Abstract
Pyrazinamide (PZA) is an important first-line tuberculosis drug that is part of the currently used short-course tuberculosis chemotherapy. PZA is a prodrug that has to be converted to the active form pyrazinoic acid by pyrazinamidase (PZase) activity, encoded by the pncA gene of Mycobacterium tuberculosis, and loss of PZase activity is associated with PZA resistance. To further define the genetic basis of PZA resistance and determine the frequency of PZA-resistant strains having pncA mutations, we sequenced the pncA gene from a panel of 59 PZA-resistant clinical isolates from Canada, the United States, and Korea. Two strains that did not contain pncA mutations and had positive PZase turned out to be falsely resistant. Three PZase-negative strains (MIC, >900 μg of PZA per ml) and one PZase-positive strain (strain 9739) (MIC, >300 μg of PZA per ml) did not have pncA mutations. The remaining 53 of the 57 PZA-resistant isolates had pncA mutations, confirming that pncA mutation is the major mechanism of PZA resistance. Various new and diverse mutations were found in the pncA gene. Interestingly, 20 PZA-monoresistant strains and 1 multidrug-resistant isolate from Quebec, Canada, all had the same pncA mutation profile, consisting of an 8-nucleotide deletion and an amino acid substitution of Arg140→Ser. Strain typing indicated that these strains are highly related and share almost identical IS6110 patterns. These data strongly suggest the spread of a PZA-monoresistant strain, which has not previously been described.
Pyrazinamide (PZA) is an important first-line tuberculosis (TB) drug. Along with isoniazid (INH), rifampin (RMP), and ethambutol (EMB), PZA is part of the currently used short-course treatment regimen, also called DOTS (for directly observed therapy, short course) recommended by the World Health Organization (21). PZA plays a unique role in achieving this shortened therapy, because PZA is believed to kill a population of semidormant tubercle bacilli residing in an acidic environment (e.g., as in active inflammation sites with low pH) in vivo that may not be affected by other TB drugs (12). Despite its role in shortening the TB therapy, PZA has no apparent activity against tubercle bacilli under normal pH conditions (18); the activity is only present at acidic pH (9). We have recently shown that the role of acid pH is to enhance accumulation of pyrazinoic acid (POA), the active moiety of PZA, in tubercle bacilli, whereas little POA accumulates in the bacterial cells at neutral pH (24). Structurally, PZA is an analog of nicotinamide. Like isoniazid (23), PZA is a prodrug. It requires conversion to POA by bacterial pyrazinamidase (PZase) in order to affect the tubercle bacilli (5, 14). Loss of PZase activity is observed in Mycobacterium tuberculosis strains that are resistant to PZA (5), and indeed, there is a very good correlation between PZA resistance and loss of this enzyme activity (8, 10, 11, 19). More details on PZA are given in a recent review by one of us (2).
To determine the genetic basis of PZA resistance, we have identified the PZase gene (pncA) from M. tuberculosis (14) and have shown in a previous study that pncA mutations appear to be a major mechanism of PZA resistance (15). Forty-one of 42 PZA-resistant strains were found to have pncA mutations (15). Subsequent studies have confirmed these findings (4, 6, 7, 10, 15). However, a study by Sreevatsan et al. reported that only 72% of 67 PZA-resistant strains had pncA mutations (16). It is not clear whether the lower percentage of PZA-resistant strains with pncA mutations is due to incorrect PZA susceptibility testing such that a portion of “PZA-resistant” strains are actually susceptible (falsely resistant) or is a genuine finding. In fact, the currently used methods for PZA susceptibility testing are unreliable and problematic (3) because of insufficient standardization of the available tests (1). A rapid molecular test for PZA resistance based on detecting pncA mutations could circumvent the problems of conventional PZA susceptibility testing. An accurate picture of the percentage of PZA-resistant strains having pncA mutations is useful not only for understanding the mechanism of PZA resistance but also for developing a PCR-based test for rapid detection of PZA resistance by detecting pncA mutations. To achieve these goals, we have in the present study analyzed more PZA-resistant clinical isolates of M. tuberculosis in terms of the correlation between PZA resistance and pncA mutations. New and diverse pncA mutations were again found in PZA-resistant strains. An interesting finding is that many clinical isolates from Quebec, Canada, are PZA monoresistant and they all share the same pncA mutation profile. IS6110 fingerprinting analysis indicated that these strains are highly related and suggest and active transmission of the disease by a PZA-monoresistant M. tuberculosis strain.
