Key Points
Question
Is computerized, dynamic, posturography-assisted vestibular retraining associated with reduced disability in patients with stable unilateral vestibular deficits?
Findings
In a cohort study of 13 participants with stable unilateral vestibular deficits, participant-reported measures of disability improved after posturography-assisted vestibular retraining, especially for those with moderate to severe disability at baseline.
Meaning
This study suggests that posturography-assisted retraining shows promise as a treatment for patients with stable unilateral vestibular deficits, a group that frequently does not respond satisfactorily to other treatments.
Abstract
Importance
Individuals with persistent unilateral vestibular deficits experience loss of quality of life and increased risk of falling, and they have few well-supported options for effective treatment.
Objectives
To evaluate whether vestibular retraining using computerized dynamic posturography is associated with reduced participant-reported disability for patients with an objectively assessed unilateral peripheral vestibular deficit and to assess the feasibility of conducting a randomized clinical trial of vestibular retraining using computerized dynamic posturography.
Design, Setting, and Participants
This single-group cohort study was conducted from April 29 to July 23, 2021, in a tertiary neurotology clinic among 13 individuals with a stable unilateral vestibular deficit present for more than 6 months, confirmed with videonystagmography and vestibular evoked myogenic potential testing. Statistical analysis was performed from July 7, 2021, to January 25, 2022.
Interventions
Twelve twice-weekly sessions of posturography-assisted vestibular retraining with prescribed weight shifting tasks guided by an interactive display.
Main Outcomes and Measures
Change in scores on the Dizziness Handicap Inventory (DHI), the Activities-Specific Balance Confidence (ABC) Scale, and the Falls Efficacy Scale–International (FES-I), which participants completed before and after retraining to measure their perception of their disability. They also completed posturography measurements. Secondary outcomes included tolerability of the intervention and rate of completion of the full protocol.
Results
A total of 13 participants (8 men [62%]; median age, 51 years [range, 18-67 years]) were enrolled. All 13 participants completed the intervention and all follow-up. After treatment, the median changes in scores were −16 points (95% CI, −20 to 2) for the DHI, −9 (95% CI, −14 to 1) for the FES-I, and 11.9 (95% CI, 0-17.3) for the ABC Scale. Eight participants (62%) improved by greater than the minimum clinically important difference (MCID) for the DHI, whereas 4 (31%) exceeded the MCID for the ABC Scale, and 3 (23%) exceeded the MCID for the FES-I. Participants with moderate to severe disability at baseline (n = 7) had a larger magnitude of improvement in DHI scores than those with mild disability (n = 6) (−18 [95% CI, −78 to 2] vs −1 [95% CI, −8 to 16]). Six of the 7 patients (86%) with moderate to severe disability improved by greater than the MCID for DHI, wherease 4 of 7 patients (57%) improved by greater than the MCID for the ABC Scale, and 3 of 7 patients (43%) improved by greater than the MCID for the FES-I.
Conclusions and Relevance
This cohort study suggests that computerized, dynamic posturography-assisted retraining was associated with clinically meaningful improvements in participant-reported disability among those with stable unilateral vestibular deficit and moderate to severe disability. Further studies should compare posturography-assisted vestibular retraining with conventional physical therapy rehabilitation techniques.
Trial Registration
ClinicalTrials.gov Identifier: NCT04875013
This cohort study evaluates whether vestibular retraining using computerized dynamic posturography is associated with reduced participant-reported disability for patients with an objectively assessed unilateral peripheral vestibular deficit.
Introduction
Balance disorders are common, with a lifetime prevalence of 17% to 30%,1 and are associated with an elevated risk of falling.2 They can lead to anxiety, reduced independence, and withdrawal from normal activities.3,4
Vestibular rehabilitation exercises, which involve prescribed movement of the head and eyes intended to promote compensation for vestibular deficits, were first described in 1946.5 Similar exercises are still commonly prescribed for patients with vestibular symptoms of diverse causes and have consistently been shown to be better than no treatment6,7; however, studies of vestibular rehabilitation exercises have had variable results. Some studies have found effectiveness as high as 92%,8 whereas in several other studies, 33% to 63% of patients reported no benefit associated with vestibular rehabilitation exercises.9,10,11
Most studies of rehabilitation interventions for vestibular deficits have looked at patient groups with diverse causes of vestibular symptoms, including Ménière disease and benign paroxysmal positional vertigo (BPPV), which are characterized by distinct natural histories and fluctuating symptoms. Studies that have included patients with fluctuating symptoms of vertigo caused by ongoing vestibular irritative pathologic characteristic may not be generalizable to patients with stable peripheral vestibular deficits.
Most patients with dizziness due to a peripheral vestibular deficit experience improvement in their symptoms, either spontaneously or with physical therapy or vestibular rehabilitation12; however, some experience persistent impairment.4 Patients with persistent symptoms of imbalance and evidence of a nonfluctuating unilateral vestibular deficit are left with few options to improve their vestibular capacity and quality of life.
Computerized dynamic posturography (CDP) was first described more 30 years ago,8,13 but it is still not commonly used clinically. Computerized dynamic posturography has been demonstrated to be effective at distingushing patients with dizziness from individuals without symptoms,14 and many clinicians recognize that CDP test protocols can provide useful objective measures of global balance function; however, there is a lack of consensus concerning the utility of CDP as a vestibular-specific assessment tool.15,16,17 Rehabilitation interventions using CDP are uncommon, although this modality has shown promise in a small number of studies for indications such as central vestibulopathy18 and Parkinson disease19,20 as well as for reducing fall risk in elderly individuals.19,21,22,23
The present study recruited patients who reported imbalance that affected their day-to-day activities and whose symptoms were present for greater than 6 months. Objective determination of a peripheral vestibular deficit was a requirement for eligibility. Patients were excluded if they demonstrated clinical or audiometric evidence of a fluctuating peripheral vestibulopathy such as a perilymphatic fistula, active Ménière disease, or otosyphilis. Participants consented to take part in 12 twice-weekly sessions of CDP-assisted vestibular retraining using real-time visual feedback responsive to the participant’s postural sway and center of gravity.
The objectives of this study were to investigate the association of CDP-assisted vestibular retraining with subjective assessments of balance disability. The primary outcome measures were change in scores on 3 validated questionnaires after CDP-assisted vestibular retraining. Secondary outcomes included tolerability of the intervention and rate of completion of the full protocol. We plan to use these latter outcomes in the design of a subsequent randomized clinical trial.
