Abstract
Background.
Understanding tuberculosis (TB) epidemiology among children and adolescents informs treatment and prevention efforts and efforts to eliminate disparities in TB incidence and mortality. We sought to describe the epidemiology of children and adolescents with TB disease in the United States (U.S.), including TB incidence rates by parental country of birth and for U.S. territories and freely associated states, which have not been previously described.
Methods.
We analyzed data for children aged <15 and adolescents aged 15–17 years with TB disease reported to the National Tuberculosis Surveillance System during 2007–2017, and calculated TB incidence rates using population estimates from the U.S. Census Bureau.
Findings.
During 2010–2017, 6,072 TB cases occurred among children and adolescents; of these, 85% (5,175/6,072) occurred in the 50 U.S. states or the District of Columbia and 15% (897/6,072) in U.S.-Affiliated Islands. In U.S. States, 68% (3,520/5,175) cases occurred among U.S.-born persons overall, including 76% (2,977/3896) among children and 42% (543/1,279) among adolescents. The incidence rate among children and adolescents was 1.0 per 100,000 person-years during 2007–2017 and declined 47.8% (95% CI −51.4%, −44.1%) during this period. We observed disproportionately high TB rates among children and adolescents of all non-white racial/ethnic groups, those living in U.S.-Affiliated Islands, and children born in or with parents from TB-endemic countries.
Interpretations.
Overall, TB incidence among children and adolescents in the U.S. is low and steadily declining, but additional efforts are needed to eliminate stark disparities in incidence and mortality.
Funding.
Centers for Disease Control and Prevention
Introduction
Globally, approximately one million cases of tuberculosis (TB) disease and 233,000 TB-related deaths occurred among children aged <15 years during 2018 (1). TB in children and adolescents is clinically and epidemiologically heterogeneous, making care and prevention challenging. TB is often difficult to diagnose in children because of nonspecific symptoms and limited sensitivity and specificity of laboratory tests for both latent TB infection (LTBI) and TB disease. Children aged <5 years are also more likely to progress to TB disease after infection and have the highest rates of severe, disseminated forms of TB, such as meningitis, compared to older age groups (2-7). Adolescents aged 15–17 years are more likely to develop infectious forms of pulmonary TB than younger children (3,8). Understanding heterogeneity in TB burden and clinical presentation in children and adolescents is critical to inform TB care and prevention efforts.
While TB rates in the United States (U.S.) are the lowest ever recorded, TB continues to affect many communities unequally, especially communities of color, Indigenous persons, persons born outside the U.S., and persons experiencing homelessness (9-13). In children and adolescents, current practice guidelines recommend TB testing for contacts of people with TB and those who were born or traveled outside the U.S. (14). While rates are 10–20 times higher among children and adolescents born outside of the U.S. than among those born in the U.S., more than two-thirds of children and adolescents with TB are U.S.-born, and therefore it is important to identify determinants other than origin of birth in these groups (15,16).
Previous studies suggest that U.S.-born children with non-U.S.–born parents may be at increased risk of TB (16,17). Approximately two-thirds of U.S.-born children with TB reported during 2009–2010 had at least one non-U.S.–born parent (15,16). Additionally, several large TB clusters with a high proportion of children have been reported in the freely associated states of the Republic of the Marshall Islands and the Federated States of Micronesia, and among Marshallese persons living in the U.S. (18,19). However, rates of TB among U.S.-born children by parental country of birth and among children and adolescents in the U.S. territories and freely associated states have not been systematically reported. Additionally, detailed analyses of national estimates of TB disease counts and incidence rates among children and adolescents were last published in 2010 and 2007, respectively (15,20).
In the present analysis, we describe the clinical characteristics and epidemiology of TB among children and adolescents reported to the U.S. National Tuberculosis Surveillance System (NTSS) during 2007–2017, including overall burden and trends in TB incidence rates by sociodemographic groups, country of birth for children and their parents, and reporting jurisdiction, including U.S. territories and freely associated states.
Methods
We considered all verified cases of TB disease reported to NTSS among children aged <15 years and adolescents aged 15–17 years between January 1, 2007 and December 31, 2017, excluding those with missing information for sex or country of birth. A verified TB case is one that has been reviewed at the local level (e.g., state or county) by a TB control official who is familiar with the NTSS surveillance definitions and has verified that NTSS criteria for a TB case are met. Verified cases include laboratory-confirmed TB and clinically diagnosed TB without laboratory confirmation. NTSS captures demographic, clinical, and laboratory characteristics of all TB cases reported in the 50 U.S. States and District of Columbia (D.C.) (hereafter referred to collectively as U.S. States), and in U.S.-Affiliated Islands which include five U.S. territories (American Samoa [AS], Guam, Commonwealth of the Northern Mariana Islands [CNMI], Puerto Rico [PR], and the U.S. Virgin Islands [USVI]) and three freely associated states (Federated States of Micronesia [FSM], Republic of the Marshall Islands [RMI], and Republic of Palau) (13,21). Under the Compacts of Free Association (COFA), the United States provides economic assistance to the sovereign nations of RMI, FSM, and Palau (22,23). CDC provides financial and technical support to public health programs in these jurisdictions. In this manuscript, the phrase “reported to NTSS” is used to refer to aggregate TB case data from all reporting jurisdictions, unless otherwise specified. In 2009, NTSS implemented new variables including how TB was initially identified (e.g., contact investigations, targeted testing of persons with risk factors, and evaluation for TB symptoms), country of birth for parents or guardians for children aged <15 years, and whether the child lived outside the U.S. for ≥2 months (Supplemental Table 1).
Consistent with U.S. Census Bureau definitions, we considered persons who were U.S. citizens at birth to be U.S.-born (i.e., anyone born in a U.S. state or territory, or born abroad to at least one U.S. citizen parent). All others were considered non-U.S.–born, including persons born in freely associated states (i.e., FSM, RMI, and Palau) (24).
To avoid gaps or overlaps with previous pediatric reports using NTSS data, we reported rates starting in 2007 and TB case counts starting in 2010 (15,16). We used Poisson models offset with log population size to obtain point estimates and 95% confidence intervals (CI) of overall and stratum-specific incidence rates, incidence rate ratios, and 10-year trends in incidence rates. We obtained population estimates from the U.S. Census Bureau’s American Community Survey (ACS) public use microsample (PUMS) 1-year data files for 2007–2017 compiled by the Integrated Public Use Microdata Series (IPUMS), or U.S. Census Bureau modeled estimates when ACS data were unavailable (i.e., for U.S.-Affiliated Islands) (Supplementary Material) (25-28). We conducted all analysis using SAS (Version 9.3, Cary, NC) and reported results from U.S.-Affiliated Islands separately from U.S. States unless otherwise specified. These data were collected as part of routine public health surveillance and therefore did not require institutional board review at CDC.
Role of the funding source.
Authors were salaried by the CDC and did not receive specific funding for this study. The data in this study are collected and managed by the CDC, however, authors were responsible for study design, analysis, interpretation and writing, of this report.
Results
During 2007–2017, 121,582 TB cases were reported to NTSS. We excluded 373 with missing age, sex, or country of birth. Of the remaining 121,209 TB cases, 9,276 (7.7%) occurred among children and adolescents aged <18 years. Both annual TB counts and proportion of TB that occurred among children and adolescents decreased from 2007 (n=1,125, 8.2%) to 2017 (n=701, 7.3%).
Characteristics of children and adolescents with TB, 2010–2017
During 2010–2017, 6,072 cases of TB were reported to NTSS among children and adolescents; of these, 85% (5,175/6,072) were reported from U.S. States and 15% (897/6,072) from U.S.-Affiliated Islands (Table 1). In U.S.-Affiliated Islands, the majority (83%, 747/897) of TB was reported from either RMI (42%, 378/897) or FSM (41%, 369/897) (data not shown). Among children and adolescents with TB in U.S. States, 68% (3,520/5,175) occurred among U.S.-born persons; among children aged <15 years, 76% (2,977/3,896) were U.S.-born and among adolescents aged 15–17 years, 42% (543/1,279) were U.S.-born. Nearly half (47%, 1,659/3,520) of U.S.-born children and adolescents with TB were Hispanic compared with 27% (444/1,655) of non-U.S.–born persons. Non-U.S.–born children and adolescents with TB were most commonly of Asian (36%, 591/1,655) or Black (30%, 489/1,655) race.
