Abstract
BACKGROUND AND OBJECTIVES:
Rates of chlamydia and gonorrhea among adolescents continue to rise. We aimed to evaluate if a universal testing program for chlamydia and gonorrhea improved testing rates in an urban general pediatric clinic and an urban family medicine clinic within a system of federally qualified health care centers and evaluated the feasibility, cost, and logistic challenges of expanding implementation across 28 primary care clinics within a federally qualified health care centers system.
METHODS:
A universal testing quality improvement program for male and female patient 14 to 18 years old was implemented in a general pediatrics and family medicine clinic in Denver, Colorado. The intervention was evaluated by using a controlled pre-post quasi-experimental design. The difference in testing rates due to the intervention was assessed by using a difference-in-differences regression model weighted with the inverse probability of treatment.
RESULTS:
In total, 15 541 pediatric encounters and 5420 family medicine encounters were included in the analyses. In pediatrics, the unadjusted testing rates increased from 32.0% to 66.7% in the intervention group and from 20.9% to 28.9% in the comparison group. For family medicine, the rates increased from 38.5% to 49.9% in the intervention group and decreased from 26.3% to 24.8% in the comparison group. The intervention resulted in an adjusted increase in screening rates of 25.2% (P < .01) in pediatrics and 11.8% (P < .01) in family medicine. The intervention was well received and cost neutral to the clinic .
CONCLUSIONS:
Universal testing for chlamydia and gonorrhea in primary care pediatrics and family medicine is a feasible approach to improving testing rates .
Chlamydia trachomatis and Neisseria gonorrhoeae are the 2 most reported sexually transmitted infections (STIs) in the United States.1 Despite considerable prevention and screening interventions, rates of chlamydia and gonorrhea continue to rise. According to the Centers for Disease Control and Prevention, from 2014 to 2018, there was a 19% and 63% increase in chlamydia and gonorrhea infections in the United States, respectively.1 Similarly, the Denver metropolitan area reported a 24% increase in chlamydia infections and a 150% increase in gonorrhea infections from 2014 to 2018.2,3 The highest prevalence of chlamydia and gonorrhea is among adolescents and young adults.1,4 In a study in which chlamydia and gonorrhea positivity rates were evaluated among >40 000 12- to 24-year-olds in a large system of primary care clinics in the Denver metropolitan area, 15.5% of female patients and 12.3% of male patient tested positive for chlamydia.5 The greatest increases have been among the Hispanic population, followed by the Black population.3
Many chlamydia and gonorrhea infections are asymptomatic, and screening may be the only way to detect these infections.6,7 Current US Preventive Task Force recommendations include annual screening of sexually active female patients aged <25 years and men who have sex with men and selective screening of those deemed to be high risk.8 Similarly, the America Academy of Pediatrics recommends annual screening of sexually active female patients who are <25 years of age.9 Thus, choosing to screen a patient requires that the provider obtain an accurate sexual history from the patient and determine risk status. This is challenging with any population, but is specifically difficult with adolescents and is infrequently completed by providers.10 We previously found that >7% of tested patients who were documented as not sexually active by a medical provider had chlamydia or gonorrhea infections.5 Thus, the recommended approach results in undertesting, particularly for adolescents and male patients. Additionally, bias and stigma may result from providers deciding risk status, which is particularly concerning given the significant health disparities evident in STI testing and infection rates.5,11,12
Despite efforts to improve testing locally and nationally, testing rates remain low.13 Such efforts have included ensuring confidentiality for adolescents, integrated health educators, provider education, patient education, provider incentives, and improving access to reproductive health services.14,15 Of interventions designed to improve testing, universal testing or opt-out approaches have the greatest impact on testing rates.14 These programs have also been shown to be acceptable by adolescents, be cost-effective, and reduce STI-associated complications.16–18 Universal testing likely results in more equitable care, decreases stigma, and reduces provider bias.18 To date, most studies evaluating universal testing approaches have been restricted to female patients.16,19–22 These studies have largely occurred in selective clinical environments, including adolescent specialty clinics,21 STI and family planning clinics, children’s hospitals,19 detention centers, and emergency departments.23–25 Given that most adolescents and young adults receive medical care in primary care pediatric and family medicine clinics,26 understanding whether universal testing is effective in these settings, along with the implementation challenges, is essential to inform future care models.
In this quality improvement project, we aimed to evaluate whether a universal testing program for chlamydia and gonorrhea improved testing rates for male and female patients in an urban general pediatric clinic and an urban family medicine clinic within a large system of federally qualified health care centers (FQHCs) compared with comparison clinics.
