Abstract
Alcohol consumption is one of the most prevalent correlates of antiretroviral therapy (ART) adherence, yet causal processes underlying this association remain largely unexplored. The goal of this systematic review was to develop a conceptual model that describes the causal effect of alcohol consumption on ART nonadherence. We reviewed 230 studies that examined the association between alcohol consumption and ART adherence with three primary aims: (1) to replicate and extend previous reviews of the literature, (2) to summarize and critique study designs capable of answering questions about temporal overlap and (3) to summarize potential mechanisms of action. A model of alcohol-associated ART nonadherence was proposed to guide future work, integrating general theories of ART adherence and theory on the psychological and behavioral effects of alcohol intoxication. The conceptual model describes two mechanistic processes—prospective memory impairment and interactive toxicity beliefs/avoidance behaviors—involved in alcohol-associated intentional and unintentional nonadherence, respectively. This model can be used to guide future research on the causal processes involved in the frequently observed correlation between alcohol consumption and adherence.
Keywords: HIV, ART, Alcohol, Adherence
Approximately 38 million people are living with Figure 2 HIV (PWH) globally, with 1.7 million new infections in 2018 (World Health Organization [WHO], 2019). While incidence and HIV-related deaths have fallen by almost 40% since 2000, the disease continues to be a major public health problem, having caused more than 35 million deaths since the onset of the epidemic (WHO, 2019). The decrease in HIV incidence and HIV-related deaths over the last two decades is largely attributable to increased access to antiretroviral therapy (ART). When taken as prescribed, ART suppresses HIV replication, decreases plasma HIV RNA levels (i.e., “viral load”) to undetectable levels, and reduces the risk of onward transmission to zero (J. Cohen, 2011; M. Cohen et al., 2011; Rodger et al., 2016; Rodger, 2018). ART “treatment as prevention” (TasP) has thus become the primary method for reducing transmission, spurring campaigns to increase HIV testing, treatment, and viral suppression worldwide (e.g., UNAIDS’ 90-90-90 campaign; U = U; Joint United Nations Program on HIV/AIDS, 2018). The TasP movement has launched public health efforts to increase universal HIV testing, and the initiation of ART as close in time as possible to diagnosis. These universal test-and-treat (UTT) models have proven feasible in sub-Saharan Africa – achieving high population-level viral suppression and reduced HIV incidence (Havlir et al., 2020). There are, however, special populations for which the benefits of UTT are attenuated. For example, men categorized as hazardous drinkers (i.e., score ≥ 8 on the Alcohol Use Disorders Identification Test [AUDIT]), were less likely to link to HIV care and initiate ART in one of the trials associated with the Universal Test and Treat Trial (UT3C) Consortium (Sabapathy et al., 2017). The success of TasP thus rests on the critical assumption of linkage to HIV care, and acceptable levels of medication adherence.
Figure 2.
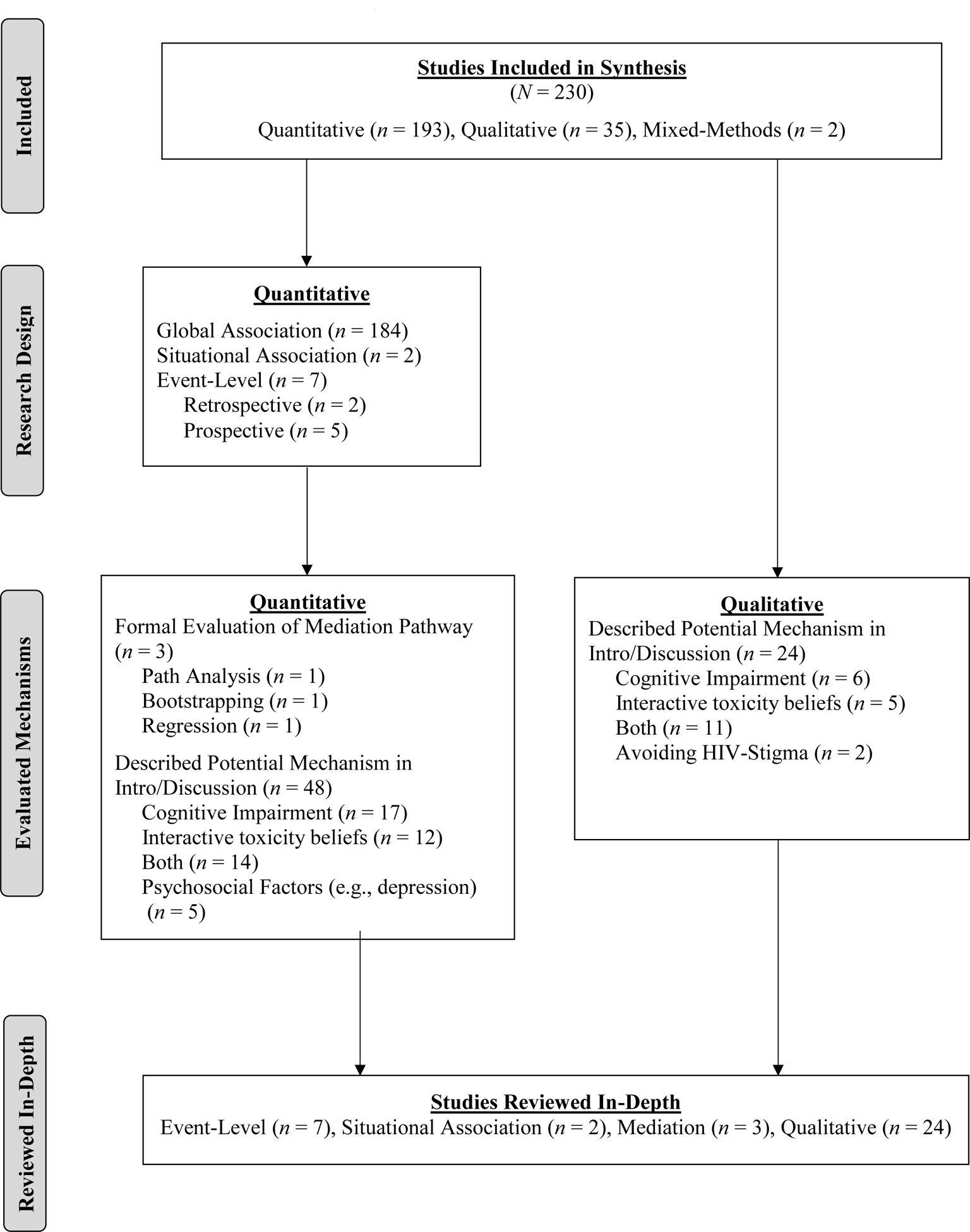
Flow Diagram of Studies Reviewed In-Depth
The importance of adherence in HIV treatment and prevention
Taking medications as prescribed (i.e., being adherent) is the single most important patient-related factor in HIV treatment success, and the most common cause of treatment failure and disease progression (Volberding & Deeks, 2010). Historically, 95% has been the level of adherence necessary for optimal population-level viral suppression (Viswanathan et al., 2015). However, newer ART regimens have been shown to be more forgiving – with 80%−90% adherence observed as sufficient for virologic control (Gordon et al., 2015; Viswanathan et al., 2015). Even so, findings from the annual cross-sectional survey associated with the U.S. National HIV Surveillance System (N = 11,914 PWH), reveal that viral rebound is significantly more likely among PWH who report <100% adherence in the last 30 days compared to PWH who report 100% adherence (adjusted Prevalence Ratio[aPR]=1.88, 95% confidence interval [CI] = 1.58, 2.23; p < 0.001; Craw et al., 2020), and real time adherence monitoring among PWH who report < 80% adherence has revealed that the odds of viral rebound increase by 25% (p < 0.01) with each day of nonadherence beyond 48 hours (Haberer et al., 2015). A meta-analysis of 84 observational studies across 20 countries found that the mean proportion of individuals reporting ≥90% ART adherence was only 62% (Ortego et al., 2011), underscoring the identification of risk factors for nonadherence as important for successful HIV treatment-related outcomes.
Correlates of ART adherence
Medication adherence is complex and influenced by multiple factors related to the patient, the treatment, the disease, the provider, and the patient-provider relationship (Ammassari et al., 2002). Over the past two decades, numerous correlates of ART adherence have been identified in the HIV literature (Shubber et al., 2016; Mills et al., 2006; Engler et al., 2018; Atkinson & Petrozzino, 2009; Fogarty et al., 2002; Ammassari et al., 2002; Langebeek et al., 2014). In the most comprehensive of these reviews, Langebeek et al., (2014) conducted a meta-analysis of 207 studies involving 103,836 HIV-infected patients. The review aimed to characterize the magnitude of the association between ART adherence and sociodemographic (e.g., age, financial constraints), interpersonal (e.g., social support, stigma), disease (e.g., time since diagnosis), treatment (e.g., time on ART), and patient (e.g., substance use, depressive symptoms, self-efficacy)-related factors. The strongest correlate was adherence self-efficacy (Standardized Mean Difference [SMD] = .60, 95% CI = .47, .73) and the second strongest correlate was current substance use (SMD = −.39, 95% CI = −.52, .25), both of which were characterized as “medium” in effect size. Indeed, alcohol consumption has been increasingly recognized as enhancing the risk for poor treatment-related outcomes at nearly every stage of the HIV care continuum (Vagenas et al., 2015).
Alcohol use among people living with HIV (PWH)
The prevalence of at-risk alcohol use (i.e., drinking that exceeds 3 drinks [4 for men] on a single day and 7 drinks [14 for men] in a week; Current Reviews Editorial Staff, 2018) among PWH has been found to range from 8% to 42%, with drinkers disproportionately represented among PWH given alcohol’s association with behaviors (e.g., condomless sex, ART nonadherence) that increase risk for onward transmission (Williams et al., 2016). In the United States (U.S.), studies suggest that 66% of PWH report consuming alcohol in the previous year (Blair et al., 2014), 8% – 29% report at-risk drinking (Marshall et al., 2015; Sullivan et al., 2011), 30% report current binge drinking (>5 drinks in past 30 days; Kelly et al., 2016), and 10% – 35% currently meet or have previously met criteria for alcohol use disorder (AUD; Justice et al., 2016). These levels of at-risk and binge (i.e., alcohol consumption that results in blood alcohol concentration (BAC) levels of at least 0.08 g/dL, which typically occurs after 4 drinks for women/5 drinks for men over 2 hours; National Institute on Alcohol Abuse and Alcoholism, 2011) alcohol consumption are higher compared to the U.S. general population (Center for Disease Control [CDC], 2018), although when PWH are compared to demographically similar HIV-uninfected samples, the levels of consumption may be more equivalent (Park et al., 2016). In sub-Saharan Africa, the region of the world most affected by HIV, prevalence of at-risk and binge alcohol consumption has been found to be higher among PWH compared to people who are HIV-uninfected (Brandt, 2009; Nouaman et al., 2018), although consumption varies widely be region. For example, the prevalence of binge drinking among PWH in ranges from 14% in Senegal to 82% in Zambia, with 7%−12% of PWH screening positive on the Alcohol Use Disorders Identification Test (AUDIT) in South Africa and Nigeria respectively (Farley et al., 2010; Myer et al., 2008).
PWH who are at-risk drinkers, compared to those who abstain, experience a significant increase in risk for engagement in sexual risk behavior (Shuper et al., 2009), lack of viral suppression (Chander et al., 2006), less ART utilization (Chander et al., 2006), taking ART medications off schedule (Cook et al., 2001), sub-optimal adherence to ART (Hendershot et al., 2009), and mortality (Braithwaite et al., 2005). Understanding the association between alcohol consumption and adherence thus has the potential to decrease onward transmission and improve HIV treatment-related outcomes.
Alcohol use and adherence to ART
As of December 2019, five systematic reviews (Costa et al., 2018, 2018; Ge et al., 2018; Grodensky et al., 2012; Vagenas et al., 2015) and two meta-analyses (Hendershot et al., 2009; Velloza et al., 2019) have been published on the association between alcohol consumption and ART adherence. Of these reviews, the two most comprehensive are the 2009 meta-analysis by Hendershot et al. and the 2012 systematic review by Grodensky et al., having summarized 40 and 47 studies, respectively. Hendershot et al. (2009) found that people who consume alcohol were ~50% less likely (OR = .55; 95% CI = .49, .61) to be classified as adherent (variably defined) compared to abstainers or those who drank relatively less. The odds of nonadherence were most extreme when at-risk drinkers were compared to abstainers or those who drank relatively less (OR = .47, 95% CI = .41, .55). These results did not include event-level studies, limiting the ability to draw conclusions about temporal overlap of the two behaviors.
Grodensky et al. (2012) largely replicated Hendershot et al.’s (2009) findings, and extended them by summarizing separately event-level studies that were designed to examine temporal overlap. Only two event-level studies had been published on the topic at the time (Braithwaite et al., 2008, 2005), both of which used Timeline Followback (TLFB) data from the Veterans Aging Cohort Study (VACS), but combined ART medications and other medications into one adherence outcome variable. Braithwaite et al. (2008) found that whereas abstainers missed doses to prescribed medications on 2% of surveyed days, binge drinkers missed doses on 11% of drinking days, 7% of postdrinking days, and 4% of nondrinking days. Braithwaite and Bryant (2010) also investigated the relative influence of alcohol consumption on adherence and survival among PWH and found a clinically significant decline in adherence on days when a threshold of two standard drinks were consumed, even when adjusted for individual difference factors. Braithwaite’s analyses have been interpreted as evidence of alcohol-related cognitive impairment, which was described as a “causal mechanism linking alcohol consumption to nonadherence” (p. 1651). The assumption that the acute cognitive and/or behavioral effects of alcohol consumption have a causal effect on medication adherence is not currently supported by experimental data however, nor is there a theoretical model that describes hypothesized mechanistic pathways. It is unknown at this time to what degree alcohol intoxication accounts for nonadherence on any given nonadherence event, and the cumulative impact of alcohol-associated nonadherence events over time.
Purpose and focus of the present review
Most reviews and meta-analyses on alcohol consumption and ART adherence assume a causal process, and even go so far as to conclude that “it is now clearly time to move from assessment to intervention” (Azar et al., 2010, p.10). Our interpretation of the status of the literature is consistent with Hendershot et al. (2009), who noted over ten years ago that while the association between alcohol and nonadherence is “replicable and reliable,” the “causal nature of the association is difficult to speak to (p. 8).” In order for ART adherence interventions to adequately address alcohol consumption, and result in clinically-meaningful behavior change, the temporal ordering and mechanisms underlying the association must be clarified (Kazdin, 2007; Tryon, 2018). Indeed, a theory-based conceptual framework explicating the processes by which alcohol use results in nonadherence is critical for identifying intervention content most likely to result in behavior change (Hagger et al., 2020).
In this review we synthesize relevant literature toward the end of developing a theoretical model of the mechanisms underlying the association between alcohol intoxication and ART adherence. To this end, we characterized the studies reviewed by their research design and the information that each design can yield regarding a causal connection between alcohol consumption and ART adherence, and the mechanism(s) that may underlie it. Critical in this regard is the idea of “temporality.” In this context, temporality refers to temporal overlap, or that alcohol consumption is occurring prior to the behavior of interest (i.e., adherence or non-adherence to an ART regimen), and that the acute pharmacological effects of alcohol, such as intoxication or hangover, co-occur with adherence/non-adherence during the same event. Table 1 summarizes the research designs that have been used to examine the association between alcohol consumption and ART adherence. Experimental studies, which feature ideal temporality, are not listed in Table 1 because there have been, to our knowledge, no published experimental studies of alcohol intoxication and ART adherence. The research designs that are included in Table 1 are listed in descending order of temporal overlap, and as Table 1 shows, have respective associated value in generating hypotheses about causal relations between alcohol consumption and ART adherence and mechanism(s) that may underlie them.
Table 1.
