Abstract
Cystic fibrosis (CF) is one of the most commonly diagnosed genetic disorders. Clinical characteristics include progressive obstructive lung disease, sinusitis, exocrine pancreatic insufficiency leading to malabsorption and malnutrition, liver and pancreatic dysfunction, and male infertility. Although CF is a life-shortening disease, survival has continued to improve to a median age of 44.4 years due to earlier diagnosis through routine newborn screening, promulgation of evidence-based guidelines to optimize nutritional and pulmonary health, and development of CF-specific interdisciplinary care centers. Future improvements in health and quality of life for individuals with cystic fibrosis are likely with the recent development of mutation-specific modulator therapies. In this review, we will cover the current understanding of the disease manifestations, diagnosis, and management as well as common complications seen in individuals with CF.
Introduction
Cystic fibrosis (CF) is a common life-shortening autosomal recessive genetic disorder, characterized by pulmonary manifestations, specifically chronic and progressive obstructive lung disease, sinusitis, malabsorption due to pancreatic exocrine insufficiency leading to malnutrition, liver disease (biliary cirrhosis), and CF-related diabetes mellitus (CFRD). Earlier diagnosis through newborn screening, improved therapies to optimize lung health and nutritional status, as well as aggressive treatment of chronic respiratory infections and lung transplantation for end-stage lung disease have led to significant improvements in survival. CF was a uniformly fatal disease in childhood at the time of its initial description in 1938, (1) but the predicted median survival is currently 44.4 years. Currently, over 50% of people living with CF are 18 years and older, (2) resulting in the evolution of the disease from exclusively a disease of childhood to a disease where affected individuals transition to adulthood and adult providers. With the advent of newer therapies targeting the basic genetic defect that causes the disease and the expansion of the age and genetic variants for which these therapies are indicated, there is promise of continued improvement of quality of life as well as overall health and survival. Individuals with CF benefit from coordination of care between their primary care providers and their interdisciplinary CF care team, in addition to routine visits with both. This review will cover the current understanding of the disease manifestations, diagnosis, and management as well as common complications seen in individuals with CF.
Epidemiology
CF is one of the most common genetic disorders among Caucasians, with an incidence of 1:3,200 individuals. (3) The incidence does vary significantly by race/ethnicity with an incidence of 1:13,500 in people of Hispanic background, 1:15,000 in people of African descent and 1:35,000 in people of Asian descent. (4) It is estimated that one in every 35 Americans is a carrier of CF. (5) Based on statistics from the 2018 Annual Data Report published by the Cystic Fibrosis Foundation (CFF), there are an estimated 30,000 affected individuals in the United States living with CF. (2) Worldwide, there are an estimated 70,000 people, with the highest prevalence in North America, Europe and Australia. Annually, approximately 1,000 new cases are diagnosed in the United States (although the rate of new cases may be declining related to pre-conception screening), (6) and the incidence is equal in males and females. Since 2010, all 50 states screen neonates for cystic fibrosis, (7) and as a result 60% of new diagnoses occur via newborn screening. (2)
Pathogenesis
CF is a multisystem disorder that results from deleterious genetic variants in the CFTR gene located on the chromosome 7q31.2, which encodes for the cystic fibrosis transmembrane conductance regulator (CFTR) protein. Defects in this protein lead to absent or malfunctioning chloride channels in the apical membranes of the lung surface or glandular epithelium resulting in the formation of thick and sticky mucus, leading to chronic lung infections, pancreatic and liver dysfunction, and reduced fertility. CF also results in abnormal chloride channel function in the sweat glands, resulting in excessive salt loss in sweat, an observation first studied by Dr. Paul di Sant’Agnese after caring for infants with CF presenting with dehydration during a heat wave in New York City in 1948. (8) This clinical observation paved the way for the pilocarpine iontophoresis sweat test for CF diagnosis. (9)
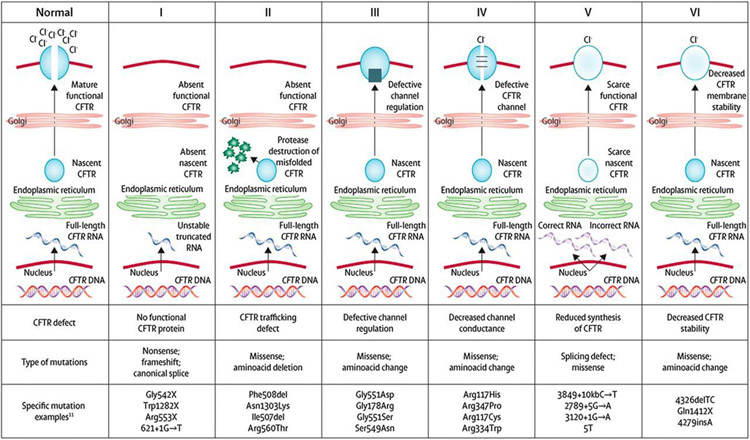
CF is an autosomal recessive disorder and for individuals to have CF, they must inherit 2 deleterious CFTR variants. To date, there are more than 2,000 different CFTR variants reported, some of which are confirmed to cause CF, and others with more putative links to the disease. (10)(11) They are classified into six distinct groups that reflect abnormalities of CFTR protein synthesis, structure and function (Figure 1). The most common CF-causing variant is F508del (p.Phe508del). F508del is a Class II mutation, meaning that the CFTR protein is created, but misfolding prevents it from reaching the cell surface (“trafficking defect”). Overall, 44.2% of individuals with CF are homozygous for F508del, an additional 40.5% have one copy of F508del and another variant, and 15.3% have 2 non-F508del variants. (2) The specific CFTR variants that an individual carries determines the amount of functioning CFTR protein present, and is partially correlated with phenotypic severity and organ involvement. (12)
Figure 1.
Cystic fibrosis transmembrane conductance regulator (CFTR) mutation classes. CFTR mutations have been grouped into 6 distinct classes based on abnormalities of CFTR synthesis, structure, and function. Reprinted with from: Boyle MP, DeBoeck K. A new era in the treatment of cystic fibrosis: correction of the underlying CFTR defect. Lancet Respir Med. 2013;1(2):158–163.
Clinical Presentations
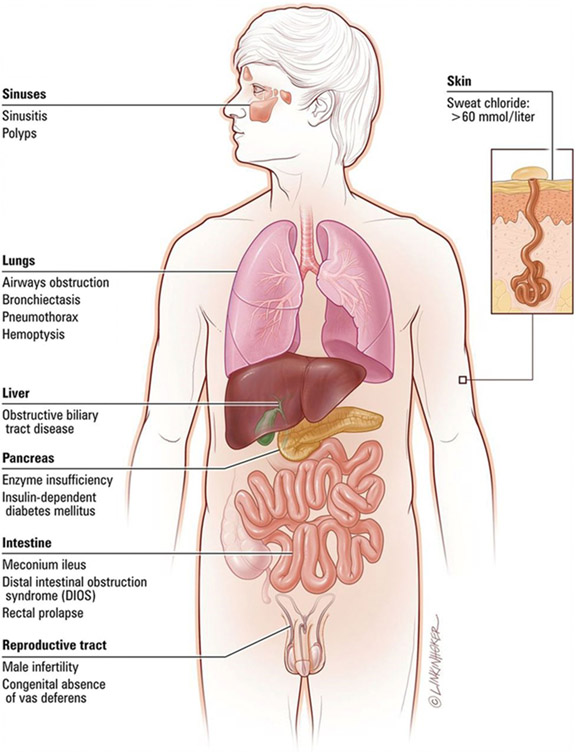
The classic manifestations of CF include a triad of (1) recurrent sinus and pulmonary infections, (2) steatorrhea, and (3) malnutrition, which in its most severe form presents as failure to thrive. In the lungs, mucous plugging from dehydrated thick secretions results in inflammation, chronic infection, progressive small airways obstruction and the development of bronchiectasis, which is an abnormal, permanent enlargement of the bronchi (Figure 2). Bronchiectasis leads to decreased ability to clear secretions, causing increased rates of infections, which further dilates and damages the airways. In addition, the effects of diminished or absent chloride channel function can result in dysfunction in several other organ systems (Figure 3). Pancreatic involvement includes both pancreatic exocrine insufficiency, which results in fat, protein and carbohydrate malabsorption and subsequent malnutrition, as well as insulin insufficiency and the development of CFRD.
