Abstract
Introduction:
There is an anatomic explanation for upper lip and midfacial tethering resulting in lack of motion in facial synkinesis.
Objective:
To measure the effect of perinasal chemodenervation on dental show in the synkinetic population and clarify the anatomic relationship of perinasal musculature.
Methods:
Literature search was performed on anatomy of the perinasal modiolus, and anatomic evaluation was performed through human anatomic specimen dissection. Photographic outcomes were observed in synkinetic patients receiving chemodenervation to smile antagonists with and without perinasal muscle injections and assessed through naive observer survey. Retrospective outcomes for all patients receiving perinasal chemodenervation was collected utilizing Facial Clinimetric Evaluation Scale, Sunnybrook Facial Grading System (FGS), Facial Disability Index (FDI), and the Synkinesis Assessment Questionnaire.
Results:
Anatomic dissections demonstrated muscular confluence spanning the nasal sidewall and upper lip tethering the soft tissue to bone. Thirty-four of 53 chemodenervation patients received perinasal Botox experiencing improvement in synkinetic symptoms of the upper lip, nose, and improved dental show as noted on paired t-test for FGS (p = 0.00096), and FDI social p = 0.015) also supported by naive observer surveys (p = 0.03).
Conclusions:
Human anatomic specimen dissections support a perinasal confluence of musculature with bony attachments that can be successfully treated with chemodenervation in facial synkinesis patients.
Key Points
Question: In people with facial synkinesis, can botulinum toxin injections around the nose improve a person's smile?
Findings: Human anatomic specimen dissection demonstrates that patients with facial synkinesis have a lack of upper lip motion with smiling caused by tethering, often confused with paralysis.
Meaning: Patients reported improved smile after botulinum toxin in the muscles around the nose, supporting an anatomic theory of upper lip tethering by overactive muscles next to the nose and lip.
Introduction
The management of facial paralysis has evolved over several decades. Our surgical mindset and algorithms for facial reanimation have become something akin to an art. We continue to make great strides in the refinement of surgical interventions for both dynamic and static reanimation from upper eyelid loading to facial reanimation.1–6 Recently, the nonsurgical interventions after facial paralysis and subsequent recovery have prompted their own intense discussions. Management of the synkinetic patient has led to innovations in facial balancing utilizing chemodenervation and supports facial retraining therapy for quality of life.7,8
Chemical denervation (chemodenervation) using botulinum toxin is a well-established treatment for providing balance, improved appearance, symmetry, and function to the synkinetic and hyperkinetic face.9,10 Early techniques for chemodenervation in the synkinetic patient targeted the contralateral nonaffected musculature to provide symmetry, but today focuses on the affected ipsilateral muscles.9,11,12 Advancements in recent years have found success in targeting the smile antagonists: depressor anguli oris (DAO), platysma, and mentalis muscles. Synkinesis in these muscles causes a tethering or downward pull of the oral commissure, resulting in strained expressions of displeasure or frowning.
Analysis of the midface in our synkinetic population led us to ask whether the medial cheek and upper lip were not moving because of persistent weakness or tethering. We then attempted to achieve further improvement in a patient's emotional expression by injecting targets other than the smile antagonists, namely the perinasal musculature. This muscular system includes the levator labii superioris alaeque nasi (LLSAN), nasalis muscle (NM), depressor septi (DS), and myrtiformis muscle (MM). We noted a number of patients who failed to improve acceptably until this perinasal muscle group was addressed in combination with the smile antagonists (Fig. 1).
Fig. 1.

Photos before and after botulinum injection. Preinjection of smile antagonists only (A, E); postinjection of smile antagonists only (B, F). Preinjection of smile antagonists and perinasal muscles (C, G); postinjection of smile antagonists and perinasal muscles (D, H). Upper patient's synkinetic side is her right; lower patient's synkinetic side is her left. Note improvements in dental show (vertical lift) with the addition of the perinasal chemodenervation.
