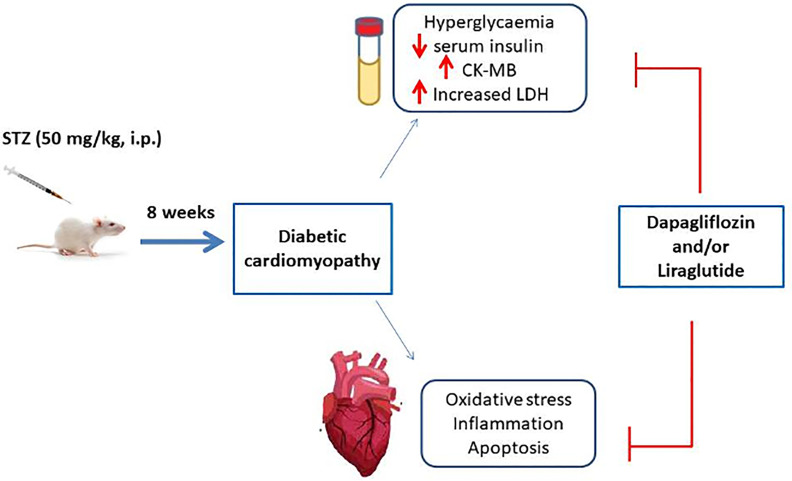
Abstract
The current study aims to assess the protective effects of dapagliflozin (Dapa; a sodium-glucose cotransporter-2 inhibitor) and/or liraglutide (Lira; a glucagon-like peptide 1 agonist) in an experimental model of diabetic cardiomyopathy (DCM). A single dose of streptozotocin (STZ) was administrated to male Sprague–Dawley rats by intraperitoneal injection at a dose of 50 mg/kg to induce diabetes mellitus (DM). Dapa (1 mg/kg, orally), Lira (0.4 mg/kg, s.c.), and Dapa–Lira combination were administrated for 8 weeks once-daily. Blood samples were evaluated for glucose level and biochemical markers of cardiac functions. Cardiac tissue was dissected and assessed for redox homeostasis (malondialdehyde (MDA), glutathione (GSH), and catalase (CAT)), pro-inflammatory mediators (NF-κB and tumor necrosis factor-α (TNF-α)), and apoptotic effectors (caspase-3). Moreover, the effect of treatments on the cardiac cellular structure was studied. Dapa and/or Lira administration resulted in significant improvement of biochemical indices of cardiac function. Additionally, all treatment groups demonstrated restoration of oxidant/antioxidant balance. Moreover, inflammation and apoptosis key elements were markedly downregulated in cardiac tissue. Also, histological studies demonstrated attenuation of diabetes-induced cardiac tissue injury. Interestingly, Dapa–Lira combination treatment produced a more favorable protective effect as compared to a single treatment. These data demonstrated that Dapa, Lira, and their combination therapy could be useful in protection against DM-accompanied cardiac tissue injury, shedding the light on their possible utilization as adjuvant therapy for the management of DM patients.
Keywords: dapagliflozin (PubChem CID: 9887712), liraglutide (PubChem CID: 16134956), diabetes risk, cardiomyopathy, rats
Introduction
Diabetes mellitus (DM) is a complex chronic metabolic disease whose incidence is escalating globally (1, 2). Type 1 DM (T1DM) is caused by progressive T cell-mediated immune damage of pancreatic β cells resulting in persistent hyperglycemia (3). On the other hand, type 2 DM (T2DM) is associated with deficient insulin secretion due to compromised β-cell function accompanied by peripheral insulin secretion. However, recent reports proposed β-cell as a key contributor to the pathogenesis of T1DM via evading the immune attack, highlighting the contribution of β-cell stress responses to disease onset (4). Thereby, several therapeutic interventions that aimed at improving glycemic control and ameliorating pressure exerted on β cells in T2DM have been evaluated in the context of T1DM (5). Among these strategies, glucagon-like peptide-1 (GLP-1) analogs and sodium-glucose cotransporter-2 (SGLT2) inhibitors have recently shown some benefit as adjuvant therapy with insulin in the treatment of patients with T1DM (6). Further, intensive insulin therapy in T1DM increases the occurrence of abdominal obesity, dyslipidemia, and hypertension, putting them at higher risk of cardiovascular disorders. Hence, antihyperglycemic classes including GLP-1 analogs and SGLT2 inhibitors hold promise as additional adjunctive therapy options that complement insulin efficacy, reduce the risk of weight gain, and improve overall glycemic control (7).
Dapagliflozin (Dapa) is an SGLT2 inhibitor that prevents renal glucose reabsorption in proximal tubules. Thus, it reduces the blood glucose level only when it exceeds a reduced renal threshold, decreasing the incidence of hypoglycemia. Additionally, urinary loss of glucose helps weight reduction (8). Liraglutide (Lira) is a synthetic long-acting GLP-1 receptor agonist that has a high structural similarity to human GLP-1. It acts by reducing glucagon secretion, suppressing appetite, slowing gastric emptying, and helping weight loss (9).
Several clinical trials were conducted to assess the utility of using SGLT2 inhibitors and GLP-1 receptor agonists as adjuncts to insulin therapy for T1DM patients. However, the results were not conclusive. This can be explained by the small sample size and the unsatisfactory glucose-lowering efficacy of these agents (10). Dapa has now been licensed for clinical use as adjuvant therapy to insulin in Europe and Japan. However, it has not been approved in the United States due to the increased risk of diabetic ketoacidosis (DKA) (11). However, a risk mitigation strategy has been developed for reducing DKA in T1DM patients treated with SGLT2 inhibitors (12). On the other hand, the use of Lira in combination with insulin resulted in a smaller HbA1c decrease; therefore, it was not considered for a license. Nevertheless, using various combinations of inhibitors of SGLT2 and agonists of GLP-1 receptor is currently under investigation to test whether this approach would yield better therapeutic outcomes in T1DM patients. In this context, a clinical trial conducted by Kuhadiya et al. demonstrated significant improvement in glycemic control and body weight when Dapa was added to Lira and insulin for the treatment of T1DM patients (13). However, two patients developed DKA. With the reported suppressive effect Lira on ketogenesis (14), using a lower dose of SGLT2 inhibitors in combination can achieve the needed balance between clinical benefit and increased risk of DKA. Indeed, the combined beneficial effects of SGLT2 inhibitors and GLP-1 receptor agonists on metabolic indices and vascular complications need further investigations for further establishment of this combination in the treatment of T1DM patients.
Uncontrolled DM is accompanied by a lot of complications that affect various body organs. Among diabetes-associated organ complications, diabetic cardiomyopathy (DCM) is a major leading cause of death in diabetic patients (15). It is characterized by diastolic and systolic dysfunction and pathological cardiac remodeling that may end in heart failure. Various preclinical studies delineated multiple intracellular pathways that are implicated in the pathogenesis of DCM including endoplasmic reticulum stress, oxidative stress, impaired calcium handling, increased lipid utilization, and activation of inflammatory pathways (16). The use of SGLT2 inhibitors and GLP-1 receptor agonists in T2DM has improved associated disorders in various preclinical (17), and clinical studies (18). However, the effect of these antidiabetic drugs on organ injury in T1DM needs further investigation. The present study aimed to study the protective effects of Dapa and/or Lira in an experimental model of DCM. Further, the potential underlying molecular mechanisms have been evaluated.
Materials and Methods
Experimental Design and Treatments
All used protocols were approved by the guidelines of the Ethics Committee at the Faculty of Medicine, Mansoura University, Mansoura, Egypt. Fifty male Sprague–Dawley (SD) rats were obtained from the laboratory animal unit of the Urology and Nephrology Center, Mansoura, Egypt, and kept under required conditions of temperature (21°C ± 2°C), humidity (50% ± 10%), and light (12 h light/dark cycle) with ad libitum access to distilled water and a standard rat diet. After 1-week acclimatization, a single intraperitoneal injection of streptozotocin (STZ) at a dose of 50 mg/kg was used to induce T1DM as previously described (19). Hyperglycemia incidence was confirmed 3 days later via assessment of blood glucose level using a glucometer (Accu-Check, Roche, Mannheim, Germany). Rats were considered diabetic when blood glucose level was greater than 250 mg/dl. The diabetic rats were further randomly assigned into subgroups (n = 10 for each group), including a saline group, a Dapa group (FORXIGA, AstraZeneca, Mississauga, ON, Canada, 1 mg/kg/day, orally), a Lira group (VICTOZA, Novo Nordisk, Bagsværd, Denmark, 0.4 mg/kg/day, s.c.), and a Dapa+Lira group. Each rat was given treatment for 8 weeks. An additional group of rats (n = 10) was used as the normal control group and received saline.
At the end of the experimental period, blood glucose level was assessed as previously mentioned. Animals were sacrificed under anesthesia, and blood samples were withdrawn from the retro-orbital plexus and centrifuged at 3,000 rpm for 10 min to separate the serum, which was further used for biochemical analysis. The hearts were rapidly isolated. One part was fixed in 10% formalin for histopathological examination and immunohistochemistry (IHC) analysis. Another part was washed with ice-cold saline, rapidly frozen in liquid nitrogen, and stored at −80°C for protein and RT-PCR assays.