Summary of Research Designs Used in Studies Reviewed on Alcohol Consumption and ART Adherence.
| Design | Description | Example | What It Can Yield | What It Cannot Yield |
|---|---|---|---|---|
| Longitudinal Event Level | Prospective assessment of the occurrence of a specific behavior on an occasion and whether alcohol was present during the behavior. Typically assess past 24-hour adherence, and number/timing of drinks consumed over the past 24 hours, every day for a period of weeks or months. | Daily Diary Study | Possible to obtain reliable/accurate information on precise temporal ordering of events with relatively little recall error. Also provides information on the relative likelihood of a behavior in the presence or absence of alcohol. | Despite good temporality, still correlational. Clear causal inferences based on data generated from it are not warranted. |
| Retrospective Event Level | Retrospective assessment of the occurrence of a specific behavior on an occasion and whether alcohol was present during the behavior. The occurrence of nonadherence and the presence of alcohol is assessed for each day over a specified past period of time. Variations include the last time 1–5 times the event occurred, or the occurrence of events over the last 30–90 days. | Critical Incident studies; Multiple event studies; Timeline Followback (TLFB) | Information on the co-occurrence of alcohol use and behavior of interest. Also provides information on the relative likelihood of a behavior in the presence or absence of alcohol. | Causal inferences between 2 or more variables; More subject to recall bias or memory failure than prospective event level studies. |
| Situational Associational | An assessment of whether some behavior has tended to occur or has ever occurred in conjunction with the behavior of interest. Typically use of single item to assess whether alcohol use tends to co-occur with ART non-adherence, whose outcome is used to predict an aggregate measure of frequency of ART adherence. | Endorsement of a single item such as “I tend skip taking my HIV medications if I will be drinking” | General information on whether the co-occurrence of two behaviors at one point in time predicts some outcome of interest. | Precise information on temporality. |
| Longitudinal Global Association | Correlation of an aggregate measure of one variable over some period of time with an aggregate measure of a second variable, measured at and over a second period of time. | Correlation of a measure of average frequency of alcohol use over the last 3 months at baseline, with self-report of ART adherence in the previous 30 days at 6-month follow-up. | In early stages of research on a phenomenon a “signal” that that two variables may co-occur and perhaps are causally connected. The signal is that follow-up research seems warranted. | Any information on temporality. |
| Cross-sectional Global Association | Correlation of an aggregate measure of one variable over some period of time with an aggregate measure of a second variable, measured at, and sometimes over, the same period of time. | Correlation of a measure of average frequency of alcohol use over the 30 days with self-report of ART adherence over the last 30 days. | In early stages of research on a phenomenon a “signal” that that two variables may co-occur. The signal is that follow-up research seems warranted. | Any information on temporality. |
To move the field towards a more mechanistic and theory-informed understanding of the alcohol-ART adherence relation, the overall goal of this review was to develop a conceptual model that describes the causal effect of alcohol consumption on ART nonadherence. Such a model could be used to design experimental and event-level studies capable of testing hypotheses about the causal effects of alcohol consumption on nonadherence, and the proportion of the variance in nonadherence events that can be attributed to these effects. To accomplish this goal, we conducted a broad systematic review of the literature on alcohol use and ART adherence with three primary aims: Aim 1: To replicate and extend previous reviews of global association studies, by summarizing changes in the frequency of measurement, and noting any advances and/or consistencies with Grodensky et al. (2012) and Hendershot et al. (2009). Aim 2: To separately summarize and critique situational and event-level studies—designs capable of answering questions about temporal overlap. Aim 3: To extract and summarize potential mediators and mechanisms of the alcohol consumption-ART adherence relation from any quantitative study that conducted a statistical test of mediation, and qualitative studies that discussed mechanisms of action.
METHOD
The methods for this systematic review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. The protocol was pre-registered at Prospero (registration # CRD42018092336) on April 6th, 2018.
Study Inclusion Criteria
Inclusion criteria for the quantitative global association studies in Aim 1 were as follows: (1) included a sample of HIV-positive adults who were prescribed ART, (2) examined the association between alcohol consumption and ART adherence, with a clearly defined measure of each, (3) reported an adjusted statistical parameter of alcohol consumption as a correlate of ART adherence in a multivariate model, and (4) published in an English-language peer-reviewed journal. ART adherence was operationalized as medication-taking behavior, as opposed to a pharmacological outcome of adherence behavior (e.g., viral load). For Aim 2, only situational and event-level studies were included consistent with the definitions provided in Table 1. For Aim 3, because we anticipated finding a low number of quantitative studies focused on mediation and/or mechanisms of action, we also searched for qualitative studies that could inform this aim. Inclusion criteria for the qualitative studies were as follows: (1) included a sample of HIV-positive adults who were prescribed ART, (2) examined the association between alcohol use and ART adherence, and (3) published in an English-language peer-reviewed journal.
Search Strategy
An electronic search of all peer-reviewed, English-language articles published through December 31st, 2019 was conducted using the PubMed, PsycINFO, PsycArticles, CINAHL, and SCOPUS databases. The following Boolean search terms were used in each database: (HIV OR “human immunodeficiency virus” OR HIV-1 OR AIDS OR “acquired immunodeficiency syndrome” OR PWHA OR PWH) AND (alcohol OR alcoholism OR “alcohol use” OR liquor OR drinking OR “alcohol drinking” OR AUD OR “substance use” OR ethanol) AND (adherence OR nonadherence OR compliance OR noncompliance OR “medication adherence” OR medication OR “treatment refusal” OR treatment) AND (HAART OR “highly active antiretroviral therapy” OR antiretroviral OR “antiretroviral therapy” OR ARV OR ART OR “combination therapy” OR “HIV treatment”). Reference lists of articles were reviewed in-depth for additional relevant citations not captured by the electronic search. We also cross-referenced the results of our review with those of Grodensky et al. (2012) and Hendershot et al. (2009).
Data Extraction
The titles and abstracts of all records identified by this search strategy were independently screened by two coders to determine eligibility for full-text review. To resolve any discrepancies in eligibility for full-text review, the two coders met and discussed their rationale for categorizing each discrepant record and came to an agreement on the most appropriate categorization. This process was repeated for any article that was identified through the reference list of articles reviewed in-depth and those that were cross-referenced from the Grodensky et al., (2012) and Hendershot et al., (2009) reviews. Each full-text record was independently reviewed by one member of the team of five coders (co-authors AS, JR, JF, DM, & MF) to determine eligibility. A randomly selected subset (25%) of both the quantitative and qualitative articles were coded by a second, independent coder to assess for agreement in classifying the full-text article for study eligibility. An inter-coder reliability analysis yielded an overall agreement of 95%. Subsequently, all records categorized as eligible to be included in the synthesis were reviewed in detail by two independent coders and all information relevant to Aims 1–3 were extracted from the full-text. Any discrepancies between the data extracted from the records were discussed and resolved in team meetings attended by all five coders while consulting with the full-text article.
Aim 1.
In order to replicate and extend the Grodensky et al. (2012) and Hendershot et al. (2009) reviews, and summarize changes in the frequency of measurement, data extracted from all quantitative global association studies included: study design, geographic location in which the study was conducted, study sample, measurement of alcohol consumption, time frame over which alcohol consumption was assessed, measurement or ART adherence, time frame over which ART adherence was assessed, overlap in the time frame over which alcohol consumption and ART adherence were assessed, study results, and any proposed mechanisms by which alcohol consumption was theorized to influence ART adherence.
Aim 2.
Situational and event-level studies were coded separately and in greater depth. Additional data extracted from these studies included: funding agency, sample demographics, study eligibility criteria, recruitment strategies, method of event-level or situational data-collection, temporal overlap between alcohol use and ART adherence measurement, approach to data analysis, global association analysis results, and event-level analysis results.
Aim 3.
Any quantitative study that conducted a statistical test of mediation, reporting both direct and indirect effects, was also coded in greater depth. Data extracted for these studies were the same as the studies included in Aim 2 with the addition of the following: measurement of the mediator, statistical test of mediation, and results of the mediation analysis. As noted above, all quantitative studies were also coded for any mention of proposed mechanisms by which alcohol consumption was theorized to influence ART adherence in the introduction and/or discussion section.
All of the qualitative studies were coded for geographic location in which the study was conducted, study sample, primary themes/findings, and proposed mechanism by which alcohol consumption influenced ART adherence. Any qualitative study that explicitly discussed potential mechanisms of action was flagged for more in-depth review. Data extracted from these studies included: geographic location in which the study was conducted, funding agency, study sample, sample demographics, study eligibility criteria, recruitment methodology, interview questions assessing alcohol consumption, interview questions assessing ART adherence, proposed mechanism by which alcohol consumption influenced adherence, qualitative data analysis approach, primary themes/findings, and supporting quotes.
RESULTS
The results of the electronic literature search yielded a total of 3,544 unique records. After screening the titles and abstracts of these records, 747 were identified as suitable for a full-text review, including 15 that were identified by either the reference sections of studies that were reviewed in-depth or articles that the electronic search did not capture but were found when cross-referencing the Grodensky et al. (2012) and Hendershot et al. (2009) reviews. A total of 230 articles met eligibility criteria to be included in the final synthesis. Figure 1 describes the study-selection process using the PRISMA flow diagram. Of the 193 quantitative articles that met eligibility criteria for review in Aim 1, only 5% (n = 9) described event-level or situational association studies eligible for review in Aim 2. Although 25% (n = 48) of the quantitative articles proposed a potential mechanism of action in the introduction or discussion section, only 2% (n = 3) conducted a mediation analysis for review in Aim 3. Of the 35 eligible qualitative articles, 69% (n = 24) discussed potential mechanisms of action and were also reviewed in Aim 3. Due to the large number of studies that met eligibility criteria (N = 230), we provide summaries of only the studies that were reviewed in-depth for Aims 2 (Table 2) and 3 (Table 3).
Figure 1.
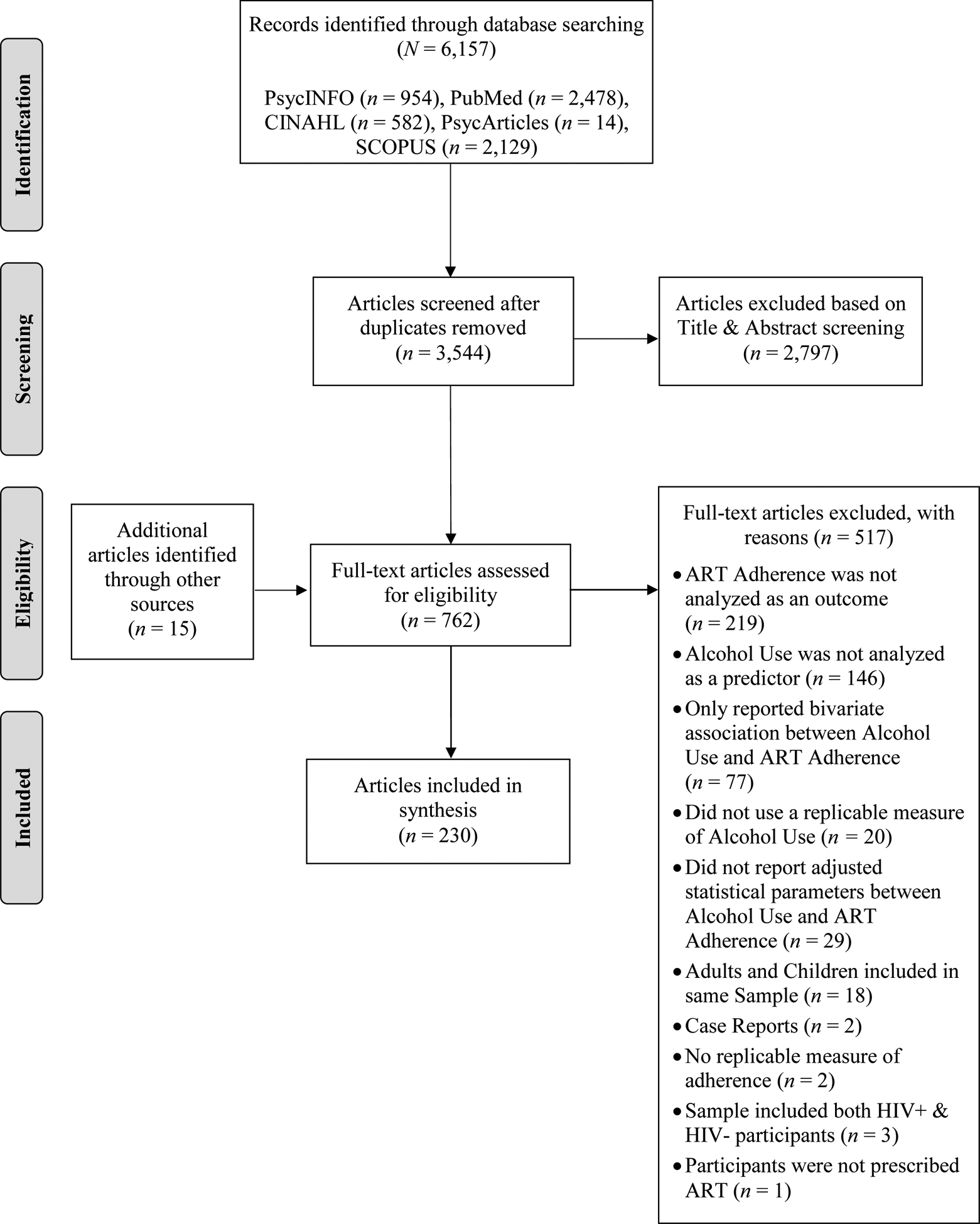
PRISMA Flow Diagram
Table 2.
Findings from Quantitative Studies that Conducted an Event Level Analysis Examining Alcohol Consumption and ART Adherence Among PLWH.
| First author (Year) | Study Design | Mechanism+ | Participant Characteristics | Covariates | Event-Level Results |
|---|---|---|---|---|---|
| Kalichman et al., (2013) | Prospective event-level (interactive text-diary) | Interactive toxicity beliefs | 70% male; M age = 45.9; 93% Black; PLWH in Atlanta, US (N = 178) | AUDIT; Drug Use | Participants who reported skipping ART while drinking had significantly more days with concurrent alcohol use and missed ART (aOR = 2.9; 95% CI = 1.09, 7.68, p < .05). |
| Kalichman et al., (2014) | Prospective event-level (interactive text-diary) | Forgetting; Interactive toxicity beliefs | 77% male; M age = 46.1; 93% Black; PLWH in Atlanta, US (N = 183) | Marital status, employment status, age | Participants categorized as non-adherent (<85%) were significantly more likely to miss an ART dose while drinking compared to participants categorized as adherent (aOR = 3.26; 95% CI = 1.82, 5.61, p < .01). |
| Parsons et al., (2008) | Retrospective event-level (TLFB) | Cognitive impairment; Interactive toxicity beliefs; Disruption in routine | 78% male; M age = 43.7; 58% Black; PLWH in New York City, US (N = 272) | Cognitive, alcohol, medication side effects, and regimen complexity factors | Participants were ~9 times more likely to report medication nonadherence on days alcohol was consumed compared to days in which alcohol was not consumed (aOR = 8.78, 95% CI = 7.16, 10.77, p < .001). On drinking days, each drink increased the odds of nonadherence by 20% (aOR = 1.2; 95% CI = 1.18, 1.22, p < .001). |
| Pellowski et al., (2016a) | Prospective event-level (daily diary) | NA | 81% male; 96% Black; PLWH in Atlanta, US (N = 57) | Income; Education | Alcohol use on a specific day predicted missing a dose of ART on that same day (β = 0.73, p = 0.02). Participants who drank alcohol and experienced hunger on a specific day, were least likely to miss a dose of ART that day (β = −1.92, p < 0.001). |
| Pellowski et al., (2016b) | Prospective event-level (daily diary) | Cognitive impairment; Interactive toxicity beliefs | 81% male; 96% Black; PLWH in Atlanta, US (N = 57) | Interactive toxicity beliefs; AUDIT; Depressive symptoms; Medication concerns | In a multilevel model predicting daily doses of missed ART, quantity of alcohol consumed on a given day did not predict missing a dose of HIV medication on that day (β = 0.15, p = 0.17). However, alcohol-ART interactive toxicity beliefs significantly predicted missed daily doses of ART (β = 0.45, p <.05). |
| Schensul et al., (2017) | Retrospective event-level (TLFB) | Forgetting; Interactive toxicity beliefs; Failure to renew medications | 100% male; M age = 43; PLWH in Mumbai, India (N = 922) | None for daily analyses | Participants who drank alcohol on a given day were significantly less likely to be adherent to ART on that same day (Z = 11.3, p < 0.001) and the following day (Z = 9.5, p < 0.001), compared to participants who did not drink any alcohol. |
| Sileo et al., (2016) | Prospective event-level (daily diary) | Perceived risk of heavy drinking | 70% female; M age = 32.9; PLWH in Cape Town, South Africa (N = 74) | Age; Gender; Education; SES | Participants who drank any alcohol on a given day were ~three times more likely to report missing ART that day compared to days in which they did not drink (aOR = 3.18, 95% CI = 2.25, 4.49, p < 0.001). Each additional drink consumed above a participant’s average number of drinks was associated with a 6% increase in likelihood of missing ART that day (aOR = 1.06, 95% CI = 1.02, 1.11, p = 0.005). Participants who reported low-moderate levels (aOR = 4.29, 95% CI = 2.81, 6.56, p < 0.001) and high-very high levels (aOR = 2.31, 95% CI = 1.56, 3.42, p < 0.001) of alcohol use were 2–4 times more likely to report missing ART that day compared to days in which no alcohol was consumed. |
Note.