Table 1.
Demographic and clinical characteristics of persons aged <18 years with tuberculosis (TB) reported to the National Tuberculosis Surveillance System (NTSS) in U.S. States and U.S.-Affiliated Islands, 2010–2017
| Reported in U.S. States1 | Reported in U.S.-Affiliated Islands2 |
Total (N=6,072) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| U.S.-born3 (n=3,520) |
Non-U.S.–born (n=1,655 |
Total (n=5,175) |
Total (n=897) |
||||||||
| n | % | n | % | n | % | p-value4 | n | % | N | % | |
| Age Category | |||||||||||
| <15 years | 2,977 | (85%) | 919 | (56%) | 3,896 | (75%) | <.0001 | 754 | (84%) | 4,650 | (77%) |
| <1 year | 448 | (13%) | 26 | (2%) | 474 | (9%) | <.0001 | 55 | (6%) | 529 | (9%) |
| 1–4 years | 1,503 | (43%) | 253 | (15%) | 1,756 | (34%) | <.0001 | 293 | (33%) | 2,049 | (34%) |
| 5–14 years | 1026 | (29%) | 640 | (39%) | 1,666 | (32%) | <.0001 | 406 | (45%) | 2,072 | (34%) |
| 15–17 years | 543 | (15%) | 736 | (45%) | 1,279 | (25%) | <.0001 | 143 | (16%) | 1,422 | (23%) |
| Female Sex at Birth | 1,725 | (49%) | 784 | (47%) | 2,509 | (48%) | 0.2727 | 424 | (47%) | 2933 | (48%) |
| Race/Ethnicity of Patient 5 | |||||||||||
| Asian | 468 | (13%) | 591 | (36%) | 1,059 | (20%) | <.0001 | 46 | (5%) | 1105 | (18%) |
| Black | 861 | (25%) | 489 | (30%) | 1,350 | (26%) | <.0001 | 0 | (0%) | 1,350 | (22%) |
| Hispanic | 1,659 | (47%) | 444 | (27%) | 2,103 | (41%) | <.0001 | 7 | (1%) | 2,110 | (35%) |
| Native American or Alaska Native | 107 | (3%) | 0 | (0%) | 107 | (2%) | <.0001 | 0 | (0%) | 107 | (2%) |
| Native Hawaiian or other Pacific Islander | 100 | (3%) | 47 | (3%) | 147 | (3%) | 0.9896 | 834 | (93%) | 981 | (16%) |
| Two or more races | 36 | (1%) | 9 | (1%) | 45 | (1%) | 0.0849 | 1 | (0%) | 46 | (1%) |
| White | 277 | (8%) | 65 | (4%) | 342 | (7%) | <.0001 | 0 | (0%) | 342 | (6%) |
| Nativity of Primary Parents/Guardians 6 | |||||||||||
| Both U.S.-born | 604 | (20%) | 40 | (4%) | 644 | (17%) | <.0001 | 25 | (3%) | 669 | (14%) |
| Both non-U.S.–born | 1205 | (41%) | 526 | (57%) | 1,731 | (44%) | <.0001 | 372 | (49%) | 2,103 | (45%) |
| Non-U.S.–born and U.S.-born | 284 | (10%) | 19 | (2%) | 303 | (8%) | <.0001 | 9 | (1%) | 312 | (7%) |
| U.S.-born and unknown | 280 | (9%) | 15 | (2%) | 295 | (8%) | <.0001 | 3 | (0%) | 298 | (6%) |
| Non-U.S.–born and unknown | 355 | (12%) | 224 | (24%) | 579 | (15%) | <.0001 | 137 | (18%) | 716 | (15%) |
| Both unknown | 249 | (8%) | 95 | (10%) | 344 | (9%) | 0.0654 | 208 | (28%) | 552 | (12%) |
| Patient lived outside U.S. for ≥2 months 6 | |||||||||||
| Yes | 346 | (12%) | 668 | (73%) | 1,014 | (26%) | <.0001 | 55 | (7%) | 1069 | (23%) |
| No | 2,383 | (80%) | 143 | (16%) | 2,526 | (65%) | <.0001 | 384 | (51%) | 2,910 | (63%) |
| Unknown | 248 | (8%) | 108 | (12%) | 356 | (9%) | 0.0017 | 315 | (42%) | 671 | (14%) |
| Primary Reason Evaluated for TB 7 | |||||||||||
| TB symptoms | 1220 | (35%) | 698 | (42%) | 1,918 | (37%) | 457 | (51%) | 2375 | (39%) | |
| Contact investigation | 1456 | (41%) | 159 | (10%) | 1,615 | (31%) | <.0001 | 306 | (34%) | 1,921 | (32%) |
| Abnormal chest x-ray | 550 | (16%) | 360 | (22%) | 910 | (18%) | <.0001 | 108 | (12%) | 1018 | (17%) |
| Immigration medical exam | 0 | (0%) | 208 | (13%) | 208 | (4%) | <.0001 | 6 | (1%) | 214 | (4%) |
| Incidental lab result | 152 | (4%) | 72 | (4%) | 224 | (4%) | 0.9576 | 14 | (2%) | 238 | (4%) |
| Targeted testing | 95 | (3%) | 104 | (6%) | 199 | (4%) | <.0001 | 2 | (0%) | 201 | (3%) |
| Other8 | 16 | (1%) | 22 | (1%) | 38 | (1%) | 0.0006 | 0 | (0%) | 38 | (1%) |
| TB Disease Verification Criteria 9 | |||||||||||
| Positive culture | 1266 | (36%) | 766 | (46%) | 2,032 | (39%) | <.0001 | 187 | (21%) | 2,219 | (37%) |
| Nucleic acid amplification test | 63 | (2%) | 26 | (2%) | 89 | (2%) | 0.5723 | 6 | (1%) | 95 | (2%) |
| Positive smear, absent culture | 22 | (1%) | 2 | (0%) | 24 | (0%) | 0.0137 | 6 | (1%) | 30 | (1%) |
| Clinical case definition | 1,759 | (50%) | 756 | (46%) | 2,515 | (49%) | 0.004 | 355 | (40%) | 2,870 | (47%) |
| Provider diagnosis | 410 | (12%) | 105 | (6%) | 515 | (10%) | <.0001 | 343 | (38%) | 858 | (14%) |
| Initial Chest X-ray 10 | |||||||||||
| Abnormal | 2,806 | (80%) | 1,317 | (80%) | 4,123 | (80%) | 0.82 | 804 | (90%) | 4,927 | (81%) |
| Normal | 640 | (18%) | 311 | (19%) | 951 | (18%) | 0.616 | 59 | (7%) | 1010 | (17%) |
| Not Done | 66 | (2%) | 26 | (2%) | 92 | (2%) | 0.4359 | 22 | (3%) | 114 | (2%) |
| HIV Status at diagnosis | |||||||||||
| Positive | 4 | (0%) | 14 | (1%) | 18 | (0%) | <.0001 | 1 | (0%) | 19 | (0%) |
| Negative | 2,135 | (61%) | 1319 | (80%) | 3,454 | (67%) | <.0001 | 479 | (53%) | 3,933 | (65%) |
| Unknown | 1,381 | (39%) | 322 | (20%) | 1,703 | (33%) | <.0001 | 417 | (47%) | 2,120 | (35%) |
| Site of Disease 11 | |||||||||||
| Pulmonary only | 2,406 | (68%) | 1135 | (69%) | 3,541 | (68%) | 0.879 | 679 | (76%) | 4,220 | (70%) |
| Extrapulmonary only | 772 | (22%) | 382 | (23%) | 1,154 | (22%) | 0.3563 | 167 | (19%) | 1,321 | (22%) |
| Both pulmonary and extrapulmonary | 339 | (10%) | 137 | (8%) | 476 | (9%) | 0.1157 | 51 | (6%) | 527 | (9%) |
| Drug Resistance 12 | |||||||||||
| DST Done | 1240 | (98%) | 750 | (98%) | 1,990 | (98%) | 0.7652 | 176 | (94%) | 2,166 | (98%) |
| INH resistance | 114 | (9%) | 59 | (8%) | 173 | (9%) | 0.3009 | 5 | (3%) | 178 | (8%) |
| RIF resistance | 14 | (1%) | 16 | (2%) | 30 | (2%) | 0.076 | 3 | (2%) | 33 | (2%) |
| Any first line resistance (INH, RIF, PZA, EMB) | 198 | (16%) | 102 | (14%) | 300 | (15%) | 0.1527 | 6 | (3%) | 306 | (14%) |
| MDR13 | 11 | (1%) | 14 | (2%) | 25 | (1%) | 0.0642 | 2 | (1%) | 27 | (1%) |
| XDR14 | 1 | (0%) | 0 | (0%) | 1 | (0%) | >0.999 | 0 | (0%) | 1 | (0%) |
| TB Treatment Outcome | |||||||||||
| Completed | 3,256 | (93%) | 1,544 | (93%) | 4,800 | (93%) | 0.3048 | 721 | (80%) | 5,521 | (91%) |
| Died | 14 | (0%) | 0 | (0%) | 14 | (0%) | 0.0073 | 18 | (2%) | 32 | (1%) |
| Other outcome15 | 32 | (1%) | 24 | (1%) | 56 | (1%) | 0.0794 | 19 | (2%) | 75 | (1%) |
| Missing | 218 | (6%) | 87 | (5%) | 305 | (6%) | 0.1822 | 139 | (16%) | 444 | (7%) |
Percentages may not add to 100% due to rounding
Abbreviations - DC: District of Columbia; DST: Drug susceptibility testing; EMB: Ethambutol; HIV: Human Immunodeficiency Virus; INH: Isoniazid; MDR: Multi-drug resistant TB; NTSS: National Tuberculosis Surveillance System; PZA: Pyrazinamide; RIF: Rifampicin; TB: Tuberculosis; XDR: Extensively Drug Resistant TB;
U.S. States reporting areas include 50 U.S. States, New York City, and the District of Columbia.