Biochemical Measurements
The serum biochemical profiles, including insulin (Cloud-Clone Corp., Houston, TX, USA), creatine kinase-MB (CK-MB) (bioMérieux Diagnostics, Milan, Italy), lactate dehydrogenase (LDH; Spectrum Diagnostic Company, Cairo, Egypt) were evaluated according to the manufacturers’ instructions.
Histological Studies
Dissected organ specimens were fixed and dehydrated in ascending grades of ethanol. Thereafter, cardiac specimens were embedded in paraffin wax. Then, 5-µm-thick sections were prepared from paraffin blocks. Sections were further stained with H&E to be assessed for histopathological alterations. The examination was performed by a qualified observer without the identification of the experimental groups. All records were performed using Olympus light microscope equipped with a digital camera (Tokyo, Japan). For morphometric analysis, semiquantification of myocardial injury was performed (20, 21),. Each slide was inspected for cardiac pathological changes in three high-power fields using the following scoring system for grading the cardiomyopathy severity: 0 = no damage, 1 = mild lesion, 2 = moderate lesion, and 3 = severe lesion, with (1+) for the presence of myocardial fiber swelling and interstitial edema, (1+) for disorganization of myocardial fiber with or without fibroblastic proliferation, (1+) for perinuclear vacuolization or myocardial fiber vacuolization, (1+) for myocardial fibers myocytolysis/necrosis, and 0 when there was no damage noted.
Preparation of Cardiac Tissue Homogenate and Assessment of Oxidative Stress
Cardiac tissue was homogenized in phosphate-buffered saline (PBS) to prepare 10% (w/v) homogenate using Omni-125 handheld homogenizer (Omni International, Kennesaw, GA, USA). The homogenates were further spun at 5,000g for 15 min at 4°C. Oxidative stress biomarkers were then assessed in freshly prepared supernatants. Levels of malondialdehyde (MDA), a marker of lipid peroxidation, and the antioxidant reduced glutathione (GSH) in addition to the activity of the antioxidant enzyme catalase (CAT) were measured in prepared tissue homogenates assayed using commercially available kits by Bio Diagnostic (Giza, Egypt) according to the manufacturer’s protocols.
Assessment of Cardiac Inflammatory Cytokine Levels by ELISA
Levels of interleukin-1β (IL-β) and interleukin-6 (IL-6) in cardiac tissue homogenates prepared from different experimental groups were assessed by the ELISA method according to the manufacturer’s instructions (Cloud-Clone Corp., Houston, TX, USA).
Real-Time PCR
Total RNA was extracted from cardiac tissues from all experimental groups using Direct-zol RNA Miniprep Plus (ZYMO RESEARCH CORP., Irvine, CA, USA, Cat# R2072). Extracted RNA was assessed for quantity and quality by spectrophotometry using Beckman dual spectrophotometer (Brea, CA, USA).
Extracted RNA was then utilized for reverse transcription into complementary DNA (cDNA) using the SuperScript IV One-Step RT-PCR kit (Thermo Fisher Scientific, Waltham, MA, USA, Cat# 12594100). cDNA amplification was performed using a 48-well plate StepOne instrument (Applied Biosystems, Foster City, CA, USA). The thermal profile included the following: reverse transcription for 10 min at 45°C, RT inactivation and initial denaturation by 40 cycles of 10 s at 98°C, and an amplification step for 10 s at 55°C followed by 30 s at 72°C. Data were then expressed in cycle threshold (Ct) for the housekeeping gene and the target genes. ΔΔCt method was used to normalize variation in the target genes expression by referring to the expression value of mean critical threshold (CT) of a housekeeping gene. Primer sequence for tumor necrosis factor-α (TNF-α) gene was forward 5′-TAC TGA ACT TCG GGG TGA TTG GTC C-3′ and reverse 5′-CAG CCT TCT CCC TTG AAG AGA ACC-3′, for caspase-3 gene was forward 5′-ATGGACAACAACGAAACCTC-3′ and reverse 5′-TTAGTGATAAAAGTACAGTTCTT-3, and for β-actin housekeeping gene was forward 5′-CTAAGGCCAACCGTGAAAAG-3′ and reverse 5′-GCCTGGATGGCTACGTACA-3′. The relative quantitation (RQ) of each target gene is performed based on the calculation of the 2−ΔΔCt method.
Immunohistochemistry
Sections were dewaxed, rehydrated, and washed, followed by incubation in 3% hydrogen peroxide (H2O2, 3%) for 10 min and then blocking with 5% bovine serum albumin (BSA) in Tris-buffered saline (TBS). Thereafter, immunolocalization was performed by incubation with antibodies for NF-κB/p56 (Thermo Fisher Scientific Inc., Waltham, MA, USA), TNF-α (sc-52746, Santa Cruz, Paso Robles, CA, USA), and cleaved caspase-3 (GB11532, Wuhan Servicebio Biotechnology, Wuhan, China) at 4°C overnight. Then, sections were washed in TBS 3 times, followed by incubation with secondary antibodies. After washing in TBS, diaminobenzidine/peroxidase was used for development and hematoxylin for counter-staining. The sections were then mounted and examined using Olympus light microscope equipped with a digital camera (Tokyo, Japan). For IHC quantitative assessment, an immunoreactive score (IRS) was used. It provides a scale of 0–12 representing IRS index (0–1 = negative, 2–3 = mild, 4–8 = moderate, and 9–12 = strongly positive). IRS is obtained by multiplication between staining intensity grading (0–3) and positive cells proportion grading (0–4) (22) and quantified using the QuPath program (0.1.2) (23).
Statistical Analysis
Analysis and graphical representation of data were accomplished by GraphPad prism statistical software (version 8, USA). Results are expressed as mean ± SE, and statistical analysis was performed using one-way ANOVA followed by a post-hoc test (Tukey–Kramer). Two-way ANOVA was used to calculate statistical significance for NF-κB/p56 nuclear and cytoplasmic expression among various experimental groups. Statistical significance was considered at p < 0.05.
Results
Effects of Dapagliflozin–Liraglutide Treatment on Blood Glucose and Serum Insulin Levels
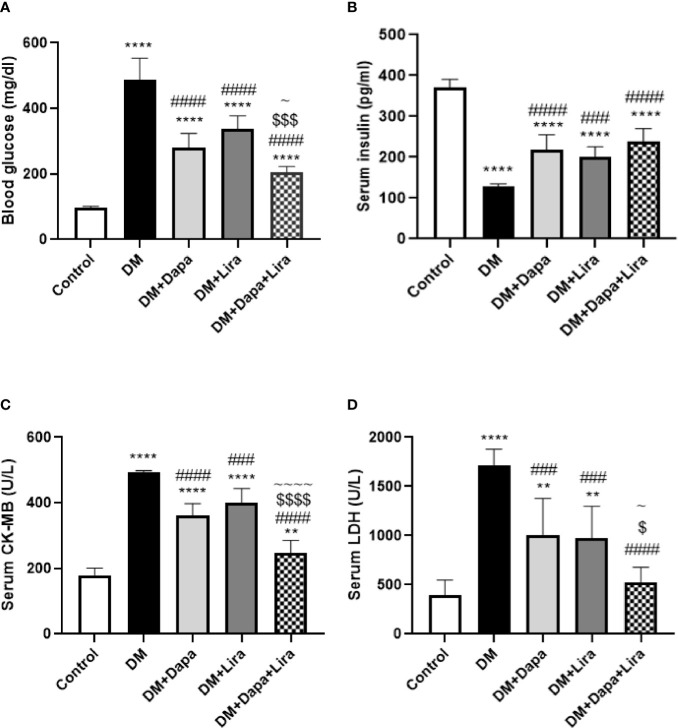
Induction of experimental DM resulted in a 4.99-fold (p < 0.0001) increase in blood glucose level accompanied by a 65.3% reduction (p < 0.0001) in serum insulin as compared to normal control. Treatment with Dapa and Lira for 8 weeks resulted in 42.5% and 30.9% significant decrease (p < 0.0001) in blood glucose level and 1.69- and 1.55-fold (p < 0.0001, p < 0.001) increase in serum insulin level, respectively, as compared to the diabetic group. Combined treatment with Dapa and Lira significantly improved blood glucose level (p < 0.05, p < 0.0001, respectively) and produced non-significant elevation in serum insulin level as compared to single treatment groups ( Figures 1A, B ).
Figure 1.
Effect of dapagliflozin (Dapa) and liraglutide (Lira) or their combination on (A) blood glucose level, (B) serum creatine kinase-MB (CK-MB), and (C) serum lactate dehydrogenase (LDH). Data are represented as mean ± SE, n = 6. ** significance in comparison with control group at p < 0.01, **** at p < 0.0001, ### significance in comparison with diabetic group at p < 0.001, #### at p < 0.0001, ~ significance in comparison with DM+Dapa at p < 0.05, ~~~~ significance in comparison with DM+Dapa at p < 0.0001, $ significance in comparison with DM+Lira group at p < 0.05, $$$ at p < 0.001, $$$$ at p < 0.0001.
