Abstract
Background
While guidelines recommend that children with asthma should receive asthma education, it is not known if education delivered in the home is superior to usual care or the same education delivered elsewhere. The home setting allows educators to reach populations (such as the economically disadvantaged) that may experience barriers to care (such as lack of transportation) within a familiar environment.
Objectives
To perform a systematic review on educational interventions for asthma delivered in the home to children, caregivers or both, and to determine the effects of such interventions on asthma‐related health outcomes. We also planned to make the education interventions accessible to readers by summarising the content and components.
Search methods
We searched the Cochrane Airways Group Specialised Register of trials, which includes the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, CINAHL, AMED and PsycINFO, and handsearched respiratory journals and meeting abstracts. We also searched the Education Resources Information Center database (ERIC), reference lists of trials and review articles (last search January 2011).
Selection criteria
We included randomised controlled trials of asthma education delivered in the home to children, their caregivers or both. In the first comparison, eligible control groups were provided usual care or the same education delivered outside of the home. For the second comparison, control groups received a less intensive educational intervention delivered in the home.
Data collection and analysis
Two authors independently selected the trials, assessed trial quality and extracted the data. We contacted study authors for additional information. We pooled dichotomous data with fixed‐effect odds ratio and continuous data with mean difference (MD) using a fixed‐effect where possible.
Main results
A total of 12 studies involving 2342 children were included. Eleven out of 12 trials were conducted in North America, within urban or suburban settings involving vulnerable populations. The studies were overall of good methodological quality. They differed markedly in terms of age, severity of asthma, context and content of the educational intervention leading to substantial clinical heterogeneity. Due to this clinical heterogeneity, we did not pool results for our primary outcome, the number of patients with exacerbations requiring emergency department (ED) visit. The mean number of exacerbations requiring ED visits per person at six months was not significantly different between the home‐based intervention and control groups (N = 2 studies; MD 0.04; 95% confidence interval (CI) ‐0.20 to 0.27). Only one trial contributed to our other primary outcome, exacerbations requiring a course of oral corticosteroids. Hospital admissions also demonstrated wide variation between trials with significant changes in some trials in both directions. Quality of life improved in both education and control groups over time.
A table summarising some of the key components of the education programmes is included in the review.
Authors' conclusions
We found inconsistent evidence for home‐based asthma educational interventions compared to standard care, education delivered outside of the home or a less intensive educational intervention delivered at home. Although education remains a key component of managing asthma in children, advocated in numerous guidelines, this review does not contribute further information on the fundamental content and optimum setting for such educational interventions.
Plain language summary
Home‐based educational interventions for families of children with asthma
Asthma is a common childhood illness causing wheezing, coughing and difficulty in breathing. Guidelines on the care of children with asthma recommend that children and families should receive education on how to manage their condition. The current review looked at 12 studies with a total of 2342 children comparing asthma education received at home with either usual care or a less intensive home‐based education programme. Eleven out of 12 trials were conducted in North America, within urban or suburban settings involving socioeconomically disadvantaged families. A table summarising some of the key components of the education programmes is presented in the review.
The included studies varied in the characteristics of children (e.g. age, severity of asthma), the education delivered and the way each outcome was reported. This made it difficult to compare the results and provide overall conclusions and we did not pool results for most of the outcomes. There was also diversity in the findings of the individual trials. We were able to combine the results of two studies reporting the average number of emergency department visits per child, which was not different at six months between the home education group and the group receiving the usual care. Only one trial contributed to our other primary outcome, exacerbations (flare‐ups) requiring a course of oral corticosteroids. Hospital admissions also demonstrated wide variation between trials with significant changes in some trials in both directions. Quality of life improved in both education and control groups over time.
Summary of findings
Summary of findings for the main comparison. Education versus control for children with asthma.
| Education versus control for children with asthma | ||||||
| Patient or population: children with asthma Settings: Intervention: education versus control | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Education versus control | |||||
| Exacerbations leading to emergency department visits | See comment | See comment | Not estimable | 985 (8 studies) | ⊕⊝⊝⊝ very low1,2,3,4 | There was too much clinical heterogeneity to pool this outcome |
| Mean exacerbations requiring a course of oral corticosteroids | See comment | See comment | Not estimable | 500 (2 studies) | ⊕⊝⊝⊝ very low1,2,3,4 | There was too much clinical heterogeneity to pool this outcome |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Downgraded two because there was so much heterogeneity in the study populations; we decided not to pool this outcome
2 Downgraded one due to limitation in populations which were predominantly North American socioeconomically disadvantaged children
3 We did not downgrade for indirectness because we felt that there were several reasonably sized RCTS for this outcome. That we did not pool is reflected in downgrading for inconsistency.
4 We did not downgrade for publication bias as most of the trials reported minimal difference in the outcomes of children in the intervention/control groups and therefore we do not think that trials showing no evidence of treatment effect are less likely to be published than others.
Summary of findings 2. Education versus other home‐based education for children with asthma.
| Education versus other home‐based education for children with asthma | ||||||
| Patient or population: patients with children with asthma Settings: Intervention: education versus other home‐based education | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Education versus other home‐based education | |||||
| Exacerbations leading to ED visits | See comment | See comment | Not estimable | 181 (1 study) | ⊕⊝⊝⊝ very low1,2,3 | There was only one trial contributing to this outcome so we were unable to pool |
| Mean exacerbations requiring a course of oral corticosteroids | See comment | See comment | Not estimable | 193 (1 study) | ⊕⊝⊝⊝ very low1,2,3 | There was only one trial contributing to this outcome so we were unable to pool |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio; ED: Emergency Department | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Downgraded one due to limitation in populations which were predominantly North American socioeconomically disadvantaged children. 2We did not downgrade for publication bias as most of the trials reported minimal difference in the outcomes of children in the intervention/control groups and therefore we do not think that trials showing no evidence of treatment effect are less likely to be published than others. 3 We downgraded one for imprecision as only one trial contributed to this outcome.
Background
Description of the condition
Asthma is a chronic condition affecting the airways causing wheezing, coughing and difficulty in breathing. It is the most common chronic condition affecting children and is estimated to affect over 300 million people worldwide (GINA 2008). Although a cure does not currently exist, symptoms can be controlled.
Asthma impacts on sufferers' quality and enjoyment of life. They may be less able to undertake strenuous activities and often display increased levels of depression and anxiety (Clayton 2005). Asthma places a financial burden on both the patient and the state from direct medical costs and reduced productivity resulting from days lost at work or school (Wu 2007).
People across the international spectrum of economic development are affected (de Oliveira 1999). Low socioeconomic groups experience higher levels of asthma morbidity (British Guideline on the Management of Asthma; de Oliveira 1999). In addition, people from minority groups have poorer asthma outcomes and typically make more asthma‐related visits to emergency departments (Bailey 2009).
Description of the intervention
Asthma education extends beyond simply providing information. It aims to integrate knowledge, improve self management and produce behaviour change. Education is recommended as an integral part of asthma management (NAEPP 2007; British Guideline on the Management of Asthma; GINA 2008). Education can be delivered to individuals or groups in a number of ways, such as personalised action plans, computer/video games, internet‐enabled packages, role play, problem‐solving, lectures, workshops and booklets. Education may be delivered by a variety of instructors including clinicians, nurses, other allied health professionals and community health workers.
How the intervention might work
Specific components of education interventions may affect behaviour change by:
reinforcing basic information about asthma to embed understanding;
emphasising adherence to prescribed long‐term controller medication;
emphasising the importance of avoiding environmental triggers;
providing self monitoring techniques to help patients identify and respond appropriately to worsening asthma;
providing written action plans to help patients respond correctly to exacerbations; and
improving communication between patients and clinicians.
There are existing reviews showing benefits of education. Wolf 2002 conducted a large systematic review showing that asthma education aimed at two to 18 year olds gave modest improvements in airflow and self efficacy and modest reductions in days off school and emergency department visits. Bhogal 2006 showed that symptom‐based written action plans are better than peak flow‐based ones for preventing acute care visits in children. Gibson 2002 showed that peak flow or symptom‐based self monitoring, coupled with regular medical review and a written action plan led to improved health outcomes in adults. Boyd 2009 showed that asthma education aimed at children and their carers who have attended the emergency department for acute exacerbations resulted in fewer subsequent visits to the emergency department and hospital admissions.
Why it is important to do this review
Education programmes delivered outside of the home may be poorly attended due to a lack of transportation or childcare for siblings. Children with asthma from low‐income families may be more likely to experience such barriers to care. These children also tend to have more severe disease and morbidity at baseline and may derive additional benefit from educational interventions (Brown 2002). Some education programmes attempt to capture a wider audience by employing school‐based delivery, but this does not address obstacles to attendance by caregivers and preschool children. Home‐based education offers an alternative solution to improving access to education.
Asthma education delivered in the home is distinct from that delivered in clinics or schools in factors relating to the educator, the family members receiving it and the environment. Community health workers may provide the education. Although their expertise in asthma management may not be as extensive as that of health professionals, the families may be more responsive to these workers who often share the same socioeconomic status, language and/or culture. Interventions at home could be delivered to additional family members. They may feel more relaxed at home and therefore be more receptive to ideas (Shelledy 2009). Additionally, it provides an opportunity for educators to offer case‐specific guidance on improvements to the living environment (Bryant‐Stephens 2008).
Wolf 2002 recommended that self management education should be routinely used as part of standard asthma therapy in children. This review included a heterogeneous group of education programmes provided in multiple settings. The current review will focus on trials involving asthma education delivered at home to children and/or their caregivers. It will also provide an updated review of home‐based education, which was recommended for young children in the most recent asthma guidelines from the United States (NAEPP 2007) but with a limited quality of evidence (level C).
Objectives
To assess the effects of educational interventions for asthma, delivered in the home to children, their parents or both, on asthma‐related outcomes.
To make the education interventions accessible to readers by summarising the content and components.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled clinical trials (RCTs). We planned to include quasi‐randomised trials (e.g. participants allocated by day of week or hospital number).
Types of participants
We included children and adolescents between two and 18 years of age with an existing diagnosis of asthma. We also included trials with children aged one to two years old if the majority of the children were older than two. We accepted both doctor‐diagnosed asthma or asthma identified against objective criteria for asthma symptoms.
Types of interventions
Inclusion criteria
We included any type of self management education programme delivered in the home of the child or adolescent. We included self management programmes delivered to children, their parents or both. We only included interventions aimed at changing behaviour including one or more of the following methods: providing information about asthma symptoms, medication and inhaler technique; symptom or lung function monitoring; provision or development of personalised action plan; development of coping strategies; improving communication between clinician and patient.
We included control groups that received either usual care, waiting list or a less intensive education programme than the intervention arm, such as information only. We analysed data from usual care control groups separately from those who received a less intensive home‐based educational intervention.
Exclusion criteria
We excluded education that provided only information with no face‐to‐face education programmes, e.g. just giving the child or parent a booklet, smoking cessation programmes for parents and education interventions delivered to physicians, nurses or other healthcare providers rather than the child or carer. We excluded programmes primarily aimed at, and providing, environmental modification (i.e. provision of vacuum cleaners with high‐efficiency particulate air (HEPA) filters, HEPA air purifiers, ventilation fans, remediation of mould‐contaminated carpet or wallboard, professional pest control, roach or rodent traps, anti‐allergy mattress or pillow covers) to reduce exposure to indoor allergens. Although this kind of environmental remediation may have a positive effect on asthma outcomes, these studies tend to focus on remediation with education as a secondary measure and may be better addressed as a separate review rather than as a subgroup of this review. We did not exclude trials based on language.
Types of outcome measures
Outcome measures were not a criterion for exclusion in the review.
Primary outcomes
Exacerbations leading to emergency department visits.
Exacerbations requiring a course of oral corticosteroids.
Secondary outcomes
Functional health status (quality of life, days of restricted activity, nights of disturbed sleep, day symptoms).
Days off school or caregiver days off work.
Exacerbations leading to hospitalisations.
Lung function (FEV1 (forced expiratory volume in one second) and PEF (peak expiratory flow)).
Withdrawals from intervention or usual care.
Search methods for identification of studies
Electronic searches
We identified trials using the Cochrane Airways Group Specialised Register of trials, which is derived from systematic searches of bibliographic databases including the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, CINAHL, AMED and PsycINFO, and handsearched respiratory journals and meeting abstracts (please see the Airways Group search methods for further details). We searched all records in the Specialised Register coded as 'AST' using the following terms:
educat* or train* or instruct* or teach* or taught or coach* or learn* or behav* or self‐monitor* or "self monitor*" or self‐manag* or "self manag*" or self‐car* or "self car*" or patient‐cent* or "patient cent*" or patient‐focus* or "patient focus*" or "management plan*" or "management program*"
In addition, we searched the Education Resources Information Center database (ERIC) using the terms home* AND asthma*, reference lists of trials and review articles. The latest search was January 2011.
We searched both databases from the date of their inception and there was no restriction on the basis of language.
Searching other resources
We reviewed reference lists of all primary studies and review articles for references to additional studies. We contacted authors of identified trials where possible and asked them to identify other published and unpublished studies.
Data collection and analysis
Selection of studies
One review author (EJW) went through the search to remove references clearly unrelated to the scope of the review based on title alone. Two review authors (EJW and PL for Airways Register search; EJW and MH for ERIC search) independently examined the titles and abstracts of the remaining reports. We retrieved the full text of the potentially relevant references and assigned each reference to a study identifier. Two of authors (EJW, PL) independently compared each study against inclusion criteria and we resolved discrepancies by consensus.
Data extraction and management
We extracted information from each study for the following characteristics.
Design (design, total duration study, number of study centres and location, withdrawals, date of study).
Participants (N, mean age, age range, gender, asthma severity, diagnostic criteria, baseline lung function, sociodemographics, caregivers' education, ethnicity, inclusion criteria, exclusion criteria).
Interventions (total number of intervention and control groups. For each intervention or control group: treatment, programme topics, setting, session type, number of sessions, session length, educator, time span of intervention, self management strategy, educational strategy, instructional methods/tools, additional information and net treatment, incentives, cost).
Outcomes (outcomes specified and collected, time points reported).
Aims.
Risk of bias.
Two authors (EJW, MH or PL) extracted data from the studies and resolved any discrepancies by discussion and consensus, or by consulting a third party where necessary. For each trial, one author (EJW or PL) transferred data from data collection forms into Review Manager 5.1 and this was checked by the other.
Assessment of risk of bias in included studies
We assessed risk of bias in included studies as high, low or unclear using the Cochrane Collaboration's 'Risk of bias' tool and the following headings: 1) sequence generation; 2) allocation concealment; 3) blinding; 4) incomplete outcome data; 5) selective outcome reporting; 6) other bias.
Unit of analysis issues
Studies that compare two types of education intervention with a control may yield important comparison results between two education types (Wolf 2002). We therefore entered both intervention arms separately in the meta‐analyses and split the control group in half (to avoid double‐counting) rather than combining the two arms (Higgins 2008).
Dealing with missing data
We requested additional data including missing numerical data and information required for the risk of bias assessment from trialists.
Analyses based on change scores were preferred, but we used final values where change scores were not available.
Assessment of heterogeneity
We reported heterogeneity using the I2 statistic and descriptively by examining the clinical characteristics of the population and interventions.
Assessment of reporting biases
We reported the proportion of participants contributing to each outcome in comparison to the total number randomised. We intended to examine funnel plots but there were insufficient trials.
Data synthesis
We planned to combine dichotomous data using the Mantel‐Haenszel fixed‐effect odds ratio using 95% confidence intervals. We planned to combine continuous data with either mean difference (MD) or standardised mean difference (SMD) using a fixed‐effect model and 95% confidence intervals.
We presented absolute differences in a 'Summary of findings' table for the primary outcomes which we produced using GRADE methodology.
Subgroup analysis and investigation of heterogeneity
We planned to investigate heterogeneity based on the following predefined subgroups.
Children (2 to 12) versus adolescents (12 to 18).
Mild/moderate versus severe asthma.
Short time frame trials < 6 months versus long time frame > 6 months.
Physician or nurse versus community health worker.
We planned to apply a test for interaction between subgroup estimates (Altman 2003).
Sensitivity analysis
We planned to assess the sensitivity of our primary outcomes to the degree of risk of bias. We planned to compare the results of fixed and random‐effects models. If combining change scores and final value scores we planned to look at baseline imbalance.
Results
Description of studies
Results of the search
The search of the Airways Register returned 1584 references. We discarded 487 references that were clearly not relevant to the review question on the basis of title alone. We examined the remaining 1097 references. There were 130 initial disagreements which were resolved through discussion. We retrieved 235 full‐text documents in total identified from the search. We identified five references from contacting authors and reference lists of included studies. We contacted the author of an abstract who identified the full text which was in press (Seid 2010). We contacted the author of a www.clincaltrial.gov entry who informed us that the trial had been published in full (Galbreath 2008) and one study that had previously been excluded on the basis of title and abstract was identified from a previous review (Gorelick 2006). We imported these 348 references into Review Manager 5.1, grouped the references into studies and discarded duplicate references, although these groupings evolved during the process of data extraction. Ultimately we identified 12 unique studies for inclusion. A PRISMA diagram can be found in Figure 1.
1.
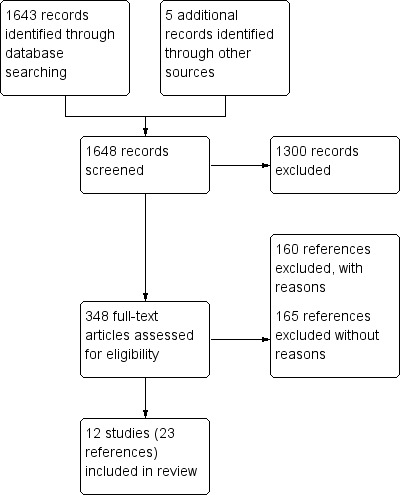
Study flow diagram.
The search in the ERIC database returned 59 references. Six studies were identified as potentially eligible. Three of these had been returned by the Airways Register search, whilst the other three were ultimately excluded.
Included studies
There were 12 included randomised controlled trials reporting data on children who had received education delivered in the home. There were a total of 2342 participants. Full details can be found in Characteristics of included studies. Key characteristics of the participants and education programme content are summarised in Table 3.
1. Summary of characteristics of included studies.
| Study | No./length sessions, where | Educator | Programme length (follow‐up time) |
% severe (how identified) (inclusion criteria relating to HCU/meds) |
Mean age (range) |
Social indicators (education status refers to caregiver) |
Basic education1, printed materials and homework | Medication/ inhaler technique | Self management | Monitoring2 or telecare | Written action plan | Trigger ID/ environmental | Other |
|
Brown 2002 N = 101 |
8 x 90‐minute home weekly, USA |
Nurse | < 6 months (12 months) | 4 (as per medication regimen) (Healthcare visit for asthma in past year and on daily asthma medication) |
4.2 (1 to 7) | Low‐income families < high school 28%, high school qualification 50%, > high school 22% 90% African American |
√√ | √ | √ | √ | √ | Wee Wheezers | |
|
Brown 2006 N = 137 |
1 x clinic, 1 x home, USA |
Nurse | < 6 months (6 months) | 37 (NHLBI) (Moderate to severe asthma or had visited the ED in the past year) |
< 18 | < high school 18%, high school qualification 32%, > high school 50% 30% African American, 59% White |
√ | √ | √ | √ | √ | Stressed monitoring and self evaluation | |
|
Butz 2006 N = 221 |
6 x 1‐hour home, USA |
Nurse | 6 months (12 months) | 14 (NAEPP) (regular nebuliser use and ED visit/hospitalisation in past year) |
4.5 (2 to 9) | Low‐income families < high school 24%, high school qualification 28%, > high school 38% on MedicAid 80% African American 89% |
√ | √ | √ | √ | √ | * The control group receive a home visit education programme and the additional intervention for the intervention group is a nebuliser‐based intervention. nebuliser group only: Wee Wheezers, A+ Asthma Club Program |
|
| Butz 2010 | 4 x 30 to 45 minutes home, accompanied to primary care clinic visits x 6 months, USA | Nurse/health educator | 8 weeks (12 months) | 13 (NHLBI) >= 1 asthma ED visits or hospitalisation in preceding year |
8.0 (6 to 12) | Caregiver education < high school graduate 32.0%; high school graduate or more education 68.0% Income < USD 20,000: 57.1%; >= USD 20,000: 42.9% |
√√ | √ | √ | √ | √ | Asthma communication education based on Chronic Care Model and other communication concepts | |
|
Dolinar 2000 N = 56 |
1 x 2‐hour home, Canada |
Principal investigator (nurse) | < 6 months (3 months) | 'Stable asthma' with a diagnosis for over 6 months. Those presenting with an acute exacerbation were excluded. | 5 (1 to 10) | High school 38%, college/university 63% Family income < CAD 20,000 (Cdn) 13% |
√√ | √ | √ | √ | √ | Air Force Asthma | |
| Fisher 2009 | 2 x home, biweekly telephone calls x 3 months, then monthly USA |
3 African American women from same neighbourhoods as participants | 2 years (2 years) | Not specified but recruited after hospitalisation | 4.9 (2 to 8) | No high school diploma 33%, high school qualification 40%, some college 23%, college graduate 4% | √ | √ | √ | √ | √ | √ | Transtheoretical Model (behaviour change strategy) |
|
Galbreath 2008 N = 473 |
4 x home at 1, 2, 3 and 6 months, 6 to 7 telephone sessions, USA |
Registered nurses for education over the phone and 24 hour hotlines. Pulmonary therapist |
6 (12 months) | 31 (NAEPP) 48 (GINA 2002) (ED visit or hospitalisation or 4 x GP visits or 6+ canisters of beta‐agonist or diagnosis of moderate‐severe asthma in past year) |
9.5 (5 to 17) | Medicaid or SCHIP 56%, black/other 18%, white 15%, Hispanic 68% | √ | √ | √ | √ | √ | √ | |
|
Gorelick 2006 N = 352 |
1 x 60 minutes home then 5 x 30 minutes home, plus several telephone calls over 6 months USA |
Case manager (nurse or social worker) | 6 months (6 months) | 14 (NAEPP) (current ED visit for asthma, treated with 1 inhaled bronchodilator, and history of physician diagnosed asthma or wheezing treated with beta‐agonists) |
6.8 (2 to 18) | 60% public insurance black 69%, white 21%, Latino 8% |
√√ | √ | √ | √ | √ | √ | Fight Asthma Milwaukee (FAM) Allies Coalition |
|
Kamps 2008 N = 15 |
6 x 1 hour weekly home, USA |
Licensed psychologists or masters‐level psychology graduate students | < 6 months (12 months) | Moderate to severe persistent (NHLBI) (Prescribed ICS) |
9 (7‐12) | Half of participants recruited from urban and half from suburban areas Intervention: high school qualification mothers 0% fathers 14%, some college mothers100% fathers 86%; control: no high school diploma mothers 50% fathers 57%, high school qualification mothers 25 fathers 14%, some college mothers 25 fathers 29%. 20% African American, 53% European American, 27% Hispanic American |
√√ | √ | √ | √ | √ | * The control group also receive a standard comprehensive education programme based on Air Wise programme and they watched Clubhouse Kids Learn About Asthma. The Clubhouse Kids Learn Asthma More intense group received a behavioural management technique intervention to improve adherence to corticosteroids. Unique barriers to adherence were identified and discussed and written solutions were provided. |
|
|
Mitchell 1986 N = 368 |
6 x 1 hour monthly home, New Zealand |
Community child health nurse | 6 months (18 months) | Children who had been admitted to hospital for asthma (Patients who had been discharged from hospital in the past year) |
5.8 (2 to 14) | European children significantly more advantaged than Polynesian children | √ | √ | √ | √ | |||
|
Otsuki 2009 ABC N = 250 (total) |
5 x 30 to 40 minutes home, USA |
Trained asthma educators | < 6 months (18 months) | Physician diagnosed asthma, 2 x ED visits or 1 x hospitalisations in the preceding year and on asthma controller medications | 7.14 (2 to 12) | Medicaid 89%, caregiver completed high school 69% 98% African American |
√√ | √ | √ discussed strategies | √ | Identification of barriers to health care and discussion of beliefs and concerns | ||
| Otsuki 2009 AMF | 5 x 30 to 40 minutes home, USA |
Trained asthma educators | < 6 months (18 months) | " | 6.83 (2 to 12) | Medicaid 89%, caregiver completed high school 69% 98% African American |
√√ | √ | √ | √ electronic medication/ feedback monitors | √ | Identification of barriers to health care and discussion of beliefs and concerns. Goal‐setting, reinforcement of adherence goals and importance of rewards | |
|
Seid 2010 N = 252 (total) |
5 x 45 to 60 minutes, weekly, USA |
Bilingual, bicultural bachelors educated asthma home visitors | < 6 months (9 months) | 33 | 7.37 (2 to 14) | 85% from subsidised community clinics (most low‐income) No diploma 73%, high school graduate 8%, > college19% Hispanic 83%, white 4%, black, 8% |
√√ | √ | √ | √ | √ | ||
| Seid 2010 problem solving | 6 x 45 to 60 minutes, weekly, USA |
Bilingual, bicultural masters educated asthma home visitors | < 6 months (9 months) |
33 | 7.37 (2 to 14) | " | √√ | √ | √ | √ | √ | Problem‐solving skill training, rapport building |
1. Basic education on concepts of asthma
2. Monitoring of asthma management by a health professional or electronic device
ED: Emergency Department; ICS: inhaled corticosteroid; NAEPP: National Asthma Education and Prevention Program; NHLBI: National Heart, Lung, and Blood Institute guidelines; SCHIP: State Children's Health Insurance Program administered by the United States Department of Health and Human Services that matches funds to states for health insurance to families with children. Designed to cover uninsured children in families with incomes that are modest but too high to qualify for Medicaid.
Setting and populations
Ten studies took place in the USA (Brown 2002; Butz 2006; Gorelick 2006; Brown 2006; Galbreath 2008; Kamps 2008; Fisher 2009; Otsuki 2009; Butz 2010; Seid 2010), one in Canada (Dolinar 2000) and one in New Zealand (Mitchell 1986). The latter study divided the population into two ethnic groups; Polynesian and European children (Mitchell 1986). Patients were recruited mostly from the Emergency Department (ED) in four studies (Brown 2006; Gorelick 2006; Otsuki 2009; Butz 2010), mostly outpatient clinics in five studies (Dolinar 2000; Brown 2002; Butz 2006; Kamps 2008; Seid 2010) and following a hospital admission in two studies (Mitchell 1986; Fisher 2009). One study recruited patients by various methods including referrals, reviewing patient lists and the media (Galbreath 2008). Eleven of the studies were conducted between 2000 to 2010, while one was much older (Mitchell 1986). The studies varied in size from 15 to 316 participants.
Seven studies reported participants with a range of asthma severity from mild to severe, with the latter group varying from 4% to 37% (Brown 2002; Butz 2006; Brown 2006; Gorelick 2006; Galbreath 2008; Butz 2010; Seid 2010). Kamps 2008 enrolled participants with moderate to severe asthma, while Dolinar 2000 described the participants as having stable asthma. The Mitchell 1986 cohort had frequent asthma attacks and Otsuki 2009 enrolled patients prescribed an asthma controller medication. All studies included patients who had a health care utilisation visit in the past year (either ED or hospitalisation), except Dolinar 2000 who excluded patients with an exacerbation requiring a visit to the ED in the previous year. A table of control group rates for ED visits and hospitalisations provides an indicator of severity and can be found in Table 4.
