect of acute exposure to hydrogen peroxide on the production of phytoplankton and bacterioplankton in a mesohumic lake. Photochem. Photobiol. 1997;66(4):471–478. [Google Scholar]
- 198.Hopwood M.J., et al. Experiment design and bacterial abundance control extracellular H2O2 concentrations during four series of mesocosm experiments. Biogeosciences. 2020;17(5):1309–1326. [Google Scholar]
- 199.Morris J.J., et al. Facilitation of robust growth of Prochlorococcus colonies and dilute liquid cultures by “helper” heterotrophic bacteria. Appl. Environ. Microbiol. 2008;74(14):4530–4534. doi: 10.1128/AEM.02479-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Morris J.J., et al. Dependence of the cyanobacterium Prochlorococcus on hydrogen peroxide scavenging microbes for growth at the ocean’s surface. PLoS One. 2011;6(2) doi: 10.1371/journal.pone.0016805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Scanlan D.J., et al. Ecological genomics of marine picocyanobacteria. Microbiol. Mol. Biol. Rev. 2009;73(2):249–299. doi: 10.1128/MMBR.00035-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Coe A., et al. Survival of Prochlorococcus in extended darkness. Limnol. Oceanogr. 2016;61(4):1375–1388. [Google Scholar]
- 203.Biller S.J., et al. Heterotroph interactions alter Prochlorococcus transcriptome dynamics during extended Periods of darkness. mSystems. 2018;3(3) doi: 10.1128/mSystems.00040-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Ma L., et al. Degradation of hydrogen peroxide at the ocean’s surface: the influence of the microbial community on the realized thermal niche of Prochlorococcus. ISME J. 2018;12:473–484. doi: 10.1038/ismej.2017.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Samuilov V.D., et al. Hydrogen peroxide inhibits the growth of cyanobacteria. Biochemistry-Moscow. 1999;64(1):47–53. [PubMed] [Google Scholar]
- 206.Drabkova M., Admiraal W., Marsalek B. Combined exposure to hydrogen peroxide and light – selective effects on cyanobacteria, green algae, and diatoms. Environ. Sci. Technol. 2007;41:309–314. doi: 10.1021/es060746i. [DOI] [PubMed] [Google Scholar]
- 207.Drabkova M., et al. Selective effects of H2O2 on cyanobacterial photosynthesis. Photosynthetica. 2007;45(3):363–369. [Google Scholar]
- 208.Tichy M., Vermaas W. In vivo role of catalase-peroxidase in Synechocystis sp. Strain PCC 6803. J. Bacteriol. 1999;181(6):1875–1882. doi: 10.1128/jb.181.6.1875-1882.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Hollibaugh J.T. Oxygen and the activity and distribution of marine Thaumarchaeota. Environmental Microbiology Reports. 2017;9(3):186–188. doi: 10.1111/1758-2229.12534. [DOI] [PubMed] [Google Scholar]
- 210.Stahl D.A., Torre J.R.d.l. Physiology and diversity of ammonia-oxidizing archaea. Annu. Rev. Microbiol. 2012;66(1):83–101. doi: 10.1146/annurev-micro-092611-150128. [DOI] [PubMed] [Google Scholar]
- 211.Tolar B.B., et al. Ammonia oxidation in the ocean can be inhibited by nanomolar concentrations of hydrogen peroxide. Front. Mar. Sci. 2016;3(237) [Google Scholar]
- 212.Qin W., et al. Influence of oxygen availability on the activities of ammonia-oxidizing archaea. Environ Microbiol Rep. 2017;9(3):250–256. doi: 10.1111/1758-2229.12525. [DOI] [PubMed] [Google Scholar]
- 213.Bayer B., et al. Proteomic response of three marine ammonia-oxidizing archaea to hydrogen peroxide and their metabolic interactions with a heterotrophic alphaproteobacterium. mSystems. 2019;4(4) doi: 10.1128/mSystems.00181-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Chen C., et al. Growth, physiochemical and antioxidant responses of overwintering benthic cyanobacteria to hydrogen peroxide. Environ. Pollut. 2016;219:649–655. doi: 10.1016/j.envpol.2016.06.043. [DOI] [PubMed] [Google Scholar]