Figure 2.

Chest computed tomographic scan of an adolescent girl with cystic fibrosis demonstrates significant bronchiectasis (white open arrows) and mucous plugging (white asterisks). Reprinted from: Paranjape SM, Mogayzel PJ. Cystic fibrosis. Pediatr Rev. 2014;35(5).
Figure 3.
Common clinical manifestations of cystic fibrosis. Reprinted with permission from Link Studio LLC.
In infants and young children, other presentations may also be indicative of CF. In utero, ultrasonographic evidence of hyperechogenic or dilated bowel are suggestive of intestinal obstruction, which have been reported in 50% to 78% of fetuses affected with CF. (13)(14) Postnatally, delayed meconium passage or meconium ileus is present in 11.9% of infants <1 year of age with CF (2), and results from thick GI secretions that become adherent to the intestinal mucosa leading to bowel obstruction. Meconium ileus is often accompanied by abdominal distension and dilated loops of bowel on imaging and a reported 30% cases of meconium ileus are complicated by intestinal perforation and peritonitis. (2) Approximately 20% of untreated children (6 months to 3 years) have rectal prolapse, which is secondary to malabsorption, malnutrition, and bulky stools as opposed to constipation. (15) Other clinical presentations during the neonatal period may include prolonged jaundice secondary to biliary stasis or bile duct obstruction and hemorrhagic disease of the newborn owing to vitamin K deficiency. Throughout infancy and early childhood, individuals may also present with salt depletion syndrome characterized by a hyponatremic, hypochloremic, hypokalemic metabolic alkalosis, and edema/acrodermatitis due to hypoproteinemia from malabsorption. (16)
Typical respiratory findings in older children, adolescents, and adults who newly present with CF may include recurrent sinusitis, bronchitis, or pneumonias, asthma that is poorly responsive to standard management, nasal polyposis or digital clubbing on physical exam, as well as bronchiectasis on lung imaging studies. (16) Common gastrointestinal symptoms may include malnutrition, poor growth, steatorrhea, intestinal obstruction, chronic constipation, rectal prolapse, and liver disease. (17) Individuals with pancreatic sufficient CF (who are more likely to be diagnosed later in life due to appropriate weight gain) can present with pancreatitis secondary to progressive pancreatic inflammation, although the exact etiology is unclear. (18) Finally, over 98% of men with CF are infertile as a result of obstructive azootemia secondary to congenital bilateral absence of the vas deferens and may be diagnosed during an infertility work-up. (19) Newborn screening is expected to reduce, but not eliminate late clinical presentations given the expected rate of false negatives associated with screening. Clinicians should include cystic fibrosis in the differential for unexplained recurrent respiratory bacterial infections (pneumonia, bronchitis, persistent cough, and/or sinusitis) and/or failure to thrive.
SCREENING
Newborn screening (NBS) for CF was first introduced in the 1980s, and is performed by measuring an immunoreactive trypsinogen (IRT) level in screening neonatal blood spots. In neonates with CF, mucus plugs partially block the pancreatic ducts that lead into the small intestine, preventing trypsinogen from reaching the intestine and being converted to the pancreatic enzyme trypsin, even in infants with CF who are pancreatic sufficient. If an infant has an elevated IRT level, almost all U.S. state laboratories perform confirmatory CFTR variant testing (IRT/DNA) with one state laboratory currently repeating the IRT measurement (IRT/IRT). Screening is considered positive if the IRT level remains persistently elevated between the ages of 7 and 14 days of life, or if at least one deleterious CFTR variant is identified on genetic testing. A positive NBS result will trigger notification of either a neonatal intensive care provider or primary care provider, and the infant should be referred to a CFF-accredited center for definitive evaluation and sweat testing within 72 hours of a positive screening result. (20) Care centers may be located on the CFF website (www.cff.org/ccd/).
The benefits of diagnosing asymptomatic infants with CF includes increased attention to early lung health to slow lung disease progression, optimization of nutritional status with early enzyme replacement and aggressive nutritional counseling, as well as providing psychosocial support to families to help prevent or delay serious complications. (16) Possible risks of NBS include an increased number of medical interventions, exposure to respiratory pathogens through attending CF clinic, financial hardships given the cost of CF-related therapies, possible iatrogenic complications (e.g., early exposure to therapies with side effects), and caregiver anxiety or guilt stemming from false-positive screening results due to perinatal asphyxia or other perinatal problems. (16)(21) False positive rates may also be higher in African-American children as they have higher IRT levels than Caucasians (22), but have a much lower risk of CF. NBS can also be falsely negative, particularly in neonates with meconium ileus (23) or those screened via IRT/DNA, as this testing may be less sensitive for picking up mutations in minority populations. (22) With current NBS practices, the possibility exists for a positive NBS and the identification of CFTR variants that do not meet clinical criteria for CF diagnosis in individuals with normal or intermediate sweat chloride testing, a syndrome known as CFTR-Related Metabolic Syndrome (CRMS)/ Cystic Fibrosis Screen Positive, Inconclusive Diagnosis (CFSPID). (24)
DIAGNOSIS
The CFF published consensus guidelines in 2017 establishing that a diagnosis of CF can be made if an individual has a clinical presentation consistent with the disease i.e. (1) a positive newborn screening; (2) clinical features consistent with CF (the presence of characteristic phenotypes such as of chronic, recurrent sinus and pulmonary disease, nutritional and gastrointestinal abnormalities, urogenital abnormalities in males (e.g., absence of the vas deferens), and/or salt depletion syndromes, or (3) a positive family history of CF and evidence of CFTR dysfunction (e.g., sweat chloride concentration ≥60mmol/L). (24)(25)
While prenatal screening and NBS have allowed for earlier detection of CF in asymptomatic individuals, the quantitative pilocarpine iontophoresis sweat test remains the gold standard for the diagnosis of CF. The sweat test, developed by Lewis Gibson and Robert Cooke in 1959, (9) specifically measures the amount of chloride in a person’s sweat. Sweat chloride testing should be performed as soon as possible after a positive NBS result. It can be performed as early as 48 hours after birth (as sweat sodium levels are transiently elevated in the first 24 hours), (26) but should be undertaken as soon as possible after 10 days of age and ideally by 4 weeks of age. Infants should weigh more than 2 kg or be corrected to 36 weeks gestation to increase the likelihood of adequate sweat collection. (24) Infants with meconium ileus and any infant or child with symptoms suggestive of cystic fibrosis such as recurrent bacterial respiratory infections and/or failure to thrive should receive sweat chloride testing regardless of age or NBS results. Any abnormal sweat test result should be repeated on a separate date, or confirmed with genetic testing.