2. Control group event rates.
| Study | Control group risk EMERGENCY DEPARTMENT VISITS | Control group risk HOSPITALISATIONS (period reported, from baseline) |
| Brown 2006 | 38% (0 to 6 months) | ‐ |
| Butz 2006 | 47% (0 to 6 months) | 13% (0 to 6 months) |
| Dolinar 2000 | 10% (0 to 6 months) | ‐ |
| Fisher 2009 | 54% (0 to 2 years) | 59% (0 to 2 years) |
| Gorelick 2006 | 38% (0 to 6 months) | ‐ |
| Mitchell 1986 Europeans | 5% (0 to 6 months) | 21% (6 months) 16% (6 to 18 months) |
| Mitchell 1986 Polynesians | 13% (0 to 6 months) | 27% (6 months) 33% (6 to 18 months) |
| Otsuki 2009 | ‐ | 17% (6 months) 12% (12 to 18 months) |
| Seid 2010 | 15% (3 to 9 months) | 8% (3 to 9 months) |
The trials enrolled participants with a range of age groups. Three trials included infants less than two years which was outside of the inclusion criteria for this review. However, we included them as the majority of participants would have been over two years (Dolinar 2000; Brown 2002; Brown 2006). The majority of participants were children (up to 12 years old) rather than teenagers (12 to 18 years old).
The studies were predominantly conducted in urban or suburban settings involving vulnerable populations. The exceptions were Dolinar 2000 where the majority of families were above the low‐income level in Canada and Mitchell 1986 who recruited and analysed data for European and Polynesian children separately, since the Polynesian children were previously reported to have lower socioeconomic status and differences in asthma management and outcomes (Mitchell 1981; Mitchell 1984). Eight studies reported high levels of participants on Medicaid or public insurance (Brown 2002; Butz 2006; Gorelick 2006; Galbreath 2008; Fisher 2009; Otsuki 2009; Butz 2010; Seid 2010) or attending subsidised community clinics (Seid 2010). Eight studies included greater than 50% participants from ethnic minorities (Brown 2002; Butz 2006; Gorelick 2006; Galbreath 2008; Fisher 2009; Otsuki 2009; Butz 2010; Seid 2010), one study was conducted in a predominantly white population (Brown 2006) while the remaining three trials did not report the ethnic groups of participants.
Interventions
A summary of the educational interventions for each study is displayed in Table 3. Eight studies included four to six‐weekly to monthly home visits that generally lasted 30 to 60 minutes each (Mitchell 1986; Butz 2006; Gorelick 2006; Galbreath 2008; Kamps 2008; Otsuki 2009; Butz 2010; Seid 2010), one study delivered eight home visits lasting 90 minutes (Brown 2002), while another three studies only had one or two home visits (Dolinar 2000; Brown 2006; Fisher 2009). Three studies (Gorelick 2006; Galbreath 2008; Fisher 2009) included additional telephone sessions. The educator who provided the home sessions assisted in one or more primary care clinic visits in two studies (Brown 2006; Butz 2010).
The shortest follow‐up was three months (Dolinar 2000) followed by two trials with six months follow‐up (Brown 2006; Gorelick 2006), while the remaining nine studies collected follow‐up data for nine to 24 months from initial enrolment.
Five studies employed nurses to deliver asthma education (Mitchell 1986; Dolinar 2000; Brown 2002; Brown 2006; Butz 2006) and four studies involved either nurses, social workers or health educators (Gorelick 2006; Fisher 2009; Otsuki 2009; Butz 2010). Galbreath 2008 used nurses for the telephonic intervention and employed pulmonary therapists for the home visits, while Kamps 2008 employed licensed psychologists or master's level psychology students. Seid 2010 provided in‐home asthma education through bachelor’s level, bilingual, bicultural home visitors, and the problem‐skills training intervention component was delivered by bilingual and bicultural bachelor’s or master’s level health educators.
All programmes provided basic education on the concepts of asthma, such as pulmonary anatomy and physiology as well as the disease process. Six of the studies provided printed materials (such as booklets) and/or homework to complete after the educational sessions (Dolinar 2000; Brown 2002; Gorelick 2006; Kamps 2008; Otsuki 2009; Seid 2010). All programmes reviewed asthma medications along with inhaler technique and reviewed strategies for self management of the disease. One study used electronic devices to provide objective feedback to inform patients and families on medication adherence (adherence monitoring arm of Otsuki 2009), while another study kept track of nebuliser use electronically to measure study outcomes (Butz 2006). Galbreath 2008 provided active disease monitoring and management via scheduled telephone calls and access to a 24‐hour hotline. Written action plans were reviewed and/or provided in 10 out of 12 studies (Dolinar 2000; Brown 2002; Butz 2006; Brown 2006; Gorelick 2006; Galbreath 2008; Fisher 2009; Otsuki 2009; Butz 2010; Seid 2010). All but one study (Otsuki 2009) specifically mentioned educational interventions that reviewed asthma triggers, measures to reduce environmental allergens or both.
Most interventions were based on already‐existing asthma educational programmes, some founded on theories of learning. Brown 2002 used the Wee Wheezers programme (Wilson 1996b), developed using principles of social learning theory by a team of paediatricians, pulmonologists, psychologists, public health educators and educational video specialists. Brown 2002 modified the scripts and handouts to make them culturally appropriate and target low‐literacy level parents. Butz 2006 also made use of Wee Wheezers as well as the A+ Asthma Club Programme (Schneider 1997), the latter of which uses the PRECEDE (Predisposing, Reinforcing, Enabling Constructs in Educational Diagnosis and Evaluation) planning model. Additional programmes included the Air Force Asthma Program developed by the Ontario Lung Association (Dolinar 2000), the National Jewish Asthma Disease Management Program and Pulmonary Therapies LLC programme (Galbreath 2008), the Fight Asthma Milwaukee Allies (Gorelick 2006), and the Air Wise Program and The Clubhouse Kids Learn Asthma interactive computer program (Kamps 2008). Butz 2010 designed an asthma communication education programme based on the chronic care model (Wagner 2001; Bodenheimer 2002) and other studies examining clinician‐parent‐child communication (Halterman 2001; Tates 2001). The community health workers in Fisher 2009 used the Transtheoretical Model to assess participants' readiness for change and adopted asthma management behaviours accordingly. They also used a "nondirective supportive style" in their interactions. Kamps 2008 used behaviour management techniques to promote adherence, specifically the Exchange Program for Improving Medication Adherence (Rapoff 1999). Similarly, Seid 2010 employed problem‐solving skill training based on a concept by D'Zurilla (D'Zurilla 1971; D'Zurilla 1986) and a protocol previously tested on mothers of children with cancer (Varni 1999). Seid 2010 based the care co‐ordination component of their intervention on the Robert Wood Johnson Foundation's Allies Against Asthma community health worker model (Friedman 2006). The NHLBI guidelines were explicitly stated as the basis of asthma management in five trials (Brown 2002; Brown 2006; Galbreath 2008; Fisher 2009; Seid 2010), while the Air Wise Program used in Kamps 2008 was affiliated with the NHLBI.
Three studies involved two intervention and one control group (Gorelick 2006; Otsuki 2009; Seid 2010). All intervention groups were included in this review except the intensive primary care linkage arm of the Gorelick 2006 trial, since there was no home education component.
Control groups
Control groups received routine care in nine trials, which may have involved asthma education but not provided in the home setting (Mitchell 1986; Dolinar 2000; Brown 2002; Brown 2006; Gorelick 2006; Galbreath 2008; Fisher 2009; Otsuki 2009; Seid 2010) and form the basis of the first comparison in this review. The remaining three studies provided controls with home‐based general asthma education, while providing additional education for specific therapies or skills in the intervention group (nebuliser therapy education, asthma communication education and strategies to improve adherence to inhaled corticosteroids for Butz 2006, Butz 2010 and Kamps 2008, respectively). These three studies form the basis of our second comparison of education versus a less intensive educational intervention.
Compliance
The full intervention was delivered to over 70% of participants in all trials except Brown 2006 in which 39% of adults and children completed the programme.
Outcomes
The primary outcomes of the trials varied. For Brown 2006, it was time to first relapse for asthma (ED or unscheduled visit for asthma). Butz 2010 defined caregiver reported symptom days and nights over the past 30 days as the primary outcome; Butz 2006 looked at asthma severity, number of ED visits and medication usage (email communication with author); Dolinar 2000 used parental coping, caregiver perception of change and quality of life; Fisher 2009 reported hospitalisations at 12 months; Galbreath 2008 chose time to first asthma‐related events, quality of life and rates of healthcare utilisation; Gorelick 2006 used proportion of patients experiencing an ED visit over six months; Kamps 2008 reported adherence to inhaled corticosteroids using an electronic monitor (MDILog); Otsuki 2009 chose ED visits; and Seid 2010 measured health‐related quality of life. Two studies did not define primary outcomes (Mitchell 1986; Brown 2002).
For full details of outcomes reported in each study, see Characteristics of included studies.
We obtained additional data from three trialists (Butz 2006; Gorelick 2006; Butz 2010).
Aims
The overall aims of the studies varied (Table 5), testing different interventions to improve various outcomes (listed in the section above).
3. Aims of studies.
| Study | Aims |
| Mitchell 1986 | In New Zealand readmission rates are higher in Polynesian children than in European children. The study was designed to find out if community child health nurses in the patients home could reduce school absenteeism, encourage visits to the GP and reduce the number of readmissions to hospital and teach parents when and how to seek medical help for an attack not responding to usual treatment. |
| Dolinar 2000 | Does home‐based education resource influence parental coping, perception of asthma change and quality of life? |
| Brown 2002 | "A home‐based program may be the most developmentally appropriate and ecologically valid methods of delivering asthma education to low income inner city families". Wanted to evaluate efficacy in terms of parental participation and effectiveness in terms of decreasing morbidity and increasing the caregiver's quality of life and asthma management skills. |
| Butz 2006 | "Low‐income minority children have disproportionately high morbidity and mortality rates" and "tend to rely on hospital emergency rooms as primary source of asthma care". Therefore aimed to decrease ED visits/hospital admissions by helping parents understand when the child's asthma was worsening and train them specifically in home nebuliser use. |
| Gorelick 2006 | Evaluated the impact of educating children in the emergency department and then following up with home‐based education interventions compared to standard care alone. |
| Brown 2006 | A "structured comprehensive asthma education program delivered by an experienced asthma nurse educator within a local asthma coalition would be an effective method of reducing asthma relapse in patients who had moderate‐sever persistent asthma." |
| Galbreath 2008 | A telephonic and home‐visiting asthma education delivered by a respiratory therapist designed to decrease healthcare utilisation and generate cost savings. |
| Kamps 2008 | Designed to increase adherence to asthma treatment regimens. |
| Otsuki 2009 | To evaluate a feedback of electronically monitored adherence and education programme in reducing ED visits ad asthma mediation adherence, symptoms, hospitalisations and courses of oral steroids. |
| Fisher 2009 | Hypothesised that "community health workers may help reduce disproportionate asthma health burden among children from low‐income families". Used a non‐professional asthma coach to try and reduce rehospitalisation. |
| Seid 2010 | Problem‐solving may be useful in helping families, especially lower socioeconomic status families improve their asthma management behaviours to improve quality of life. |
| Butz 2010 | Hypothesised that a programme designed to improve clinician‐caregiver communication would be associated with reduced symptom days and nights and increased compliance with appropriate controller medication in inner‐city children. |
ED: Emergency Department
Excluded studies
We recorded the reasons for exclusion of 160 studies after retrieving the full‐text documents (see Characteristics of excluded studies). We excluded 26 studies aimed primarily at environmental remediation or which supplied participants with allergen‐reducing materials (such as mattress encasings and HEPA (high‐efficiency particulate air) filters); 25 studies conducted on adults; 66 studies delivering the education outside the home; 12 studies providing education primarily via multimedia but without face‐to‐face; in‐person education delivered in the home; three studies delivering an exercise‐based intervention; three studies providing a low‐intensity information‐only intervention; one study using a self help kit; one study involving a decision‐making programme aimed at reducing substance misuse; three studies aimed at education physicians or nurses; and one study for multiple reasons (randomisation not described, location of education delivered not specified, no extractable data and unable to contact the study author). Nineteen studies were not randomised controlled trials.
There was one potentially eligible trial identified that is still ongoing (Rand 2006)
Risk of bias in included studies
Full details of risk of bias judgements can be found in Characteristics of included studies. Graphical representations of our judgements of the risk of bias can be found in Figure 2.
2.
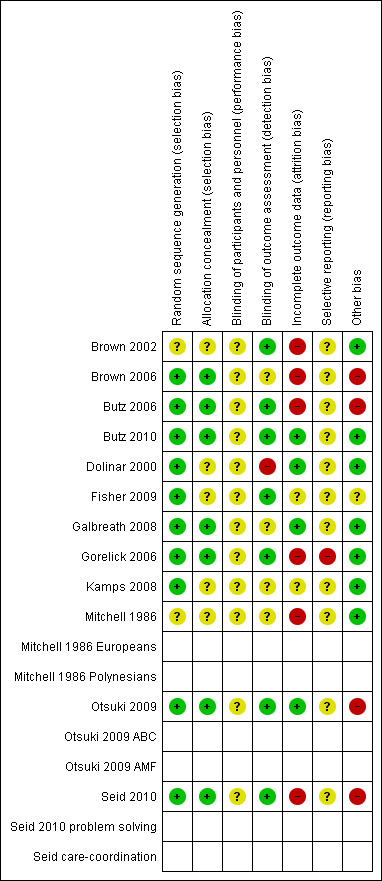
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Ten studies reported full details of adequate sequence generation and we judged them to be of low risk of bias (Dolinar 2000; Butz 2006; Brown 2006; Gorelick 2006; Galbreath 2008; Kamps 2008; Fisher 2009; Otsuki 2009; Butz 2010; Seid 2010). Two studies were reported as randomised, but gave no description of the methods used and we therefore judged them to be at unclear risk of bias (Mitchell 1986; Brown 2002).
Seven studies were low risk of bias with full details of allocation sequence concealment reported (Brown 2006; Butz 2006; Gorelick 2006; Galbreath 2008; Otsuki 2009; Butz 2010; Seid 2010). The author confirmed allocation concealment in one study (Butz 2006). There were insufficient details for the remaining five studies for us to reach a firm conclusion so we judged them to be at unclear risk of bias (Mitchell 1986; Dolinar 2000; Brown 2002; Kamps 2008; Fisher 2009).
Blinding
The nature of the intervention precludes the possibility of blinding patients or the educator and therefore all the studies were judged to be at unclear risk of performancebias. However, it is possible to blind the people who collected or analysed the data. We judged seven studies to be at low risk of bias with respect to blinding of data collectors (Brown 2002; Butz 2006; Gorelick 2006; Fisher 2009; Otsuki 2009; Butz 2010; Seid 2010). Three studies did not describe blinding, so we judged them to be at unclear risk of bias (Mitchell 1986; Brown 2006; Kamps 2008). Dolinar 2000 was high risk of bias as it was described as non‐blinded. The lack of blinding would likely have a greater impact on the more subjective outcomes (e.g. quality of life) than more objective measures (e.g. ED visits).
Incomplete outcome data
We judged four studies to be at low risk of bias due to incomplete outcome data as indicated by withdrawals/losses to follow‐up in Table 6 (Dolinar 2000; Galbreath 2008; Otsuki 2009; Butz 2010). Butz 2010 and Dolinar 2000 had similar losses to follow‐up in both education and control groups, Galbreath 2008 obtained comprehensive health care utilisation data for 99% of participants and Otsuki 2009 had consistent losses to follow‐up across treatment arms which averaged around 10%. We judged six studies to be at high risk of bias with respect to incomplete outcome data (Mitchell 1986; Brown 2002; Brown 2006; Butz 2006; Gorelick 2006; Seid 2010). Brown 2002 had a high number of withdrawals from the treatment arm (n = 6) compared to none on the control arm, Butz 2006 had unbalanced losses of pharmacy records, Brown 2006 had 21% lost to follow‐up in the intervention group at six months compared to six percent in the control group, Gorelick 2006 reported the baseline characteristics of those lost to follow‐up or with incomplete data (22%) but there were more lost in the treatment versus the control group, while Seid 2010 had high and unbalanced losses to follow‐up and refusal to comply, which we assume was related to the nature of the interventions. Mitchell 1986 did not mention how many people contributed data, so we judged this to be at a high risk of bias. The remaining two studies were at unclear risk of bias (Kamps 2008; Fisher 2009). Fisher 2009 unbalanced follow‐up survey data but reviewed hospital records for all patients. The risk of bias was unclear because records were only available from one hospital in the city, albeit the latter was where the majority of admissions likely occurred. Kamps 2008 was a small pilot study with poor follow‐up, so although the losses were balanced between arms, the reported data were on limited participants.
4. Completers/ withdrawals.
| Study | % completed full education programme | Partial completion | Completed no sessions | Lost to follow‐up | Withdrawals |
| Brown 2002 | 71% | 11% | 7% | ‐ | 11% |
| Brown 2006 | 38%* | 9% received only clinic visit* 15% received only home visit* |
39%* | Intervention group 21%
Control group 6% (reported for children) |
Not reported * these data are from both adults and children |
| Butz 2006 | Most completed | ‐ | ‐ | Nebuliser: 14% excluded from follow‐up (10% no pharmacy data, 2% died, 3% lost). SAE 23% excluded (18% no pharmacy data, 1% died, 4% lost) | Not reported |
| Butz 2010 | ‐ | More intense group received mean 3.29 out of 4 visits Control received mean 2.27 out of 3 visits |
‐ | More intense group 17% Control group 14% |
Not reported |
| Dolinar 2000 | 100% | ‐ | ‐ | 5% | 0 |
| Fisher 2009 | ‐ | ‐ | 4% (no substantive contact) | Hospitalisations available for all participants. 83% completed surveys |
Not reported |
| Galbreath 2008 | 70% completed at least 80% of the intervention ‐ although this was for adults and children | ‐ | ‐ | 35% | 2 |
| Gorelick 2006 | Average of 4 (out of 6) home visits per patient (average of 2 missed visits per patient); average 2.3 calls per patient | 72% had at least 1 home visit | ‐ | 22% | |
| Kamps 2008 | 100% | ‐ | ‐ | 2 months 33% 6 months 60% 12 months 67% |
3 |
| Mitchell 1986 | 68% | 26% | 6% | Not reported | Not reported |
| Otsuki 2009 | ABC 71%; AMF 63% | ‐ | ‐ | ˜10% | 1 person deceased in each group |
| Seid 2010 | 67% completed all 5 visits | Completed 4.0 (CC) and 3.8 (CC + PST) visits on average | ‐ | CC intervention: 20% lost (7% refused), PST intervention 32% lost (19% refused) | PST 19% and CC 6% "refused" |
ABC: Asthma Basic Care AMF: Adherence Monitoring with Feedback SAE: serious adverse event PST: problem‐solving skill training
For consideration of the number of patients withdrawing and those lost to follow‐up, please see Effects of interventions and Characteristics of included studies.
Selective reporting
We judged 11 studies to be at unclear risk of bias for selective outcome reporting since there was no published protocol (Mitchell 1986; Dolinar 2000; Brown 2002; Butz 2006; Brown 2006; Galbreath 2008; Kamps 2008; Otsuki 2009; Fisher 2009; Seid 2010; Butz 2010). Five trials (Butz 2006; Galbreath 2008; Otsuki 2009; Butz 2010; Seid 2010) were registered in clinicaltrials.gov but all had one or more secondary outcomes in the protocols that were not reported in the final publications, and were therefore deemed unclear risk of bias. The final study (Gorelick 2006) measured hospital admissions but did not report this in the results and the trialists were unable to provide these data through correspondence. We therefore judged Gorelick 2006 at high risk of selective reporting bias.
Other potential sources of bias
The main additional source of bias identified in these studies was recall bias for outcomes where the patient or parent had to report the number of events experienced over time. We judged seven studies to be at low risk of recall bias (Mitchell 1986; Dolinar 2000; Brown 2002; Gorelick 2006; Galbreath 2008; Kamps 2008; Butz 2010). Dolinar 2000 and Kamps 2008 only reported outcomes that by their nature have to be measured by self report and we therefore judged them to be at low risk of bias. Four studies reviewed medical records for healthcare utilisation (Mitchell 1986; Brown 2002; Galbreath 2008; Butz 2010) while Gorelick 2006 collected both self reported ED visits as well as those in their computerised tracking system. We judged Fisher 2009 to be an unclear risk, once again due to the retrieval of hospitalisations from one hospital only. We judged four studies to be at high risk of bias for outcomes that were self reported, but could have been verified (e.g. ED visits, hospitalisations; Butz 2006; Brown 2006; Otsuki 2009; Seid 2010). Otsuki 2009 compared self reported adherence to pharmacy records for inhaled corticosteroid usage and showed that the self reports overestimated adherence compared to pharmacy records, which highlights the potential for recall bias in these situations and the need for collecting accurate data where possible.
Effects of interventions
Home‐based education versus usual care or a less intensive, non‐home‐based education
Primary outcome: Exacerbations leading to Emergency Department (ED) visits
Six studies involving 985 patients reported the number of patients with one or more asthma exacerbations resulting in ED visit(s) (Mitchell 1986; Dolinar 2000; Brown 2006; Gorelick 2006; Fisher 2009; Seid 2010). The studies were not pooled due to heterogeneity in the study populations, outcome definitions, follow‐up time and differences in the control group event rate (Table 4). Brown 2006 reported unscheduled urgent visits to a physician office and ED visits for asthma as a single outcome and Fisher 2009 reported the subset of ED visits that did not result in hospitalisations. Three studies measured outcomes at six months (Mitchell 1986; Brown 2006; Gorelick 2006), one reported data at three months (Dolinar 2000) and one at 24 months (Fisher 2009). Only one study (Mitchell 1986) showed a significant effect favouring controls over the intervention for the European children subgroup (Analysis 1.1). Seid 2010 reported the number of patients experiencing asthma exacerbations at three months and nine months from baseline. Seid 2010 also reported odds ratios for patients with ED visits adjusted for the baseline level of the outcome and covariates (including age, race/ethnicity, Spanish language and mother's education), which were not significant for either of the intervention groups compared to controls.
1.1. Analysis.
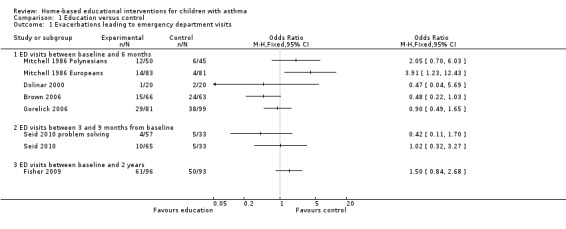
Comparison 1 Education versus control, Outcome 1 Exacerbations leading to emergency department visits.
Three studies involving 525 patients reported the mean number of acute visits for asthma (Brown 2002; Gorelick 2006; Otsuki 2009). The pooled data for two studies on 430 people at six months was not statistically significant (mean difference (MD) 0.04; 95% confidence interval (CI) ‐0.20 to 0.27; Analysis 1.2). Brown 2002 reported only means without standard deviations and incorporated ED and clinic visits for acute asthma as a single outcome, so we could not pool these data. Although there was a significant decrease over time in the mean number of acute asthma visits at 12 months in both groups, there was no significant net treatment effect. Otsuki 2009 reported mean ED visits in the previous six months at six‐month intervals from 0 to 18 months of follow‐up. At 18 months, the results (for the previous six months) revealed no significant difference between either intervention groups (basic asthma education or intervention with additional adherence monitoring with feedback) and the control group. However, the authors analysed the decrease in ED visits over the entire 18‐month period, and showed that the rate of decrease in ED visits over time was faster in both the combined treatment groups and the adherence feedback group alone than the control group (Otsuki 2009).
1.2. Analysis.
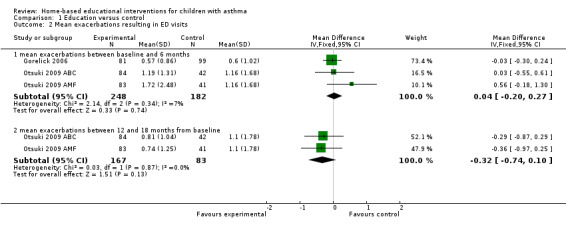
Comparison 1 Education versus control, Outcome 2 Mean exacerbations resulting in ED visits.
Galbreath 2008 reported an adjusted annual asthma exacerbation rate per patient leading to ED visits showing no significant difference between groups.
Primary outcome: Exacerbations requiring oral corticosteroids (OCS)
One study involving 250 patients reported the mean number of exacerbations requiring a course of oral corticosteroids every six months from baseline to 18 months (Otsuki 2009). At six and 18 months, the pooled results for the two intervention arms (basic education and adherence monitoring) were not statistically significant (MD ‐0.18; 95% CI ‐0.63 to 0.26 and MD ‐0.72; 95% CI ‐1.51 to 0.07 respectively; Analysis 1.3). However, the authors once again demonstrated that the rates of decrease in mean courses of OCS over 18 months follow‐up were faster for either intervention groups compared to usual care (Otsuki 2009). Galbreath 2008 reported no statistically significant difference in the number of oral corticosteroid bursts, but the numbers were not provided to allow pooling of results.
1.3. Analysis.
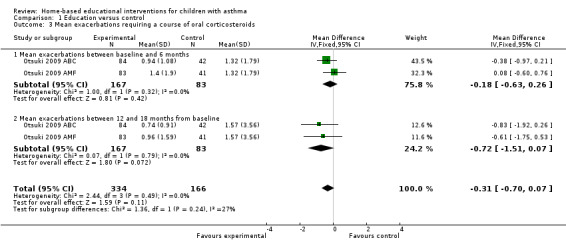
Comparison 1 Education versus control, Outcome 3 Mean exacerbations requiring a course of oral corticosteroids.
Secondary outcome: Quality of life
Five studies on 727 participants measured quality of life (Dolinar 2000; Brown 2002; Gorelick 2006; Galbreath 2008; Seid 2010). Given the difference in instrument scores and missing data in some trials, we present a narrative synthesis below.
Three studies involving 331 patients used the Paediatric Asthma Caregiver's Quality of Life Questionnaire (PACQLQ) to assess quality of life (Dolinar 2000; Brown 2002; Galbreath 2008). Juniper et al developed both the PACQLQ (Juniper 1996a) and Paediatric Quality of Life Questionnaire (PAQLQ; Juniper 1996b), which were validated as evaluative and discriminative instruments for children seven to 17 years old. The PAQLQ contains 23 items in the domains of activity limitation, symptoms and emotional function, administered to the child (Juniper 1996b) while the PACQLQ contains 13 items concerning activity limitations and emotional function administered to the caregiver (Juniper 1996a). Brown 2002 reported improvement in scores among the caregivers of both intervention and control arms. However an intervention effect was only noted in the younger patient subgroup (one to three years old). The remaining two studies failed to show a significant effect of the intervention as measured by the PACQLQ (Dolinar 2000; Galbreath 2008) although in Galbreath 2008 the quality of life improved over time in all groups.
Galbreath 2008 also administered the PAQLQ to children. Galbreath 2008 reported no statistically significant group differences in the quality of life scores at follow‐up (adjusted for baseline differences in demographics; P = 0.40), although quality of life improved from baseline in both groups. Seid 2010 reported overall health‐related quality of life in 165 children using the PedsQL Total (23 items on physical and psychosocial health) administered to the parent and, if applicable, the child. Seid 2010 also reported quality of life using the PedsQL Asthma (28 items on asthma symptoms, treatment problems, worry and communication) administered to the parent and, if applicable, the child. There was no statistically significant difference in the primary outcome PedsQL Total for parent‐reported symptom scores for control versus either intervention groups at three months. At nine months, there was no statistically significant difference between control versus the care co‐ordination intervention, but there was a statistically significant difference in control versus care co‐ordination with additional problem‐solving skills training in the PedsQL Total administered to parents (adjusted MD 4.05; 95% CI 0.63 to 7.4).
Gorelick 2006 had parents/caregivers complete the Integrated Therapeutics Group Child Asthma Short Form (ITG‐CASF), containing 10 items specific to asthma, at baseline and six months. This tool was previously validated for use in the ED (Gorelick 2004). The mean change in scores from baseline improved in both intervention and control groups but the difference between groups was not statistically significant.