U.S.-Affiliated Island reporting areas include U.S. Territories: American Samoa, Guam, the Commonwealth of the Northern Mariana Islands, Puerto Rico, and the U.S. Virgin Islands; and freely associated states: Federated States of Micronesia, Republic of the Marshall Islands, and Republic of Palau).
Consistent with U.S. Census Bureau definitions, anyone who was a U.S. citizen at birth (i.e., born in the U.S. or a U.S. territory, or born abroad to at least one parent who is a U.S. citizen) were considered to be U.S.-born; all others were considered non-U.S.–born, including those born in the freely associated states, RMI, FSM and Palau; https://www.census.gov/topics/population/foreign-born/about.html#par_textimage;
For the statistical test for differences between U.S.-born and non-U.S.–born children and adolescents
Self-reported or reported by parent/guardian. Hispanic individuals may be of any race, including two or more races. Excludes 13 children missing race or ethnicity.
Among n=4,650 children aged <15 years; NTSS does not collect this information for persons aged ≥15 years.
“Situation or reason that led to the initial suspicion that the patient might have TB disease”; Excludes n=67 children with missing or unknown reason for evaluation.
Other reasons evaluated include employment/administrative testing (e.g., testing at schools), testing for healthcare workers
Disease verification criteria categories are hierarchical in the order listed. For definitions see https://www.cdc.gov/tb/programs/rvct/instructionmanual.pdf
Excludes n=21 children with missing or unknown chest x-ray results
Excludes n=4 missing site of TB disease
Among n=2,219 children with a positive culture for M. tuberculosis and drug susceptibility results
Resistant to at least isoniazid and rifampin
Resistant to at least isoniazid and rifampin plus any fluoroquinolone and at least one second-line injectable (i.e., amikacin, kanamycin, or capreomycin)
Other outcomes include adverse treatment event (n=3), lost to follow-up (n=16), refused (n=14), or other (n=42)
Diagnostic evaluations for TB symptoms prompted a plurality of TB diagnoses (37%, 1,918/5,175) in children and adolescents in U.S. States; however, diagnoses were more likely to be made following contact investigations among the U.S.-born compared with non-U.S.–born (41%; 1,220/3,520 vs. 10%; 159/1,655) (Table 1). Culture confirmation of disease among children and adolescents was uncommon (39%, 2,032/5,175) in U.S. States, but adolescents were more likely to have culture confirmation than children (69% vs. 30%, data not shown). In total, 93% (4,800/5,175) of all children and adolescents completed treatment. Thirty-two deaths occurred; 18 among children and adolescents diagnosed in the U.S.-Affiliated Islands and 14 among the U.S.-born diagnosed in U.S. States.
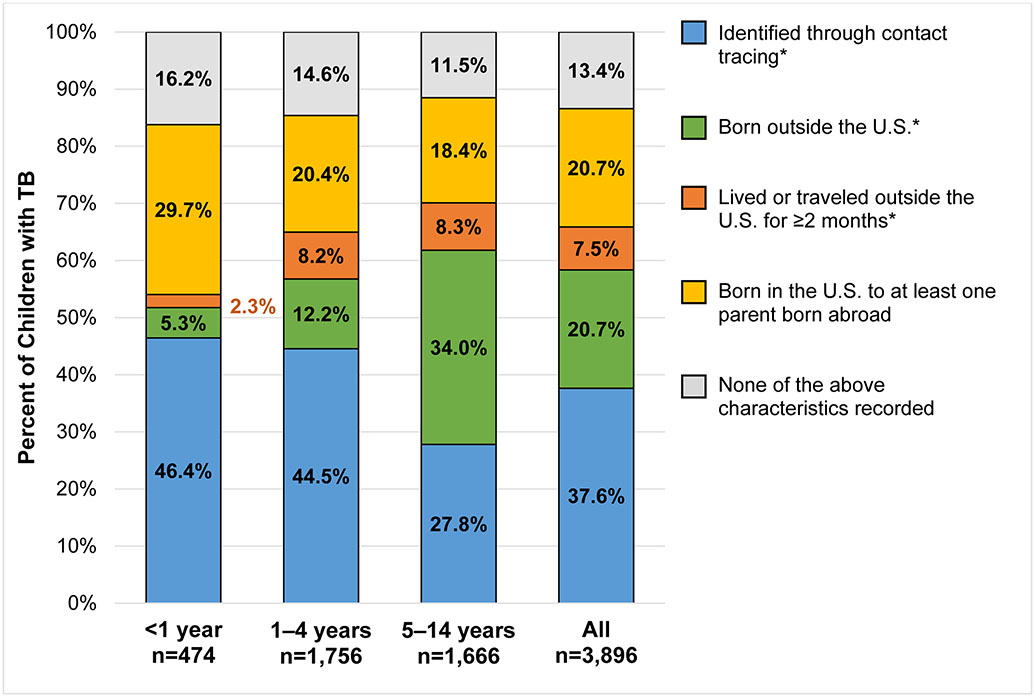
Approximately two-thirds (2,565/3,896) of children aged <15 years with TB in U.S. States from 2010–2017 would have been recommended for TB testing under current targeted testing guidelines – 38% (1,465/3,896) were identified through contact tracing, and 21% (807/3,896) and 8% (294/3,896) were born or traveled outside the U.S. for ≥2 months, respectively (Figure 1). An additional 21% (806/3,896) did not meet guidelines for testing but did have at least one parent born abroad. The remaining 13% (524/3,896) had none of these characteristics reported.
Figure 1. Characteristics of children aged <15 years with tuberculosis (TB), reported in U.S. States and District of Columbia, 2010–2017.