Effects of Dapagliflozin–Liraglutide Treatment on Biomarkers of Cardiac Injury
Serum levels of LDH and CK-MB were significantly increased by 2.47- and 4.31-fold (p < 0.0001 and p < 0.0001), respectively, in the DM group compared to the normal group. However, these levels were significantly reduced by 26.5% and 41.2% (p < 0.0001, p < 0.001), respectively, in the Dapa-treated group and by 18.7% and 43.2% (p < 0.001), respectively, in the Lira-treated group when compared to the diabetic group. Combined Dapa–Lira therapy produced more reduction in the levels of these cardiac markers by 31.6% and 47.7% (p < 0.0001, p < 0.05, respectively), as compared to the DM+Dapa group and by 38% and 45.9% (p < 0.0001, p < 0.05, respectively) as compared to the DM+Lira group ( Figures 1C, D ).
Effects of Dapagliflozin–Liraglutide Treatment on Type 1 Diabetes Mellitus-Induced Cardiac Histological Changes
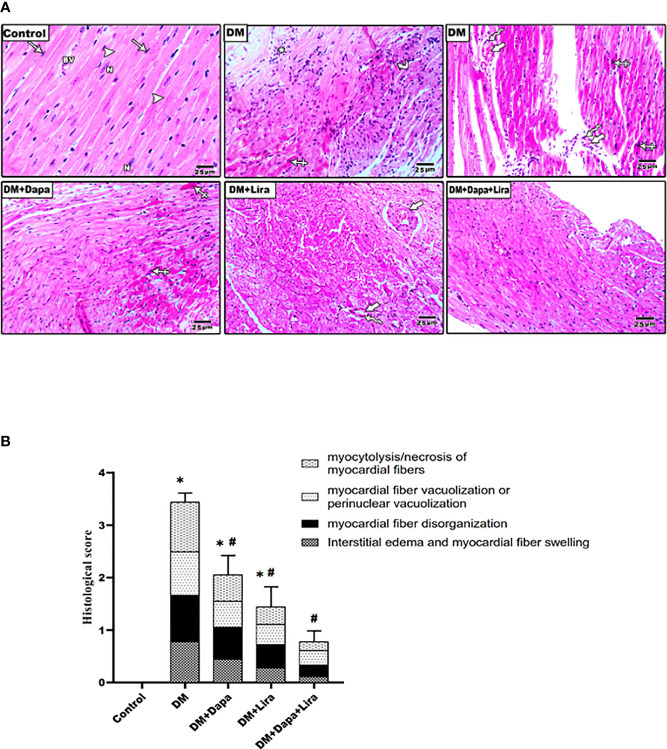
As shown in Figure 2A , H&E-stained heart sections from the control group demonstrated the normal histological structure of the cardiac muscle fibers. Sections from diabetic rat hearts showed disarray of the cardiac myocytes with myocardial fiber disorganization, myocardial fiber necrosis, and mild chronic inflammatory cells in the subpericardium, apoptotic myocyte with hypereosinophilic cytoplasm, pyknotic nuclei and increased intermyocyte, interstitial edema, and perivascular chronic inflammatory cells. The degree of injury appears to be moderate in the DM+Dapa-treated group and mild in the DM+Lira-treated group. The normal structure in cardiac sections from the DM+Dapa+Lira group was almost restored. Additionally, these results were further ascertained by morphometric analysis shown in Table 1 and Figure 2B .
Figure 2.
(A) Representative photomicrographs of H&E-stained cardiac sections from different experimental groups. Normal control rats showed normal histological structure of the cardiac muscle diabetic group demonstrating disarray of the cardiac myocytes with myocardial fiber disorganization (curved arrow), myocardial fiber necrosis with chronic inflammatory cells in the subpericardium (astrix), apoptotic myocyte with hypereosinophilic cytoplasm, pyknotic nuclei (crossed arrow), increased intermyocyte (thick arrows), and perivascular chronic inflammatory cells (zigzag arrows) marked interstitial edema. Treatment groups (DM+Dapa) and (DM+Lira) showed less structural injury. Combination therapy group (DM+Dapa+Lira) showed almost restoration of normal cardiac structure. ×400 bar 25. (B) A graph showing histological score for cardiomyopathy. Data are expressed as mean ± SEM (n = 6). *p < 0.05 versus control and # p < 0.05 versus diabetic group. Dapa, dapagliflozin; Lira, liraglutide.
Table 1.
Histopathological scores for myocardial necrosis among different experimental groups.
| Myocardial fiber swelling and interstitial edema | Disorganization of myocardial fiber with or without fibroblastic proliferation | Perinuclear vacuolization or myocardial fiber vacuolization | Myocardial fibers myocytolysis/necrosis | Total severity score | |
|---|---|---|---|---|---|
| Control | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| DM | 0.78 ± 0.14* | 0.89 ± 0.08* | 0.83 ± 0.09* | 0.94 ± 0.06* | 3.44 ± 0.17* |
| DM+Dapa | 0.44 ± 0.13*# | 0.66 ± 0.11*# | 0.50 ± 0.12*# | 0.50 ± 0.13*# | 2.06 ± 0.37*# |
| DM+Lira | 0.28 ± 0.10*# | 0.45 ± 0.12*# | 0.39 ± 0.12*# | 0.33 ± 0.11*# | 1.44 ± 0.38*# |
| DM+Dapa+Lira | 0.06 ± 0.03# | 0.23 ± 0.10# | 0.28 ± 0.11# | 0.17 ± 0.09# | 0.78 ± 0.21# |
Tissue pathological changes within each slide were inspected and scored in three high-power fields based on severity and extent of myocardial injury. A semiquantitative scoring from 0 to 3 or more was used, with no damage = 0 and severe damage = 3 or more. Values represent the average score for each animal. Results are considered significantly different when p < 0.05. Data are expressed as mean ± SEM (n = 6).
DM, diabetes mellitus; Dapa, dapagliflozin; Lira, liraglutide.
*p < 0.05 versus control.
#p < 0.05 versus diabetic group.
Effects of Dapagliflozin–Liraglutide Treatment on Oxidative Stress Markers in Cardiac Tissue
Cardiac tissue levels of MDA demonstrated a marked increase (p < 0.0001) in the DM group in comparison with the normal control group. Meanwhile, a significant decrease in the treated groups (DM+Dapa and DM+Lira groups) (p < 0.0001) was observed as compared to that of the untreated DM group. Additionally, the combination treatment produced a significant decrease in MDA as compared to the DM+Lira group (p < 0.05). In contrast, the level of the antioxidant GSH and activity of antioxidant enzyme CAT were significantly decreased in cardiac tissues of the DM group (p < 0.0001) in comparison with the normal control group. However, a significant increase in the levels of these antioxidant moieties was observed in the treated groups (DM+Dapa and DM+Lira groups) when compared to the untreated DM group (p < 0.0001). Also, the combination therapy markedly increased tissue GSH (p < 0.0001) as compared to the DM+Lira group as well as tissue CAT (p < 0.05) compared to the DM+Dapa and DM+Lira groups ( Figures 3A–C ).
Figure 3.
