Abstract
Background:
Exposure to phthalates during pregnancy may alter DNA methylation in the placenta, a crucial organ for the growth and development of the fetus.
Objectives:
We studied associations between urinary concentrations of phthalate biomarkers during pregnancy and placental DNA methylation.
Methods:
We measured concentrations of 11 phthalate metabolites in maternal spot urine samples collected between 22 and 29 gestational weeks in 202 pregnant women. We analyzed DNA methylation levels in placental tissue (fetal side) collected at delivery. We first investigated changes in global DNA methylation of repetitive elements Alu and LINE-1. We then performed an adjusted epigenome-wide association study using IlluminaHM450 BeadChips and identified differentially methylated regions (DMRs) associated with phthalate exposure.
Results:
Monobenzyl phthalate concentration was inversely associated with placental methylation of Alu repeats. Moreover, all phthalate biomarkers except for monocarboxy-iso-octyl phthalate and mono(2-ethyl-5-hydroxyhexyl) phthalate were associated with at least one DMR. All but three DMRs showed increased DNA methylation with increased phthalate exposure. The largest identified DMR (22 CpGs) was positively associated with monocarboxy-iso-nonyl phthalate and encompassed heat shock proteins (HSPA1A, HSPA1L). The remaining DMRs encompassed transcription factors and nucleotide exchange factors, among other genes.
Conclusions:
This is the first description of genome-wide modifications of placental DNA methylation in association with pregnancy exposure to phthalates. Our results suggest epigenetic mechanisms by which exposure to these compounds could affect fetal development. Of interest, four identified DMRs had been previously associated with maternal smoking, which may suggest particular sensitivity of these genomic regions to the effect of environmental contaminants.
Keywords: Placenta, DNA methylation, Pregnancy exposure, Phthalates, Alu and LINE-1
1. Introduction
The placenta plays an important role in the paradigm of the developmental origins of health and disease (DOHaD), a concept focusing on the role of the prenatal environment in determining the development of disease later in life (Gillman 2005). Apart from transporting nutrients and waste products between mother and fetus, the placenta affects the programming of the fetal phenotype. There is growing evidence for epigenetics playing an important role in this process (Maccani and Marsit 2009; Robinson et al. 2019). Placental epigenetic mechanisms can be sensitive to environmental factors, such as chemicals, several of which have been associated with different developmental disorders, birth defects, and child health problems (Kishi and Grandjean 2020). The placental unique epigenetic landscape could also serve as a “molecular archive” of the fetal developmental environment (Heijmans et al. 2009).
Herein we focused on phthalates, a family of non-persistent chemicals abundant in the environment due to their broad spectrum of applications including in solvents, as plasticizers and additives in polyvinyl chloride plastics or personal care products (Latini 2005). Some phthalates can cross the placental barrier and their metabolites have been detected in the placental tissue (Mose et al. 2007b, 2007a). There is also growing epidemiological evidence that pregnancy exposure to several phthalates may be associated with different placental epigenetic endpoints (reviewed by Vlahos et al. 2019), DNA methylation being one of them (reviewed by Dutta et al., 2020; Strakovsky and Schantz 2018). Nevertheless, epidemiological studies on associations between phthalate exposure during pregnancy and DNA methylation marks in placenta are scarce (LaRocca et al. 2014; Zhao et al. 2015b, 2016). These studies relied on a candidate gene approach in normal or complicated pregnancies (i.e., fetal growth restriction newborns) and included two genes at most. LaRocca et al. focused on the imprinted H19 and insulin like growth factor 2 (IGF2) genes (n = 179, LaRocca et al. 2014) while Zhao et al. on IGF2 and growth-related aryl-hydrocarbon receptor repressor (AHRR) genes (n = 181, Zhao et al. 2016) or LINE-1 repetitive elements (n = 119, Zhao et al. 2015b). The only existing epigenome-wide study focused on 16 early terminated pregnancies, which limits the general-izability of the findings to fully developed pregnancies (Grindler et al. 2018). In the present study we hypothesized that pregnancy exposure to phthalates impacts DNA methylation profiles in placenta. Therefore, we investigated the associations between maternal concentrations of 11 phthalate metabolites and genome-wide DNA methylation in placentas collected at birth. We assessed global DNA methylation relying on repetitive elements Alu and LINE-1 and performed an adjusted epigenome-wide association study (EWAS) using IlluminaHM450 BeadChips. We also identified differentially methylated regions (DMRs) associated with phthalate exposure.
2. Methods
2.1. Study design and population
We relied on a subsample of 202 mother-son pairs from the French mother–child cohort EDEN (Etude des Déterminants pré et postnatals du développement et de la santé de l’Enfant) recruited between 2003 and 2006 (Heude et al. 2016). Recruitment of pregnant women at the Nancy and Poitiers University hospitals took place before their 24th week of gestation. Exclusion criteria were: maternal diabetes before pregnancy, multiple fetuses, intention to deliver outside the university hospital or to move out of the study region within the next three years, and inability to speak French. Out of the 2,002 enrolled participants, 1,301 had placental samples collected and, for 668 individuals, placental DNA methylation was assessed. Out of those, 202 women delivering a boy had phthalate metabolite concentrations assessed in urine (Botton et al. 2016; Chevrier et al. 2012) and available information on covariates (Supplementary Fig. 1). Phthalate metabolites assessment in urine was restricted to pregnancies with male fetuses in the context of a previous project focusing on their associations with male congenital malformations (Chevrier et al. 2012). The characteristics [maternal smoking status, maternal pre-pregnancy body mass index (BMI), maternal and gestational age] of the 798 mother-son pairs excluded from this study were similar to those of the included participants (results not shown).
The EDEN cohort received approval from the ethics committee (CCPPRB) of Kremlin Bicêtre and from the French data privacy institution “Commission Nationale de l’Informatique et des Libertés”. Written consent was obtained from the mother for herself and for the offspring. The involvement of the Centers for Disease Control and Prevention (CDC) laboratory did not constitute engagement in human subjects’ research.
2.2. Assessment of phthalate metabolites in maternal urine
Between 22 and 29 gestational weeks, women were asked to collect a spot sample of a first morning urine void at home, before a study visit. Women who did not collect their urine at home, collected a spot sample at the hospital during the visit. Urine samples were aliquoted and stored on dry ice at −80 °C before shipment to the National Center for Environmental Health laboratory at the CDC in Atlanta, Georgia, for the assessment of phthalate metabolite concentrations. Eleven phthalate metabolites were measured: monoethyl phthalate (MEP), mono-iso-butyl phthalate (MiBP), mono-n-butyl phthalate (MnBP), monobenzyl phthalate (MBzP), mono(3-carboxypropyl) phthalate (MCPP), monocarboxy-iso-octyl phthalate (MCOP), monocarboxy-iso-nonyl phthalate (MCNP), and four metabolites of di-2-ethylhexyl phthalate (DEHP): mono(2-ethylhexyl) phthalate (MEHP), mono(2-ethyl-5-hydroxyhexyl) phthalate (MEHHP), mono(2-ethyl-5-oxohexyl) phthalate (MEOHP), mono(2-ethyl-5-carboxypentyl) phthalate (MECPP). Metabolite concentrations were quantified using online solid phase extraction-high performance liquid chromatography-isotope dilution-tandem mass spectrometry (Ye et al. 2005). Creatinine, a marker of urine dilution, was also measured.
2.3. Phthalate metabolite concentrations, imputation and standardization
Urinary concentrations (μg/L) below the limit of detection (LOD) were substituted by instrumental reading values. If the instrumental reading values equaled zero (i.e., indicating no signal) they were replaced by the lowest instrumental reading value specific for each phthalate and divided by . Concentrations were then standardized on sampling conditions (gestational age at collection, day of sampling, hour of sampling, year of sample analysis at the CDC, and duration of storage at room temperature before freezing), and creatinine concentrations using a method based on regression residuals (Mortamais et al. 2012) and applied previously in the EDEN cohort (Botton et al. 2016; Philippat et al. 2014). To limit the impact of outliers, phthalate metabolite concentrations were log2-transformed. The sum of DEHP metabolite concentrations (ΣDEHP) was calculated by summing molar concentrations of MEHP, MEHHP, MEOHP, and MECPP.
2.4. Placental tissue collection and DNA extraction
Placental tissue samples were obtained at delivery by the midwife or the technician of the study using a standardized procedure. Samples of around 5 mm3 were collected from the fetal side, a few centimeters from the insertion of the cord, and immediately frozen at −80 °C. DNA was extracted using the QIAsymphony instrument (Qiagen, Germany) following the manufacturer’s protocol. DNA concentration was determined by Nanodrop (ThermoFisher Scientific, USA) measurement and fluorescent quantification using PicoGreen (ThermoFisher Scientific, France). No sample was discarded due to low DNA concentration.
2.5. Placental DNA methylation assessment and quality control
As previously described (Abraham et al. 2018; Jedynak et al. 2021), whole-genome DNA methylation was measured at > 485,000 CpGs using the Infinium HumanMethylation450 BeadChips (Illumina, San Diego, CA, USA) following standard manufacturer’s protocols. Raw intensities of fluorescent signals were processed with the Chip Analysis Methylation Pipeline (ChAMP) V2.14 (Morris et al., 2014; Tian et al., 2017). All samples but one passed initial quality control with an average of > 98% valid data points (detection p-value < 0.01). Filtering included removal of probes with detection p-values above 0.01 (52,692 probes), low numbers of measured events [beadcount < 3 in at least 5% of samples (44 probes)], probes not targeting a CpG (2,034 probes), probes associated with SNPs (50,829 probes, Zhou et al. 2017) or unspecific probes (9 probes, Nordlund et al. 2013). Methylation levels of individual CpGs were reported as continuous averaged β-value, representing the proportion of methylated alleles for each methylation site ranging from 0 (indicating that the site is completely unmethylated) to 1 (completely methylated) and were normalized in ChAMP using Beta MIxture Quantile (BMIQ) normalization (Teschendorff et al. 2013). To reduce the influence of outliers, methylation beta values above the 75th percentile + three interquartile ranges (IQRs) or below the 25th percentile − three IQRs were removed (in total, 0.39% of methylation values in our sample of 202 participants). 379,904 methylation sites remained after quality control, normalization, and filtering of outliers (Fig. 1). For each individual, pyrosequencing was used to measure methylation levels of four CpG sites of repetitive Alu elements (Alu) and long interspersed nucleotide elements 1 (LINE-1) (Yang 2004). One individual had no information on Alu methylation due to low signal-to-noise ratio and was removed from the analysis, yielding a final sample size of n = 201 for this study.
Fig. 1.