METHODS
Setting
The intervention was implemented at a Denver Health and Hospital Authority (DH) pediatric clinic and a family medicine clinic from April to August 2019. DH is an integrated safety-net health care system located in Denver, Colorado, is composed of 27 FQHCs (9 multispecialty clinics and 18 school-based health centers) and a level 1 trauma center, and encompasses Denver Public Health and the regional STI clinic.27 Similar to other FQHCs, nearly three-quarters of patients have Medicaid or are self-pay.28 The intervention pediatric clinic had 14 providers, and the intervention family medicine clinic had 16 providers. The intervention clinics were high-volume clinics that had historically been successful with early adoption of innovated care models. Comparison clinics were selected to closely resemble the 2 intervention clinics in terms of clinic size and volume, complexity, and types of patients served but did not have the intervention implemented during the time period. It was noted that the intervention and comparison clinics differed significantly with respect to patient demographics. Preimplementation data were evaluated from January 1, 2018, to the intervention start (April 2019 for female patients in the pediatric clinic and August 2019 for male patients in the pediatric clinic and male and female patients in the family medicine clinic), and postintervention data were evaluated through February 1, 2020. The comparison pediatric clinic had 11 providers, and the comparison family medicine clinic had 17 providers.
Intervention
The universal testing intervention included adolescents aged 14 to 18 years old at the time of the encounter presenting for well-child care. The intervention began with female patients at the pediatric clinic in April 2019 and expanded to male patients in pediatrics and to male and female patients at the family medicine clinic in August 2019. A time line is shown in Fig 1.
FIGURE 1.
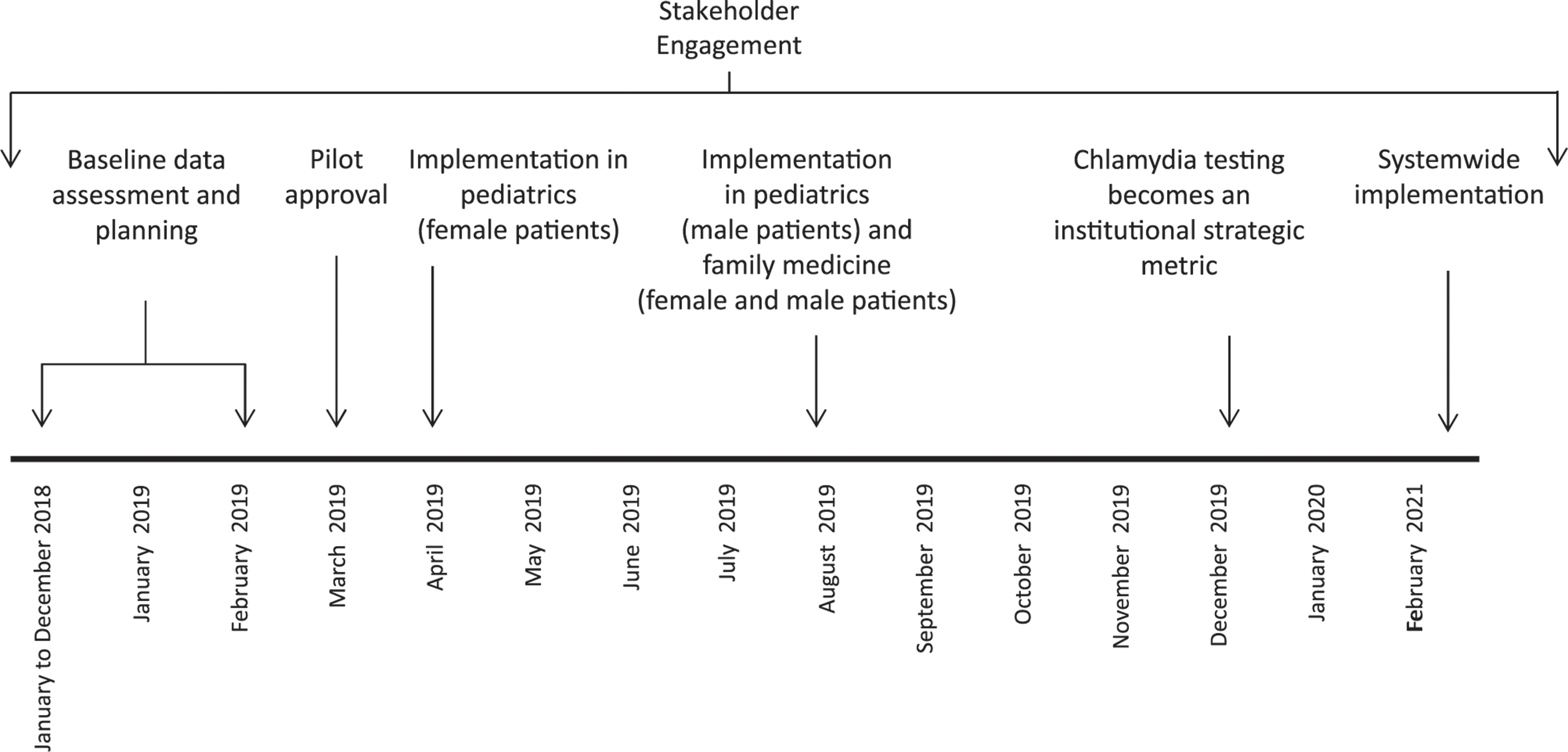
Time line of the universal gonorrhea and chlamydia intervention.