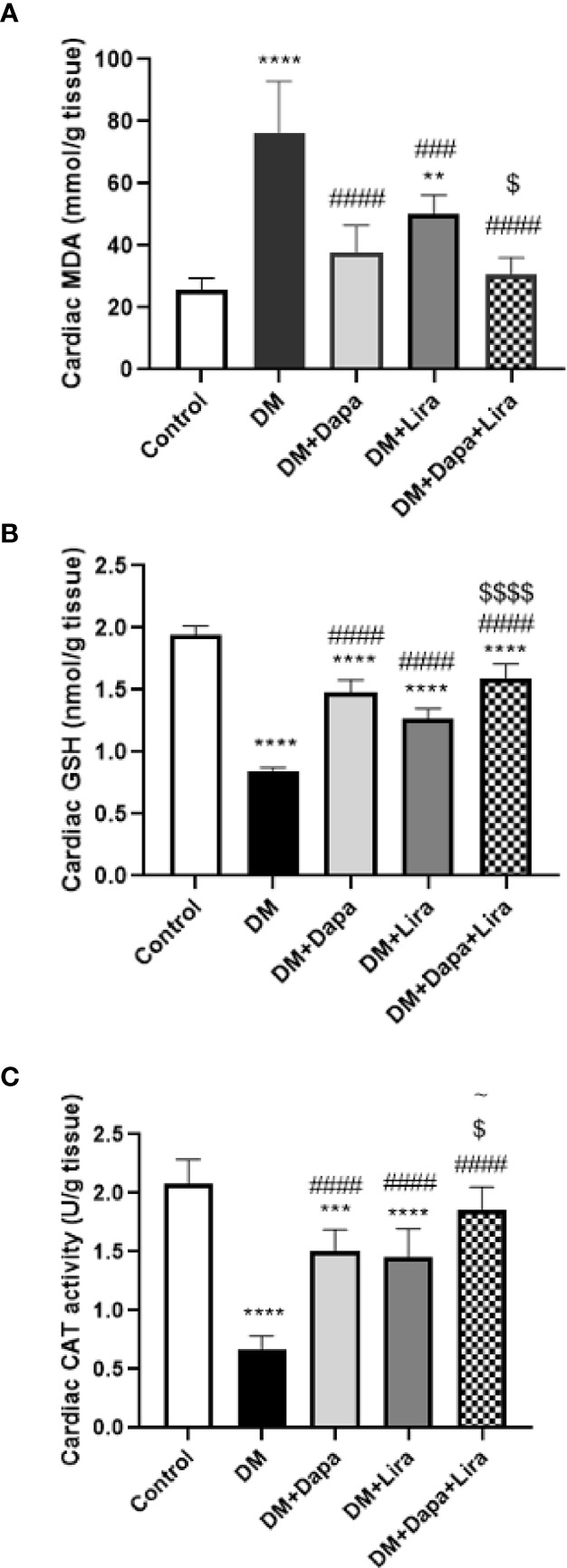
Effect of dapagliflozin (Dapa) and liraglutide (Lira) or their combination on oxidative stress biomarkers in rat heart tissues: (A) tissue malondialdehyde (MDA), (B) tissue reduced glutathione (GSH), and (C) tissue catalase activity (CAT). Data are represented as mean ± SE, n = 6. ** significance in comparison with control group at p < 0.01, *** at p < 0.001, **** at p < 0.0001, ### significance in comparison with diabetic group at p < 0.001, #### at p < 0.0001, ~ significance in comparison with DM+Dapa at p < 0.05, $ significance in comparison with DM+Lira group at p < 0.05, $$$$ at p < 0.0001.
Effect of Dapagliflozin–Liraglutide Treatment on mRNA Expression of TNF-α and Caspase-3 in Cardiac Tissue
TNF-α mRNA levels were significantly increased in the cardiac tissue of the DM group (p < 0.0001) compared to normal control. On the other hand, the pro-inflammatory cytokine mRNA levels were markedly decreased in the treated groups (DM+Dapa and DM+Lira groups, p < 0.0001) compared to the DM group. Additionally, the combination treatment resulted in a significant decrease in TNF-α mRNA level when compared with single treatment, p < 0.0001 ( Figure 4A ).
Figure 4.
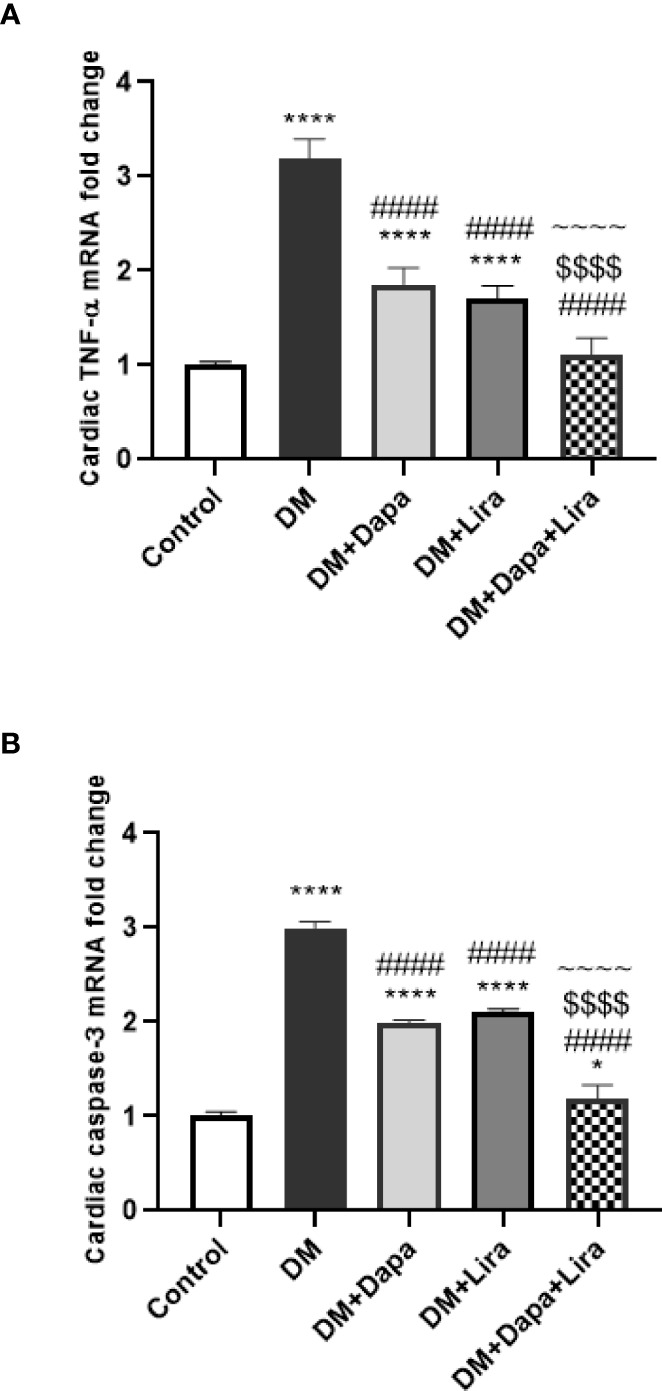
Effect of dapagliflozin (Dapa) and liraglutide (Lira) or their combination on mRNA expression of (A) tissue TNF-α and (B) tissue caspase-3. Data are represented as mean ± SE, n = 5. **** significance in comparison with control group at p < 0.0001, #### significance in comparison with diabetic group at p < 0.0001, ~~~~ significance in comparison with DM+Dapa at p < 0.0001, $$$$ significance in comparison with DM+Lira group at p < 0.0001.
Regarding caspase-3, the mRNA levels of the apoptotic enzyme were significantly elevated in cardiac tissues of the DM group, p < 0.0001, in comparison with those of the normal control group. However, a marked decrease in its level was observed in groups treated with Dapa or Lira, p < 0.0001, as compared to the untreated DM group. Further, the combination therapy resulted in a more significant reduction in caspase-3 mRNA level compared to single treatment groups, p < 0.0001 ( Figure 4B ).
Effect of Dapagliflozin–Liraglutide Treatment on Cardiac Tissue Inflammatory Cytokines IL-1β and IL-6
Cardiac tissue of the DM group demonstrated significantly increased levels of inflammatory cytokines IL-1β and IL-6 (p < 0.0001) as compared to normal control. However, the pro-inflammatory cytokine levels were significantly decreased in cardiac tissue of the DM+Dapa group (p < 0.01, p < 0.0001, respectively) and DM+Lira group (p < 0.0001) as compared to the untreated DM group. Also, the combination treatment group demonstrated a significant decrease in cardiac IL-1β levels (p < 0.0001) when compared with the DM+Dapa group and in cardiac IL-6 levels (p < 0.0001) when compared with treatment groups (DM+Dapa and DM+Lira groups) ( Figure 5 ).
Figure 5.
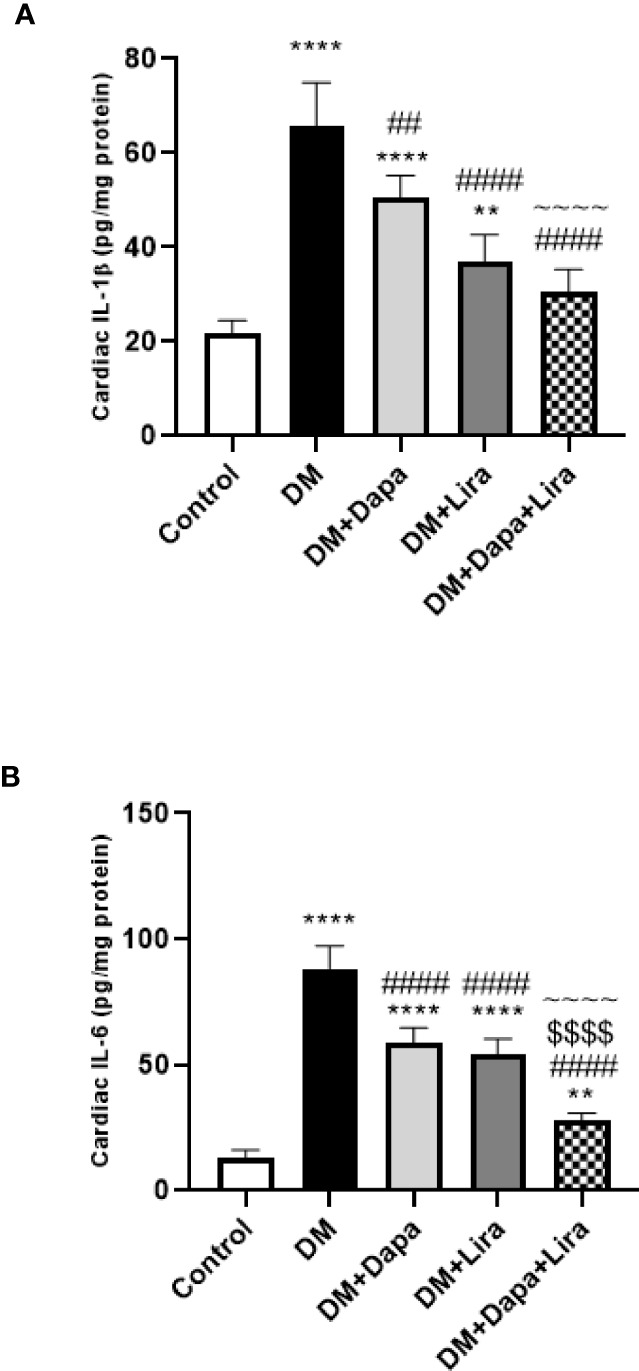
Effect of dapagliflozin (Dapa) and liraglutide (Lira) or their combination on protein levels of (A) tissue IL-1β and (B) tissue IL-6. Data are represented as mean ± SE, n = 5. ** significance in comparison with control group at p < 0.01, **** at p < 0.0001, ## significance in comparison with diabetic group at p < 0.01, #### at p < 0.0001, ~~~~ significance in comparison with DM+Dapa at p < 0.0001, $$$$ significance in comparison with DM+Lira group at p < 0.0001.
