Abstract
Object:
A convergence of clinical research suggests that the temporal pole (TP) plays an important, and potentially under appreciated, role in the genesis and propagation of seizures in temporal lobe epilepsy (TLE). Understanding the role of the TP in TLE is becoming increasingly important because selective surgical resections for medically intractable TLE spare TP cortex. The purpose of this study was to characterize the role of TP cortex in TLE using dense electrocorticographic (ECoG) recordings in patients undergoing invasive monitoring for medically intractable TLE.
Methods:
Chronic ECoG recordings were obtained in 10 consecutive patients using an array customized to provide dense coverage of the TP as part of invasive monitoring to localize the epileptogenic zone. All patients would eventually undergo cortico-amygdalohippocampectomy. A retrospective review of clinical records including ECoG, neuroimaging, neuropathology, and clinical outcomes was performed.
Results:
In seven patients (70%), the TP was involved at seizure onset, in seven patients (70%) there were interictal discharges from the TP, and in one case early spread to the TP. Seizure onset in the TP did not necessarily correlate with preoperative neuroimaging abnormalities of the TP.
Conclusions:
These data demonstrate that TP cortex commonly plays a crucial role in temporal lobe seizure networks. Seizure onset from the TP would not have been predicted by available neuroimaging data or interictal discharges. These findings illustrate the importance of thoroughly considering the role of the TP prior to resective surgery for TLE, particularly when selective mesial resection is being considered.
Keywords: Epilepsy, Seizures, Anterior Temporal Lobe, Epilepsy Surgery, Temporal Lobe Epilepsy, Electrocorticography
Introduction
Temporal lobe epilepsy (TLE) is the most common type of surgically remediable intractable epilepsy in adults. Of appropriately selected patients with medically intractable TLE who undergo cortico-amygdalohippocampectomy (CAH), 60–80% demonstrate long-term seizure freedom and up to 95% have improvement in seizure control16,23,34. In a significant proportion of patients with TLE, seizures arise from the mesial temporal lobe, which is associated with a syndrome defined as mesial temporal lobe epilepsy (MTLE). MTLE is typified in patients with neuroimaging findings of mesial temporal sclerosis (MTS), a characteristic clinical semiology, neuropsychological evidence of mesial temporal dysfunction, and anterior and mid-inferomedial temporal ictal and interictal discharges on scalp EEG9,25. Patients with findings characteristic of MTLE may be candidates for more selective mesial temporal resections, such as selective amygdalohippocampectomy (SAH) or the more recent laser interstitial thermal therapy (LITT)11,15,19,35. Notably, the indications to undergo CAH or a more selective mesial approach such as SAH or LITT varies widely among epilepsy surgery centers. Furthermore, while there is clear evidence that in appropriately selected patients mesial resection is effective for achieving seizure freedom3,32, some studies suggest lower seizure freedom after selective mesial resection, particularly LITT23,35. Patients with incomplete improvement after selective mesial resection or ablation likely have extrahippocampal seizure genesis sites. Understanding which patients have extrahippocampal seizure genesis is key to determining the correct surgical resection strategy for patients with TLE.
The temporal pole (TP) has been proposed as an important site of seizure genesis within temporal lobe seizure networks9,21. Intracerebral recording studies with concurrent recording of hippocampus and TP suggest that the TP becomes involved in seizures either concurrently or before the hippocampus in approximately 50% of patients9. However, exactly what role TP cortex plays in temporal lobe seizure networks and TLE remains poorly understood. One barrier to understanding the role of the TP in temporal lobe seizure networks is that many groups place relatively few electrodes on the TP during intracranial recording studies used to localize seizures2. Standard electrocorticography implantation strategies typically include an anteromedial strip electrode12 or temporopolar strip electrodes, which both leave much of the TP uncovered. Alternatively, groups using stereo-EEG, may place only one depth electrode in the TP9. With any of these intracranial recording strategies coverage of the TP is relatively sparse, and it is possible that ictal onset in the TP could be missed.
Given the relative lack of information of the role of the TP in TLE, the purpose of this study was to examine the role of TP cortex in temporal lobe seizure networks. Based on previous studies9, we hypothesized that the TP would be involved in seizure onset regardless of whether or not neuroimaging data would have supported a role for the TP. To accomplish this, we reviewed recordings from the TP in 10 consecutive patients who ultimately underwent CAH using an electrode array specialized to provide high-density recordings of the TP2.
Materials Methods
Patients and preoperative evaluation
Ten consecutive patients who underwent cortico-amygdalohippocampectomy (CAH) after dense intracranial recording of the ATL were included as subjects for this study (Table 1). Each subject was demonstrated to have medically intractable epilepsy after multiple medication trials and thus were candidates for surgical evaluation. To maintain as much homogeneity of the study population as possible, each patient exhibited the following characteristics: 1) seizures of temporal lobe origin, however, the epileptogenic zone could not be satisfactorily estimated using non-invasive methods, 2) dense ATL recording with either a specialized ATL recording array (see below) or two dedicated ATL strip electrodes in addition to a standard anteromedial strip electrode12, and 3) ultimately underwent CAH. Thus, patients with spatially sparse ATL recording limited to a standard anteromedial strip electrode or patients who underwent lesionectomy without CAH, were not included.