MATERIALS AND METHODS
Strains, PZA susceptibility testing, and PZase assay.
Thirty PZA-resistant strains, 21 of which were monoresistant, were from Quebec, Canada. These Canadian strains were collected over 3 years between 1990 and 1992 from 15 hospitals in 10 different regions of Quebec. The PZA-resistant M. tuberculosis strains were identified by using the pH 6.0 liquid medium in the BACTEC radiometric method with PZA concentrations of 100, 300, and 900 μg/ml for all of the U.S. strains (1), 100 and 300 μg of PZA per ml by the same method for the Canadian strains, or Lowenstein-Jensen medium at pH 5.6 with 100 and 500 μg of PZA per ml for the South Korean strains (strain designations starting with K). PZA resistance was defined as resistance to at least 100 μg of PZA per ml for the Lowenstein-Jensen method and the BACTEC method. For PZA susceptibility testing, susceptible strain H37Rv and PZA-resistant strain BCG were included as susceptible and resistant controls. PZase activity was assayed using the Wayne method (14) and confirmed using the C14-pyrazinamide method (17). A PZase-positive culture (PZA-susceptible M. tuberculosis strain H37Rv) and a PZase-negative culture (BCG Pasteur) were included as controls for the PZase assay.
Genomic DNA, PCR, and DNA sequencing.
M. tuberculosis cultures were grown in 7H9 liquid medium with albumin-dextrose-catalase enrichment (Difco) at 37°C for 3 to 4 weeks. Genomic DNA isolation and PCR were performed as described previously (22). The pncA forward primer 5′GTCGGTCATGTTCGCGATCG3′ was from bp −105 upstream of pncA, which contains putative promoter region, and the reverse primer 5′GCTTTGCGGCGAGCGCTCCA3′ was from 60 bp downstream of stop codon of the M. tuberculosis pncA gene (558 bp) (accession number U59967) (14). The expected size of the pncA PCR products was 720 bp. The pncA PCR products were run on Tris-borate-EDTA–0.8% agarose gel, and the DNA was isolated using a Qiagen kit according to manufacturer's instructions. The gel-purified PCR products were directly sequenced in an ABI automatic DNA sequencer (model 377), using the above-described forward and reverse primers.
IS6110 fingerprinting.
Mycobacterial genomic DNAs from various PZA-resistant strains were digested with PvuII and then run on a Tris-borate-EDTA–0.8% agarose gel. The IS6110 probe used in the Southern hybridization was a 245-bp PCR DNA fragment amplified by PCR using INS-1 (5′CGTGAGGGCATCGAGGTGGC3′) and INS-2 (5′GCGTAGGCGTCGGTGACAAA3′) primers as described previously (20). The 245-bp PCR product was labeled with [32P]dCTP using a random primer labeling kit (GIBCO BRL). The Southern blotting procedure was performed as described previously (22).
RESULTS
Identification of pncA mutations in PZA-resistant M. tuberculosis clinical isolates.