Secondary outcome: Symptoms
Four studies involving 711 participants reported information relating to asthma symptoms, but we were unable to pool data for this outcome due to missing data, the heterogeneity of tools used to measure symptoms and underlying differences in the control group exacerbation rate (Table 4) (Brown 2002; Galbreath 2008; Otsuki 2009; Seid 2010). Brown 2002 reported the mean for asthma symptom score (using a sub‐scale of the PAQLQ) and the mean symptom‐free days. Although follow‐up analyses were able to show that there was a benefit for symptoms and symptom‐free days in the younger children (one to three years old) and not in the older children, overall there was no treatment effect on either outcome.
Galbreath 2008 measured symptom scores using the Lara Asthma Symptom Scale (LASS) and stated that symptom scores decreased over time in all groups, but did not show a significant treatment effect. Otsuki 2009 measured caregiver reports of symptoms every six months (baseline, 6, 12 and 18 months), calculating the mean asthma symptoms in the previous 30 days. None of the mean values for symptom scores were different at each time point between either intervention groups versus controls, but Otsuki 2009 reported differences in the rate of improvement in symptoms over time for the asthma basic care versus control group ‐ the intervention improving faster than the control group over the first 12 months. Using ordinal scales, Seid 2010 reported the percentage of children experiencing daytime symptoms (< twice a week, three to six times a week and every day) and night‐time symptoms (< once/week, > once/week) at baseline, three months and nine months after baseline. The odds ratios adjusted for baseline levels and covariates showed no significant different between either intervention groups compared with controls for daytime symptoms, but statistically improved night‐time symptoms at three months but not nine months for the care co‐ordination/problem‐solving skills group compared to controls (odds ratio (OR) 0.33; 95% CI 0.13 to 0.82; Seid 2010).
Secondary outcome: Night‐time awakening
No study reported night‐time awakening.
Secondary outcome: Days missed from school
One study with 259 participants reported no statistically significant difference between groups in the number of school days missed (Mitchell 1986; Analysis 1.4). One study combining 239 adults and children reported on days missed from school or work, also showing no group difference (Brown 2006).
1.4. Analysis.
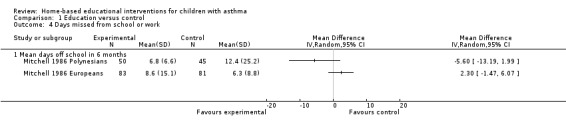
Comparison 1 Education versus control, Outcome 4 Days missed from school or work.
Secondary outcome: Hospitalisations
Five studies on 1012 participants reported the number of patients experiencing one or more hospitalisations (Mitchell 1986; Dolinar 2000; Fisher 2009; Otsuki 2009; Seid 2010). However, the data were not combined due to clinical heterogeneity and follow‐up periods (Analysis 1.5). Fisher 2009 demonstrated reduced hospitalisations at 24 months in the intervention group in (OR 0.4; 95% CI 0.22 to 0.71) and the Europeans in Mitchell 1986 had increased admissions between six and 18 months (OR 2.45; 95% CI 1.25 to 4.83). All the other studies reported no group difference. None of the patients in Dolinar 2000 had a hospital admission.
1.5. Analysis.
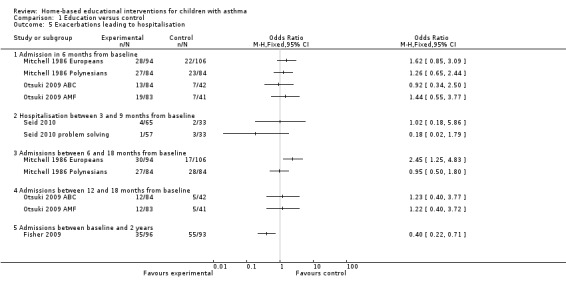
Comparison 1 Education versus control, Outcome 5 Exacerbations leading to hospitalisation.
Two studies on 569 participants reported the mean hospitalisations, although we did not pool due to heterogeneity (Mitchell 1986; Galbreath 2008). Mitchell 1986 had heterogeneity between study populations so we did not to pool this outcome (Analysis 1.6). Mitchell 1986 reported mean hospital admissions at both six months and between six and 18 months. Only the European children showed a significant effect favouring controls in the latter time period. Galbreath 2008 reported no significant treatment effect for the adjusted rates of hospital admissions per patient per year.
1.6. Analysis.
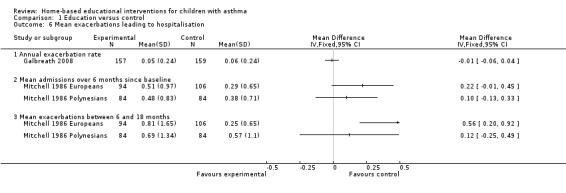
Comparison 1 Education versus control, Outcome 6 Mean exacerbations leading to hospitalisation.
Secondary outcome: Lung function
No studies reported this outcome.
Education versus another type of home‐based education
Three studies reported on a home‐based education intervention compared to another, less intensive type of home‐based education as a control group (Butz 2006; Kamps 2008; Butz 2010).
Primary outcome: Exacerbations leading to ED visits
Butz 2006 showed fewer patients in the nebuliser‐targeted education group (27/95) reporting at least one ED visit at six months compared to the less intensive education group (40/86), which was a statistically significant (OR 0.46; 95% CI 0.25 to 0.84; Analysis 2.1). Butz 2010 reported no statistically significant difference between groups for the decrease in mean number of ED visits at 12 months.
2.1. Analysis.
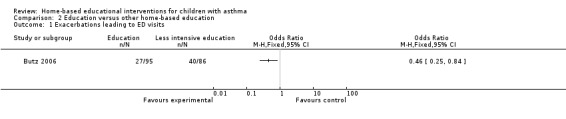
Comparison 2 Education versus other home‐based education, Outcome 1 Exacerbations leading to ED visits.
Primary outcome: Exacerbations requiring oral corticosteroids (OCS)
Butz 2006 reported no statistical differences between intervention and control groups for the mean number of oral corticosteroid prescriptions at 12 months. Butz 2010 reported no significant differences for the mean number of oral corticosteroids filled at 12 months (Analysis 2.2).
2.2. Analysis.
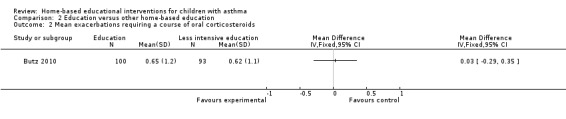
Comparison 2 Education versus other home‐based education, Outcome 2 Mean exacerbations requiring a course of oral corticosteroids.
Secondary outcome: Quality of life
Kamps 2008 measured physical function and psychosocial health with the generic PedsQL and asthma‐related quality of life with the PedsQL Asthma Module (with sections on asthma symptoms, treatment problems, worry, and communication) administered to both child and caregiver. No significant treatment effects were observed using repeated measures analyses of covariance and pooled time series analysis using data from baseline (n = 15), two (n = 10), six (n = 6) and 12 months (n = 5) (Kamps 2008).
Secondary outcome: Symptoms
Kamps 2008 measured caregiver‐reported and child‐reported asthma symptoms using PedsQL Asthma Module and found no significant difference in treatment effect at baseline and two, six and 12 months. Repeated‐measures ANCOVAS did not yield significant interactions or main effects for symptom scores. Butz 2010 reported no statistically significant differences between groups for the decrease in mean number of symptoms during both the day and at night measured with a Likert‐type scale at 12 months. We did not pool the data due to differences in measurement tools.
Secondary outcome: Night‐time awakening
No study reported this outcome.
Secondary outcome: Days missed from school
No study reported this outcome.
Secondary outcome: Hospitalisations
A single study on 181 participants reported the number of patients experiencing at least one hospitalisation for the previous six months at 12 months follow‐up (Butz 2006).There were fewer hospitalisations in the group receiving the additional nebuliser use training compared to the less intensive education, which was a statistically significant difference (OR 0.30; 95% CI 0.09 to 0.98; Analysis 2.3). Butz 2010 reported no difference in the mean number of hospitalisations at 12 months (Analysis 2.4).
2.3. Analysis.
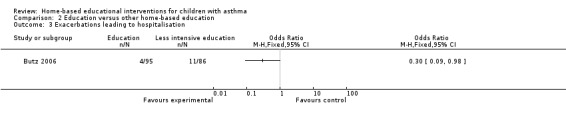
Comparison 2 Education versus other home‐based education, Outcome 3 Exacerbations leading to hospitalisation.
2.4. Analysis.
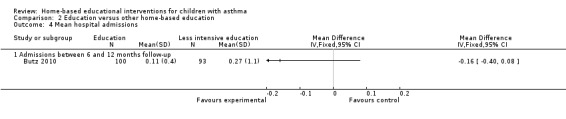
Comparison 2 Education versus other home‐based education, Outcome 4 Mean hospital admissions.
Secondary outcome: Lung function
Kamps 2008 did not report any significant differences in lung function (FEF (forced expiratory flow) 25% to 75%) using repeated measures analyses of covariance and pooled time series analysis at two months (n = 10), six months (n = 6) and 12 months (n = 6).
Cost
Galbreath 2008 reported the cost of the four home visits to be USD 206 and USD 531 for the telephonic intervention, totaling USD 737 per participant.
Withdrawals
We decided not to pool these data due to heterogeneity in the trial design (such as the number of education sessions) and reporting of the number of withdrawals. Instead we created Table 6 to reflect the number of patients who completed all or some of the education, patients lost to follow‐up and those who withdrew. This table should be viewed and interpreted with caution. Each study had its own definition of completing the programme and in some case people may have missed some sessions yet still be registered as completing the programme. The programmes also varied in number of sessions.
Subgroup analysis
We were unable to perform any of the prespecified subgroup analyses due to the lack of studies. Even if we had more included studies, performing subgroup analysis would be unwise due to the heterogeneity in the studies. We could not subgroup by age as most studies were on two to 12 year olds, except for three studies which included a range of ages spanning childhood and adolescence (Brown 2006; Gorelick 2006; Galbreath 2008). None of the studies could be divided into mild/moderate versus severe as all included children had a range of asthma severities. Fisher 2009 was the only intervention running for longer than six months. We had planned to subgroup by physician or nurse versus community health worker. However, all studies employed nurses combined with either a social worker, a trained health educator or a pulmonary therapist, except Fisher 2009 who employed a community health worker, Kamps 2008 who used a psychologist or a psychology graduate student, Otsuki 2009 an asthma educator, and Seid 2010 who employed bilingual, bicultural graduate asthma visitors.
Discussion
Summary of main results
We included 2342 patients from 12 trials in this review. Of these, eleven trials were conducted in North America. Ten studies were in urban or suburban settings involving vulnerable populations. We summarised the components of the home‐based educational interventions in Table 3. We were unable to pool many of the outcomes due to clinical heterogeneity of the populations, interventions and timing of outcome assessment. The control event rates for Emergency Department (ED) visits and hospitalisations were quite different between trials. It is possible that trials with a higher control group event rate (poorly controlled asthma) would more likely achieve a decrease in admissions/ED visits, leading to difficulties in pooling (see Table 4 and further discussion below). Overall the effect of home‐based education is heavily dependent on the context of the trial (including but not limited to; aims of study, focus of the education, characteristics of the population) so deriving and interpreting average outcomes across these studies is not meaningful.
Overall completeness and applicability of evidence
Implementation, feasibility and applicability of home‐based educational interventions
Many of the included studies tested commercially‐available asthma educational interventions covering similar programme topics, although these were delivered differently and with different emphasis on components of education. These studies showed that while home‐based interventions are feasible, additional resources and trained personnel are required, which may not be easily applied in real‐world settings, especially without adequate financial support. None of the studies analysed cost‐effectiveness. Economic data may strengthen the case for such interventions to be included in policy and healthcare budgets, concurrent with more evidence supporting clinical effectiveness. Administrators and policy‐makers may want to consider whether children with asthma and their caregivers are able to attend asthma clinics to receive education or whether some families can only be reached by visiting their home.
Our review also draws attention to another issue pertaining to the feasibility of interventions: the ability to retain participants. Although the participants who provided follow‐up data generally completed most of the education sessions, there appeared to be a higher attrition rate in the education group compared to the control groups in almost half of the trials. While high and unbalanced withdrawal rates are common in trials with high demands on participants' time, unbalanced withdrawal rates may also provide a biased indication of the effectiveness of home‐based education, depending on whether the intervention retained children who were more or less likely to benefit than those who dropped out.
Although most of our included trials employed health professionals (specifically, nurses), it may be possible to improve feasibility and reduce costs of interventions by employing lay workers from the community to deliver the education sessions. Fisher 2009 demonstrated that a programme delivered by community health workers was not only feasible, but also reduced hospitalisations. Community health workers have successfully delivered education in other trials, albeit in programmes primarily aimed at reducing environmental allergens thus beyond the scope of our review (Hovell 2002; Krieger 2002; Krieger 2005). A study by Flores 2009, excluded from this review, employed lay asthma educators in the form of parent mentors and suggested that this was an inexpensive and effective intervention for reducing asthma exacerbations and ED visits over 12 months, leading to overall cost savings.
Two studies in our review require careful interpretation with regards to the applicability of the interventions and results (Mitchell 1986; Butz 2006). Butz 2006 was focused on a population of children using nebulisers to deliver medications at home, which is not currently standard practice for most children with asthma and is not usually recommended in current guidelines (NAEPP 2007; British Guideline on the Management of Asthma; GINA 2008). Butz 2006 was the only trial among three with a home‐based control education group that showed a significant treatment effect for reducing ED visits and hospital admissions. This may relate to more severe asthma in children enrolled who were using nebulisers, and hence the greater likelihood of needing ED visits or hospital admissions for acute asthma (nearly half of the control group had ED visits in this study). Mitchell 1986 was conducted more than two decades ago, when asthma management was different and guidelines were less widely recognised and disseminated (Brouwer 2008).
Quality of the evidence
A criticism of existing research on education for childhood asthma is that no randomised controlled trials (RCTs) have evaluated what are the specific components of the programmes that are effective. Rather, most studies have tested educational interventions featuring a comprehensive strategy (Brouwer 2008; Boyd 2009). Our review has highlighted the clinical heterogeneity present in randomised trials of home‐based educational interventions.
Heterogeneity in educational programmes
The diversity in educational programmes (e.g. focus, intensity and duration of education), as well as the variation in the control education and in the assessment of outcomes, led to substantial clinical heterogeneity. Because of this, we did not pool many of the data and were unable to draw definite conclusions from the narrative syntheses. Several sources of heterogeneity are highlighted below.
The aims of the studies varied from reducing ED visits and readmission rates to improving adherence or quality of life. Some studies appeared to focus on one aspect of education (e.g. adherence to steroids or improving nebuliser use) or more specifically on improving an outcome (e.g. reducing readmissions or increasing primary care follow‐up). We attempted to summarise the aims of the trials in Table 5 although these were not always explicit in the trial. It is impossible to untangle what impact the differences in the stated aims have on the education delivered and outcomes.
Most trials reported programmes covering comparable education topics (e.g. written action plans, self monitoring) and we tried to capture any differences in Table 3. We were unable to tease out the individual components of the interventions that made a difference to the outcomes measured.
The trials in our review provided a similar intensity of sessions, with a few exceptions. Three studies delivered only a single education session (Dolinar 2000; Brown 2006; Gorelick 2006) while the most intense intervention provided visits every three months for two years (Fisher 2009). Although the latter, more intense trial demonstrated significantly reduced hospital admissions, there are many other possible explanations for this (e.g. this trial also had the highest control group event rate) (Fisher 2009). Therefore we cannot draw definitive conclusions on the effect of programme intensity.
Control group event rates and population differences
Heterogeneity was present in the control groups, as each trial and institution likely had different standards of usual care, and the severity of asthma and trial duration was heterogeneous. Although not an outcome in our review, there were three deaths in the trial by Butz 2006, two in Otsuki 2009 and two adults died in Galbreath 2008, which may be an indication of asthma severity or a higher‐risk population. In the case of the three trials with a home‐based education control group (Butz 2006; Kamps 2008; Butz 2010), there may have been a decreased ability to detect a difference between groups due to the controls receiving less intense education in the home setting.
Children with asthma are likely to have different levels of access to care, which may affect outcomes. For example, a child may benefit from education if the caregiver learns when to seek medical attention before an exacerbation becomes too severe, but if a primary care provider is not accessible they may end up having to make a non‐urgent ED visit. The end result is an educational intervention that has outcomes that become difficult to interpret. To investigate this further we looked at the event rates for ED visits and hospitalisations in the control groups (Table 4). From the trials reporting ED visits as an outcome, four had relatively high control group ED visit rates (38% to 54%; Butz 2006; Brown 2006; Gorelick 2006; Fisher 2009) and these trials may be more likely to show a decrease in ED visits than the three reporting lower rates of ED visits (5% to 15%; Mitchell 1986; Dolinar 2000; Seid 2010). However, most of the individual trial results were not statistically significant for ED visits (except Butz 2006 and Mitchell 1986, with their unique populations and limited applicability of results, as previously described) and we were unable to draw conclusions. The statistically significant difference in hospitalisations observed in Fisher 2009 may be explained by the high hospitalisation control group event rate (59%) compared to the lower event rates for the other studies (8% to 33%; Table 4).
Some outcome measures improved over time in both intervention and control groups
In many of the included studies, outcomes such as quality of life scores improved in both groups over the trial period (Brown 2002; Gorelick 2006; Galbreath 2008; Seid 2010). This may be due to recruitment factors (patients are enrolled after an ED visit or hospital admission, when they are potentially at their sickest and the majority will get better) or the natural evolution of the disease (younger children getting better with age). This highlights the importance of observing data over extended periods of time to assess whether change is truly due to the intervention and whether the effects are long‐lasting after the trial period.
Potential biases in the review process
Although we attempted to apply a systematic process for including and excluding studies in this review and followed the criteria prespecified in our protocol, the final decisions are open to interpretation or criticism. In order to reduce clinical heterogeneity, we excluded some trials that had mixed interventions, such as those with more education delivered outside of the home (e.g. Flores 2009) or more focus on environmental allergen reduction (e.g. Jones 2001; see Characteristics of excluded studies for more information).
Agreements and disagreements with other studies or reviews
The current review brings together new trials that were not included in previous systematic reviews (Coffman 2008; Boyd 2009; Wolf 2002). Some of these RCTs were also missing from the last update of the National Asthma Education and Prevention Program (NAEPP) guidelines in 2007 (NAEPP 2007), which revealed the need for more research at the time and recommended home‐based education for young children based on evidence from two RCTs (Jones 2001; Brown 2002) only one of which was included in this current review. Previous reviews have supported asthma self management education in children to improve outcomes such as reduced asthma‐related morbidity and acute health care utilisation. Wolf 2002 included 32 trials examining asthma self management educational interventions in children and adolescents that took place in various settings (including home, clinic and school). Only one study from our review was included in Wolf's review (Mitchell 1986). Wolf 2002 noted that the majority of the trials were poorly reported and lacked sufficient data for meta‐analyses. Likewise, Coffman 2008 meta‐analysed 37 trials conducted solely in the United States in the clinical setting on education to decrease acute care services use and Boyd 2009 included 38 trials on education for children who had attended the ED. Altogether, the pooled trials in these reviews demonstrated statistically significant decreased ED use in terms of the risk of subsequent ED visits (risk ratio (RR) 0.73; 95% confidence interval (CI) 0.65 to 0.81; Boyd 2009) as well as the mean number of ED visits (standardised mean difference (SMD) ‐0.21; 95% CI ‐0.33 to ‐0.09; Wolf 2002 and SMD ‐0.17; 95% CI ‐0.31 to ‐0.03; Coffman 2008). In Coffman 2008, the reduced odds of ED visits was just outside of statistical significance (odds ratio (OR) 0.78; 95% CI 0.61 to 1.01). The other primary outcome in the current review (exacerbations requiring a course of oral corticosteroids) was not previously reported, but hospital admissions were shown to decrease in two reviews (Boyd 2009 and Coffman 2008), quality of life scores improved in one review (mean difference (MD) 0.13, 95% 0.73 to 0.99; Boyd 2009) and days of restricted activity decreased in another (SMD ‐0.29; 95% CI ‐0.49 to ‐0.08; Wolf 2002). To reiterate an earlier point, these reviews included a heterogenous group of educational interventions. It is unclear which components of the programmes contributed to the significant benefits observed.
These previous reviews also examined interventions conducted within a wide range of settings. Coffman 2008 observed favourable results in trials where comprehensive education occurred in the clinical setting. They hypothesised that this may have permitted access to medical charts and information, allowing for a more individualised intervention. There may also be more support for school‐based asthma education and a future Cochrane Review will address this question.
Authors' conclusions
Implications for practice.
We included 2342 patients from 12 trials in this review, most of which involved a vulnerable urban population of North American children with asthma hypothesised to benefit from home‐based asthma educational interventions. We were unable to pool many of the outcomes due to clinical heterogeneity. Overall the effect of home‐based education is heavily dependent on the context (aims, control group event rate, type and intensity of education etc.) of that education so trying to derive an average outcome across the studies is not meaningful. We found inconsistent evidence for home‐based asthma educational interventions compared to standard care, education delivered outside of the home or a less intensive educational intervention delivered at home. Although education remains a key component of managing asthma in children, advocated in numerous guidelines, our review does not contribute further information on the fundamental content and optimum setting for such educational interventions. We cannot rule out the possibility that home‐based education may be beneficial under some circumstances, where resources and funding permit.
Implications for research.
Asthma self management education is recommended in guidelines and we did not find evidence to either support or reject the notion that home‐based education may be beneficial. There are still important questions that could benefit from well‐designed trials to address the following issues:
-
Defining the exact components of education that are linked with improved asthma knowledge and outcomes. This will involve addressing the heterogeneity of trials and may involve:
comparing similar programmes or programmes with slight variations (for example, with differences in duration, number of visits, types of educators, length of follow‐up);
designing trials with multiple intervention arms to tease out which components of education are effective;
standardising reporting of outcomes ‐ for example, reporting a minimum set of outcomes (such as those specified in this review) and using the same validated quality of life questionnaire(s).
Cost‐effectiveness: while cost data were not well‐reported in the included trials, further work is needed to establish whether the extra costs of delivering education in homes ties in with a reduction in costly outcomes such as acute health care visits. Demonstration of cost‐effectiveness, along with clinical effectiveness, would support the widespread adoption of such programmes by health systems financing them.
What's new
| Date | Event | Description |
|---|---|---|
| 5 September 2014 | Amended | PLS title amended. Reference to withdrawn protocol removed |
History
Protocol first published: Issue 4, 2010 Review first published: Issue 10, 2011
| Date | Event | Description |
|---|---|---|
| 11 April 2013 | Amended | NIHR acknowledgement added |
Acknowledgements
We would like to thank Susan Hansen who helped design the search strategy, Liz Stovold for additional searches and the editors and peer referees who provided helpful comments. We would like to thank Dr Butz and Dr Gorelick for providing additional information and Dr Galbreath and Dr Seid for providing copies of their publications in print.
CRG Funding Acknowledgement: The National Institute for Health Research (NIHR) is the largest single funder of the Cochrane Airways Group.
Disclaimer: The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the NIHR, NHS or the Department of Health.
Appendices
Appendix 1. Calculation of number of ED visits for Mitchell 1986
Intervention group
Europeans: 83 (total) x 48% (asthma attack not responding to Tx at home) x 34% Tx @ hospital = 14 Polynesians: 50 (total) x 49% (asthma attack not responding to Tx at home) x 47% Tx @ hospital = 12
Control group
Europeans: 81 (total) x 46% (asthma attack not responding to Tx at home) x 11% Tx @ hospital = 4 Polynesians: 45 (total) x 44% (asthma attack not responding to Tx at home) x 30% Tx @ hospital = 6
Data and analyses
Comparison 1. Education versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Exacerbations leading to emergency department visits | 8 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 ED visits between baseline and 6 months | 5 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 ED visits between 3 and 9 months from baseline | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 ED visits between baseline and 2 years | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Mean exacerbations resulting in ED visits | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 mean exacerbations between baseline and 6 months | 3 | 430 | Mean Difference (IV, Fixed, 95% CI) | 0.04 [‐0.20, 0.27] |
| 2.2 mean exacerbations between 12 and 18 months from baseline | 2 | 250 | Mean Difference (IV, Fixed, 95% CI) | ‐0.32 [‐0.74, 0.10] |
| 3 Mean exacerbations requiring a course of oral corticosteroids | 2 | 500 | Mean Difference (IV, Fixed, 95% CI) | ‐0.31 [‐0.70, 0.07] |
| 3.1 Mean exacerbations between baseline and 6 months | 2 | 250 | Mean Difference (IV, Fixed, 95% CI) | ‐0.18 [‐0.63, 0.26] |
| 3.2 Mean exacerbations between 12 and 18 months from baseline | 2 | 250 | Mean Difference (IV, Fixed, 95% CI) | ‐0.72 [‐1.51, 0.07] |
| 4 Days missed from school or work | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.1 Mean days off school in 6 months | 2 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Exacerbations leading to hospitalisation | 7 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.1 Admission in 6 months from baseline | 4 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Hospitalisation between 3 and 9 months from baseline | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.3 Admissions between 6 and 18 months from baseline | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.4 Admissions between 12 and 18 months from baseline | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.5 Admissions between baseline and 2 years | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Mean exacerbations leading to hospitalisation | 3 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.1 Annual exacerbation rate | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Mean admissions over 6 months since baseline | 2 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.3 Mean exacerbations between 6 and 18 months | 2 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 2. Education versus other home‐based education.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Exacerbations leading to ED visits | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Mean exacerbations requiring a course of oral corticosteroids | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Exacerbations leading to hospitalisation | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Mean hospital admissions | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 Admissions between 6 and 12 months follow‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Brown 2002.