Effect of Dapagliflozin–Liraglutide Treatment on Immunostaining of NF-κB/p56, TNF-α, and Cleaved Caspase-3 in Cardiac Tissue
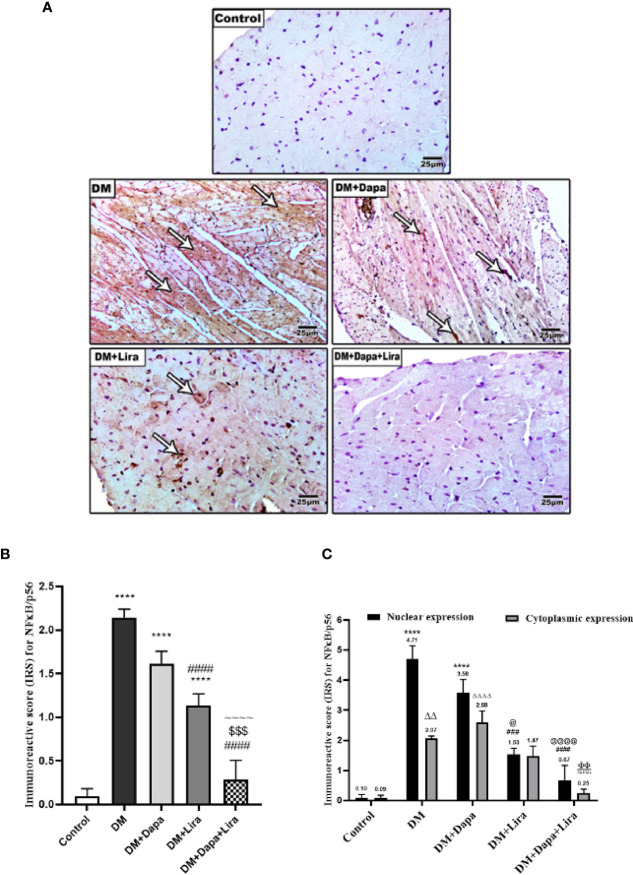
Results from IHC further reinforce RT-PCR findings. Indeed, immunostaining of cardiac tissues from different experimental groups demonstrated increased immunostaining of NF-κB/p56 in cardiac tissue of the diabetic group (p < 0.0001) when compared to normal control. The immunostaining was markedly decreased in treatment groups (DM+Dapa and DM+Lira groups, p < 0.0001) compared to the diabetic group. Additionally, the combination treatment resulted in a significant decrease in immunostaining of NF-κB/p56 in cardiac tissue compared with the DM+Dapa group (p < 0.0001) and DM+Lira group (p < 0.001) ( Figures 6A, B ). Further, the IRS score was used to compare the nuclear and cytoplasmic expression of NF-κB/p56 among different experimental groups. As shown in Figure 6C , the nuclear expression of NF-κB/p56 in the diabetic group was significantly different as compared to normal control (p < 0.0001). Treatment with Lira resulted in a significant reduction in NF-κB/p56 nuclear expression compared to the diabetic group (p < 0.001). Further, combination treatment resulted in a significant reduction in nuclear expression of NF-κB/p56 as compared to the DM+Dapa-treated group (p < 0.0001).
Figure 6.
(A) Representative photomicrographs of NF-κB/p56 immuno-stained cardiac sections from different experimental groups, ×200 bar 50. (B) A graph showing immunoreactive score for NF-κB/p56 cellular expression. Data are represented as mean ± SE, n = 6. **** significance in comparison with control group at p < 0.0001, #### significance in comparison with diabetic group at p < 0.0001, ~~~~ significance in comparison with DM+Dapa at p < 0.0001, $$$ significance in comparison with DM+Lira group at p < 0.001. (C) A graph showing immunoreactive score for NF-κB/p56 differentially in the nucleus and cytoplasm. Data are expressed as mean ± SEM (n = 6). **** p < 0.0001 versus control, #### p < 0.0001, ### p < 0.001 versus diabetic group and p < 0.0001, @ p < 0.05 versus DM+Dapa for nuclear expression, ΔΔp < 0.01, ΔΔΔΔp < 0.0001 versus control, ΦΦ p < 0.01 versus diabetic group, πππp < 0.001 versus DM+Dapa for cytoplasmic expression. Dapa, dapagliflozin; Lira, liraglutide.
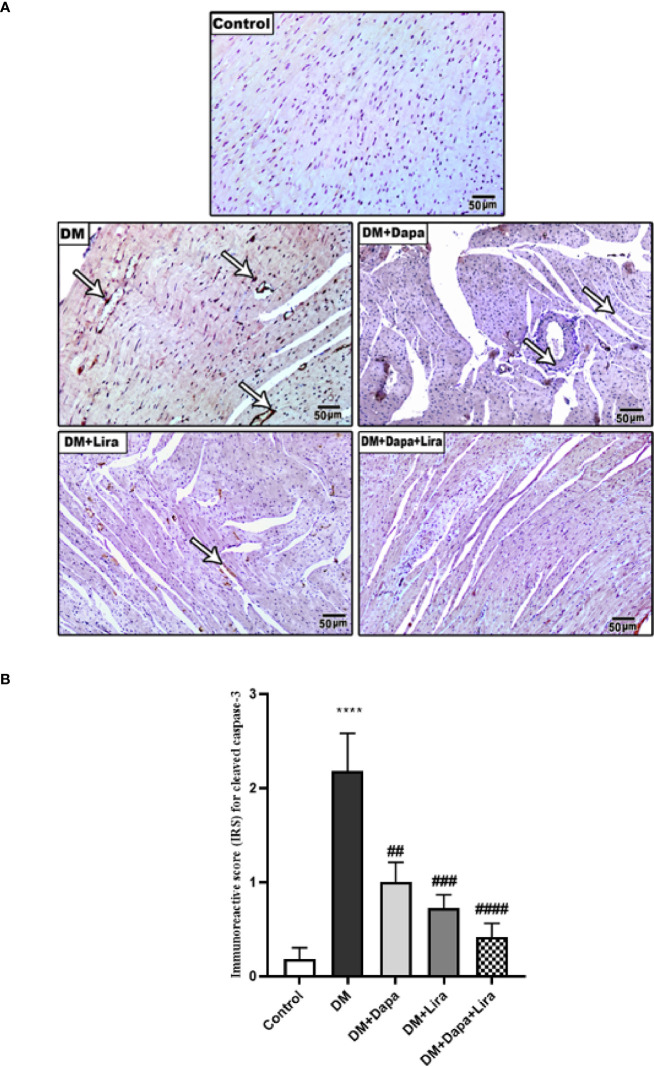
Immunostaining of TNF-α and cleaved caspase-3 was significantly increased in cardiac tissue of the diabetic group (p < 0.0001) compared to normal control. However, immunostaining of TNF-α and cleaved caspase-3 was significantly decreased in cardiac tissue of treatment groups (DM+Dapa and DM+Lira groups and combination treatment groups, p < 0.0001) compared to the diabetic group ( Figures 7 , 8 ).
Figure 7.
