This systematic review and meta-analysis investigates the prevalence of diabetic retinopathy and its progression in pregnant women with preexisting type 1 or type 2 diabetes.
Key Points
Question
How many pregnant women with preexisting type 1 or type 2 diabetes are affected by diabetic retinopathy (DR) and experience progression of DR during pregnancy?
Findings
In this systematic review and meta-analysis that included 18 observational studies of pregnant women with preexisting type 1 or type 2 diabetes, the prevalence of any DR and proliferative DR in early pregnancy was 52.3% and 6.1%, respectively. Meanwhile, DR progression rate ranged from 6.3% to 37.0%.
Meaning
Pregnant women with preexisting type 1 or type 2 diabetes had a higher rate of any DR and proliferative DR and a relatively higher DR progression rate than nonpregnant women; therefore, close follow-up should be maintained during pregnancy to prevent vision loss in this growing population.
Abstract
Importance
Diabetic retinopathy (DR) may be worsened by pregnancy in pregnant women with preexisting type 1 diabetes (T1D) or type 2 diabetes (T2D). Conflicting findings from previous studies have resulted in inconsistencies in guidelines regarding DR management in pregnancy. Global estimates of DR prevalence and progression in pregnancy are therefore required to provide clearer information about the overall true burden of DR in this population.
Objective
To estimate the prevalence of DR and its progression rate in pregnant women with preexisting T1D or T2D diagnosed before pregnancy.
Data Sources
For this systematic review and meta-analysis, conducted from November 27, 2018, to June 29, 2021, a systematic literature search was conducted in MEDLINE/Ovid, Embase/Ovid, and Scopus databases to identify English-language articles that were published from inception through October 2020.
Study Selection
Observational studies that reported on DR and its changes in pregnant women with preexisting T1D and T2D.
Data Extraction and Synthesis
Two independent reviewers extracted relevant data from each included study. Data were pooled using a random-effects model with the Freeman-Tukey double arcsine transformation. This study followed the Meta-analysis of Observational Studies in Epidemiology (MOOSE) reporting guidelines.
Main Outcomes and Measures
Prevalence of any DR, proliferative DR (PDR), and DR progression rates.
Results
A total of 18 observational studies involving 1464 pregnant women with T1D and 262 pregnant women with T2D were included in the analysis. The pooled prevalence of any DR and PDR in early pregnancy was 52.3 (95% CI, 41.9-62.6) and 6.1 (95% CI, 3.1-9.8) per 100 pregnancies, respectively. The pooled progression rate per 100 pregnancies for new DR development was 15.0 (95% CI, 9.9-20.8), worsened nonproliferative DR was 31.0 (95% CI, 23.2-39.2), progression from nonproliferative DR to PDR was 6.3 (95% CI, 3.3-10.0), and worsened PDR was 37.0 (95% CI, 21.2-54.0). DR progression rates per 100 pregnancies were similar between the T1D and T2D groups, except for the development of new DR (T1D groups: 15.8; 95% CI, 10.5-21.9; T2D groups: 9.0; 95% CI, 4.9-14.8). A global trend toward a lower DR progression rate was observed after the 1989 St Vincent Declaration.
Conclusions and Relevance
Results of this systematic review and meta-analysis suggest that women with T1D and T2D had a similar risk of DR progression during pregnancy. Despite improvements in the management of diabetes and diabetes during pregnancy, DR prevalence and progression in pregnant women with diabetes remains higher than the nonpregnant population with diabetes, highlighting the need to improve DR management in pregnancy.
Introduction
Diabetic retinopathy (DR) is projected to develop in approximately one-third of all people with diabetes during their lifetime, and it is the leading cause of irreversible blindness in those of reproductive age.1 A recent population-based meta-analysis reported that women were at greater risk of developing blindness due to DR than men in all age groups, and this risk increased if these women became pregnant.2 Although the changes that occurred during pregnancy were mostly temporary, any progression to sight-threatening disease was not reversible, and the risk of this progression persisted for up to 12 months post partum.3,4
In 1989, the St Vincent Declaration (SVD) established an optimized standard of care for pregnant women with diabetes (ie, gestational and preexisting type 1 diabetes [T1D] or type 2 diabetes [T2D]) to achieve similar pregnancy outcomes as women without diabetes.5 These outcomes included reducing the rate of maternal complications, such as diabetes-related blindness in pregnant women with preexisting diabetes and preeclampsia, and reducing the rate of adverse fetal and neonatal complications, such as congenital malformations, perinatal mortality, and preterm delivery. Despite this, it is difficult to determine the true disease burden of DR in pregnant women with preexisting T1D or T2D owing to the very wide range of reported DR prevalence (8%-63%6,7,8) and progression rates (5%-41.5%9,10) in this group. These disparate findings have resulted in a worldwide lack of consensus for guidelines regarding the management of DR in pregnancy.
More precise estimates of DR prevalence and its progression rate in pregnant women with preexisting T1D or T2D from high-quality studies are needed to inform the development of robust management guidelines on DR in pregnancy. Therefore, we collected evidence from available observational studies to determine DR prevalence as well as its rate of progression in pregnant women with preexisting T1D or T2D.
Methods
This systematic review and meta-analysis, conducted from November 27, 2018, to June 29, 2021, was registered on the PROSPERO database and was performed in accordance with Meta-analysis of Observational Studies in Epidemiology (MOOSE) reporting guidelines.11,12 A systematic search was performed by 2 independent reviewers (F.W., R.K.) to retrieve English-language articles from MEDLINE/Ovid, Embase/Ovid, and Scopus. Any disagreement was settled by a third reviewer (L.L.L.). Keywords that were used included “pregnant” or “pregnancy” and “diabetic retinopathy” (eTable 1 in the Supplement). As there was great variability in the definition of diabetes in pregnancy, the diabetes type was not restricted in search strategies, with the final decision to include or exclude a study based on its abstract or full text. The final search was conducted on December 31, 2020. This review had 2 objectives: (1) to determine the prevalence of DR in pregnancy and (2) to determine the progression rate of DR during pregnancy. This study was approved by the University of Melbourne’s Medicine and Dentistry Human Ethics Subcommittee, which waived the requirement for patient informed consent owing to the use of aggregated deidentified patient data.
Inclusion and Exclusion Criteria
Any observational study, whether prospective or retrospective, that investigated and reported the number of pregnant women with preexisting diabetes (either T1D or T2D or a mixture of both types that was diagnosed before pregnancy) with or without DR and evaluated as primary or nonprimary outcomes was included. Ethnicity and race data were collected where possible; however, as these data were only available in 5 studies, we were unable to report on these variables.