The anatomy and consequent function of this musculature remains controversial in the literature and may often be ignored. Anatomic studies that have focused on providing a more accurate and in-depth understanding of this area describe the presence of deep premaxillary perinasal muscles such as the MM and the synergetic sphincter function of a perinasal modiolus.13–15 These may be very important in the dysfunction of facial expression involving upper lip and oral commissure synkinesis.
In this study, we sought to understand the anatomic relationship of perinasal musculature and how it relates to limitations on dental show in the synkinetic population. We performed a literature review of facial muscle anatomy to explain these novel results as supported by our own human anatomic specimen analysis. We discuss our early findings of the improvement in midfacial and upper lip dysfunction in synkinetic smiles and other subjective improvements through chemodenervation of the perinasal musculature on 34 patients.
Methods
This study was approved by the Louisiana State University Health Sciences Center (New Orleans) IRB. Retrospective chart review was performed on a cohort of patients in a multidisciplinary setting, August 2018–June 2020, with a facial plastic surgeon and facial nerve trained physical therapist focusing on the synkinetic and reanimated population. Patients were evaluated using the Facial Clinimetric Evaluation Scale,16 Sunnybrook Facial Grading System (FGS),17 Facial Disability Index (FDI),18 and the Synkinesis Assessment Questionnaire.19 Smile quality represented by dental show from upper lip elevation (vertical movement) or increased buccal corridor (lateral movement) (Fig. 2) was evaluated using photographs of the patient's smiles.
Fig. 2.
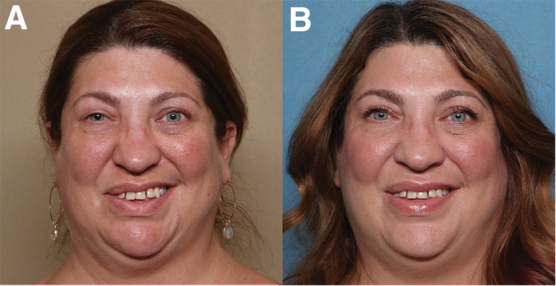
Postinjection of botulinum toxin A into smile antagonist only (A) versus smile antagonists and perinasal muscles (B). The synkinetic side is the patient's right. Note improvements in dental show (vertical lift) and buccal corridor (lateral motion) with the addition of the perinasal chemodenervation, also improving the symmetry of her nasolabial crease.
Chemodenervation was performed utilizing Onabotulinum toxin or Botox, 100 U vials, diluted with 2 cc of injectable saline. Initially, multiple injection patterns were attempted along the upper lip, ala, and nasal sidewall. Ultimately, the most consistent pattern to yield improvements included an initial dose of 1 U (titrated up or down depending on outcome with a range of 0.5–1.25 U) medially into the deeper musculature of the upper lip toward the columellar base (DS, MM) and identical dosing to the inferior lateral nasal sidewall, above the alar crease (LLSAN, NM) (Fig. 3). To evaluate the improvements in smile, pre- and postchemodenervation photos were compared.
Fig. 3.

Photo, botulinum injection of DS/MM (A), transverse bundle of the NM and alar extension of LLSAN (B). DS, depressor septi nasi; LLSAN, levator labii superioris alaeque nasi; MM, myrtiformis muscle; NM, nasalis muscle.
For a smaller cohort of patients (n = 9) within the main cohort, we retrospectively observed the therapeutic effect from two regimens of chemodenervation, targeting the smile antagonists only and the other targeting the smile antagonists and the perinasal musculature (Fig. 1). A forced choice survey was sent out to naive observers. In this survey, observers were presented with two smile photos for each of the nine subjects. One photo was from postbotulinum toxin with smile antagonists only and the other photo was postbotulinum toxin with smile antagonists and perinasal muscles. Subject's eyes were blocked for privacy to ensure that rating was focused on the mouth and oral commissure position only.
A random integer generator was used to determine which photo was presented first for each subject and respondents were asked to choose which smile was better. We then looked at patient-reported outcomes for all patients receiving perinasal chemodenervation through paired t-test using R Software.