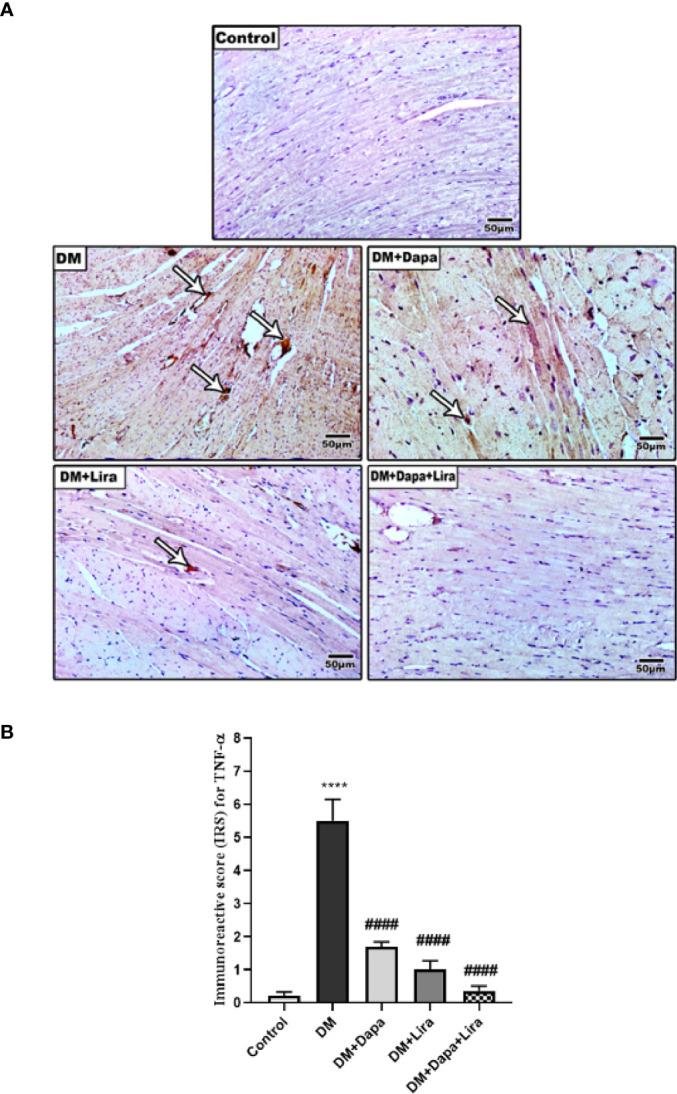
(A) Representative photomicrographs of TNF-α immuno-stained cardiac sections from different experimental groups, ×200 bar 50. (B) A graph showing immunoreactive score for TNF-α cellular expression. Data are represented as mean ± SE, n = 6. **** significance in comparison with control group at p < 0.0001, #### significance in comparison with diabetic group at p < 0.0001. Dapa, dapagliflozin; Lira, liraglutide.
Figure 8.
(A) Representative photomicrographs of cleaved caspase-3 immuno-stained cardiac sections from different experimental groups. ×200 bar 50. (B) A graph showing immunoreactive score for cleaved caspase-3 cellular expression. **** Significance in comparison with control group at p < 0.0001, #### significance in comparison with diabetic group at p < 0.0001, ### significance in comparison with diabetic group at p < 0.001, ## significance in comparison with diabetic group at p < 0.01.
Discussion
Despite using insulin as the mainstay for T1DM treatment, non-insulin adjunct therapy is a new trend. The purpose of this therapy is to slow down autoimmune processes, to regulate glucagon secretion, and to protect pancreatic β cells. Thereby, the use of this therapy has improved glycemic control, provided nephroprotection, and protected vascular endothelium in both clinical and experimental studies The present study demonstrated the protective efficacy of a selective SGLT2 inhibitor Dapa and a long-acting GLP-1 receptor agonist Lira and their combination therapy in DM-accompanied organ injury (24).
Oxidative stress plays a crucial role in the pathogenesis of DM and its induced complications. Disruption of the normal homeostasis of free radicals has been implicated in the impairment of pancreatic β-cell function (25). Further, oxidation of glucose and non-enzymatic glycation of proteins trigger free radical formation, which in turn causes damage to macromolecules, cellular machinery, and antioxidant enzymes (26). Indeed, several in vivo and in vitro experimental studies reported that diabetes-accompanied metabolic abnormalities cause mitochondrial superoxide overproduction. This event is considered a central and major mediator of diabetes tissue damage via activation of key pathogenic pathways involved in the pathogenesis of diabetic organ complications (27). Besides, oxidative stress inflammation is a common feature of DM. Both effectors reportedly share the same stimulus, reactive oxygen species (ROS). Of note, oxidative stress triggers inflammatory cascades, which in turn promote ROS production, creating a vicious cycle that increases the complexity of diabetes-associated multi-organ complications (28). Furthermore, DM-related oxidative stress and inflammation can trigger cellular apoptosis leading to cellular damage and organ failure (29). In agreement, we previously reported signs of oxidative stress, inflammation, and apoptosis in diabetic tissue using the same experimental model (30–33). Currently, our data were consistent as evident by an increased tissue biomarker of oxidative stress, MDA, and reduced cellular antioxidant defense, GSH and CAT. This was accompanied by increased tissue pro-inflammatory cytokine expression TNF-α as well as upregulated expression of the apoptotic enzyme, caspase-3.
In addition to its role as a crucial modulator of the inflammation process via regulation of the expression of hundreds of genes involved in cellular inflammatory events, NF-κB is also a redox-sensitive nuclear factor that subsequently modulates a large number of processes to maintain tissue homeostasis. Thereby, NF-κB possesses a strategic position at the crossroad between inflammation and oxidative stress, emphasizing its role as a potential target for the management of DM-accompanied organ injury (34). Herein, our data showed upregulated expression of NF-κB along with increased NF-κB/p56 immunostaining in rat diabetic cardiac tissues.
Accumulating evidence demonstrated therapeutic and protective efficacies of Dapa and Lira in T2DM-associated tissue injury. Chen et al. reported that Dapa administration protected against DM-induced oxidative stress in lens (35). Wei et al. demonstrated that Dapa improved pancreatic β-cell function in db/db mice (36). Tang et al. reported antioxidant and anti-inflammatory effects of Dapa with subsequent inhibition of glomerulosclerosis and liver fibrosis in db/db mice (37). In T2DM patients, Dapa administration resulted in beneficial outcomes pertaining to the microvascular sequelae including improvement of the renal resistive index, arterial stiffness, and systemic endothelial function (38, 39). With respect to Lira, it has been found to slow down memory function decline (40), improve cardiovascular and kidney outcomes, and reduce mortality in T2DM patients (41). Moreover, Lira treatment ameliorated the severity of T2DM complicated with non-alcoholic fatty liver disease (42). In experimental models of T2DM, Lira treatment protected against cognitive deficits (43) and exerted a renoprotective effect (44) via autophagy activation and endoplasmic reticulum stress attenuation (45). Moreover, Lira administration has modulated the gut microbiome and ameliorated fatty liver in db/db mice (46). Interestingly, a recent study using Dapa–Lira combined therapy showed beneficial metabolic and neuroprotective effects in diet-induced diabetic mice (17). Additionally, a clinical trial performed by Petrie et al. demonstrated that Dapa administration reduced cardiovascular morbidity and mortality in patients with heart failure independent of DM (47). Qin et al. reported protective effects of Dapa against the development of ventricular arrhythmia in pulmonary artery hypertension rats. The mechanism involves modulation of TLR4/NF-κB signaling pathway (48). Similarly, Lira has been reported to protect against myocardial pyroptosis in diabetic rats via activation of Sirt1/AMPK signaling pathways (49).
Given the aforementioned evidence and due to their multiple beneficial effects, Dapa and Lira seem to be a very attractive treatment option in T1DM. Indeed, using Dapa in combination with insulin and Lira resulted in a marked improvement in blood glucose and weight loss in T1DM patients (13). Moreover, Dapa treatment demonstrated an anti-atherogenic effect in T1DM mice (50). Interestingly, administration of Dapa or Lira to T1DM mice enhanced β-cell proliferation and decreased β-cell apoptosis (51). Similarly, our data revealed that administration of Dapa and/or Lira to diabetic animals attenuated accompanying cardiac tissue injury via modulating key elements of oxidative stress, inflammation, and apoptosis. Combination treatment was more effective as compared to sole treatment.
In conclusion, the present study demonstrated beneficial protective effects of Dapa and/or Lira administration against T1DM-related cardiac injury. The mechanisms underlying these effects are attenuating oxidative stress, downregulated inflammation, and apoptosis. Both non-insulin pharmacological drugs could be used as adjunct therapy given their beneficial protective effects on body organs during the diabetic course ( Figure 9 ).
Figure 9.
Graphical abstract.
Data Availability Statement
Data are available upon request from the corresponding author.
Ethics Statement
The animal study was reviewed and approved by the Research Ethics Committee, Faculty of Medicine, Mansoura University, Egypt.
Author Contributions
Conceptualization, ME-Sha, ME-A, M-She; Funding acquisition, HE, M-She; Investigation, ME-Sha, ME-A, ME, HE, DE, ME-She, SA and NE; Methodology; ME-Sha, ME-A, ME, HE, DE, ME-She; Resources, ME-Sha, ME-A, ME, HE, DE, ME-She, SA and NE; Software, ME-Sha, ME-A, ME, HE, DDE, ME-She, SA and NE; Visualization, HE, ME-She, SA; Writing – original draft, ME-Sha, MA-A, DDE, ME-She, NE; Writing – review & editing, ME-Sha, ME-A, DE, ME-Sha, NE.