The final included articles were then assessed again for their relevance to each objective. Depending on the outcomes reported, some articles contributed to just one of the review objectives whereas others contributed to both. Studies in which DR was measured at any trimester up to delivery were included for a prevalence analysis. Studies that did not specifically report DR prevalence but presented count data that could be used to calculate prevalence were also included. Studies where DR prevalence could not be calculated were excluded. For a progression analysis, studies were included if DR was assessed at least twice (once at ≤22 weeks’ gestation and another assessment thereafter up to 12 weeks post partum). Studies that described the interval change in participants’ DR status (stable, progressed, or regressed), enabling the calculation of a progression rate, were included. Studies that recruited women at more than 22 weeks’ gestation and/or only performed 1 retinal examination during pregnancy were excluded.
Data Extraction and Methodology and Reporting Quality Assessment
Two reviewers (F.W., S.L.R.) assessed the literature independently and extracted the following information: year of publication, country, study design, participant characteristics, sample size, year of participants’ first retinal examination, the retinal assessment method used, how DR severity/status was graded, and the outcome data of interest. Although most studies reported their sample as the number of women, some reported their sample as the number of pregnancies. Therefore, this review calculated the outcome estimates as the rate per pregnancy. For studies that reported the number of included women only, it was assumed that each woman was studied only once (1 pregnancy) in their data.
For prevalence analysis, the outcomes measured were the number of pregnancies with (1) any DR and (2) proliferative DR (PDR). The number of women and the corresponding total number of pregnancies assessed (the denominator) were extracted based on gestational age at the time of examination. This review focused on the prevalence of DR at early pregnancy (≤22 weeks’ gestation) and around delivery (from 23 weeks’ gestation through to 12 weeks post partum). If a study reported data at more than 1 time point in any of these time categories, data from the earliest and latest visits during pregnancy were chosen for early pregnancy and around delivery, respectively. Meanwhile, progression analysis focused on changes that occurred between these 2 periods. Outcome measures included the number of pregnant women with (1) development of new DR (from a baseline of no DR), (2) progression by the equivalent of 1-step in DR severity as measured on the 5-step severity scale of the Early Treatment Diabetic Retinopathy Study (ETDRS; from a baseline of nonproliferative DR [NPDR]),13 (3) from no DR to PDR, (4) from any NPDR to PDR, and (5) PDR worsening marked by the development of new neovascularization or vitreous hemorrhages, reactivation of treated PDR, or requiring laser treatment.
Study methodology and reporting quality were assessed independently by 2 investigators (F.W., R.K.). Any disagreement was resolved by a third reviewer (S.L.R.). Studies were appraised for the quality of their extracted data for the purposes of each objective with respect to a list of attributes; method of appraisal was adapted from Yau et al14 (eTables 2-3 in the Supplement). Studies with well-described data and rigorous ophthalmic reports were defined by a score of 9 or more points (maximum, 13 points) for prevalence analysis and a score of 12 or more points (maximum, 19 points) for progression analysis.
Statistical Analysis
Command metaprop-one in Stata SE, version 16.1 (StataCorp) was used for all meta-analyses by which the reported binomial count data are transformed with the Freeman-Tukey double arcsine transformation. The Dersimonian-Laird random-effects model was used to calculate the pooled estimate of prevalence and progression rates with exact 95% CIs. These pooled rates were estimated using data only from those studies with similar enough methods and sufficiently high reporting quality (ie, a quality score of ≥9 points for prevalence analysis and ≥12 points for progression analysis). A subsequent pooled rate estimation was performed on only those studies with similar DR grading schemes (ie, studies that used the ETDRS grading system or its modifications, including the modified Airlie House, Wisconsin Epidemiologic Study of Diabetic Retinopathy [WESDR],15 and modified WESDR methods). Pooled estimates of rates and 95% CIs were presented in forest plots.
The presence of heterogeneity was assessed with the χ2 test and quantified via the I2 test. A significant and considerable heterogeneity was defined by an I2 value of 75% or greater coupled with a χ2 test statistic that corresponded to a 2-sided P value < .05.16,17 Subgroup analyses were performed for potential causes of heterogeneity,16 such as study region, DR grading methods, and when the study was conducted in relation to the SVD (before or after).
Results
A total of 1219 articles and 6 articles were identified from the systematic search and citation searching, respectively (eFigure 1 in the Supplement). After removing duplicates, 688 articles were screened for the title and abstract, leaving 67 articles for full-text retrieval. Of these, 39 articles were assessed for eligibility. After assessment, only 18 studies8,9,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33 had sufficiently well-reported data and rigorous ophthalmic reports (17 studies8,9,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32 for prevalence analysis and 16 studies8,9,18,19,20,21,22,23,24,25,26,27,28,29,30,33 for progression analysis) so that pooled estimates could be calculated. An overview of the included articles’ characteristics and their quality scores are shown in the Table and eTables 4 to 5 in the Supplement.
Table. Characteristics of Included Studies.