To further define the molecular basis of PZA resistance and to determine the frequency of pncA mutations among PZA-resistant strains, we analyzed 59 PZA-resistant M. tuberculosis clinical isolates for potential mutations in the pncA gene by PCR sequencing (Table 1). Two strains (11830 and 10274) which were initially reported as resistant to PZA by using the BACTEC method at a PZA concentration of 100 μg/ml were in fact susceptible to PZA (falsely resistant) (MIC, <100 μg/ml) upon retesting. Fifty-three of 57 genuinely PZA-resistant strains had various pncA mutations, as shown in Table 1. The nature of the pncA mutations ranged from nucleotide transitions or transversions causing amino acid substitutions to nucleotide insertions or deletions causing nonsense polypeptides. It is remarkable that 17 new and diverse mutations were found in the 558-bp-long pncA gene (Table 1). Some of the strains had the same type of mutations but different IS6110 patterns, indicating that they are actually different strains which happened to acquire the same type of mutation. For example, strains T63168 and M52997 had the same mutation of Gly97→Ser, yet IS6110 analysis indicated that they are different TB strains (Fig. 1, lanes 1 and 2). Similarly, strains H2374 and H1033 both had a single nucleotide G insertion at position 420, but IS6110 typing showed that they are different strains (Fig. 1, lanes 3 and 4). Three PZA-resistant PZase-negative strains (11552, F57636, and M49586), for which the MICs were greater than 900 μg/ml, did not have any mutations in the pncA gene. Further sequence analysis of the pncA upstream region (2 to 3 kb before the start codon), which contains the putative pncA promoter, also failed to reveal any mutations (data not shown). This suggests that there could be a pncA regulatory gene and that mutation of this gene could affect the expression of the pncA gene. One PZA-resistant strain (strain 9739) with positive PZase and for which the PZA MIC was 300 μg/ml did not have any pncA mutation.
TABLE 1.
Characteristics of PZA-resistant clinical isolates of M. tuberculosis
| Straina | Resistance to other drugsc | PZA MIC (μg/ml) | PZase activity |
pncA mutation(s)b
|
|
|---|---|---|---|---|---|
| Nucleotide change(s) | Amino acid change(s) | ||||
| 9869 | INH, RMP, SM | >300 | − | −11 promoter mutation A→G | |
| 10347 | Monoresistant | >300 | − | C to A at 418, 8-bp deletion at 446 | Arg140→Ser, frameshift∗ |
| 9131 | Monoresistant | >300 | − | C to A at 418, 8-bp deletion at 446 | Arg140→Ser, frameshift |
| 11041 | Monoresistant | >300 | − | C to A at 418, 8-bp deletion at 446 | Arg140→Ser, frameshift |
| 11135 | Monoresistant | >300 | − | C to A at 418, 8-bp deletion at 446 | Arg140→Ser, frameshift |
| 9721 | Monoresistant | >300 | − | C to A at 418, 8-bp deletion at 446 | Arg140→Ser, frameshift |
| 9769 | Monoresistant | >300 | − | C to A at 418, 8-bp deletion at 446 | Arg140→Ser, frameshift |
| 11823 | Monoresistant | >300 | − | C to A at 418, 8-bp deletion at 446 | Arg140→Ser, frameshift |
| 9132 | Monoresistant | >300 | − | C to A at 418, 8-bp deletion at 446 | Arg140→Ser, frameshift |
| 9579 | Monoresistant | >300 | − | C to A at 418, 8-bp deletion at 446 | Arg140→Ser, frameshift |
| 10800 | Monoresistant | >300 | − | C to A at 418, 8-bp deletion at 446 | Arg140→Ser, frameshift |
| 10350 | Monoresistant | >300 | − | C to A at 418, 8-bp deletion at 446 | Arg140→Ser, frameshift |