Results from sweat chloride testing can be categorized into diagnostic, intermediate, and unlikely. Diagnostic sweat chloride values are ≥60 mmol/L, and require a confirmatory second sweat test or 2 identified CF-causing genetic variants to make the diagnosis. Intermediate values are between 30-59 mmol/L, and sweat chloride testing should be repeated periodically in these individuals and further evaluation at a CF center should be considered. A diagnosis of CF can still be made in an individual with an intermediate value if the individual has 2 identified CF-causing genetic variants. Individuals with an intermediate sweat chloride value of 30-59 mmol/L and 0-1 CF-causing genetic variants may be diagnosed with a CFTR-related disorder depending on clinical presentation and family history. Individuals with sweat chloride values <30 are unlikely to have CF, but if 2 CF-causing genetic variants are identified, they should still be diagnosed with CF. (24)
In conjunction with sweat testing, genetic testing is now widely available to help confirm a diagnosis of CF, particularly for cases with intermediate sweat chloride values. Identifying an individual’s specific CF-causing variants is needed for prescribing CFTR modulator therapies, which are approved for particular CFTR variants. A diagnosis by genotype can be made with the identification of 2 known pathogenic variants on separate chromosomes. Most individuals with CF can be diagnosed through commercial laboratories, which test for the most common CFTR variants, (27) but complete sequencing of the CFTR gene may be necessary to help confirm diagnosis in individuals with clinically atypical presentations. (16) Clinical information related to specific CFTR variants can be found online (www.cftr2.org).
The era of genetic testing has expanded our understanding of CFTR dysfunction, but has also added complexity to the diagnosis of CF, as there are individuals with CF phenotypes without known CF-causing mutations, as well as individuals with detected mutations who remain asymptomatic. The limitations of sweat chloride testing and genetic testing may require performing both tests in selected patients where there is strong clinical suspicion of CF. (28)
CF Management
In the United States, individuals with CF should be evaluated at a minimum of quarterly in a CFF-accredited care center, (29) which can provide interdisciplinary, patient/family-centered care. Infants under 6 months of age should be evaluated monthly and then every 1-2 months in the second six months of life (20) These regular visits allow for education surrounding airway clearance methods, infection prevention, monitoring of age-appropriate growth and weight gain, and in older children, the assessment of lung function.
Therapies to Maintain Optimal Lung Health
CF is characterized by viscous airway secretions that lead to chronic mucous obstruction, inflammation, and recurrent infections that result in long term damage to the airways (bronchiectasis) and lung parenchyma. (16) Chronic cough and sputum production are characteristic symptoms. Management of respiratory symptoms focuses on maintaining lung function and preventing the development of bronchiectasis and parenchymal destruction. (30) In addition to encouraging a smoke-free environment, children with CF are often prescribed several therapies, which will likely be lifelong and should be initiated shortly after diagnosis.
Clearance of Airway Secretions
A critical aspect of maintaining lung health is airway clearance therapy (ACT). By removing airway mucus, ACT helps to decrease the respiratory bacterial load along with irritants, leading to improved gas exchange and a decrease in airway obstruction. (16) Twice daily ACT as maintenance is recommended for all patients with CF, regardless of symptoms or disease severity, and is increased in frequency during acute CF pulmonary exacerbations (PEx). (31) Commonly used ACT modalities include manual percussion, positive expiratory pressure devices, and high-frequency chest wall oscillation (achieved through an inflatable vest that performs chest physical therapy by vibrating at a high frequency). No form of ACT has been demonstrated to be superior to any other form, and ACT choice should be personalized. Exercise should be encouraged as an adjunctive therapy, but should not be used as a substitute for airway clearance. (31) Nebulized agents that thin the viscous mucus of CF are commonly used with ACT, and include recombinant human rhDNase (dornase alfa/Pulmozyme) and hypertonic saline.
Chronic Airway Infections
In addition to patient education and infection control measures, aggressive management of chronic airway infections has been shown to prevent lung function decline. (32) Management includes frequent respiratory cultures (oropharyngeal or sputum), including surveilling for Staphylococcus aureus (particularly methicillin-resistant S. aureus) and Pseudomonas aeruginosa. (16) Receiving microbiology laboratories should be made aware of the patient’s CF diagnosis in order to assess for the respiratory pathogens commonly seen in CF. The initial acquisition of P. aeruginosa is typically treated with anti-pseudomonal antibiotics such as nebulized tobramycin (TOBI nebs) in an attempt to achieve eradication. (33) Nebulized antibiotics such as tobramycin or aztreonam (Cayston) can also be used as suppressive therapy for individuals with chronic infection or colonization with P. aeruginosa and/or other gram-negative organisms. This suppressive therapy is administered every other month to decrease the risk of antibiotic resistance. Other organisms, including Burkholderia cepacia complex, nontuberculous mycobacteria (Mycobacterium avium complex and Mycobacterium abscessus) and fungal pathogens (Aspergillus fumigatus) are also monitored, as they can have significant impact on CF lung disease. Of note, individuals with CF are also prone to developing a hypersensitivity reaction to Aspergillus, known as allergic bronchopulmonary aspergillosis or ABPA, which can affect lung function and requires management with corticosteroid therapy. (34)
Chronic Airway Inflammation
Cystic fibrosis lung disease is caused by a combination of infection and inflammation. The routine use of oral or inhaled corticosteroids in CF is not indicated unless used for another inflammatory comorbidity such as asthma or ABPA. (30) Chronic airway inflammation is managed with either high-dose ibuprofen or azithromycin. (35) Although ibuprofen has proven benefits in CF, the risk of gastrointestinal bleeding and need for monitoring serum levels has limited its use. (36) Azithromycin has been demonstrated to result in improved lung function and a reduced number of PEx, and is typically given orally three times per week. (37) However, there is concern that individuals with unrecognized mycobacterial infections receiving azithromycin long-term may develop resistance; screening using mycobacterial cultures is recommended before initiating treatment. (30)
Therapies to Maintain Optimal Nutritional Status
Decline in pulmonary status is the hallmark of CF, however poor growth is one of its earliest manifestations. The combined effects of decreased intake, malabsorption and increased metabolic demands contribute to the poor growth seen as early as infancy. Malnutrition has been associated with increased morbidity and mortality in CF. (38) CFF guidelines recommend that all children achieve a weight-for-length at or above the 50th percentile by 2 years old, and that all children and adolescents aged 2-20 years maintain a body mass index at or above the 50th percentile. (20)(39) Education about the role of enteral tube feeding in optimizing nutritional status should be provided to caregivers and patients throughout their lifetime. (40) Infants with CF should receive breastmilk if possible, and otherwise should receive standard infant formula (rather than hydrolyzed protein formulas). (20) Infants under 2 years old should be supplemented with table salt, up to ¼ teaspoon per day by 6 months of age, (20) due to ongoing salt losses. Infants with CF should have their fecal elastase measured after diagnosis to assess pancreatic functional status, as 85% of individuals with CF are exocrine pancreatic insufficient (fecal elastase <200 ug/g). (2) Pancreatic insufficiency leads to malabsorption, which presents as bulky, malodorous stools, malnutrition, and ultimately failure to thrive.