| Source, location | Study period | Subset characteristics | Sample sizea | Diabetes duration, mean (SD) [range], y | DR grading method | Fundus photography | Quality score prevalence analysisb |
Quality score progression rate analysisc |
|---|---|---|---|---|---|---|---|---|
| Arun and Taylor,18 2008, British Isles | 1989-1998 | NA | 59 | 14.4 (8) | Eurodiab | Yes | 10 | 14 |
| Axer-Siegel et al,9 1996, Israel | 1989-1994 | NA | 65 | 13.6 (6.4) [2-29] | Otherd | Yes | 12 | 16 |
| Buchbinder et al,19 2000, US | 1997-1999 | Insulin lispro group | 12 | 15.6 (8.2) | Modified Airlie House | Yes | 10 | 12 |
| Regular insulin group | 42 | 14.6 (8.2) | ||||||
| Chew et al,20 1995, US | 1983 | NA | 155 | 11.9 (7.0) | Modified Airlie House | Yes | 12 | 17 |
| Dibble et al,21 1982, US | NR | NA | 55 | 12.44 | Other | Yes | 10 | 12 |
| Klein et al,22 1990, US | 1982-1986 | NA | 133 | 11 (6.9) | Modified Airlie House | Yes | 12 | 17 |
| Laatikainen et al,23 1980, Finland | 1978-1979 | NA | 73 | (1-28) | Other | Yes | 9 | 13 |
| Lapolla et al,24 1998, Italy | NR | NA | 16 | 11.2 (7.1) | ETDRS | Yes | 11 | 16 |
| Lauszus et al,25 2003, Denmark | 1993-1997 | NA | 103 | NR | WESDR | Yes | 13 | 19 |
| Loukovaara et al,31 2003, Finland | 1998-2002 | Insulin lispro group | 36 | 16.5 (7.9) | ETDRS | Yes | 12 | NA |
| Regular insulin group | 33 | 14.7 (6.2) | ||||||
| McElvy et al,26 2001, US | 1978 | NA | 205 |
|
Modified Airlie House | Yes | 10 | 13 |
| Moloney and Drury,32 1982, British Isles | 1979-1981 | NA | 53 | 10.2 (0.8) | Other | Yes | 11 | NA |
| Phelps et al,27 1986, US | 1977-1982 | NA | 38 |
|
Modified Airlie House | Yes | 11 | 14 |
| Rahman et al,33 2007, Saudi Arabia | 1998-2002 | NA | 54 | 12.4 (6.4) [2-24] | ETDRS | No or not described | 7 | 12 |
| Rosenn et al,28 1992, US | 1978 | NA | 154 |
|
Other | Yes | 9 | 12 |
| Vestgaard et al,8 2010, Denmark | 2004-2006 | NA | 102 |
|
Modified WESDR | Yes | 10 | 14 |
| Rasmussen et al,29 2010, Denmark | 2003-2008 | NA | 160 |
|
Modified WESDR | Yes | 10 | 16 |
| Hampshire et al,30 2013, British Isles | 2006-2009 | T1D | 76 | NR | UK NSCG | Yes | 10 | 13 |
| T2D | 102 | NR |
Abbreviations: DR, diabetic retinopathy; ETDRS, Early Treatment Diabetic Retinopathy Study; NA, not applicable; NPDR, nonproliferative diabetic retinopathy; NR, not reported; NSCG, National Screening Committee guidelines; PDR, proliferative diabetic retinopathy; ST, sight-threatening; T1D, type 1 diabetes; T2D, type 2 diabetes; WESDR, Wisconsin Epidemiologic Study of Diabetic Retinopathy.
Number of pregnancies.
Quality score with respect to a prevalence analysis (maximum possible score of 13 points; detailed quality scoring presented in eTable 4 in the Supplement).
Quality score with respect to a progression rate analysis (maximum possible score of 19 points; detailed quality scoring presented in eTable 4 in the Supplement).
Other includes trained and accredited grader and diabetes physician.
These 18 studies were undertaken in 12 countries, most of which were well-resourced countries. Fifteen studies8,9,19,20,21,22,23,24,25,26,27,28,29,31,32 (83%) were a prospective cohort study design and 3 studies18,30,33 (17%) were retrospective cohort studies. A study by Hampshire et al30 observed women with either T1D or T2D but presented their results so that data could be extracted and analyzed separately for women with T1D and T2D. Two studies19,31 recruited their participants based on the type of insulin used and presented them as 2 separate groups (ie, insulin lispro group or regular insulin group) allowing these groups to be analyzed separately in our study; therefore, the total of 18 included studies provided a total of 21 subsets of women for analysis. Overall, a total of 1464 pregnant women with T1D and 262 pregnant women with T2D contributed data.
DR Prevalence in Pregnancy
Eighteen subsets (1543 pregnancies) from 16 studies8,9,18,19,20,21,22,23,24,25,26,27,28,29,30,32 fulfilled the criteria for prevalence analysis of any DR, and a similar, but not identical, set of 18 subsets (1494 pregnancies) from the same 16 studies (which differed by 2 subsets from 1 study that were not included in the first set)8,9,18,19,20,21,22,23,24,25,26,27,28,29,31,32 fulfilled criteria for PDR prevalence in pregnancy. The overall any DR prevalence per 100 pregnancies in early pregnancy was 52.3 (95% CI, 41.9-62.6; I2 = 93.95%; P < .001) and around delivery was 57.8 (95% CI, 45.0-70.2; I2 = 94.46%; P < .001) (eFigure 2 in the Supplement). For PDR, the prevalence per 100 pregnancies in early pregnancy was 6.1 (95% CI, 3.1-9.8; I2 = 83.40%; P < .001) and around delivery was 8.2 (95% CI, 4.3-12.9; I2 = 84.08%; P < .001) (eFigure 3 in the Supplement).
Pooled estimates of any DR and PDR prevalence in early pregnancy and around delivery calculated using only studies with a similar DR grading scheme are presented in eFigures 4 to 5 in the Supplement. Pooled prevalence per 100 pregnancies of any DR were 55.5 (95% CI, 38.9-71.6; I2 = 95.97%; P < .001) and 59.5 (95% CI, 36.1-80.8; I2 = 97.09%; P < .001) in early pregnancy and around delivery, respectively. Only 1 study29 of T2D pregnancies fulfilled our quality score criteria for these analyses, and discernible differences in the prevalence were found between this T2D study29 and T1D studies8,19,20,22,24,25,26,27 (early pregnancy χ2 = 148.94; around delivery χ2 = 109.55; P values <.001). The T2D study had significantly and substantially lower any DR prevalence at both time points (early pregnancy: 9.4; 95% CI, 5.8-14.9 per 100 pregnancies; late pregnancy: 17.5; 95% CI, 12.4-24.1 per 100 pregnancies). The PDR prevalence in early pregnancy in the T2D study was also much lower than the prevalence in T1D studies.
Considerable and significant heterogeneity was also observed between T1D studies (I2 = 80.60%; P < .001) in the estimation of PDR prevalence at around delivery; therefore, subgroup analyses were performed for this outcome (eTable 6 in the Supplement). The considerable heterogeneity between T1D studies in the PDR prevalence was markedly reduced (I2 values ≤12.3% in all subgroups) after accounting for the study region, study era, or DR grading methods individually. It is important to note that all 3 studies20,26,27 with eye examinations that predate the SVD were from the US region and all 3 studies24,25,31 that were performed after the SVD were in Europe; thus, the high heterogeneity between T1D studies could be ascribed to either of these factors. With respect to DR grading methods, per 100 pregnancies, the pooled prevalence in studies that used the modified Airlie House Classification (16.9; 95% CI, 13.3-20.8) was significantly higher than in studies that used ETDRS (1.3; 95% CI, 0-6.4) or WESDR (3.9; 95% CI, 1.1-9.6; P < .001), suggesting that differing DR grading methods might also play a role in the high heterogeneity within the T1D studies.
