Abstract
This cross-sectional study assesses differences in the accuracy of oxygen saturation measured by pulse oximetry among Black and White pediatric patients.
Introduction
Oxygen saturation measured by pulse oximetry (SpO2) is used for triage and management of acute illness in hospitalized children. Adult data indicate that pulse oximetry does not detect hypoxemia as frequently in Black patients as in White patients.1,2 We aimed to investigate the frequency of occult hypoxemia in children and assess variability by patient race.
Methods
This cross-sectional study conducted at a single center in Ann Arbor, Michigan, analyzed patients aged 17 years or younger admitted between January 1, 2015, and December 31, 2020, who self-identified or were identified by a parent as Black or White. We extracted patient race, age, SpO2, arterial blood gas (ABG) data, respiratory support, vasoactive support, and diagnosis information from electronic health records. The University of Michigan Medical School Institutional Review Board exempted the study from approval and informed consent because it constituted secondary research. This study followed the STROBE reporting guideline.
We compared SpO2 readings with arterial oxygen saturation (SaO2) readings directly measured by ABG if the ABG measurement was performed within 10 minutes at the same respiratory support. Exclusion criteria are presented in the eTable in the Supplement. We used the Vasoactive Infusion Score3 to adjust for cardiovascular severity of illness.
We evaluated the mean pulse oximeter bias (SpO2 – SaO2) and frequency of occult hypoxemia (SpO2 ≥92% and SaO2 <88%) by patient race. Statistical analyses were conducted using Stata, version 16 (StataCorp LLC). Significance was set at 2-sided P < .05 (eMethods and eAppendix in the Supplement).
Results
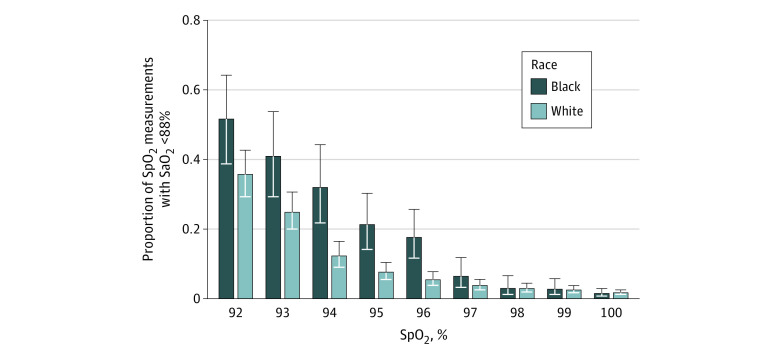
We analyzed 9023 SpO2-SaO2 pairs from 1061 total patients (878 [82.8%] White; 183 [17.2%] Black; 572 [53.9%] male; mean [SD] age, 7 [6] years) (Table). The median time between SpO2 and SaO2 readings was 4 minutes (range, 0 to 9 minutes) for both groups. The overall mean (SD) pulse oximeter bias was 3.5% (5.0%) among White patients and 4.3% (5.0%) among Black patients (P < .001). The frequency of occult hypoxemia was 5.8% (95% CI, 4.6%-7.3%) among White patients and 9.6% (95% CI, 6.3%-14.5%) among Black patients (Figure). After adjusting for age, sex, SpO2, and Vasoactive Infusion Score, the odds ratio for occult hypoxemia among SpO2 measurements for Black patients was 2.16 (95% CI, 1.36-3.44) compared with White patients. At the patient level, 134 of 860 White patients (15.6%; 95% CI, 13.3%-18.2%) and 38 of 180 Black patients (21.1%; 95% CI, 15.7%-27.7%) had occult hypoxemia episodes. After adjusting for the same covariates, the odds ratio for occult hypoxemia was 1.79 (95% CI, 1.07-3.02) for Black patients compared with White patients.
Table. Characteristics of the Study Sample by Race.
| Characteristic | Hospitalizations, No. (%)a | P valueb | ||
|---|---|---|---|---|
| White | Black | Total | ||
| Hospitalizationsc | 934 (83.4) | 186 (16.6) | 1120 (100) | NA |
| Unique patients | 878 (82.8) | 183 (17.2) | 1061 (100) | NA |
| Sex | ||||
| Female | 426 (45.6) | 90 (48.4) | 516 (46.1) | .54 |
| Male | 508 (54.4) | 96 (51.6) | 604 (53.9) | |
| Patient age | ||||
| <6 mo | 152 (16.3) | 33 (17.7) | 185 (16.5) | .98 |
| 6 to <12 mo | 83 (8.9) | 16 (8.6) | 99 (8.8) | |
| 12 to <24 mo | 75 (8.0) | 12 (6.5) | 87 (7.8) | |
| 2 to <5 y | 152 (16.3) | 29 (15.6) | 181 (16.2) | |
| 5 to <12 y | 207 (22.2) | 42 (22.6) | 249 (22.2) | |
| 12 to 17 y | 265 (28.4) | 54 (29.0) | 319 (28.5) | |
| Maximum respiratory support | ||||
| None | 35 (3.7) | 8 (4.3) | 43 (3.8) | .002 |
| Supplemental oxygen | 313 (33.5) | 36 (19.4) | 349 (31.2) | |
| Noninvasive positive pressure | 43 (4.6) | 11 (5.9) | 54 (4.8) | |
| Invasive mechanical ventilation | 543 (58.1) | 131 (70.4) | 674 (60.2) | |
| Maximum VIS | ||||
| 0-5 | 551 (59.0) | 115 (61.8) | 666 (59.5) | .51 |
| 6-10 | 120 (12.8) | 18 (9.7) | 138 (12.3) | |
| 11-20 | 111 (11.9) | 26 (14.0) | 137 (12.2) | |
| >20 | 152 (16.3) | 27 (14.5) | 179 (16.0) | |
| SpO2-SaO2 pairs, No./total No. (%)d | 7018/9023 (77.8) | 2005/9023 (22.2) | 9023/9023 (100) | NA |
| SpO2 values, No./total No. (%) | ||||
| <88% | 626/7018 (8.9) | 248/2005 (12.4) | 874/9023 (9.7) | .71 |
| ≥88% | 6392/7018 (91.1) | 1757/2005 (87.6) | 8149/9023 (90.3) | |
| SpO2 – SaO2, mean (SD) | 3.5 (5.0) | 4.3 (5.0) | 3.7 (5.0) | <.001 |
| SaO2 <88% and SpO2 ≥88%, No./total No. (%) | ||||
| SpO2 of 88%-91% | 188/334 (56.3) | 74/106 (69.8) | 262/440 (59.6) | NA |
| SpO2 of 92%-96% | 248/1845 (13.4) | 127/433 (29.3) | 375/2278 (16.5) | |
| SpO2 >96% | 104/4213 (2.5) | 32/1218 (2.6) | 136/5431 (2.5) | |
| Occult hypoxemia, No./total No. (%)e | 352/6058 (5.8) | 159/1651 (9.6) | 511/7709 (6.6) | .03 |
Abbreviations: NA, not applicable; SaO2, arterial oxygen saturation; SpO2, oxygen saturation measured by pulse oximetry; VIS, Vasoactive Infusion Score.