The anatomic evaluation of perinasal musculature responsible for our results was performed bilaterally on two unpreserved donor specimens. The perinasal muscles were exposed through transcutaneous and intraoral approaches. The superficial perinasal muscles of facial expression were exposed by removing the overlying skin and subcutaneous fat between the midpupillary lines over the midface and premaxilla. The intraoral mucosal approach allowed for observation of the relationship between the orbicularis oris muscle (OO) superficially and the deep musculature originating from the maxilla. Fiber directionality and bony insertion was noted to infer normal muscular function and resultant synkinetic dysfunction.
Results
There were 109 chemodenervation visits in 53 distinct patients with 34 of those patients undergoing perinasal chemodenervation. Three patients were male, 31 were female, mean age was 55 years (range 14–76 years), with etiologies including Bell's palsy (14), Ramsey Hunt (4), acoustic neuroma (12), vestibular nerve section (1), facial neuroma (1) and trauma (2). Time of onset to initial chemodenervation session ranged from 9 months to 35 years (average 5.2 years)
Nine of the 34 patients had previously undergone chemodenervation of smile antagonists without perinasal injection and subsequently had denervation of the perinasal muscles added to their regimen, with recognized increase in vertical dental show (lip elevation), buccal corridor (lateral movement) (Figs. 1 and 2), and smile symmetry. To support further improvement in smile formation in the perinasal injection group, a forced choice survey was sent out to naive observers, which received 36 complete respondents. Results showed that the naive observers reliably chose the photograph with the addition of perinasal botulinum toxin (p = 0.03).20
The 34 patients who underwent perinasal muscle injections in the DS, NM, MM, and LLSAN showed marked improvement in dental show when pre- and post-treatment photos were analyzed. Subjective findings with perinasal injection included decreased nostril collapse and improved nasal respiration. Also noted is that the overall position (height) of the affected ala/nostril was improved with perinasal injection (Fig. 4). These observational improvements were supported by the scores on the FGS, which improved significantly by an average of 7.64 (p = 0.00096). Furthermore, the patients did report subjective improvements that were significant on the FDI social scale. This measure improved by an average of six points (p = 0.015). The other subjective measures did not show significant change.
Fig. 4.

Improvement in symmetry of the position of the ala on the affected side before (A) and after (B) injection of smile antagonist and perinasal muscles. Synkinetic side is patient's right.
The donor dissection was performed with specific attention to the insertion, origin, confluence, and muscular fiber directionality of each muscle. Immediately apparent under the skin were LLSAN, levator labii superioris (LLS), and the NM with its transverse (compressor naris/CN) and alar (dilator naris/DN) components (Fig. 5A). These more superficial muscles were released at the superior lateral lip and reflected superiorly. We then dissected through the nasalis portion of the superficial orbicularis oris (SOON). This allowed us to recognize the confluence of the superficial lip elevators to the deeper muscles traveling horizontally across the upper lip creating a sling of musculature around the nasal ala, the perinasal modiolus (Fig. 5B).
Fig. 5.
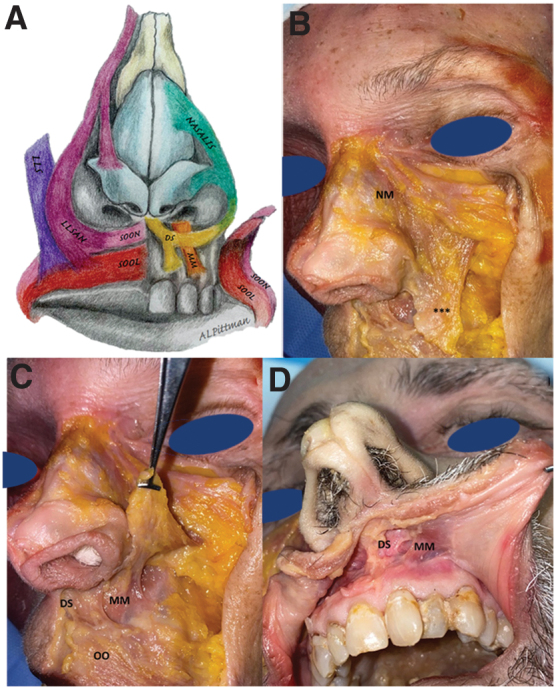
(A) Artistic rendition of the relevant muscles of facial expression, including the LLS, LLSAN, SOON, SOOL, DS, MM, NM, and the confluence into a perinasal modiolus. (B–D) Anatomic dissection of muscles of facial expression. (B) NM, ***confluence of nasalis, LLSAN, and LLS. (C) Superficial muscles reflected superiorly to show the confluence to deep upper lip muscles and their bony attachments. (D) Cadaveric dissection of the upper lip premaxillary musculature (DS and MM), transoral approach demonstrating attachment to the perialar area and medial cheek/nasal sidewall musculature creating a perinasal sling/modiolus. SOOL, superficial orbicularis oris labial component; SOON, superficial orbicularis oris nasal component.