DR Progression Rate in Pregnancy
Sixteen studies8,9,18,19,20,21,22,23,24,25,26,27,28,29,30,33 fulfilled our criteria for progression analysis. Sixteen subsets (675 pregnancies) from 15 studies8,9,18,19,20,21,22,23,24,25,26,27,28,29,33 provided data for progression from no DR to any DR, 18 subsets (673 pregnancies) from 16 studies8,9,18,19,20,21,22,23,24,25,26,27,28,29,30,33 provided data for worsened NPDR by at least 1 DR level. Sixteen subsets (524 pregnancies) from 14 studies9,18,19,20,21,22,23,24,25,27,28,29,30,33 contributed data for progression from NPDR to PDR, and 12 subsets (116 pregnancies) from 11 studies8,9,19,21,22,23,25,26,27,28,33 for worsened PDR. Figures 1, 2, 3, and 4 show the overall DR progression rate per 100 pregnancies from early pregnancy to around delivery. Among the included articles, only 1 study19 presented progression from no DR to PDR (1 of 16 pregnancies with T1D in the regular insulin subset).
Figure 1. Pooled Progression Rate From None to Any Diabetic Retinopathy From Early Pregnancy to Around Delivery Using Studies With Similar Quality, by Diabetes Type.
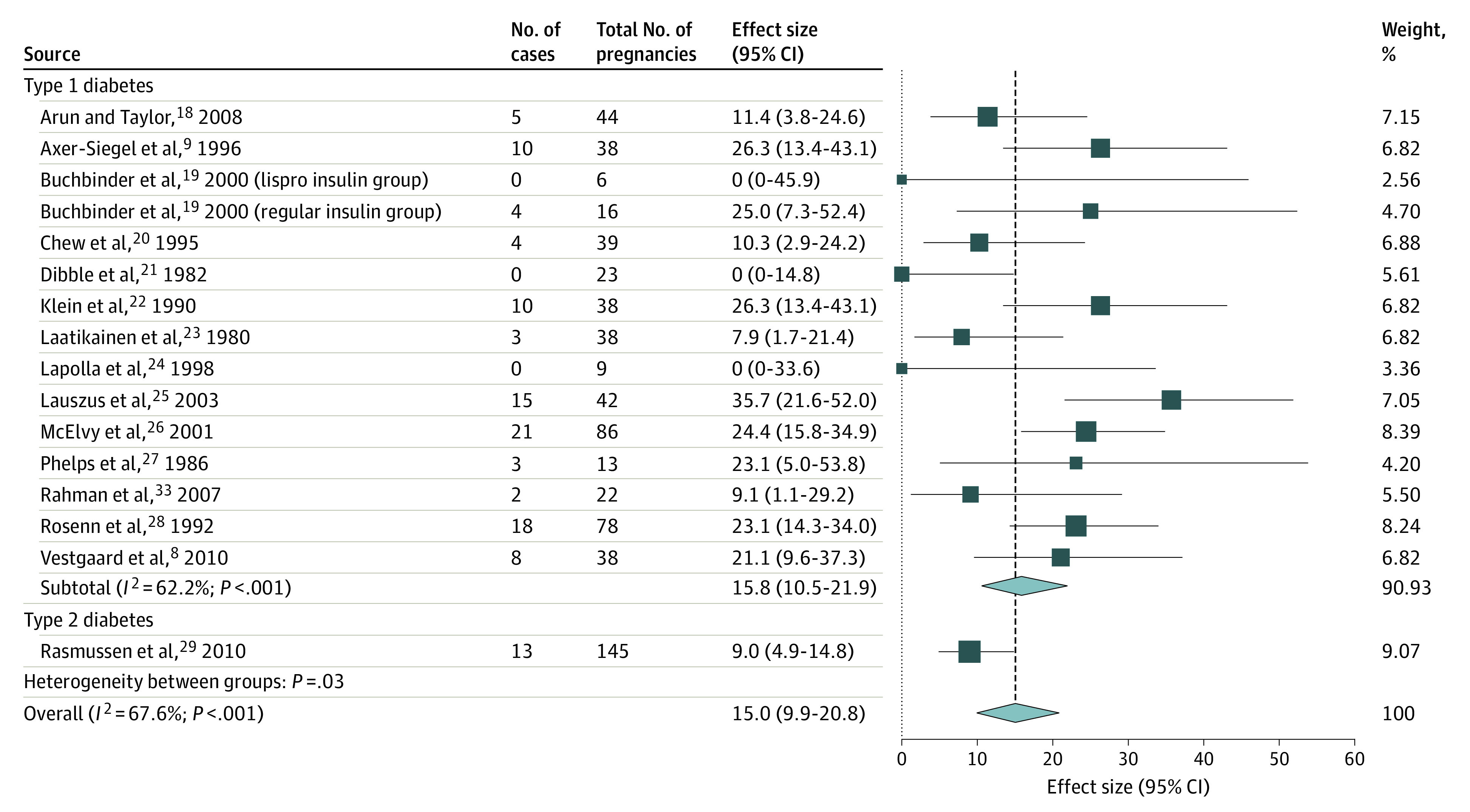
Weights are from random-effects analysis.
Figure 2. Pooled Progression Rate of Worsened Nonproliferative Diabetic Retinopathy (DR) by at Least 1 DR Level From Early Pregnancy to Around Delivery Using Studies With Similar Quality, by Diabetes Type.