| 11243 | Monoresistant | >300 | − | C to A at 418, 8-bp deletion at 446 | Arg140→Ser, frameshift |
| 9811 | Monoresistant | >300 | − | C to A at 418, 8-bp deletion at 446 | Arg140→Ser, frameshift |
| 9004 | Monoresistant | >300 | − | C to A at 418, 8-bp deletion at 446 | Arg140→Ser, frameshift |
| 10348 | Monoresistant | >300 | − | C to A at 418, 8-bp deletion at 446 | Arg140→Ser, frameshift |
| 10257 | Monoresistant | >300 | − | C to A at 418, 8-bp deletion at 446 | Arg140→Ser, frameshift |
| 10003 | Monoresistant | >300 | − | C to A at 418, 8-bp deletion at 446 | Arg140→Ser, frameshift |
| 11743 | Monoresistant | >300 | − | C to A at 418, 8-bp deletion at 446 | Arg140→Ser, frameshift |
| 10611 | Monoresistant | >300 | − | C to A at 418, 8-bp deletion at 446 | Arg140→Ser, frameshift |
| 9155 | Monoresistant | >300 | − | C to A at 418, 8-bp deletion at 446 | Arg140→Ser, frameshift |
| 9953 | INH, RMP, EMB | >300 | − | C to A at 418, 8-bp deletion at 446 | Arg140→Ser, frameshift |
| W39117 | INH, RMP, EMB, Oflox | >900 | − | A to G at 29 | Gln10→Arg∗ |
| H3628 | INH, RMP | >900 | − | A to C at 35 | Asp12→Ala |
| K10451 | NT | >500 | − | C to A at 137 | Ala46→Glu |
| K10437 | NT | >500 | − | C to T at 137 | Ala46→Val |
| F29684 | INH, RMP, EMB | >900 | − | C to T at 137 | Ala46→Val |
| W5457 | NT | >900 | − | C to T at 137 | Ala46→Val |
| A7259 | INH, RMP, SM | >900 | − | C to T at 137 | Ala46→Val |
| A7183 | INH, RMP, SM | >900 | − | C to T at 137 | Ala46→Val |
| 8989 | NT | >900 | − | C to T at 137 | Ala46->Val |
| K10552 | NT | >100 | − | A to C at 158 | Asp53→Ala∗ |
| PZA-R9717 | Monoresistant, in vitro mutant | >900 | − | G to A at 203 | Trp68→stop∗ |
| A7157 | INH, RMP, SM | >900 | − | T to G at 213 | His71→Glu∗ |
| A7153 | INH, RMP, EMB, SM | 300 | − | A to G at 245 | His82→Arg∗ |
| K10400 | NT | >500 | − | T to G at 254 | Leu85→Arg |
| T63168 | INH, RMP, EMB, SM | >900 | − | G to A at 289 | Gly97→Ser∗ |
| M52997 | INH, RMP, EMB, SM | >900 | − | G to A at 289 | Gly97→Ser |
| 10426 | INH, RMP, SM | >300 | − | C to T at 401 | Ala134→Val |
| K10551 | NT | >500 | − | G to C at 413 | Cys138→Ser |
| K10428 | NT | >500 | − | G to A at 415 | Val139→Met∗ |
| W76757 | INH, RMP, EMB, SM | >300 | − | A to C at 422 | Gln141→Pro |
| K10447 | NT | >500 | − | T to G at 464 | Val155→Gly |
| 099-3-CIP8 | RMP, Oflox | 300 | − | C to A at 503 | Thr168→Asn∗ |
| 10467 | INH, RMP, SM | >300 | − | G insertion at 52∗ | Frameshift |
| 11627 | SM | >300 | − | A insertion at 193∗ | Frameshift |
| A7156 | INH, RMP, SM | >900 | − | A insertion at 193 | Frameshift |
| K10429 | NT | >500 | − | G deletion at 301∗ | Frameshift |
| K10452 | NT | >500 | − | C deletion at 341∗ | Frameshift |
| H2374 | RMP | >900 | − | G insertion at 420∗ | Frameshift |
| H1033 | INH, RMP, SM, Oflox | >900 | − | G insertion at 420 | Frameshift |
| K10436 | NT | >100 | − | GG insertion at 428∗ | Frameshift |
| T61823 | INH, RMP, SM | >900 | − | GG insertion at 420, T to G at 385 | Val130→Gly∗ |
| 11552 | INH | >300 | − | No mutation | |
| M49586 | NT | >900 | − | No mutation | |
| F57636 | INH, RMP | >900 | − | No mutation | |
| 9739 | NT | 300 | + | No mutation | |
| 10274 | NT | 100 | + | No mutation | |
| 11830 | NT | 100 | + | No mutation | |
Strain designations with only numbers indicate strains from Quebec, Canada Strain designations starting with K indicate strains from South Korea, and strain designations starting with other letters indicate U.S. strains.