All studies described potential mechanisms in the introduction and/or discussion sections. Sample demographic information reported at follow-up is presented when possible, otherwise baseline sample information is presented. PLWH = people living with HIV; AUDIT = Alcohol Use Disorder Identification Test; ART = Antiretroviral Therapy; aOR = Adjusted Odds Ratio; 95% CI = 95% Confidence Interval; SES = Socioeconomic Status; US= United States; NA = Not Applicable; TLFB = Timeline Followback
Table 3.
Findings from Qualitative Studies of General and Alcohol-specific Barriers to ART Adherence Among PLWH.
| First author (Year) | Study Design | Mechanism+ | Participant Characteristics | General Study Results | Alcohol & ART-Adherence Mechanism Results |
|---|---|---|---|---|---|
| Adeniyi et al., (2018) | Semi-structured interviews | Forgetting | 100% female; 48.6% between age 21–30; PLWH (N = 177) from maternity facilities in South Africa | Barriers to ART adherence included: side-effects, travel away from primary clinic, forgetfulness, stigma and health systems factors. | Some participants reported that alcohol use, especially over weekends or festive periods, made them forget to take their HIV medications. |
| Axelsson et al., (2015) | Semi-structured interviews | Forgetting; Interactive toxicity beliefs | 61% female; M age = 39.8; PLWH (N = 28) from HIV clinics in Lesotho | Barriers to ART adherence included: interruptions of daily routines or leaving the house without medicine, while facilitators of ART adherence included use of alarms and social support. | Alcohol was identified as a barrier to ART adherence by 4/28 participants. Reasons included skipping medication due to concerns about interactions with alcohol and forgetfulness caused by alcohol. |
| Balcha et al., (2011) | FGDs & semi-structured interviews | Interactive toxicity beliefs | 64% female; Age 26–37; PLWH (N = 14) and healthcare providers (N = 3) from hospitals in Ethiopia | Barriers to ART adherence included: financial constraints, stigma, and sociocultural factors, while disclosure, community support, and universal access to ART facilitated ART adherence. | Providers were described as recommending against alcohol use while on ART. Some participants reported a preference for missing doses of ART rather than abstaining from alcohol. |
| Barnett et al., (2013) | Semi-structured interviews | Forgetting; Interactive toxicity beliefs | 90% female; PLWH (N = 10) and healthcare providers (N = 11) from an HIV clinic in South Africa | Barriers to ART adherence included: side-effects, not using condoms, a lack of understanding around medication timing, time delay between medication and food intake, and large pill sizes. | Drinking was identified as a reason for ART failure by one participant, however, alcohol use was the most common reason for ART failure cited by providers. Patients were reported to often forget to take medication while drinking or actively avoid mixing alcohol with medications. |
| Bukenya et al., (2019) | Semi-structured interviews | Forgetting; Interactive toxicity beliefs | 60% female; M age = 41.7; PLWH (N = 30) from Uganda | Barriers to ART adherence included: travel for work or social activities, stigma, receiving little or no continuous ART adherence education, alcohol consumption, use of alternative ‘HIV cure’ medicines, ART side effects, treatment fatigue, belief that long-term ART or God can ‘cure HIV’, and food insecurity. | Two participants reported forgetting their ART due to alcohol use. No female participants reported using alcohol, reporting total cessation due to toxicity beliefs. |
| Conroy et al., (2017) | Semi-structured interviews | Forgetting; Interactive toxicity beliefs | 50% female; M age = 35.5; PLWH from HIV clinics in South Africa (N = 24 couples with at least one HIV-positive partner) | Several patterns of partner influence on alcohol use and ART emerged: Partners discouraged their significant other from mixing ART and alcohol, partners helped manage alcohol and ART use, and partners encouraged adherence to ART no matter what. | PLWH and their partners acknowledged that drinking alcohol can cause unintentional nonadherence. Some (16.7%) participants also endorsed interactive toxicity beliefs. |
| Conroy et al., (2019) | Semi-structured interviews | Forgetting; Interactive toxicity beliefs; Social support | 50% female; M age = 38; PLWH from HIV clinics in Malawi (N = 25 couples with at least one HIV-positive partner) | Relationship factors such as food insecurity, intimate partner violence, and extramarital relationships worsened the negative consequences of alcohol use on ART adherence. Women encouraged their partners to reduce alcohol use and offered adherence support when men were drinking. Men’s alcohol use was a barrier to supporting wives’ ART adherence. | Participants reported that alcohol use by men weakens the relationship and social support systems necessary for ART adherence. Women reported being more likely to experience IPV when men are drinking, which causes missed ART doses. Couples reported men forget to take medication while drinking. |
| Conroy et al., (2020) | Semi-structured interviews | Forgetting; Interactive toxicity beliefs; Partner support | 50% female; PLWH from HIV clinics in Malawi (N = 23 couples with at least one HIV-positive partner) | Alcohol use was described as a major barrier to ART adherence and was also viewed as the cause of couple and family violence, extramarital partnerships, food insecurity, and poverty. | Wives identified alcohol use as a barrier to ART adherence and endorsed interactive toxicity beliefs as a reason that was given by HIV counselors. |
| Dahab et al., (2008) | Semi-structured interviews | Forgetting | 100% male; PLWH (N = 6) and healthcare providers (N = 5) from an HIV clinic in South Africa | Barriers to ART adherence included: alcohol use, being away from home, stigma, use of traditional medicines, and lack of belief in the existence of HIV and/or one’s own status. | Alcohol use was identified as a primary barrier to ART adherence by causing people to forget to take the medication. |
| Dickson-Gomez et al., (2015) | Semi-structured interviews | Forgetting; Interactive toxicity beliefs | PLWH (N = 29) and healthcare providers (N = 13) from HIV clinics in El Salvador | PLWH who use substances experience barriers to accessing medical care including: a lack of knowledge of HIV and effective treatments, inconsistent linkage to care, and stigma within health care settings. | Participants described stopping ART when drinking alcohol. Many providers believed and told patients that alcohol in combination with ART was contraindicated and could cause severe side effects (including death). Some patients reported forgetting associated with long-term cognitive impairment from alcohol use. |
| Fitzgerald et al., (2010) | Semi-structured interviews | Interactive toxicity beliefs | 100% male; PLWH (N = 8) from an HIV clinic in South Africa | Alcohol was reported to influence disclosure, uptake, and adherence to ART. Participants reported self-imposed delays to enroll in HIV treatment while attempting to reduce alcohol use. | Some participants expressed interactive toxicity beliefs and were concerned whether they could initiate ART based on their alcohol consumption. |
| Hershow et al., (2018) | Semi-structured interviews | Forgetting; Timing | 40% female; Median age = 38; PLWH (N = 30) with alcohol use disorder from an HIV clinic in Vietnam | Barriers to reducing alcohol use included: availability/affordability of alcohol, social norms, lack of treatment, using alcohol to cope with HIV-related problems. Participants who reduced alcohol use and maintained high ART adherence reported social support as a buffer against social pressure to drink and drinking to cope. | Common reasons for missing ART doses included forgetfulness while under the influence of alcohol and perceived inability to take the medication when at a social event during their typical dosing time. |
| Laws et al., (2015) | Semi-structured interviews | Interactive toxicity beliefs | 31.3% female; PLWH (N = 32) from the US | Participants generally reported limited and inaccurate biomedical understanding of HIV and ART. Participants expressed that strict adherence to ART regimens was important, with some endorsing periodic treatment interruption as beneficial. | Participants reported that ART medications should not be taken while drinking or using illicit drugs because they can “block” the medication. Some participants reported that they received these instructions from their doctor. |
| Lyimo et al., (2012) | Semi-structured interviews | Interactive toxicity beliefs | 59.1% female; M age = 34; PLWH (N = 61) from HIV clinics in Tanzania | Facilitators of adherence included: social-support and assistance of home-based care providers. Barriers to ART adherence included: alcohol use, food insecurity, stigma and disclosure concerns, and the clinics dispensing too few pills. | Participants reported alcohol use was strictly prohibited to patients on ART, or that ART cannot be taken after alcohol had been consumed. Alcohol use was also reported as contributing to inconsistent adherence behaviors. |
| Madhombiro et al., (2018) | Focus group discussions | Forgetting; Interactive toxicity beliefs; Disinhibition | 41% female; M age = 40; PLWH (N = 39) from HIV clinics in Zimbabwe | Major themes included: alcohol has negative effects on HIV treatment, PLWH who drink should be understood and take responsibility for reducing alcohol use, stigma interferes with HIV disclosure and disclosure of drinking to providers, and there is support available to those who wish to reduce alcohol use. | Participants reported awareness of both the direct and indirect effects of alcohol use on ART non-adherence Participants endorsed beliefs that alcohol and ART medications should not be mixed due to negative side-effects. Additionally, they perceived alcohol to negatively influence ART adherence by causing one to forget to take medications. |
| Moucheraud et al., (2019) | Semi-structured interviews | Forgetting | 63.5% female; M age = 41.5; PLWH (N = 148) and healthcare providers (N = 49) from HIV clinics in Tanzania and Uganda | Barriers to adherence identified by PLWH included: distance from pill supply, food insecurity, pill burden/side effects, access to care. Barriers identified by providers included alcohol/alcoholism, stigma, and adherence education. | Although < 5% of participants identified alcohol as a barrier to ART adherence, most providers identified alcohol as a barrier to adherence based on patients forgetting healthcare appointments when intoxicated. |
| Mukumbang et al., (2017) | Semi-structured interviews and focus group discussions | Forgetting | 56% female; PLWH (N = 45) and healthcare providers (N = 20) from HIV clinics in Zambia | Barriers to retention in HIV care included: individual (e.g., alcohol, side effects), interpersonal (e.g., stigma), institutional (e.g., drug shortages), and community (e.g., food insecurity) factors. | Heavy drinking was reported to affect ART adherence by causing participants to forget to take medications. |
| Nkosi et al., (2016) | Focus group discussions | Forgetting; Interactive toxicity beliefs | 100% male; PLWH (N = 27) from HIV clinics in South Africa | Major themes regarding masculinity, alcohol use, and ART adherence included: construction of a health-oriented masculinity that challenged masculine norms, masculinity and HIV-related stigma, and power dynamics in patient-provider relationships that left men feeling powerless. | Participants reported that providers discouraged them from drinking alcohol due to interactive toxicity beliefs, and that healthcare professionals advised against consuming alcohol while on ART because it can cause patients to forget to take ART medications. Participants were resistant to following provider advice to abstain from alcohol while on ART. |
| Oliveira Serra et al., (2017) | Focus group discussions | Interactive toxicity beliefs; Stigma | 44% female; Age range 28–70; PLWH (N = 25) from an HIV clinic in Brazil | Participants were not aware of the effects of using alcohol and other drugs on the reduction of immunity, did not endorse interactive toxicity beliefs, nor beliefs about alcohol and immune functioning. | Participants reported that there are no problems with mixing alcohol with ART, despite hearing otherwise from healthcare providers. |
| Pengpid et al., (2014) | Semi-structured interviews | Interactive toxicity beliefs | 42% female; M age = 37; PLWH (N = 26) from HIV clinics in South Africa | Participants reported developing routines to drink alcohol around the times they took ART to reduce negative consequences, and reducing alcohol use when initiating ART or following education by health care staff. | Participants reported engaging in interactive toxicity avoidance behaviors (e.g., avoiding taking ART while drinking). |
| Sankar et al., (2007) | Semi-structured interviews | Interactive toxicity beliefs | 25% female; M age = 43.3; PLWH (N = 82) and healthcare providers (N = 17) from HIV clinics in the US. | Almost the entire sample (85–90%) endorsed interactive toxicity beliefs, and 51% reported not taking ART while drinking. Participants who were categorized as heavy drinkers were less likely to endorse toxicity beliefs. | Four potential explanatory models of the alcohol/ART interaction were identified: (a) Alcohol reduces the effectiveness of ART; (b) Alcohol is toxic and should never be mixed with medication; (c) Alcohol can worsen HIV by impairing the immune system; and (d) Alcohol has no impact on HIV or ART. |
| Schensul et al., (2017) | Semi-structured interviews | Forgetting; Interactive toxicity beliefs | 100% male; PLWH (N = 50) from HIV clinics in India | Barriers and facilitators to adherence identified by PLWH included: alcohol use, social support (e.g., transportation to the HIV clinic, emotional support), and psychological factors. | The association between alcohol and ART adherence was explained by: forgetting to take ART when drinking and skipping ART due to interactive toxicity beliefs. |
| Sileo et al., (2019) | Semi-structured interviews | Forgetting; Interactive toxicity beliefs | 100% male; M age = 34; PLWH (N = 30) from fishing communities in Uganda | Alcohol was identified, unsolicited, as the primary barrier to ART adherence. Men reported that alcohol use occurs in social and occupational settings, is heavily influenced by peers, is normative in fishing communities, and is used to relieve stress. All men reported that PLWH should reduce or abstain from alcohol use. | Alcohol use was identified as a barrier to ART adherence via cognitive impairment and intentionally skipping doses while drinking. Participants described reducing alcohol consumption after HIV-diagnosis based on recommendations from care providers. |
| Sorsdahl et al., (2019) | Focus group discussions | Forgetting; Interactive toxicity beliefs | 57% female; PLWH (N = 23) from hospitals in South Africa | General barriers to adherence included: wait-times at HIV clinics, ability to attend clinic appointments, stigma, and ART side effects. | Participants reported that alcohol consumption was related to unintentional missed doses related to forgetting and/or failing to prioritize ART during drinking events and/or feeling too hungover to take their ART. Other participants reported intentional nonadherence due to the belief that mixing alcohol with ART was harmful. |
Note.
All studies described potential mechanisms in the introduction, results, and/or discussion sections. Sample demographic information reported at follow-up is presented when possible, otherwise baseline sample information is presented. PLWH = people living with HIV; ART = Antiretroviral Therapy; US = United States; IPV = intimate partner violence
Aim 1: Replicate and extend previous reviews of the global association literature.