Our hypothesis is that CDP-assisted vestibular retraining therapy will improve participant-reported measures of disability and objective sensory organization test and limits of stability measurements. The participant-reported questionnaires form the basis of the current study.
Methods
Participants
In this cohort study, candidate participants were screened from April 29 to July 23, 2021, in a tertiary neurotology clinic based on their medical records: eligible patients were aged between 18 and 80 years and reported feelings of imbalance characterized by symptoms of imbalance present for more than 6 months that negatively affected their day-to-day activities. To be included in the study, the symptoms of imbalance were clinically assessed to be caused by a stable vestibular deficit rather than an active or irritative vestibulopathy based on the criteria of the Bárány Society International Classification of Vestibular Disorders consensus classification of vestibular symptoms.24 Objective determination of unilateral peripheral vestibular deficit required at least 1 of the following: (1) unilateral weakness during videonystagmography, as defined by a 25% or greater difference between ears using a videonystagmogram; (2) significant cervical or ocular vestibular evoked myogenic potential (VEMP) interaural asymmetry, or absent cervical or ocular VEMP responses in one ear with intact responses in the other ear.25 This study was approved by the Clinical Research Ethics Board at the University of British Columbia. All participants provided written consent. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
We excluded individuals who exhibited fluctuating symptoms of an active vestibulopathic cause within the last 6 months, such as active Ménière disease (characterized by fluctuating hearing loss, tinnitus, and vertiginous exacerbations lasting ≥20 minutes according to American Academy of Otolaryngology–Head and Neck Surgery criteria26); patients with concurrent diagnosis of BPPV; or patients with clinical and audiometric evidence of a perilymphatic fistula or otosyphilis. We also excluded those with a deficit that precluded providing informed consent or completing the rehabilitation exercises, such as orthopedic or neurologic deficits. Those meeting the eligibility criteria were contacted by telephone and invited to enroll in the study.
Intervention and Assessments
Consenting participants were invited to the clinic for their baseline assessment, where they completed the following 3 questionnaires: the Dizziness Handicap Inventory (DHI),27 the Activities-Specific Balance Confidence (ABC) Scale,28 and the Falls Efficacy Scale–International (FES-I).29 During this visit, participants also completed a sensory organization test and a limits of stability test.
Participants completed 12 biweekly sessions of CDP-guided vestibular retraining exercises in the clinic. These exercises were designed in accordance with the accepted principles of vestibular rehabilitation to promote compensation (or habituation) and substitution.6,30 Participants were challenged to shift their weight forward and backward and right to left as directed by an interactive display or to maintain their balance while the support surface moved. The display also provided a visual representation of the center of gravity as a biofeedback aid for their postural control. The exercises grew progressively more difficult over the course of the treatment protocol. The exercise programs were predetermined, and each participant received the same protocol except to account for the laterality of their deficit. On completion of all 12 sessions of retraining exercises, the participants again completed the DHI, ABC Scale, and FES-I and performed the sensory organization test and limits of stability tests.
Statistical Analysis
Statistical analysis was performed from July 7, 2021, to January 25, 2022. The questionnaires were scored according to published instructions29 and reported as the median change and 95% CI. Participants were stratified into those with moderate to severe disability (DHI scores >30) and those with mild disability (DHI scores ≤30),31 as well as those with or without previous vestibular rehabilitation. Estimates of the minimum clinically important difference (MCID) were adopted from published psychometric studies. We used an MCID of 3 for the DHI,32 10 for the ABC Scale,33 and 8.2 for the FES-I.34 Spearman rank-order correlation was used to estimate the correlation between the 3 instruments. Analysis was performed using Prism 9, version 9.2.0 (GraphPad Software).
Results
Participants
We enrolled 13 participants (8 men [62%]; median age, 51 years [range, 18-67 years]) with stable unilateral vestibular deficits (Table 1). Five participants had a deficit on the left side and 9 had a deficit on the right (1 had an abnormal ocular VEMP on one side and an abnormal videonystagmogram on the other side). Seven participants showed a vestibular deficit by results of videonystagmogram with normal VEMPs, 1 had abnormal cervical VEMP and ocular VEMP but normal videonystagmography response, and 5 had abnormal VEMPS and videonystagmography results. All 13 completed the full course of retraining sessions and follow-up.
Table 1. Participant Demographic Characteristics and Vestibular Test Results.
| Characteristic | No./total No. (%) |
|---|---|
| Age, median (range), y | 51 (18-67) |
| Sex | |
| Female | 5/13 (38) |
| Male | 8/13 (62) |
| Previous vestibular rehabilitation | 9/13 (69) |
| Abnormal vestibular test results | |
| Videonystagmography | 12/13 (92) |
| vHIT | 1/11 (9) |
| oVEMP | 6/13 (46) |
| cVEMP | 3/12 (25) |
Abbreviations: cVEMP, cervical vestibular evoked myogenic potential; oVEMP, ocular vestibular evoked myogenic potential; vHIT, video head impulse test.
Before treatment, the median DHI score was 40 (range, 12-80), the median ABC scale score was 74.4 (range, 39.4-96.3), and the median FES-I score was 31 (range, 16-54). Six of 13 participants had DHI scores at baseline of less than 30, indicating mild disability, whereas 4 had DHI scores indicating moderate disability and 3 had DHI scores indicating severe disability. Using Spearman rank-order correlation, Dizziness Handicap Inventory scores correlated with ABC Scale scores (r = −0.8375 [95% CI, −0.9518 to −0.5187]) and FES-I scores (r = 0.8008 [95% CI, 0.4324-0.9401]).
Participant-Reported Change After Retraining
After treatment, DHI, FES-I, and ABC Scale scores improved, with median changes in scores of −16 points (95% CI, −20 to 2) for the DHI, −9 (95% CI, −14 to 1) for the FES-I, and 11.9 (95% CI, 0-17.3) for the ABC Scale (Table 2 and Figure, A). Eight participants (62%) had improvements greater than the MCID for the DHI, whereas 4 participants (31%) exceeded the MCID for the ABC Scale and 3 participants (23%) exceeded the MCID for the FES-I.