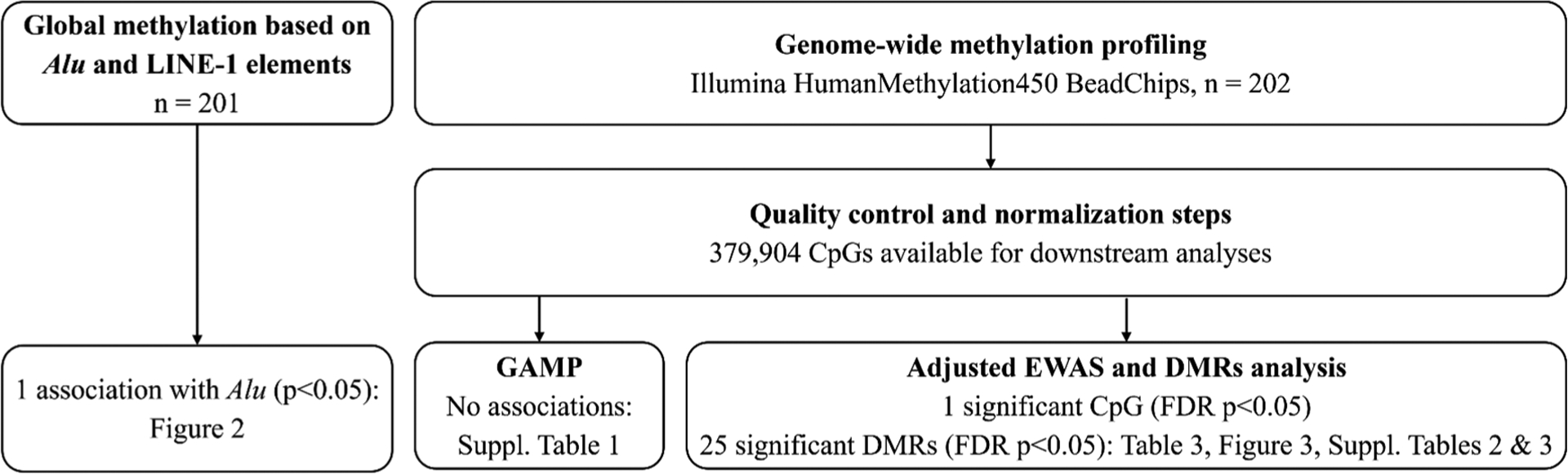
Workflow of the statistical analyses performed in this study. Abbreviations: DMR = differentially methylated region. FDR = false discovery rate. GAMP = global analysis of methylation profiles.
2.6. Placental cell heterogeneity estimation
We applied a recently proposed method to obtain reference-based estimates of placental cell composition (Yuan et al. 2021). In their work, Yuan et al. measured methylation profiles of six reference cell types [endothelial, Hofbauer, nucleated red blood cells (nRBC), stromal, syncytiotrophoblasts, and trophoblasts] in term placental tissues. Taking advantage of this cell-type specific reference provided in the R package planet, we applied the Robust Partial Correlations method implemented in the R package EpiDISH (Teschendorff et al. 2017) to the methylation data collected for a bigger EDEN sample (n = 668) that our study sample was derived from. This allowed us to obtain reference-based estimates of cell composition that were then used in our regression models as adjustment factors. If zero estimates were obtained for a cell type, they were considered as below the LOD and their values were imputed using the impCoda function from the R package robCompositions designed for compositional data and relying on an iterative regression-based procedure after KNN-initialization (Hron et al. 2010; Templ et al. 2011) (Supplementary Fig. 2).
2.7. Statistical analyses
2.7.1. Adjustment factors
We a priori selected factors that may affect both phthalate exposure and methylation marks in the placenta or the DNA methylation only. This included recruitment site (Nancy, Poitiers), maternal age (continuous), maternal pre-pregnancy BMI (< 18.5 kg/m2, ≥ 18 - < 25 kg/m2, ≥ 25 kg/m2), maternal active smoking in the three months preceding pregnancy and during pregnancy (did not smoke, smoked before pregnancy, smoked before and during pregnancy, other), maternal education level (< two years after high school, high school + two years, ≥ high school + three years), parity (nulliparous, ≥ one child), and season of conception (January-March, April-June, July-September, October-December). All analyses were additionally adjusted for technical factors related to the DNA methylation measurements [batch, plate, chip, and placental cell proportions for the epigenome-wide (EWAS) and global analysis of methylation profiles (GAMP); batch and plate for the study of repetitive Alu and LINE-1 elements]. Our analyses were not adjusted for gestational age at birth since it may be in the pathway between phthalate exposure and DNA methylation.
2.7.2. Associations with the global DNA methylation
We fitted one adjusted robust linear regression per phthalate biomarker to test the associations with global placental DNA methylation represented by the median methylation level of repetitive elements Alu or LINE-1. In the GAMP analysis we approximated the density and cumulative distribution functions of the methylation distribution using B-spline basis functions in order to characterize methylation profiles for each individual (Zhao et al. 2015a). Then we used the obtained B-spline coefficients as representatives of the individual overall methylation distribution. The variance component score test from the kernel machine framework served to test the adjusted associations between B-spline coefficients and concentrations of each phthalate biomarker. Using this method, we were able to evaluate whether pregnancy phthalate exposure changed the overall profile or distribution of DNA methylation for each individual instead of examining phthalates’ effect on each CpG individually.
2.7.3. Associations with the CpG-specific DNA methylation
We performed an adjusted EWAS to assess associations between each phthalate metabolite concentration and DNA methylation at the level of individual CpG sites using robust linear regression (MASS R package Venables and Ripley 2002). p-values were calculated using Wald test from the survey R package (Lumley 2004) and corrected for false discovery rate (FDR) taking into account the number of CpGs tested for each chemical (Benjamini and Hochberg 1995). FDR corrected p-values below 0.05 were considered as statistically significant. Genomic inflation factor (λ) and Q-Q plots were generated using the QCEWAS R package (Van der Most et al. 2017) and the Bayesian inflation factor (BIF) was calculated using the bacon R/Bioconductor package (van Iterson et al., 2017). Gene annotations were based on Illumina’s v1.2 annotation for the hg19 reference genome from the IlluminaHumanMethylation450kanno.ilmn12.hg19 R/Bioconductor package (Hansen 2016) and information from the University of California, Santa Cruz (UCSC, https://genome.ucsc.edu) database.
2.7.4. Analysis of differentially methylated regions (DMRs)
To identify DMRs associated with phthalate biomarker concentrations we used the comb-p Python module (Pedersen et al. 2012). Using sliding windows, it combines p-values for CpGs detected in the EWAS accounting for their spatial correlations across the genome with a Stouffer-Liptak-Kechris correction (Kechris et al. 2010). Regional p-values are then adjusted for multiple testing by Šidák correction (Šidák 1967). DMRs with Šidák-corrected p-value below 0.05 and including at least two probes (p-value < 0.001 to initiate a region) at a maximum distance of 500 bp were considered significant. Basic information on genes encompassed by the DMRs identified as associated with the phthalate exposure were retrieved from the GeneCards Human Gene Database (Stelzer et al. 2016).
2.8. Research data and code
All analyses were conducted using R v. 4.0.5 (R Core Team and R Foundation for Statistical Computing 2020), RStudio v. 1.3.1106 (RStudio Team 2020) and Python v. 3.7.4 (van Rossum and Drake 2009). The data used in this study can only be provided upon reasonable request after approval by the EDEN steering committee. The code is available upon request to the corresponding authors. The statistical analysis plan for this study was pre-registered online (osf.io/2apqw).
3. Results
3.1. Study population characteristics and phthalate biomarker concentrations
Median maternal age was 29.1 years and median gestational duration was 40.0 weeks (Table 1). The minimal frequency of detection of phthalate metabolites was 98.5% and most of them were detected in 100% of the urine samples (Table 2). MEP was the most abundant biomarker (median standardized concentration: 117.8 μg/L) followed by MnBP (44.7 μg/L), MiBP (38.4 μg/L), and MECPP (38.1 μg/L). We observed strong correlations [Spearman’s coefficient (rho) = 0.67] between the standardized concentrations of MCPP and MnBP and very strong correlations (0.84 ≤ rho ≤ 0.98) between the four individual DEHP metabolites (Supplementary Fig. 3).
Table 1.
Population characteristics for the 202 mother-son pairs included in the study and recruited between 2003 and 2006.
| Characteristics | Distribution | |
|---|---|---|
| n (%) | Median [25th, 75th centiles] | |
| Center of recruitment | ||
| Nancy | 103 (51.0%) | |
| Poitiers | 99 (49.0%) | |
| Season of conception | ||
| January-March | 44 (21.8%) | |
| April-June | 41 (20.3%) | |
| July-September | 57 (28.2%) | |
| October-December | 60 (29.7%) | |
| Maternal active smoking in the 3 months preceding pregnancy and during pregnancy | ||
| Did not smoke | 127 (62.9%) | |
| Smoked before pregnancy | 19 (9.4%) | |
| Smoked before and during pregnancy | 26 (12.9%) | |
| Othera | 30 (14.9%) | |
| Parity | ||
| Nulliparous | 88 (43.6%) | |
| ≥ 1 child | 114 (56.4%) | |
| Maternal level of education | ||
| < 2 years after high school | 93 (46.0%) | |
| high school + 2 years | 43 (21.3%) | |
| ≥ high school + 3 years | 66 (32.7%) | |
| Maternal pre-pregnancy BMI b | ||
| Underweight (< 18.5 kg/m2) | 19 (9.4%) | |
| Normal weight (≥ 18 - < 25 kg/m2) | 135 (66.8%) | |
| Overweight and obesity (≥ 25 kg/m2) | 48 (23.8%) | |
| Maternal age (years) | 29.1 [25.6;33.0] | |
| Gestational age at delivery (weeks) c | 40.0 [38.9;41.0] | |
Abbreviations: BMI = body mass index. LMP = last menstrual period.
Category “Other” referred to women that smoked at some point during pregnancy (during 1 or 2 out of 3 trimesters) but not during the whole pregnancy, or to women that smoked before pregnancy and at some point during pregnancy but not during the whole pregnancy (e.g., women who smoked in the 3 months preceding pregnancy and during the first trimester but quit smoking afterwards).
Categorized according to the World Health Organization definitions.
Based on the date of the LMP or gestational duration assessed by the obstetrician if it differed from the LMP-based estimate by more than 2 weeks.
Table 2.
Maternal urinary phthalate biomarker concentrations (n = 202).
| Phthalate biomarker | Molecular weight | LOD (μg/L) | > LOD (%) | Measured concentrations | Standardized concentrationsa | ||||
|---|---|---|---|---|---|---|---|---|---|
| Percentiles (μg/L) | Percentiles (μg/L) | ||||||||
| 5th | 50th | 95th | 5th | 50th | 95th | ||||
| Monoethyl phthalate (MEP) | Low | 0.6 | 100.0 | 23.1 | 121.5 | 1098.0 | 27.9 | 117.8 | 749.0 |
| Mono-iso-butyl phthalate (MiBP) | Low | 0.2 | 100.0 | 10.8 | 47.0 | 220.8 | 13.2 | 38.4 | 151.6 |
| Mono-n-butyl phthalate (MnBP) | Low | 0.2 | 100.0 | 11.5 | 60.0 | 625.5 | 14.2 | 44.7 | 689.2 |
| Monobenzyl phthalate (MBzP) | High | 0.3 | 100.0 | 3.4 | 19.6 | 102.9 | 4.9 | 17.7 | 89.8 |
| Mono(3-carboxypropyl) phthalate (MCPP) | High | 0.2 | 100.0 | 0.6 | 2.6 | 13.1 | 0.7 | 2.2 | 11.2 |
| Mono(2-ethylhexyl) phthalate (MEHP) | High | 0.5 | 98.5 | 0.9 | 8.6 | 42.6 | 1.3 | 7.2 | 30.1 |
| Mono(2-ethyl-5-hydroxyhexyl) phthalate (MEHHP) | High | 0.2 | 100.0 | 6.0 | 31.2 | 118.8 | 7.0 | 25.5 | 98.3 |
| Mono(2-ethyl-5-oxohexyl) phthalate (MEOHP) | High | 0.2 | 100.0 | 5.0 | 24.6 | 83.0 | 5.7 | 20.2 | 76.8 |
| Mono(2-ethyl-5-carboxypentyl) phthalate (MECPP) | High | 0.2 | 100.0 | 11.7 | 43.1 | 176.9 | 12.2 | 38.1 | 142.6 |
| Molar sum of DEHP metabolites (ΣDEHP)b | High | NA | NA | 0.1 | 0.4 | 1.4 | 0.1 | 0.3 | 1.1 |
| Monocarboxy-iso-octyl phthalate (MCOP) | High | 0.2 | 99.5 | 1.0 | 3.6 | 19.4 | 1.1 | 3.8 | 18.8 |
| Monocarboxy-iso-nonyl phthalate (MCNP) | High | 0.2 | 99.5 | 0.5 | 1.7 | 16.8 | 0.6 | 1.4 | 10.5 |
Phthalate metabolite concentrations are displayed in μg/L for all compounds except for ΣDEHP for which the concentrations are presented as μmol/L. Abbreviations: LOD = limit of detection. NA = not applicable.