Of the 184 global association studies identified by this review, 135 were cross sectional and 49 were longitudinal; 53 of the studies were published between 1998–2009 and 131 were published between 2010–2019. We compared studies that were published pre- and post-2009 on the type of alcohol consumption and ART adherence measures used, the number of global and event-level studies included, and the general conclusions reached from the included studies. Consistent with previous reviews, there was considerable heterogeneity in the assessment of both alcohol use (see Figure 3) and ART adherence (see Figure 4), as well as wide variability in the time frames over which both were assessed (e.g., alcohol use over the previous year, ART adherence over the previous 30-days). The frequency of the measures used to assess alcohol consumption has changed since 2009, with an increase in the use of validated measures (e.g., the AUDIT), and a decrease in the use of non-specific dichotomous categorizations (see Figure 3). For example, prior to 2010, 26% of the global association studies available in the literature used a dichotomous drinker/nondrinker measure, compared to 18% of the global association published between 2010–2018. Similarly, only 6% of studies published prior to 2010 used the AUDIT, and no studies used the AUDIT-C, whereas after 2010, 31% of the global association studies used the AUDIT and 12% used the AUDIT-C. Similar changes were observed in the adherence measures, with a decrease in the use of percent adherent and dichotomous measures over time, from 38% to 26% and 11% to 5%, respectively (see Figure 4). There was a concomitant, but small, increase in the use of visual analog scale (VAS) ratings of adherence (from 2% to 11%), unannounced pill counts (from 4% to 7%), multiple measures of adherence (from 9% to 12%), and pharmacy refills (from 0% to 2%), indicating a subtle shift towards more objective measurement. The primary methodological change over time however was the increase in the number of event-level studies from zero (pre-2010) to seven (five unique studies). The conclusions that can be drawn from the 1998–2009 and 2010–2019 subgroups of global association studies are remarkably similar—the global association studies generally revealed a significant and negative association between alcohol use and ART adherence, which was observed regardless of study design and/or mode of measurement.
Figure 3. Frequency of Alcohol Use Measures.
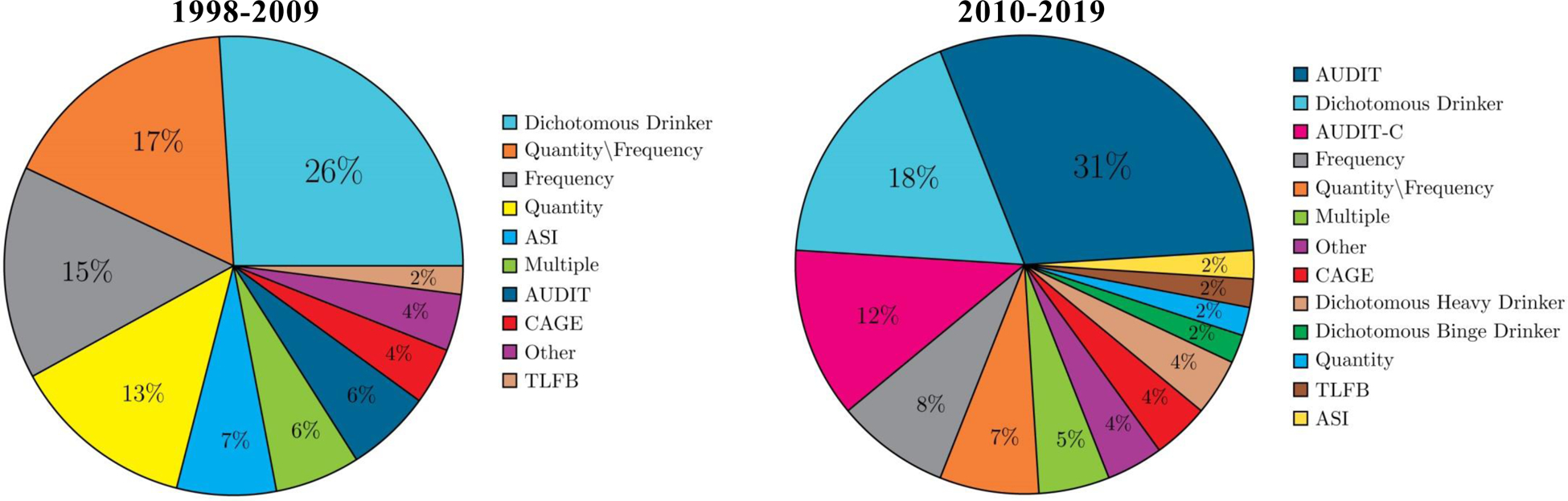
Note. AUDIT = Alcohol Use Disorder Identification Test, AUDIT-C = Alcohol Use Disorder Identification Test-Consumption, ASI = Addiction Severity Index, CAGE = Cut, Annoyed, Guilty, Eye-Openers Alcohol Screening Questionnaire, TLFB = Timeline Followback.
Figure 4. Frequency of ART Adherence Measures.
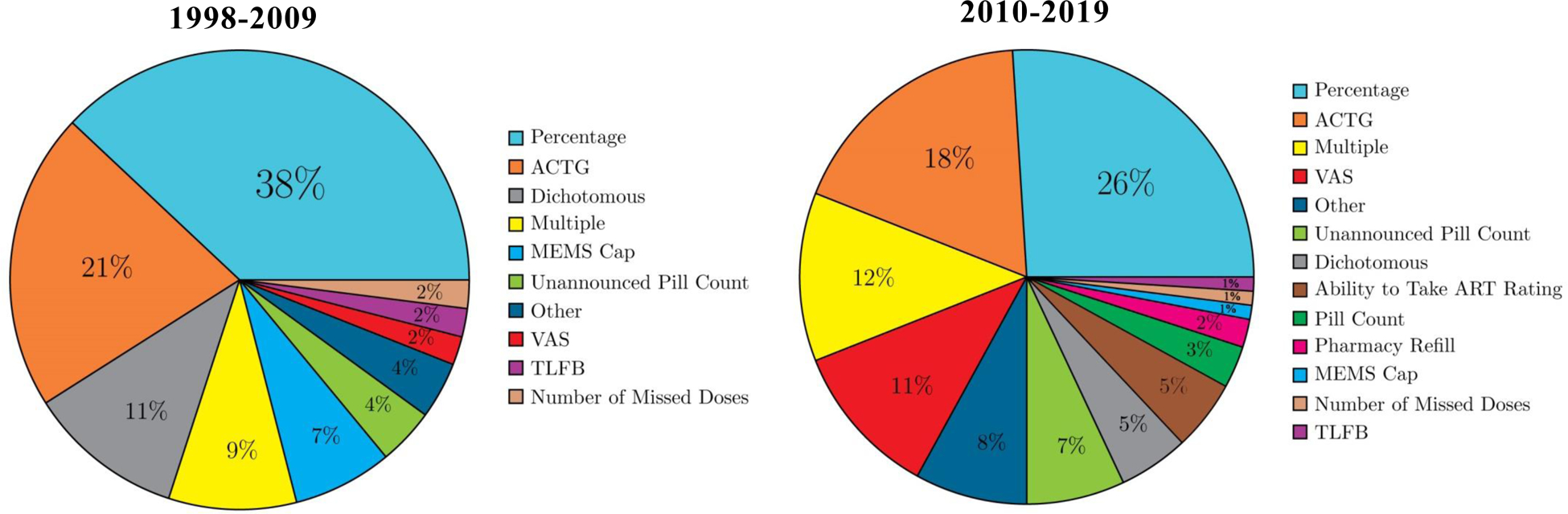
Note. ACTG = AIDS Clinical Trials Group, MEMS Cap = Medication Event Monitoring System Cap, TLFB = Timeline Followback, VAS = Visual Analog Scale.
Aim 2: Summarize and critique study designs capable of answering questions about temporal overlap.
Our search revealed two publications, representing one unique situational association study (Kalichman, Horne, et al., 2019; Kalichman, Katner, et al., 2019) and seven publications, representing five unique event-level studies (Kalichman et al., 2013, 2014; Parsons et al., 2008; Pellowski, Kalichman, Cherry, et al., 2016; Pellowski, Kalichman, Kalichman, et al., 2016; J. Schensul et al., 2017; Sileo et al., 2016). The two publications that examined the situational association between alcohol use and ART adherence asked 124 PWH who reported current alcohol consumption to report if they had “practiced” one of six different alcohol-ART avoidance behaviors (e.g., “I skip taking my HIV medications if I will be drinking”). Participants with suppressed viral load were significantly less likely to endorse skipping ART while consuming alcohol, compared to participants with unsuppressed viral load, although only a minority of participants endorsed this item (7% of participants who were virally suppressed and 18% of participants who were unsuppressed).
A total of seven publications, representing five unique studies, assessed discrete drinking events and examined the event-level co-occurrence of alcohol consumption and ART adherence (Kalichman et al., 2013, 2014; Parsons et al., 2008; Pellowski, Kalichman, Cherry, et al., 2016; Pellowski, Kalichman, Kalichman, et al., 2016; J. Schensul et al., 2017; Sileo et al., 2016). Three of the event-level studies were conducted in the U.S., one study was conducted in South Africa (Sileo et al., 2016), and one was conducted in India (J. Schensul et al., 2017). Two of the studies involved the collection of retrospective daily data using the TLFB (Parsons et al., 2008; J. Schensul et al., 2017), and the remaining three collected prospective daily data using cell-phone-based text messages and/or phone interviews, sometimes supplemented with unannounced pill counts (Kalichman et al., 2013, 2014) or Wisepill monitoring (Pellowski, Kalichman, Cherry, et al., 2016; Pellowski, Kalichman, Kalichman, et al., 2016).
Parsons et al. (2008) were the first to publish findings on the day-level association between alcohol use and ART adherence among a sample of 272 predominantly male (78%) and Black (58%) at-risk drinkers. Using retrospective daily data collected via the TLFB, Parsons et al. (2008) examined the association between the number of standard drinks consumed on a given day and the odds of ART nonadherence on that same day. Multilevel modeling was used to estimate the odds of ART nonadherence at the within-person level, while also accounting for several between-subjects factors (e.g., attitudes towards HIV medication adherence, demographics). Results indicated that the odds of ART nonadherence on a given day increased by 1.20 (95% CI = 1.18, 1.22) for each standard drink consumed. In analyses in which alcohol use was treated as a dichotomous variable, the odds of nonadherence on days in which alcohol was consumed were 8.78 times higher (95% CI = 7.16, 10.77) compared with days in which alcohol was not consumed. These findings were consistent with the other event-level study that used the TLFB with a sample of HIV-positive men from India (J. Schensul et al., 2017). Results also supported a strong and significant association between alcohol consumption and ART adherence, with nonadherence significantly more likely to occur on drinking days compared to nondrinking days, and significantly more likely on binge drinking days (defined as six or more drinks), compared to non-binge drinking days (J. Schensul et al., 2017).
The three other event-level studies were prospective, cell-phone-based, daily diary studies. Pellowski and colleagues (2016) enrolled 59 PWH (68% men) who reported experiencing food insecurity in the previous month. Alcohol consumption was assessed via daily text message surveys, and ART adherence was assessed via daily electronic medication monitoring (Wisepill) for a period of 45 days. Consuming alcohol on a given day was significantly associated with missing a dose of medication on that same day (Pellowski, Kalichman, Cherry, et al., 2016). In another analysis of these data, the association between quantity of alcohol consumed on a given day and medication nonadherence on that same day was no longer significant in a multivariate multilevel model that included a variable measuring alcohol-ART interactive toxicity beliefs, which were significantly, and negatively, associated with adherence (Pellowski, Kalichman, Kalichman, et al., 2016).
A longitudinal measurement burst design in which ten consecutive days of daily data were collected six times during a 12-month period, was used to investigate interactive toxicity beliefs in a sample of 178 PWH (30% women; Kalichman et al., 2013). In one analysis of these data, three days of daily alcohol consumption assessments were compared to three days of retrospective self-reported adherence data (Kalichman et al., 2013). Participants who reported at baseline that that they skip or stop their ART when drinking (i.e., adherence avoidance behaviors), were almost three times more likely to have days with concurrent alcohol consumption and missed medications, compared with participants who did not report adherence avoidance behaviors (aOR = 2.90; 95% CI = 1.09,7.68; Kalichman et al., 2013). In another analysis of these data, Kalichman et al. (2014) compared participants who had taken at least 85% of their prescribed ART (assessed by monthly unannounced cell-phone-based pill counts) over the course of the study with participants who had taken less than 85% of prescribed ART. Those categorized as less than 85% adherent were 3.26 times (95% CI = 1.82, 5.61) more likely to report missing medication on days alcohol was consumed in analyses adjusted for marital status, employment status, and age. There were no analyses in either publication directly comparing adherence on drinking days to adherence on non-drinking days.
A 42-day daily diary study of 74 (70% women) PWH from South Africa did make this comparison, and found that participants who consumed any alcohol (aOR = 3.18, 95% CI = 2.25, 4.49), low to moderate levels of alcohol (for men: 1–4 drinks; for women: 1–3.3 drinks; aOR = 4.29, 95% CI = 2.81, 6.56), and binge-levels of alcohol (for men 6+ drinks; for women 3.4+ drinks; aOR = 2.31, 95% CI = 1.56, 3.42) on a given day were 2–4 times more likely to report missing ART on that same day, compared with days in which no alcohol was consumed (Sileo et al., 2016). The analyses controlled for sociodemographic variables (gender, age, education, SES, and marital status) and time. Furthermore, the results were similar in analyses that controlled for each person’s average level of consumption, with each additional drink beyond what the participant typically drinks associated with significantly increased odds for ART nonadherence (aOR = 1.06, 95% CI = 1.02, 1.11).
It is noteworthy that only 5% of the quantitative studies in this review collected event-level data, and of these, three studies collected prospective daily data— the only design capable of examining the co-occurrence of alcohol and ART nonadherence over time. Even among the three daily diary studies, only one included analyses that compared adherence on drinking and non-drinking days (Sileo et al., 2016). This study also stands out for the examination of different levels of alcohol consumption and the statistical control for each person’s average level of consumption. It is studies like Sileo et al. (2016) that permit conclusions about temporal overlap and provide a template for replication in other samples of PWH.
Aim 3: Extract and summarize potential mediators and mechanisms of the alcohol use-ART relation from the reviewed studies.
There were only three articles that examined a statistical mediator of the alcohol consumption – ART adherence relation, and we could not find an article that formally examined a mechanism – i.e., the steps or processes that explain the ways in which alcohol has an effect on ART adherence (Kazdin, 2007). However, we did find several quantitative and qualitative articles that discussed potential mechanisms of action. We summarize first the studies that conducted a statistical test of mediation, followed by a summary of the most commonly proposed mechanisms of action.
Summary of quantitative studies that conducted a mediation analysis.
We identified three studies that conducted a mediation analysis in which the direct and indirect association of alcohol consumption on ART adherence was examined via an intervening variable (Kekwaletswe et al., 2017; Malow et al., 2013; Tucker et al., 2004). All three of the studies were conducted with cross-sectional data, which prevented conclusions about temporal ordering and the direction of the associations. The predictions about the role of the mediating variable also appeared to be determined from the data available rather than generated a priori based on commonly applied theory of health behavior. It is difficult to interpret these findings beyond the idea that the variables are all correlated in some way. Indeed, in any mediation analysis, a significant mediation statistic can occur for several reasons (e.g., the statistically significant mediator is simply a correlate of the dependent variable, or a correlate of the true mediator; Fiedler et al., 2011), and cannot, especially without an a priori theoretical rationale, be taken as evidence of a causal process. We therefore focus the remainder of the review on studies that are relevant to testing theoretical mechanisms of action.
Summary of studies that describe a potential mechanism of action.
Of the 230 records that met eligibility criteria to be included in this review, 31% (n = 72; 48 quantitative studies, 24 qualitative studies) discussed a potential mechanism by which alcohol use influences ART adherence. The mechanisms described primarily involved the negative effect of alcohol consumption on the ability to remember to take medications (i.e., “forgetting”; n = 23 studies), and alcohol-ART interactive toxicity beliefs (n = 17 studies); 25 studies described both mechanisms in the same article. These two mechanisms explain two different types of adherence, unintentional (forgetting) and intentional (toxicity beliefs and associated behaviors), a distinction that has been recognized in the broader adherence literature for some time (Clifford et al., 2008), and which is addressed in more detail in the Discussion section.
Cognitive impairment/forgetting.