| Methods | Study design: parallel, randomised, controlled trial Recruitment setting: clinics associated with a University School of Medicine or a children's hospital in Atlanta, Georgia, USA Study duration and start date: 12 months, September 1997 to June 1999 |
|
| Participants | N (completed) = 101 (98) Mean age (range): 4.2 (1.1 to 7.0) Gender (% male): 63% Asthma severity: mild intermittent (19%), mild persistent (56%), moderate persistent (21%), severe persistent (4%) Diagnostic criteria: NAEPP Concurrent treatment: 85% had been previously prescribed one of more daily anti‐inflammatories (cromolyn (43%), ICS (78%), antileukotriene modifier (6%), LABA (6%). In addition, some children had been prescribed theophylline (8%) and/or a brief course of OCS (26%) and all but one of the children had been prescribed SABA (98%, 16% were using SABA only) Socioeconomic indicators: parents received no high school (28%), high school qualification (50%), some college (22%) Ethnicity: 90% African American Eligibility criteria: children between 1 and 6.99 years of age at study entry and had made a healthcare visit for asthma in the preceding year and who were prescribed daily asthma medication. The primary care giver had to speak English and have no know involvement with illegal drugs. Those who refused or could not be contacted were excluded. |
|
| Interventions | Educator: registered nurses trained in the Wee Wheezers at Home programme. Nurses attended supervisory sessions twice a month and focused on their cases and received ongoing training. The same nurse conducted all 8 sessions with a family. Audience: families (86% mothers) and their children. Others present in the household were also invited to participate Where delivered: home‐based INTERVENTION GROUP N (completed): 55 (49) N, duration and frequency of education sessions: 8 weekly sessions of 90 minutes Educational/self management strategy: Based on Wee Wheezers at Home programme. The teaching script of which was adapted for low‐literacy levels (5th grade) and adapting for child audience and ensuring cultural appropriateness of materials. The material was delivered over 8 sessions rather than 4. Each 90‐minute session consisted of the caregiver and nurse jointly completing a checklist of the child's symptoms for the previous week (5 minutes), a discussion of the previous weeks homework (5 minutes), the session topics (60 minutes), a review of concepts learned during that session (5 minutes) and assigning homework (5 minutes). Example of caregiver/child activities include tracing the airflow on a picture of a child with the lungs drawn, identifying and colouring asthma cues and environmental triggers in a colouring book, practising belly breathing, keeping an asthma diary, watching videos about asthma management and practicing the use of a peak flow meter. Educational materials used/provided: printed materials, homework and occasional videotapes Programme topics: session 1) basic concepts of asthma; 2) developmentally appropriate involvement of child in asthma self management plan and asthma cues; 3) asthma medication and non‐medication techniques for managing asthma symptoms as part of action plan and working together with child to administer medicines; 4) symptoms of asthma attacks, review of asthma action plan, children with chronic health problems; 5) symptom prevention including trigger identification, environmental control measures and use of preventative medication; 6) communication about asthma to teachers, physicians and family members; 7) review of asthma concepts; 8) review of communication about asthma. Incentives: at the baseline visit, parents gave consent and received USD 25 and were given USD 25 for each of 2 further data collection visits Compliance: 20% of the 49 families who completed the education received some or no lessons CONTROL GROUP: received no education but did receive the data collection visits. Families of control group were offered one educational home visit after completion of data collection. N (completed): 46 (46) Net treatment: complete education programme |
|
| Outcomes | Outcomes measured: asthma morbidity: a rating of how much patents were bothered by symptoms, the number of symptom‐free days since most recent asthma visit, the number of acute visits for asthma exacerbations. Caregivers quality of life: measured by questionnaire on how much caregiver was bothered or worried by their child's symptoms. Caregivers' rating of the level of child's participation in administering asthma medication and symptoms prevention and treatment. Outcomes reported: as stated Time points: baseline, 3 (actually about 19 weeks) and 12 months. The number of acute asthma visits were collected fore the previous year at baseline and 12 months. |
|
| Notes | Funding: National Institute of Nursing Research grant R01NR04431 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomised". Groups balanced by medical site and season of enrolment. Comment: not described |
| Allocation concealment (selection bias) | Unclear risk | "Families were informed of their group assignment via a letter" Comment: unclear who assigned participants to their groups |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | It was not possible to blind participants or personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | A graduate student blind to group assignment abstracted asthma‐related information from the child's medical records. Project social worker collected data from families was blinded, although may have become aware of group assignment in certain instances. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | One control group family and 3 intervention families could not be contacted at 3 months and two control families could not be contacted at 12 months. Six families withdrew on assignment to treatment group and this can be assumed to be related to the treatment. Comment: only have outcome data for the 49/55 families who completed the intervention. Complete data for all the control patients. |
| Selective reporting (reporting bias) | Unclear risk | No protocol published, but assume all of the measured outcomes reported |
| Other bias | Low risk | Observational used over self reports where possible |
Brown 2006.
| Methods | Study design: parallel, randomised, controlled trial Recruitment setting: community hospital Grand Rapids, USA Study duration and start date: 2004 |
|
| Participants | N (completed) = 248 (239) adults and children; 137 (129) children Mean age (range): adults and children, but data were presented separately for those under 18 years Gender (% male): 46% Asthma severity: mild intermittent (23%), mild persistent (21%), moderate persistent (19), severe persistent (37%) Diagnostic criteria: NHLIB Expert Panel 2 Guidelines or had visited the ED at least once in the last year (71%) Concurrent treatment: 80% on inhaled corticosteroids Socioeconomic indicators: no high school diploma ˜18%, high school qualification ˜ 32%, some college ˜50% Ethnicity: 30% African American, 59% white, 11% other Eligibility criteria: moderate to severe persistent asthma or had visited the ED at least once in the last year. Although moderate to severe asthma was an eligibility criteria, patients with mild intermittent of mild persistent were also included. |
|
| Interventions | Educator: trained asthma nurse educator Audience: parent and child Where delivered: primary care clinic/home INTERVENTION GROUP N (completed): 120 (117, 66 children) N, duration and frequency of education sessions: 1 session in primary care clinic within 3 weeks of initial ED visit, 1 session at home 6 weeks after first session. Educational/self management strategy: 1) optimising medical therapy based on NHLBI guidelines; 2) optimising understanding of asthma management and control by stressing self evaluation and monitoring; 3) developing or refining individually tailored AMP; 4) conducting follow‐up home visit to identify potential asthma triggers and reinforce recent changes in treatment and management Programme topics: before ED discharge patients received age‐appropriate instruction from respiratory therapist on the use of inhaler/spacer/PEF meter from the asthma nurse educator who called to arrange a follow‐up appointment with the primary care physician within 5 days. At the clinic appointment, patient, parent and asthma nurse educator worked with the primary care physician to review current treatment, develop written action plan and provide education about appropriate response to further asthma exacerbations. The home visit included review of current medication and inhaler, spacer and PEF meter techniques, asthma management plan and encouraged distribution of the plan to school, day‐care etc. Basic education relating to triggers, early warning signs and prevention was also given, along with an in‐home environmental evaluation. Incentives: 2 USD 10 grocery vouchers Compliance: 39% of the intervention group did not comply with any of the post ED activities CONTROL GROUP: standard management consistent with NHLBI 2 guidelines. Received instruction by respiratory therapist on proper use of inhaler and spacer, and if age appropriate, peak expiratory flow meter. Written discharge instructions including recommendation to contact PCP within 3 to 5 days to schedule follow‐up appointment. If no regular PCP, referral made using hospital‐affiliated paediatric clinic for children. ED physician dictation faxed to PCP. N (completed): 128 (122, 63 children) Net treatment: appointment with primary care provider facilitated by the nurse educator and home visit |
|
| Outcomes | Outcomes measured: primary outcome was time to first asthma relapse (asthma‐related visit to ED or unscheduled urgent visit to physician office during 6‐month follow‐up period); secondary outcomes were total number of ED visits and hospitalisations during 6 months, self reported compliance with spacer and PEF, use of asthma management plan, self reported actions taken to reduce exposure to asthma triggers, missed work or school days Outcomes reported: as above Time points: follow‐up data collected by telephone call at 2 and 6 months after enrolment |
|
| Notes | Funding: grant from the Centers for disease control and the Butterworth Foundation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were stratified by age and "randomised using computer‐generated random numbers..." Patients were enrolled consecutively from selected ED shifts representing a broad range of time of day and day of week |
| Allocation concealment (selection bias) | Low risk | "... followed by the use of sealed opaque envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not possible to blind patients or educators |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Loss to follow‐up was 21% in group and 6% in the control arm. Comment: the loss to follow‐up was high and unbalanced, and there were more losses in the intervention arm which is related to the treatment and people's willingness to comply |
| Selective reporting (reporting bias) | Unclear risk | No protocol published, but assume all of the measured outcomes reported |
| Other bias | High risk | ED visits and hospitalisations were self reported |
Butz 2006.
| Methods | Study design: parallel, randomised, controlled trial Recruitment setting: pediatric primary care (30%), pulmonary/allergy clinics (50%) and ED practices (20%) associated with the University of Maryland medical System and the Johns Hopkins Hospital, Baltimore, USA Study duration and start date: October 2001 to December 2003 (recruitment) |
|
| Participants | N (completed) = 221 (181) Mean age (range): 4.6 (2 to 9) Gender (% male): 65% Asthma severity: mild intermittent (5%), mild persistent (61%), moderate persistent (21%), severe persistent (14%) Diagnostic criteria: national guidelines Concurrent treatment: number of SABA prescriptions in the past 6 months from baseline from pharmacy data mean (SD) 1.8 (1.9), Number of OCS prescriptions in the past 6 months from baseline from pharmacy data mean (SD) 0.6 (0.9), number of ICS prescriptions in the past 6 months from baseline from pharmacy data mean (SD) 0.9 (1.4) Baseline lung function: Socioeconomic indicators: no high school diploma (24%), high school qualification (39%), some college or trade school or college graduate (38%). Annual household income less than USD 20,000 48%, more than or equal to USD 20,000 40% (sic). Medicaid health insurance 80%. Ethnicity: African American 89%, other 11% Eligibility criteria: children resident in Baltimore aged 2 to 9 years with a previous medical diagnosis of asthma. Children should have experienced daytime asthma symptom at least 2 or more times a week within the past 30 days, night‐time asthma symptom at least 2 or more times a week within the past 30 days, use of a nebuliser to administer asthma medication within the past 30 days, 1 or more ED visits for asthma within the past 12 months or hospitalisation for asthma in the past 12 months. Exclusion criteria were low or no nebuliser use in the prior 30 days and children newly diagnosed as having asthma. |
|
| Interventions | Educator: 3 community health nurses with paediatric asthma training. Supervised monthly by a paediatric nurse asthma specialist. Audience: parents and child Where delivered: home NEBULISER INTERVENTION GROUP N (completed): 110 (95) N, duration and frequency of education sessions: 6 x 1‐hour sessions over 6 months Educational/self management strategy: The parent component of the educational intervention included teaching comparison of a child's normal breathing to breathing patterns noted during an acute asthma episode. Parents taught to recognise each asthma symptoms (cough, wheeze, inability to talk and signs including intercostal retractions and use of a PFM in children over 5 years of age, so that they could make accurate treatment decisions. Specific nebuliser‐use education targeted accurate medication dispensing including measuring accurate amount of medication, pouring medication in nebuliser cup, the frequency of changing nebuliser mask and tubing, and the cleaning and maintenance of the nebuliser device. The programme was based on the Wee Wheezers Program and the A+ Asthma Club Program and teaching paediatric symptom identification in children with asthma, and recommendations for nebuliser therapy Educational materials used/provided: home visit checklist used by nurse (available in the public health nursing paper) Compliance: number of sessions attended mean (SD): 5.6 (1.2) STANDARD ASTHMA EDUCATION CONTROL GROUP: a less intensive intervention group. Received basic asthma education, comparable to education received during non‐urgent care visits. Facilitating access to acute asthma care, encouraging parents to obtain WAP from healthcare provider, addressed dose and frequency of current medication, teaching the use of a PFM to children over 5 years old. No symptom identification or nebuliser use was taught. 3 asthma education visits Mean (SD) of completed home visits: 2.9 (0.5) N (completed): 111 (86) Net treatment: specific quick relief and controller medication use, symptom identification and nebuliser use technique |
|
| Outcomes | Outcomes measured: symptom frequency, appropriate nebulizer and asthma medication use, ED visits and hospitalisations (reported as events in the past 6 months) Outcomes reported: as above Time points: baseline, 12 months |
|
| Notes | Funding: grant NR05060 from the National Institute for Nursing Research Further information on education available from study authors: yes |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised "based on even or odd digits from a random digit list" |
| Allocation concealment (selection bias) | Low risk | Project co‐ordinator and principal investigator did not order of allocation (email communication with author) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not possible to blind patients or educators |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Project co‐ordinator and co‐investigators all blinded (email communication with author) |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Nebuliser intervention: 14% excluded from follow‐up (10% no pharmacy data, 2% died, 3% lost). SAE 23% excluded (18% no pharmacy data, 1% died, 4% lost. |
| Selective reporting (reporting bias) | Unclear risk | No protocol published, but assume all of the measured outcomes reported |
| Other bias | High risk | Self reports were verified except for hospitalisations |
Butz 2010.
| Methods | Study design: parallel, randomised, controlled trial Recruitment setting: paediatric ED (72%), paediatric community practices (28%), Johns Hopkins Hospital, Baltimore, USA Study duration and start date: December 2004 to December 2006 (recruitment) |
|
| Participants | N (completed) = 231(193) Mean age (range): 8.02 (6 to 12) Gender (% male): 60.6% Asthma severity: mild intermittent (22.6%), mild persistent (48.3%), moderate persistent (16.1%), severe persistent (13.0%) Diagnostic criteria: NHLBI Concurrent treatment: controller medication use (68%), SABA canister equivalents in past 12 months (mean 2.61; SD 2.8), Oral corticosteroid fills past 12 months (mean 1.17; SD 1.5), inhaled corticosteroid canisters past 12 months (mean 1.82; SD 2.6), LABA past 12 months (mean 0.44; SD 1.5), ratio controller to total asthma medications fills for 12 months (mean 0.41; SD 0.3) Baseline lung function: Socioeconomic indicators: income < USD 20,000 (57.1%); income >= USD 20,000 (42.9%); caregiver education < high school graduate (32.0%); high school graduate or more education (68.0%) Ethnicity: African American (92.6%), white (3.5%), other/missing (3.9%) Eligibility criteria: children 6 to 12 years old with physician‐diagnosed asthma, currently used controller and/or SABA medication, with one or more asthma ED visits or hospitalisation in preceding year and no specialty care within past year. |
|
| Interventions | Educator: trained nurse/health educator Audience: parents and child Where delivered: home ASTHMA COMMUNICATION INTERVENTION N (completed): 121 (100). Mean 3.29 (SD 1.2) out of 4 visits. N, duration and frequency of education sessions: 4 home visits x 30 to 45 minutes each, over 8 weeks Educational/self management strategy: communication skills education (role play, cue cards for enhanced communication with clinician), assistance in arranging clinician appointments, reinforcement of medication device technique. Also received control group education intervention (see below) Educational materials used/provided: written asthma educational materials (same as control) as well as one‐page cue card to enhance caregiver to clinician communication Compliance: mean 3.29 (SD 1.2) out of 4 home visits; 60% had 1 clinic visit, 27% had 2 clinic visits STANDARD ASTHMA EDUCATION CONTROL GROUP: 3 asthma education visits, each 30 minutes over 8 weeks. Teaching topics: asthma triggers, medications, standard device training for peak‐flow meter and inhaler/spacer technique, reducing barriers to regular follow‐up asthma care. Families received written educational materials. No home environment assessment performed. N (completed): 110 (93). Mean 2.27 (SD 1.1) out of 3 visits. Net treatment: asthma communication education (communication skills education, assistance in arranging clinician appointments, reinforcement of medication device technique with child) |
|
| Outcomes | Outcomes measured: morbidity measures (caregiver reported symptom days and nights over past 30 days, asthma severity using symptoms and rescue medication frequency algorithm from NHLBI, activity limitation from asthma, number of ED and clinician visits, number of hospitalisations), pharmacy‐based medication use (appropriate controller and SABA medications based on pharmacy records over 12 months, oral corticosteroid prescription fills, appropriate controller med use (ratio of controller:total asthma medications)), caregiver rating of communication with PCP (4‐item, 5‐point Likert‐type scale), and characteristics of home and clinician visits (checklists for completed home visits and psychosocial issues; for intervention group, checklists used to examine content of communication between clinician and caregiver) Outcomes reported: as above Time points: baseline, 12 months |
|
| Notes | Funding: grant NR008544 from the National Institute for Nursing Research | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random digits generated by STATA (email communication with Dr. Butz) |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not possible to blind patients or educators |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Research staff remained blinded for all follow‐up surveys |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat analysis; ‐intervention 83% lost to follow‐up and control 86% lost to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | No protocol published, but assume all of the measured outcomes reported |
| Other bias | Low risk | Although most health care utilisation outcome self reported, ED and clinician visits verified by child’s clinician |
Dolinar 2000.
| Methods | Study design: parallel, randomised, controlled trial Recruitment setting: paediatric outpatient office in Sudbury, Ontario, Canada Study duration and start date: |
|
| Participants | N (completed) = 40 families with 56 children. Intervention delivered to parents. Mean age (range): 5 years (1 to 10) Gender (% male): 43% Asthma severity: collected, but not reported Socioeconomic indicators: well‐supported financially and most families had 2 parents. 67% earned over CAD 40,000 Ethnicity: Eligibility criteria: Parents with at least 1 child, 10 years old or younger with a diagnosis of asthma for greater than 6 months. Parents must have responsibility for the management of the child's asthma, no previous participation in asthma health education programme, avoid any other education programme, and be able to read, write and communicate in English |
|
| Interventions | Educator: principal investigator of the project Audience: parents Where delivered: home‐based INTERVENTION GROUP N (completed): 20 (18) families N, duration and frequency of education sessions: a single, 2‐hour session Educational/self management strategy: education session based on the Air Force Asthma Program Educational materials used/provided: childhood asthma education booklet representing conventional care Programme topics: provides information on asthma and decreases concerns related to the care of a child with asthma. Reinforcement of rational for therapy, compliance and follow‐up. Assist the parent in the day‐to‐day management of their child’s asthma and smoking cessation and alternative coping strategies to enhance respiratory health. CONTROL GROUP: received childhood asthma education booklet representing conventional care. The content of the education was the same for both groups, but the mode of delivery varied. N (completed): 20 (17) families Net treatment: |
|
| Outcomes | Outcomes measured: parental coping measured by Hymovich’s Parent Perception Inventory (PPI), quality of life measured by the Paediatric Asthma Caregiver Quality of Life Questionnaire (PACQLQ) and change in asthma measured by the Caregiver Perception of Change (CPC) survey Outcomes reported: final values not reported apart from for caregiver perception of change Time points: 3‐month follow‐up |
|
| Notes | Cost: no detailed costing done, but asthma educator costs CAD 30 to 34 per hour inclusive of transport costs Funding: Ontario Lung Association awarded a fellowship to fund project |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Families were randomly assigned to one of the treatment arms using the Moses‐Oakford method, and were assigned in blocks to assure equal numbers in each group" |
| Allocation concealment (selection bias) | Unclear risk | "Consecutive asthmatic patients and their families were recruited by the office staff and referred to the researcher, unaware of the allocation schedule." Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not possible to blind patients or educators |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not described, but study described as "non‐blinded" so assumed not done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 5 families lost to follow‐up; 2 from experimental, 3 from control |
| Selective reporting (reporting bias) | Unclear risk | No protocol published, but assume all of the measured outcomes reported |
| Other bias | Low risk | Only self reports possible |
Fisher 2009.
| Methods | Study design: parallel, randomised, controlled trial Recruitment setting: St Louis Children's hospital (SLCH; hospitalised children), Missouri, USA Study duration and start date: April 1997 to March 2001 |
|
| Participants | N (completed) = 191 (189); intervention delivered to parents Mean age (range): 4.9 (2 to 8) Gender (% male): 59% Asthma severity: mean hospitalisations in the previous year; treatment 0.47 (0.86) usual care 0.49 (0.79). Mean ED visits in the previous year treatment 1.07 (1.81) usual care 0.94 (1.39). Parents rating of symptoms in the previous week of randomisation (1 = very often to 3 = never) treatment 2.28 (0.52) usual care 2.35 (0.50) Diagnostic criteria: not specified Concurrent treatment: not specified Baseline lung function: not specified Socioeconomic indicators (parents' education): no high school diploma 33%, high school qualification 40%, some college 23%, college graduate 4% Ethnicity: predominantly African American Eligibility criteria: parents of children 2 to 8 years old who had been hospitalised for asthma at SLCH, received Medicaid, diagnosis of asthma made by admitting physician, resident of predominantly African American population defined by zip codes in St Louis City and county, phone number on record. Excluded if the phone was disconnected, 10 phone calls went unanswered or refusal to participate. |
|
| Interventions | Educator: 3 trained asthma educators, African American women from the same neighbourhood as the participants who were high school educated and full‐time university employees
Educators received 3 months initial training in: asthma disease process, asthma action plans, communication techniques, social support, behaviour change strategies (including Transtheoretical Model). Weekly training thereafter with supervision meetings with nurse, psychologist and expert in Transtheoretical Model. Audience: parents of children with asthma Where delivered: in the home or a ‘neutral site, i.e. local fast food restaurant’ INTERVENTION GROUP N (completed): 97 (96) N, duration and frequency of education sessions: 2 home visits and telephone calls biweekly for 3 months, then monthly for duration of 2‐year intervention Educational/self management strategy: 1st and 2nd visit was a review of 7 key asthma management behaviours (see below) with parent and assessment of their readiness to adopt them (using Transtheoretical Model). Subsequent visits consisted of problem‐solving and adoption of 7 key behaviours. Emphasis on use of asthma action plan. If child was re‐hospitalised during the study, coaches reinitiate biweekly contact with parents. Coaches also discussed general stressors such as moving residence, social service resources, housing, illness of parent and new jobs. Coaches categorised parent’s readiness to adopt management behaviours using the Transtheoretical Method, according to stage pre‐contemplation, contemplation, preparation, action or maintenance. Use of asthma action plan emphasised at all stages. Other 6 behaviours discussed in order of parent’s readiness to adopt them and individually tailored. The intervention was "implemented in a flexible manner that followed a nondirective supportive style" tailored to individual. The style was co‐operative and accepting of feelings and choices, i.e. coaches said they would call back in a few weeks to "check in with you" not "check up on you". Coaches' approach was reliable and persistent, but non‐demanding. Educational materials used/provided: not specified Programme topics: 1) use of an Asthma Action Plan; 2) administration of asthma controller medications; 3) administration of asthma‐reliever medications at first symptoms; 4) attendance at asthma monitoring visits with a primary care provider every 3 to 4 months; 5) development of a collaborative partnership with the primary care provider; 6) minimisation of exposure to second‐hand tobacco smoke; 7) minimisation of exposure to cockroach allergen Incentives: USD 10 for completing baseline survey, USD 10 for completing evaluations surveys by telephone each at 6, 12 and 18 months. USD 50 given on completion of final survey and home visit at 24 months. Compliance: 4% of patients did not participate in any education or phone calls, 86% had contacts through more than 4 of 8 quarters, and the mean number of contacts was 21 over the 2 years CONTROL GROUP: standard inpatient care pathway including asthma education and discharge planning, asthma action plan and suggested to attend a follow‐up appointment with the primary care provider within 1 week of discharge N (completed): 94 (93) Net treatment: education sessions delivered in the home or at a neutral site and telephone calls |
|
| Outcomes | Outcomes measured: hospitalisations, coaches records, telephone surveys (details not described) Outcomes reported: as above and ED visits not followed by hospitalisations Time points: hospitalisations were monitored from the hospital admissions register monthly and reported at 24 months. Telephone surveys: 0, 6, 12, 18, 24 months. |
|
| Notes | Cost: not specified Funding: NHLBI, NIEHS, peers for progress of the American Physicians Foundation and Eli Lily grants |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "On the basis of random numbers, under supervision by the project statistician" "Those randomized to the asthma coach group were assigned to a coach based on openings in their case load" |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not possible to blind patients or educators |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Those abstracting hospitalization data from charts were blind to condition and to the nature of the comparison between coaching and usual care" "...survey workers who were blinded to condition conducted computer‐assisted surveys with parents by telephone." All emergency care and hospitalisations due to asthma were scanned electronically using case numbers, addresses, names of guardians/children and child's date of birth |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Two withdrawals due to transfer of child custody "St Louis has a second paediatric hospital, Cardinal Glennon Children’s Hospital but its records were not reviewed" "it is assumed that in the study cohort more than 95% of readmissions would be to SLCH, providing nearly complete ascertainment of all readmissions among study participants" Comment: there might have been hospitalisations in the other hospital that have gone unrecorded. The authors discuss the possibility that the true figure may be lower. |
| Selective reporting (reporting bias) | Unclear risk | No protocol published, but assume all of the measured outcomes reported |
| Other bias | Unclear risk | Same rational as above regarding incomplete capture of hospitalisations with SLCH records only |
Galbreath 2008.
| Methods | Study design: parallel, randomised, controlled trial. STAMP trial Recruitment setting: 7 centres in Texas, USA Study duration and start date: August 2003 to May 2006 |
|
| Participants | N (completed) = 473 (301) Mean age (range): ˜9.5 (5 to 17). Adults and children were enrolled in this study, but data were presented separately Gender (% male): 59% Asthma severity: mild intermittent 6.7%; mild persistent 28.8%; moderate persistent 33.6%; severe persistent 30.9% Diagnostic criteria: NAEPP and GINA 2002 Concurrent treatment: patents on short‐acting beta2‐agonists (95%); long‐acting beta2‐agonists (38%); oral corticosteroids (5%); theophylline (< 1%); leukotriene inhibitors (41%); ICS alone (32%) Baseline lung function: FEV1 %predicted control group 97.4 (15.9); AM group 97 (19.5); ADM 100.3 (21.5) Socioeconomic indicators: Medicaid or SCHIP (56%); enrolled in indigent programme (2%); private insurance (36%); uninsured (6%) Ethnicity: black/other 17%; Caucasian 15%; Hispanic 68% Eligibility criteria: age 5 to 64 years with a physician diagnosis of asthma and access to telephone, access to a primary care provider (those without were provided telephone numbers for suitable clinics) AND one or more of the following: One hospitalisation, emergency department visit with a diagnosis of asthma within the previous 12 months or 4 or more office visits with a diagnosis of asthma within the previous 12 months or 6 or more canisters of inhaled beta2‐agonist in the preceding 12 months or physician diagnosis of moderate to severe persistent asthma based on symptoms and/or pulmonary function testing. Exclusion criteria Other lung diseases with a possible reactive component, any disease other than asthma requiring long‐term systemic corticosteroids, enrolment in any other asthma disease management programme, plan to move out of local area within the next 18 months. |
|
| Interventions | Educator: trained programme nurse Audience: There were 2 intervention groups of different intensity Intervention group: augmented disease management. Telephone disease management plus home visits. N (completed): 157 (94) Where delivered: telephone calls to child's home and home visits N, duration and frequency of education sessions: 6 or 7 telephone calls focusing on disease management and 4 home visits at 1, 2, 3 and 6 months Educational/self management strategy: telephone call 1. Evaluate existing self management strategy, health status and educational needs. Provide individual advice and training and developed written action plan. Subsequent calls reviewed written action plan and provided more advice and training. 24‐hour hotline available which patients were encouraged to call if they experienced symptoms. Programme nurses faxed reports/recommendations to primary care provider who ultimately directed care. Patients also received 4 home visits from a pulmonary therapist. A locally developed programme structure around a national guideline based list of education topics. Provided hands‐on instruction in use of equipment, reviewed an encouraged individualised asthma action plan and conducted a home environmental evaluation. Educational materials used/provided: an asthma action plan was provided if the child did not already have one Compliance: 70% completed at least 80% of the programme ‐ although these data were for adults and children CONTROL GROUP: routine care. Spacers and peak flow meters were made available to control group on request N (completed): 159 (2) |
|
| Outcomes | Outcomes measured: primary outcomes: time to first asthma‐related emergency department visit or inpatient hospitalisation, AQLQ/PAQLQ overall score, and rates of asthma‐related utilisation (inpatient admissions, ED visits and urgent office visits for asthma), respectively. Secondary outcomes: rate of initiation of controller medications, number of oral corticosteroid bursts prescribed during office visits, asthma symptom scores, and number of school days missed
Symptoms measured on the Lara Asthma Symptom Scale and quality of life measure on PAQLQ and PACQLQ Outcomes reported: although "asthma symptom diaries" was in the initial protocol, authors decided not to use these pre‐enrolment due to concerns over feasibility, reliability and validity Time points: 6 and 12 months. HCU outcomes collected through medical record review and telephone calls at 2‐monthly intervals. |
|
| Notes | Cost: the telephone‐only programme cost USD 531 per patient, and the augmented programme that included home visits cost USD 737 per patient Funding: Grant from US department of health and human services and the CDC There was also an adult arm of the trial where the same education was conducted in patents of 18 to 64 years of age |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Individuals who met inclusion criteria and signed a consent form were randomly assigned to 1 of the 3 study groups, using a sequence of randomly permuted blocks generated with the statistical package Stata." |
| Allocation concealment (selection bias) | Low risk | "The randomisation sequence was transferred to a series of consecutively numbered, sealed cardboard randomisation boxes that contained a printed sheet in English and Spanish describing the participant's study group assignment as well as a holding chamber (spacer) for those randomly assigned to either of the intervention groups." "Boxes were packaged so that blinded research staff could not identify the group assignment from the sound or weight of the box. Non blinded study coordinators were available to answer participant questions about the study". |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not possible to blind patients or educators |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Data collected by trained research staff blinded to intervention group. Medical record abstraction was performed by trained study staff who were blinded to study group |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "All participants were telephonically polled regarding healthcare utilization every 2 months throughout the trial. Study staff requested medical records for both inpatient and outpatient encounters from all identified providers and healthcare facilities for the entire duration of a participant's enrolment in the trial." "comprehensive healthcare utilization data were obtained for 99% of participants" |
| Selective reporting (reporting bias) | Unclear risk | "Our initial protocol used symptom‐free days as 1 of the primary outcomes. However, before enrolment of study patients, we made a decision not to use asthma symptom diaries, based on concerns about feasibility, reliability, and validity." Comment: assumed done |
| Other bias | Low risk | Comprehensive healthcare utilisation data collected through the use of medical record review |
Gorelick 2006.