Measured concentrations were standardized on sampling conditions (hour of sampling, day of sampling, year of sample analysis at the CDC, gestational age at collection, duration of storage at room temperature before freezing) and creatinine concentration using a method based on regression residuals (Mortamais et al. 2012; Philippat et al. 2014).
ΣDEHP was calculated by summing molar concentrations of MEHP, MEHHP, MEOHP, and MECPP.
3.2. Associations of phthalate biomarker urinary concentrations with global DNA methylation
Average methylation level was 16.2% (±1.1%) for Alu and 26.4% (±1.9%) for LINE-1. MBzP urinary concentrations were negatively associated with global methylation of the Alu element [β = 0.099, 95% confidence interval (95 %CI): 0.195; 0.002 for each doubling of MBzP concentration, Fig. 2]. None of the phthalate biomarker concentrations was significantly associated with the global methylation levels of the LINE-1 repetitive element nor with the overall profile or distribution of DNA methylation in the GAMP (Supplementary Table 1).
Fig. 2.
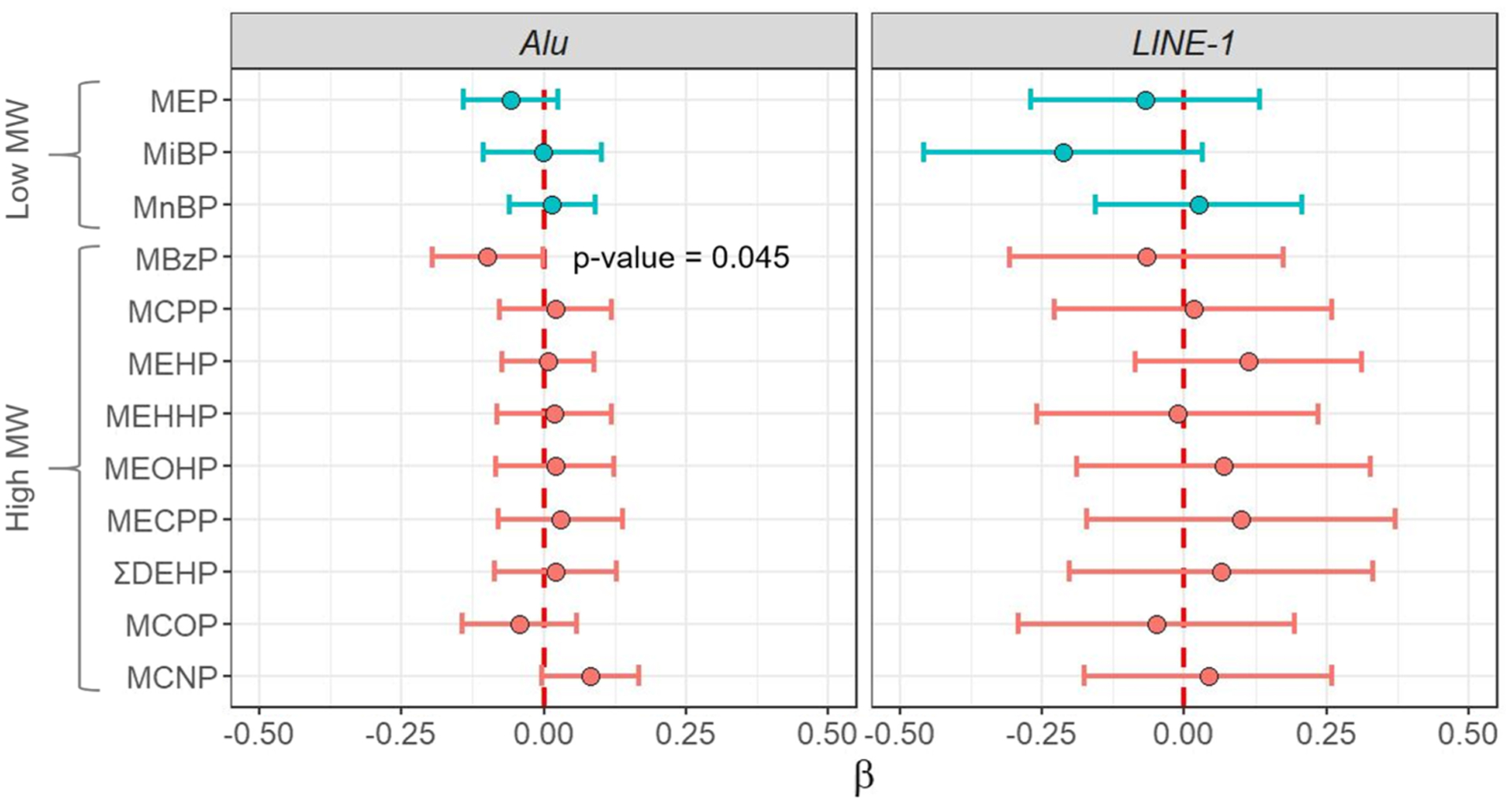
Adjusted associations between urinary concentrations of phthalates biomarkers and methylation levels of the repetitive elements Alu and LINE-1 during pregnancy (n = 201). Circles represent β regression coefficient estimates reported with 95% CIs and correspond to a change in the global DNA methylation level for doubling of the urinary biomarker concentration. Colors represent biomarkers of low (blue) or high (red) molecular weight phthalates. Regression models were adjusted for recruitment center, maternal active smoking in the three months preceding pregnancy and during pregnancy, maternal age, parity, maternal education level, maternal pre-pregnancy BMI, season of conception, batch and plate. Abbreviations: BMI = body mass index. CI = confidence interval. MBzP = monobenzyl phthalate. MCNP = monocarboxy-iso-nonyl phthalate. MCOP = monocarboxy-iso-octyl phthalate. MCPP = mono(3-carboxypropyl) phthalate. MECPP = mono(2-ethyl-5-carboxypentyl) phthalate. MEHHP = mono(2-ethyl-5-hydroxyhexyl) phthalate. MEHP = mono(2-ethylhexyl) phthalate. MEOHP = mono(2-ethyl-5-oxohexyl) phthalate. MEP = monoethyl phthalate. MiBP = mono-iso-butyl phthalate. MnBP = mono-n-butyl phthalate. MW = molecular weight. ΣDEHP = molar sum of di(2-ethylhexyl) phthalate metabolites (MEHP, MEHHP, MEOHP, MECPP).
3.3. Associations between phthalate biomarker concentrations and individual CpG methylation levels
The p-value distributions of the CpGs included in the EWAS were close to the theoretical distribution as indicated by the BIF values (ranging from 0.98 to 1.05 depending on the phthalate), and were notably smaller compared to the genomic inflation factor values (0.90–1.33, Supplementary Fig. 4). We identified only one CpG (cg16039342) that was positively methylated (β = 0.02, 95 %CI: 0.01; 0.02) in association with an increase of MEHP concentration (FDR-corrected p-value < 0.05). The identified CpG mapped to the olfactomedin 2 (OLFM2) gene located on chromosome 19.
3.4. Regional DNA methylation analysis
The regional analysis identified 25 DMRs associated with phthalate biomarker concentrations during pregnancy (Šidák-corrected p-value < 0.05, Table 3). These 25 DMRs contained 131 CpGs and encompassed 22 protein coding genes, one RNA gene, and four intergenic regions (Fig. 3, Table 3, Supplementary Tables 2 and 3). For 22 (88%) detected DMRs, DNA methylation levels increased with increased phthalate biomarker concentrations.
Table 3.
DMRs associated with pregnancy concentrations of phthalate metabolites (25 DMRs, Šidák-corrected p-value < 0.05, n = 202, 379,904 CpGs).
| Phthalate metabolite | Molecular weight | Genea | DMR (chromosome:start-end) | No. of CpGs | SLK p-value | Šidák p-value | Direction of association |
|---|---|---|---|---|---|---|---|
| Monoethyl phthalate (MEP) | Low | FDFT1 | chr8:11659961–11660110 | 6 | 4.02E–09 | 1.03E–05 | + |
| Monoethyl phthalate (MEP) | Low | GCA | chr2:163200476–163200638 | 6 | 4.27E–10 | 1.00E–06 | + |
| Mono-iso-butyl phthalate (MiBP) | Low | REPIN1 | chr7:150065170–150065256 | 6 | 1.70E–12 | 7.51E–09 | + |
| Mono-n-butyl phthalate (MnBP) | Low | FOXS1 | chr20:30433405–30433513 | 2 | 4.37E–07 | 1.53E–03 | + |
| Mono-n-butyl phthalate (MnBP) | Low | HS3ST3B1/MGC12916 | chr17:14206871–14207037 | 6 | 2.91E–08 | 6.67E–05 | + |
| Mono-n-butyl phthalate (MnBP) | Low | SLC17A9 | chr20:61583979–61584073 | 6 | 1.85E–09 | 3.53E–06 | + |
| Mono-n-butyl phthalate (MnBP) | Low | SPIB | chr19:50931515–50931623 | 3 | 1.17E–08 | 4.12E–05 | + |
| Mono-n-butyl phthalate (MnBP) | Low | chr12:115135238–115135369 | 5 | 3.95E–12 | 7.50E–09 | + | |
| Mono-n-butyl phthalate (MnBP) | Low | chr3:185000648–185000761 | 3 | 3.75E–08 | 1.26E–04 | + | |
| Mono(2-ethylhexyl) phthalate (MEHP) | High | EPS8L1 | chr19:55598874–55599030 | 2 | 2.30E–08 | 5.59E–05 | + |
| Mono(2-ethylhexyl) phthalate (MEHP) | High | MYH3 | chr17:10541530–10541621 | 2 | 6.81E–07 | 2.84E–03 | + |
| Mono(2-ethylhexyl) phthalate (MEHP) | High | OLFM2 | chr19:9965044–9965172 | 2 | 6.95E–09 | 2.06E–05 | + |
| Mono(2-ethylhexyl) phthalate (MEHP) | High | ZSCAN16 | chr6:28092239–28092421 | 7 | 3.35E–09 | 6.99E–06 | − |
| Mono(2-ethyl-5-carboxypentyl) phthalate (MECPP) | High | FGF12 | chr3:192445514–192445539 | 3 | 1.07E–06 | 1.62E–02 | + |
| Mono(2-ethyl-5-oxohexyl) phthalate (MEOHP) | High | TCF21 | chr6:134210138–134210308 | 7 | 4.75E–09 | 1.06E–05 | + |
| Mono(2-ethyl-5-oxohexyl) phthalate (MEOHP) | High | TIAM2 | chr6:155537901–155538056 | 5 | 3.50E–08 | 8.58E–05 | + |
| Mono(2-ethyl-5-oxohexyl) phthalate (MEOHP) | High | chr6:32828996–32829145 | 4 | 1.70E–08 | 4.34E–05 | + | |
| Monobenzyl phthalate (MBzP) | High | NAALAD2 | chr11:89867653–89867820 | 5 | 1.74E–07 | 3.96E–04 | − |
| Monobenzyl phthalate (MBzP) | High | RAPGEFL1 | chr17:38347603–38347817 | 4 | 2.97E–10 | 5.26E–07 | + |
| Monobenzyl phthalate (MBzP) | High | SOX21 | chr13:95364510–95364676 | 6 | 1.17E–08 | 2.68E–05 | − |
| Mono(3-carboxypropyl) phthalate (MCPP) | High | ART5 | chr11:3663491–3663843 | 8 | 1.47E–12 | 1.80E–09 | + |
| Mono(3-carboxypropyl) phthalate (MCPP) | High | chr12:115135333–115135369 | 5 | 5.33E–11 | 1.01E–07 | + | |
| Monocarboxy-iso-nonyl phthalate (MCNP) | High | CA5B | chrX:15756372–15756408 | 4 | 4.65E–08 | 4.90E–04 | + |
| Monocarboxy-iso-nonyl phthalate (MCNP) | High | HSPA1A/HSPA1L | chr6:31782873–31783546 | 22 | 9.83E–18 | 5.55E–15 | + |
| Monocarboxy-iso-nonyl phthalate (MCNP) | High | PARP10 | chr8:145061291–145061319 | 2 | 9.85E–09 | 1.34E–04 | + |
EWAS regression models, on which the DMR analysis was based, were adjusted for recruitment center, maternal active smoking in the three months preceding pregnancy and during pregnancy, maternal age, parity, maternal education level, maternal pre-pregnancy BMI, season of conception, batch, plate, chip, and estimated placental cell-type proportions.