Categories are hierarchical in the order listed in the legend above (i.e., category ‘lived or traveled outside the U.S. for ≥ 2 months’ does not include children and adolescents identified through contact tracing or those born outside the U.S.). Starred (*) characteristics are currently covered under U.S. targeted testing guidelines. The category, “none of the above characteristics recorded” includes n=136 (3.5%) children for whom nativity of both parents was unknown or missing, and n=119 (3.1%) children who had one U.S.–born parent and one parent of unknown or missing nativity status.
TB incidence among children and adolescents, 2007–2017
During 2007–2017, 8,030 TB cases were reported among an estimated 73.6 million children and adolescents living in U.S. States (Table 2). Overall, TB incidence among children and adolescents was 1.0 per 100,000 person-years (PY). Overall incidence rates were highest in children aged ≤1 year (2.2 per 100,000 PY), lowest among children aged 7–12 years (0.5 per 100,000 PY) and intermediate among adolescents aged 15–17 years (1.4 per 100,000 PY) (Supplemental Figure 1A). Although age-specific incidence rates were at least 10 times higher among non-U.S.–born children and adolescents compared with U.S.-born children, age-related trends in rates were consistent (Supplemental Figure 1B-1C).
Table 2.
Incidence rates of tuberculosis (TB) among children and adolescents aged <18 years in U.S. States and D.C., 2007–2017
| Overall (2007–2017) | 2007 | 2017 | % Change (2017 v. 2007) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pop. aged <18 years1 (2012) |
n TB | Rate2 | (95% CI) | Rate Ratio3 |
(95% CI) | Rate | (95% CI) | Rate | (95% CI) | % Change4 |
(95% CI) | |
| Total aged < 18 years 5 | 73,632,197 | 8,030 | 1.0 | (1.0, 1.0) | - | 1.4 | (1.3, 1.5) | 0.8 | (0.7, 0.8) | −47.8% | (−51.4%, −44.1%) | |
| Age Category (years) | ||||||||||||
| <1 | 3,675,358 | 798 | 1.9 | (1.8, 2.0) | 3.4 | (3.2, 3.7) | 2.8 | (2.3, 3.4) | 1.4 | (1.1, 1.9) | −57.9% | (−66.3%, −47.3%) |
| 1–4 | 16,136,463 | 2,797 | 1.6 | (1.5, 1.6) | 2.8 | (2.7, 3.0) | 2.1 | (1.9, 2.4) | 1.1 | (0.9, 1.3) | −52.9% | (−58.2%, −46.9%) |
| 5–14 | 41,339,646 | 2,508 | 0.6 | (0.5, 0.6) | ref | 0.8 | (0.7, 0.9) | 0.5 | (0.4, 0.6) | −44.2% | (−50.8%, −36.8%) | |
| 15–17 | 12,480,730 | 1,927 | 1.4 | (1.3, 1.4) | 2.5 | (2.3, 2.6) | 1.8 | (1.6, 2.1) | 1.2 | (1.0, 1.4) | −33.8% | (−42.5%, −23.7%) |
| Sex at Birth | ||||||||||||
| Female | 35,961,951 | 3,905 | 1.0 | (1.0, 1.0) | ref | 1.4 | (1.3, 1.6) | 0.8 | (0.7, 0.9) | −47.7% | (−52.7%, −42.1%) | |
| Male | 37,670,246 | 4,125 | 1.0 | (1.0, 1.0) | 1.0 | (1.0, 1.1) | 1.3 | (1.2, 1.4) | 0.8 | (0.7, 0.9) | −48.0% | (−52.8%, −42.7%) |
| Race/Ethnicity 6 | ||||||||||||
| Asian | 3,255,938 | 1,555 | 4.4 | (4.2, 4.6) | 34.8 | (31.6, 38.4) | 5.4 | (4.7, 6.4) | 1.3 | (1.1, 1.5) | −44.7% | (−52.8%, −35.2%) |
| Black | 10,137,456 | 2,145 | 1.9 | (1.8, 2.0) | 15.2 | (13.9, 16.8) | 2.9 | (2.6, 3.3) | 1.4 | (1.2, 1.7) | −52.4% | (−58.5%, −45.4%) |
| Hispanic | 17,544,826 | 3,380 | 1.8 | (1.7, 1.8) | 14.1 | (12.9, 15.4) | 2.8 | (2.6, 3.1) | 1.3 | (1.1, 1.5) | −62.1% | (−66.1%, −57.8%) |
| Native American/Native Alaskan | 568,812 | 133 | 2.2 | (1.8, 2.6) | 17.1 | (14.2, 20.7) | 1.6 | (0.8, 3.1) | 2.7 | (1.6, 4.5) | 39.8% | (−18.8%, 140.4%) |
| Native Hawaiian/Pacific Islander | 127,473 | 191 | 14.4 | (12.5, 16.6) | 114.0 | (96.6, 134.4) | 16.4 | (10.2, 26.3) | 24.8 | (17.4, 35.3) | 50.3% | (−5.3%, 138.5%) |
| Two or more races | 3,030,887 | 56 | 0.2 | (0.1, 0.2) | 1.3 | (1.0, 1.8) | 0.2 | (0.1, 0.5) | 0.1 | (0.1, 0.3) | 22.4% | (−47.6%, 185.7%) |
| White | 38,765,384 | 543 | 0.1 | (0.1, 0.1) | ref | 0.2 | (0.2, 0.2) | 0.1 | (0.0, 0.1) | −55.8% | (−66.3%, −41.9%) | |
| Nativity 7 | ||||||||||||
| U.S.-born | 71,061,556 | 5,424 | 0.7 | (0.7, 0.7) | ref | 0.9 | (0.9, 1.0) | 0.6 | (0.5, 0.6) | −47.9% | (−52.2%, −43.3%) | |
| Non-U.S.–born | 2,570,641 | 2,606 | 9.0 | (8.6, 9.3) | 12.9 | (12.3, 13.5) | 12.1 | (10.9, 13.4) | 7.0 | (6.1, 8.1) | −39.3% | (−46.2%, −31.4%) |
| Nativity & World Area of Birth 8 | ||||||||||||
| U.S.-born | ||||||||||||
| U.S. State | 70,332,800 | 5,380 | 0.7 | (0.7, 0.7) | ref | 0.9 | (0.9, 1) | 0.6 | (0.5, 0.6) | −48.3% | (−52.6%, −43.7%) | |
| U.S. Island Area, Oceania9 | 188,416 | 24 | 1.1 | (0.7, 1.6) | 1.6 | (1.1, 2.4) | 3.2 | (1.5, 7.2) | 1.3 | (0.4, 4.0) | −33.9% | (−81.2%, 132.0%) |
| Non-U.S.–born | ||||||||||||
| Latin America | 1,322,256 | 893 | 6.0 | 8.6 | (8, 9.2) | 8.6 | (7.3, 10.1) | 5.5 | (4.3, 7) | −36.2% | (−48.3%, −21.2%) | |
| Asia | 781,065 | 938 | 10.8 | (10.1, 11.5) | 15.5 | (14.5, 16.6) | 13.9 | (11.4, 16.9) | 6.8 | (5.3, 8.8) | −51.2% | (−60.2%, −40.2%) |
| Europe | 227,336 | 51 | 1.9 | (1.4, 2.5) | 2.7 | (2.0, 3.5) | 4.0 | (2.3, 6.8) | 0.0 | (0.0, 0.0) | −88.7% | (−96.0%, −67.9%) |
| Africa | 178,612 | 641 | 32.3 | (29.9, 34.9) | 46.4 | (42.8, 50.4) | 59.7 | (48.7, 73.3) | 19.9 | (15.1, 26.4) | −61.0% | (−69.4%, −50.2%) |
| Oceania | 17,535 | 67 | 30.3 | (23.9, 38.6) | 43.7 | (34.3, 55.6) | 36.3 | (18.2, 72.6) | 37.8 | (18, 79.4) | −10.5% | (−57.6%, 89.3%) |
Population estimates are from the U.S. Census Bureau ACS PUMS 1-year estimates, 2007–2017; Population estimate at midpoint (2012) displayed for each demographic group.