Table 1 reveals the chlamydia and gonorrhea workflow for an adolescent presenting for a well-child visit. Patients had a separate confidential encounter created in the electronic health record (EHR) (Epic, Verona, WI) during check-in and were provided an adolescent questionnaire and information sheet that described confidential care and universal testing (Supplemental Information). In the questionnaire, patients were asked about sexual activity and concerns about STIs as well as other adolescent-specific topics. The questionnaire was used as a guide to stimulate conversation between the provider, the adolescent, and the parent or guardian.
TABLE 1.
Universal Chlamydia Testing Intervention Components and Workflow for Male and Female Patients 14–18 Years of Age Presenting for Well-Child Care in Pediatrics and Family Medicine Clinics
| Tasks by Role | |
|---|---|
|
| |
| Before check-in (clerk) | 1. Confidential encounter created in addition to nonconfidential encounter in the EHR (Epic)a
2. Preregistration of patient |
| Check-in (clerk) | 1. Confidential phone No. entered into demographics in confidential encounter 2. Adolescent signs consent for treatment in confidential encounter 3. Adolescent screening questionnaire with parent cover sheet that explains confidential care and universal screening is handed to the patient and parentb |
| Rooming (medical assistant) | 1. Adolescent and parent brought back to the examination room 2. Confidential chlamydia and/or gonorrhea laboratory test ordered and signed in the confidential encounterc 3. Vital signs, hearing and vision status, and urine collected; standard scripting is used to explain urine collectionb 4. Confidential phone No. verifiedc 5. Specimen sent to the laboratory before the patient leaves the clinic |
| Visit (provider) | 1. Well-child check, including confidential portion, completed as normal 2. Integrated health educator counsels patient when needed 3. All confidential notes, laboratory tests, and medications documented in confidential encounter 4. All nonconfidential notes, laboratory tests, immunizations, and medications documented in nonconfidential encounter 5. Billing for confidential and nonconfidential visits is completed, following an institutionally approved algorithmb |
| After visit (medical assistant, nurse, health educator, or provider) | 1. Result follow-up workflow is determined by individual clinics, 2. Treatment of positive results and ordering of follow-up laboratorytests per institutional guidelinesb |
In our Epic system, confidential laboratory tests and medications cannot be ordered in a nonconfidential encounter.
Document is available to use and adapt, free of charge, with written permission of the authors.
This will be changed to a best practice alert for medical assistants as part of rooming in the near future. The best practice alert contains a field for a confidential phone No. and back-up contact.
During check-in, the medical assistant separated the adolescent from the caregiver to verify a confidential phone number, explain testing for chlamydia and gonorrhea using standard language, and collect a urine sample (Supplemental Information). Confidential contact information was documented in the confidential section of the demographics in the EHR and the standard documentation template for adolescents. The medical assistant ordered the confidential chlamydia and gonorrhea test as part of standard rooming orders and sent the specimen to the laboratory.
The provider completed the visit, including confidential portions, per normal routine. All nonconfidential portions of the well-child visit were documented in the nonconfidential encounter, whereas confidential information, laboratory tests, and medications were documented in the confidential encounter. Providers billed for visits according to an institutional billing algorithm that protects confidentiality regardless of insurance type.
Implementation Strategies
Before designing the intervention, a baseline assessment of chlamydia and gonorrhea testing and positivity rates and health disparities was completed to define the scope of the problem. Input from stakeholders, including providers, clerical staff supervisors, medical assistants, nurse program managers, administration, laboratory services, finance, billing and coding, and others, was obtained and used to adapt the program (Supplemental Table 7). Administrative approval was granted by the Directors of Service for Pediatrics and Family Medicine and the Pediatric Quality Improvement Committee.
Clinical staff meetings were held to discuss the background and definition of the problem and clinical issues, including feasibility, cost, data collection, and workflows, to set the groundwork for the importance of the intervention at each site. Clinic teams had a practice champion, the clinic team lead, who was responsible for the development and execution of the workflow at the local site and problem-solving in real time to address site-specific issues. Within the confines of the program, clinics completed their own internal plan-do-study-act cycles to work out challenges with local workflow.
Statistical Analysis
The intervention was evaluated by using a controlled pre-post quasi-experimental design. Data from January 1, 2018, to February 1, 2020, from the 2 intervention sites and 2 comparison clinics were electronically abstracted from the EHR. Abstracted data included demographics, encounters, and laboratory test results. All encounters for patients aged 14 to 18 years (inclusive) at the encounter date were included. A lower age of 14 years, rather than 15 years, was chosen because chlamydia and gonorrhea infection rates within DH begin increasing at age 14 years.