Abbreviations: BMI = body mass index. DMR = differentially methylated region. SLK = Stouffer-Liptak-Kechris correction.
University of California, Santa Cruz Genome Browser (https://genome.ucsc.edu).
Fig. 3.
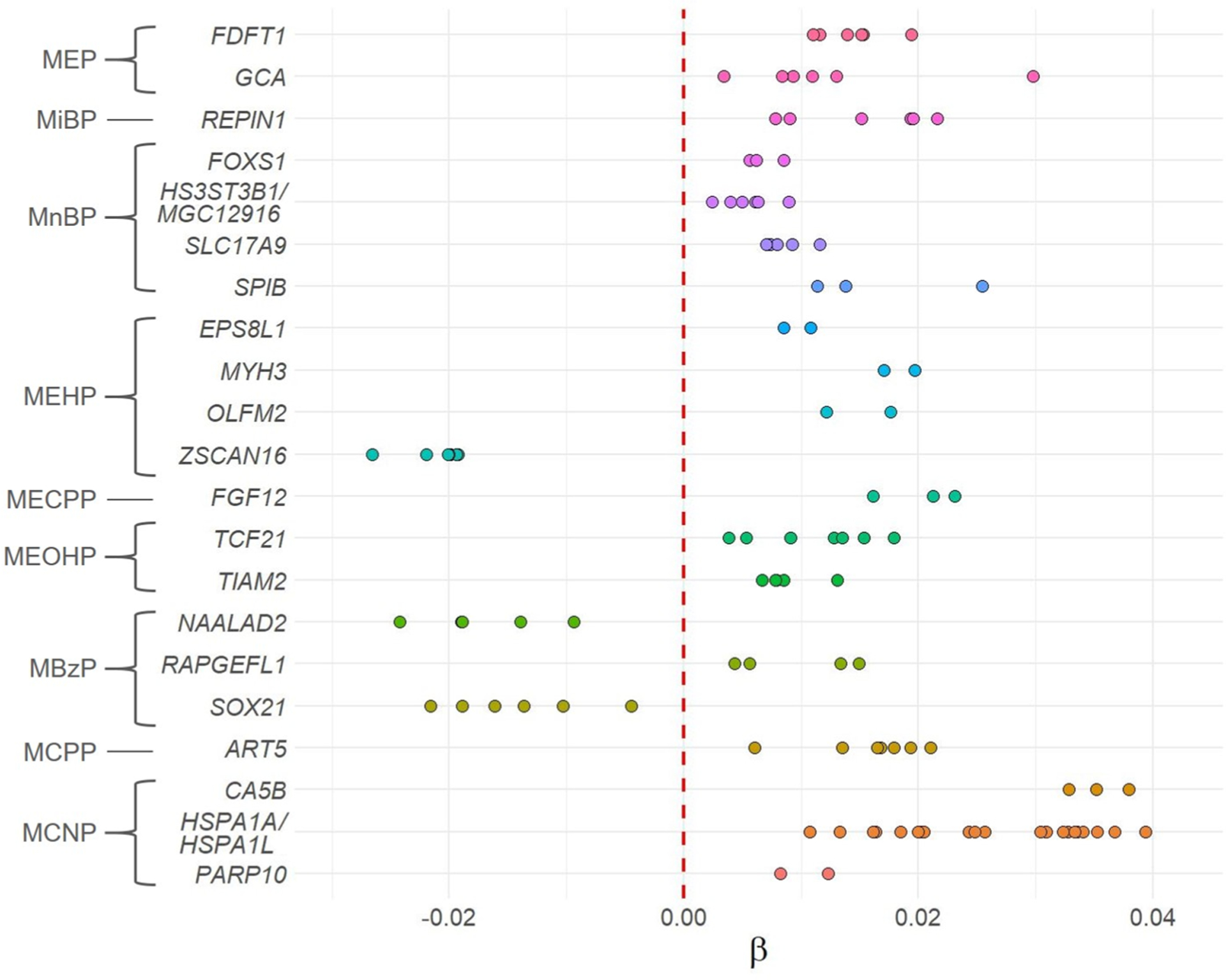
β regression coefficient estimates according to genes encompassed by DMRs identified as associated with phthalate biomarkers (Šidák-corrected p-value < 0.05, n = 202, 379,904 CpGs). Colors represent genes. Circles represent CpGs mapping to genes within identified DMRs. β coefficient estimates correspond to a change in the DNA methylation level for doubling of the urinary exposure concentration. EWAS regression models, on which the DMR analysis was based, were adjusted for recruitment center, maternal active smoking in the three months preceding pregnancy and during pregnancy, maternal age, parity, maternal education level, maternal pre-pregnancy BMI, season of conception, batch, plate, chip, and estimated placental cell-type proportions. Abbreviations: BMI = body mass index. DMR = differentially methylated region. MBzP = monobenzyl phthalate. MCNP = monocarboxy-iso-nonyl phthalate. MCPP = mono (3-carboxypropyl) phthalate. MECPP = mono(2-ethyl-5-carboxypentyl) phthalate. MEOHP = mono(2-ethyl-5-oxohexyl) phthalate. MEP = monoethyl phthalate. MiBP = mono-iso-butyl phthalate. MnBP = mono-n-butyl phthalate. MEHP = mono(2-ethylhexyl) phthalate.
Seven phthalate biomarkers were positively associated with placental DNA methylation while two (MBzP and MEHP) showed both positive and negative associations with DNA methylation. MEP was positively associated with two DMRs mapping to two genes: grancalcin (GCA) and farnesyl-diphosphate farnesyltransferase 1 (FDFT1). MiBP concentrations were associated with increased methylation of the replication initiator 1 (REPIN1). MnBP concentrations were positively associated with six DMRs that encompassed four protein coding genes including forkhead box S1 (FOXS1), heparan sulfate-glucosamine 3-sulfotransferase 3B1 (HS3ST3B1), spi-B transcription factor (SPIB), solute carrier family 17 member 9 (SLC17A9), an RNA gene (MGC12916) coding uncharacterized protein MGC12916, and two intergenic regions.
DEHP metabolites were associated with eight DMRs. MEHP metabolite was positively associated with epidermal growth factor receptor pathway substrate 8 like 1 (EPS8L1), myosin heavy chain 3 (MYH3), and OLFM2. It was negatively associated with zinc finger and SCAN domain containing 16 (ZSCAN16) gene methylation. MEOHP concentrations were linked with increased DNA methylation of two genes: transcription factor 21 (TCF21), TIAM Rac1 associated GEF 2 (TIAM2), and one intergenic region, while MECPP concentrations were positively associated with only one DMR that encompassed the fibroblast growth factor 12 (FGF12).
Pregnancy concentrations of MCNP were positively associated with the largest identified DMR (22 probes) and encompassed the heat shock protein (HSP) family A member 1A (HSPA1A) and HSPA1 like (HSPA1L) genes. This phthalate biomarker was also positively associated with carbonic anhydrase 5B (CA5B) and poly(ADP-ribose) polymerase family member 10 (PARP10). MCPP concentrations were positively associated with the methylation of ADP-ribosyltransferase 5 (ART5) gene and of one intergenic region. Finally, MBzP was positively associated with methylation of rap guanine nucleotide exchange factor like 1 (RAPGEFL1) and negatively associated with N-acetylated alpha-linked acidic dipeptidase 2 (NAALAD2) and SRY-box transcription factor 21 (SOX21).
4. Discussion
Herein we explored the epigenome-wide associations between concentrations of phthalate exposure biomarkers during pregnancy and placental DNA methylation. As for global DNA methylation, increased maternal concentrations of MBzP were associated with decreased methylation of the repetitive Alu element. With regard to other analyses, in the following discussion we will focus on the DMR results as biological functions are associated rather with genomic regions than with single CpGs (Svendsen et al. 2016). All studied phthalate biomarkers except for MCOP and MEHHP were associated with at least one DMR. For most of the identified regions (n = 22, 88%), DNA methylation levels were increased. Identified DMRs encompassed 23 genes encoding heat shock proteins, transcription factors, and nucleotide exchange factors, among others.
4.1. Low molecular weight (LMW) phthalates
LMW phthalates are frequently found in cosmetics and personal care products such as shampoos, perfumes, aftershaves, or lotions (Dodson et al. 2012). Pregnancy concentrations of LMW phthalate metabolites, including MEP, MnBP, and MiBP [either individually or the molar sum of the three metabolites (i.e., ∑ LMW)], have been previously associated with differential DNA methylation in placenta in a study considering two candidate imprinted genes: H19 and IGF2 (n = 179, LaRocca et al. 2014). The authors reported DNA methylation loss within the IFG2 DMR0 associated with increased pregnancy concentrations of MEP and within the H19 and IFG2 DMR0 associated with increased ∑LMW phthalate biomarker concentrations. There were only eight common probes between LaRocca et al. and our study (one for IFG2 DMR0 and seven for IFG2 DMR2) and we did not identify any of them to be associated with MEP (p-values not corrected for FDR ≥ 0.17). However, in our study 91 additional CpGs mapped to the IGF2 gene and for four of them increased MEP concentrations were associated with DNA methylation change (p-value not corrected for FDR < 0.05, data not shown); however, in contrast to LaRocca et al., these associations were positive. Result discrepancies between the two studies may come from different methodologies used for DNA methylation assessment (pyrosequencing vs. BeadChip technology), distinct populations, timing of urine collection (< 16 gestational weeks in LaRocca et al. compared to 22–29 weeks in our study), and different phthalate biomarker levels (geometric mean of MEP concentration equaled 76.2 μg/L in LaRocca et al. compared to 124.1 μg/L in the present study). The set of adjustment factors also differed between the two studies (LaRocca et al. adjusted only for child sex, maternal smoking, and maternal age).