Table 2. Participant-Reported Measures Before and After Treatment.
| Instrument | Score, median (range) | Difference in score, median (95% CI) | Achieved MCID, No. (%) | |
|---|---|---|---|---|
| Before treatment (N = 13) | After treatment (N = 13) | |||
| DHI total | 40 (12 to 80) | 24 (10 to 62) | −16 (−20 to 2) | 8 (62) |
| DHI-p | 12 (0 to 26) | 10 (2 to 20) | −2 (−10 to 2) | NA |
| DHI-e | 12 (0 to 22) | 8 (2 to 18) | −4 (−12 to 2) | NA |
| DHI-f | 14 (0 to 36) | 10 (0 to 24) | −4 (−6 to 2) | NA |
| ABC Scale | 74.4 (39.4 to 76.3) | 86.3 (56.3 to 98.8) | 11.9 (0 to 17.3) | 4 (31) |
| FES-I | 31 (16 to 54) | 22 (16 to 32) | −9 (−14 to 1) | 3 (23) |
Abbreviations: ABC Scale, Activities-Specific Balance Confidence Scale; DHI, Dizziness Handicap Inventory; DHI-e, emotional component of DHI; DHI-f, functional component of DHI; DHI-p, physical component of DHI; FES-I, Falls Efficacy Scale–International; MCID, minimum clinically important difference; NA, not applicable.
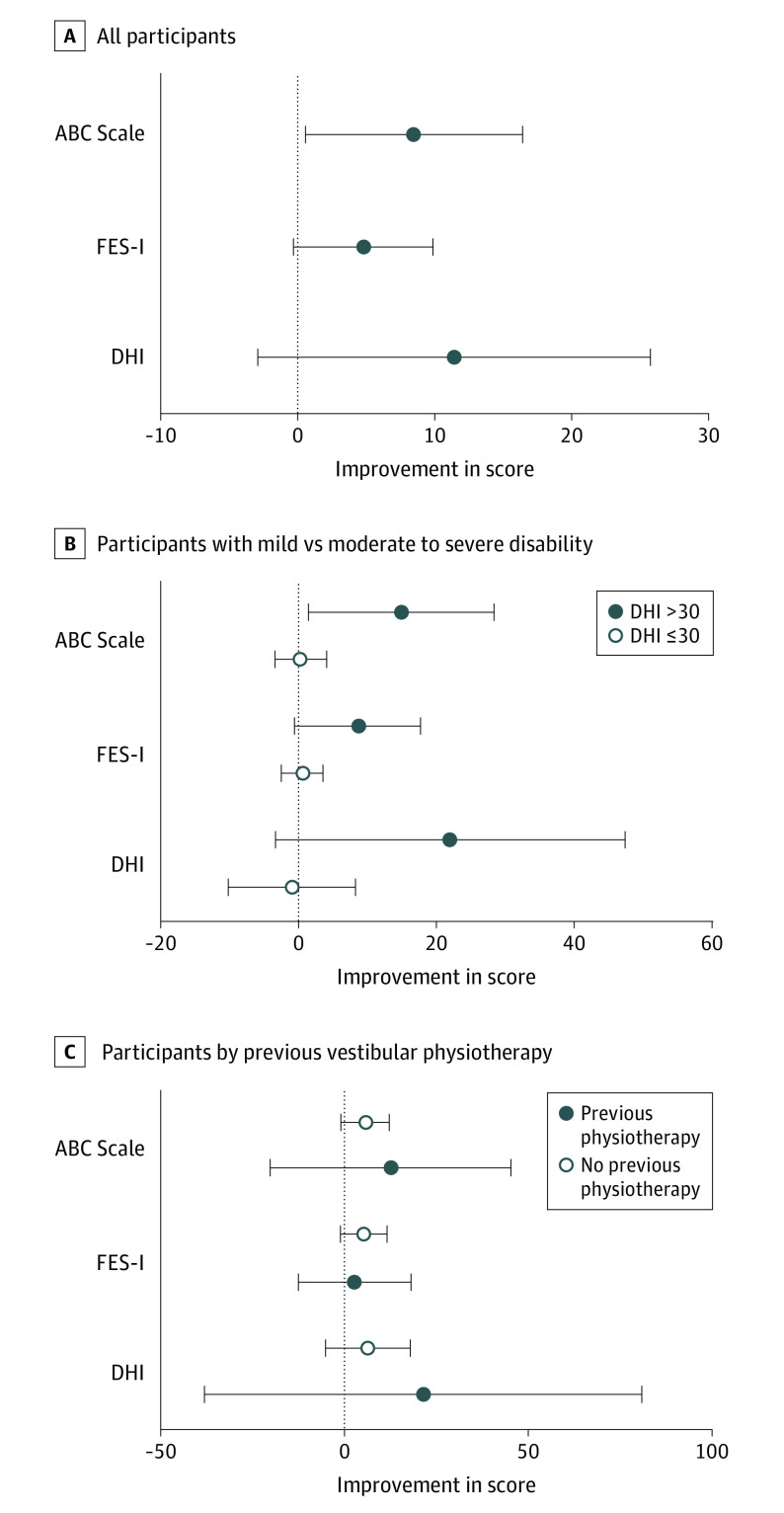
Figure. Median Improvement in Scores for the Dizziness Handicap Inventory (DHI), Falls Efficacy Scale–International (FES-I), and Activities-Specific Balance Confidence (ABC) Scale.
A, Magnitude of improvement for all participants. B, Participants with mild disability (DHI score ≤30) at baseline compared with those with moderate to severe disability (DHI score >30) at baseline. C, Participants with previous vestibular physiotherapy compared with those with no previous physiotherapy. Horizontal bars indicate 95% CIs.
Given that some participants had mild symptoms and, as measured by the 3 instruments in this study, had little room for improvement, we performed post hoc analysis of only those who reported moderate to severe disability according to the DHI (Table 2). Six participants (3 women and 3 men, with a median age of 60 years [range, 22-67 years]) had mild disability and 7 participants (2 women and 5 men, with a median age of 41 years [range, 18-65 years]) had moderate to severe disability. Among those with moderate to severe disability at baseline, the median magnitude of improvement in all scores was greater than for those with mild disability (Figure, B). Participants with moderate to severe disability at baseline had a larger magnitude of improvement in DHI scores than those with mild disability (−18 [95% CI, −78 to 2] vs −1 [95% CI, −8 to 16]). For the subgroup with moderate to severe disability, 6 of 7 (86%) had improvement exceeding the MCID for the DHI. The DHI measures self-perceived disability in 3 domains: physical, emotional, and functional. For those with baseline DHI scores greater than 30, there was improvement of −14 points (95% CI, −36 to −2) in the functional domain, whereas improvement in the physical domain was −4 (95% CI, −24 to 2) and improvement in the emotional domain was −10 (95% CI, −18 to 6) (Table 3).