| Methods | Study design: 3‐arm parallel, randomised, controlled trial Recruitment setting: Children's Hospital of Wisconsin ED Study duration and start date: February 2003 to May 2004 |
|
| Participants | N (completed) = 234 (180) Mean age (range): 6.8 years (study criteria 2 to 17 years) Gender (male): 66% Asthma severity: mild intermittent 30%; mild persistent 28%; moderate persistent 28%; severe persistent 14% Diagnostic criteria: NAEPP (1997) Concurrent treatment (uses controller medications): 60% Socioeconomic indicators: 60% public insurance Ethnicity: black 69%; white 21%; Latino 8% Eligibility criteria: residents of Wisconsin, aged 2 to 18 years of age, treated at the ED for acute asthma (defined as wheezing or respiratory distress treated with at least 1 inhaled bronchodilator treatment in a patient with physician‐diagnosed asthma or history of wheezing treated with beta‐agonists). Exclusions: Non‐English speaking caregivers and participants with other chronic diseases (e.g. CF, bronchopulmonary dysplasia), tracheostomy, who had previously been enrolled in the study, enrolled in ED Allies tracking system (web‐based computer database of ED visits for asthma or wheezing illnesses), or previously received care co‐ordination or case management. |
|
| Interventions | Educator: case manager (nurse or social worker) Audience: families Where delivered: home (and telephone calls) INTERVENTION GROUP (intensive primary care linkage and care co‐ordination/case management ‐ CC/CM) N (completed): 118 (81) N, duration and frequency of education sessions: 6 home sessions (average 4/patient; 1st visit 60 minutes and subsequent 30 minutes), several phone calls (average 2.3 calls/patient) Educational/self management strategy: Patients received standard education and discharge planning in ED same as control group Intensive primary care linkage: copy of ED chart and letter recommending asthma care plan faxed to PCP office. Research co‐ordinator called PCP office to notify of ED visit and inquire if follow‐up scheduled. Subjects called day after ED visit and asked if follow‐up arranged; could get assistance in making appointment (also called on days 3, 5, 7 until appointment reported); day 14 contacted again to see if follow‐up visit made; if no PCP, given list or instructed to call insurance carrier for list. CC/CM: patients then enrolled in Flight Asthma Milwaukee (FAM) Allies coalition. This programme helped co‐ordinate health and social services across different agencies and clinicians for children with asthma and their families. Patients were assigned to a nurse or social worker case manager (depending on patients' health insurance cover) who then: a) performed standardised asthma needs assessment and environmental and smoking assessments; b) identified and addressed family asthma goals by using a personalised care plan; c) provided asthma education by using the FAM Allies asthma toolkit and additional materials; and d) made referrals to community and other services as appropriate. Educational materials used/provided: FAM Allies asthma toolkit Programme topics: FAM Toolkit covers the following topics: trigger management, medications and delivery devices, self management tools (written action plan and asthma diaries, peak flow meters) Incentives: none Compliance: 72% (85/118) had at least 1 home visit; average of 4 successful visits per patient and 2 missed per patient. 69% (81/118) completed all telephone follow‐up at 1, 3 and 6 months CONTROL GROUP: Standard education and discharge planning in ED, including: Mastering Asthma (videotape) shown during ED visit, assessment and teaching of proper use of peak‐flow meter and metered‐dose inhaler with spacer device, acute asthma medications for current exacerbation, instructions to follow‐up with primary caregiver within 7 days, written asthma care plan based on chronic symptoms N (completed): 116 (99) Net treatment: intensive primary care linkage and care co‐ordination/case management | |
| Outcomes | Outcome measured: ED visits for asthma (self reported and through web‐based tracking system), number of hospitalisations for asthma, use of controller medications, Integrated Therapeutics Group Child Asthma Short Form (ITG‐CASF) quality of life score, smoking status of family and caregivers Outcome reported: ED visits for asthma (self reported and tracking system), use of controller medications, ITG‐CASF quality of life score, smoking status of family and caregivers | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated list |
| Allocation concealment (selection bias) | Low risk | Sealed, opaque envelope, sequentially numbered study packet |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not possible to blind patients or educators |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Single‐blind (person collecting data through telephone interviews was blinded) |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 69% completed in CC/CM; 85% completed in control. The 77 patients lost to follow‐up or excluded from analysis (including 2nd intervention arm of intensive primary linkage only) were similar to those completing the study with respect to age, chronic asthma severity, ED visits in previous 12 months. However, lost to follow‐up were more likely to have public insurance and be non‐white. Although the authors attempted to describe the baseline characteristics of all those lost to follow‐up or with incomplete data, there was an imbalance in the control group (15%) versus the intervention group (31%). |
| Selective reporting (reporting bias) | High risk | Hospital admissions in previous 6 months not reported although measured |
| Other bias | Low risk | Provide self reported ED visits as well as those obtained through web‐based tracking system |
Kamps 2008.
| Methods | Study design: parallel, randomised, 3‐arm controlled trial Recruitment setting: 1 university asthma allergy clinic in an urban medical centre and 1 private practice asthma allergy clinic in a suburban area in New Orleans, USA Study duration and start date: |
|
| Participants | N (completed) = 15 Withdrawals: from 20 eligible randomised children, 2 were run as pilot participants and 3 participants dropped out during treatment, so 5 were excluded from analysis Mean age (range): 9 years (7 to 12) Gender (% male): intervention 57%; control 75% Asthma severity: moderate to severe persistent Diagnostic criteria: National Heart, Lung, and Blood Institute, National Asthma Education and Prevention Program criteria Concurrent treatment: inhaled corticosteroids (beclomethasone or fluticasone) Baseline lung function FEF 25%‐75%, mean (± SD): intervention 76.4 (17.8); control 54.2 (37.5) Socioeconomic indicators: intervention: no high school diploma % mother (father) 0 (0)%, high school qualification 0 (14)%, some college 100 (86)%; control: no high school diploma 50 (57)%, high school qualification 25 (14)%, some college 25 (29)%. Household income intervention < USD 30,000 29%, USD 30,000 to 50,000 14%, > USD 50,000 57%; control < USD 30,000 62%, USD 30,000 to 50,000 13%, > USD 50,000 25% Ethnicity: 20% African American, 53% European American, 27% Hispanic American Eligibility criteria: moderate‐severe persistent asthma as determined by their physician according to NHLBI guidelines and had been prescribed an inhaled corticosteroid (beclomethasone or fluticasone). Participants should have been less than 70% adherent to medication during run‐in. Participants who were adherent to medication regimen by electronic monitoring during 2 weeks run‐in period were excluded. |
|
| Interventions | Educator: 2 licensed psychologists and 2 masters‐level graduate students in psychology. There was a manual for each session and educators completed a checklist of tasks and treatment met regularly to discuss implementation. Adherence data reviewed and discussed with children/parents at each session Audience: children and parents Where delivered: home‐based INTERVENTION GROUP N (completed): 7 (7) N, duration and frequency of education sessions: 6 weekly sessions, approximately 60 minutes in length Educational/self management strategy: standard care plus a comprehensive asthma education programme. Targeted adherence improvement strategies such as focused education, monitoring, contingency management and discipline techniques. Aimed at improving adherence to ICS. Adherence to inhaled corticosteroids was measured by MDILog (records date, time of activation of a MDI) Pulmonary function tests taken by spirometer Educational materials used/provided: 'The Clubhouse Kids Learn About Asthma' computer program Programme topics: session 1) taught about treatment through interactive computer program The Clubhouse Kids Learn About Asthma and written material covering normal lung function, physiology of asthma, medications, trigger reduction strategy; session 2) monitoring skills and adherence improvement strategies such as taking medication with regularly scheduled activities; session 3) behavioural management techniques to promote adherence; session 4) barriers to adherence for individual families and written solutions provided; session 5) adherence‐related cognitive restructuring component – children's thought related to taking their asthma medication were examined; session 6) review of adherence improvement strategies. Incentives: none Compliance: the majority of families did not attend all follow‐up sessions CONTROL GROUP: standard care plus a comprehensive asthma education programme, on topic from Air Wise Program. Six sessions of approximately 60 minutes including lung anatomy, identification of asthma triggers and prevention of asthma attacks, treatment and monitoring of symptoms. Also watched The Clubhouse kids Learn About Asthma. Parents and children learned about the importance of communication with service providers and were taught relaxation techniques and coping strategies for stress/asthma management. No targeted adherence strategies. N (completed): 8 (8) Net treatment: targeted adherence improvement strategies given, such as focused education, monitoring, contingency management and discipline techniques Adherence data reviewed with children and parents |
|
| Outcomes | Outcomes measured: adherence, pulmonary lung function Outcomes reported: reported only FEF 25%‐75% as it is the most sensitive measure Time points: adherence: measured continually by electronic device at baseline, at each of the 6 visits, 2 weeks after the intervention (called 2 month) and then each month for the following 10 months Pulmonary lung function: each week of baseline and then once a month for 12 months paediatric QoL: once during baseline and the each month for 12 months healthcare cost data collected each month for 12 months |
|
| Notes | Cost: expenses related to asthma management incurred by families over the full 12 months; intervention USD 111.63; control USD 214.43, although there was a large standard deviation for the particularly high month in the control group which may reflect a particularly high cost and skew the cost for this treatment Funding: National Institute of Child Health and Human Development Grant number HD34784 Based on the thesis of Jodie L Kamps; we did not look at the thesis in the process of writing this review |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratified by age < 9/6 and > 9.7 year prior to randomisation. "A randomisation table was developed by a statistics consultant prior to participant recruitment to assign children to a group…" |
| Allocation concealment (selection bias) | Unclear risk | "… and we assigned children to groups based on this table." |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not possible to blind patients or educators |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Very small study with poor follow‐up. However, the losses to follow‐up were balanced between arms. |
| Selective reporting (reporting bias) | Unclear risk | No protocol published, but assume all of the measured outcomes reported |
| Other bias | Low risk | Only self reports possible |
Mitchell 1986.
| Methods | Study design: parallel, randomised, controlled trial Recruitment setting: patients discharged from paediatric medical ward of Auckland Hospital for asthma Study duration and start date: April 1983 to April 1984 |
|
| Participants | N (completed) = 200 (164), European children and 168 (95) Polynesian children Mean age (range): (2 to 14) Gender (% male): Same age and sex ratio across European and Polynesian children Asthma severity: "In this study the children were having frequent attacks of asthma (an average of 13 each year, lasting on average two days), were missing an average of three and a half weeks of school because of asthma, and by the completion of the study had had an average of 5‐3 admissions to hospital for asthma." Concurrent treatment: "European children were taking a larger number of medications for asthma than Polynesians (1‐8 (1‐2) v 1‐4 (1‐3), respectively, P<0‐001), and were significantly more likely to be taking cromoglycate, inhaled steroids, and sympathomimetics." Baseline lung function: Socioeconomic indicators: European children significantly more advantaged than Polynesian children Ethnicity: either European or Polynesian Eligibility criteria: excluded if child was less than 2 years old, they lived outside of the hospital catchment area, had had a previous life‐threatening attack or they were not either Polynesian of European |
|
| Interventions | Educator: community child health nurse Audience: children and their families Where delivered: home INTERVENTION GROUP N (completed): European 83; Polynesian 50 N, duration and frequency of education sessions: 6 monthly sessions Educational/self management strategy: basic asthma management with emphasis on reducing environmental triggers and encouraging patient to visit GP rather than ED. No attempt made to influence type of treatment or follow‐up that the patient received. Educational materials used/provided: Programme topics: 1) Explanation of anatomy, pulmonary physiology, pathophysiology of lung and factors that can provoke asthma 2) description of drugs used in asthma 3) emphasis of importance of avoiding stimuli that may provoke asthma and controlling patient's environment 4) check on drug compliance and correct use of aerosols 5) encouraged to attend follow‐up clinic visit to either paediatrician at outpatient clinic or GP and to consult GP rather than A&E. Incentives: Compliance: "Of the returns, eight (6%) had no visits as the families could not be located, 35 (26%) had some but not all six of the monthly visits, and 92 (68%) had all six of the monthly visits." CONTROL GROUP: not described, assume no intervention N (completed): European 81; Polynesian 45 Net treatment: |
|
| Outcomes | Outcomes measured: self administered postal questionnaire (6 months after discharge from hospital), school absenteeism. Days off school, exacerbations leading to hospitalisation, GP and other treatment outside of the home. Outcomes reported: as above Time points: number of readmissions, duration of readmission at 6 and 18 months |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomised" |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not possible to blind patients or educators |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of those extracting data from hospital charts was not described. Self reported outcomes, except hospitalisations. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Not stated |
| Selective reporting (reporting bias) | Unclear risk | No protocol published, but assume all of the measured outcomes reported |
| Other bias | Low risk | Used hospital records to confirm readmissions and length of hospital stay |
Mitchell 1986 Europeans.
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
Mitchell 1986 Polynesians.
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
Otsuki 2009.
| Methods | Study design: 3‐arm, parallel, randomised, controlled trial Recruitment setting: Johns Hopkins Paediatric ED, Baltimore, USA Study duration and start date: |
|
| Participants | N (completed) = 250 (204) Mean age (range): 7.0 (2 to 12) Gender (% male): 62% Asthma severity: physician diagnosed asthma, 2 x ED visits or 1 x hospitalisations in the preceding year and on asthma controller medications Diagnostic criteria: physician diagnosed asthma Concurrent treatment: leukotriene modifiers (24%); inhaled corticosteroids 72% Socioeconomic indicators: Medicaid (86%); caregiver completed high school (69%); household income < USD 10,000 per year (38%) Ethnicity: 98% black Eligibility criteria: physician diagnosed asthma, 2 ED visits or one hospitalisation for asthma in the previous year, resident in Baltimore City and prescribed asthma controller medication |
|
| Interventions | Educator: trained asthma educators Audience: parent and child Where delivered: home INTERVENTION GROUP: Asthma Basic Care Group (ABC) N (completed): n = 84, 92% completed 6 month and 96% completed 18‐month surveys N, duration and frequency of education sessions: 5 x 30 to 45 minutes sessions at weeks 1, 2, 3, 4 and 8 weeks after randomisation Educational/self management strategy: 5 core components 1) review of prescribed asthma regimen and training in medication, spacer and peak flow technique; 2) development of an asthma action plan; 3) identification of barriers to accessing health care and problem‐solving to remove them; 4) discussion of beliefs and concerns about asthma and medications; 5) provision of written asthma education materials. Educational materials used/provided: written asthma education materials and asthma action plan. INTERVENTION GROUP: Adherence Monitoring with Feedback Group (AMF) N (completed): n = 83, 87% completed 6‐month and 80% completed 18‐month surveys N, duration and frequency of education sessions: Educational/self management strategy: received the ABC programme as described above plus the following: 1) objective feedback of medication adherence from an electronic adherence monitor and the asthma educator was trained to provide support in a non‐threatening way; 2) families were encouraged to set asthma control goals (e.g. no coughing at night); 3) the importance of positive reinforcement such as verbal praise and low‐cost rewards was emphasised. The educator worked with families to identify barriers when goals were not achieved; 4) families were taught strategies to monitor adherence and asthma symptoms by using behavioural charts and symptom diaries, the educator highlighted relationships between improvements in symptoms and adherence where possible. Educational materials used/provided: written asthma education materials and asthma action plan Incentives: none but parents were encouraged to provide low‐cost rewards and verbal praise as incentives for adherence CONTROL GROUP: usual care N (completed): n = 83, 92% completed 6‐month and 93% completed 18‐month surveys Compliance: 67% completed all 5 visits. Completed 4.0 and 3.8 visits on average for the ABC and AMF intervention groups respectively |
|
| Outcomes | Outcomes measured: caregiver‐reported frequency of asthma symptoms, ED visits, hospitalisation, courses of OCS, adherence to ICS and number of ICS refills Outcomes reported: as above Time points: baseline, 6, 12 and 18 months Patients were encouraged to seek care from their primary care provider |
|
| Notes | Funding: NHLBI grant | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomised" via blocked randomisation schema |
| Allocation concealment (selection bias) | Low risk | Assignment place in sealed envelopes which were opened after completion of baseline surveys |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not possible to blind patients or educators |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Research staff who conducted telephone surveys were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The numbers lost to follow‐up at each survey were balanced between treatment arms and consistently around 10% which is to be expected |
| Selective reporting (reporting bias) | Unclear risk | No protocol published, but assume all of the measured outcomes reported |
| Other bias | High risk | Although adherence was monitored both by self reports and through pharmacy records, hospitalisations and ED visits were only recorded via self reports |
Otsuki 2009 ABC.
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
Otsuki 2009 AMF.
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
Seid 2010.
| Methods | Study design: 3‐arm, parallel, randomised, controlled trial Recruitment setting: San Diego, California. Families recruited from Federally Qualified Health Centers, commercial HMO, school/daycare, local asthma initiatives, or self referred Study duration and start date:11 June 2004 to 16 October 2007 |
|
| Participants | N (completed) = 252 (211 completed at least 1 follow‐up) Mean age (range): 7.37 (2 to 14) Gender (% male): 61.1% Asthma severity: mild 27.0%, moderate 40.5%, severe 32.5% Diagnostic criteria: persistent asthma (mild, moderate, severe) as per NHLBI criteria using symptoms, activity level, exacerbations Concurrent treatment: Baseline lung function: Socioeconomic indicators: 84% recruited from Federally Qualified Health Centres (subsidised community clinics treating generally un/underinsured on sliding scale fee); most patients low income; mother's education < 6th grade 26%, 7th to 9th grade 23%, 10th to 12th grade 24%, high school graduate 8%, some college 13%, college graduate 5%, graduate/professional degree 0.4%; father's education < 6th grade 28%, 7th to 9th grade 25%, 10th to 12th grade 21%, high school graduate 8%, some college 11%, college graduate 7%, graduation/professional degree 0.5% Ethnicity: Hispanic 83% (Spanish only 56%), non‐Hispanic white 4%, non‐Hispanic black 8%, other 4% Eligibility criteria: 2 to 14 years with physician‐diagnosed persistent asthma, whose parents spoke English or Spanish |
|
| Interventions | Educator: CC = 2 bilingual, bicultural bachelor's level asthma home visitors; PST = bilingual, bicultural, master's level health educator Audience: PST = primary caregiver, although children encouraged to participate Where delivered: INTERVENTION GROUP: care co‐ordination (CC); care co‐ordination + problem‐solving skill training (CC + PST) N (completed): CC = 81 (71); PST = 84 (60) N, duration and frequency of education sessions: CC = 5, 45 to 60 minutes sessions, weekly; PST = 6, 45 to 60 minutes sessions, weekly Educational/self management strategy: CC = structured set of educational interventions with written material, based on NHLBI guidelines, Robert Wood Johnson Foundation's Allies Against Asthma community health worker model; PST = based on D'Zurilla's conceptualisation and adapted from comprehensive protocol used in previous trial of PST in mothers and children with cancer Educational materials used/provided: CC = written materials on programme topics (described below); PST = treatment manual, worksheets for each step, cartoon handouts to reinforce main ideas Programme topics: CC = what is asthma, asthma medications and devices, asthma action plan, how to recognise and respond to symptom onset, how to reduce irritants and allergens in home. Referred families when needed to existing health insurance enrolment assistance, smoking cessation, other community support services; provided PCP with summaries of interventions, updates on progress, and noting family difficulties and needs; PST = session 1 rapport building, understanding medical and social situation, presenting overview of PST curriculum, assigning first homework; session 2 review homework, introduced idea of developing alternative solutions, assigned homework (defining and evaluating options); session 3 review homework, developed action plan, assigned homework (implementing action plan); session 4 to 6 depended on outcome of actions, focusing on alternative plans if results of action plan not satisfactory to client or on additional problems if results satisfactory Compliance: treatment fidelity (percent of prescribed intervention behaviours performed) 98.4% CC, 97.5% CC + PST; intervention fidelity (percent of sessions delivered) 91.6% CC, 71.8% CC + PST; in PST group, 23.8% received no PST sessions, 52.4% received all PST sessions CONTROL GROUP: received ongoing asthma care from their place of care; after T3 follow‐up, offered CC + PST intervention N (completed): 87 (73) Net treatment: care co‐ordination +/‐ problem‐solving skills training |
|
| Outcomes | Outcomes measured: parent‐reported child generic HRQOL using PedsQL total, asthma symptoms using PedsQL asthma, utilisation (recall of ED, inpatient, urgent doctor's appointments for asthma over past 6 months at T1, 3 months at T2, 6 months at T3) Outcomes reported: PedsQL total parent, child; PedQL asthma parent, child; daytime symptoms, night‐time symptoms, ED visits, visits to hospital, unscheduled office visits Time points: baseline (T1), post‐intervention (about 3 months after baseline, T2), 6 month follow‐up (about 9 months after baseline, T3) |
|
| Notes | Cost: Funding: grant from Maternal and Child Health Bureau of the Health Resource and Services Administration; 2nd author holds copyright and trademark of PedsQL |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Blocked randomisation, stratified by site of care and disease severity; prepared randomisation lists created by statistician |
| Allocation concealment (selection bias) | Low risk | Randomisation lists concealed until intervention assignment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not possible to blind patients or educators |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Research staff blinded to intervention group administered surveys |
| Incomplete outcome data (attrition bias) All outcomes | High risk | CC intervention: 20% lost (7% refused), PST intervention 32% lost (19% refused) These are unbalanced and assumed to be due to the nature of the intervention |
| Selective reporting (reporting bias) | Unclear risk | No protocol published, but assume all of the measured outcomes reported |
| Other bias | High risk | Parent‐reported healthcare utilisation |
Seid 2010 problem solving.
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
Seid care‐coordination.