As for tissues other than placenta, a study performed in the CHAMACOS cohort reported negative associations between MiBP and MnBP assessed in early pregnancy (13 weeks) and methylation of cord blood LINE-1, and between MEP and ∑LMW assessed in late pregnancy (26 weeks) and Alu methylation (n = 239, Huen et al. 2016). In our study we did not observe any association between LMW phthalates and methylation of the repetitive elements and the discrepancy may be related to different biological matrices used (cord blood vs. placenta) or different exposure assessment windows (13 or 26 gestational weeks in Huen et al. compared to 22–29 weeks in our study).
Although not affecting global DNA methylation, we found MEP concentrations being positively associated with two DMRs encompassing FDFT1 and GCA genes. FDFT1 encodes an enzyme important in cholesterol biosynthesis (O’Leary et al., 2016) and GCA encodes a calcium-binding protein abundant in neutrophils and macrophages and plays a role in the innate immune response (Stelzer et al. 2016). Both FDFT1 and GCA genes have been previously associated with maternal smoking. Placental DNA methylation of FDFT1 has been shown to be associated with pregnancy tobacco use (direction and magnitude of the association depended on the smoking status) in a previous study on the EDEN cohort (n = 668, Rousseaux et al. 2020). Moreover, FDFT1 has been found to be up-regulated in lung tissue obtained from smokers with lung adenocarcinoma (Pintarelli et al. 2019). As for GCA, one study with a relatively small sample size showed an increase of its placental methylation associated with maternal smoking during pregnancy (n = 36, Suter et al. 2011), however an aforementioned study with a bigger sample size carried on the EDEN cohort did not replicate this result (n = 668, Rousseaux et al. 2020). Since cigarette smoke may contain diethyl phthalate (DEP) (Moldoveanu and St. Charles, 2007), the parent compound of MEP, this could partially explain the observed association between this phthalate metabolite and differential methylation of GCA and FDFT1 previously linked with maternal smoking. Our results could also suggest that these genomic locations are particularly sensitive to environmental exposure.
In our population, another LMW phthalate, MnBP, was positively associated with six DMRs. Maternal MnBP urinary concentrations have been previously associated with higher expression of inflammation-related genes in placenta in a study relying on a candidate gene approach (n = 2469, Wang et al. 2020b). In our study, we have not detected differential methylation of any of inflammation-related genes, which may be explained by the fact that gene expression does not necessarily correlate with DNA methylation levels. Instead, we identified an increase of DNA methylation of four protein coding genes (FOXS1, HS3ST3B1, SLC17A9, SPIB), one long non-coding RNA gene (MGC12916), and two intergenic regions. FOXS1 and SPIB encode transcription factors, HS3ST3B1 protein plays a role in nucleotide binding, and SLC17A9 is a transmembrane protein involved in the transport of small molecules.
Lastly, we found maternal MiBP concentrations being associated with increased placental DNA methylation of REPIN1 gene encoding protein facilitating DNA binding. To the best of our knowledge, none of the genes identified in our study as associated with pregnancy MnBP or MiBP concentrations has been previously described in the context of epigenetic modifications or functioning of the placenta.
4.2. High molecular weight (HMW) phthalates
HMW phthalates are used as plasticizers in products such as food packaging, plastic bags, vinyl plastics used in flooring, toys, and intravenous tubing (Hauser and Calafat 2005).
4.2.1. DEHP metabolites
DEHP and its metabolite MEHP may alter placental homeostasis by disrupting trophoblast differentiation, invasion, oxidative stress response, immuno-modulation, and endocrine function (reviewed by Martínez-Razo et al. 2021). Regarding potential effect of DEHP metabolites on the placental DNA methylation, LaRocca et al. showed MEOHP and ΣDEHP to be negatively associated with IGF2 DMR0 methylation (LaRocca et al. 2014). After stratification for sex, they also reported negative associations between MECPP, MEHHP, and MEHP and IGF2 DMR0 methylation in females. In our study restricted to males, after analysis of all CpGs mapping to the IGF2 gene (99 CpGs), we found a few positive associations with MECPP (3 CpGs), MEHHP (2), MEHP (3), MEOHP (2), and ΣDEHP (3) and one negative association with MEHP (p-values not corrected for FDR < 0.05, data not shown). However, none of these CpGs was located within the IGF2 DMR0.
Two other studies investigated associations between DEHP metabolites and placental genes methylation. One study using placentas from fetal growth restricted and normal growth newborns focused on candidate imprinted genes IGF2 and AHRR (n = 181, Zhao et al. 2016). The authors found maternal urinary concentrations of MEHHP, MEOHP, and the molar sum of MEHHP, MEHP, and MEOHP to be negatively associated with IGF2 methylation. The second study on fetal growth restricted infants reported decrease in methylation of placental LINE-1 element in association with urinary phthalate DEHP metabolites concentrations (MEHHP and molar sum of MEHHP, MEHP, and MEOHP) (n = 65, Zhao et al. 2015b). In our study, we have not identified any of these genes to be associated with DEHP metabolites, which may be explained by the fact that associations observed by Zhao et al. were present only in the growth restricted and not in normal growth newborns.
As for tissues other than placenta, a few studies investigated associations between maternal urinary concentrations of DEHP metabolites and DNA methylation in cord blood. A study carried on the CHAMACOS cohort and focusing on ten candidate imprinted genes reported positive associations between MECPP, MEHHP, MEOHP, and ΣDEHP and DNA methylation averaged across seven CpGs of maternally expressed 3 (MEG3) gene (n = 296, Tindula et al. 2018). No effect was reported for the MEG3 expression. Inversely, a study focusing on candidate genes playing a role in metabolism, growth, or development showed ΣDEHP to be associated with decreased cord blood methylation of peroxisome proliferator-activated receptor alpha (PPARA), a gene encoding nuclear receptor that regulates fatty acid metabolism. In our study we did not observe differential methylation of either MEG3 or PPARA, which may be explained by different biological matrices (cord blood vs. placenta), distinct methodologies of DNA methylation assessment (pyrosequencing vs. BeadChip) or distinct populations and adjustment factors or timing of exposure assessment (8–14 gestational weeks in Montrose et al. and averaged concentrations from 13th and 26th weeks in Tindula et al. compared to 22–29 weeks in our study). Lastly, an epigenome-wide study on the CHAMACOS cohort identified 27 cord blood DMRs predominantly positively associated with phthalate biomarkers (n = 336, Solomon et al. 2017). Identified DMRs were primarily associated with individual and summary measurements of DEHP metabolites assessed in the 26th week of gestation. They encompassed several genes related to hormonal balance, male fertility, metabolic health and cancer, however none of them was common with our study. Instead, we found all DEHP biomarkers except for MEHHP and ΣDEHP to be associated with at least one DMR. MEHP was positively associated with three genes: EPS8L1, MYH3, and OLFM2. OLFM2 is involved in smooth muscle differentiation and was the only gene also detected in the EWAS. MYH3 encodes one of the myosin heavy chains playing a role in motor activity while the exact function of the protein encoded by the EPS8L1 gene is unknown. In contrast, MEHP was associated with decreased DNA methylation of the ZSCAN16 gene coding DNA-binding transcription factor. ZSCAN16 methylation has been shown to be increased in association with high total phthalate exposure (sum of concentrations of 23 metabolites including MEHP) in first trimester placentas of women undergoing elective terminations (among 244 other genes none of which was identified in our study) (Grindler et al. 2018). However, the latter result should be interpreted with caution since Grindler et al. relied on 16 placentas only while analyzing as many as 834,015 CpG sites; they also did not formally correct for multiple comparisons and used a relatively low threshold to detect significant DMRs (p-value < 0.005). Moreover, the authors did not adjust their analyses for potential confounders. ZSCAN16 differential DNA methylation has been also associated with maternal smoking in a study conducted in the EDEN cohort (n = 668, Rousseaux et al. 2020). However, in contrast to our results, this association was positive. To the best of our knowledge, the presence of DEHP or any of its metabolites in tobacco smoke has not been reported. Nevertheless, it has been demonstrated that DEHP exposure may induce production of reactive oxygen species (ROS) in both in vitro models as well as in pregnant women with high levels of DEHP metabolites detected in urine (reviewed by Martínez-Razo et al. 2021). Moreover, high pregnancy urinary concentrations of DEHP metabolites have been linked with placental overexpression of metallothioneins, proteins that show cellular antioxidative properties (Li et al. 2016). Because tobacco smoke is also a well-known factor related to ROS production and was recently linked to oxidative damages in the placenta (reviewed by Suter and Aagaard 2020), the common effect of DEHP metabolites and smoking on the ZSCAN16 methylation may be explained by the production of ROS linked to these two factors. Our results may also suggests particular sensitivity of this locus to environmental exposures.
In the present study, concentrations of another DEHP metabolite, MEOHP, were associated with increased DNA methylation of two genes (TCF21 and TIAM2) and one intergenic region. The protein encoded by TIAM2 gene is a nucleotide exchange factor suspected to play a role in neural cell development (O’Leary et al., 2016). The impact of epigenetic modifications of this gene in placenta or cord blood have not been studied so far. TCF21 is a transcription factor and a tumor suppressor. Increased placental methylation of this gene was associated with maternal smoking in the EDEN cohort (n = 668, Rousseaux et al. 2020). Again, the common mechanism linking maternal MEOHP concentrations and smoking status with increased placental DNA methylation of the TCF21 gene may be related to the production of ROS. Finally, MEOHP have been previously shown to be negatively associated with the expression of the inflammation-related genes (CRP, MCP-1, CD68) in female placentas in a study relying on a candidate approach (n = 2469, Wang et al. 2020b). In our study restricted to boys we did not identify such associations.
The last association we detected for DEHP metabolites was positive and involved pregnancy MECPP concentrations and DNA methylation of FGF12, a gene from the FGF family involved in a variety of biological processes including embryonic development, cell growth, morphogenesis, tissue repair, and tumor growth and invasion (O’Leary et al., 2016). The specific function of the FGF12 gene has not yet been determined nor has it been previously described in the context of epigenetic modifications or functioning of the placenta.
4.2.2. HMW phthalates other than DEHP metabolites
To date, none of the studies on placenta reported differential DNA methylation associated with maternal concentrations of HMW phthalates other than DEHP metabolites (LaRocca et al. 2014; Zhao et al. 2015b, 2016). As for cord blood, a study by Montrose et al. showed MBzP and MCPP being negatively associated with methylation of PPARA (MBzP) and IGF2, PPARA, and LINE-1 (MCPP). However, after stratification for sex, some of these associations appeared to be female-specific or were not retained (Montrose et al. 2018), what may partially explain why we did not observe such associations in our study restricted to boys. Two other studies focused on the cord blood DNA methylation of LINE-1 and Alu repetitive elements. They reported negative associations between MBzP concentrations assessed in early pregnancy and LINE-1 methylation (Huen et al. 2016) as well as between MnBP concentrations and Alu methylation in males and MBzP and Alu methylation in females (n = 106, Huang et al. 2018). The latter association was also detected in our study restricted to boys which may suggest a potential mechanism through which exposure to benzylbutyl phthalate, the parent compound of MBzP, may affect placental health.