Funding
The authors acknowledge the support provided by the Researchers Supporting program (TUMA, Project-2021-3), AlMaarefa University, Riyadh, Saudi Arabia. The present study was funded by Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2022R171), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1. Pappachan JM. Efficacy and Cardiovascular Safety of Antidiabetic Medications. Curr Drug Saf (2021) 16(2):115–21. doi: 10.2174/1574886316666210112153429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Asseri SM, Elsherbiny NM, El-Sherbiny M, Sherif IO, Alsamman AM, Maysarah NM, et al. Glycyrrhizic Acid Ameliorates Submandibular Gland Oxidative Stress, Autophagy and Vascular Dysfunction in Rat Model of Type 1 Diabetes. Sci Rep (2022) 12(1):725. doi: 10.1038/s41598-021-04594-w [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 3. Pang H, Luo S, Xiao Y, Xia Y, Li X, Huang G, et al. Emerging Roles of Exosomes in T1DM. Front Immunol (2020) 11:593348. doi: 10.3389/fimmu.2020.593348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Roep BO, Thomaidou S, van Tienhoven R, Zaldumbide A. Type 1 Diabetes Mellitus as a Disease of the β-Cell (Do Not Blame the Immune System?). Nat Rev Endocrinol (2020) 17(30):150–61. doi: 10.1038/s41574-020-00443-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brawerman G, Thompson PJ. Beta Cell Therapies for Preventing Type 1 Diabetes: From Bench to Bedside. Biomolecules (2020) 10(12):1–20. doi: 10.3390/biom10121681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Aeng E, Boucher M, Severn M. Newer Drugs for Type 2 Diabetes: An Emerging Adjunctive Therapy to Insulin for Type 1 Diabetes? In: CADTH Issues in Emerging Health Technologies. Ottawa (ON: Canadian Agency for Drugs and Technologies in Health; (2016). Available at: http://www.ncbi.nlm.nih.gov/books/NBK538377/. [PubMed] [Google Scholar]
- 7. Bode BW, Garg SK. The Emerging Role Of Adjunctive Noninsulin Antihyperglycemic Therapy In The Management Of Type 1 Diabetes. Endocr Pract (2016) 22(2):220–30. doi: 10.4158/EP15869.RA [DOI] [PubMed] [Google Scholar]
- 8. Shih J-Y, Lin Y-W, Fisch S, Cheng J-T, Kang N-W, Hong C-S, et al. Dapagliflozin Suppresses ER Stress and Improves Subclinical Myocardial Function in Diabetes: From Bedside to Bench. Diabetes (2021) 70(1):262–7. doi: 10.2337/db20-0840 [DOI] [PubMed] [Google Scholar]
- 9. Yang M, Ma X, Xuan X, Deng H, Chen Q, Yuan L. Liraglutide Attenuates Non-Alcoholic Fatty Liver Disease in Mice by Regulating the Local Renin-Angiotensin System. Front Pharmacol (2020) 11:432. doi: 10.3389/fphar.2020.00432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kim YJ, Hwang SD, Lim S. Effects of Sodium-Glucose Cotransporter Inhibitor/Glucagon-Like Peptide-1 Receptor Agonist Add-On to Insulin Therapy on Glucose Homeostasis and Body Weight in Patients With Type 1 Diabetes: A Network Meta-Analysis. Front Endocrinol (Lausanne) (2020) 11:553. doi: 10.3389/fendo.2020.00553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Goyal I, Sattar A, Johnson M, Dandona P. Adjunct Therapies in Treatment of Type 1 Diabetes. J Diabetes (2020) 12(10):742–53. doi: 10.1111/1753-0407.13078 [DOI] [PubMed] [Google Scholar]
- 12. Goldenberg RM, Gilbert JD, Hramiak IM, Woo VC, Zinman B. Sodium-Glucose Co-Transporter Inhibitors, Their Role in Type 1 Diabetes Treatment and a Risk Mitigation Strategy for Preventing Diabetic Ketoacidosis: The STOP DKA Protocol. Diabetes Obes Metab (2019) 21(10):2192–202. doi: 10.1111/dom.13811 [DOI] [PubMed] [Google Scholar]
- 13. Kuhadiya ND, Ghanim H, Mehta A, Garg M, Khan S, Hejna J, et al. Dapagliflozin as Additional Treatment to Liraglutide and Insulin in Patients With Type 1 Diabetes. J Clin Endocrinol Metab (2016) 101(9):3506–15. doi: 10.1210/jc.2016-1451 [DOI] [PubMed] [Google Scholar]
- 14. Garg M, Ghanim H, Kuhadiya ND, Green K, Hejna J, Abuaysheh S, et al. Liraglutide Acutely Suppresses Glucagon, Lipolysis and Ketogenesis in Type 1 Diabetes. Diabetes Obes Metab (2017) 19(9):1306–11. doi: 10.1111/dom.12944 [DOI] [PubMed] [Google Scholar]
- 15. Wang Z, Zhu Y, Zhang Y, Zhang J, Ji T, Li W, et al. Protective Effects of AS-IV on Diabetic Cardiomyopathy by Improving Myocardial Lipid Metabolism in Rat Models of T2DM. BioMed Pharmacother (2020) 127:110081. doi: 10.1016/j.biopha.2020.110081 [DOI] [PubMed] [Google Scholar]
- 16. Tan Y, Zhang Z, Zheng C, Wintergerst KA, Keller BB, Cai L. Mechanisms of Diabetic Cardiomyopathy and Potential Therapeutic Strategies: Preclinical and Clinical Evidence. Nat Rev Cardiol (2020) 17(9):585–607. doi: 10.1038/s41569-020-0339-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Millar P, Pathak N, Parthsarathy V, Bjourson AJ, O’Kane M, Pathak V, et al. Metabolic and Neuroprotective Effects of Dapagliflozin and Liraglutide in Diabetic Mice. J Endocrinol (2017) 234(3):255–67. doi: 10.1530/JOE-17-0263 [DOI] [PubMed] [Google Scholar]
- 18. Gordon M, Meagher P, Connelly KA. Effect of Empagliflozin and Liraglutide on the Nucleotide-Binding and Oligomerization Domain-Like Receptor Family Pyrin Domain-Containing 3 Inflammasome in a Rodent Model of Type 2 Diabetes Mellitus. Can J Diabetes (2020) 45(6):553–6. doi: 10.1016/j.jcjd.2020.11.003 [DOI] [PubMed] [Google Scholar]
- 19. Eisa NH, Khodir AE, El-Sherbiny M, Elsherbiny NM, Said E. Phenethyl Isothiocyanate Attenuates Diabetic Nephropathy via Modulation of Glycative/Oxidative/Inflammatory Signaling in Diabetic Rats. BioMed Pharmacother (2021) 142:111666. doi: 10.1016/j.biopha.2021.111666 [DOI] [PubMed] [Google Scholar]
- 20. Saad SY, Najjar TA, Alashari M. Cardiotoxicity of Doxorubicin/Paclitaxel Combination in Rats: Effect of Sequence and Timing of Administration. J Biochem Mol Toxicol (2004) 18(2):78–86. doi: 10.1002/jbt.20012 [DOI] [PubMed] [Google Scholar]
- 21. Zhang R, Xu L, Zhang D, Hu B, Luo Q, Han D, et al. Cardioprotection of Ginkgolide B on Myocardial Ischemia/Reperfusion-Induced Inflammatory Injury via Regulation of A20-NF-κb Pathway. Front Immunol (2018) 9:2844. doi: 10.3389/fimmu.2018.02844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fedchenko N, Reifenrath J. Different Approaches for Interpretation and Reporting of Immunohistochemistry Analysis Results in the Bone Tissue - a Review. Diagn Pathol (2014) 9:221. doi: 10.1186/s13000-014-0221-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bankhead P, Loughrey MB, Fernández JA, Dombrowski Y, McArt DG, Dunne PD, et al. QuPath: Open Source Software for Digital Pathology Image Analysis. Sci Rep (2017) 7(1):16878. doi: 10.1038/s41598-017-17204-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Otto-Buczkowska E, Jainta N. Pharmacological Treatment in Diabetes Mellitus Type 1 - Insulin and What Else? Int J Endocrinol Metab (2018) 16(1):e13008. doi: 10.5812/ijem.13008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Francescato MP, Stel G, Geat M, Cauci S. Oxidative Stress in Patients With Type 1 Diabetes Mellitus: Is it Affected by a Single Bout of Prolonged Exercise? PloS One (2014) 9(6):e99062. doi: 10.1371/journal.pone.0099062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Asmat U, Abad K, Ismail K. Diabetes Mellitus and Oxidative Stress-A Concise Review. Saudi Pharm J (2016) 24(5):547–53. doi: 10.1016/j.jsps.2015.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Giacco F, Brownlee M. Oxidative Stress and Diabetic Complications. Circ Res (2010) 107(9):1058–70. doi: 10.1161/CIRCRESAHA.110.223545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yuan T, Yang T, Chen H, Fu D, Hu Y, Wang J, et al. New Insights Into Oxidative Stress and Inflammation During Diabetes Mellitus-Accelerated Atherosclerosis. Redox Biol (2019) 20:247–60. doi: 10.1016/j.redox.2018.09.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nna VU, Abu Bakar AB, Ahmad A, Eleazu CO, Mohamed M. Oxidative Stress, NF-κb-Mediated Inflammation and Apoptosis in the Testes of Streptozotocin-Induced Diabetic Rats: Combined Protective Effects of Malaysian Propolis and Metformin. Antioxid (Basel) (2019) 8(10):1–23. doi: 10.3390/antiox8100465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Alzahrani S, Zaitone SA, Said E, El-Sherbiny M, Ajwah S, Alsharif SY, et al. Protective Effect of Isoliquiritigenin on Experimental Diabetic Nephropathy in Rats: Impact on Sirt-1/Nfκb Balance and NLRP3 Expression. Int Immunopharmacol (2020) 87:106813. doi: 10.1016/j.intimp.2020.106813 [DOI] [PubMed] [Google Scholar]