Some percentages may not sum to 100% owing to rounding.
P values for the patient-level analysis were calculated using the χ2 test or analysis of variance; P values for the SpO2 measurement-level analysis were calculated using mixed models to adjust for clustering within patients.
Sex, age, maximum respiratory support, and VIS are reported for each hospitalization.
The sample included SpO2 and SaO2 directly measured by arterial blood gas analysis. Pairs were measured within 10 minutes at the same level of respiratory support.
Occult hypoxemia was defined as SaO2 less than 88% in the presence of SpO2 of 92% or greater.
Figure. Racial Differences in the Proportion of Oxygen Saturation Measured by Pulse Oximetry (SpO2)–Arterial Oxygen (SaO2) Pairs Demonstrating Occult Hypoxemia.
Vertical lines indicate 95% CIs.
Discussion
In this study, 21.1% of Black children experienced arterial hypoxemia despite a normal SpO2 reading; occult hypoxemia occurred more frequently than in White children. Among SpO2-Sao2 pairs, the odds of the SpO2 reading failing to detect arterial hypoxemia were more than twice as great if the pair had come from a Black child. Underrecognition and undertreatment of hypoxemia in Black children may contribute to racial disparities in respiratory illness outcomes.4,5,6 We observed higher mean pulse oximeter bias among all children than has been reported in adults.2
This study has limitations. Because pediatric ABG values are rarely obtained outside critical care settings, all study patients were likely critically ill. Clinically significant changes could have occurred in the 10-minute window between obtaining SpO2 and SaO2 values. Black children required more respiratory support than White children, potentially confounding the results, although the findings were not different in adjusted analysis. In addition, self-reported race encompasses many phenotypes. Future research may explore more nuanced associations between pulse oximetry accuracy and skin pigmentation and whether pulse oximeter bias is associated with outcomes. Further evaluation of racial differences in pediatric pulse oximetry accuracy in children and investigation of potential clinical outcomes are warranted.
eMethods. Exclusion Criteria
eTable. Grouping of International Classification of Disease, Version 10 Codes Used to Exclude Patients With Ductal-Dependent Cardiac Lesions From Analysis
eAppendix. Stata Log Files
References
- 1.Sjoding MW, Dickson RP, Iwashyna TJ, Gay SE, Valley TS. Racial bias in pulse oximetry measurement. N Engl J Med. 2020;383(25):2477-2478. doi: 10.1056/NEJMc2029240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Valbuena VSM, Barbaro RP, Claar D, et al. Racial bias in pulse oximetry measurement among patients about to undergo extracorporeal membrane oxygenation in 2019-2020: a retrospective cohort study. Chest. Published online September 27, 2021. doi: 10.1016/j.chest.2021.09.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gaies MG, Gurney JG, Yen AH, et al. Vasoactive-inotropic score as a predictor of morbidity and mortality in infants after cardiopulmonary bypass. Pediatr Crit Care Med. 2010;11(2):234-238. doi: 10.1097/PCC.0b013e3181b806fc [DOI] [PubMed] [Google Scholar]
- 4.Akinbami LJ, Moorman JE, Simon AE, Schoendorf KC. Trends in racial disparities for asthma outcomes among children 0 to 17 years, 2001-2010. J Allergy Clin Immunol. 2014;134(3):547-553.e5. doi: 10.1016/j.jaci.2014.05.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dadlez NM, Esteban-Cruciani N, Khan A, Douglas LC, Shi Y, Southern WN. Risk factors for respiratory decompensation among healthy infants with bronchiolitis. Hosp Pediatr. 2017;7(9):530-535. doi: 10.1542/hpeds.2017-0034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O’Halloran AC, Holstein R, Cummings C, et al. Rates of influenza-associated hospitalization, intensive care unit admission, and in-hospital death by race and ethnicity in the United States from 2009 to 2019. JAMA Netw Open. 2021;4(8):e2121880. doi: 10.1001/jamanetworkopen.2021.21880 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Exclusion Criteria
eTable. Grouping of International Classification of Disease, Version 10 Codes Used to Exclude Patients With Ductal-Dependent Cardiac Lesions From Analysis
eAppendix. Stata Log Files