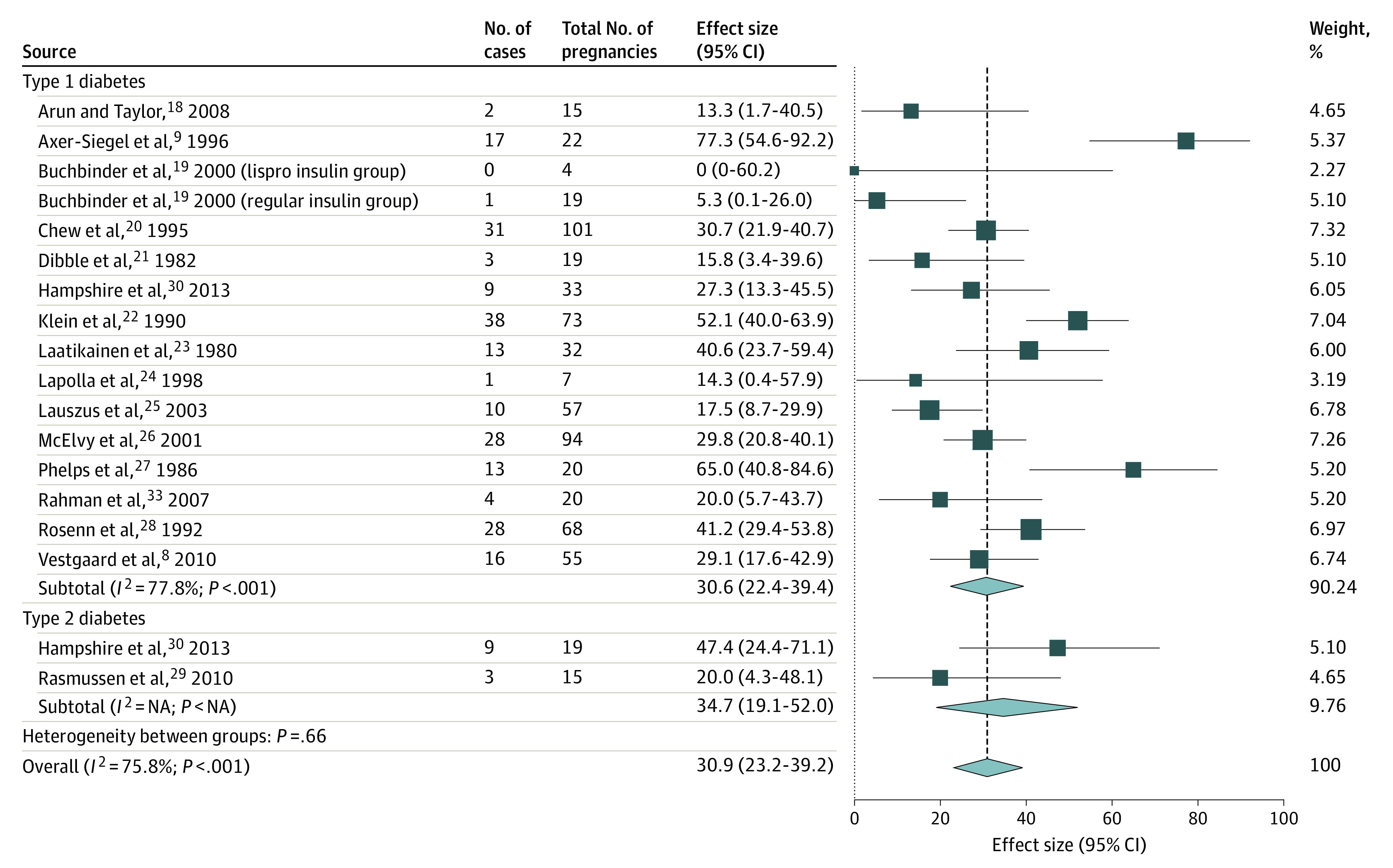
Weights are from random-effects analysis. NA indicates not applicable owing to insufficient included studies in this subgroup for this outcome.
Figure 3. Pooled Sight-Threatening Progression Rate From Nonproliferative Diabetic Retinopathy to Proliferative Diabetic Retinopathy From Early Pregnancy to Around Delivery Using Studies With Similar Quality, by Diabetes Type.

Weights are from random-effects analysis. NA indicates not applicable owing to insufficient included studies in this subgroup for this outcome.
Figure 4. Pooled Sight-Threatening Progression Rate of Worsened Proliferative Diabetic Retinopathy From Early Pregnancy to Around Delivery Using Studies With Similar Quality, by Diabetes Type.
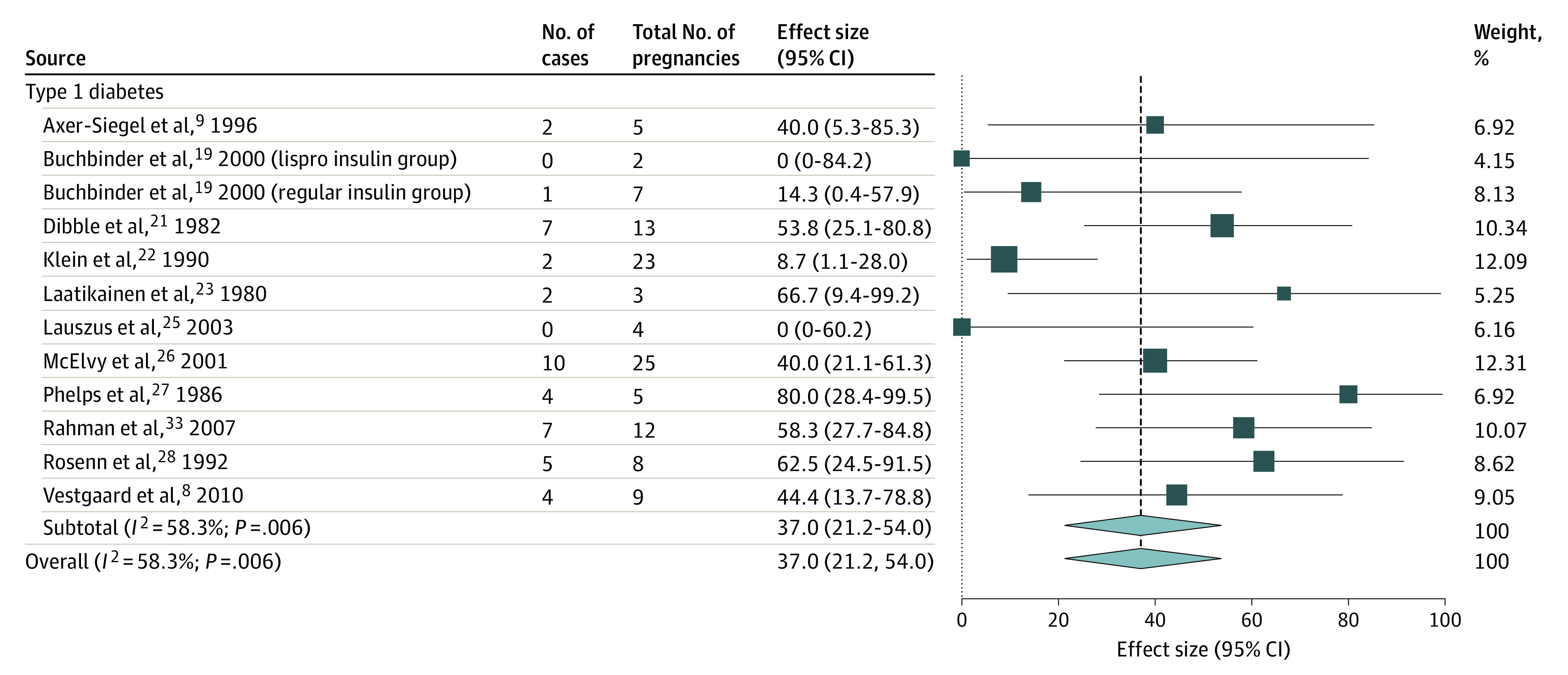
Weights are from random-effects analysis.