- 31. Alzahrani S, Ajwah SM, Alsharif SY, Said E, El-Sherbiny M, Zaitone SA, et al. Isoliquiritigenin Downregulates miR-195 and Attenuates Oxidative Stress and Inflammation in STZ-Induced Retinal Injury. Naunyn Schmiedebergs Arch Pharmacol (2020) 393(12):2375–85. doi: 10.1007/s00210-020-01948-5 [DOI] [PubMed] [Google Scholar]
- 32. Elsherbiny NM, Ahmad S, Naime M, Elsherbini AM, Fulzele S, Al-Gayyar MM, et al. ABT-702, an Adenosine Kinase Inhibitor, Attenuates Inflammation in Diabetic Retinopathy. Life Sci (2013) 93(2–3):78–88. doi: 10.1016/j.lfs.2013.05.024 [DOI] [PubMed] [Google Scholar]
- 33. Elsherbiny NM, Al-Gayyar MMH, Abd El Galil KH. Nephroprotective Role of Dipyridamole in Diabetic Nephropathy: Effect on Inflammation and Apoptosis. Life Sci (2015) 143:8–17. doi: 10.1016/j.lfs.2015.10.026 [DOI] [PubMed] [Google Scholar]
- 34. Muriach M, Flores-Bellver M, Romero FJ, Barcia JM. Diabetes and the Brain: Oxidative Stress, Inflammation, and Autophagy. Oxid Med Cell Longev (2014) 2014:102158. doi: 10.1155/2014/102158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chen Y-Y, Wu T-T, Ho C-Y, Yeh T-C, Sun G-C, Kung Y-H, et al. Dapagliflozin Prevents NOX- and SGLT2-Dependent Oxidative Stress in Lens Cells Exposed to Fructose-Induced Diabetes Mellitus. Int J Mol Sci (2019) 20(18):1–14. doi: 10.3390/ijms20184357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wei R, Cui X, Feng J, Gu L, Lang S, Wei T, et al. Dapagliflozin Promotes Beta Cell Regeneration by Inducing Pancreatic Endocrine Cell Phenotype Conversion in Type 2 Diabetic Mice. Metabolism (2020) 111:154324. doi: 10.1016/j.metabol.2020.154324 [DOI] [PubMed] [Google Scholar]
- 37. Tang L, Wu Y, Tian M, Sjöström CD, Johansson U, Peng X-R, et al. Dapagliflozin Slows the Progression of the Renal and Liver Fibrosis Associated With Type 2 Diabetes. Am J Physiol Endocrinol Metab (2017) 313(5):E563–76. doi: 10.1152/ajpendo.00086.2017 [DOI] [PubMed] [Google Scholar]
- 38. Solini A, Giannini L, Seghieri M, Vitolo E, Taddei S, Ghiadoni L, et al. Dapagliflozin Acutely Improves Endothelial Dysfunction, Reduces Aortic Stiffness and Renal Resistive Index in Type 2 Diabetic Patients: A Pilot Study. Cardiovasc Diabetol (2017) 16(1):138. doi: 10.1186/s12933-017-0621-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nashawi M, Sheikh O, Battisha A, Ghali A, Chilton R. Neural Tone and Cardio-Renal Outcomes in Patients With Type 2 Diabetes Mellitus: A Review of the Literature With a Focus on SGLT2 Inhibitors. Heart Fail Rev (2020) 26(3):643–52. doi: 10.1007/s10741-020-10046-w [DOI] [PubMed] [Google Scholar]
- 40. Vadini F, Simeone PG, Boccatonda A, Guagnano MT, Liani R, Tripaldi R, et al. Liraglutide Improves Memory in Obese Patients With Prediabetes or Early Type 2 Diabetes: A Randomized, Controlled Study. Int J Obes (Lond) (2020) 44(6):1254–63. doi: 10.1038/s41366-020-0535-5 [DOI] [PubMed] [Google Scholar]
- 41. Kristensen SL, Rørth R, Jhund PS, Docherty KF, Sattar N, Preiss D, et al. Cardiovascular, Mortality, and Kidney Outcomes With GLP-1 Receptor Agonists in Patients With Type 2 Diabetes: A Systematic Review and Meta-Analysis of Cardiovascular Outcome Trials. Lancet Diabetes Endocrinol (2019) 7(10):776–85. doi: 10.1016/S2213-8587(19)30249-9 [DOI] [PubMed] [Google Scholar]
- 42. Tian F, Zheng Z, Zhang D, He S, Shen J. Efficacy of Liraglutide in Treating Type 2 Diabetes Mellitus Complicated With non-Alcoholic Fatty Liver Disease. Biosci Rep (2018) 38(6):1–9. doi: 10.1042/BSR20181304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yang Y, Fang H, Xu G, Zhen Y, Zhang Y, Tian J, et al. Liraglutide Improves Cognitive Impairment via the AMPK and PI3K/Akt Signaling Pathways in Type 2 Diabetic Rats. Mol Med Rep (2018) 18(2):2449–57. doi: 10.3892/mmr.2018.9180 [DOI] [PubMed] [Google Scholar]
- 44. Yamada S, Tanabe J, Ogura Y, Nagai Y, Sugaya T, Ohata K, et al. Renoprotective Effect of GLP-1 Receptor Agonist, Liraglutide, in Early-Phase Diabetic Kidney Disease in Spontaneously Diabetic Torii Fatty Rats. Clin Exp Nephrol (2021) 25(4):365–75. doi: 10.1007/s10157-020-02007-2 [DOI] [PubMed] [Google Scholar]
- 45. Zhao X-Y, Yu T-T, Liu S, Liu Y-J, Liu J-J, Qin J. Effect of Liraglutide on Endoplasmic Reticulum Stress in the Renal Tissue of Type 2 Diabetic Rats. World J Diabetes (2020) 11(12):611–21. doi: 10.4239/wjd.v11.i12.611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Liu Q, Cai B-Y, Zhu L-X, Xin X, Wang X, An Z-M, et al. Liraglutide Modulates Gut Microbiome and Attenuates Nonalcoholic Fatty Liver in Db/Db Mice. Life Sci (2020) 261:118457. doi: 10.1016/j.lfs.2020.118457 [DOI] [PubMed] [Google Scholar]
- 47. Petrie MC, Verma S, Docherty KF, Inzucchi SE, Anand I, Belohlávek J, et al. Effect of Dapagliflozin on Worsening Heart Failure and Cardiovascular Death in Patients With Heart Failure With and Without Diabetes. JAMA (2020) 323(14):1353–68. doi: 10.1001/jama.2020.1906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Qin T, Kong B, Dai C, Xiao Z, Fang J, Shuai W, et al. Protective Effects of Dapagliflozin on the Vulnerability of Ventricular Arrhythmia in Rats With Pulmonary Artery Hypertension Induced by Monocrotaline. Bioengineered (2022) 13(2):2697–709. doi: 10.1080/21655979.2021.2017652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zhang Z, Wang X, Yang L, Yang L, Ma H. Liraglutide Ameliorates Myocardial Damage in Experimental Diabetic Rats by Inhibiting Pyroptosis via Sirt1/AMPK Signaling. Iran J Basic Med Sci (2021) 24(10):1358–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Terasaki M, Hiromura M, Mori Y, Kohashi K, Nagashima M, Kushima H, et al. Amelioration of Hyperglycemia With a Sodium-Glucose Cotransporter 2 Inhibitor Prevents Macrophage-Driven Atherosclerosis Through Macrophage Foam Cell Formation Suppression in Type 1 and Type 2 Diabetic Mice. PloS One (2015) 10(11):e0143396. doi: 10.1371/journal.pone.0143396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sarnobat D, Moffett CR, Tanday N, Reimann F, Gribble FM, Flatt PR, et al. Antidiabetic Drug Therapy Alleviates Type 1 Diabetes in Mice by Promoting Pancreatic α-Cell Transdifferentiation. Biochem Pharmacol (2020) 182:114216. doi: 10.1016/j.bcp.2020.114216 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available upon request from the corresponding author.