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
A&E : accident and emergency ABC : Asthma Basic Care AM: Asthma management ADM: Augmented disease management (in Galbreath, DM plus in‐home visits by a respiratory therapist), AMF: Adherence Monitoring with Feedback AMP: asthma management plan AQLQ: Asthma Quality of Life Questionnaire CC: care co‐ordination CDC: Centers for Disease Control and Prevention CF: cystic fibrosis CM: case management ED: emergency department FEV1: forced expiratory volume in one second GCSE: General Certificate of Secondary Education, academic qualification, generally taken in a number of subjects by students aged 14 to 16 in secondary education in England, Wales, and Northern Ireland GINA: Global Initiative for Asthma GP: general practitioner HCU: healthcare utilisation High School Diploma ‐ a diploma awarded for the completion of high school. In the United States a high school is an upper secondary school which educates children from grade nine (14 years old) or 10 (15) through grade 12 (17 or 18) HRQOL: health‐related quality of life ICS: inhaled corticosteroid ITT ‐ intention‐to‐treat analysis LABA: long‐acting ß2 agonist NAEPP: National Asthma Education and Prevention Program NHLBI: National Heart, Lung, and Blood Institute guidelines NIEHS: National Institute of Environmental Health Sciences NS: not stated OCS: oral corticosteroids PACQLQ: Paediatric Asthma Caregiver's Quality of Life Questionnaire PAQLQ: Paediatric Quality of Life Questionnaire PCP: primary care provider PEF: peak expiratory flow PFM: peak flow meter PST: problem‐solving skill training QoL: quality of life SABA: short‐acting ß2 agonist SAE: serious adverse event SCHIP: State Children's Health Insurance Program administered by the United States Department of Health and Human Services that matches funds to states for health insurance to families with children. Designed to cover uninsured children in families with incomes that are modest but too high to qualify for Medicaid. SD: standard deviation STAMP: South Texas Asthma Management Project WAP: written action plan
Where we report means for baseline characteristics, we took the mean across all arm where necessary
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Adgate 2008 | Focus on removing allergen through cleaning procedures |
| Agertoft 1998 | The education component was provided in the clinic and the training was using the turbuhaler at home |
| Agrawal 2005 | Patients in the intervention group were given an individualised written home‐management plan, but the standard education administered to both intervention and control groups was not delivered at home |
| Akkaya 1997 | Education delivered in an outpatient clinic |
| Aleman Mendez 1992 | No data available to extract, unsure of where education was delivered, randomisation not described and could not contact author |
| Alexander 1972 | Education delivered in groups in treatment rooms in care homes |
| Alexander 1988 | Appointments held in the allergy division in a medical centre |
| Andersen 2007 | Published as abstract only. Appears to be education delivered by the internet. Author did not confirm that there was no face to face education delivered in the home. |
| Arbes 2003 | Provided insect bait and professional cleaning |
| Arbes 2004 | Provided insect bait and professional cleaning |
| Barnes 2008 | Provided home‐cleaning |
| Bateman 2000 | Education mainly in adults. Full text not published. |
| Becker 2003 | Education delivered to small groups, i.e. outside home |
| Bender 1997 | An observational study looking at the "medical, demographic, and psychologic characteristics of asthmatic children and adults who dropped out of a year‐long medication trial" rather than a randomised controlled trial |
| Bender 2003 | Delivered in patient education centres. Study looking at reasons for attrition in CAMP trial |
| Bonner 2002 | Although this intervention included a baseline interview delivered at home and home visits and telephone calls to check compliance, the education component of this study was delivered in group sessions outside of the home |
| Bonsignore 2008 | Delivered exercise training and monitoring in a gym centre |
| Bramson 1996 | Commentary piece, not a randomised controlled trial |
| Brewin 1995 | Education delivered in the hospital |
| Brosco 2005 | This is an ongoing observational study designed to document prevalence of asthma in preschool children, identify barriers to optimal asthma care, assess clinics and improve asthma outcomes |
| Bryant 2001 | Removal of common indoor asthma triggers |
| Bryant‐Stephens 2004 | The active group received education plus environmental remediation whereas the control group received observational home visits only |
| Bryant‐Stephens 2008a | The comparison group was the same home‐based education plus environmental remediation ‐ i.e. a higher intensity intervention |
| Burkhart 2001 | Education component delivered outside of the home. Weekly telephone calls encourage adherence to peak flow monitoring |
| Burkhart 2007 | Sessions conducted in child‐friendly interview rooms at the university |
| Butz 2005a | Education delivered in workshops |
| Butz 2007 | Not delivered in the home |
| Bynum 2001 | Education delivered by video teleconference calls at local clinics |
| Callahan 2003 | Provided mattress encasings and HEPA filters |
| Callahan 2004 | Not a RCT |
| CAMP | Describes recruitment into education programme, not a RCT |
| Catov 2005 | Not a randomised controlled trial |
| Chan 2003 | Web‐based education |
| Chan 2007 | Clinic and web‐based education |
| Chiang 2004 | Group parent education sessions delivered on an outpatient basis |
| Clark 1986 | Education delivered in hospital clinics |
| Claus 2004 | Education delivered to small groups |
| Cohen 1979 | Single asthma discussion group |
| Colland 2004 | Study aimed at encouraging parents and children to recognise prodromal signs and to double the amount of medication at the first prodromal sign. Visits conducted at an outpatient clinic. |
| Cote 1997 | Participants aged over 16 |
| Dahl 1990 | Web‐based education delivered at school/home |
| Deaves 1993 | Not a randomised controlled trial |
| Delaronde 2005 | Quasi‐randomised ‐ some of the patients had a choice about which group they were allocated. Results data included adult data. |
| Donaghy 1995 | Education delivered in the clinic. Also patients in the range 13 to 50 years old. |
| Eggleston 2004 | Provided HEPA filter |
| Eggleston 2005 | Provided cockroach/rodent extermination, mattress/pillow encasings etc. |
| Evans 1999 | Education component delivered to groups |
| Finkelstein 2000 | Adults |
| Finkelstein 2002 | No education component delivered other than environmental awareness |
| Flores 2009 | Group work |
| Friedman 1999 | Assume telecommunication study is excluded. However, we could not find the full‐text article to check properly. |
| Gardida 2002 | Education delivered in schools |
| Greineder 1995 | Education delivered outside of the home |
| Greineder 1999 | Education delivered outside of the home |
| Griffiths 2004 | Education delivered outside of the home |
| Guendelman 2002 | Education delivered in the clinic, game played at home |
| Holzheimer 1998 | Education delivered outside of the home. Children were given a book to take home at the end of the intervention. |
| Homer 2000 | Computer game played in the hospital clinic |
| Homer 2005 | Education delivered to multidisciplinary teams from practices (physician, nurse and front office staff person) rather than children or parents of children with asthma |
| Horner 2004 | Education delivered in school |
| Horner 2006 | Education delivered in school |
| Horner 2008 | Education delivered in school |
| Hovell 1994 | Compared groups randomised to receive counselling to reduce a child's exposure to environmental tobacco smoke |
| Hovell 2002 | Both groups received same asthma education, but the more intense group also received counselling of the parent in reducing the child to environmental tobacco smoke. Although one could argue that this is simply a more intense form of education, we felt that the trial was asking a different clinical question than intended by our review. |
| Hughes 1991 | Study aimed at improving the home environment and reducing exposure to tobacco smoke. Also mainly delivered outside of the home. |
| Huss 2003 | Web‐based education |
| Indinnimeo 2009 | Not delivered in the home |
| Jain 1991 | Yoga training given during a hospital stay |
| Jan 2007 | Web‐based education |
| Jenkinson 1988 | Study old and not aimed at behaviour change, no separate paediatric data or outcomes useful to our review |
| Jerant 2009 | Adults |
| Jones 2001 | Aimed at reduce a child's exposure to environmental tobacco smoke. We felt that this was a sufficiently different clinical question to that being asked by this review |
| Joseph 2000 | Education delivered in a hospital meeting room |
| Joseph 2005 | Education delivered in school |
| Kamps 2004 | A commentary article |
| Karnick 2007 | Delivered at the clinic |
| Kay Bartholomew 2006 | Education delivered in school |
| Kercsmar 2006 | Received environmental remediation including a water filter and mould/damp ventilation |
| Khan 2004 | Single phone call delivered education |
| Klinnert 2005 | Trial is in infants less than 2 years old and main focus was environmental remediation |
| Kokubu 1999 | Adults |
| Kotses 1996 | Adults |
| Krieger 2009 | Provided mattress encasements |
| Krishna 2003 | Education via computer package available in consultation and waiting rooms |
| Kuijer 2007 | Adults |
| La Roche 2006 | Delivered to groups of patients at outpatient clinic |
| LeBaron 1985 | Education delivered in the office |
| Lecheler 1988 | Intervention comparing interval and continuous running training with not educational component |
| Lee 2010 | Adults ‐ via keywords |
| Letz 2004 | Education delivered in the clinic |
| Lewis 1987 | Sessions that started out as individual families and then moved into group sessions, therefore delivered outside of the home |
| Lewis 1994 | Education given in lectures |
| Li 2006 | Patients not allocated randomly |
| Lieberman 2001 | Review of video games for different diseases, some of which were asthma. Not a randomised controlled trial. |
| Linicome 2001 | Part of PAC‐PORT II trial delivering education to healthcare providers |
| Liu 2001 | Patients randomised to 3 intervention groups, however the control group was not randomised, but selected from a neighbouring hospital |
| Liu 2007 | Adults |
| Mandhane | Education delivered in school |
| Marabini 2002 | Adults |
| McCarthy 2002 | Education component delivered in groups |
| McConnell 2005 | Provided mattress encasings and trained families in cleaning |
| McGhan 2010 | Education delivered in school |
| McNabb 1985 | Education delivered in a clinic |
| McPherson 2006 | Game played at home |
| Mendes 2010 | An aerobic exercise intervention |
| Meszaros 2003 | Adults |
| Mildenhall 1997 | Adults |
| Morgan 2004 | Provided environmental remediation including mattress encasings and HEPA filters |
| Mosnaim 2008 | Education/positive messages delivered by MP3 players rather than face‐to‐face |
| Moudgil 1998 | An observational study |
| Nishioka 2006 | Not a randomised controlled trial |
| Nokela 2010 | Adults |
| O'Connor 1996 | Part of Morgan et al, NEJM, 2004. Excluded because it was an environmental intervention with little or no education. |
| Page 1999 | A RCT is not described in this paper |
| Parker 2008 | Environmental remediation given |
| Perrin 1992 | Delivered in a clinic |
| Persky 1999 | Emphasis on environmental remediation |
| Petro 2005 | Adults |
| Put 2003 | Adults and delivered outside of the home |
| Rakos 1985 | Education was in the form of a self help kit |
| Rand 2005a | Delivered in school |
| Rhee 2008 | This study aimed to help decision‐making and reduce risk by reducing substance misuse among participants |
| Ronchetti 1997 | Delivered in groups in a clinic |
| Rubin 1986 | Computer game used in the clinic |
| Schatz 2006 | Adults |
| Schmidt 1993 | Not randomised |
| Shames 2004 | Video game plus a telephone hotline |
| Shegog 2001 | Education delivered on a university campus |
| Shields 1990 | Telephone calls were reinforcement rather than education ‐ which was delivered in the ED |
| Shields 2004 | Education delivered at school |
| Slader 2006 | Adults |
| Smith 2004 | The telephone call to the parents home did not deliver education (which was administered previously in the emergency department), just encouraged and helped the parents to make a follow‐up appointment |
| Sommaruga 1995 | Adults |
| Steurer‐Stey 2010 | Adults and delivered in outpatients |
| Stevens 2002 | Education delivered in an outpatient clinic |
| Sublett 2000 | Adults |
| Sun 2010 | Adults |
| Szczepanski 2010 | Delivered in groups |
| Tagaya 2005 | No education delivered in the home. There was a booklet to take home. |
| Takaro 2004 | Emphasis on providing environmental remediation |
| Takaro 2004a | Provided environmental remediation such as mattress encasements |
| Talabere 1990 | We could not obtain separate data for the children educated at home from this thesis |
| Thoonen 2002 | Adults |
| Tong 2002 | Patients not allocated randomly |
| Tsoukleris 2007 | An observational study |
| Urek 2005 | Adults |
| Valery 2007 | Much of the education was delivered outside of the home |
| van Es 2001 | Education delivered by doctors in group/individual sessions at the clinic and additional education provided by an asthma nurse at the same visit |
| Vazquez 1993 | Delivered in the clinic |
| Walders 2006 | Education delivered in the clinic. Patients in the experimental group were granted access to an hotline where they could ask an asthma nurse for advice; this was not deemed to be an education intervention. |
| Warschburger 2003 | Education delivered to groups of parents at the clinic |
| Weiss 2003 | Education provided to physician |
| Wensley 2001 | Self management education delivered outside the home, although the participants were randomised to either symptom‐based or PFM‐based monitoring at home |
| Willems 2004 | Education component was minimal/absent |
| Williams 2006 | Environmental remediation the main focus, supplied mattress casings etc. |
| Wilson 1996a | Small group sessions therefore not in the child's home |
| Wise 2007 | Internet telehealth care intervention |
| Yoon 1989 | Adults |
| Zhao 2005 | Patients not allocated randomly |
| Zorc 2005 | Emergency department based |
CAMP: Childhood Asthma Management Program ED: Emergency Department HEPA: high‐efficiency particulate air (filter) NEJM: New England Journal of Medicine PFM: peak flow meter RCT: randomised controlled trial
Characteristics of studies awaiting assessment [ordered by study ID]
Adams 2004.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Boone 2002.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Cameron 1989.
| Methods | Booklet explained by a nurse in 4 sessions and the showing of a videotaped dramatisation of the same information |
| Participants | Intervention group N = 16 parents Control group N = 15 parents |
| Interventions | |
| Outcomes | Six‐month follow‐up |
| Notes | "Preliminary report shows that the parents of both groups had similar levels of knowledge of asthma at the initial test. On retesting at the six‐month follow‐up, the parents in both groups did significantly better than on the initial test. However, the experimental group's improvement was statistically better than that of the controls (P = 0.003). More important are the changes in attitude and behaviour implied by the higher rate of casualty visits, and the higher rate of attacks identified in cases as compared with controls. The fall in admissions among cases, while controls had a steady rate of admissions in both the year of the study and in the preceding year, has positive economic implications that are especially exciting in a developing country such as ours (AU)." |
Kelly 2005.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Mishra 2005.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Rand 2004.
| Methods | This appears to be an intervention about a mobile asthma education unit called a 'breathmobile' |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Strunk 2005.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Taylor‐Fishwick 2005.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Yang 2005.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Yildiz 2002.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Characteristics of ongoing studies [ordered by study ID]
Rand 2006.
| Trial name or title | Increasing Adherence to Asthma Medication in Urban Teens |
| Methods | Randomised, parallel, open‐label trial |
| Participants | Estimated enrolment 226 10 to 15 years |
| Interventions | Participants will be randomly assigned to 1) Self management or 2) Motivational interviewing plus self management training. The duration of the intervention condition will be 5 home visits over 2 months. Follow‐up measures will be collected from families at 3 and 6 months post‐randomisation. |
| Outcomes | Primary outcome measure: adherence to asthma controller therapy as measured by electronic medication monitoring Secondary outcome measures: number of symptom‐free days, emergency department utilisation and hospitalisation, caregiver/adolescent quality of life |
| Starting date | May 2006. Estimated completion Jan 2012. |
| Contact information | Cynthia Rand crand@jhmi.edu |
| Notes |
Contributions of authors
EJW: Screening search results, co‐ordinating review team, data extraction, data entry and drafting review.
MH: Screening ERIC search results, data extraction, commenting on draft.
PL: Screening search results, data extraction, data entry, drafting review, providing clinical input.
Sources of support
Internal sources
St George's University of London, UK.
External sources
-
NIHR, UK.
Programme grant
Declarations of interest
None known.
Edited (no change to conclusions)
References
References to studies included in this review
Brown 2002 {published data only}
- Brown JV, Bakeman R, Celano MP, Demi AS, Kobrynski L, Wilson SR. Home‐based asthma education of young low‐income children and their families. Journal of Pediatric Psychology 2002;27(8):677‐88. [DOI] [PubMed] [Google Scholar]
- Brown JV, Demi AD, Wilson SR, Lee SY, Bakeman R, Celano M, et al. A home‐based asthma education program for low‐income families and their young asthmatic children. American Journal of Respiratory and Critical Care Medicine. 2000; Vol. 161 (Suppl 3):A902.
- Brown JV, Demi AS, Celano MP, Bakeman R, Kobrynski L, Wilson SR. A home visiting asthma education program: challenges to program implementation. Health Education and Behavior 2005;32(1):42‐56. [DOI] [PubMed] [Google Scholar]
Brown 2006 {published data only}
- Brown MD, Reeves MJ, Meyerson K, Korzeniewski SJ. Randomized trial of a comprehensive asthma education program after an emergency department visit. Annals of Allergy, Asthma, and Immunology 2006;97(1):44‐51. [DOI] [PubMed] [Google Scholar]
- Reeves MJ, Brown MD, Meyerson K, Korzeniewski S. A randomized controlled trial of an asthma education program following an emergency department (ED) visit for asthma in children and adults. Annals of Allergy, Asthma, and Immunology. 2005; Vol. C47:A22. [DOI] [PubMed]
Butz 2006 {published data only}
- Butz AM, Syron L, Johnson B, Spaulding J, Walker M, Bollinger ME. Home‐based asthma self‐management education for inner city children. Public Health Nursing 2005;22(3):189‐99. [DOI] [PubMed] [Google Scholar]
- Butz AM, Tsoukleris MG, Donithan M, Hsu VD, Zuckerman I, Mudd KE, et al. Effectiveness of nebulizer use‐targeted asthma education on underserved children with asthma. Archives of Pediatrics and Adolescent Medicine 2006;160(6):662‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Butz 2010 {published data only}
- Butz A, Kub J, Donithan M, et al. Influence of caregivers and provider communication on symptom days and medication use for inner‐city children with asthma. Journal of Asthma 2010;47:478‐85. [DOI] [PubMed] [Google Scholar]
Dolinar 2000 {published data only}
- Dolinar R E. Influence of a home‐based asthma health education program on quality of life and coping in parents of children with asthma. Dissertation 1997.
- Dolinar RM, Kumar V, Coutu‐Wakulczyk G, Rowe BH. Pilot study of a home‐based asthma health education program. Patient Education and Counseling 2000;40(1):93‐102. [DOI] [PubMed] [Google Scholar]
Fisher 2009 {published data only}
- Fisher EB, Strunk RC, Highstein GR, Kelley‐Sykes R, Tarr KL, Trinkaus K, et al. A randomized controlled evaluation of the effect of community health workers on hospitalization for asthma: the asthma coach. Archives of Pediatrics and Adolescent Medicine 2009;163(3):225‐32. [DOI] [PubMed] [Google Scholar]
Galbreath 2008 {published data only}
- Freeman G. Asthma in a decentralized patient population: is traditional disease management enough?. Clinicaltrials.gov 2005:http://www.clinicaltrials.gov/ct/show/ NCT00124085.
- Galbreath AD, Smith B, Wood PR, Inscore S, Forkner E, Vazquez M, et al. Assessing the value of disease management: impact of 2 disease management strategies in an underserved asthma population. Annals of Allergy, Asthma and Immunology 2008;101(6):599‐607. [DOI] [PubMed] [Google Scholar]
Gorelick 2006 {published data only}
Kamps 2008 {published data only}
- Kamos JL. Improving adherence to inhaled corticosteroids in children with asthma. Dissertation 2002:110 p.
- Kamps JL, Rapoff MA, Roberts MC, Varela RE, Barnard M, Olson N. Improving adherence to inhaled corticosteroids in children with asthma: a pilot of a randomized clinical trial. Children's Health Care 2008;37(4):261‐77. [Google Scholar]
Mitchell 1986 {published data only}
- Mitchell EA, Ferguson V, Norwood M. Asthma education by community child health nurses. Archives of Disease in Childhood 1986;61(12):1184‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mitchell 1986 Europeans {published data only}
- Mitchell EA, Ferguson V, Norwood M. Asthma education by community child health nurses. Archives of Disease in Childhood 1986;61(12):1184‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mitchell 1986 Polynesians {published data only}
- Mitchell EA, Ferguson V, Norwood M. Asthma education by community child health nurses. Archives of Disease in Childhood 1986;61(12):1184‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Otsuki 2009 {published data only}
- Otsuki M, Eakin MN, Rand CS, Butz AM, Hsu VD, Zuckerman IH, et al. Adherence feedback to improve asthma outcomes among inner‐city children: a randomized trial. Pediatrics 2009;124(6):1513‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuki M, Kalesan B, Butz A, Rand C, Riekert K. Longitudinal evaluation of a randomized controlled trail of an adherence promoting intervention for inner‐city children. American Thoracic Society International Conference, May 18‐23, 2007, San Francisco, California, USA. 2007:A18.
- Rand CS. Adherence intervention for minority children with asthma. CRISP 2000, 1 March 2005 End date.
- Rand CS. Adherence intervention for minority children with asthma. Clinicaltrials.gov NCT00233181.
Otsuki 2009 ABC {published data only}
- Otsuki M, Eakin MN, Rand CS, Butz AM, Hsu VD, Zuckerman IH, et al. Adherence feedback to improve asthma outcomes among inner‐city children: a randomized trial. Pediatrics 2009;124(6):1513‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Otsuki 2009 AMF {published data only}
- Otsuki M, Eakin MN, Rand CS, Butz AM, Hsu VD, Zuckerman IH, et al. Adherence feedback to improve asthma outcomes among inner‐city children: a randomized trial. Pediatrics 2009;124(6):1513‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Seid 2010 {published data only}
- Seid M. Problem‐solving skills training to improve care for children with asthma. http://www.clinicaltrials.gov/ct/show/NCT00250588 2005.
- Seid M, Varni JW, Gidwani P, Gelhard LR, Slymen DJ. Problem‐solving skills training for vulnerable families of children with persistent asthma: report of a randomized trial on health‐related quality of life outcomes. Journal of Pediatric Psychology 2010;35(10):1133‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Seid 2010 problem solving {published data only}
- Seid M, Varni JW, Gidwani P, Gelhard LR, Slymen DJ. Problem‐solving skills training for vulnerable families of children with persistent asthma: report of a randomized trial on health‐related quality of life outcomes. Journal of Pediatric Psychology 2010; Vol. 35, issue 10:1133‐43. [DOI] [PMC free article] [PubMed]
Seid care‐coordination {published data only}
- Seid M, Varni JW, Gidwani P, Gelhard LR, Slymen DJ. Problem‐solving skills training for vulnerable families of children with persistent asthma: report of a randomized trial on health‐related quality of life outcomes. Journal of Pediatric Psychology 2010; Vol. 35, issue 10:1133‐43. [DOI] [PMC free article] [PubMed]
References to studies excluded from this review
Adgate 2008 {published data only}
Agertoft 1998 {published data only}
- Agertoft L, Pedersen S. Importance of training for correct Turbuhaler use in preschool children. Acta Paediatrica 1998;87(8):842‐7. [DOI] [PubMed] [Google Scholar]
Agrawal 2005 {published data only}
- Agrawal SK, Singh M, Mathew JL, Malhi P. Efficacy of an individualized written home‐management plan in the control of moderate persistent asthma: a randomized, controlled trial. Acta Paediatrica 2005;94(12):1742‐6. [DOI] [PubMed] [Google Scholar]
Akkaya 1997 {published data only}
- Akkaya E, Yilmaz A, Ece F, Bayramguler B, Baran A, Akakca A. Effects of patient education to the life quality in asthma patients: 3 years experience. European Respiratory Journal 1997;10(Suppl 25):194S. [Google Scholar]
Aleman Mendez 1992 {published data only}
- Aleman Mendez S, Sanchez Palacios A. An integrated approach to the psychological features of the asthmatic child. Allergologia et Immunopathologia 1992;20(6):240‐5. [PubMed] [Google Scholar]
Alexander 1972 {published data only}
- Alexander AB, Miklich DR, Hershkoff H. The immediate effects of systematic relaxation training on peak expiratory flow rates in asthmatic children. Psychosomatic Medicine 1972;34(5):388‐94. [DOI] [PubMed] [Google Scholar]
Alexander 1988 {published data only}
- Alexander JS, Younger RE, Cohen RM, Crawford LV. Effectiveness of a nurse‐managed program for children with chronic asthma. Journal of Pediatric Nursing 1988;3(5):312‐7. [PubMed] [Google Scholar]
Andersen 2007 {published data only}
- Andersen UM. Does a www‐based interactive computer program change asthma outcomes, quality of life and asthma knowledge. Journal of Allergy and Clinical Immunology 2007;119(Suppl 1):S9. [Google Scholar]
Arbes 2003 {published data only}
Arbes 2004 {published data only}
Barnes 2008 {published data only}
Bateman 2000 {published data only}
- Bateman ED, Kruger MJ. A computer‐based home‐monitoring disease management programme, Pulmassist Plus® (PAP), achieves significant improvement in quality of life, and healthcare costs in moderate and severe asthma. American Journal of Respiratory and Critical Care Medicine 2000;161(Suppl 3):A457. [Google Scholar]
- Bateman ED, Kruger MJ. Improvements in prescriptions and health care utilisation in patients with moderate or severe asthma enrolled in a 12 month study with the Pulmassist Plus® (PAP) disease management programme. American Thoracic Society 2000 International Conference; May 5‐10; Toronto, Canada. 2000; Vol. 161 (Suppl 3):A787.
Becker 2003 {published data only}
- Becker AB, Whitters D, Gillespie CA, Filuk SE, McColm JE, Thomas NJ, et al. Impact of a randomized asthma education program on asthma control in children. Journal of Allergy and Clinical Immunology 2003;111(Suppl 2):S212. [Google Scholar]
Bender 1997 {published data only}
- Bender BG, Ikle DN, DuHamel T, Tinkelman D. Retention of asthmatic patients in a longitudinal clinical trial. Journal of Allergy and Clinical Immunology 1997;99(2):197‐203. [DOI] [PubMed] [Google Scholar]
Bender 2003 {published data only}
- Bender B G, Ellison M C, Gleason M, Murphy J R, Sundstrom D A, Szefler S J. Minimizing attrition in a long‐term clinical trial of pediatric asthma. Annals of Allergy, Asthma, and Immunology 2003;91(2):168‐76. [DOI] [PubMed] [Google Scholar]
Bonner 2002 {published data only}
- Bonner S, Zimmerman BJ, Evans D, Irigoyen M, Resnick D, Mellins RB. An individualized intervention to improve asthma management among urban Latino and African‐American families. Journal of Asthma 2002;39(2):167‐79. [DOI] [PubMed] [Google Scholar]
Bonsignore 2008 {published data only}
- Bonsignore MR, Grutta S, Cibella F, Cuttitta G, Messineo B, Veca M, et al. Effects of 12 week aerobic training in children with mild intermittent asthma. Proceedings of the American Thoracic Society. 2006:A162 [Poster C72].
- Bonsignore MR, Grutta S, Cibella F, Scichilone N, Cuttitta G, Interrante A, et al. Effects of exercise training and montelukast in children with mild asthma. Medicine and Science in Sports and Exercise 2008;40(3):405‐12. [DOI] [PubMed] [Google Scholar]
Bramson 1996 {published data only}
- Bramson R. Self‐management of asthma. Journal of Family Practice 1996;43(1):21‐2. [PubMed] [Google Scholar]
Brewin 1995 {published data only}
- Brewin AM, Hughes JA. Effect of patient education on asthma management. British Journal of Nursing 1995;4(2):81‐2, 99‐101. [DOI] [PubMed] [Google Scholar]
Brosco 2005 {published data only}
- Brosco J P. HealthSpark 2: improving asthma care for preschool children. Clinicaltrials.gov 2005. [URL: http://www.clinicaltrials.gov/ct/show/NCT00304304]
Bryant 2001 {published data only}
- Bryant S, Tyra MD. Reducing asthma triggers in the asthmatic child's bedroom: a randomized, controlled study using lay home visitors. Annual Meeting of the American Public Health Association, Philadelphia (October 22, 2001) 2001.
Bryant‐Stephens 2004 {published data only}
- Bryant‐Stephens T. Asthma and environmental control: the results of a randomized, controlled study to remove asthma triggers from the bedrooms of children with persistent asthma. Chest 2004;126(Suppl 4):761S. [Google Scholar]
Bryant‐Stephens 2008a {published data only}
- Bryant‐Stephens T, Li Y. Outcomes of a home‐based environmental remediation for urban children with asthma. Journal of the National Medical Association 2008;100(3):306‐16. [DOI] [PubMed] [Google Scholar]
Burkhart 2001 {published data only}
- Burkhart PV. Effect of contingency management on adherence to peak flow monitoring in school‐age children with asthma. PhD Thesis, University of Pittsburgh 1996:276 p.
- Burkhart PV, Dunbar‐Jacob JM, Fireman P, Rohay J. Children's adherence to recommended asthma self‐management. Pediatric Nursing 2002;28(4):409‐14. [PubMed] [Google Scholar]
- Burkhart PV, Dunbar‐Jacob JM, Rohay JM. Accuracy of children's self‐reported adherence to treatment. Journal of Nursing Scholarship 2001;33(1):27‐32. [DOI] [PubMed] [Google Scholar]
- Burkhart PV, Rayens MK. Self‐concept and health locus of control: factors related to children's adherence to recommended asthma regimen. Pediatric Nursing 2005;31(5):404‐9. [PubMed] [Google Scholar]
- Burkhart PV, Ward HJ. Children's self‐reports of characteristics of their asthma episodes. Journal of Asthma 2003;40(8):909‐16. [DOI] [PubMed] [Google Scholar]
Burkhart 2007 {published data only}
- Burkhart P. Promoting children's adherence to asthma self‐management. Computer Retrieval of Information on Scientific Projects 2002:31 July 2004 End date.
- Burkhart PV, Rayens MK, Oakley MG, Abshire DA, Zhang M. Testing an intervention to promote children's adherence to asthma self‐management. Journal of Nursing Scholarship 2007;39(2):133‐40. [DOI] [PubMed] [Google Scholar]
- Burkhart PV, Rayens MK, Revelette WR, Ohlmann A. Improved health outcomes with peak flow monitoring for children with asthma. Journal of Asthma 2007;44(2):137‐42. [DOI] [PubMed] [Google Scholar]
Butz 2005a {published data only}
- Butz A, Pham L, Lewis L, Lewis C, Hill K, Walker J, et al. Rural children with asthma: impact of a parent and child asthma education program. Journal of Asthma 2005;42(10):813‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butz AM. Teaching children with asthma and who live in a rural setting how to self‐manage their asthma. Clinicaltrials.gov 2005:http://www.clinicaltrials.gov/ct/show/ NCT00218803.
Butz 2007 {published data only}
- Butz A. Improving asthma communication in minority families. clinicaltrials.gov 2005:http://www.clinicaltrials.gov/ct/show/ NCT00133666.
- Butz A, Land C, Walker J, Bollinger M E. Improving asthma communication with inner city parents, does it work?. Journal of Allergy and Clinical Immunology 2007;119(1 Suppl):S66 [257]. [Google Scholar]
Bynum 2001 {published data only}
- Bynum A, Hopkins D, Thomas A, Copeland N, Irwin C. The effect of telepharmacy counseling on metered‐dose inhaler technique among adolescents with asthma in rural Arkansas. Telemedicine Journal and E‐Health 2001;7(3):207‐17. [DOI] [PubMed] [Google Scholar]
Callahan 2003 {published data only}
Callahan 2004 {published data only}
- Callahan KA, Rand CS, Grant CS, Curtin‐Brosnan JM, Swartz LJ, Diette GB, et al. Providing an in‐home allergen control intervention; a comparison of behavioural changes in inner‐city homes of children with asthma [Abstract]. Journal of Allergy and Clinical Immunology 2004; Vol. 113, issue 2 Suppl:S102.
CAMP {published data only}
- Childhood Asthma Management Program Research Group. Recruitment of participants in the childhood Asthma Management Program CAMP. I. Description of methods. Journal of Asthma 1999;36(3):217‐37. [PubMed] [Google Scholar]
Catov 2005 {published data only}
- Catov JM, Marsh GM, Youk AO, Huffman VY. Asthma home teaching: two evaluation approaches. Disease Management 2005;8(3):178‐87. [DOI] [PubMed] [Google Scholar]
Chan 2003 {published data only}
- Chan DS, Callahan CW, Sheets SJ, Moreno CN, Malone FJ. An Internet‐based store‐and‐forward video home telehealth system for improving asthma outcomes in children. American Journal of Health‐System Pharmacy 2003;60(19):1976‐81. [DOI] [PubMed] [Google Scholar]
Chan 2007 {published data only}
- Callahan CW. Asthma in‐home monitoring (AIM) trial. Clinicaltrials.gov 2006:http://www.clinicaltrials.gov/ct/show/ NCT00282516.