Pancreatic Enzyme Replacement Therapy
Pancreatic enzyme replacement therapy (PERT) should be initiated in those with a diagnostic fecal elastase or two CFTR variants associated with pancreatic insufficiency as well as those with unequivocal signs or symptoms of malabsorption. Individuals who have exocrine pancreatic insufficiency require enzyme replacement with every meal, snack, and enteral tube feeding, ranging from 2,000 to 2,500 units/kg of lipase per meal, to a maximum of 10,000 units/kg/day. (29)(41) Immobilized lipase cartridges may be used for patients on continuous enteral tube feeding to help hydrolyze fats in enteral formulas. Exceeding recommended dosages generally does not result in improved nutrient absorption, and supratherapeutic dosing is associated with fibrosing colonopathy, an uncommon complication characterized by foreshortening and strictures of the right colon. (42) Individuals with CF can achieve age-appropriate growth with optimized PERT. (43)
Fat-Soluble Vitamin Replacement Therapy
Pancreatic insufficiency results in malabsorption of fat and associated fat-soluble vitamins A, D, E, and K. Vitamin A deficiency can be associated with night blindness and ocular xerosis, as well as dermatological manifestations, such as follicular hyperkeratosis. (16) Vitamin D deficiency may result in rickets, osteopenia, and osteoporosis, which can result in fractures; recommendations for individuals with CF specify maintaining a goal of a serum 25-hydroxyvitamin D level of at least 30 ng/ml. (44) Vitamin E deficiency may result in peripheral neuropathy, myopathy, and hemolysis while Vitamin K deficiency is associated with coagulopathy and may also contribute to bone disease. (16) Supplemental vitamin therapy should begin after diagnosis and annual monitoring of serum Vitamin A, D, and E levels by CF providers is recommended. (20)
CFTR Modulator Therapies
CFTR modulators are the first therapies to target the basic defect in CF by directly acting on the CFTR protein. They are categorized into 3 types: potentiators, correctors, and amplifiers. (45) Ivacaftor (Kalydeco), the first approved modulator therapy, is a potentiator, which helps improve chloride flow through the CFTR protein at the cell surface for patients with Class III-V mutations. Correctors, such as lumacaftor and tezacaftor, help the CFTR protein to form correctly to allow the protein to move, or traffic, to the cell surface. When added to potentiators, correctors such as lumacaftor/ivacaftor (Orkambi) or tezacaftor/ivacaftor (Symdeko) work to improve the amount of protein that reaches the cell surface for patients with Class II mutations. Kalydeco is currently Federal Drug Administration (FDA)-approved for individuals aged ≥6 months, Orkambi for those aged ≥2 years with homozygous F508del variants, and Symdeko for those aged ≥6 years with homozygous F508del or several other specific CF-variants.
In 2019, the FDA approved the use of a triple-combination therapy, elexacaftor/tezacaftor/ivacaftor (Trikafta), for individuals 12 and older with at least one F508del variant. This new therapy will allow nearly 90% of individuals with CF to have a highly effective therapy for the underlying cause of their disease. Trikafta has been shown in clinical trials to show dramatic improvement in key measures of disease, including increased lung function, reduced PEx, decreased sweat chloride values, increased BMI and improved patient-reported quality of life. Amplifiers are expected to increase the amount of CFTR protein that a cell makes, but are under development and not currently available clinically. (46)
Diagnosis and Management of Common Pulmonary and Extrapulmonary Complications of CF
Pulmonary exacerbation
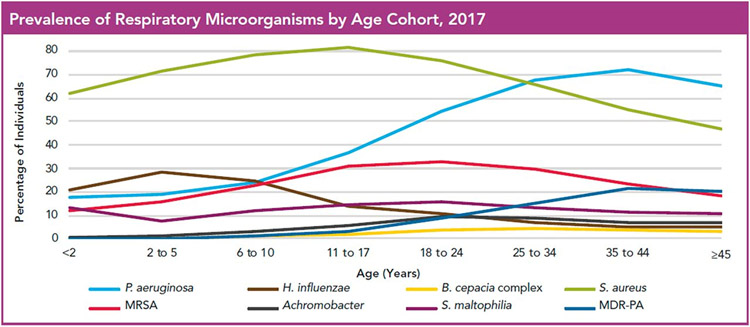
One of the most common complications of CF lung disease is episodic acute worsening of symptoms, referred to as pulmonary exacerbations (PEx). PEx are characterized by increased respiratory symptoms, including coughing, sputum production, and/or wheezing, a decline in pulmonary function measures (specifically FEV1), fatigue, decreased appetite, and weight loss. (47) Fevers are not commonly seen with PEx. (16) The frequency of PEx varies among individuals, but contributes to the long-term lung function decline of most people with CF. Treatment typically includes antibiotics and increased frequency of ACT to help clear secretions from the airways. Antibiotic therapy and mode of delivery (enteral, inhaled, and/or intravenous) is dictated by the severity of the exacerbation and previous/current respiratory culture results (Figure 4).
Figure 4.
Prevalence of bacterial pathogens in cystic fibrosis. MDR-PA=multidrug-resistant Pseudomonas aeruginosa, MRSA=methicillin-resistant Staphylococcus aureus. Reprinted from: Cystic Fibrosis Foundation Patient Registry © 2017 Cystic Fibrosis Foundation.
Hemoptysis
Hemoptysis is reported to occur in 3% of individuals with CF annually. (2) While often seen with severe lung disease, it can also be a manifestation of a PEx. (48) CF-related hemoptysis is most commonly a result of chronic infection and inflammation, leading to erosion of hypertrophied bronchial arteries into the airways. (49) Vitamin K deficiency, either from malabsorption or liver disease, can contribute to hemoptysis. Managing scant to moderate hemoptysis (≤240 mL) includes evaluation, likely antibiotic therapy for PEx management, and consideration of limiting certain exacerbating therapies, such as ibuprofen, hypertonic saline, DNAse, and ACT. (48) Massive hemoptysis (>240 mL) is considered life-threatening and management includes appropriate stabilization and discontinuation of anti-inflammatory and airway clearance measures. The treatment for massive hemoptysis or significant recurrent hemoptysis is bronchial artery embolization, if the site of bleeding can be identified. (48)
Pneumothorax
Pneumothoraces occur secondary to air trapping. High alveolar pressure forces air into the lower pressure interstitial spaces, leading to air leak into the pleural space, resulting in symptoms of acute chest pain and dyspnea. (16) The prevalence of having at least one pneumothorax is 3.4% among individuals with CF. (50) Pneumothoraces are more likely in adults and those with advanced lung disease. (50) The initial diagnostic test is chest radiography, but computer tomography may be required to define the extent of a pneumothorax in severe lung disease. (16) Small pneumothoraces may be observed or treated with needle aspiration, but large pneumothoraces require chest tube placement and hospitalization. (48) Pleurodesis is an option for recurrent pneumothoraces, but may complicate later lung transplantation. Following development of a pneumothorax, individuals should refrain from devices that use positive pressure (including vest therapy) as well as pulmonary function testing for at least two weeks, as these can hinder the resolution of the pneumothorax or lead to recurrence. (48)
Chronic Rhinosinusitis and Nasal Polyposis
The epithelium of the sinuses also possess defective CFTR protein, so chronic pan-sinus disease is almost universal among individuals with CF. (51) In contrast, the prevalence of nasal polyposis is more variable and increases with age. (52) Both rhinosinusitis and nasal polyposis result from mucous obstruction of the sinus ostia. Clinical presentations may include chronic headache and facial pressure. Long-term findings may include broadening of the nasal bridge and septal deformation from chronic nasal obstruction. (16) Medical management includes saline nasal irrigation to aid with mucus clearance and nasal steroids to decrease inflammation. Surgical treatment for severe and recurrent disease can improve mucus clearance, but may not necessarily improve lung function. (53)(54)
Distal Intestinal Obstruction Syndrome
Distal Intestinal Obstruction Syndrome (DIOS) is a common gastrointestinal complication in CF. It presents as partial or complete small bowel obstruction secondary to viscous fecal impaction in the distal intestine. Clinical manifestations include abdominal pain and distension, emesis, and a history of decreased stooling. Pathophysiology may be related to CFTR-dependent bile acid secretion and uptake within the distal ileum. (55) Known risk factors for DIOS include dehydration, dietary changes, suboptimal fat absorption (ie inadequate PERT dosing), immobilization, bacterial overgrowth, a prior episode of DIOS, and constipating medications. (55)(56) Differential diagnosis for DIOS includes intussusception, constipation, intestinal adhesions from prior abdominal surgery, volvulus, inflammatory bowel disease and appendicitis. (16)(56) Abdominal radiography, history, and physical examination are usually sufficient to make the diagnosis, but other causes of bowel obstruction should be considered. Management typically includes rehydration and osmotic laxative therapy. More severe cases may require inpatient admission, intravenous fluids, complete bowel rest, and the use of large volumes of polyethylene glycol. Near complete obstruction may require sodium meglumine diatrizoate (Gastrografin) enemas with retrograde lavage and visualization of the terminal ileum by an experienced radiologist. With early diagnosis and implementation of appropriate medical management, surgical interventions are generally not required for DIOS. (16)