All rates are reported per 100,000 person-years
Incidence rate ratio for relative differences between demographic groups overall (2007–2017)
8-year percent change in rate for 2017 compared to 2007;
Does not include children or adolescents with TB reported from U.S. territories or freely associated states (See Table 3).
Self-reported or reported by parent/guardian. Hispanic may be of any race, including two or more races. n=27 cases aged <18 years occurred among those with unknown race/ethnicity
Consistent with U.S. Census Bureau definition: https://www.census.gov/topics/population/foreign-born/about.html#par_textimage
A total of n=36 children and adolescents with TB not shown (n=20 U.S.-born, born abroad to parents who are U.S. citizens; n=3 non-U.S.–born children born in North America, n=13 non-U.S.–born, with unknown world area of birth)
Combined based on collapsed U.S. Census Bureau data categories; U.S. Island Areas: American Samoa, Guam, Commonwealth of the Northern Mariana Islands, US Virgin Islands, Puerto Rico, Republic of the Marshall Islands, Federated States of Micronesia, Palau; Oceania: Fiji, New Zealand, Australia, Tonga, Samoa, Oceania Not Specified, or at Sea
Incidence varied substantially between racial/ethnic groups; rates were 14.4 per 100,000 PY among Native Hawaiian and Pacific Islander children and adolescents, more than three times as high as any other racial or ethnic group and 114.0 (95% CI 96.6, 134.4) times higher than non-Hispanic white children and adolescents. Rates among all other single race/ethnicity groups were at least 14 times higher than among non-Hispanic white children and adolescents (Table 2).
Incidence was 12.9 (95% CI 12.3, 13.5) times higher among children and adolescents born outside the U.S. compared with U.S.-born; nonetheless, incidence among non-U.S.–born children varied significantly according to their birth region. The highest rates (>40 per 100,000 PY) occurred among children born in Africa or Oceania (Table 2).
TB incidence among children and adolescents in U.S. States declined 47.8% (95% CI −51.4%, −44.1%) from 1.4 per 100,000 PY in 2007 to 0.8 per 100,000 PY in 2017. While rates decreased across all age categories, the greatest declines occurred among children aged <5 years (Table 2). Rates also decreased among all racial/ethnic groups, except Native Americans/Alaska Natives, Native Hawaiian/Pacific Islanders, and children and adolescents of two or more races. The greatest decreases were observed among Hispanics (62.1%, [95% CI −66.1%, −57.8%]) followed by Blacks (52.4%, [95% CI −58.5%, −45.4%]).
While consistent decreases in incidence occurred among both U.S.-born (−47.9%, [95% CI −52.2%, −43.3%]) and non-U.S.–born (−39.3%, [95% CI −46.2%, −31.4%]) children and adolescents, the most precipitous occurred among those born in Africa (−61.0%, [95% CI (−69.4%, −50.2%]) and Europe (−88.7%, [95% CI –96.0%, −67.9%]). Smaller, but significant decreases occurred among children and adolescents born in Latin America (−36.2%, [95% CI −48.3%, −21.1%]) and Asia (−51.2%, [95% CI −60.2%, −40.2%]). There were no significant changes among those born in Oceania (−10.5%, [95% CI −57.6%, 89.3%]).
Rates were substantially higher in U.S.-Affiliated Islands than U.S. States (11.7 vs. 1.0 per 100,000 PY, respectively), but varied widely by reporting jurisdiction (Table 3, Supplemental Figure 2). Incidence in RMI and FSM exceeded 150 per 100,000 PY, while rates in PR and AS were lower than in the U.S. States (<1.0 per 100,000 PY). Despite small populations, FSM (n=526) and RMI (n=469) ranked third and sixth in absolute number of TB cases reported among children and adolescents, respectively, whereas the U.S. States with the greatest numbers of cases were California (n=1,483), Texas (n=1,227), New York (n=516), and Florida (n=490). TB incidence rates among children and adolescents in U.S. States ranged from 0.1 per 100,000 PY in Wyoming to 3.9 per 100,000 PY in Alaska.
Table 3.
Incidence rates of tuberculosis (TB) among children and adolescents aged <18 years in U.S.-Affiliated Islands and selected U.S. States reported to the National Tuberculosis Surveillance System (NTSS), 2007–2017
| Reporting Area | Population aged <18 years (2012)1 |
n TB | Rate2 | (95% CI) |
|---|---|---|---|---|
| U.S.-Affiliated Islands | 1,022,536 | 1,246 | 11.7 | (11.1, 12.4) |
| American Samoa (AS) | 21,656 | 1 | 0.6 | (0.1, 4.3) |
| Commonwealth of Northern Mariana Islands (CNMI) | 18,034 | 23 | 16.3 | (10.8, 24.5) |
| Federated States of Micronesia (FSM) | 42,508 | 526 | 159.7 | (146.6, 173.9) |
| Guam (GU) | 55,572 | 201 | 45.3 | (39.5, 52.1) |
| Palau (PL) | 5,684 | 11 | 24.8 | (13.8, 44.9) |
| Puerto Rico (PR) | 836,637 | 15 | 0.2 | (0.1, 0.3) |
| Republic of the Marshall Islands (RMI) | 29,819 | 469 | 195.3 | (178.4, 213.8) |
| U.S. States and DC 3 | 73,632,197 | 8,030 | 1.0 | (1.0, 1.0) |
| Distribution by number of TB cases | ||||
| California (Max) | 9,229,544 | 1,483 | 1.5 | (1.4, 1.5) |
| Virginia (Q3) | 1,855,004 | 164 | 0.8 | (0.7, 0.9) |
| Alaska (Median) | 184,564 | 79 | 3.9 | (3.1, 4.8) |
| Iowa (Q1) | 721,858 | 26 | 0.3 | (0.2, 0.5) |
| Wyoming (Min) | 136,250 | 1 | 0.1 | (0, 0.5) |
| Distribution by TB incidence rate | ||||
| Alaska (Max) | 184,564 | 79 | 3.9 | (3.1, 4.8) |
| Arizona (Q3) | 1,619,974 | 207 | 1.1 | (1, 1.3) |
| Alabama (Median) | 1,125,653 | 94 | 0.8 | (0.6, 0.9) |
| Vermont (Q1) | 122,488 | 6 | 0.4 | (0.2, 1) |
| Wyoming (Min) | 136,250 | 1 | 0.1 | (0, 0.5) |
Population estimates for Puerto Rico and U.S. States come from the U.S. Census Bureau’s American Community Survey, Public Use Microsample Data 1-year estimates; Population estimates for AS, GU, NMI obtained from https://www.census.gov/newsroom/releases/archives/2010_census/press-kits/island-areas.html and FSM, RMI, PL obtained from: https://www.census.gov/data-tools/demo/idb/informationGateway.php
TB incidence rate per 100,000 person-years
Total for all 50 U.S. States and D.C. combined. Selected states shown below for comparison to U.S.-Affiliated. Islands. States were selected based on the distribution of number of reported TB cases and TB incidence rates among children and adolescents. Data for all reporting jurisdictions are shown in Supplemental Figure 2.
TB incidence by nativity of child and parents for children aged <15 years in U.S. States, 2010–2017
During 2010–2017, TB rates varied both by birthplace of the child and that of their parents. While incidence was highest among children born outside the U.S. (6.5 per 100,000 PY), rates among U.S.-born children with at least one parent born outside the U.S. were substantially higher than among those without (1.7 per 100,000 PY vs. 0.3 per 100,000 PY, respectively) (Table 4, Figure 2). U.S.-born children with two parents born outside the U.S. had higher incidence (2.4 per 100,000 PY) than those with only one parent born outside the U.S. (1.0 per 100,000 PY); these rates were 8.5 (95% CI 7.7, 9.3) and 3.5 (95% CI 3.0, 4.0) times higher than among children with two U.S.-born parents (Table 4).
Table 4.