Our second approach to the deeper perinasal muscles was performed intraorally, utilizing a superior vestibular incision.13 The DS, MM, and DN were visible, running in a superior–inferior axis with attachments to the maxilla above the alveolar ridge, the nasal base, and medial midface confluent with and decussating into the muscular sling of the OO, the lip elevators and CN (Fig. 5C, D). Our dissection revealed evidence of a muscular aponeurosis extending from superior-lateral to the nasal dorsum around the entire nasal alar complex to the premaxillary bone inferiorly (Fig. 5). Pulling inferiorly on the medial aspect of this deep premaxillary muscle composed of the DS and MM, we were able to depress the nasal tip, nostril sill, and ala.
Discussion
Facial balancing with chemodenervation seems to be transitioning from the well-accepted contralateral botulinum toxin for improved symmetry to the ipsilateral facial musculature focusing on creating symmetry by minimizing aberrant contractility. Upon initial consultation, the synkinetic patient will often describe their attempts at smiling as continued paralysis; however, the simultaneous contraction of smile antagonists (DAO, mentalis, buccinator, and platysma) actually inhibits the upward deflection of the corner of the mouth, which is actually antagonistic muscle function.9,11,12,21,22 We have found that looking beyond these smile antagonists, one can also find a hypertonicity and fixation of the upper lip and medial cheek with attempted animation.
There is no current study that discuss the contribution of the midfacial musculature as a contribution to smile inhibition in the synkinetic state. Our current study was able to demonstrate an anatomic cause for lack of dental show in patients with synkinesis through human anatomic specimen dissections and supported by botulinum toxin injection outcomes.
It is important to delineate in this population, tethering and fixation versus continued midfacial paresis or hypotonicity. There are some patients, even in the spectrum of partial recovery, who have continued flaccid paralysis of the midface and upper lip. These tissues appear more globally ptotic without a strained or fixed appearance. In synkinetic patients, there appears to be a “tethering” of the midface and perinasal region. This concept of tethering is supported by postinjection elevation of the nasal ala and a more horizontal position of the nasal sill (Fig. 4) and reports of improved nasal respiration.
Discussion of the “modiolus alae naris”13–15,23 exists in facial aesthetic surgery. The perinasal musculature of the medial cheek, including but not limited to the lip elevators, extends into the nasal sidewall (LLSAN) and the area just lateral to the alar crease (LLSAN, LLS, and NM). These muscles blend into a modiolus at the alar crease and medial cheek with the muscles of the upper lip (OO and SOON), nasal sill (DS), and premaxilla (DS, MM, and DN), creating a sling fixating the upper lip in an inferior position.14
Although we have appeared to improve the position of the oral commissure with smile antagonist injection,24 the smile antagonist contribution to upper lip mobility seems to be limited. Upper lip synkinesis appears to be better addressed by targeting the perialar and nasal base musculature. This perinasal confluence of musculature appears to be robustly synkinetic in some patients, and with many of these muscles having at least one attachment to the maxilla, it seems to anchor the midfacial tissue in place. Our dissection confirmed the existence of a fibromuscular aponeurosis extending from superior and lateral to the nasal dorsum around the entire nasal complex to the premaxillary bone inferiorly, confirming a modiolus alae naris (Fig. 5).