Of the studies that mentioned a mechanism, 71% (n = 17) of the qualitative studies and 65% (n = 31) of the quantitative studies mentioned a mechanism consistent with alcohol consumption-related “forgetting.” Participants expressed this idea repeatedly in interviews and focus groups with HIV patients and providers. Although none of the qualitative studies were designed to specifically probe for evidence of this mechanism, the ways in which alcohol consumption was described as inducing forgetfulness were remarkably consistent across studies conducted worldwide. Also noteworthy is that of the qualitative studies that were designed to gather open-ended feedback about general barriers to ART adherence (Adeniyi et al., 2018; Axelsson et al., 2015; Barnett et al., 2013; Bukenya et al., 2019; Dahab et al., 2008; Moucheraud et al., 2019; Mukumbang et al., 2017), rather than alcohol-specific barriers, alcohol consumption and alcohol-induced forgetting emerged as one of a few primary barriers to adherence. In these studies, all conducted in sub-Saharan Africa, alcohol was frequently described as “mak(ing) you forget things” (Dahab et al., 2008, p. 3), especially taking ART as-prescribed (“when I got drunk I used to forget to take my drugs,” Mukumbang et al., 2017, p. 5; “I was drinking beer and was forgetting an entire week,” Axelsson et al., 2015, p.14; “I take alcohol excessively and not only forget to take food, but also drugs,” Bukenya et al., 2019, p.4) and attending medical appointments (“they get drunk and forget their appointment dates,” Moucheraud et al., 2019, p.409). In the qualitative studies specifically designed to examine alcohol-related adherence barriers (Conroy et al., 2017; Conroy et al., 2020; Hershow et al., 2018; Madhombiro et al., 2018; Nkosi et al., 2016; S. Schensul et al., 2017; Sileo et al., 2019; Sorsdahl et al., 2019), alcohol consumption was again described as inducing forgetfulness that leads to medication nonadherence as described by couples from Malawi (“when I drank beer that day it was very difficult for me to remember to take my medications,” Conroy et al., 2017 p. 206), men and women in Zimbabwe (“you can forget to take your medication when you are drunk,” Madhombiro et al., 2018 p. 49), hazardous-drinking PWH from Vietnam (“drinking wine had made me forget to take medicine,” Hershow et al., 2018, p. 6), and men living with HIV in South Africa (“If you drink you are likely to forget to take your treatment,” Nkosi et al., 2016, p. 372), India (“I forget because I drink,” S. Schensul et al., 2017, p. 721), and Uganda (Sileo et al., 2019).
Interactive Toxicity Beliefs.
The belief that alcohol is toxic when combined with ART was mentioned in a majority (67%; n = 16) of the qualitative and quantitative 54% (n = 26) studies that described a mechanism. This belief was prevalent among both patients and providers in several different regions of the world, leading to intentional avoidance of ART when consuming alcohol. Again, the qualitative studies on general barriers to adherence were consistent in identifying beliefs about the interactive toxicity of alcohol and ART as among the most frequently cited reasons for nonadherence among providers and PWH in South Africa (Barnett et al., 2013; Fitzgerald et al., 2010), Tanzania (Lyimo et al., 2012), Ethiopia (Balcha et al., 2011), Uganda (Bukenya et al., 2019), and the U.S. (Laws et al., 2015). In qualitative studies specifically designed to examine alcohol-related ART adherence barriers among drinkers, a majority of the participants in the studies endorsed toxicity beliefs and associated behaviors, which were again remarkably consistent across diverse settings. At-risk drinkers from South Africa (“I don’t take ARVs when I have been drinking. I feel that I don’t want to mix my treatment with alcohol,” Pengpid & Peltzer, 2014, p. 390; “the pills that we are taking do not work with alcohol,” Sorsdahl et al., 2019, p. 1051), light and moderate drinkers from the U.S. (“My doctor told me they don’t mix. You’re not supposed to take alcohol when taking the HAART medication – period,” Sanker et al., 2007, p. 198), drinkers from El Salvador (“It’s not because I forget or because I didn’t bring it with me. It’s because you can’t take it with alcohol,” Dickson-Gomez et al., 2015, p. 1727), Zimbabwe (“alcohol and medication are not compatible,” “there will be competition between alcohol and medication,” Madhombiro et al., 2018, p. 49), and Uganda (“since I am drunk, if I swallow my medication I would go through a very bad experience,” Sileo et al., 2019, p. 38), couples from Malawi (“My partner drinks alcohol and the medication cannot be combined with alcohol,” Conroy et al., 2020, p. 1603), and South Africa (“most of them who were doing that have died, which means they are dangerous once you stop taking them or mix them with alcohol,” Conroy et al., 2017, p. 1887), men living with HIV in South Africa (“alcohol effects ARVs” Nkosi et al., 2016, p. 372), India (“Yes, when I drink that day, I skip the medicine. I have a fear, if I take the medicine then it will have an effect on my health,” S. Schensul et al., 2017, p. 721), and Uganda (“It is not that I just forget to take my medication, but just fear that since I have taken some alcohol…I would go through a very bad experience,”; Sileo et al., 2019, p. 38) all described the fear of mixing alcohol with ART and ways in which it results in intentional nonadherence. Of note, the strength and influence of toxicity beliefs may vary by drinking quantity and frequency, with heavier drinkers less likely to endorse toxicity beliefs and behaviors compared with infrequent or moderate drinkers (Laws et al., 2015).
In summary, alcohol was described as having an effect on adherence via two distinct pathways: unintentional nonadherence associated with forgetting, and intentional nonadherence associated with toxicity beliefs. The ways in which these mechanisms were described were highly consistent across many different study populations, recruited from countries all over the world. A model of the causal process by which alcohol consumption results in nonadherence should thus include each as potential mechanism of action.
DISCUSSION
We conducted a systematic review of 230 studies that examined the association between alcohol consumption and ART adherence. The global association studies were consistent in showing a significant, and negative, correlation between the two variables. The increase in the number of event-level studies observed, the majority of which were published after the Hendershot et al. (2009) and Grodensky et al. (2012) reviews, is encouraging and provides some insight into the co-occurrence of alcohol consumption and nonadherence on a given day. These studies revealed that nonadherence is 2–4 times more likely on days in which alcohol is consumed, compared with the days in which alcohol is not consumed. The qualitative studies reviewed were also remarkably consistent in describing the cognitive effects of alcohol consumption on adherence, and the influence of toxicity beliefs. We next describe existing theory on ART adherence and the acute effects of alcohol consumption on human behavior, and subsequently propose a model that synthesizes both with the research reviewed in this paper. The derived model emphasizes the research findings about possible mechanisms underlying the inverse relation between alcohol consumption and ART adherence.
General theories of adherence.
Theoretical models used to guide general medication adherence interventions (Conn, Enriquez, et al., 2016; Conn, Ruppar, et al., 2016) are similar to those used in the ART adherence literature (Amico et al., 2018; Munro et al., 2007), and map on to well-established theories of health behavior such as the Information-Motivation-Behavioral Skills (IMB) model, Social Cognitive Theory (SCT), and the Theory of Planned Behavior (TPB). There is significant overlap in the core constructs of these theories, which focus on beliefs and knowledge about medications, motivation and self-efficacy for taking medications as prescribed, and intentions and goal setting for achieving optimal adherence. While no single theory has emerged as superior, models that integrate aspects of knowledge, expectancies, beliefs, motivation, and skills tend to produce the largest intervention effects (Conn, Enriquez, et al., 2016; Conn, Ruppar, et al., 2016).
In a review of health behavior theories that have been applied to ART adherence (Munro et al., 2007), the IMB model (Fisher et al., 2008) emerged as unique given that it was developed specifically to explain HIV-related behavior and to guide the development of ART adherence support strategies. The IMB model suggests that ART adherence information (e.g., regimen complexity, side effects, correct utilization) and adherence motivation (e.g., personal attitudes and beliefs about the outcome of suboptimal adherence, social beliefs about significant other’s support for adherence and motivation to comply with these beliefs) directly and indirectly predict adherence behavior (i.e., taking ART as prescribed) via self-efficacy for ART adherence behavioral skills (e.g., self-cueing and self-administering ART medications). The IMB model also describes several moderating factors, one of which is “acute impairments in psychological functioning” consistent with substance use, although it does not systematically integrate the effects of alcohol consumption on human behavior – which is characteristic of all the health behavior theories described above. We thus describe next theory on the acute effects of alcohol on behavior, and subsequently integrate this theory with Fisher et al.’s (2008) IMB model.
The acute effects of alcohol consumption on human behavior.
We argue that of the numerous pharmacological effects of alcohol consumption on human behavior, those on cognitive functioning are most pertinent to medication adherence. In this regard, it is well-established that at low to moderate doses (blood alcohol concentrations [BACs] of .07%) or higher, alcohol consumption reliably impairs memory and other neurocognitive functions (e.g., Cromer et al., 2010; Mintzer, 2007). The immediate effect in the context of medication adherence is that individuals are more likely to forget to take their medications as prescribed when their BACs are in the .07% range. This “forgetting,” or more precisely not remembering to remember, can also be conceived of as a prospective memory failure, which has been shown in experimental studies to be acutely affected by alcohol consumption (Leitz et al., 2009; Montgomery et al., 2011; Paraskevaides et al., 2010; Smith-Spark et al., 2016; Walter & Bayen, 2016), and uniquely related to medication adherence among PWH (Avci et al., 2018; Woods et al., 2007).
An integrated conceptual model of alcohol consumption and ART adherence.
The IMB model and theory on the acute effects of alcohol ingestion can be combined to understand how alcohol consumption affects intentional and unintentional ART nonadherence, and how this effect fits within the broader general adherence literature. Figure 5 presents the IMB model of ART adherence as described by Fisher et al. (2008). This model succinctly categorizes the many correlates of ART adherence identified in the literature, and how they are related to adherence behavior and HIV-related outcomes. We propose that the path from adherence-related behavioral skills to adherence behavior is moderated by alcohol consumption. When alcohol is consumed, it can influence adherence behavior in one of two ways: (1) via the cognitive effects of alcohol consumption theorized to cause a prospective memory failure, leading to unintentional ART nonadherence or (2) via medication-related beliefs about mixing alcohol and ART (i.e., interactive toxicity beliefs), leading to intentional ART nonadherence. We further propose that the main effect of alcohol consumption on intentional and unintentional nonadherence is moderated by several person-level factors including provider beliefs, level of routine or busyness in everyday life, the timing and complexity of the ART regimen, and the presence of other daily medications.
Figure 5.
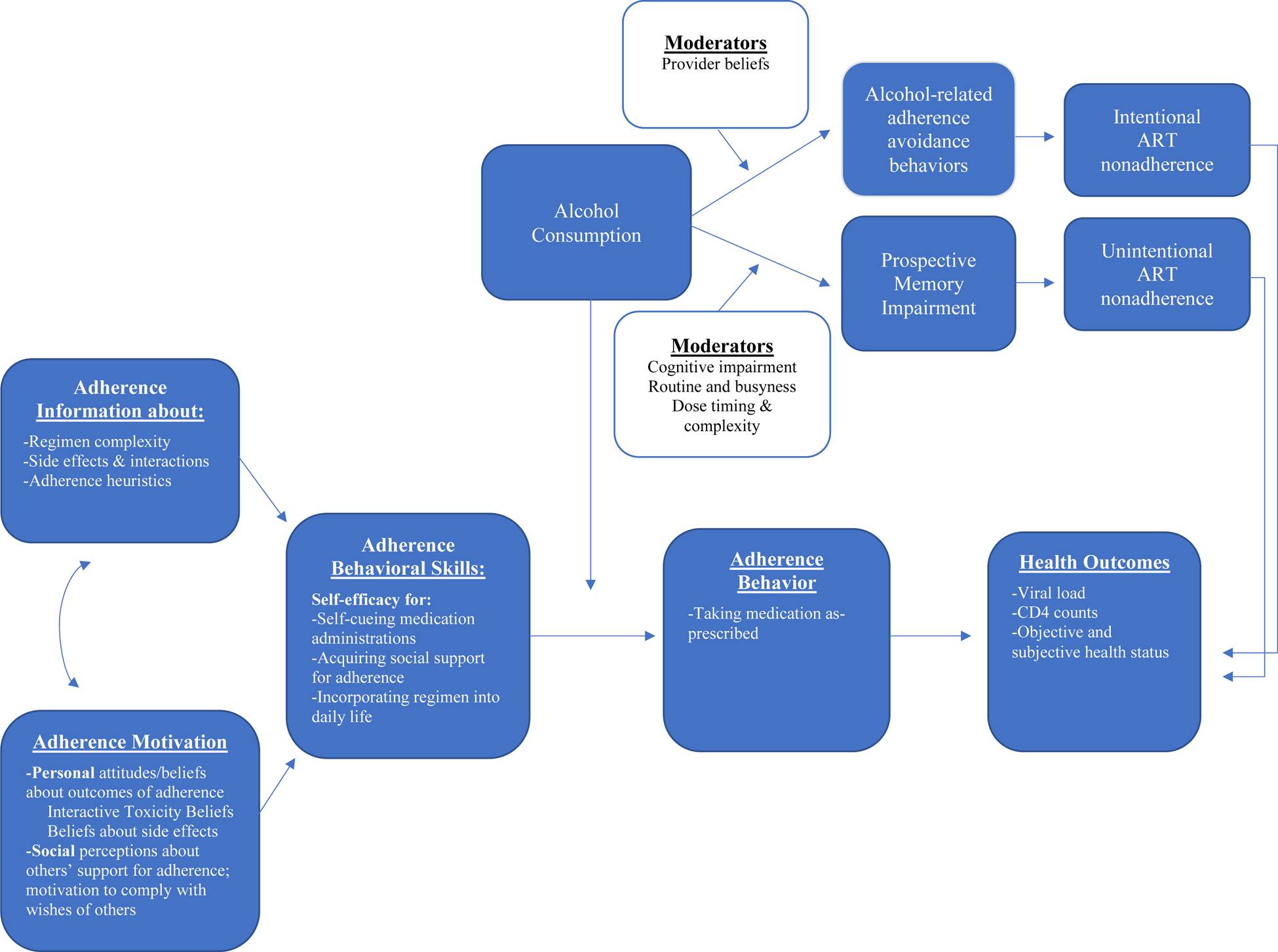
Integrated Model of Alcohol Consumption and ART Adherence
These two proposed mechanisms, and their differential role in unintentional and intentional ART nonadherence, are consistent with the broader adherence literature, which has shown unintentional and intentional nonadherence to be governed by distinct processes (Clifford et al., 2008). Unintentional nonadherers are more likely to endorse “forgetting” as a reason for not taking medication as-prescribed, compared to intentional nonadherers, who are more likely to endorse concerns about the medication and its side effects (Clifford et al., 2008). We discuss next the two alcohol-related mechanisms of unintentional and intentional nonadherence specific to the HIV literature. However, it is important to note that these mechanisms are likely relevant to other chronic diseases for which alcohol has been identified as a significant correlate of nonadherence (e.g., diabetes, hypertension, depression; Grodensky et al., 2012), the implications of which are discussed below.
Alcohol consumption and unintentional nonadherence: Prospective memory as a mechanism of action.
We were unable to locate a publication that has formally examined cognitive impairment from alcohol consumption as a mechanism of the alcohol-unintentional nonadherence relation. There is however literature suggesting that the “forgetting” so commonly reported by PWH who consume alcohol is a particular type of cognitive impairment, known as a prospective memory failure, that is influenced by both alcohol consumption and HIV status, and uniquely implicated in medication-taking behavior. Prospective memory, or remembering to remember, is the “neurocognitive capacity to successfully form, maintain, and execute an intention at a particular point in the future,” (Zogg et al., 2012, p. 48). Prospective memory is a process that involves several stages, the first of which is the formation of an intention (e.g., I must take my medication at 9 a.m.), followed by a delay (e.g., minutes, hours) in which attention is allocated towards competing activities of daily life. The environment is continuously monitored for a relevant cue that signals intention retrieval, and, once detected, the content of the intention (e.g., take my medication at 9 a.m.) is recalled and then ultimately executed (e.g., the pill is extracted from the medicine bottle and swallowed). Impairment in this prospective memory process is prevalent among PWH, and is uniquely associated with ART nonadherence even when controlling for global cognitive impairment (Woods et al., 2008). Additionally, alcohol administration studies have shown that low to moderate doses of alcohol (i.e., BAC = .02% – .07%) significantly impair performance on prospective memory tasks among undergraduate social drinkers (Leitz et al., 2009; Montgomery et al., 2011; Paraskevaides et al., 2010; Smith-Spark et al., 2016; Walter & Bayen, 2016), although there are no experimental studies on the effects of alcohol consumption on prospective memory impairment among PWH. We view this as a significant gap in the literature given that alcohol consumption has been shown to have unique acute effects on PWH when compared to HIV-uninfected controls (Justice et al., 2016; Shuper et al., 2018).