SM, streptomycin; Oflox, ofloxacin; NT, not tested.
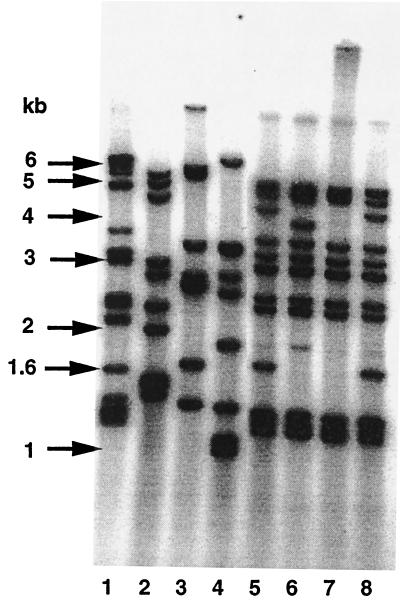
FIG. 1.
IS6110 strain typing analysis of PZA-resistant M. tuberculosis strains. Lanes 1 and 2, strains T63168 and M52997, respectively, which share the same mutation of Gly97→Ser. Lanes 3 and 4, H2374 and H1033, respectively, which share the same mutation of nucleotide G insertion at position 420. Lanes 5 to 8, 10350, 10003, 9155, and 10257, which all share the same pncA mutation profile of an 8-bp deletion at nucleotide position 446 followed by Arg140→Ser.
Active transmission of a PZA-monoresistant strain in Quebec, Canada.
Twenty-one of 27 PZA-resistant clinical isolates from Quebec, Canada, were found to have the same type of pncA mutations, which consisted of an 8-bp deletion at nucleotide position 446 followed by an amino acid substitution of Arg140 to Ser. One such strain, 9953, is a multidrug-resistant (MDR) strain, which is resistant to INH, RMP, and EMB in addition to PZA. This suggests that this MDR strain initially had PZA monoresistance but later acquired resistance to other drugs. IS6110 strain typing showed that the PZA-monoresistant strains that had the characteristic 8-bp deletion and an amino acid substitution shared almost identical banding pattern (Fig. 1, lanes 5 to 8), indicating that these strains are highly related and were derived from a single source.
DISCUSSION
The present study has shown that 53 of 57 PZA-resistant M. tuberculosis clinical isolates had mutations in the pncA gene, indicating that pncA mutation is the major mechanism of PZA resistance in M. tuberculosis. The nature of the pncA mutations includes (i) substitution of amino acids due to nucleotide transitions or transversions or (ii) nucleotide insertions or deletions leading to nonsense polypeptides. The distribution of pncA mutations was dispersed along the gene, as found in previous studies. So far, a remarkably diverse array of 120 types of mutations had been identified, including 87 mutations leading to amino acid substitutions or stop codons, 30 nucleotide deletions or insertions including an insertion of IS6110 into the pncA gene, and 3 putative promoter mutations, in PZA-resistant strains from six independent studies (4, 6, 7, 10, 15, 16). Yet again, 17 new and diverse pncA mutations were found in this study. The highly diverse mutation profile in the pncA gene observed in PZA-resistant strains is unique among all drug resistance genes in M. tuberculosis. While the cause for this remarkable diversity of pncA mutations is unclear, it is possible that this could be due to adaptive mutagenesis or to a deficiency in DNA mismatch repair mechanisms in M. tuberculosis (12). Furthermore, the possibility that the pncA gene might be located in a hot spot of mutation in the genome cannot be ruled out. Another explanation relates to the nonessential nature of the pncA gene, as shown in this study with the spread of a PZA-monoresistant strain, such that it can accumulate various mutations without affecting the viability of the organism. In contrast, in the case of RMP, streptomycin, kanamycin, EMB, and quinolone resistance, not all mutations in the target genes lead to a viable organism, such that only a limited array of mutations can be tolerated without losing the function of vital enzymes and fitness of the organism.