- Chan DS, Callahan CW, Hatch‐Pigott VB, Lawless A, Proffitt HL, Manning NE, et al. Internet‐based home monitoring and education of children with asthma is comparable to ideal office‐based care: results of a 1‐year asthma in‐home monitoring trial. Pediatrics 2007;119(3):569‐78. [DOI] [PubMed] [Google Scholar]
Chiang 2004 {published data only}
- Chiang LC, Huang JL, Yeh KW, Lu CM. Effects of a self‐management asthma educational program in Taiwan based on PRECEDE‐PROCEED model for parents with asthmatic children. Journal of Asthma 2004;41(2):205‐15. [DOI] [PubMed] [Google Scholar]
Clark 1986 {published data only}
- Clark NM, Feldman CH, Evans D, Duzey O, Levison MJ, Wasilewski Y, et al. Managing better: children, parents, and asthma. Patient Education and Counseling 1986;8(1):27‐38. [DOI] [PubMed] [Google Scholar]
- Clark NM, Feldman CH, Evans D, Levison MJ, Wasilewski Y, Mellins RB. The impact of health education on frequency and cost of health care use by low income children with asthma. Journal of Allergy and Clinical Immunology 1986;78(1 Pt 1):108‐15. [DOI] [PubMed] [Google Scholar]
Claus 2004 {published data only}
- Claus R, Michael H, Josef L, Jan‐Torsten T, Marion S. Internet based patient education evaluation of a new tool for young asthmatics. European Respiratory Journal 2004;24(Suppl 48):383s. [Google Scholar]
Cohen 1979 {published data only}
- Cohen HI, Harris C, Green HW, Goodriend‐Resnik S. Cost‐benefit analysis of asthma self‐management educational program in children. Journal of Allergy and Clinical Immunology 1979;63(3):155‐6. [Google Scholar]
Colland 2004 {published data only}
- Colland VT, Essen‐Zandvliet LE, Lans C, Denteneer A, Westers P, Brackel HJL. Poor adherence to self‐medication instructions in children with asthma and their parents. Patient Education and Counseling 2004;55(3):416‐21. [DOI] [PubMed] [Google Scholar]
Cote 1997 {published data only}
- Cote J, Cartier A, Robichaud P, Boutin H, Malo JL, Rouleau M, et al. Influence on asthma morbidity of asthma education programs based on self‐management plans following treatment optimization. American Journal of Respiratory and Critical Care Medicine 1997;155(5):1509‐14. [DOI] [PubMed] [Google Scholar]
Dahl 1990 {published data only}
- Dahl J, Gustafsson D, Melin L. Effects of a behavioral treatment program on children with asthma. Journal of Asthma 1990;27(1):41‐6. [DOI] [PubMed] [Google Scholar]
Deaves 1993 {published data only}
- Deaves D. An assessment of the value of health eduction in the prevention of childhood asthma. Journal Advanced Nursing 1993;18:354‐63. [DOI] [PubMed] [Google Scholar]
Delaronde 2005 {published data only}
- Delaronde S, Peruccio DL, Bauer BJ. Improving asthma treatment in a managed care population. American Journal of Managed Care 2005;11(6):361‐8. [PubMed] [Google Scholar]
- Peruccio D, Bauer B, Delaronde S. Improving asthma treatment and quality of life in a managed care population. Journal of Allergy and Clinical Immunology. 2005; Vol. 115 (Suppl 2):S63. [PubMed]
Donaghy 1995 {published data only}
- Donaghy D. The asthma specialist and patient education. Professional Nurse 1995;11(3):160‐2. [PubMed] [Google Scholar]
Eggleston 2004 {published data only}
- Eggleston PA, Butz A, Rand A, Swartz L, Callahan K, Curtin‐Brosnan J, et al. Home environmental treatment improves asthma in inner city children [Abstract]. Journal of Allergy and Clinical Immunology 2004; Vol. 113, issue Suppl 2:S179.
Eggleston 2005 {published data only}
Evans 1999 {published data only}
- Evans R 3rd, Gergen PJ, Mitchell H, Kattan M, Kercsmar C, Crain E, et al. A randomized clinical trial to reduce asthma morbidity among inner‐city children: results of the National Cooperative Inner‐City Asthma Study. Journal of Pediatrics 1999;135(3):332‐8. [DOI] [PubMed] [Google Scholar]
- Sullivan SD, Weiss KB, Lynn H, Mitchell H, Kattan M, Gergen PJ, et al. The cost‐effectiveness of an inner‐city asthma intervention for children. Journal of Allergy and Clinical Immunology 2002;110(4):579‐81. [DOI] [PubMed] [Google Scholar]
Finkelstein 2000 {published data only}
- Finkelstein J. Inhaled steroid adherence in moderate & severe asthma. CRISP (Computer Retrieval of Information on Scientific Projects) 2000.
Finkelstein 2002 {published data only}
- Finkelstein JA, Fuhlbrigge A, Lozano P, Grant EN, Shulruff R, Arduino KE, et al. Parent‐reported environmental exposures and environmental control measures for children with asthma. Archives of Pediatrics and Adolescent Medicine 2002;156(3):258‐64. [DOI] [PubMed] [Google Scholar]
Flores 2009 {published data only}
- Flores G, Bridon C, Torres S, Perez R, Walter T, Brotanek J, et al. Improving asthma outcomes in minority children: a randomized, controlled trial of parent mentors. Pediatrics 2009;124:1522‐32. [DOI] [PubMed] [Google Scholar]
Friedman 1999 {published data only}
- Friedman R. Impact of a telecommunication system in childhood asthma. CRISP (Computer Retrieval of Information on Scientific Projects) 1999.
Gardida 2002 {published data only}
- Gardida A, Rojas M, Tavera C, Catalan M. Evaluation of an educational program to control asthma in school age children in the Morelos state, Mexico [Spanish]. Revista del Instituto Nacional de Enfermedades Respiratorias 2002;15(1):27‐30. [Google Scholar]
Greineder 1995 {published data only}
- Greineder DK, Loane KC, Parks P. Reduction in resource utilization by an asthma outreach program. Archives of Pediatric and Adolescent Medicine 1995;149(4):415‐20. [DOI] [PubMed] [Google Scholar]
Greineder 1999 {published data only}
- Greineder D K, Loane K C, Parks P. A randomized controlled trial of a pediatric asthma outreach program. Journal of Allergy and Clinical Immunology 1999;103(3 Pt 1):436‐40. [DOI] [PubMed] [Google Scholar]
Griffiths 2004 {published data only}
- Griffiths C, Foster G, Barnes N, Eldridge S, Tate H, Begum S, et al. Specialist nurse intervention to reduce unscheduled asthma care in a deprived multiethnic area: the east London randomised controlled trial for high risk asthma (ELECTRA). BMJ 2004;328(7432):144. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guendelman 2002 {published data only}
- Guendelman S, Meade K, Benson M, Chen YQ, Samuels S. Improving asthma outcomes and self‐management behaviors of inner‐city children: a randomized trial of the Health Buddy interactive device and an asthma diary. Archives of Pediatrics and Adolescent Medicine 2002;156(2):114‐20. [DOI] [PubMed] [Google Scholar]
- Guendelman S, Meade K, Chen YQ, Benson M. Asthma control and hospitalizations among inner‐city children: results of a randomized trial. Telemedicine Journal & E‐Health 2004;10(Suppl 2):114‐20. [PubMed] [Google Scholar]
- Guendelman S, Meade K, Chen YQ, Benson M. Asthma control and hospitalizations among inner‐city children: results of a randomized trial. Telemedicine Journal and E‐Health. 2004; Vol. 10 (Suppl 2):s6‐s14. [PubMed]
Holzheimer 1998 {published data only}
- Holzheimer L, Mohay H, Masters B. Evaluation of asthma self management materials for young children. Australian and New Zealand Journal of Medicine. 1995; Vol. 25:451.
- Holzheimer L, Mohay H, Masters IB. Educating young children about asthma: comparing the effectiveness of a developmentally appropriate asthma education video tape and picture book. Child: Care, Health & Development 1998;24(1):85‐99. [DOI] [PubMed] [Google Scholar]
Homer 2000 {published data only}
- Homer C, Susskind O, Alpert HR, Owusu MS, Schneider L, Rappaport LA, et al. An evaluation of an innovative multimedia educational software program for asthma management: report of a randomized, controlled trial. Pediatrics 2000;106(1 Pt 2):210‐5. [PubMed] [Google Scholar]
Homer 2005 {published data only}
- Homer CJ, Forbes P, Horvitz L, Peterson LE, Wypij D, Heinrich P. Impact of a quality improvement program on care and outcomes for children with asthma. Archives of Pediatric and Adolescent Medicine 2005;159(5):464‐9. [DOI] [PubMed] [Google Scholar]
Horner 2004 {published data only}
- Horner S D. Effect of education on school‐age children's and parents' asthma management. Journal for Specialists in Pediatric Nursing 2004;9(3):95‐102. [DOI] [PubMed] [Google Scholar]
Horner 2006 {published data only}
- Horner SD, Rew DL, Brown SA, Fouladi RT. Promoting asthma self‐management among rural families and children. Proceedings of the American Thoracic Society 2006:A52 [Poster 108].
Horner 2008 {published data only}
- Horner SD, Fouladi RT. Improvement of rural children's asthma self‐management by lay health educators. Journal of School Health 2008;78(9):506‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horner SD, Rew DL, Brown SA. Enhancing asthma management among rural Mexican American, white and African American school‐aged children and their parents. American Thoracic Society International Conference, May 18‐23, 2007, San Francisco, California, USA. 2007:Poster #714.
Hovell 1994 {published data only}
Hovell 2002 {published data only}
Hughes 1991 {published data only}
- Hughes DM, McLeod M, Garner B, Goldbloom RB. Controlled trial of a home and ambulatory program for asthmatic children. Pediatrics 1991;87(1):54‐61. [PubMed] [Google Scholar]
Huss 2003 {published data only}
- Huss K, Winkelstein M, Nanda J, Naumann PL, Sloand ED, Huss RW. Computer game for inner‐city children does not improve asthma outcomes. Journal of Pediatric Health Care 2003;17(2):72‐8. [DOI] [PubMed] [Google Scholar]
Indinnimeo 2009 {published data only}
- Indinnimeo L, Bonci E, Capra L, Grutta S, Monaco F, Paravati F, et al. Clinical effects of a long‐term educational program for children with asthma ‐ Aironet. A 1‐yr randomized controlled trial. Pediatric Allergy and Immunology 2009;20:654‐9. [DOI] [PubMed] [Google Scholar]
Jain 1991 {published data only}
- Jain SC, Rai L, Valecha A, Jha UK, Bhatnagar SO, Ram K. Effect of yoga training on exercise tolerance in adolescents with childhood asthma. Journal of Asthma 1991;28(6):437‐42. [DOI] [PubMed] [Google Scholar]
Jan 2007 {published data only}
- Jan RL, Wang JY, Huang MC, Tseng SM, Su HJ, Liu LF. An internet‐based interactive telemonitoring system for improving childhood asthma outcomes in Taiwan. Telemedicine Journal and e‐Health 2007;13(3):257‐68. [DOI] [PubMed] [Google Scholar]
Jenkinson 1988 {published data only}
- Jenkinson D, Davison J, Jones S, Hawtin P. Comparison of effects of a self management booklet and audiocassette for patients with asthma. BMJ 1988;297(6643):267‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jerant 2009 {published data only}
- Jerant A. A randomized trial of home self‐efficacy enhancement. Computer Retrieval of Information on Scientific Projects 2003; Vol. 31 December 2007 End date.
- Jerant A, Moore‐Hill M, Franks P. Home‐based, peer‐led chronic illness self‐management training: findings from a 1‐year randomized controlled trial. Annals of Family Medicine 2009;7(4):319‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jones 2001 {published data only}
- Jones JA, Wahlgren DR, Meltzer SB, Meltzer Eli O, Clark NM, Hovell MF. Increasing asthma knowledge and changing home environments for Latino families with asthmatic children. Patient Education and Counseling 2001;42(1):67‐79. [DOI] [PubMed] [Google Scholar]
Joseph 2000 {published data only}
- Joseph CLM, Havstad S, Ownby DR, Johnson CC, Tilley BC. "What's the 411 on asthma?" Asthma education for African American teenagers. American Journal of Respiratory and Critical Care Medicine 2000;161(Suppl 3):A621. [Google Scholar]
Joseph 2005 {published data only}
- Joseph CL, Havstad S, Anderson EW, Brown R, Johnson CC, Clark NM. Effect of asthma intervention on children with undiagnosed asthma. Journal of Pediatrics 2005;146(1):96‐104. [DOI] [PubMed] [Google Scholar]
Kamps 2004 {published data only}
- Kamps AWA. Unscheduled care for people with asthma in a multi‐ethnic area is reduced following educational outreach programme by specialist nurses. Evidence Based Healthcare 2004;8(4):190‐1. [Google Scholar]
Karnick 2007 {published data only}
- Johnson RD, Karnick P, Seals G, Margellos H, Silva A, Whitman S, et al. Randomized study of the impact of intensive asthma education (IAE), with/without case management (CM), on the health of inner city asthmatic children: preliminary results of the Chicago Asthma Initiative (IAE). Pediatric Research. 2002; Vol. 51 (4):212A #1234.
- Karnick P, Margellos‐Anast H, Seals G, Whitman S, Aljadeff G, Johnson D. The pediatric asthma intervention: a comprehensive cost‐effective approach to asthma management in a disadvantaged inner‐city community. Journal of Asthma 2007;44(1):39‐44. [DOI] [PubMed] [Google Scholar]
Kay Bartholomew 2006 {published data only}
- Kay Bartholomew L, Sockrider M, Abramson S L, Swank PR, Czyzewski DI, Tortolero SR, et al. Partners in school asthma management: evaluation of a self‐management program for children with asthma. Journal of School Health 2006;76(6):283‐90. [DOI] [PubMed] [Google Scholar]
Kercsmar 2006 {published data only}
- Kercsmar CM, Dearborn DG, Schluchter M, Xue L, Kirchner HL, Sobolewski J, et al. Reduction in asthma morbidity in children as a result of home remediation aimed at moisture sources. Environmental Health Perspectives 2006; Vol. 114, issue 10:1574‐80. [DOI] [PMC free article] [PubMed]
Khan 2004 {published data only}
- Khan MSR, O'Meara M, Henry RL. Background severity of asthma in children discharged from the emergency department. Journal of Paediatrics and Child Health 2003;39(6):432‐5. [DOI] [PubMed] [Google Scholar]
- Khan MSR, O'Meara M, Stevermuer TL, Henry RL. Randomized controlled trial of asthma education after discharge from an emergency department. Journal of Paediatrics and Child Health 2004;40(12):674‐7. [DOI] [PubMed] [Google Scholar]
- Khan S, O'Meara M, Hurst T, Henry RL. Randomised controlled trial of asthma education by telephone after discharge from an emergency department. European Respiratory Journal. 2003; Vol. 22 (Suppl 45):P2294.
Klinnert 2005 {published data only}
- Klinnert MD, Liu AH, Pearson MR, Ellison MC, Budhiraja N, Robinson JL. Short‐term impact of a randomized multifaceted intervention for wheezing infants in low‐income families. Archives of Pediatrics and Adolescent Medicine 2005;159(1):75‐82. [DOI] [PubMed] [Google Scholar]
- Klinnert MD, Liu AH, Pearson MR, Tong S, Strand M, Luckow A, et al. Outcome of a randomized multifaceted intervention with low‐income families of wheezing infants. Archives of Pediatrics and Adolescent Medicine 2007;161(8):783‐90. [DOI] [PubMed] [Google Scholar]
- Klinnert MD, Liu AH, Price M, Ellison MC, Budhiraja N. Short‐term impact of a multi‐faceted intervention for wheezy infants at risk for asthma. Journal of Allergy and Clinical Immunology. 2004; Vol. 113 (Suppl 2):S302.
Kokubu 1999 {published data only}
- Kokubu F, Nakajima S, Ito K, Makino S, Kitamura S, Fukuchi Y, et al. Hospitalization reduction by an asthma tele‐medicine system. Arerugi ‐ Japanese Journal of Allergology 2000;49(1):19. [PubMed] [Google Scholar]
- Kokubu F, Suzuki H, Sano Y, Kihara N, Adachi M. Tele‐medicine system for high‐risk asthmatic patients. Arerugi ‐ Japanese Journal of Allergology 1999;48(7):700‐12. [PubMed] [Google Scholar]
Kotses 1996 {published data only}
- Kotses H, Stout C, McConnaughy K, Winder J A, Creer TL. Evaluation of individualized asthma self‐management programs. Journal of Asthma 1996;33(2):113‐8. [DOI] [PubMed] [Google Scholar]
Krieger 2009 {published data only}
- Krieger J, Takaro TK, Song L, Beaudet N, Edwards K. A randomized controlled trial of asthma self‐management support comparing clinic‐based nurses and in‐home community health workers: the Seattle‐King County Healthy Homes II Project. Archives of Pediatrics & Adolescent Medicine 2009;163(2):141‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger JW, Song L, Takaro TK, Stout J. Asthma and the home environment of low‐income urban children: preliminary findings from the Seattle‐King County healthy homes project. Journal of Urban Health 2000;77(1):50‐67. [DOI] [PMC free article] [PubMed] [Google Scholar]
Krishna 2003 {published data only}
- Krishna S, Francisco BD, Andrew Balas E, Konig P, Graff GR, Madsen RW. Internet‐enabled interactive multimedia asthma education program: a randomized trial. Pediatrics 2003;111(3):503‐10. [DOI] [PubMed] [Google Scholar]
Kuijer 2007 {published data only}
- Kuijer RG, Ridder DTD, Colland VT, Schreurs KMG, Sprangers MAG. Effects of a short self‐management intervention for patients with asthma and diabetes: evaluating health‐related quality of life using then‐test methodology. Psychology and Health 2007;22(4):387‐411. [Google Scholar]
La Roche 2006 {published data only}
- Roche MJ, Koinis‐Mitchell D, Gualdron L. A culturally competent asthma management intervention: a randomized controlled pilot study. Annals of Allergy, Asthma and Immunology 2006;96(1):80‐5. [DOI] [PubMed] [Google Scholar]
LeBaron 1985 {published data only}
- LeBaron S, Zeltzer LK, Ratner P, Kniker WT. A controlled study of education for improving compliance with cromolyn sodium®: the importance of physician‐patient communication. Annals of Allergy 1985;55(6):811‐8. [PubMed] [Google Scholar]
Lecheler 1988 {published data only}
- Lecheler J, Biberger A, Seligmann C, Dorsch U, Hasse‐Dorsch I. Sports therapy in the treatment of pediatric bronchial asthma. Comparison of interval and continuous training. Praxis und Klinik der Pneumologie 1988;42(7):475‐8. [PubMed] [Google Scholar]
Lee 2010 {published data only}
- Lee JK, Yang YH. Evaluation of an education program for patients with asthma who use inhalers [Korean]. Journal of Korean Academy of Nursing 2010;40(2):202‐12. [DOI] [PubMed] [Google Scholar]
Letz 2004 {published data only}
- Letz K, Smits W. A randomized trial comparing peak expiratory flow versus symptom self‐management plans for children with persistent asthma. Journal of Allergy and Clinical Immunology. 2004; Vol. 113 (Suppl 2):S286.
- Letz KL, Schlie AR, Smits WL. A randomized trial comparing peak expiratory flow versus symptom self‐management plans for children with persistent asthma. Pediatric Asthma Allergy and Immunology 2004;17(3):177‐90. [Google Scholar]
Lewis 1987 {published data only}
- Lewis MA, Sota A, Rachelefsky G, Lewis CE, Quinones H, Richards W. ACT‐asthma control y tratamiento para ninos: a progress report. Health Education Quarterly 1987;14(3):281‐90. [DOI] [PubMed] [Google Scholar]
Lewis 1994 {published data only}
- Lewis MA, Rachelefsky G, Lewis CE, Leake B, Richards W. The termination of a randomized clinical trial for poor Hispanic children. Archives of Pediatric and Adolescent Medicine 1994;148:364‐7. [DOI] [PubMed] [Google Scholar]
Li 2006 {published data only}
- Li S, Zhang Z. Influence of self‐care education on self‐care ability and quality of living of asthma patients [Chinese]. Chinese Nursing Research 2006;20(4A):885‐7. [Google Scholar]
Lieberman 2001 {published data only}
- Lieberman D A. Management of chronic pediatric diseases with interactive health games: theory and research findings. Journal of Ambulatory Care Management 2001;24(1):26‐38. [DOI] [PubMed] [Google Scholar]
Linicome 2001 {published data only}
- Linicome V, Lozano P, Jones C, Bryant J E, Finkelstein J, Shulruff R, et al. The nurse‐patient collaboration: experiences with a self‐management support intervention for asthma. Journal of Allergy and Clinical Immunology 2001; Vol. 107, issue 2:S73.
Liu 2001 {published data only}
- Liu C Y, Feekery C. Can asthma education improve clinical outcomes? an evaluation of a pediatric asthma education program. Journal of Asthma 2001;38(3):269‐78. [DOI] [PubMed] [Google Scholar]
Liu 2007 {published data only}
- Liu WT, Wang CH, Huang CD, Kuo H P. A novel mobile phone‐based self‐care system improves asthma control [Abstract]. American Thoracic Society International Conference, May 18‐23, 2007, San Francisco, California, USA. 2007:A93.
Mandhane {published data only}
- Mandhane PJ, McGhan SL, Sharpe HM, Wong E, Hessel PA, Befus AD, et al. A child's asthma quality of life rating does not significantly influence management of their asthma. Pediatric Pulmonology 2010;45:141‐8. [DOI] [PubMed] [Google Scholar]
Marabini 2002 {published data only}
- Marabini A, Brugnami G, Curradi F, Anulli R, Casciola G, Pettinari L, et al. Short‐term effectiveness of an asthma patient education program. American Journal of Respiratory and Critical Care Medicine 1998;157(Suppl 3):A737. [Google Scholar]
- Marabini A, Brugnami G, Curradi F, Casciola G, Stopponi R, Pettinari L, et al. Short‐term effectiveness of an asthma educational program: results of a randomized controlled trial. Respiratory Medicine 2002;96(12):993‐8. [DOI] [PubMed] [Google Scholar]
- Marabini A, Brugnami G, Curradi F, Siracusa A. Does an asthma education program improve quality of life? A two‐year randomized trial. Journal of Asthma 2005;42(7):577‐81. [DOI] [PubMed] [Google Scholar]
- Siracusa A, Anulli R, Brugnami G, Casciola G, Curradi F, Marabini A, et al. Effectiveness of an asthma education programme: a one year follow‐up study. European Respiratory Journal. 1998; Vol. 12 (Suppl 29):P193.
McCarthy 2002 {published data only}
- McCarthy MJ, Herbert R, Brimacombe M, Hansen J, Wong D, Zelman M. Empowering parents through asthma education. Pediatric Nursing 2002;28(5):465‐73. [PubMed] [Google Scholar]
McConnell 2005 {published data only}
McGhan 2010 {published data only}
- McGhan SL, Wong E, Sharpe HM, Hessel PA, Mandhane P, Boechler VL, et al. A children's asthma education program: Roaring Adventures of Puff (RAP), improves quality of life. Canadian Respiratory Journal 2010;17(2):67‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
McNabb 1985 {published data only}
- McNabb WL, Wilson‐Pessano SR, Hughes GW, Scamagas P. Self‐management education of children with asthma: AIR WISE. American Journal of Public Health 1985;75(10):1219‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
McPherson 2006 {published data only}
- Glazebrook C, McPherson A, Forster D, James C, Crook I, Smyth A. The asthma files: randomised controlled trial of interactive multimedia program to promote self‐management skills in children. American Thoracic Society 100th International Conference, May 21‐26, 2004, Orlando. 2004; Vol. D29:P506.
- McPherson A, Glazebrook C, Smyth S. Effectiveness of a multimedia education program for children with asthma. European Respiratory Journal. 2003; Vol. 22 (Suppl 45):P2299.
- McPherson AC, Glazebrook C, Forster D, James C, Smyth A. A randomized, controlled trial of an interactive educational computer package for children with asthma. Pediatrics 2006;117(4):1046‐54. [DOI] [PubMed] [Google Scholar]
Mendes 2010 {published data only}
- Mendes F, Cukier A, Stelmach R, Martins M, Carvalho C. Effect of aerobic training on autonomic modulation in asthmatic patients [Abstract]. European Respiratory Society Annual Congress, Barcelona, Spain. 2010:E2161.
Meszaros 2003 {published data only}
- Meszaros A, Orosz M, Magyar P, Mesko A, Vincze Z. Evaluation of asthma knowledge and quality of life in Hungarian asthmatics. Allergy 2003;58(7):624‐8. [DOI] [PubMed] [Google Scholar]
Mildenhall 1997 {published data only}
- Mildenhall S, McLean M, Pearce S, Noble M, Harrison BDW. Evaluation of a home based coping skills training programme for high risk asthma sufferers. Thorax 1997;52(Suppl 6):A46 P62. [Google Scholar]
Morgan 2004 {published data only}
Mosnaim 2008 {published data only}
- Mosnaim GS, Cohen MS, Rhoads CH, Rittner SS, Powell LH. Use of MP3 players to increase asthma knowledge in inner‐city African‐American adolescents. International Journal of Behavioral Medicine 2008;15(4):341‐6. [DOI] [PubMed] [Google Scholar]
- Mosnaim GS, Rhoads C, Cohen M. Adolescents disease empowerment and persistency technology (ADEPT) increases asthma knowledge among inner city African American teenagers using MP3 players. Journal of Allergy and Clinical Immunology. 2007; Vol. 119 (Suppl 1):S285 [1118].
Moudgil 1998 {published data only}
- Moudgil H, Honeybourne D. Differences in asthma management between white European and Indian subcontinent ethnic groups living in socioeconomically deprived areas in the Birmingham (UK) conurbation. Thorax 1998;53(6):490‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moudgil H, Honeybourne D. Evaluating the asthma education of white European and Indian subcontinent asthmatics. Thorax 1997;52(Suppl 6):A47 P64. [Google Scholar]
- Moudgil H, Honeybourne D. Reducing the need for urgent health care: impact of asthma education in white European and Indian subcontinent groups. American Journal of Respiratory and Critical Care Medicine. 1998; Vol. 157 (Suppl 3):A632.
Nishioka 2006 {published data only}
Nokela 2010 {published data only}
- Nokela M, Heibert Arnlind M, Ehrs PO, Krakau I, Forslund L, Wikstrom Jonsson E. The influence of structured information and monitoring on the outcome of asthma treatment in primary care: a cluster randomized study. Respiration 2010;79(5):388‐94. [DOI] [PubMed] [Google Scholar]
O'Connor 1996 {published data only}
- O'Connor G T. Trial of interventions to reduce asthma morbidity. CRISP (Computer Retrieval of Information on Scientific Projects) 1996; Vol. End date: 31 July 2004.
Page 1999 {published data only}
- Page P, Lengacher C, Holsonback C, Himmelgreen D, Pappalardo LJ, Lipana MJ, et al. Quality of care‐risk adjustment outcomes model: testing the effects of a community‐based educational self‐management program for children with asthma. Nursing Connections 1999;12(3):47‐58. [PubMed] [Google Scholar]
Parker 2008 {published data only}
- Parker EA, Israel BA, Robins TG, Mentz G, Xihong L, Brakefield‐Caldwell W, et al. Evaluation of Community Action Against Asthma: a community health worker intervention to improve children's asthma‐related health by reducing household environmental triggers for asthma. Health Education and Behavior 2008;35(3):376‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
Perrin 1992 {published data only}
- Perrin JM, MacLean WE Jr, Gortmaker SL, Asher KN. Improving the psychological status of children with asthma: a randomized controlled trial. Developmental and Behavioural Pediatrics 1992;13(4):241‐7. [PubMed] [Google Scholar]
Persky 1999 {published data only}
- Persky V, Coover L, Hernandez E, Contreras A, Slezak J, Piorkowski J, et al. Chicago community‐based asthma intervention trial: feasibility of delivering peer education in an inner‐city population. Chest 1999;116(4 (Suppl 1)):216S‐23S. [DOI] [PubMed] [Google Scholar]
Petro 2005 {published data only}
- Petro W, Schulenburg JMVD, Greiner W, Weithase J, Schulke A, Metzdorf N. Efficacy of a disease management programme in asthma [Effizienz eines Disease Managment Programmes bei Asthma]. Pneumologie 2005;59(2):101‐7. [DOI] [PubMed] [Google Scholar]
Put 2003 {published data only}
- Put C, Bergh O, Lemaigre V, Demedts M, Verleden G. Evaluation of an individualised asthma programme directed at behavioural change. European Respiratory Journal 2003;21(1):109‐15. [DOI] [PubMed] [Google Scholar]
- Put CL, Verleden G, Bergh O, Demedts M. Evaluation of an individualized asthma program aiming at behavioral change. Annual Thoracic Society 97th International Conference; San Francisco CA ,May 18‐23. 2001.