The pooled progression rate per 100 pregnancies for new DR development was 15.0 (95% CI, 9.9-20.8), worsened NPDR was 31.0 (95% CI, 23.2-39.2), progression from NPDR to PDR was 6.3 (95% CI, 3.3-10.0), and worsened PDR was 37.0 (95% CI, 21.2-54.0). Among the pooled progression rates, a high heterogeneity was observed in estimating the progression rate of worsened NPDR (Figure 2) that could not be explained by the difference in the diabetes type nor any other subgroup analysis. It can be seen from eTable 7 in the Supplement that although significant heterogeneity could be accounted for by the different study regions and study eras of the included studies, considerable heterogeneity was still observed within the subgroups of US studies (I2 = 80.1%; P < .001) and studies that predate the SVD (I2 = 79.9%; P < .001).
Meanwhile, significant heterogeneity between the diabetes types was only seen in the progression from none to any DR per 100 pregnancies (progression rate 15.8; 95% CI, 10.5-21.9 in the T1D groups; 9.0; 95% CI, 4.9-14.8 in the T2D groups; χ21 = 4.692; P = .03) (Figure 1), verifying the decision to pool studies of women with both diabetes types into a single pooled estimate. No T2D studies were eligible or available to provide data to measure worsening of PDR during pregnancy for comparison.
Discussion
In this systematic review and meta-analysis, which was based on data from high-quality studies from around the world, we found that the pooled prevalence of any DR during pregnancy (52.3%-57.8%) was higher than the pooled prevalence estimated in the nonpregnant diabetic population (34.6%).14 Although the pooled prevalence of PDR in early pregnancy was similar (pregnant vs nonpregnant populations: 6.1% vs 6.81%), the pooled prevalence of PDR around delivery was also higher (8.2%). Considering that pregnant women in the included studies were younger than the comparative population by approximately 20 to 30 years, our results suggest that impaired vision in this pregnant population would have an even greater societal impact. However, in our meta-analysis, considerable heterogeneity was observed (I2 values ≥83.40%; P values <.001), suggesting that there was clinical or methodological diversity among the included studies.
Analyses limited to only studies with sufficiently similar quality and DR grading schemes showed slightly higher rates but more homogeneous results, especially after categorizing these included studies by the diabetes type in the study population (except for the PDR prevalence around delivery, which continued to show heterogeneity). Unfortunately, only 1 study29 could be found with a good quality score and compatible DR grading scheme to provide the corresponding data for women with T2D. All prevalence measures estimated from this single T2D study were significantly lower than the pooled prevalence measures from the T1D studies. This aligns with findings from the nonpregnant diabetic population where prevalence of any DR, and PDR was significantly higher in people with T1D than in those with T2D.14
Our meta-analysis also estimated the rates of DR progression from early pregnancy to around delivery. Using studies with similar quality, we found that the pooled progression rate was higher in pregnant women with preexisting DR (ranging from 6.3% for progression from NPDR to PDR up to 37.0% for worsened PDR) than in those without DR in early pregnancy. Unlike in the prevalence estimations, no significant difference was found between the progression rate in T1D and T2D studies, with one notable exception: the rate for the development of new DR was substantially higher in women with T1D than in women with T2D. Taken together, these findings suggest that women with T1D have a higher risk of developing new DR during pregnancy, but that once a woman has DR, her risk of DR progression is similar in pregnancy irrespective of her diabetes type.
Our analysis showed that the high heterogeneity in our estimates was probably due to the difference in the study methodology (eg, DR grading schema) and study population characteristics (ie, diabetes type). Other factors, such as study region and study era (before vs after SVD) may have also played a role. Our results revealed that the prevalence in the US before SVD was much higher than in Europe after SVD (16.9% vs 2.3%), however, unraveling the interaction between study region and study era was not possible owing to the lack of studies with discordant combinations of these factors. However, as the SVD resulted in several improvements in the management of pregnancy with preexisting diabetes, such as the implementation of a multidisciplinary team in antenatal clinics, pregnancy outcomes have improved34,35,36,37; and thus, DR during pregnancy may also be associated with a similar improvement.
High heterogeneity was also observed in the pooled rate of progression to worsening NPDR, which could not be explained by our predefined subgroup analyses. The reported rate of worsening NPDR ranged widely from 0.0% in a subset of pregnant women using insulin lispro in US19 to 77.3% in an Israeli cohort.9 The wide variation in the reported rates may be related to other factors (eg, proportion of patients with preexisting DR) that may cause these wide variations. Owing to these clinical and methodological diversities, firm conclusions could not be made.
In this study, we used the Freeman-Tukey double arcsine transformation method to calculate the pooled estimates. This model is widely used for calculating pooled proportions and has several advantages compared with the traditional transformation models (ie, logit transformation), such as improved stabilization of variances and handling of zero event studies. However, recent reports have shown that the back transformation of pooled estimates from Freeman-Tukey double arcsine transformation measures may produce misleading results if there are widely disparate study-specific denominators.38,39 Schwarzer and colleagues39 presented an example of 5 studies ranging from 29 participants to more than 200 000 participants, where the Freeman-Tukey double arcsine transformation method produced vastly different pooled estimates. This is unlikely to affect our meta-analyses of denominators ranging between 12 to 205 participants, as shown in (eTable 8 in the Supplement), where the outcomes using both of these analytic methods were found to be almost identical.
Limitations
This study had some limitations. First, it was evident that the available data in this field is scarce, which made it difficult to assimilate the results and use them to guide clinical practice. Second, differences in the study era, study geographic region, or DR grading methods resulted in differing reported results. Third, it was also evident that most included studies were from affluent countries. This discrepancy may highlight the possibility of a higher DR burden in the global population, particularly because patients in less affluent settings have limited access to diabetes and eye care in pregnancy. This lack of access has been associated with more severe diabetes, worse pregnancy outcomes, and higher cases of diabetes-related complications than those patients in affluent settings.40
Conclusions
Results of this systematic review and meta-analysis suggest that the risk of DR progression in pregnant women with preexisting DR was similar between women with T1D and T2D. Therefore, equal attention should be given to monitoring DR during pregnancy in those already known to have DR, irrespective of diabetes type.
eTable 1. Systematic Review Search Strategy
eTable 2. Methodological and Reporting Quality Scoring
eTable 3. Determination of Score Thresholds for High-Quality Studies
eTable 4. Quality Score of Included Studies
eTable 5. Characteristics of Pregnant Women in Each Study Population
eTable 6. Pooled Prevalence of Proliferative Diabetic Retinopathy Around Delivery
eTable 7. Pooled Progression Rate of Nonproliferative Diabetic Retinopathy Worsening by at Least 1 Level
eTable 8. Comparison of Pooled Estimates Between Freeman-Tukey Double Arcsine Transformation and Random Intercept Mixed-Effects Logistic Regression Model
eFigure 1. Systematic Search and Selection of Eligible Literature
eFigure 2. Forest Plots of Prevalence of any DR Using Studies With Similar Quality, by Type of Diabetes
eFigure 3. Forest Plots of Prevalence of PDR Using Studies With Similar Quality, by Type of Diabetes
eFigure 4. Forest Plots of Prevalence of any DR Using Studies With Similar Quality and DR Grading Scheme, by Diabetes Type.