Table 3. Participant-Reported Measures Stratified by Baseline Severity of Disability.
| Instrument | Score, median (range) | Difference in score, median (95% CI) | Achieved MCID, No. (%) | |
|---|---|---|---|---|
| Before treatment | After treatment | |||
| DHI ≤30 before treatment (n = 6) | ||||
| DHI total | 17 (12 to 24) | 16 (6 to 40) | −1 (−8 to 16) | 2 (33) |
| DHI-p | 7 (0 to 12) | 7 (0 to 12) | 0 (0 to 0) | NA |
| DHI-e | 6 (0 to 12) | 6 (0 to 12) | 0 (0 to 12) | NA |
| DHI-f | 4 (0 to 8) | 4 (4 to 16) | −1 (−4 to 10) | NA |
| ABC Scale | 91.9 (84.4 to 96.3) | 94.4 (81.3 to 96.9) | 0.3 (−3.8 to 6.3) | 0 |
| FES-I | 18 (16 to 32) | 18 (16 to 32) | 0 (−6 to 2) | 0 |
| DHI >30 before treatment (n = 7) | ||||
| DHI total | 52 (40 to 80) | 34 (2 to 62) | −18 (−78 to 2) | 6 (86) |
| DHI-p | 16 (6 to 26) | 12 (6 to 20) | −4 (−24 to 2) | NA |
| DHI-e | 18 (6 to 22) | 8 (0 to 18) | −10 (−18 to 6) | NA |
| DHI-f | 24 (14 to 36) | 10 (0 to 24) | −14 (−36 to −2) | NA |
| ABC Scale | 55.0 (39.4 to 74.4) | 75.6 (56.3 to 98.8) | 20.6 (1.3 to 43.8) | 4 (57) |
| FES-I | 34 (23 to 54) | 26 (17 to 36) | −8 (−23 to 5) | 3 (43) |
Abbreviations: ABC Scale, Activities-Specific Balance Confidence Scale; DHI, Dizziness Handicap Inventory; DHI-e, emotional component of DHI; DHI-f, functional component of DHI; DHI-p, physical component of DHI; FES-I, Falls Efficacy Scale–International; MCID, minimum clinically important difference; NA, not applicable.
Six of 7 participants (86%) with moderate to severe disability had improvement that exceeded the MCID for the DHI, and 3 of 7 participants (43%) had improvement that exceeded the MCID for the FES-I. It was important to ensure that treatment was not associated with worsening of self-reported disability for those who entered the study with mild disability as measured by the DHI. Those with a DHI score of 30 or less at baseline showed negligible change in scores for the DHI (−1 [95% CI, −8 to 16]), the FES-I (0 [95% CI, −6 to 2]), and the ABC Scale (0.3 [95% CI, −3.8 to 6.3]) after treatment (Table 3 and Figure, B).
Nine participants had undergone previous vestibular physiotherapy, but there was no association between previous physiotherapy and response to treatment (Figure, C). Individuals who had received previous vestibular physiotherapy had changes in scores of −6 (95% CI, −20 to 6) for the DHI, −5 (95% CI, −14 to 1) for the FES-I, and 5.6 (95% CI, −3.1 to 13.8) for the ABC Scale, whereas those without previous physiotherapy had changes of −5 (95% CI, −78 to 0) for the DHI, 0 (95% CI, −17 to 5) for the FES-I, and 3.8 (95% CI, 0.63-43.8) for the ABC Scale. Six of the 9 participants (67%) who had previously received vestibular physiotherapy had improvements exceeding the MCID for the DHI, whereas 2 of 9 participants (22%) had meaningful improvement on the FES-I and 3 of 9 participants (33%) had meaningful improvement on the ABC Scale.
Discussion
In this study, we report the change in participant-reported vestibular disability after vestibular therapy using a CDP-assisted retraining protocol. We enrolled only patients with symptoms associated with a persistent, stable unilateral vestibular deficit lasting longer than 6 months. This protocol was chosen to limit confounding by pathologic conditions whose natural histories are associated with variable symptoms or by spontaneous resolution of symptoms common in acute phases of vestibular deficit, which are likely to cause overestimation of the improvement associated with an intervention.
Modern physical therapy rehabilitation techniques seek to promote vestibular compensation, adaptation, and substitution, with the aim of improving postural stability and reducing the sensation of dizziness.6,35 Rehabilitation programs of various methods have been shown to be effective for improving patient-reported measures of imbalance.6,12,36,37,38,39,40 However, there is little evidence showing the relative effectiveness of different rehabilitation modalities, nor is there evidence suggesting which vestibular pathologic conditions are amenable to which treatment.6
Patients with dizziness are a diverse group with varying underlying pathologic conditions, which makes them a challenging population to study. Dizziness can arise due to disruption of the central vestibular system by tumors or infection or by damage or dysfunction of the peripheral vestibular organs. Proprioceptive defects, psychological distress, neurologic disease, orthopedic deficits, and age are associated with dizziness symptoms. Accurate diagnosis of the underlying pathologic condition can be time consuming and requires access to specialist health care professionals, so studies of vestibular rehabilitation with larger cohorts frequently enroll highly heterogeneous patient groups.
Enrollment Criteria in the Literature
Yardley et al9,10,12 recruited a diverse group of patients to study exercise-based therapy in a series of publications. These randomized clinical trials enrolled large cohorts and robustly demonstrated that rehabilitation exercises are beneficial for patients with dizziness. However, these studies did not obtain precise clinical diagnoses and, thus, could not assess whether patients with some causes of dizziness experienced more of a benefit associated with rehabilitation than others. In fact, except for 1 study,10 the distribution of pathologic conditions within the cohort was not described.