- 215.Kim J.-G., et al. Hydrogen peroxide detoxification is a key mechanism for growth of ammonia-oxidizing archaea. Proc. Natl. Acad. Sci. Unit. States Am. 2016;113(28):7888–7893. doi: 10.1073/pnas.1605501113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Friedman L.E., Gilmore T.D., Finnerty J.R. Intraspecific variation in oxidative stress tolerance in a model cnidarian: differences in peroxide sensitivity between and within populations of Nematostella vectensis. PLoS One. 2018;13(1) doi: 10.1371/journal.pone.0188265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Hopwood M.J., et al. Photochemical vs. Bacterial control of H2O2 concentration across a pCO2 gradient mesocosm experiment in the subtropical north atlantic. Front. Mar. Sci. 2018;5(105) [Google Scholar]
- 218.Müller R., et al. UV-radiation and elevated temperatures induce formation of reactive oxygen species in gametophytes of cold-temperate/Arctic kelps (Laminariales, Phaeophyceae) Phycol. Res. 2012;60(1):27–36. [Google Scholar]
- 219.Goyen S., et al. A molecular physiology basis for functional diversity of hydrogen peroxide production amongst Symbiodinium spp. (Dinophyceae) Mar. Biol. 2017;164(3) [Google Scholar]
- 220.Jang C.J., et al. Response of the ocean mixed layer depth to global warming and its impact on primary production: a case for the North Pacific Ocean. ICES (Int. Counc. Explor. Sea) J. Mar. Sci. 2011;68(6):996–1007. [Google Scholar]
- 221.Lopes A.R., et al. Transgenerational exposure to ocean acidification induces biochemical distress in a keystone amphipod species (Gammarus locusta) Environ. Res. 2019;170:168–177. doi: 10.1016/j.envres.2018.12.040. [DOI] [PubMed] [Google Scholar]
- 222.Hennon G.M.M., et al. The impact of elevated CO2 on Prochlorococcus and microbial interactions with ‘helper’ bacterium Alteromonas. ISME J. 2018;12:520–531. doi: 10.1038/ismej.2017.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Zepp R.G., Faust B.C., Hoigne J. Hydroxyl radical formation in aqueous reactions (ph 3-8) of iron(ii) with hydrogen-peroxide - the photo-fenton reaction. Environ. Sci. Technol. 1992;26(2):313–319. [Google Scholar]
- 224.Myhre Jensen E., et al. Trends in de-lousing of Norwegian farmed salmon from 2000–2019—consumption of medicines, salmon louse resistance and non-medicinal control methods. PLoS One. 2020;15(10) doi: 10.1371/journal.pone.0240894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Duchene L. Global Aquaculture Advocate; 2017. In Sea Lice Fight, Salmon Farmers Phasing Out Hydrogen Peroxide. [Google Scholar]
- 226.Escobar-Lux R.H., et al. Ecotoxicology and Environmental Safety; 2020. Short-term exposure to hydrogen peroxide induces mortality and alters exploratory behaviour of European lobster (Homarus gammarus) p. 204. [DOI] [PubMed] [Google Scholar]
- 227.Escobar-Lux R.H., et al. vol. 4. 2019. pp. 626–637. (The Effects of Hydrogen Peroxide on Mortality, Escape Response, and Oxygen Consumption of Calanus spp.F. Facets). [Google Scholar]
- 228.Abele-Oeschger D., Buchner T. Effect of environmental hydrogen peroxide accumulation on filtration rates of the intertidal bivalve clam Cerastoma edule. Verh. Dtsch. Zool. Ges. 1995;88:93. [Google Scholar]
- 229.Vera L.M., Migaud H. Hydrogen peroxide treatment in Atlantic salmon induces stress and detoxification response in a daily manner. Chronobiol. Int. 2016;33(5):530–542. doi: 10.3109/07420528.2015.1131164. [DOI] [PubMed] [Google Scholar]
- 230.Helgesen K.O., et al. First report of reduced sensitivity towards hydrogen peroxide found in the salmon louse Lepeophtheirus salmonis in Norway. Aquaculture Reports. 2015;1:37–42. [Google Scholar]
- 231.Huisman J., et al. Cyanobacterial blooms. Nat. Rev. Microbiol. 2018;16(8):471–483. doi: 10.1038/s41579-018-0040-1. [DOI] [PubMed] [Google Scholar]
- 232.Taranu Z.E., et al. Acceleration of cyanobacterial dominance in north temperate‐subarctic lakes during the Anthropocene. Ecol. Lett. 2015;18(4):375–384. doi: 10.1111/ele.12420. [DOI] [PubMed] [Google Scholar]
- 233.Chapra S.C., et al. Climate change impacts on harmful algal blooms in US freshwaters: a screening-level assessment. Environ. Sci. Technol. 2017;51(16):8933–8943. doi: 10.1021/acs.est.7b01498. [DOI] [PubMed] [Google Scholar]