Incidence rates of tuberculosis (TB) by nativity of child and nativity of parents for children with TB aged <15 years in U.S. States and DC, 2010–20171
| Nativity of Child |
Nativity of Parents2 | n TB | Population aged<15 years3 |
TB Incidence Rate (95% CI)4 |
Rate Ratio (95% CI) |
|---|---|---|---|---|---|
| Non-U.S.–Born | All nativities | 919 | 1,765,819 | 6.5 (6.1, 6.9) | 23.1 (20.9, 25.6) |
| U.S.-born | Both non-U.S.–born | 1,205 | 6,310,790 | 2.4 (2.3, 2.5) | 8.5 (7.7, 9.3) |
| U.S.-born | One U.S.-born, one non-U.S.–born | 284 | 3,629,607 | 1.0 (0.9, 1.1) | 3.5 (3, 4) |
| U.S.-born | Both U.S.-born | 604 | 26,819,512 | 0.3 (0.3, 0.3) | ref. |
Variable was introduced in 2009 and only collected for persons with TB aged <15 years; Data are shown for children aged <15 years from 2010-2017 when variable was collected regularly; Does not include children with TB reported from U.S.-Affiliated Islands
Only children with known nativity for two parents/guardians are shown. Children with at least one unknown or missing nativity for parent/guardian not shown to prevent misclassification between NTSS numerators and U.S. Census Bureau population estimates
Annualized (average) population estimate
TB incidence rate per 100,000 person-years
Figure 2. Number of tuberculosis (TB) cases and TB rates by country of birth among non-U.S.–born children aged <15 years and by parental country of birth among U.S.-born children with at least one non-U.S.–born parent in U.S. States and District of Columbia, 2010–2017.
Countries are shown by descending case count (bars) within U.S. Census Bureau world regions (Latin America, Asia, Africa, and Oceania); All countries with at least 25 children who were non-U.S.–born or had non-U.S.–born parents from that country (total bar height) are shown. For U.S.-born children with at least one non-U.S.–born (non-USB) parent, includes children who have two non-U.S.–born parents, children with one non-U.S.- and one U.S.-born parent, and children with one non-U.S.–born parent and one parent with unknown nativity. For the n=100 children with two non-U.S.–born parents from different countries, children are counted twice for each country of birth for their parents (e.g., for a child with one parent born in El Salvador and one parent born in Ecuador, the child will appear in the calculations and totals for both El Salvador and Ecuador)
Abbreviations: USB=U.S.-born;
Among children born outside the U.S., TB burden varied by country of birth (Figure 2). While children born in Mexico (n=99), Ethiopia (n=78), Philippines (n=61), Myanmar (n=60), and Haiti (n=42) accounted for the largest absolute number of TB cases, incidence rates were highest among children born in RMI (149.4 per 100,000 PY), Somalia (139.4 per 100,000 PY), Myanmar (79.0 per 100,000 PY), Malaysia (66.0 per 100,000 PY), and Ethiopia (41.2 per 100,000 PY).
Similarly, TB incidence among U.S.-born children with non-U.S.–born parents varied substantially by parents’ birth country (Figure 2). While the most common birth countries for non-U.S.–born parents of U.S.-born children were Mexico (n=832), Guatemala (n=111), Vietnam (n=85), Philippines (n=71), and India (n=70), rates were highest among children with parents from RMI (78.2 per 100,000 PY), FSM (27.5 per 100,000 PY), Myanmar (11.9 per 100,000 PY), and Somalia (11.9 per 100,000 PY), for whom rates exceeded those among non-U.S.–born children.
Discussion
Although overall TB incidence among children and adolescents during 2007–2017 was low at 1.0 case per 100,000 PY and TB rates in these age groups decreased substantially (48%) during this period, substantial heterogeneity in burden exists. During this examination of national surveillance data, we observed disproportionately high rates of TB among children and adolescents of all non-white racial/ethnic groups, children and adolescents living in U.S.-Affiliated Islands, and children born in or with parents from TB-endemic countries. In addition, we observed disproportionately high mortality among children and adolescents with TB in U.S.-Affiliated Islands compared to U.S. States. These wide-ranging and pervasive disparities likely reflect structural inequality that gives rise to disproportionate exposure, vulnerability to infection and disease, and unequal access to prompt diagnosis and treatment. Our findings suggest that U.S. TB care and prevention strategies are succeeding in reducing overall TB burden among children and adolescents, but that more attention and possibly new approaches are needed to address the stark disparities in TB incidence and mortality in these age groups.
TB in children and adolescents is preventable and curable. In the context of historically low TB incidence in the U.S. and limited sensitivity and specificity of available diagnostic tests for TB infection and disease, epidemiologic information may be useful in ascertaining risk to inform TB testing decisions (14). In addition to those with compatible signs or symptoms and those with medical conditions that convey higher risk for progression to TB, current clinical practice guidelines recommend TB testing for contacts of persons with TB, as well as children and adolescents immigrating from, or who have traveled to, TB-endemic countries (14). Our findings are consistent with previous studies in the U.S. and Canada, and reinforce the importance of contact with a person with TB disease and birth or travel outside the U.S. as potential markers of increased TB risk and indicators for targeted testing (15,20,29). Thirty-one percent of children and adolescents with TB in U.S. States were diagnosed through contact tracing; 32% were non-U.S.–born, for whom rates of TB were roughly 13 times higher than among U.S.-born children and adolescents; and more than a quarter (26%) of children with TB lived outside the U.S. for two or more months. Taken together, targeted testing characteristics accounted for two-thirds (66%) of children with TB in U.S. States. Nevertheless, one-third of TB in children occurred outside of these groups, highlighting the opportunity to improve TB care and prevention efforts through consideration of additional characteristics, such as parental place of birth.
In our analysis, 21% of children with TB in U.S. States had at least one non-U.S.–born parent, but were not known contacts of persons with TB, and had neither been born nor lived outside the U.S. For children aged <1 year, this figure was 30%. We observed increasing TB rates with increasing numbers of non-U.S.–born parents for children with non-U.S.–born parents; rates were 8.5 and 3.5 times higher among children with two and one non-U.S.–born parent, respectively, compared to children with two U.S.-born parents. These findings are consistent with previous evidence among young children where TB rates among U.S.-born children with at least one non-U.S.–born parent were six times higher than those with two U.S.-born parents (17).
Importantly, TB burden in children varied widely by their country of birth and that of their parents. Rates in U.S.-born children with parents from RMI, FSM, Somalia, and Myanmar exceeded the overall rate in non-U.S.–born children. In contrast, children born in and with parents from several countries, including Mexico and Guatemala, contributed a large number of cases but had relatively low rates of TB. Variation in TB burden by country of birth of the children and their parents underscores the importance of local context, as the epidemiology, demography, and determinants of health may vary widely between settings. In addition to approaches already recommended to reduce TB burden, including overseas screening of U.S.-bound immigrants and current guidelines for targeted testing for LTBI, clinicians and public health professionals may find these data helpful to identify and reach children at highest risk for TB and optimize the predictive value of TB testing in the context of the populations they serve. The California Department of Public Health and California TB Controllers Association, for example, have developed a Pediatric TB Risk Assessment Tool and User Guide to help California providers identify asymptomatic children and adolescents who may benefit from TB testing (30). In addition to characteristics covered under current targeted testing guidelines, the guide also notes that TB testing can be considered in children and adolescents “with frequent exposure to adults at high risk of TB infection,” which, in some contexts, might include consideration of parental birthplace or extensive parental travel to areas with high TB rates. The user guide also encourages local TB control programs and clinics to further customize the pediatric TB risk assessment tool according to local recommendations. Our findings support the role of local epidemiology in devising TB risk assessments for children and adolescents.
We observed pervasive racial/ethnic and geographic disparities in TB incidence and mortality among children and adolescents. Every racial/ethnic group examined had significantly increased TB incidence compared to non-Hispanic white children and adolescents, suggesting potentially broad influences of structural and social determinants of health that drive increased risk of TB exposure, infection, or disease progression among historically marginalized children and adolescents. Despite small population sizes, Native Hawaiians/Pacific Islanders living in U.S. States and persons in U.S.-Affiliated Islands arguably bear the most disproportionate burden of TB among children and adolescents. In U.S. States, TB rates among Native Hawaiian/Pacific Islander children and adolescents were more than 100 times higher than their non-Hispanic white counterparts. Additionally, incidence among children living in the U.S. with parents from RMI and FSM were higher than any other country. In RMI and FSM, TB incidence rates among children and adolescents were at epidemic levels, exceeding 150 per 100,000 PY. Children and adolescents living in RMI and FSM accounted for 11% of TB disease and more than half of all TB deaths during 2010–2017, despite representing <0.1% of the estimated population.