Table 1:
Clinical Characteristics, Surgery, and Seizure Outcome
| Patient/Group | Sex | Age | Febrile seizures | Epilepsy onset | Epilepsy laterality | Surgical intervention | Aura | Scalp EEG IEDs | Scalp EEG seizure pattern | Post-op outcome class | F/U (Mo) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 – A | M | 21 | No | 16 | R | R CAH | Deja vu | F8=T4 | Several F8 maximal sharp waves, then right fronto-centro- temporal theta/delta | I | 2 |
| 2 – A | M | 29 | Yes | 18 | L | L CAH | Right foot tingling | T3 or SP1 | SP1 sharp waves and delta | I | 40 |
| 3 – A | F | 50 | No | 1 | L | L CAH + lesionectomy | None | F8=T4 | Artifact obscured | II | 21 |
| 4 – A | M | 31 | No | 23 | R | R CAH | Nausea, hearing things | F8 or T4 | Right lateralized or regional temporal delta | II | 43 |
| 5 – A | M | 26 | Yes | 21 | R | R CAH + OF resection | Dizzy | FP2>F8 | Artifact obscured | III | 8 |
| 6 – A | M | 31 | No | 24 | L | L CAH | Smell, warmth, tingling | T3 | Left lateralized polyfrequency | I | 46 |
| 7 – A | M | 47 | No | 35 | L | L CAH | Abdominal | F7>T3 | Left regional temporal theta | I | 27 |
| 8 – B | M | 26 | No | 21 | R | R CAH | Present, but not described | T6 | T6 and right fronto-temporal alpha | II | 57 |
| 9 – B | M | 35 | No | 33 | L | L CAH | Abdominal | T3>T5 (and SP1) | Left regional temporal theta | I | 32 |
| 10 – B | M | 51 | No | 33 | L | L CAH | None | T3 (and SP1) | Left regional temporal theta | I | 38 |
Clinical characteristics, surgical plan, and seizure outcome for all patients in study. Group A is patients 1–7 and Group B is patients 8–10. Age and Epilepsy onset numbers are indicated in years. Postoperative score is indicated by Engel class I-IV. The scalp EEG pattern refers to initial changes related to the seizure. SP: sphenoidal electrodes. The ‘=’ symbol indicates isopotential at the two listed electrode sites. The ‘>’ symbol indicates two populations of IEDs with one electrode site predominating.
The study group consisted of 10 subjects (nine men, one woman; ages 21 – 52, mean age 34.7). Epilepsy was lateralized to the left hemisphere in five subjects and the right hemisphere in five subjects. Age of onset ranged from one to 35. Two subjects (#2 and #5) had a history of febrile seizures.
After determination of medical intractability, each subject underwent preoperative epilepsy surgery work-up per the University of Iowa Comprehensive Epilepsy Center Protocol. This included MR imaging at 3Tesla with high resolution epilepsy protocol, interictal FDG PET, inpatient video-EEG monitoring, neuropsychological examination, and, in some cases, a Wada test. The epileptogenic zone could not be satisfactorily estimated using non-invasive methods in any of the subjects. Therefore, further evaluation was performed using intracranial grid, strip, and depth electrodes in each subject (see below).
Ultimately, after localization of the ictal onset zone and estimation of the epileptogenic zone, each patient underwent a tailored CAH. In addition to CAH, one subject had additional frontal lesionectomy (Patient #3) and one subject had additional orbitofrontal resection (Patient #5), which were both deemed necessary on the basis of intracranial recordings. All CAHs were performed by a single surgeon team (H.K. and M.H.). The CAH technique consisted of an approach through the middle temporal gyrus with resection of the hippocampus, amygdala, and parahippocampal gyrus. A tailored resection of the hippocampus was performed in each subject guided by intraoperative ECoG. After resection of the mesial structures, the anterior temporal lobe and temporopolar cortex was disconnected in each case. The posterior margin of the anterior temporal disconnection was determined by extraoperative ECoG in each case.
Intracranial electrode implantation and recording
Chronic intracranial recordings were performed in all patients to estimate the extent of the epileptogenic zone in the temporal lobe and to determine whether or not extratemporal brain structures were involved. Thus, intracranial recordings were performed which in all cases included grid, strip, and depth electrodes. To place electrodes, a frontotemporal craniotomy was performed on the side determined by noninvasive diagnostic tests to contain seizure onset. Electrode coverage was determined in each case on clinical grounds based on findings from seizure semiology, MRI, video EEG, and FDG PET. Frameless stereotaxy was used to place depth electrodes in all subjects and each subject had at least a hippocampal and amygdala depth electrode placed.
All subjects included in the present study had dense subdural electrode coverage of the anterior temporal lobe, including the TP. Recently, we have developed a specialized electrode array that provides dense and consistent subdural electrode coverage from the TP for clinical recording purposes2. The design and implantation of this array has been previously described2 and is summarized in Figure 1. Thus, our criteria for dense recordings of the TP and inclusion in the present study was implantation of a customized TP electrode array or placement of at least three strip electrodes covering the TP (one standard anteromedial strip electrode and two ATL strip electrodes).
Figure 1:
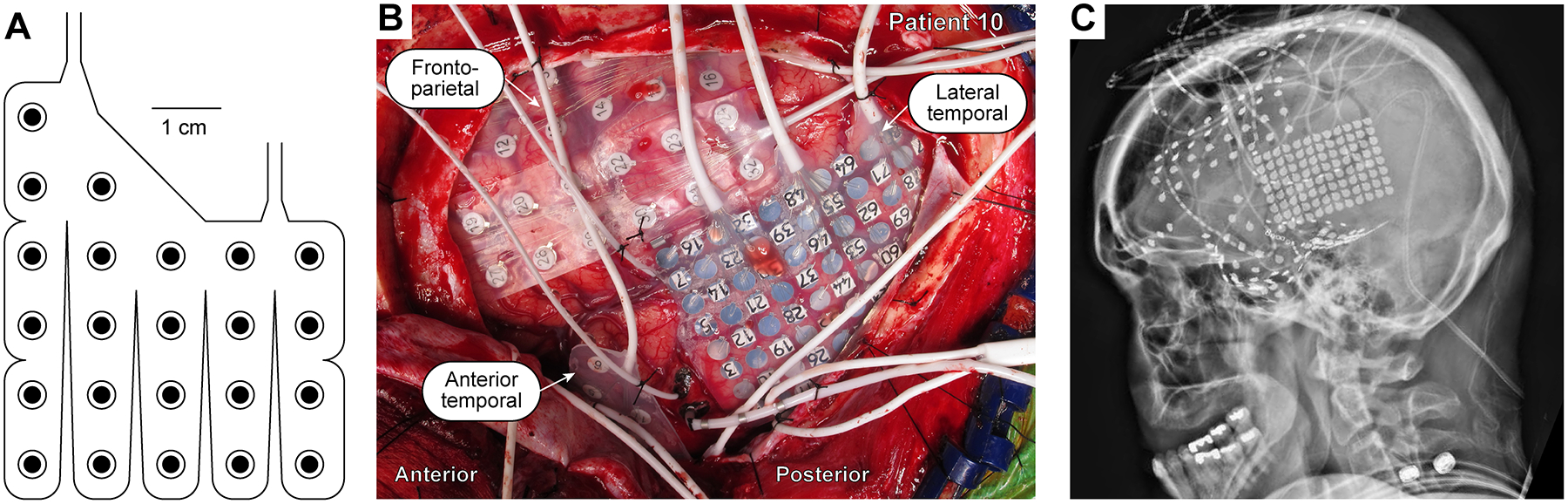
Intracranial recording from the ATL. A: Schematic of a customized 23-contact ATL electrode array. B: Intraoperative photograph depicting placement of recording arrays, including the ATL array.