It is intriguing that we were unable to identify any mutations in the pncA gene or in the putative pncA promoter region in three PZA-resistant strains with negative PZase. This suggests that there could be a pncA-regulatory gene and that mutation of this gene could affect expression of pncA, thereby causing PZA resistance. Identification of this pncA-regulatory gene may be useful for designing a molecular test for better detection of PZA-resistant strains. In addition, we identified one PZase-positive, PZA-resistant strain, strain 9739, that did not have any pncA mutation. From the published reports on this topic (4, 6, 7, 10, 14, 15), it appears that such highly resistant M. tuberculosis strains with positive PZase activity and no pncA mutations are rare. However, this suggests that a new mechanism of PZA resistance without affecting PZase activity or expression may exist. Mutations leading to modification or amplification of the POA target or to enhanced POA efflux could potentially cause PZA resistance. However, these alternative resistance mechanisms have yet to be identified. Strain 9739 may provide an opportunity to study alternative mechanisms of PZA resistance.
In this study, we found that two PZase-positive strains, initially identified as PZA-resistant based on the single-concentration test with 100 μg/ml by the BACTEC method, turned out to be susceptible to PZA (100 μg/ml) upon retesting. Sequence analysis showed that these strains did not have any pncA mutations. In view of this potential false-resistance problem, we would like to stress that the findings reported here support the previous suggestion (2) to use 300 instead of 100 μg of PZA per ml in a single-concentration test by the BACTEC method in pH 6.0 medium. The laboratory at the National Jewish Medical and Research Center in Denver, Colo., has been using this technique for many years with three PZA concentrations (100, 300, and 900 μg/ml) for a quantitative test to determine the MIC (1) and with 300 μg/ml for the single-concentration qualitative test (2). The 300-μg/ml PZA cutoff is less vulnerable to variations in the pH values that are likely to occur from different batches of the medium, as well as more tolerant to slight increases in the inoculum size that could increase the MIC of PZA.
The transmission of a PZA-monoresistant TB strain in Quebec, Canada, is interesting. In the Province of Quebec, the average annual prevalence of PZA monoresistance for the past 5 years was 2.8% (L. Thibert, unpublished data). In this study, 21 PZA-monoresistant strains, which were isolated from different individual patients in 10 different geographic regions of Quebec, all had the same mutation profile—an 8-bp deletion at nucleotide position 446 followed by an amino acid substitution of Arg140→Ser. These patients are not known to have taken PZA monotherapy or PZA prophylaxis. IS6110 typing indicated that these strains are highly related and share almost identical IS6110 patterns. The finding of a slight difference in the IS6110 pattern (Fig. 1) among the 20 PZA-monoresistant strains and one MDR strain with the same mutation profile suggests that this clone has probably been in that area for some time. PZA monoresistance is unusual, as PZA is not used alone to treat TB, and often, if any single drug resistance occurs it is usually INH resistance. The finding that one MDR TB strain from Quebec also had the same type of mutation as the 20 PZA-monoresistant strains strongly suggests that the PZA-monoresistant strain emerged first and then was followed by an accumulation of other mutations leading to resistance to INH, RMP, and EMB. In this case, detection of pncA mutation helped to identify the transmission of a PZA-monoresistant TB strain. While INH-resistant, catalase-peroxidase-defective strains may have impaired ability to cause disease, it is clear from this study that PZA-resistant strains lacking the PZase enzyme are still fully capable of causing active disease. This is the first demonstration of an active transmission of TB due to a PZA-monoresistant strain by using a molecular epidemiology approach. It remains to be determined if PZA-monoresistant strains from other parts of Canada are related to those characterized in this study in terms of pncA mutation and IS6110 profile.