The extent to which alcohol-associated prospective memory impairment affects ART adherence likely depends, at least in part, on the level of routine in one’s everyday life, the timing of the ART dose, the complexity of the ART regimen, and the presence and complexity of other pharmacological treatments. The literature on alcohol use and ART adherence has not addressed the role of these factors, in particular the presence of multiple concurrent medication regimens for comorbid conditions, which we view as an important direction for future research (Back & Marzolini, 2020; Mata-Marín et al., 2019; Ware et al., 2019). Regarding the timing of the ART dose, the role of acute intoxication, and its effects on prospective memory processes that could affect ART adherence, is certainly different if the ART dose has already been administered when the drinking event is initiated (e.g., a once-a-day pill is taken in the morning, and alcohol consumption is initiated in the evening; Marcum & Gellad, 2012). The routine disruption and cognitive effects of intoxication can however persist the day following a drinking event (i.e., hangover effects), causing a prospective memory failure that could affect medication adherence the following day. Indeed, event-level data on alcohol and adherence among PWH have shown significant, negative effects on both the day-of and day-after a drinking event (Braithwaite et al., 2008). Alcohol administration studies with PWH that examine the effect of a controlled dose of alcohol on prospective memory tasks, coupled with daily diary studies that account for regimen complexity, timing, and comorbid pharmacological treatments, are needed in order to fully test the model presented in Figure 5 (Justice et al., 2018; Manzano-García et al., 2018).
Alcohol consumption and intentional nonadherence: Toxicity beliefs and adherence avoidance behavior as a mechanism of action.
Kalichman and colleagues have led the field in the exploration of interactive toxicity beliefs and intentional nonadherence, demonstrating that between 10% – 43% of HIV-positive drinkers report intentionally skipping or stopping their ART medication when consuming alcohol (Kalichman et al., 2009; Kalichman, Katner, et al., 2019). The prevalence of interactive toxicity beliefs among PWH who report intentionally skipping or stopping ART while drinking is predictably high – about 80% endorse that mixing HIV medications and alcohol is not safe and/or that ART does not work well when consuming alcohol (Kalichman et al., 2013). These beliefs have been reliably linked to intentional nonadherence in daily diary studies – PWH who endorse interactive toxicity beliefs are significantly more likely to miss doses of ART on drinking days (Kalichman et al., 2013; Pellowski, Kalichman, Kalichman, et al., 2016). Conceptually, when alcohol is consumed, toxicity beliefs activate what Kalichman, Katchner, and colleagues (2019) term “alcohol-ART avoidance behaviors”–e.g., intentionally stopping or skipping HIV medications while drinking. Although the temporal ordering of alcohol consumption, toxicity beliefs, alcohol-ART avoidance behaviors, and ART adherence has not been established empirically, a preliminary serial mediation model (Kalichman, Katner, et al., 2019) indicates that this sequence would likely be supported with intensive longitudinal data.
Limitations
This review has several limitations. First, we did not conduct a quantitative synthesis of the findings, opting instead to provide a descriptive summary of the included studies with a focus on event-level findings and the identification of potential mechanisms of action. Our findings thus cannot be directly compared with Hendershot et al. (2009) and we cannot comment on if the magnitude of the association between alcohol consumption and ART adherence has changed in the past ten years. However, given that the methods for investigating the association between alcohol consumption and ART adherence have changed little since 2009, an updated meta-analysis may be of limited utility until more event-level data are published. Second, the studies reviewed were highly variable in terms of the measurement of alcohol consumption and ART adherence, sample composition, study design, and primary research question. Although we report here broad conclusions about the consistency with which alcohol consumption was correlated with ART adherence across studies, and attempted to more heavily weight event-level data, the heterogeneity of the literature, and the low percentage of event-level studies should be considered in the interpretation of our findings. Finally, given the lack of quantitative studies focused on mediators and/or mechanisms, the conceptual model we proposed is based on qualitative data and theoretical speculation present in the discussion sections of the reviewed manuscripts. It should thus be considered a preliminary characterization of potential mechanistic processes and used as an impetus for hypothesis generation and continued research.
Research and Clinical implications
The literature on alcohol consumption and ART adherence has proliferated over the past two decades – providing numerous correlational studies that consistently show an inverse association between the two variables. A renewed focus on the causal processes and contextual factors that contribute to an alcohol-associated nonadherence event would significantly advance this line of research. Experimental and intensive longitudinal data could be used to test and refine the model described in Figure 5, providing PWH, and the providers who treat them, with more precise and nuanced messages about alcohol’s effect on HIV treatment-related outcomes. Additionally, the growing literature on alcohol interventions for PLH could advance our understanding of the mechanisms underlying the alcohol-ART adherence relation and provide some evidence of a causal effect if changes in ART adherence in the experimental condition could be accounted for by changes to drinking behavior. The proposed model could also be used to include variables relevant to mechanistic processes in the design of future alcohol intervention RCTs. This would allow for analyses that could examine the degree to which changes in ART adherence are predicted by the processes such as prospective memory impairment and/or toxicity beliefs.
The mechanisms described in this review also have implications for nonadherence to treatments for medical conditions other than HIV. Multiple studies have shown alcohol use to be correlated with nonadherence to treatment for diabetes, hypertension, and depression (Grodensky et al., 2012) – diseases that are among the most widespread and costly in the U.S. (CDC, 2019). The role of prospective memory in particular is likely to be relevant to any chronic disease for which regular adherence to medications is required – especially in populations for which prospective memory is already impaired (e.g., aging populations). Future work is needed to clarify the extent to which findings from the HIV literature can be applied to people living with other chronic diseases.
As the field moves towards studies that can better answer questions about causality and the mechanisms of action proposed in Figure 5, we have a few implementable recommendations for HIV care providers: (1) it is important to assess for interactive toxicity beliefs and provide accurate information about the importance of taking ART even when alcohol is consumed. Kalichman and colleagues have developed a three-item scale (Kalichman et al., 2009), based on qualitative data from PWH, that has been used in several research studies (Kalichman et al., 2015; Pellowski, Kalichman, Kalichman, et al., 2016), and could be feasibly integrated into HIV care. The scale, especially if administered at treatment-initiation, could be used to alert providers to the presence of interactive toxicity beliefs, and prompt a corrective discussion. (2) Screening for, and offering evidence-based treatment of, at-risk alcohol consumption has many benefits for PWH, and would likely improve HIV-related outcomes via increased adherence to ART. While there are, at present, few efficacious interventions for alcohol use among PWH (Madhombiro et al., 2019; Scott-Sheldon et al., 2017), there is some evidence that screening, brief intervention, and self-monitoring via daily cell-phone based assessments can significantly reduce quantity and frequency of alcohol use among PWH (Aharonovich et al., 2017; Chander et al., 2015; Hasin et al., 2013; Kahler et al., 2018; Papas et al., 2011; Velasquez et al., 2009). These interventions can be feasibly integrated into HIV care and, at a minimum, alert the patient and the provider to the potential role of alcohol consumption in the treatment of the patient’s HIV. (3) For patients who report “forgetting” to take medication as a common consequence of alcohol consumption, creating an individualized action plan for taking medications prior to the drinking event and/or increasing the level of routinization in the patient’s daily life may help decrease alcohol-associated unintentional nonadherence. While it is premature to directly address prospective memory processes in ART adherence, helping patients understand how and why they “forget” to take medications while drinking may increase self-monitoring of adherence in anticipation of and/or during drinking events.
Conclusions
Alcohol consumption is associated with both intentional and unintentional nonadherence to ART. The mechanisms underlying this association have yet to be fully established, although theory and correlational studies indicate two processes worthy of continued research: prospective memory impairment and interactive toxicity beliefs/avoidance behaviors. A comprehensive model of alcohol-associated ART nonadherence was proposed as a guide for future work, integrating general models of ART adherence and theory on acute alcohol intoxication. This model can be refined as the literature increases in nuance and precision, providing an evidence-based framework for the a priori specification of mechanisms of action (Hagger et al., 2020), and the subsequent development and implementation of efficacious interventions capable of decreasing the impact of alcohol consumption on HIV treatment and prevention.
Acknowledgements:
This work was supported by the National Institute on Alcohol Abuse and Alcoholism (NIAAA) under the following grants: R34AA026246 (Co-PIs: Woolf-King, Maisto), K01AA02167 (PI: Woolf-King), and K05AA016928 (PI: Maisto)
Footnotes
No potential conflict of interest was reported by the authors.
References
- Adeniyi OV, Ajayi AI, Ter Goon D, Owolabi EO, Eboh A, & Lambert J (2018). Factors affecting adherence to antiretroviral therapy among pregnant women in the Eastern Cape, South Africa. BMC Infectious Diseases, 18(1), 175. 10.1186/s12879-018-3087-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aharonovich E, Stohl M, Cannizzaro D, & Hasin D (2017). HealthCall delivered via smartphone to reduce co-occurring drug and alcohol use in HIV-infected adults: A randomized pilot trial. Journal of Substance Abuse Treatment, 83, 15–26. 10.1016/j.jsat.2017.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amico KR, Mugavero M, Krousel-Wood MA, Bosworth HB, & Merlin JS (2018). Advantages to Using Social-Behavioral Models of Medication Adherence in Research and Practice. Journal of General Internal Medicine, 33(2), 207–215. 10.1007/s11606-017-4197-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ammassari A, Trotta MP, Murri R, Castelli F, Narciso P, Noto P, Vecchiet J, D’Arminio Monforte A, Wu AW, Antinori A, & AdICoNA Study Group. (2002). Correlates and predictors of adherence to highly active antiretroviral therapy: Overview of published literature. Journal of Acquired Immune Deficiency Syndromes (1999), 31 (Suppl 3), S123–127. 10.1097/00126334-200212153-00007 [DOI] [PubMed] [Google Scholar]
- Atkinson MJ, & Petrozzino JJ (2009). An evidence-based review of treatment-related determinants of patients’ nonadherence to HIV medications. AIDS Patient Care and STDs, 23(11), 903–914. 10.1089/apc.2009.0024 [DOI] [PubMed] [Google Scholar]
- Avci G, Sheppard DP, Tierney SM, Kordovski VM, Sullivan KL, & Woods SP (2018). A systematic review of prospective memory in HIV disease: From the laboratory to daily life. The Clinical Neuropsychologist, 32(5), 858–890. 10.1080/13854046.2017.1373860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelsson JM, Hallager S, & Barfod TS (2015). Antiretroviral therapy adherence strategies used by patients of a large HIV clinic in Lesotho. Journal of Health, Population, and Nutrition, 33, 10. 10.1186/s41043-015-0026-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azar MM, Springer SA, Meyer JP, & Altice FL (2010). A systematic review of the impact of alcohol use disorders on HIV treatment outcomes, adherence to antiretroviral therapy and health care utilization. Drug and Alcohol Dependence, 112(3), 178–193. 10.1016/j.drugalcdep.2010.06.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Back D, & Marzolini C (2020). The challenge of HIV treatment in an era of polypharmacy. Journal of the International AIDS Society, 23(2), e25449. 10.1002/jia2.25449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balcha TT, Jeppsson A, & Bekele A (2011). Barriers to antiretroviral treatment in Ethiopia: A qualitative study. Journal of the International Association of Physicians in AIDS Care, 10(2), 119–125. 10.1177/1545109710387674 [DOI] [PubMed] [Google Scholar]
- Barnett W, Patten G, Kerschberger B, Conradie K, Garone DB, van Cutsem G, & Colvin CJ (2013). Perceived adherence barriers among patients failing second-line antiretroviral therapy in Khayelitsha, South Africa. Southern African Journal of HIV Medicine, 14(4), 166–169. [Google Scholar]
- Blair JM, Fagan JL, Frazier EL, Do A, Bradley H, Valverde EE, McNaghten A, Beer L, Zhang S, Huang P, Mattson CL, Freedman MS, Johnson CH, Sanders CC, Spruit-McGoff KE, Heffelfinger JD, Skarbinski J, & National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention, CDC. (2014). Behavioral and clinical characteristics of persons receiving medical care for HIV infection—Medical Monitoring Project, United States, 2009. MMWR Supplements, 63(5), 1–22. [PubMed] [Google Scholar]
- Braithwaite RS, & Bryant KJ (2010). Influence of Alcohol Consumption on Adherence to and Toxicity of Antiretroviral Therapy and Survival. Alcohol Research & Health, 33(3), 280–287. [PMC free article] [PubMed] [Google Scholar]
- Braithwaite RS, Conigliaro J, McGinnis KA, Maisto SA, Bryant K, & Justice AC (2008). Adjusting alcohol quantity for mean consumption and intoxication threshold improves prediction of nonadherence in HIV patients and HIV-negative controls. Alcoholism, Clinical and Experimental Research, 32(9), 1645–1651. 10.1111/j.1530-0277.2008.00732.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braithwaite RS, McGinnis KA, Conigliaro J, Maisto SA, Crystal S, Day N, Cook RL, Gordon A, Bridges MW, Seiler JFS, & Justice AC (2005). A temporal and dose-response association between alcohol consumption and medication adherence among veterans in care. Alcoholism, Clinical and Experimental Research, 29(7), 1190–1197. 10.1097/01.alc.0000171937.87731.28 [DOI] [PubMed] [Google Scholar]
- Brandt R (2009). The mental health of people living with HIV/AIDS in Africa: A systematic review. African Journal of AIDS Research: AJAR, 8(2), 123–133. 10.2989/AJAR.2009.8.2.1.853 [DOI] [PubMed] [Google Scholar]
- Bukenya D, Mayanja BN, Nakamanya S, Muhumuza R, & Seeley J (2019). What causes non-adherence among some individuals on long term antiretroviral therapy? Experiences of individuals with poor viral suppression in Uganda. AIDS Research and Therapy, 16. 10.1186/s12981-018-0214-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Center for Disease Control and Prevention. (2019). About Chronic Diseases | CDC. https://www.cdc.gov/chronicdisease/about/index.htm