The primary outcome measure was the proportion of encounters in which the patient had chlamydia and gonorrhea testing completed at the encounter date or within the previous 365 days. Secondary outcome measures included (1) the percentage of chlamydia and gonorrhea tests that were positive for chlamydia or gonorrhea and (2) the prevalence of chlamydia and gonorrhea. Prevalence was calculated as the number of positive chlamydia or gonorrhea results per 100 patients. Only chlamydia and gonorrhea tests completed by nucleic acid amplification testing were included (Aptima Combo 2 Assay [Panther System]; Hologic, Inc, Marlborough, MA). Test results from all body sites and DH locations (including those not ordered at the intervention or comparison clinics) were included because providers were able to see these results, and we expected they would appropriately influence ordering behavior.
Separate analyses for encounters that occurred at pediatric and family medicine clinics were conducted. Patients who received care at the intervention and comparison clinics were compared by using the χ2 test for proportions and the t test for continuous variables.
The analysis was conducted in SAS (SAS Institute, Inc, Cary, NC), and the graphs were generated in R. We estimated the difference in testing rates due to the intervention using a difference-in-differences regression model for encounter-level data. We recognized that there were significant differences in the composition of our control and intervention cohorts. To balance the cohorts, we obtained the propensity score by regressing the receipt of screening on age, race, ethnicity, sex, and insurance type. We subsequently estimated the inverse probability of testing on the basis of the propensity score, which was incorporated as a weight in the difference-in-differences model. We also controlled for age at the time of testing, race (Black, white, Asian American, or other), ethnicity (Hispanic or non-Hispanic), sex (male or female), and insurance type (public, private, or uninsured). A retrospective power analysis revealed that in using our sample sizes of 15 541 and 5420 for pediatrics and family medicine clinics, respectively, we had sufficient power to detect a difference of 2% in screening rate at an α of .05 and power of 90% for both analyses for the primary outcome measure.
Internal costs to the clinic, including direct costs, personnel costs, and laboratory test costs, were evaluated every 3 months during the intervention.
The project was reviewed by the Quality Improvement Committee of DH, which is authorized by the Colorado Multiple Institutional Review Board at the University of Colorado, Denver, Colorado, and the DH Ethic Committee, Denver, Colorado, and was exempted because it was determined not to be human subjects research.
RESULTS
Characteristics of Adolescents Presenting for Care
The baseline demographic characteristics of patients who received care at the intervention and comparison clinics are presented in Table 2. Significant differences in sex, race, ethnicity, and insurance type were found between intervention and comparison clinics. Sex was defined as the sex assigned at birth because that was reliably obtained from the EHR. In the pediatric clinic, there were 10137 encounters for adolescents in the preintervention phase and 5404 in the postintervention phase. In the family medicine clinic there were 3992 encounters for adolescents in the preintervention phase and 1436 in the postintervention phase. The numbers of chlamydia and gonorrhea tests ordered by clinical site and intervention phase are shown in Table 3.
TABLE 2.
Baseline Demographics of Patients at Intervention and Comparison Clinics
| Pediatrics (n = 15 541) |
Family Medicine (n = 5420) |
|||||
|---|---|---|---|---|---|---|
| Comparison (n = 5528) | Intervention (n = 10 013) | P a | Comparison (n = 2368) | Intervention (n = 3085) | P | |
|
| ||||||
| Age, y, mean (SD) | 15.8 (1.3) | 15.8 (1.3) | .41 | 16.0 (1.4) | 16.1 (1.4) | .36 |
| Sex, n (%) | <.01 | .18 | ||||
| Female | 3071 (55.6) | 5968 (59.6) | 1415 (59.6) | 1875 (61.4) | ||
| Male | 2457 (44.5) | 4045 (40.4) | 959 (40.4) | 1179 (38.6) | ||
| Race, n (%) | <.01 | <.01 | ||||
| Asian American | 184 (3.3) | 142 (1.4) | 228 (9.6) | 18 (0.6) | ||
| Black | 555 (10.0) | 533 (5.3) | 868 (36.6) | 537 (17.6) | ||
| White | 4143 (75.0) | 6986 (69.8) | 1041 (43.9) | 1818 (59.5) | ||
| Other or unknown | 646 (11.7) | 2352 (23.5) | 237 (10.0) | 681 (22.3) | ||
| Ethnicity, n (%) | <.01 | <.01 | ||||
| Hispanic | 3456 (62.5) | 8007 (80.0) | 845 (35.6) | 2030 (66.5) | ||
| Non-Hispanic | 2072 (37.5) | 2006 (20.0) | 1529 (64.4) | 1024 (33.5) | ||
| Insurance, n (%) | <.01 | <.01 | ||||
| Commercial | 1299 (23.5) | 1037 (10.3) | 461 (19.4) | 436 (14.3) | ||
| Publicb | 3962 (71.7) | 8283 (82.7) | 1772 (74.6) | 2303 (75.4) | ||
| Uninsured | 267 (4.8) | 693 (6.9) | 141 (5.9) | 315 (10.3) | ||
Calculated by using χ2 analyses.