eFigure 5. Forest Plots of Prevalence of PDR Using Studies With Similar Quality and DR Grading Scheme, by Type of Diabetes
eReferences
References
- 1.Klein BE. Overview of epidemiologic studies of diabetic retinopathy. Ophthalmic Epidemiol. 2007;14(4):179-183. doi: 10.1080/09286580701396720 [DOI] [PubMed] [Google Scholar]
- 2.Flaxman SR, Bourne RRA, Resnikoff S, et al. ; Vision Loss Expert Group of the Global Burden of Disease Study . Global causes of blindness and distance vision impairment 1990-2020: a systematic review and meta-analysis. Lancet Glob Health. 2017;5(12):e1221-e1234. doi: 10.1016/S2214-109X(17)30393-5 [DOI] [PubMed] [Google Scholar]
- 3.Morrison JL, Hodgson LA, Lim LL, Al-Qureshi S. Diabetic retinopathy in pregnancy: a review. Clin Exp Ophthalmol. 2016;44(4):321-334. doi: 10.1111/ceo.12760 [DOI] [PubMed] [Google Scholar]
- 4.Diabetes Control and Complications Trial Research Group . Effect of pregnancy on microvascular complications in the diabetes control and complications trial. Diabetes Care. 2000;23(8):1084-1091. doi: 10.2337/diacare.23.8.1084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Diabetes care and research in Europe: the St Vincent Declaration. Diabet Med. 1990;7(4):360. doi: 10.1111/j.1464-5491.1990.tb01405.x [DOI] [PubMed] [Google Scholar]
- 6.Vääräsmäki M, Anttila M, Pirttiaho H, Hartikainen AL. Are recurrent pregnancies a risk in type 1 diabetes? Acta Obstet Gynecol Scand. 2002;81(12):1110-1115. [PubMed] [Google Scholar]
- 7.Makwana T, Takkar B, Venkatesh P, et al. Prevalence, progression, and outcomes of diabetic retinopathy during pregnancy in Indian scenario. Indian J Ophthalmol. 2018;66(4):541-546. doi: 10.4103/ijo.IJO_1062_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vestgaard M, Ringholm L, Laugesen CS, Rasmussen KL, Damm P, Mathiesen ER. Pregnancy-induced sight-threatening diabetic retinopathy in women with type 1 diabetes. Diabet Med. 2010;27(4):431-435. doi: 10.1111/j.1464-5491.2010.02958.x [DOI] [PubMed] [Google Scholar]
- 9.Axer-Siegel R, Hod M, Fink-Cohen S, et al. Diabetic retinopathy during pregnancy. Ophthalmology. 1996;103(11):1815-1819. doi: 10.1016/S0161-6420(96)30421-1 [DOI] [PubMed] [Google Scholar]
- 10.Temple RC, Aldridge VA, Sampson MJ, Greenwood RH, Heyburn PJ, Glenn A. Impact of pregnancy on the progression of diabetic retinopathy in type 1 diabetes. Diabet Med. 2001;18(7):573-577. doi: 10.1046/j.1464-5491.2001.00535.x [DOI] [PubMed] [Google Scholar]
- 11.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of Observational Studies in Epidemiology: a proposal for reporting. Meta-analysis of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283(15):2008-2012. doi: 10.1001/jama.283.15.2008 [DOI] [PubMed] [Google Scholar]
- 12.Widyaputri F, Rogers S, Kandasamy R, Lim L. Diabetic retinopathy in pregnant women with preexisting diabetes—systematic review and meta-analysis. Accessed March 3, 2021. https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42020119643. [DOI] [PMC free article] [PubMed]
- 13.Early Treatment Diabetic Retinopathy Study Research Group . Grading diabetic retinopathy from stereoscopic color fundus photographs—an extension of the modified Airlie House classification. ETDRS report number 10. Early Treatment Diabetic Retinopathy Study Group. Ophthalmology. 1991;98(5)(suppl):786-806. doi: 10.1016/S0161-6420(13)38012-9 [DOI] [PubMed] [Google Scholar]
- 14.Yau JW, Rogers SL, Kawasaki R, et al. ; Meta-Analysis for Eye Disease (META-EYE) Study Group . Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012;35(3):556-564. doi: 10.2337/dc11-1909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klein R, Klein BE, Magli YL, et al. An alternative method of grading diabetic retinopathy. Ophthalmology. 1986;93(9):1183-1187. doi: 10.1016/S0161-6420(86)33606-6 [DOI] [PubMed] [Google Scholar]
- 16.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557-560. doi: 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Higgins JPT, Thomas J, Chandler J, et al. , eds. Cochrane Handbook for Systematic Reviews of Interventions. 2nd ed. John Wiley & Sons; 2019. [Google Scholar]
- 18.Arun CS, Taylor R. Influence of pregnancy on long-term progression of retinopathy in patients with type 1 diabetes. Diabetologia. 2008;51(6):1041-1045. doi: 10.1007/s00125-008-0994-z [DOI] [PubMed] [Google Scholar]
- 19.Buchbinder A, Miodovnik M, McElvy S, et al. Is insulin lispro associated with the development or progression of diabetic retinopathy during pregnancy? Am J Obstet Gynecol. 2000;183(5):1162-1165. doi: 10.1067/mob.2000.108893 [DOI] [PubMed] [Google Scholar]
- 20.Chew EY, Mills JL, Metzger BE, et al. Metabolic control and progression of retinopathy. the Diabetes in Early Pregnancy study. National Institute of Child Health and Human Development Diabetes in Early Pregnancy Study. Diabetes Care. 1995;18(5):631-637. doi: 10.2337/diacare.18.5.631 [DOI] [PubMed] [Google Scholar]
- 21.Dibble CM, Kochenour NK, Worley RJ, Tyler FH, Swartz M. Effect of pregnancy on diabetic retinopathy. Obstet Gynecol. 1982;59(6):699-704. [PubMed] [Google Scholar]
- 22.Klein BE, Moss SE, Klein R. Effect of pregnancy on progression of diabetic retinopathy. Diabetes Care. 1990;13(1):34-40. doi: 10.2337/diacare.13.1.34 [DOI] [PubMed] [Google Scholar]
- 23.Laatikainen L, Larinkari J, Teramo K, Raivio KO. Occurrence and prognostic significance of retinopathy in diabetic pregnancy. Metab Pediatr Ophthalmol. 1980;4(4):191-195. [PubMed] [Google Scholar]