- Center for Disease Control and Prevention. (2018). CDC - Data and Maps—Alcohol. https://www.cdc.gov/alcohol/data-stats.htm
- Chander G, Hutton HE, Lau B, Xu X, & McCaul ME (2015). Brief Intervention Decreases Drinking Frequency in HIV-Infected, Heavy Drinking Women: Results of a Randomized Controlled Trial. Journal of Acquired Immune Deficiency Syndromes (1999), 70(2), 137–145. 10.1097/QAI.0000000000000679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chander G, Lau B, & Moore RD (2006). Hazardous Alcohol Use: A Risk Factor for Non-Adherence and Lack of Suppression in HIV Infection. Journal of Acquired Immune Deficiency Syndromes (1999), 43(4), 411–417. 10.1097/01.qai.0000243121.44659.a4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifford S, Barber N, & Horne R (2008). Understanding different beliefs held by adherers, unintentional nonadherers, and intentional nonadherers: Application of the Necessity-Concerns Framework. Journal of Psychosomatic Research, 64(1), 41–46. 10.1016/j.jpsychores.2007.05.004 [DOI] [PubMed] [Google Scholar]
- Cohen J (2011). HIV Treatment as Prevention. Science, 334(6063), 1628–1628. 10.1126/science.334.6063.1628 [DOI] [PubMed] [Google Scholar]
- Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, Hakim JG, Kumwenda J, Grinsztejn B, Pilotto JHS, Godbole SV, Mehendale S, Chariyalertsak S, Santos BR, Mayer KH, Hoffman IF, Eshleman SH, Piwowar-Manning E, Wang L, … Fleming TR (2011). Prevention of HIV-1 Infection with Early Antiretroviral Therapy. New England Journal of Medicine, 365(6), 493–505. 10.1056/NEJMoa1105243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conn VS, Enriquez M, Ruppar TM, & Chan KC (2016). Meta-analyses of Theory Use in Medication Adherence Intervention Research. American Journal of Health Behavior, 40(2), 155–171. 10.5993/AJHB.40.2.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conn VS, Ruppar TM, Enriquez M, & Cooper P (2016). Medication adherence interventions that target subjects with adherence problems: Systematic review and meta-analysis. Research in Social & Administrative Pharmacy: RSAP, 12(2), 218–246. 10.1016/j.sapharm.2015.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conroy AA, Ruark A, McKenna SA, Tan JY, Darbes LA, Hahn JA, & Mkandawire J (2020). The Unaddressed Needs of Alcohol-Using Couples on Antiretroviral Therapy in Malawi: Formative Research on Multilevel Interventions. AIDS and Behavior, 24(6), 1599–1611. 10.1007/s10461-019-02653-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conroy AA, Leddy A, Johnson M, Ngubane T, van Rooyen H, & Darbes L (2017). “I told her this is your life”: Relationship Dynamics, Partner Support, and Adherence to Antiretroviral Therapy among South African Couples. Culture, Health & Sexuality, 19(11), 1239–1253. 10.1080/13691058.2017.1309460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook RL, Sereika SM, Hunt SC, Woodward WC, Erlen JA, & Conigliaro J (2001). Problem drinking and medication adherence among persons with HIV infection. Journal of General Internal Medicine, 16(2), 83–88. 10.1111/j.1525-1497.2001.00122.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa JM, Torres TS, Coelho LE, & Luz PM (2018). Adherence to antiretroviral therapy for HIV/AIDS in Latin America and the Caribbean: Systematic review and meta-analysis. Journal of the International AIDS Society, 21(1), e25066. 10.1002/jia2.25066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craw JA, Beer L, Tie Y, Jaenicke T, Shouse RL, & Prejean J (2020). Viral Rebound Among Persons With Diagnosed HIV Who Achieved Viral Suppression, United States. Journal of Acquired Immune Deficiency Syndromes (1999), 84(2), 133–140. 10.1097/QAI.0000000000002321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cromer JR, Cromer JA, Maruff P, & Snyder PJ (2010). Perception of alcohol intoxication shows acute tolerance while executive functions remain impaired. Experimental and Clinical Psychopharmacology, 18(4), 329–339. 10.1037/a0019591 [DOI] [PubMed] [Google Scholar]
- Current Reviews Editorial Staff. (2018). Drinking Patterns and Their Definitions. Alcohol Research: Current Reviews, 39(1), 17–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahab M, Charalambous S, Hamilton R, Fielding K, Kielmann K, Churchyard GJ, & Grant AD (2008). “That is why I stopped the ART”: Patients’ & providers’ perspectives on barriers to and enablers of HIV treatment adherence in a South African workplace programme. BMC Public Health, 8(1), 1–6. 10.1186/1471-2458-8-63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson-Gomez J, Bodnar G, Petroll A, Johnson K, & Glasman L (2015). HIV Treatment for Alcohol and Non-Injection Drug Users in El Salvador. Qualitative Health Research, 25(12), 1719–1732. 10.1177/1049732314568322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler K, Lènàrt A, Lessard D, Toupin I, & Lebouché B (2018). Barriers to antiretroviral therapy adherence in developed countries: A qualitative synthesis to develop a conceptual framework for a new patient-reported outcome measure. AIDS Care, 30(sup1), 17–28. 10.1080/09540121.2018.1469725 [DOI] [PubMed] [Google Scholar]
- Farley J, Miller E, Zamani A, Tepper V, Morris C, Oyegunle M, Lin M, Charurat M, & Blattner W (2010). Screening for hazardous alcohol use and depressive symptomatology among HIV-infected patients in Nigeria: Prevalence, predictors, and association with adherence. Journal of the International Association of Physicians in AIDS Care, 9(4), 218–226. 10.1177/1545109710371133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiedler K, Schott M, & Meiser T (2011). What mediation analysis can (not) do. Journal of Experimental Social Psychology, 47(6), 1231–1236. 10.1016/j.jesp.2011.05.007 [DOI] [Google Scholar]
- Fisher JD, Amico KR, Fisher WA, & Harman JJ (2008). The information-motivation-behavioral skills model of antiretroviral adherence and its applications. Current HIV/AIDS Reports, 5(4), 193–203. [DOI] [PubMed] [Google Scholar]
- Fitzgerald M, Collumbien M, & Hosegood V (2010). “No one can ask me ‘Why do you take that stuff?’”: Men’s experiences of antiretroviral treatment in South Africa. AIDS Care, 22(3), 355–360. 10.1080/09540120903111536 [DOI] [PubMed] [Google Scholar]
- Fogarty L, Roter D, Larson S, Burke J, Gillespie J, & Levy R (2002). Patient adherence to HIV medication regimens: A review of published and abstract reports. Patient Education and Counseling, 46(2), 93–108. 10.1016/s0738-3991(01)00219-1 [DOI] [PubMed] [Google Scholar]
- Ge S, Sanchez M, Nolan M, Liu T, & Savage CL (2018). Is Alcohol Use Associated With Increased Risk of Developing Adverse Health Outcomes Among Adults Living With Human Immunodeficiency Virus: A Systematic Review. Journal of Addictions Nursing, 29(2), 96–118. 10.1097/JAN.0000000000000220 [DOI] [PubMed] [Google Scholar]
- Gordon LL, Gharibian D, Chong K, & Chun H (2015). Comparison of HIV Virologic Failure Rates Between Patients with Variable Adherence to Three Antiretroviral Regimen Types. AIDS Patient Care and STDs, 29(7), 384–388. 10.1089/apc.2014.0165 [DOI] [PubMed] [Google Scholar]
- Grodensky CA, Golin CE, Ochtera RD, & Turner BJ (2012). Systematic review: Effect of alcohol intake on adherence to outpatient medication regimens for chronic diseases. Journal of Studies on Alcohol and Drugs, 73(6), 899–910. 10.15288/jsad.2012.73.899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberer JE, Musinguzi N, Boum Y, Siedner MJ, Mocello AR, Hunt PW, Martin JN, & Bangsberg DR (2015). Duration of Antiretroviral Therapy Adherence Interruption Is Associated With Risk of Virologic Rebound as Determined by Real-Time Adherence Monitoring in Rural Uganda. Journal of Acquired Immune Deficiency Syndromes (1999), 70(4), 386–392. 10.1097/QAI.0000000000000737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagger MS, Moyers S, McAnally K, & McKinley LE (2020). Known knowns and known unknowns on behavior change interventions and mechanisms of action. Health Psychology Review, 14(1), 199–212. 10.1080/17437199.2020.1719184 [DOI] [PubMed] [Google Scholar]
- Hasin DS, Aharonovich E, O’Leary A, Greenstein E, Pavlicova M, Arunajadai S, Waxman R, Wainberg M, Helzer J, & Johnston B (2013). Reducing heavy drinking in HIV primary care: A randomized trial of brief intervention, with and without technological enhancement. Addiction (Abingdon, England), 108(7), 1230–1240. 10.1111/add.12127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havlir D, Lockman S, Ayles H, Larmarange J, Chamie G, Gaolathe T, Iwuji C, Fidler S, Kamya M, Floyd S, Moore J, Hayes R, Petersen M, & Dabis F (2020). What do the Universal Test and Treat trials tell us about the path to HIV epidemic control? Journal of the International AIDS Society, 23(2), e25455. 10.1002/jia2.25455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendershot CS, Stoner SA, Pantalone DW, & Simoni JM (2009). Alcohol use and antiretroviral adherence: Review and meta-analysis. Journal of Acquired Immune Deficiency Syndromes (1999), 52(2), 180–202. 10.1097/QAI.0b013e3181b18b6e [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershow RB, Zuskov DS, Vu Tuyet Mai N, Chander G, Hutton HE, Latkin C, Vuong ND, Sripaipan T, Lancaster KE, Ha TV, & Go VF (2018). “[Drinking is] Like a Rule That You Can’t Break”: Perceived Barriers and Facilitators to Reduce Alcohol Use and Improve Antiretroviral Treatment Adherence among People Living with HIV and Alcohol Use Disorder in Vietnam. Substance Use & Misuse, 53(7), 1084–1092. 10.1080/10826084.2017.1392986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joint United Nations Program on HIV/AIDS. (2018). Undetectable = untransmittable—Public health and HIV viral load suppression. https://www.unaids.org/sites/default/files/media_asset/undetectable-untransmittable_en.pdf
- Justice AC, Gordon KS, Skanderson M, Edelman EJ, Akgün KM, Gibert CL, Lo Re V, Rimland D, Womack JA, Wyatt CM, Tate JP, & VACS Project Team. (2018). Nonantiretroviral polypharmacy and adverse health outcomes among HIV-infected and uninfected individuals. AIDS (London, England), 32(6), 739–749. 10.1097/QAD.0000000000001756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justice AC, McGinnis KA, Tate JP, Braithwaite RS, Bryant KJ, Cook RL, Edelman EJ, Fiellin LE, Freiberg MS, Gordon AJ, Kraemer KL, Marshall BDL, Williams EC, & Fiellin DA (2016). Risk of mortality and physiologic injury evident with lower alcohol exposure among HIV infected compared with uninfected men. Drug and Alcohol Dependence, 161, 95–103. 10.1016/j.drugalcdep.2016.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahler CW, Pantalone DW, Mastroleo NR, Liu T, Bove G, Ramratnam B, Monti PM, & Mayer KH (2018). Motivational interviewing with personalized feedback to reduce alcohol use in HIV-infected men who have sex with men: A randomized controlled trial. Journal of Consulting and Clinical Psychology, 86(8), 645–656. 10.1037/ccp0000322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalichman SC, Amaral CM, White D, Swetsze C, Pope H, Kalichman MO, Cherry C, & Eaton L (2009). Prevalence and clinical implications of interactive toxicity beliefs regarding mixing alcohol and antiretroviral therapies among people living with HIV/AIDS. AIDS Patient Care and STDs, 23(6), 449–454. 10.1089/apc.2008.0184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalichman SC, Grebler T, Amaral CM, McNerey M, White D, Kalichman MO, Cherry C, & Eaton L (2013). Intentional non-adherence to medications among HIV positive alcohol drinkers: Prospective study of interactive toxicity beliefs. Journal of General Internal Medicine, 28(3), 399–405. 10.1007/s11606-012-2231-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalichman SC, Grebler T, Amaral CM, McNerney M, White D, Kalichman MO, Cherry C, & Eaton L (2014). Viral suppression and antiretroviral medication adherence among alcohol using HIV-positive adults. International Journal of Behavioral Medicine, 21(5), 811–820. 10.1007/s12529-013-9353-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalichman SC, Horne R, Katner H, & Hernandez D (2019). Perceived sensitivity to medicines, alcohol interactive toxicity beliefs, and medication adherence among people living with HIV who drink alcohol. Journal of Behavioral Medicine, 42(3), 392–400. 10.1007/s10865-018-9987-7 [DOI] [PubMed] [Google Scholar]
- Kalichman SC, Kalichman MO, Cherry C, Hoyt G, Washington C, Grebler T, Merely C, & Welles B (2015). Intentional Medication Non-Adherence Due to Interactive Toxicity Beliefs among HIV Positive Active Drug Users. Journal of Acquired Immune Deficiency Syndromes (1999), 70(5), 503–509. 10.1097/QAI.0000000000000776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalichman SC, Mathews C, Banas E, & Kalichman MO (2019). Treatment adherence in HIV stigmatized environments in South Africa: Stigma avoidance and medication management. International Journal of STD & AIDS, 30(4), 362–370. 10.1177/0956462418813047 [DOI] [PubMed] [Google Scholar]
- Kalichman SC, Katner H, Hill M, Kalichman MO, & Hernandez D (2019). Alcohol-Related Intentional Antiretroviral Nonadherence among People Living with HIV: Test of an Interactive Toxicity Beliefs Process Model. Journal of the International Association of Providers of AIDS Care (JIAPAC), 18, 1–9. 10.1177/2325958219826612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazdin AE (2007). Mediators and mechanisms of change in psychotherapy research. Annual Review of Clinical Psychology, 3, 1–27. 10.1146/annurev.clinpsy.3.022806.091432 [DOI] [PubMed] [Google Scholar]
- Kekwaletswe CT, Jordaan E, Nkosi S, & Morojele NK (2017). Social Support and the Mediating Roles of Alcohol Use and Adherence Self-Efficacy on Antiretroviral Therapy (ART) Adherence Among ART Recipients in Gauteng, South Africa. AIDS and Behavior, 21(7), 1846–1856. 10.1007/s10461-016-1595-3 [DOI] [PubMed] [Google Scholar]
- Kelly SG, Plankey M, Post WS, Li X, Stall R, Jacobson LP, Witt MD, Kingsley L, Cox C, Budoff M, & Palella FJ (2016). Associations between Tobacco, Alcohol, and Drug Use with Coronary Artery Plaque among HIV-Infected and Uninfected Men in the Multicenter AIDS Cohort Study. PloS One, 11(1), e0147822. 10.1371/journal.pone.0147822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langebeek N, Gisolf EH, Reiss P, Vervoort SC, Hafsteinsdóttir TB, Richter C, Sprangers MAG, & Nieuwkerk PT (2014). Predictors and correlates of adherence to combination antiretroviral therapy (ART) for chronic HIV infection: A meta-analysis. BMC Medicine, 12, 142. 10.1186/PREACCEPT-1453408941291432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laws MB, Rose GS, Beach MC, Lee Y, Rogers WS, Velasco AB, & Wilson IB (2015). Patient-Provider Concordance with Behavioral Change Goals Drives Measures of Motivational Interviewing Consistency. Patient Education and Counseling, 98(6), 728–733. 10.1016/j.pec.2015.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitz JR, Morgan CJA, Bisby JA, Rendell PG, & Curran HV (2009). Global impairment of prospective memory following acute alcohol. Psychopharmacology, 205(3), 379–387. 10.1007/s00213-009-1546-z [DOI] [PubMed] [Google Scholar]
- Lyimo RA, de Bruin M, van den Boogaard J, Hospers HJ, van der Ven A, & Mushi D (2012). Determinants of antiretroviral therapy adherence in northern Tanzania: A comprehensive picture from the patient perspective. BMC Public Health, 12(1), 1–8. 10.1186/1471-2458-12-716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhombiro M, Marimbe-Dube B, Dube M, Kaiyo-Utete M, Paradzai A, Chibanda D, Rusakaniko S, van der Watt A, & Seedat S (2018). Perceptions of alcohol use in the context of HIV treatment: A qualitative study. HIV/AIDS: Research and Palliative Care; Macclesfield, 10, 47–55. 10.2147/HIV.S150095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhombiro M, Musekiwa A, January J, Chingono A, Abas M, & Seedat S (2019). Psychological interventions for alcohol use disorders in people living with HIV/AIDS: A systematic review. Systematic Reviews, 8(1), 244. 10.1186/s13643-019-1176-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malow R, Dévieux JG, Stein JA, Rosenberg R, Jean-Gilles M, Attonito J, Koenig SP, Raviola G, Sévère P, & Pape JW (2013). Depression, substance abuse and other contextual predictors of adherence to antiretroviral therapy (ART) among Haitians. AIDS and Behavior, 17(4), 1221–1230. 10.1007/s10461-012-0400-1 [DOI] [PubMed] [Google Scholar]