Cystic Fibrosis-Related Diabetes Mellitus
Cystic fibrosis leads to disruption of both the exocrine and endocrine functions of the pancreas. Cystic Fibrosis-Related Diabetes Mellitus (CFRD) results from ongoing obstructive damage to the pancreas from thick secretions, which in turn results in fatty infiltration of the pancreas and islet cell destruction. (16) CFRD typically presents after the first decade, and occurs in up to 20% of adolescents and at least 50% of adults with CF. (57) CFRD is typically diagnosed in patients with pancreatic insufficiency and has been associated with increased morbidity and mortality through worse nutritional status and decreased lung function. (58)(59). CFRD is often asymptomatic, and unexplained decreases in growth, weight, or lung function may be related to occult CFRD. Annual screening conducted via 2-hour oral glucose tolerance test should be initiated at age 10 years. (60) Studies have found that fasting levels of hyperglycemia or increased levels of glycated hemoglobin are not sufficiently sensitive for the diagnosis of CFRD, therefore HbA1c levels should not be used for screening for CFRD. (61) However, HbA1c levels can be used to monitor glucose control in individuals with CFRD. While microvascular complications such as retinopathy, microalbuminuria, and neuropathy may occur with CFRD, similar to other forms of diabetes, ketoacidosis is uncommon. (16)(60) The management of CFRD is focused on glycemic control through insulin therapy; oral anti-hyperglycemic agents are not as effective as insulin in improving long-term outcomes. Nutritional management remains focused on maintaining a high calorie diet while attempting to limit intake of processed carbohydrates to avoid hyperglycemia.
Cystic Fibrosis Liver Disease
Liver disease accounts for 3.4% of overall CF mortality. (2) Approximately 3% of individuals develop CF-related cirrhosis (primarily individuals with pancreatic insufficiency), with a median age of diagnosis of 10 years. (62)(63) Clinical manifestations include cholestasis, cholelithiasis, cirrhosis, portal hypertension, and in severe cases, end-stage liver disease. The pathophysiology is thought to be related to the role of CFTR in promoting bile flow, with abnormal flow leading to cholestasis and biliary fibrosis. (64) Males as well as carriers of Alpha-1 antitrypsin Z allele are at increased risk of developing advanced liver disease. (64)(65) Periodic screening and evaluation for Cystic Fibrosis Liver Disease (CFLD) is critical, as many individuals remain asymptomatic even with advanced cirrhosis. Annual screening of individuals should include assessment of liver function (AST, ALT, and GGT), an ultrasound evaluation and/or evaluation by a gastroenterologist for other causes of liver disease, as indicated. The presence of CFLD should be considered with at least 2 of the following: (1) abnormal physical examination findings such as hepatosplenomegaly, (2) abnormalities of liver function test results above normal reference ranges on at least 3 consecutive determinations during a 12-month period, (3) ultrasound with Doppler evidence of abnormal liver echotexture or portal hypertension, or (4) tissue biopsy has ruled out other causes of liver disease. (66 While there are currently no proven therapies to prevent development or progression of CF-related cirrhosis, ursodiol is frequently used in the management of hyperbilirubinemia in CFLD.
Bone Disease
Another complication of CF is cystic fibrosis-related bone disease, which manifests as low bone density and increased rates of fractures. Poor bone health is likely a result of a combination of factors including malabsorption of fat-soluble vitamins such as vitamin D and K, in addition to poor nutritional status and chronic lung inflammation. Prevention includes optimizing nutritional status as well as encouraging weight-bearing exercise, while dual X-ray absorptiometry is used for screening at-risk patients. Treatment includes aggressive management of potential pulmonary or endocrine co-morbidities, and may include bisphosphonates for those with severe osteopenia. (67)
Depression/Anxiety
Living with a chronic illness, such as CF, can be both challenging and isolating, placing individuals with CF at higher risk for mental health issues. Approximately 15% of all individuals with CF report having either an anxiety disorder or depression, and 41% of individuals with CF report having both conditions. (2) It is recommended that all children with CF who are 7-11 years old be clinically assessed for depression and anxiety when a caregiver reports clinically elevated symptoms of depression or anxiety, or when there is significant concern for the child exhibiting symptoms of depression or anxiety. Annual screening for depression and anxiety in individuals with CF should begin at 12 years old using the PHQ-9 and GAD-7, respectively. Annual screening is also recommended for caregivers of children with CF. (68) Early identification of mental health difficulties is critical to helping ensure individuals receive referrals to appropriate mental health services in order to receive treatment and maintain their overall health and quality of life.
Preventative Care
Children with CF should receive routine well-child care according to the American Academy of Pediatrics guidelines, including all vaccinations. Annual influenza vaccination is recommend for children ≥ 6 months, as well as for all household members. The use of palivizumab should be considered in all children with CF under 2 years old as prophylaxis against respiratory syncytial virus. (20) Providers should encourage a smoke-free environment for all children with CF and caregivers should be informed of the health effects associated with second-hand smoke exposure.
Transition from Pediatric to Adult CF Care Centers
As most individuals with CF are living into adulthood, the topic of transition from pediatric care centers to adult care centers will continue to be important for patients and their families. One step identified to improve the transition process includes introducing the ideas of self-care skills and transition to adult care during the teenage years. Readiness assessments for self-management skills, including the Transition Readiness Assessment Questionnaire (TRAQ) (69) and transition toolsets have been used to assess patients’ readiness to transition to more independent management of their disease. These discussions should also include educational/vocational plans, behavioral risk counseling, screening for depression/anxiety and reproductive health and family planning. Finally, implementation of formal transition-focused visits may be helpful to introduce patients to their new care team in a familiar clinic setting. (70)
Lung Transplantation
Pulmonary disease continues to account for almost 60% of CF-related mortality. (2) Lung transplantation is a surgical option that can extend and improve the quality of life of individuals with CF, but involves extensive evaluation prior to transplant as well as adherence with therapies and lifestyle recommendations to optimize the success of transplanted lungs. Lung transplant confers a survival benefit, (71) with recent reports indicating that individuals with CF are experiencing 9.5 years median survival following lung transplantation. (72) CF providers are recommended to discuss disease trajectory and treatment options including risk and benefits of lung transplantation with individuals with advanced lung disease, and referral to lung transplant centers should be discussed with individuals whose FEV1 <50% predicted or is rapidly declining (>20% decline in FEV1 over 12 months). (73) Overall, 6.3% of CF transplants performed in 2018 were among individuals younger than age 18, (2) and criteria for referral are similar to those for adults over 18 years of age. (73) Discussion about lung transplantation may be viewed as another transition by individuals with CF, and should be facilitated by education, communication and support for the individual and their family. (73)
Conclusion
Individuals affected by CF are living longer and healthier lives, and survival is expected to continue to improve with earlier diagnosis through routine newborn screening, promulgation of evidence-based guidelines, interdisciplinary care centers, and the use of mutation-specific modulator therapies. The primary goals of treatment remain optimization of pulmonary function and nutritional status, and incremental advances in these therapies have had a profound effect on health and quality of life for individuals with CF. Building partnerships with individuals and their families requires recognition of the emotional, social and financial effects of this lifelong disease and effective communication and coordination among primary care physicians and CF care center teams.