C: X-ray imaging of a patient implanted with the ATL array.
Anatomical definitions of the TP vary widely throughout in the literature1,8,9,14. For the purposes of this study, we considered the TP to be that region of the anterior temporal lobe that is anterior to the rhinal sulcus9. We chose this definition because it is consistent with previous clinical studies of the electrophysiologic properties of the TP in TLE patients9.
Chronic recording was performed over the course of one to four weeks. The duration of monitoring was determined based on the time necessary to record ictal events. Depending on which imaging data was available for each given patient, electrodes were localized to the brain surface using a combination of intraoperative photographs, axial CT images with 1 mm slices, PA and lateral skull films, and MRI.
Clinical and electrophysiological seizure analysis
The clinical and electrophysiological characteristics of each seizure were recorded with concurrent video and intracranial recordings. After electrode implantation, recording commenced immediately and continued without interruption until resection period. All intracranial recording data and seizure semiology were analyzed by a board certified clinical electrophysiologist and epileptologist (M.A.W.).
Each subject’s seizure network was characterized based on the involvement of the TP. Based on analysis of multiple seizures, each patient’s seizure network was classified as one of the following 1) ictal onset in the TP (Group A) or ictal onset outside the TP in either 1) hippocampus, 2) amygdala, or 3) other temporal neocortical (Group B). Patients with concurrent ictal onset in the TP and other structures (e.g. the hippocampus), were also classified as having ictal onset in the TP. The time from seizure onset to the onset of TP involvement was documented for characteristic seizures. To characterize the relationship between seizure involvement in the TP and the mesial temporal structures, hippocampal, amygdala, and other temporal neocortex onset was also documented for all subjects.
Neuroimaging and neuropathological analysis
Clinical and electrophysiological data were correlated to available neuroimaging and neuropathological specimens. Preoperative MR imaging was retrospectively analyzed by two board, CAQ-certified neuroradiologists (T.M. and A.C.) who were blinded to all other clinical data (i.e. semiology, EEG, and PET). During MR imaging analysis, the neuroradiologists investigated the presence of 1) ATL abnormalities, 2) hippocampal sclerosis, and 3) anatomical lesions anywhere in the brain. Interpretation of neuropathological specimens was performed by one neuropathologist (P.K.) to determine etiology of epilepsy. When appropriate, the ILAE classification of cortical dysplasias was applied in pathologic diagnosis5.
Case grouping
Patients were divided into two groups for ease of comparison. Those who had ictal onset of any clinically significant seizure from the TP were placed into Group A. Patients who did not have onset of any clinically significant seizures from the TP were placed in Group B.
Results
Electrophysiological results
Seizure onset
Electrophysiological results are summarized for all subjects in Table 2. For seven of the 10 patients, the ictal activity of at least one clinically significant seizure type was detected at onset from an electrode on TP cortex. For two patients in Group A (Patient #3 and Patient #5), onset was widespread, but included the TP. An example of a seizure starting in the TP and corresponding electrode localization is depicted in Figure 2. The subject had extensive subdural electrode coverage of subfrontal and subtempotal cortex, including the ATL (Fig. 2A). The seizure started at a single electrode on TP cortex (see TP06, Fig. 2B) and seizure activity at this electrode did not propagate for 8 s. The seizure then spread rapidly throughout all of temporopolar cortex and the superior temporal sulcus. There was late propagation to the amygdala, hippocampus, and orbitofrontal cortex (Fig. 2B).
Table 2:
Interictal Epileptiform Discharges, Seizures Onset, and Seizure Spread Patterns in All Patients
| Patient/Group | TP IEDs | Intracranial EEG seizure onset | Intracranial EEG Seizure propagation | Subclinical TP | |||||
|---|---|---|---|---|---|---|---|---|---|
| TP onset | HPC onset | Amygdala onset | Other neocortical onset | TP propagation | HPC propagation | Amygdala propagation | |||
| 1 – A | + | + | - | - | - | NA | - | 10 s | NA |
| 2 – A | + | + | - | - | + | NA | - | - | NA |
| 3 – A | - | + | + | + | - | NA | NA | NA | NA |
| 4 – A | + | + | - | - | - | NA | - | - | NA |
| 5 – A | - | + | + | - | - | NA | NA | - | NA |
| 6 – A | + | + | - | - | + | NA | - | - | NA |
| 7 – A | + | + | - | - | + | NA | 10 s | 10 s | - |
| 8 – B | - | - | - | - | + | 5 s | - | 4–10 s | + |
| 9 – B | + | - | - | + | - | - | - | NA | - |
| 10 – B | + | - | - | + | - | - | - | NA | - |
Intracranial recording findings of IEDs, seizure onset site, and seizure spread patterns in all patients. Group A is patients 1–7 and Group B is patients 8–10.