- 234.Paerl H.W., Otten T.G., Kudela R. Mitigating the expansion of harmful algal blooms across the freshwater-to-marine continuum. Environ. Sci. Technol. 2018;52(10):5519–5529. doi: 10.1021/acs.est.7b05950. [DOI] [PubMed] [Google Scholar]
- 235.Paerl H.W. Mitigating harmful cyanobacterial blooms in a human- and climatically-impacted world. Life. 2014;4(4):988–1012. doi: 10.3390/life4040988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Skurlatov Y.I., Ernestova L.S. The impact of human activities on freshwater aquatic systems. Acta Hydrochim. Hydrobiol. 1998;26(1):5–12. [Google Scholar]
- 237.Barroin G., Feuillade M. Hydrogen peroxide as a potential algicide for Oscillatoria rubescens D.C. Wat. Res. 1986;20(5):619–623. [Google Scholar]
- 238.Weenink E.F., et al. Combatting cyanobacteria with hydrogen peroxide: a laboratory study on the consequences for phytoplankton community and diversity. Front. Microbiol. 2015;6:714. doi: 10.3389/fmicb.2015.00714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Matthijs H.C., et al. Selective suppression of harmful cyanobacteria in an entire lake with hydrogen peroxide. Water Res. 2012;46(5):1460–1472. doi: 10.1016/j.watres.2011.11.016. [DOI] [PubMed] [Google Scholar]
- 240.Piel T., et al. Suppressing cyanobacteria with hydrogen peroxide is more effective at high light intensities. Toxins. 2019;12(1) doi: 10.3390/toxins12010018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Matthijs H.C.P., et al. Existing and emerging cyanocidal compounds: new perspectives for cyanobacterial bloom mitigation. Aquat. Ecol. 2016;50(3):443–460. [Google Scholar]
- 242.Dziga D., et al. Microbial degradation of microcystins. Chem. Res. Toxicol. 2013;26(6):841–852. doi: 10.1021/tx4000045. [DOI] [PubMed] [Google Scholar]
- 243.Huo X., et al. Exposure of Microcystis aeruginosa to hydrogen peroxide under light: kinetic modeling of cell rupture and simultaneous microcystin degradation. Environ. Sci. Technol. 2015;49(9):5502–5510. doi: 10.1021/acs.est.5b00170. [DOI] [PubMed] [Google Scholar]
- 244.Ndungu L.K., et al. Hydrogen peroxide measurements in subtropical aquatic systems and their implications for cyanobacterial blooms. Ecol. Eng. 2019;138:444–453. [Google Scholar]
- 245.Wagstaff B.A., et al. bioRxiv; 2020. Dissecting the toxicity and mitigating the impact of harmful Prymnesium blooms in the UK waters of the Norfolk Broads. 2020.03.26.010066. [DOI] [PubMed] [Google Scholar]
- 246.Burson A., et al. Termination of a toxic Alexandrium bloom with hydrogen peroxide. Harmful Algae. 2014;31:125–135. doi: 10.1016/j.hal.2013.10.017. [DOI] [PubMed] [Google Scholar]
- 247.Häkkinen P.J., Anesio A.M., Granéli W. Hydrogen peroxide distribution, production, and decay in boreal lakes. Can. J. Fish. Aquat. Sci. 2004;61:1520–1527. [Google Scholar]
- 248.Morris J.J. Microbiology. University of Tennessee; Knoxville: 2011. The ‘helper’ phenotype: a symbiotic interaction between Prochlorococcus and hydrogen peroxide scavenging microorganisms; p. 187. [Google Scholar]
- 249.Abele D., Ferreyra G.A., Schloss I. H2O2 accumulation from photochemical production and atmospheric wet deposition in Antarctic coastal and off-shore waters of Potter Cove, King George Island, South Shetland Islands. Antarct. Sci. 1999;11(2):131–139. [Google Scholar]
- 250.Resing J., et al. Palmer L.T.E.R. Hydrogen peroxide in the Palmer LTER region: II. Water column distribution. Antarct. J. U. S. 1993;28:227–228. [Google Scholar]
- 251.Zika R.G., Saltzman E.S., Cooper W.J. Hydrogen peroxide concentrations in the Peru upwelling area. Mar. Chem. 1985;17:265–275. [Google Scholar]
- 252.Clark C.D., et al. Hydrogen peroxide measurements in recreational marine bathing waters in Southern California, USA. Water Res. 2010;44(7):2203–2210. doi: 10.1016/j.watres.2009.12.044. [DOI] [PubMed] [Google Scholar]