Starting at the ascending process of the maxilla, the muscles under consideration of the nasal sidewall include the LLSAN and the NM. The LLSAN originates from the frontal process of the maxilla, nasal bones and medial canthus and divides into two muscular processes, the alaris and labiocolumellar components. The alaris extends to the cephalic margin of the lateral crus and the labiocolumellar component around the ala to the philtral column to act as a dilator and tip depressor.
Inferior to the LLSAN, the NM functions to contract and dilate the nasal cartilages. The transverse (compressor) of the NM is separate and distinct from the more vertical alar (dilator) sections of the muscle. The CN originates from the maxilla near the incisive fossa and inserts into the fibrotic aponeurosis of the nasal dorsum and nasal bone. The DN can be considered a separate muscle,13 spanning from the maxilla above the canine to the fibrotic attachments of the lateral ala. The injection pattern described at the inferior nasal sidewall limits effect to the alar portion of the LLSAN and NM, avoiding the labiocolumellar component passing further laterally. Injection further laterally could possibly affect the labiocolumellar LLSAN or the LLS resulting in lip drop.
The DS was extensively described by de Souza Pinto in 1998.25 The origin of DS is the maxilla superior to the incisors extending superior-medially through the membranous septum, nasal septum, nasal tip ligaments, as well as laterally to the alar division of the NM, essentially creating a sling. DS was seen in our dissection in the medial aspect of the deep premaxillary muscle running superior into the septum and nasal base, intimately associated with the MM lateral to it.
The MM is described as originating from the maxillary bone with attachments to the upper lip and posterior nostril floor.26 Daniel described it as a muscle running from the nasal base to the premaxilla between the DS and the DN and considered a depressor and expander of the nostril.13,14 It has been alternatively described and omitted in Gray's Anatomy. Our dissection found evidence of the MM as a premaxillary muscle that was deep and distinct from the OO, lateral to but intimately associated with the DS medially and DN and LLSAN laterally. Interestingly, the MM appears to have attachments to the deep surface of the OO (Fig. 5C, D).
The OO is described as having deeper and superficial components.13,27 The deeper circumoral component functions as the oral constrictor. The superficial component is divided into a labial and nasal bundle. The superficial labial component extends from the modiolus to the dermis of the philtrum. The superficial nasal OO originates from the lip elevators and the zygomaticus complex toward the columellar base to function as a tip depressor and nostril. It is possible that accentuation of these two separate and superficial bundles is what we see as the horizontal semicircular line extending from the columella and beginning to wrap around the ala in our synkinesis patients (Fig. 1A, C).
We typically place our medial lip injection deep into the orbicularis oris, reducing the risk of oral incompetence and directly addressing the muscles with bony attachments (MM and DS).
The cumulative process of synkinesis therapy is a titration method to any patient's individual deficit. We performed multiple injection patterns to include small unit doses along the length of superior upper lip. The best success was had with deeper injections, which we believe was more directly addressing the DS and the MM. More superficial or lateral injections appeared to weaken the OO or lip elevators. The more medial the injection was performed, essentially at the flare of the medial crus at the nasal sill (Fig. 3A), the more this seemed to protect against the unfortunate outcome of lip drop. The target of the second injection at the inferior nasal side wall, just superior to the alar crease, is often anatomically demonstrated by a visible dimple or contraction with animation. A small dose injection to this area addresses both the alar component LLSAN and the NM (Fig. 3B).
We have found that these two injection sites will address the larger muscles that make up the perinasal sphincter alleviating the visible anchoring and tethering of the upper lip, nasal ala, and midfacial soft tissues in our synkinetic population.
Limitations
Our study is beneficial in its clarification of an additional target for denervation in synkinetic patients; however, it is limited by its retrospective nature and a low number of patients in the antagonist only group. The human anatomic specimen analysis was limited by the current COVID-19 pandemic and its effect on availability of unpreserved donor specimens.