Figure 2:
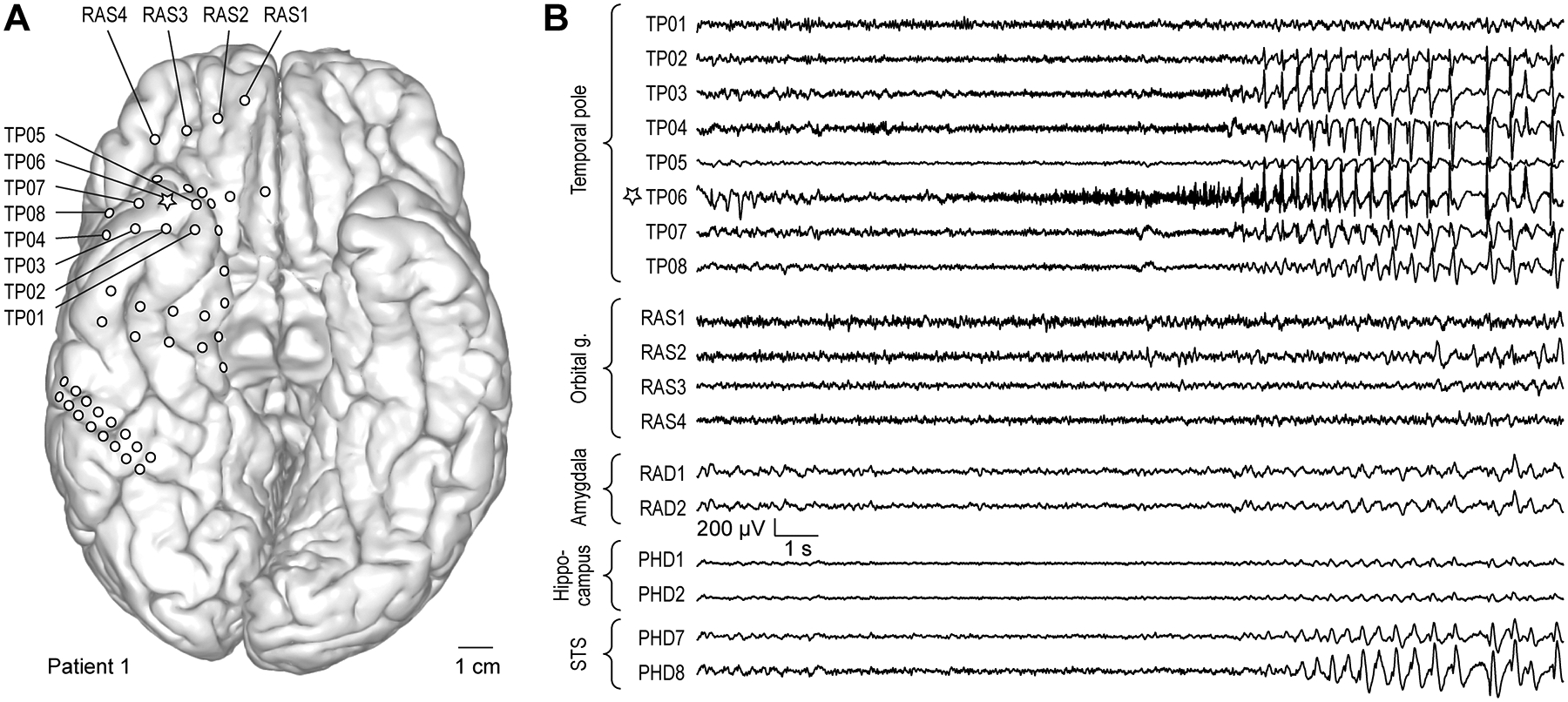
Exemplary seizure. A: Anatomical reconstruction of ventral brain surface demonstrating location of implanted epicortical electrode arrays in a representative patient (#1). Star denotes the recording site where seizure onset was observed (TP06). B: Exemplary recordings from epicortical electrodes implanted over TP and orbital gyrus, and depth electrodes implanted in the amygdala, hippocampus and superior temporal sulcus (STS).
Importantly, most Group B patients (with seizure onset outside the TP) had evidence of hyperexcitability in temporopolar cortex as evidenced by the presence of clinically silent seizure activity or early seizure spread to the TP. Patient #8, for example, had clinically silent seizure activity arising from the TP electrodes. Each of these recordings lasted less than 6s and were not associated with symptoms. Patient #8 also had early spread of ictal activity to the TP within 5s, which occurred prior to propagation to mesial temporal structures (amygdala or hippocampus).
Interictal discharges
All temporopolar electrode sites were evaluated for the presence of interictal epileptiform discharges (IEDs; results summarized in Table 2). In total, 7 out of 10 patients had IEDs from TP cortex. For Group A, 5/7 patients had IEDs arising from the TP. In Group B, 2/3 patients had IEDs arising from the TP. Thus, the presence of IEDs localized to the temporopolar cortex was not necessarily associated with seizure onset from the TP.
Neuroimaging correlation
Both interictal FDG PET and MR imaging data were reviewed for all patients. These findings are summarized in Table 3. Hypometabolism was observed in all patients who underwent interictal FDG PET (9/10, one patient did not have a PET study). Two neuroradiologists (T.M. and A.C.) blinded to all clinical data (including the presence of IEDs, ictal onset, and FDG PET results) reviewed preoperative MR imaging for evidence of MTS, temporopolar abnormal MRI signal, and anatomical lesions for all patients in the study. Ultimately, 5/10 of patients had evidence of MTS of neuroimaging. In Group A, 3/7 patients had MR imaging evidence for MTS, whereas in Group B 2/3patients had MR evidence for MTS. Three patients (patients #5, 6, and 7) had abnormalities of TP cortex visible on MR imaging. Of patients with discernable TP abnormalities, two were in Group A (2/7 and one was in Group B (1/3). Detection of TP abnormalities varied slightly between neuroradiologists (Table 3), with one more patient with TP abnormality being detected by one neuroradiologist. Two examples of TP abnormalities detected on MR imaging are depicted in Figure 3. In each example, there is high FLAIR signal of TP cortex and underlying white matter.
Table 3:
FDG PET, MR Imaging, and Pathological Analysis
| Patient/Group | PET hypometabolism | PET laterality | MRI MTS | MRI TPa | MRI AL | Lesion Location | Pathology | Pathology MTS | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| NR1 | NR2 | NR1 | NR2 | NR1 | NR2 | ||||||
| 1 – A | Y | R | - | _ | - | - | - | + | R amygdala enlargement | FCDIIa | No |
| 2 – A | Y | BL | - | _ | - | - | - | + | L amygdala enlargement | FCDIb | No |
| 3 – A | Y | L | L | L | - | - | - | - | - | FCDIIa | No |
| 4 – A | N/A | N/A | - | _ | - | - | + | + | R amygdala, periamygdaloid and temporal piriform cortex, hippocampal head | DNT | No |
| 5 – A | Y | R | R | R | R | R | - | - | - | FND | Yes |
| 6 – A | Y | L | - | _ | L | L | - | + | L amygdala and hippocampal head | FCDIb | No |
| 7 – A | Y | L | L | L | - | - | + | + | L PHG | FCDIIIa | Yes |
| 8 – B | Y | R | - | _ | - | - | + | + | R fusiform gyrus | GG; FCDIIIb | No |
| 9 – B | Y | L | L | L | - | L | - | - | Left hippocampal sclerosis | None | Yes |
| 10 – B | Y | L | L | L | - | - | - | - | Left hippocampal sclerosis | None | Yes |
Neuroimaging and pathology findings in all study patients. Group A are patients 1–7 and Group B are patients 8–10. MRI findings of MTS, temporal pole abnormalities, or anatomical lesions are denoted as either R, L, or BL (if present, with R, L, or BL denoting Right, Left, or Bilateral, respectively) or – (not present). One MRI interpretation is provided for each patient by two different neuroradiologists. NR1 and NR2 denotes which neuroradiologist interpretation is depicted. TPa: Temporal pole abnormalities. MRI AL: Anatomical lesion on MR imaging. FCD: Focal cortical dysplasia. DNT: Dysembryoplastic neuroepithelial tumor. FND: Focal nodular dysplasia. GG: Ganglioglioma.