The increasing emergence and spread of drug-resistant M. tuberculosis worldwide pose serious threat to the control of TB. The rapid detection of drug-resistant strains represents an important part of the TB control strategy. While drug susceptibility testing could be reliably done for most TB drugs, PZA susceptibility testing is difficult and may produce inconsistent results and frequent false-resistance reports (3). Because of this, many clinical microbiology laboratories do not perform PZA susceptibility testing, and most drug resistance epidemiology surveys do not have PZA resistance data. Our finding of pncA mutations as a major mechanism of PZA resistance provides promise for developing a molecular test for rapid detection of PZA resistance based on detecting pncA mutations.
ACKNOWLEDGMENTS
This work was supported by research grants from the Potts Memorial Foundation and by NIH RO1AI40584 and RO1AI44063.
We thank S. J. Kim for providing some of the PZA-resistant strains.
REFERENCES
- 1.Heifets L B. Drug susceptibility tests in the management of chemotherapy of tuberculosis. In: Heifets L B, editor. Drug susceptibility in the chemotherapy of mycobacterial infections. Boca Raton, Fla: CRC Press; 1991. pp. 89–122. [Google Scholar]
- 2.Heifets L B. Pyrazinamide. In: Yu V L, Merigan T C, Barriere S L, editors. Antimicrobial therapy and vaccines. Baltimore, Md: Williams and Wilkins; 1999. pp. 668–676. [Google Scholar]
- 3.Hewlett D, Horn D L, Alfalla C. Drug-resistant tuberculosis: inconsistent results of pyrazinamide susceptibility testing. JAMA. 1995;273:916–917. [PubMed] [Google Scholar]
- 4.Hirano K, Takahashi M, Kazumi Y, Fukasawa Y, Abe C. Mutation in pncA is a major mechanism of pyrazinamide resistance in Mycobacterium tuberculosis. Tuber Lung Dis. 1997;78:117–122. doi: 10.1016/s0962-8479(98)80004-x. [DOI] [PubMed] [Google Scholar]
- 5.Konno K, Feldman F M, McDermott W. Pyrazinamide susceptibility and amidase activity of tubercle bacilli. Am Rev Respir Dis. 1967;95:461–469. doi: 10.1164/arrd.1967.95.3.461. [DOI] [PubMed] [Google Scholar]
- 6.Lemaitre N, Sougakoff W, Truffot-Pernot C, Jarlier V. Characterization of new mutations in pyrazinamide-resistant strains of Mycobacterium tuberculosis and identification of conserved regions important for the catalytic activity of the pyrazinamidase. Antimicrob Agents Chemother. 1999;43:1761–1763. doi: 10.1128/aac.43.7.1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marttila H J, Marjamaki M, Vyshnevskaya E, Vishnevskiy B I, Otten T F, Vasilyef A V, Viljanen M K. pncA mutations in pyrazinamide-resistant Mycobacterium tuberculosis isolates from northwest Russia. Antimicrob Agents Chemother. 1999;43:1764–1766. doi: 10.1128/aac.43.7.1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McClatchy J K, Tsang A Y, Cernich M S. Use of pyrazinamidase activity in Mycobacterium tuberculosis as a rapid method for determination of pyrazinamide susceptibility. Antimicrob Agents Chemother. 1981;20:556–557. doi: 10.1128/aac.20.4.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McDermott W, Tompsett R. Activation of pyrazinamide and nicotinamide in acidic environments in vitro. Am Rev Tuberc. 1954;70:748–754. doi: 10.1164/art.1954.70.4.748. [DOI] [PubMed] [Google Scholar]