Practice Gap.
In light of the significant increase in the median age of survival for individuals with cystic fibrosis (CF), both pediatric and adult providers should be familiar with the current recommendations related to optimization of lung health and nutritional status.
Providers should also be aware of the development of modulator therapies that target the different basic genetic defects of the disease, which have allowed for personalized therapies that promise continued improvement in outcomes. As of 2019, approximately 90% of individuals with CF have mutations that would benefit from modulator therapy.
Clinicians should be familiar with the clinical presentation, diagnosis, and current management of CF as well as the more common disease-related morbidities.
Objectives.
After completing this article, readers should be able to:
Describe the common clinical manifestations of CF, as well as the laboratory and genetic studies needed to diagnose CF.
Recognize the most common presentations of cystic fibrosis-related morbidities.
Describe the current recommendations for long-term maintenance of optimal lung health, nutritional status, and other involved organ systems in children with CF.
Summary.
On the basis of consensus, (22) the diagnosis of cystic fibrosis (CF) is based on (1) a positive newborn screening; (2) clinical features consistent with CF (the presence of one or more characteristic phenotypic features of chronic, recurrent sinopulmonary disease, nutritional and gastrointestinal abnormalities, male urogenital abnormalities (e.g., absence of the vas deferens), and salt depletion syndromes, or (3) a positive family history of CF, and laboratory demonstrated evidence of CFTR dysfunction, such as elevation of sweat chloride concentration (≥60mmol/L)
On the basis of consensus, (50) annual oral glucose tolerance tests are recommended for people with CF older than 9 years to screen for CF-related diabetes mellitus.
On the basis of research evidence, (25) the long-term therapies to maintain optimal lung health for children and adults with CF include control of chronic airways infection and inflammation, clearance of mucous secretions, and, where clinically applicable, treatments aimed at the basic CF genetic defect.
Abbreviations:
- ACT
airway clearance therapy
- ABPA
allergic bronchopulmonary aspergillosis
- CF
cystic fibrosis
- CFF
Cystic Fibrosis Foundation
- CFLD
cystic fibrosis liver disease
- CFRD
cystic fibrosis-related diabetes mellitus
- CFTR
cystic fibrosis transmembrane conductance regulator
- DIOS
distal intestinal obstruction syndrome
- IRT
immunoreactive trypsinogen
- NBS
newborn screening
- PERT
pancreatic enzyme replacement therapy
- PEx
pulmonary exacerbations
Footnotes
Author Disclosure: Drs. Dickinson and Collaco have no financial disclosures relevant to this article. This commentary does not contain a discussion of an unapproved/investigative use of commercial products/devices.
References
- 1.Andersen DH. Cystic fibrosis of the pancreas and its relation to celiac disease. Am J Dis Child. 1938;56(2):344–399 [Google Scholar]
- 2.Cystic Fibrosis Foundation Patient Registry. 2018 Patient Registry Annual Data Report. Bethesda, Maryland. Bethesda, Maryland. [Google Scholar]
- 3.Hamosh A, FitzSimmons SC, Macek M, Knowles MR, Rosenstein BJ, Cutting GR. Comparison of the clinical manifestations of cystic fibrosis in black and white patients. J Pediatr. 1998;132(2):255–259 [DOI] [PubMed] [Google Scholar]
- 4.Rohlfs EM, Zhou Z, Heim RA, et al. Cystic fibrosis carrier testing in an ethnically diverse US population. Clin Chem. 2011;57(6):841–848 [DOI] [PubMed] [Google Scholar]
- 5.Carrier Testing for Cystic Fibrosis. Cystic Fibrosis Foundation.[internet] Https://www.cff.org/What-is-CF/Testing/Carrier-Testing-for-Cystic-Fibrosis/. Accessed August 1, 2019 [Google Scholar]
- 6.Scotet V, Duguépéroux I, Saliou P, et al. Evidence for decline in the incidence of cystic fibrosis: A 35-year observational study in Brittany, France. Orphanet J Rare Dis. 2012;7:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wagener J, Zemanick E, Sontag M. Newborn screening for cystic fibrosis. Curr Opin Pediatr. 2012;24(3):329–335 [DOI] [PubMed] [Google Scholar]
- 8.di Sant’agnese P, Darling RC, Perera GA, Shea E. Abnormal Electrolyte Composition of sweat in cystic fibrosis of the pancreas: clinical significance and relationship to the disease. Pediatrics. 1953;12(5):549–563 [PubMed] [Google Scholar]
- 9.Gibson LE, Cooke RE. A Test for Concentration of Electrolytes in Sweat in Cystic Fibrosis of the Pancreas Utilizing Pilocarpine by Iontophoresis. Pediatrics. 1959;23(3):545–549 [PubMed] [Google Scholar]
- 10.CFTR2@Johns Hopkins. Home page [Internet]. Http://cftr2.org/. Updated August 2016. Accessed July 31, 2019 [Google Scholar]
- 11.Dorfman R, for the CFMD/CFTR1 Team. Cystic fibrosis mutation database [Internet]. Http://www.genet.sickkids.on.ca/cftr/app. Updated April 2011. Accessed July 2017, 2019 [Google Scholar]
- 12.McCague AF, Raraigh KS, Pellicore MJ, et al. Correlating Cystic Fibrosis Transmembrane Conductance Regulator Function with Clinical Features to Inform Precision Treatment of Cystic Fibrosis. Am J Respir Crit Care Med. 2019;199(9):1116–1126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Irish MS, Ragi JM, Karamanoukian H, Borowitz DS, Schmidt D, Glick PL. Prenatal diagnosis of the fetus with cystic fibrosis and meconium ileus. Pediatr Surg Int Berl. 1997;12(5-6):434–436 [DOI] [PubMed] [Google Scholar]
- 14.De Oronzo MA. Hyperechogenic fetal bowel: An ultrasonographic marker for adverse fetal and neonatal outcome? J Prenat Med. 2011;5(1):9–13 [PMC free article] [PubMed] [Google Scholar]
- 15.Rentea RM, St Peter SD. Pediatric Rectal Prolapse. Clin Colon Rectal Surg. 2018;31(2):108–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paranjape SM, Mogayzel PJ. Cystic Fibrosis. Pediatr Rev.2014;35(5):194–205 [DOI] [PubMed] [Google Scholar]
- 17.Sabharwal S Gastrointestinal Manifestations of Cystic Fibrosis. Gastroenterol Hepatol. 2016;12(1):43–47 [PMC free article] [PubMed] [Google Scholar]