Figure 3:
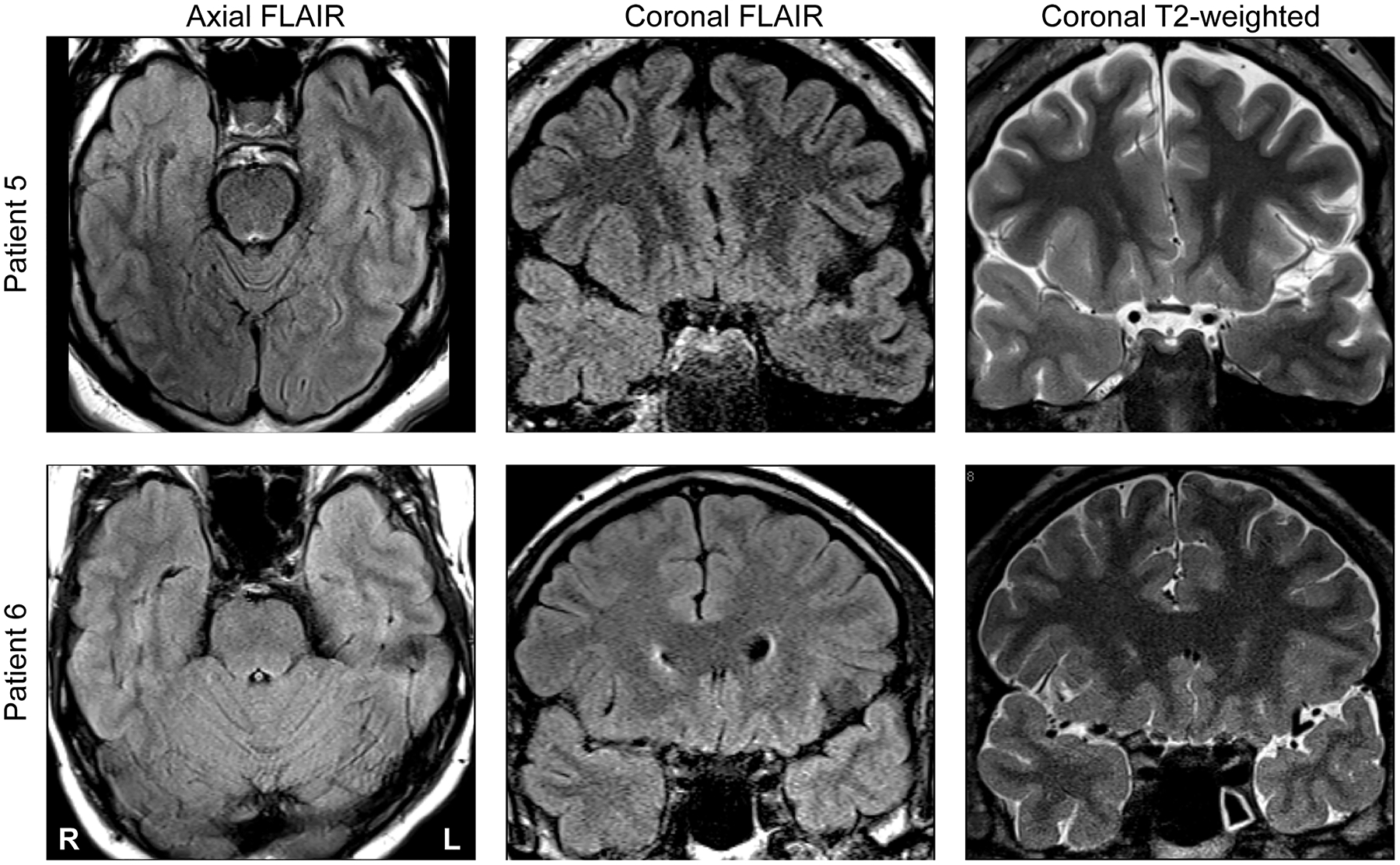
Neuroimaging findings in two representative patients (#5 and #6; top and bottom row, respectively). Axial FLAIR, coronal FLAIR and coronal T2-weighted scans are shown in left, middle and right columns, respectively. In each patient, there is clear atrophy and FLAIR signal abnormality of temporopolar cortex manifest on MR imaging.
Neuroradiologists also examined MR imaging for structural lesions. When examined by T.M., three patients had structural lesions evident on MRI: 2/7 in Group A and 1/3 in Group B. When examined by A.C., 6/10 patients had structural lesions evident on MRI: 5/7 Group A patients and 1/3 Group B patients. MR imaging in Patient #4 demonstrated an intrinsic nonenhancing heterogeneously high T2 signal mass in the right mesial temporal lobe, consistent with a DNT. MR imaging in Patient #7 demonstrated a mesial temporal mass involving the hippocampus and amygdala, suggestive of cortical dysplasia. MR imaging in Patient #8 revealed an intrinsic T1 isointense and T2 hypointense lesion thought to represent a cavernoma (later diagnosed as a ganglioglioma). Other anatomical lesions detected involved subtle FLAIR signal change in the amygdala, which may explain why these abnormalities were not detected by both neuroradiologists.
Pathological correlation
Pathologic diagnosis was performed by a neuropathologist (P.K.) and the ILAE classification system was applied when appropriate. The results are summarized in Table 3. All patients in Group A had focal cortical dysplasias that could be classified by the ILAE classification system or focal nodular dysplasia (Patient #5, see Table 3). In contrast, only one patient in Group B had a focal cortical dysplasia (Patient #8, FCDIIIb and concomitant ganglioglioma). Two tumors were diagnosed: a ganglioglioma in Patient #8 (Group B) and a dysembryoplastic neuroepithelial tumor (DNT) in Patient #4 (Group A). In Group A, 2/7 patients had pathologically confirmed MTS and in Group B, 2/3 patients had pathologically confirmed MTS.