- 10.Mestdagh M, Fonteyne P A, Realini L, Rossau R, Jannes G, Mijs W, De Smet K A L, Portaels F, Van Den Eeckhout E. Relationship between pyrazinmide resistance, loss of pyrazinamidase activity, and mutations in the pncA locus in multidrug-resistant clinical isolates of Mycobacterium tuberculosis. Antimicrob Agents Chemother. 1999;43:2317–2319. doi: 10.1128/aac.43.9.2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miller M, Thibert L, Desjardins F, Siddiqi S, Dascal A. Testing of susceptibility of Mycobacterium tuberculosis to pyrazinamide: comparison of Bactec method with pyrazinamidase assay. J Clin Microbiol. 1995;33:2468–2470. doi: 10.1128/jcm.33.9.2468-2470.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mitchison D A. The action of antituberculosis drugs in short course chemotherapy. Tubercle. 1985;66:219–225. doi: 10.1016/0041-3879(85)90040-6. [DOI] [PubMed] [Google Scholar]
- 13.Mizrahi V, Andersen S. DNA repair in Mycobacterium tuberculosis. What have we learnt from the genome sequence? Mol Microbiol. 1998;29:1331–1339. doi: 10.1046/j.1365-2958.1998.01038.x. [DOI] [PubMed] [Google Scholar]
- 14.Scorpio A, Zhang Y. Mutations in pncA, a gene encoding pyrazinamidase/nicotinamidase, cause resistance to the antituberculous drug pyrazinamide in tubercle bacillus. Nat Med. 1996;2:662–667. doi: 10.1038/nm0696-662. [DOI] [PubMed] [Google Scholar]
- 15.Scorpio A, Lindholm-Levy P, Heifets L, Gilman R, Siddiqi S, Cynamon M, Zhang Y. Characterization of pncA mutations in pyrazinamide-resistant Mycobacterium tuberculosis. Antimicrob Agents Chemother. 1997;41:540–543. doi: 10.1128/aac.41.3.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sreevatsan S, Pan X, Zhang Y, Kreiswirth B, Musser J M. Mutations associated with pyrazinamide resistance in pncA of Mycobacterium tuberculosis complex organisms. Antimicrob Agents Chemother. 1997;41:636–640. doi: 10.1128/aac.41.3.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun Z H, Zhang Y. Reduced pyrazinamidase and the natural resistance of Mycobacterium kansasii to the antituberculosis drug pyrazinamide. Antimicrob Agents Chemother. 1999;43:537–542. doi: 10.1128/aac.43.3.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tarshis M S, Weed W A. Lack of significant in vitro sensitivity of Mycobacterium tuberculosis to pyrazinamide on three different solid media. Am Rev Tuberc. 1953;67:391–395. doi: 10.1164/art.1953.67.3.391. [DOI] [PubMed] [Google Scholar]
- 19.Trivedi S S, Desai S G. Pyrazinamidase activity of Mycobacterium tuberculosis—a test of sensitivity to pyrazinamide. Tubercle. 1987;68:221–224. doi: 10.1016/0041-3879(87)90058-4. [DOI] [PubMed] [Google Scholar]
- 20.van Soolingen D, Hermans P W M, de Haas E W, Soll D R, van Embden J D A. Occurrence and stability of insertion sequence in Mycobacterium tuberculosis complex strains: evaluation of an insertion sequence-dependent DNA polymorphism as a tool in the epidemiology of tuberculosis. J Clin Microbiol. 1991;29:2578–2586. doi: 10.1128/jcm.29.11.2578-2586.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.World Health Organization. WHO Report on the Tuberculosis Epidemic. Stop TB at the source. Geneva, Switzerland: Tuberculosis Programme, World Health Organization; 1995. [Google Scholar]
- 22.Zhang Y, Garcia M J, Lathigra R, Allen B, Moreno C, van Embden J D A, Young D. Alterations in the superoxide dismutase gene of an isoniazid-resistant strain of Mycobacterium tuberculosis. Infect Immun. 1992;60:2160–2165. doi: 10.1128/iai.60.6.2160-2165.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang Y, Heym B, Allen B, Young D, Cole S. The catalase-peroxidase gene and isoniazid resistance of Mycobacterium tuberculosis. Nature (London) 1992;358:591–593. doi: 10.1038/358591a0. [DOI] [PubMed] [Google Scholar]
- 24.Zhang Y, Scorpio A, Nikaido H, Sun Z H. Role of acid pH and deficient efflux of pyrazinoic acid in the unique susceptibility of Mycobacterium tuberculosis to pyrazinamide. J Bacteriol. 1999;181:2044–2049. doi: 10.1128/jb.181.7.2044-2049.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]