- 18.Shwachman H, Lebenthal E, Khaw KT. Recurrent acute pancreatitis in patients with cystic fibrosis with normal pancreatic enzymes. Pediatrics. 1975;55(1);86–95. [PubMed] [Google Scholar]
- 19.Boyd JM, Mehta A, Murphy DJ. Fertility and pregnancy outcomes in men and women with cystic fibrosis in the United Kingdom. Hum Reprod. 2004;19(10):2238–2243 [DOI] [PubMed] [Google Scholar]
- 20.Borowitz D, Robinson KA, Rosenfeld M, et al. Cystic Fibrosis Foundation Evidence-Based Guidelines for Management of Infants with Cystic Fibrosis. J Pediatr. 2009;155(6, Supplement):S73–S93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rock MJ, Mischler EH, Farrell PM, Bruns WT, Hassemer DJ, Laessig RH. Immunoreactive trypsinogen screening for cystic fibrosis: Characterization of infants with a false-positive screening test. Pediatr Pulmonol. 1989;6(1):42–48 [DOI] [PubMed] [Google Scholar]
- 22.Ross LF. Newborn Screening for Cystic Fibrosis: A Lesson in Public Health Disparities. J Pediatr. 2008;153(3):308–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rusakow LS, Abman SH, Sokol RJ, Seltzer W, Hammond KB, Accurso FJ. Immunoreactive trypsinogen levels in infants with cystic fibrosis complicated the quantitative pilocarpine iontophoresis sweat test by meconium ileus. Screening. 1993;2(1):13–17 [Google Scholar]
- 24.Farrell PM, White TB, Ren CL, et al. Diagnosis of Cystic Fibrosis: Consensus Guidelines from the Cystic Fibrosis Foundation. J Pediatr. 2017;181:S4–S15.e1 [DOI] [PubMed] [Google Scholar]
- 25.Farrell PM, Rosenstein BJ, White TB, et al. Guidelines for Diagnosis of Cystic Fibrosis in Newborns through Older Adults: Cystic Fibrosis Foundation Consensus Report. J Pediatr. 2008;153(2):S4–S14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hardy JD, Davison SH, Higgins MU, Polycarpou PN. Sweat test in the newborn period. Arch Dis Child. 1973;48(4):316–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Palomaki GE, Fitzsimmons SC, Haddow JE. Clinical sensitivity of prenatal screening for cystic fibrosis via CFTR carrier testing in a United States panethnic population. Genet Med. 2004;6(5):405–414 [DOI] [PubMed] [Google Scholar]
- 28.Mishra A, Greaves R, Massie J. The relevance of sweat testing for the diagnosis of cystic fibrosis in the genomic era. Clin Biochem Rev. 2005(26)4:135–153 [PMC free article] [PubMed] [Google Scholar]
- 29.Lahiri T, Hempstead SE, Brady C, et al. Clinical Practice Guidelines From the Cystic Fibrosis Foundation for Preschoolers With Cystic Fibrosis. Pediatrics. 2016:peds.2015-1784 [DOI] [PubMed] [Google Scholar]
- 30.Mogayzel PJ, Naureckas ET, Robinson KA, et al. Cystic Fibrosis Pulmonary Guidelines: Chronic Medications for the Maintenance of Lung Health. Am J Respir Crit Care Med. 2013;187(7):680–689 [DOI] [PubMed] [Google Scholar]
- 31.Flume PA, Robinson KA, O’Sullivan BP, et al. Cystic Fibrosis Pulmonary Guidelines: Airway Clearance Therapies. Respir CARE. 2009;54(4):16. [PubMed] [Google Scholar]
- 32.Cohen-Cymberknoh M, Showeyov D, Kerem E. Managing cystic fibrosis: strategies that increase life expectancy and improve quality of life. Am J Respir Crit Care Med. 2011;183(11):1463–1471 [DOI] [PubMed] [Google Scholar]
- 33.Mogayzel PJ, Naureckas ET, Robinson KA, et al. Cystic Fibrosis Foundation Pulmonary Guideline. Pharmacologic Approaches to Prevention and Eradication of Initial Pseudomonas aeruginosa Infection. Ann Am Thorac Soc. 2014;11(10):1640–1650 [DOI] [PubMed] [Google Scholar]
- 34.Stevens DA, Moss RB, Kurup VP, et al. Allergic bronchopulmonary aspergillosis in cystic fibrosis-state of the art: Cystic Fibrosis Foundation Consensus Conference. Clin Infect Dis. 2003(37):S225–64. [DOI] [PubMed] [Google Scholar]
- 35.Dinwiddie R Anti-inflammatory therapy in cystic fibrosis. J Cyst Fibros. 2005. (2):45–8. [DOI] [PubMed] [Google Scholar]
- 36.Lands LC, Stanojevic S. Oral non-steroidal anti-inflammatory drug therapy for lung disease in cystic fibrosis. Cochrane Database of Systematic Reviews 2016, Issue 4. Art. No.: CD001505. DOI: 10.1002/14651858.CD001505.pub4.29. [DOI] [PubMed] [Google Scholar]
- 37.Southern KW, Barker PM, Solis-Moya A, Patel L. Macrolide antibiotics for cystic fibrosis. Cochrane Database Syst Rev. 2004;(2) [DOI] [PubMed] [Google Scholar]
- 38.Corey M, McLaughlin FJ, Williams M, Levison H. A comparison of survival, growth, and pulmonary function in patients with cystic fibrosis in Boston and Toronto. J Clin Epidemiol. 1988;41(6):583–591. [DOI] [PubMed] [Google Scholar]
- 39.Stallings VA, Stark LJ, Robinson KA, Feranchak AP, Quinton H. Evidence-Based Practice Recommendations for Nutrition-Related Management of Children and Adults with Cystic Fibrosis and Pancreatic Insufficiency: Results of a Systematic Review. J Am Diet Assoc. 2008;108(5):832–839 [DOI] [PubMed] [Google Scholar]
- 40.Schwarzenberg SJ, Hempstead SE, McDonald CM, et al. Enteral tube feeding for individuals with cystic fibrosis: Cystic Fibrosis Foundation evidence-informed guidelines. J Cyst Fibros. 2016;15(6):724–735 [DOI] [PubMed] [Google Scholar]
- 41.Borowitz D, Grand R, Durie P et al. Use of pancreatic enzyme supplements for patients with cystic fibrosis in the context of fibrosing colonopathy. J Pediatr. 1995;127(5):681–684 [DOI] [PubMed] [Google Scholar]
- 42.Reichard KW, Vinocur CD, Franco M et al. Fibrosing colonopathy in children with cystic fibrosis. J Pediatr Surg. 1997;32(2):237–241 [DOI] [PubMed] [Google Scholar]
- 43.Schibli S, Durie P, Tullis E. Proper usage of pancreatic enzymes. Curr Opin Pulm Med. 2002;8(6):542–546. [DOI] [PubMed] [Google Scholar]
- 44.Tangpricha V, Kelly A, Stephenson A, et al. An Update on the Screening, Diagnosis, Management, and Treatment of Vitamin D Deficiency in Individuals with Cystic Fibrosis: Evidence-Based Recommendations from the Cystic Fibrosis Foundation. J Clin Endocrinol Metab. 2012;97(4):1082–1093 [DOI] [PubMed] [Google Scholar]
- 45.Grasemann H CFTR Modulator Therapy for Cystic Fibrosis. N Engl J Med. 2017;377(21):2085–2088 [DOI] [PubMed] [Google Scholar]