- 253.Zika R.G., et al. Spatial and temporal variations of hydrogen peroxide in Gulf of Mexico waters. Geochem. Cosmochim. Acta. 1985;49:1173–1184. [Google Scholar]
- 254.Yuan J., Shiller A.M. Distribution of hydrogen peroxide in the northwest Pacific Ocean. G-cubed. 2005;6(9):1–3. [Google Scholar]
- 255.Croot P.L., et al. Influence of the ITCZ on H2O2 in near surface waters in the equatorial Atlantic Ocean. Geophys. Res. Lett. 2004;31(23) [Google Scholar]
- 256.Moore C.A., Farmer C.T., Zika R.G. Influence of the Orinoco River on hydrogen peroxide distribution and production in the eastern Caribbean. J. Geophys. Res.: Oceans. 1993;98(C2):2289–2298. [Google Scholar]
- 257.Miller W.L., Kester D.R. Peroxide variations in the sargasso sea. Mar. Chem. 1994;48:17–29. [Google Scholar]
- 258.Steigenberger S., Croot P.L. Identifying the processes controlling the distribution of H2O2 in surface waters along a meridional transect in the eastern Atlantic. Geophys. Res. Lett. 2008;35(3) [Google Scholar]
- 259.Johnson K.S., et al. Hydrogen peroxide in the western Mediterranean Sea: a tracer for vertical advection. Deep-Sea Res. 1989;36(2):241–254. [Google Scholar]
- 260.Sarthou G., et al. Fe and H2O2 distributions in the upper water column in the Indian sector of the Southern Ocean. Earth Planet Sci. Lett. 1997;147(1–4):83–92. [Google Scholar]
- 261.Yuan J.C., Shiller A.M. Hydrogen peroxide in deep waters of the north pacific ocean. Geophys. Res. Lett. 2004;31(1) [Google Scholar]
- 262.Hopwood M.J., et al. Hydrogen peroxide in deep waters from the mediterranean sea, South Atlantic and South Pacific oceans. Sci. Rep. 2017;7(1):43436. doi: 10.1038/srep43436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Cooper W.J., Saltzman E.S., Zika R.G. The contribution of rainwater to variability in surface ocean hydrogen peroxide. J. Geophys. Res.: Oceans. 1987;92(C3):2970–2980. [Google Scholar]
- 264.Zika R., et al. H2O2 levels in rainwater collected in south Florida and the Bahama Islands. J. Geophys. Res.: Oceans. 1982;87(C7):5015–5017. [Google Scholar]
- 265.Burridge L.E., et al. vols. 420–421. 2014. pp. 180–186. (The Acute Lethality of Three Anti-sea Lice Formulations: AlphaMax®, Salmosan®, and Interox®Paramove™50 to Lobster and Shrimp. Aquaculture). [Google Scholar]
- 266.Hansen B.H., et al. Acute hydrogen peroxide (H2O2) exposure does not cause oxidative stress in late-copepodite stage of Calanus finmarchicus. J. Toxicol. Environ. Health. 2017;80(16–18):820–829. doi: 10.1080/15287394.2017.1352182. [DOI] [PubMed] [Google Scholar]
- 267.Matthews R.S. Artemia salina as a test organism for measuring superoxide-mediated toxicity. Free Radic. Biol. Med. 1995;18(5):919–922. doi: 10.1016/0891-5849(94)00205-x. [DOI] [PubMed] [Google Scholar]
- 268.Smit M.G.D., et al. Time and concentration dependency in the potentially affected fraction of species: the case of hydrogen peroxide treatment of ballast water. Environ. Toxicol. Chem. 2008;27(3):746–753. doi: 10.1897/07-343.1. [DOI] [PubMed] [Google Scholar]
- 269.Kanda T., Murata H., Kuroki A. Toxicity of removal agents of red tide plankton to the fishes with special reference to the toxicity of hydrogen peroxide, iron sulfate, and iron chloride. Suisan zoshoku. 1989;37(3):221–224. [Google Scholar]
- 270.Yang C.Z., Albright L.J., Yousif A.N. Oxygen-radical-mediated effects of the toxic phytoplankter Heterosigma carterae on juvenile rainbow trout Oncorhynchus mykiss. Dis. Aquat. Org. 1995;23(2):101–108. [Google Scholar]
- 271.Reichwaldt E.S., et al. Acute toxicological response of Daphnia and Moina to hydrogen peroxide. J. Environ. Eng. 2012;138(5):607–611. [Google Scholar]
- 272.Peixoto G.V., et al. Safe disposal of E. coli DH5α strain from liquid broth and solid substrate. J. Environ. Stud. 2020;23(1):1–12. [Google Scholar]
- 273.Abele-Oeschger D., Tüg H., Röttgers R. Dynamics of UV-driven hydrogen peroxide formation on an intertidal sandflat. Limnol. Oceanogr. 1997;42(6):1406–1415. [Google Scholar]