As for other studies involving MBzP, it has been previously shown to alter placental expression of inflammation-related genes (TNF-α, MCP-1, and CD68) in males (n = 2469, Wang et al. 2020b), but none of these genes was differentially methylated in our study. Instead, we identified an increase of the DNA methylation of the RAPGEFL1 gene and a decrease of methylation of two other genes (NAALAD2 and SOX21) associated with this phthalate. RAPGEFL1 is a nucleotide exchange factor showing signal transducer activity and NAALAD2 has neuropep-tide cleaving function (Stelzer et al. 2016), but with no proven role in placental functioning. The third gene associated with MBzP was the transcription factor SOX21 that encodes a protein regulating placentation and differentiation of the trophoblast (Mrema et al. 2013; Ullah et al. 2020), among other tissues. Placental SOX21 was found to be downregulated in association with total phthalates (sum of concentrations of 23 metabolites including MBzP) in the above cited study on women undergoing elective pregnancy terminations during first trimester (n = 16, Grindler et al. 2018). Therein, decreased SOX21 expression was accompanied by an increase of DNA methylation of one CpG mapping to this gene, while in our study we observed DNA methylation loss within this gene.
Another DMR identified in our study was positively associated with MCNP concentrations and was the largest (22 CpGs) among those detected. This DMR encompasses HSPA1A and HSPA1L genes encoding heat shock proteins responsible for various physiological processes (e.g., protein refolding and degradation) and involved in response to cellular stress. Increased DNA methylation of HSPA1A/ HSPA1L genes has been reported in intrauterine growth restriction placentas, compared to their normal twin counterparts (n = 8, Roifman et al. 2016). Additionally, increased placental HSPA1A mRNA and protein levels have been reported in preeclamptic pregnancies (n = 8, Wang et al. 2020a) and in placental vascular disease (n = 62, Liu et al. 2008). Of interest, MCNP has been previously associated with the placenta weight to birth weight ratio in another study from the EDEN cohort relying on 457 mother-son pairs (Philippat et al. 2019), which may suggest an effect of this phthalate on fetal growth and development, potentially mediated by the epigenetic modifications in placenta. We observed two additional positive associations for MCNP with CA5B and PARP10 genes encoding proteins showing carbonate dehydratase activity and responsible for gene transcription regulation, respectively (O’Leary et al., 2016). To the best of our knowledge, none of these genes has been studied in the context of placental function.
Lastly, we detected a positive association between MCPP concentration and DNA methylation of one intergenic region and the ART5 gene encoding protein responsible for protein function regulation. Again, there are no studies linking epigenetic modifications of ART5 to placental outcomes.
5. Strengths and limitations
The present study is the first genome-wide analysis of differentially methylated probes and regions in placenta collected at birth in relation to pregnancy exposure to phthalates. In our analyses, we corrected for the number of CpGs tested for each chemical but we did not account for the number of tested phthalate metabolites, which might have led to identification of false positive associations. It should be noted though that for 11 studied phthalate metabolites, all except for MEP showed moderate (MCNP, MCOP, MBzP, MiBP), strong (MCPP, MnBP) or very strong (DEHP metabolites) correlation with at least one other phthalate metabolite. When this was taken into account, the effective number of independent exposures (formula adapted from Li et al. 2012) dropped from 11 to six (data not shown). Taken together with the lack of other epigenome-wide studies on the effects of phthalates on placental DNA methylation, cautious interpretation of the results is required and replication studies are needed to confirm our findings. Moreover, the fact that the observed changes in DNA methylation cannot be directly translated to gene expression (Lim et al. 2017) may impede the interpretation of how our results may link to pregnancy complications or health outcomes later in life.
In this study, we followed two statistical approaches providing complementary information. The EWAS produces CpG-specific effect estimates that can be directly compared with the estimates observed in other studies and that can be used in meta-analyses. On the other hand, DMR analysis takes into account the location of the differentially methylated CpGs in the genome and their potential interdependence. Although the EDEN mother–child cohort is well established and provides information on a broad range of potential confounders, residual confounding by factors not considered in our analysis (e.g., genetics, ancestry, or maternal behaviors such as diet or physical activity during pregnancy) cannot be excluded. Phthalate exposure was assessed only for boys which, while not being a source of bias, limits the generaliz-ability of our conclusions for female offspring. This is especially important because previous studies have reported sex-specific effects of some phthalates on methylation and expression of genes in placenta and cord blood (Huang et al. 2018; LaRocca et al. 2014; Montrose et al. 2018). Finally, phthalate biomarkers were assessed in a single spot urine sample. Given the short half-life of phthalates (Casas et al. 2018) and temporal variability in behaviors linked to exposure (e.g., food intake, use of personal care products), we cannot rule out exposure misclassification, attenuation bias, and power reduction (Perrier et al. 2016). Exposure misclassification depends on the temporal variability of urinary concentrations which, as shown in Supplementary Table 4, varies across phthalate metabolites. The highest attenuation bias is expected for the metabolites with the highest temporal variability (i.e., with the lowest intraclass correlation coefficient) which, for our study, include the metabolites with the highest molecular weight (MCNP, MCOP, MCPP, MECPP, MEHHP, MEHP, and MEOHP) (Supplementary Table 4).
6. Conclusions
Herein we explored the epigenome-wide effects of pregnancy exposure to selected phthalates on DNA methylation in placentas collected at birth. We found that MBzP concentrations may be associated with decreased methylation of repetitive Alu elements. Moreover, most of the studied phthalates were associated with increased DNA methylation of several DMRs. For two metabolites (MBzP and MEHP), decreased methylation of three DMRs was also observed. Identified regions encompassed 23 genes encoding heat shock proteins, transcription factors, and nucleotide exchange factors, among others. Of interest, four genes have been previously identified as associated with maternal smoking, suggesting that these genomic regions might be particularly sensitive to the effect of environmental contaminants. Presented results suggest epigenetic mechanisms by which pregnancy exposure to phthalates could affect fetal development; however, additional studies are needed to confirm our results.
Supplementary Material
Acknowledgements
We are indebted to the midwife research assistants for data collection and to P. Lavoine and J. Sahuquillo for checking, coding and data entry and to L. Giorgis-Allemand for data management.
The authors thank the EDEN mother-child cohort study group, whose members are I. Annesi-Maesano, J.Y. Bernard, M.A. Charles, P. Dargent-Molina, B. de Lauzon-Guillain, P. Ducimetière, M. de Agostini, B. Foliguet, A. Forhan, X. Fritel, A. Germa, V. Goua, R. Hankard, B. Heude, M. Kaminski, B. Larroquey, N. Lelong, J. Lepeule, G. Magnin, L. Marchand, C. Nabet, F Pierre, R. Slama, M.J. Saurel-Cubizolles, M. Schweitzer, and O. Thiebaugeorges.
We also would like to acknowledge M. Silva, E. Samandar, J. Preau, and T. Jia (Centers for Disease Control and Prevention, Atlanta, USA) for their work on quantification of phthalate biomarkers. We are grateful to all the participating families, practitioners and researchers who took part in this study.
We wish to thank S. Rousseaux, director of the EpiMed facility at the Institute for Advanced Biosciences, University Grenoble Alpes, for her constructive suggestions and comments about this manuscript.
Funding/ Support
Paulina Jedynak was supported by the French National Agency for Research (ANR, grant n◦ ANR-15-IDEX-02) and Milan Jakobi was supported by the ANR grant n◦ ANR-18-CE36-0005. DNA methylation measurements were obtained through funding from the Fondation de France (n° 2012-00031593 and 2012-00031617) and the ANR (n◦ ANR-13-CESA-0011). Phthalates measurements were obtained thanks to a grant funded by ANSES (EST-2010/2/126). The EDEN cohort has been funded by the Foundation for Medical Research (FRM), ANR, National Institute for Research in Public Health (IRESP: TGIR cohorte santé 2008 program), French Ministry of Health (DGS), French Ministry of Research, Inserm Bone and Joint Diseases, National Research (PRO-A) and Human Nutrition National Research Programs, Paris-Sud University, Fondation Nestlé, French National Institute for Population Health Surveillance (InVS), French National Institute for Health Education (INPES), the European Union Seventh Framework Program (FP7/2007-2013, HELIX, ESCAPE, ENRIECO, Medall projects), Diabetes National Research Program [through a collaboration with the French Association of Diabetic Patients (AFD)], French Agency for Environmental Health Safety (now ANSES), Mutuelle Générale de l’Education Nationale (MGEN), French National Agency for Food Security and the French-speaking Association for the Study of Diabetes and Metabolism (ALFEDIAM). Some computations were performed using the GRICAD infrastructure (https://gricad.univ-grenoble-alpes.fr), which is partly supported by the Equip@Meso project (grant n° ANR-10-EQPX-29-01) of the Programme Investissements d’Avenir supervised by the ANR.
Role of the Funder/ Sponsor
The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review or approval of the manuscript; decision to submit the manuscript for publication.
Abbreviations:
- BIF
Bayesian inflation factor
- BMI
body mass index
- BMIQ
Beta MIxture Quantile
- CDC
Centers for Disease Control and Prevention
- CDF
cumulative distribution function
- ChAMP
Chip Analysis Methylation Pipeline
- CI
confidence interval
- DEHP
di(2-ethylhexyl) phthalate
- ΣDEHP
molar sum of DEHP metabolites (MEHP, MEHHP, MEOHP, MECPP)
- DEP
diethyl phthalate
- DMR
differentially methylated region
- DOHaD
developmental origins of health and disease
- EDEN
Etude des Déterminants pré et postnatals du développement et de la santé de l’Enfant
- FDR
false discovery rate
- GAMP
global analysis of methylation profiles
- HMW
high molecular weight
- HSP
heat shock protein
- IQR
interquartile ranges
- LINE-1
long interspersed nucleotide element 1
- LMP
last menstrual period
- LMW
low molecular weight
- LOD
limit of detection
- MBzP
monobenzyl phthalate
- MCNP
monocarboxy-iso-nonyl phthalate
- MCOP
monocarboxy-iso-octyl phthalate
- MCPP
mono(3-carboxypropyl) phthalate
- MECPP
mono(2-ethyl-5-carboxypentyl) phthalate
- MEHHP
mono(2-ethyl-5-hydroxyhexyl) phthalate
- MEHP
mono(2-ethylhexyl) phthalate
- MEOHP
mono(2-ethyl-5-oxohexyl) phthalate
- MEP
monoethyl phthalate
- MiBP
mono-iso-butyl phthalate
- MnBP
mono-n-butyl phthalate
- MW
molecular weight
- nRBC
nucleated red blood cells
- ROS
reactive oxygen species
- SLK
Stouffer-Liptak-Kechris correction
- UCSC
University of California, Santa Cruz
Footnotes
CRediT authorship contribution statement
Paulina Jedynak: Formal analysis, Investigation, Writing – original draft, Writing – review & editing. Jörg Tost: Data curation, Formal analysis, Resources. Antonia M. Calafat: Formal analysis, Resources. Ekaterina Bourova-Flin: Methodology. Lucile Broséus: Methodology. Florence Busato: Data curation, Formal analysis, Resources. Anne Forhan: Data curation. Barbara Heude: Conceptualization, Data curation, Resources. Milan Jakobi: Data curation, Software. Joel Schwartz: Formal analysis, Methodology. Rémy Slama: Funding acquisition. Daniel Vaiman: Formal analysis, Resources, Methodology. Johanna Lepeule: Conceptualization, Data curation, Formal analysis, Resources, Funding acquisition, Investigation, Methodology, Software, Supervision, Writing – review & editing Claire Philippat: Conceptualization, Formal analysis, Funding acquisition, Investigation, Supervision, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: Disclaimer
Publisher's Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention (CDC). Use of trade names is for identification only and does not imply endorsement by the CDC, the Public Health Service, or the U.S. Department of Health and Human Services.