While we highlight heterogeneity in TB burden by race/ethnicity, geography, and place of birth, we note that this heterogeneity is likely attributable to underlying social, policy, and environmental conditions. We lack data in our analysis to determine causes of the stark disparities in TB burden among children and adolescents. However, TB occurrence is widely recognized to be inextricably linked with social deprivation and living conditions. Previous work suggests that coarse, area-level socioeconomic status explained more than half of racial disparities in adult TB, and a 1994 study of children in New York showed TB rates were closely tied to neighborhood crowding (31,32). In addition, research on a diverse array of health outcomes has identified additional factors that contribute to population-level health disparities (33,12,31,34-36). These factors may include food security and nutrition, access to economic and material resources, residential segregation, exposure to secondhand smoke, indoor and outdoor air quality, lasting effects of historical trauma (e.g., slavery, colonization, displacement, etc.), and healthcare policy, access, and infrastructure (e.g., insurance coverage, availability of providers, lack of translation services, etc.) (9,35,37-40). Additionally, physiologic responses to acute and chronic stress attributable to poverty, racism, stigma, and other forms of social trauma and deprivation can impact immune function and may shape population distribution of disease (33,40-44). In addition to efforts to prevent and treat TB among children and adolescents at highest risk, efforts to elucidate and ameliorate the underlying social and structural drivers of increased risk should be explored.
This analysis has limitations. Variables capturing parental nativity and international travel were introduced in 2009 only for children aged <15 years, limiting our ability to draw conclusions about trends for these variables. Second, information about other potential TB exposures, such as international travel or TB contacts who are not parents are not systematically captured. NTSS does not collect information on health insurance, household crowding, nutrition, or individual or household socioeconomic indicators such as income or education. Consequently, we could not examine whether observed disparities might be explained by these factors. Finally, for approximately 31% of children, nativity of one or both parents is unknown. Therefore, incidence rates based on parental nativity likely underestimate rates among children with at least one non-U.S.–born parent.
Conclusions
Although overall TB rates among children and adolescents are low and steadily declining, substantial heterogeneity exists in burden and trends across geographies and sociodemographic groups. Given limited sensitivity and specificity of TB diagnostics in children and current strategies for targeted TB testing in low-incidence areas, variations in TB epidemiology among children and adolescents could be used to optimize the predictive value of TB testing by prioritizing groups with the highest rates. Furthermore, strategies to eliminate TB in the U.S. should account for the contextual factors that markedly increase TB risk in particular populations, so that disparities in TB-associated morbidity and mortality among children and adolescents do not persist or worsen, even as overall TB burden declines.
Supplementary Material
Research in context.
Evidence before this study
While tuberculosis (TB) rates in the United States (U.S.) are the lowest ever recorded, TB continues to affect many communities unequally, especially communities of color, indigenous persons, persons born outside the U.S., and persons experiencing homelessness. Among children and adolescents, previous national estimates suggest that although TB incidence rates among persons born outside of the U.S. are 10–20 times higher compared to those who are U.S.-born, more than two-thirds of children and adolescents with TB are U.S.-born. Therefore, it is important to identify and address determinants other than origin of birth in these groups. Additionally, national rates of TB among U.S.-born children stratified by parental country of birth and rates among children and adolescents in the U.S. territories and freely associated states have not been systematically reported. Finally, national estimates of TB disease counts and incidence rates among children and adolescents were last reported in 2010 and 2007, respectively.
Added value of this study
Using data from the National Tuberculosis Surveillance System and U.S. Census Bureau, this study summarizes the epidemiology and clinical characteristics of TB disease occurring in children and adolescents in the U.S. during 2007–2017, including overall burden and trends in TB incidence rates by sociodemographic groups, country of birth for both children and their parents, and reporting jurisdiction, including U.S. territories and freely associated states. Overall TB incidence among children and adolescents was low (1.0 case per 100,000 PY) and decreased substantially (−48%) during 2007–2017, however, substantial heterogeneity exists in burden and trends across geographies and sociodemographic groups. We observed disproportionately high rates of TB among children and adolescents of all non-white racial/ethnic groups, children and adolescents living in U.S. territories and freely associated states, and children born in or with parents from TB-endemic countries. Finally, compared to U.S. States, we observed disproportionately high mortality among children and adolescents with TB in the freely associated states of the Republic of the Marshall Islands and Federated States of Micronesia.
Implications of all the available evidence
Although overall TB incidence among children and adolescents in the U.S. is low and steadily declining, additional attention and possibly new approaches are needed to address the stark disparities in TB incidence and mortality among these groups. Given limited sensitivity and specificity of TB diagnostics in children and current strategies for targeted TB testing in low-incidence areas, variations in TB epidemiology among children and adolescents could be used to optimize the predictive value of TB testing by prioritizing groups with the highest rates. Furthermore, strategies to eliminate TB in the U.S. should account for the contextual factors that markedly increase TB risk in particular populations, so that disparities in TB-associated morbidity and mortality among children and adolescents do not persist or worsen, even as overall TB burden declines.
Acknowledgements.
The authors would like to acknowledge Bob Pratt, Adam Langer, Andrew Hill for their assistance with accessing and interpreting the data.
Role of the funding source.
Authors were salaried by the CDC and did not receive specific funding for this study. The data in this study are collected and managed by the CDC, however, authors were responsible for study design, analysis, interpretation and writing, of this report.
Disclaimer.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Abbreviations.
- ACS
American Community Survey
- CDC
U.S. Centers for Disease Control and Prevention
- CI
95% Confidence Interval
- DC
District of Columbia
- FSM
Federated States of Micronesia
- IPUMS
Integrated public-use microsample data
- MDR TB
Multi-drug resistant tuberculosis
- NTSS
National Tuberculosis Surveillance System
- PR
Puerto Rico
- PRCS
Puerto Rico Community Survey
- PUMS
Public-use microsample data
- PY
Person-years
- RMI
Republic of the Marshall Islands
- RR
Rate Ratio
- RVCT
Report of Verified Case of Tuberculosis
- TB
Tuberculosis
- USVI
United States Virgin Islands
- XDR TB
Extensively drug resistant tuberculosis
Footnotes
Declaration of interest. The authors have no conflicts of interest relevant to this article to disclose.
Financial Disclosure Statement. The authors have indicated they have no financial relationships relevant to this article to disclose.