Includes Medicaid and sliding-scale public insurance programs.
TABLE 3.
Number of Patient Encounters by Clinic and Intervention Period: Gonorrhea and Chlamydia Tests Ordered During Encounters
| Preintervention Period |
Postintervention Period |
||||
|---|---|---|---|---|---|
| Clinic Type | Clinic | Encounters | Tests Ordered, n (%) | Encounters | Tests Ordered, n (%) |
|
| |||||
| Pediatric | Comparison | 3625 | 758 (20.9) | 1903 | 550 (28.9) |
| Intervention | 6512 | 2086 (32.0) | 3501 | 2335 (66.7) | |
| Family medicine | Comparison | 1733 | 456 (26.3) | 641 | 159 (24.8) |
| Intervention | 2259 | 869 (38.5) | 795 | 397 (49.9) | |
Chlamydia and Gonorrhea Testing Rates in Unadjusted Analyses
The unadjusted proportion of adolescents who had chlamydia and gonorrhea testing completed on the encounter date is shown in Fig 2. In pediatrics, testing rates increased from 32.0% preintervention to 66.7% post intervention in the intervention group and from 20.9% preintervention to 28.9% post intervention in the comparison group. For family medicine, testing rates increased from 38.5% preintervention to 49.9% post intervention in the intervention group and decreased slightly from 26.3% preintervention to 24.8% post intervention in the comparison group. Using an unadjusted difference-in-differences model, we estimated the magnitude of impact of the intervention to be 26.7% (P < .01) for the pediatric clinic and 13.0% (P < .01) for the family medicine clinic.
FIGURE 2.

Percentage of encounters in which patients had gonorrhea and chlamydia testing completed in the pre- and postintervention periods by month. Raw data are represented by circles, whereas the adjusted model outcomes are shown with a solid line. Vertical lines denote the beginning of the intervention. A, Pediatric testing rates by month. The figure has 2 vertical lines, one denoting when the intervention was initially implemented for only female patients at the intervention clinic and the second for when the intervention was implemented for all patients. B, Family medicine testing rates by month.
Chlamydia and Gonorrhea Testing Rates in Adjusted Analyses
Table 4 contains the estimated treatment effect attributed to the intervention in both the unadjusted and adjusted difference-in-differences regression models. When we adjusted for age at the time of visit, race, ethnicity, insurance status, and sex, we found the treatment effect to be 26.9% (P < .01) for pediatrics and 11.7% for family medicine (P < .01). After further adjusting to balance covariates by weighting using the inverse probability of treatment and controlling for the covariates, we found the treatment effect to be 25.2% for pediatrics and 11.8% for family medicine. The coefficients n the covariates can be found in Supplemental Tables 8 and 9. In both models, age, sex, insurance status, and race were found to be significant. The results of the adjusted model are overlaid with the raw monthly screening rates in Fig 2.
TABLE 4.
Mean Estimate of Intervention Effect for Difference-in-Differences Regression Model
| Pediatrics |
Family Medicine |
|||
|---|---|---|---|---|
| Model | Estimate, % | P | Estimate, % | P |
|
| ||||
| Unadjusted | 26.7 | <.01 | 13.0 | <.01 |
| Adjusted (unweighted)a | 26.9 | <.01 | 11.7 | <.01 |
| Adjusted (weighted)b | 25.2 | <.01 | 11.8 | <.01 |
Adjusted for covariates only.
Adjusted for covariates and inverse probability of treatment weighting by using propensity scores.
Chlamydia and Gonorrhea Infections
In general, we saw decreases in the percentage of positive chlamydia test results for both the family medicine and pediatric intervention clinics between the pre- and postintervention periods (Table 5). The percentage of positive gonorrhea test results increased at the pediatric intervention clinic but decreased at the family medicine intervention clinic compared with before implementation. The prevalence of chlamydia and gonorrhea increased in the family medicine intervention group between the pre and postintervention periods (Table 6), whereas the prevalence of chlamydia and gonorrhea decreased in the family medicine control groups. In pediatrics, the prevalence of gonorrhea increased in both the intervention and control groups, whereas the prevalence of chlamydia decreased in the intervention group and increased in the control group.
TABLE 5.