Appendix A. Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.envint.2021.107054.
References
- Abraham E, Rousseaux S, Agier L, Giorgis-Allemand L, Tost J, Galineau J, Hulin A, Siroux V, Vaiman D, Charles M-A, Heude B, Forhan A, Schwartz J, Chuffart F, Bourova-Flin E, Khochbin S, Slama R, Lepeule J, 2018. Pregnancy exposure to atmospheric pollution and meteorological conditions and placental DNA methylation. Environ. Int 118, 334–347. 10.1016/j.envint.2018.05.007. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y, 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Series B Stat. Methodol 57 (1), 289–300. 10.1111/j.2517-6161.1995.tb02031.x. [DOI] [Google Scholar]
- Botton J, Philippat C, Calafat AM, Carles S, Charles M-A, Slama R, the EDEN mother-child cohort study group, 2016. Phthalate pregnancy exposure and male offspring growth from the intra-uterine period to five years of age. Environ. Res 151, 601–609. 10.1016/j.envres.2016.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casas M, Basagaña X, Sakhi AK, Haug LS, Philippat C, Granum B, Manzano-Salgado CB, Brochot C, Zeman F, de Bont J, Andrusaityte S, Chatzi L, Donaire-Gonzalez D, Giorgis-Allemand L, Gonzalez JR, Gracia-Lavedan E, Grazuleviciene R, Kampouri M, Lyon-Caen S, Pañella P, Petraviciene I, Robinson O, Urquiza J, Vafeiadi M, Vernet C, Waiblinger D, Wright J, Thomsen C, Slama R, Vrijheid M, 2018. Variability of urinary concentrations of non-persistent chemicals in pregnant women and school-aged children. Environ. Int 121, 561–573. 10.1016/j.envint.2018.09.046. [DOI] [PubMed] [Google Scholar]
- Chevrier C, Petit C, Philippat C, Mortamais M, Slama R, Rouget F, Calafat AM, Ye X, Silva MJ, Charles M-A, Cordier S, 2012. Maternal urinary phthalates and phenols and male genital anomalies. Epidemiology 23 (2), 353–356. 10.1097/EDE.0b013e318246073e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodson RE, Nishioka M, Standley LJ, Perovich LJ, Brody JG, Rudel RA, 2012. Endocrine disruptors and asthma-associated chemicals in consumer products. Environ. Health Perspect 120, 935–943. 10.1289/ehp.1104052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta S, Haggerty DK, Rappolee DA, Ruden DM, 2020. Phthalate exposure and long-term epigenomic consequences: A review. Front Genet. Front. Genet 11 10.3389/fgene.2020.00405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillman MW, 2005. Developmental Origins of Health and Disease. N. Engl. J. Med 353, 1848–1850. 10.1056/NEJMe058187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grindler NM, Vanderlinden L, Karthikraj R, Kannan K, Teal S, Polotsky AJ, Powell TL, Yang IV, Jansson T, 2018. Exposure to phthalate, an endocrine disrupting chemical, alters the first trimester placental methylome and transcriptome in women. Sci. Rep 8 (1) 10.1038/s41598-018-24505-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen K, 2016. IlluminaHumanMethylation450kanno.ilmn12.hg19: Annotation for Illumina’s 450k methylation arrays.
- Hauser R, Calafat AM, 2005. Phthalates and human health. Occup. Environ. Med 62, 806–818. 10.1136/oem.2004.017590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heijmans BT, Tobi EW, Lumey LH, Slagboom PE, 2009. The epigenome: archive of the prenatal environment. Epigenetics 4, 526–531. 10.4161/epi.4.8.10265. [DOI] [PubMed] [Google Scholar]
- Heude B, Forhan A, Slama R, Douhaud L, Bedel S, Saurel-Cubizolles M-J, Hankard R, Thiebaugeorges O, De Agostini M, Annesi-Maesano I, Kaminski M, Charles M-A, the EDEN mother-child cohort study group, 2016. Cohort Profile: The EDEN mother-child cohort on the prenatal and early postnatal determinants of child health and development. Int. J. Epidemiol 45 (2), 353–363. 10.1093/ije/dyv151. [DOI] [PubMed] [Google Scholar]
- Hron K, Templ M, Filzmoser P, 2010. Imputation of missing values for compositional data using classical and robust methods. Comput. Stat. Data Anal 54 (12), 3095–3107. 10.1016/j.csda.2009.11.023. [DOI] [Google Scholar]
- Huang L-L, Zhou B, Ai S-H, Yang P, Chen Y-J, Liu C, Deng Y-L, Lu Q, Miao X-P, Lu W-Q, Wang Y-X, Zeng Q, 2018. Prenatal phthalate exposure, birth outcomes and DNA methylation of Alu and LINE-1 repetitive elements: A pilot study in China. Chemosphere 206, 759–765. 10.1016/j.chemosphere.2018.05.030. [DOI] [PubMed] [Google Scholar]
- Huen K, Calafat AM, Bradman A, Yousefi P, Eskenazi B, Holland N, 2016. Maternal phthalate exposure during pregnancy is associated with DNA methylation of LINE-1 and Alu repetitive elements in Mexican-American children. Environ. Res 148, 55–62. 10.1016/j.envres.2016.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Iterson M, van Zwet EW, Heijmans BT, 2017. Controlling bias and inflation in epigenome- and transcriptome-wide association studies using the empirical null distribution. Genome Biol. 18:19. 10.1186/s13059-016-1131-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jedynak P, Tost J, Calafat AM, Bourova-Flin E, Busato F, Forhan A, Heude B, Jakobi M, Rousseaux S, Schwartz J, Slama R, Vaiman D, Philippat C, Lepeule J, 2021. Pregnancy exposure to synthetic phenols and placental DNA methylation - An epigenome-wide association study in male infants from the EDEN cohort. Environ. Pollut 290, 118024. 10.1016/j.envpol.2021.118024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kechris KJ, Biehs B, Kornberg TB, 2010. Generalizing moving averages for tiling arrays using combined p-value statistics. Stat. Appl. Genet. Mol. Biol 9 (1) 10.2202/1544-6115.1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishi R, Grandjean P, 2020. Health impacts of developmental exposure to Environmental chemicals. Springer, Singapore. [Google Scholar]
- LaRocca J, Binder AM, McElrath TF, Michels KB, 2014. The impact of first trimester phthalate and phenol exposure on IGF2/H19 genomic imprinting and birth outcomes. Environ. Res 133, 396–406. 10.1016/j.envres.2014.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latini G, 2005. Monitoring phthalate exposure in humans. Clin. Chim. Acta 361, 20–29. 10.1016/j.cccn.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Li B, Xu X, Zhu Y, Cao J, Zhang Y, Huo X, 2016. Neonatal phthalate ester exposure induced placental MTs, FATP1 and HFABP mRNA expression in two districts of southeast China. Sci. Rep 6:21004. 10.1038/srep21004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M-X, Yeung JMY, Cherny SS, Sham PC, 2012. Evaluating the effective numbers of independent tests and significant p-value thresholds in commercial genotyping arrays and public imputation reference datasets. Hum. Genet 131, 747–756. 10.1007/s00439-011-1118-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim YC, Li J, Ni Y, Liang Q, Zhang J, Yeo GSH, Lyu J, Jin S, Ding C, 2017. A complex association between DNA methylation and gene expression in human placenta at first and third trimesters. PLoS One 12. 10.1371/journal.pone.0181155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Li N, You L, Liu X, Li H, Wang X, 2008. HSP70 is associated with endothelial activation in placental vascular diseases. Mol. Med 14, 561–566. 10.2119/2008-00009. [DOI] [PubMed] [Google Scholar]
- Lumley T, 2004. Analysis of complex survey samples. J. Stat. Softw 9 10.18637/jss.v009.i08. [DOI] [Google Scholar]
- Maccani MA, Marsit CJ, 2009. Epigenetics in the placenta. Am. J. Reprod. Immunol 62, 78–89. 10.1111/j.1600-0897.2009.00716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Razo LD, Martínez-Ibarra A, Vázquez-Martínez ER, Cerbón M, 2021. The impact of di-(2-ethylhexyl) phthalate and mono(2-ethylhexyl) phthalate in placental development, function, and pathophysiology. Environ. Int 146, 106228. 10.1016/j.envint.2020.106228. [DOI] [PubMed] [Google Scholar]
- Moldoveanu S, St. Charles F, 2007. Differences in the chemical composition of the particulate phase of inhaled and exhaled cigarette mainstream smoke. Beitr. Tab. Int./ Contrib. Tob. Res 22, 290–302. 10.2478/cttr-2013-0834. [DOI] [Google Scholar]
- Montrose L, Padmanabhan V, Goodrich JM, Domino SE, Treadwell MC, Meeker JD, Watkins DJ, Dolinoy DC, 2018. Maternal levels of endocrine disrupting chemicals in the first trimester of pregnancy are associated with infant cord blood DNA methylation. Epigenetics 13 (3), 301–309. 10.1080/15592294.2018.1448680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris TJ, Butcher LM, Feber A, Teschendorff AE, Chakravarthy AR,Wojdacz TK, Beck S, 2014. ChAMP: 450k Chip Analysis Methylation Pipeline. Bioinformatics 30 (3), 428–430. 10.1093/bioinformatics/btt684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortamais M, Chevrier C, Philippat C, Petit C, Calafat AM, Ye X, Silva MJ, Brambilla C, Eijkemans MJC, Charles M-A, Cordier S, Slama R, 2012. Correcting for the influence of sampling conditions on biomarkers of exposure to phenols and phthalates: a 2-step standardization method based on regression residuals. Environ. Health 11 (1). 10.1186/1476-069X-11-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mose T, Knudsen LE, Hedegaard M, Mortensen GK, 2007a. Transplacental transfer of monomethyl phthalate and mono(2-ethylhexyl) phthalate in a human placenta perfusion system. Int. J. Toxicol 26, 221–229. 10.1080/10915810701352721. [DOI] [PubMed] [Google Scholar]
- Mose T, Mortensen GK, Hedegaard M, Knudsen LE, 2007b. Phthalate monoesters in perfusate from a dual placenta perfusion system, the placenta tissue and umbilical cord blood. Reprod. Toxicol 23, 83–91. 10.1016/j.reprotox.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Mrema EJ, Rubino FM, Brambilla G, Moretto A, Tsatsakis AM, Colosio C, 2013. Persistent organochlorinated pesticides and mechanisms of their toxicity. Toxicology 307, 74–88. 10.1016/j.tox.2012.11.015. [DOI] [PubMed] [Google Scholar]