References
- 1.World Health Organization. Global tuberculosis report 2018. 2018. [Google Scholar]
- 2.Seddon JA, Jenkins HE, Liu L, Cohen T, Black RE, Vos T, et al. Counting children with tuberculosis: why numbers matter. The International Journal of Tuberculosis and Lung Disease. 2015;19(12):9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seddon J a. Epidemiology and disease burden of tuberculosis in children:a global perspective. Infection and Drug Resistance. 2014;(Dovepress; ):153–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Loeffler AM. Pediatric Tuberculosis. Vol. 18, Seminars in Respiratory Infections. 2003. p. 272–91. [DOI] [PubMed] [Google Scholar]
- 5.Marais BJ, Schaaf HS. Tuberculosis in children. Cold Spring Harbor Perspectives in Medicine. 2014;4(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perez-Velez CM, Marais BJ. Tuberculosis in Children. New England Journal of Medicine. 2012;367(4):348–61. [DOI] [PubMed] [Google Scholar]
- 7.Lewinsohn DM, Leonard MK, Lobue PA, Cohn DL, Daley CL, Desmond E, et al. Official American thoracic society/Infectious diseases society of America/Centers for disease control and prevention clinical practice guidelines: diagnosis of tuberculosis in adults and children. Clinical Infectious Diseases. 2017;64(2):111–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Snow KJ, Nelson LJ, Sismanidis C, Sawyer SM, Graham SM. Incidence and prevalence of bacteriologically confirmed pulmonary tuberculosis among adolescents and young adults: a systematic review. Epidemiology and Infection. 2018. Jun 15;146(08):946–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bloss E, Holtz TH, Jereb J, Redd JT, Podewils LJ, Cheek JE, et al. Tuberculosis in indigenous peoples in the U.S., 2003-2008. Public Health Reports. 2011;126(5):677–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khan AD, Magee E, Grant G. Tuberculosis - United States, 1993-2010. MMWR Surveill Summ. 2013;62 Suppl 3:149–54. [PubMed] [Google Scholar]
- 11.Cain KP, Benoit SR, Winston CA, Mac Kenzie WR. Tuberculosis among foreign-born persons in the United States. JAMA - Journal of the American Medical Association. 2008;300(4):405–12. [DOI] [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention. Establishing a Holistic Framework to Reduce Inequities in HIV , Viral Hepatitis , STDs , and Tuberculosis in the United States. Center for Disease Control and Prevention. 2010;32. [Google Scholar]
- 13.National Center for HIV/AIDS,Viral Hepatitis, STD and TP, Centers for Disease Control and Prevention (CDC). Reported tuberculosis in the United States - 2016. 2016. [Google Scholar]
- 14.Kimberlin DW, Brady MT, Jackson MA, Long SS Eds, editor. Tuberculosis. In: Red Book: 2018 Report of the Committee on Infectious Diseases. American Academy of Pediatrics; 2018. p. 829–53. [Google Scholar]
- 15.Menzies HJ, Winston CA, Holtz TH, Cain KP, Mac Kenzie WR. Epidemiology of tuberculosis among US- And foreign-born children and adolescents in the United States, 1994-2007. American Journal of Public Health. 2010;100(9):1724–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Winston CA, Menzies HJ. Pediatric and Adolescent Tuberculosis in the United States, 2008-2010. Pediatrics. 2012;130(6):e1425–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pang J, Teeter LD, Katz DJ, Davidow AL, Miranda W, Wall K, et al. Epidemiology of Tuberculosis in Young Children in the United States. Pediatrics. 2014;133(3):e494–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rothfeldt LL, Patil N, Haselow DT, Williams SH, Wheeler JG, Mukasa LN. Notes from the Field : Cluster of Tuberculosis Cases Among Marshallese Persons Residing in Arkansas — 2014–2015. MMWR Morbidity and Mortality Weekly Report. 2016. Aug 26;65(33):882–3. [DOI] [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention (CDC). Two simultaneous outbreaks of multidrug-resistant tuberculosis--Federated States of Micronesia, 2007-2009. MMWR Morbidity and mortality weekly report. 2009. Mar 20;58(10):253–6. [PubMed] [Google Scholar]
- 20.Winston CA, Menzies HJ. Pediatric and Adolescent Tuberculosis in the United States, 2008-2010. Pediatrics. 2012;130(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Report of Verified Case of Tuberculosis (RVCT) Manual. 2009.
- 22.99th Congress. Compact of Free Association - Republic of Palau [Internet]. Sect. [H.J. Res. 626], PUBLIC LAW 99-658 Nov, 1986. Available from: https://www.doi.gov/oia/islands/palau
- 23.99th Congress. Compact of Free Association Act of 1985 (COFA). https://www.doi.gov/oia/about/compact; 1985.
- 24.U.S. Census Bureau. About the Foreign-Born Population [Internet]. Available from: https://www.census.gov/topics/population/foreign-born/about.html
- 25.U.S. Census Bureau. American Community Survey - Public Use Microdata Sample (PUMS) Documentation [Internet]. 2018. Available from: https://www.census.gov/programs-surveys/acs/technical-documentation/pums.html
- 26.Ruggles Steven, Genadek Katie, Goeken Ronald, Grover Josiah and MS. Integrated Public Use Microdata Series (IPUMS): Version 7.0 [dataset] [Internet]. Minneapolis: University of Minnesota, 2017. 2017. Available from: 10.18128/D010.V7.0 [DOI] [Google Scholar]
- 27.U.S. Census Bureau. Press Kit: 2010 Census Island Areas [Internet]. Available from: https://www.census.gov/newsroom/releases/archives/2010_census/press-kits/island-areas.html
- 28.U.S. Census Bureau. International Database [Internet]. Available from: https://www.census.gov/programs-surveys/international-programs/about/idb.html
- 29.Dhawan V, Bown J, Lau A, Langlois-Klassen D, Kunimoto D, Bhargava R, et al. Towards the elimination of paediatric tuberculosis in high-income, immigrant-receiving countries: a 25-year conventional and molecular epidemiological case study. ERJ Open Research. 2018. Apr 1;4(2):00131–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.California Department of Public Health. California Pediatric TB Risk Assessment and User Guide [Internet]. Available from: https://www.cdph.ca.gov/Programs/CID/DCDC/CDPHDocumentLibrary/TBCB-CA-Pediatric-TB-Risk-Assessment.pdf
- 31.Cantwell MF, McKenna MT, McCray E, Onorato IM. Tuberculosis and race/ethnicity in the United States: impact of socioeconomic status. American journal of respiratory and critical care medicine. 1998;157(4 Pt 1):1016–20. [DOI] [PubMed] [Google Scholar]
- 32.Drucker E, Alcabes P, Sckell B, Alcabes P, Bosworth W. Childhood tuberculosis in the Bronx, New York. The Lancet. 1994;343(8911):1482–5. [DOI] [PubMed] [Google Scholar]
- 33.Williams DR, Lawrence JA, Davis BA. Racism and Health: Evidence and Needed Research. Annual Review of Public Health. 2019;40(1):null. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.CSDH. Closing the gap in a generation. Health Equity Through Action on the Social Determinants of Health. Final Report of the Commission on Social Determinants of Health. 2008. [DOI] [PubMed] [Google Scholar]
- 35.Hargreaves JR, Boccia D, Evans CA, Adato M, Petticrew M, Porter JDH. The social determinants of tuberculosis: from evidence to action. American journal of public health. 2011;101(4):654–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lienhardt C From exposure to disease: the role of environmental factors in susceptibility to and development of tuberculosis. Epidemiologic reviews. 2001;23(2):288–301. [DOI] [PubMed] [Google Scholar]
- 37.Lönnroth K, Jaramillo E, Williams BG, Dye C, Raviglione M. Drivers of tuberculosis epidemics: The role of risk factors and social determinants. Social Science and Medicine. 2009;68(12):2240–6. [DOI] [PubMed] [Google Scholar]
- 38.Pedrazzoli D, Boccia D, Dodd PJ, Lönnroth K, Dowdy DW, Siroka A, et al. Modelling the social and structural determinants of tuberculosis: opportunities and challenges. The International Journal of Tuberculosis and Lung Disease. 2017. Sep 1;21(9):957–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Acevedo-Garcia D Residential segregation and the epidemiology of infectious diseases. Vol. 51, Social Science and Medicine. 2000. p. 1143–61. [DOI] [PubMed] [Google Scholar]
- 40.Sotero M A Conceptual Model of Historical Trauma: Implications for Public Health Practice and Research. Journal of Health Disparities Research and Practice. 2006;1(1 (Fall)):93–108. [Google Scholar]
- 41.Asad AL, Clair M. Racialized legal status as a social determinant of health. Social Science and Medicine. 2018;199:19–28. [DOI] [PubMed] [Google Scholar]
- 42.Aiello AE, Simanek AM, Galea S. Population levels of psychological stress, herpesvirus reactivation and HIV. AIDS and Behavior. 2010;14(2):308–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hatzenbuehler ML, Phelan JC, Link BG. Stigma as a fundamental cause of population health inequalities. American Journal of Public Health. 2013;103(5):813–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Colen CG, Ramey DM, Cooksey EC, Williams DR. Racial disparities in health among nonpoor African Americans and Hispanics: The role of acute and chronic discrimination. Social Science and Medicine. 2018;199:167–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.