We were also limited by inconsistent capturing of patient reported outcome measures (PROM) data on all patients. Furthermore, the sensitivity of our surveys is limited when there is some improvement with the initial injection pattern, followed by further enhancement with an advanced pattern. We feel that currently utilized patient-reported outcome measures may not be sensitive enough to pick up these subtle changes.
Conclusion
Success with perinasal chemodenervation mandates that the practitioner is able to delineate the difference between hypertonicity of the perinasal musculature and tethering versus continued flaccid paralysis. Perinasal chemodenervation has shown improvements in facial synkinesis patients with regard to dental show, midface symmetry, and may be an important treatment adjunct in those demonstrating midfacial hypertonicity.
Authors' Contributions
M.H. and L.T.H. had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design by L.T.H. and S.M. Acquisition of data by L.T.H., M.H., S.M., and T.L. Analysis and interpretation of data by L.T.H., L.M., and S.M. Drafting of article by M.H., L.T.H., and T.L. Critical revision of the article for important intellectual content by all authors. Statistical analysis by L.M., S.M., and L.T.H. Administrative, technical, or material support by J.M., L.T.H., S.M., and M.H. Supervision by L.T.H., J.M., and S.M.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
No Funding was received for this study.
References
- 1. Artsi EB, Ullrich K, Brusasco L, Malhotra R.. Long-term outcomes of upper eyelid loading with platinum segment chains for lagophthalmos: Am J Ophthalmol. 2020;214:188–195. [DOI] [PubMed] [Google Scholar]
- 2. Bladen J, Norris J, Malhotra R.. Cosmetic comparison of gold weight and platinum chain insertion in primary upper eyelid loading for lagophthalmos. Ophthal Plast Reconstr Surg. 2012;28(3), 2012. [DOI] [PubMed] [Google Scholar]
- 3. Murphey A, Clinkscales W, Oyer S. Masseteric nerve transfer for facial nerve paralysis a systematic review and meta-analysis. JAMA Facial Plast Surg. 2018;20(2):104–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Klebuc M. Facial reanimation using the masseter-to-facial nerve transfer. Plast Reconstr Surg. 2011;127(5):1909–1915. [DOI] [PubMed] [Google Scholar]
- 5. Okazaki M, Kentaro T, Noriko U, et al. One-stage dual latissimus dorsi muscle flap transfer with a pair of vascular anastomoses and double nerve suturing for long-standing facial paralysis. J Plast Reconstr Aesthet Surg. 2015;68(6):e113–e119. [DOI] [PubMed] [Google Scholar]
- 6. Biglioli F, Bayoudh W, Colombo V, Pedrazzoli M, Rabbiosi D. Double innervation (facial-masseter) on the gracilis flap, in the mid face reanimation in the management of facial paralysis—a new concept (French). Ann Chir Plast Esthét. 2013;58(2):89–95. [DOI] [PubMed] [Google Scholar]
- 7. Landingham S, Diels J, Lucareli M. Physical therapy for facial nerve palsy—applications for the physician. Curr Opin Ophthalmol. 2018;29(5):469–475. [DOI] [PubMed] [Google Scholar]
- 8. Lindsay RW, Robinson M, Hadlock TA. Comprehensive facial rehabilitation improves function in people with facial paralysis: a 5-year experience at the Massachusetts Eye and Ear Infirmary. Phys Ther. 2010;90(3):391–397. [DOI] [PubMed] [Google Scholar]