- Nordlund J, Bäcklin CL, Wahlberg P, Busche S, Berglund EC, Eloranta M-L, Flaegstad T, Forestier E, Frost B-M, Harila-Saari A, Heyman M, Jónsson ÓG, Larsson R, Palle J, Rönnblom L, Schmiegelow K, Sinnett D, Söderhäll S, Pastinen T, Gustafsson MG, Lönnerholm G, Syvänen A-C, 2013. Genome-wide signatures of differential DNA methylation in pediatric acute lymphoblastic leukemia. Genome Biol. 14 (9), r105. 10.1186/gb-2013-14-9-r105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Leary NA, Wright MW, Brister JR, Ciufo S, Haddad D, McVeigh R, Rajput B, Robbertse B, Smith-White B, Ako-Adjei D, Astashyn A, Badretdin A, Bao Y, Blinkova O, Brover V, Chetvernin V, Choi J, Cox E, Ermolaeva O, Farrell CM, Goldfarb T, Gupta T, Haft D, Hatcher E, Hlavina W, Joardar VS, Kodali VK, Li W, Maglott D, Masterson P, McGarvey KM, Murphy MR, O’Neill K, Pujar S, Rangwala SH, Rausch D, Riddick LD, Schoch C, Shkeda A, Storz SS, Sun H, Thibaud-Nissen F, Tolstoy I, Tully RE, Vatsan AR, Wallin C, Webb D, Wu W, Landrum MJ, Kimchi A, Tatusova T, DiCuccio M, Kitts P, Murphy TD, Pruitt KD, 2016. Reference sequence (RefSeq) database at NCBI: current status, taxonomic expansion, and functional annotation. Nucleic Acids Res. 44 (D1), D733–D745. 10.1093/nar/gkv1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen BS, Schwartz DA, Yang IV, Kechris KJ, 2012. Comb-p: software for combining, analyzing, grouping and correcting spatially correlated P-values. Bioinformatics 28, 2986–2988. 10.1093/bioinformatics/bts545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrier F, Giorgis-Allemand L, Slama R, Philippat C, 2016. Within-subject pooling of biological samples to reduce exposure misclassification in biomarker-based studies. Epidemiology 27, 378–388. 10.1097/EDE.0000000000000460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippat C, Botton J, Calafat AM, Ye X, Charles M-A, Slama R, 2014. Prenatal exposure to phenols and growth in boys. Epidemiology 25, 625–635. 10.1097/EDE.0000000000000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippat C, Heude B, Botton J, Alfaidy N, Calafat AM, Slama R, 2019. Prenatal exposure to select phthalates and phenols and associations with fetal and placental weight among male births in the EDEN cohort (France). Environ. Health Perspect 127 (1), 017002. 10.1289/EHP3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pintarelli G, Noci S, Maspero D, Pettinicchio A, Dugo M, De Cecco L, Incarbone M, Tosi D, Santambrogio L, Dragani TA, Colombo F, 2019. Cigarette smoke alters the transcriptome of non-involved lung tissue in lung adenocarcinoma patients. Sci. Rep 9 (1) 10.1038/s41598-019-49648-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team, 2020. R Foundation for Statistical Computing. R: A language and environment for statistical computing. [Google Scholar]
- Robinson WP, Peñaherrera MS, Konwar C, Yuan V, Wilson SL, 2019. Epigenetic modifications in the human placenta. In: Human Reproductive and Prenatal Genetics. Elsevier, 293–311. [Google Scholar]
- Roifman M, Choufani S, Turinsky AL, Drewlo S, Keating S, Brudno M, Kingdom J, Weksberg R, 2016. Genome-wide placental DNA methylation analysis of severely growth-discordant monochorionic twins reveals novel epigenetic targets for intrauterine growth restriction. Clin Epigenetics 8 (1). 10.1186/s13148-016-0238-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousseaux S, Seyve E, Chuffart F, Bourova-Flin E, Benmerad M, Charles M-A, Forhan A, Heude B, Siroux V, Slama R, Tost J, Vaiman D, Khochbin S, Lepeule J, the EDEN Mother-Child Cohort Study Group, 2020. Immediate and durable effects of maternal tobacco consumption alter placental DNA methylation in enhancer and imprinted gene-containing regions. BMC Med 18 (1). 10.1186/s12916-020-01736-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RStudio Team, 2020. RStudio: integrated development for R. RStudio, Inc. [Google Scholar]
- Šidák Z, 1967. Rectangular confidence regions for the means of multivariate normal distributions. J. Am. Stat. Assoc 62 (318), 626–633. 10.1080/01621459.1967.10482935. [DOI] [Google Scholar]
- Solomon O, Yousefi P, Huen K, Gunier RB, Escudero-Fung M, Barcellos LF, Eskenazi B, Holland N, 2017. Prenatal phthalate exposure and altered patterns of DNA methylation in cord blood. Environ. Mol. Mutagen 58 (6), 398–410. 10.1002/em.22095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stelzer G, Rosen N, Plaschkes I, Zimmerman S, Twik M, Fishilevich S, Stein TI, Nudel R, Lieder I, Mazor Y, Kaplan S, Dahary D, Warshawsky D, Guan-Golan Y, Kohn A, Rappaport N, Safran M, Lancet D, 2016. The GeneCards Suite: from gene data mining to disease genome sequence analyses. Curr. Protoc. Bioinform 54 (1) 10.1002/cpbi.5. [DOI] [PubMed] [Google Scholar]
- Strakovsky RS, Schantz SL, Dolinoy D, 2018. Impacts of bisphenol A (BPA) and phthalate exposures on epigenetic outcomes in the human placenta. Environ. Epigenet 4 (3) 10.1093/eep/dvy022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suter M, Ma J, Harris AS, Patterson L, Brown KA, Shope C, Showalter L, Abramovici A, Aagaard-Tillery KM, 2011. Maternal tobacco use modestly alters correlated epigenome-wide placental DNA methylation and gene expression. Epigenetics 6 (11), 1284–1294. 10.4161/epi.6.11.17819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suter MA, Aagaard KM, 2020. The impact of tobacco chemicals and nicotine on placental development. Prenat. Diagn 40 (9), 1193–1200. 10.1002/pd.5660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svendsen AJ, Gervin K, Lyle R, Christiansen L, Kyvik K, Junker P, Nielsen C, Houen G, Tan Q, 2016. Differentially methylated DNA regions in monozygotic twin pairs discordant for rheumatoid arthritis: an epigenome-wide study. Front. Immunol 7 10.3389/fimmu.2016.00510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Templ M, Hron K, Filzmoser P, 2011. robCompositions: An R-package for robust statistical analysis of compositional data. In: Compositional Data Analysis. John Wiley & Sons, Ltd:Chichester, UK, 341–355. [Google Scholar]
- Teschendorff AE, Breeze CE, Zheng SC, Beck S, 2017. A comparison of reference-based algorithms for correcting cell-type heterogeneity in Epigenome-Wide Association Studies. BMC Bioinf. 18:105. 10.1186/s12859-017-1511-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teschendorff AE, Marabita F, Lechner M, Bartlett T, Tegner J, Gomez-Cabrero D, Beck S, 2013. A beta-mixture quantile normalization method for correcting probe design bias in Illumina Infinium 450k DNA methylation data. Bioinformatics 29 (2), 189–196. 10.1093/bioinformatics/bts680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Y, Morris TJ, Webster AP, Yang Z, Beck S, Feber A, Teschendorff AE, 2017. ChAMP: updated methylation analysis pipeline for Illumina BeadChips. Bioinformatics 33 (24), 3982–3984. 10.1093/bioinformatics/btx513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tindula G, Murphy SK, Grenier C, Huang Z, Huen K, Escudero-Fung M, Bradman A, Eskenazi B, Hoyo C, Holland N, 2018. DNA methylation of imprinted genes in Mexican-American newborn children with prenatal phthalate exposure. Epigenomics 10 (7), 1011–1026. 10.2217/epi-2017-0178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullah R, Naz A, Akram HS, Ullah Z, Tariq M, Mithani A, Faisal A, 2020. Transcriptomic analysis reveals differential gene expression, alternative splicing, and novel exons during mouse trophoblast stem cell differentiation. Stem Cell Res. Ther 11 (1) 10.1186/s13287-020-01848-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Most PJ, Küpers LK, Snieder H, Nolte I, 2017. QCEWAS: automated quality control of results of epigenome-wide association studies. Bioinformatics 33, 1243–1245. 10.1093/bioinformatics/btw766. [DOI] [PubMed] [Google Scholar]
- van Rossum G, Drake F, 2009. Python 3 reference manual.
- Venables WN, Ripley BD, 2002. Modern Applied Statistics with S, 4. ed. New York, Springer. [Google Scholar]
- Vlahos A, Mansell T, Saffery R, Novakovic B, 2019. Human placental methylome in the interplay of adverse placental health, environmental exposure, and pregnancy outcome. PLoS Genet 15, e1008236. 10.1371/journal.pgen.1008236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Gao F, Zhao X, Cai Y, Jin H, 2020a. Integrated analysis of the transcriptome-wide m6A methylome in preeclampsia and healthy control placentas. PeerJ 8; doi: 10.7717/peerj.9880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J-Q, Hu Y-B, Gao H, Sheng J, Huang K, Zhang Y-W, Mao L-J, Zhou SS, Cai X-X, Zhang L-J, Wang S-F, Hao J-H, Yang L-Q, Tao F-B, 2020b. Sex-specific difference in placental inflammatory transcriptional biomarkers of maternal phthalate exposure: a prospective cohort study. J. Expo Sci. Environ. Epidemiol 30 (5), 835–844. 10.1038/s41370-020-0200-z. [DOI] [PubMed] [Google Scholar]
- Yang AS, 2004. A simple method for estimating global DNA methylation using bisulfite PCR of repetitive DNA elements. Nucleic Acids Res. 32, 38e–338e. 10.1093/nar/gnh032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye X, Kuklenyik Z, Needham LL, Calafat AM, 2005. Quantification of urinary conjugates of bisphenol A, 2,5-dichlorophenol, and 2-hydroxy-4-methoxybenzophenone in humans by online solid phase extraction–high performance liquid chromatography–tandem mass spectrometry. Anal. Bioanal. Chem 383, 638–644. 10.1007/s00216-005-0019-4. [DOI] [PubMed] [Google Scholar]
- Yuan V, Hui D, Yin Y, Peñaherrera MS, Beristain AG, Robinson WP, 2021. Cell-specific characterization of the placental methylome. BMC Genom. 22:6. 10.1186/s12864-020-07186-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao N.i., Bell DA, Maity A, Staicu A-M, Joubert BR, London SJ, Wu MC, 2015a. Global analysis of methylation profiles from high resolution CpG data. Genet. Epidemiol 39 (2), 53–64. 10.1002/gepi.21874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Chen J, Wang X, Song Q, Xu H-H, Zhang Y-H, 2016. Third trimester phthalate exposure is associated with DNA methylation of growth-related genes in human placenta. Sci. Rep 6 10.1038/srep33449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Shi H-J, Xie C-M, Chen J, Laue H, Zhang Y-H, 2015b. Prenatal phthalate exposure, infant growth, and global DNA methylation of human placenta. Environ. Mol. Mutagen 56 (3), 286–292. 10.1002/em.21916. [DOI] [PubMed] [Google Scholar]
- Zhou W, Laird PW, Shen H, 2017. Comprehensive characterization, annotation and innovative use of Infinium DNA methylation BeadChip probes. Nucleic Acids Res. 45 (e22) 10.1093/nar/gkw967. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