In studies that reported diagnoses, many studies enrolled participants with pathologic conditions that could confound analysis.10,38,41 One of these conditions is Ménière disease, which is characterized by variable episodes of vertigo and periods of remission. Thus, if enrollment criteria required participants to be symptomatic on entry to the study, the natural progression of Ménière disease would be associated with some of these participants experiencing less severe symptoms at a later time regardless of treatment. Another frequently included diagnosis in the vestibular rehabilitation literature is BPPV, which is typically self-limiting and can be treated successfully. Benign paroxysmal positional vertigo is unlikely to cause persistent symptoms of imbalance as it is not associated with unilateral vestibular weakness during videonystagmogram testing, although some studies have described persistent VEMP abnormalities in patients with a history of BPPV.42 Our study excluded patients with a diagnosis of Ménière disease or BPPV.
Another important variable is the interval since the onset of symptoms. Symptoms of imbalance often resolve or improve spontaneously. In one study, 33% of those in the control group showed significant improvement without any specialized care.10 In another study, nearly three-quarters of respondents who declined to enroll reported they were no longer dizzy.12 Studies of compensation in the acute phase of vestibular impairment saw improvement regardless of the modality of treatment40,43 or even without treatment.37
In addition to variability in patient characteristics, the rehabilitation interventions that are described in the literature are similarly variable. Some studies prescribed simple exercises involving nodding and shaking of the head,10,12,38,39,44 whereas others added exercises including standing on one leg on firm or soft surfaces and bending and/or reaching tasks.40,41 Some of these rehabilitation interventions were performed at home with minimal supervision,10,12,38 whereas others were performed under the supervision of a trained physiotherapist who adjusted the exercises to meet the needs of patients.36 For home studies, it is difficult to assess adherence, and, in the studies with supervised protocols, the details of the customized programs were often not reported.36,41
Because of these limitations in much of the published literature on vestibular rehabilitation—namely, heterogeneity of participants, inclusion of patients in the acute phase of their illness, inclusion of patients with diagnoses of pathologic conditions that are associated with naturally variable symptoms, lack of adequate controls, failure to identify and control for the severity of cogent comorbid ailments, lack of reporting or stratification by diagnosis, and variability in treatment protocols—the literature does not provide adequate guidance on how to manage patients with stable, persistent peripheral unilateral vestibular deficits.
One study by Giray et al36 does address this patient group. Their study enrolled 42 individuals with a diagnosis of a chronic, decompensated unilateral vestibular deficit and randomized them to either a customized exercise program or no treatment. In that study, the untreated group showed no improvement in participant-reported outcomes, whereas the exercise group showed significant improvement after treatment for all 3 questionnaires used. The lack of improvement among untreated controls is in contrast with the studies mentioned previously, which showed significant amelioration of symptoms without treatment.10,37,45 This finding is consistent with the authors’ clinical experience that achieving satisfactory outcomes in patients with stable, persistent unilateral vestibular deficits is difficult. Giray et al36 enrolled patients at a minimum of 30 days after onset of symptoms; our study had even more stringent criteria for persistent disorder—a minimum of 6 months.
CDP-Assisted Vestibular Retraining
Given the limitations of conventional rehabilitation exercises in treating patients with a stable unilateral vestibular deficit, we sought to evaluate CDP-assisted vestibular retraining for this group. We included only those with a deficit confirmed by either videonystagmogram or VEMP, and we excluded those with diagnoses known to be associated with variable symptoms or that are readily resolved spontaneously. Vestibular rehabilitation using CDP has been reported to improve balance and reduce falls among elderly individuals without a diagnosed vestibular impairment,46,47,48 among patients after surgical removal of vestibular schwannoma,49 among patients with central vestibulopathy,18 and among those wth Parkinson disease.19 Computerized dynamic posturography–assisted rehabilitation, in conjunction with home-based exercises, was superior to no treatment for patients with an acute peripheral vestibular disorder.18 To our knowledge, CDP-assisted vestibular retraining has not been evaluated for individuals with stable unilateral vestibular deficit.
In our present single-group pilot study, patients with persistent unilateral vestibular deficit received 12 sessions of CDP-assisted vestibular retraining. The exercises they performed were designed to facilitate compensation via the plasticity of the nervous system and promote sensory substitution, whereby the individual would integrate sensory cues from vision, proprioception, and intact contralateral vestibular organs to substitute for lost vestibular function.50 The participants achieved this outcome through repetitive and provocative movements according to visual prompts and by maintaining postural control during dynamic movements of the platform. Participants were provided with visual biofeedback of their center of pressure during all exercises, which is thought to facilitate reweighting of sensory inputs from defective sources and increase weighting to sensory inputs that are intact.51,52
We found that, for individuals with moderate to severe disability, 12 twice-weekly sessions of CDP-assisted vestibular retraining was associated with changes in DHI, ABC Scale, and FES-I scores, indicating improvement. Many participants demonstrated improvements that were clinically meaningful based on MCID estimates derived from the literature. We adopted the DHI as our primary end point for this study because it is widely used to measure the association of a therapeutic intervention with vestibular deficits, and it has been shown to be responsive to changes in self-reported dizziness.32 Marchetti et al33 found that an improvement of 11 or 13 points on the DHI was associated with an improvement exceeding the minimal detectable change in 2 measurements of gait among those with balance and vestibular disorders. Our present study did not measure gait performance as an outcome; rather, we are interested in whether CDP-assisted vestibular retraining is associated with self-reported improvement in dizziness. Friscia et al32 found that an improvement of 3 points on the DHI was the optimal cutoff for significant change. Because the outcomes used in the study by Friscia et al32 were more closely associated with those in our present study, we used the cutoff assessed in this study. An improvement of greater than 3 points on the DHI corresponds to the participant changing their answer on at least 2 questions from “yes” to “sometimes” or from “sometimes” to “no.” Alternatively, such an improvement could be achieved by changing a response on 1 question from yes to no.