- 9. Shinn J, Nwabueze N, Du L, et al. Treatment patterns and outcomes in botulinum therapy for patients with facial synkinesis. JAMA Facial Plast Surg. 2019;21(3):244–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Filipo R, Spahiu I, Covelli E, Nicastri M, Bertoli GA. Botulinum toxin in the treatment of facial synkinesis and hyperkinesis. Laryngoscope. 2012;122(2):266–270. [DOI] [PubMed] [Google Scholar]
- 11. Benedetto A. Asymmetrical smiles corrected by botulinum toxin serotype A. Dermatol Surg. 2007;33(1 Spec No.):S32–S36. [DOI] [PubMed] [Google Scholar]
- 12. Markey JD, Loyo M. Latest advances in the management of facial synkinesis. Curr Opin Otolaryngol Head Neck Surg. 2017;25(4):265–272. [DOI] [PubMed] [Google Scholar]
- 13. Daniel R, Glasz T, Molnar G, Palhazi P, Saban Y, Journel B. The lower nasal base: An anatomical study. Aesthet Surg J. 2012;33(2):222–232. [DOI] [PubMed] [Google Scholar]
- 14. Figallo E. The nasal tip: a new dynamic structure. Plast Reconstr Surg. 1995;95(7):1178–1184. [DOI] [PubMed] [Google Scholar]
- 15. Vercruysse H, Van Nassauw L, San Miguel-Moragas J, et al. The effect of a Le Fort I incision on nose and upper lip dynamics: unraveling the mystery of the “Le Fort I lip.” J Cranio-Maxillo-Fac Surg. 2016;44(12):1917–1921. [DOI] [PubMed] [Google Scholar]
- 16. Kahn J, Gliklich R, Boyev K, Stewart M, Metson R, McKenna M. Validation of a patient-gradient instrument for facial nerve paralysis: the FaCE scale. Laryngoscope. 2001;111(3):387–398. [DOI] [PubMed] [Google Scholar]
- 17. Neely JG, Cherian NG, Dickerson CB, Nedzelski JM. Sunnybrook facial grading system: reliability and criteria for grading. Laryngoscope. 2010;120(5):1038–1045. [DOI] [PubMed] [Google Scholar]
- 18. Van Swearingen J, Brach J (1996). The facial disability index: reliability and validity of a disability assessment instrument for disorders of the facial neuromuscular system. Phys Ther. 1996;76(12):1288–1300. [DOI] [PubMed] [Google Scholar]
- 19. Mehta RP, Wernick-Robinson M, Hadlock TA. Validation of the Synkinesis Assessment Questionnaire. Laryngoscope. 2007;117(5):923–926. [DOI] [PubMed] [Google Scholar]
- 20. Bates D, Maechler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. J Statist Softw. 2015;67(1):1–48. [Google Scholar]
- 21. Wei LA, Diels J, Lucarelli MJ. Treating buccinator with botulinum toxin in patients with facial synkinesis, a previously overlooked target. Ophthalmic Plast Reconstr Surg. 2016;32(2):p138–p141. [DOI] [PubMed] [Google Scholar]
- 22. Patel PN, Owen SR, Norton CP, et al. Outcomes of buccinator treatment with botulinum toxin in facial synkinesis. JAMA Facial Plast Surg. 2018;20(3):196–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Iwanaga J, Watanabe K, Schmidt C, et al. Anatomical study and comprehensive review of the incisivus labii superioris muscle: application to lip and cosmetic surgery. Cureus. 2017;9(9):e1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jowett N, Malka R, Hadlock T. Effect of weakening of ipsilateral depressor anguli oris on smile symmetry in postparalysis facial palsy. JAMA Fac Plast Surg. 2017;19(1):29–33. [DOI] [PubMed] [Google Scholar]
- 25. de Souza Pinto EB, da Rocha RP, Filho WQ, et al. Anatomy of the median part of the septum depressor muscle in aesthetic surgery. Aesthetic Plast Surg. 1998;22(2):111–115. [DOI] [PubMed] [Google Scholar]
- 26. Figallo EE, Acosta JA. Nose muscular dynamics: the tip trigonum. Plast Reconstr Surg. 2001;108(5):1118–1126. [DOI] [PubMed] [Google Scholar]
- 27. Nicolau PJ. The orbicularis oris muscle: a functional approach to repair in the cleft lip. Br J Plast Surg. 1983;36(2):141–153. [DOI] [PubMed] [Google Scholar]