Number of Gonorrhea and Chlamydia Tests by Clinic and Intervention Period: Gonorrhea and Chlamydia Test Results and Percentage Positive
| Clinic Type | Clinic | Preintervention Period |
Postintervention Period |
||||
|---|---|---|---|---|---|---|---|
| Total Tests | Positive for Chlamydia, n (%) | Positive for Gonorrhea, n (%) | Total Tests | Positive for Chlamydia, n (%) | Positive for Gonorrhea, n (%) | ||
|
| |||||||
| Pediatric | Comparison | 758 | 38 (5.0) | 5 (0.7) | 550 | 24 (4.4) | 3 (0.5) |
| Intervention | 2086 | 168 (8.1) | 20 (1.0) | 2335 | 94 (4.0) | 20 (0.9) | |
| Family medicine | Comparison | 456 | 25 (5.5) | 8 (1.8) | 159 | 7 (4.4) | 1 (0.6) |
| Intervention | 869 | 70 (8.1) | 14 (1.6) | 397 | 28 (7.0) | 13 (3.3) | |
TABLE 6.
Number of Infections by Clinic and Intervention Period: Gonorrhea and Chlamydia Prevalence
| Clinic Type | Clinic | Preintervention Period |
Postintervention period |
||||
|---|---|---|---|---|---|---|---|
| Patientsa | Chlamydia Cases per 100 Patients | Gonorrhea Cases per 100 Patients | Patients | Chlamydia Cases per 100 Patients | Gonorrhea Cases per 100 Patients | ||
|
| |||||||
| Pediatric | Comparison | 4121 | 1.0 | 0.1 | 1407 | 1.4 | 0.2 |
| Intervention | 5901 | 2.7 | 0.3 | 4083 | 2.5 | 0.5 | |
| Family medicine | Comparison | 1729 | 1.5 | 0.5 | 639 | 1.1 | 0.2 |
| Intervention | 22 288 | 3.1 | 0.6 | 797 | 3.5 | 1.6 | |
Some patients had >1 encounter during the time period.
Internal financial analyses completed in August 2019 and January 2020 by using actual cost and reimbursement data indicated that the pilot intervention was cost neutral to the clinics. Numerous providers commented that they identified chlamydia or gonorrhea in patients they would not have previously tested. Providers anecdotally felt the program reduced bias and allowed for normalization of STI screening when talking to patients.
DISCUSSION
A universal chlamydia testing approach in male and female adolescents presenting for primary care improved testing rates in both pediatric and family medicine clinic settings.
Although the universal testing program improved testing rates across both clinical settings, we saw the greatest increase in the pediatric setting. Pediatric providers see a greater volume of adolescent patients and have fewer competing priorities compared with family medicine providers, which may have increased effectiveness in this setting. Testing was significantly improved for both sexes and was felt by providers to reduce stigma and bias. A key aspect of this workflow was shifting STI testing to the medical assistant standard rooming workflow rather than relying on providers to order testing and obtain specimens. The previous workflow relied on a provider first completing a sexual history to assess need for STI testing. This was a more complex and time-intensive workflow. Additionally, it resulted in youth with potentially positive results being missed because of unreliable reporting of sexual history. Universal testing created a more streamlined and equitable approach.
We saw variability in the percentage of positive test results after implementation, likely secondary to a combination of dilution effect from increased testing of asymptomatic patients, changes in demographics of patients tested, and increased community prevalence during the time frame evaluated. In future studies, researchers should evaluate the long-term effectiveness of the intervention in improving testing rates and reducing community prevalence of chlamydia and gonorrhea. Given the small number of cases detected during the intervention, we were insufficiently powered to determine differences in prevalence as a result of the intervention. Similar to Denver metropolitan area trends, the prevalence increased for all clinical sites during the evaluation period. However, prevalence trends indicate that the prevalence at intervention sites rose faster than that at comparison sites, particularly for gonorrhea. Universal testing also helped to universalize patient sexual health education, including education regarding condom use, coercion, family planning options, and expedited partner therapy.
Perhaps one of the most encouraging aspects of this work was the tremendous collaboration and enthusiasm we found for the project. Stakeholder engagement was critical for designing and implementing the intervention, particularly given the complexities of workflows for staff at different points during the patient visit. The program united staff and managers from >12 divisions at DH. Standardization of language for clerks and medical assistants, procedures for check-in and rooming, adolescent screening forms, parent education, and billing were vital for implementation. One of our greatest concerns before implementation was the potential for parent dissatisfaction with the program. To counter this concern, we created standard messaging for parents that started at check-in and extended through the entire visit (Supplemental Information). To date, this has not proven to be a challenge within our organization, and parents have been generally supportive of the program.
Financial concern was a primary barrier for initial implementation, particularly for testing within confidential encounters. To address this concern, we completed an internal financial analysis using modeling data from the previous year before the intervention and an actual financial analysis every 3 months during the intervention. Having a standard billing algorithm was important for financial feasibility. Similar to other organizations, we found the program to be cost neutral to the clinics.15–17 Although every organization will need to assess financial feasibility internally, we encourage others to complete this analysis before discounting their ability to implement a universal testing program.
Strengths of this study included the ability to implement the intervention in both family medicine and pediatric community-based settings, which are the 2 most common settings where adolescents seek care.26 Using comparison clinics permitted for pre-post analysis of effectiveness while accounting for regional changes in STI testing and infection rates. By engaging stakeholders before and during the intervention, we were able to adapt the program and secure buy-in for implementation.