Rakos 1985 {published data only}
- Rakos RF, Grodek MV, Mack KK. The impact of a self‐administered behavioral intervention program on pediatric asthma. Journal of Psychosomatic Research 1985;29(1):101‐8. [DOI] [PubMed] [Google Scholar]
Rand 2005a {published data only}
- Rand C. A+ asthma early intervention in asthma management. Clinicaltrials.gov 2005. [URL: http://www.clinicaltrials.gov/ct/show/NCT00217906]
Rhee 2008 {published data only}
- Rhee H, Hollen PJ, Belyea MJ, Sutherland MA. Decision‐making program for rural adolescents with asthma: a pilot study. Journal of Pediatric Nursing 2008;23(6):439‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ronchetti 1997 {published data only}
- Ronchetti R, Indinnimeo L, Bonci E, Corrias A, Evans D, Hindi‐Alexander M, et al. Asthma self‐management programmes in a population of Italian children: a multicentric study. Italian Study Group on Asthma Self‐Management Programmes. European Respiratory Journal 1997;10(6):1248‐53. [DOI] [PubMed] [Google Scholar]
Rubin 1986 {published data only}
- Rubin DH, Leventhal JM, Sadock RT, Letovsky E, Schottland P, Clemente I, et al. Educational intervention by computer in childhood asthma: a randomized clinical trial testing the use of a new teaching intervention in childhood asthma. Pediatrics 1986;77(1):1‐10. [PubMed] [Google Scholar]
Schatz 2006 {published data only}
- Schatz M, Gibbons C, Nelle C, Harden K, Zeiger RS. Impact of a care manager on the outcomes of higher risk asthmatic patients: a randomized controlled trial. Journal of Asthma 2006;43(3):225‐9. [DOI] [PubMed] [Google Scholar]
Schmidt 1993 {published data only}
- Schmidt S, Konning J, Szczepanski R, Gebert N, Hummelink R, Wahn U. Asthma training in practice. Evaluation of an integrated care concept including the involvement of a paediatrician in practice. Padiatrische Praxis 1993;45:635‐41. [Google Scholar]
Shames 2004 {published data only}
- Bergman DA, Mayer ML, Sharek PJ, Robinson T, Shames R. An asthma disease management program: results from a randomized clinical trial. Pediatric Research. 2001; Vol. 4(4):457A.
- Shames RS, Sharek P, Mayer M, Robinson TN, Hoyte EG, Gonzalez‐Hensley F, et al. Effectiveness of a multicomponent self‐management program in at‐risk, school‐aged children with asthma. Annals of Allergy, Asthma, and Immunology 2004;92(6):611‐8. [DOI] [PubMed] [Google Scholar]
Shegog 2001 {published data only}
- Shegog R, Bartholomew LK, Parcel GS, Sockrider MM, Masse L, Abramson SL. Impact of a computer assisted education program on factors related to asthma self‐management behavior. Journal of the American Medical Informatics Association 2001;8(1):49‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shields 1990 {published data only}
- Shields MC, Griffin KW, McNabb WL. The effect of a patient education program on emergency room use for inner‐city children with asthma. American Journal of Public Health 1990;80(1):36‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shields 2004 {published data only}
- Shields M D, Patterson E E, Brennan M P, Linskey K, Webb D, Patterson C C. A cluster randomised intervention trial of asthma clubs to improve quality of life in primary school children ‐ the school care and asthma management project (SCAMP). Thorax 2004;59(Suppl II):ii21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Slader 2006 {published data only}
- Slader CA, Reddel HK, Spencer LM, Belousova EG, Armour CL, Bosnic‐Anticevich SZ, et al. Double blind randomised controlled trial of two different breathing techniques in the management of asthma. Thorax 2006;61(8):651‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Smith 2004 {published data only}
- Smith SR, Jaffe DM, Fisher EB Jr, Trinkaus KM, Highstein G, Strunk RC. Improving follow‐up for children with asthma after an acute Emergency Department visit. Journal of Pediatrics 2004;145(6):772‐7. [DOI] [PubMed] [Google Scholar]
Sommaruga 1995 {published data only}
- Sommaruga M, Spanevello A, Migliori GB, Neri M, Callegari S, Majani G. The effects of a cognitive behavioural intervention in asthmatic patients. Thorax 1995;50(5):398‐402. [PubMed] [Google Scholar]
Steurer‐Stey 2010 {published data only}
- Steurer‐Stey C, Storch M, Steffen‐Bürgi B, Steurer J, Puhan M. A general self‐management program improves self‐efficacy but not short‐term adherence with asthma self‐management behavior: a randomized controlled trial [Abstract]. European Respiratory Society Annual Congress, Barcelona, Spain. 2010:P3989.
Stevens 2002 {published data only}
- Couriel J. A randomised control trial of an educational package and self‐management guide for pre‐school asthmatic children and their parents. National Research Register 1 June 2000.
- Stevens CA, Wesseldine LJ, Couriel JM, Dyer AJ, Osman LM, Silverman M. Parental education and guided self‐management of asthma and wheezing in the pre‐school child: a randomised controlled trial. Thorax 2002;57(1):39‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sublett 2000 {published data only}
- Sublett JL, Basile F, Wighton T, Wald JA, Vivra. The impact of expert allergy care and a nurse telephonic asthma disease management program on outcomes in asthmatics. American Journal of Respiratory and Critical Care Medicine 2000;161(Suppl 3):A904. [Google Scholar]
Sun 2010 {published data only}
- Sun HW, Wang JP, Wang SZ, Wang YY, Song YP, Yang ZH, et al. Effect of educational and psychological intervention on the quality of life of asthmatic patients. Respiratory Care 2010;55:725‐8. [PubMed] [Google Scholar]
Szczepanski 2010 {published data only}
- Szczepanski R, Jaeschke R, Spindler T, Ihorst G, Forster J, ASEV Study Group. Preschoolers' and parents' asthma education trial (P2AET)‐‐a randomized controlled study. European Journal of Pediatrics 2010;169:1051‐60. [DOI] [PubMed] [Google Scholar]
Tagaya 2005 {published data only}
- Tagaya E, Tamaoki J, Konodo M, Taira M, Nagai A, Amo M, et al. Effect of self‐management program in patients with mild to moderate asthma: a randomised controlled study. Respirology. 2006; Vol. 11(Suppl 5):PS‐1‐10.
- Tagaya E, Tamaoki J, Nagai A, Murasugi H, Igi H. The role of a self‐management program in the control of mild to moderate asthma: a randomized controlled study. Allergology International 2005;54(4):527‐31. [Google Scholar]
- Tagaya E, Tamoki J, Nagal A, Igi H. Role of self management program alone in asthma control in mild to moderate asthmatics, randomized controlled study. American Thoracic Society 2005 International Conference; May 20‐25; San Diego, California. 2005; Vol. A40:G18.
Takaro 2004 {published data only}
- Takaro TK, Krieger JW, Song L. Effect of environmental interventions to reduce exposure to asthma triggers in homes of low‐income children in Seattle. Journal of Exposure Analysis and Environmental Epidemiology 2004;14(Suppl 1):S133‐43. [DOI] [PubMed] [Google Scholar]
Takaro 2004a {published data only}
Talabere 1990 {published data only}
- Talabere LR. The effects of an asthma education program on selected health behaviors of school‐age children who have recently experienced an acute asthma episode. The Ohio State University ** PH.D. 1990:260 p.
Thoonen 2002 {published data only}
- Thoonen BPA, Schermer TRJ, Jansen M, Smeele I, Jacobs AJE, Grol R, et al. Asthma education tailored to individual patient needs can optimise partnerships in asthma self‐management. Patient Education and Counseling 2002;47(4):355‐60. [DOI] [PubMed] [Google Scholar]
Tong 2002 {published data only}
- Tong R, Pan J, Tao M. The effect of psychological intervention on childhood asthma. Chinese Mental Health Journal. 2002;16(8):555‐7. [Google Scholar]
Tsoukleris 2007 {published data only}
- Tsoukleris M, Hsu V, Vibbert CL, Bollinger ME, Butz AM. Asthma controller awareness, knowledge, health beliefs and behaviours. Journal of Allergy and Clinical Immunology 2007; Vol. 119, issue Suppl 1:S285 [1116].
Urek 2005 {published data only}
- Urek MC, Markoviu AS, TuDman Z, Urek R, Uvorisec B, Tudoric N, et al. The influence of patient education on asthma treatment. European Respiratory Journal. 2003; Vol. 2(Suppl 45):P1612.
- Urek MC, Tudoric N, Plavec D, Urek R, Koprivc‐Milenovic T, Stojic M. Effect of educational programs on asthma control and quality of life in adult asthma patients. Patient Education and Counseling 2005;58(1):47‐54. [DOI] [PubMed] [Google Scholar]
Valery 2007 {published data only}
- Valery PC, Masters IB, Clements V, Taylor B, Laifoo Y, Chang AB. A randomised controlled study on education intervention for childhood asthma by the Aboriginal and Torres Strait Islander health workers in Torres Strait region. Respirology 2007; Vol. 12, issue Suppl 4:A193.
- Valery PC, Masters IB, Taylor B, O'Rourke P, Laifoo Y, Chang AB. A randomised controlled study on education intervention for childhood asthma by indigenous health workers in the Torres Strait. Respirology. 2008; Vol. 13(Suppl 2):A67.
- Valery PC, Masters IB, Taylor B, O'Rourke, Laifoo Y, Chang AB. Education intervention for childhood asthma by indigenous health workers in the Torres Strait, Australia. Respirology. 2009; Vol. 14(Suppl 1):A41. [DOI] [PubMed]
van Es 2001 {published data only}
- Es SM, Nagelkerke AF, Colland VT, Scholten RJ, Bouter LM. An intervention programme using the ASE‐model aimed at enhancing adherence in adolescents with asthma. Patient Education and Counseling 2001;44(3):193‐203. [DOI] [PubMed] [Google Scholar]
Vazquez 1993 {published data only}
- Vazquez MI, Buceta JM. Effectiveness of self‐management programmes and relaxation training in the treatment of bronchial asthma: relationships with trait anxiety and emotional attack triggers. Journal of Psychosomatic Research 1993;37(1):71‐91. [DOI] [PubMed] [Google Scholar]
- Vazquez MI, Buceta JM. Psychological treatment of asthma: effectiveness of a self‐management program with and without relaxation training. Journal of Asthma 1993;30(3):171‐83. [DOI] [PubMed] [Google Scholar]
Walders 2006 {published data only}
- Walders N. A randomized controlled trial of a problem‐solving intervention for pediatric asthma. Dissertation 2003:261 p.
- Walders N, Kercsmar C, Schluchter M, Redline S, Kirchner HL, Drotar D. An interdisciplinary intervention for undertreated pediatric asthma. Chest 2006;129(2):292‐9. [DOI] [PubMed] [Google Scholar]
Warschburger 2003 {published data only}
- Warschburger P, Schwerin AD, Buchholz HT, Petermann F. An educational program for parents of asthmatic preschool children: short‐ and medium‐term effects. Patient Education and Counseling 2003;51(1):83‐91. [DOI] [PubMed] [Google Scholar]
Weiss 2003 {published data only}
- Weiss KB, Lozano P, Finkelstein JA, Carey V, Sullivan S, Fuhlbrigge A, et al. A randomized controlled clinical trial to improve asthma care for children through provider education and health systems change: a description of the pediatric asthma care patient outcome research team (PAC‐PORT II) study design. Health Services and Outcomes Research Methodology 2003;4(4):265‐82. [Google Scholar]
Wensley 2001 {published data only}
- Wensley D, Silverman M. Peak flow monitoring for guided self‐management in childhood asthma: a randomized controlled trial. American Journal of Respiratory and Critical Care Medicine 2004;170(6):606‐12. [DOI] [PubMed] [Google Scholar]
- Wensley DC Silverman M. The quality of home spirometry in school children with asthma. Thorax 2001;56(3):183‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Willems 2004 {published data only}
- Willems DCM, Joore MA, Hendricks JJE, Duurling RAH, Wesseling GJ, Severns JL, et al. The cost effectiveness of an information communication technology supporting self management program in asthmatics. Proceedings of the American Thoracic Society. 2006:A597 [Poster D3].
- Willems DCM, Joore MA, Hendriks HJE, Duurling AH, Wesseling GJ, Wouters EFM. The cost effectiveness of an ICT supporting self‐management program in children with asthma. European Respiratory Journal. 2005; Vol. 26(Suppl 49):Abstract No. 1189.
- Willems DCM, Joore MA, Hendriks HJE, Duurling AJ, Wesseling GJ, Wouters EFM. The feasibility of ICT supporting self management program in asthma. European Respiratory Journal 2004;24(Suppl 48):256s. [Google Scholar]
- Willems DCM, Joore MA, Nieman FHM, Hendriks JJE, Severns JL, Wouters EFM. The effects of an information communication technology supporting self management intervention on quality of life symptoms and medical consumption of asthmatics. Proceedings of the American Thoracic Society. 2006:A90 [Poster E5].
Williams 2006 {published data only}
- Williams SG, Brown CM, Falter KH, Alverson CJ, Gotway‐Crawford C, Homa D, et al. Does a multifaceted environmental intervention alter the impact of asthma on inner‐city children?. Journal of the National Medical Association 2006;98(2):249‐60. [PMC free article] [PubMed] [Google Scholar]
Wilson 1996a {published data only}
- Wilson SR, Latini D, Starr NJ, Fish L, Loes LM, Page A, et al. Education of parents of infants and very young children with asthma: a developmental evaluation of the Wee Wheezers program. Journal of Asthma 1996;33(4):239‐54. [DOI] [PubMed] [Google Scholar]
Wise 2007 {published data only}
- Shanovich KK, Pulvermacher AD, Hollman SJ, Richardson PA, Wise ME, Lee SH, et al. Nurse case management services provided to supplement a web‐based asthma education program. Journal of Allergy and Clinical Immunology. 2008; Vol. 121(2 Suppl 1):S40 [155].
- Wise M, Gustafson DH, Sorkness CA, Molfenter T, Staresinic A, Meis T, et al. Internet telehealth for pediatric asthma case management: integrating computerized and case manager features for tailoring a web‐based asthma education program. Health Promotion Practice 2007;8(3):282‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise M, Pulvermacher A, Shanovich KK, Gustafson DH, Sorkness C, Bhattacharya A. Using action research to implement an integrated pediatric asthma case management and ehealth intervention for low‐income families. Health Promotion Practice 2009;11(6):798. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yoon 1989 {published data only}
- Yoon R, McKenzie DK, Bauman A. Randomised controlled trial evaluation of an asthma education program. Australian Journal of Hospital Pharmacy 1989;19(1):51. [Google Scholar]
Zhao 2005 {published data only}
- Zhao X, Gao G. Influence of health education on quality of life of asthma patients [Chinese]. Chinese Nursing Research 2005;19(9B):1809‐10. [Google Scholar]
Zorc 2005 {published data only}
- Zorc JJ. Educational video for improving follow‐up after emergency department visit for asthma. Clinicaltrials.gov 2005. [URL: http://www.clinicaltrials.gov/ct/show/NCT00113633]
- Zorc JJ, Chew A, Allen JL, Shaw K. Beliefs and barriers to follow‐up after an emergency department asthma visit: a randomized trial. Pediatrics 2009;124(4):1135‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
Adams 2004 {published data only}
- Adams CD. Parent‐youth teamwork in pediatric asthma management. NCT00166582 2005.
- Adams CD, Joseph KE, MacLaren JE, DeMore M, Koven L, Detweiler MF, et al. Parent‐youth teamwork in pediatric asthma management. Journal of Allergy and Clinical Immunology 2004;113(Suppl 2):S159. [Google Scholar]
Boone 2002 {published data only}
- Boone D, Counsell P, Dobson C, Baker EH. Effect of computer‐assisted education on inhaler technique and knowledge in children with asthma. European Respiratory Society Annual Congress. 2002:P2062.
Cameron 1989 {published data only}
- Cameron ES, Remy L, Dillon Remy MT, Bratt DE. Childhood asthma education project. West Indian Medical Journal 1989;38(Suppl 1):20. [Google Scholar]
Kelly 2005 {published data only}
- Kelly CS, Taylor‐Fishwick JC, Harrison L, Collins‐Odoms C, Hixson J. EZ breathers: a partnership to improve asthma management using a combination of staff education and home visits in a Head Start population. Journal of Allergy and Clinical Immunology 2005;115(Suppl 2):S63. [Google Scholar]
Mishra 2005 {published data only}
- Mishra N, Rao KVR, Padhi SK. Asthma education for better compliance in disease management. Indian Journal of Allergy Asthma & Immunology 2005;19(1):25‐8. [Google Scholar]
Rand 2004 {published data only}
- Rand C. Improving asthma care for minority children in Head Start. Clinicaltrials.gov 2004:http://www.clinicaltrials.gov/ct2/show/ NCT00094276?term= NCT00094276&rank=1.
Strunk 2005 {published data only}
- Strunk R. Social support and education in asthma follow‐up (SSEA). Clinicaltrials.gov 2005. [URL: http://www.clinicaltrials.gov/ct/show/NCT00149500]
Taylor‐Fishwick 2005 {published data only}
- Taylor‐Fishwick JC, Kelly CS, Butterfoss FD, Harrison L, Hixson J, Smith L. Asthma ambassadors ‐ a home visiting program in public housing programs in Southeastern Virginia. Journal of Allergy and Clinical Immunology 2005;115(Suppl 2):S67. [Google Scholar]
Yang 2005 {published data only}
- Yang BH, Chen YC, Chiang BL, Chang YC. Effects of nursing instruction on asthma knowledge and quality of life in schoolchildren with asthma. Journal of Nursing Research 2005;13(3):174‐83. [DOI] [PubMed] [Google Scholar]
Yildiz 2002 {published data only}
- Yildiz A, Ongen G, Gemicioglu B, Oncel A, Erturan S, Musellim B. The effect of individual and group education on disease symptoms, pulmonary functions and quality of life in asthma patients. European Respiratory Journal 2002;20(Suppl 38):454s. [Google Scholar]
References to ongoing studies
Rand 2006 {published data only}
- Rand C. Motivating asthma adherence in urban teens. Clinicaltrials.gov 2006:http://www.clinicaltrials.gov/ct/show/ NCT00269282.
Additional references
Altman 2003
- Altman DG, Bland JM. Statistics notes: interaction revisited: the difference between two estimates. BMJ 2003;326(7382):219. [DOI: 10.1136/bmj.326.7382.219] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bailey 2009
- Bailey EJ, Cates CJ, Kruske SG, Morris PS, Brown N, Chang AB. Culture‐specific programs for children and adults from minority groups who have asthma. Cochrane Database of Systematic Reviews 2009, Issue 2. [DOI: 10.1002/14651858.CD006580.pub4] [DOI] [PubMed] [Google Scholar]
Bhogal 2006
- Bhogal SK, Zemek RL, Ducharme F. Written action plans for asthma in children. Cochrane Database of Systematic Reviews 2006, Issue 3. [DOI: 10.1002/14651858.CD005306.pub2] [DOI] [PubMed] [Google Scholar]
Bodenheimer 2002
- Bodenheimer T, Wagner EH, Grumbach K. Improving primary care for patients with chronic illness: the chronic care model, Part 2. JAMA 2002;288:1909‐14. [DOI] [PubMed] [Google Scholar]
Boyd 2009
- Boyd M, Lasserson TJ, McKean MC, Gibson PG, Ducharme FM, Haby M. Interventions for educating children who are at risk of asthma‐related emergency department attendance. Cochrane Database of Systematic Reviews 2009, Issue 2. [DOI: 10.1002/14651858.CD001290.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
British Guideline on the Management of Asthma
- British Thoracic Society and Scottish Intercollegiate Guidelines Network. British Guideline on the Management of Asthma. Thorax 2008;63(Suppl 4):1‐121. [DOI] [PubMed] [Google Scholar]
Brouwer 2008
- Brouwer AFJ, Brand PLP. Asthma education and monitoring; what has been shown to work. Paediatric Respiratory Reviews 2008;9:193‐200. [DOI] [PubMed] [Google Scholar]
Bryant‐Stephens 2008
- Bryant‐Stephens T, Li Y. Outcomes of a home‐based environmental remediation for urban children with asthma. Journal of the National Medical Association 2008;100(3):306‐16. [DOI] [PubMed] [Google Scholar]
Clayton 2005
- Clayton S. Paediatric asthma: overcoming barriers to an improved quality of life. British Journal of Nursing 2005;14(2):80‐5. [DOI] [PubMed] [Google Scholar]
Coffman 2008
- Coffman JM, Cabana MD, Halpin HA, Yelin EH. Effects of asthma education on children's use of acute care services: a meta‐analysis. Pediatrics 2008;121:575‐86. [DOI] [PMC free article] [PubMed] [Google Scholar]
D'Zurilla 1971
- D'Zurilla TJ, Goldfried M. Problem‐solving and behavior modification. Journal of Abnormal Psychology 1971;78:107‐26. [DOI] [PubMed] [Google Scholar]
D'Zurilla 1986
- D'Zurilla TJ. Problem‐Solving Therapy: A Social Competence Approach to Clinical Intervention. New York: Springer, 1986. [Google Scholar]
de Oliveira 1999
- Oliveira MA, Faresin SM, Bruno VF, Bittencourt AR, Fernandes ALG. Evaluation of an educational programme for socially deprived asthma patients. European Respiratory Journal 1999;14(4):908‐14. [DOI] [PubMed] [Google Scholar]
Friedman 2006
- Friedman AR, Butterfoss FD, Krieger JW, Peterson JW, Dwyer M, Wicklund K, et al. Allies community health workers: bridging the gap. Health Promotion Practice 2006;7(Suppl 2):96S‐107S. [DOI] [PubMed] [Google Scholar]
Gibson 2002
- Gibson PG, Powell H, Wilson A, Abramson MJ, Haywood P, Bauman A, et al. Self‐management education and regular practitioner review for adults with asthma. Cochrane Database of Systematic Reviews 2002, Issue 3. [DOI: 10.1002/14651858.CD001117] [DOI] [PMC free article] [PubMed] [Google Scholar]
GINA 2008
- Agostinis F, Foglia C, Landi M, Cottini M, Lombardi C, Canonica GW, et al. GINA Report, Global Strategy for Asthma Management and Prevention. Allergy 2008;63(12):1637‐9. [DOI] [PubMed] [Google Scholar]
Gorelick 2004
- Gorelick MH, Brousseau DC, Stevens MW. Validity and responsiveness of a brief, asthma‐specific quality‐of‐life instrument in children with acute asthma. Annals of Allergy, Asthma, and Immunology 2004;92:47‐51. [DOI] [PubMed] [Google Scholar]
Halterman 2001
- Halterman JS, Yoos L, Sidora K, Kitzman H, McMullen A. Medication use and health care contacts among symptomatic children with asthma. Ambulatory Pediatrics 2001;1:275‐9. [DOI] [PubMed] [Google Scholar]
Higgins 2008
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Chichester: John Wiley & Sons, 2008. [Google Scholar]
Juniper 1996a
- Juniper EF, Guyatt GH, Feeny DH, Ferrie PJ, Griffith LE, Townsend M. Measuring quality of life in the parents of children with asthma. Quality of Life Research 1996;5(1):27‐34. [DOI] [PubMed] [Google Scholar]
Juniper 1996b
- Juniper EF, Guyatt GH, Feeny DH, Ferrie PJ, Griffith LE, Townsend M. Measuring quality of life in children with asthma. Quality of Life Research 1996;5(1):35‐46. [DOI] [PubMed] [Google Scholar]
Krieger 2002
- Krieger J, Takaro TK, Allen C, Song L, Weaver M, Chai S, et al. The Seattle‐King County Healthy Homes Project: implementation of a comprehensive approach to improving indoor environmental quality for low‐income children with asthma. Environmental Health Perspectives 2002;110:311‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Krieger 2005
- Krieger JW, Takaro TK, Song L, Weaver M. The Seattle‐King County Healthy Homes Project: a randomized, controlled trial of a community health worker intervention to decrease exposure to indoor asthma triggers. American Journal of Public Health 2005;95:652‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mitchell 1981
- Mitchell EA, Elliott RB. Hospital admissions for asthma in children: a prospective study. New Zealand Medical Journal 1981;93:331‐3. [PubMed] [Google Scholar]
Mitchell 1984
- Mitchell EA, Cutler DR. Paediatric admission to Auckland Hospital for asthma from 1970‐1980. New Zealand Medical Journal 1984;97:67‐70. [PubMed] [Google Scholar]
NAEPP 2007
- Expert Panel Report 3 (EPR3): Guidelines for the Diagnosis and Management of Asthma. National Heart, Blood and Lung Institute 2007.
Rapoff 1999
- Rapoff MA. Adherence to Pediatric Medical Regimens. New York: Kluwer Academic/Plenum, 1999. [Google Scholar]
Review Manager 5.1 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager. Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Schneider 1997
- Schneider SL, Richard M, Huss K, Huss RW, Thompson LC, Butz AM, et al. Moving health care education into the community. Nursing Management 1997;28(9):40‐3. [PubMed] [Google Scholar]
Shelledy 2009
- Shelledy DC, Legrand TS, Gardner DD, Peters JI, Shelledy DC, Legrand TS, et al. A randomized, controlled study to evaluate the role of an in‐home asthma disease management program provided by respiratory therapists in improving outcomes and reducing the cost of care. Journal of Asthma 2009;46(2):194‐201. [DOI] [PubMed] [Google Scholar]
Tates 2001
- Tates K, Meeuwesen L. Doctor‐parent‐child communication: a (re) view of the literature. Social Sciences Medicine 2001;52:839‐51. [DOI] [PubMed] [Google Scholar]
Varni 1999
- Varni JW, Sahler OJ, Katz ER, Mulhern RK, Copeland DR, Noll RB, et al. Maternal problem‐solving therapy in pediatric cancer. Journal of Psychosocial Oncology 1999;16(3 & 4):41‐71. [Google Scholar]
Wagner 2001
- Wagner EH, Augstin BT, Davis CI, Hindmarsh M, Schaefer J, Bonomi A. Improving chronic illness care: translating evidence into action. Health Affairs (Millwood) 2001;20:64‐78. [DOI] [PubMed] [Google Scholar]
Wilson 1996b
- Wilson SR, Fish L, Page A, Starr‐Schneidkraut N. Wee Wheezers: an educational program for parents of children with asthma under the age of seven. Palo Alto, CA: American Institutes for Research.
Wolf 2002
- Wolf FM, Guevara JP, Grum CM, Clark NM, Cates CJ. Educational interventions for asthma in children. Cochrane Database of Systematic Reviews 2002, Issue 4. [DOI: 10.1002/14651858.CD000326] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wu 2007
- Wu F, Takaro TK. Childhood asthma and environmental interventions. Environmental Health Perspectives 2007;115(6):971‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]