- 24.Lapolla A, Cardone C, Negrin P, et al. Pregnancy does not induce or worsen retinal and peripheral nerve dysfunction in insulin-dependent diabetic women. J Diabetes Complications. 1998;12(2):74-80. doi: 10.1016/S1056-8727(97)00002-0 [DOI] [PubMed] [Google Scholar]
- 25.Lauszus FF, Klebe JG, Bek T, Flyvbjerg A. Increased serum IGF-I during pregnancy is associated with progression of diabetic retinopathy. Diabetes. 2003;52(3):852-856. doi: 10.2337/diabetes.52.3.852 [DOI] [PubMed] [Google Scholar]
- 26.McElvy SS, Demarini S, Miodovnik M, Khoury JC, Rosenn B, Tsang RC. Fetal weight and progression of diabetic retinopathy. Obstet Gynecol. 2001;97(4):587-592. doi: 10.1016/S0029-7844(00)01202-3 [DOI] [PubMed] [Google Scholar]
- 27.Phelps RL, Sakol P, Metzger BE, Jampol LM, Freinkel N. Changes in diabetic retinopathy during pregnancy. correlations with regulation of hyperglycemia. Arch Ophthalmol. 1986;104(12):1806-1810. doi: 10.1001/archopht.1986.01050240080044 [DOI] [PubMed] [Google Scholar]
- 28.Rosenn B, Miodovnik M, Kranias G, et al. Progression of diabetic retinopathy in pregnancy: association with hypertension in pregnancy. Am J Obstet Gynecol. 1992;166(4):1214-1218. doi: 10.1016/S0002-9378(11)90608-5 [DOI] [PubMed] [Google Scholar]
- 29.Rasmussen KL, Laugesen CS, Ringholm L, Vestgaard M, Damm P, Mathiesen ER. Progression of diabetic retinopathy during pregnancy in women with type 2 diabetes. Diabetologia. 2010;53(6):1076-1083. doi: 10.1007/s00125-010-1697-9 [DOI] [PubMed] [Google Scholar]
- 30.Hampshire R, Wharton H, Leigh R, Wright A, Dodson P. Screening for diabetic retinopathy in pregnancy using photographic review clinics. Diabet Med. 2013;30(4):475-477. doi: 10.1111/dme.12077 [DOI] [PubMed] [Google Scholar]
- 31.Loukovaara S, Immonen I, Teramo KA, Kaaja R. Progression of retinopathy during pregnancy in type 1 diabetic women treated with insulin lispro. Diabetes Care. 2003;26(4):1193-1198. doi: 10.2337/diacare.26.4.1193 [DOI] [PubMed] [Google Scholar]
- 32.Moloney JB, Drury MI. The effect of pregnancy on the natural course of diabetic retinopathy. Am J Ophthalmol. 1982;93(6):745-756. doi: 10.1016/0002-9394(82)90471-8 [DOI] [PubMed] [Google Scholar]
- 33.Rahman W, Rahman FZ, Yassin S, Al-Suleiman SA, Rahman J. Progression of retinopathy during pregnancy in type 1 diabetes mellitus. Clin Exp Ophthalmol. 2007;35(3):231-236. doi: 10.1111/j.1442-9071.2006.01413.x [DOI] [PubMed] [Google Scholar]
- 34.Celia B, Richard H, Chineze O, Casey E, Elizabeth P, Macauley A. The St Vincent Declaration—25 years on: review of maternal deaths reported to the Confidential Enquiry in the UK and the lessons learnt. Eur J Obstet Gynecol Reprod Biol. 2016;206:e139-e140. doi: 10.1016/j.ejogrb.2016.07.354 [DOI] [Google Scholar]
- 35.Dunne FP, Avalos G, Durkan M, et al. ; ATLANTIC DIP collaborators . ATLANTIC DIP: pregnancy outcome for women with pregestational diabetes along the Irish Atlantic seaboard. Diabetes Care. 2009;32(7):1205-1206. doi: 10.2337/dc09-1118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schaefer-Graf U, Napoli A, Nolan CJ; Diabetic Pregnancy Study Group . Diabetes in pregnancy: a new decade of challenges ahead. Diabetologia. 2018;61(5):1012-1021. doi: 10.1007/s00125-018-4545-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yoeli-Ullman R, Dori-Dayan N, Mazaki-Tovi S, et al. Towards implementation of the St Vincent Declaration: outcomes of women with pregestational diabetes. Isr Med Assoc J. 2020;22(3):137-141. [PubMed] [Google Scholar]
- 38.Lin L, Xu C. Arcsine-based transformations for meta-analysis of proportions: pros, cons, and alternatives. Health Sci Rep. 2020;3(3):e178. doi: 10.1002/hsr2.178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schwarzer G, Chemaitelly H, Abu-Raddad LJ, Rücker G. Seriously misleading results using inverse of Freeman-Tukey double arcsine transformation in meta-analysis of single proportions. Res Synth Methods. 2019;10(3):476-483. doi: 10.1002/jrsm.1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goldenberg RL, McClure EM, Harrison MS, Miodovnik M. Diabetes during pregnancy in low- and middle-income countries. Am J Perinatol. 2016;33(13):1227-1235. doi: 10.1055/s-0036-1584152 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Systematic Review Search Strategy
eTable 2. Methodological and Reporting Quality Scoring
eTable 3. Determination of Score Thresholds for High-Quality Studies
eTable 4. Quality Score of Included Studies
eTable 5. Characteristics of Pregnant Women in Each Study Population
eTable 6. Pooled Prevalence of Proliferative Diabetic Retinopathy Around Delivery
eTable 7. Pooled Progression Rate of Nonproliferative Diabetic Retinopathy Worsening by at Least 1 Level
eTable 8. Comparison of Pooled Estimates Between Freeman-Tukey Double Arcsine Transformation and Random Intercept Mixed-Effects Logistic Regression Model
eFigure 1. Systematic Search and Selection of Eligible Literature
eFigure 2. Forest Plots of Prevalence of any DR Using Studies With Similar Quality, by Type of Diabetes
eFigure 3. Forest Plots of Prevalence of PDR Using Studies With Similar Quality, by Type of Diabetes
eFigure 4. Forest Plots of Prevalence of any DR Using Studies With Similar Quality and DR Grading Scheme, by Diabetes Type.
eFigure 5. Forest Plots of Prevalence of PDR Using Studies With Similar Quality and DR Grading Scheme, by Type of Diabetes
eReferences