This study has several important limitations. Similar to all single-center studies, the results may not be generalizable to other regions or clinical settings; however, the population served by DH is comparable to other urban FQHCs, which serve 28 million patients annually.28 Intervention and comparison site demographics varied significantly; therefore, we used propensity scores to adjust for these variations. Given the small number of cases, we were underpowered to assess changes in prevalence or to assess the additional number of cases detected with universal testing. It is important to note that DH had a previously established workflow in the EHR for confidential visits. Differences in EHR capabilities, health care system finances, or stakeholder engagement levels may limit implementation in other settings. The intervention was limited to well-adolescent visits, which could have resulted in those presenting for acute visits being missed. However, at DH, when a patient presents for an acute visit but is found to be due for a well visit, the visit is typically converted to a well visit to provide preventive and acute care. Although there was much enthusiasm for this work, this was a new process, so complete compliance was not reached.
Additionally, some adolescent patients were not able to urinate or declined testing and therefore did not receive the test. Additionally, provider-based testing at acute visits and family planning services was available. Future work on adapting universal testing to acute visits is needed. Finally, although patients could opt out of testing, the actual number who opted out could not be differentiated from those who did not complete testing for other reasons.
Considering the success of universal testing approaches in improving testing rates, we recommend that systems definitively explore this option before excluding universal testing as a possible approach. Given the success of the intervention, DH is now implementing a universal testing approach for gonorrhea and chlamydia across all 28 FQHCs for male and female patients 14 to 24 years of age. Ultimately, universal testing may be an effective method to improve testing rates, reduce STI-related stigma, improve health disparities, and help combat the evolving chlamydia and gonorrhea epidemics.
Supplementary Material
FUNDING:
Dr Frost received salary support from the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health under award K23HD099925. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The National Institutes of Health had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. Funded by the National Institutes of Health (NIH).
Dr Tomcho conceptualized and designed the study, assisted with the initial analysis, drafted the initial manuscript, and reviewed and revised the manuscript; Mr Lou conceptualized and designed the study, completed the data analysis, assisted with data interpretation, and critically reviewed and revised the manuscript; Drs O’Leary and Frost conceptualized and designed the study, assisted with data interpretation, and critically reviewed and revised the manuscript; Dr Rinehart and Ms Thomas-Gale assisted with data analysis and interpretation and critically reviewed and revised the manuscript; Dr Douglas assisted with data interpretation and critically reviewed and revised the manuscript; Dr Wu assisted with study design and critically reviewed and revised the manuscript; Drs Penny and Federico assisted with study design and data interpretation and critically reviewed and revised the manuscript; and all authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
ABBREVIATIONS
- DH
Denver Health and Hospital Authority
- EHR
electronic health record
- FQHC
federally qualified health care center
- STI
sexually transmitted infection
Footnotes
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
REFERENCES
- 1.Centers for Disease Control and Prevention. Sexually transmitted diseases surveillance 2018. 2019. Available at: https://www.cdc.gov/std/stats18/toc.htm. Accessed February 4, 2020
- 2.Colorado Department of Public Health and Environment. STI and HIV/AIDS epidemiology reports. 2018. Available at: https://www.colorado.gov/pacific/cdphe/sti-and-hivaids-epidemiology-reports. Accessed January 2, 2020
- 3.Denver Public Health. Denver’s rate of STDs continues to climb. 2019. Available at: www.denverpublichealth.org/news/2019/04/rate-of-stdsin-denver-continues-to-climb. Accessed February 1, 2020
- 4.Centers for Disease Control and Prevention. STDs in adolescents and young adults. 2019. Available at: https://www.cdc.gov/std/stats18/adolescents.htm. Accessed December 26, 2019
- 5.Douglas CM, O’Leary SC, Tomcho MM, et al. Gonorrhea and chlamydia rates among 12- to 24-year-old patients in an urban health system. Sex Transm Dis.2021;48(3):161–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Farley TA, Cohen DA, Elkins W. Asymptomatic sexually transmitted diseases: the case for screening. Prev Med. 2003;36(4):502–509 [DOI] [PubMed] [Google Scholar]
- 7.Korenromp EL, Sudaryo MK, de Vlas SJ, et al. What proportion of episodes of gonorrhoea and chlamydia becomes symptomatic? Int J STD AIDS. 2002;13(2):91–101 [DOI] [PubMed] [Google Scholar]