Eight of 13 participants, and 6 of 7 participants with moderate to severe disability, experienced improvement greater than the MCID on the DHI after CDP-assisted retraining according to this threshold. The DHI measures self-perceived disability in 3 domains: physical, emotional, and functional. For those with baseline DHI scores greater than 30, there was improvement of −14 points (95% CI, −36 to −2) in the functional domain, whereas improvement in the physical domain was −4 (95% CI, −24 to 2) and improvement in the emotional domain was −10 (95% CI, −18 to 6). The functional domain is indicative of the participant’s ability to engage in activities of daily life, such as work, social activities, and travel.27 Fewer participants met the MCID threshold for the FES-I or the ABC Scale; however, it has been reported that the DHI is more responsive than the ABC Scale32 and, in turn, the ABC Scale is more responsive than the FES-I.28
Delbaere et al53 performed a longitudinal study of the FES-I for validity in predicting falls and found that those with multiple falls had a starting mean FES-I score of 26, whereas those who did not fall during the study had a mean score of less than 21. In that study, they used a receiver operator characteristic curve to propose a score cutoff of 23 for high concern of falling, while scores of 16 to 22 indicated low concern. In our present study, 9 participants’ scores prior to retraining were above the cutoff for high concern, which decreased to 6 participants after retraining; 2 of these participants improved by greater than the MCID of 8.2 points. These changes are consistent with a meaningful decrease in fall risk for these individuals.
Nine of the 13 participants in our present study had received some form of previous vestibular rehabilitation or physiotherapy without satisfactory resolution of their symptoms, whereas 4 had received no prior treatment. Of these 4 participants, 2 had mild disease according to the DHI, so it was not possible to estimate whether previous rehabilitation status was associated with outcomes. However, we are encouraged that the intervention described here is associated with improvement in self-reported vestibular disability—even for those who had previously received some rehabilitation or physiotherapy that had failed to resolve their symptoms adequately.
Limitations
This study has some limitations. The study design did not include a waiting list, sham treatment, or alternative treatment control, so we cannot estimate how much change in participant-reported disability was owing to factors other than the CDP intervention or how it compares with other treatment modalities. We enrolled only 13 participants, and only 7 had mild to moderate symptom severity. Individuals with mild impairment showed no benefit associated with the intervention, but we cannot assess whether this outcome was because of a floor or ceiling effect of the questionnaires53,54 or whether those with mild impairment do not respond to this intervention. Subsequent studies would benefit from enrolling more participants and from limiting eligibility to those with moderate to severe impairment.
Conclusions
This cohort study found that CDP-assisted vestibular retraining delivered by a supervised and standardized protocol, such that each participant received the same intervention except for adjusting for laterality of the vestibular deficit, was associated with improvement in participant-reported outcomes. This intervention requires specialized equipment, which is a barrier to access; however, if such equipment is available, replicable computer-guided vestibular retraining treatments could be offered without the need for a physical therapist to develop a customized exercise program.
Our findings suggest the use of CDP-assisted vestibular retraining as an adjunct treatment for patients with dizziness who have persistent symptoms with or without previous physical therapy. These findings support the need for, and will inform the design of, a randomized clinical trial of CDP-assisted vestibular retraining for patients with a stable unilateral vestibular deficit.
References
- 1.Murdin L, Schilder AGM. Epidemiology of balance symptoms and disorders in the community: a systematic review. Otol Neurotol. 2015;36(3):387-392. doi: 10.1097/MAO.0000000000000691 [DOI] [PubMed] [Google Scholar]
- 2.Agrawal Y, Carey JP, Della Santina CC, Schubert MC, Minor LB. Disorders of balance and vestibular function in US adults: data from the National Health and Nutrition Examination Survey, 2001-2004. Arch Intern Med. 2009;169(10):938-944. doi: 10.1001/archinternmed.2009.66 [DOI] [PubMed] [Google Scholar]
- 3.Yardley L. Contribution of symptoms and beliefs to handicap in people with vertigo: a longitudinal study. Br J Clin Psychol. 1994;33(1):101-113. doi: 10.1111/j.2044-8260.1994.tb01100.x [DOI] [PubMed] [Google Scholar]
- 4.Mira E. Improving the quality of life in patients with vestibular disorders: the role of medical treatments and physical rehabilitation. Int J Clin Pract. 2008;62(1):109-114. doi: 10.1111/j.1742-1241.2006.01091.x [DOI] [PubMed] [Google Scholar]
- 5.Cawthorne T. Vestibular injuries. J R Soc Med. 1946;39(5):270-273. doi: 10.1177/003591574603900522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McDonnell MN, Hillier SL. Vestibular rehabilitation for unilateral peripheral vestibular dysfunction. Cochrane Database Syst Rev. 2015;1(1):CD005397. doi: 10.1002/14651858.CD005397.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hall CD, Herdman SJ, Whitney SL, et al. Vestibular rehabilitation for peripheral vestibular hypofunction: an evidence-based clinical practice guideline: from the American Physical Therapy Association Neurology Section. J Neurol Phys Ther. 2016;40(2):124-155. doi: 10.1097/NPT.0000000000000120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Horak FB, Jones-Rycewicz C, Black FO, Shumway-Cook A. Effects of vestibular rehabilitation on dizziness and imbalance. Otolaryngol Head Neck Surg. 1992;106(2):175-180. doi: 10.1177/019459989210600220 [DOI] [PubMed] [Google Scholar]