Clinical outcomes
Clinical outcomes are summarized for all patients in Table 1. The average length of follow-up for all patients was 31.4 months. The average follow-up for Group A was 26.7 months and for Group B was 42.3 months. Patient outcomes were classified via the Engel Classification scale. In Group A, 4/7 patients had a Class I outcome, 2/7 patients had a Class II outcome, and 1/7 patient had a Class III outcome. In Group B, 2/3 had a class I outcome and 1/3 had a Class II outcome.
Discussion
The goal of this study was to delineate the role of the TP in TLE seizure networks. Understanding the role of the TP in TLE is crucial as the indications for CAH and SAH vary widely among epilepsy centers and excluding the TP from resection could be a cause of poor seizure freedom rates in patients who undergo SAH9. The superiority of CAH verses SAH for medically intractable TLE is a topic of intense debate; however for patients with findings suggestive of mesial TLE several studies suggest that CAH and SAH are equivalent with regard to seizure freedom18,24,29,31,33. However, some studies have also demonstrated better seizure outcomes with CAH compared to SAH, particularly in pediatric patients4,10,31. Previous studies suggest that even in patients with noninvasive imaging findings suggestive of mesial TLE, the TP can still be part of the epileptogenic zone9. Thus, understanding when the TP is part of the epileptogenic zone should be an integral part of surgical planning to achieve an appropriate surgical intervention for patients with medically intractable TLE.
Despite any controversy regarding the indications for TP resection in patients with medically intractable TLE, numerous studies suggest the role of the TP should be evaluated as part of the epileptogenic zone in patients with TLE6,9,20,21,28. Though the amygdala, hippocampus, and parahippocampal gyrus are frequently implicated as the major players in many patients with TLE, histopathologic alterations outside these structures occur frequently in patients with TLE17,26,27. Additionally, MR imaging of patients with TLE have reported abnormal anterior temporal T2 signal26, grey-white matter signal blurring in TP,17 and volume loss in the TP13. The histopathologic correlates of these imaging abnormalities remain unclear, though a variety of pathologic abnormalities have been observed in the TP in patients with TLE13,26,27. In 2012, Garbelli et. al.17 compared 7T MR imaging and myelin staining of TLE specimens and found reduced axonal density in the TP of patients with temporopolar blurring on MR imaging. Additionally, TP hypometabolism as evidenced by FDG PET is seen frequently in patients with TLE30. Taken together, these findings suggest that the TP can be significantly affected in TLE. As others have pointed out7, it is important to note that though the TP may be affected in TLE, structural changes in the TP do not confirm its involvement in the epileptogenic zone.
That said, there is also electrophysiologic evidence that the TP can play a crucial role in epileptogenesis in patients with TLE. For example, stimulation of the TP can be just as effective in inducing seizures in TLE patients as the amygdala, hippocampus, or parahippocampal gyrus22. Additionally, simultaneous onset of seizures in the amygdala, hippocampus, and hippocampal gyrus is a common phenomenon in TLE9,21, as we address in further detail below.
The main finding of this study is that temporopolar cortex plays a key role in ictal onset of clinically significant seizures in a large proportion (70% in our series) of patients with TLE in our study. The patients who exhibited ictal onset from the TP in our series were not patients expected to have TP involvement at seizure onset based on noninvasive preoperative work-up. In our study, two blinded neuroradiologists searched for both structural abnormalities of the temporopolar cortex on MR imaging and also anatomical lesions in the temporal lobe (see Table 3). Review of MR imaging by these neuroradiologists did not uniformly implicate the TP cortex in the epileptogenic zone. For example, only two of seven patients in Group A were found to have an imaging abnormality of the TP on MR imaging by both of our neuroradiologists. It is interesting to note that one of our neuroradiologists found that five of seven Group A patients had anatomical lesions while our other neuroradiologist only found anatomical lesions in two of seven Group A patients, thereby illustrating the potential for discrepancy in MR imaging interpretation even by trained neuroradiologists. In contrast, one Group B patient (patient 9) was found to have a structural abnormality in the TP on MR imaging and did not demonstrate any spread of seizure activity to the TP cortex (with onset in the amygdala). These examples illustrate the difficulty of determining whether or not the TP should be considered part of the epileptogenic zone by noninvasive means. This fact is also apparent from our FDG PET findings: every patient in our study who underwent FDG PET (9 patients, see Table 2) demonstrated hypometabolism of the TP, yet only six had ictal onset from the TP. FDG PET hypometabolism also did not seem to correlate with the presence of interictal discharges from the TP. Ultimately, the correlation between both MR imaging and FDG PET would suggest the importance of intracranial recordings from the TP in cases where the TP could potentially be involved, which others have also advocated9,21,28.
Our finding of ictal onset from the TP in 70% of TLE patients agrees with other intracranial recording studies of the TP in patients with TLE9,21. In 2005, Chabardes and colleagues9 reported 48 patients with TLE who underwent concurrent SEEG recordings from the TP and hippocampus prior to CAH. In their study 23/48 patients (48%) demonstrated involvement of the TP either before or concurrently with the hippocampus. In their series of patients with TP involvement at seizure onset, 9 had MTS and 11 had TP abnormalities on MR imaging. This is an important distinction from our smaller series in which only 2 of 7 patients with TP involvement at seizure onset have MTS. Interestingly, Chabardes et al.9 also found that patients who had TP involvement at seizure onset actually had better postoperative seizure freedom rates compared to patients where the TP was not involved at seizure onset (95 vs. 72% Engel Class I). Importantly, five of seven patients in Group A had seizure onset in the TP concurrent with another temporal lobe structure. While it is possible that recording techniques with improved temporal resolution (e.g. depth recordings of the TP) could show onset at a specific site (rather than two concurrent sites), the finding of concurrent onset in the TP demonstrates that the TP plays an intimate role at seizure onset even with distant sites within the temporal lobe.