- Manzano-García M, Pérez-Guerrero C, Álvarez de Sotomayor Paz M, Robustillo-Cortés M. de L. A., Almeida-González CV, & Morillo-Verdugo R (2018). Identification of the Medication Regimen Complexity Index as an Associated Factor of Nonadherence to Antiretroviral Treatment in HIV Positive Patients. The Annals of Pharmacotherapy, 52(9), 862–867. 10.1177/1060028018766908 [DOI] [PubMed] [Google Scholar]
- Marcum ZA, & Gellad WF (2012). Medication Adherence to Multi-Drug Regimens. Clinics in Geriatric Medicine, 28(2), 287–300. 10.1016/j.cger.2012.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall BDL, Operario D, Bryant KJ, Cook RL, Edelman EJ, Gaither JR, Gordon AJ, Kahler CW, Maisto SA, McGinnis KA, van den Berg JJ, Zaller ND, Justice AC, & Fiellin DA (2015). Drinking trajectories among HIV-infected men who have sex with men: A cohort study of United States veterans. Drug and Alcohol Dependence, 148, 69–76. 10.1016/j.drugalcdep.2014.12.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mata-Marín JA, Martínez-Osio MH, Arroyo-Anduiza CI, Berrospe-Silva M.de los Á., Chaparro-Sánchez A, Cruz-Grajales I, Cruz-Herrera JE, Uribe-Noguez LA, Gaytán-Martínez JE, & Jerónimo-Morales M (2019). Comorbidities and polypharmacy among HIV-positive patients aged 50years and over: A case–control study. BMC Research Notes, 12(1), 556. 10.1186/s13104-019-4576-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills EJ, Nachega JB, Bangsberg DR, Singh S, Rachlis B, Wu P, Wilson K, Buchan I, Gill CJ, & Cooper C (2006). Adherence to HAART: A systematic review of developed and developing nation patient-reported barriers and facilitators. PLoS Medicine, 3(11), e438. 10.1371/journal.pmed.0030438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mintzer M (2007). The acute effects of alcohol on memory: A review of laboratory studies in healthy adults. International Journal on Disability and Human Development, 6. 10.1515/IJDHD.2007.6.4.397 [DOI] [Google Scholar]
- Montgomery C, Ashmore K, & Jansari A (2011). The effects of a modest dose of alcohol on executive functioning and prospective memory. Human Psychopharmacology, 26, 208–215. 10.1002/hup.1194 [DOI] [PubMed] [Google Scholar]
- Moucheraud C, Stern AF, Ahearn C, Ismail A, Nsubuga-Nyombi T, Ngonyani MM, Mvungi J, & Ssensamba J (2019). Barriers to HIV Treatment Adherence: A Qualitative Study of Discrepancies Between Perceptions of Patients and Health Providers in Tanzania and Uganda. AIDS Patient Care and STDs, 33(9), 406–413. 10.1089/apc.2019.0053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukumbang FC, Van Belle S, Marchal B, & van Wyk B (2017). Exploring “generative mechanisms” of the antiretroviral adherence club intervention using the realist approach: A scoping review of research-based antiretroviral treatment adherence theories. BMC Public Health, 17(1), 385. 10.1186/s12889-017-4322-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro S, Lewin S, Swart T, & Volmink J (2007). A review of health behaviour theories: How useful are these for developing interventions to promote long-term medication adherence for TB and HIV/AIDS? BMC Public Health, 7(1), 104. 10.1186/1471-2458-7-104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myer L, Smit J, Roux LL, Parker S, Stein DJ, & Seedat S (2008). Common mental disorders among HIV-infected individuals in South Africa: Prevalence, predictors, and validation of brief psychiatric rating scales. AIDS Patient Care and STDs, 22(2), 147–158. 10.1089/apc.2007.0102 [DOI] [PubMed] [Google Scholar]
- National Institute on Alcohol Abuse and Alcoholism. (2011). Drinking Levels Defined. https://www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/moderate-binge-drinking
- Nkosi S, Rich EP, Kekwaletswe CT, & Morojele NK (2016). Experiences of alcohol consumption and taking antiretroviral medication among men living with HIV in Tshwane, South Africa. African Journal of AIDS Research, 15(4), 367–376. 10.2989/16085906.2016.1255651 [DOI] [PubMed] [Google Scholar]
- Nouaman MN, Vinikoor M, Seydi M, Ekouevi DK, Coffie PA, Mulenga L, Tanon A, Egger M, Dabis F, Jaquet A, & Wandeler G (2018). High prevalence of binge drinking among people living with HIV in four African countries. Journal of the International AIDS Society, 21(12). 10.1002/jia2.25202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortego C, Huedo-Medina TB, Llorca J, Sevilla L, Santos P, Rodríguez E, Warren MR, & Vejo J (2011). Adherence to highly active antiretroviral therapy (HAART): A meta-analysis. AIDS and Behavior, 15(7), 1381–1396. 10.1007/s10461-011-9942-x [DOI] [PubMed] [Google Scholar]
- Papas RK, Sidle JE, Gakinya BN, Baliddawa JB, Martino S, Mwaniki MM, Songole R, Omolo OE, Kamanda AM, Ayuku DO, Ojwang C, Owino-Ong’or WD, Harrington M, Bryant KJ, Carroll KM, Justice AC, Hogan JW, & Maisto SA (2011). Treatment outcomes of a Stage 1 cognitive-behavioral trial to reduce alcohol use among HIV-infected outpatients in western Kenya. Addiction (Abingdon, England), 106(12), 2156–2166. 10.1111/j.1360-0443.2011.03518.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paraskevaides T, Morgan CJA, Leitz JR, Bisby JA, Rendell PG, & Curran HV (2010). Drinking and future thinking: Acute effects of alcohol on prospective memory and future simulation. Psychopharmacology, 208(2), 301–308. 10.1007/s00213-009-1731-0 [DOI] [PubMed] [Google Scholar]
- Park LS, Hernández-Ramírez RU, Silverberg MJ, Crothers K, & Dubrow R (2016). Prevalence of non-HIV cancer risk factors in persons living with HIV/AIDS: A meta-analysis. AIDS (London, England), 30(2), 273–291. 10.1097/QAD.0000000000000922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons JT, Rosof E, & Mustanski B (2008). The temporal relationship between alcohol consumption and HIV-medication adherence: A multilevel model of direct and moderating effects. Health Psychology, 27(5), 628–637. 10.1037/a0012664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellowski JA, Kalichman SC, Cherry S, Conway-Washington C, Cherry C, Grebler T, & Krug L (2016). The Daily Relationship Between Aspects of Food Insecurity and Medication Adherence Among People Living with HIV with Recent Experiences of Hunger. Annals of Behavioral Medicine, 50(6), 844–853. 10.1007/s12160-016-9812-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellowski JA, Kalichman SC, Kalichman MO, & Cherry C (2016). Alcohol-antiretroviral therapy interactive toxicity beliefs and daily medication adherence and alcohol use among people living with HIV. AIDS Care, 28(8), 963–970. 10.1080/09540121.2016.1154134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pengpid S, & Peltzer K (2014). Perceptions of alcohol risk among people on antiretroviral treatment in South Africa: A qualitative study. Journal of Psychology in Africa, 24(4), 388–392. 10.1080/14330237.2014.980628 [DOI] [Google Scholar]
- Rodger AJ (2018). PARTNER-2 Study No HIV transmissions in 8 years when gay HIV+ partner has undetectable load. http://www.natap.org/2018/IAC/IAC_06.htm
- Rodger AJ, Cambiano V, Bruun T, Vernazza P, Collins S, van Lunzen J, Corbelli GM, Estrada V, Geretti AM, Beloukas A, Asboe D, Viciana P, Gutiérrez F, Clotet B, Pradier C, Gerstoft J, Weber R, Westling K, Wandeler G, … PARTNER Study Group. (2016). Sexual Activity Without Condoms and Risk of HIV Transmission in Serodifferent Couples When the HIV-Positive Partner Is Using Suppressive Antiretroviral Therapy. JAMA, 316(2), 171–181. 10.1001/jama.2016.5148 [DOI] [PubMed] [Google Scholar]
- Sabapathy K, Mubekapi-Musadaidzwa C, Mulubwa C, Schaap A, Hoddinott G, Stangl A, Floyd S, Ayles H, Fidler S, Hayes R, & HPTN 071 (PopART) study team. (2017). Predictors of timely linkage-to-ART within universal test and treat in the HPTN 071 (PopART) trial in Zambia and South Africa: Findings from a nested case-control study. Journal of the International AIDS Society, 20(4). 10.1002/jia2.25037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankar A, Wunderlich T, Neufeld S, & Luborsky M (2007). Sero-positive African Americans’ Beliefs about Alcohol and Their Impact on Anti-retroviral Adherence. AIDS and Behavior, 11(2), 195–203. 10.1007/s10461-006-9144-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schensul JJ, Ha T, Schensul S, Sarna A, & Bryant K (2017). Identifying the Intersection of Alcohol, Adherence and Sex in HIV Positive Men on ART Treatment in India Using an Adapted Timeline Followback Procedure. AIDS and Behavior, 21(2), 228–242. 10.1007/s10461-017-1916-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schensul SL, Ha T, Schensul JJ, Vaz M, Singh R, Burleson JA, & Bryant K (2017). The Role of Alcohol on Antiretroviral Therapy Adherence Among Persons Living With HIV in Urban India. Journal of Studies on Alcohol and Drugs, 78(5), 716–724. 10.15288/jsad.2017.78.716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott-Sheldon LAJ, Carey KB, Johnson BT, Carey MP, & MASH Research Team. (2017). Behavioral Interventions Targeting Alcohol Use Among People Living with HIV/AIDS: A Systematic Review and Meta-Analysis. AIDS and Behavior, 21(Suppl 2), 126–143. 10.1007/s10461-017-1886-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shubber Z, Mills EJ, Nachega JB, Vreeman R, Freitas M, Bock P, Nsanzimana S, Penazzato M, Appolo T, Doherty M, & Ford N (2016). Patient-Reported Barriers to Adherence to Antiretroviral Therapy: A Systematic Review and Meta-Analysis. PLoS Medicine, 13(11), e1002183. 10.1371/journal.pmed.1002183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuper PA, Joharchi N, Irving H, & Rehm J (2009). Alcohol as a correlate of unprotected sexual behavior among people living with HIV/AIDS: Review and meta-analysis. AIDS and Behavior, 13(6), 1021–1036. 10.1007/s10461-009-9589-z [DOI] [PubMed] [Google Scholar]
- Shuper PA, Joharchi N, & Rehm J (2018). Lower Blood Alcohol Concentration Among HIV-Positive Versus HIV-Negative Individuals Following Controlled Alcohol Administration. Alcoholism, Clinical and Experimental Research, 42(9), 1684–1692. 10.1111/acer.13816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sileo KM, Kizito W, Wanyenze RK, Chemusto H, Musoke W, Mukasa B, & Kiene SM (2019). A qualitative study on alcohol consumption and HIV treatment adherence among men living with HIV in Ugandan fishing communities. AIDS Care, 31(1), 35–40. 10.1080/09540121.2018.1524564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sileo KM, Simbayi LC, Abrams A, Cloete A, & Kiene SM (2016). The role of alcohol use in antiretroviral adherence among individuals living with HIV in South Africa: Event-level findings from a daily diary study. Drug and Alcohol Dependence, 167, 103–111. 10.1016/j.drugalcdep.2016.07.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith-Spark JH, Moss AC, & Dyer KR (2016). Do Baseline Executive Functions Mediate Prospective Memory Performance under a Moderate Dose of Alcohol? Frontiers in Psychology, 7. 10.3389/fpsyg.2016.01325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorsdahl K, Morojele NK, Parry CD, Kekwaletswe CT, Kitleli N, Malan M, Shuper PA, & Myers B (2019). “What will it take”: Addressing alcohol use among people living with HIV in South Africa. International Journal of STD & AIDS, 30(11), 1049–1054. 10.1177/0956462419862899 [DOI] [PubMed] [Google Scholar]
- Sullivan LE, Goulet JL, Justice AC, & Fiellin DA (2011). Alcohol consumption and depressive symptoms over time: A longitudinal study of patients with and without HIV infection. Drug and Alcohol Dependence, 117(2), 158–163. 10.1016/j.drugalcdep.2011.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tryon WW (2018). Mediators and Mechanisms. Clinical Psychological Science, 6(5), 619–628. 10.1177/2167702618765791 [DOI] [Google Scholar]
- Tucker JS, Orlando M, Burnam MA, Sherbourne CD, Kung FY, & Gifford AL (2004). Psychosocial Mediators of Antiretroviral Nonadherence in HIV-Positive Adults With Substance Use and Mental Health Problems. Health Psychology, 23(4), 363–370. 10.1037/0278-6133.23.4.363 [DOI] [PubMed] [Google Scholar]
- Vagenas P, Azar MM, Copenhaver MM, Springer SA, Molina PE, & Altice FL (2015). The Impact of Alcohol Use and Related Disorders on the HIV Continuum of Care: A Systematic Review. Current HIV/AIDS Reports, 12(4), 421–436. 10.1007/s11904-015-0285-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velasquez MM, von Sternberg K, Johnson DH, Carbonari JP, Green C, & Parsons JT (2009). Reducing Sexual Risk Behaviors and Alcohol Use Among HIV-Positive Men Who Have Sex With Men: A Randomized Clinical Trial. Journal of Consulting and Clinical Psychology, 77(4), 657–667. 10.1037/a0015519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velloza J, Kemp CG, Aunon FM, Ramaiya MK, Creegan E, & Simoni JM (2019). Alcohol Use and Antiretroviral Therapy Non-Adherence Among Adults Living with HIV/AIDS in Sub-Saharan Africa: A Systematic Review and Meta-Analysis. AIDS and Behavior, 24(6), 1727–1742. 10.1007/s10461-019-02716-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viswanathan S, Williams ME, Bloss EB, Stasevich TJ, Speer CM, Nern A, Pfeiffer BD, Hooks BM, Li W-P, English BP, Tian T, Henry GL, Macklin JJ, Patel R, Gerfen CR, Zhuang X, Wang Y, Rubin GM, & Looger LL (2015). High-performance probes for light and electron microscopy. Nature Methods, 12(6), 568–576. 10.1038/nmeth.3365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volberding PA, & Deeks SG (2010). Antiretroviral therapy and management of HIV infection. Lancet (London, England), 376(9734), 49–62. 10.1016/S0140-6736(10)60676-9 [DOI] [PubMed] [Google Scholar]
- Walter NT, & Bayen UJ (2016). Selective effects of acute alcohol intake on the prospective and retrospective components of a prospective-memory task with emotional targets. Psychopharmacology, 233(2), 325–339. 10.1007/s00213-015-4110-z [DOI] [PubMed] [Google Scholar]
- Ware D, Palella FJ, Chew KW, Friedman MR, D’Souza G, Ho K, & Plankey M (2019). Examination of Polypharmacy Trajectories Among HIV-Positive and HIV-Negative Men in an Ongoing Longitudinal Cohort from 2004 to 2016. AIDS Patient Care and STDs, 33(8), 354–365. 10.1089/apc.2019.0057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams EC, Hahn JA, Saitz R, Bryant K, Lira MC, & Samet JH (2016). Alcohol Use and Human Immunodeficiency Virus (HIV) Infection: Current Knowledge, Implications, and Future Directions. Alcoholism, Clinical and Experimental Research, 40(10), 2056–2072. 10.1111/acer.13204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SP, Carey CL, Moran LM, Dawson MS, Letendre SL, Grant I, & HIV Neurobehavioral Research Center (HNRC) Group. (2007). Frequency and predictors of self-reported prospective memory complaints in individuals infected with HIV. Archives of Clinical Neuropsychology: The Official Journal of the National Academy of Neuropsychologists, 22(2), 187–195. 10.1016/j.acn.2006.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SP, Moran LM, Carey CL, Dawson MS, Iudicello JE, Gibson S, Grant I, Atkinson JH, & HIV Neurobehavioral Research Center (HNRC) Group. (2008). Prospective memory in HIV infection: Is “remembering to remember” a unique predictor of self-reported medication management? Archives of Clinical Neuropsychology: The Official Journal of the National Academy of Neuropsychologists, 23(3), 257–270. 10.1016/j.acn.2007.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (WHO). (2019). HIV/AIDS. https://www.who.int/news-room/fact-sheets/detail/hiv-aids
- Zogg JB, Woods SP, Sauceda JA, Wiebe JS, & Simoni JM (2012). The Role of Prospective Memory in Medication Adherence: A Review of an Emerging Literature. Journal of Behavioral Medicine, 35(1), 47–62. 10.1007/s10865-011-9341-9 [DOI] [PMC free article] [PubMed] [Google Scholar]