- 9.Yardley L, Beech S, Zander L, Evans T, Weinman J. A randomized controlled trial of exercise therapy for dizziness and vertigo in primary care. Br J Gen Pract. 1998;48(429):1136-1140. [PMC free article] [PubMed] [Google Scholar]
- 10.Yardley L, Donovan-Hall M, Smith HE, Walsh BM, Mullee M, Bronstein AM. Effectiveness of primary care-based vestibular rehabilitation for chronic dizziness. Ann Intern Med. 2004;141(8):598-605. doi: 10.7326/0003-4819-141-8-200410190-00007 [DOI] [PubMed] [Google Scholar]
- 11.Yardley L, Kirby S. Evaluation of booklet-based self-management of symptoms in Ménière disease: a randomized controlled trial. Psychosom Med. 2006;68(5):762-769. doi: 10.1097/01.psy.0000232269.17906.92 [DOI] [PubMed] [Google Scholar]
- 12.Yardley L, Barker F, Muller I, et al. Clinical and cost effectiveness of booklet based vestibular rehabilitation for chronic dizziness in primary care: single blind, parallel group, pragmatic, randomised controlled trial. BMJ. 2012;344:e2237. doi: 10.1136/bmj.e2237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cyr DG, Moore GF, Moller CG. Clinical application of computerized dynamic posturography. Entechnology. September 1988:36-47. [PubMed] [Google Scholar]
- 14.Yardley L, Burgneay J, Nazareth I, Luxon L. Neuro-otological and psychiatric abnormalities in a community sample of people with dizziness: a blind, controlled investigation. J Neurol Neurosurg Psychiatry. 1998;65(5):679-684. doi: 10.1136/jnnp.65.5.679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Balaguer García R, Pitarch Corresa S, Baydal Bertomeu JM, Morales Suárez-Varela MM. Static posturography with dynamic tests: usefulness of biomechanical parameters in assessing vestibular patients. Acta Otorrinolaringol Esp. 2012;63(5):332-338. doi: 10.1016/j.otorri.2012.03.006 [DOI] [PubMed] [Google Scholar]
- 16.Falls C. Videonystagmography and posturography. Adv Otorhinolaryngol. 2019;82:32-38. doi: 10.1159/000490269 [DOI] [PubMed] [Google Scholar]
- 17.O’Neill DE, Gill-Body KM, Krebs DE. Posturography changes do not predict functional performance changes. Am J Otol. 1998;19(6):797-803. [PubMed] [Google Scholar]
- 18.Marioni G, Fermo S, Zanon D, Broi N, Staffieri A. Early rehabilitation for unilateral peripheral vestibular disorders: a prospective, randomized investigation using computerized posturography. Eur Arch Otorhinolaryngol. 2013;270(2):425-435. doi: 10.1007/s00405-012-1944-4 [DOI] [PubMed] [Google Scholar]
- 19.Qutubuddin AA, Cifu DX, Armistead-Jehle P, Carne W, McGuirk TE, Baron MS. A comparison of computerized dynamic posturography therapy to standard balance physical therapy in individuals with Parkinson’s disease: a pilot study. NeuroRehabilitation. 2007;22(4):261-265. doi: 10.3233/NRE-2007-22402 [DOI] [PubMed] [Google Scholar]
- 20.Rossi-Izquierdo M, Soto-Varela A, Santos-Pérez S, et al. Vestibular rehabilitation with computerised dynamic posturography in patients with Parkinson's disease: improving balance impairment. Disabil Rehabil. 2009;31(23):1907-1916. doi: 10.1080/09638280902846384 [DOI] [PubMed] [Google Scholar]
- 21.Rossi-Izquierdo M, Santos-Pérez S, Soto-Varela A. What is the most effective vestibular rehabilitation technique in patients with unilateral peripheral vestibular disorders? Eur Arch Otorhinolaryngol. 2011;268(11):1569-1574. doi: 10.1007/s00405-011-1532-z [DOI] [PubMed] [Google Scholar]
- 22.Rossi-Izquierdo M, Santos-Pérez S, Rubio-Rodríguez JP, et al. What is the optimal number of treatment sessions of vestibular rehabilitation? Eur Arch Otorhinolaryngol. 2014;271(2):275-280. doi: 10.1007/s00405-013-2423-2 [DOI] [PubMed] [Google Scholar]
- 23.Fil-Balkan A, Salci Y, Keklicek H, et al. Sensorimotor integration training in Parkinson’s disease. Neurosciences (Riyadh). 2018;23(4):208-215. doi: 10.17712/nsj.2018.3.20180021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bisdorff A, Von Brevern M, Lempert T, Newman-Toker DE. Classification of vestibular symptoms: towards an international classification of vestibular disorders. J Vestib Res. 2009;19(1-2):1-13. doi: 10.3233/VES-2009-0343 [DOI] [PubMed] [Google Scholar]
- 25.Shahnaz N, David EA. Normal values for cervical and ocular vestibular–evoked myogenic potentials using EMG scaling: effect of body position and electrode montage. Acta Otolaryngol. 2021;141(5):440-448. doi: 10.1080/00016489.2021.1887517 [DOI] [PubMed] [Google Scholar]
- 26.Lopez-Escamez JA, Carey J, Chung WH, et al. ; Classification Committee of the Barany Society; Japan Society for Equilibrium Research; European Academy of Otology and Neurotology (EAONO); Equilibrium Committee of the American Academy of Otolaryngology-Head and Neck Surgery (AAO-HNS); Korean Balance Society . Diagnostic criteria for Ménière’s disease. J Vestib Res. 2015;25(1):1-7. doi: 10.3233/VES-150549 [DOI] [PubMed] [Google Scholar]
- 27.Jacobson GP, Newman CW. The development of the Dizziness Handicap Inventory. Arch Otolaryngol Head Neck Surg. 1990;116(4):424-427. doi: 10.1001/archotol.1990.01870040046011 [DOI] [PubMed] [Google Scholar]
- 28.Powell LE, Myers AM. The Activities-Specific Balance Confidence (ABC) Scale. J Gerontol A Biol Sci Med Sci. 1995;50A(1):M28-M34. doi: 10.1093/gerona/50A.1.M28 [DOI] [PubMed] [Google Scholar]
- 29.Yardley L, Beyer N, Hauer K, Kempen G, Piot-Ziegler C, Todd C. Development and initial validation of the Falls Efficacy Scale-International (FES-I). Age Ageing. 2005;34(6):614-619. doi: 10.1093/ageing/afi196 [DOI] [PubMed] [Google Scholar]
- 30.Whitney SL, Sparto PJ. Principles of vestibular physical therapy rehabilitation. NeuroRehabilitation. 2011;29(2):157-166. doi: 10.3233/NRE-2011-0690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Whitney SL, Wrisley DM, Brown KE, Furman JM. Is perception of handicap related to functional performance in persons with vestibular dysfunction? Otol Neurotol. 2004;25(2):139-143. doi: 10.1097/00129492-200403000-00010 [DOI] [PubMed] [Google Scholar]