It is interesting to note that all Group A patients were retrospectively found to have pathologic abnormalities in the anterior temporal cortex (see Table 3), particularly cortical dysplasia. This is understandable given that ictal onset involved the TP cortex in each of these patients. Additionally, our results support the notion that the TP is more frequently involved at the pathologic and electrophysiologic levels in patients with dysplastic lesions of the mesial temporal lobe as opposed to other etiologies. Importantly, as discussed above, the TP cortex would not necessarily have been implicated in the epileptogenic zone based on MR imaging alone. Furthermore, imaging abnormalities can be seen in temporopolar cortex in TLE patients regardless of whether or not onset involves the TP17,26,27.
This study has several limitations that must be considered. A major limitation is the small sample size that precludes meaningful use of statistics. Additionally, there is a potential for selection bias as patients with TLE and concordant VEEG, FDG PET, and MR imaging (i.e. MTS) findings undergo CAH without intracranial recording studies (and would not be included in this study). Based on previous studies9, we suspect that some patients with MTS exhibit seizures that involve the TP at seizure onset; however, our study did not evaluate this possibility. Furthermore, our study population is quite heterogeneous with several of our patients retrospectively found to have cortical dysplasias or other cortical lesions. With those limitations in mind, the present results still demonstrate the frequent possibility that the TP is involved in the epileptogenic zone in TLE patients where it would not usually be suspected.
Additionally, since all of our patients underwent CAH, our study did not evaluate the relative benefits of CAH compared to SAH or treatment by selective laser ablation. It is possible that the similar outcomes seen with CAH and SAH occur because the TP is partially disconnected with certain approaches to the mesial temporal lobe. Given our results, it is possible that the lower seizure freedom rates observed with LITT23 result from the preserved connections to temporopolar cortex. Future studies are needed to determine whether tailoring resection strategies to electrophysiologic findings in the TP has value. Furthermore, it could be possible that our finding of early involvement of temporopolar cortex is a non-specific finding and also occurs in extra-temporal epilepsies. The TP has extensive connectivity with brain structures outside the temporal lobe that would also support this possibility20. However, based on previous studies9, this does not seem likely.
Conclusion
Using dense intracranial recordings of the TP, we have demonstrated ictal onset of clinically significant seizures from the TP in 7 of 10 consecutive patients with medically intractable TLE. Our findings provide evidence that TP should be given consideration as part of the epileptogenic zone in patients with TLE even when noninvasive imaging studies (e.g. MR imaging) would not suggest a role for the TP, particularly when dysplastic lesions are suspected. The presence of MR imaging abnormalities, FDG PET hypometabolism, and interictal discharges are not necessarily associated with the presence of ictal onset from the TP. Involvement of temporopolar cortex in the epileptogenic zone may be a potential reason for poor seizure outcomes after selective mesial resections, particularly LITT.
Acknowledgements
The authors wish to acknowledge Eishi Asano, M.D., Ph.D. for his insightful comments during the preparation of this manuscript. We wish to thank Tammy Bryant, R.E.E.G.T., for her assistance with data analysis.
Funding Sources
This work was supported by the National Institutes of Health (NIH F32-NS087664).
Footnotes
Portions of this work were presented at the 2016 Congress of Neurological Surgeons Annual Meeting (September 24–28, 2016, San Diego, CA) and at the American Epilepsy Society Annual Meeting (December 2–6, 2016, Houston, TX).
References
- 1.Abel TJ, Rhone AE, Nourski KV, Ando TK, Oya H, Kovach CK, et al. : Beta modulation reflects name retrieval in the human anterior temporal lobe: an intracranial recording study. J Neurophysiol 115:3052–3061, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abel TJ, Rhone AE, Nourski KV, Granner MA, Oya H, Griffiths TD, et al. : Mapping the temporal pole with a specialized electrode array: technique and preliminary results. Physiol Meas 35:323–337, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Acar G, Acar F, Miller J, Spencer DC, Burchiel KJ: Seizure outcome following transcortical selective amygdalohippocampectomy in mesial temporal lobe epilepsy. Stereotactic and functional neurosurgery 86:314–319, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Bate H, Eldridge P, Varma T, Wieshmann U: The seizure outcome after amygdalohippocampectomy and temporal lobectomy. European journal of neurology 14:90–94, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Blumcke I, Thom M, Aronica E, Armstrong DD, Vinters HV, Palmini A, et al. : The clinicopathologic spectrum of focal cortical dysplasias: a consensus classification proposed by an ad hoc Task Force of the ILAE Diagnostic Methods Commission. Epilepsia 52:158–174, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonilha L, Martz GU, Glazier SS, Edwards JC: Subtypes of medial temporal lobe epilepsy: influence on temporal lobectomy outcomes? Epilepsia 53:1–6, 2012 [DOI] [PubMed] [Google Scholar]
- 7.Caboclo LO, Garzon E, Oliveira PA, Carrete H Jr., Centeno RS, Bianchin MM, et al. : Correlation between temporal pole MRI abnormalities and surface ictal EEG patterns in patients with unilateral mesial temporal lobe epilepsy. Seizure 16:8–16, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Chabardes S, Kahane P, Minotti L, Hoffmann D, Benabid AL: Anatomy of the temporal pole region. Epileptic Disord 4 Suppl 1:S9–15, 2002 [PubMed] [Google Scholar]