- 46.Middleton PG, Mall MA, Drevinek P et al. Elexacaftor-Tezacaftor-Ivacaftor for Cystic Fibrosis with a Single Phe508del Allele. N Engl J Med. 2019;381(19):1809–1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Flume PA, Mogayzel PJ, Robinson KA, et al. Cystic Fibrosis Pulmonary Guidelines: treatment of pulmonary exacerbations. Am J Respir Crit Care Med. 2009;180(9):802–808 [DOI] [PubMed] [Google Scholar]
- 48.Flume PA, Mogayzel PJ, Robinson KA, Rosenblatt RL, Quittell L, Marshall BC. Cystic Fibrosis Pulmonary Guidelines: pulmonary complications: hemoptysis and pneumothorax. Am J Respir Crit Care Med. 2010;182(3):298–306 [DOI] [PubMed] [Google Scholar]
- 49.Brinson GM, Noone PG, Mauro MA, et al. Bronchial Artery Embolization for the Treatment of Hemoptysis in Patients with Cystic Fibrosis. Am J Respir Crit Care Med. 1998;157(6):1951–1958 [DOI] [PubMed] [Google Scholar]
- 50.Flume PA, Strange C, Ye X, Ebeling M, Hulsey T, Clark LL. Pneumothorax in Cystic Fibrosis. Chest. 2005;128(2):720–728 [DOI] [PubMed] [Google Scholar]
- 51.Chaaban MR, Kejner A, Rowe SM, Woodworth BA. Cystic Fibrosis chronic rhinosinusitis: a comprehensive review. Am J Rhinol Allergy. 2013;27(5):387–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mainz JG, Koitschev A. Pathogenesis and Management of Nasal Polyposis in Cystic Fibrosis. Curr Allergy Asthma Rep. 2012;12(2):163–174 [PubMed] [Google Scholar]
- 53.Khalfoun S, Tumin D, Ghossein M, Lind M, Hayes D, Kirkby S. Improved Lung Function after Sinus Surgery in Cystic Fibrosis Patients with Moderate Obstruction. Otolaryngol Neck Surg. 2018;158(2):381–385 [DOI] [PubMed] [Google Scholar]
- 54.Osborn AJ, Leung R, Ratjen F, James AL. Effect of Endoscopic Sinus Surgery on Pulmonary Function and Microbial Pathogens in a Pediatric Population With Cystic Fibrosis. Arch Otolaryngol Neck Surg. 2011;137(6):542. [DOI] [PubMed] [Google Scholar]
- 55.Colombo C, Ellemunter H, Houwen R, Munck A, Taylor C, Wilschanski M. Guidelines for the diagnosis and management of distal intestinal obstruction syndrome in cystic fibrosis patients. J Cyst Fibros. 2011;10:S24–S28 [DOI] [PubMed] [Google Scholar]
- 56.Rowe SM, Hoover W, Solomon GM, Sorscher EJ. (2016). Murray & Nadel's Textbook of Respiratory Medicine. 6th ed.,Elsevier, 2016 [Google Scholar]
- 57.Kelly A, Moran A. Update on cystic fibrosis-related diabetes. J Cyst Fibros. 2013;12(4):318–331 [DOI] [PubMed] [Google Scholar]
- 58.Moran A, Dunitz J, Nathan B, Saeed A, Holme B, Thomas W. Cystic Fibrosis–Related Diabetes: Current Trends in Prevalence, Incidence, and Mortality. Diabetes Care. 2009;32(9):1626–1631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Marshall BC, Butler SM, Stoddard M, Moran AM, Liou TG, Morgan WJ. Epidemiology of cystic fibrosis-related diabetes. J Pediatr. 2005;146(5):681–687 [DOI] [PubMed] [Google Scholar]
- 60.Moran A, Brunzell C, Cohen RC, Katz M, Marshall BC, Onady G, et al. Clinical care guidelines for cystic fibrosis-related diabetes: a position statement of the American Diabetes Association and a clinical practice guideline of the Cystic Fibrosis Foundation, endorsed by the Pediatric Endocrine Society. Diabetes Care. 2010;33(12):2697–708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lanng S, Hansen A, Thorsteinsson B, Nerup J set)rn, Koch C. Glucose tolerance in patients with cystic fibrosis: Five year prospective study. BMJ. 1995;311(7006):655–659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Colombo C, Battezzati PM, Crosignani A, et al. Liver disease in cystic fibrosis: A prospective study on incidence, risk factors, and outcome. Hepatology. 2002;36(6):1374–1382 [DOI] [PubMed] [Google Scholar]
- 63.Debray D, Kelly D, Houwen R, Strandvik B, Colombo C. Best practice guidance for the diagnosis and management of cystic fibrosis-associated liver disease. J Cyst Fibros. 2011;10:S29–S36 [DOI] [PubMed] [Google Scholar]
- 64.Sokol RJ, Durie PR, Group CFFHDC. Recommendations for Management of Liver and Biliary Tract Disease in Cystic Fibrosis. J Pediatr Gastroenterol Nutr. 1999;28:S1. [DOI] [PubMed] [Google Scholar]
- 65.Bartlett JR, Friedman KJ, Ling SC, et al. Genetic modifiers of liver disease in cystic fibrosis. JAMA. 2009;302(10):1076–1083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Van de Pappel IP, Bertolini A, Jonker JW, Bodewes FA et al. Diagnosis, follow-up and treatment of cystic fibrosis-related liver disease. Curr Opin Pulm Med. 2017:23(6):562–569. [DOI] [PubMed] [Google Scholar]
- 67.Aris RM, Merkel PA, Bachrach LK, et al. Guide to Bone Health and Disease in Cystic Fibrosis. J Clin Endocrinol Metab. 2005;90(3):1888–1896 [DOI] [PubMed] [Google Scholar]
- 68.Quittner AL, Abbott J, Georgiopoulos AM, et al. International Committee on Mental Health in Cystic Fibrosis: Cystic Fibrosis Foundation and European Cystic Fibrosis Society consensus statements for screening and treating depression and anxiety. Thorax. 2016;71(1):26–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wood DL, Sawicki GS, Miller MD, Smotherman C et al. The transition readiness assessment questionnaire (TRAQ): its factor structure, reliability, and validity. Acad Pediatr. 2014;14(4):415–422. [DOI] [PubMed] [Google Scholar]
- 70.McLaughlin SE, Diener-West M, Indurkhya A, Rubin H et al. Improving transition from pediatric to adult cystic fibrosis care: lessons from a national survey of current practices. Pediatrics. 2008;121(5):e1160–1166. [DOI] [PubMed] [Google Scholar]
- 71.Thabut G, Christie JD, Mal H, Fournier M et al. Survival benefit of lung transplant for cystic fibrosis since lung allocation score implementation. Am J Respir Crit Care Med. 2013;187(12):1355–1340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chambers DC, Cherik WS, Goldfarb SB, Heys D et al. The international thoracic organ transplant registry of the international society for heart and lung transplantation: thirty-fifth adult lung and heart-lung transplant report-2018: focuse theme: multiorgan transplantation. J Heart Lung Transplant. 2018;37(10):1169–1183 [DOI] [PubMed] [Google Scholar]
- 73.Ramos KJ, Smith PJ, McKone EF, Pilewski JM. Lung transplant referral for individuals with cystic fibrosis: Cystic Fibrosis Foundation consensus guidelines. J Cyst Fibros. 2019;18(3):321–333 [DOI] [PMC free article] [PubMed] [Google Scholar]