- 8.LeFevre ML; U.S. Preventive Services Task Force. Screening for chlamydia and gonorrhea: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;161(12):902–910 [DOI] [PubMed] [Google Scholar]
- 9.Hagan JF, Shaw JS, Duncan PM, eds. Bright Futures: Guidelines for Health Supervision of Infants, Children, and Adolescents. 4th ed. Elk Grove Village, IL: American Academy of Pediatrics; 2017 [Google Scholar]
- 10.Goyal MK, Witt R, Hayes KL, Zaoutis TE,Gerber JS. Clinician adherence to recommendations for screening of adolescents for sexual activity and sexually transmitted infection/human immunodeficiency virus. J Pediatr. 2014;165(2):343–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chambers LC, Khosropour CM, Katz DA, Dombrowski JC, Manhart LE, Golden MR. Racial/ethnic disparities in the lifetime risk of chlamydia trachomatis diagnosis and adverse reproductive health outcomes among women in King County, Washington. Clin Infect Dis. 2018; 67(4):593–599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Owusu-Edusei K Jr, Chesson HW, Leichliter JS, Kent CK, Aral SO. The association between racial disparity in income and reported sexually transmitted infections. Am J Public Health. 2013;103(5):910–916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nation Committee for Quality Assurance.Chlamydia screening in women (CHL). 2019. Available at: https://www.ncqa.org/hedis/measures/chlamydia-screening-inwomen/. Accessed May 26, 2020
- 14.Guy RJ, Ali H, Liu B, et al. Efficacy of interventions to increase the uptake of chlamydia screening in primary care: a systematic review. BMC Infect Dis. 2011;11:211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taylor MM, Frasure-Williams J, Burnett P,Park IU. Interventions to improve sexually transmitted disease screening in clinic-based settings. Sex Transm Dis. 2016; 43(2, suppl 1):S28–S41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Owusu-Edusei K Jr, Hoover KW, Gift TL. Cost-effectiveness of opt-out chlamydia testing for high-risk young women in the U.S. Am J Prev Med. 2016;51(2):216–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blake DR, Quinn TC, Gaydos CA. Should asymptomatic men be included in chlamydia screening programs? Cost-effectiveness of chlamydia screening among male and female entrants to a national job training program. Sex Transm Dis.2008;35(1):91–101 [DOI] [PubMed] [Google Scholar]
- 18.Hull S, Kelley S, Clarke JL. Sexually transmitted infections: compelling case for an improved screening strategy. Popul Health Manag. 2017;20 (suppl 1):S1–S11 [PubMed] [Google Scholar]
- 19.Elattma A, Laves E, Taber B, Karvonen KL,Herrera MC, Bakken EH. Using provider incentives and an opt-out strategy in a successful quality initiative to increase chlamydia screening. Jt Comm J Qual Patient Saf. 2020;46(6):326–334 [DOI] [PubMed] [Google Scholar]
- 20.Shafer MA, Tebb KP, Pantell RH, et al.Effect of a clinical practice improvement intervention on chlamydial screening among adolescent girls. JAMA. 2002; 288(22):2846–2852 [DOI] [PubMed] [Google Scholar]
- 21.Wood SM, McGeary A, Wilson M, et al. Effectiveness of a quality improvement intervention to improve rates of routine Chlamydia trachomatis screening in female adolescents seeking primary preventive care. J Pediatr Adolesc Gynecol. 2019;32(1):32–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DiVasta AD, Trudell EK, Francis M, et al. Practice-based quality improvement collaborative to increase chlamydia screening in young women. Pediatrics. 2016; 137(5):e20151082 [DOI] [PubMed] [Google Scholar]
- 23.Maraynes ME, Chao JH, Agoritsas K,Sinert R, Zehtabchi S. Screening for asymptomatic chlamydia and gonorrhea in adolescent males in an urban pediatric emergency department. World J Clin Pediatr. 2017;6(3):154–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller CA, Tebb KP, Williams JK, Neuhaus JM, Shafer MA. Chlamydial screening in urgent care visits: adolescent-reported acceptability associated with adolescent perception of clinician communication. Arch Pediatr Adolesc Med. 2007;161(8): 777–782 [DOI] [PubMed] [Google Scholar]
- 25.Schneider K, FitzGerald M, Byczkowski T,Reed J. Screening for asymptomatic gonorrhea and chlamydia in the pediatric emergency department. Sex Transm Dis. 2016;43(4):209–215 [DOI] [PubMed] [Google Scholar]
- 26.Irwin CE Jr, Adams SH, Park MJ,Newacheck PW. Preventive care for adolescents: few get visits and fewer get services. Pediatrics. 2009;123(4).Available at: https://pediatrics.aappublications.org/content/123/4/e565 [DOI] [PubMed] [Google Scholar]
- 27.Agency for Healthcare Research and Quality. Denver Health: how a safety net system maximizes its value. 2019. Available at: https://www.ahrq.gov/sites/default/files/wysiwyg/lhs/lhs_case_studies_denver_health.pdf. Accessed January 5, 2020
- 28.Health Resources and Services Administration. Denver health & hospital authority health center program awardee data. 2018. Available at: https://data.hrsa.gov/tools/data-reporting/program-data?grantNum=H80CS00218. Accessed December 3, 2019
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