- 32.Friscia LA, Morgan MT, Sparto PJ, Furman JM, Whitney SL. Responsiveness of self-report measures in individuals with vertigo, dizziness, and unsteadiness. Otol Neurotol. 2014;35(5):884-888. doi: 10.1097/MAO.0000000000000421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marchetti GF, Lin CC, Alghadir A, Whitney SL. Responsiveness and minimal detectable change of the dynamic gait index and functional gait index in persons with balance and vestibular disorders. J Neurol Phys Ther. 2014;38(2):119-124. doi: 10.1097/NPT.0000000000000015 [DOI] [PubMed] [Google Scholar]
- 34.Morgan MT, Friscia LA, Whitney SL, Furman JM, Sparto PJ. Reliability and validity of the Falls Efficacy Scale-International (FES-I) in individuals with dizziness and imbalance. Otol Neurotol. 2013;34(6):1104-1108. doi: 10.1097/MAO.0b013e318281df5d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Whitney SL, Marchetti GF, Schade AI. The relationship between falls history and computerized dynamic posturography in persons with balance and vestibular disorders. Arch Phys Med Rehabil. 2006;87(3):402-407. doi: 10.1016/j.apmr.2005.11.002 [DOI] [PubMed] [Google Scholar]
- 36.Giray M, Kirazli Y, Karapolat H, Celebisoy N, Bilgen C, Kirazli T. Short-term effects of vestibular rehabilitation in patients with chronic unilateral vestibular dysfunction: a randomized controlled study. Arch Phys Med Rehabil. 2009;90(8):1325-1331. doi: 10.1016/j.apmr.2009.01.032 [DOI] [PubMed] [Google Scholar]
- 37.Teggi R, Caldirola D, Fabiano B, Recanati P, Bussi M. Rehabilitation after acute vestibular disorders. J Laryngol Otol. 2009;123(4):397-402. doi: 10.1017/S0022215108002983 [DOI] [PubMed] [Google Scholar]
- 38.Andersson G, Asmundson GJG, Denev J, Nilsson J, Larsen HC. A controlled trial of cognitive-behavior therapy combined with vestibular rehabilitation in the treatment of dizziness. Behav Res Ther. 2006;44(9):1265-1273. doi: 10.1016/j.brat.2005.09.008 [DOI] [PubMed] [Google Scholar]
- 39.Cohen HS, Kimball KT. Increased independence and decreased vertigo after vestibular rehabilitation. Otolaryngol Head Neck Surg. 2003;128(1):60-70. doi: 10.1067/mhn.2003.23 [DOI] [PubMed] [Google Scholar]
- 40.Kammerlind ASC, Ledin TE, Ödkvist LM, Skargren EI. Effects of home training and additional physical therapy on recovery after acute unilateral vestibular loss—a randomized study. Clin Rehabil. 2005;19(1):54-62. doi: 10.1191/0269215505cr830oa [DOI] [PubMed] [Google Scholar]
- 41.Pavlou M, Lingeswaran A, Davies RA, Gresty MA, Bronstein AM. Simulator based rehabilitation in refractory dizziness. J Neurol. 2004;251(8):983-995. doi: 10.1007/s00415-004-0476-2 [DOI] [PubMed] [Google Scholar]
- 42.Chen G, Dai X, Ren X, et al. Ocular vs cervical vestibular evoked myogenic potentials in benign paroxysmal positional vertigo: a systematic review and meta-analysis. Front Neurol. 2020;11:596454. doi: 10.3389/fneur.2020.596454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barozzi S, Di Berardino F, Arisi E, Cesarani A. A comparison between oculomotor rehabilitation and vestibular electrical stimulation in unilateral peripheral vestibular deficit. Int Tinnitus J. 2006;12(1):45-49. [PubMed] [Google Scholar]
- 44.Clendaniel RA. The effects of habituation and gaze stability exercises in the treatment of unilateral vestibular hypofunction: a preliminary results. J Neurol Phys Ther. 2010;34(2):111-116. doi: 10.1097/NPT.0b013e3181deca01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brugnera C, Bittar RSM, Greters ME, Basta D. Effects of vibrotactile vestibular substitution on vestibular rehabilitation—preliminary study. Braz J Otorhinolaryngol. 2015;81(6):616-621. doi: 10.1016/j.bjorl.2015.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rossi-Izquierdo M, Gayoso-Diz P, Santos-Pérez S, et al. Short-term effectiveness of vestibular rehabilitation in elderly patients with postural instability: a randomized clinical trial. Eur Arch Otorhinolaryngol. 2017;274(6):2395-2403. doi: 10.1007/s00405-017-4472-4 [DOI] [PubMed] [Google Scholar]
- 47.Rossi-Izquierdo M, Gayoso-Diz P, Santos-Pérez S, et al. Vestibular rehabilitation in elderly patients with postural instability: reducing the number of falls—a randomized clinical trial. Aging Clin Exp Res. 2018;30(11):1353-1361. doi: 10.1007/s40520-018-1003-0 [DOI] [PubMed] [Google Scholar]
- 48.Soto-Varela A, Gayoso-Diz P, Rossi-Izquierdo M, et al. Reduction of falls in older people by improving balance with vestibular rehabilitation (ReFOVeRe study): design and methods. Aging Clin Exp Res. 2015;27(6):841-848. doi: 10.1007/s40520-015-0362-z [DOI] [PubMed] [Google Scholar]
- 49.Čakrt O, Chovanec M, Funda T, et al. Exercise with visual feedback improves postural stability after vestibular schwannoma surgery. Eur Arch Otorhinolaryngol. 2010;267(9):1355-1360. doi: 10.1007/s00405-010-1227-x [DOI] [PubMed] [Google Scholar]
- 50.Lacour M, Helmchen C, Vidal PP. Vestibular compensation: the neuro-otologist’s best friend. J Neurol. 2016;263(suppl 1):S54-S64. doi: 10.1007/s00415-015-7903-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dozza M, Chiari L, Hlavacka F, Cappello A, Horak FB. Effects of linear versus sigmoid coding of visual or audio biofeedback for the control of upright stance. IEEE Trans Neural Syst Rehabil Eng. 2006;14(4):505-512. doi: 10.1109/TNSRE.2006.886732 [DOI] [PubMed] [Google Scholar]
- 52.Sienko KH, Seidler RD, Carender WJ, Goodworth AD, Whitney SL, Peterka RJ. Potential mechanisms of sensory augmentation systems on human balance control. Front Neurol. 2018;9:944. doi: 10.3389/fneur.2018.00944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Delbaere K, Close JCT, Mikolaizak AS, Sachdev PS, Brodaty H, Lord SR. The Falls Efficacy Scale International (FES-I). a comprehensive longitudinal validation study. Age Ageing. 2010;39(2):210-216. doi: 10.1093/ageing/afp225 [DOI] [PubMed] [Google Scholar]
- 54.Talley KMC, Wyman JF, Gross CR. Psychometric properties of the Activities-Specific Balance Confidence Scale and the survey of activities and fear of falling in older women. J Am Geriatr Soc. 2008;56(2):328-333. doi: 10.1111/j.1532-5415.2007.01550.x [DOI] [PubMed] [Google Scholar]