- 9.Chabardes S, Kahane P, Minotti L, Tassi L, Grand S, Hoffmann D, et al. : The temporopolar cortex plays a pivotal role in temporal lobe seizures. Brain 128:1818–1831, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Clusmann H, Kral T, Gleissner U, Sassen R, Urbach H, Blumcke I, et al. : Analysis of different types of resection for pediatric patients with temporal lobe epilepsy. Neurosurgery 54:847–859; discussion 859–860, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Clusmann H, Schramm J, Kral T, Helmstaedter C, Ostertun B, Fimmers R, et al. : Prognostic factors and outcome after different types of resection for temporal lobe epilepsy. J Neurosurg 97:1131–1141, 2002 [DOI] [PubMed] [Google Scholar]
- 12.Cohen-Gadol AA, Spencer DD: Use of an anteromedial subdural strip electrode in the evaluation of medial temporal lobe epilepsy. Technical note. J Neurosurg 99:921–923, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Coste S, Ryvlin P, Hermier M, Ostrowsky K, Adeleine P, Froment JC, et al. : Temporopolar changes in temporal lobe epilepsy: a quantitative MRI-based study. Neurology 59:855–861, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Ding SL, Van Hoesen GW, Cassell MD, Poremba A: Parcellation of Human Temporal Polar Cortex: A Combined Analysis of Multiple Cytoarchitectonic, Chemoarchitectonic, and Pathological Markers. Journal of Comparative Neurology 514:595–623, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Drane DL, Loring DW, Voets NL, Price M, Ojemann JG, Willie JT, et al. : Better object recognition and naming outcome with MRI-guided stereotactic laser amygdalohippocampotomy for temporal lobe epilepsy. Epilepsia, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elsharkawy AE, Alabbasi AH, Pannek H, Oppel F, Schulz R, Hoppe M, et al. : Long-term outcome after temporal lobe epilepsy surgery in 434 consecutive adult patients. J Neurosurg 110:1135–1146, 2009 [DOI] [PubMed] [Google Scholar]
- 17.Garbelli R, Milesi G, Medici V, Villani F, Didato G, Deleo F, et al. : Blurring in patients with temporal lobe epilepsy: clinical, high-field imaging and ultrastructural study. Brain 135:2337–2349, 2012 [DOI] [PubMed] [Google Scholar]
- 18.Hu WH, Zhang C, Zhang K, Meng FG, Chen N, Zhang JG: Selective amygdalohippocampectomy versus anterior temporal lobectomy in the management of mesial temporal lobe epilepsy: a meta-analysis of comparative studies. J Neurosurg 119:1089–1097, 2013 [DOI] [PubMed] [Google Scholar]
- 19.Josephson CB, Dykeman J, Fiest KM, Liu X, Sadler RM, Jette N, et al. : Systematic review and meta-analysis of standard vs selective temporal lobe epilepsy surgery. Neurology 80:1669–1676, 2013 [DOI] [PubMed] [Google Scholar]
- 20.Kahane P, Bartolomei F: Temporal lobe epilepsy and hippocampal sclerosis: lessons from depth EEG recordings. Epilepsia 51 Suppl 1:59–62, 2010 [DOI] [PubMed] [Google Scholar]
- 21.Kahane P, Chabardes S, Minotti L, Hoffmann D, Benabid AL, Munari C: The role of the temporal pole in the genesis of temporal lobe seizures. Epileptic Disord 4 Suppl 1:S51–58, 2002 [PubMed] [Google Scholar]
- 22.Kahane P, Tassi L, Francione S, Hoffmann D, Russo GL, Munari C: Manifestations électrocliniques induites par la stimulation électrique intracérébrale par «chocs dans les épilepsies temporales. Neurophysiologie Clinique/Clinical Neurophysiology 23:305–326, 1993 [DOI] [PubMed] [Google Scholar]
- 23.Kang JY, Wu C, Tracy J, Lorenzo M, Evans J, Nei M, et al. : Laser interstitial thermal therapy for medically intractable mesial temporal lobe epilepsy. Epilepsia 57:325–334, 2016 [DOI] [PubMed] [Google Scholar]
- 24.Kuang Y, Yang T, Gu J, Kong B, Cheng L: Comparison of therapeutic effects between selective amygdalohippocampectomy and anterior temporal lobectomy for the treatment of temporal lobe epilepsy: a meta-analysis. Br J Neurosurg 28:374–377, 2014 [DOI] [PubMed] [Google Scholar]
- 25.Luders HO: Textbook of epilepsy surgery: CRC Press, 2008 [Google Scholar]
- 26.Meiners LC, Witkamp TD, de Kort GA, van Huffelen AC, van der Graaf Y, Jansen GH, et al. : Relevance of temporal lobe white matter changes in hippocampal sclerosis. Magnetic resonance imaging and histology. Invest Radiol 34:38–45, 1999 [DOI] [PubMed] [Google Scholar]
- 27.Mitchell LA, Jackson GD, Kalnins RM, Saling MM, Fitt GJ, Ashpole RD, et al. : Anterior temporal abnormality in temporal lobe epilepsy: a quantitative MRI and histopathologic study. Neurology 52:327–336, 1999 [DOI] [PubMed] [Google Scholar]
- 28.Munari C, Hoffmann D, Francione S, Kahane P, Tassi L, Lo Russo G, et al. : Stereo-electroencephalography methodology: advantages and limits. Acta Neurol Scand Suppl 152:56–67, discussion 68–59, 1994 [DOI] [PubMed] [Google Scholar]
- 29.Nascimento FA, Gatto LA, Silvado C, Mader-Joaquim MJ, Moro MS, Araujo JC: Anterior temporal lobectomy versus selective amygdalohippocampectomy in patients with mesial temporal lobe epilepsy. Arq Neuropsiquiatr 74:35–43, 2016 [DOI] [PubMed] [Google Scholar]
- 30.Rubin E, Dhawan V, Moeller JR, Takikawa S, Labar DR, Schaul N, et al. : Cerebral metabolic topography in unilateral temporal lobe epilepsy. Neurology 45:2212–2223, 1995 [DOI] [PubMed] [Google Scholar]
- 31.Schramm J: Temporal lobe epilepsy surgery and the quest for optimal extent of resection: a review. Epilepsia 49:1296–1307, 2008 [DOI] [PubMed] [Google Scholar]
- 32.Tanriverdi T, Olivier A, Poulin N, Andermann F, Dubeau F: Long-term seizure outcome after mesial temporal lobe epilepsy surgery: corticalamygdalohippocampectomy versus selective amygdalohippocampectomy. J Neurosurg 108:517–524, 2008 [DOI] [PubMed] [Google Scholar]
- 33.Wendling AS, Hirsch E, Wisniewski I, Davanture C, Ofer I, Zentner J, et al. : Selective amygdalohippocampectomy versus standard temporal lobectomy in patients with mesial temporal lobe epilepsy and unilateral hippocampal sclerosis. Epilepsy Res 104:94–104, 2013 [DOI] [PubMed] [Google Scholar]
- 34.Wiebe S, Blume WT, Girvin JP, Eliasziw M: A randomized, controlled trial of surgery for temporal-lobe epilepsy. New England Journal of Medicine 345:311–318, 2001 [DOI] [PubMed] [Google Scholar]
- 35.Willie JT, Laxpati NG, Drane DL, Gowda A, Appin C, Hao C, et al. : Real-time magnetic resonance-guided stereotactic laser amygdalohippocampotomy for mesial temporal lobe epilepsy. Neurosurgery 74:569–585, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]


